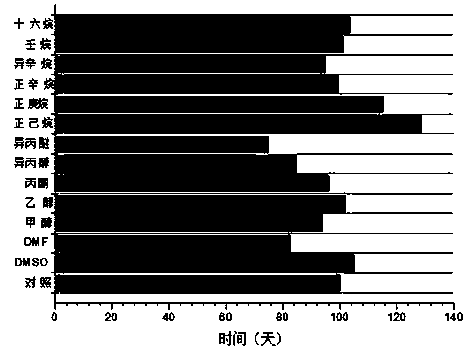
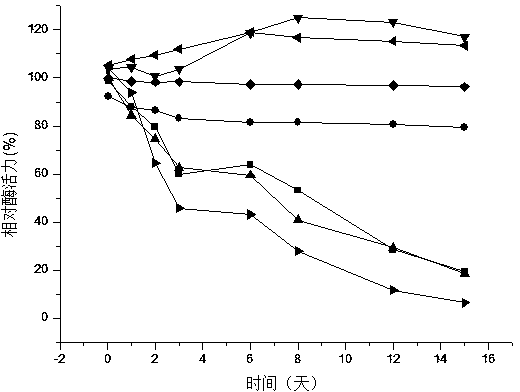
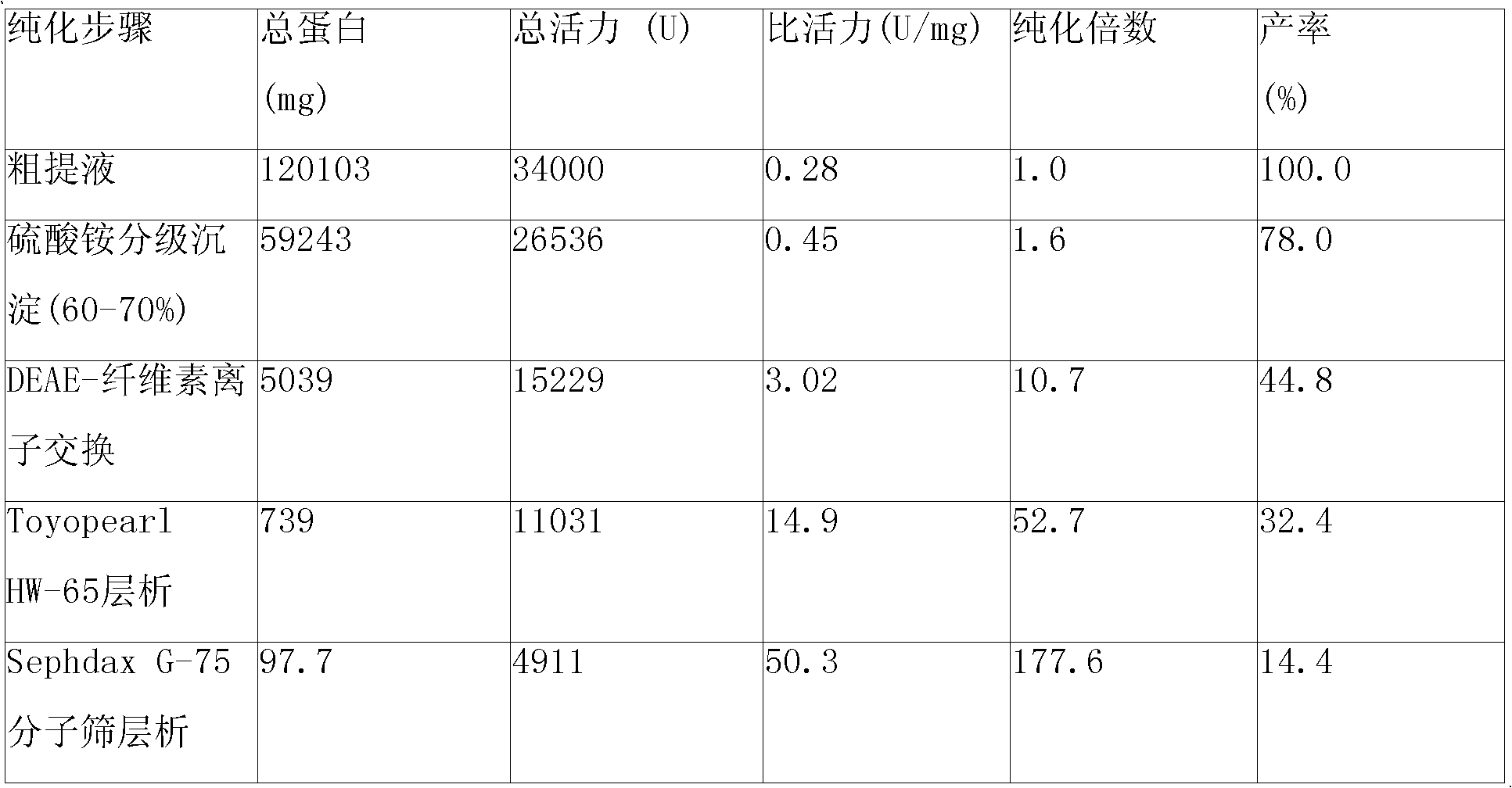
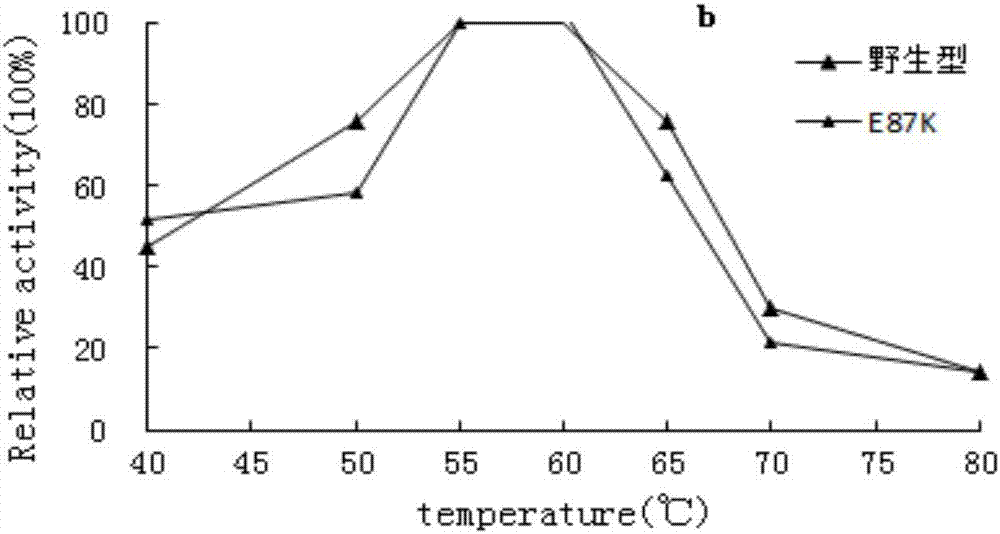
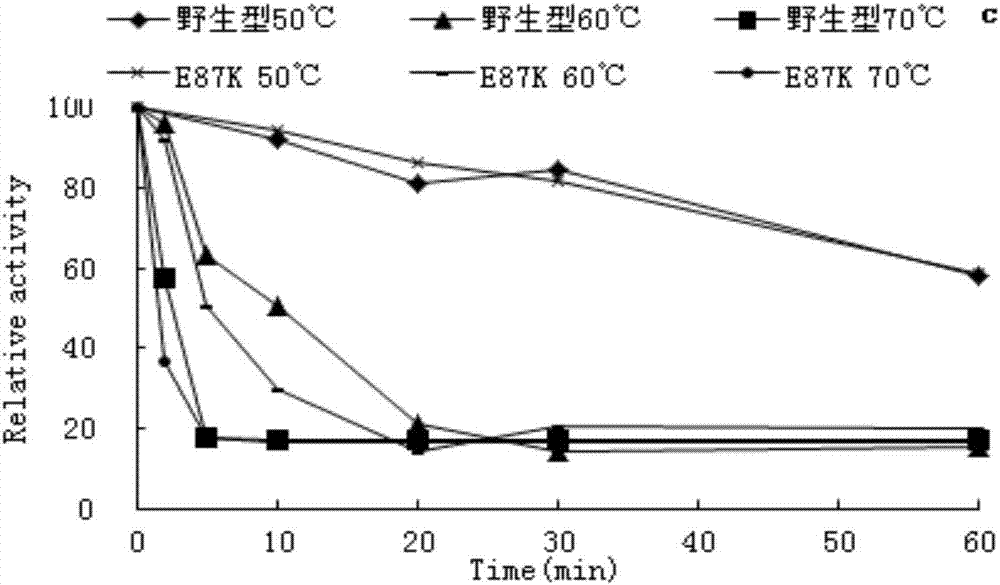
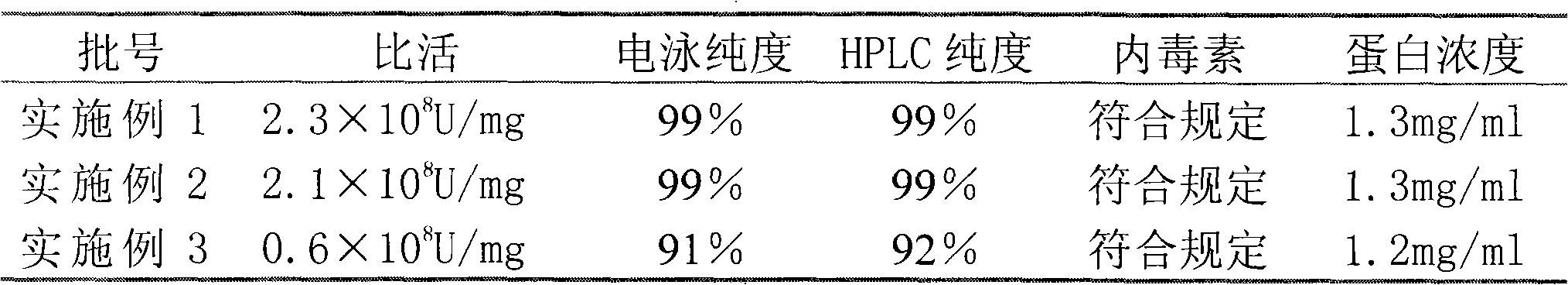
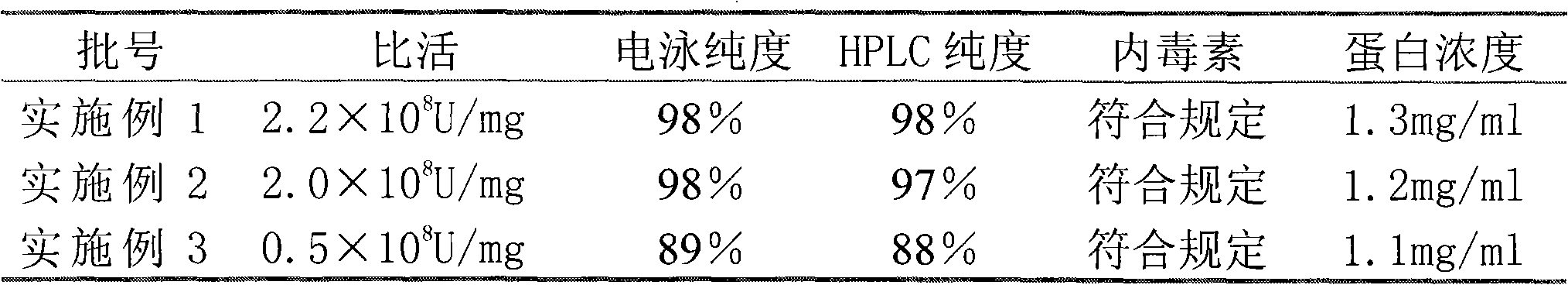
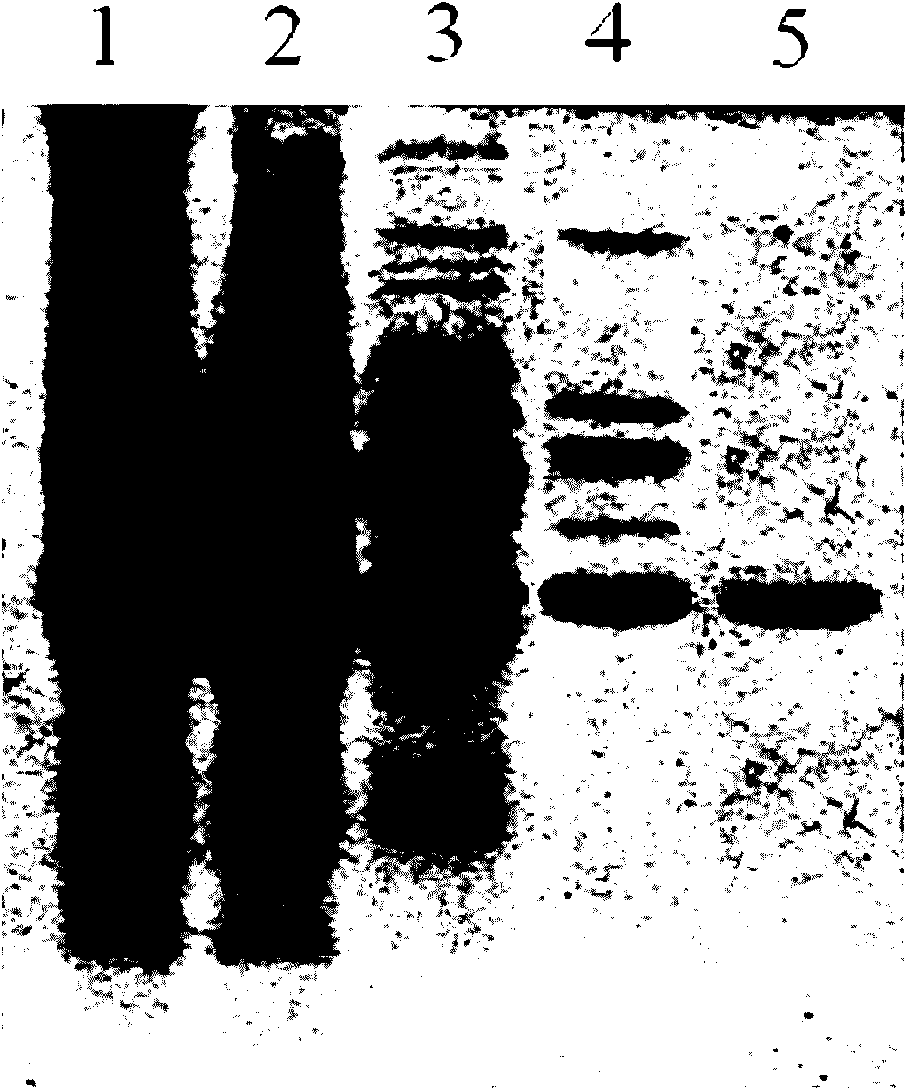Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86results about How to "Taller than alive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
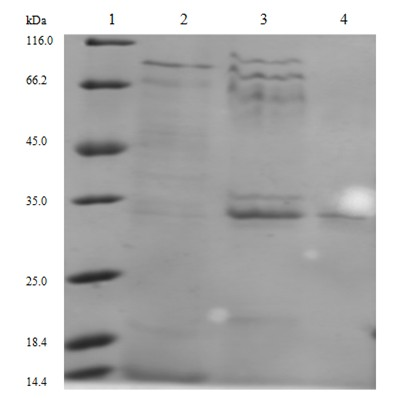
Method for preparing flavobacterium heparinum heparinase I
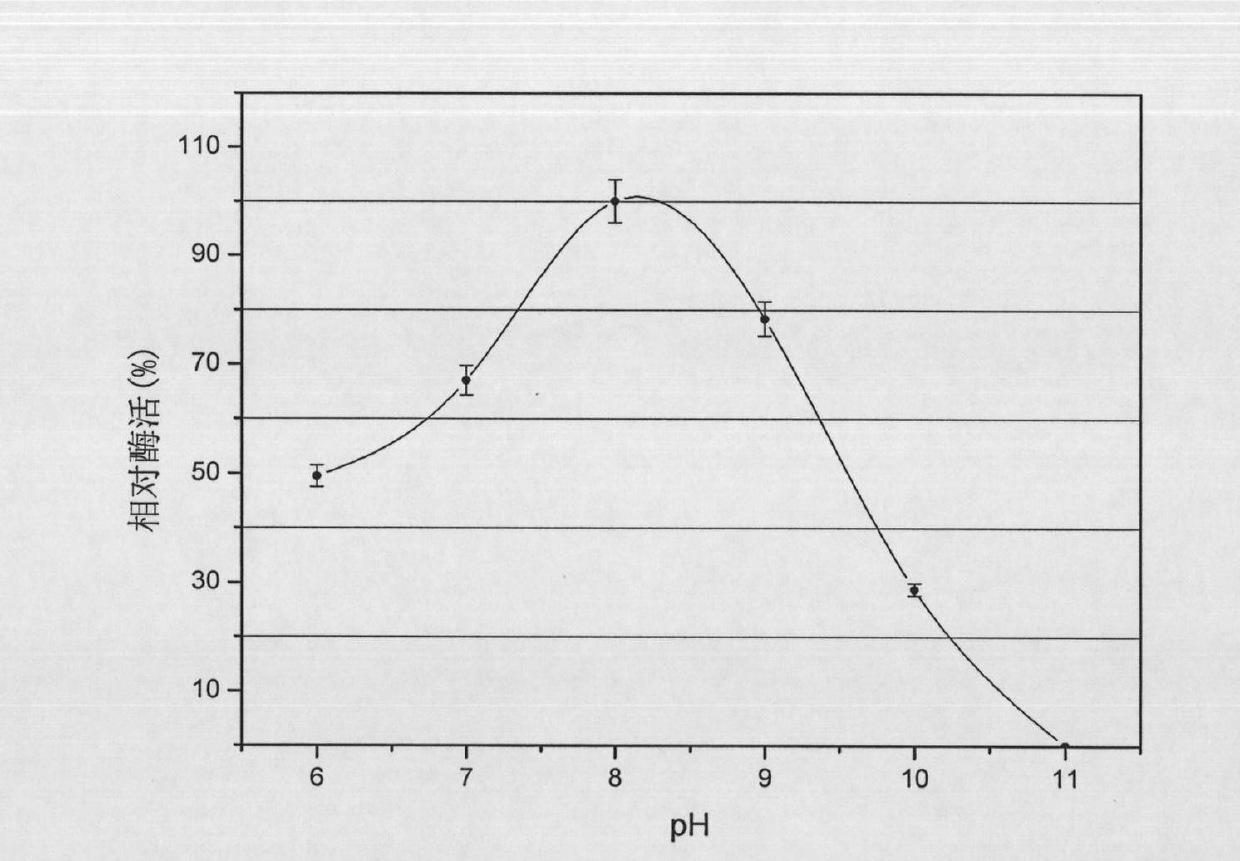
ActiveCN101886067ATaller than aliveEnsure stabilityMicroorganism based processesLyasesCentrifugationFlavobacterium heparinum
The invention provides a method for preparing heparinase I. The method for preparing the heparinase I comprises the following steps of: inoculating flavobacterium heparinum serving as a raw material to a seed culture medium for culture; then inoculating the flavobacterium heparinum to a fermentation culture medium; centrifugally collecting precipitate; performing ultrasonication on the precipitate; performing centrifugation again to obtain crude enzyme liquid of the flavobacterium heparinum heparinase I; and performing SP-sepharose FF chromatographic purification on the crude enzyme liquid for three times to obtain the high-purity flavobacterium heparinum heparinase I, wherein the SP-sepharose FF chromatographic purification for three times is protected by calcium chloride in the whole course, so that the yield of pure enzymic activity is greatly increased. The method for preparing the heparinase I has the characteristics of simple process, easy amplification, large preparation amount of products at a time, low cost of reagents and the like. The specific activity of the prepared heparinase I reaches 223 IU / mg and the yield of the pure enzymic activity reaches 30 percent. Compared with the conventional newest method, the method for preparing the flavobacterium heparinum heparinase I has the advantages of increasing the specific activity by more than two times, and the purification yield by about one time.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Method for producing recombinant human granulocyte colony stimulating factor
ActiveCN1814779ASimple production methodProduction method is stablePeptide preparation methodsCytokines/lymphokines/interferonsInclusion bodiesIon exchange
This invention relates to a method for producing recombined human granular leukocyte colony stimulating factors including: fermenting, breaking bactrriums, extracting occlusion bodies, chromatographing with molecular sieves, renaturating, exchanging anions, exchanging cations to get the raw fluid, in which, BL21 is selected as the host bacterium and the engineering bacterium type is got in high expression volume, quick reproduction and stable passage, several purification steps greatly reduce the residural toxin in the bacterium and the specific activity is increased greatly, a dialysis method is applied for the renaturation to reduce the concentration of the denaturalization agent steadily so the renaturation rate is at high level.
