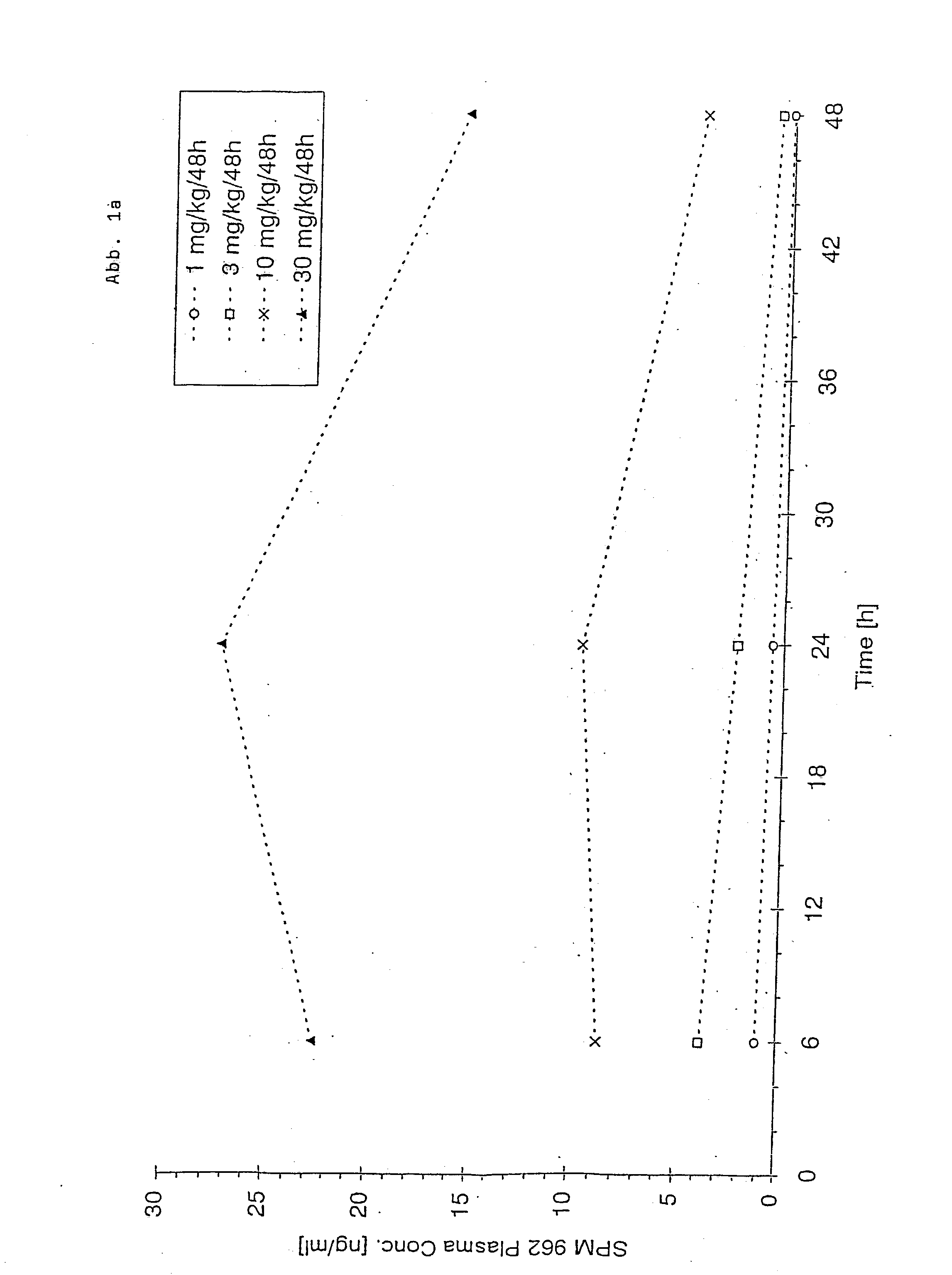
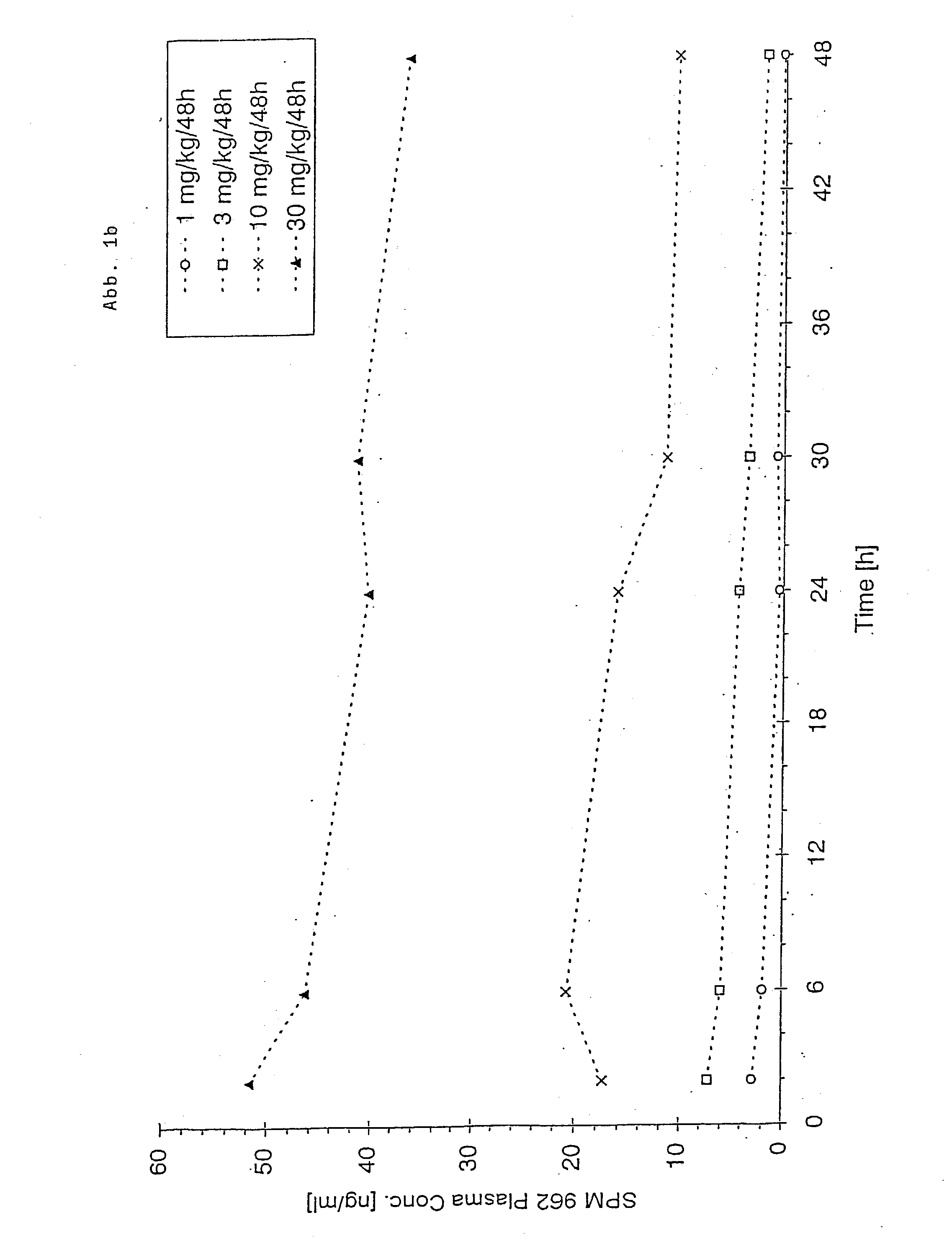
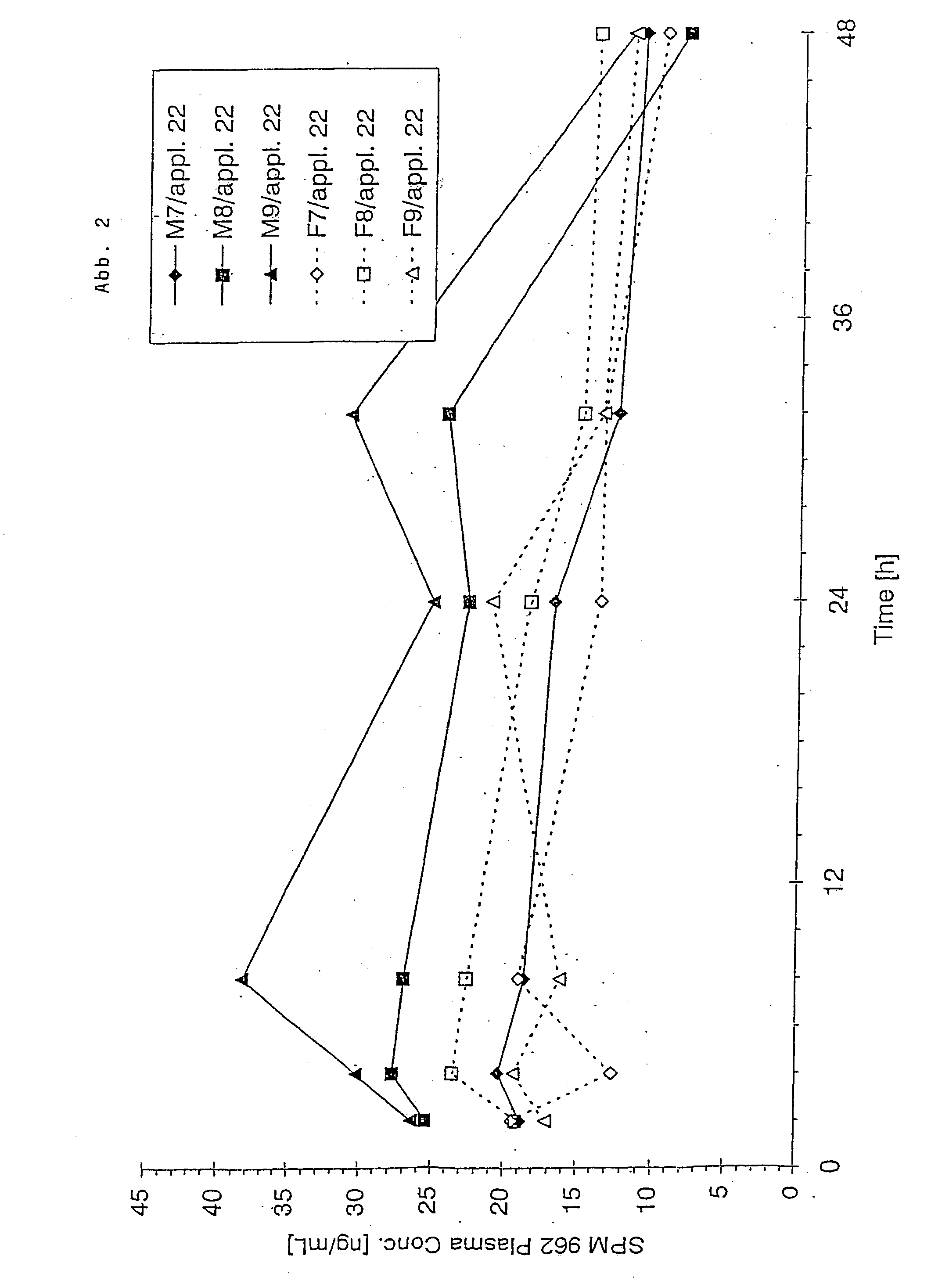
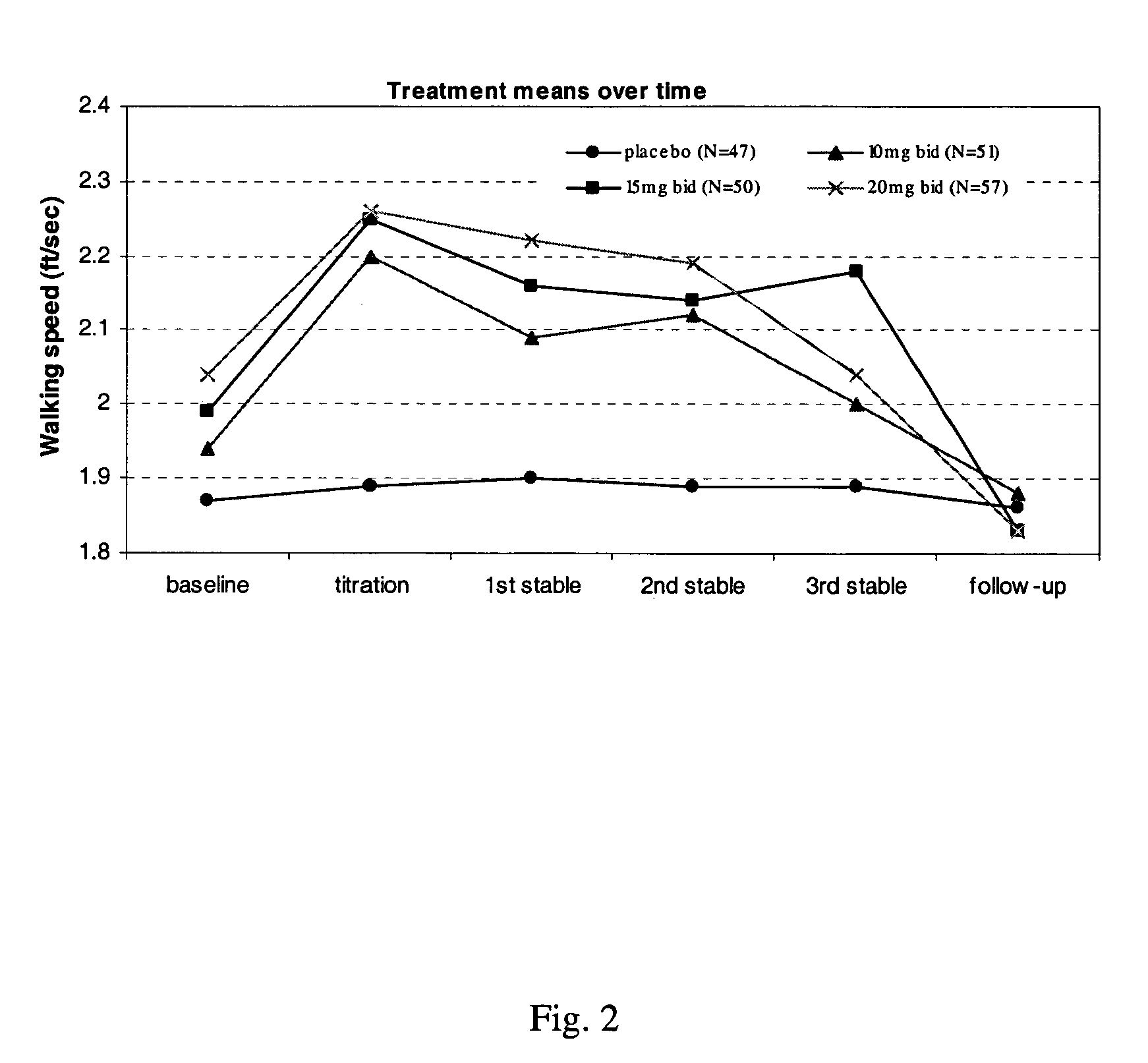
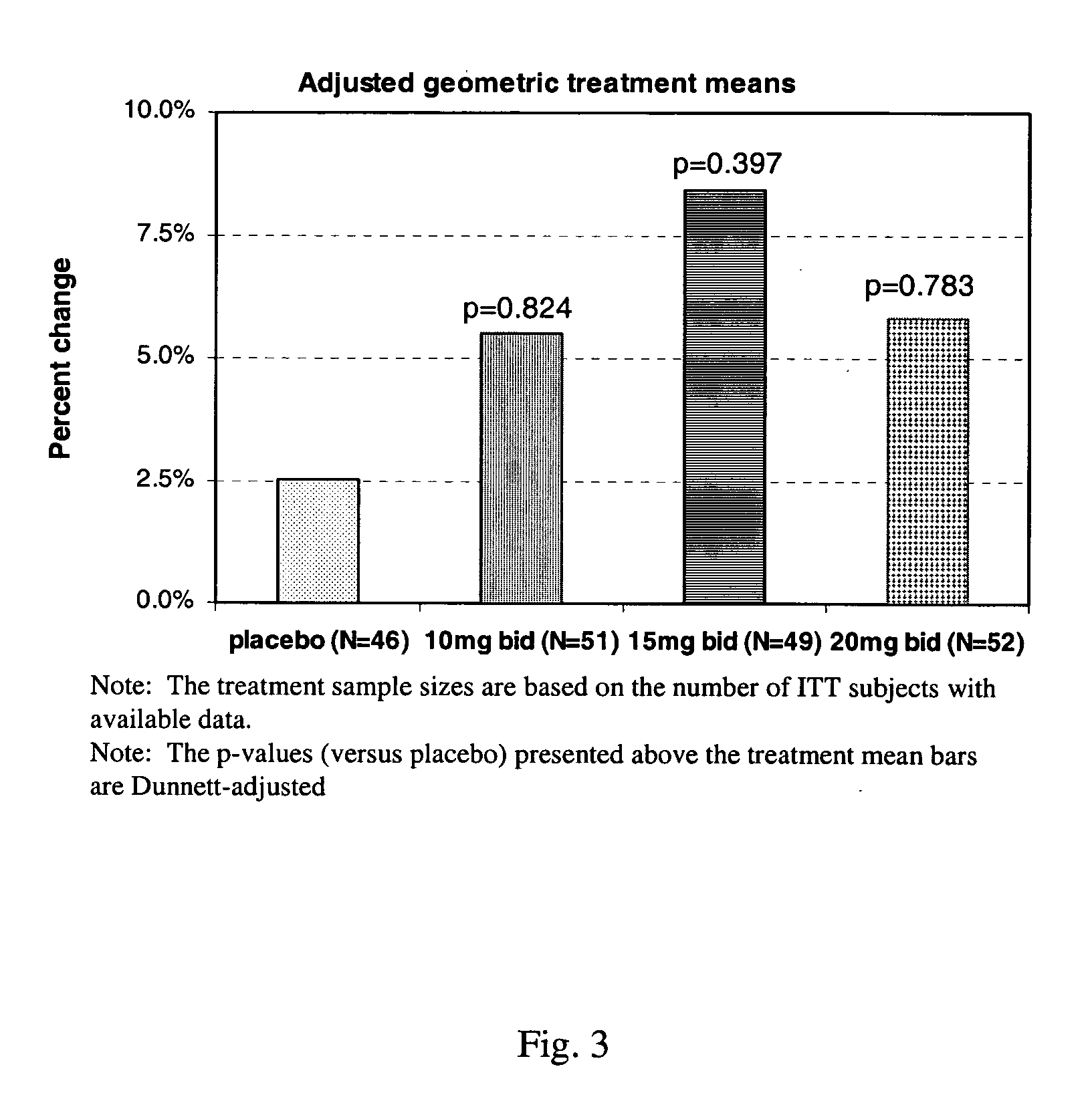
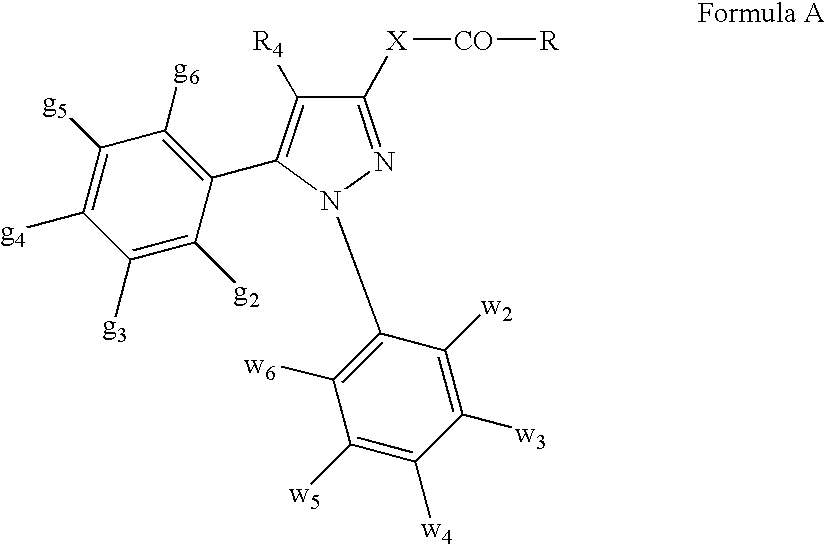
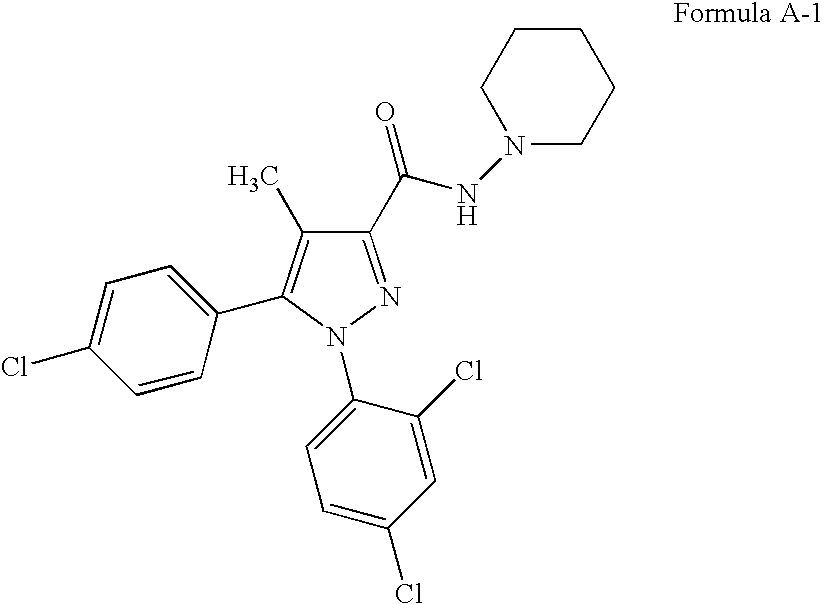
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
234 results about "Plasma levels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Normal Chloride Plasma Levels. Normal plasma chloride ranges are expressed in terms of the anion gap. The normal range should be between 8 and 16mmol – anything more than this is an indication of acidosis, renal failure, and even alcohol intoxication.
