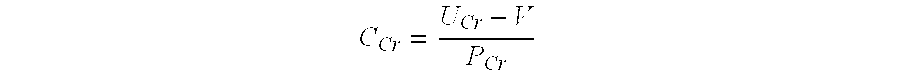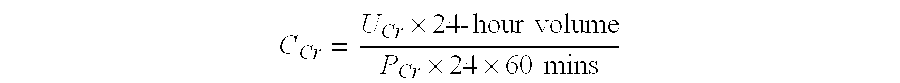Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Coagulation factor VII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
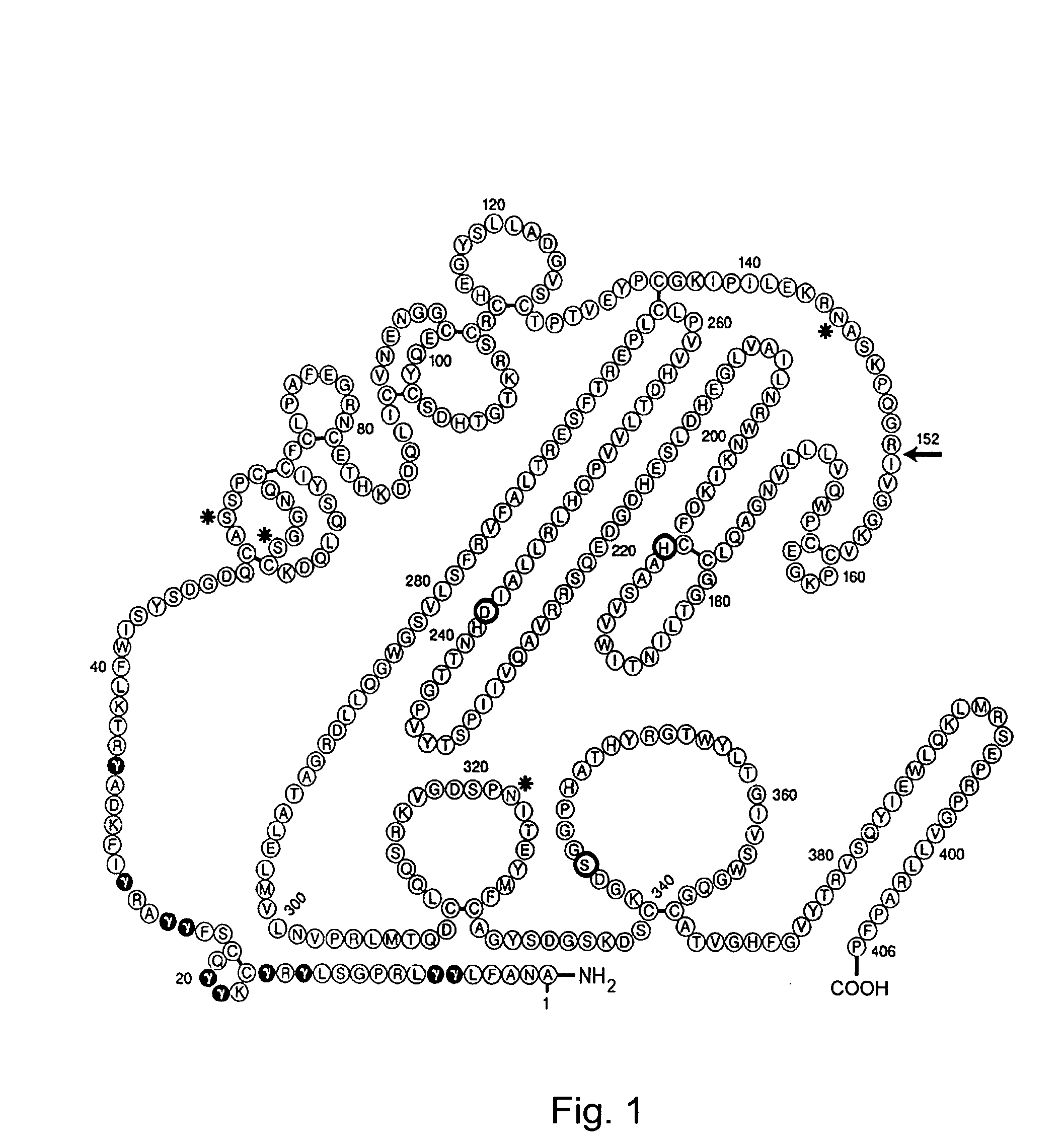
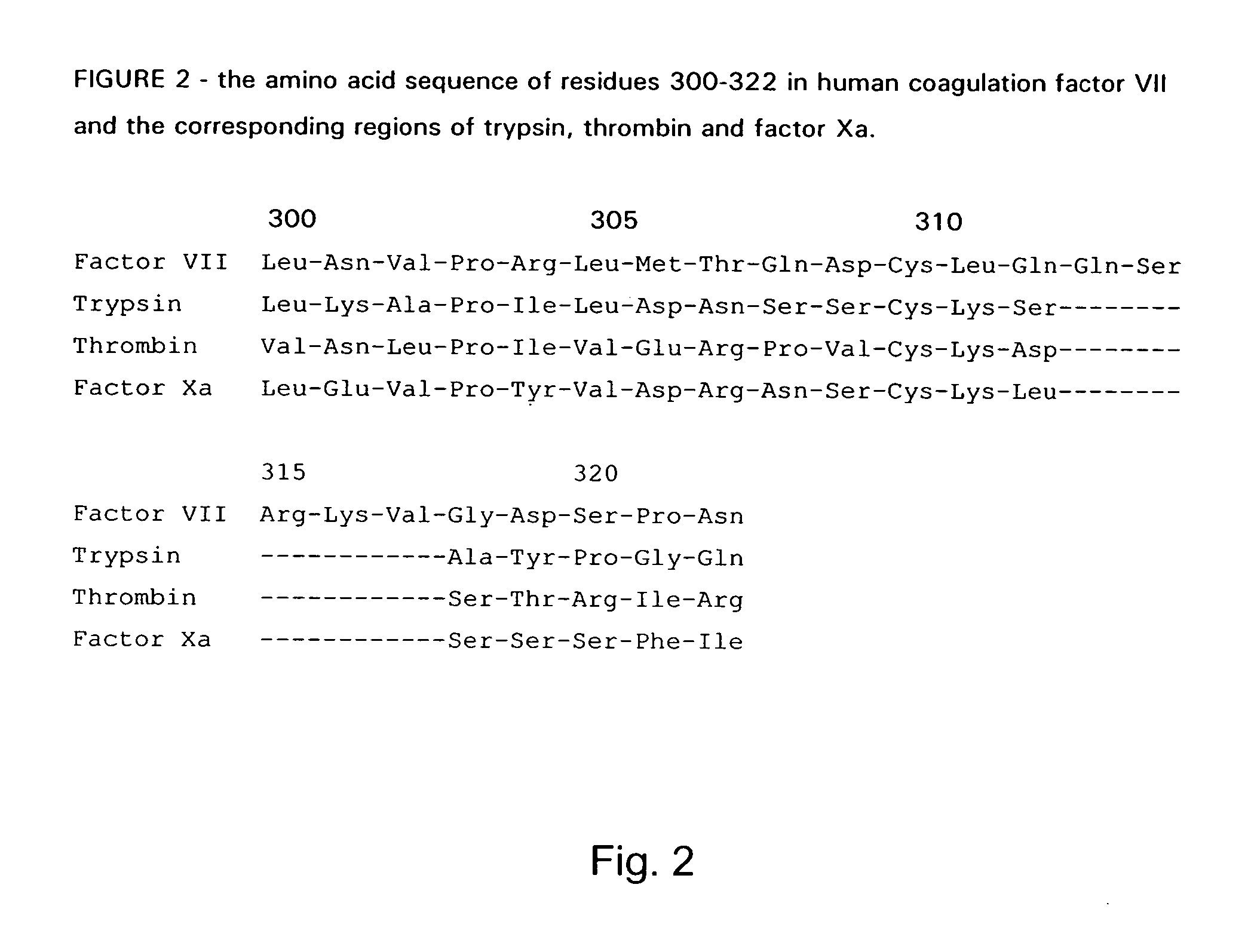
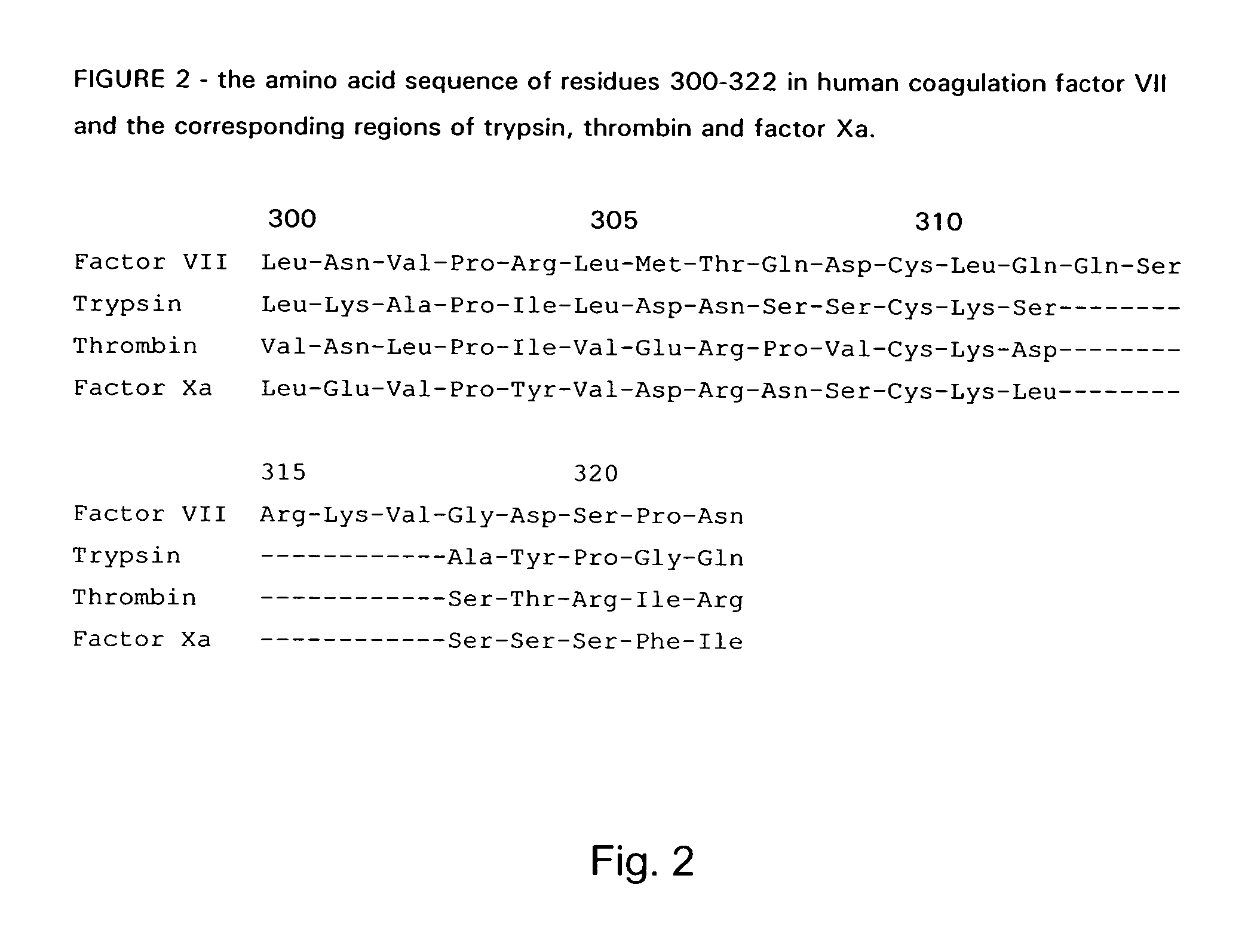
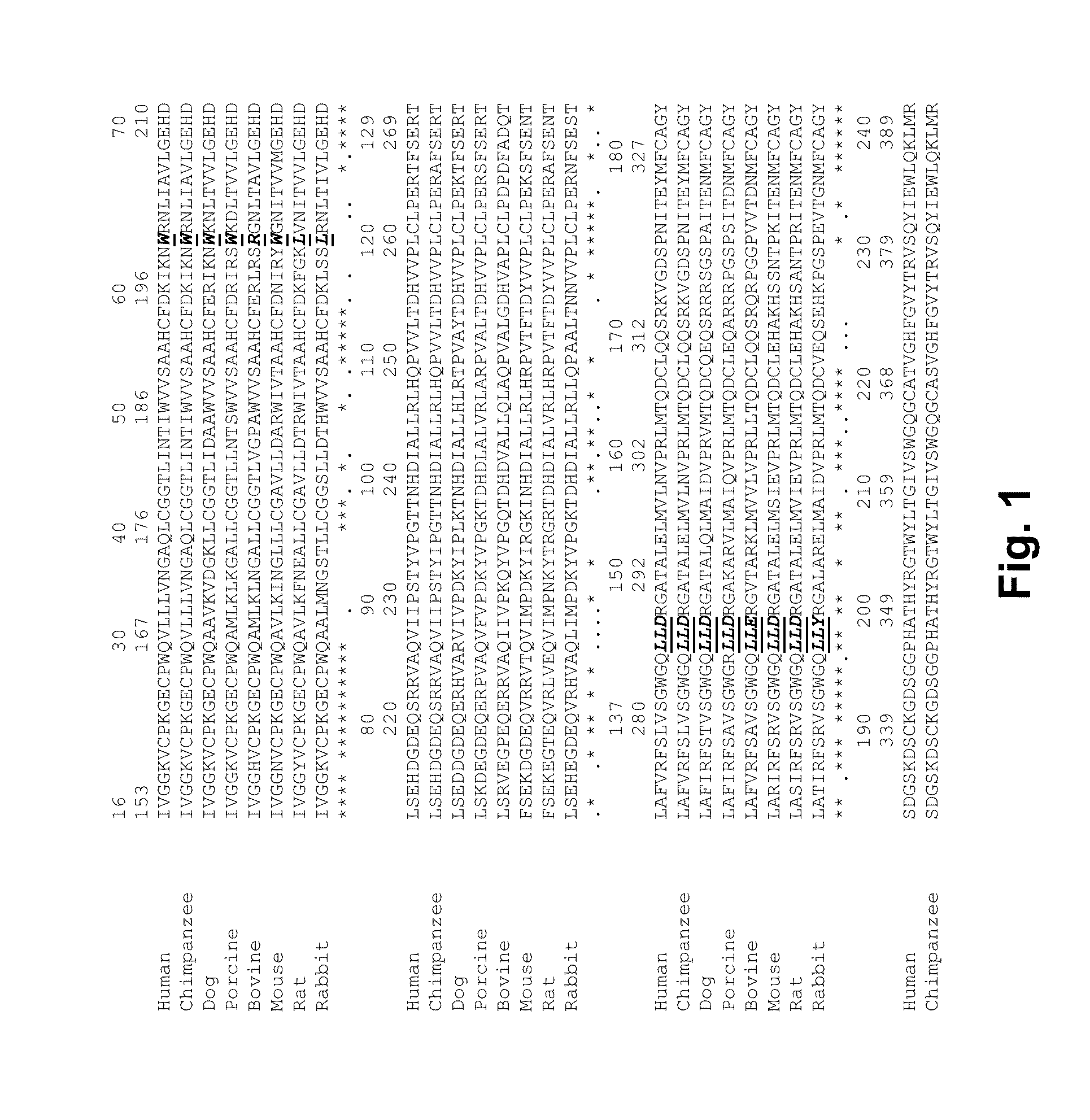
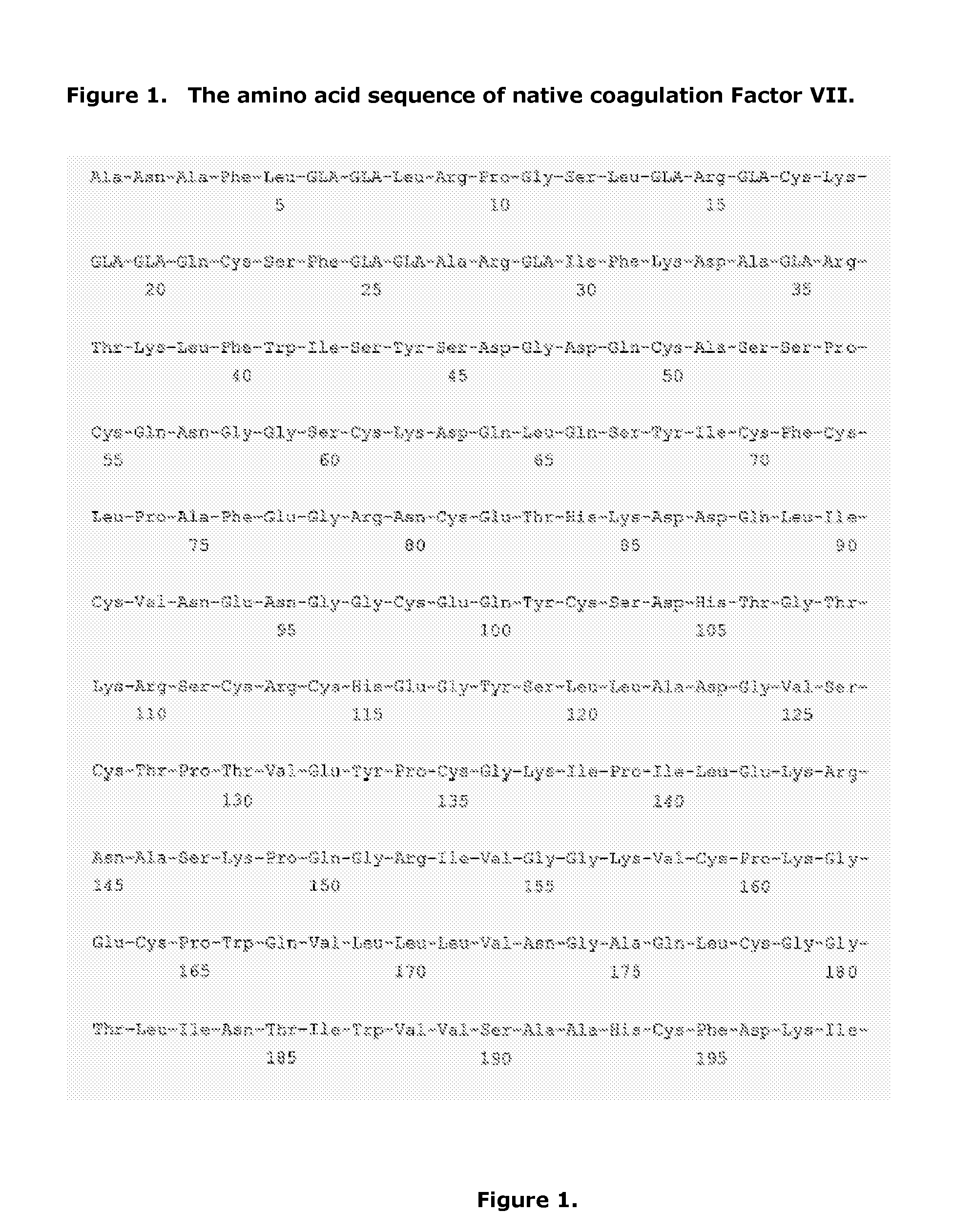
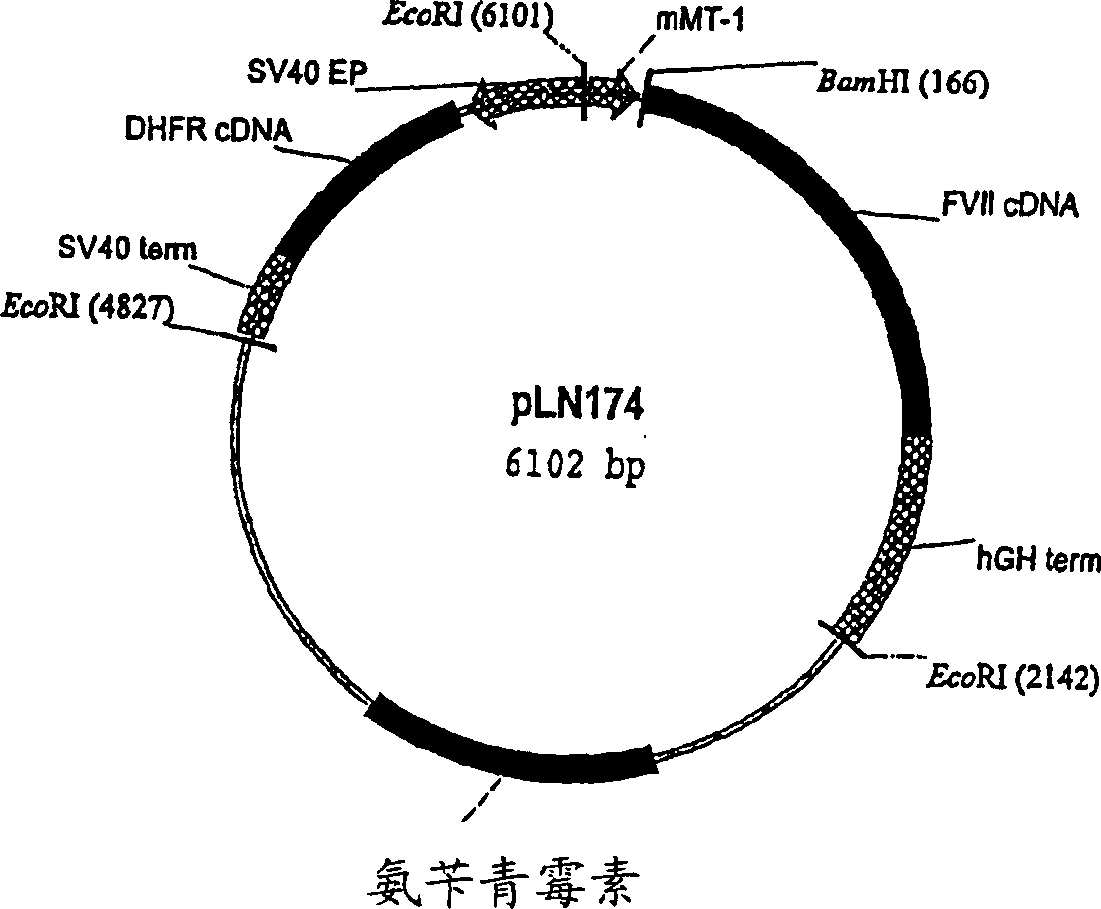
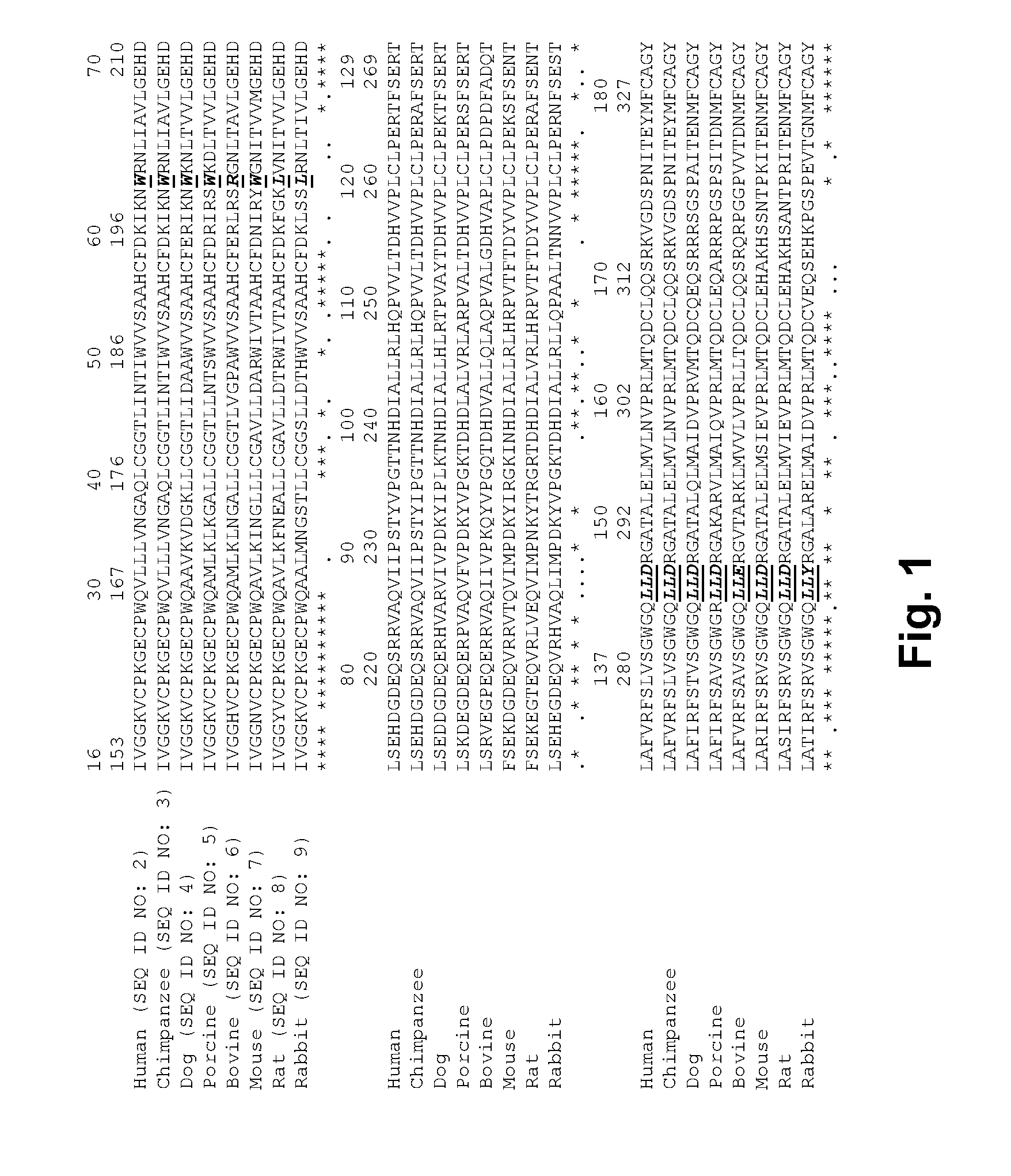
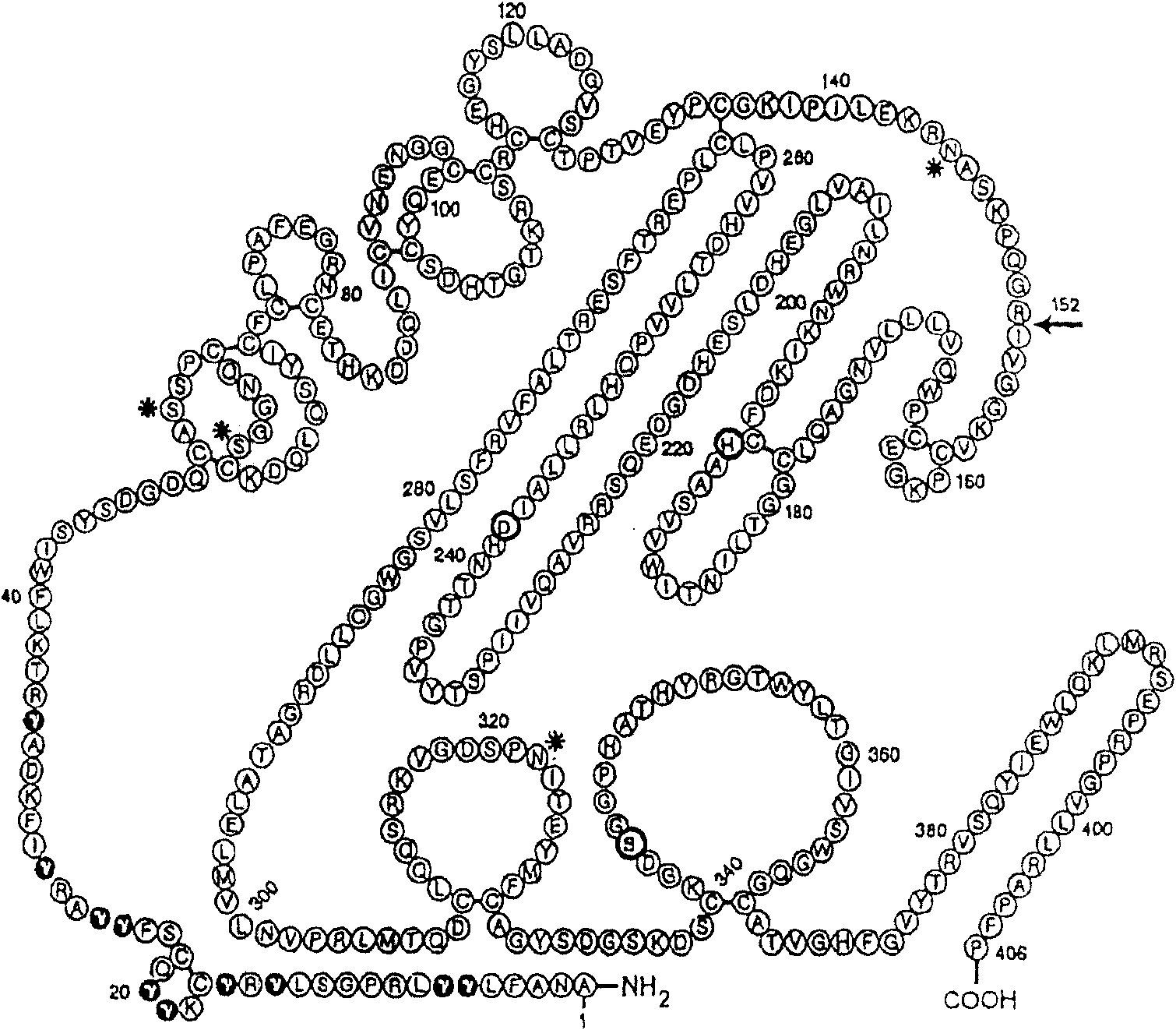
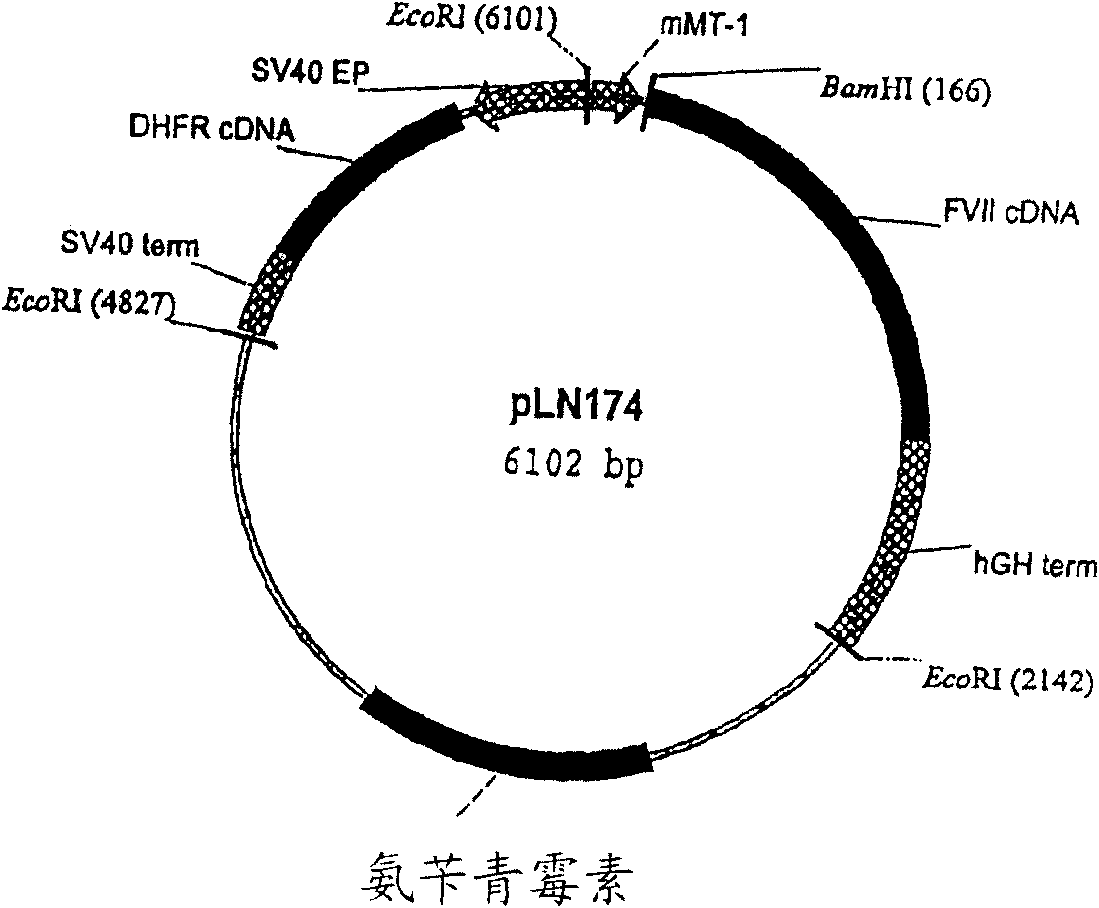
Coagulation factor VII derivatives
InactiveUS7235638B2Same and increased activityExtended half-lifeBiocidePeptide/protein ingredientsNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VII polypeptides, Factor VII derivatives as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Coagulation Factor VII polypeptides
InactiveUS20060205036A1High activitySame and increased activityPeptide/protein ingredientsMammal material medical ingredientsPolynucleotideCoagulation factor VII
The present invention relates to novel coagulation Factor VII polypeptides, polynucleotide constructs encoding such polypeptides, as well as vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Sealant or tissue generating product
