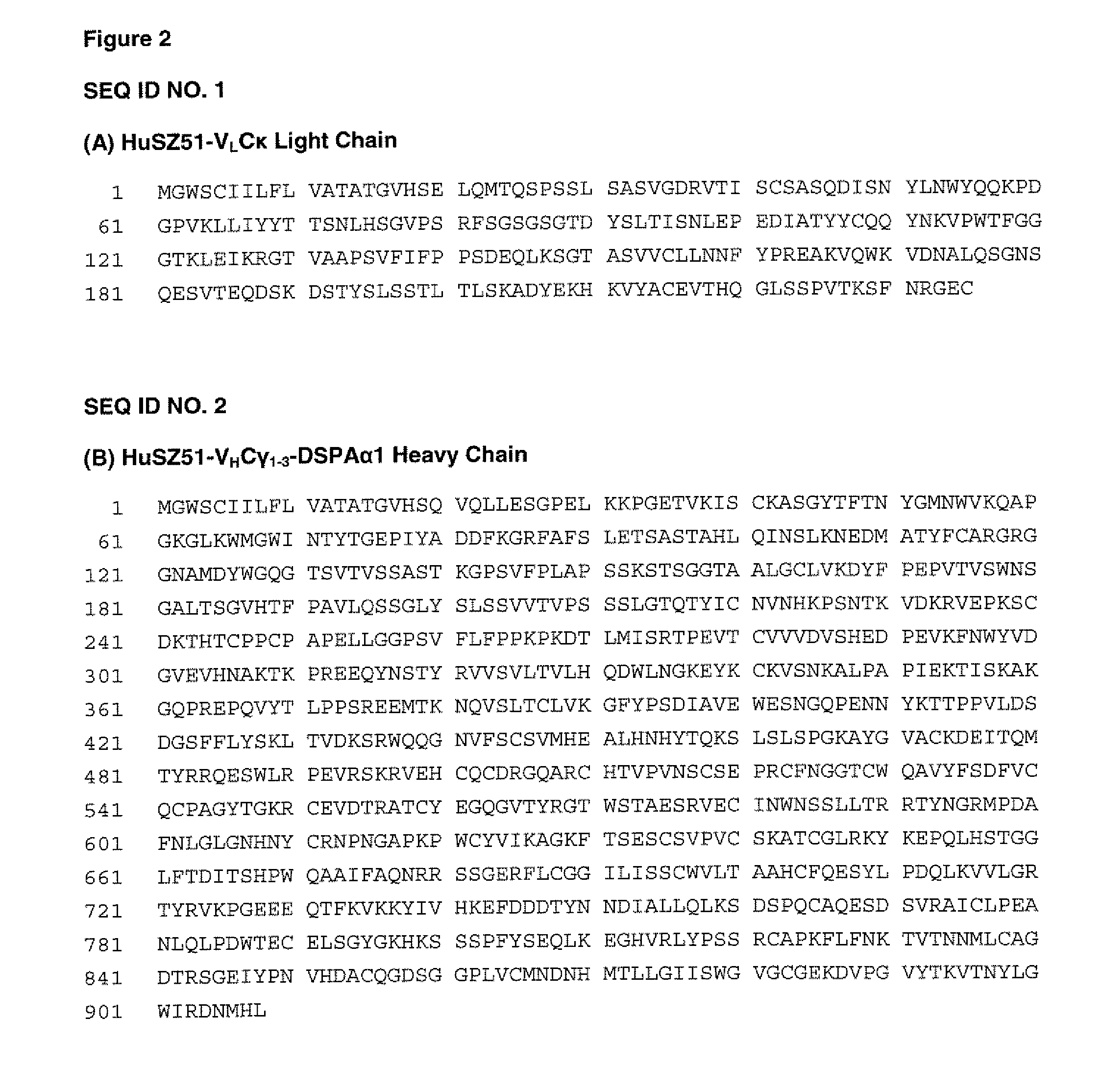
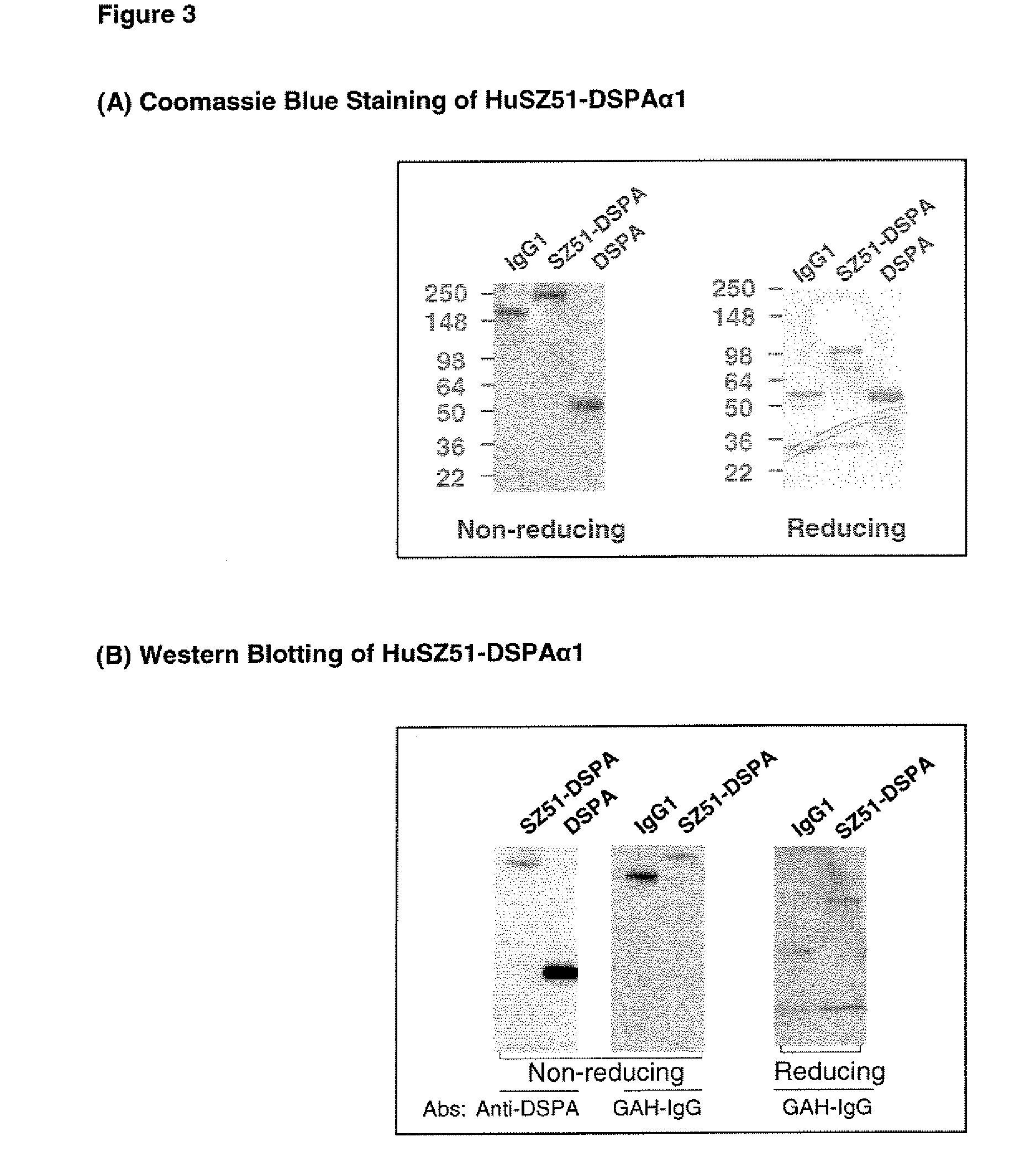
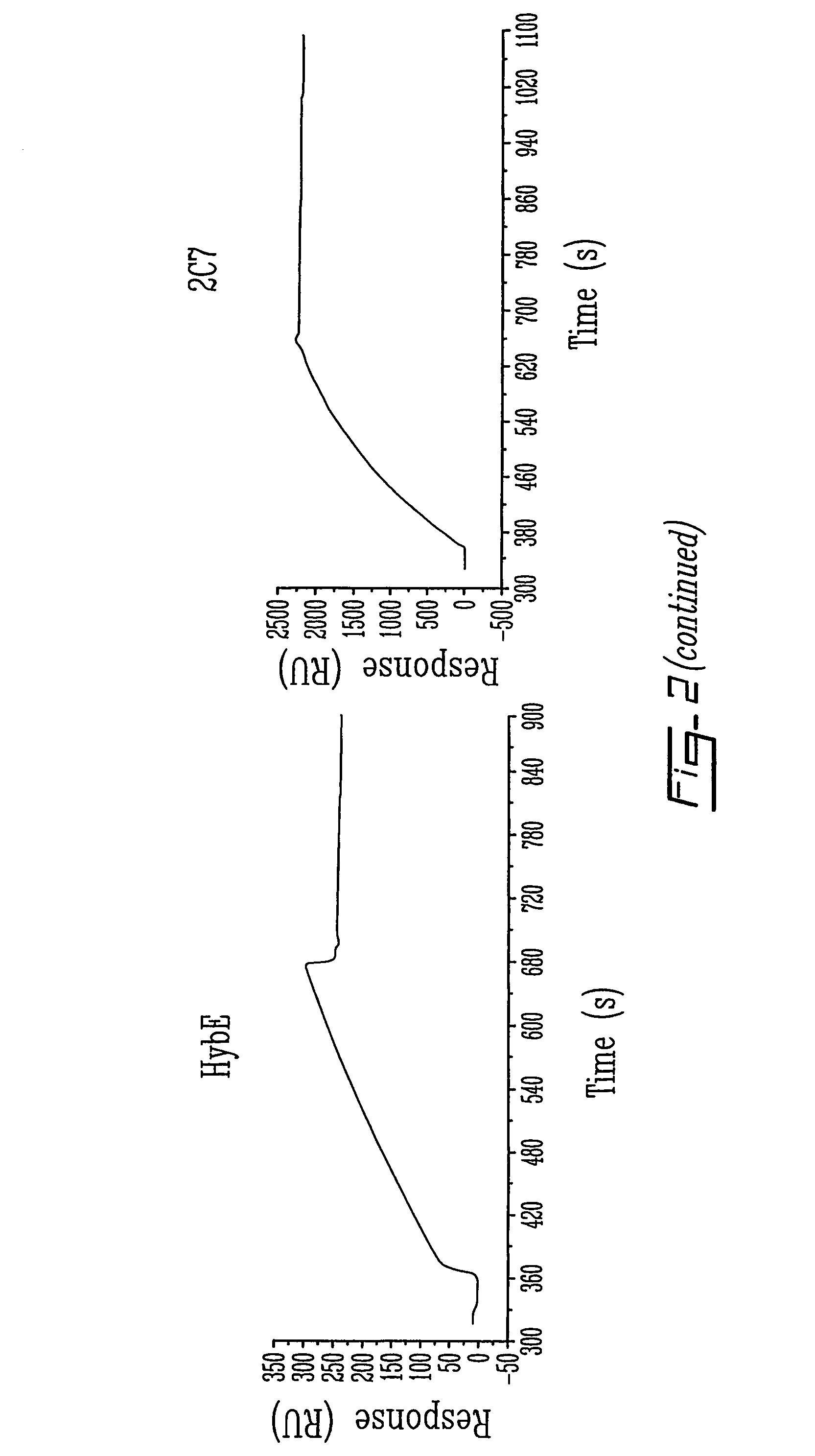
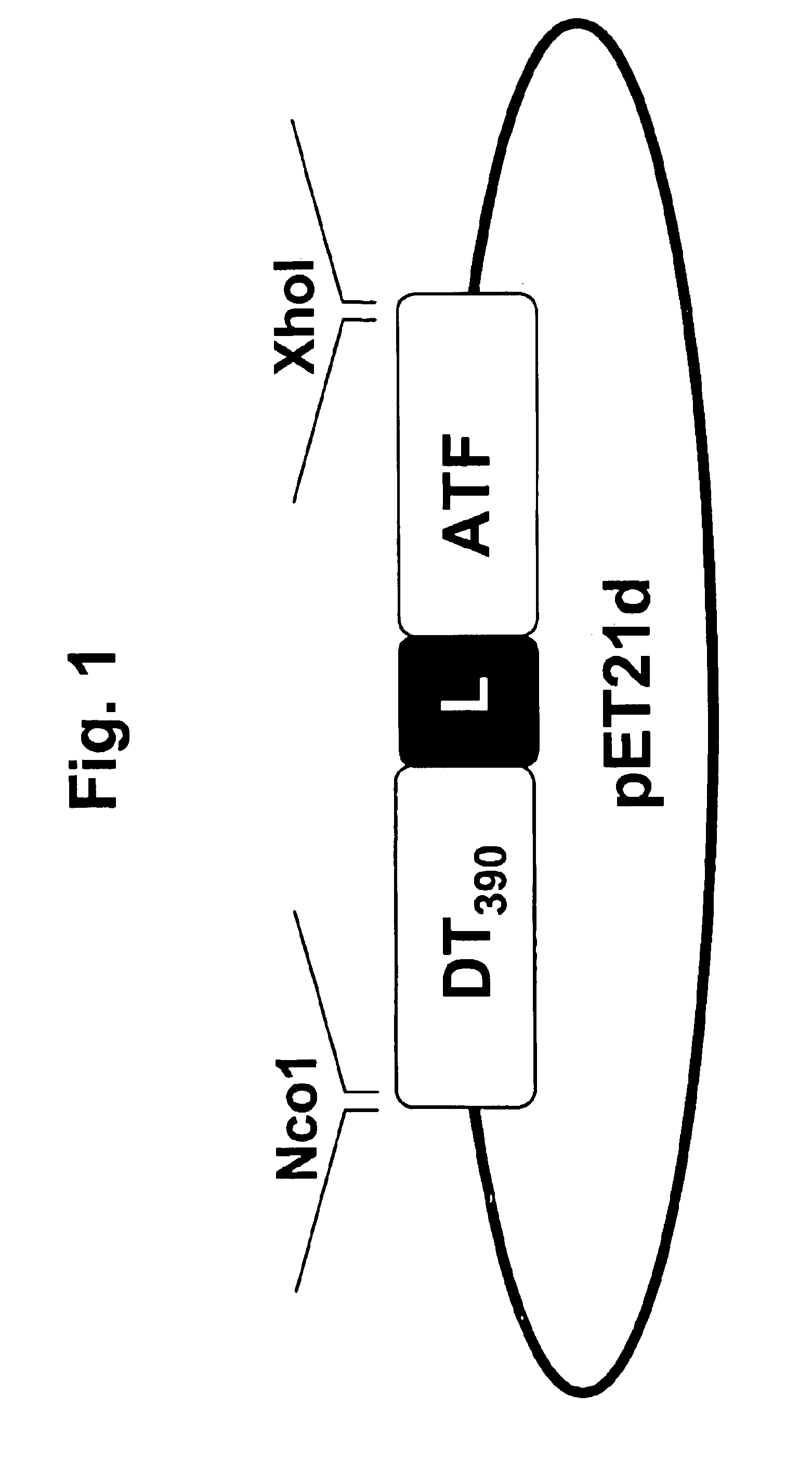
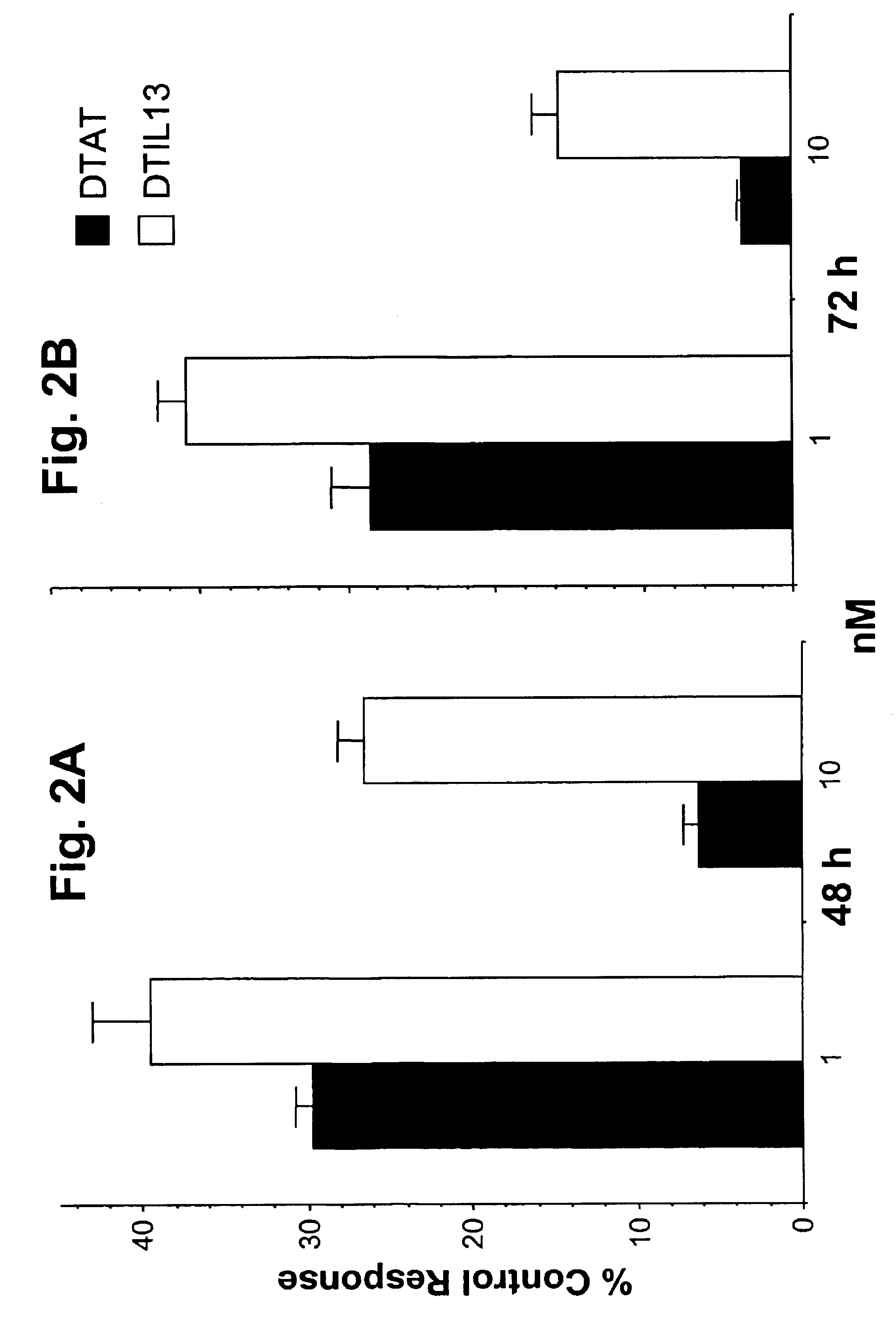