Owner:山东泉港药业有限公司
Organic solvent resistant protease producing strain, gene of organic solvent resistant protease produced thereby and application of organic solvent resistant protease
The invention relates to an organic solvent resistant protease producing strain, a gene of organic solvent resistant protease and an application of the organic solvent resistant protease, in particular relating to the organic solvent resistant protease producing strain as well as the gene of the organic solvent resistant protease thereby and the application of the organic solvent resistance protease in catalytic synthesis of small peptides in nonaqueous phases, belonging to the fields of microbiology and enzymology. Bacterial strains are named as Bacilluscereus WQ9-2 by classification, are gram-positive strains and can resist a plurality of organic solvents with a certain concentration. The protease-coding gene which is separated and cloned on organic solvent resistant protease produced by the producing strain is provided with a nucleotide sequence shown as SEQ ID NO:1, and an amino acid sequence shown as SEQ ID NO:2. The protease has the characteristics of high yield, high specific activity, wide and strong tolerance and the like. The protease has industrial application value for the catalytic synthesis of the small peptides in the nonaqueous phases.
Owner:NANJING UNIV OF TECH
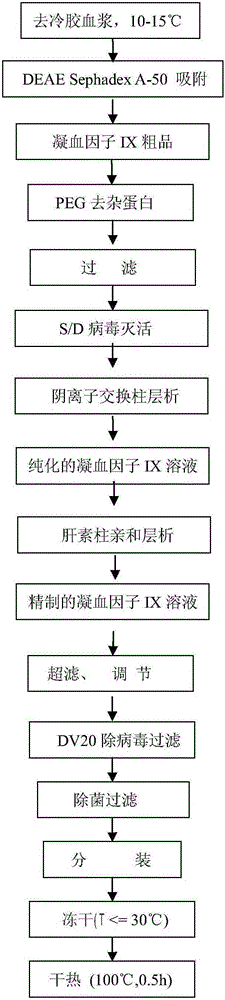
Preparation method of high-purity human coagulation factor IX
InactiveCN105175486AReduce lossesPrevent denaturation and inactivationPeptide preparation methodsUltrafiltrationPolyethylene glycol
The invention relates to a preparation method of a high-purity human coagulation factor IX, which comprises the following steps: melting refrigerated plasma, and carrying out low-temperature centrifugation; adsorbing with a DEAE (diethylaminoethanol) Sephadex A-50 gel to remove the coagulation factor IX in the cold-glue plasma; removing impure proteins in the solution by using polyethyleneglycol; carrying out S / D virus inactivation; carrying out anion exchange column chromatography to obtain a purified coagulation factor IX solution; passing through a heparin affinity column for further chromatography to obtain a high-purity coagulation factor IX solution; carrying out ultrafiltration, dialysis and concentration, and adding arginine hydrochloride and glycinate as protective agents; filtering through a 20nm filter element to remove viruses; carrying out freeze-drying; and carrying out dry heat virus inactivation. The protein protective agents are added during the gel adsorption, column chromatography and ultrafiltration dialysis, thereby lowering the activation probability of the FIX product thrombin and enhancing the qualification rate of the product. The technique has high product yield; the FIX specific activity can reach 150 IU / mg or so which is much higher than that of the traditional product; and by performing the three-step virus inactivation, the product is safe and reliable to use.
Owner:上海洲跃生物科技有限公司
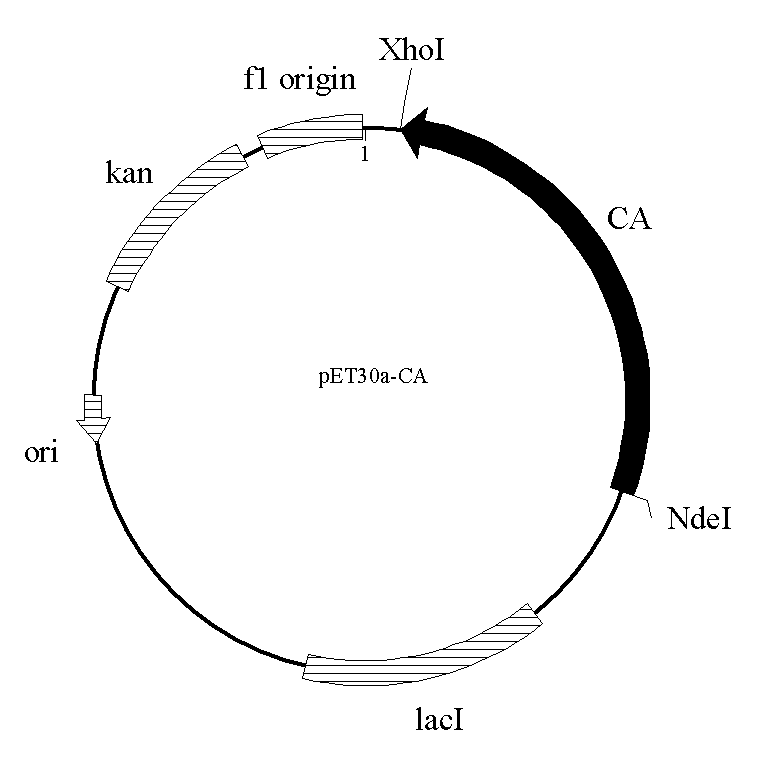
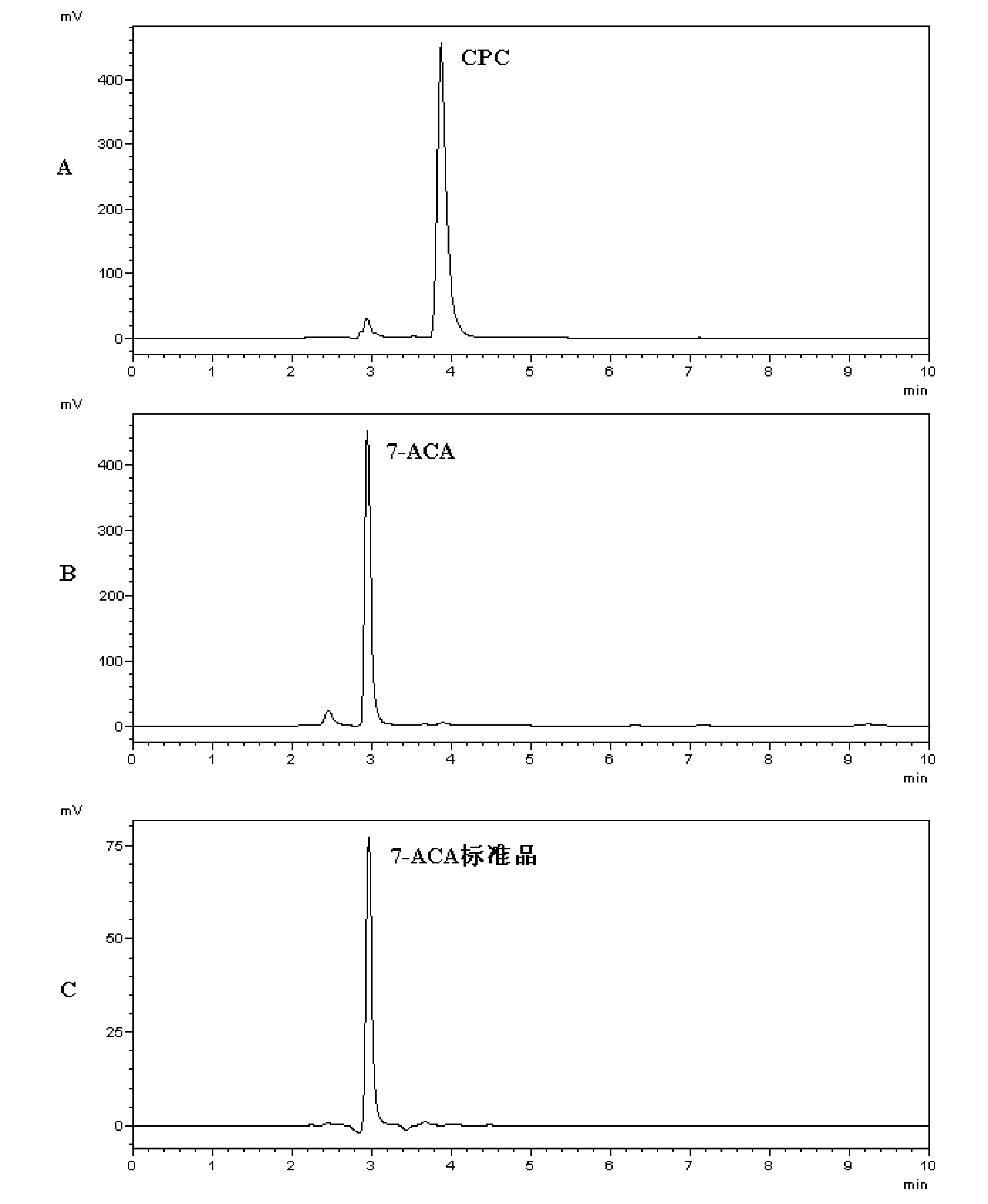
Cephalosporin acylase mutant and encoding gene and application thereof
The invention discloses a cephalosporin acylase mutant and an encoding gene and an application thereof. The protein provided by the invention is any one of the following 1)-3): 1) protein formed by an amino acid sequence shown as sequence 4 in the sequence table; 2) protein formed by an amino acid sequence shown as sequence 3 in the sequence table; 3) protein which is obtained by substitution and / or deletion and / or addition of one or several amino acid residues in the amino acid residue sequence of sequence 3 or sequence 4 in the sequence table, has the functions of cephalosporin acylase, andis derived from 1). Experiment of the invention shows that the invention provides a wild-type CPC acylase mutant; HPLC results show that the specific activity for CPC of the wild-type CPC acylase mutant of the invention is increased by 6.5 times when compared with that of wild-type enzymes. In one-step conversion, the conversion rate is up to above 98% within 3 hours.
