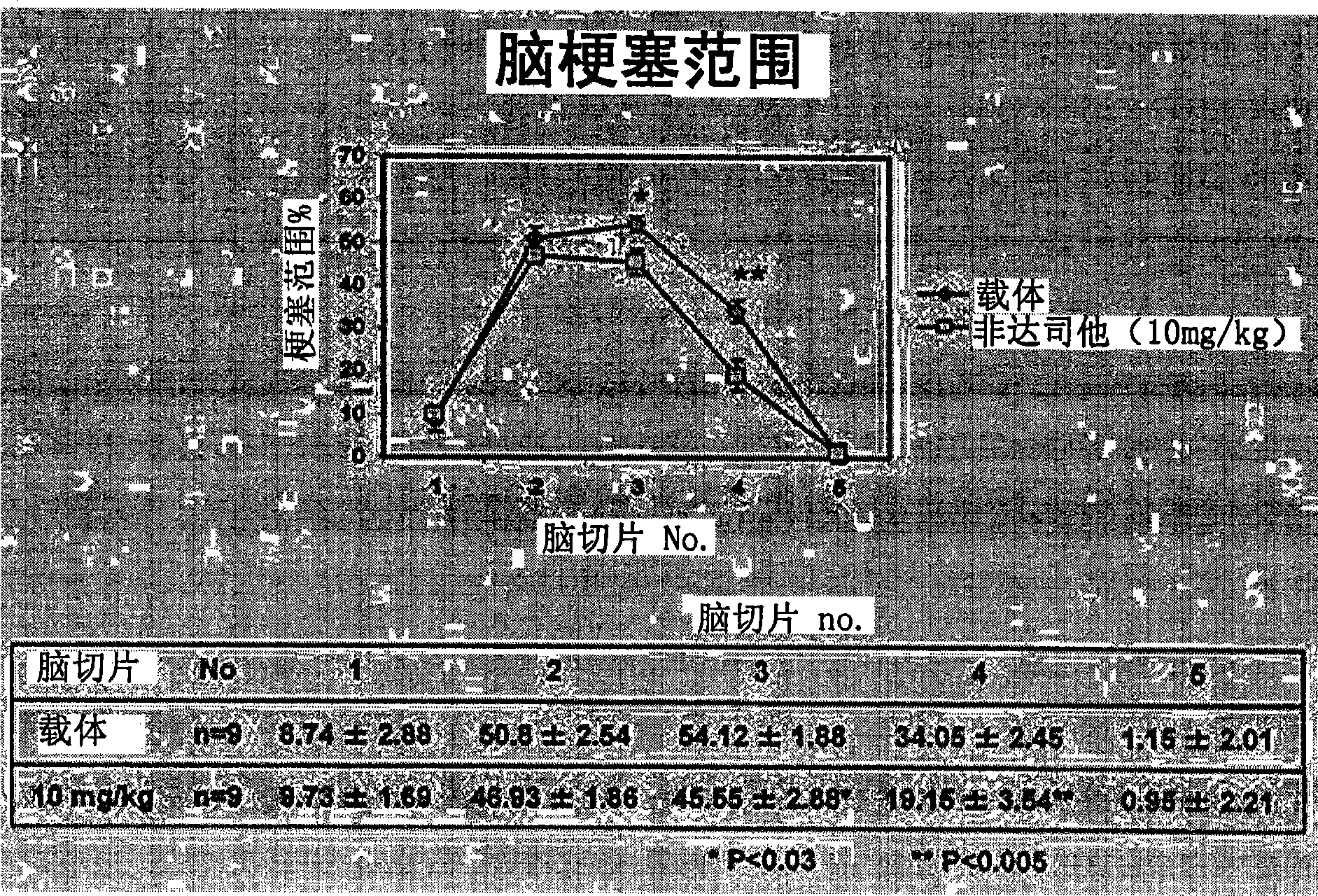
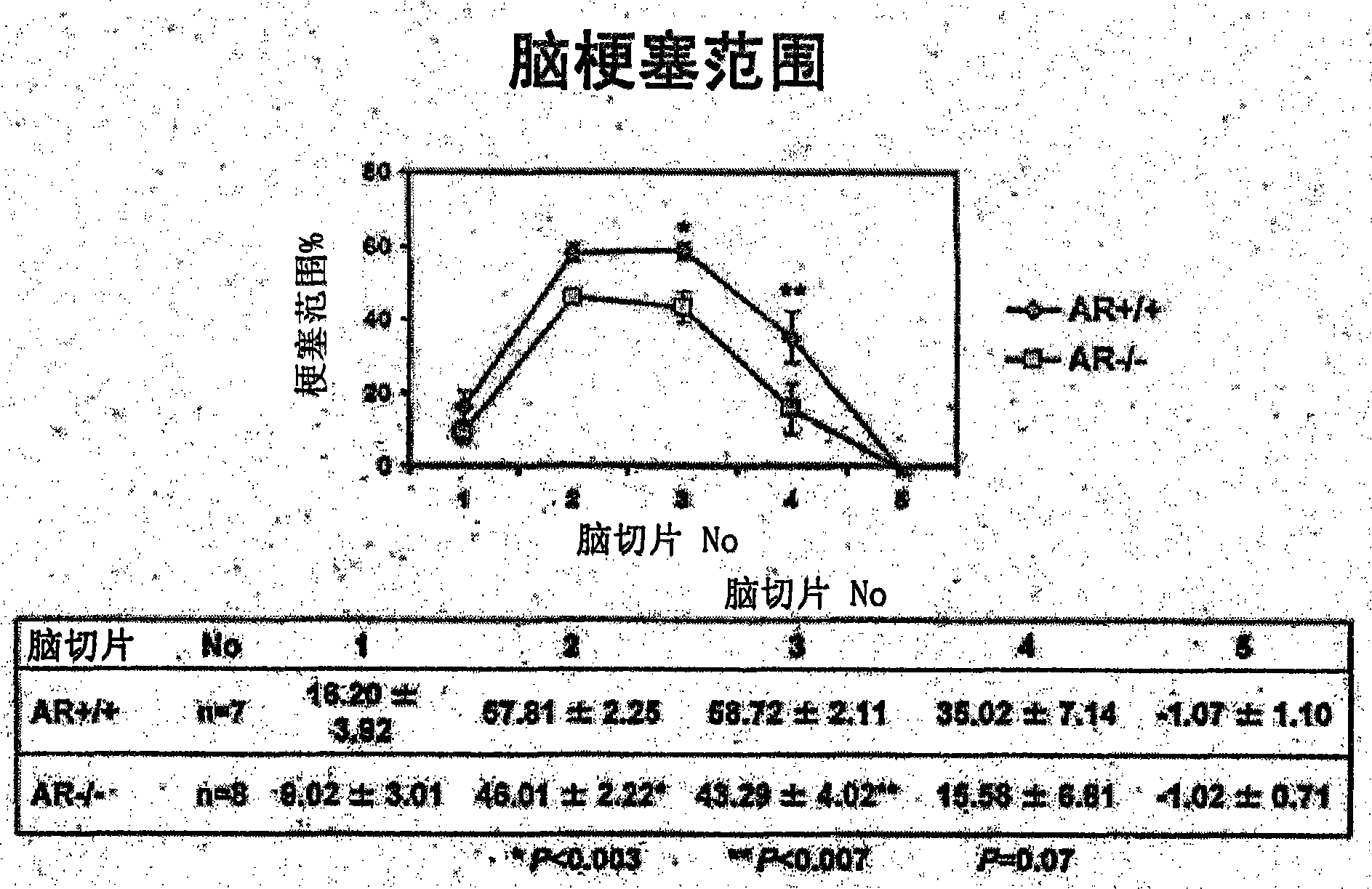
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Cerebral haemorrhages" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Genetic remedies for neurodegenerative diseases
ActiveUS20040265283A1Effective preventionEffective therapyBiocideNervous disorderCerebrovascular disorderPeripheral neuritis
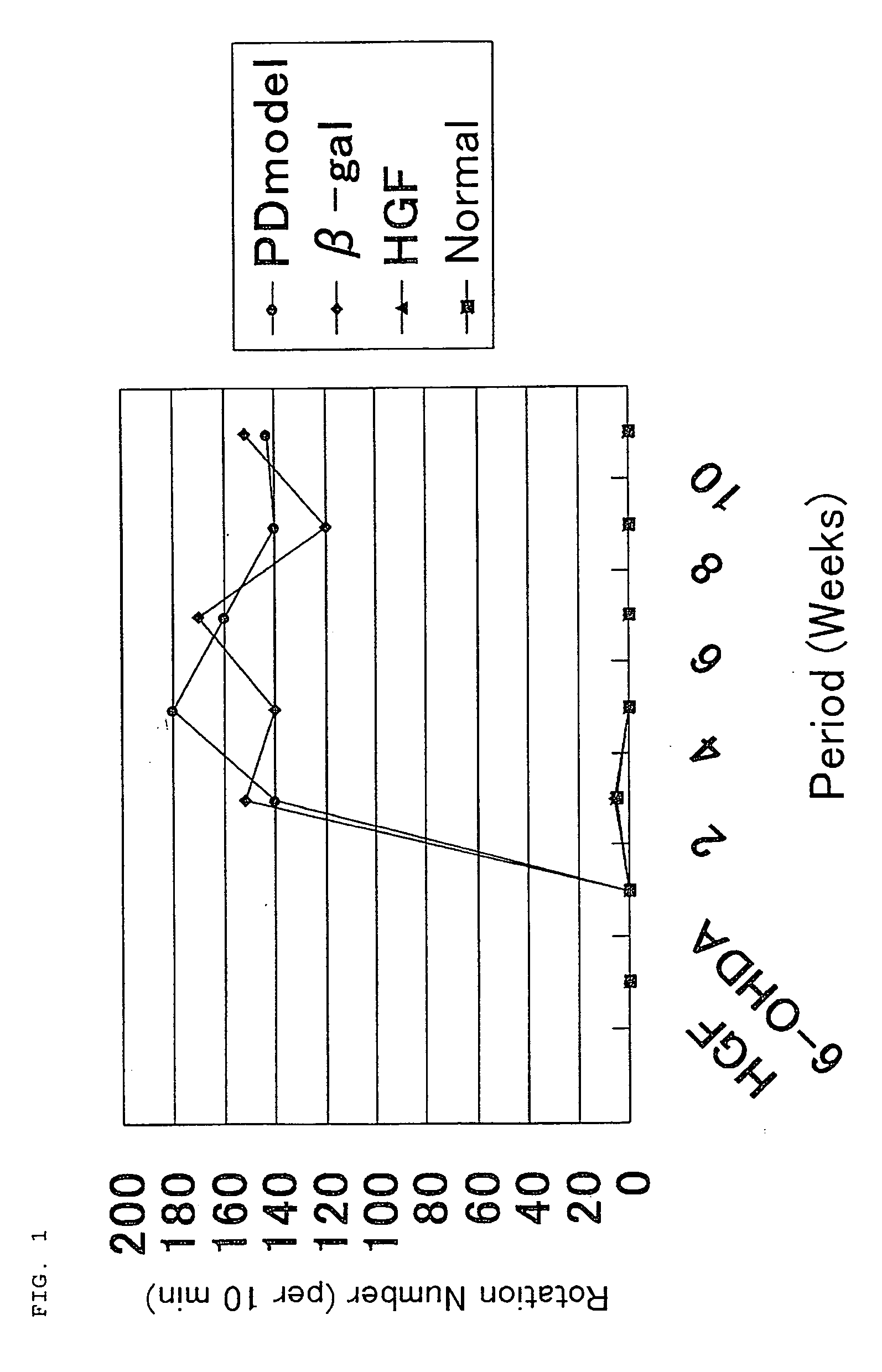
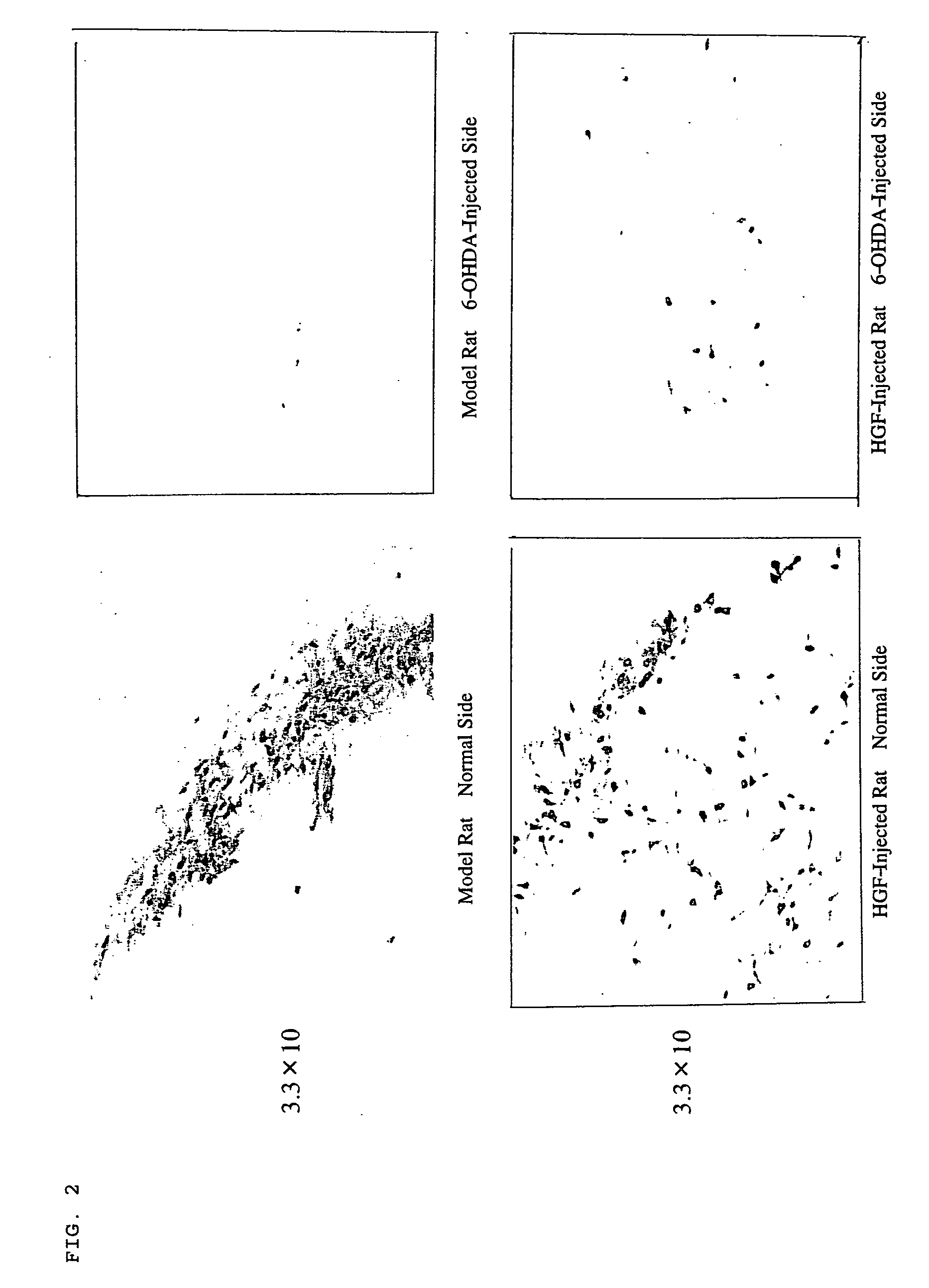
The present invention provides a therapeutic agent containing as an active ingredient an HGF gene used for therapy of neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, spinal cord injury, diabetic peripheral neuritis, and ischemic cerebrovascular disorders (cerebral infarction, cerebral haemorrhage, etc.) More specifically, the present invention provides a therapeutic agent for neurodegenerative diseases, containing a hepatocyte growth factor (HGF) gene as an active ingredient.
Owner:ANGES MG INC
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
InactiveUS20060063714A1Good storage stabilityPeptide/protein ingredientsInorganic non-active ingredientsFactor VIIaClotting factor deficiency
The invention relates to a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (e.g. human Factor VIIa) and a buffering agent; wherein the molar ratio of non-complexed calcium ions (Ca2+) to the Factor VII polypeptide is lower than 0.5. The composition may further comprise a stabilizing agent (e.g. copper or magnesium ions, benzamidine, or guanidine), a non-ionic surfactant, a tonicity modifying agent, an antioxidant and a preservative. The composition is useful for treating a Factor VII-responsive syndrome, such as bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor XI deficiency, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