InactiveUS20050244393A1Increase influenceHigh concentrationBiocideSurgical adhesivesBiotechnologyPhospholipid
The present invention is related to a sealant or a tisuue generating product comprising a (coagulated) plasma matrix, one or more growth factors, at least one phospholipid and a protein scatffold for the generation of said tissue (or the coagulation factor VII).
Owner:HENOGEN
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
InactiveUS20060063714A1Good storage stabilityPeptide/protein ingredientsInorganic non-active ingredientsFactor VIIaClotting factor deficiency
The invention relates to a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (e.g. human Factor VIIa) and a buffering agent; wherein the molar ratio of non-complexed calcium ions (Ca2+) to the Factor VII polypeptide is lower than 0.5. The composition may further comprise a stabilizing agent (e.g. copper or magnesium ions, benzamidine, or guanidine), a non-ionic surfactant, a tonicity modifying agent, an antioxidant and a preservative. The composition is useful for treating a Factor VII-responsive syndrome, such as bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor XI deficiency, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
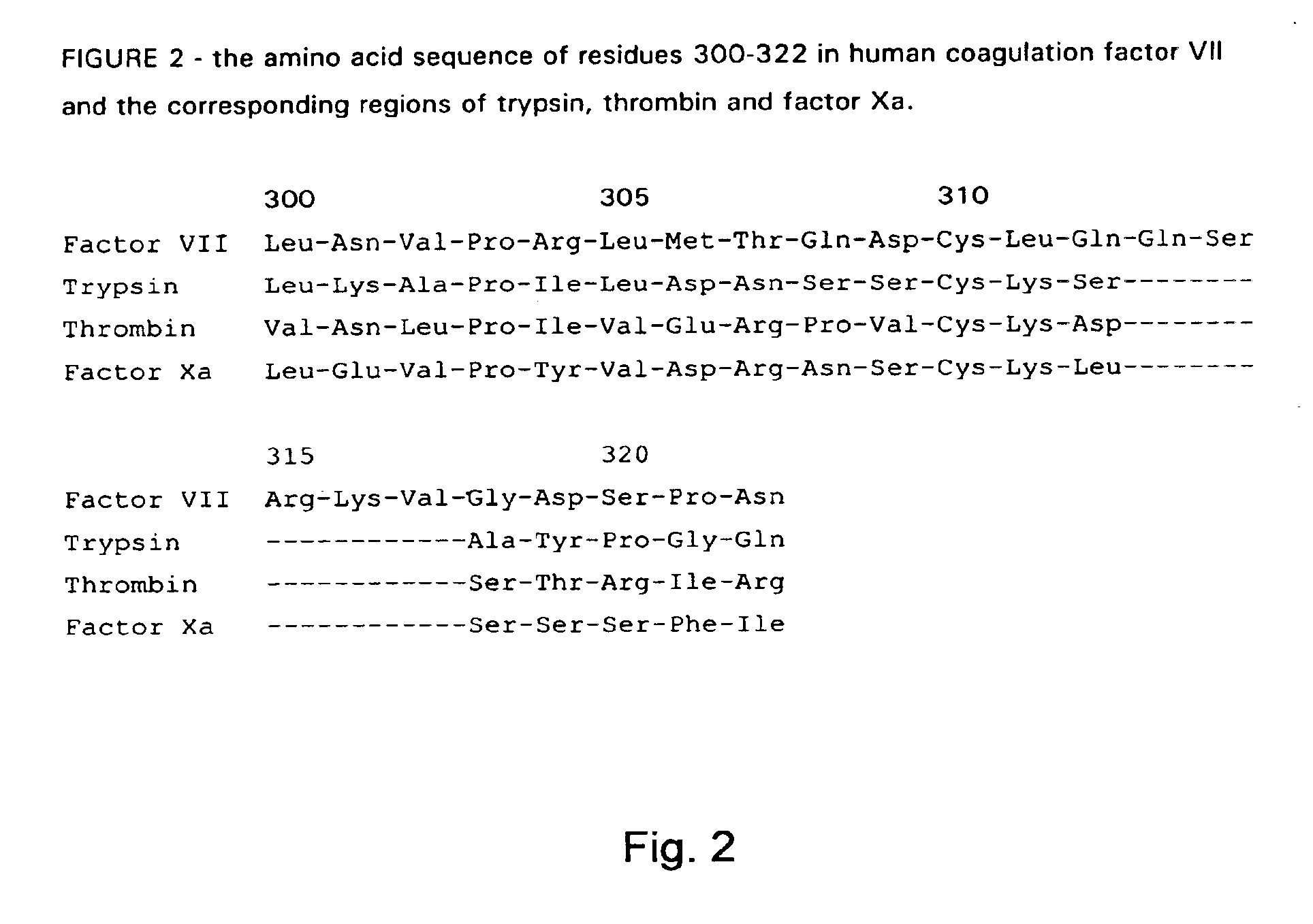
Human coagulation factor VII variants