Owner:TSINGHUA UNIV
Method for extracting superoxide dismutase
The invention relates to a method for extracting superoxide dismutase, in particular to a method for extracting superoxide dismutase from barbadosnut. The method for extracting superoxide dismutase is achieved by using seeds or leaves of barbadosnut as raw materials, and comprises the steps of the preparing crude extract, removing foreign protein, sectional salting-out ammonium sulfate, precipitating acetone, carrying out Sephadex G2100 molecular sieve column chromatography, and the like. The invention provides a new source for extracting superoxide dismutase, and optimizes technical parameters for extracting superoxide dismutase. The method for extracting superoxide dismutase can give consideration to both the specific activity and the recovery ratio of enzyme, and is beneficial to the large-scale production of enterprises.
Owner:SICHUAN UNIV
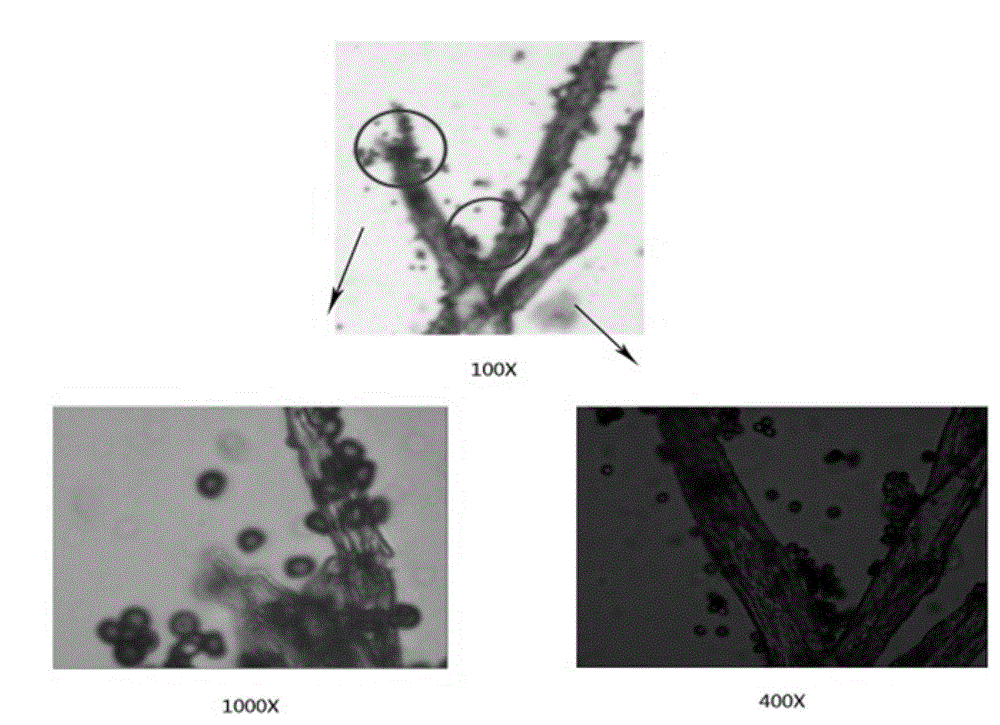
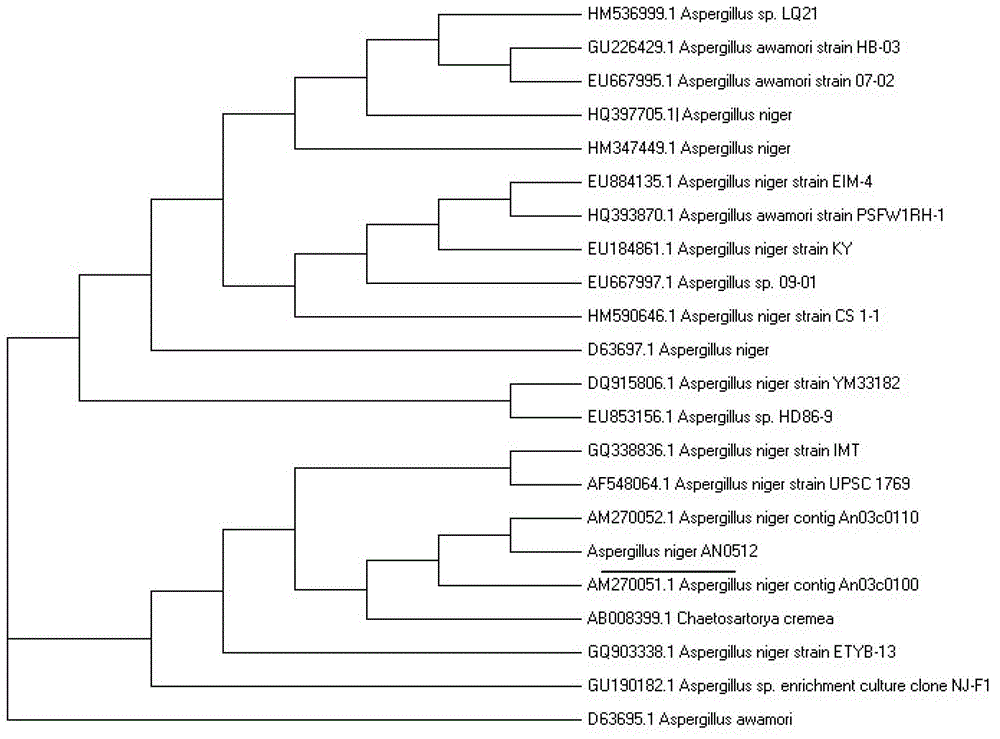
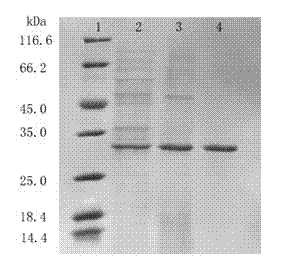
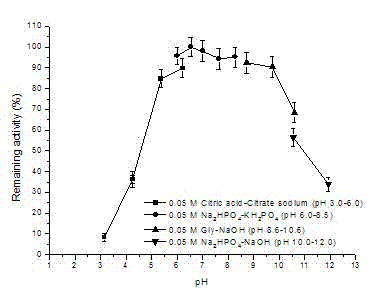
Lipase made of aspergillus niger strains, and producing method and utilization thereof
ActiveCN103060286AGood business prospectsStable temperatureHydrolasesMicroorganism based processesLaboratory cultureAmmonium sulfate
The invention discloses lipase made of aspergillus niger strains, and a producing method and utilization thereof. The lipase (Aspergillus niger) AN0512 is preserved in the China general microbiological culture collection center which is called CGMCC for short on November 19th, 2012, and the preserving number is CGMCC No.6835. The producing method includes: fermenting the lipase in a culture medium using olive oil as a main carbon source; removing thalluses to obtain lipase crude enzyme liquid; and then depositing and dialyzing, and separating the lipase crude enzyme liquid through anion exchange chromatography and molecular exclusion chromatography to obtain electrophoresis pure lipase. The lipase has a wide temperature application range, good activity when hydrogen ion concentration ranges from 4.0 to 6.0, and good acid resistance, and can be used in industries of food processing, drug preparing, chemical synthesizing and the like.
Owner:广州英赞生物科技有限公司
Lipase producing strain tolerant to organic solvent as well as genes and applications of lipase
The invention relates to a lipase producing strain tolerant to an organic solvent as well as genes of the lipase producing strain tolerant to the organic solvent and an application of the lipase producing strain tolerant to the organic solvent in catalyzing and splitting chiral compounds in an organic phase, belonging to the technical field of microbiology engineering and enzyme engineering. The classification name of the lipase producing strain is PseudomonasstutzeriLC2-8, the preservation registration number thereof is: CCTCC NO: M2020279. The lipase producing strain is a gram negative strain which can tolerate multiple organic solvents with a certain concentration. The lipase producing strain in the invention is split and cloned to the lipase coded genes tolerant to the organic solvent, which is produced by the lipase producing strain. The lipase tolerant to the organic solvent has the properties such as high yield, high specific activity, good tolerance to solvents, wide acting pH range and the like. The lipase also enjoys industrial application value such as catalyzing and splitting the chiral compounds in the organic phase and the like.
Owner:NANJING UNIV OF TECH
Fusion protein GST-SOD (superoxide dismutase)1-X-R9 as well as preparation method and application thereof
ActiveCN107400167APlay the role of targeted protection of normal tissuesTaller than alivePolypeptide with localisation/targeting motifPeptide/protein ingredientsDismutaseEscherichia coli
The invention relates to the field of fusion protein, and particularly provides fusion protein GST-SOD (superoxide dismutase)1-X-R9 as well as a preparation method and an application thereof. A linker peptide X and cell-penetrating peptide R9 are fused with a gene recombination technology according to the gene sequence of copper-zinc SOD in Genbank, SOD1-X-R9 is obtained, the synthesized SOD1-X-R9 is inserted into a pGEX4T-1 plasmid containing a GST sequence, pGEX4T-1-SOD1-X-R9 expression plasmid is obtained, and soluble protein GSTSOD1-X-R9 with high expression quantity is obtained through expression in Escherichia coli BL21 (DE3). The fusion protein GST-SOD1-X-R9 not only has activity of GST and SOD, but also can penetrate cells through R9 in a normal cellular environment and scavenging excessive free radicals in the cells, thereby protecting the cells from oxidative damage.
Owner:FUZHOU UNIV
Purifying method of interferon
ActiveCN103014101ANo significant change in purityNo significant change in activityPeptide preparation methodsFermentationInclusion bodiesInterferon alpha
The invention relates to a purifying method of interferon. The method comprises the following steps of a. performing fermentation culture by selecting engineering bacteria as bacterial strains; b. separating and washing inclusion bodies of the bacterial strains obtained from the fermentation culture to obtain refined inclusion bodies; c. performing renaturation on the refined inclusion bodies to obtain a renaturation preoduct; d. performing anionic column chromatography treatment on the renaturation product to obtain an anionic column chromatography product; d. performing cation column chromatography treatment on the anionic column chromatography product to obtain a cation column chromatography product; and f. processing the cation column chromatography product by using a reverse phase packing column chromatography, and performing desalinizing treatment to obtain the interferon.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
High-stability organic solvent-resistant lipase producing strain and lipase as well as gene and application thereof
The invention belongs to the technical fields of microbial engineering and enzyme engineering, in particular to a high-stability organic solvent-resistant lipase producing strain Burkholderiaambifaria YCJ01, a gene of the organic solvent-resistant lipase and an application of the organic solvent-resistant lipase for catalyzing and resolving chiral compound in an organic phase. The strain is a gram negative strain, the preservation registration number of the strain is CCTCCNO: M2011058, the strain has high yield of the organic solvent-resistant lipase, wider application range, good temperature stability and higher resistance to multiple organic solvents, and the strain has good application prospect during resolving of the chiral compound.