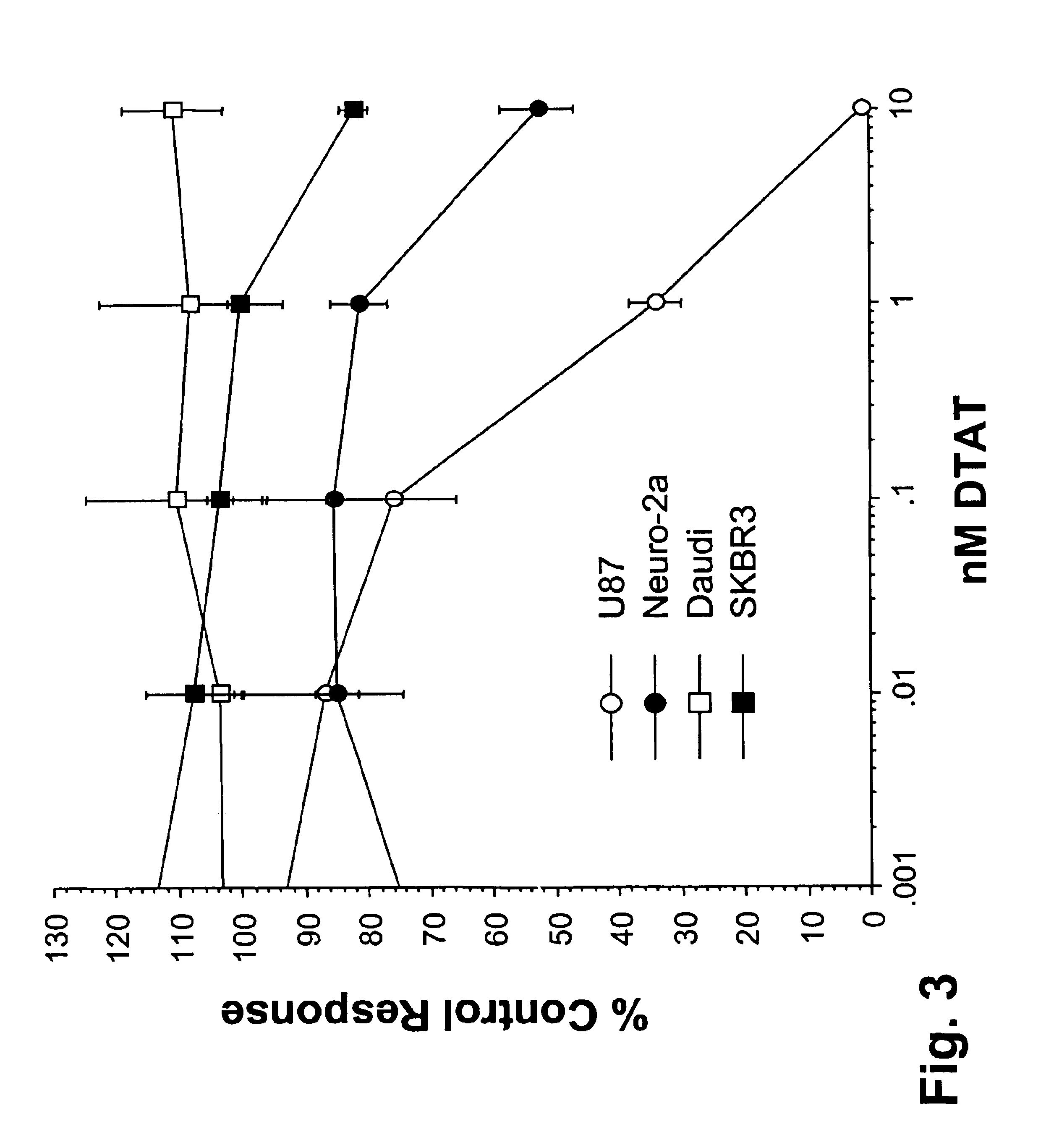
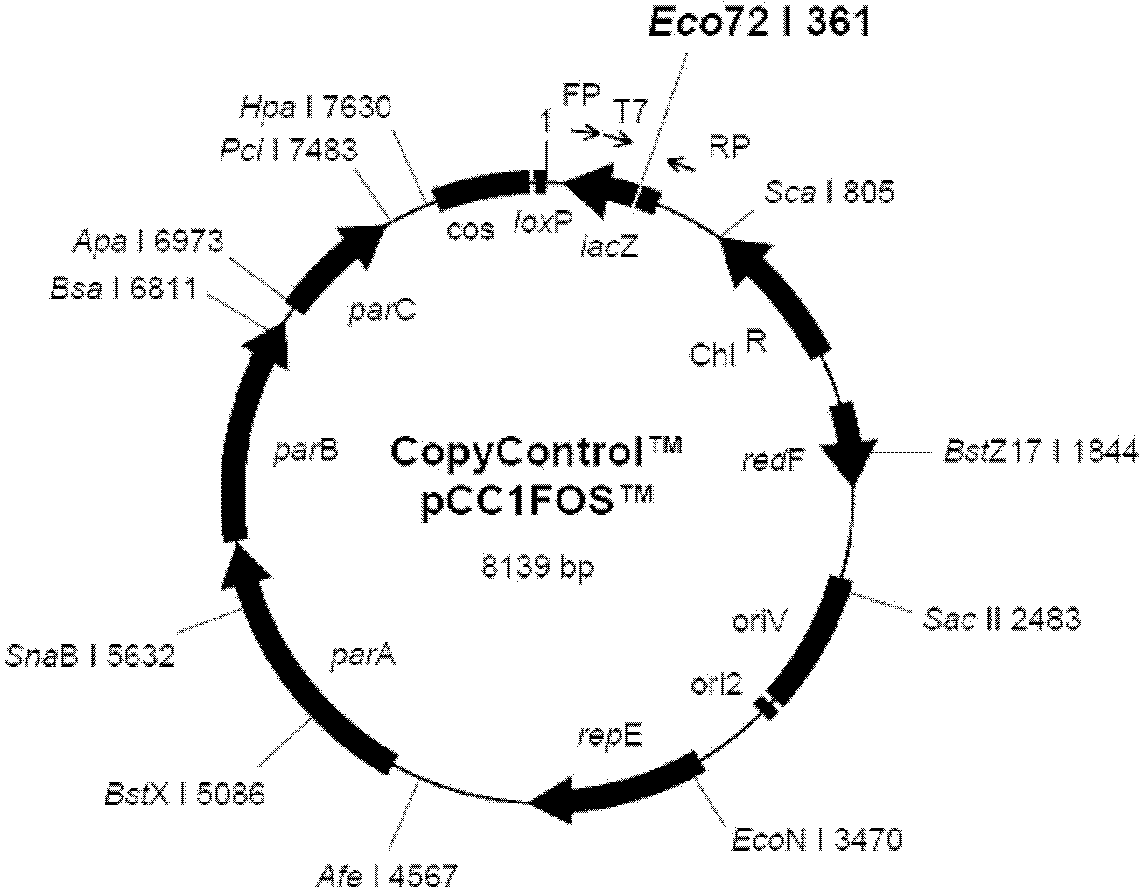
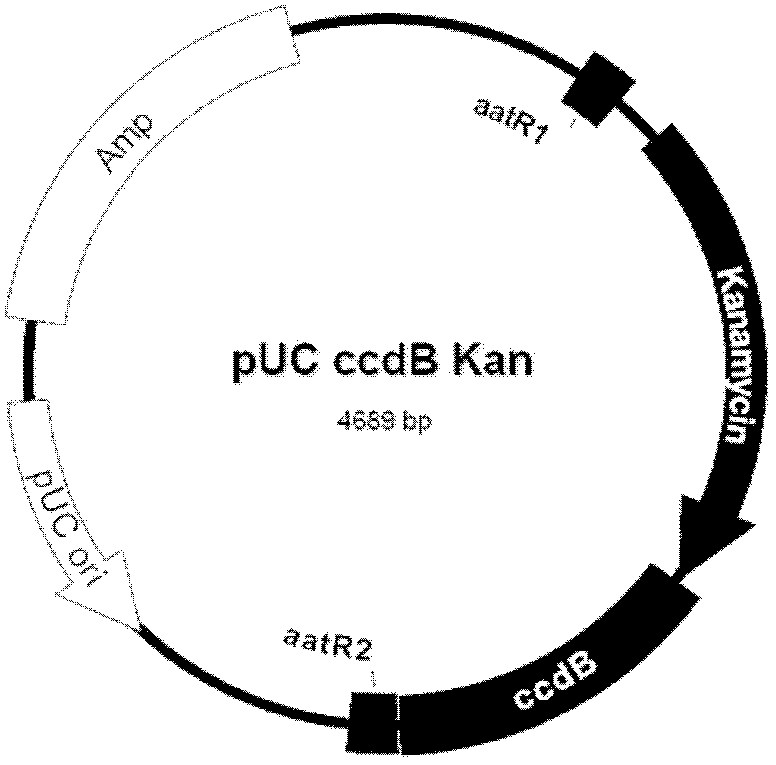
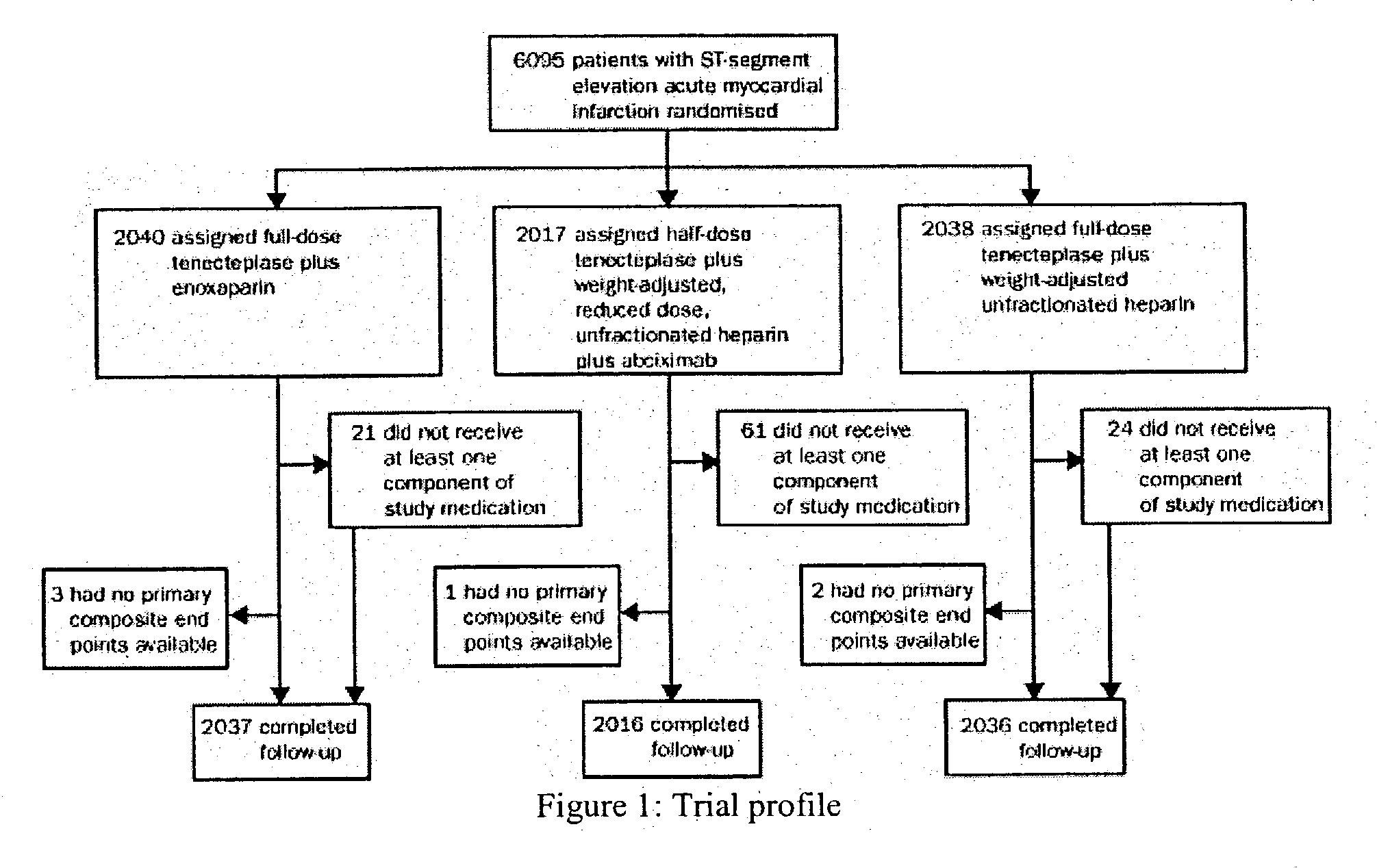
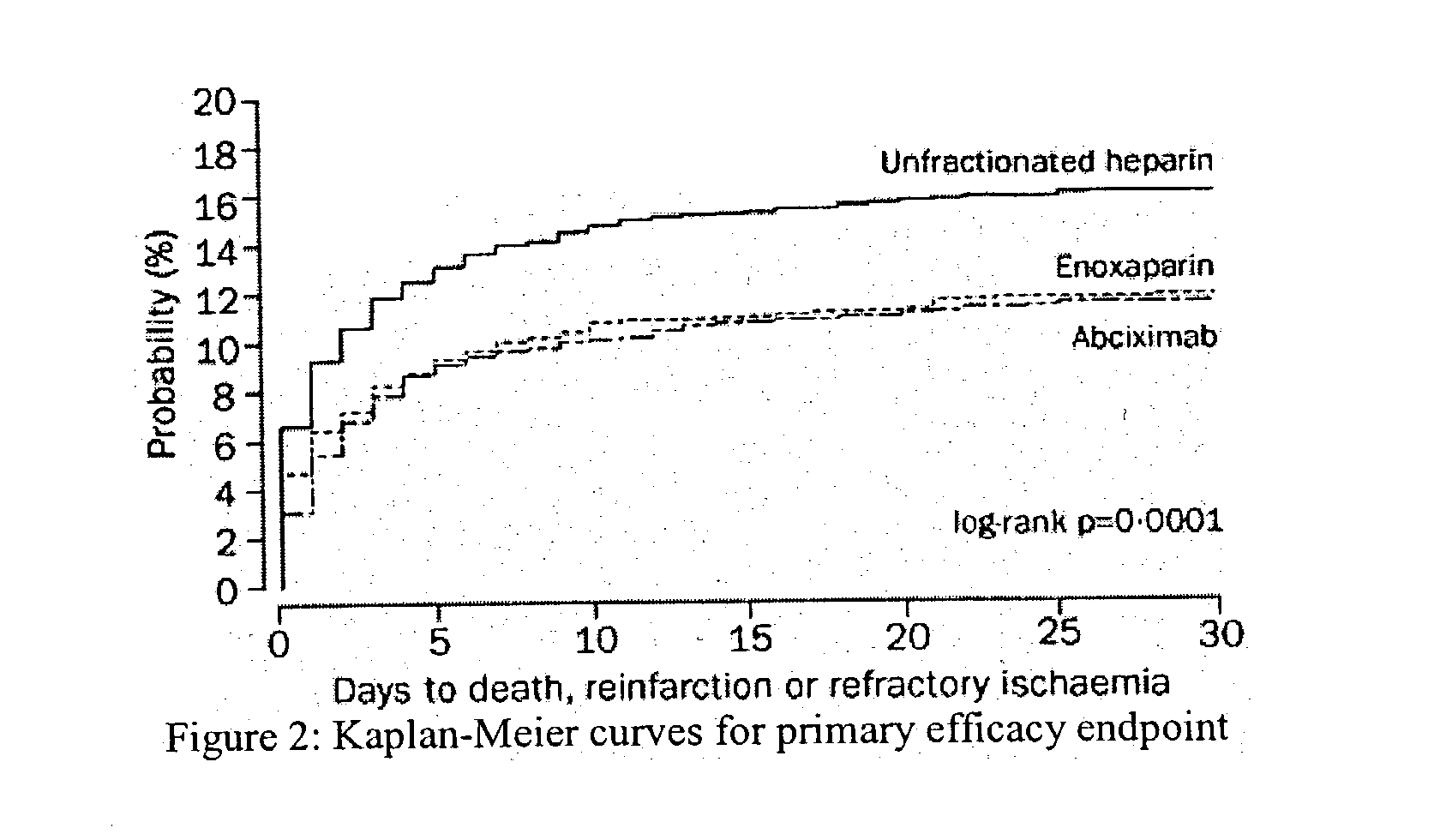