InactiveUS6905683B2Increased tissue factor-independent activityPeptide/protein ingredientsMammal material medical ingredientsProteinase activityCoagulation system
The invention concerns novel coagulation factor VII variants, wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs;with the proviso that the variant is not FVII(Ala305).The invention further concerns nucleic acids encoding the Factor VII variants; vectors and cells comprising the nucleic acid; methods for producing the variants; pharmaceutical compositions comprising a Factor VII variant wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs; use of the variants for producing a medicament for treatment or prophylaxis of bleeding disorders or enhancement of the coagulation system; and methods of treatment.
Owner:NOVO NORDISK AS
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20130157881A1Eliminate needEasy to adaptLibrary screeningDisease diagnosisPancreatic hormoneOncology
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Coagulation factor VII, CA19-9, Insulin-like growth factor-binding protein 7, C—X—C motif chemokine 6, and C—C motif chemokine 13 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
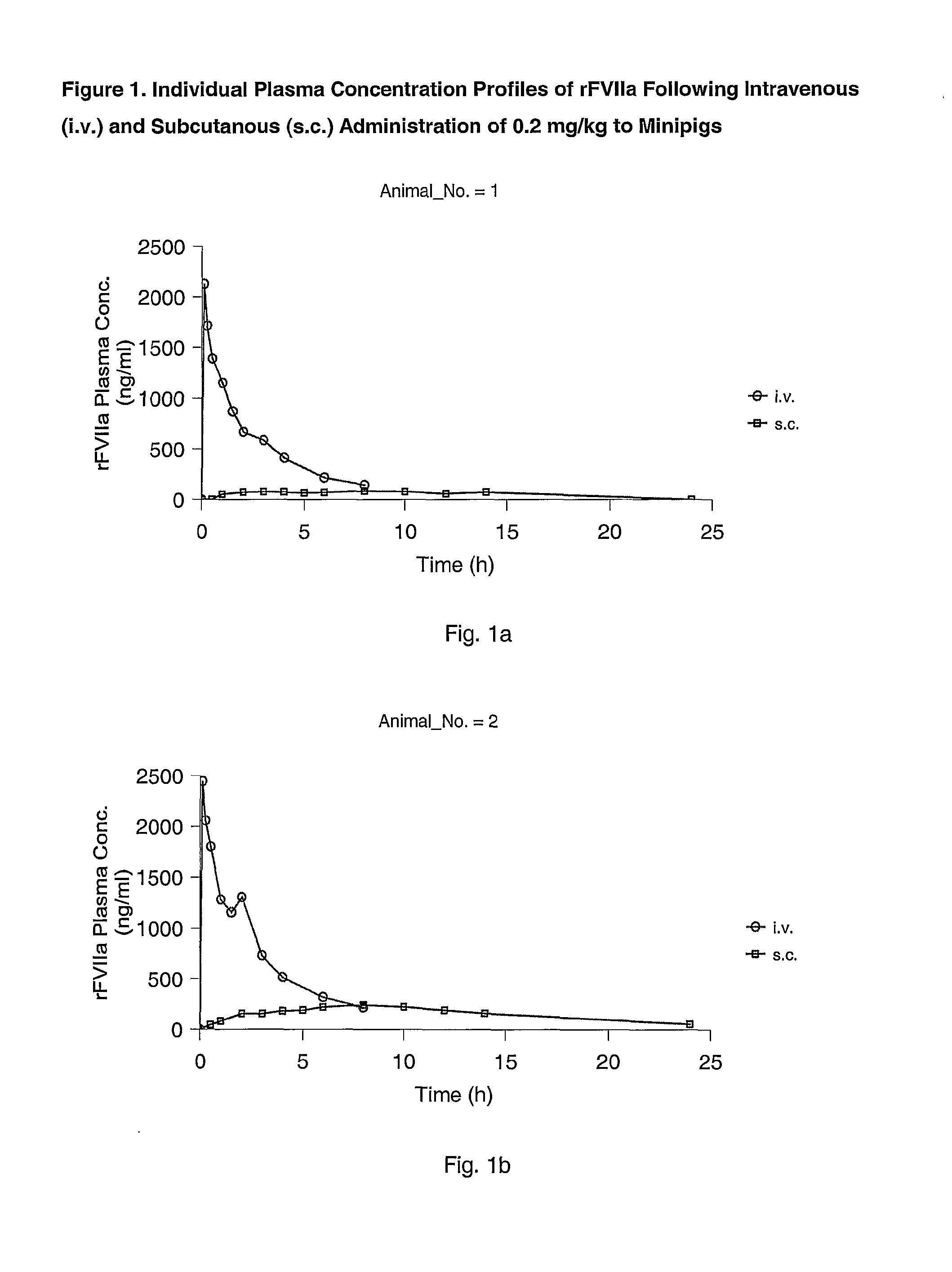
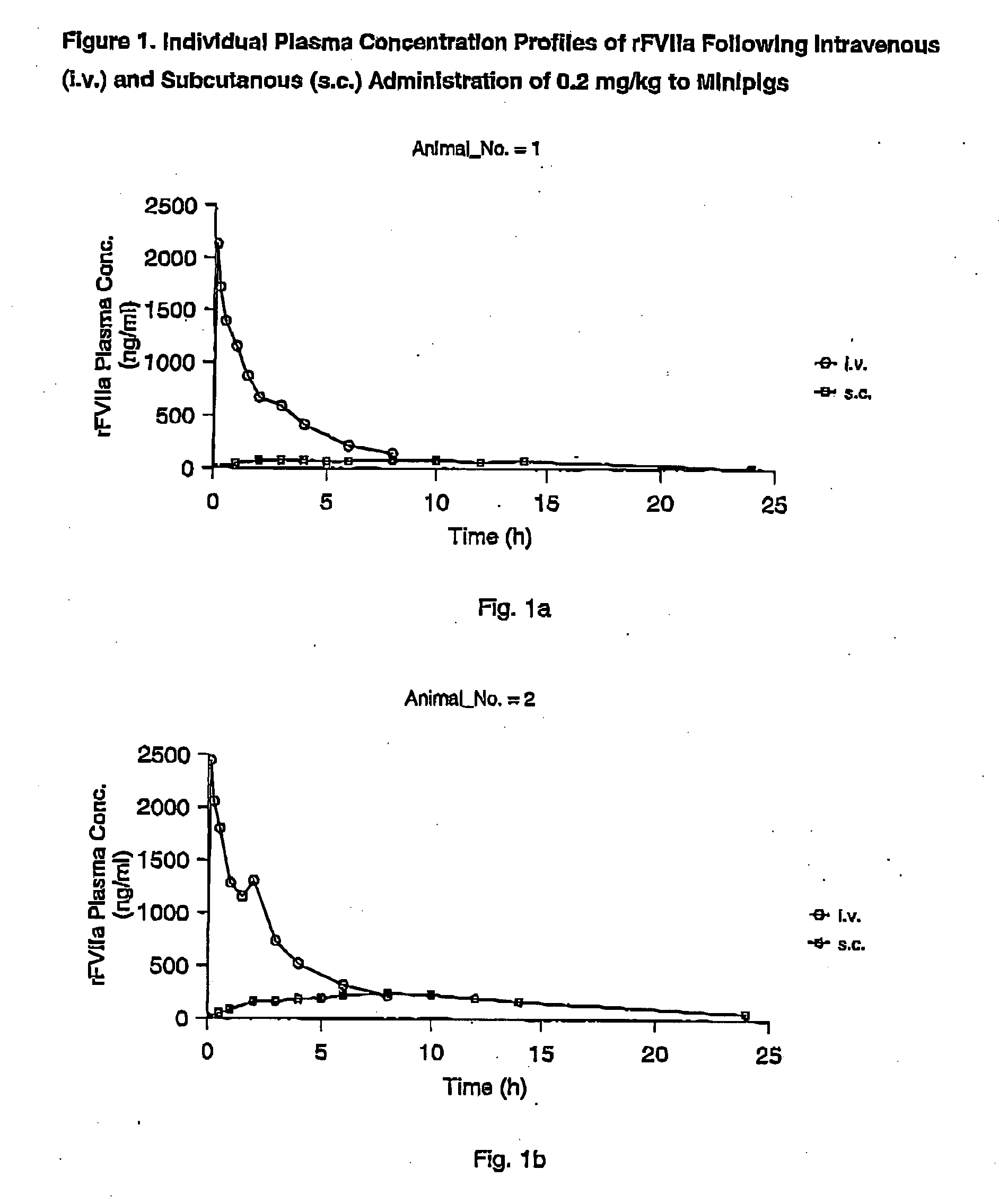
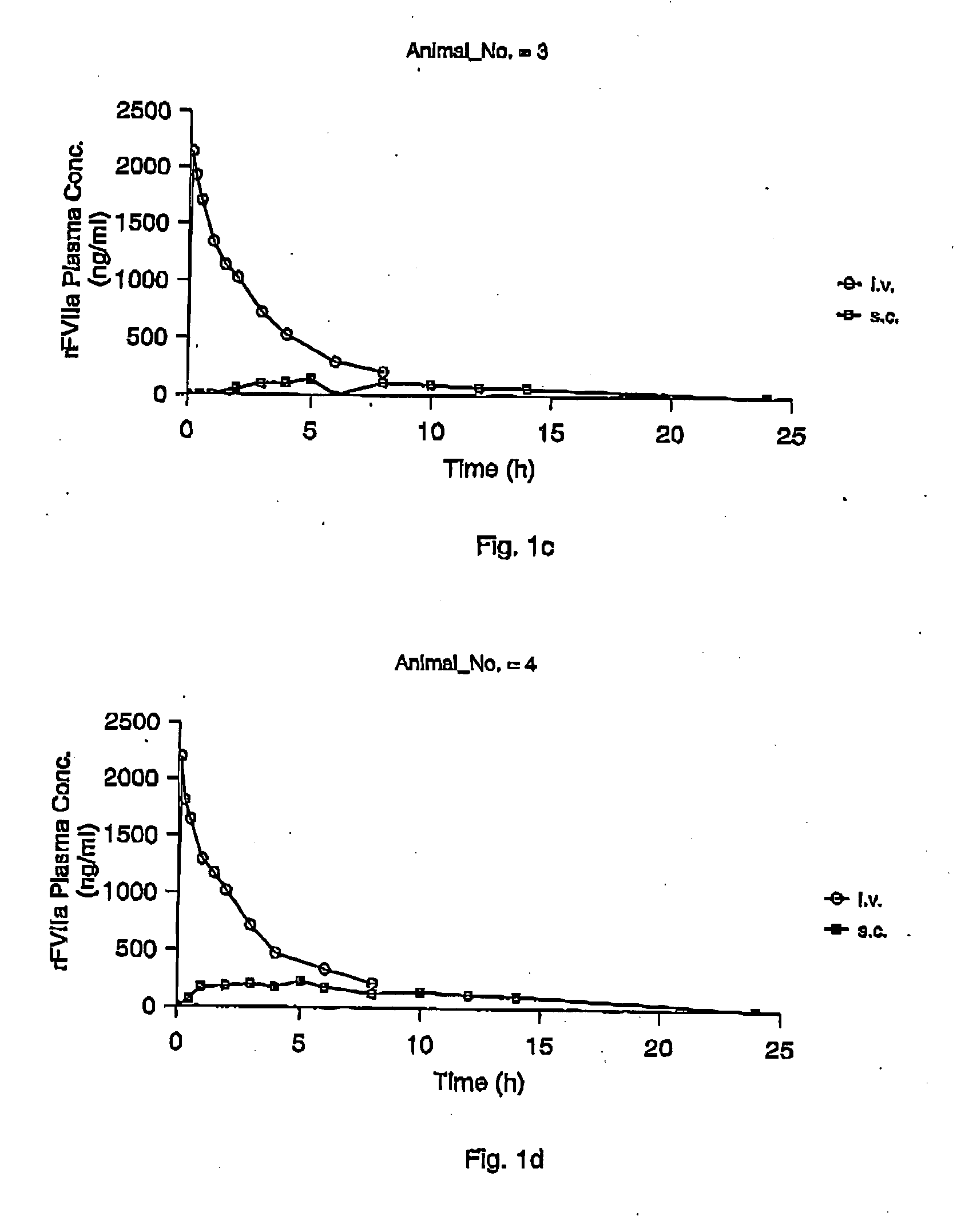
Subcutaneous administration of coagulation factor VII