Owner:NANJING UNIV OF TECH
Process for cultivating recombinant human tectotype fibrinolytic enzyme activator TNK mutant with micro-carrier
InactiveCN101096655ATaller than aliveIncrease cell densityRecombinant DNA-technologyForeign genetic material cellsCell concentrationCell strain
The invention discloses a technology for producing and recombining a human tectotype profibrinolytic activator TNK mutant with Cytopore porous microcarrier culture method, which comprises the following steps: suspending TNK-tPA engineering project CHO cell strain with the culture medium; culturing by transferring to the cell culture bottle; forming dense monolayer cell; culturing by transferring the cell to Wheaton agitator; transferring to the Celligen reactor (the culture temperature is 37 Deg. C, PH is 7.1+-0.1, the dissolved oxygen is 30-60%, the original rotating speed is 20rpm, the rotating speed increases progressively after 4 hours and reaches 40rpm when inoculating for 6 hours); culturing 20 days by replenishing the culture liquid and the fresh microcarrier; collecting when the cell concentration reaches the finite level. TNK-tPA with the invention has the big cell density, the low cost, the high expression level and the high expression product specific activity.
Owner:冯来坤
Green plant bug water-soluble trehalase, coding sequence, vector, strain and application
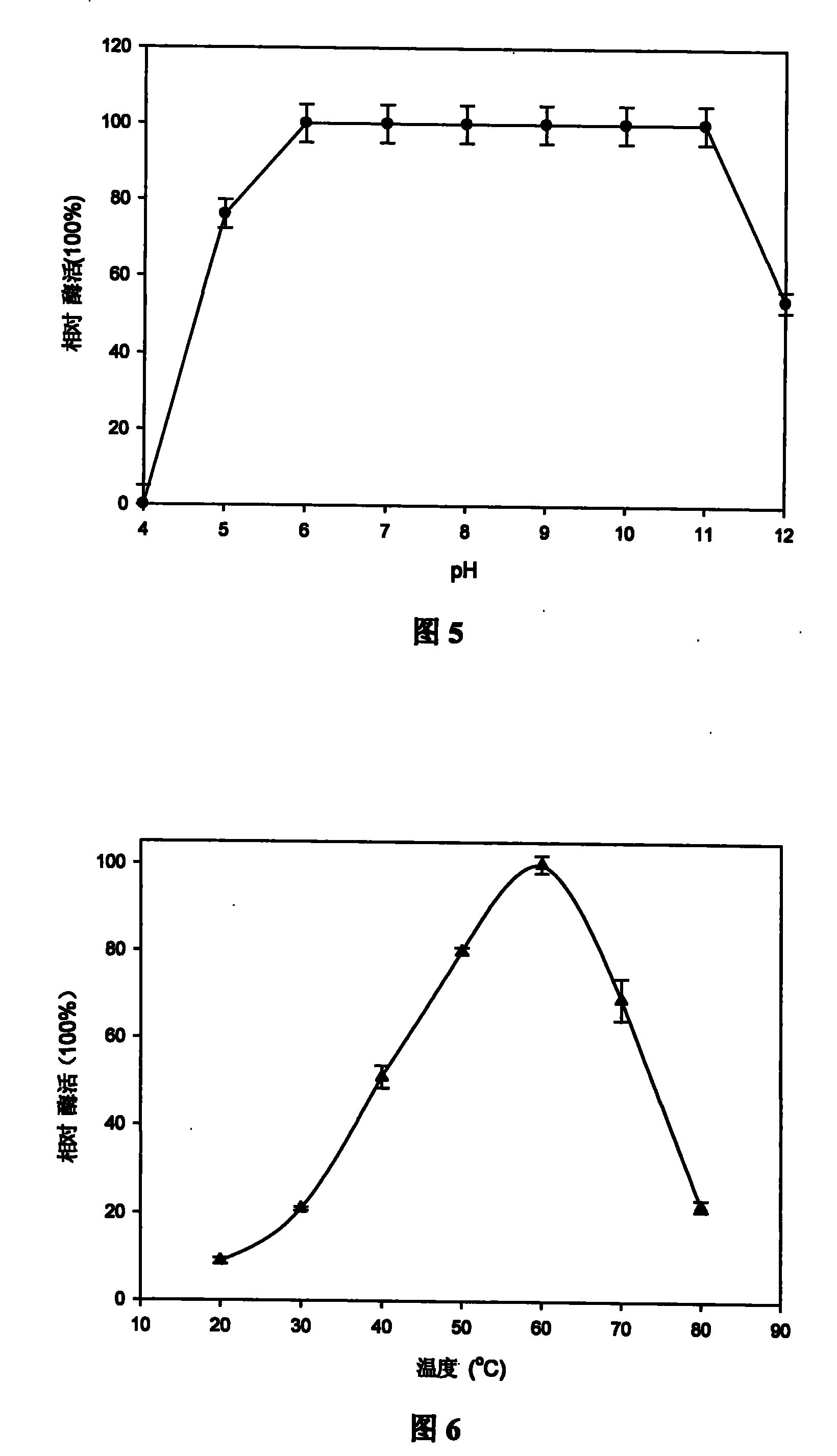
The invention discloses a DNA (deoxyribonucleic acid) sequence for coding a green plant bug water-soluble trehalase. The invention also discloses a green plant bug water-soluble trehalase, a recombinant expression vector of the DNA sequence for coding the green plant bug water-soluble trehalase, a transgenic cell system or transgenic recombinant bacterium, application of the recombinant expression vector and transgenic cell system or transgenic recombinant bacterium in producing the green plant bug water-soluble trehalase, and a preparation method of the green plant bug water-soluble trehalase. The molecular weight of the prepared zymoprotein is 71KD, the optimum pH value is 7.0, the optimum temperature is 55 DEG C, and the zymoprotein can degrade trehalose into glucose. The purified trehalase can be used for qualitative and quantitative work of trehalose content in industry and some other fields, and can also provide foundation for researching and developing insect trehalase inhibitors.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Organic solvent resisting lipase, application thereof and bacteria for producing same
The invention aims at providing bacteria for producing organic solvent resisting lipase, the organic solvent resisting lipase produced from the bacteria and the application of the organic solvent resisting lipase to the enzymatic reaction for the synthesis of the biodiesel in the organic phase, in particular to the ester exchange reaction for the synthesis of the biodiesel. The bacteria of pseudomonas aeruginosa LX1 is obtained through screening, the organic solvent resisting LX1 lipase produced from the pseudomonas aeruginosa LX1 has the amino acid sequence showed in the sequence identifier (SEQ ID) No. 2, and the organic solvent resisting LX1 lipase encoding gene has the nucleotide sequence showed in the SEQ ID No. 2. The organic solvent resisting LX1 lipase is used for catalyzing the substrate of soybean oil and exchanging the soybean oil and the ester methanol in the organic solvent tert-butanol system or the solvent-free system to synthesize the clean energy of biodiesel which has the conversion rate of 80-90 percent.
Owner:NANJING UNIV OF TECH
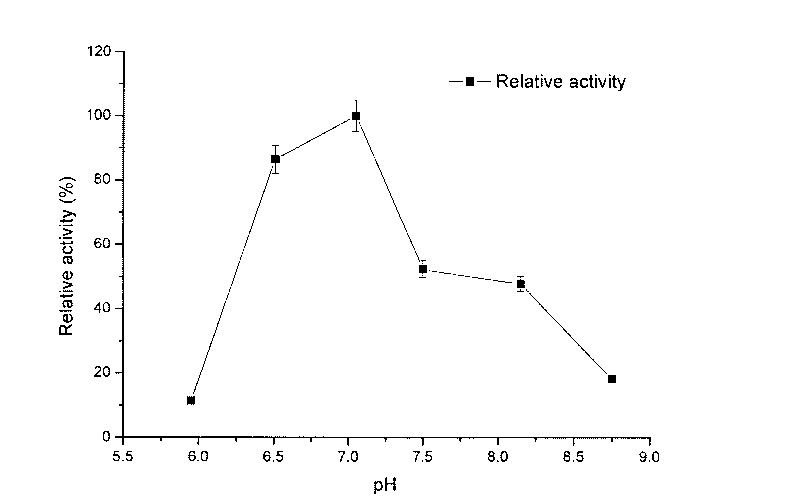
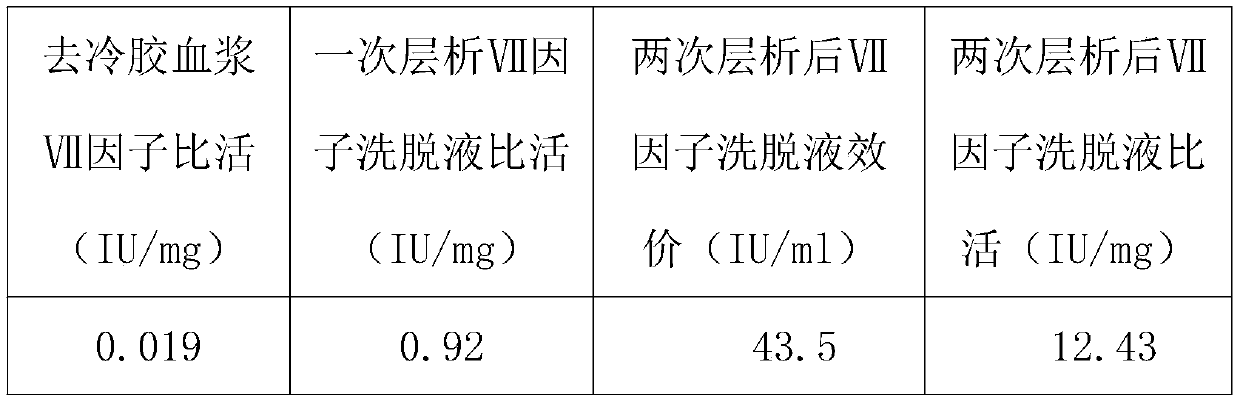
Method for purifying human coagulation factor VII from human plasma
ActiveCN111500563ATaller than aliveIncrease profitPeptidasesMonosodium glutamateAnion-exchange chromatography
The invention discloses a method for purifying a human coagulation factor VII from human plasma. The method comprises the following steps: (1) removing cold gel plasma, (2) performing anion exchange chromatography for the first time, (3) filtering the eluent, and adjusting the conductivity and pH value of the product, and (4) carrying out ion exchange chromatography for the second time. Accordingto the method, the human coagulation factor VII can be separated from the cold-gel-free plasma by adopting sodium glutamate as an eluent through first ion exchange chromatography, and the human coagulation factor VII can be further purified through two times of chromatography, so that the utilization rate of the plasma is greatly improved, and the human coagulation factor VII can achieve relatively high specific activity.