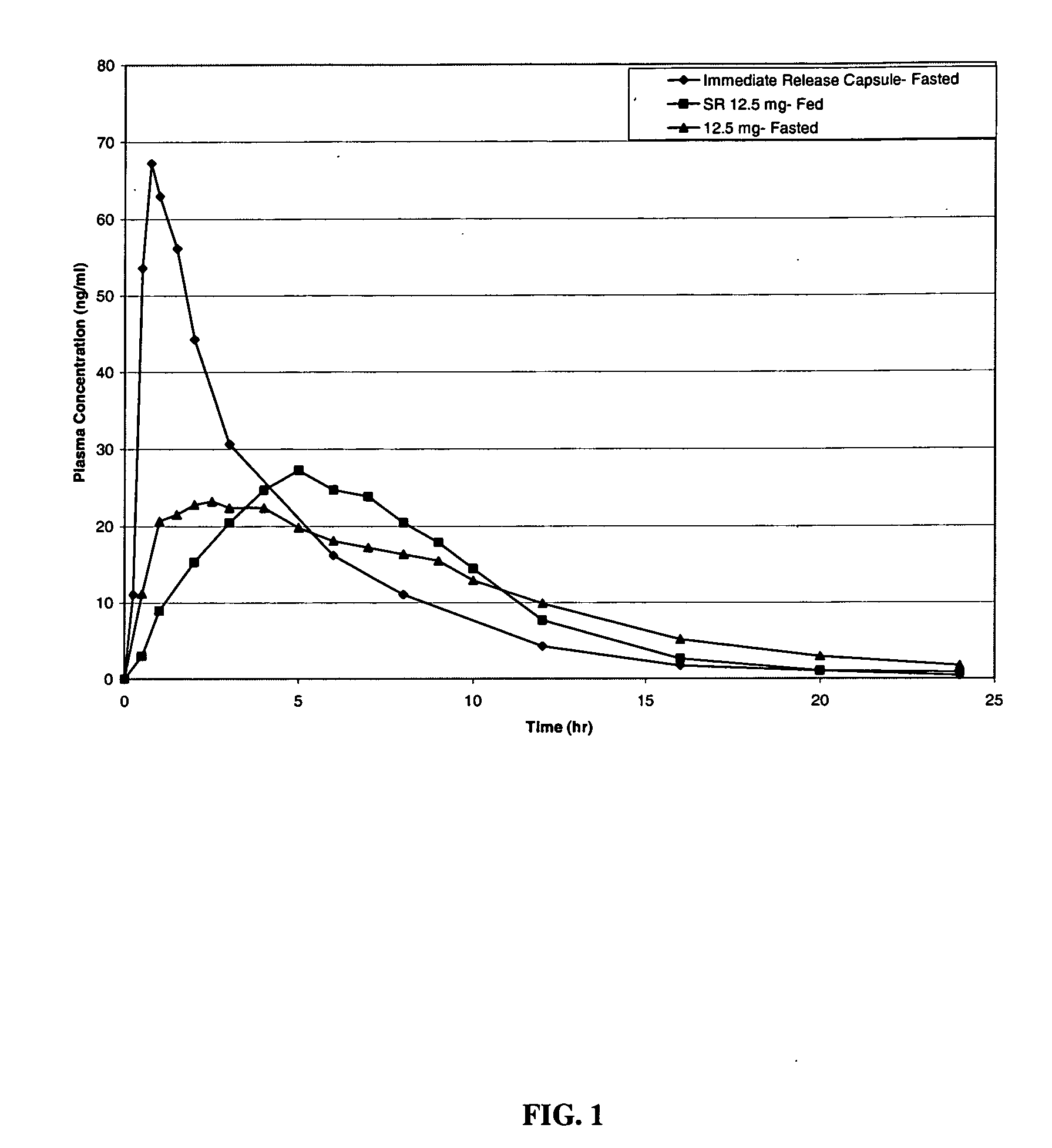
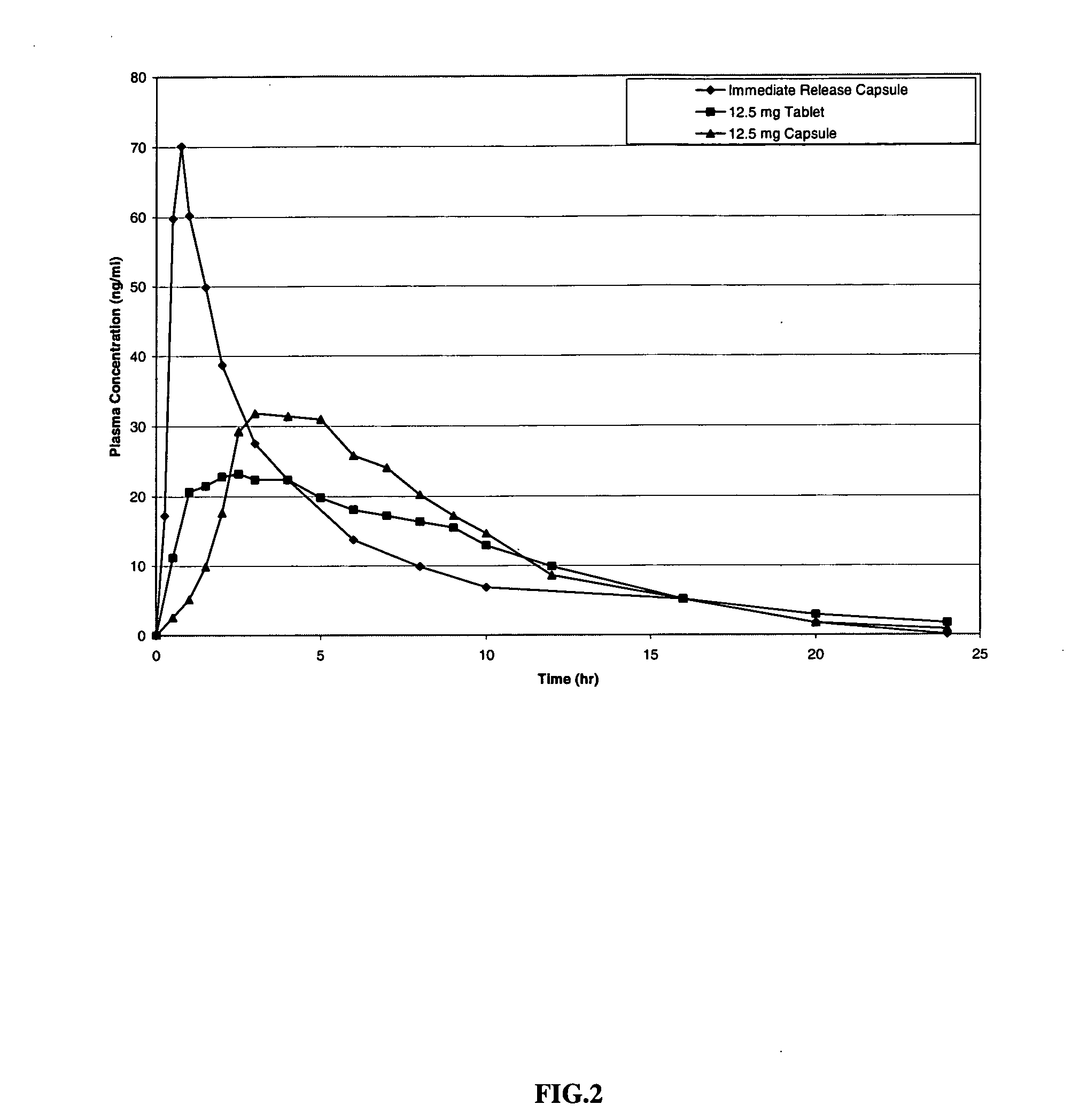
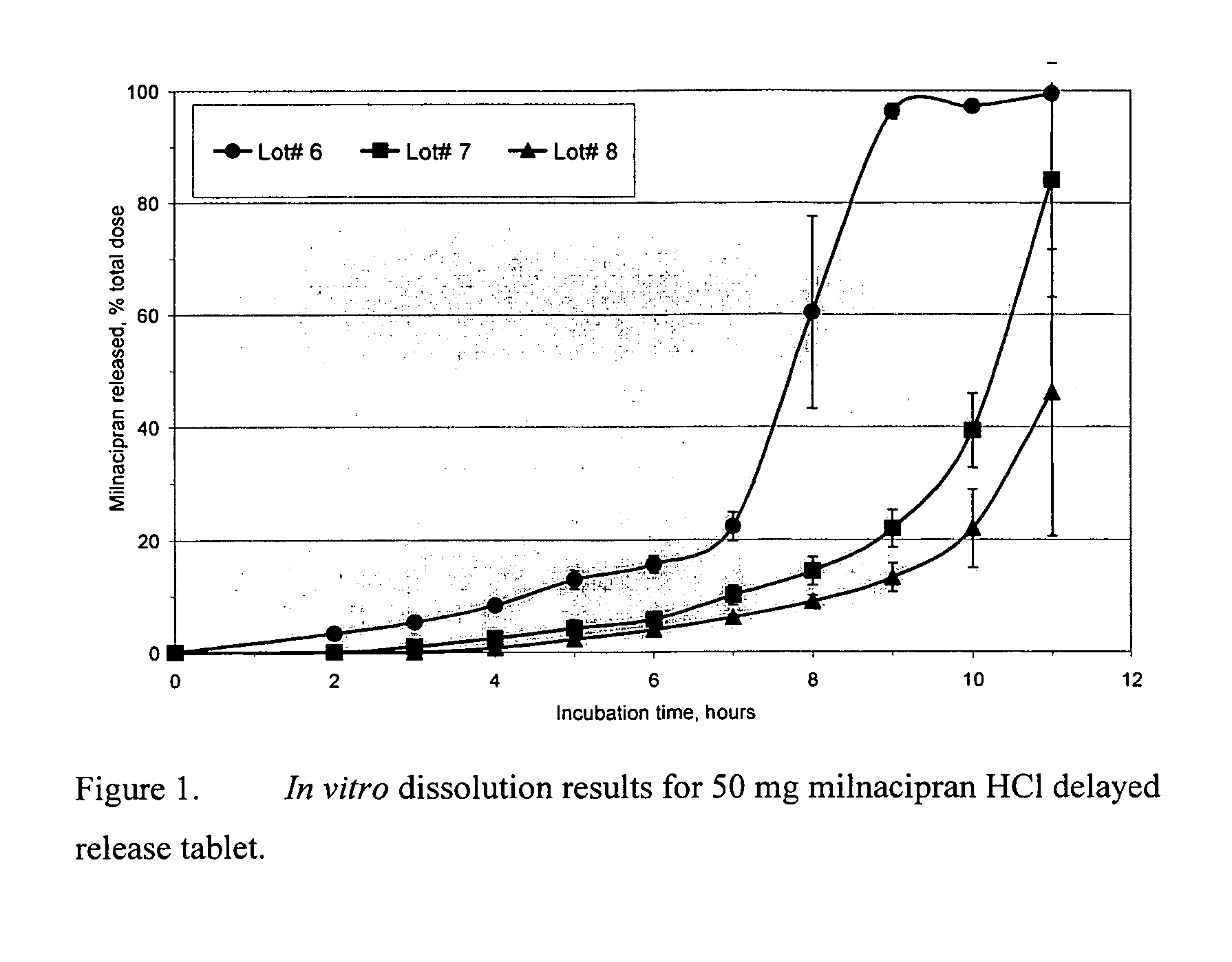
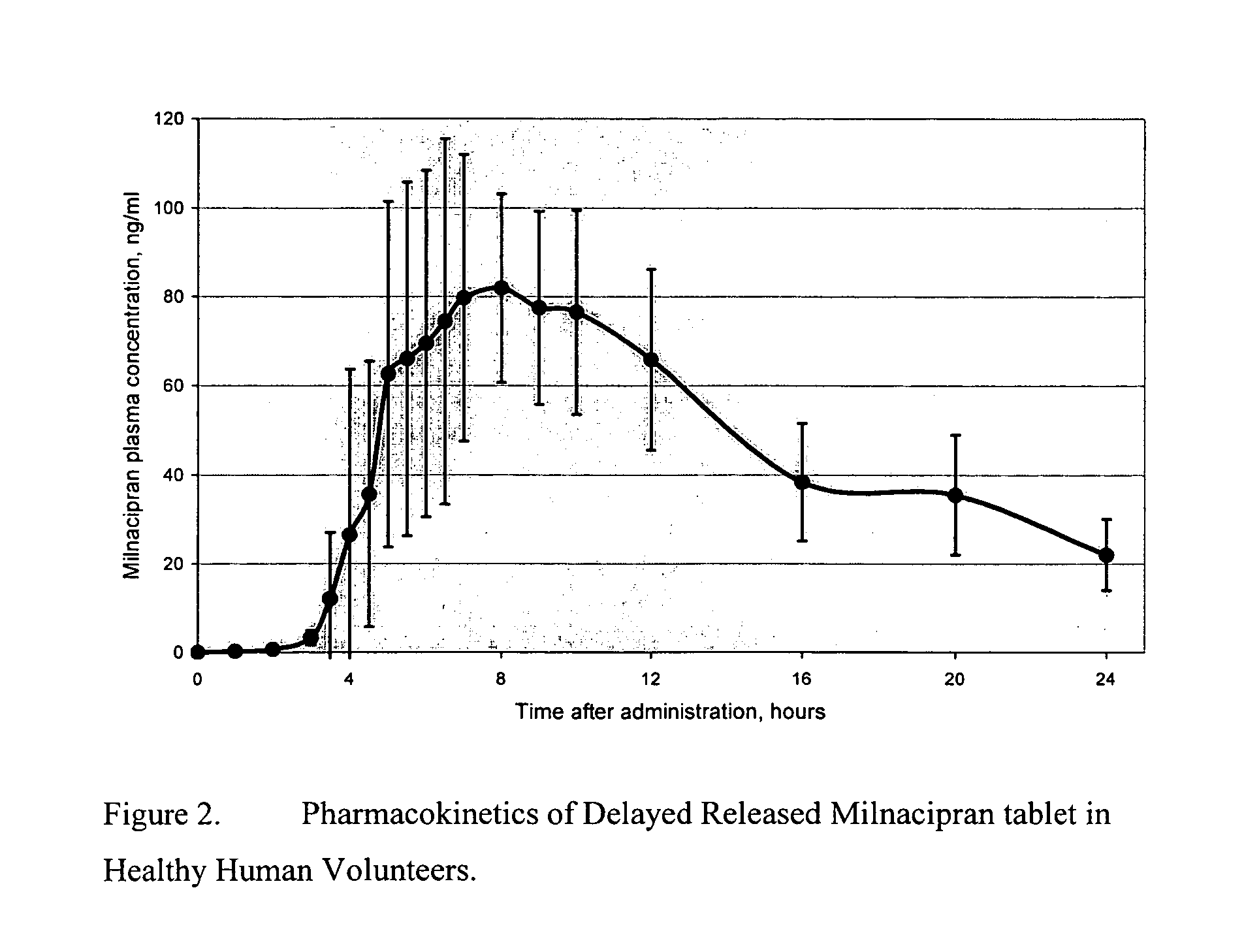
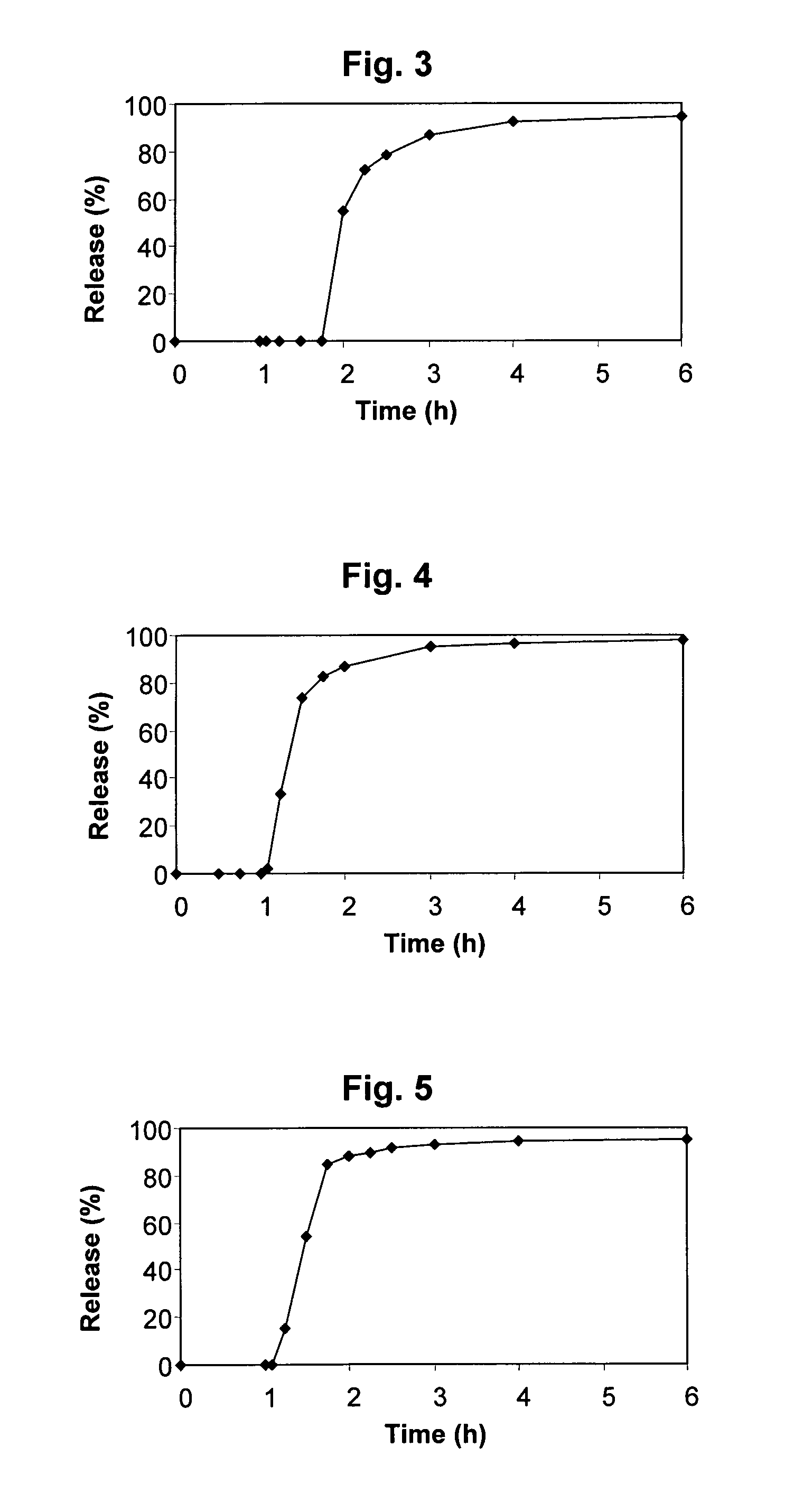
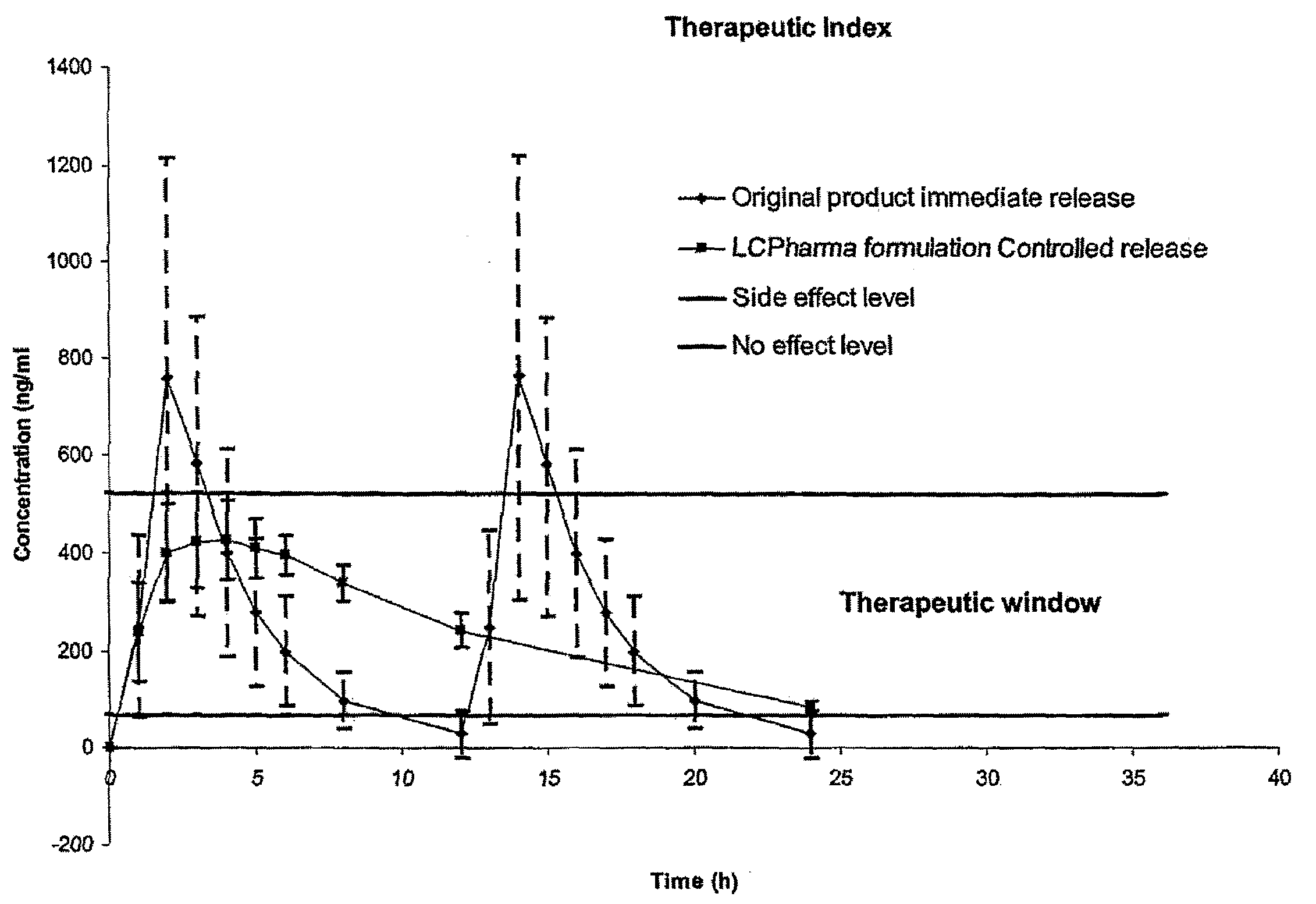
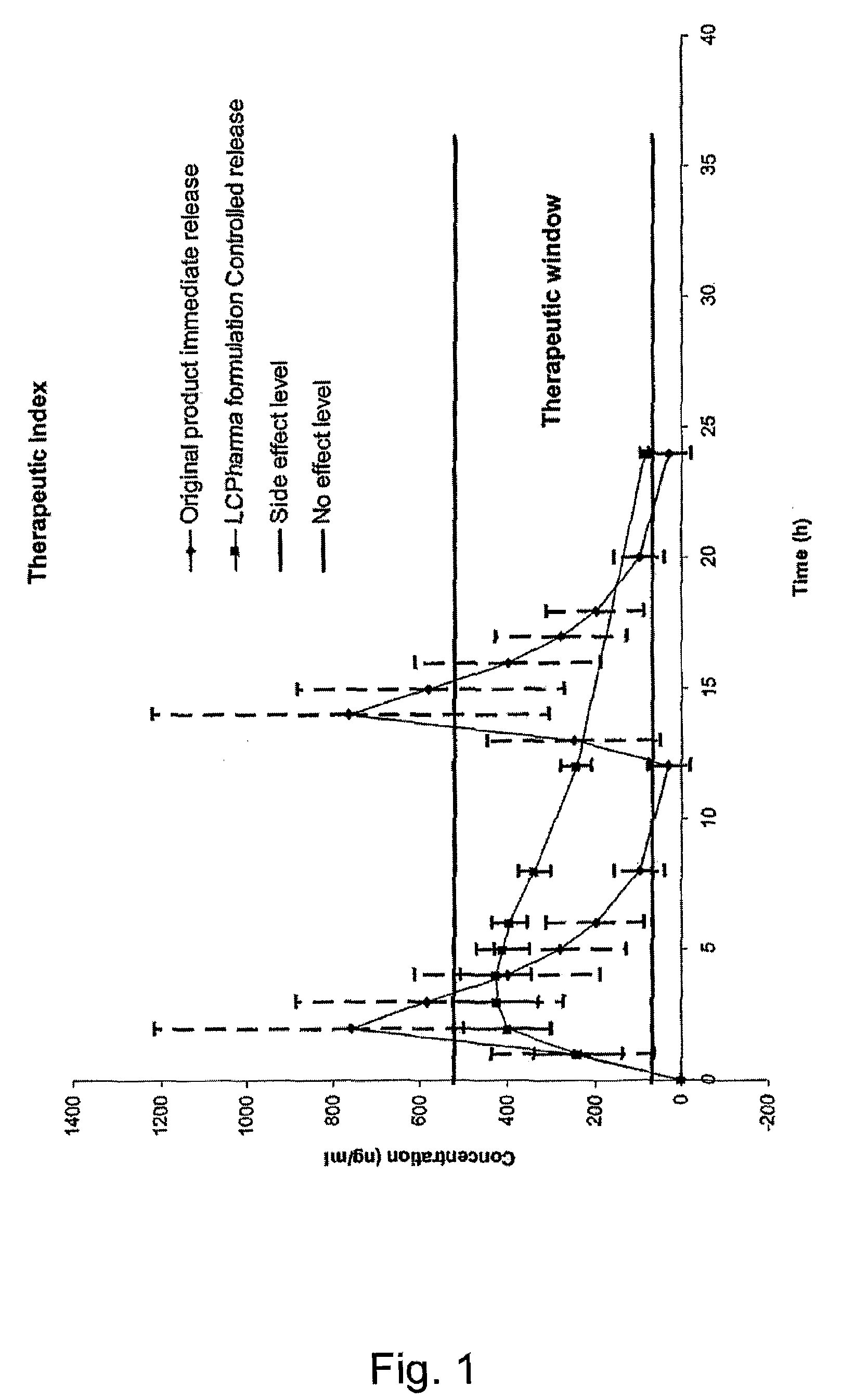
Modified release compositions of milnacipran
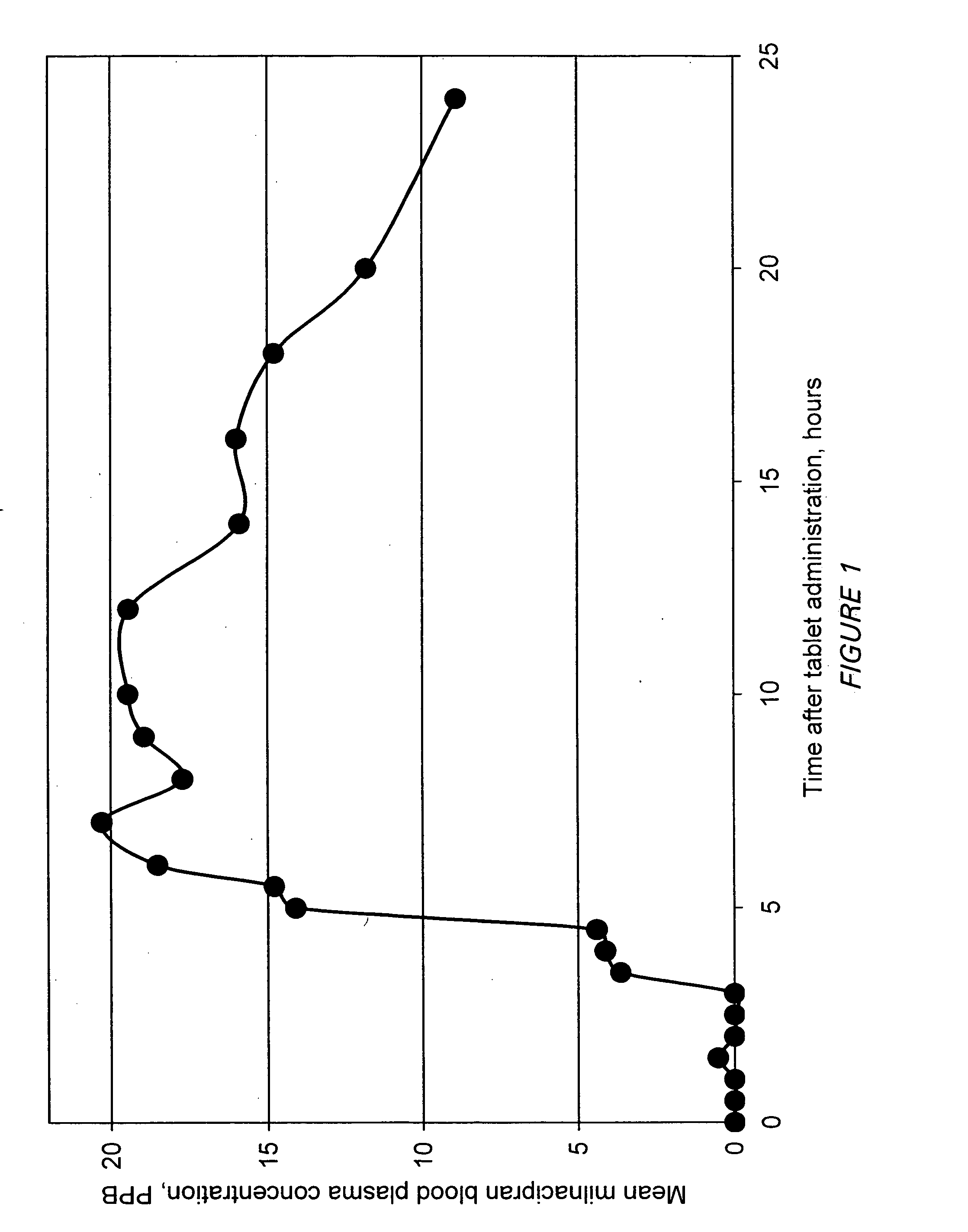
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
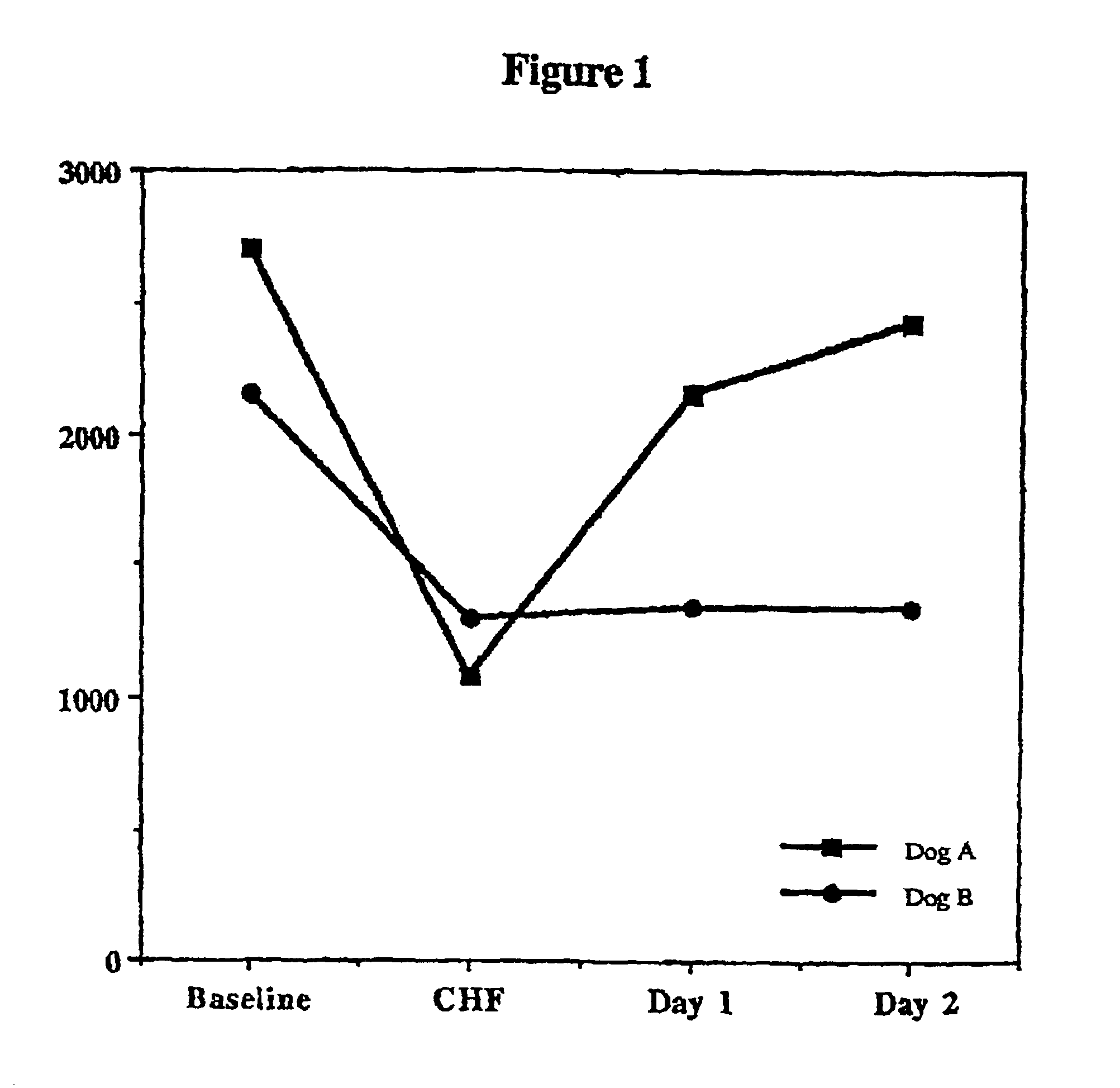
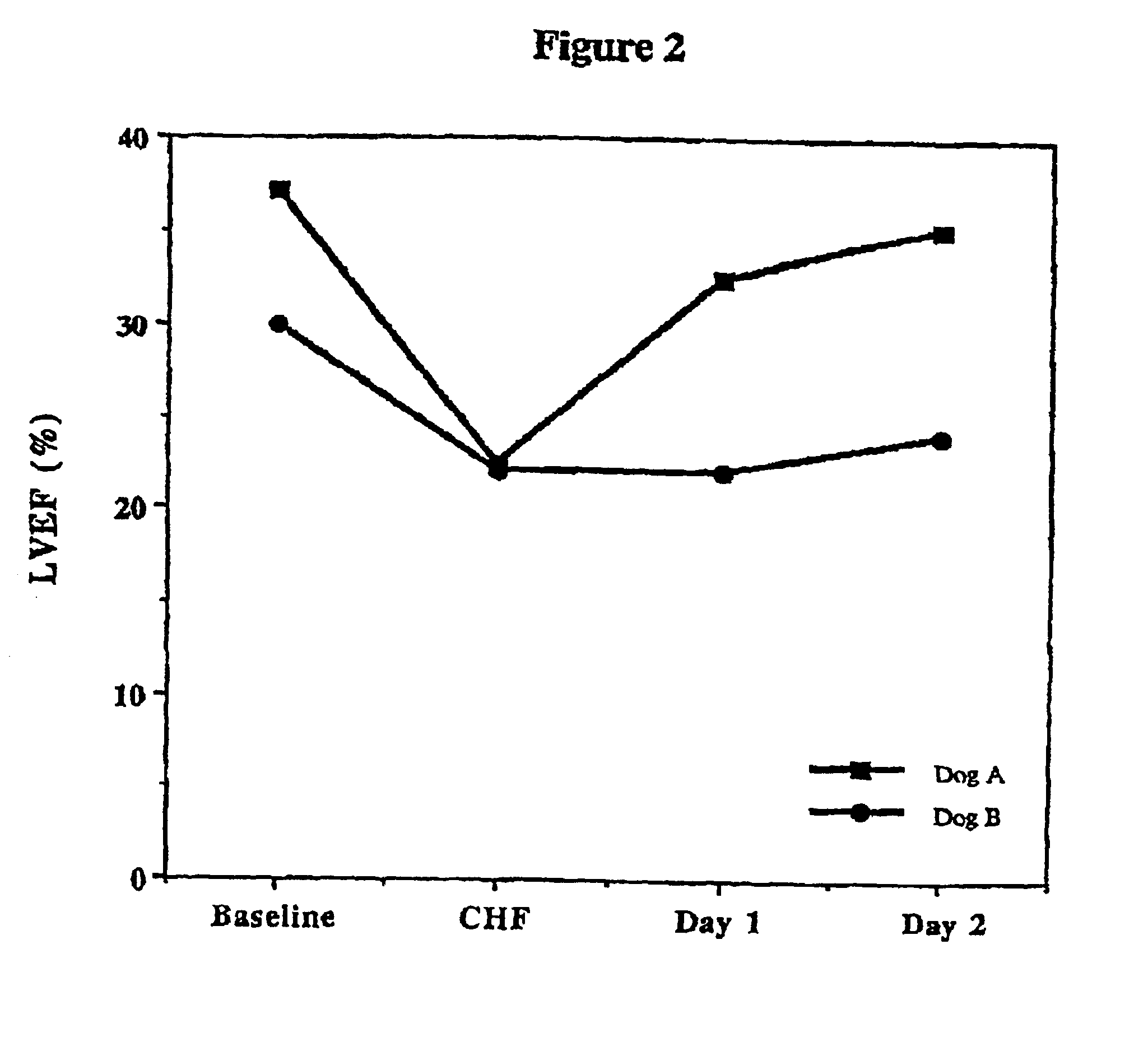
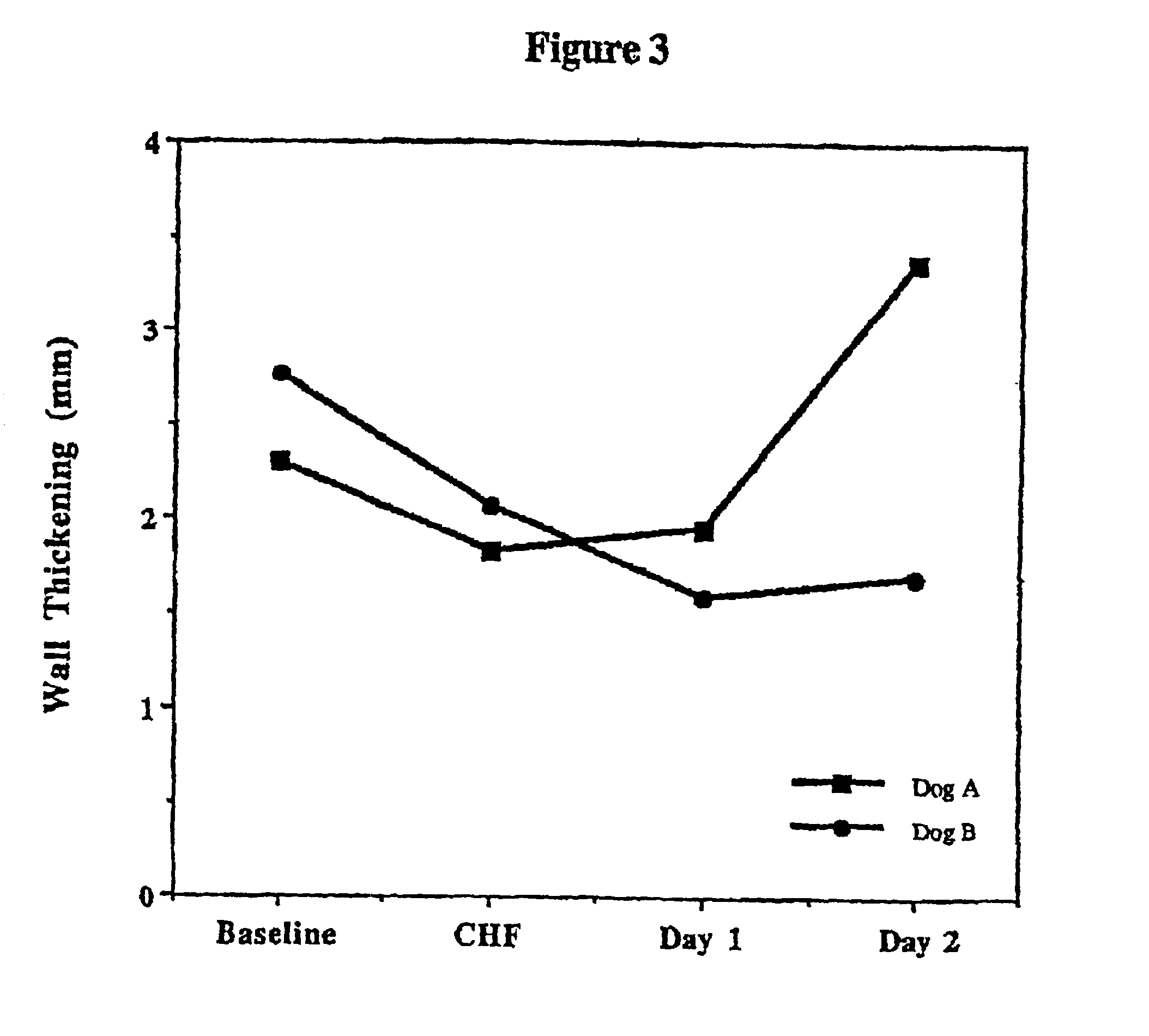
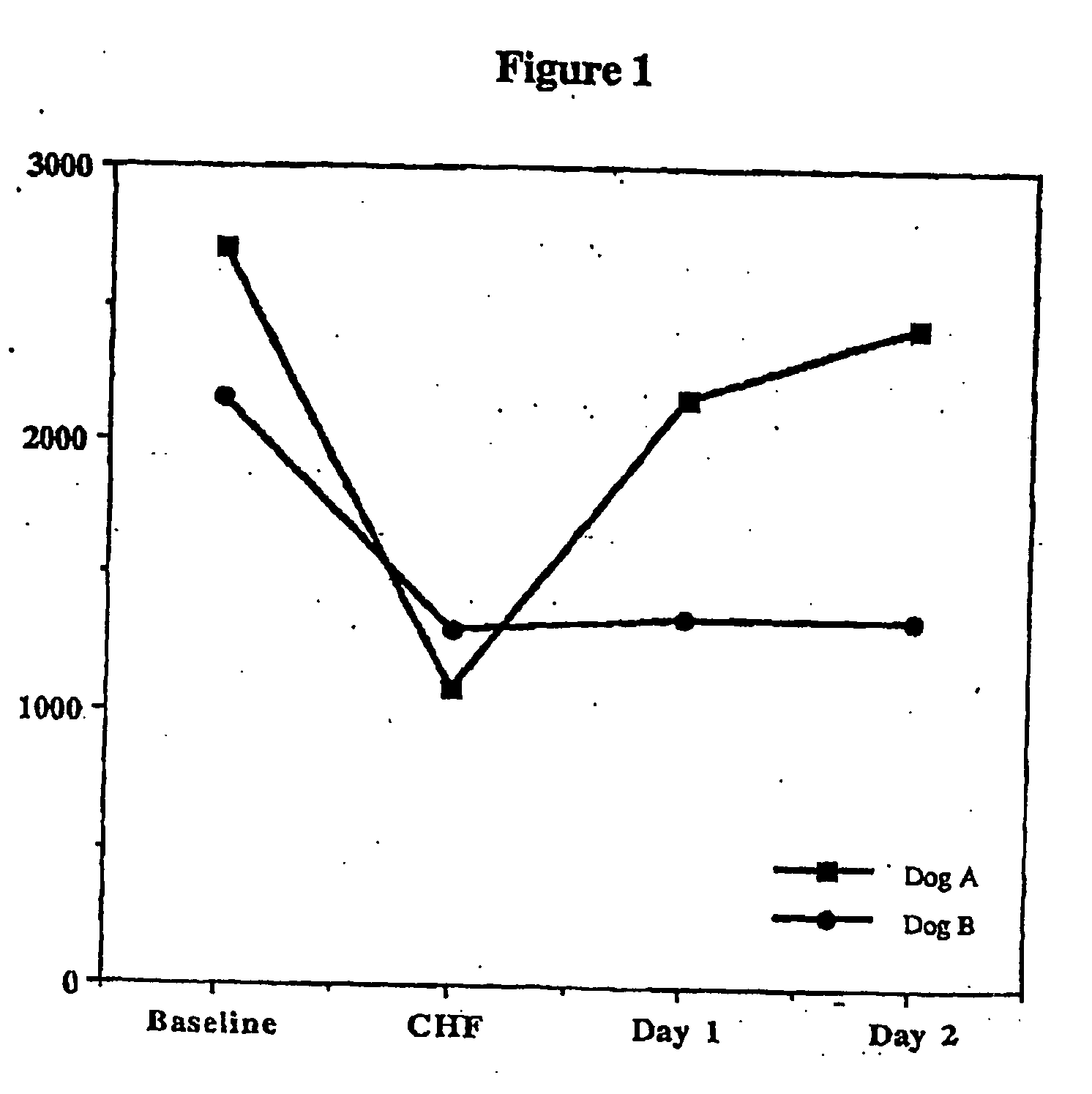
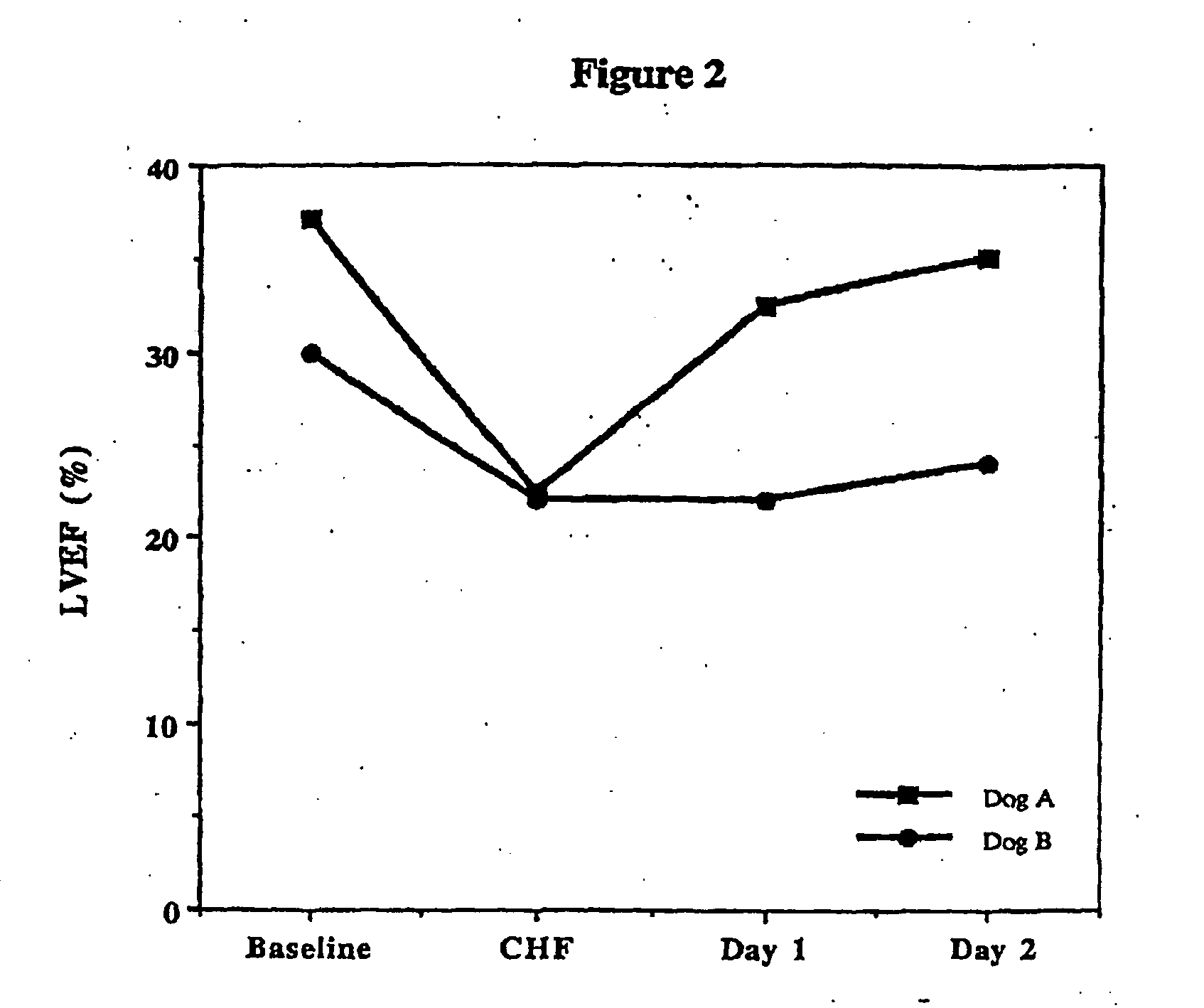
Treatment of hibernating myocardium and diabetic cardiomyopathy with a GLP-1 peptide
InactiveUS6894024B2Suppress plasma blood levelReducing norepinepherine levelPeptide/protein ingredientsMetabolism disorderHigh energyMortality rate
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:ASTRAZENECA PHARMA LP
Implantable device for continuous delivery of interferon
An implantable device includes a reservoir containing a suspension of an interferon in an amount sufficient to provide continuous delivery of the interferon at a therapeutically effective rate of 1 ng / day to 600 μg / day to maintain and achieve therapeutic blood or plasma levels of the interferon throughout a substantial period of the administration period.