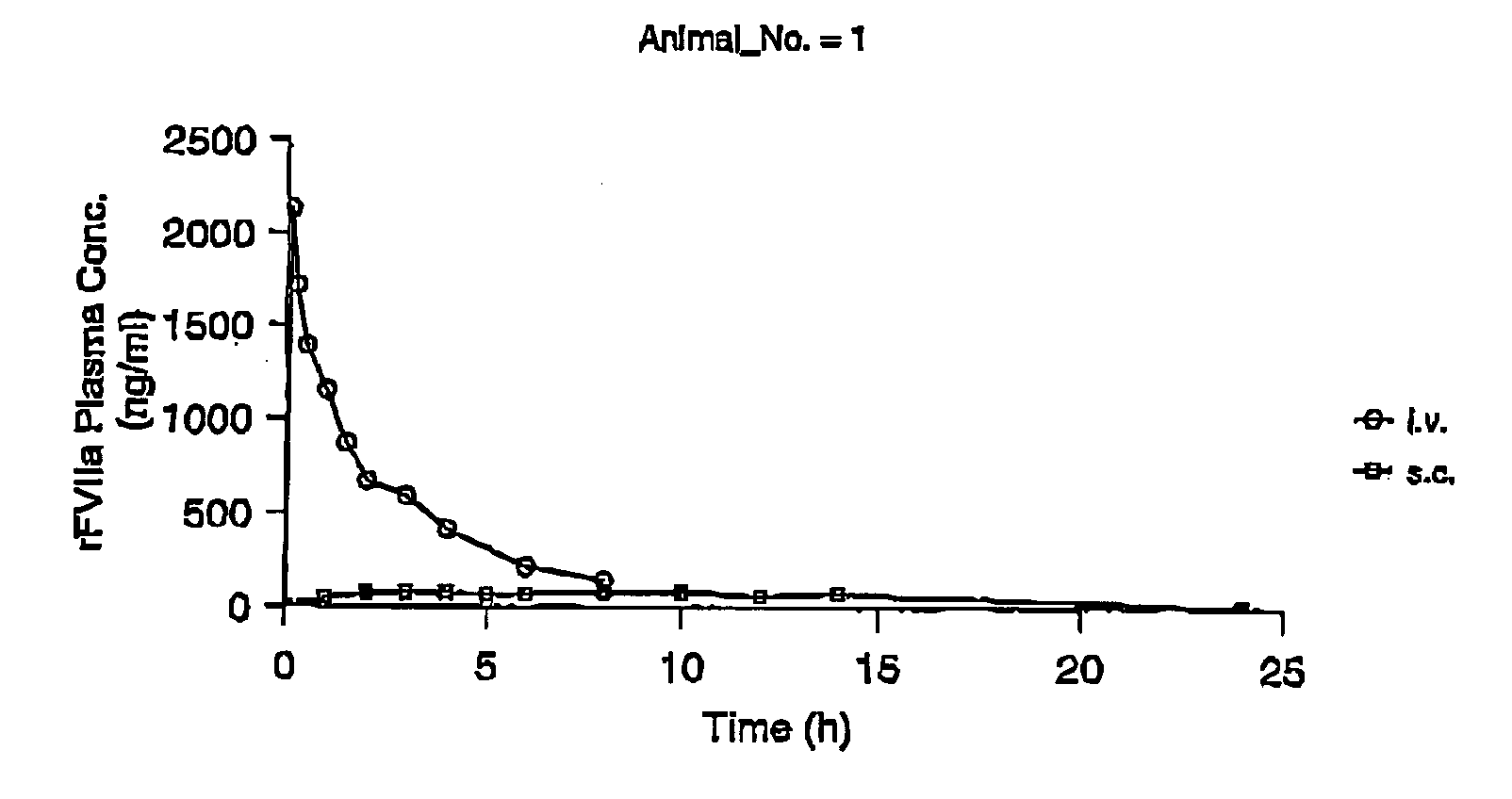
InactiveUS7786070B2Acceptable absorptionImprove the level ofPeptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBiological half-life
The invention relates to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, said medicament being for subcutaneous, intramuscular or intradermal administration, and to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, wherein said medicament, when administered subcutaneously, intradermally or intramuscularly, shows a prolonged biological half-life.
Owner:NOVO NORDISK HEALTH CARE AG
Human coagulation factor VII variants
InactiveUS20050204406A1Peptide/protein ingredientsMammal material medical ingredientsDiseaseProteinase activity
Owner:NOVO NORDISK AS
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
Nucleic acids encoding human coagulation factor VII variants
InactiveUS7416860B2Peptide/protein ingredientsMammal material medical ingredientsDrug biological activityHuman coagulation factor VII
The invention concerns nucleic acids encoding coagulation Factor VII variants in which the Leu residue in position 305 has been replaced by another amino acid residue. The invention further concerns the expression of such nucleic acids resulting in a Factor VII variant having increased biological activity.