Traditional Chinese medicine enteric oral liquor using leech extractive as active component, and its preparation method
InactiveCN1911250AAnthropod material medical ingredientsPill deliveryMedicinal herbsUpper gastrointestinal
An orally taken enteric Chinese medicine in the form of tablet, capsule, dripping pill, or soft capsule for treating unstable angina pectoris, acute myocardial infarction, cerebral haemorrhage, etc and preventing deep phlebothrombosis is prepared from leech. Its preparing process is also disclosed.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
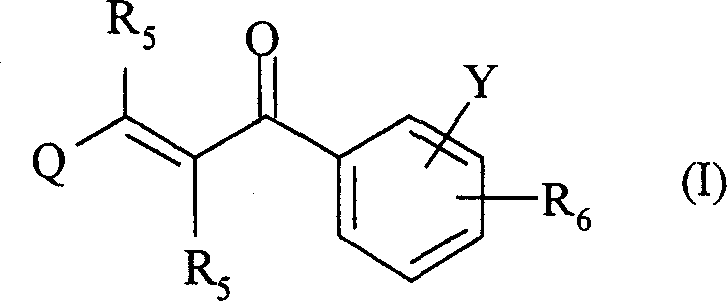
2-propylene-1-ones as hsp70 inducer
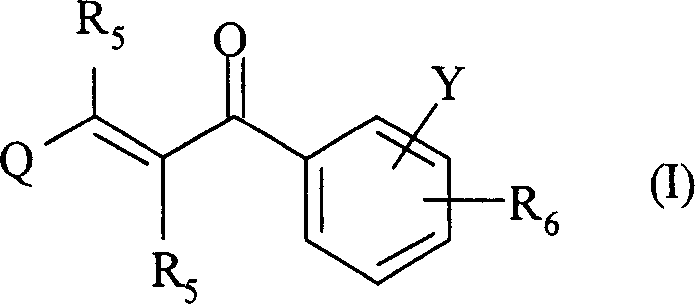
The present invention relates to novel compounds of 2-propene-1-one series, of general formula (I), their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, pharmaceutically acceptable salts, pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them, wherein R5, R6, Q and Y are as defined in the specification. The present invention also relates to a process for preparing such compounds, compositions containing such compounds, and use of such compound and composition in medicine. The compounds of the general formula (I) induce HSP-70 and are useful for the treatment of diseases accompanying pathological stress in a living mammalian organism, including a human being, such as stroke, myocardial infarction, inflammatory disorder, hepatotoxicity, sepsis, diseases of viral origin, allograft rejection, tumourous diseases, gastric mucosal damage, brain haemorrhage, endothelial dysfunctions, diabetic complications, neuro-degenerative diseases, post-traumatic neuronal damage, acute renal failure, glaucoma and aging related skin degeneration.