Owner:HUALAN BIOLOGICAL ENG CHONGQING
Preparation method of human blood coagulation factor VIII and human blood coagulation factor VIII product
ActiveCN107880112AHigh recovery rateTaller than aliveFactor VIIPeptide preparation methodsWhole blood productIon chromatography
The invention discloses a preparation method of a human blood coagulation factor VIII and a human blood coagulation factor VIII product and relates to the field of blood products. The preparation method of the human blood coagulation factor VIII comprises the following steps: dissolving a cryoprecipitate with a 0.015-0.025mol / L tromethamine solution in a mass ratio of (3-5): 1 by reasonably processing the cryoprecipitate; and carrying out separation and purification by means of a polyethylene glycol precipitating method combined with ion exchange chromatography, wherein the recovery ratio of the human blood coagulation factor VIII and the specific activity of a final product can be improved effectively, the yield reaches 180-240IU / L plasma, and the specific activity is not lower than 100IU / mg proteins. The prepared human blood coagulation factor VIII product is rich in vWF factors and the proportion of the vWF factors and the human blood coagulation factor VIII is close to 1: 1. Besides treating hemophiliac A, the human blood coagulation factor VIII can be also used for treating patients with angiohemophilia, and the human blood coagulation factor VIII has good stability and heat resistance.
Owner:HUALAN BIOLOGICAL ENG INC +2
High-stability organic solvent-resistant lipase producing strain and lipase as well as gene and application thereof
The invention belongs to the technical fields of microbial engineering and enzyme engineering, in particular to a high-stability organic solvent-resistant lipase producing strain Burkholderiaambifaria YCJ01, a gene of the organic solvent-resistant lipase and an application of the organic solvent-resistant lipase for catalyzing and resolving chiral compound in an organic phase. The strain is a gram negative strain, the preservation registration number of the strain is CCTCCNO: M2011058, the strain has high yield of the organic solvent-resistant lipase, wider application range, good temperature stability and higher resistance to multiple organic solvents, and the strain has good application prospect during resolving of the chiral compound.
Owner:NANJING TECH UNIV
Process for producing D(-)-tartaric acid by biotransformation
InactiveCN101845465AEasy to useSolution to short lifeMicroorganism based processesOn/in organic carrierMicroorganismCarrageenan
The invention provides a process for producing a D(-)-tartaric acid by biotransformation. The process comprises the following steps of: A, extracting cis-epoxysuccinic acid hydrolase from microorganisms capable of degrading epoxysuccinic acid, and purifying the cis-epoxysuccinic acid hydrolase; B, immobilizing the purified cis-epoxysuccinic acid hydrolase; C, performing catalytic hydrolysis of the epoxysuccinic acid by using the immobilized enzyme as a biocatalyst to produce the D(-)-tartaric acid. By employing the properly purified enzyme for immobilization, the specific activity of the catalytic is increased greatly, so the reaction time and required equipment volume are reduced and consequently the production cost is reduced. The method for immobilizing the enzyme combines a carrageenan immobilizing method with strong mechanical strength and a protein (gelatin and enzyme) covalent crosslinking method with strong stability and toughness, so the immobilized enzyme has better service performance and a longer service life. As a result, the production cost of the D(-)-tartaric acid is reduced.
Owner:ZHEJIANG GENEBEST PHARMA
Method for producing recombinant human granulocyte colony stimulating factor
ActiveCN1313612CReduced residue levelsTaller than aliveFermentationGenetic engineeringBiotechnologyIon exchange
This invention relates to a method for producing recombined human granular leukocyte colony stimulating factors including: fermenting, breaking bactrriums, extracting occlusion bodies, chromatographing with molecular sieves, renaturating, exchanging anions, exchanging cations to get the raw fluid, in which, BL21 is selected as the host bacterium and the engineering bacterium type is got in high expression volume, quick reproduction and stable passage, several purification steps greatly reduce the residural toxin in the bacterium and the specific activity is increased greatly, a dialysis method is applied for the renaturation to reduce the concentration of the denaturalization agent steadily so the renaturation rate is at high level.
Owner:山东泉港药业有限公司
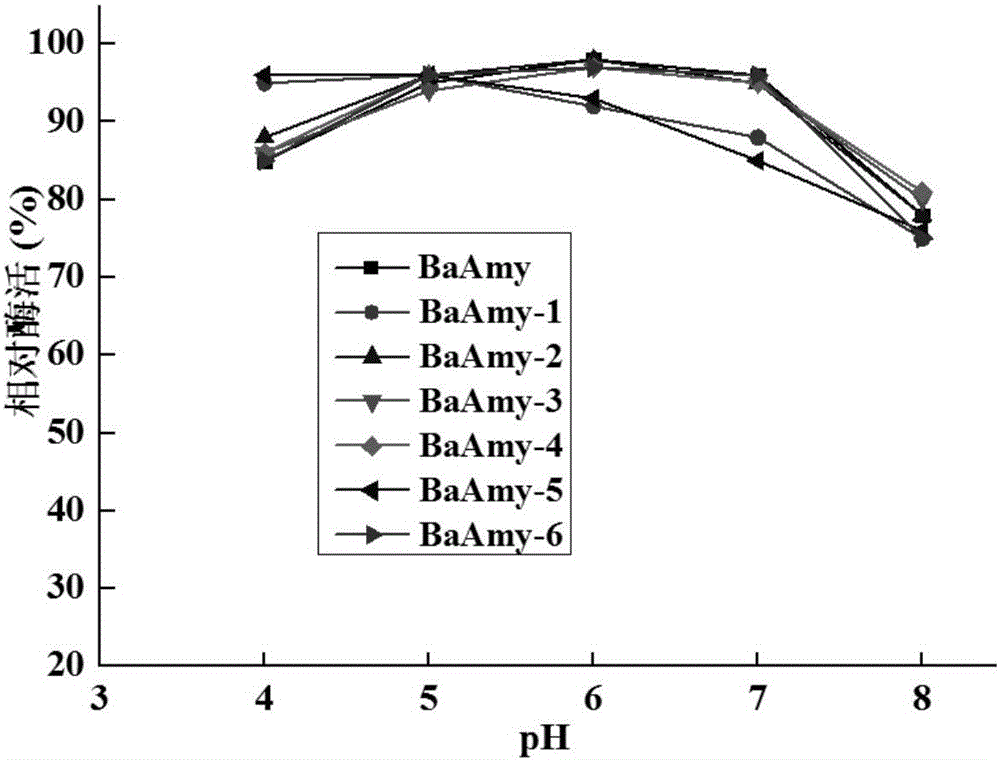
Alpha-amylase BaAmy mutant capable of improving specific activity, and encoding gene and application thereof
The invention relates to the field of gene engineering, in particular to an alpha-amylase BaAmy mutant capable of improving specific activity, and an encoding gene and application thereof. The mutant is a mutant of acidic alpha-amylase BaAmy with an amino acid sequence as shown in SEQ ID NO.8, wherein mutation sites are any one or more of the 37th, 85th, 191st, 241st, 279th, 291st, 319th and / or 333rd amino acid. The specific activity of the mutated alpha-amylase is increased by 45 to 100 percent relative to the specific activity of the original alpha-amylase, and foundation is laid for industrialized application of acidic bacillus acidic alpha-amylase.
Owner:GUANGDONG VTR BIO TECH
Acidic trehalase TreA and gene and application thereof
InactiveCN108841808AAcid resistantTaller than aliveFungiMicroorganism based processesBiotechnologyTrehalase
The invention belongs to the technical field of agricultural genes and in particular relates to acidic trehalase TreA sourced from a fungus and a gene and application thereof. The amino acid sequenceof the trehalase TreA is as shown in SEQ ID NO.1 or SEQ ID NO.2. The invention provides a novel trehalase gene. The trehalase encoded by the trehalase gene has high properties and can be applied to industries of insect control, foot, starch sugar, medicines and the like.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Method for removing viruses in preparation of antitoxin and antiserum
InactiveCN102512449AQuality improvementQuality, including increased protein concentrationSerum immunoglobulinsMammal material medical ingredientsEnzymatic digestionIon exchange
The invention provides a method for removing viruses in preparation of antitoxin and antiserum. The method comprises the following steps of: diluting immunological blood plasma and adjusting pH value; then digesting by stomach enzyme; heating to denaturize; adsorbing by alum; concentrating / desalinating by ultrafiltration; performing ion exchange chromatography to obtain collected liquid; removing bacteria and filtering; and then subpackaging to obtain an antitoxin and antiserum product. Thus, the viruses which probably exist in raw material serum can be removed simultaneously in the process of preparing the antitoxin and antiserum. The virus removal method for the antitoxin and antiserum provided by the invention has the characteristics of simplicity, quickness economy and practicality; the viruses which probably exist in the raw material serum can be removed completely during production to guarantee the quality of the product.
