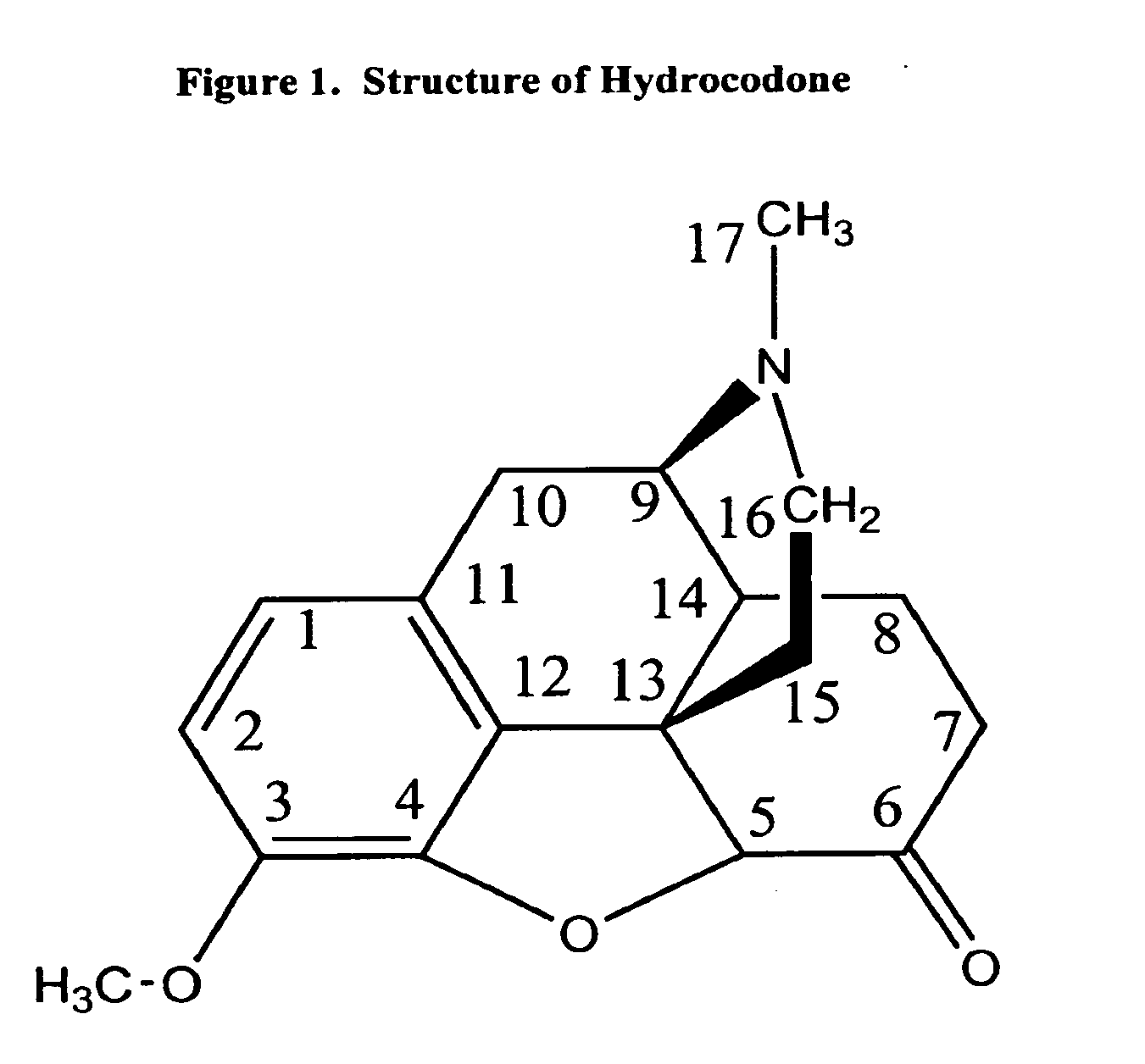
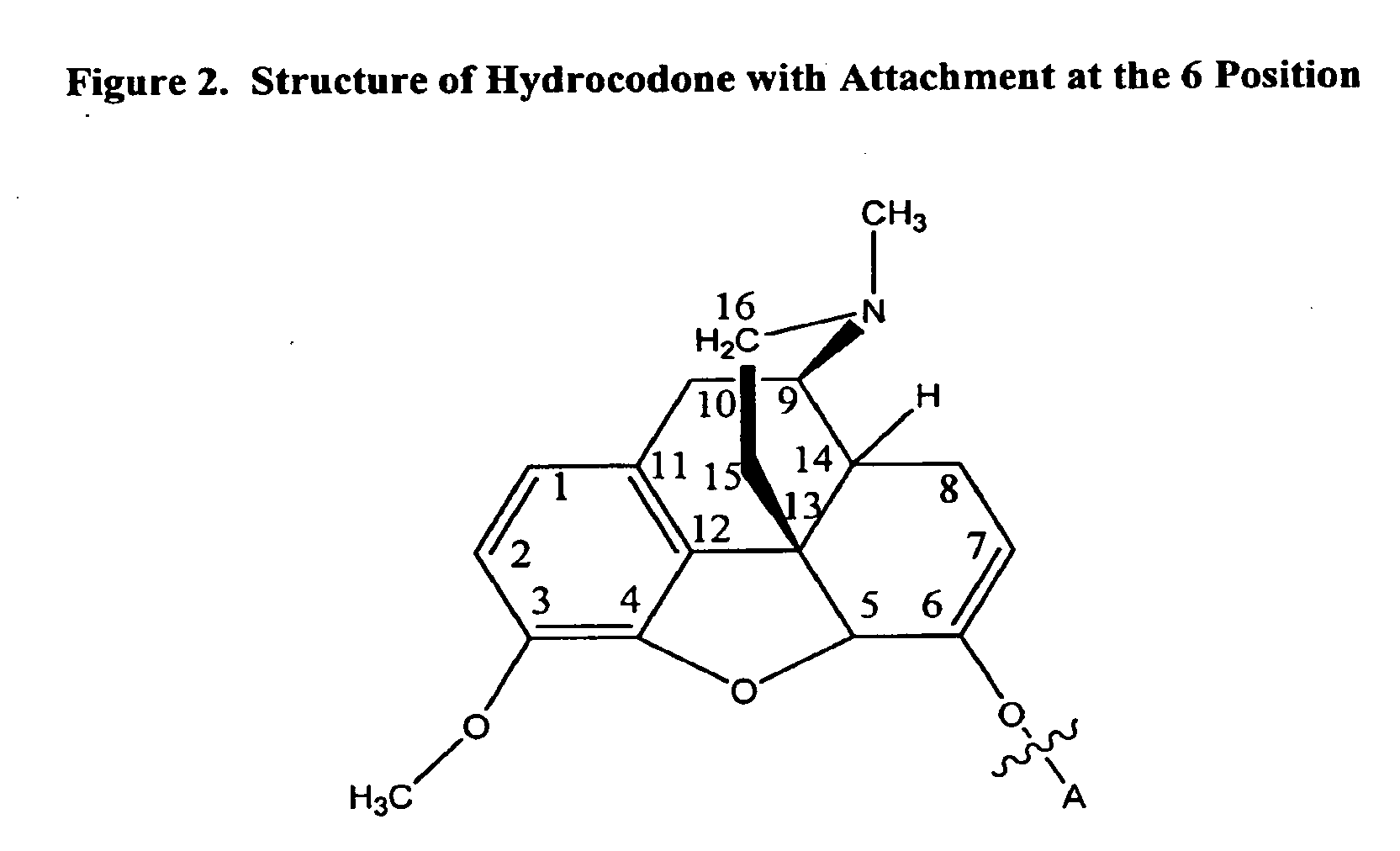
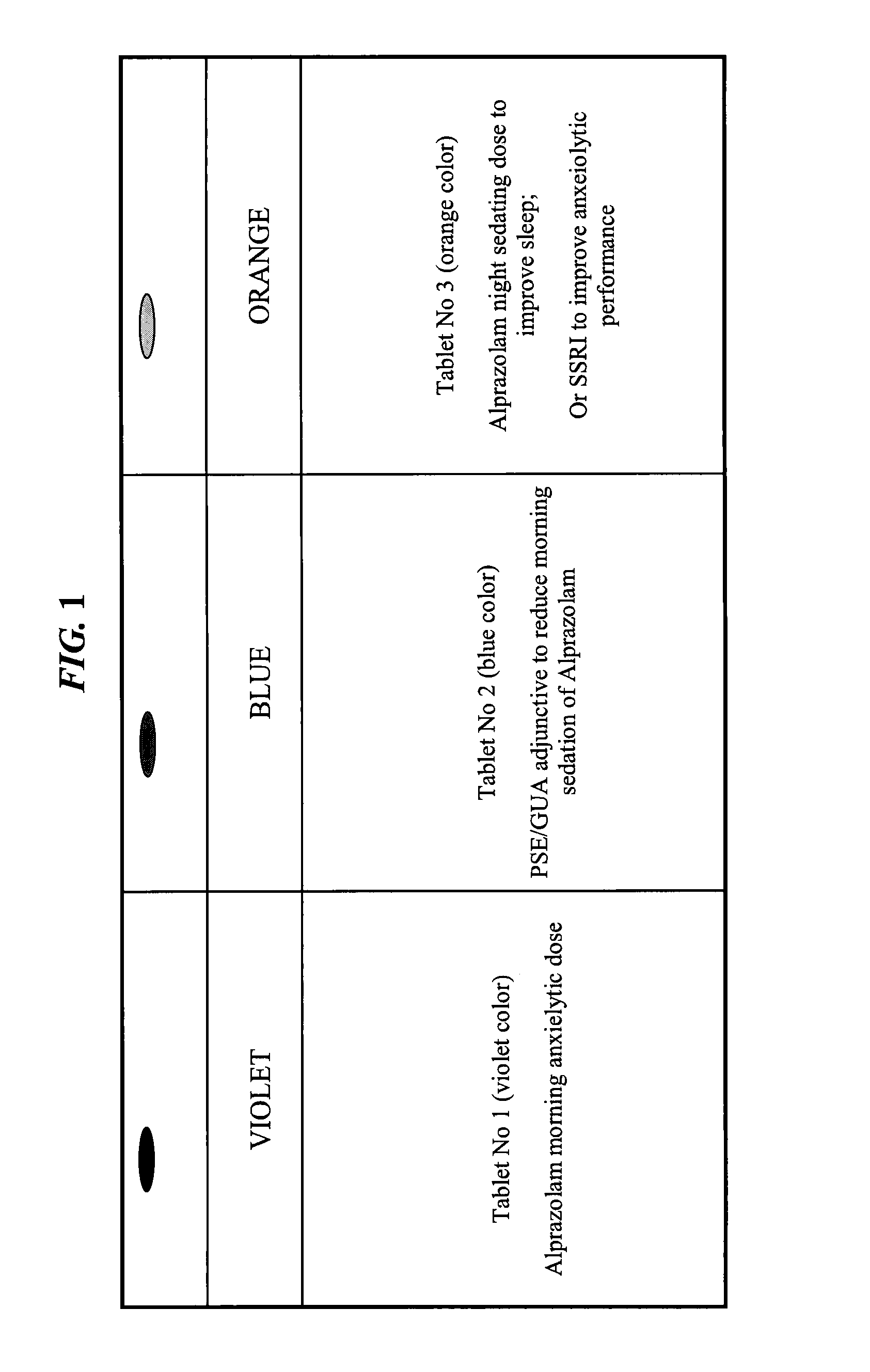
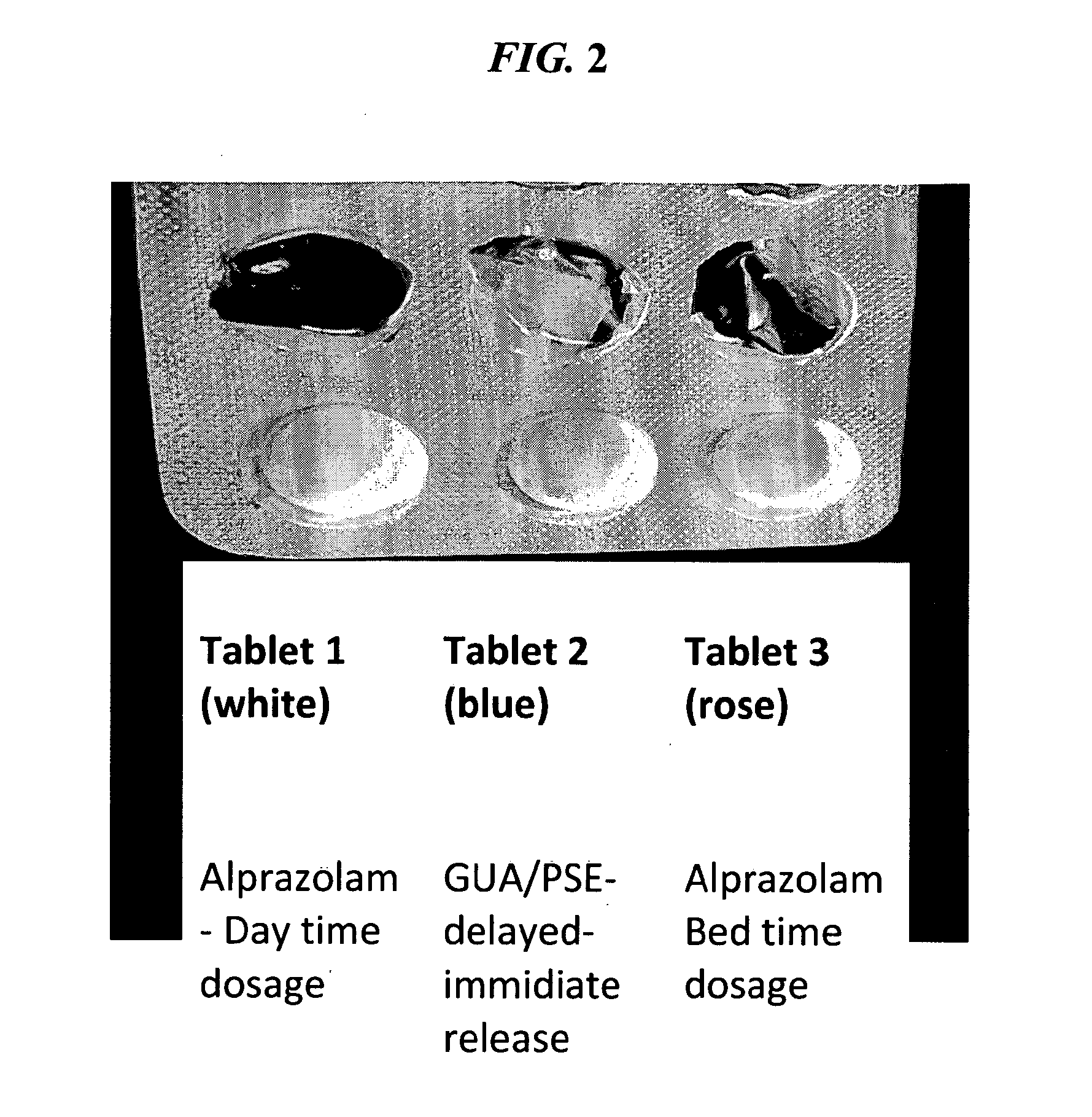
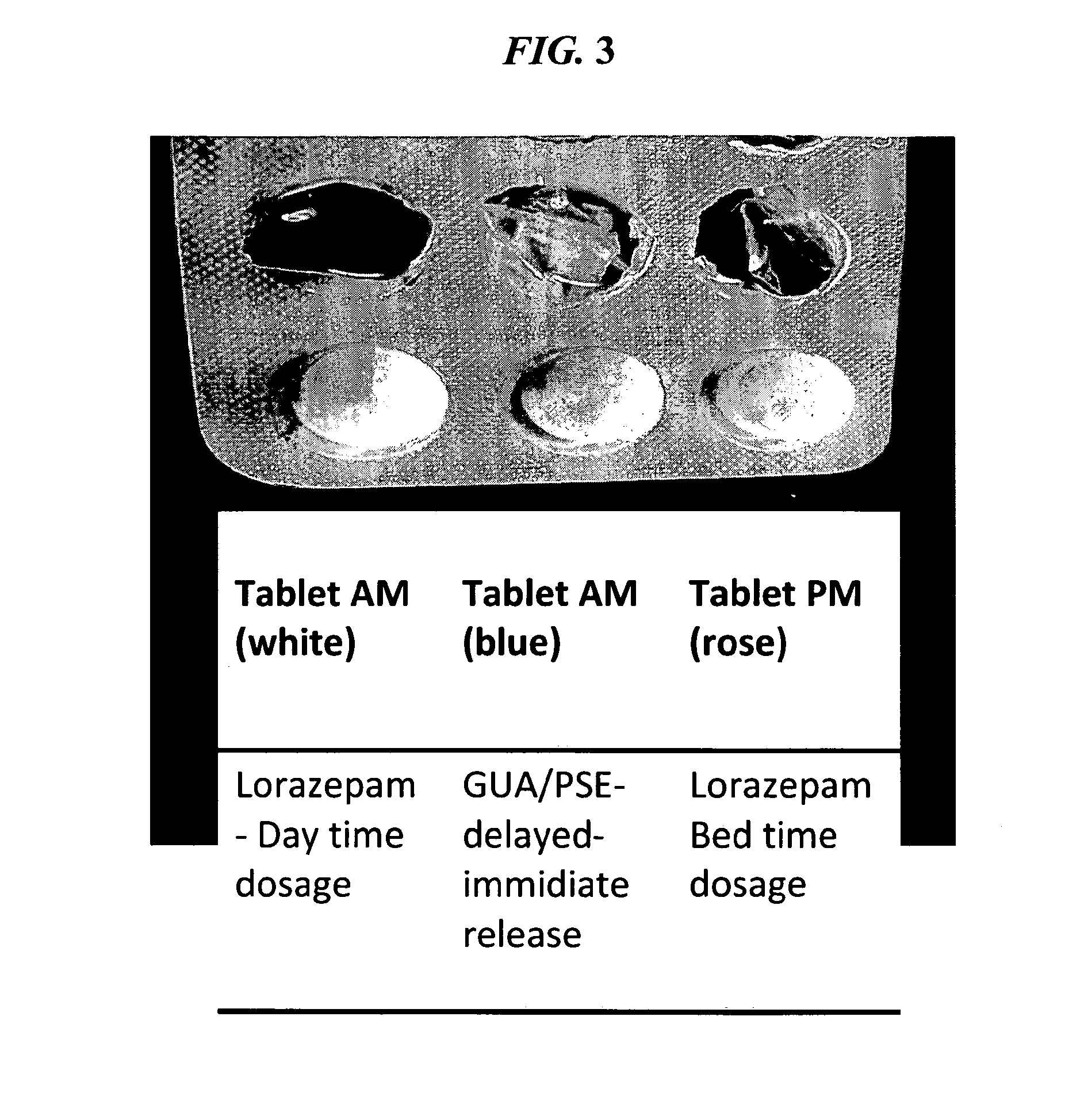
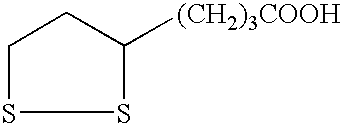
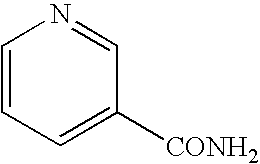
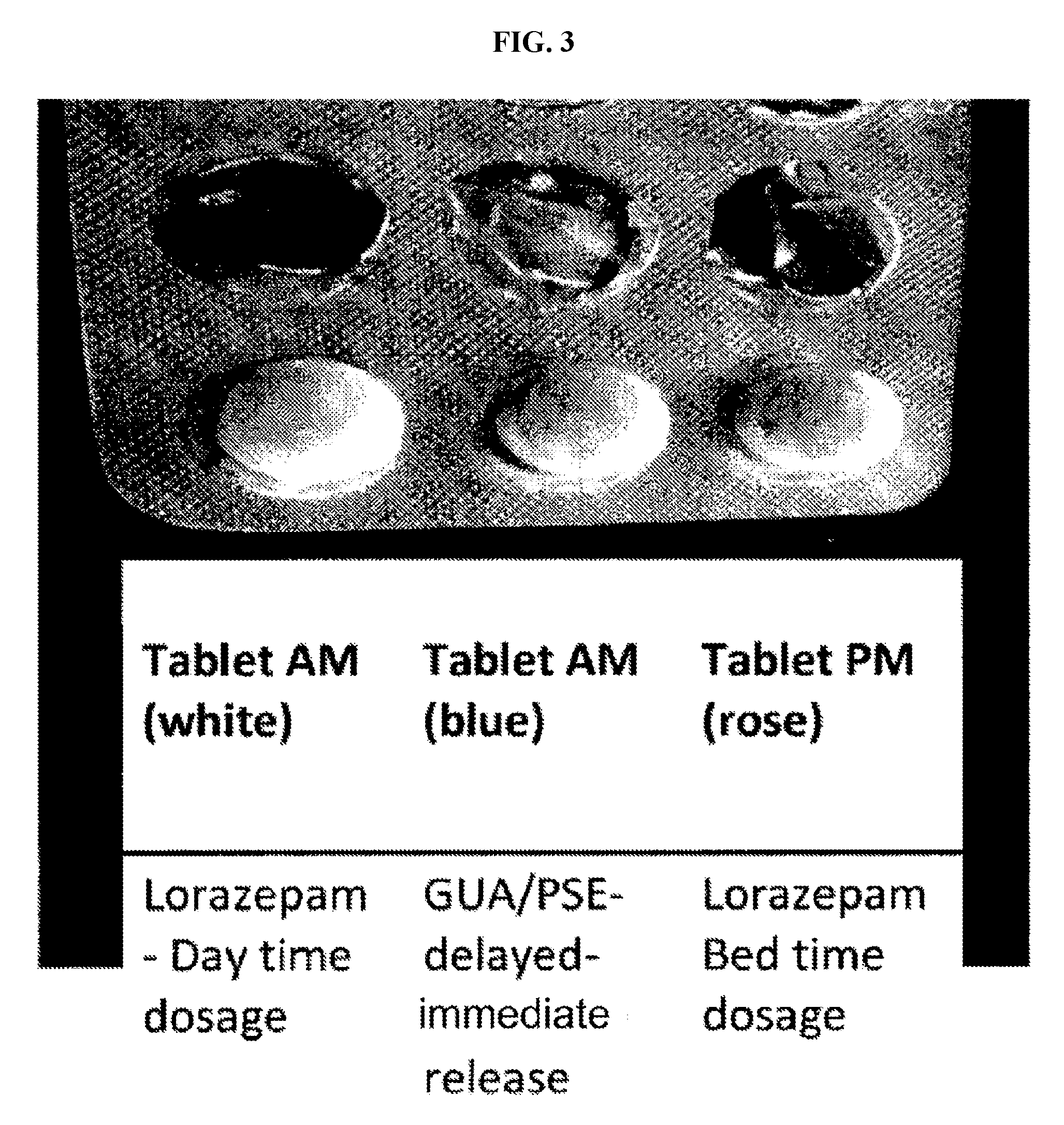
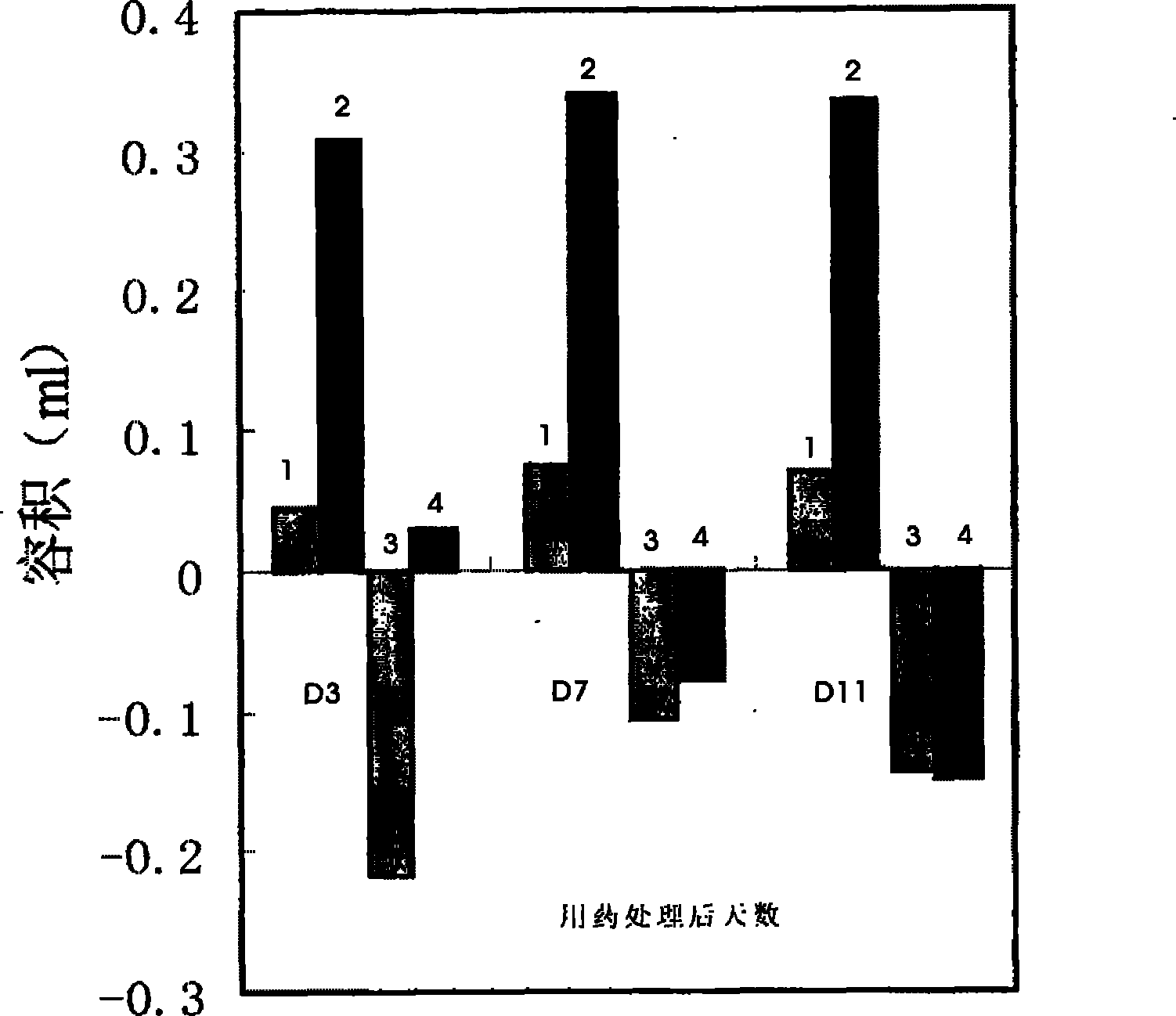
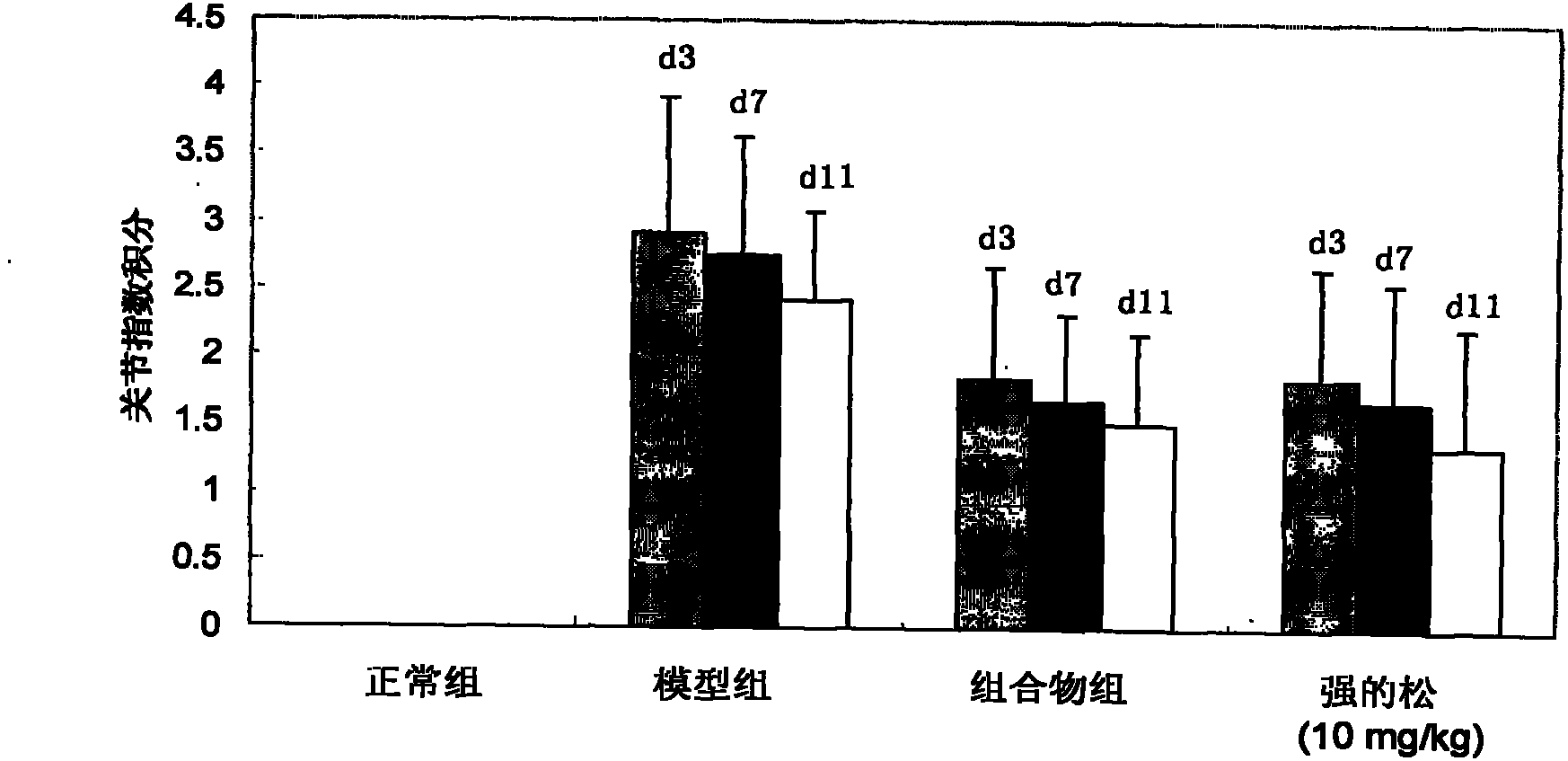
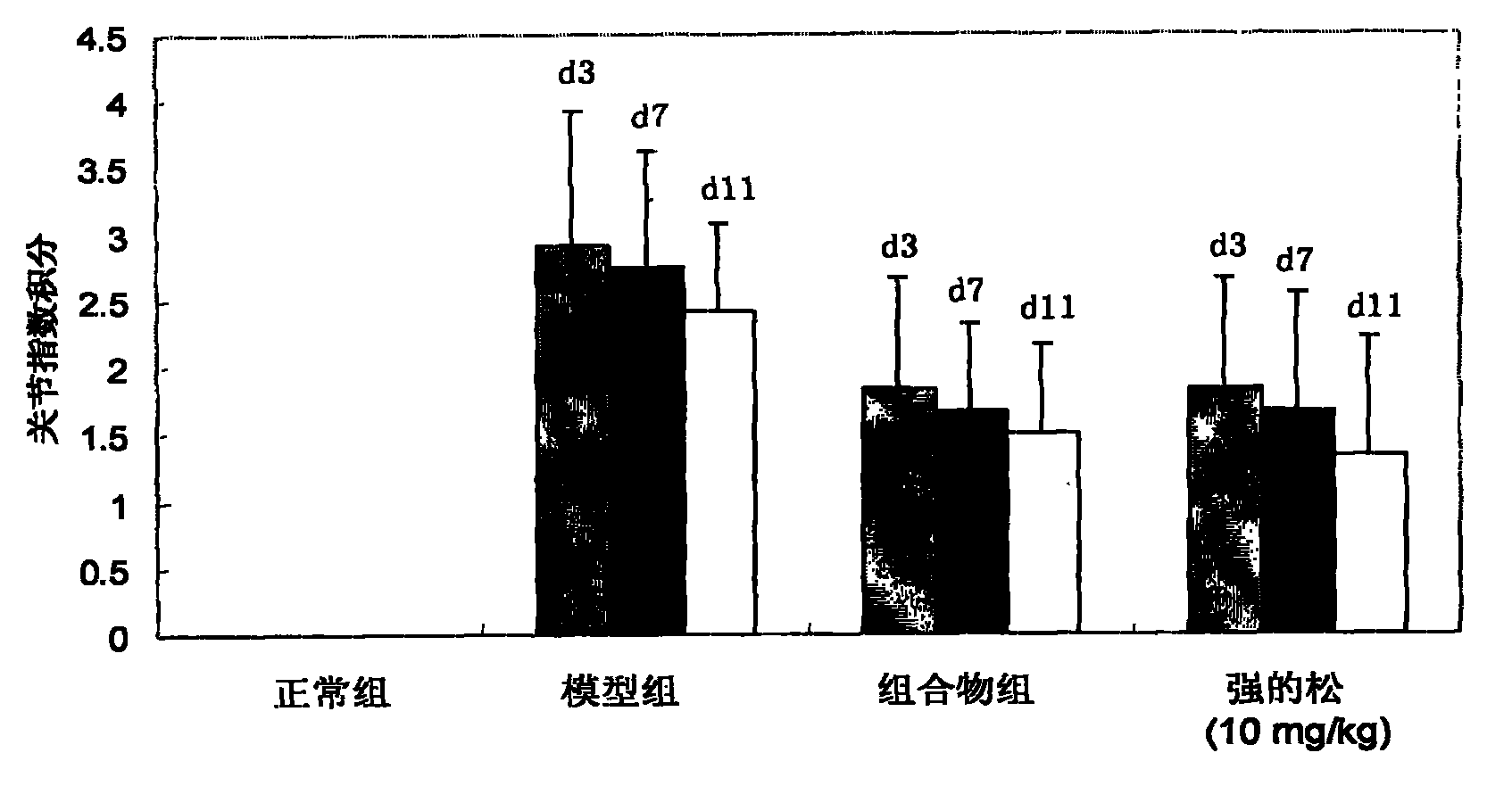
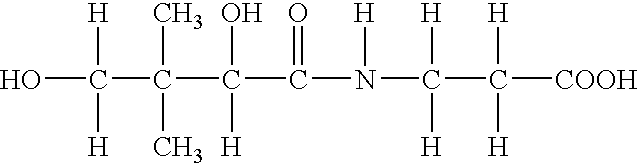Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Daytime somnolence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Somnolence is often viewed as a symptom rather than a disorder by itself. However, the concept of somnolence recurring at certain times for certain reasons constitutes various disorders, such as excessive daytime sleepiness, shift work sleep disorder, and others; and there are medical codes for somnolence as viewed as a disorder.
Modified release compositions of milnacipran
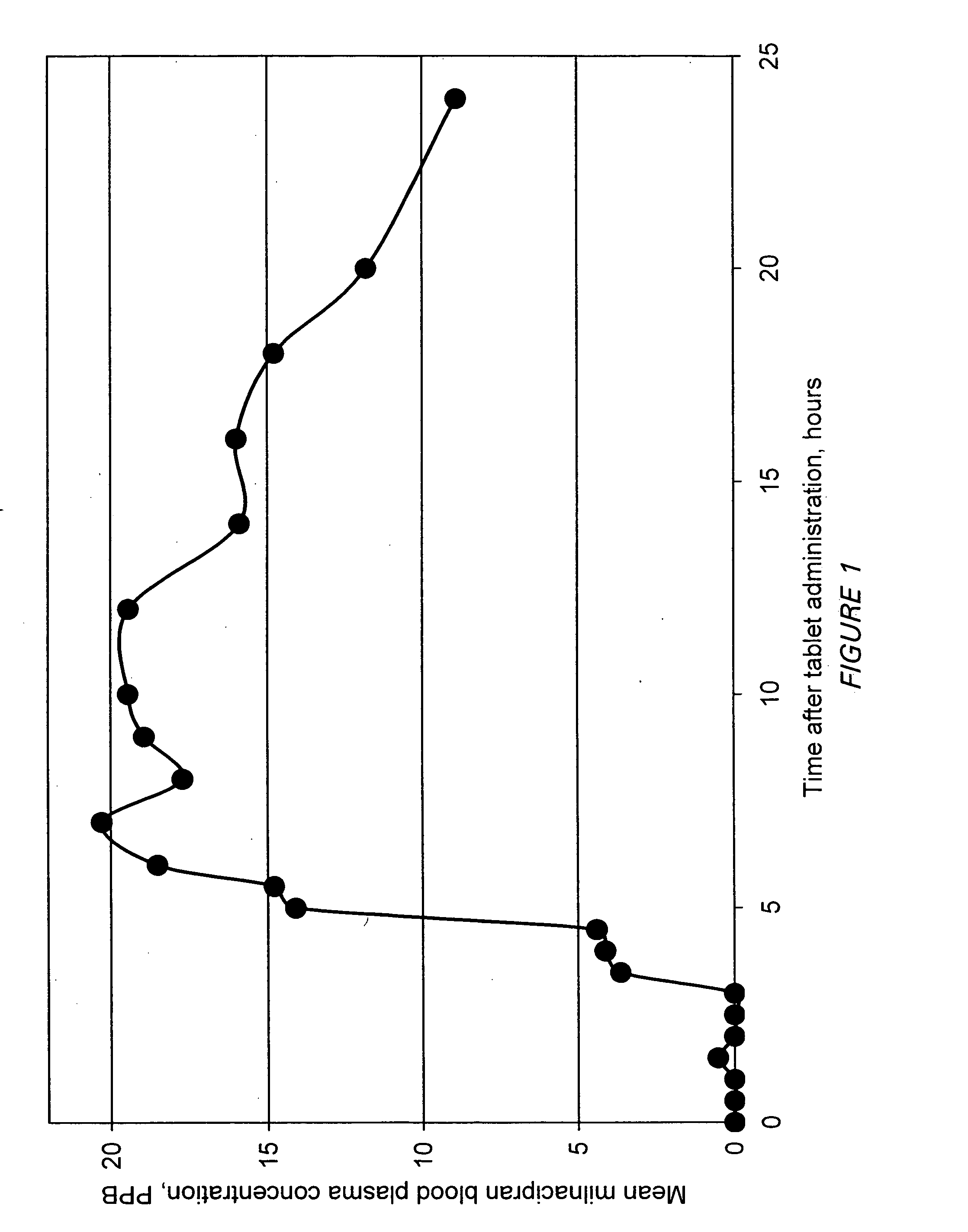
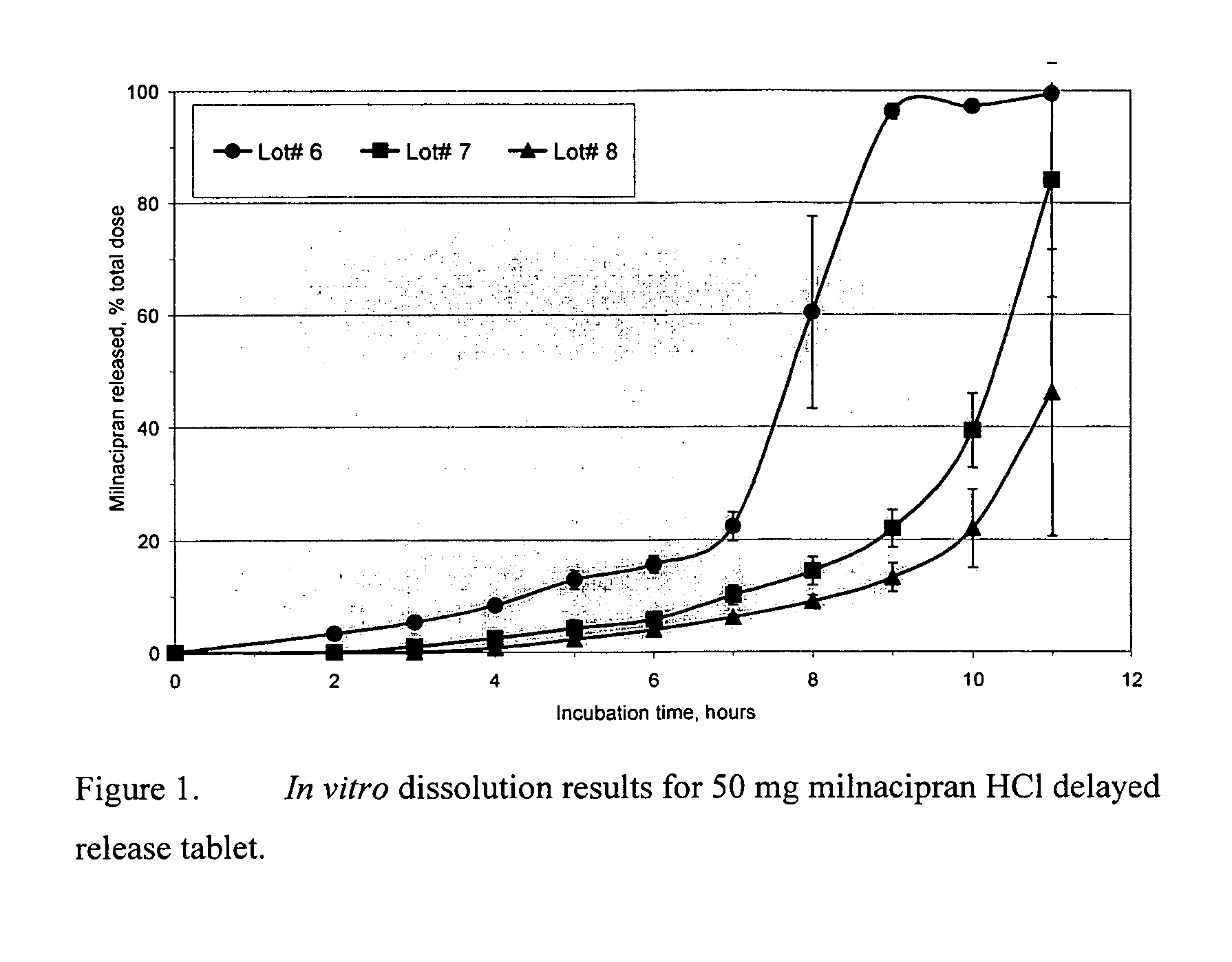
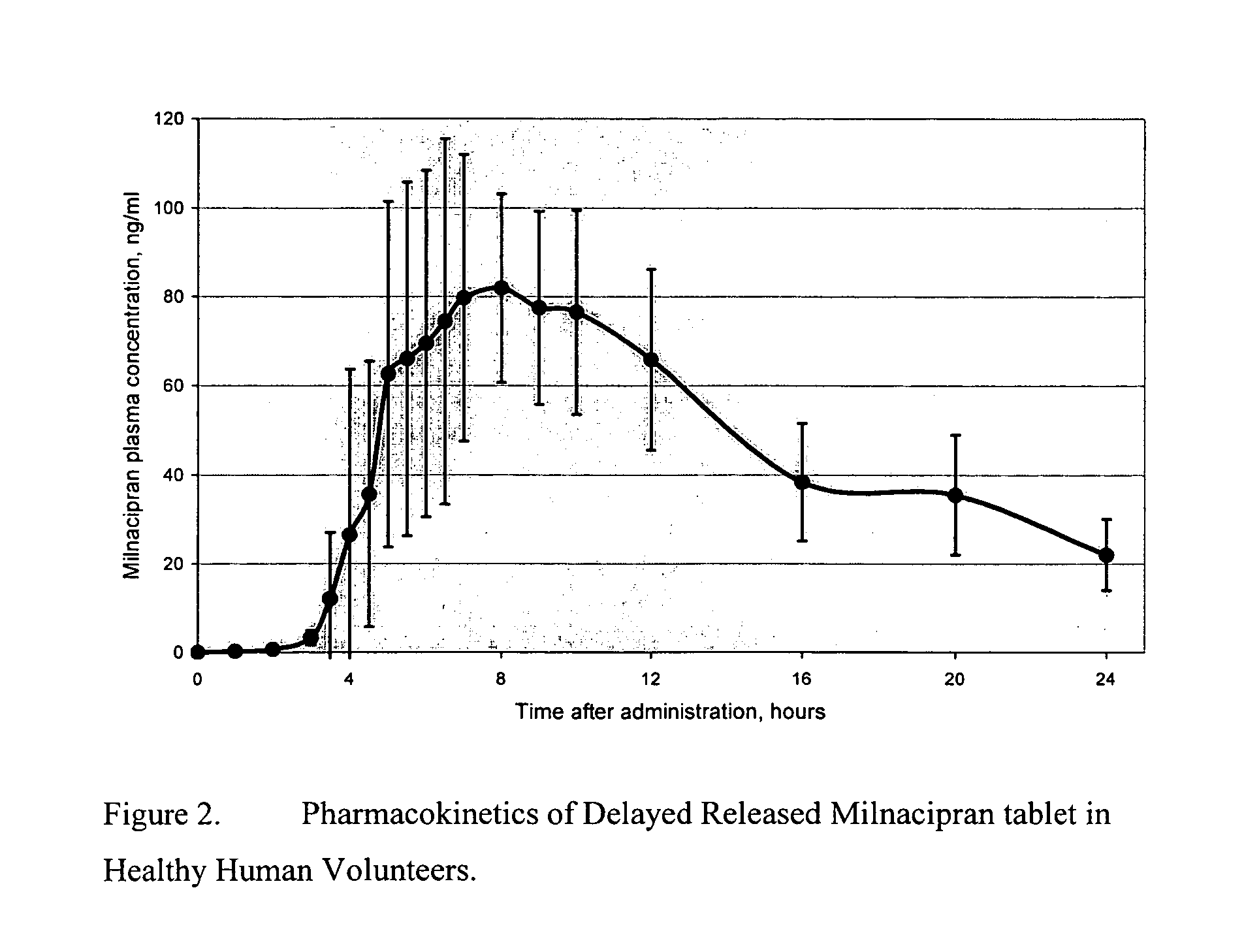
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Pharmaceutical Compositions and Related Methods of Treatment