Owner:TORRENT PHARMA LTD
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
Aza-peptides
Provided are peptides comprising at least one azaamino acid and having β-sheet breaking ability, useful in the treatment and prevention of diseases such as alzheimer's disease, Dementia Pugilistica (including head trauma), Hereditary Cerebral Haemorrhage with amyloidosis of the Dutch type (HCHWA-D) and Vascular Dementia with amyloid angiopathy.
Owner:LAB SERONO SA
Nutritional powder with walnut oil powder and tartary buckwheat powder
InactiveCN101744244AFull of nutritionPrevent cardiovascular diseaseFood preparationEdible oils/fats production/working-upPolygonum fagopyrumFood additive
The invention provides a nutritional powder with walnut oil powder and tartary buckwheat powder. The nutritional powder comprises 50wt%-85wt% of tartary buckwheat powder, 11wt%-46wt% of walnut oil powder and the balance food additives. The invention also provides a preparation method of the nutritional powder with walnut oil powder and tartary buckwheat powder. Walnut oil powder which contains linoleic acid, linolenic acid and various fat-soluble vitamins is added in the nutritional powder of the invention, wherein the linoleic acid of walnut oil powder also contains seven of the eight kinds of necessary amino acid for the human health, has rich nutrition, has important functions of effectively preventing cardiovascular disease and preventing and curing cerebral apoplexy and cerebral haemorrhage and also has effects for preventing freckles and senile plaques; and after eaten for long time, the nutritional powder has functions that the skin can become delicate and smooth, the face can become white, tender and pink and the effect of beauty and weight reduction can be realized.
Owner:万锦荣
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
Application of whole hemp extract in preparation of drug for preventing and controlling cardiovascular and cerebrovascular diseases
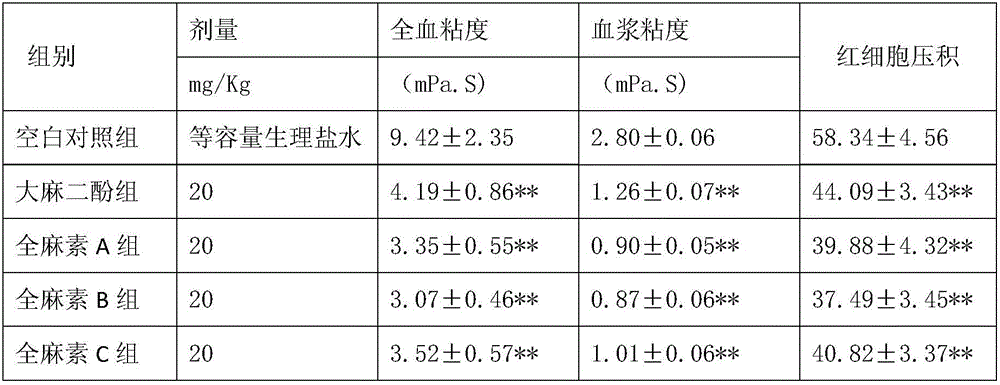
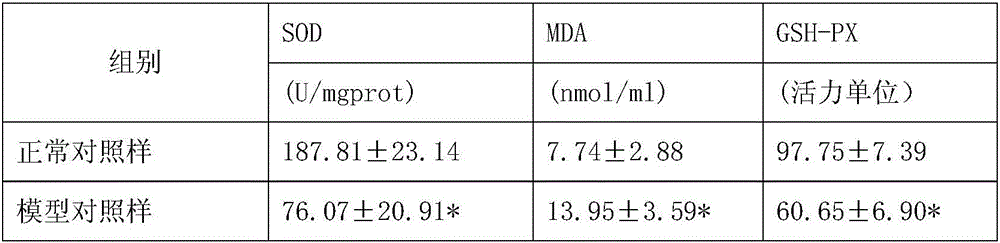
InactiveCN105943613AExcellent function and effectReduce volumeNervous disorderCardiovascular disorderCannabisDisease
The invention discloses application of a whole hemp extract in preparation of drugs for preventing and treating cardiovascular and cerebrovascular diseases. The whole hemp extract is prepared by evenly mixing 0.3-99.7 parts of an industrial hemp and fructus cannabis extract and 0.3-99.7 parts of an industrial hemp and cannabis sativa L extract. Experiments prove that the whole hemp extract has the effects of improving the activity of SOD, GSH-PX and other anti-free-radical enzymes, scavenging free radicals and protecting cardio and cerebral blood vessels and tissues and preventing and controlling occurrence and development of the cardiovascular and cerebrovascular diseases. The whole hemp extract can be used for preparing the drugs for preventing and treating the cardiovascular and cerebrovascular diseases, such as cerebral ischemia, myocardial ischemia, neuronal damage caused by hypoxia-reoxygenation, cerebral infarction (stroke), cerebral thrombosis, cerebral hemorrhage, a coronary heart disease and myocardial infarction and has the good application prospect in treatment or prevention of the cardiovascular and cerebrovascular diseases.
Owner:云南瑞酚生物科技有限公司
Traditional Chinese medicine composition for treating dyskinesia and preparation method thereof
InactiveCN107519449AConsolidate and improve curative effectGood effectOrganic active ingredientsNervous disorderPharmaceutical drugMassage
The invention discloses an external Chinese medicinal composition for treating motor dysfunction and a preparation method thereof. Its raw materials include Chinese medicinal materials such as calamus calamus, maple lotus, ivy, windproof, and multiflorum multiflorum; compared with the prior art, the present invention has obvious characteristics in principles, methods, prescriptions, and medicines, and is mainly used for stroke (cerebral hemorrhage) , cerebral infarction, etc.) and brain atrophy caused by paralysis, limb numbness, muscle atrophy, motor dysfunction, etc. have preventive and therapeutic effects. The present invention adopts medicine for external use combined with massage therapy, and in the course of medication, adjusts medication according to the specific condition of the patient, so as to achieve the best therapeutic effect.