Owner:INTARCIA THERAPEUTICS INC
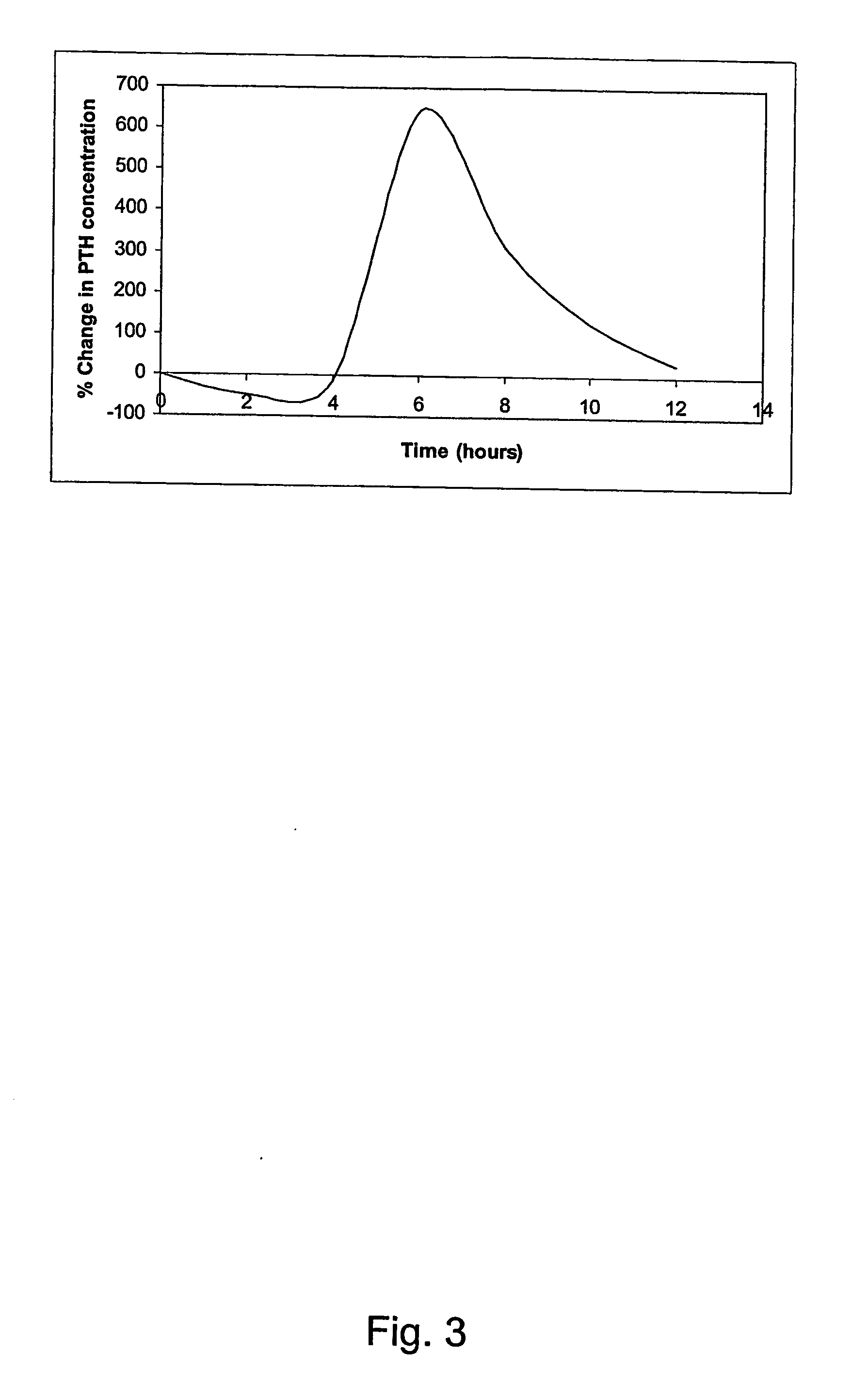
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
InactiveUS20070155664A1Quick releaseSufficient amountOrganic active ingredientsPeptide/protein ingredientsRegimenBlood plasma
A pharmaceutical composition for oral administration comprising PTH, wherein the in vitro release of PTH-when tested in a dissolution test of pharmacopoeia standard-is delayed with at least 2 hours and once the release starts, at least 90% w / w such as, e.g., at least 95% or at least 99% of all PTH contained in the composition is released within at the most 2 hours. The composition may also comprises a calcium containing compound and / or a vitamin, D. In particular, PTH is administered in combination with a calcium-containing compound for the treatment or prevention of bone-related diseases, so that I) an effective amount of a calcium-containing compound is administered to lower the plasma level of endogenous PTH, and II) an effective amount of PTH is administered to obtain a peak concentration of Pm once the endogeneous PTH level is lowered. This present a potential therapeutic or prophylactic regimen for bone-related disorders including osteoporosis.
Owner:NYCOMED DANMARK AS
Hydroxybutyrate ester and medical use thereof
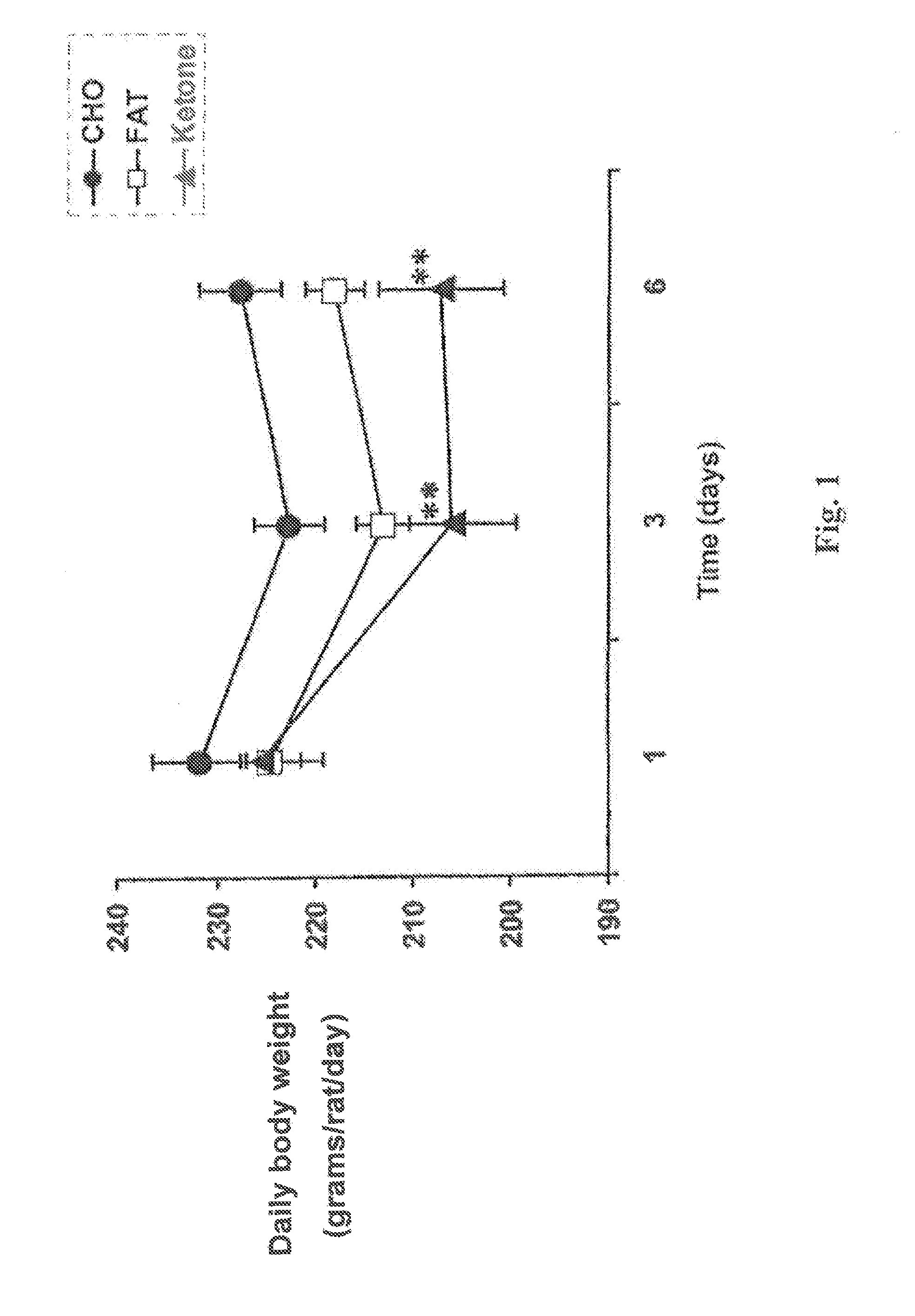
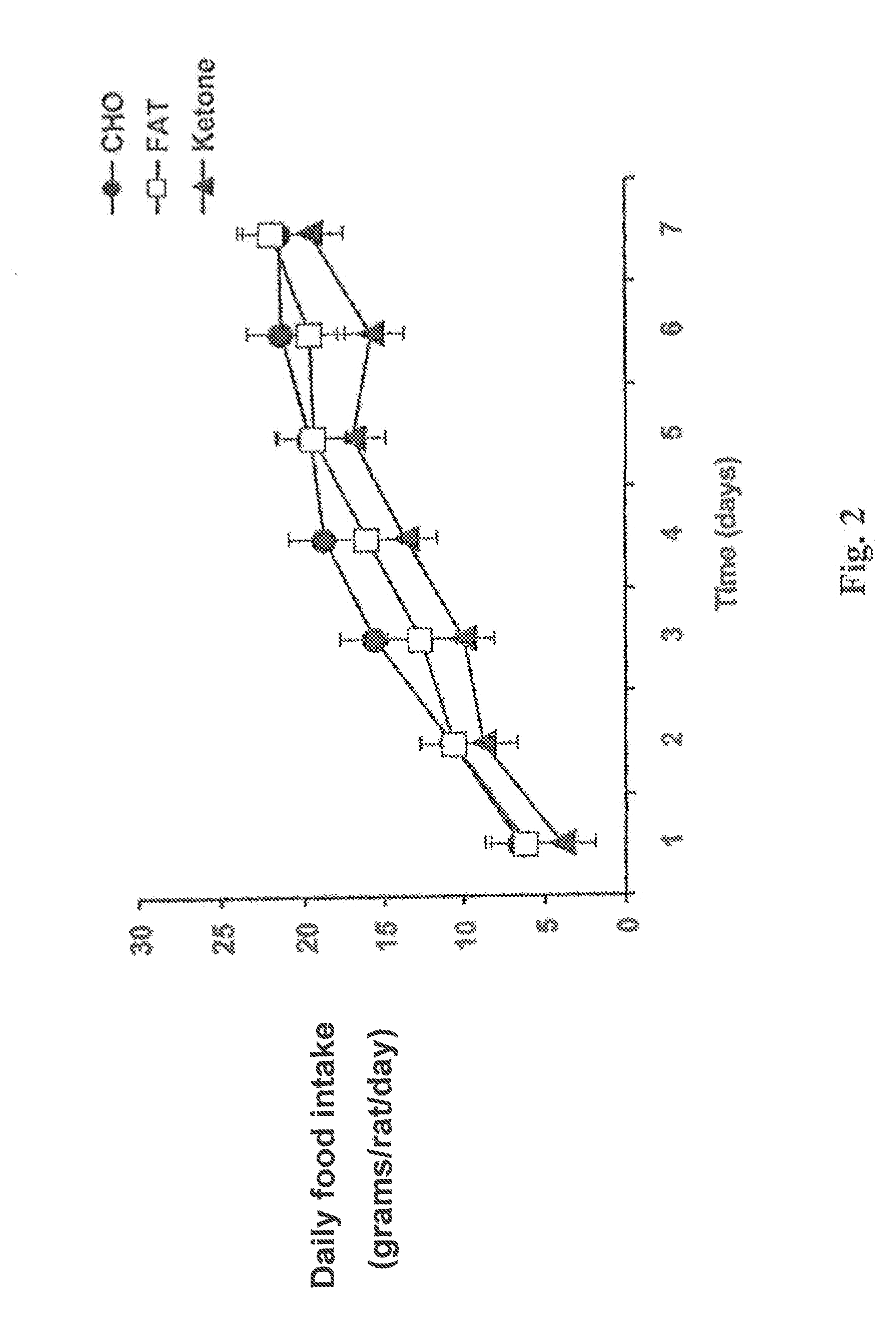
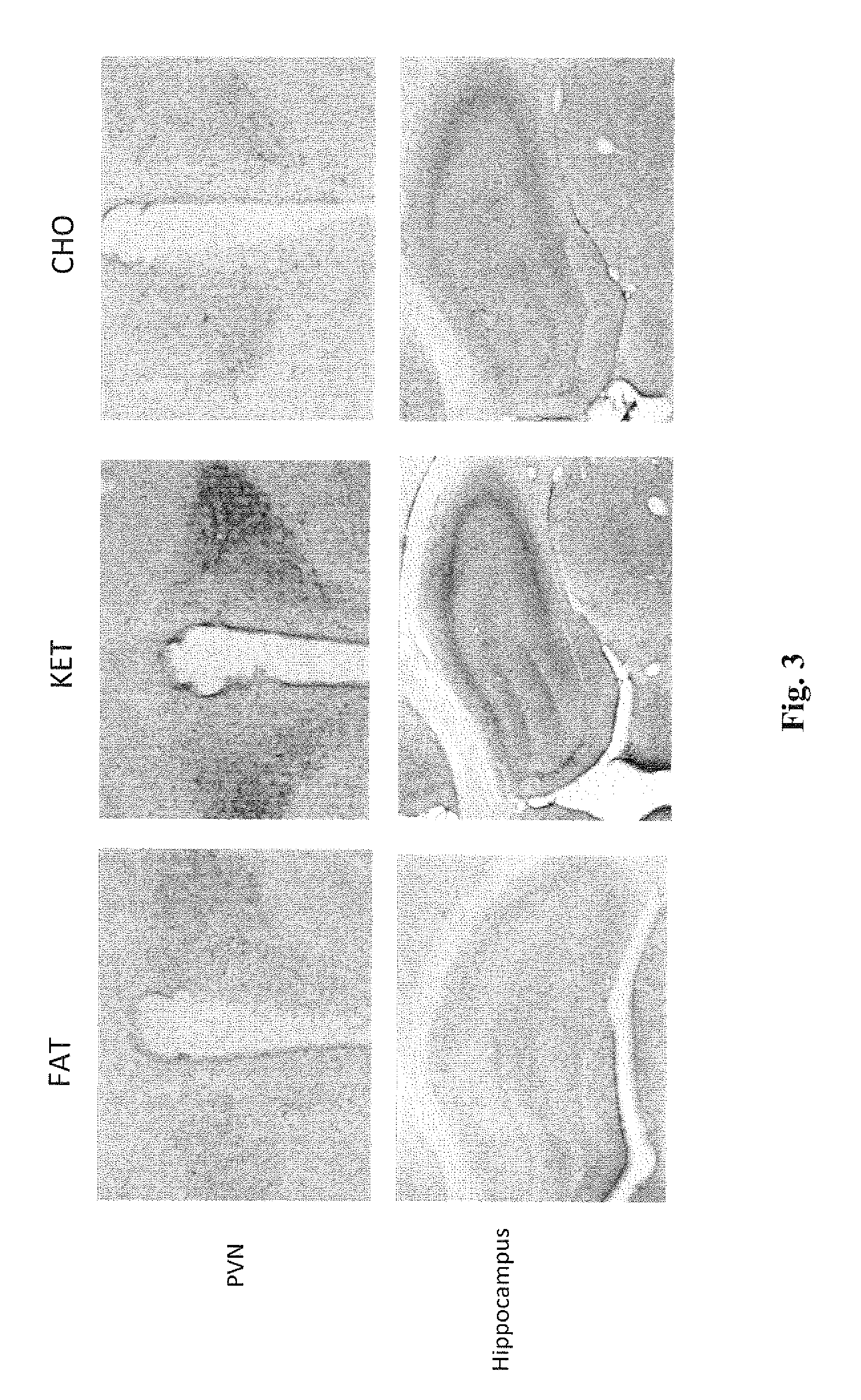
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:UNITED STATES OF AMERICA +1
Novel pharmaceutical compositions administering n-0923
The invention relates to a pharmaceutical composition for administering the dopamine agonist N-0923 in depot form. The invention makes available for the first time a depot form of N-0923, which achieves a therapeutically significant plasma level over a period of at least 24 hours after administration to a patient. As a result of poor oral bio-availability and the short plasma half-life, N-0923 was previously administered either by an intravenous drip or by transdermal systems. Preferred embodiments of said invention are oily suspensions, containing the active ingredient N-0923 in a solid phase, in addition to anhydrous pharmaceutical preparations of N-0923.