InactiveUS20080021074A1Decrease and prevent sleepinessDecrease and prevent and lethargyBiocideNervous disorderAdrenergic receptor agonistsClumsiness
Pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed. Pharmaceutical compositions comprising tizanidine and modafinil are disclosed. Methods for reducing somnolence, sleepiness, lethargy, dizziness, drowsiness, somnolence, tiredness, lightheadedness, increased weakness, confusion, unsteadiness, clumsiness, or a combination of the symptoms thereof in a human patient; treating pain; and attenuating muscle spasticity, using pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed.
Owner:QUESTCOR PHARMA
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
Compositions and methods for enhancing analgesic potency of covalently bound-compounds, attenuating its adverse side effects, and preventing their abuse
InactiveUS20100144645A1Lower potentialAmenable to synthesizing conjugatesBiocideNervous disorderChemical MoietyOpioid antagonist
The invention generally relates to compositions and methods with covalently bound compounds, such as controlled substances covalently attached to a chemical moiety, and opioid antagonists or covalently bound opioid antagonists to enhance analgesic potency and / or attenuate one or more adverse effects of covalently bound compounds, including adverse side effect(s) in humans such as nausea, vomiting, dizziness, headache, sedation (somnolence), physical dependence or pruritis. This invention relates to compositions and methods for selectively enhancing the analgesic potency of a covalently bound compound and simultaneously attenuating anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects associated with the administration of a covalently bound compound. The methods of the invention comprise administering to a subject an analgesic or sub-analgesic amount of a covalently bound compound and an amount of excitatory opioid receptor antagonist such as naltrexone or nalmefene effective to enhance the analgesic potency of a covalently bound compound and attenuate the anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects of covalently bound compound. The invention also relates to the addition of covalently-bound opioid antagonists to the compositions containing covalently bound compounds such that if the compositions are subjected to manipulation by illicit chemists, the opioid antagonist is released effectively reducing or eliminating the euphoric effect of the covalently bound compounds.