Owner:玉溪九洲生物技术有限责任公司
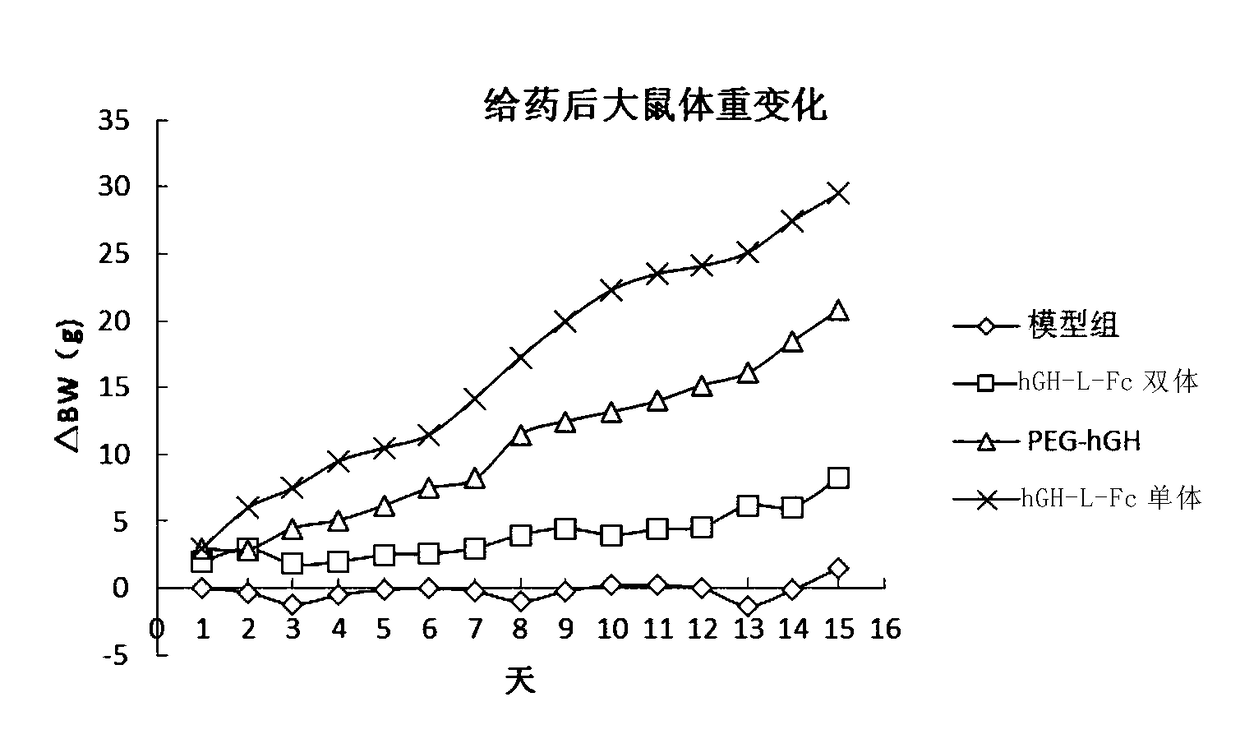
Recombinant long-acting HGH (human growth hormone) fusion protein and preparation and application thereof
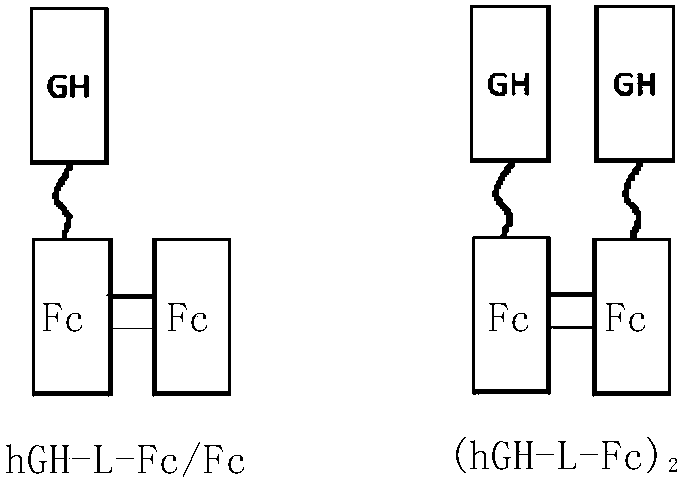
InactiveCN108794634AEasy to makeEasy to purifyPeptide/protein ingredientsAntibody mimetics/scaffoldsCrystallographyHuman growth hormone
The invention provides a recombinant long-acting HGH (human growth hormone) fusion protein and preparation and application thereof, particularly provides a fusion protein having the structure as shownas the formula I, wherein X refers to GH element, Y1 and Y2 refer to Fc elements respectively, L refers to None or linker peptide, ' ' refers to a covalent bond (such as a peptide bond), '... 'represents n interchain disulfide bonds between Y1 andY2, and n is 1, 2, 3, 4, 5, or 6.
Owner:SYNDEGEN SHANGHAI BIOTECH
Organic solvent resisting proteinase high-yield bacterium, gene and application of the organic solvent resisting proteinase
The invention discloses a tolerance organic solvent protease high yield bacterial strain, protease gene and the protease application in organic phase catalysis peptides synthesis. The bacterial strain sorting naming is Pseudomonas aeruginosa PT121, preserving registration number is CCTCC M 208029, and is gram negative bacterial strain and various organic solvent which can resist certain concentration. The organic solvent-tolerance protease encode gene produced from the bacterium is separated and cloned by the invention which comprises nucleotide sequence showed as SEQ ID NO:1 and amino acid sequence showed as SEQ ID NO:2. The tolerance organic solvent protease has the habitude of high yield, high specific activity, strong solvent tolerance, wide acting pH range and high temperature resistant. The protease is provided with organic phase catalysis peptides synthesis etc. industry applications value.
Owner:NANJING TECH UNIV
Preparation method and application of high-specific-activity amylase mutant
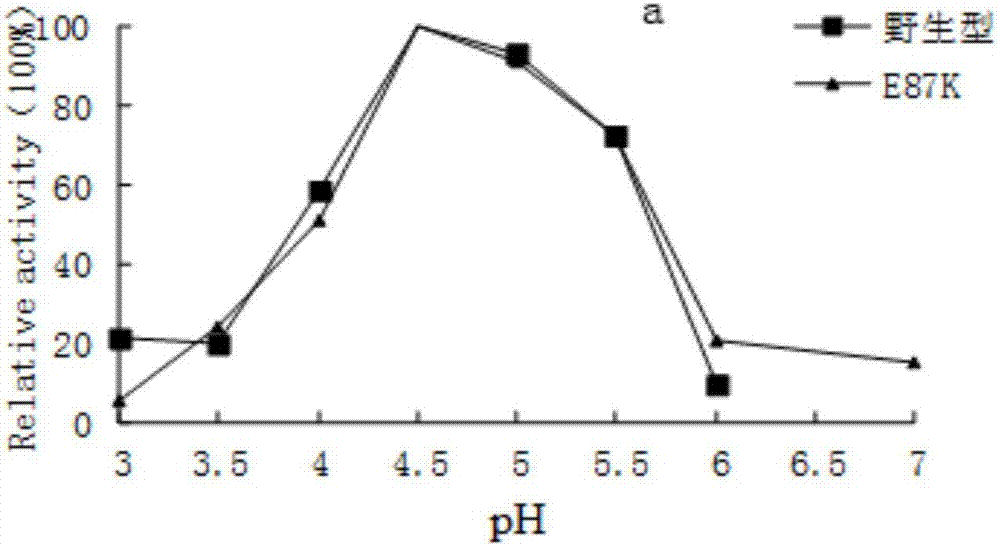
ActiveCN107201351ATaller than aliveShorten retrofit timeFungiMicroorganism based processesBiotechnologyWild type
The invention provides a high-specific-activity high-catalytic-efficiency amylase mutant and a preparation method and application thereof and relates to the field of gene engineering. Acid amylase derived from Talaromyces leycettanus JCM12802 is used as a female parent, and the wild type is subjected to site-directed mutagenesis by means of molecular biological techniques. Under the modification condition, the amylase mutant has significantly higher catalytic efficiency and specific activity than the wild (before mutation) female parent. By using the scheme, it is possible to greatly increase the specific activity and acting conditions of amylase, and basis is provided for the amylase in industrial production field. The scheme is of important guidance for increasing the catalytic efficiency and specific activity of amylase and other enzymes.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
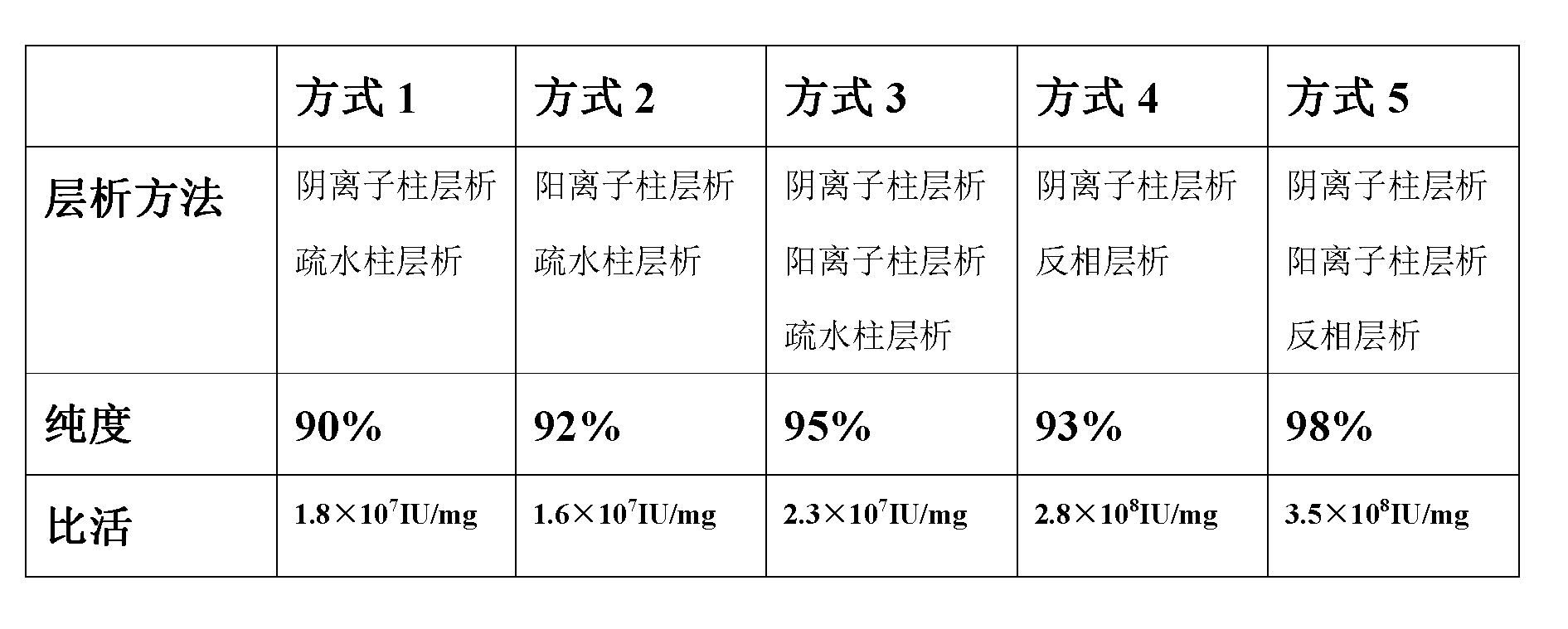
Purifying method of recombinant human granulocyte colony stimulating factors
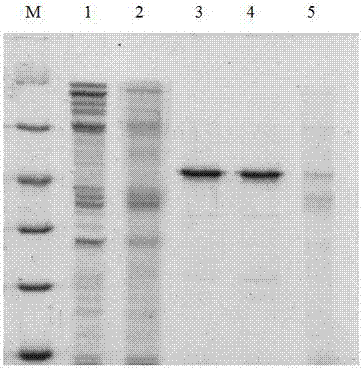
InactiveCN101597319AHigh purityHigh activityPeptide preparation methodsGranulocyte colony-stimulating factorElectrophoreses
The invention relates to a purifying method of recombinant human granulocyte colony stimulating factors and applies a fine separating step of opposite-phrase filling to a purifying technology of the recombinant human granulocyte colony stimulating factors for the first time. Compared with the prior art, the method adopts the opposite-phrase filling for the first time to finely separate the recombinant human granulocyte colony stimulating factors, the electrophoresis purity is increased to 99 percent from 90 percent, the HPLC purity is increased to 99 percent from 90 percent, and the specific activity is increased to 2.3*10U / mg from 6.1*10U / mg. The purifying technology of the recombinant human granulocyte colony stimulating factors is adopted to greatly increase the purity, the specific activity and the stability of the recombinant human granulocyte colony stimulating factors and can effectively reduce the clinical side effect of the recombinant human granulocyte colony stimulating factors.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Method for producing alpha interferon and dedicated bacteria therefor
ActiveCN101531967AGood genetic stabilityInhibition of replicationBacteriaMicroorganism based processesEscherichia coliBiotechnology