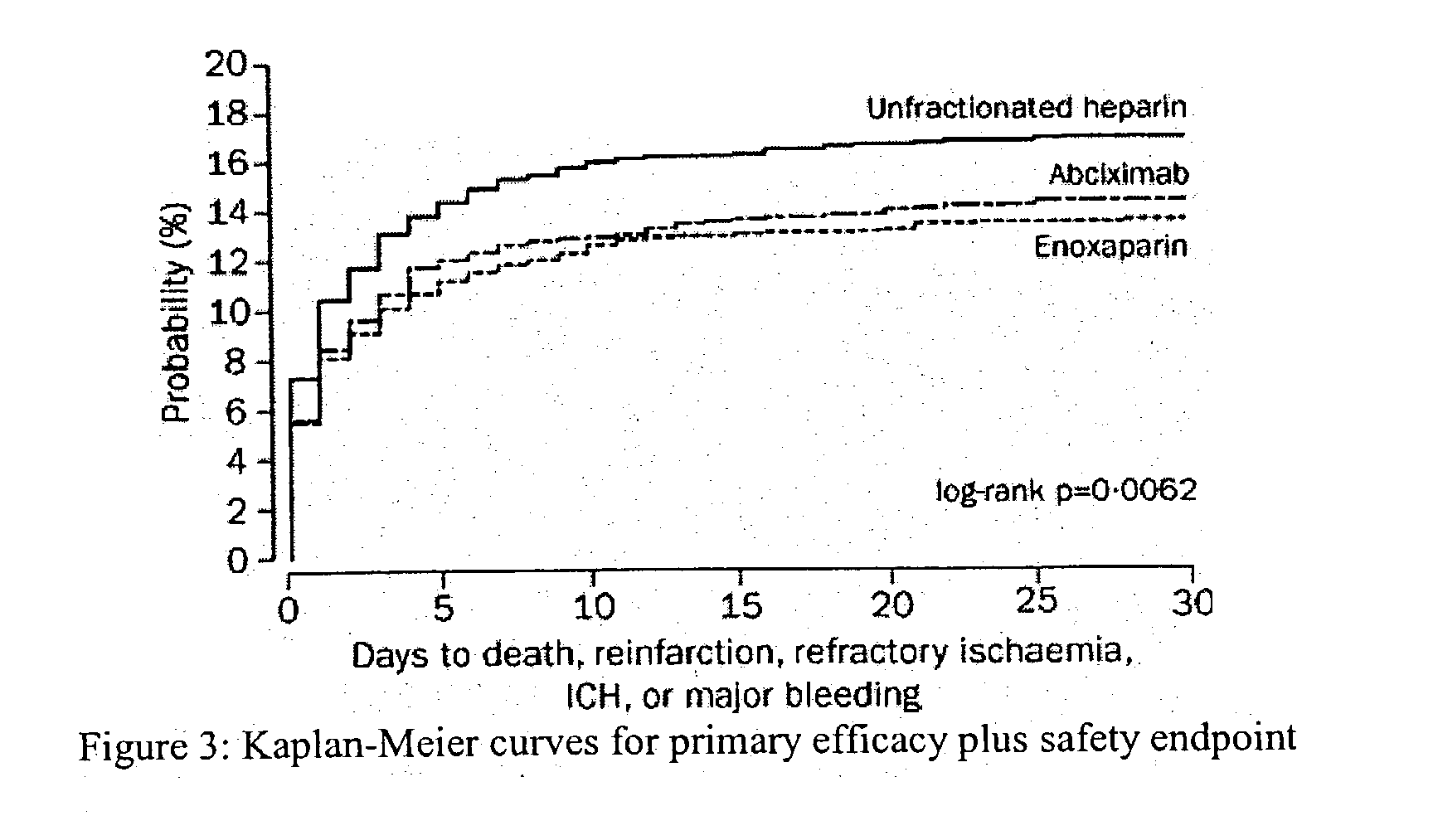
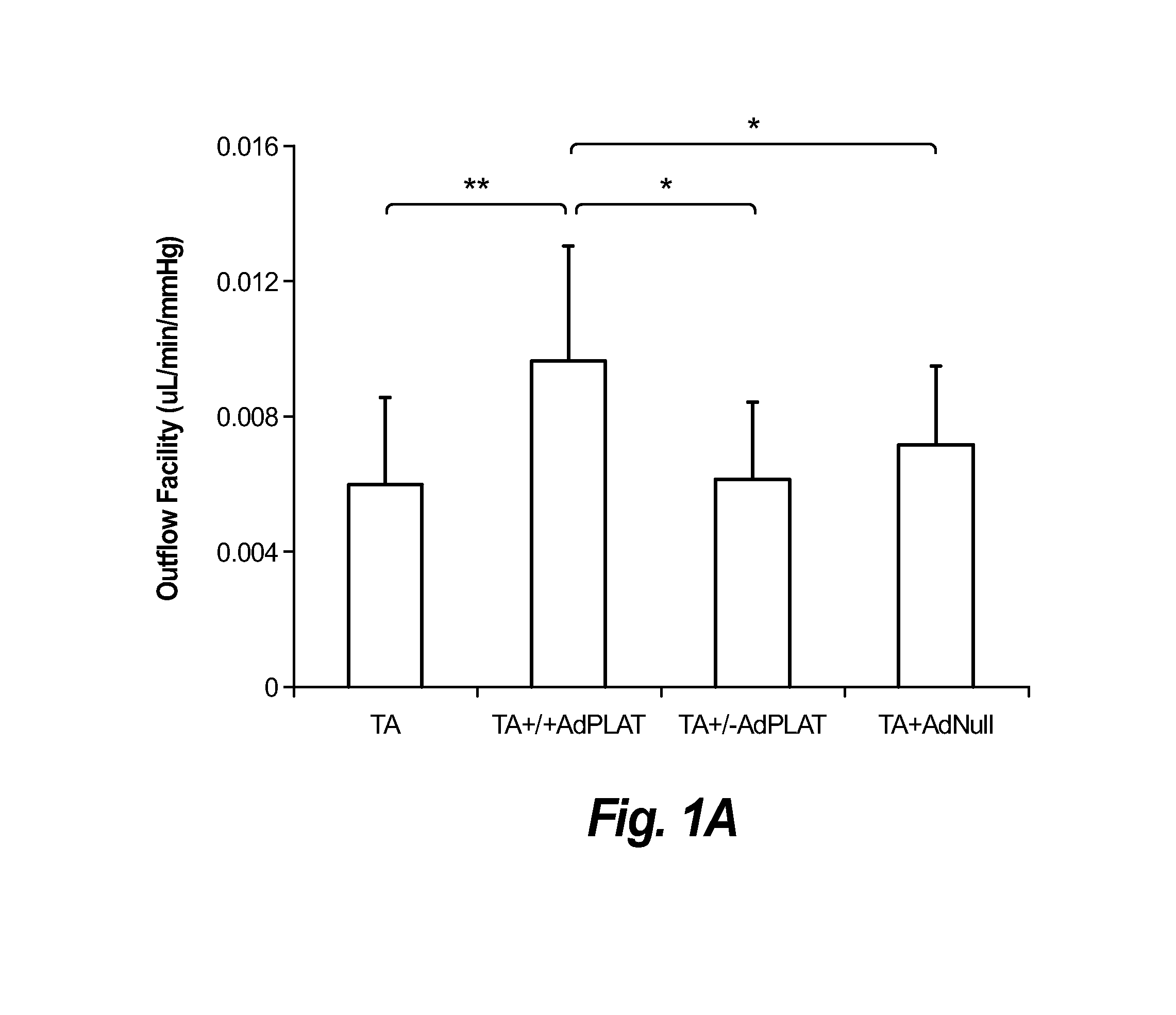
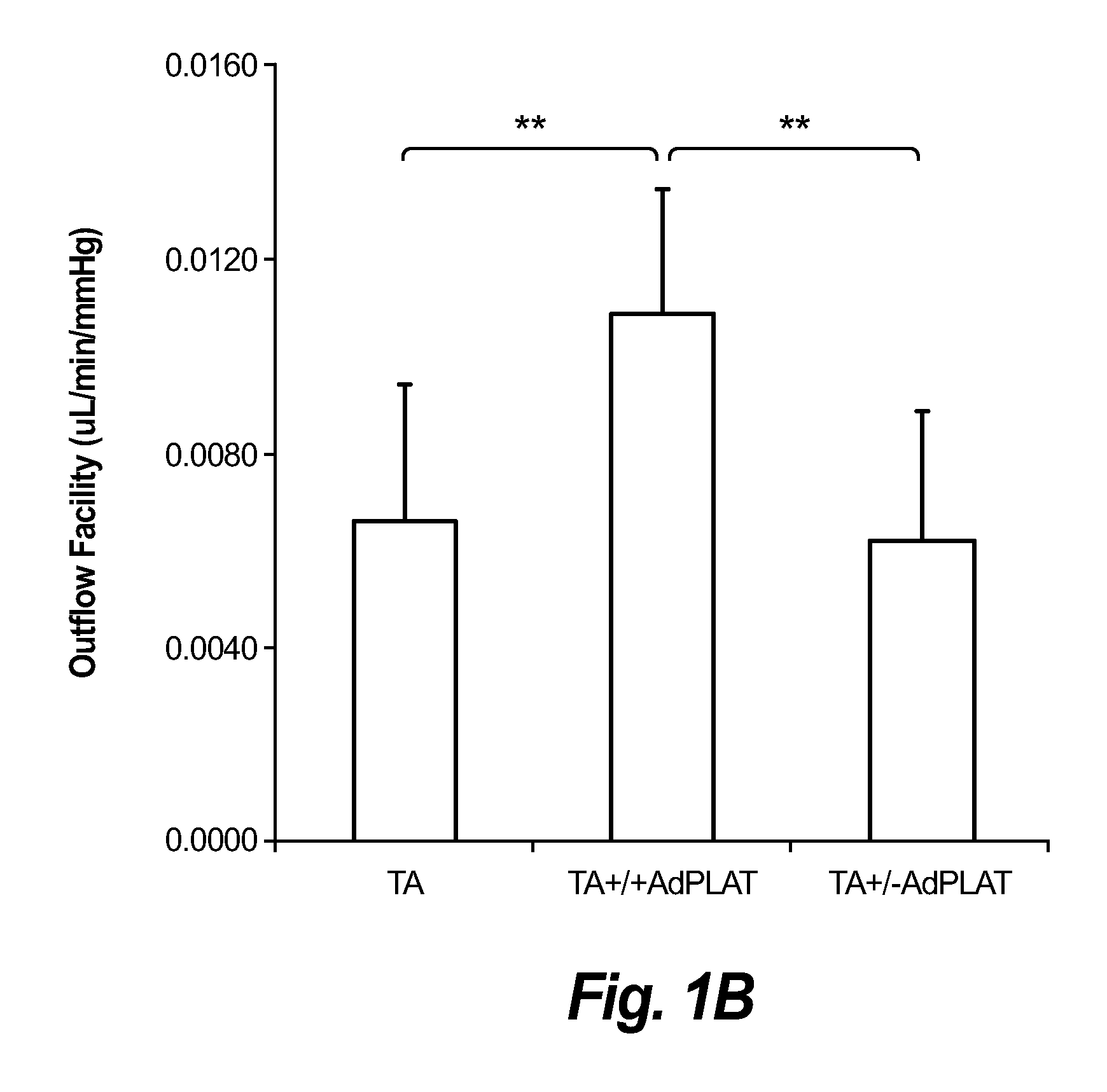
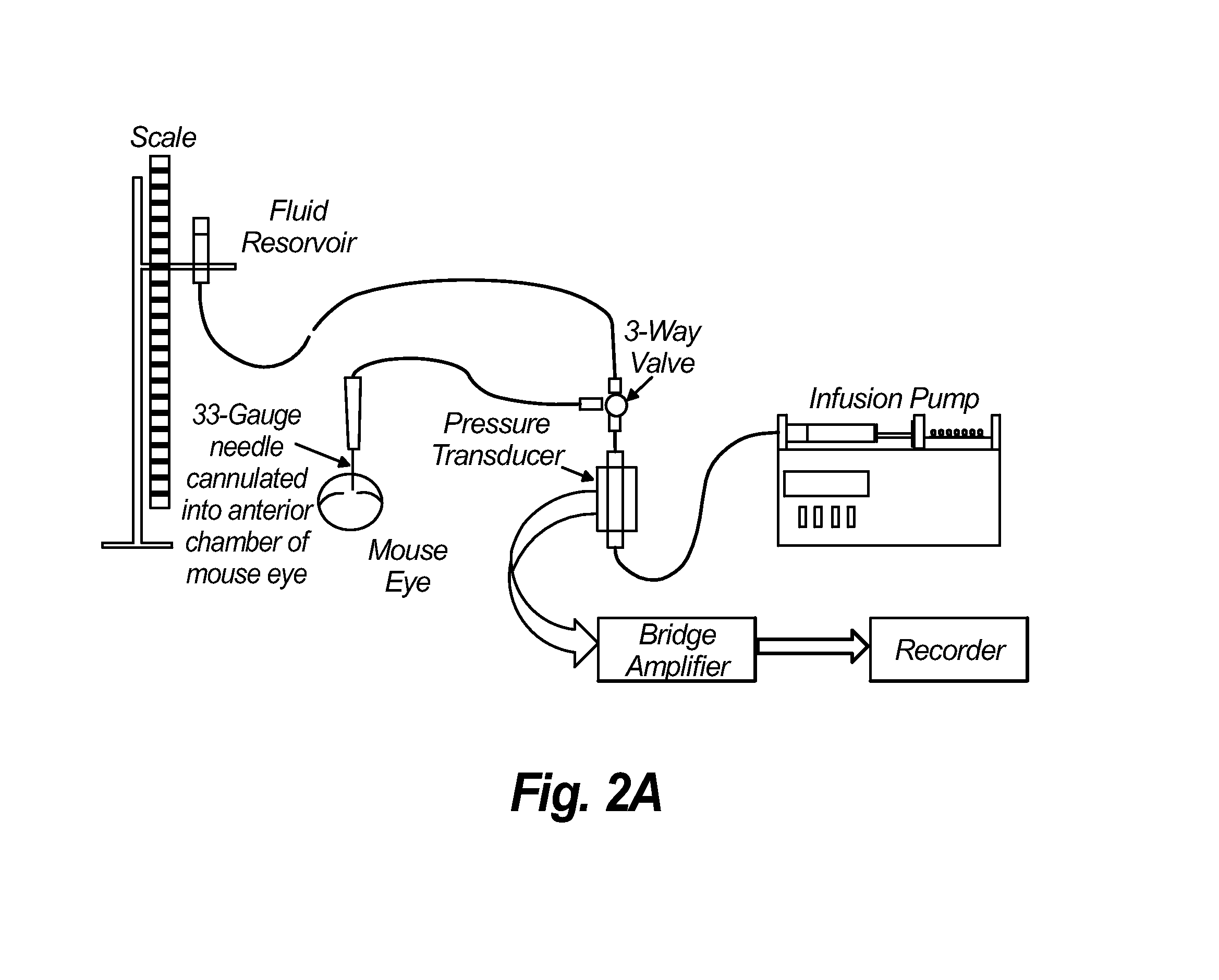
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Tissue plasminogen activator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tissue plasminogen activator (abbreviated tPA or PLAT) is a protein involved in the breakdown of blood clots. It is a serine protease (EC 3.4.21.68) found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Human tPA has a molecular weight of ~70 kDa in the single-chain form.