Owner:UCB SA
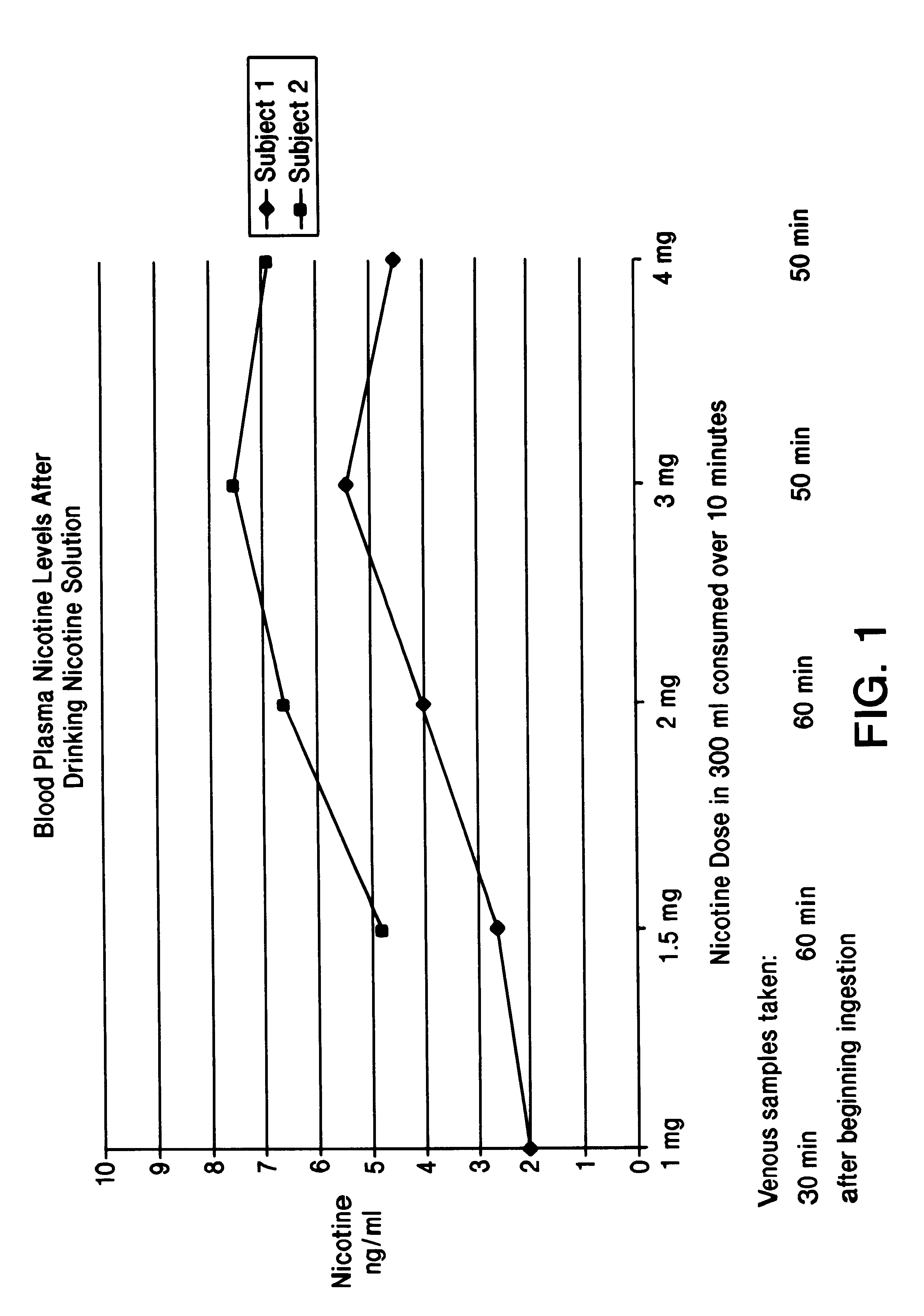
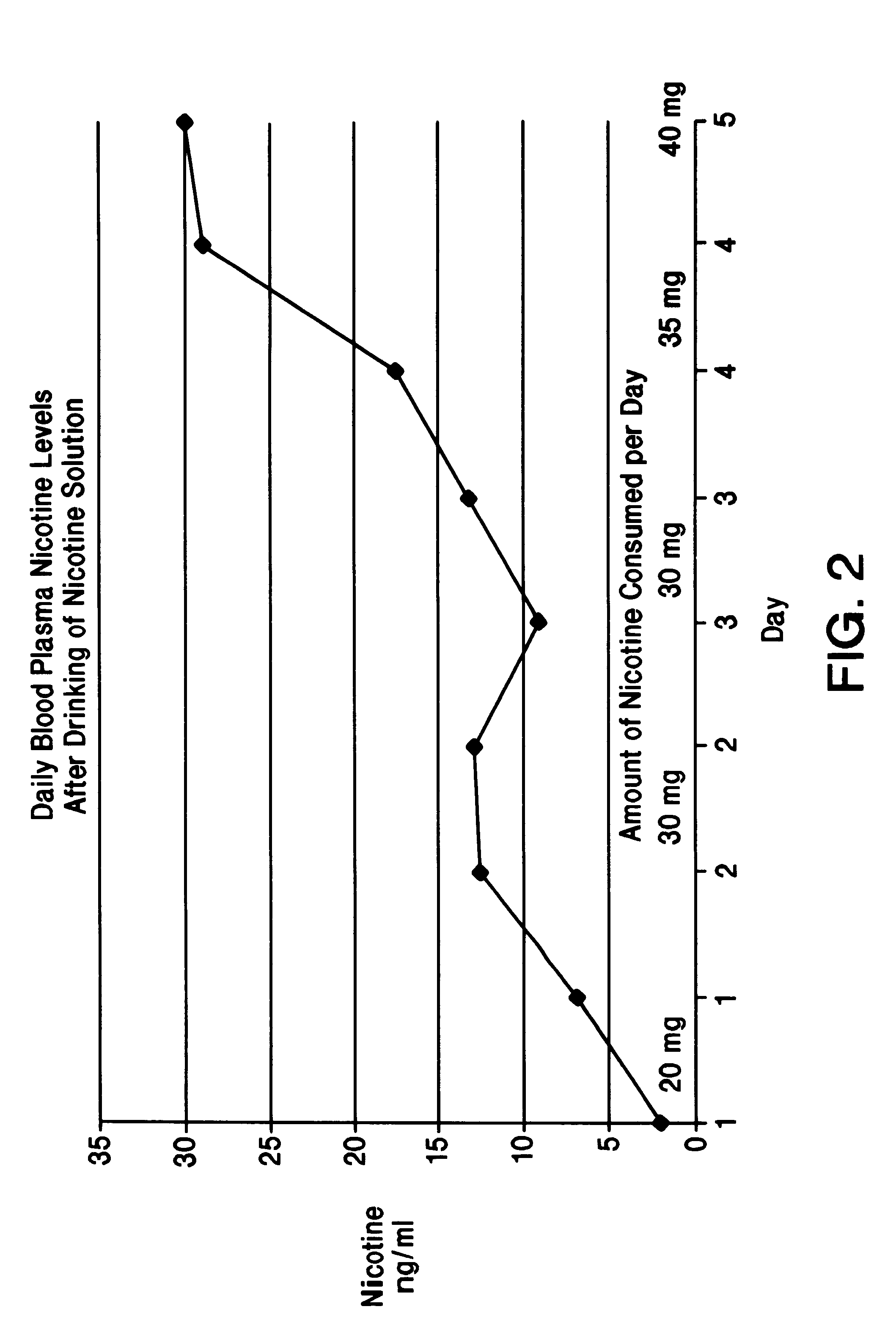
Solution containing nicotine
InactiveUS6211194B1Reduce the possibilityReducing craving for cigarettesBiocidePowder deliveryDiseaseAttention deficits
A nicotine method and solution which utilizes an acidic solution containing nicotine. The solution is for use to treat various medical conditions, such as to reduce the need of a user of smoking tobacco to smoke tobacco, to reduce attention deficit disorder symptoms in a person who has attention deficit disorder, and / or to reduce Alzheimer's disease symptoms in a person who has Alzheimer's disease. The solution is palatable and may be introduced into the person by the person drinking it. Subsequent to drinking, the blood plasma levels are sufficient to reduce the need to smoke tobacco, to reduce attention deficit disorder symptoms, and / or to reduce Alzheimer's disease symptoms.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
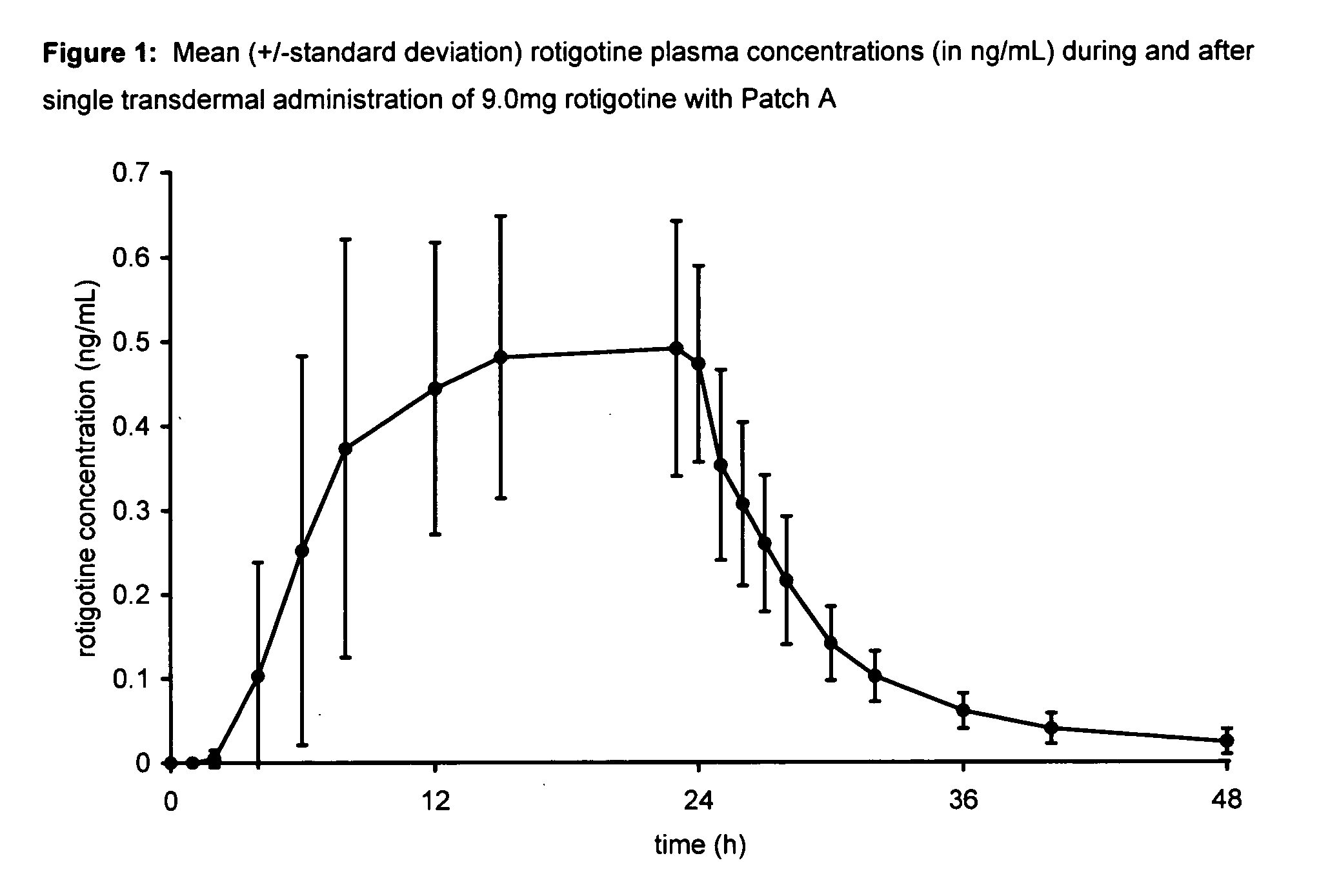
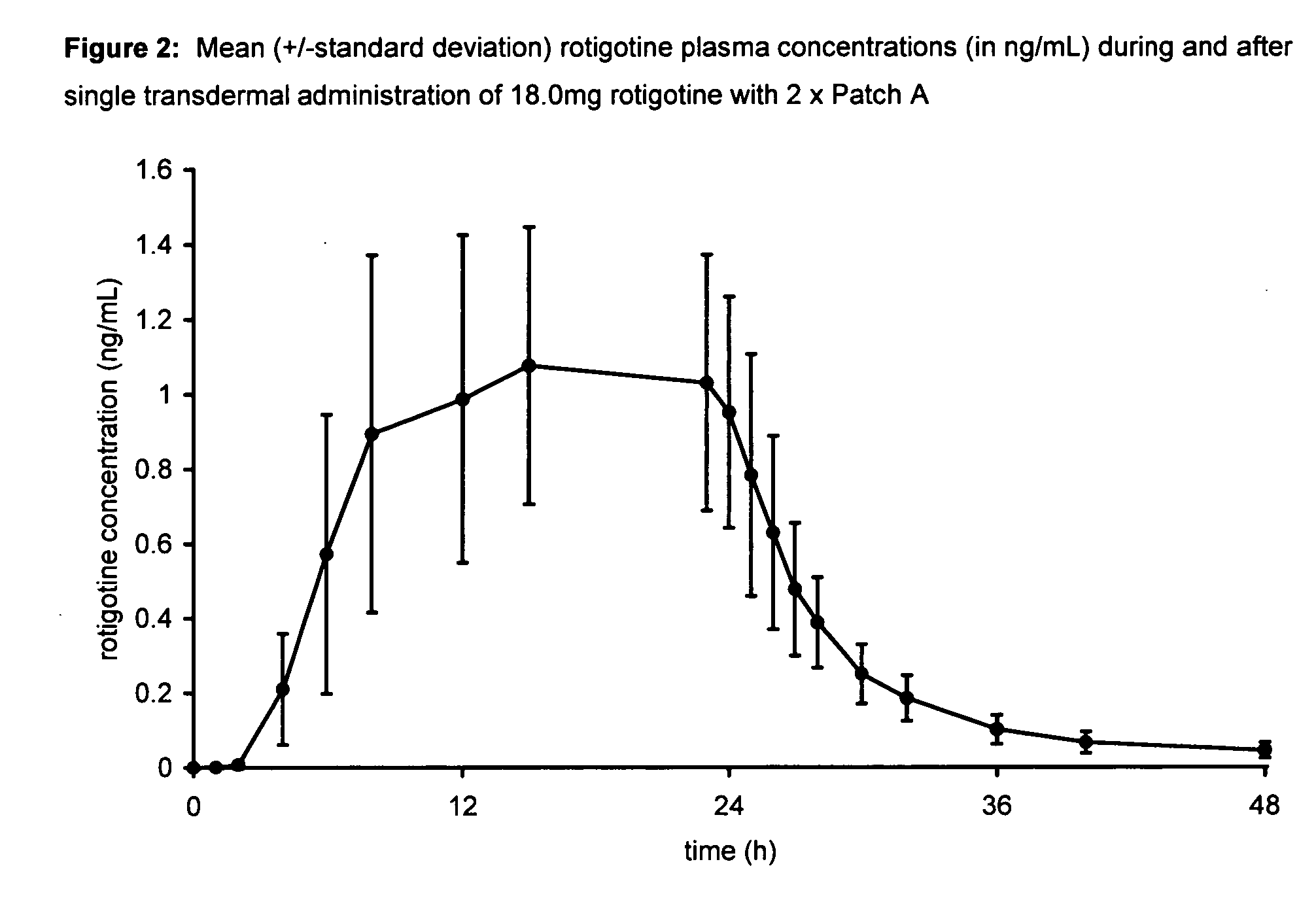
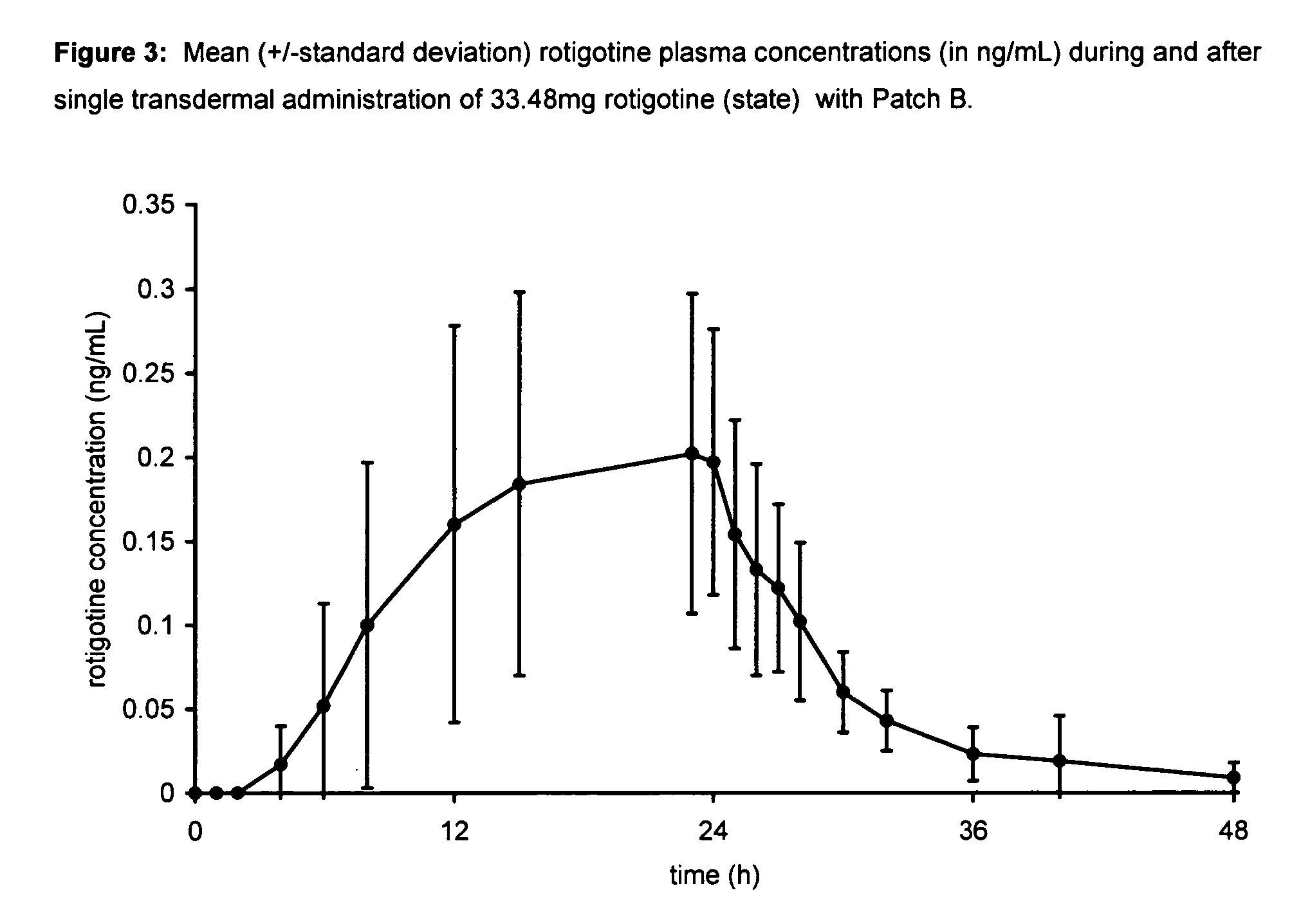
Transdermal therapeutic system for parkinson's disease inducing high plasma levels of rotigotine
This invention provides the use of a silicone-based transdermal therapeutic system having an area of 10 to 40 cm2 and containing 0.1 to 3.15 mg / cm2 of rotigotine as active ingredient, for the preparation of an anti-Parkinson medicament which induces a mean plasma concentration of rotigotine in the range of 0.4 to 2 ng / ml 24 h after administration.
Owner:UCB SA +1
Sustained release aminopyridine composition
ActiveUS20050276851A1Imparts chemical stabilityImparts physical stabilityBiocideNervous disorderDiseaseBlood plasma
A pharmaceutical composition which comprises a therapeutically effective amount of a aminopyridine dispersed in a release matrix, including, for example, a composition that can be formulated into a stable, sustained-release oral dosage formulation, such as a tablet which provides, upon administration to a patient, a therapeutically effective plasma level of the aminopyridine for a period of at least 12 hours, preferably 24 hours or more and the use of the composition to treat various neurological diseases.
Owner:ACORDA THERAPEUTICS INC
Transdermal therapeutic system for Parkinson's Disease
InactiveUS20060263419A1Effective treatmentRelieve symptomsBiocideAdhesive dressingsBULK ACTIVE INGREDIENTBlood plasma
The invention provides a transdermal therapeutic system (TTS) containing rotigotine as the active ingredient. The TTS is useful in the treatment of Parkinson's Disease because it induces a pharmacokinetic profile where the rotigotine plasma level is high and stable.