Owner:SHIRE PLC
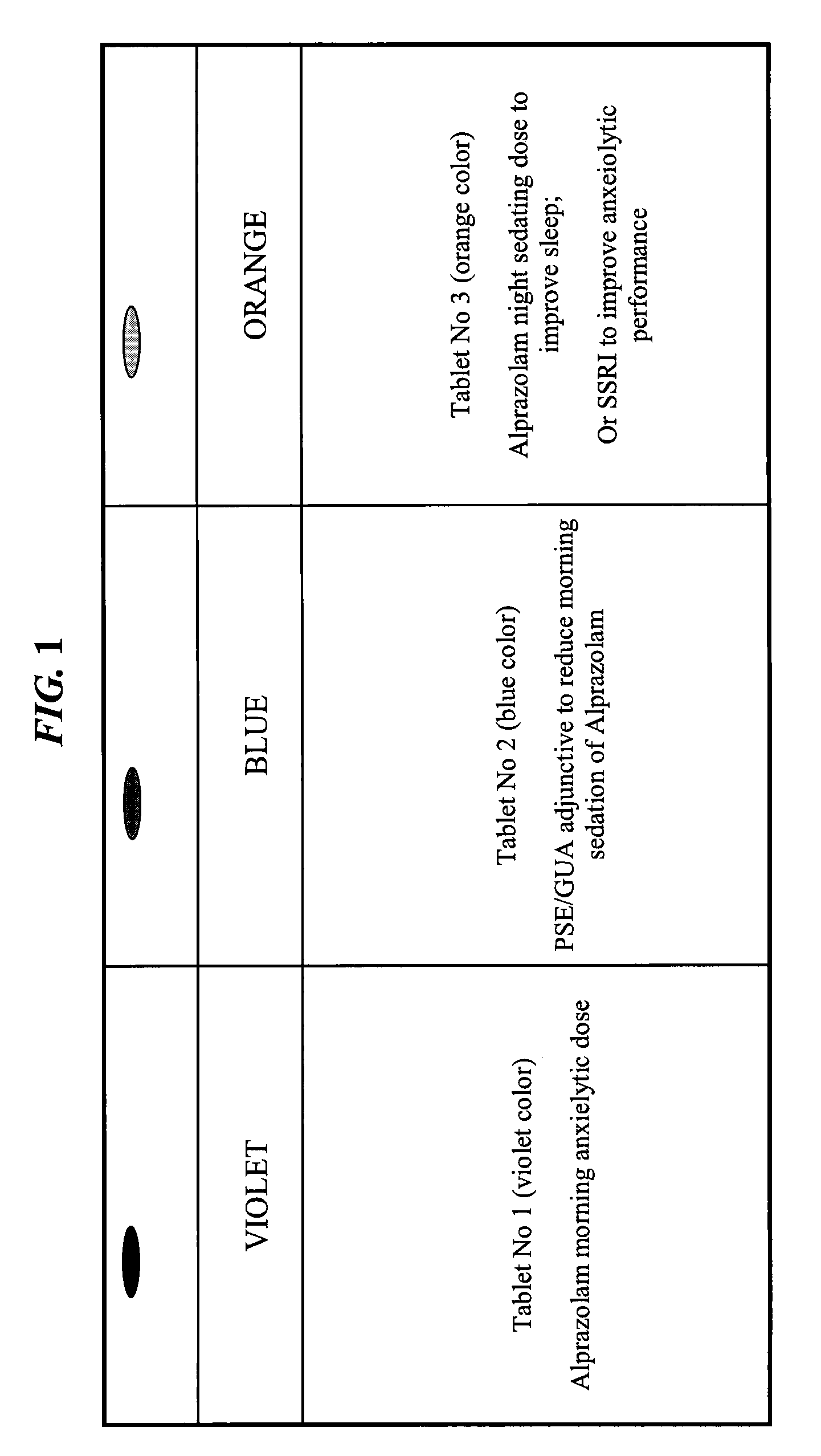
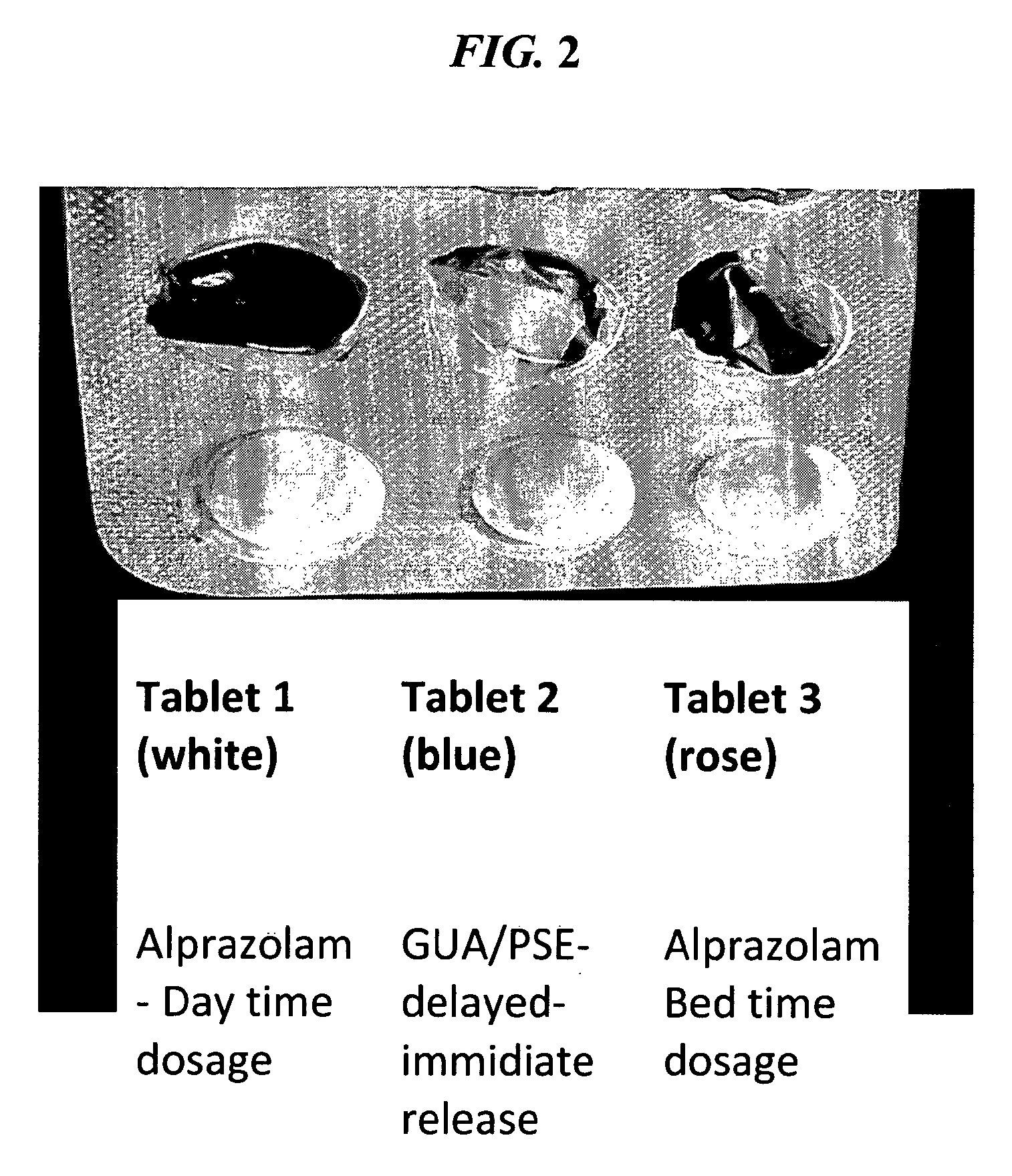
CNS pharmaceutical compositions and methods of use
ActiveUS20140256709A1Eliminate side effectsReduce dependenceBiocideNervous disorderSide effectOral medication
The present invention is directed to CNS pharmaceutical compositions and methods of use. The pharmaceutical compositions comprise a CNS active agent and preferably at least two vagal neuromodulators, one of which is a mechanoreceptor stimulator. The vagal neuromodulators are preferably in an amount sufficient to reduce a somnolence side-effect of the CNS active agent without changing its therapeutic efficacy / activity. The invention further encompasses a method of reducing CNS active agent side-effects. The method typically comprises oral administration of at least one CNS active agent to a patient at the conventionally accepted dose; and administration of at least two vagal neuromodulators to the patient so that at least one neuromodulator is administered or released from dosage form after the CNS active agent is administered and / or released.
