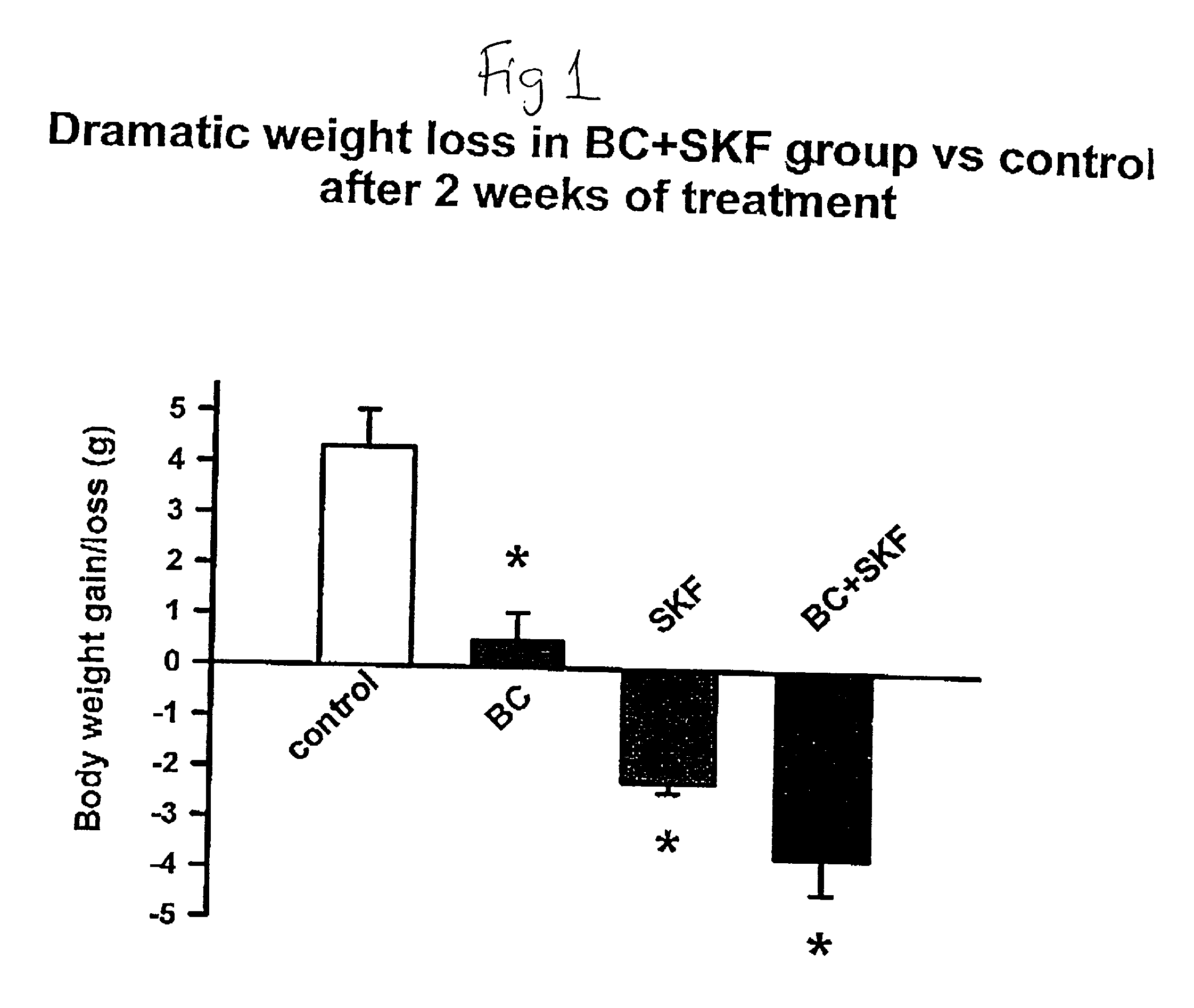Method and composition for the treatment of lipid and glucose metabolism disorders
a lipid and glucose metabolism, composition technology, applied in the direction of heterocyclic compound active ingredients, biocide, animal husbandry, etc., can solve the problems of side effects, no truly effective or practical treatment has been found for controlling obesity or other lipid metabolism disorders, and the use of hmg-coa enzyme inhibitors is sometimes accompanied by side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
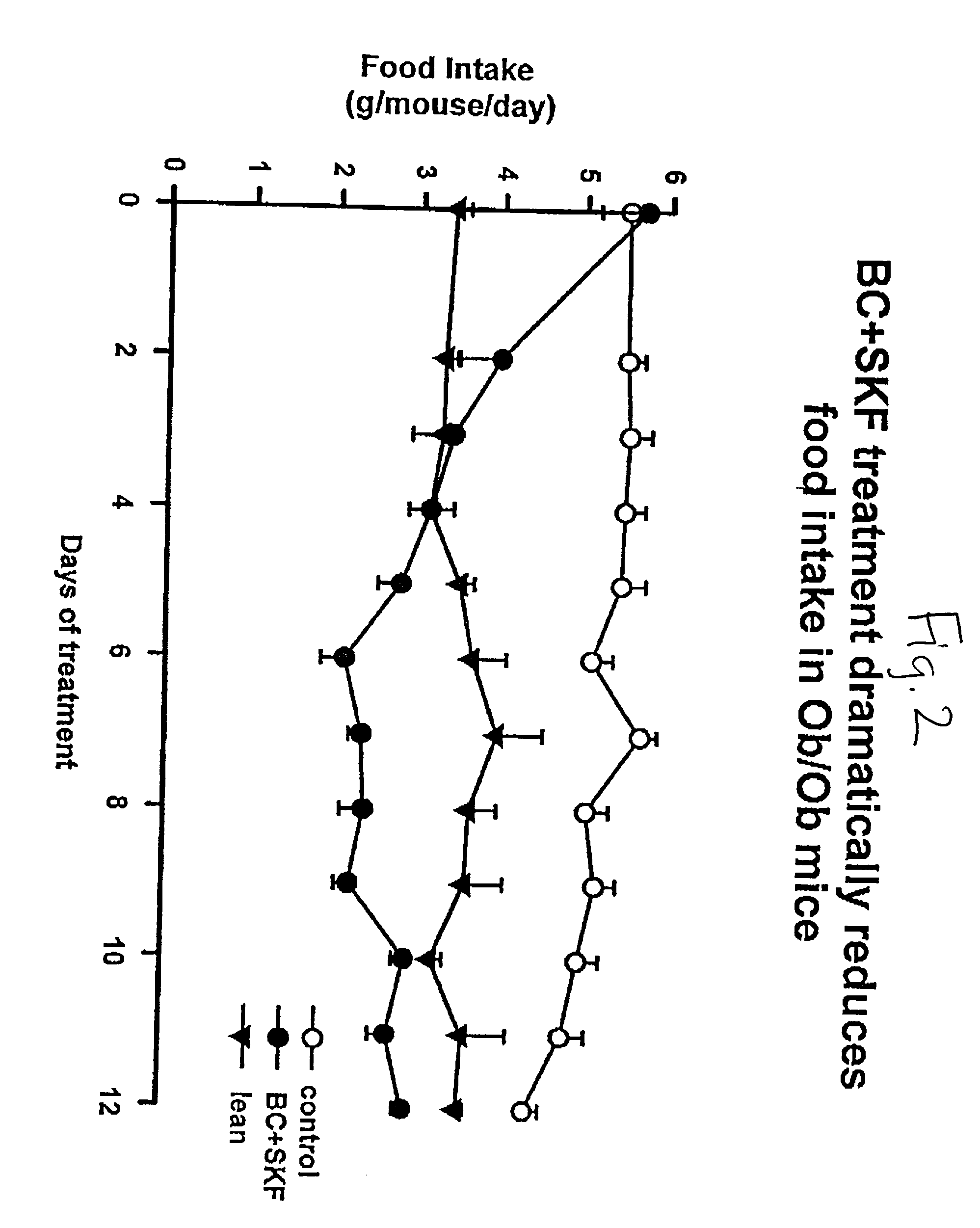
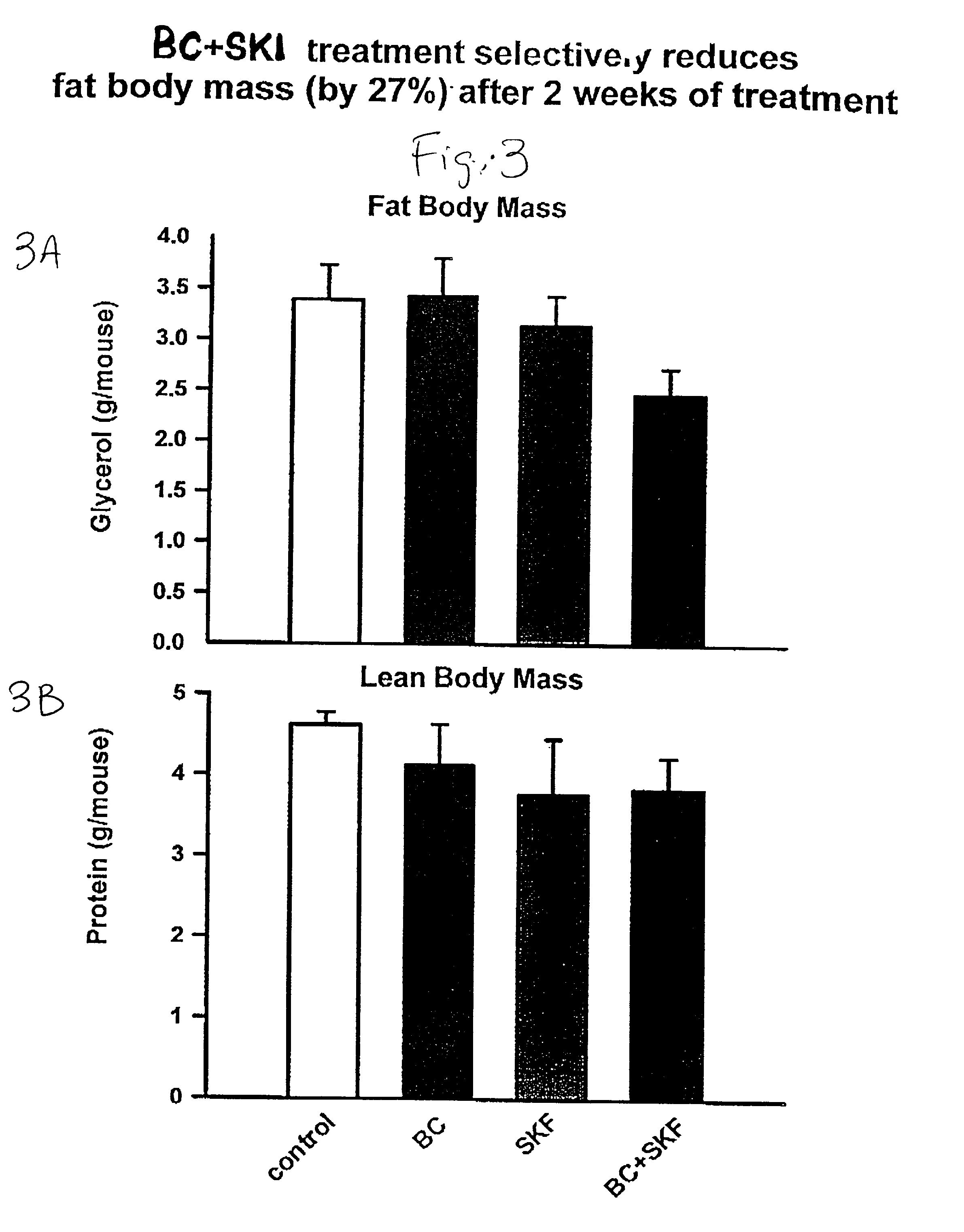
[0108] Different groups of 6-week old C57BL / 6 ob / ob mice (lacking a functional leptin protein) were treated with either bromocriptine ("BC") (10 mg / kg BW), SKF38393 ("SKF") (10 mg / kg BW), both drugs, or vehicle for two weeks at 1 hour after light onset (HALO). Animals were held on 12-hour daily photoperiods and allowed to feed ad libitum. Food consumption was monitored daily for 3 days before the initiation of treatment throughout the 14-day treatment period. Animals were sacrificed between 1 and 3 HALO on the day following the final treatment (i.e., 24-26 hours after last injection) and plasma