Owner:LTS LOHMANN THERAPIE-SYST AG +1
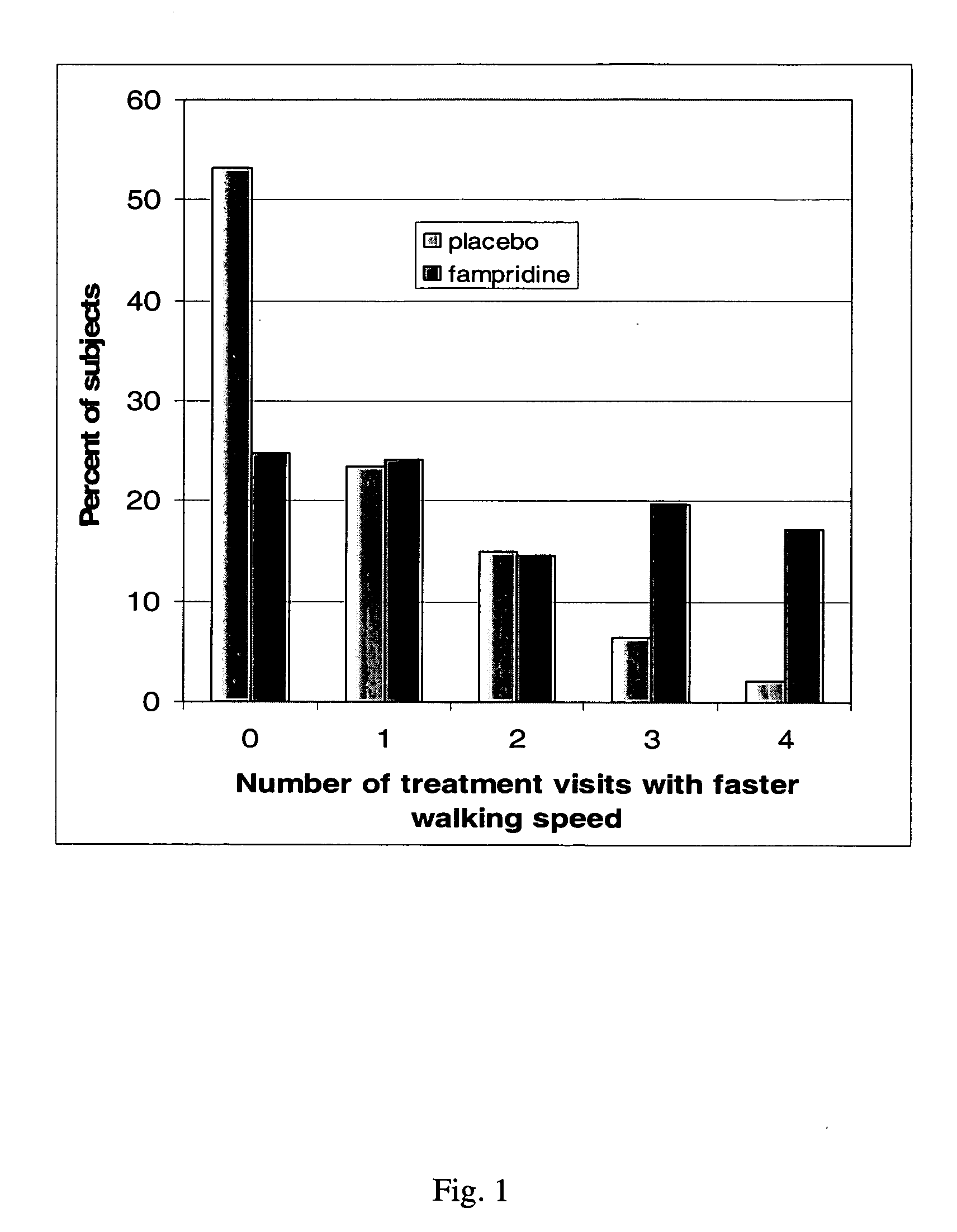
Method of using sustained release aminopyridine compositions
ActiveUS20050228030A1Undesirable to releaseImparts chemical and physical stabilityBiocideNervous disorderBlood plasmaDisease cause
A pharmaceutical composition which comprises a therapeutically effective amount of a aminopyridine dispersed in a release matrix, including, for example, a composition that can be formulated into a stable, sustained-release oral dosage formulation, such as a tablet which provides, upon administration to a patient, a therapeutically effective plasma level of the aminopyridine for a period of at about 12 hours and the use of the composition to treat various neurological diseases, including multiple sclerosis. A method of selecting individuals based on responsiveness to a treatment, including, for example, identifying individuals who responded to treatment with a sustained release fampridine composition.
Owner:ACORDA THERAPEUTICS INC
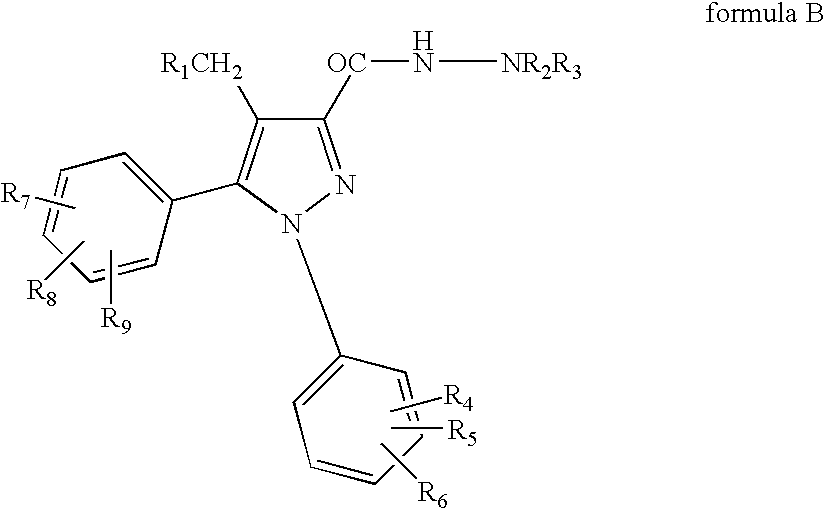
Combinations of substituted azetidinones and CB1 antagonists
The present invention provides compositions, therapeutic combinations and methods including: (a) at least one selective CB1 antagonist; and (b) at least one substituted azetidinone or substituted β-lactam sterol absorption inhibitor which can be useful for treating vascular conditions, diabetes, obesity, metabolic syndrome and lowering plasma levels of sterols or 5α-stanols.
Owner:SCHERING CORP
N-acyl sulfamic acid esters useful as hypocholesterolemic agents
PCT No. PCT / US97 / 06725 Sec. 371 Date Aug. 10, 1998 Sec. 102(e) Date Aug. 10, 1998 PCT Filed Apr. 21, 1997 PCT Pub. No. WO97 / 44314 PCT Pub. Date Nov. 27, 1997The instant invention is new compounds of Formula I their use as cerebrovascular agents in diseases such as stroke, peripheral vascular disease, restenosis, and as agents for regulating plasma cholesterol concentrations, for treating hypercholesterolemia and atherosclerosis, and for lowering the serum or plasma level of Lp(a). A pharmaceutical composition is also claimed.
Owner:WARNER-LAMBERT CO
Transdermal delivery of hormones without the need of penetration enhancers
ActiveUS20050142175A1High plasma levelInhibit ovulationBiocideOrganic active ingredientsEthinyl oestradiolHormones regulation
The present invention relates to a patch comprising a drug-containing layer with low content of hormones, such as gestodene, and optionally an estrogen (e.g. ethinyl estradiol). Upon administering the patch to a woman, plasma levels of at least 1.0 ng / ml of Gestodene is achieved at steady state conditions without the need of incorporating penetration enhancers or permeation enhancers in the drug-containing layer. Satisfactorily plasma levels of the hormones is also achieved throughout a period of at least 1 week, making the patch applicable for being used in female contraception with the concept of administering the patch ones weekly.
Owner:LUYE PHARMA SWITZERLAND AG
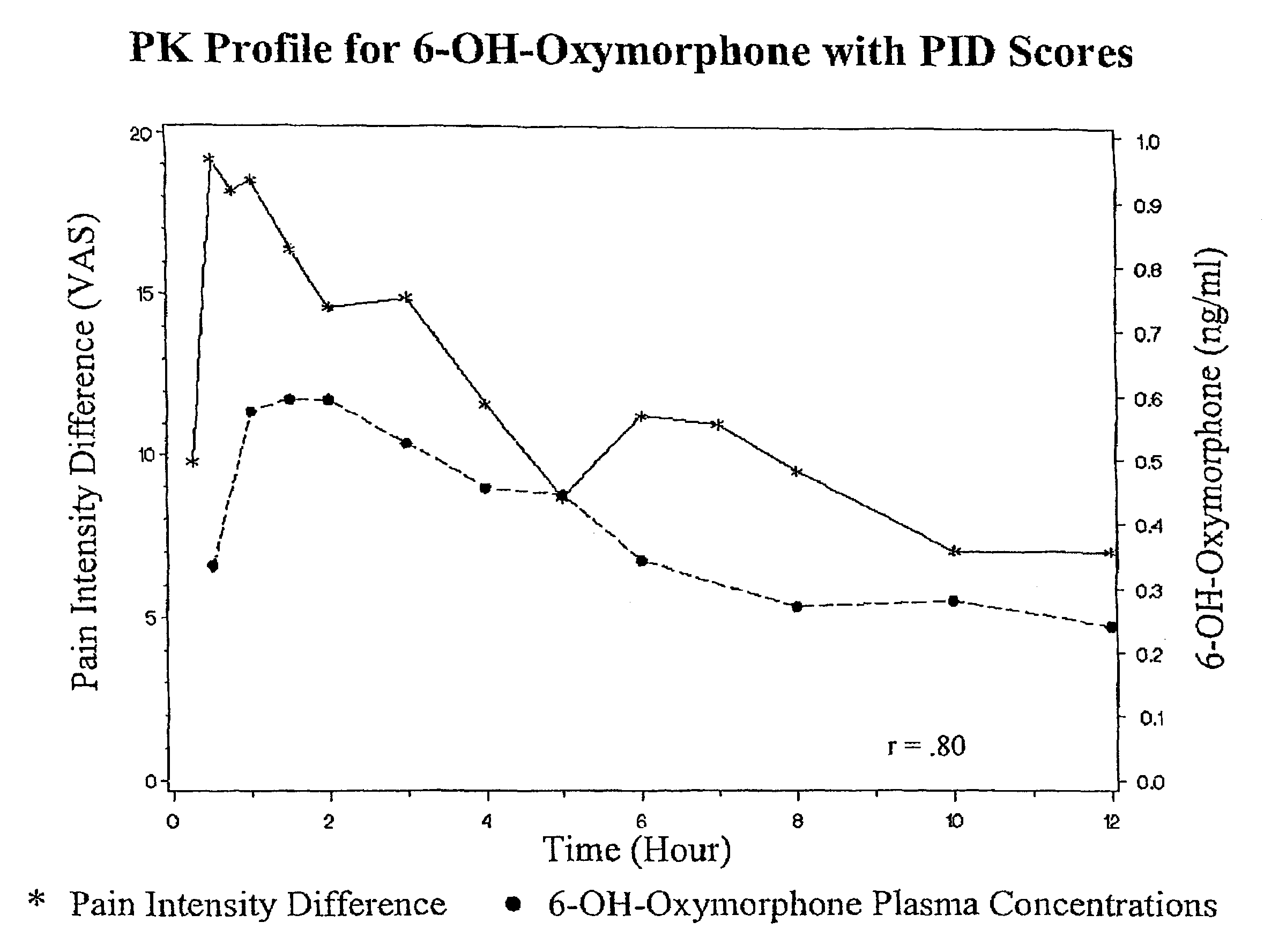
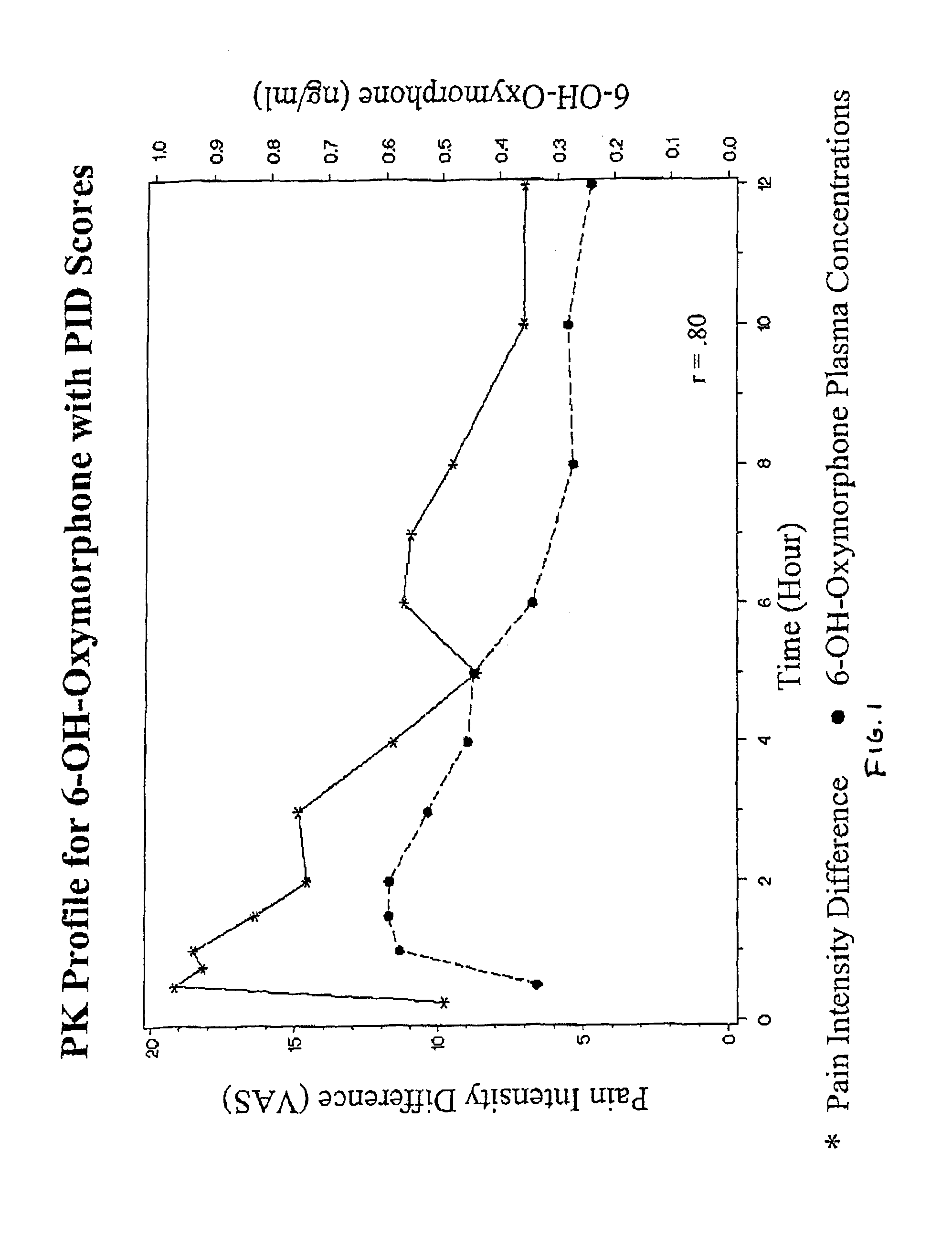
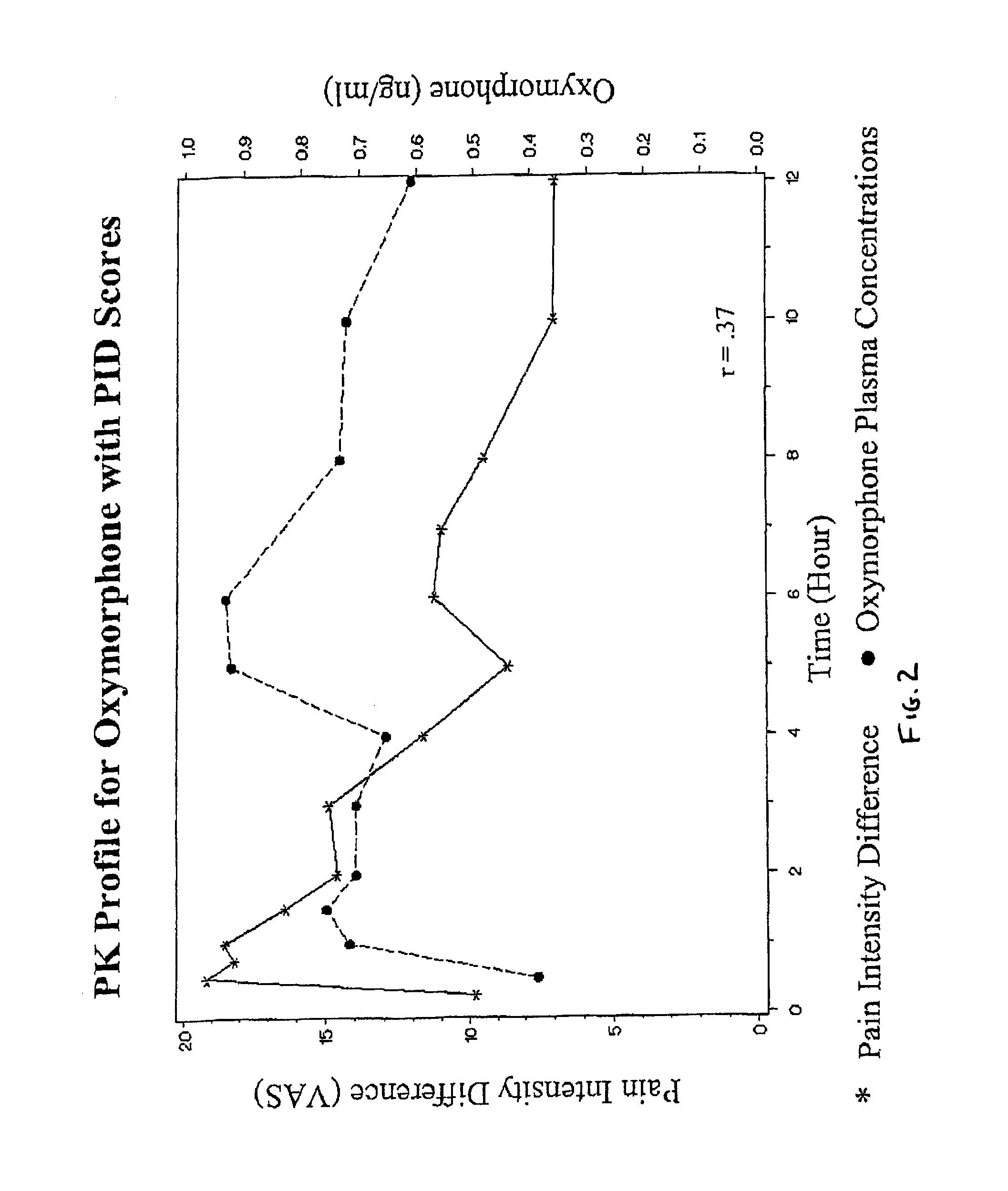
Oxymorphone controlled release formulations
The invention pertains to a method of relieving pain by administering a controlled release pharmaceutical tablet containing oxymorphone which produces a mean minimum blood plasma level 12 to 24 hours after dosing, as well as the tablet producing the sustained pain relief.
Owner:ENDO PHARMA INC
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Uses of ion channel modulating compounds
Methods, formulations, dosing regimes, and routes of administration for the treatment or prevention of arrhythmias, including the treatment or prevention of atrial fibrillation. In these methods, the disease or condition is treated or prevented by administering one or more ion channel modulating compounds to a subject, where the ion channel modulating compound or compounds produce specific plasma levels in the subject. The ion channel modulating compounds may be cycloalkylamine ether compounds, particularly cyclohexylamine ether compounds.
Owner:CORREVIO INT SARL
Method for obtaining apolar and polar extracts of curcuma and applications thereof
InactiveUS6440468B1Avoid solvent lossLower Level RequirementsBiocideMetabolism disorderLipid formationFiltration
A process for obtaining the apolar extract comprises: (a) extracting the rhizomes with an organic solvent; (b) filtration and evaporation to dryness of the extract; (c) dissolution of the oleoresin obtained in hot conditions, precipitation while allowing to cool down and filtration of the solid; (d) drying and recrystallizing the solid in order to obtain a product having a purity in curcuminoids higher than 90%. Obtaining the polar extract comprises: (a') extraction of the rhizomes with water at 50-70° C. and (b') filtration and evaporation of the water. Application of the compositions and preparations as catchers of free radicals and antiageing agents, as well as reducing agents to reduce the plasma levels of lipid peroxides in human beings are disclosed.
Owner:A S A C PHARMA INT A I E
Methods for the Preparation of Injectable Depot Compositions
ActiveUS20130177603A1Simple methodConstant and effective plasma levelBiocideNervous disorderMedicineGlycolic acid
Injectable depot compositions, comprising a biocompatible polymer which is a polymer or copolymer based on lactic acid and / or lactic acid plus glycolic acid having a monomer ratio of lactic to glycolic acid in the range from 48:52 to 100:0, a water-miscible solvent having a dipole moment of about 3.7-4.5 D and a dielectric constant of between 30 and 50, and a drug, were found suitable for forming in-situ biodegradable implants which can evoke therapeutic drug plasma levels from the first day and for at least 14 days.
Owner:LAB FARM ROVI SA
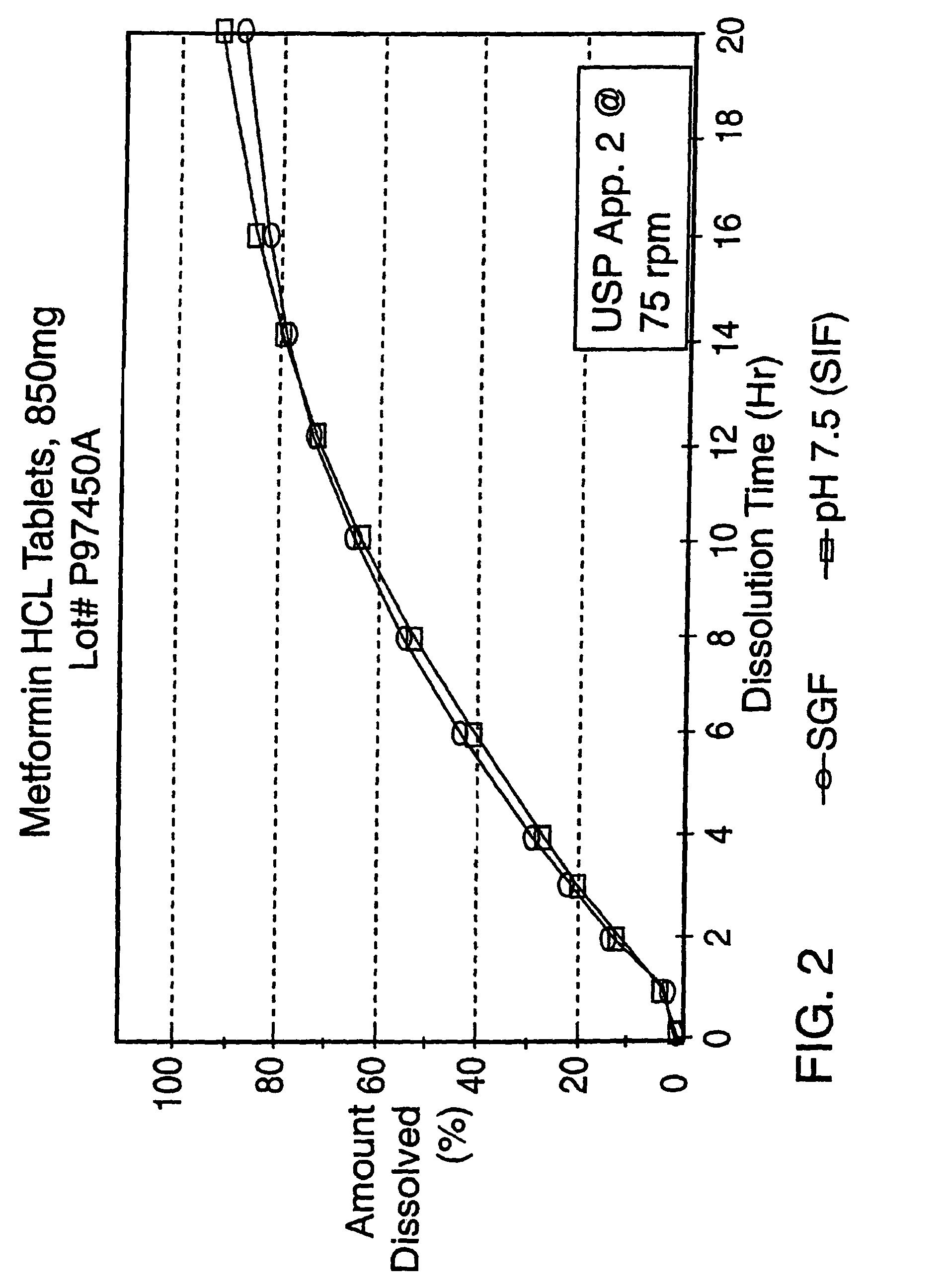
Controlled release metformin formulations
InactiveUS7919116B2Reduced bioavailabilityImprove bioavailabilityOrganic active ingredientsBiocideControlled releaseSustained release drug
Sustained release pharmaceutical formulations comprising an antihyperglycemic drug or a pharmaceutically acceptable salt thereof are disclosed. The formulations provide therapeutic plasma levels of the antihyperglycemic drug to a human patient over a 24 hour period after administration.