Owner:TARGIA PHARMA
Chinese medicinal composition for curing somnolence and preparation method thereof
InactiveCN101890142AImprove self-coordinationEffective treatmentNervous disorderMammal material medical ingredientsSleep paralysisSalvia miltiorrhiza
The invention discloses a novel Chinese medicinal composition for curing somnolence and a preparation method thereof. The Chinese medicinal composition mainly comprises the following raw material medicaments: tangerine peel, immature bitter orange, raw raidx astragali, white paeony root, Chinese angelica, common yam rhizome, campanumaea pilosula, ginseng, motherwort herb, storax, nutgrass galingale rhizome, camphor, Szechwan Chinaberry Fruit, chrysanthemum, salvia miltiorrhiza bunge, fineleaf schizonepeta herb, divaricate saposhnikovia root, incised notopterygium rhizome, medicated leaven, malt, radish seed, peach seed, safflower, south dodder seed, twotooth achyranthes root and the like. The Chinese medicinal composition can be prepared into any common oral preparation by the conventional Chinese medicinal preparation method. The Chinese medicinal composition can obviously improve symptoms such as somnolence, drowsiness, cataplexy, sleep paralysis, hypnoagogic hallucination and the like. The Chinese medicinal composition has the advantages of exact clinical effect, obvious curative effect, quick response, low cost, basically no toxic or side effect and the like because the Chinese medicinal composition is basically combined by edible and pharmaceutical medicaments specified in national formulary.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Heart fire-clearing upset-relieving health-care tea and preparation method thereof
The invention provides a heart fire-clearing upset-relieving health-care tea, which is characterized by being prepared from the following materials: Chinese angelica, szechuan lovage rhizome, prepared rehmannia root, turmeric root-tuber, rhizoma zingiberis preparatum, red peony root, white paeony root, peach seed, carthamus, thinleaf milkwort root-bark, lotus leaf, semen nelumbinis, mix-fried licorice, Chinese date and ginkgo seed. The preparation method comprises the following steps: cleaning the raw materials; mixing the cleaned raw materials; adding water into the mixture; after boiling the mixture for 20 to 60 minutes, filtering to remove slag and obtaining the heart fire-clearing upset-relieving health-care tea. In the tea, the raw materials jointly exert effects of promoting qi circulation and removing obstruction in the collateral, clearing heart and inducing resuscitation, activating blood and dissolving stasis and warming inside and relieving pains, so the tea can treat sadness, palpitation due to fright, mind absence, somnolence, poor memory, stagnation of the liver-qi, and qi stagnation and blood stasis. The tea can be drunk directly after being boiled, has health-care function, and can relieve long-term depression, ease mind, and be used as a daily health-care product.
Owner:周大红
Enzyme cofactor combination for supplementing pyruvate dehydrogenase and alpha ketogluterate dehydrogenase complexes
The present invention provides a method and pharmaceutical composition for preventing or treating the development of syndromes related to dysfunctional energy metabolism, such as neuropathy, spontaneous nocturnal muscle cramps associated with neuropathy, seizuring, diabetes mellitus; pediatric hypoglycemia; myopathy; muscle fatigue; muscle spasms; somnolence; reduced mental acuity; exercise intolerance and myocardial insufficiency, which are due to cofactor deficiencies associated with the pyruvate dehydrogenase complex and the alpha-ketogluterate dehydrogenase (a-ketogluterate dehydrogenase) complex in humans or other mammals in need thereof. In particular, a method of preventing or treating at least one syndrome related to defective glucose metabolism in humans or other mammals is provided comprised of administering a combination of enzyme cofactors containing therapeutically effective amounts of thioctic acid, niacinamide, pantothenate, riboflavin and thiamine. Also provided is a pharmaceutical composition comprised of a carrier and the combination of cofactors.
Owner:HOWARD JAMES R
Dry powder formulations of antihistamine for nasal administration
InactiveUS20080038358A1Reduce morbidityNot impart bitter tastePowder deliveryBiocideSide effectAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
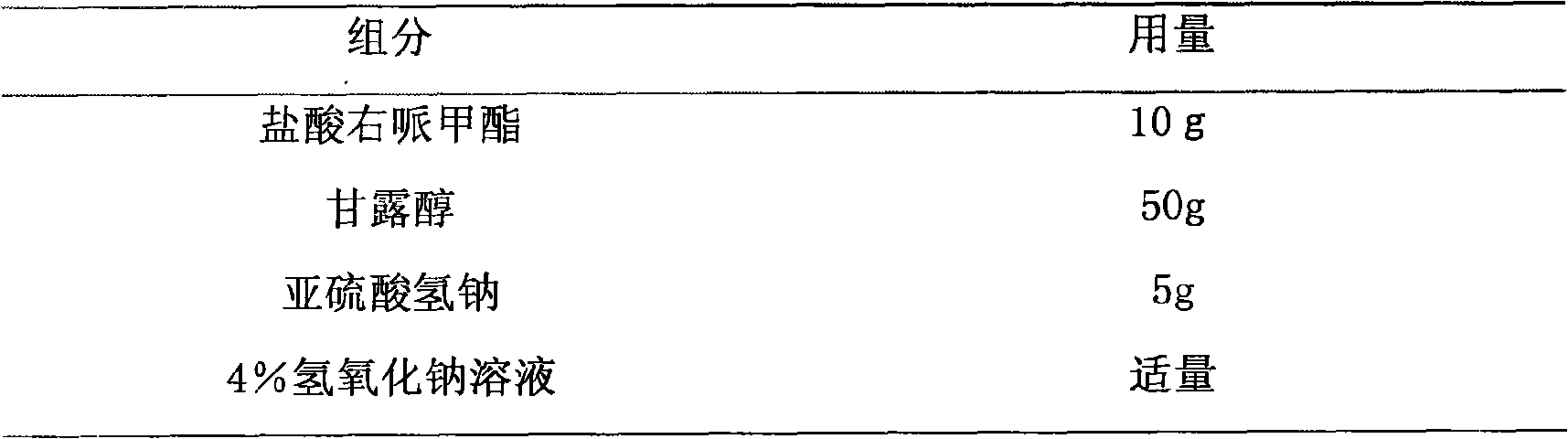
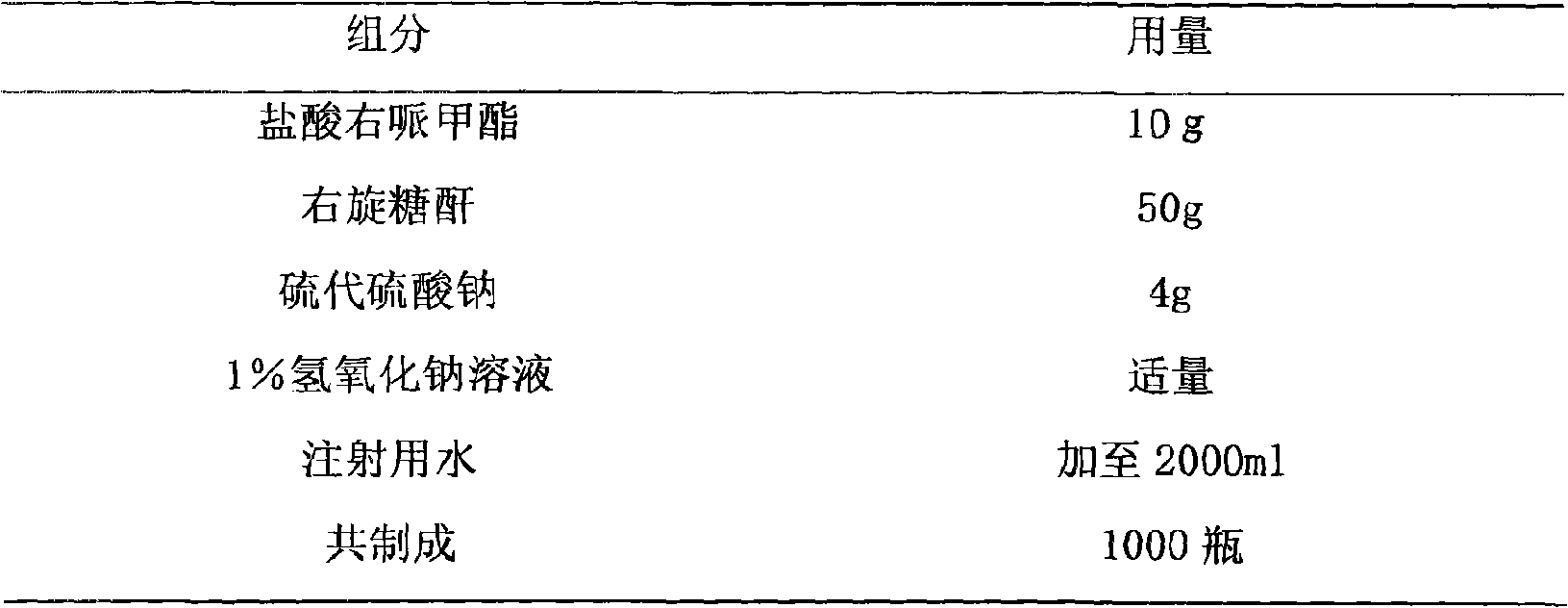
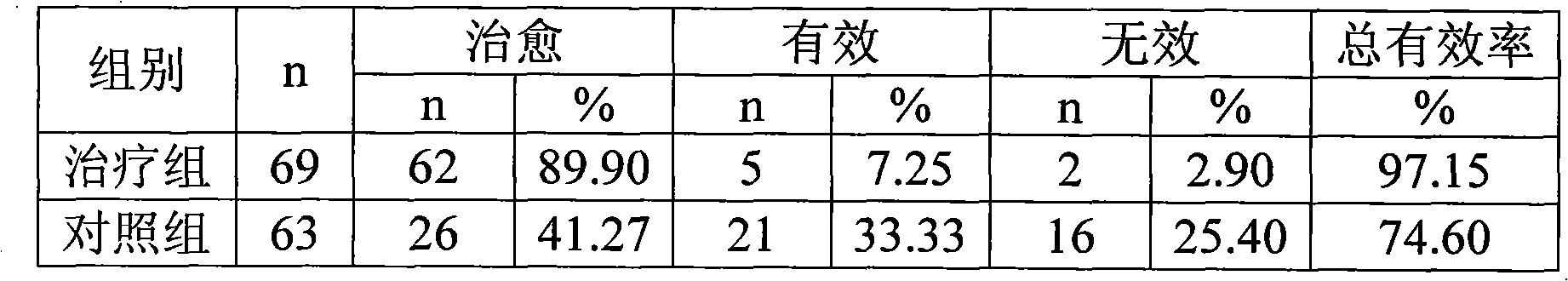
Methylphenidatefrozen dry powder preparation for injection and preparation process thereof
InactiveCN101683342AEliminate drowsinessEliminate burnoutOrganic active ingredientsPowder deliveryDaytime somnolenceHyperkinetic syndrome
The invention relates to a methylphenidatefrozen dry powder preparation for injection and a preparation process thereof; the preparation takes the salt of the methylphenidatefrozen as drug constituents and forms the drug composite by mixing acceptable accessories pharmaceutically; the preparation method is that: the salt of the methylphenidatefrozen is taken as raw material, the accessories with specific kinds and proportion are added, and the dry powder preparation for intravenous injection is prepared and developed by adopting the illustrative technological measures. The dry powder preparation is used for treating attention deficiency hyperkinetic disorder (hyperkinetic syndrome of children and mild brain functional maladjustment), and eliminating somnolence, lassitude and respiratory depression caused by soporifics.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
American cockroach medicinal composition for curing gastritis and peptic ulcers and preparation method thereof
InactiveCN101837023AGood curative effectEasy to prepareOrganic active ingredientsAnthropod material medical ingredientsDiseaseBULK ACTIVE INGREDIENT
The invention discloses an American cockroach medicinal composition for curing gastritis and peptic ulcers. The medicinal composition is mainly prepared from an active ingredient mixture prepared by mixing an American cockroach extract and omeprazole. The weight part ratio of the American cockroach extract to the omeprazole is equal to 0.8-1.2g: 0.33-80mg. A preparation method of the American cockroach medicinal composition for curing the gastritis and the peptic ulcers comprises the following steps of: preparing the American cockroach extract, mixing active ingredients, and preparing medicaments. The medicinal composition has simple preparation method, fully exerts effects of the American cockroach extract, and effectively improves curative effects on the gastritis and the peptic ulcers. The American cockroach medicinal composition has no responses such as headache, diarrhea, astriction, stomachache, sicchasia, emesis skin eruption, dizziness, somnolence and insomnia and the like, and basically does not increase transaminase or bilirubin.