Owner:贵州骏驰黔江中医药科技发展有限公司
Chinese herb for inhibiting tumor and its preparation
InactiveCN1895554ALittle side effectsEnhance immune functionOrganic active ingredientsCapsule deliveryCordycepsOphiopogon japonicus
Owner:李 俊杰
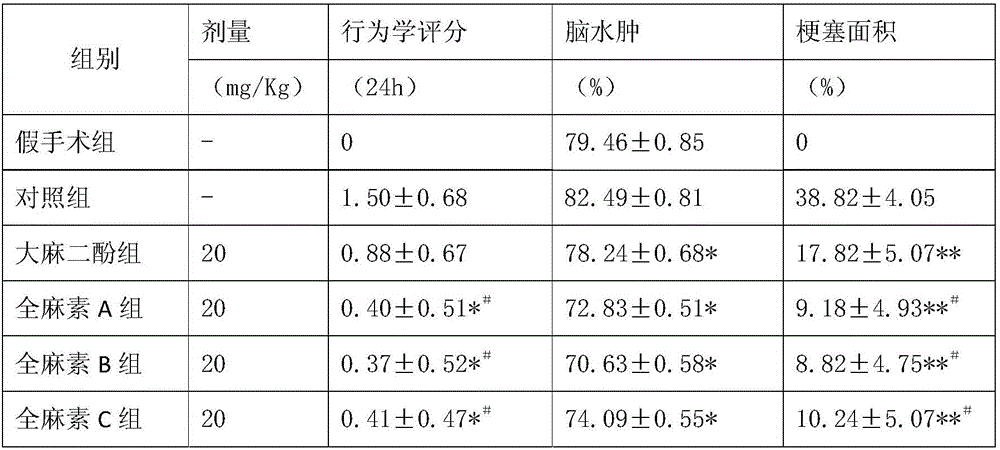
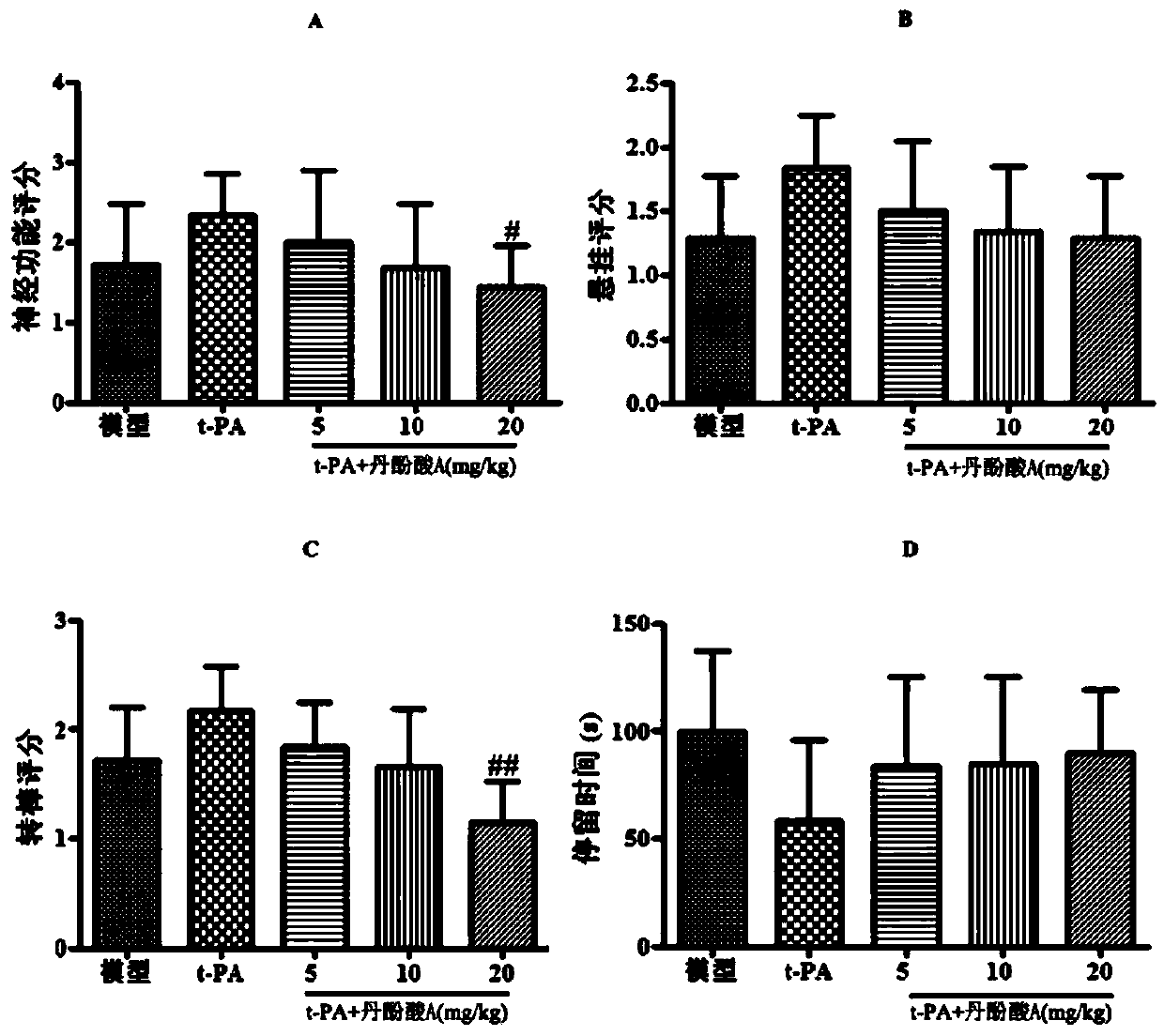
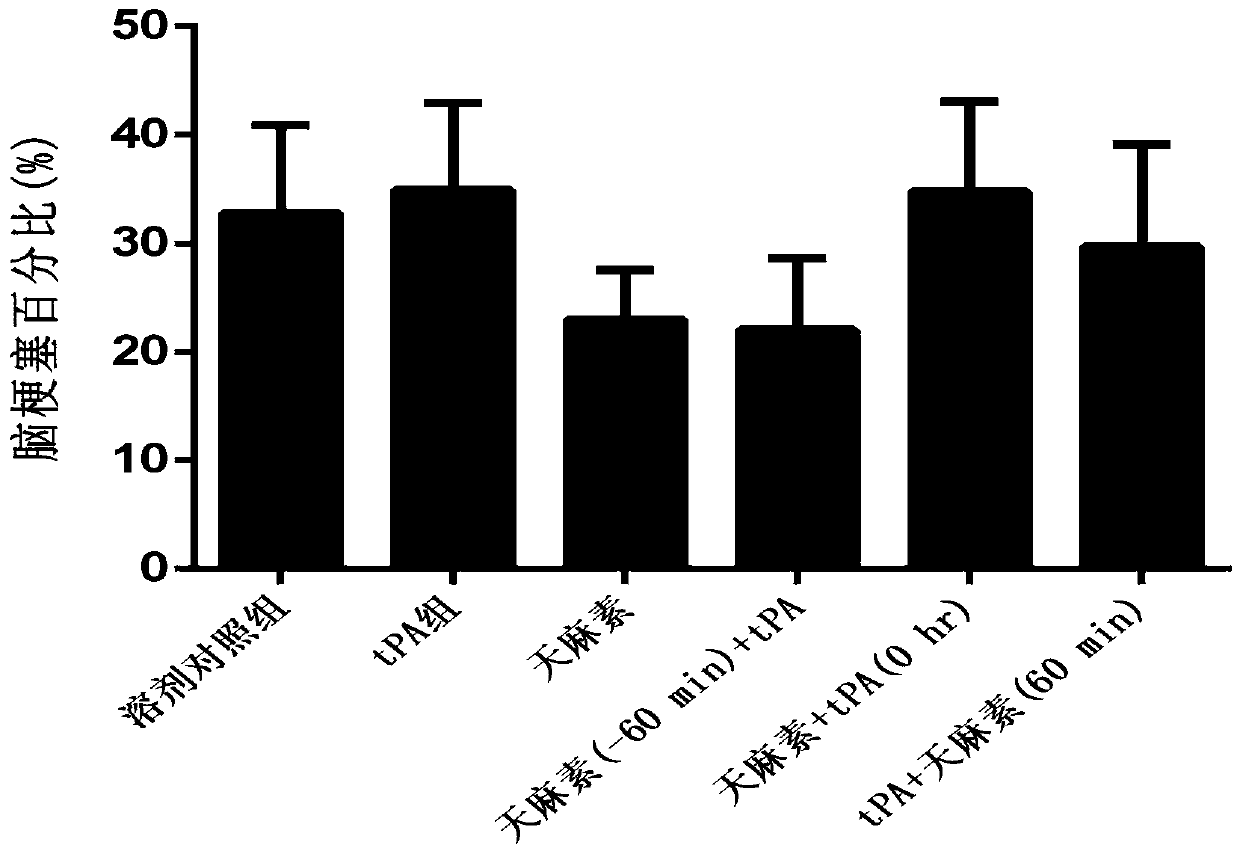
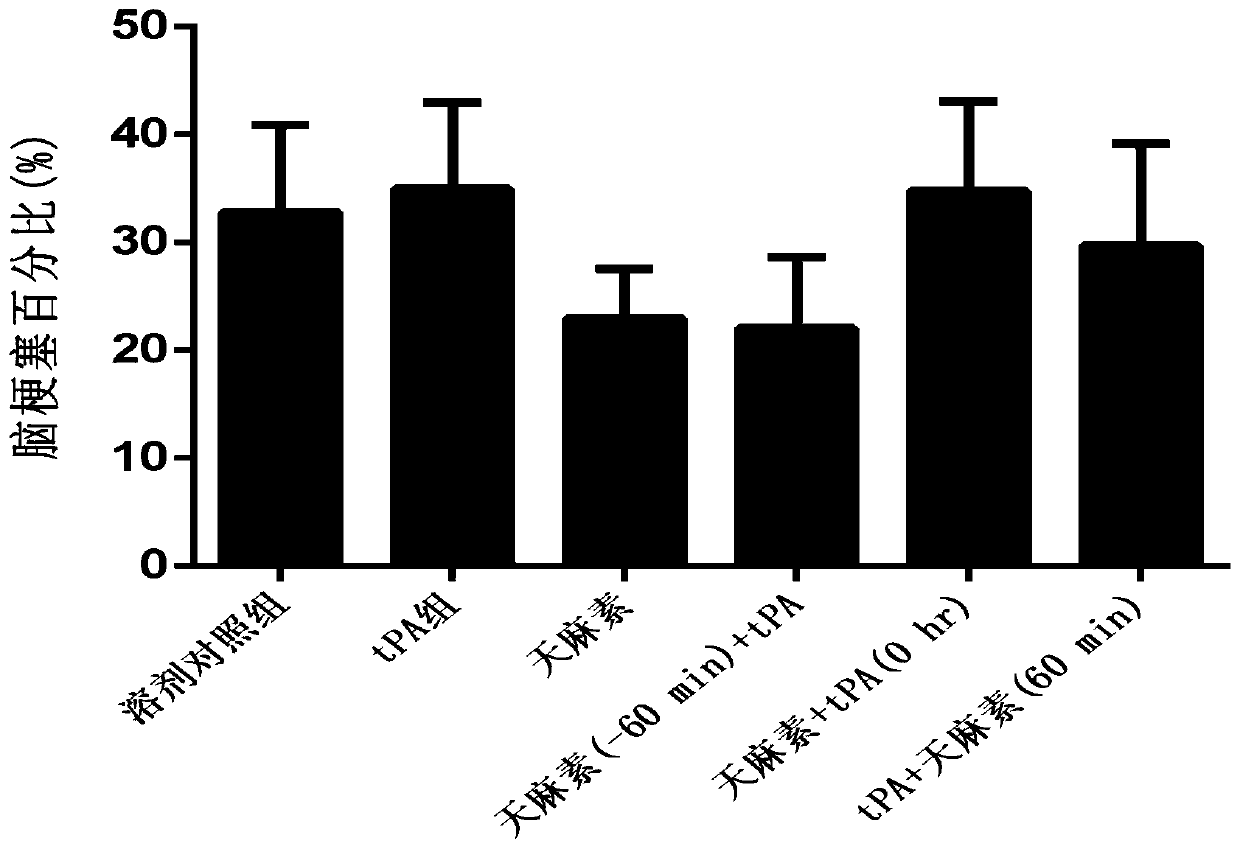
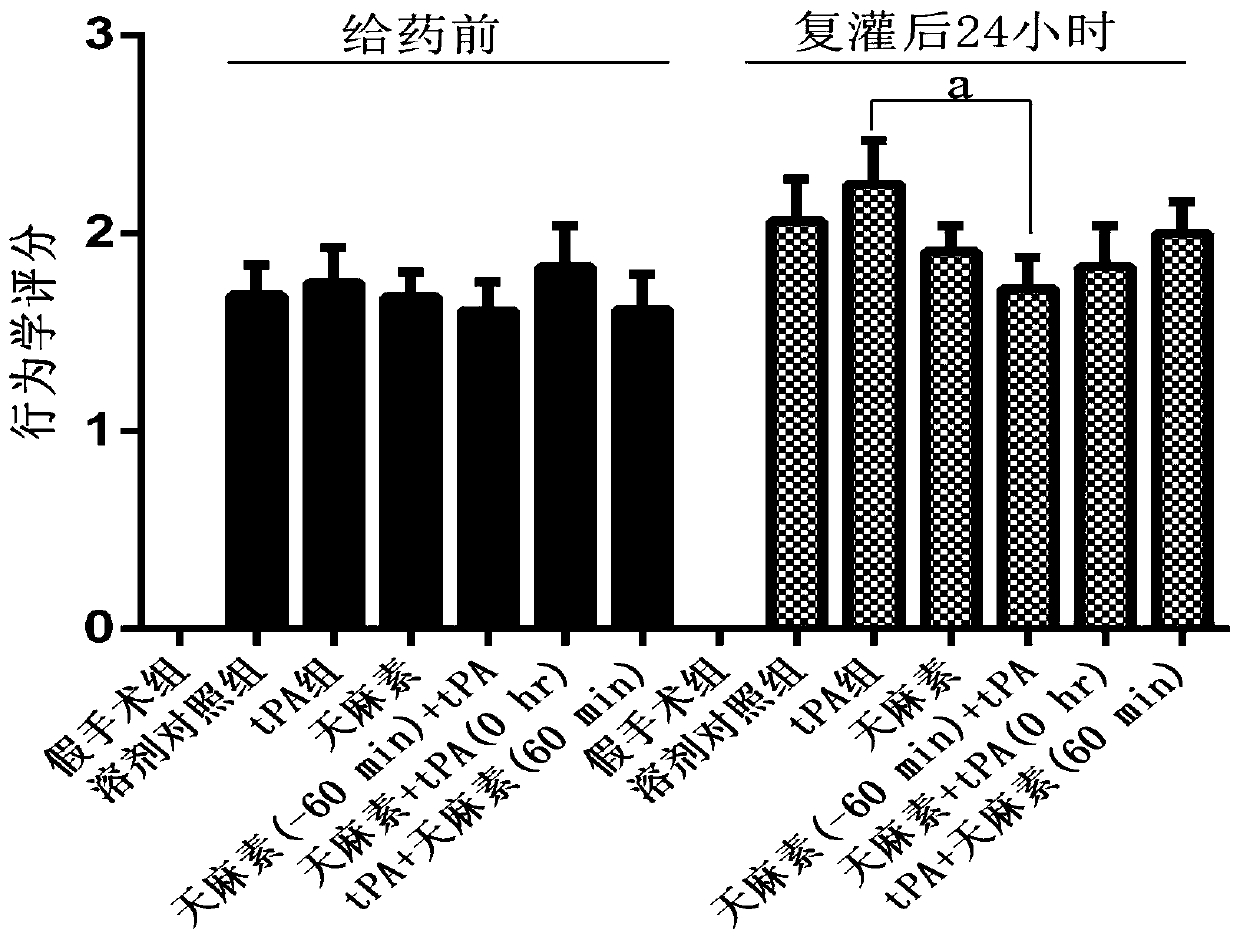
Application of salvianolic acid A in preparation of anti-cerebral hemorrhage drug
PendingCN111084770AReduce riskReduce exudationOrganic active ingredientsBlood disorderThrombolytic drugCerebral embolus
The invention discloses application of salvianolic acid A in preparation of a drug for treating cerebral hemorrhage. Cerebral hemorrhage comprises simple cerebral hemorrhage stroke, hemorrhage after cerebral embolism and hemorrhage caused by thrombolytic drug treatment and other factors. Experimental research finds that salvianolic acid A can significantly reduce the cerebral infarction volume andencephaledema of rats, improve neurological functions and protect blood-brain barrier damage caused by thrombolytic drugs such as t-PA, thereby reducing the risk and degree of hemorrhagic transformation when the thrombolytic drugs are used. The salvianolic acid A can be used for preparing the drug for treating cerebral hemorrhage and hemorrhage caused by thrombolytic drug treatment, relieving secondary hemorrhage in ischemic areas and improving prognosis of patients.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Strawberry and honey beverage and its production
A strawberry fruit-honey beverage is proportionally prepared from strawberry fruit, honey and VC through washing and cutting strawberry fruits to obtain small blocks, squeezing to obtain juice, mixing it with honey and VC, boiling at 70-80 deg.C for 30 min, and cooling. It can be used to prevent enterogastric disease, anemia and scurvy, and treat hypertension, hypercholesteremia, atherosclerosis, coronary heart disease and cerebral haemorrhage.
Owner:TIANJIN CHINESE & BRITISH NANOMETER TECH DEV
Chinese medicine for cardiac and encephalic ischemia
InactiveCN1490046ASymptoms improvedGood blood pressureUnknown materialsCapsule deliveryDiseaseContractility
A Chinese medicine for treating cardiac or cerebral ischemia is prepared from evodia fruit or rhubarb through immersing the evodia fruit in liquorice root solution for 2 hr, drying in air, pulverizing by 120 meshes and loading in capsules, or proportionally mixing rhubarb with bran, parching for 2 min, pulverizing by 120 meshes and loading in capsules. Its advantage is high curative effect.