Owner:ANDRX LABS
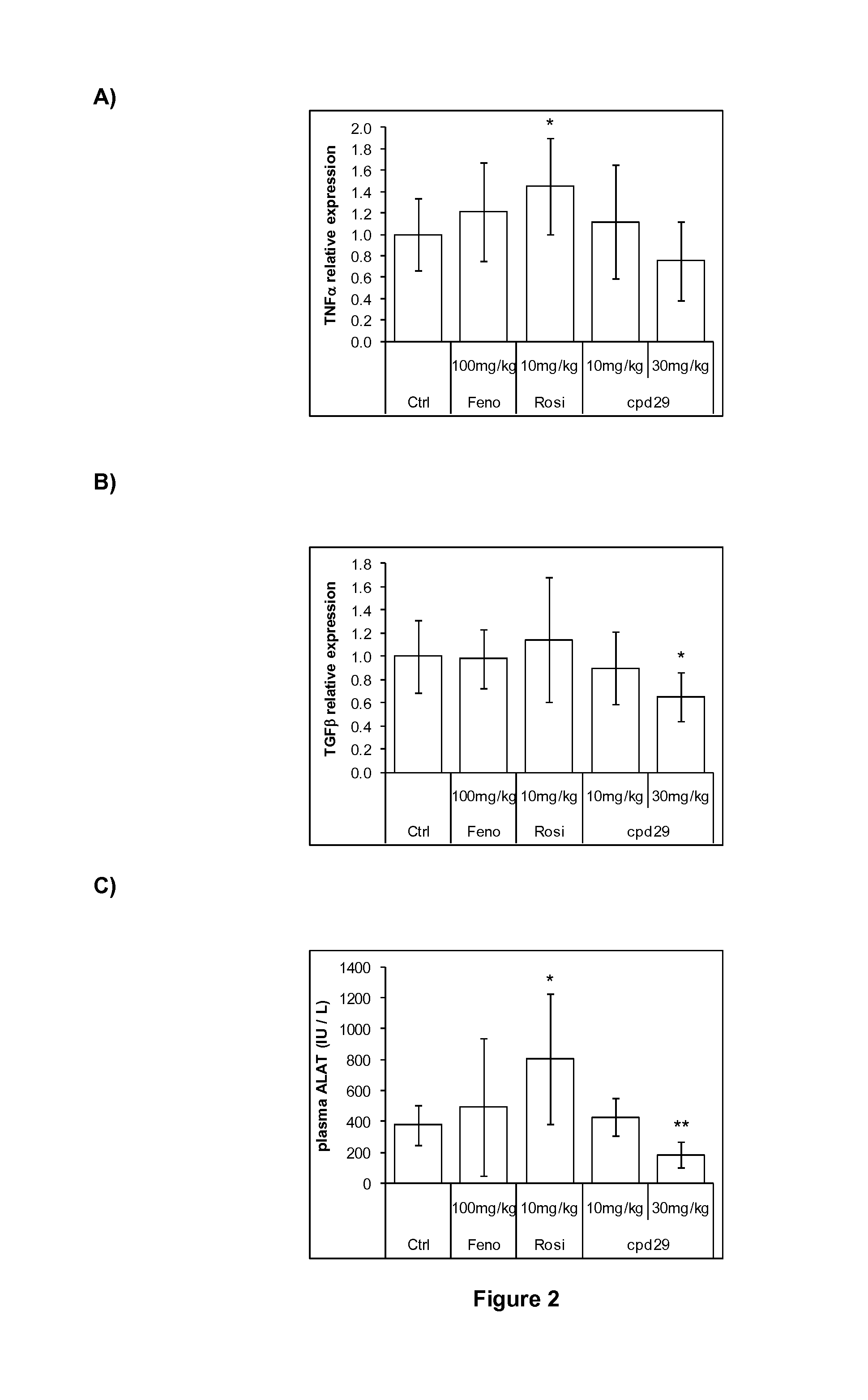
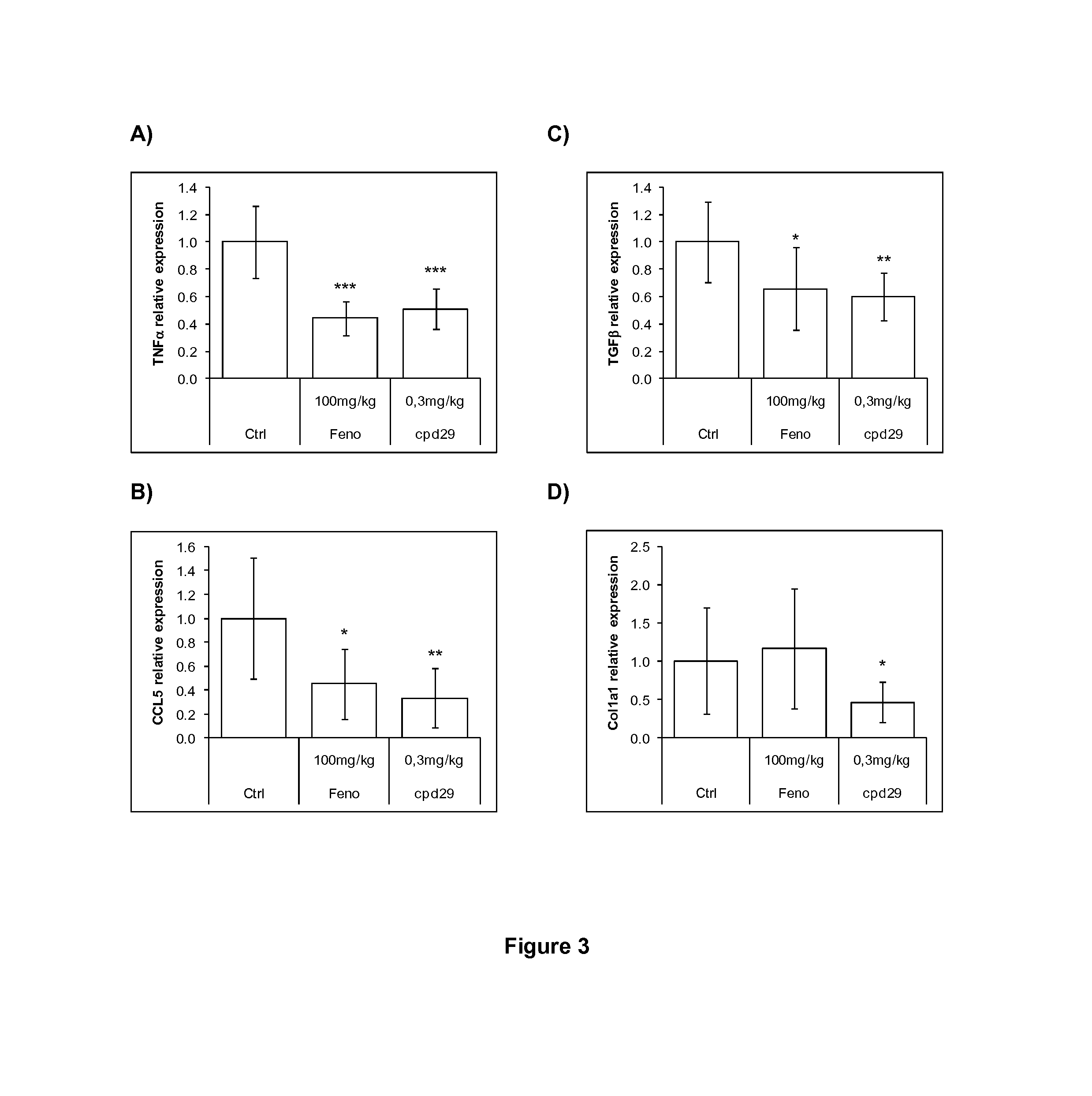
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as aminotransferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
Hydroxybutyrate ester and medical use thereof
ActiveUS20110237666A1Increase alertnessImprove cognitive functionBiocideNervous disorderFatty acidAnimal subject
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
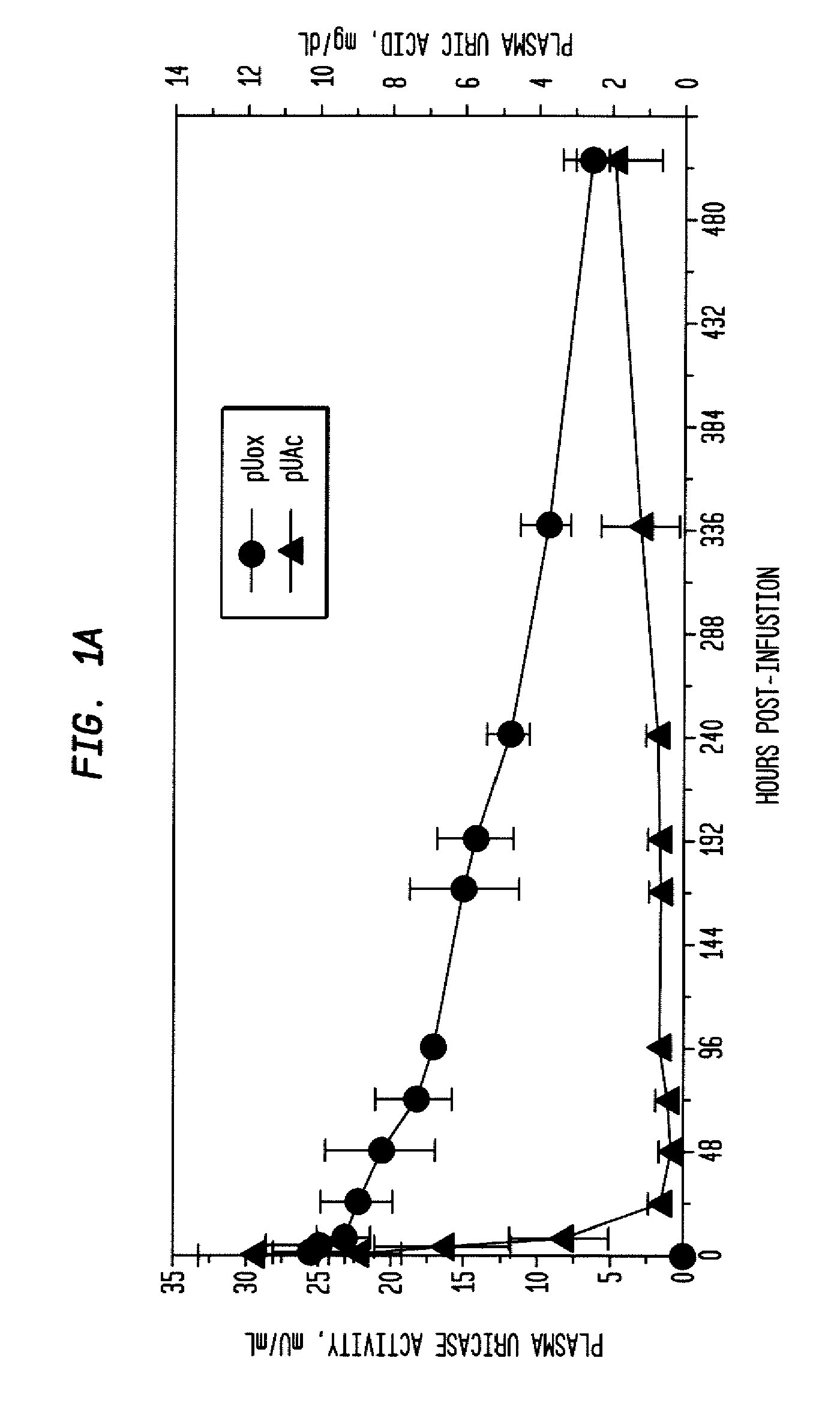
Methods for lowering elevated uric acid levels using intravenous injections of PEG-uricase
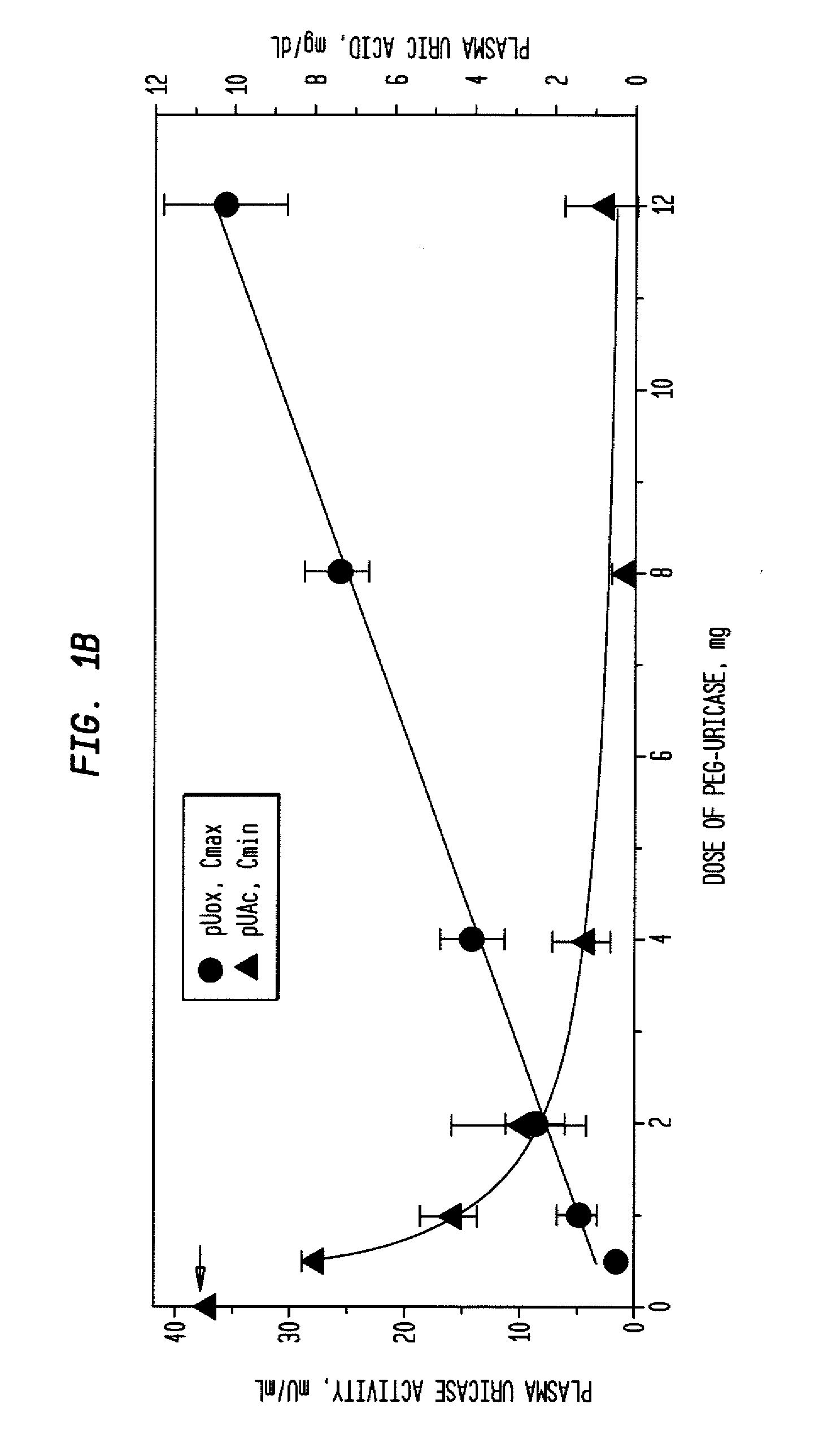
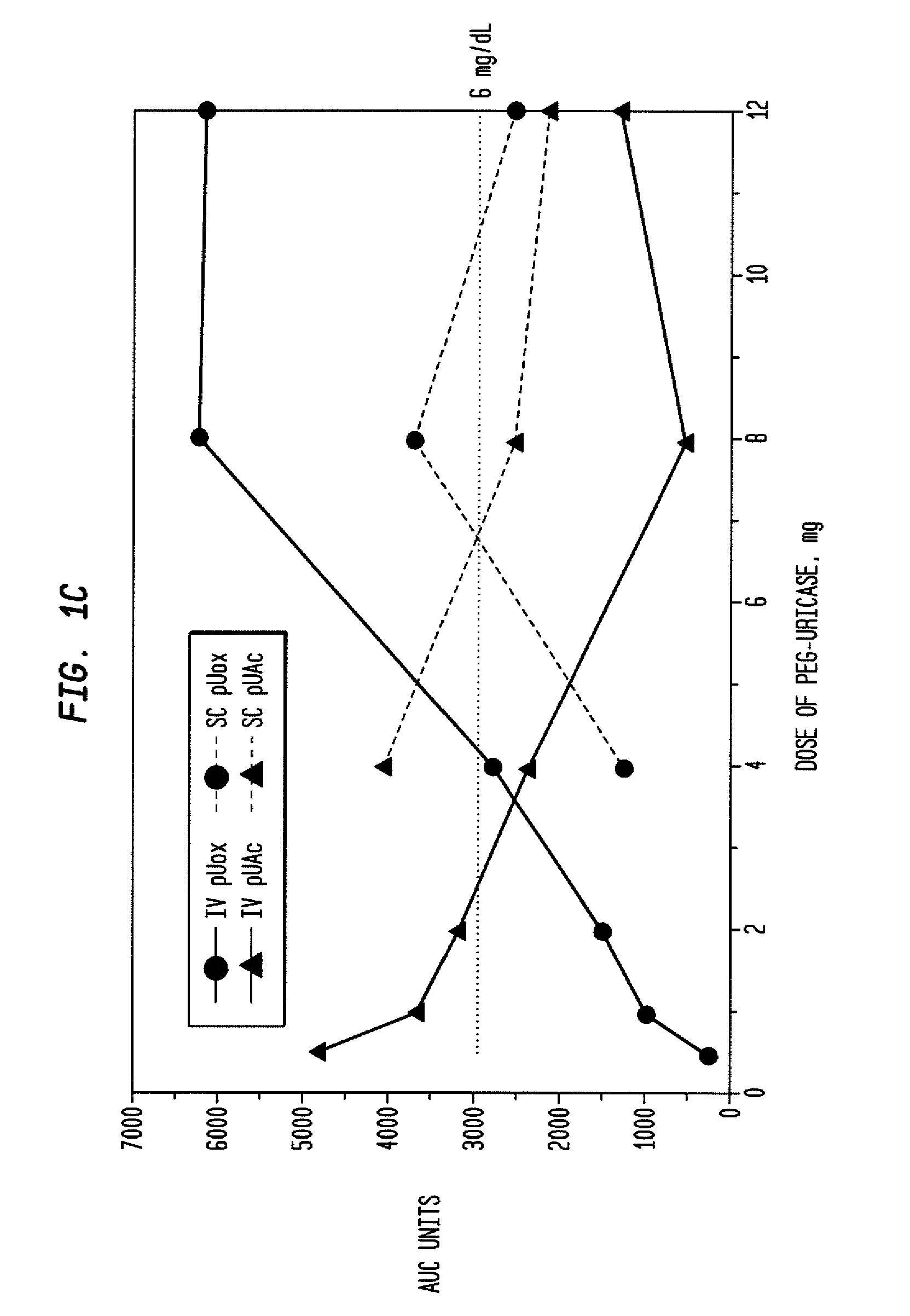
InactiveUS20080159976A1Lower uric acid levelsPeptide/protein ingredientsSkeletal disorderIntravenous therapyBlood plasma
Owner:HORIZON THERAPEUTICS USA INC
Long acting parasiticidal composition containing a salicylanilide compound, a polymeric species and at least one other anti-parasitic compound
ActiveUS20050118221A1Improve bioavailabilityEfficient ConcentrationBiocidePharmaceutical delivery mechanismPolyethylene glycolBlood plasma
A composition prepared for treating animals suffering from parasites which parasites are known to be susceptible to at least one of the avermectins, milbemycins or salicylanilides, comprises for example ivermectin in an amount of from 0.1 to 10%(w / v), a solvent selected from the group consisting of glycerol formal, propylene glycol, polyethylene glycol and combinations thereof, and a salicylanilide such as closantel in a required dosage amount for the animal to be treated, typically about 2.5 mg / kg live weight of the animal to be treated, a polymeric species selected from the group consisting of polyvinylpyrrolidone and polyoxypropylene / polyoxyethylene block copolymers, the said polymeric species improving the bioavailability of closantel to the extend that blood plasma levels of the said compound greater than about 20 ppm over period of treatment are achievable.
Owner:NORBROOK LABORATORIES LIMITED
Release of statins in the intestine
InactiveUS20100055173A1Reduced food effect on the releaseBiocideCapsule deliveryIntestinal structureLower Gastrointestinal Tract
The present invention provides a controlled absorption formulation in which modified release of the active ingredient preferentially occurs in the lower gastrointestinal tract, including the colon. The formulation supports a significantly higher bioavailability of the active ingredient in the body of the subject than that can be achieved from the currently used conventional formulation, such that therapeutically significant plasma levels of statin are maintained for an extended period after administration. The formulation preferably features a core, a subcoat surrounding the core comprising at least one water soluble hydrophilic carrier and an outer coating. The core is optionally and preferably in the form of a tablet.