Owner:耿福能
Method of treating the chemotoxic effects of chemotherapeutic drugs on brain tissue
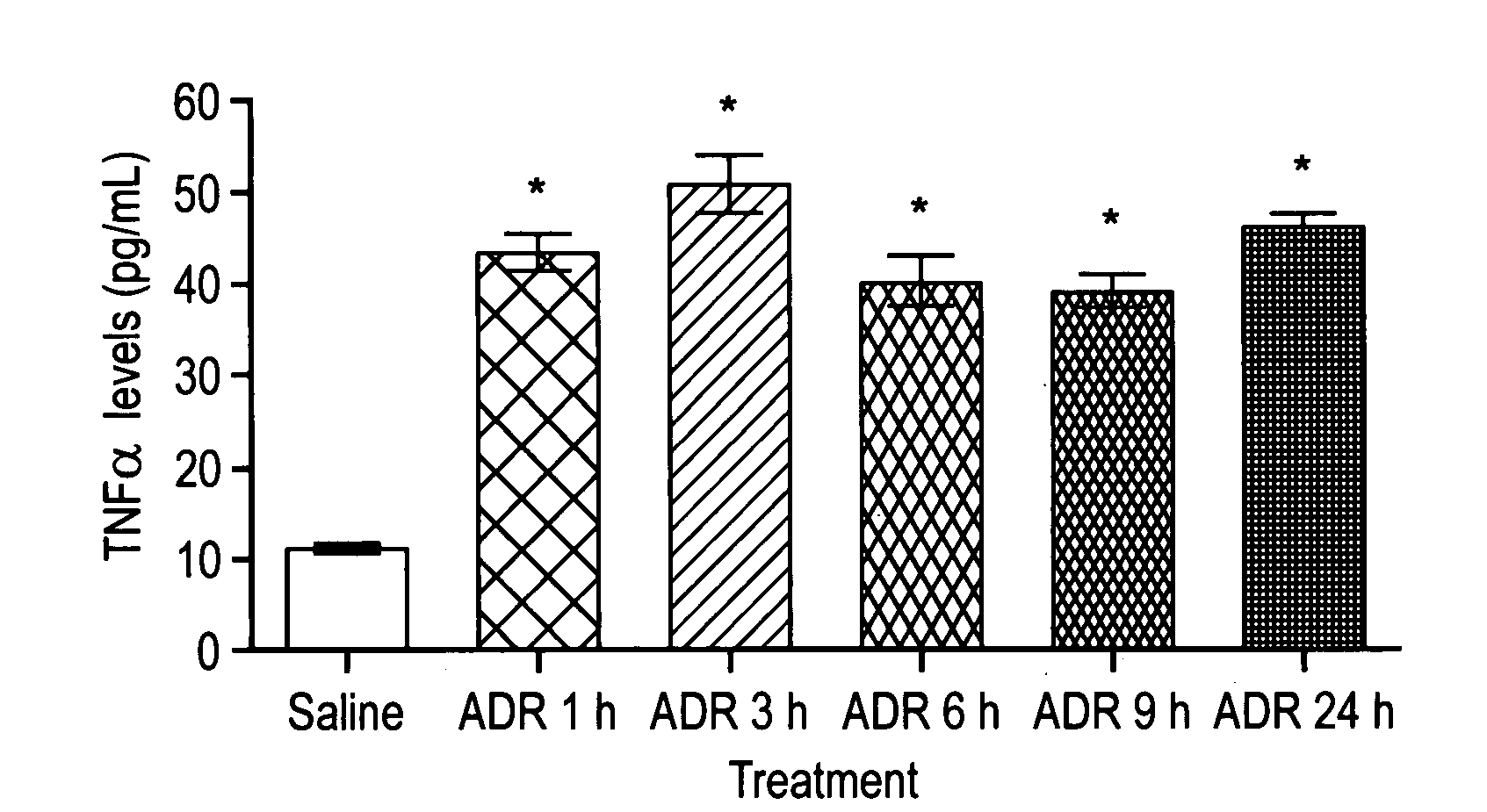
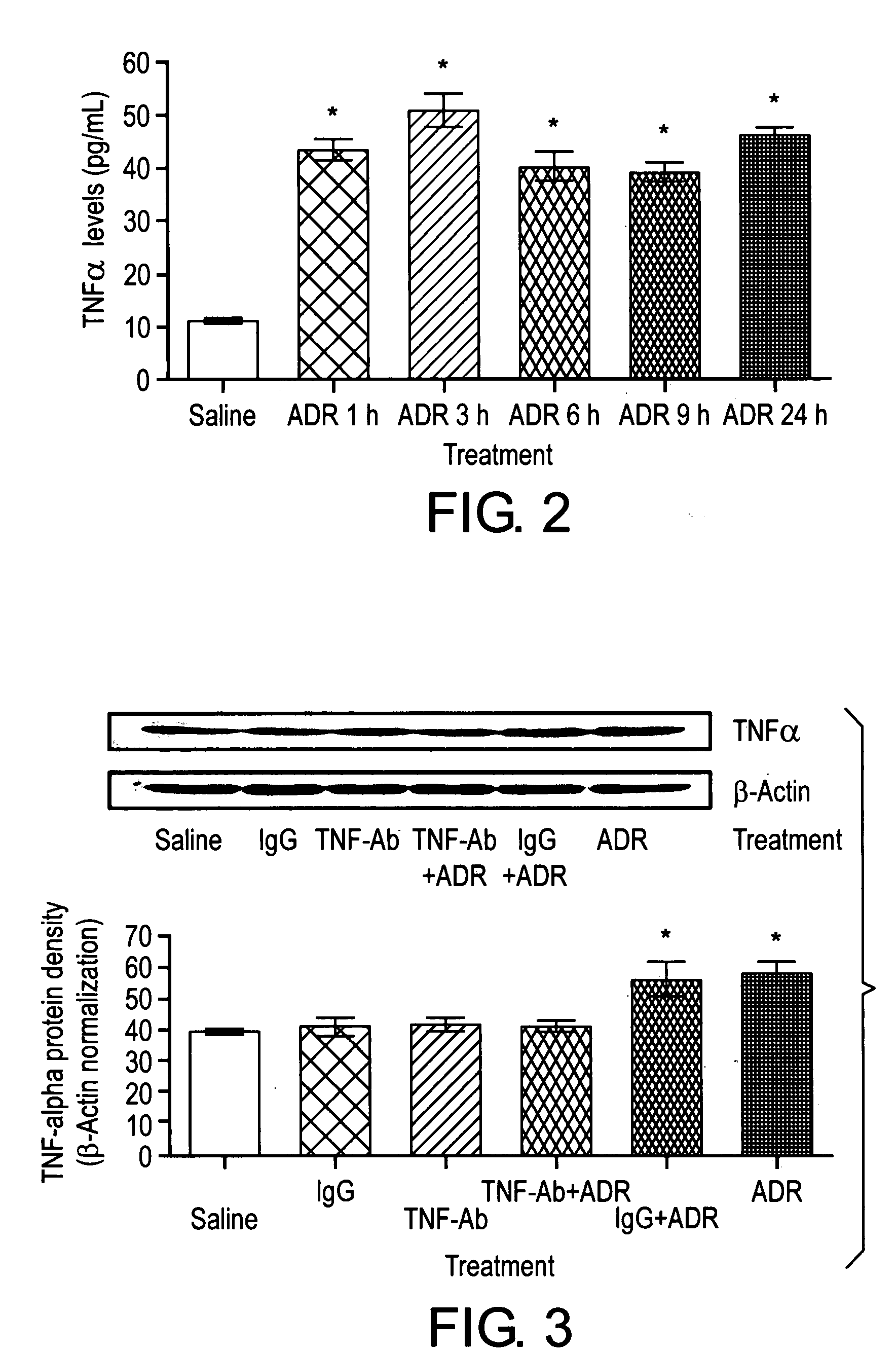
This invention is related to a novel method of treating the chemotoxic effects on the central nervous system of chemotherapeutic drugs, namely Adriamycin, by administering a therapeutically effective amount of anti-Tumor Necrosis Factor-α antibody to a patient to alleviate the symptoms of the effects. The antibody operates to decrease the serum level of Tumor Necrosis Factor-α, prevents the p53 translocation to mitochondria and prevents the decline in mitochondrial respiration of brain tissues and thereby relieves the symptoms of somnolence caused by chemotherapeutic drugs.
Owner:UNIV OF KENTUCKY RES FOUND
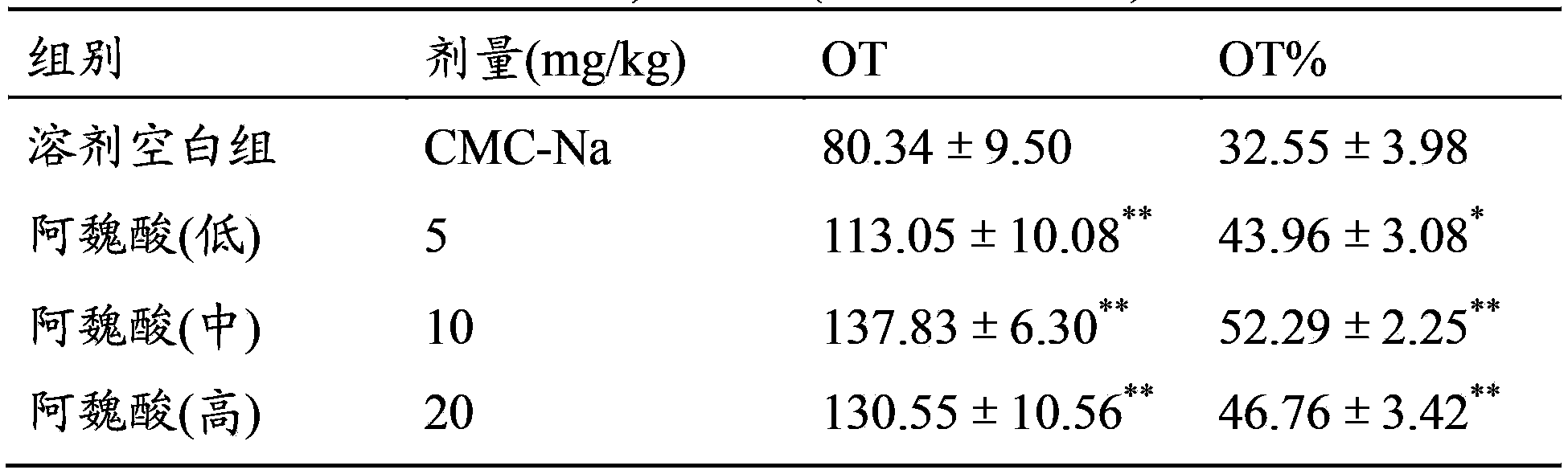
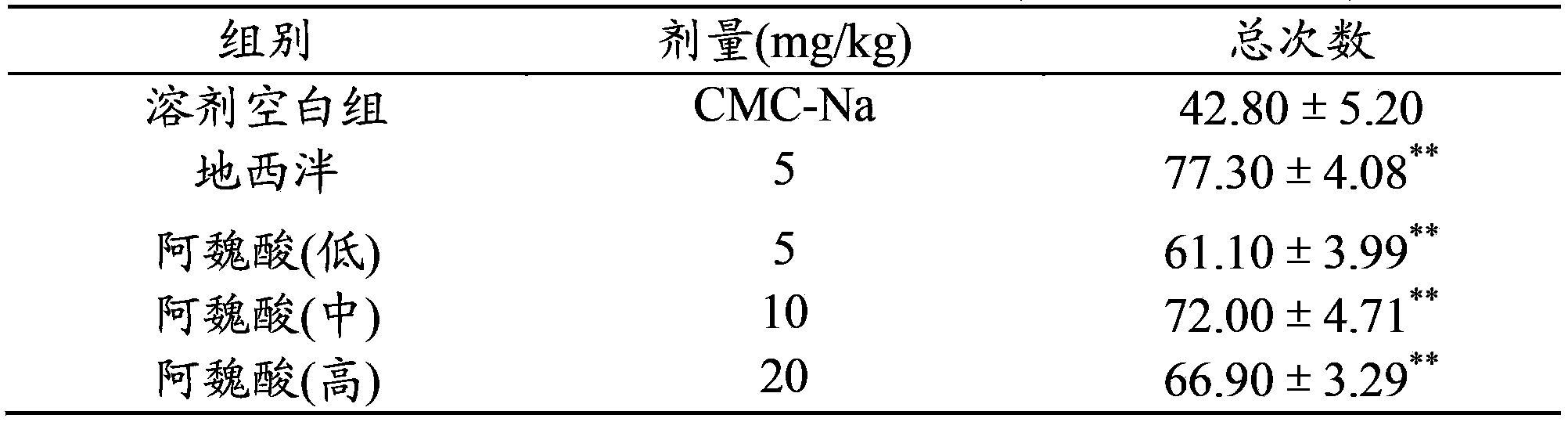
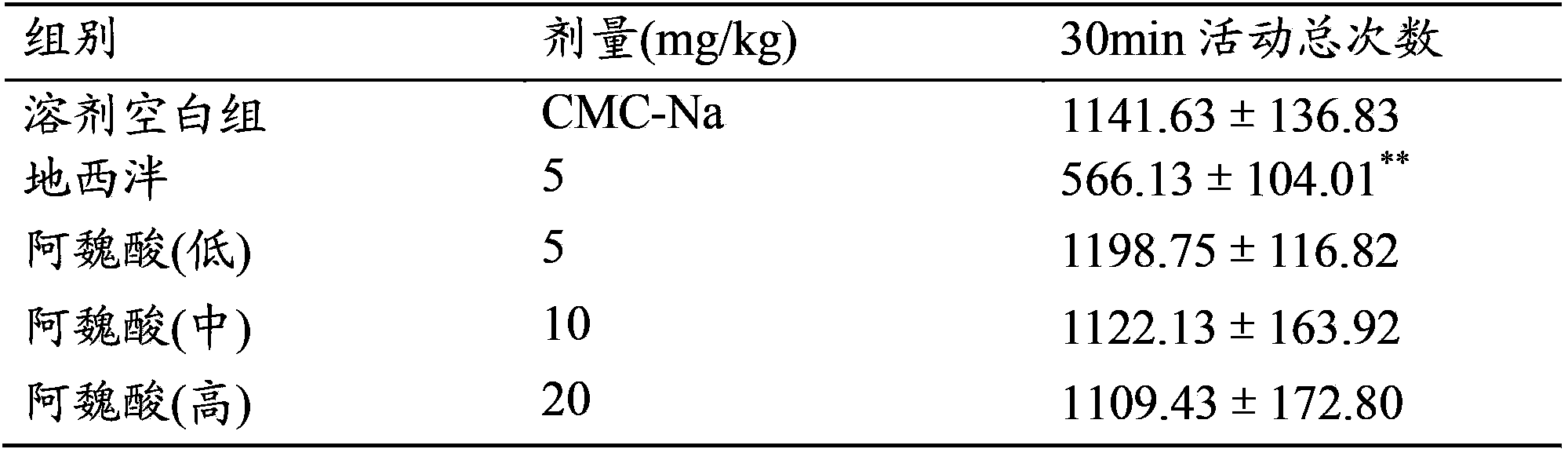
Use of ferulic acid in preparation of drug for resisting mammal anxiety
InactiveCN104083349ASignificant anxiolytic effectNo sedationOrganic active ingredientsNervous disorderSide effectAnti-Anxiety Effect
The invention provides a use of ferulic acid in preparation of a drug for resisting mammal anxiety and provides a novel antianxiety drug having good effects and small side effects. Ferulic acid is an effective component of the antianxiety drug. Results of mouse elevated plus-maze test, hole-board test and spontaneous activity test on ferulic acid shows that ferulic acid has substantial antianxiety effects and no calmness effect and thus adverse reactions such as fatigue and somnolence caused by calmness effects of the existing antianxiety drug are avoided.