Owner:董运祥
Novel thrombolytic molecules and a process therefor
ActiveUS20100015123A1Promote absorptionDissolve thrombusPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein moleculesCerebrovascular disorder
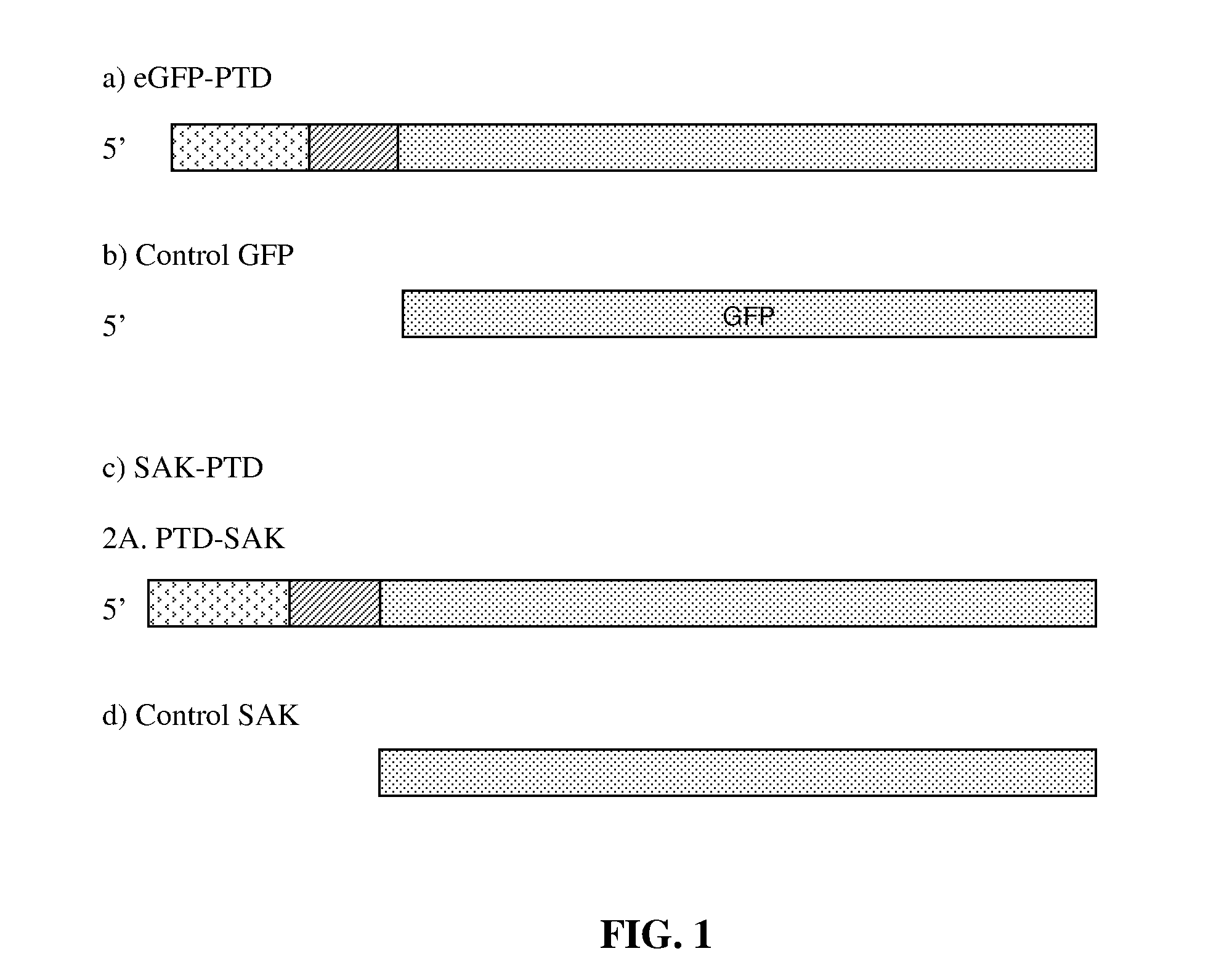
New thrombolytic protein molecules such as recombinant staphylokinase or streptokinase, urokinase, tissue plasminogen activator and the like, and suitable variants thereof, for targeting to brain tissue or any other tissue by either fusing to, or by synthesizing the candidate thrombolytic molecule(s) with a protein sequence comprising a strong amphipathic alpha helix containing protein transduction domain. Thrombolytic protein molecule(s) so engineered with the protein transduction domain is useful for enhanced uptake of such protein thrombolytic molecule(s) across the cell membranes and tissues including the blood brain barrier and find their use in the treatment of vascular thrombosis including cerebrovascular disorders caused by cerebral thrombosis or cerebral haemorrhage when used a as a therapeutic. The design and processes for cloning, expression, purification and protein transduction of such proteins across cell membranes.
Owner:BHARAT BIOTECH INTERNATIONAL
Beta-Amyloid Inhibitors and Use Thereof
InactiveUS20070293422A1Suitable for treatmentImprove propertiesNervous disorderPeptide/protein ingredientsCerebral haemorrhagesAmyloid angiopathy
Peptides and derivatives or analogs thereof are provided for having β-amyloid aggregation inhibitory activity, useful in the treatment and prevention of diseases such as Alzheimer's disease, Dementia pugilistica (including head trauma), Hereditary Cerebral Haemorrhage with amyloidosis of the Dutch type (HCHWA-D) and vascular dementia with amyloid angiopathy.
Owner:LAB SERONO SA
Use of imidazolone compounds for treating cardiovascular and cerebrovascular diseases
The invention relates to a medicine field, in particular relates to application of (Z)-1-methyl-1, 5-dihydro-2-amido-5-(4-(mesyl) benzal)-4H-imidazole-4-ketone and pharmaceutically acceptable salt thereof in the treatment of cardiovascular and cerebrovascular diseases of mammals; the compound and the salt of the compound can effectively prohibit formation of thrombus and are used for preventing or curing diseases such as coronary heart disease, atherosclerosis, artery and vein thrombosis, angina, myocardial infarction, coronary heart disease, myocarditis, ischemic cardiomyopathy, myocardial infarction, cardiovascular complications of diabetes, cerebral haemorrhage, cerebral thrombosis, cerebral embolism, cerebral infarction, brain stroke, lacunar infarction, transient ischemic attack, cerebral arteriosclerosis and peripheral vascular diseases such as obstructive vasculitis, aortoarteritis, hypercholesterolemia, or high blood lipids, etc.
Owner:CHINA PHARM UNIV
Chimeric fusion proteins
ActiveUS8968728B2Promote absorptionDissolve thrombusPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein moleculesCerebrovascular disorder
Owner:BHARAT BIOTECH INTERNATIONAL
Naodesheng preparation and its new preparation method
A composite Chinese medicine in the form of dripping pill and soft capsule for treating cerebral arteriosclerosis, ischemic cerebral apoplexy and cerebral haemorrhage sequelae, and its preparing process are disclosed.
Owner:FUKANGREN BIO PHARMA
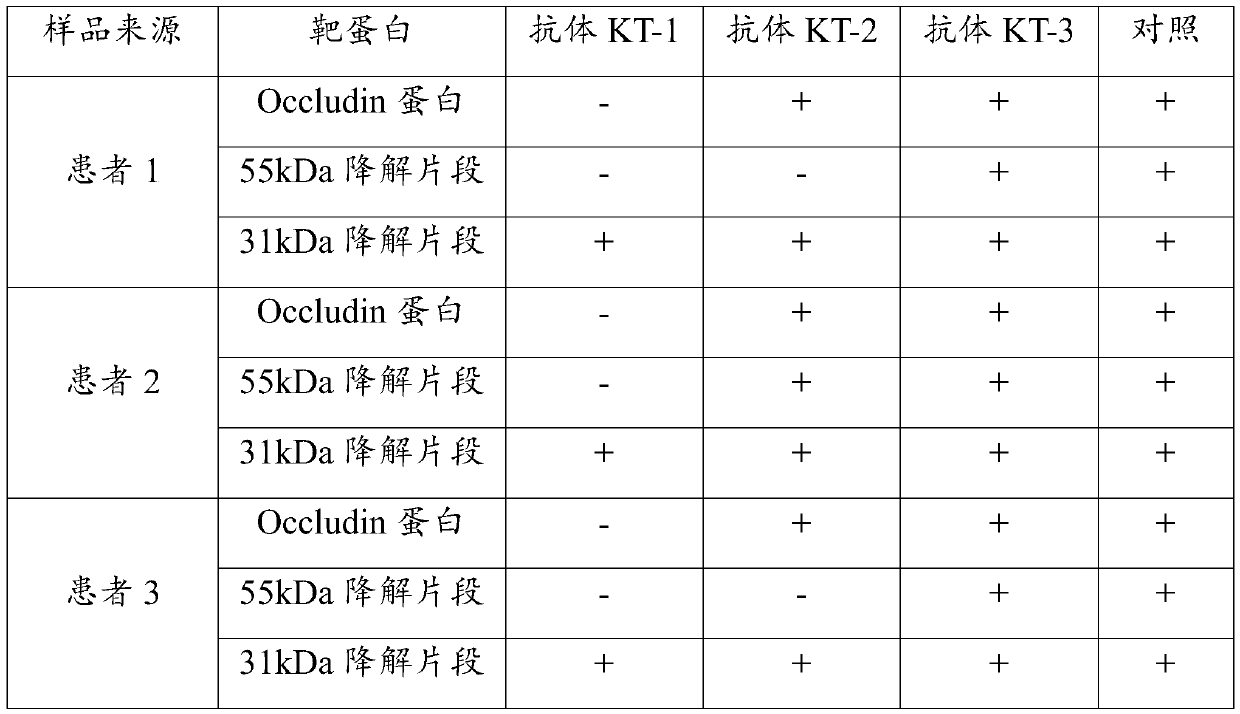
Specific epitope peptide of occludin 31kDa degradation fragment and application of specific epitope peptide
ActiveCN110467665AStrong specificityLow detection limitImmunoglobulins against animals/humansDisease diagnosisPeptideIschemic brain
The invention discloses a specific epitope peptide of an occludin 31kDa degradation fragment and application of the specific epitope peptide. The specific epitope peptide comprises an amino acid sequence shown in the site 323-334 of SEQ ID No.1. An antibody generated by an antigen prepared from the specific epitope peptide is only capable of recognizing the occludin 31kDa degradation fragment, butcannot recognize an occludin 55kDa degradation fragment or undegraded occluding, and has the advantages of being good in specificity and low in detection limit. By adopting the specific epitope peptide, a corresponding novel detection technique is provided for rapidly and efficiently testing the level of a 31-kDa occludin fragment in serum of a patient suffering from acute ischemic stroke at a super early stage, and predicting cerebral hemorrhage in recanalization treatment on acute ischemic stroke.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Traditional Chinese medicine for treating cerebral haemorrhage
The invention relates to a traditional Chinese medicine for treating cerebral haemorrhage. The component proportion of the medicament is as follows: radix paeoniae rubrathe 20g, peach seed 20g, Radix Aconiti Praeparata 30g, windproof 30g, rhizoma Gastrodiae 30g, chuanxiong root 20g, antelope horn 10g, cinnamon 10g, radix glycyrrhizae preparata 10g and notopterygium root 10g. The accuracy of the ratio of the fixed dosages is plus or minus 10% of the above components. Indication of the traditional Chinese medicine is cerebral haemorrhage with the symptoms of eye-mouth deviation, hemiplegia, complete paralysis, gatism, limb ache and swelling. The traditional Chinese medicine has an obvious effect on treating cerebral haemorrhage and is free of any toxic or side effect.