Owner:NOVO NORDISK AS
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
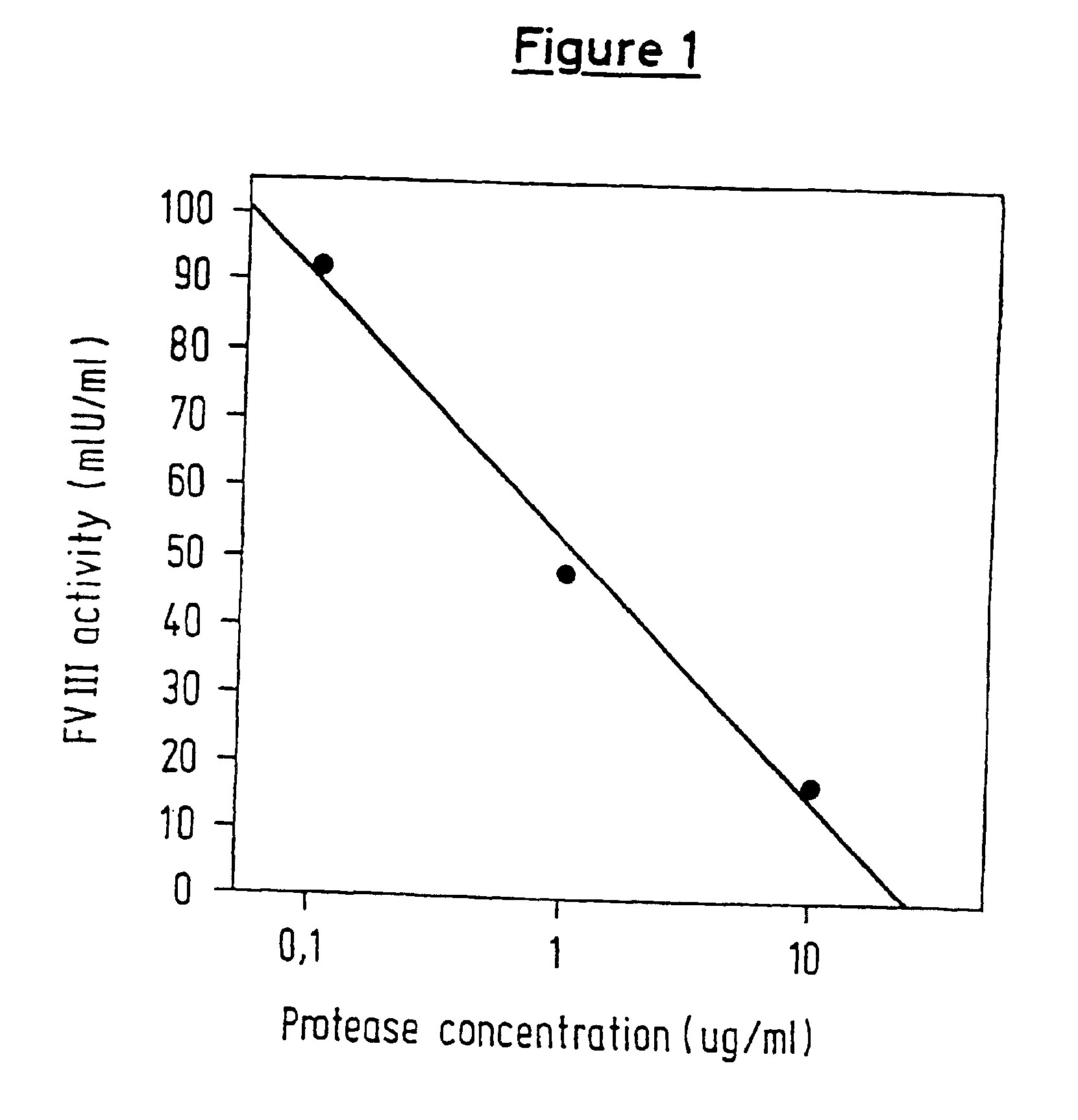
Protease for activating clotting factor VII
InactiveUS6911334B2High amidolytic activityHigh activityPeptide/protein ingredientsHydrolasesZymogenStaining
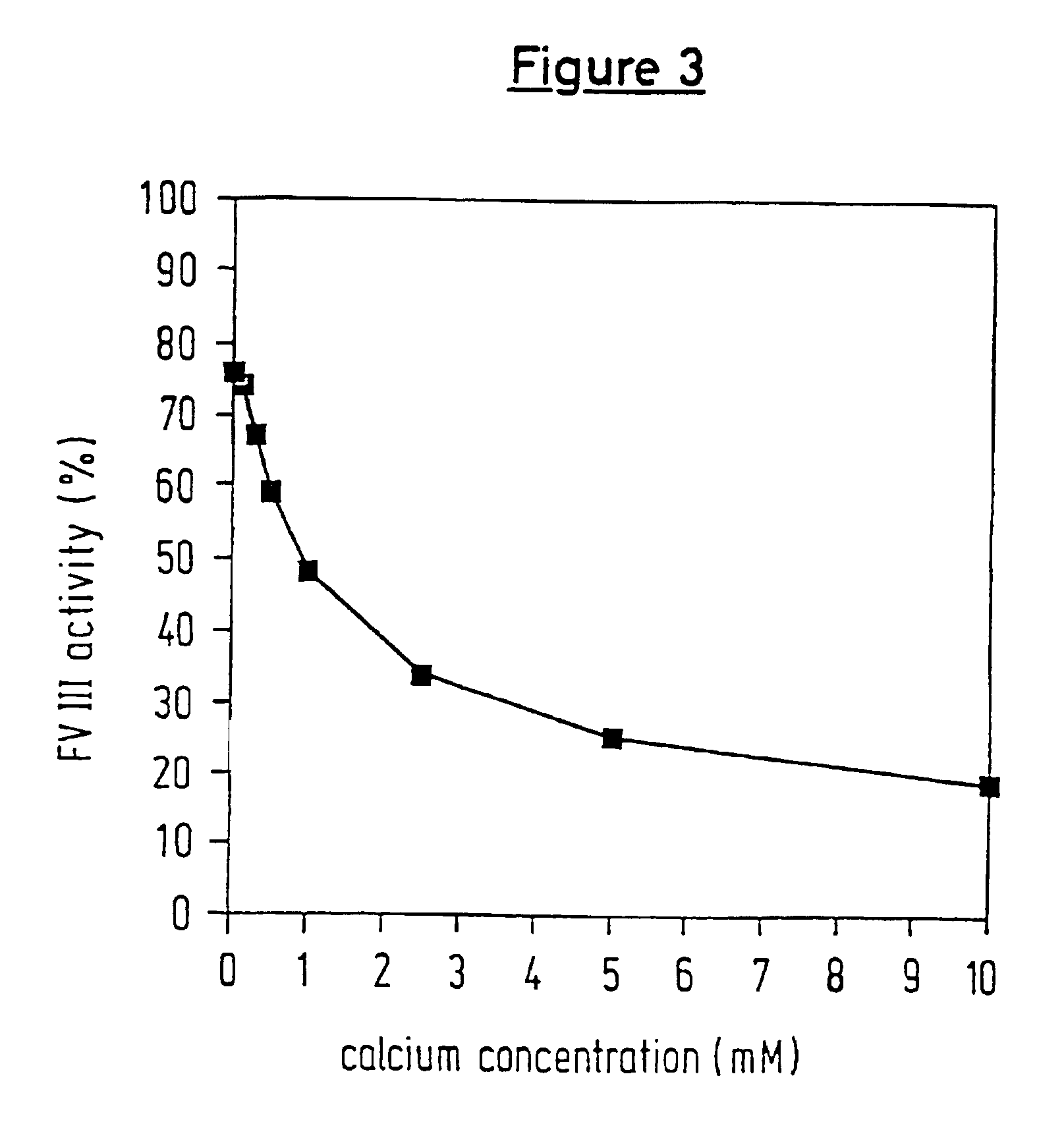
A protease for activating the blood clotting factor VII is described, wherein the protease is inhibited by the presence of aprotinin and is increased in its activity by calcium ions and / or heparin or heparin-related substances, and wherein in SDS-PAGE, on subsequent staining in the non-reduced state, the protease has one or more brands in the molecular weight range from 50 to 75 kDa, and in the reduced state, the protease has a band at 40 to 55 kDa and one or more bands in the molecular weight range from 10 to 35 kDa. The proenzyme of this protease is also characterized. Further, a process for obtaining this protease and its use in hemorrhage prophylaxis or hemostasis is described. Moreover, a test system for the qualitative and quantitative detection of a protease which activates the blood clotting factor VII is described.
Owner:CSL BEHRING GMBH
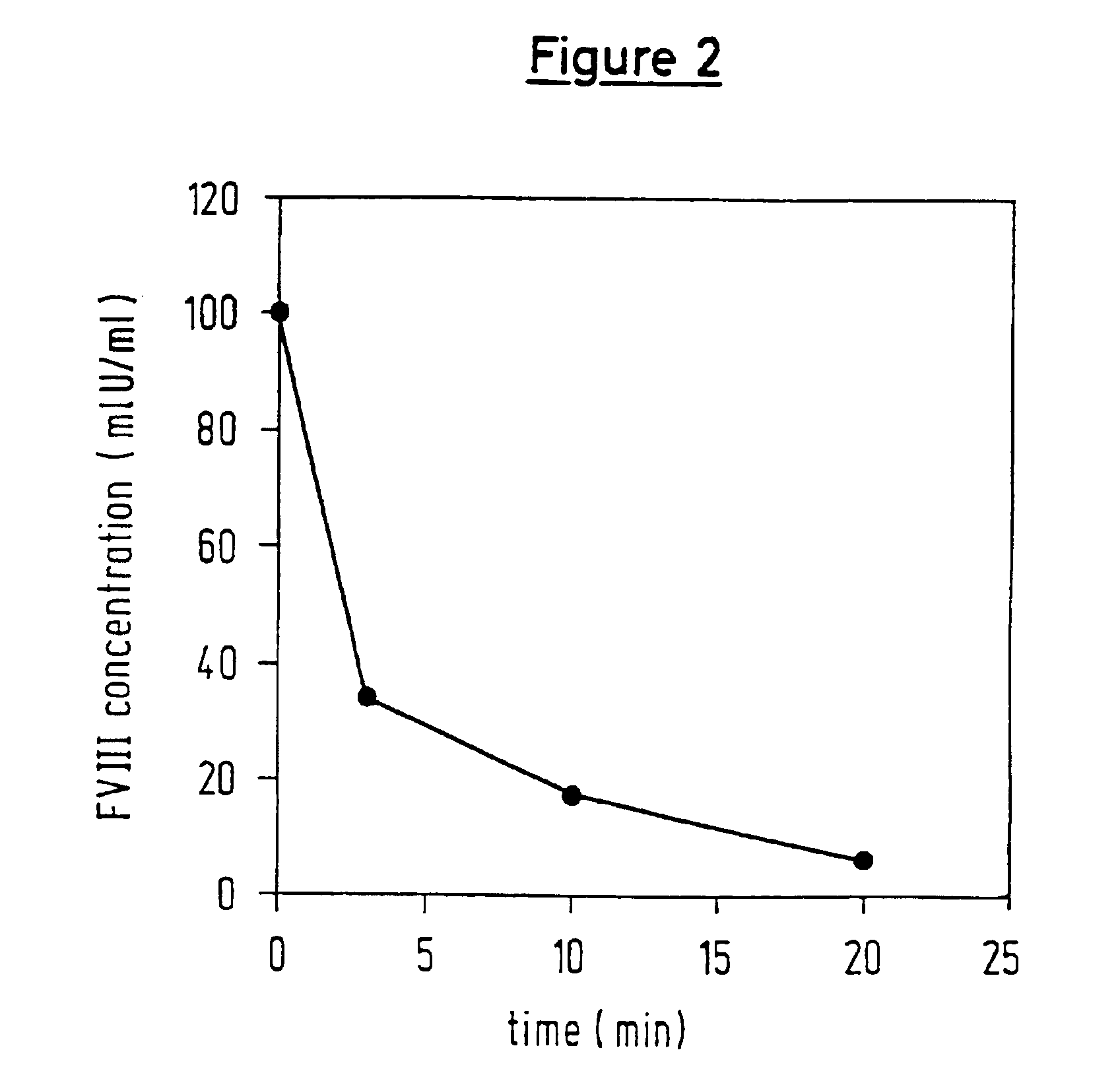
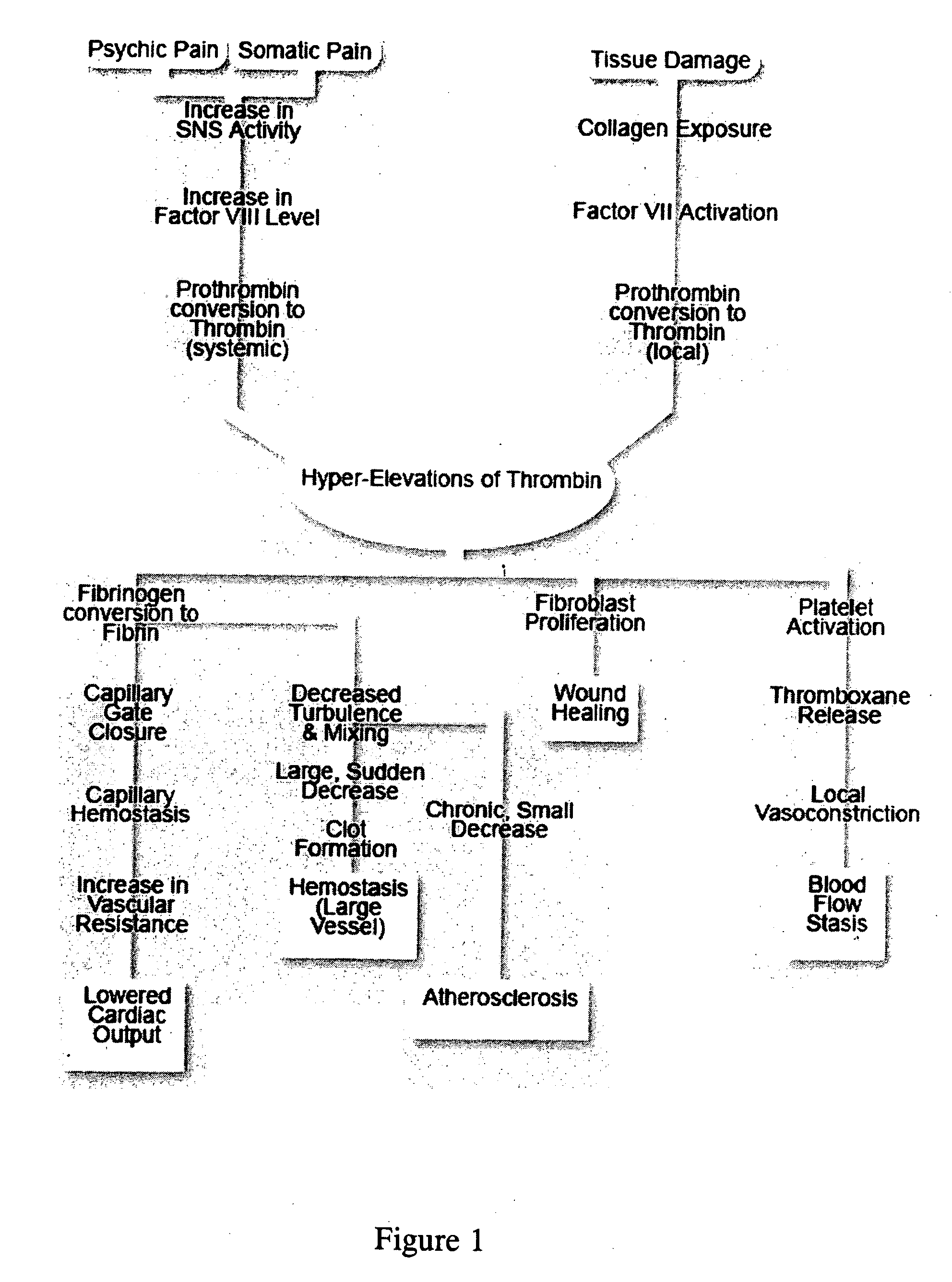
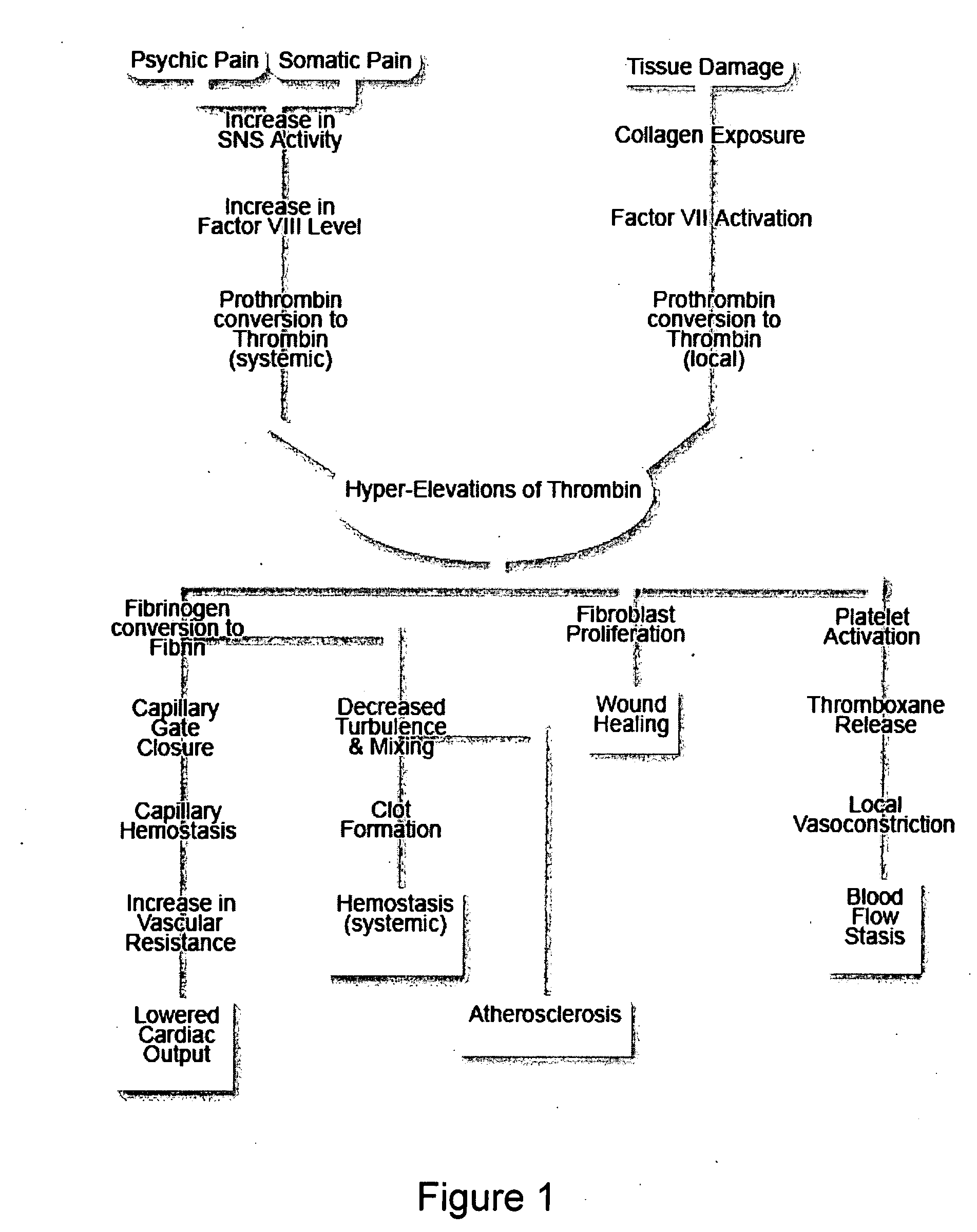
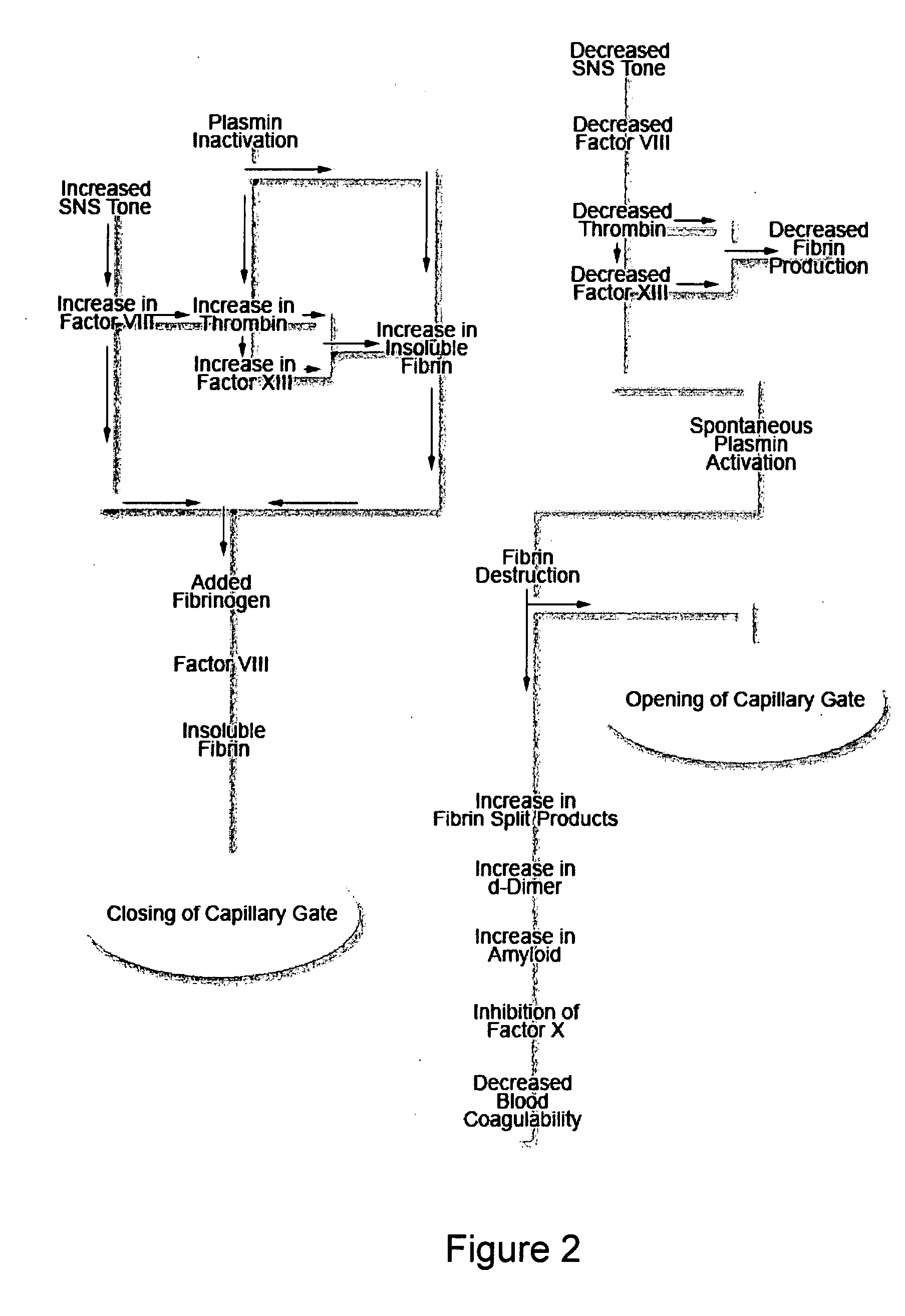
Therapies and compositions for controlling the stress mechanism and for stabilizing hemostasis in an organism
A theory has been presented that provides a simplified explanation of a cohesive mechanism of embryological development, hemostasis, coagulation, wound repair and tissue maintenance that operates continuously in the animal body to oppose the effects of stress. The theory endeavors to fit all known facts, and is based on the hypothesis that coagulation Factors VII and VIII are respectively local and systemic stress agents that regulate thrombin activity and