Methods for producing soluble, biologically-active disulfide-bond containing eukaryotic proteins in bacterial cells
InactiveUS6027888AEfficient productionFold preciselyPeptide/protein ingredientsMicroorganismsDisulfide bondingZymogen
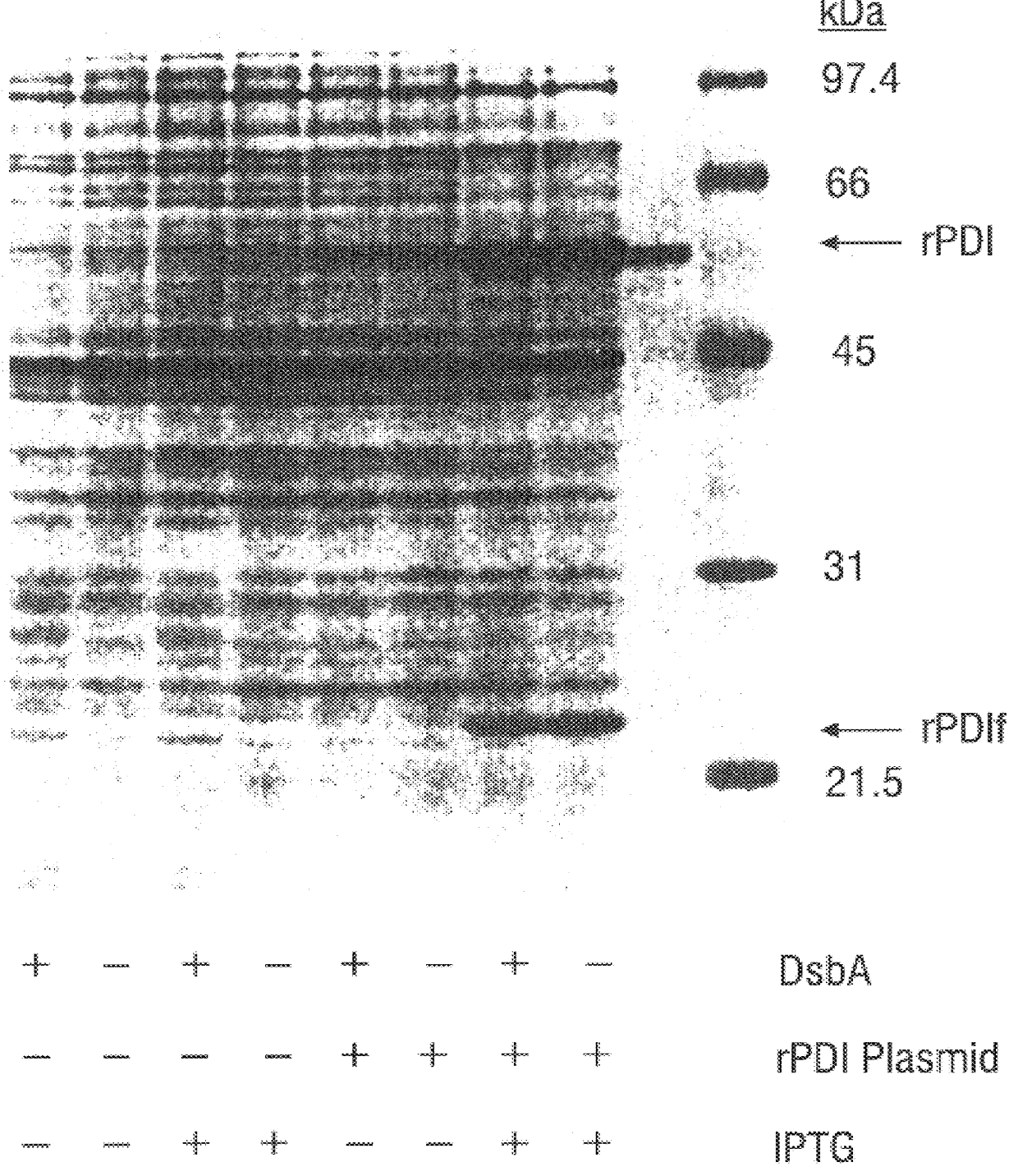
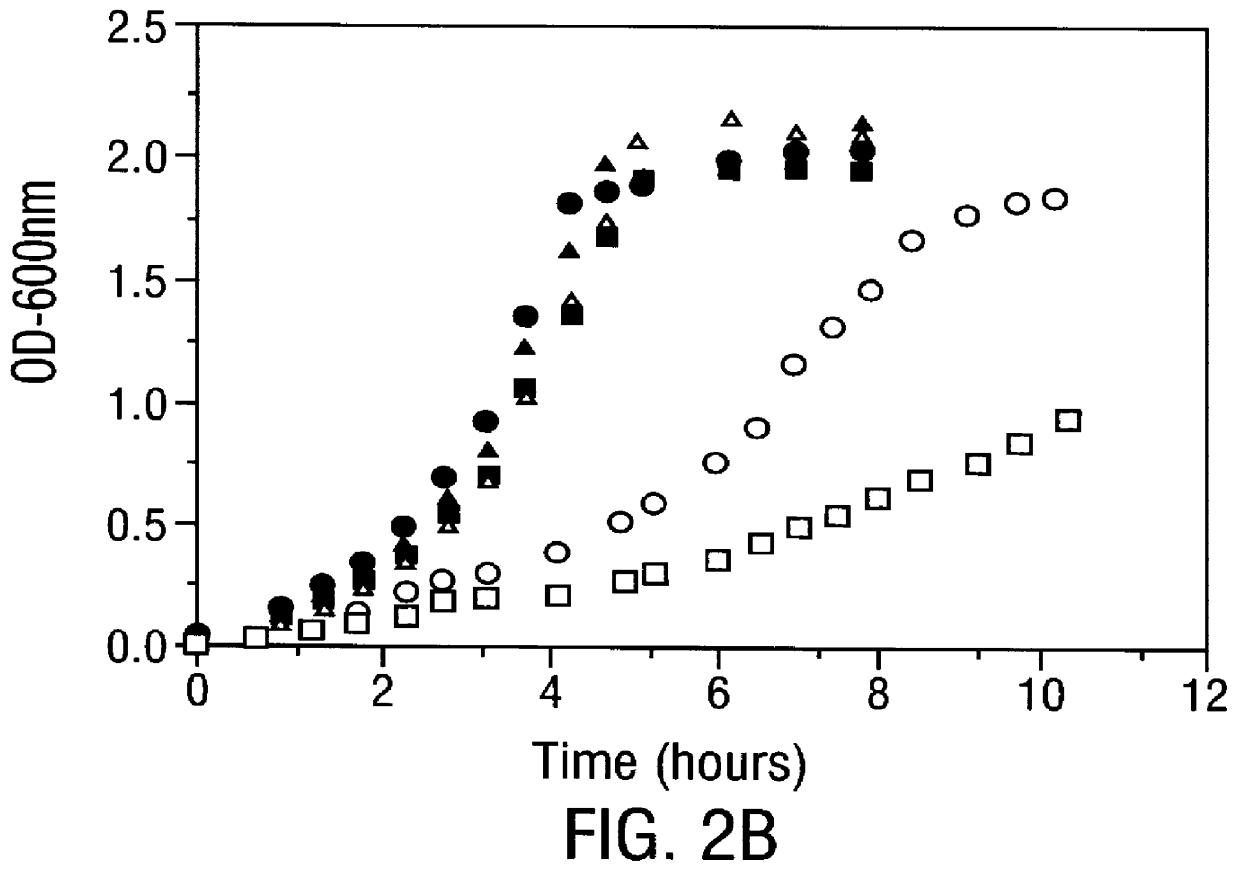
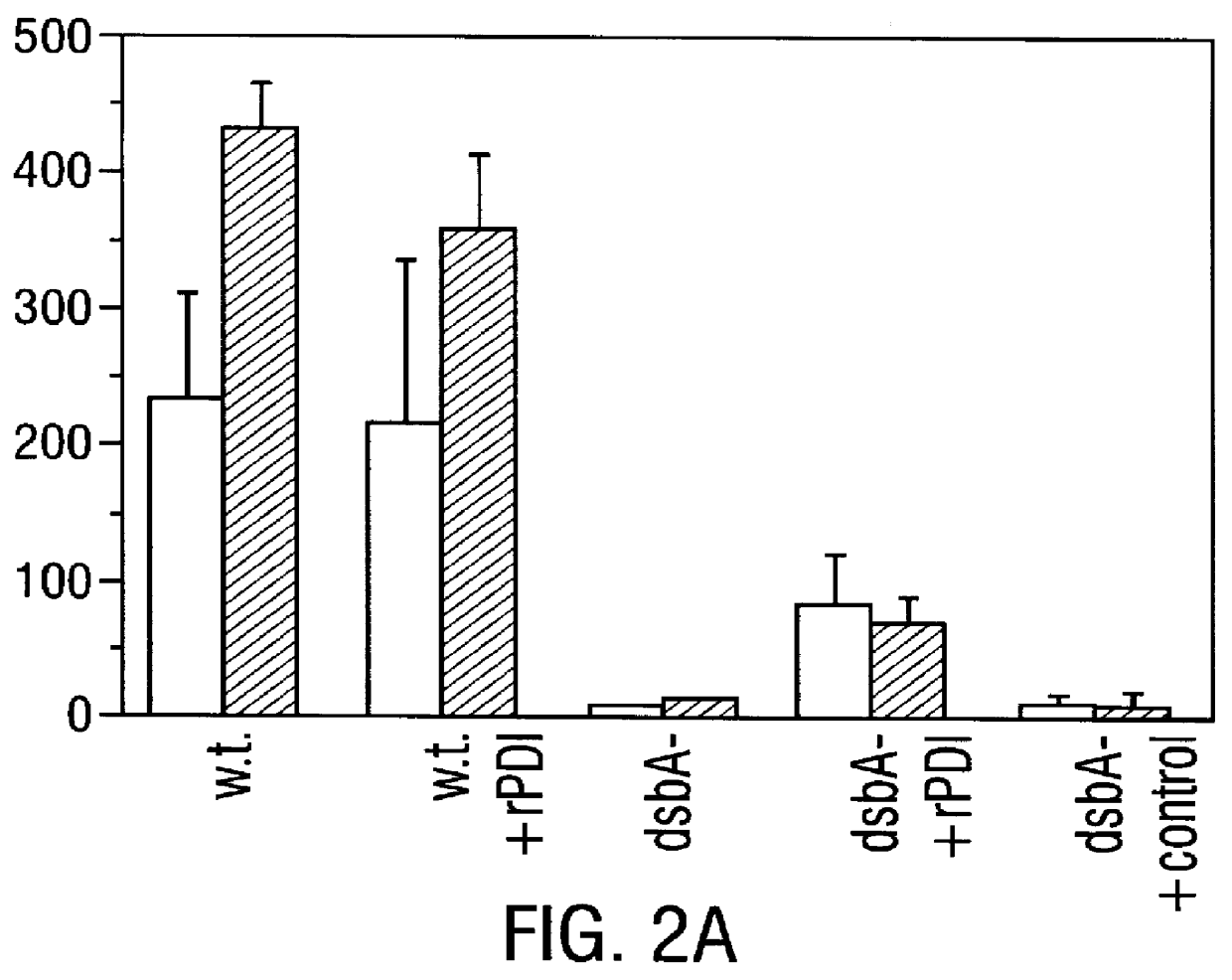
Disclosed are methods of producing eukaryotic disulfide bond-containing polypeptides in bacterial hosts, and compositions resulting therefrom. Co-expression of a eukaryotic foldase and a disulfide bond-containing polypeptide in a bacterial host cell is demonstrated. In particular embodiments, the methods have been used to produce mammalian pancreatic trypsin inhibitor and tissue plasminogen activator (tPA) in soluble, biologically-active forms, which are isolatable from the bacterial periplasm. Also disclosed are expression systems, recombinant vectors, and transformed host cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and methods for enhancing structural and functional nervous system reorganization and recovery
InactiveUS20060104969A1Improve the degradation problemSimple structurePeptide/protein ingredientsInorganic phosphorous active ingredientsNervous systemDelivery vehicle
The present invention provides methods and compositions for enhancing recovery in a subject suffering from damage to the nervous system. In particular, the invention includes a method for promoting recovery and / or reorganization in the nervous system of a subject in need of enhancement of recovery and / or reorganization of the nervous system as a result of ischemic, hemorrhagic, neoplastic, degenerative, or traumatic damage by focally administering a composition comprising a proteolysis-enhancing agent such as tissue plasminogen activator (tPA), plasmin, or a PAI inhibitor to the nervous system of the subject. In some embodiments an additional active agent is also administered. The composition can be delivered using a variety of techniques including injection, via infusion pump, from an implantable microchip, or using a polymeric delivery vehicle. The composition can be administered, for example, to one or more subdivisions or areas of the brain, the spinal cord, or to one or more nerves or nerve tracts innervating diverse regions of the body. The invention also includes a drug delivery device for implantation into the nervous system to promote nervous system reorganization and / or recovery following ischemic, hemorrhagic, neoplastic, traumatic or degenerative damage, the drug delivery device comprising a biocompatible polymer and a proteolysis-enhancing agent such as tissue plasminogen activator (tPA), plasmin, or a PAI inhibitor, wherein the proteolysis-enhancing agent is released from the polymer in an amount effective to promote structural reorganization of the nervous system. In some embodiments the biocompatible polymer is a hydrogel.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Process for generating plasmin in the vitreous of the eye and inducing separation of the posterior hyaloid from the retina
InactiveUS6899877B2Inhibiting retinal tearReducing and preventing effectSenses disorderEye implantsUrokinase Plasminogen ActivatorVascular proliferation
A process for inhibiting vascular proliferation separately introduces components into the eye to generate plasmin in the eye in amounts to induce complete posterior vitreous detachment where the vitreoretinal interface is devoid of cortical vitreous remnants. The process administers a combination of lysine-plasminogen, at least one recombinant plasminogen activator selected from the group consisting of urokinase, streptokinase, tissue plasminogen activator, chondroitinase, pro-urokinase, retavase, metaloproteinase, and thermolysin and a gaseous adjuvant to form a cavity in the vitreous. The composition is introduced into the vitreous in an amount effective to induce crosslinking of the vitreous and to induce substantially complete posterior vitreous detachment from the retina without causing inflammation of the retina. The gaseous adjuvant material is introduced into the vitreous simultaneously with or after the lysine-plasminogen and recombinant plasminogen activator such as recombinant urokinase to compress the vitreous against the retina while the composition induces the complete posterior vitreous detachment.