Owner:刘树彬
Mulberry leaf tea and preparation technology thereof
The invention relates to mulberry leaf tea and a preparation technology thereof. The raw materials of the mulberry leaf tea mainly comprise 50-100 parts of frosted mulberry leaves, 10-20 parts of folium eucommiae, 10-30 parts of oat, 30-50 parts of soybeans and 10-30 parts of chrysanthemums. The mulberry leaf tea is prepared through the following steps of cleaning the raw materials, drying the cleaned materials, crushing the dried materials, and performing mixing. According to the mulberry leaf tea prepared by the technology, the mouth feel is improved, the blood sugar is effectively reduced, diabetes is prevented, blood viscosity is reduced, and miocardial infarction and cerebral haemorrhage are effectively prevented from generating.
Owner:DANYANG HUADU GARDENING
Application of gastrodin in preparing drugs for treating cerebral hemorrhage
PendingCN110179808AProtect the brainOrganic active ingredientsPeptide/protein ingredientsDrugCentral nervous system
The present invention relates to the technical field of medicines, specifically relates to a use of gastrodin in preparing drugs for cerebral hemorrhage, particularly provides a use in preparing the drugs for reducing hemorrhagic transformation caused after a cerebral ischemia rt-PA thrombolytic therapy, and provides the use of the gastrodin in preparing the drugs for reducing the hemorrhagic transformation caused after the cerebral ischemia rt-PA thrombolytic therapy. The use suggests that a clinical early use of an gastrodin injection has a certain inhibitory effect for the existing hemorrhagic transformation caused by the use of the rt-PA thrombolytic therapy to treat ischemic stroke, can relatively extend time window of the rt-PA thrombolytic therapy, strives for more treatment time for patients with the clinical cerebral stroke, further reduces damages to central nervous system, enables prognosis to be better, reduces occurrence of sequelae and improves life quality of the patients.
Owner:KPC PHARM INC
Medicament for treating brain injury sequelae
The invention relates to a medicament for treating brain injury sequelae. The medicament is prepared by the following substances in ratios by weight: 50-100 parts of blood centipede, 50-150 parts of panax japonicus, 50-100 parts of edible tulip and 10-20 parts of asarum insigne diels. The medicament for treating brain injury sequelae disclosed by the invention can treat traumatic brain injury sequelae, cerebral haemorrhage, oedema, dizziness and headache.
Owner:范信
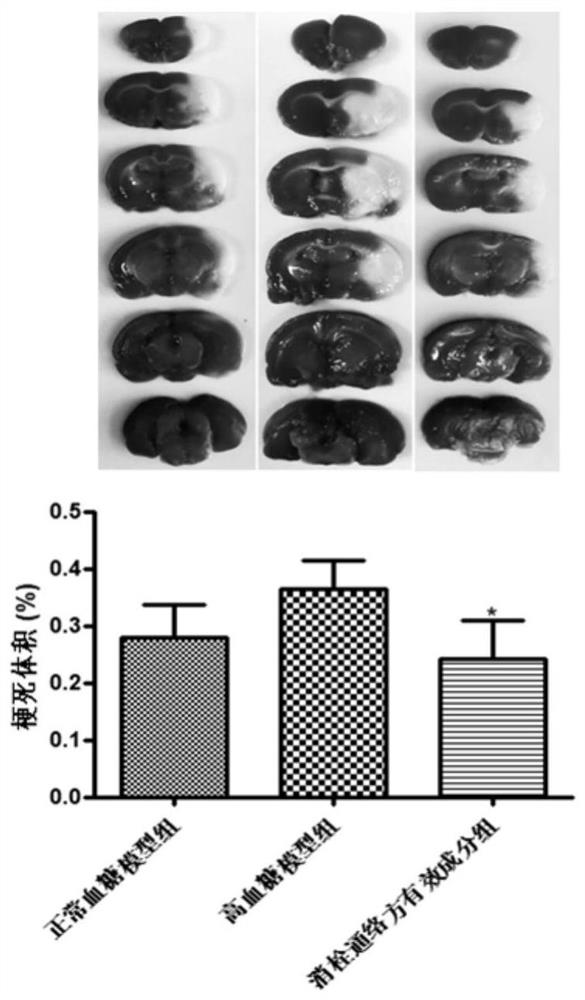
Application of effective component group of thrombus-eliminating collateral-dredging formula in treating cerebral hemorrhage
PendingCN111658744APrevent hemorrhagic transformationReduce mortalityNervous disorderHydroxy compound active ingredientsBrain edemaThrombus
The invention discloses an application of an effective component group of a thrombus-eliminating collateral-dredging formula in preparation of a medicine for treating cerebral hemorrhage. The cerebralhemorrhage comprises simple cerebral hemorrhage stroke, cerebral ischemia hemorrhage caused by hyperglycemia or hypertension after cerebral embolism, and hemorrhage caused by thrombolytic medicine treatment and other factors. Experimental research finds that an effective component group formed by active screening of components obtained by different extraction methods of the thrombus-eliminating and collateral-dredging formula can significantly reduce the volume of cerebral infarction and encephaledema of rats, improve neurological functions and protect blood-brain barrier damage caused by hyperglycemia, thereby reducing the risk and degree of hemorrhagic transformation during hyperglycemia. The effective component group of the thrombus-eliminating and collateral-dredging formula can be used for preparing medicines for treating cerebral hemorrhage and hemorrhage transformation caused by hyperglycemia, relieving secondary hemorrhage in ischemic areas and improving prognosis of patients.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Traditional Chinese medicine for treating cerebral apoplexy
InactiveCN105147966AHydroxy compound active ingredientsCardiovascular disorderCerebral paralysisFormulary
The invention aims to provide traditional Chinese medicine for treating cerebral apoplexy. The traditional Chinese medicine is characterized by comprising the following medicine: salvia miltiorrhiza, violet magnolia, evening primrose, kudzuvine root, ligusticum wallichii, kelp, mustard leaf, radix et herba rumicis maritimi, asarum, rhizoma anemarrhenae, achyranthis root, hemerocallis fulva Linn., bee pollen, gingko, pomelo, tangerine, orange, lychee, lemon, banana, borneol, and camellia oil, wherein salvia miltiorrhiza, violet magnolia, evening primrose, kudzuvine root, ligusticum wallichii, kelp, and mustard leaf serve as monarch drugs to perform the efficacies of activating blood circulation to expel bruises, clearing channels to relieve pain, eliminating annoyance, and refreshing; radix et herba rumicis maritimi, asarum, rhizoma anemarrhenae, achyranthis root, hemerocallis fulva Linn., and bee pollen serve as ministerial drugs to perform the efficacies of tonifying deficiency, dredging blood vessels, and stabilizing blood pressure; gingko, pomelo, tangerine, orange, lychee, lemon, banana, borneol, and camellia oil serve as adjuvant drugs contributing to human body vasoconstriction. All the medicine are combined to achieve the efficacy of repairing cranial nerves, so as to not only fast dissolve thrombus and dredge blood vessels to recover normal blood circulation, but also can feed nutrition to damaged brain cells continuously, activate the activity of the damaged brain cells, and quickly recover the conduction function of the cranial nerves. Therefore, the traditional Chinese medicine can be used for effectively treating symptoms such as cerebral apoplexy, cerebral thrombosis, cerebral infarct and cerebral haemorrhage.