synergize each other's effects. Stress Theory may explain the etiologies of several hitherto mysterious disease syndromes, and the stress mechanism lo and may play a more pervasive role in disease than is generally appreciated. The theory offers fresh avenues for research and clinical strategy.
Owner:LEWIS S COLEMAN MD
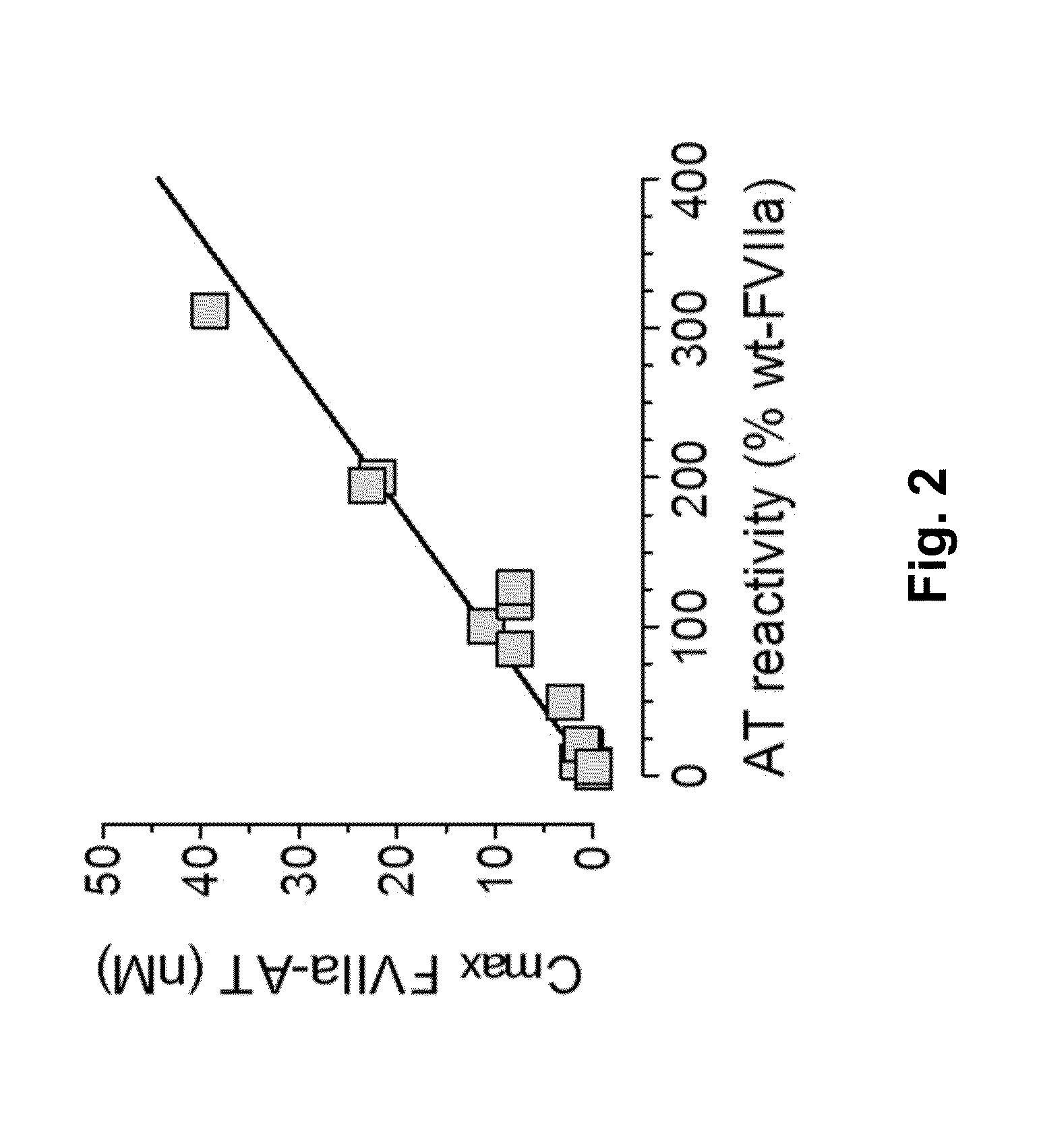
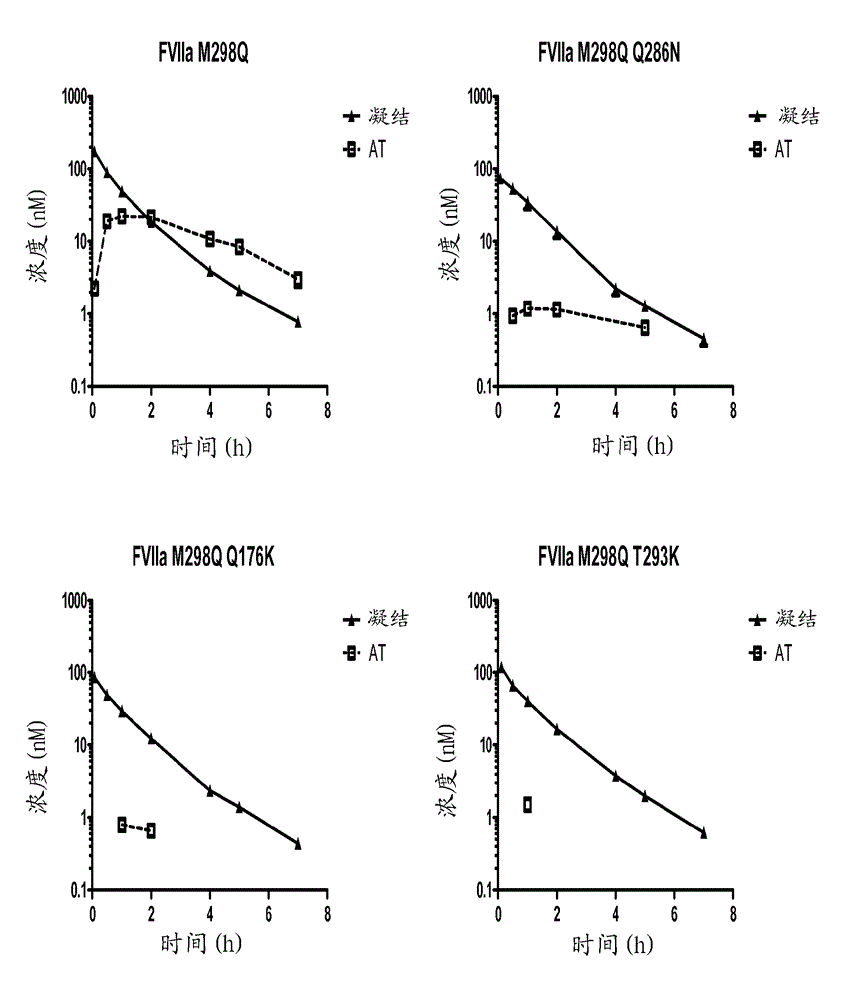
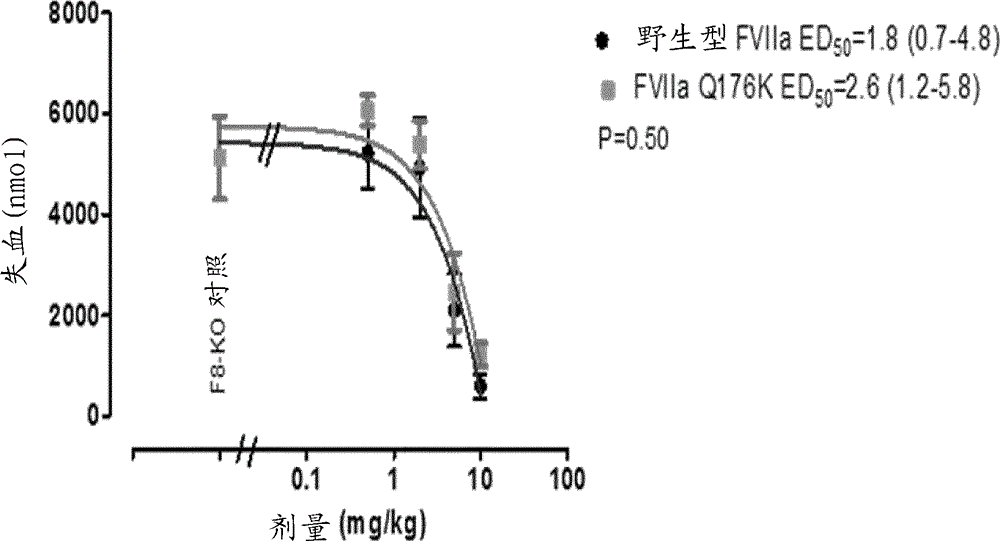
Coagulation factor vii polypeptides
InactiveUS20150105321A1Function increaseEnhanced and little and no loss of proteolytic activityPeptide/protein ingredientsMammal material medical ingredientsNucleotideFactor ii
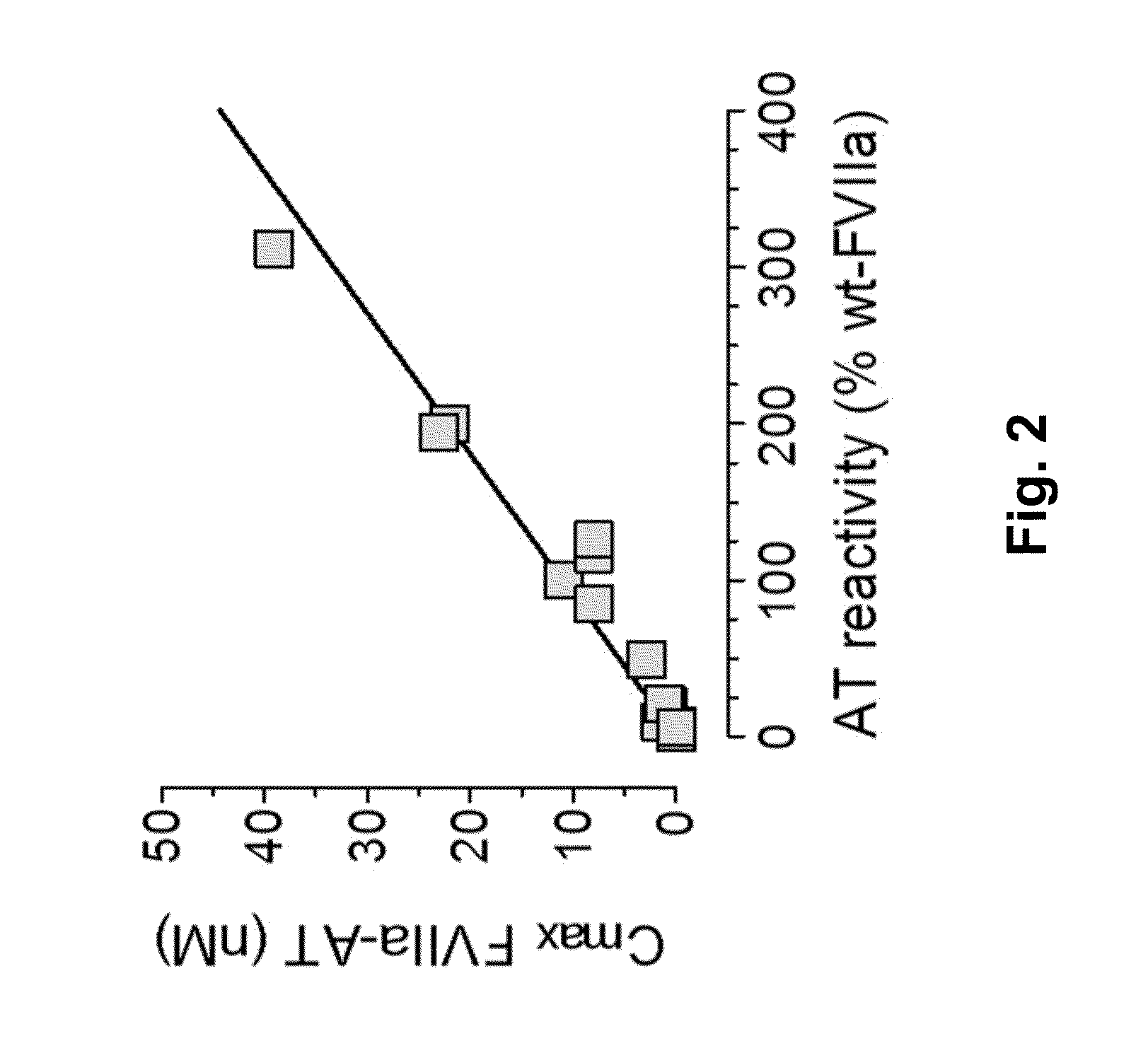
The present invention relates to modified coagulation Factor VII polypeptides exhibiting increased resistance to antithrombin inactivation and enhanced proteolytic activity. The present invention also relates to polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Purification of Coagulation Factor VII Polypeptides
InactiveUS20080268521A1Limiting and even avoiding auto-activationLimiting and even avoiding and auto-degradationPeptidasesCombinatorial chemistryImproved method
Owner:NOVO NORDISK AS
Coagulation factor vii derivatives
The present invention relates to novel human coagulation Factor VII polypeptides, Factor VII derivatives as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Coagulation factor VII polypeptides
InactiveUS9370583B2Function increaseEnhanced and little and no loss of proteolytic activityPeptide/protein ingredientsPharmaceutical non-active ingredientsNucleotideFactor ii
The present invention relates to modified coagulation Factor VII polypeptides exhibiting increased resistance to antithrombin inactivation and enhanced proteolytic activity. The present invention also relates to polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma
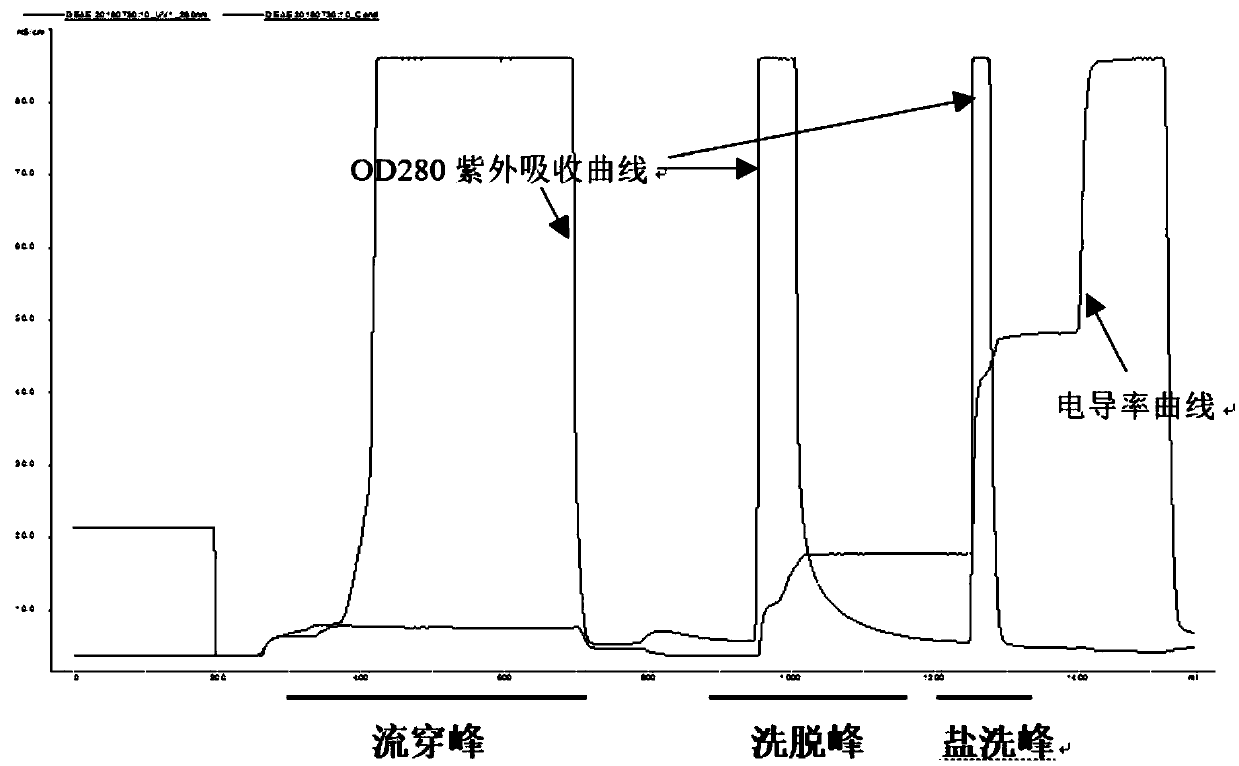