Owner:MINU
Flow electroporation chamber and method
InactiveUS20050019311A1Low affinityIncreased oxygen deliveryBiocideOther blood circulation devicesStreptokinaseMedicine
The present invention relates to a method and apparatus for the encapsulation of substances and drugs into cells and platelets. The present invention is also related to the incorporation of thrombus dissolving drugs, such as tissue plasminogen activator and streptokinase into platelets using the apparatus described herein. The treated platelets can then be used to treat patients suffering from a thrombus blocking a blood vessel. The present invention is also related to a preparation of red blood cells that has a stable right shift of the oxygen dissociation curve.
Owner:HOLADAY JOHN W +4
Substituted azetidinones
Owner:EXITHERA PHARMA
Peptide Inhibition of Lung Epithelial Apoptosis and Pulmonary Fibrosis
ActiveUS20090227515A1High expressionBlock bleo induced mouse lung fibrosisPolypeptide with localisation/targeting motifCell receptors/surface-antigens/surface-determinantsIn vivoPulmonary fibrosis
During lung injury, p53 expression increases, inducing plasminogen activator inhibitor-1 (PAI-1) while inhibiting expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR), resulting in apoptosis of lung epithelial cells (LECs). In the bleomycin lung injury model, p53 and PAI-1 are induced while uPA and uPAR are inhibited. A 20 residue peptide DGIWKASFTTFTVTKYWFYR termed PP-1 (the Cav-1 scaffolding domain) or peptide NYHYLESSMTALYTLGH, termed PP-2, protected LECs from bleomycin-induced apoptosis in vitro and in vivo and prevented subsequent pulmonary fibrosis by attenuating lung epitheilial damage. Pharmaceutical compositions, peptide multimers and deliverable