Owner:BEIJING UNIV OF CHINESE MEDICINE
CNS pharmaceutical compositions and methods of use
ActiveUS9579299B2Eliminate side effectsLow toxicityNervous disorderInorganic active ingredientsSide effectActive agent
The present invention is directed to CNS pharmaceutical compositions and methods of use. The pharmaceutical compositions comprise a CNS active agent and preferably at least two vagal neuromodulators, one of which is a mechanoreceptor stimulator. The vagal neuromodulators are preferably in an amount sufficient to reduce a somnolence side-effect of the CNS active agent without changing its therapeutic efficacy / activity. The invention further encompasses a method of reducing CNS active agent side-effects. The method typically comprises oral administration of at least one CNS active agent to a patient at the conventionally accepted dose; and administration of at least two vagal neuromodulators to the patient so that at least one neuromodulator is administered or released from dosage form after the CNS active agent is administered and / or released.
Owner:TARGIA PHARMA
Peony extract-methotrexate composition, preparation method thereof and use thereof
InactiveCN101926860AEffective in treating rheumatoidReduce or avoid liver damageOrganic active ingredientsAntipyreticTreatment effectMedicine
The invention provides a peony extract-methotrexate composition, a preparation method thereof and use thereof. The peony extract-methotrexate composition is prepared from a peony extract and methotrexate and has a better rheumatoid treatment effect than methotrexate and the mixture of the methotrexate and total glucosides of paeony. Meanwhile, the composition can reduce or avoid adverse reactions caused by the methotrexate, such as liver damage, somnolence, inappetence and the like.
Owner:NINGBO LIWAH PHARM CO LTD
Medicine for clearing heat and calming nerves and preparation method thereof
InactiveCN102247527AHigh content of active ingredientsReduce dosageHeavy metal active ingredientsNervous disorderSalvia miltiorrhizaCelastrus orbiculatus
Owner:LIAONING HUAXIN PHARMA
Spleen-Qi-nourishing and dampness-removing particles containing effective traditional Chinese medicinal ingredients, and preparation method thereof
ActiveCN106729190AImprove sleepingEliminates dampness and resolves phlegmNervous disorderDigestive systemSucroseAdditive ingredient
The invention provides spleen-Qi-nourishing and dampness-removing particles containing effective traditional Chinese medicinal ingredients, and a preparation method thereof. The particles contain lotus leaf extracts, yam extracts, poria cocos extracts, burdock root extracts, eucommia ulmoides extracts, hawthorn extracts, semen coicis extracts, red bean extracts, red dates extracts, roasted malt extracts, soluble starch, sucrose, dextrin, distilled water and 75% ethyl alcohol. Compared with the prior art, the spleen-Qi-nourishing and dampness-removing particles contain multiple effective traditional Chinese medicinal ingredients, and can be used for nourishing Qi and spleen, removing dampness, resolving phlegm, conditioning a phlegm-damp type body, eliminating local edema, and improving symptoms of hyperhidrosis, profuse sputum, tiredness and somnolence caused by insufficiency of the spleen; the spleen is nourished whilst water dampness is removed.
Owner:WANNAN MEDICAL COLLEGE
Detecting undiagnosed sleep disordered breathing using daytime sleepiness and nighttime obstructive sleep apnea (OSA) severity
PendingUS20210282707A1Raise the possibilityOvercomes shortcomingPhysical therapies and activitiesDrug and medicationsPhysical medicine and rehabilitationExcessive daytime sleepiness
An apparatus and method for detecting undiagnosed sleep disordered breathing uses daytime sleepiness and nighttime Obstructive Sleep Apnea (OSA) severity. This involves detecting people with excessive daytime sleepiness (and high likelihood of falling asleep during the day) caused by OSA through objective and subjective daytime and nighttime monitoring. Screening is provided for those who are most likely to be suffering a daytime impact of their sleep apnea and thus most likely to respond positively to a potential diagnosis, and notifying these people is also provided.
Owner:KONINKLJIJKE PHILIPS NV
Traditional Chinese medicine health tea
InactiveCN108029819ANormal metabolismWith qi and depressionTea substituesSide effectAdditive ingredient
The invention discloses traditional Chinese medicine health tea. The traditional Chinese medicine health tea is prepared from the following raw materials in parts by weight: 50-70 parts of roses, 50-70 parts of jasmine flowers, 50-70 parts of seville orange flowers, 50-70 parts of lotus leaves, 30-50 parts of wolfberry fruits and 5-20 parts of dried orange peel. The traditional Chinese medicine health tea adopts common medicinal and edible traditional Chinese medicines as raw materials, is safe and reliable, has no toxic and side effects and no injury to the digestive system and the absorptionsystem of a human body, can give full play to medicinal effects of every traditional Chinese medicine component by compatible application of the raw materials and has the efficacies of beautifying skin, reducing lipid, producing a refreshing effect, regulating the flow of qi, relieving stagnation, resolving phlegm and removing dampness by diuresis. Honey in the traditional Chinese medicine healthtea is mainly used for flavoring and added on the preference of drinkers. The traditional Chinese medicine health tea is capable of promoting normal metabolism of a human body, has obvious weight reducing effects and also has good efficacies of regulating the flow of qi, relieving stagnation, resolving phlegm and removing dampness by diuresis on patients who have fatty liver disease due to accumulation of phlegm-dampness in the body and have the following clinical manifestations: fat body, dull pain in chest and hypochondrium, dizziness, choking sensation in chest, somnolence, tiredness and the like.
Owner:河南明珍方医药科技有限公司
Ophthalmic anti-somnolence lens, device and method
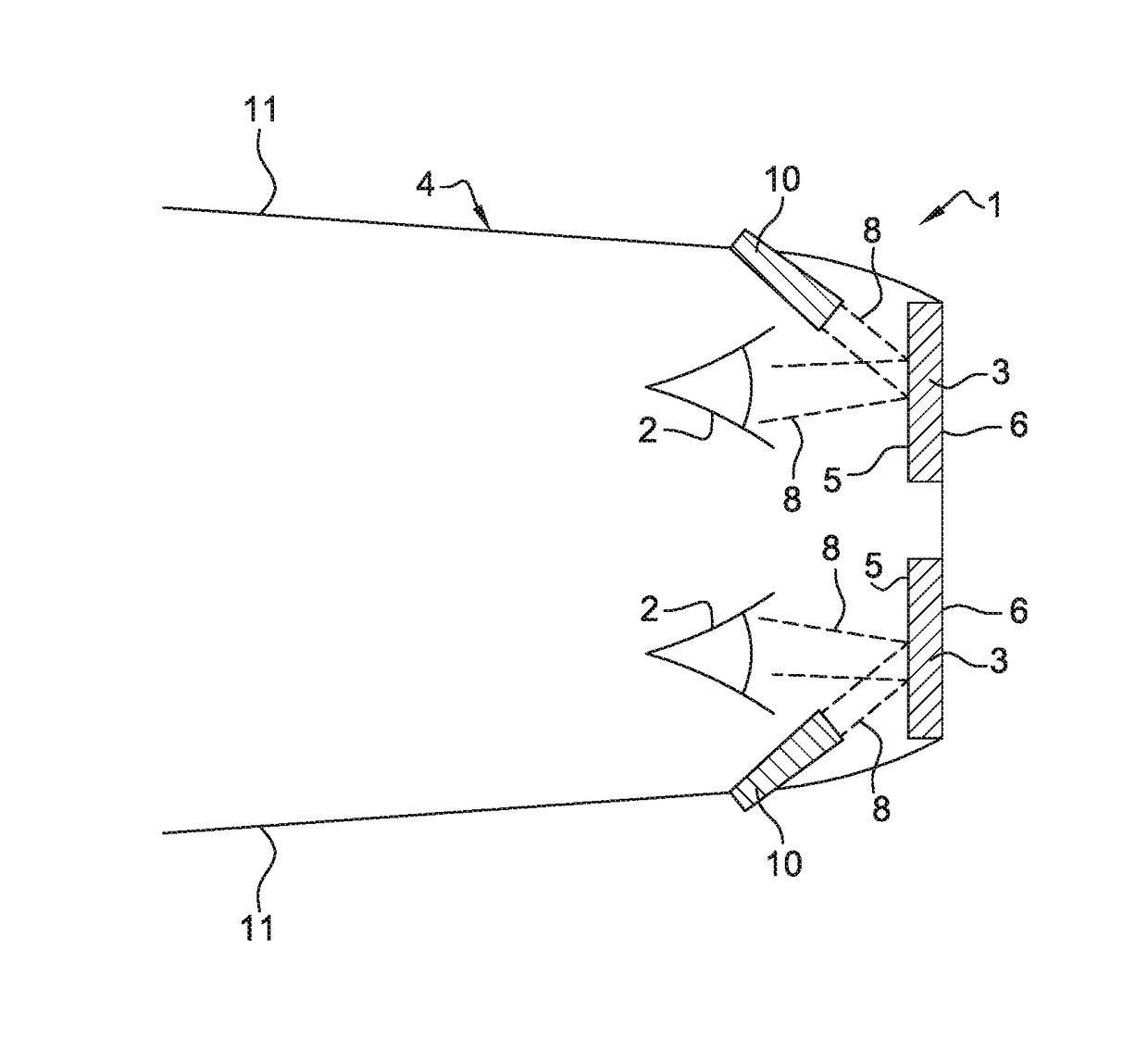
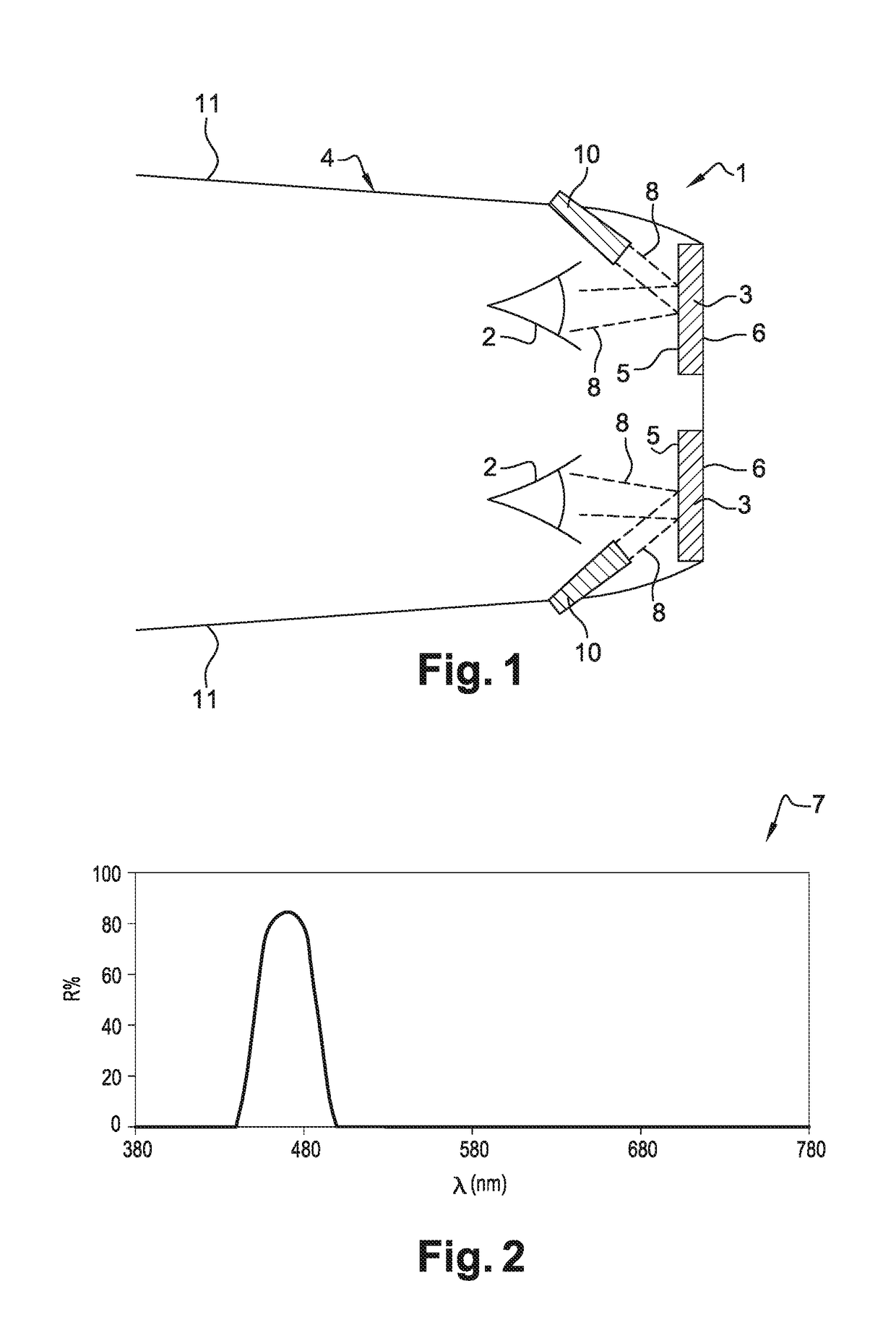
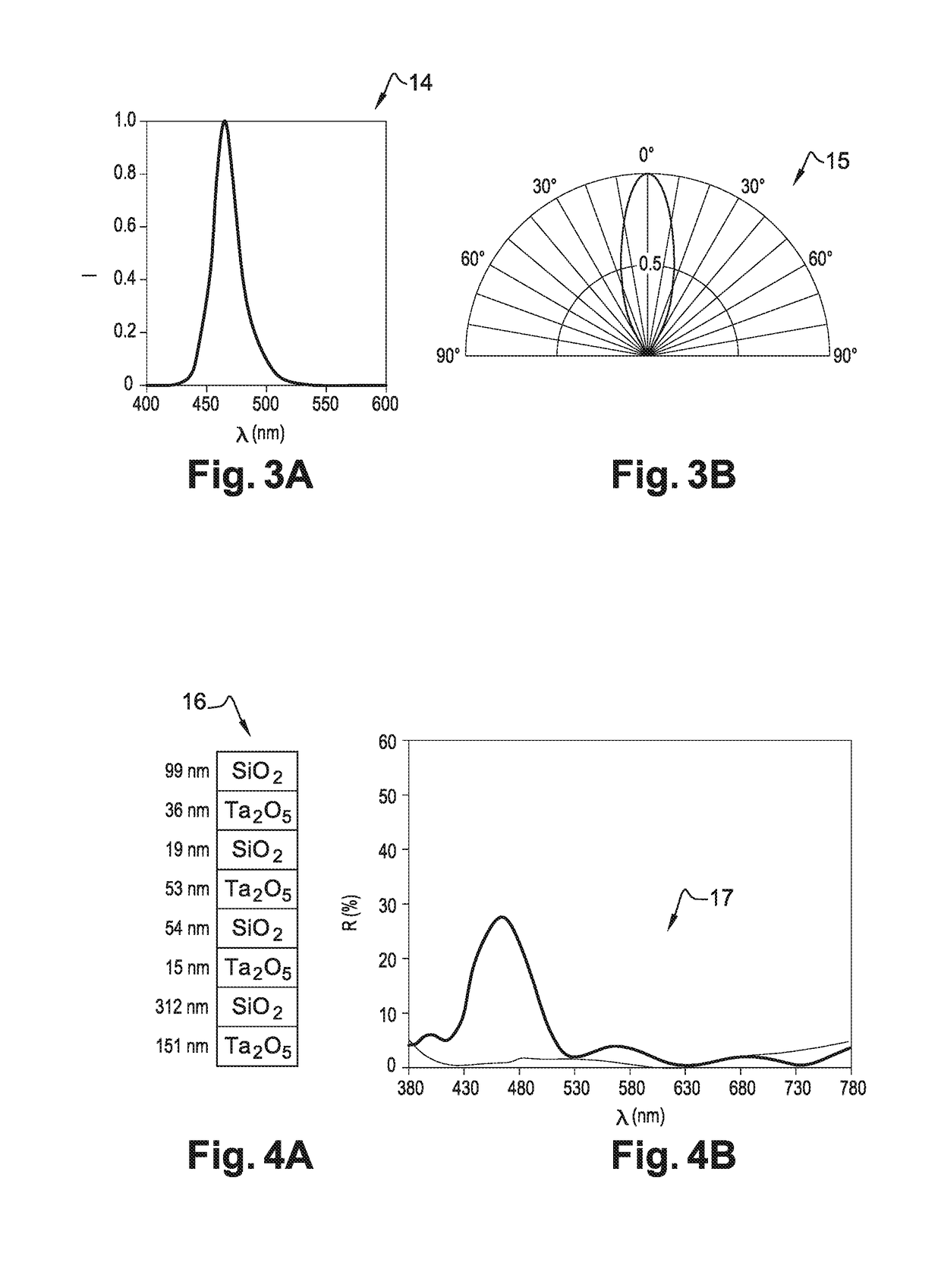
ActiveUS10048515B2Improved estheticImprove eyesightSpectales/gogglesNon-optical adjunctsDaytime somnolenceEngineering
The ophthalmic lens includes a substrate, the substrate having a rear face and a front face, and at least one external interferential coating, the at least one interferential coating being such that it enables to selectively reflect light arriving on the rear face with a local maximum of reflection of at least 10% for a wavelength within the range between 450 nm and 490 nm.