Owner:DEXCEL PHARMA TECH
Pharmaceutical Compositions Comprising Sirolimus and/or an Analogue Thereof
InactiveUS20080275076A1Improve safety/efficacy ratioReduce impactAntibacterial agentsBiocideParticulatesSide effect
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising sirolimus (rapamycin) and / or derivatives and / or analogues thereof. Compositions of the invention exhibit an acceptable bioavailability of sirolimus and / or a derivative and / or an analogue thereof. The pharmaceutical compositions of the invention are designed to release sirolimus in a controlled manner so that the plasma levels stays within the narrow therapeutic window that exist for this class of substances. An extended release profile, where the peak concentration has been reduced without loosing significant bioavailability, together with less variable absorption, is expected to improve the safety / efficacy ratio of the drug. Furthermore, compositions according to the invention provide for a significant reduced food effect and a delayed release of sirolimus is expected to reduce the number of gastro-intestinal related side effects.
Owner:LIFECYCLE PHARMA AS
Methods of modulation of branched chain acids and uses thereof
ActiveUS20120220661A1Decrease in blood plasma levelImprove enzymatic activityBiocideMetabolism disorderScavengerBlood plasma
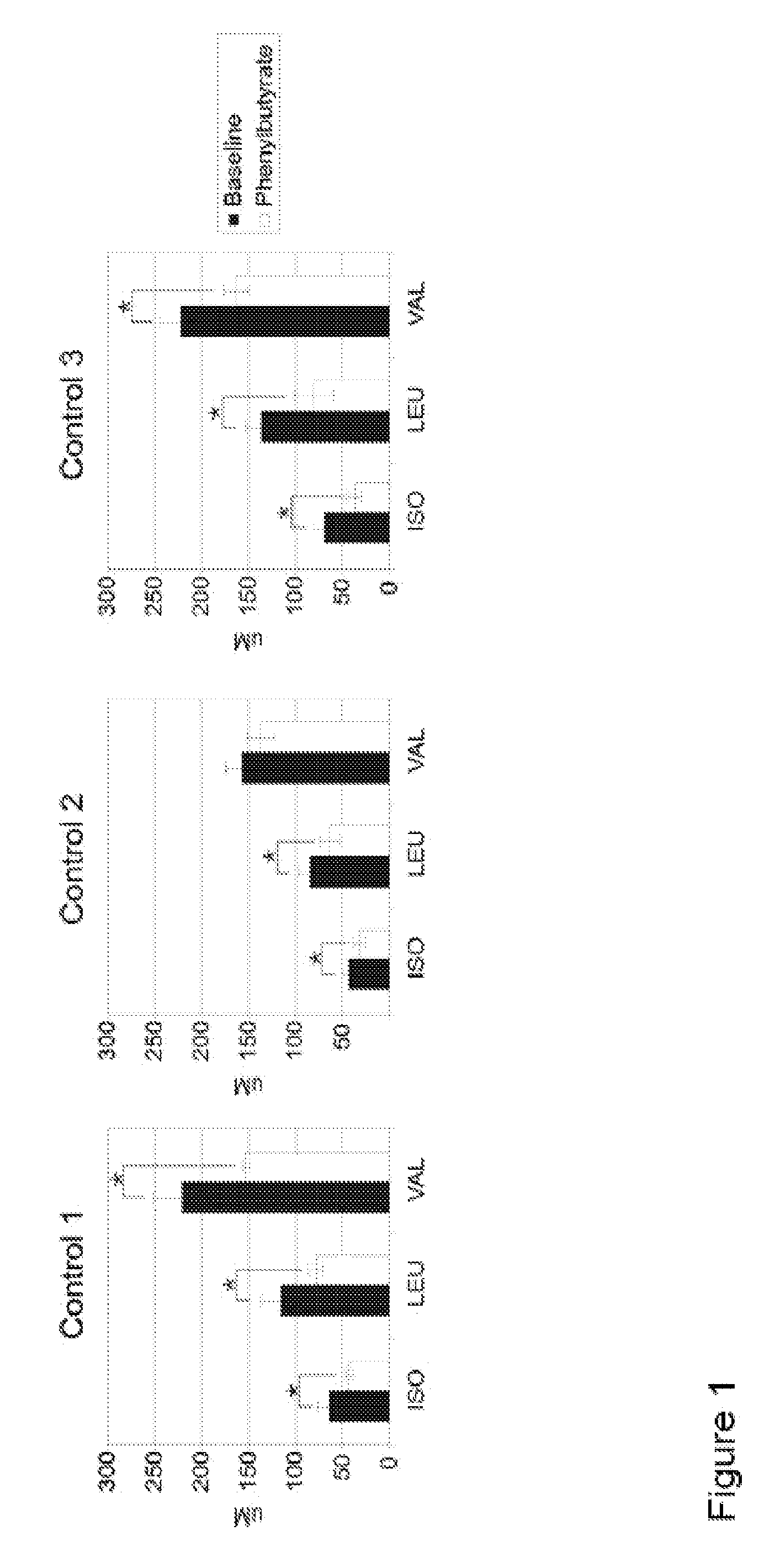
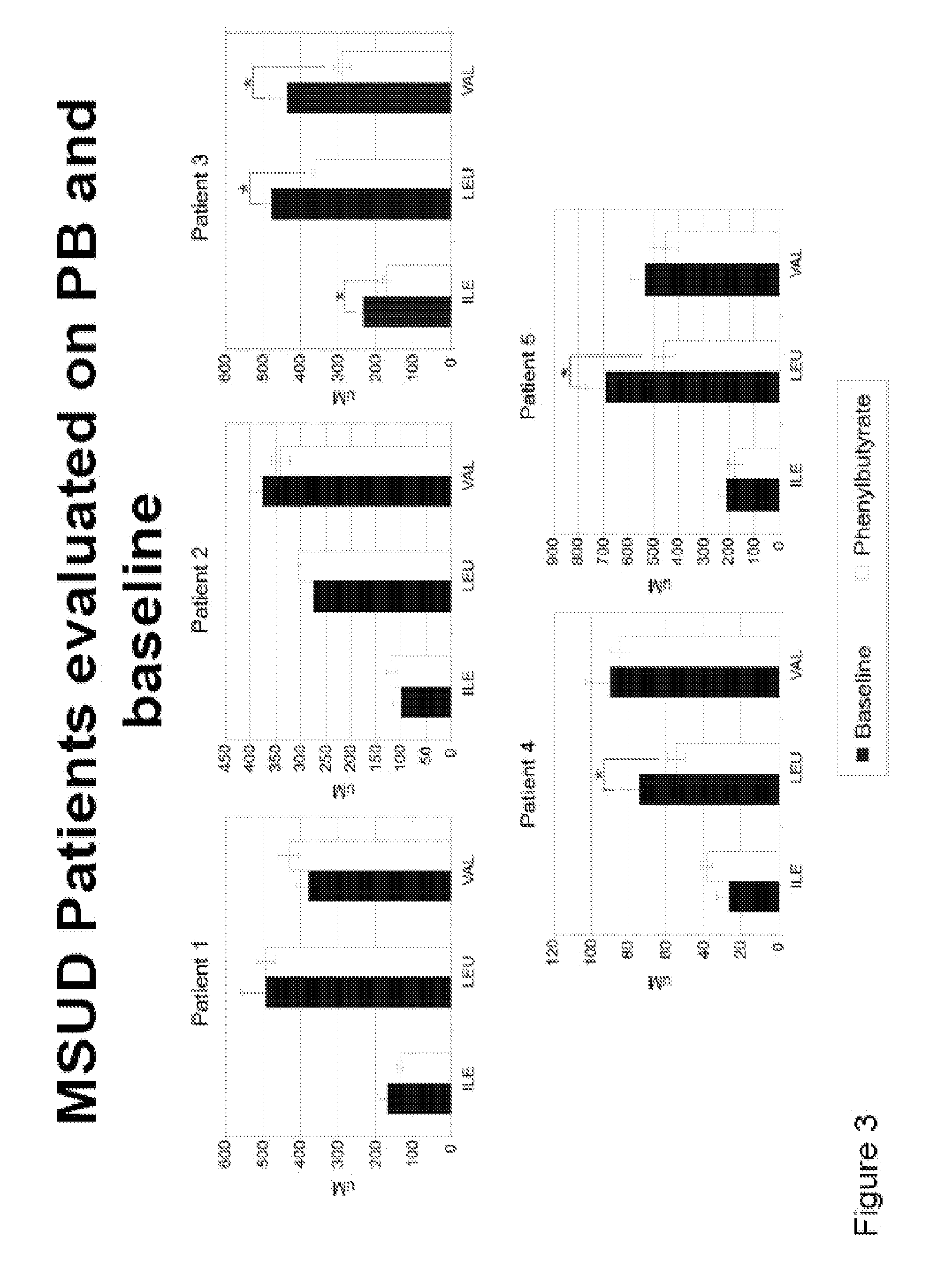
A method of modulating plasma levels of branched chain amino acids and branched chain alpha-keto acids is disclosed, wherein an ammonia scavenger compound or a salt thereof, for example phenylbutyrate or an even numbered congener thereof or a salt thereof, is administered to an individual in need thereof. In various methods, a decrease in plasma levels of branched chain amino acids and branched chain alpha-keto acids is effected to treat individuals suffering from an inborn error in metabolism of amino acids, such as Maple Syrup Urine Disease, for example.
Owner:BAYLOR COLLEGE OF MEDICINE
Sustained release oral dosage forms of gabapentin
InactiveUS20050158380A1Extended gastric residence timeGood sustained releaseOrganic active ingredientsBiocideGabapentinSustained Release Tablet
The present invention relates to sustained release oral dosage forms of gabapentin and at least one rate controlling polymer, and a process for the preparation of the sustained release oral dosage forms, and a process for the preparation thereof. The sustained release tablet includes gabapentin or a pharmaceutically acceptable salt or hydrates thereof and at least one rate-controlling polymer such that the tablet provides therapeutically effective plasma levels of gabapentin for a period of up to about 12 hours.
Owner:RANBAXY LAB LTD
Treatment of hibernating myocardium with a GLP-1 peptide
InactiveUS20050096276A1Suppress plasma blood levelEase ischemic stressPeptide/protein ingredientsMetabolism disorderCatecholamineHigh energy
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:COOLIDGE THOMAS +1
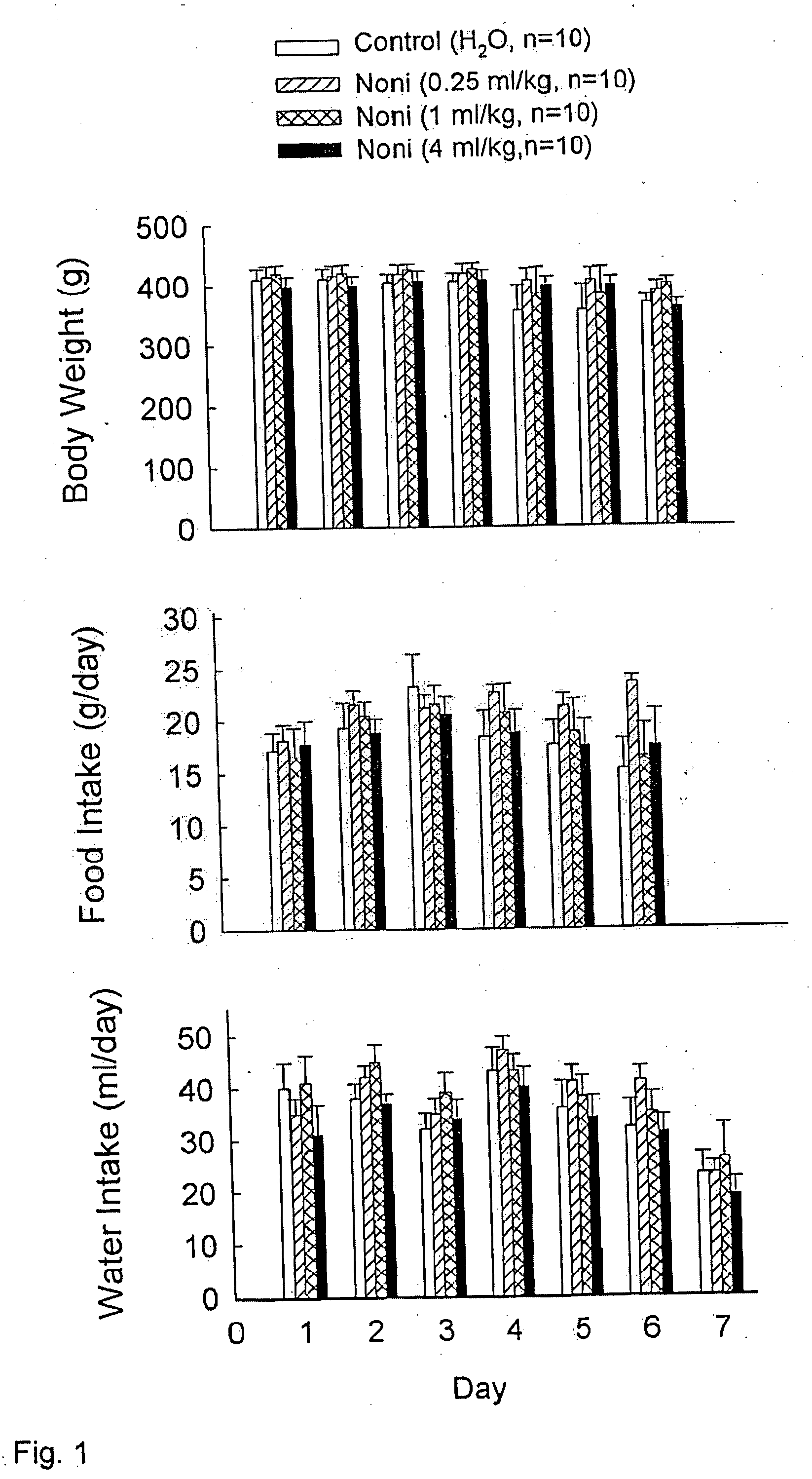
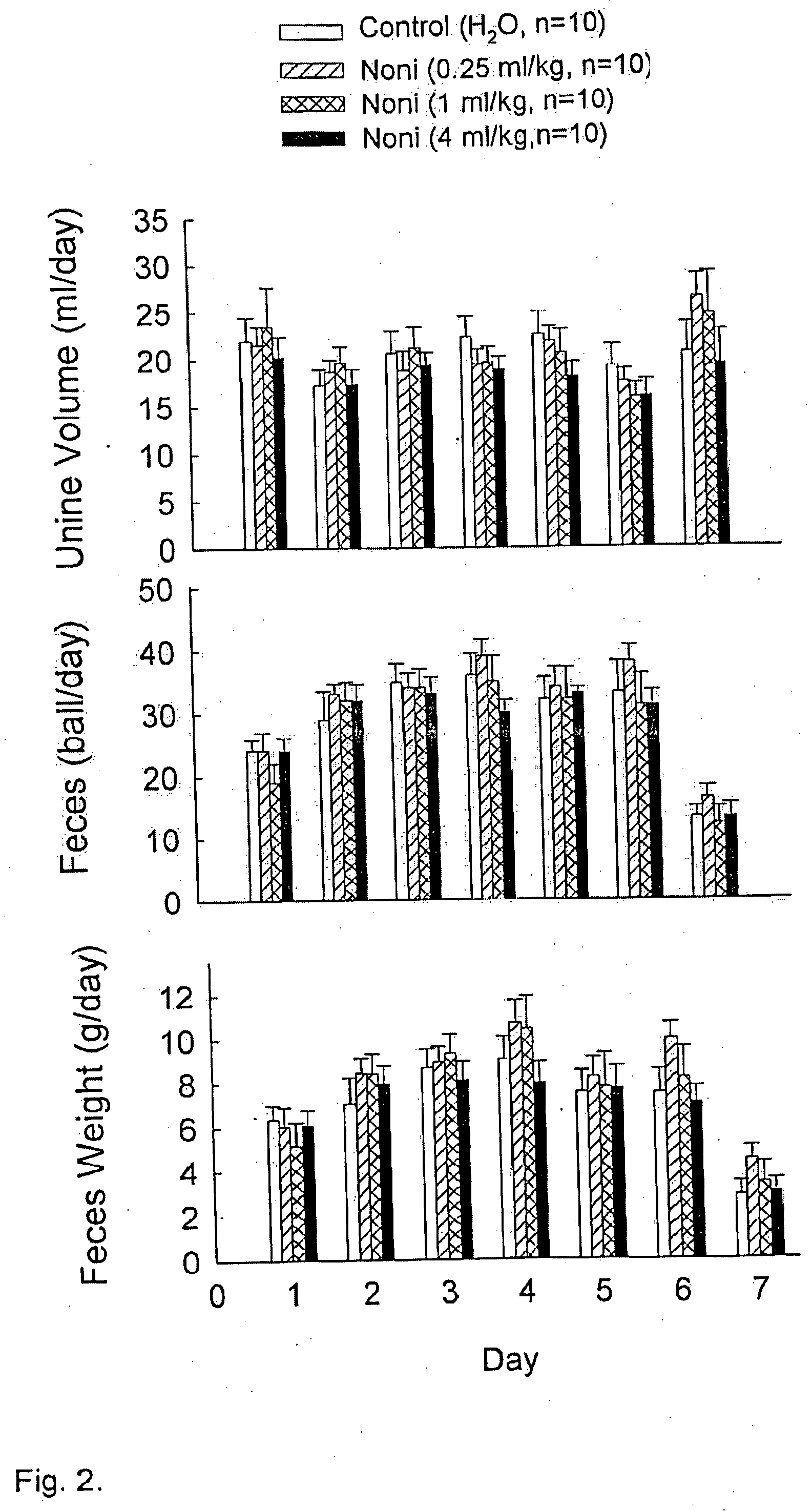
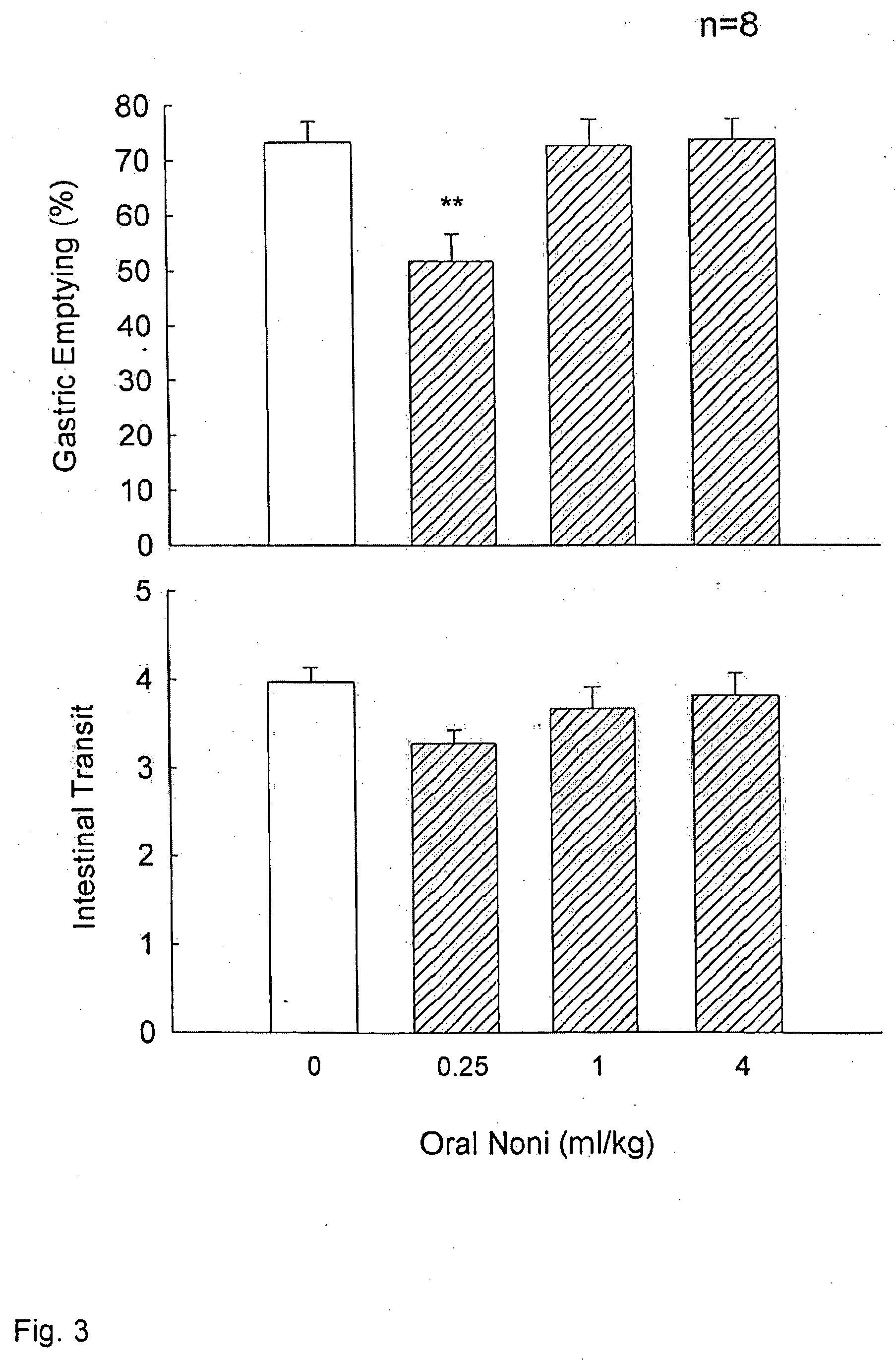
Morinda citrifolia-based formulations and methods for weight management
InactiveUS20060088611A1Inhibit gastric emptyingImprove concentrationBiocideMetabolism disorderWeight managementBlood plasma
The present invention relates to formulations and methods for weight management utilizing processed Morinda citrifolia products or extracts. Specifically, the present invention relates to formulations, which may be used for weight loss, regulating gastric motility and regulating plasma levels of cholecystokinin.
Owner:TAHITIAN NONI INT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com