Method for purifying human coagulation factor VII from human plasma
A technology of human blood coagulation factor and human plasma, which is applied in the field of bioengineering, can solve the problems of decreased titer of blood coagulation factor VII, achieve high specific activity, reduce pollution, and improve utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
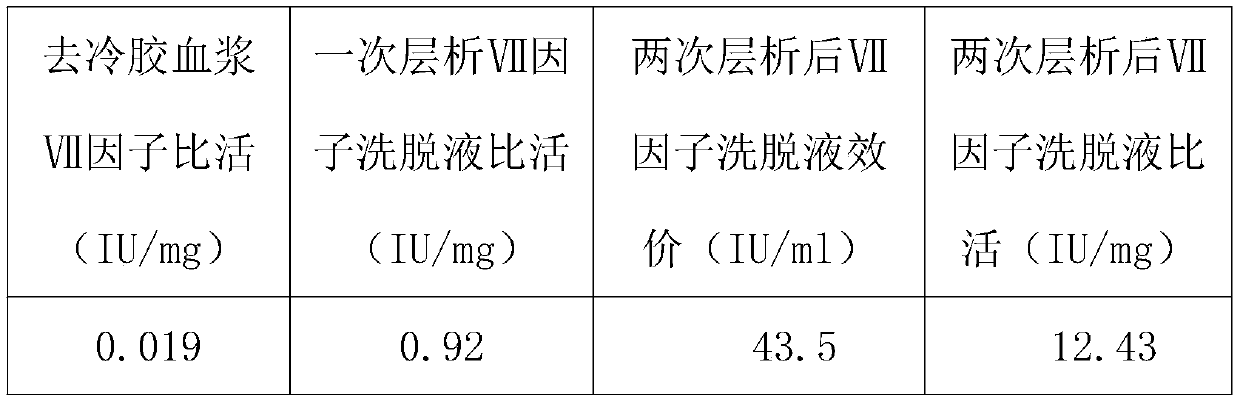
[0021] A method for purifying human blood coagulation factor VII from human plasma, comprising the following steps:
[0022] S1-de-cold plasma:
[0023] Thaw the fresh frozen plasma, and continuously centrifuge it at a temperature of 0-3°C to remove the cold glue. cold-depleted plasma;
[0024] S2- Preparation before the first chromatography:
[0025] UniGel-80Q ion exchange packing chromatography column preparation, equilibrate UniGel-80Q gel packing with a buffer containing 0.01mol / L~0.02mol / L sodium citrate and 0.1mol / L~0.15mol / L sodium chloride, The pH of the buffer solution is 6.80-7.80, after treatment, wait for sample loading.
[0026] S3-first anion exchange chromatography:
[0027] Adjust the plasma conductivity to 10-13mS / cm, the pH value to 7.00-8.00, and the protein content to 45g / L±5g / L, and load the adjusted gel-free plasma on the UniGel-80Q gel chromatography column to make the blood coagulation factor The target protein of VII is adsorbed in the UniGel-80Q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com