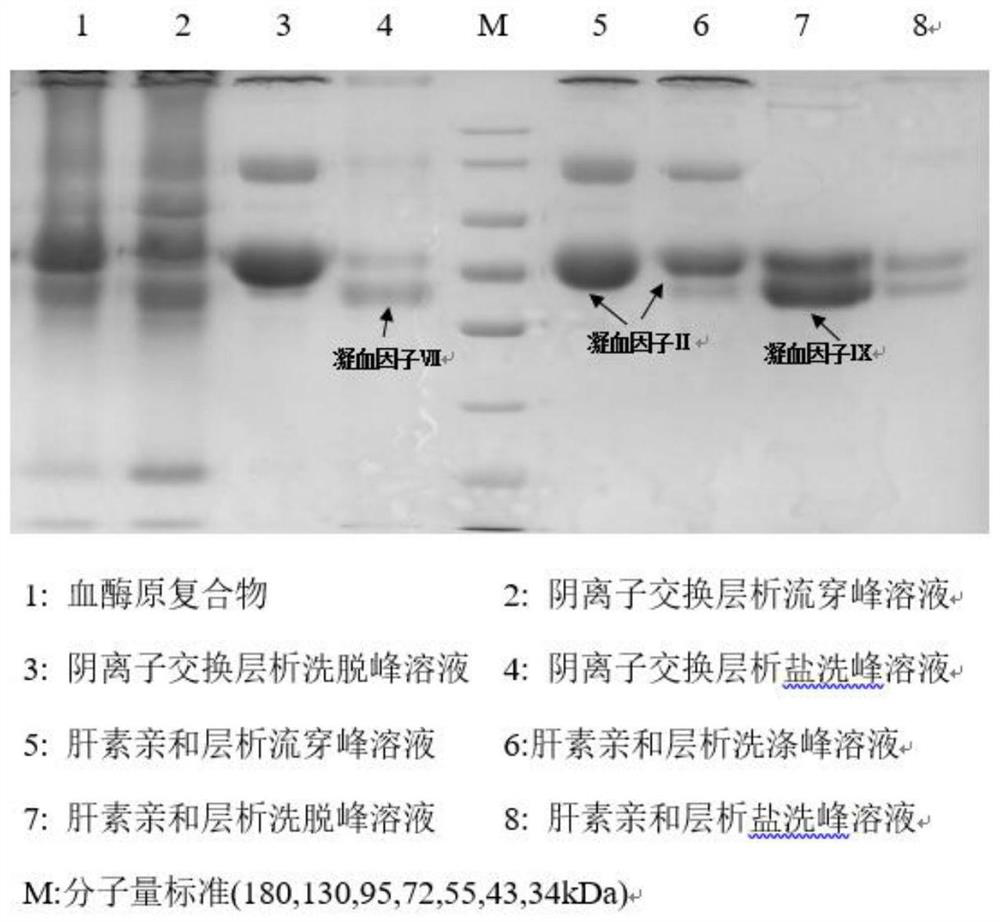
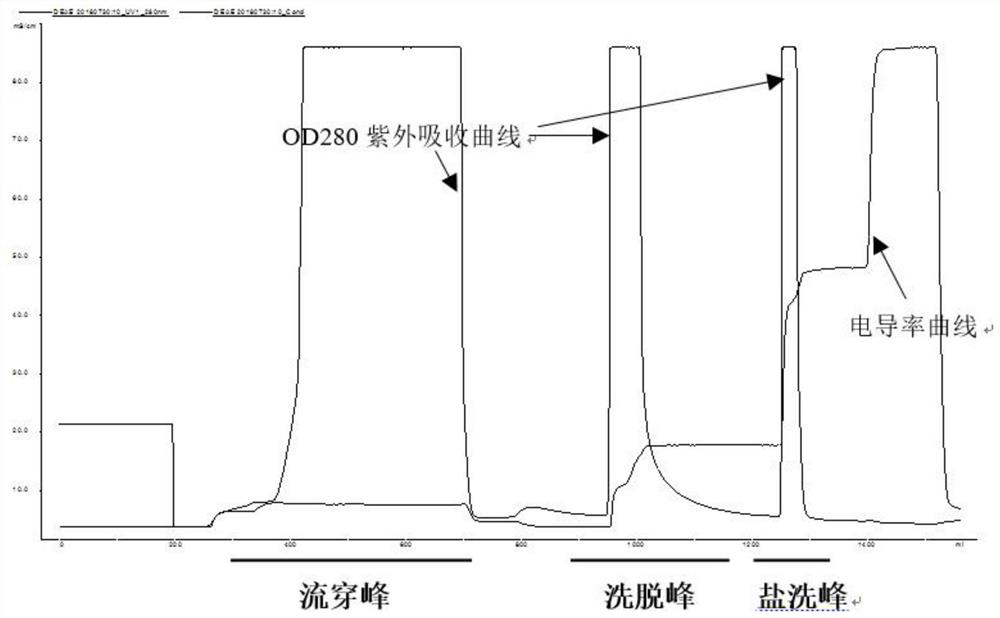
ActiveCN109651502AAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Subcutaneous administration of coagulation Factor VII
InactiveUS20080145914A1Acceptable absorptionImprove the level ofPeptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBiological half-life
The invention relates to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, said medicament being for subcutaneous, intramuscular or intradermal administration, and to the use of a Factor VIIa for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, wherein said medicament, when administered subcutaneously, intradermally or intramuscularly, shows a prolonged biological half-life.
Owner:NOVO NORDISK AS
Coagulation Factor VII Polypeptides
InactiveUS20100197597A1High activityEnhancement of the normal haemostatic systemPeptide/protein ingredientsMetabolism disorderNucleotidePolynucleotide
The present invention relates to novel coagulation Factor VII polypeptides, polynucleotide constructs encoding such polypeptides, as well as vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Coagulation factor vii polypeptides
InactiveCN104704118ALong durationSmall dosePeptide/protein ingredientsPharmaceutical non-active ingredientsPharmaceutical drugPolynucleotide
The present invention relates to modified coagulation Factor VII (Factor VII) polypeptides having coagulant activity as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Expression of gamma-carboxylated polypeptides in gamma-carboxylation deficient host sytems
The present invention relates to a novel method for preparing gamma-carboxylated poly-peptides, including coagulation Factors VII, IX, X and Protein C. The present invention also relates to novel host cells and recombinant vectors to be used in this improved method for preparing gamma-carboxylated polypeptides.