Owner:王艳娣
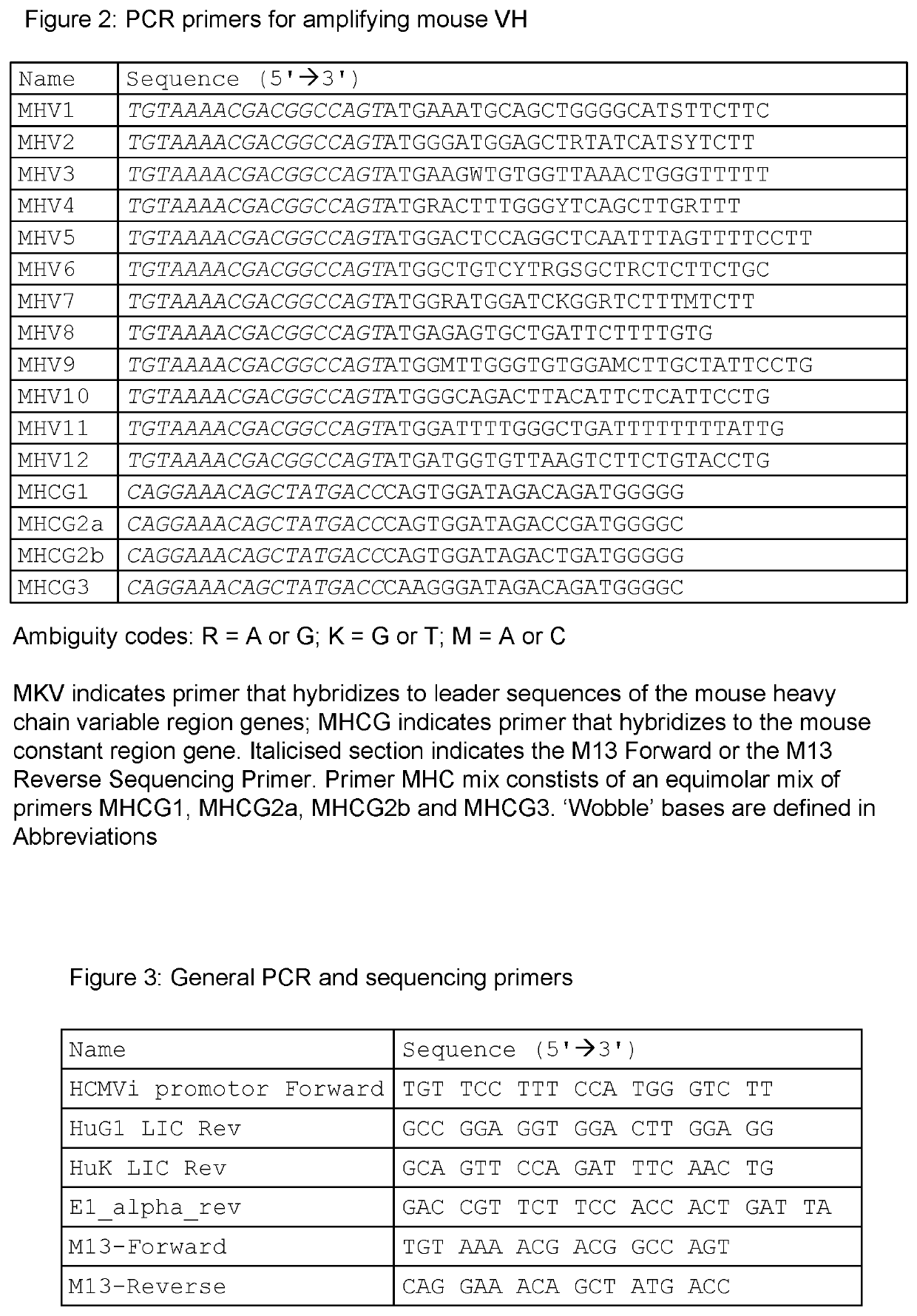
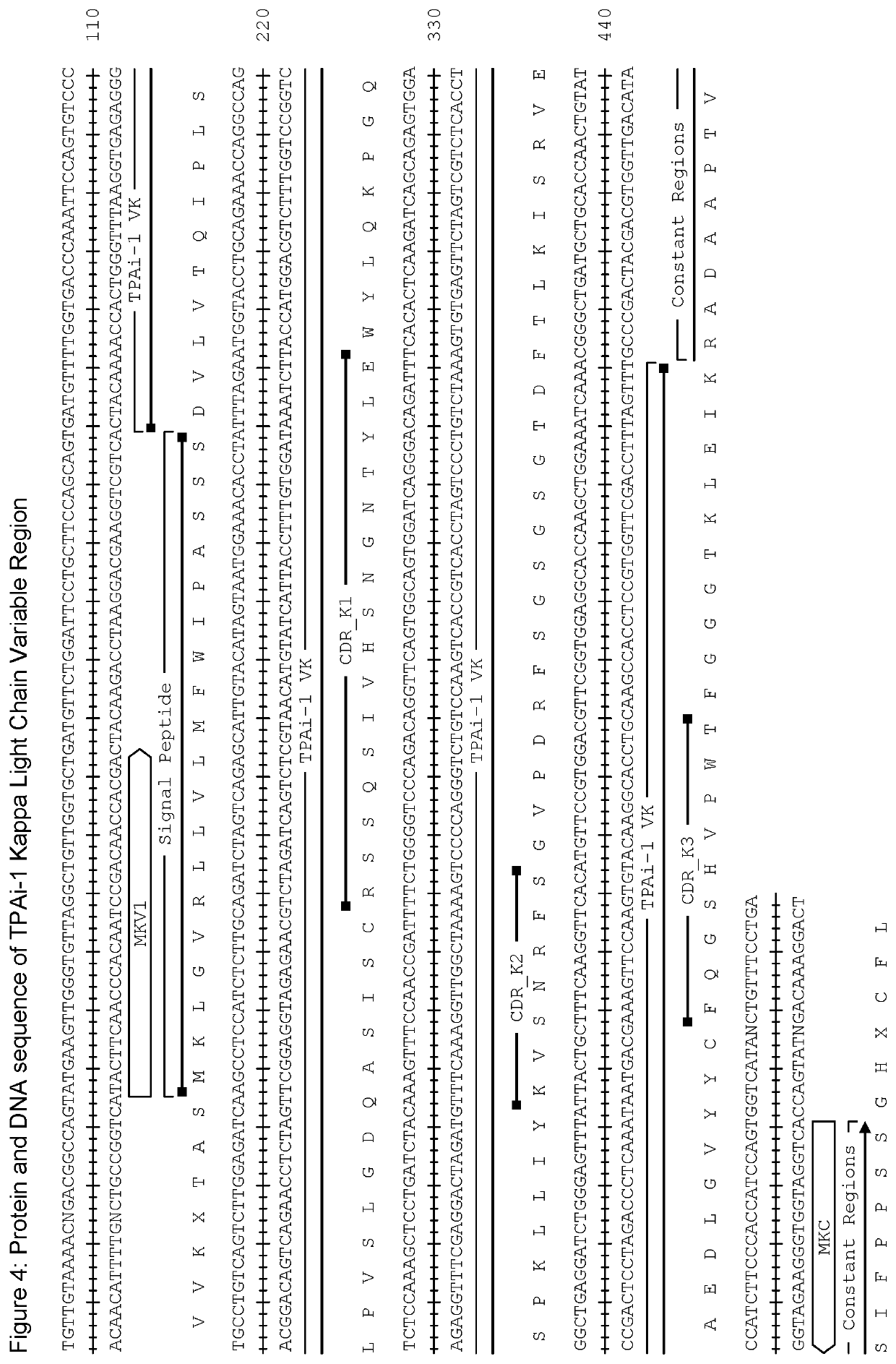
Tissue plasminogen activator antibodies and method of use thereof
PendingUS20210395393A1Peptide/protein ingredientsAntibody ingredientsPLG - PlasminogenAntiendomysial antibodies
The present invention provides tissue plasminogen activator antibody molecules and their uses. More particularly, the presently-disclosed invention provides humanised antibody molecules which specifically bind tissue plasminogen activator (TPA) and their use in treating TPA induced haemorrhage, in particular treating systemic haemorrhage such as brain haemorrhage after treatment of ischemic stroke or myocardial infarction, or systemic bleeding after TPA treatment of pulmonary embolism, ischemic stroke or myocardial infarction.
Owner:EMSTOPA LTD
Bingliushui traditional Chinese medicament
The present invention provides new medicine preparation and new therapy. The icy water Chinese medicine preparation of the present invention can kill bacteria, eliminate various foreign matter in blood vessels, regulate and improve the functions of internal organs and cure various diseases radically. The said preparation may be used in treat cerebral haemorrhage, cerebral thrombus, cerebral infarction, sciatica, protrusion of lumber intervertebral disc and many other diseases with high curative effect.
Owner:李秀坤
Cerebral micro-hemorrhage treatment equipment
PendingCN113425364AImprove damage repair abilityCatheterDiagnostic recording/measuringInjury brainCerebral damage
The invention discloses cerebral micro-hemorrhage treatment equipment in the technical field of cerebral micro-hemorrhage treatment equipment. The cerebral micro-hemorrhage treatment equipment comprises the following steps: step 1, selecting 36 cerebral hemorrhage patients, and dividing the 36 patients into a test group and a control group, wherein 18 patients are in each group; and step 2, applying conventional drug treatment to all patients by referring to domestic and foreign guidelines, and adding upper limb ischemia adaptation training treatment to the patients in the test group on the basis of conventional treatment. Aiming at the current situation that cerebral micro-hemorrhage lacks effective treatment measures, the cerebral micro-hemorrhage treatment equipment is based on a human body endogenous protection theory, and provides a simple, convenient, feasible, safe, noninvasive and easy-to-popularize treatment means for accelerating brain injury repair after cerebral micro-hemorrhage from the perspective of improving the brain injury repair ability.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Preventive or therapeutic agent for cerebral ischemic injury or cerebral ischemia reperfusion in stroke
InactiveCN101389332AGood effectLong-term administrationOrganic active ingredientsOrganic chemistryImidazolidineCerebral ischaemia
Owner:SANWAKAGUKU KENKYUSHO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com