The invention discloses a method for producing alpha interferon and a dedicated bacterial strain therefor. The bacterial strain is obtained by introducing the following genes into a host strain: (1) DNA molecules with the nucleotide sequence as shown in the sequence one in the sequence table; (2) DNA molecules whose functional protein is hybridized with and provided with the same coding with the DNA sequence as prescribed in (1) under strict conditions; and (3) DNA molecules whose functional protein has over 90% of homology with and has the same coding with the DNA sequence as prescribed in (1). The method for preparing the alpha interferon comprises the following step of fermenting the recombined strains to obtain the alpha interferon. The Escherichia coli DH5a / pBV220 / Sw IFN-aCCMCC NO.2845 of the invention has good genetic stability, stable production performance, and the fermentation expressive recombined alpha interferon has high specific activity, high yield and high safety. The method of the invention is used for preparing the alpha interferon, thus the operation is simple, the cost is low and the efficiency is high. Therefore, the method for preparing alpha interferon of the invention has wide application prospect.
Owner:中科拜克(天津)生物药业有限公司
Hemocoagulase extracted from bothrops atrox and preparation method and application thereof
PendingCN108611341AReduce risk of exposureFewer steps in the preparation processHydrolasesPeptide/protein ingredientsBenzamidinePharmaceutical drug
The invention provides a hemocoagulase extracted from bothrops atrox and also provides a preparation method of the hemocoagulase. The method comprises the following steps of: pretreating the bothropsatrox; sequentially passing the diethylaminoethyl cross-linked dextran gel A-25 ion exchange chromatography, benzamidine agarose gel 4-FF (HS) affinity chromatography, and cross-linked dextran gel G-75 gel filtration chromatography; and obtaining hemocoagulase. The invention also provides the application of the bothrops atrox hemocoagulase. in the preparation of a medicament for treating bleedingdisorder. Compared with the prior art, the preparation process steps are less, the period is short, and the exposure risk of the process environment is reduced; the product yield is high, the processhas strong ability to remove impurities, the yield of the product can be obtained more than 35% and the purity is over 98%; the process stability is high and the product quality is good, and the process is beneficial to clinical medication safety.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
A method for preparing urokinase
ActiveCN105087531BImprove adsorption capacitySimple structurePeptidasesURINARY TRYPSIN INHIBITORUrokinase Plasminogen Activator
The invention relates to a urinary protein enriching method which comprises the steps of directly adsorbing urinary proteins in urine in urinals or urinating buckets with filter cloth bags loaded with modified silica gel, and transporting the urinary protein adsorbed filter cloth bags to working points for subsequent treatment. According to the method, the isoelectric point property of specific urinary proteins is utilized, and urinary proteins such as urinary trypsin inhibitors, human urinary kininogenase, urokinase and the like are effectively absorbed directly by using the modified silica gel, macroporous resin, chitin, ionic resin and the like, so that a urine collecting step is avoided. The method is free of obvious influence on sanitary conditions of toilets, and the cost for urine transportation and a series of environmental problems resulting from urine transportation are greatly reduced.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for purifying interferon protein
InactiveCN101659690ALittle side effectsHigh purityPeptide preparation methodsElectrophoresesSpecific activity
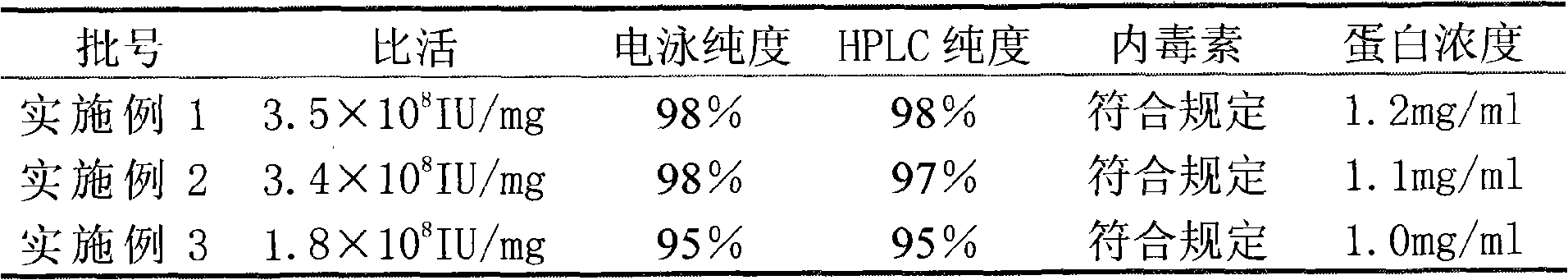
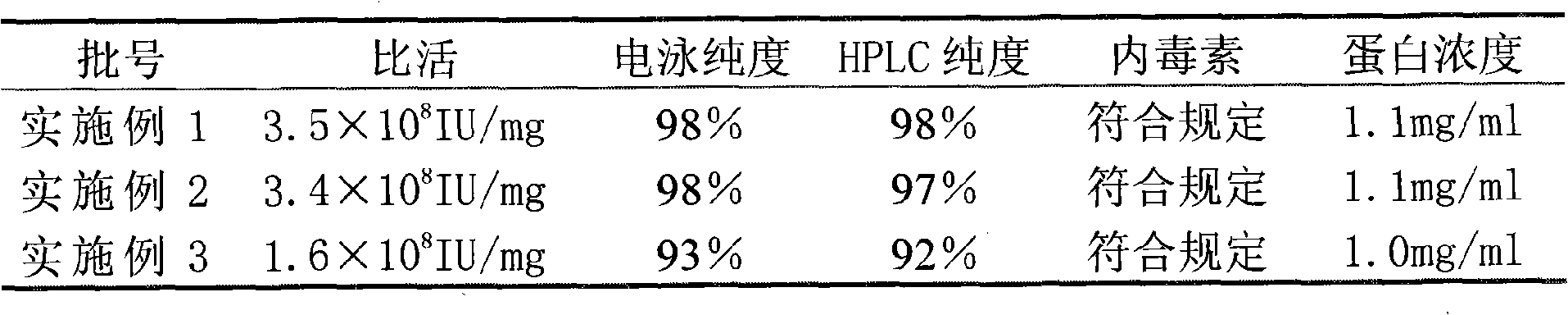
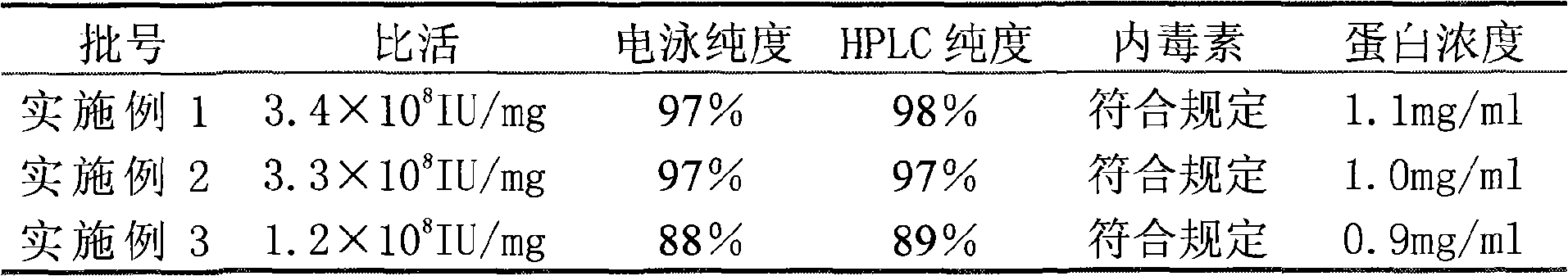
The invention relates to a method for purifying interferon protein. The method adopts a reverse phase filler refining and separating purification step in a purification process. Compared with the prior art, the method purifies an interferon by using a reverse phase filler refining and separating method for the first time and improves the electrophoresis purity of the interferon to 98 percent from90 percent, HPLC purity to 98 percent from 90 percent and specific activity of the interferon to 3.5*10<8>U / mg to 0.9*10<7>U / mg. The purification process of the recombinant interferon can greatly improve the purity, specific activity and stability of human interferons and effectively reduce the side effects of the interferons in clinic.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com