Purifying method of recombinant human granulocyte colony stimulating factors
A colony-stimulating factor and granulocyte technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., to achieve the effects of reducing side effects, improving protein purity, and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A semi-preparative MKF-RP column was used.
[0019] Sample: 200ml of cationic chromatography product, protein concentration 2mg / ml.
[0020] Prepare eluent A as buffer containing 50mmol NaCl and 20mmol Tris-HCl (pH 8), and eluate B as containing 50mmol NaCl solution 20mmol Tris-HCl 60% acetonitrile (pH 8).
[0021] First elute with eluent A, then use gradient elution of 15%-90% eluent B, and collect sample peaks in 10-55 minutes.
[0022] The collected samples were evaporated at low temperature to remove organic matter, then concentrated by dialysis and stored at 4°C.
Embodiment 2
[0024] XK 50 / 30 column was used, packed with SBC MCL GEL type reversed-phase preparative liquid chromatography packing material.
[0025] Sample: 500ml of anion chromatography product, protein concentration 2.2mg / ml.
[0026] The eluent A is 20% phosphate buffer, the eluent B is 30% methanol solution containing 0.02% tween80, and the flow rate is 15ml / min.
[0027] Gradient elution, eluting with 20%-75% eluent B in 10-80 minutes, and collecting sample peaks. When collecting samples, collect the middle section. The collected samples were evaporated at low temperature to remove organic matter, then concentrated by dialysis and stored at 4°C.
Embodiment 3
[0029] Use Butyl-Sepharose 4 Fast Flow, XK50H16 chromatography column.
[0030] Sample: 500ml of anion chromatography product, protein concentration 1.5mg / ml. Add ammonium sulfate to 0.8M.
[0031] Equilibrium solution: 20mmol / ml Tris-HCl, pH8.0, 0.8M ammonium sulfate
[0032] Pre-eluent: 20mmol / ml Tris-HCl, pH8.0, 0.5M ammonium sulfate
[0033] Eluent: 20mmol / ml Tris-HCl, pH8.0, 0.2M ammonium sulfate
[0034] Collect the eluted part of the eluate and dialyze to remove salt.
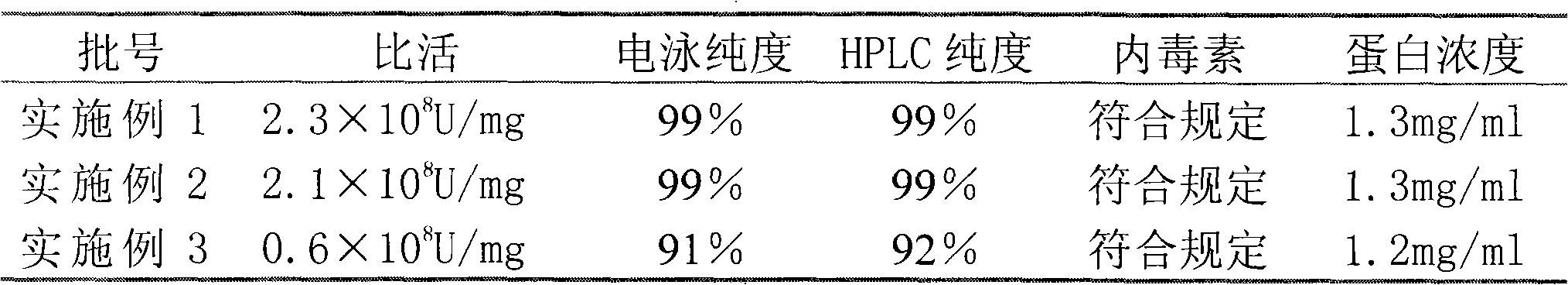
[0035] Under the condition of 4 DEG C, the samples of Examples 1, 2, and 3 were stably investigated, and the investigation results were as follows:
[0036] 0 months
[0037]
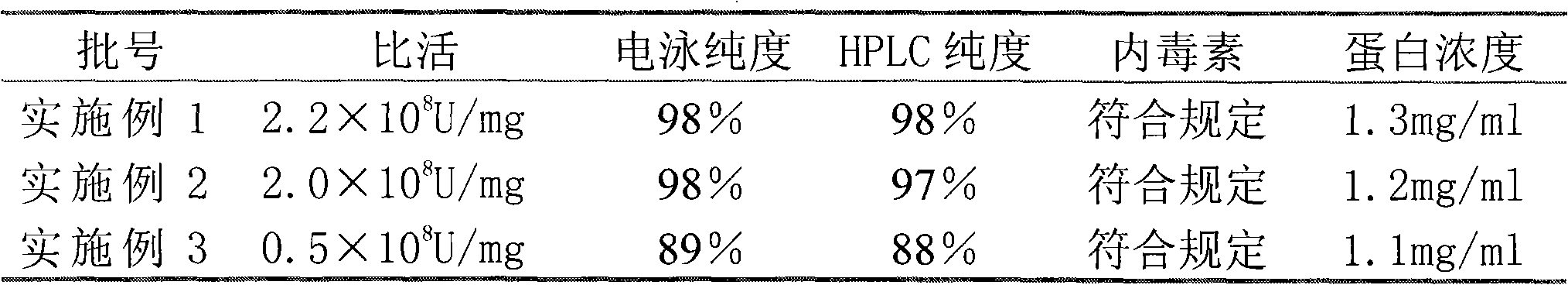
[0038] March
[0039]
[0040] June
[0041]
[0042] December
[0043]
[0044] The above data shows that compared with Example 3, the samples of Example 1 and 2 obtained by the reverse packing method have electrophoretic purity and HPLC purity greater than 97% within 12 months, and the specific activity reach...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com