Owner:NOVO NORDISK AS
A method for simultaneously separating and purifying blood coagulation factors ix, x and ⅶ from human plasma
ActiveCN109651502BAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Coagulation factor vii polypeptides
InactiveUS20150307865A1Increase heightGood treatment effectPeptide/protein ingredientsPharmaceutical non-active ingredientsNucleotideFactor ii
The present invention relates to modified coagulation Factor VII (Factor VII) polypeptides having coagulant activity as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Coagulation factor vii polypeptides
InactiveUS20160120992A1Function increaseEnhanced and little and no loss of proteolytic activityPeptide/protein ingredientsMammal material medical ingredientsPolynucleotideProteolysis
The present invention relates to modified coagulation Factor VII polypeptides exhibiting increased resistance to antithrombin inactivation and enhanced proteolytic activity. The present invention also relates to polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing such polynucleotides, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
Method of providing anesthesia
InactiveUS20070191430A1Preserve integrityInduce “damping” of turbulence and mixing in bloodBiocideEther/acetal active ingredientsEtiologyWhole body
A theory has been presented that provides a simplified explanation of a cohesive mechanism of embryological development, hemostasis, coagulation, wound repair and tissue maintenance that operates continuously in the animal body to oppose the effects of stress. The theory endeavors to fit all known facts, and is based on the hypothesis that coagulation Factors VII and VIII are respectively local and systemic stress agents that regulate thrombin activity and synergize each other's effects. Stress Theory may explain the etiologies of several hitherto mysterious disease syndromes, and the stress mechanism and may play a more pervasive role in disease than is generally appreciated. The theory offers fresh avenues for research and clinical strategy.
Owner:COLEMAN LEWIS S
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Coagulation factor VII, CA19-9, Insulin-like growth factor-binding protein 7, C—X—C motif chemokine 6, and C—C motif chemokine 13 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Long-acting coagulation factor vii and methods of producing same
Polypeptides comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotropin attached to the carboxy terminus but not to the amino terminus of a coagulation factor and polynucleotidesencoding the same are disclosed. Pharmaceutical compositions and pharmaceutical formulations comprising the polypeptides and polynucleotides of the disclosure and methods of using and producing sameare also disclosed.
Owner:OPKO BIOLOGICS
Coagulation factor VII derivatives
The present invention relates to novel human blood coagulation factor VII polypeptide, factor VII derivative and polynucleotide construct encoding the polypeptide, vector and host cell containing and expressing the polynucleotide, pharmaceutical composition, use and treatment method.
Owner:NOVO NORDISK HEALTH CARE AG
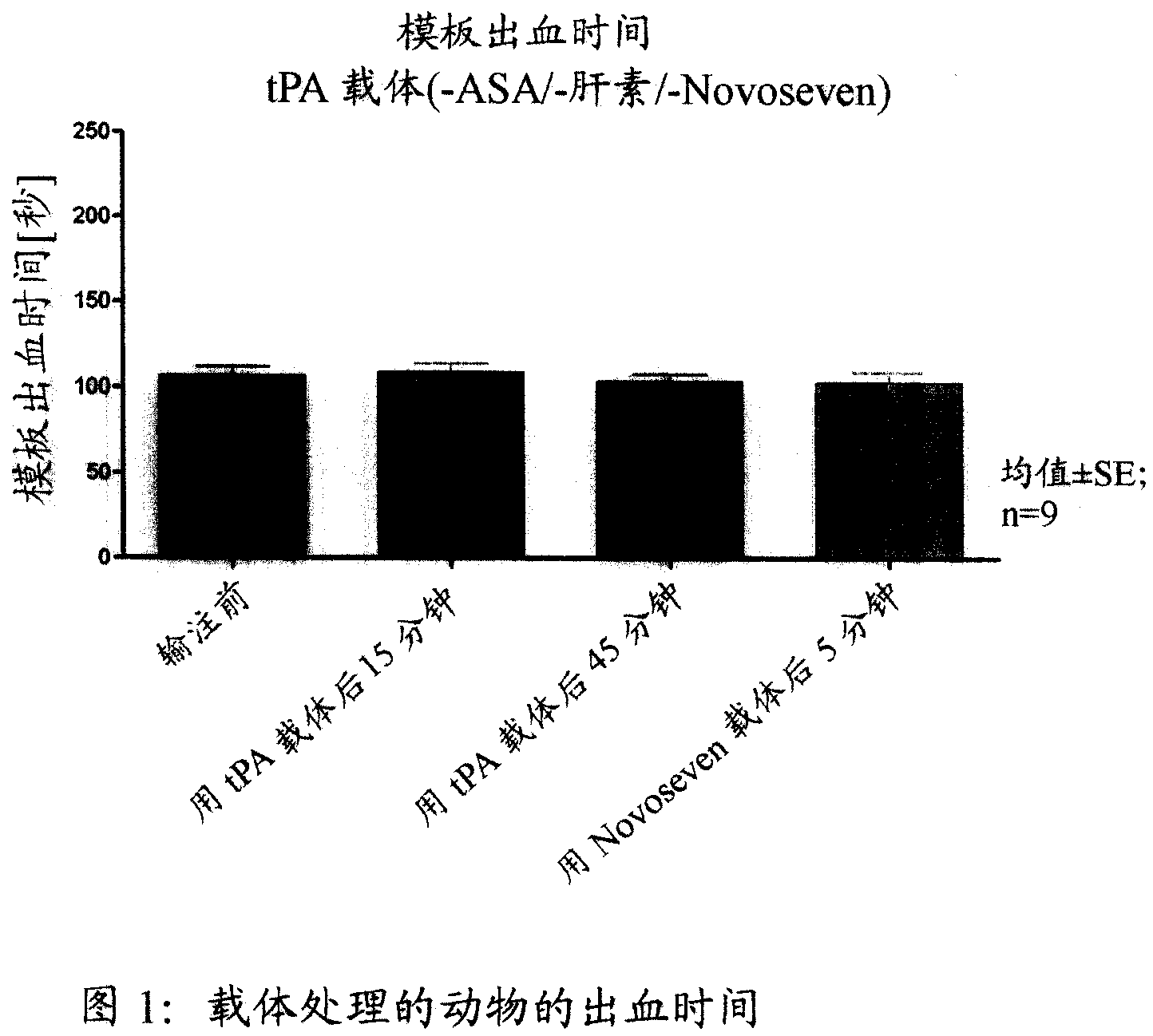
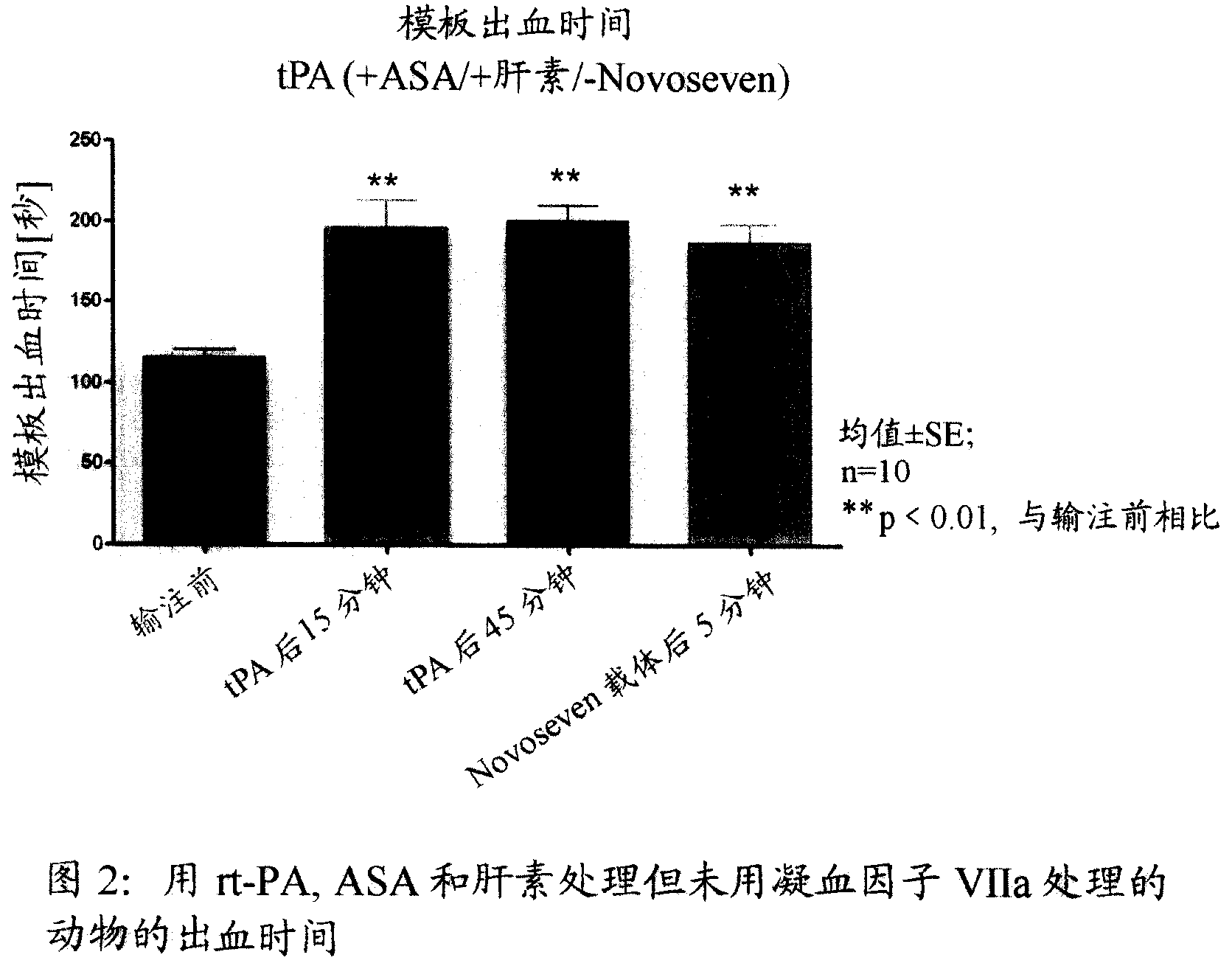
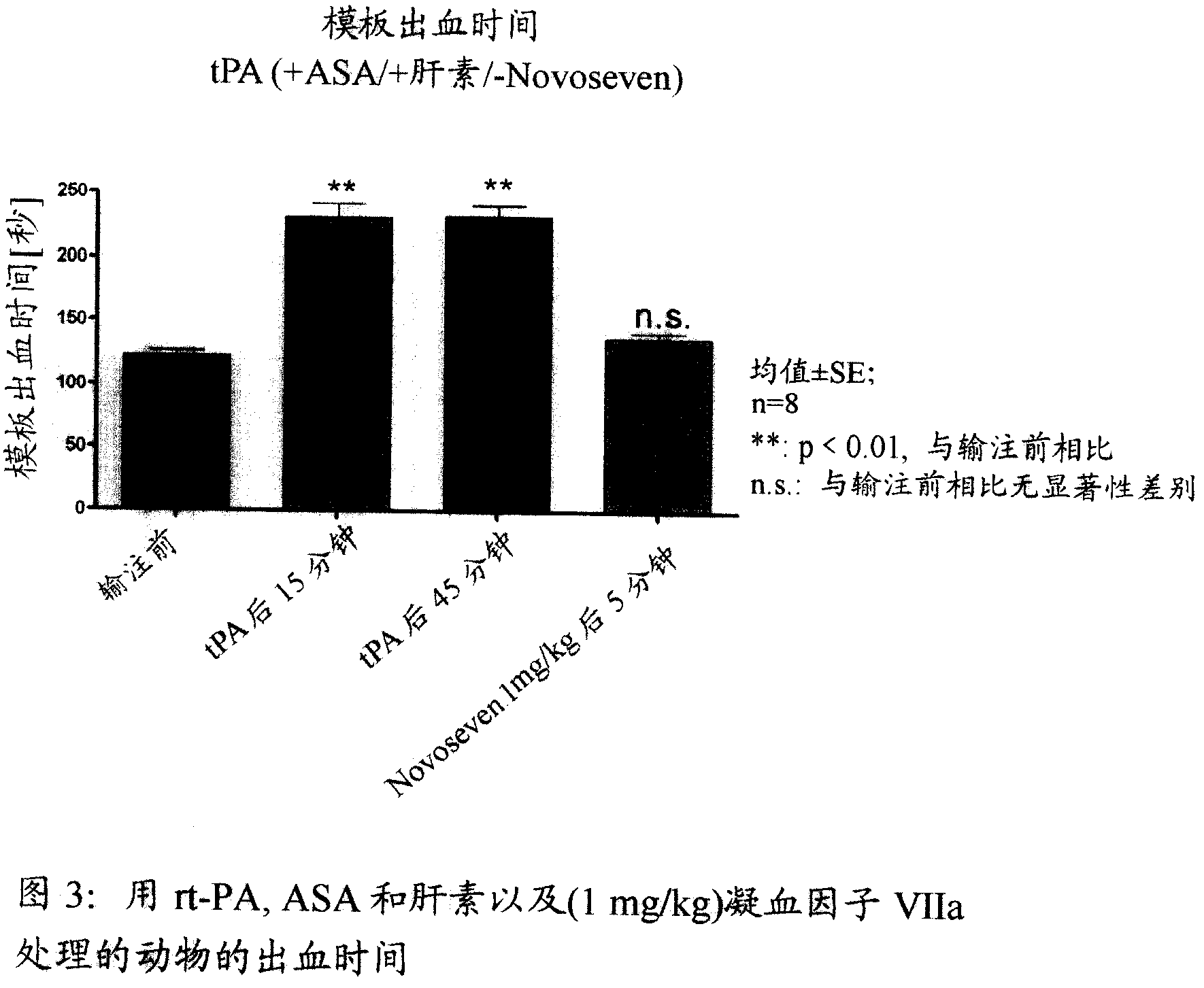
Activated plasma thromboplastin component VII for treating massive haemorrhage caused by thrombolysis therapy
The invention relates to a method for treating massive hemorrhage resulted from thrombolytic therapy / fibrinolytic therapy through administrating an active coagulation factor VII (VIIa) and functional derivatives thereof with an effective amount to the volunteers. The massive hemorrhage includes intracranial hemorrhage.
Owner:BOEHRINGER INGELHEIM PHARM KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com