Owner:ESSILOR INT CIE GEN DOPTIQUE
Middle-aged and elderly health-care food for improving sleep and protecting eyesight and preparation method thereof
ActiveCN104886574AImprove blurDizziness noNatural extract food ingredientsFood ingredient functionsBlurred visionGanoderma atrum
The invention belongs to the technical field of food, and in particular relates to a middle-aged and elderly health-care food for improving sleep and protecting eyesight and a preparation method thereof. The formula of the middle-aged and elderly health-care food for improving sleep and protecting eyesight comprises the following components by weight percent: 5-10 percent of xanthophyll, 20-50 percent of Ganoderma atrum extract, 1-5 percent of melatonin and 20-50 percent of grassleaf sweelflag rhizome extract. The formula is scientific and reasonable, has the functions of calming the mind and nerves, relieving anxiety, promoting sleep, and improving sleep quality, and can not cause dizzness, somnolence and addiction which are caused by common drugs after awaking. In addition, the health-care food also has the effects of relieving asthenopia and hypopsia caused by insomnia, improving blurred vision and protecting the eyesight.
Owner:南京贝杉国际贸易有限公司
Traditional Chinese medicine for treating damp-abundance spleen trapped type somnolence disease
InactiveCN105343843AQuick resultsNo dependenciesNervous disorderPlant ingredientsMonkshoodsSide effect
The invention relates to the field of traditional Chinese medicine, in particular to a traditional Chinese medicine for treating a damp-abundance spleen trapped type somnolence disease. The traditional Chinese medicine is prepared from 10-20 g of Chinese yams, 10-20 g of medcinal evodia fruit, 8-18 g of Chinese starjasmine stems, 8-18 g of fiveleaf gynostemma herb, 8-18 g of semen coicis, 8-18 g of gorgon euryale seeds, 8-18 g of radix stephaniae tetrandrae, 8-18 g of officinal magnolia bark, 8-18 g of rhizoma atractylodis macrocephalae, 8-18 g of prepared common monkshood daughter roots, 5-15 g of cortex erythrinae, 5-15 g of poria cocos, 5-15 g of heterophyllous wing seedtree roots, 5-15 g of paniculate swallowwort roots, 3-9 g of cocklebur fruit, 2-10 g of radix codonopsis, 2-10 g of java amomum fruit, 2-10 g of pericarpium citri reticulatae, 2-10 g of licorice roots, 2-10 g of cortex cinnamomi and 2-10 g of ginger. According to clinical experiments, the cure rate of the traditional Chinese medicine for treating the damp-abundance spleen trapped type somnolence disease is high than 96.92 percent and can reach up to 99.23 percent, the average cure rate is 98.15 percent, the total effective rate is 100 percent, and the traditional Chinese medicine is very suitable for treating the damp-abundance spleen trapped type somnolence disease. In addition, the traditional Chinese medicine is quick in efficacy, short in cure cycle, and the traditional Chinese medicine composition is prepared from pure traditional Chinese medicines and is free of toxic and side effects and free of concerns about the drug dependence.
Owner:JINAN SHUNXIANG MEDICINE SCI & TECH
Method of reducing somnolence in patients treated with tizanidine
InactiveUS20090017109A1Least overall somnolenceRaise the possibilityBiocidePowder deliveryImmediate releaseDaytime somnolence
An article and method for reducing somnolence in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release multiparticulate composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:PELLEGRINI CARA A +1
Peptic particle and preparing method thereof
InactiveCN1456292AImprove stabilityAvoid discomfortDigestive systemUnknown materialsCyclodextrinGLYCYRRHIZA EXTRACT
A process for preparing Chinese medicing "Pingwei particles" for treating distension of gastral cavity, anorexia, vomiting, nausea, somnolence, etc includes such steps as pulverizing liquorice root, decocting 3 Chinese-medicinal materials including atractyldes rhizome to obtain volatile oil, mixing the volatile oil with cyclodextrin, concentrating the decoction, drying, adding additive, mixing all together, granulating and loading in capsules. Its advantage is high curative effect and safety.
Owner:毛友昌
Method of reducing somnolence in patients treated with tizanidine
InactiveUS20120027851A1Least overall somnolenceRaise the possibilityBiocidePowder deliveryImmediate releaseDaytime somnolence
An article and method for reducing somnolence in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release multiparticulate composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:KING GEORGE HLDG LUXEMBOURG IIA S A R L
Traditional Chinese medicine for treating liver stagnation and spleen deficiency type post-stroke depression
InactiveCN102139086BReliable and reliableSafeNervous disorderPlant ingredientsSide effectTherapeutic effect
The invention relates to a traditional Chinese medicine for treating liver constraint and spleen deficiency type post-stroke depression. The traditional Chinese medicine has obvious treatment effects on symptoms such as deprementia, chest hypochondriac pain, lassitude, upset irritability, abdominal distension and diarrhea and the like of the liver constraint and spleen deficiency type post-strokedepression, improves spirit and emotions, promotes the recovery of neural functions of stroke patients, and does not have obvious toxic or side effects such as dizziness, hypodynamia, somnolence, drymouth, constipation, hypotension, abnormal liver functions, epilepsy, cardiac arrhythmia, toxicosis and the like. The technical scheme is that: the traditional Chinese medicine is prepared form the following raw materials by weight: 10 to 14g of bupleurum, 9 to 11g of turmeric root-tuber, 10 to 14g of Chinese angelica, 10 to 14g of white paeony root, 13.5 to 16.5g of fried largehead atractylodes rhizome, 9 to 11g of Indian buead, 18 to 22g of codonopsis pilosula, 9 to 11g of tangerine peel, 9 to 11g of bamboo shavings, 13.5 to 16.5g of gardenia, 4.5 to 5.5g of mint and 2.7 to 3.3g of liquoricroot. The traditional Chinese medicine is prepared into decoction by the conventional soaking, decocting and filtering or is prepared by directly blending market-sold single Chinese medicinal formulaparticles. The traditional Chinese medicine has the advantages of high reliability, high safety, high flexibility, no dependence and the like.
Owner:HENAN UNIV OF CHINESE MEDICINE
Traditional Chinese medicine for treating somnolence caused by yang-qi deficiency
InactiveCN105343846AQuick resultsShort curing cycleNervous disorderPteridophyta/filicophyta medical ingredientsSide effectCurculigo orchioides
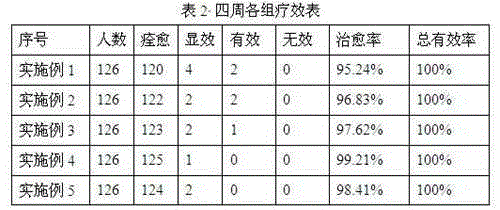
The invention relates to the field of traditional Chinese medicine, in particular to a traditional Chinese medicine for treating somnolence caused by yang-qi deficiency. The traditional Chinese medicine is prepared from 10-20 g of cynomorium songaricum, 10-20 g of evodia, 8-18 g of cibotium barometz, 8-18 g of epimedium, 8-18 g of coix seeds, 8-18 g of gordon euryale seeds, 8-18 g of curculigo orchioides, 8-18 g of radix astragali preparata, 8-18 g of rhizoma chuanxiong, 8-18 g of radix aconite lateralis preparata, 5-15 g of aralia chinensis L., 5-15 g of poria cocos, 5-15 g of astragalus complanatus bunge, 5-15 g of radix cynanchi panicullati, 3-9 g of fructus xanthii, 2-10 g of radix codonopsis, 2-10 g of atractylodes macrocephala koidz, 2-10 g of tangerine peel, 2-10 g of liquorice, 2-10 g of lindera aggregate and 2-10 g of dried ginger. Clinical experiments for treating somnolence caused by yang-qi deficiency show that the cure rate is higher than 95.24% and can reach 99.21% at most, the average cure rate is 97.46%, the total effective rate is 100%, and the traditional Chinese medicine is quite suitable for treating somnolence caused by yang-qi deficiency. In addition, the traditional Chinese medicine is quick in effect and short in cure period, is prepared from pure traditional Chinese machine, and is free of toxic and side effects and free of worries of medicine dependence.
Owner:JINAN SHUNXIANG MEDICINE SCI & TECH
Traditional Chinese medicine for treating blood stasis type somnolence
InactiveCN105412820AQuick resultsShort curing cycleNervous disorderMammal material medical ingredientsSide effectMyrrh
The invention relates to the field of traditional Chinese medicines, and in particular relates to a traditional Chinese medicine for treating blood stasis type somnolence. The traditional Chinese medicine is prepared from the following components by weight: 10-20 g of caulis spatholobi, 10-20 g of radix puerariae, 8-18 g of myrrh, 8-18 g of frankincense, 8-18 g of dalbergia wood, 8-18 g of the root bark of the peony tree, 8-18 g of beautiful sweetgum fruits, 8-18 g of curcuma zedoary, 5-15 g of the seeds of cowherb, 5-15 g of radix curcumae longae, 5-15 g of radix paeoniae rubra, 5-15 g of cassia twig, 5-15 g of radix bupleuri, 5-15 g of hematoxylon, 2-10 g of musk, 2-10 g of clove, 2-10 g of peach kernels, 2-10 g of Armeniaca mume, 2-10 g of radish seeds, 2-10 g of chastetree fruits, 2-10 g of gentiana macrophylla, 2-10 g of salsola ruthenica, 2-10 g of hematoxylon, and 2-10 g of rhizoma chuanxiong. The traditional Chinese medicine has the cure rate higher than 95.35% for the blood stasis type somnolence proved through clinical experiment, the highest cure rate is 100%, the average cure rate is 97.91%, the total effective rate is 100%, and the traditional Chinese medicine is very suitable for treating the blood stasis type somnolence, rapid in taking effect, and short in cure cycle; the traditional Chinese medicine composition is prepared from pure traditional Chinese medicinal materials, is free of toxic and side effects, and is free of the trouble of drug dependence.
Owner:JINAN SHUNXIANG MEDICINE SCI & TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com