was collected for the analyses of insulin, glucose, and lipids while the carcasses were solubilized in ethanolic KOH and analyzed for protein and lipid content. Bromocriptine and SKF38393, individually, were ineffective in reducing body weight gain where as SKF, but not BC, reduced food consumption (19%, P<0.01). However, the combined treatment of bromocriptine and SKF38393 (BC / SKF) decreased...
example 3
[0109] The effects of BC / SKF treatment on circadian rhythms of key metabolic enzyme activities, serum metabolites and hormones regulating metabolism were examined. Obese C57BL / 6J mice were treated for 2 weeks at 1 hour after light onset with BC (10 mg / kg BW) and SKF (20 mg / kg BW) or vehicle. Mice were then sacrificed every 4 hours over a 24 hr period for the analyses of serum hormones and metabolites and hepatic enzymatic activities. Serum glucose, free fatty acid (FFA) and hepatic glucose-6-phosphatase (G6Pase) activity were greatest during the light period of the day showing that this time period is the daily peak for lipolysis and hepatic glucose production in mice. BC / SKF treatment significantly reduced blood glucose (51%), FFA(56%) and G6Pase activity (38%) during this light period. Moreover, serum levels of the lipolytic and gluconeogenic hormones thyroxine and corticosterone were also highest during the light period and their levels were significantly reduced by 51% and 53%, ...
example 4
[0110] The effect of in vivo BC / SKF treatment on glucose induced insulin release was studied in vitro. Obese (ob / ob) and lean (+ / +) C57BL / 6J mice were treated daily for 2 weeks with BC (10 mg / kg) plus SKF (20 mg / kg) or vehicle only. Mice were sacrificed 25 hours after the final treatment and islets were isolated for static incubation with glucose. The BC / SKF treatment of obese mice reduced blood glucose (173.+-.14 mg / dl, P<0.01), plasma total glycerol 162.+-.9 vs. 386.+-.33 mg / dl, P<0.01), and plasma total cholesterol (143.+-.5 vs. 184.+-.5 mg / dl, P<0.01) relative to obese controls. The plasma free fatty acid and insulin levels of treated mice were also reduced by 20-30% compared with that in obese controls. In control ob / ob mice, the insulin release from isolated islets stimulated by 10 mM glucose was the same as that by 8 mM glucose (1.6.+-.0.2 vs. 1.9.+-.0.5 ng / islet / h), while in BC / SKF treated ob / ob mice, 15 mM glucose induced a significant increase of insulin release compared w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com