polypeptides comprising the above peptides are dislcosed. The peptides and functional variants, peptide multimers, cell-targeted polyepeptides and pharmaceutical compositions are used in methods for inhibiting apoptosis of injured or damaged lung epithelial cells and for treating acute lung injury and consequent pulmonary fibrosis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
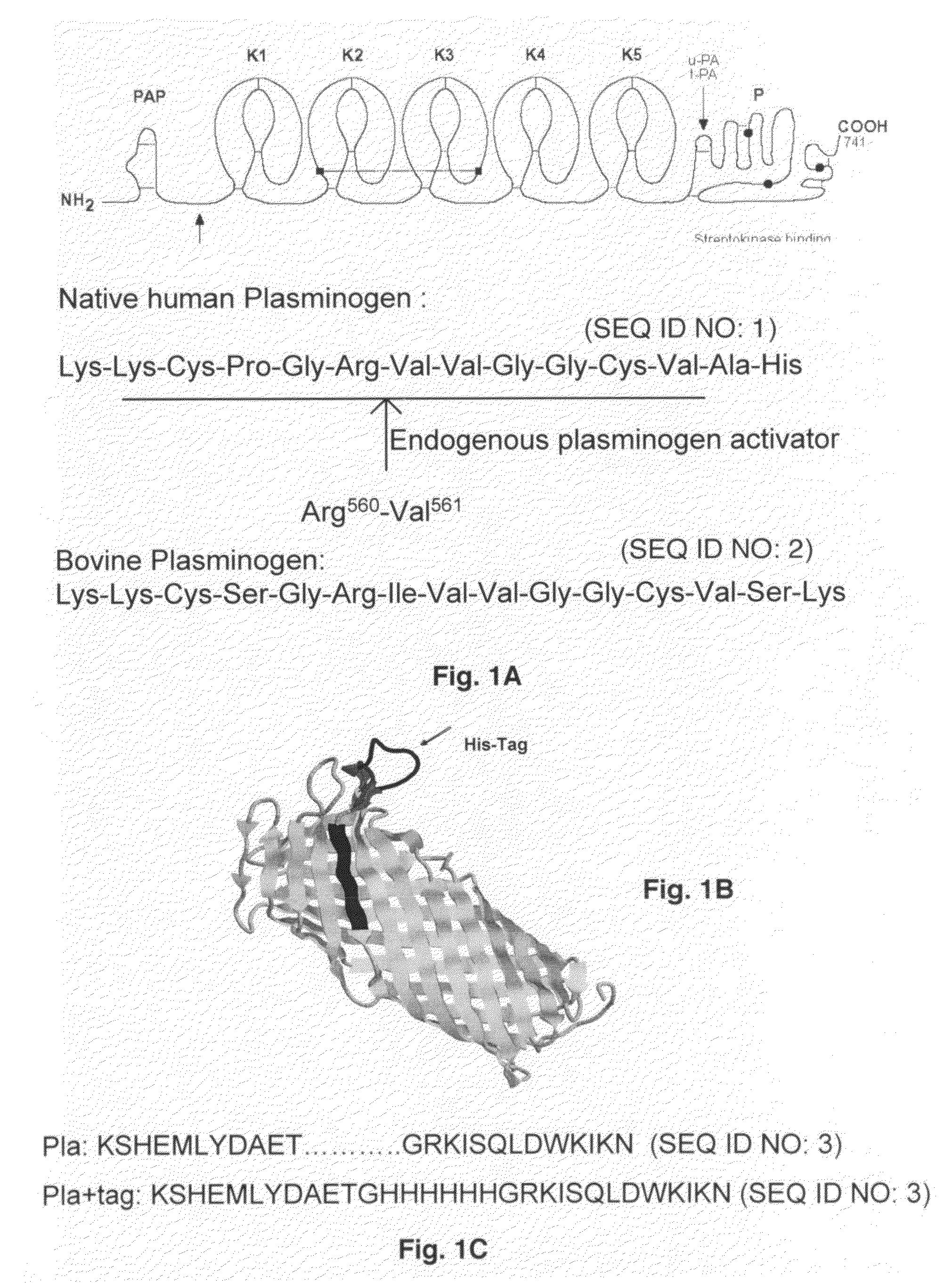
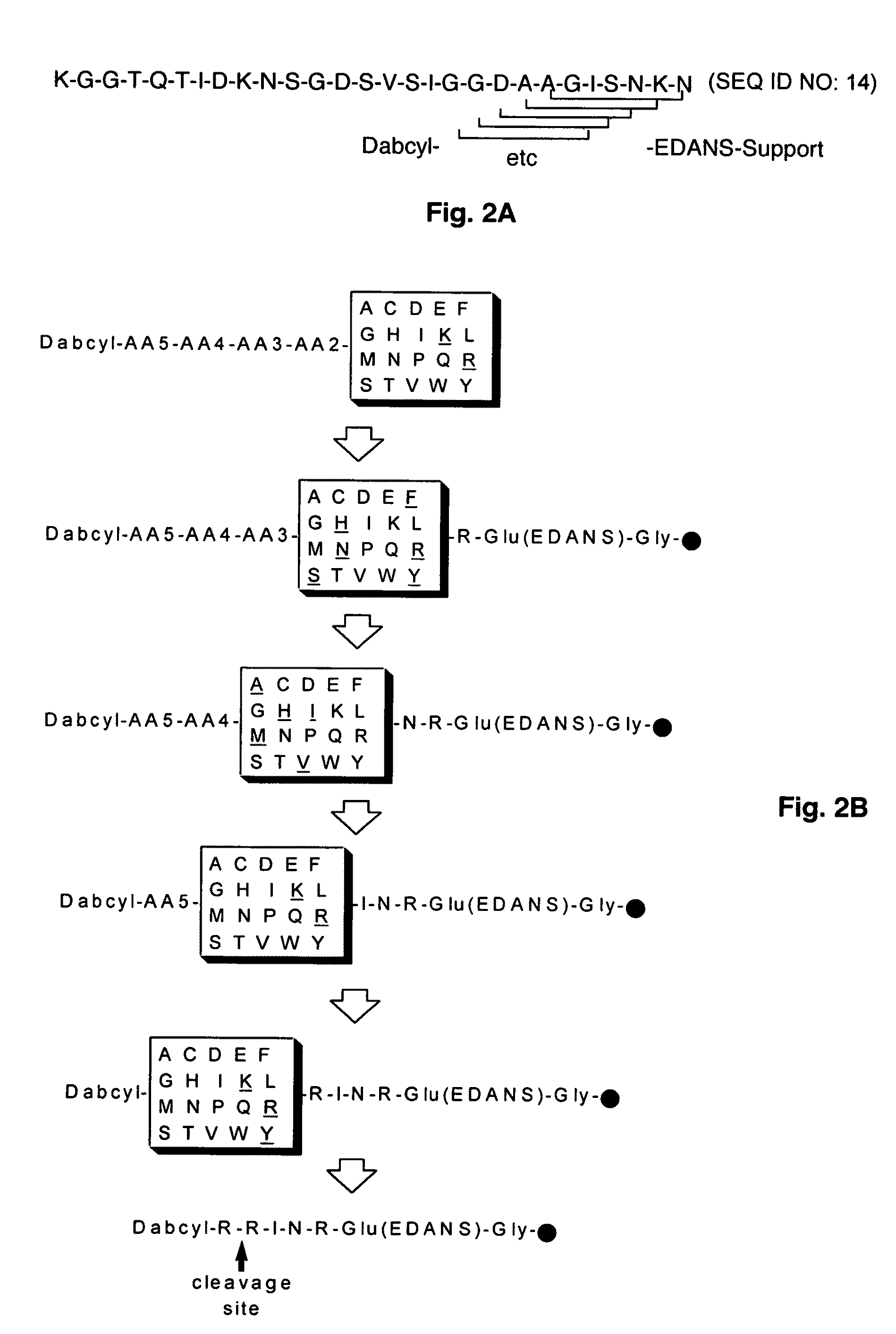
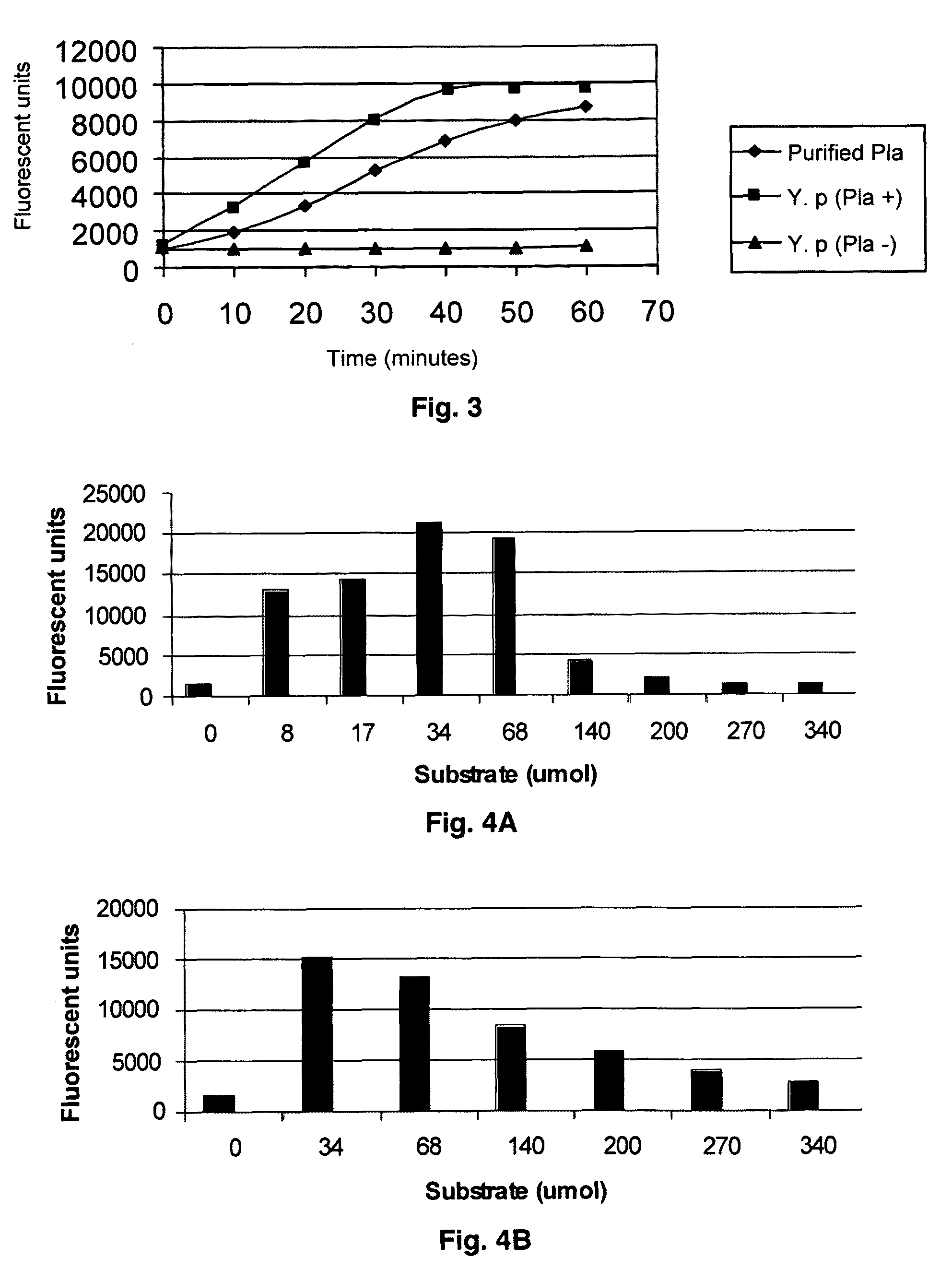
Substrate peptide sequences for plague plasminogen activator and uses thereof
ActiveUS8030447B2Antibacterial agentsMicrobiological testing/measurementProteinase activityPeptide sequence
The present invention is directed to peptide sequences that were identified from combinatorial libraries and could serve as substrates of plague plasminogen activator (Pla). Another aspect of the present invention is drawn to peptides derived from the substrates for Pla as a result of chemical modifications leading to specific inactivation of the proteolytic activity of Pla. Additionally, the present invention is directed to the use of the substrates identified herein in the detection of bacteria expressing omptin family of proteases which includes Y. pestis. Furthermore, the present invention is also directed to the use of the inhibitors identified herein in the prevention and treatment of infection caused by these bacteria.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
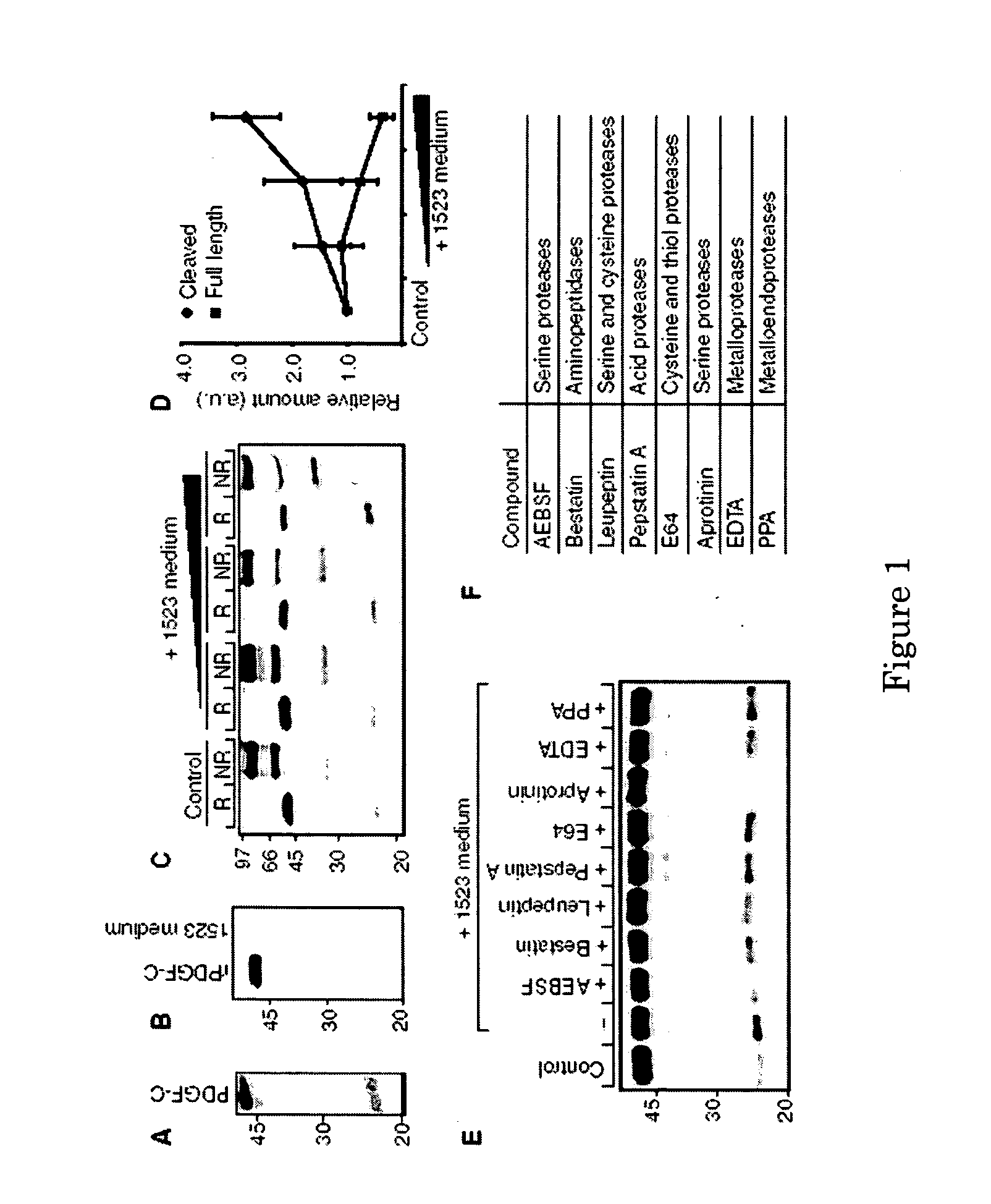
Methods and compositions for PDGF-C activation and inhibition
InactiveUS20060104978A1Inhibits downstream signallingPromote angiogenesisImmunoglobulins against growth factorsAntibody ingredientsTPA InhibitorsBiological activation
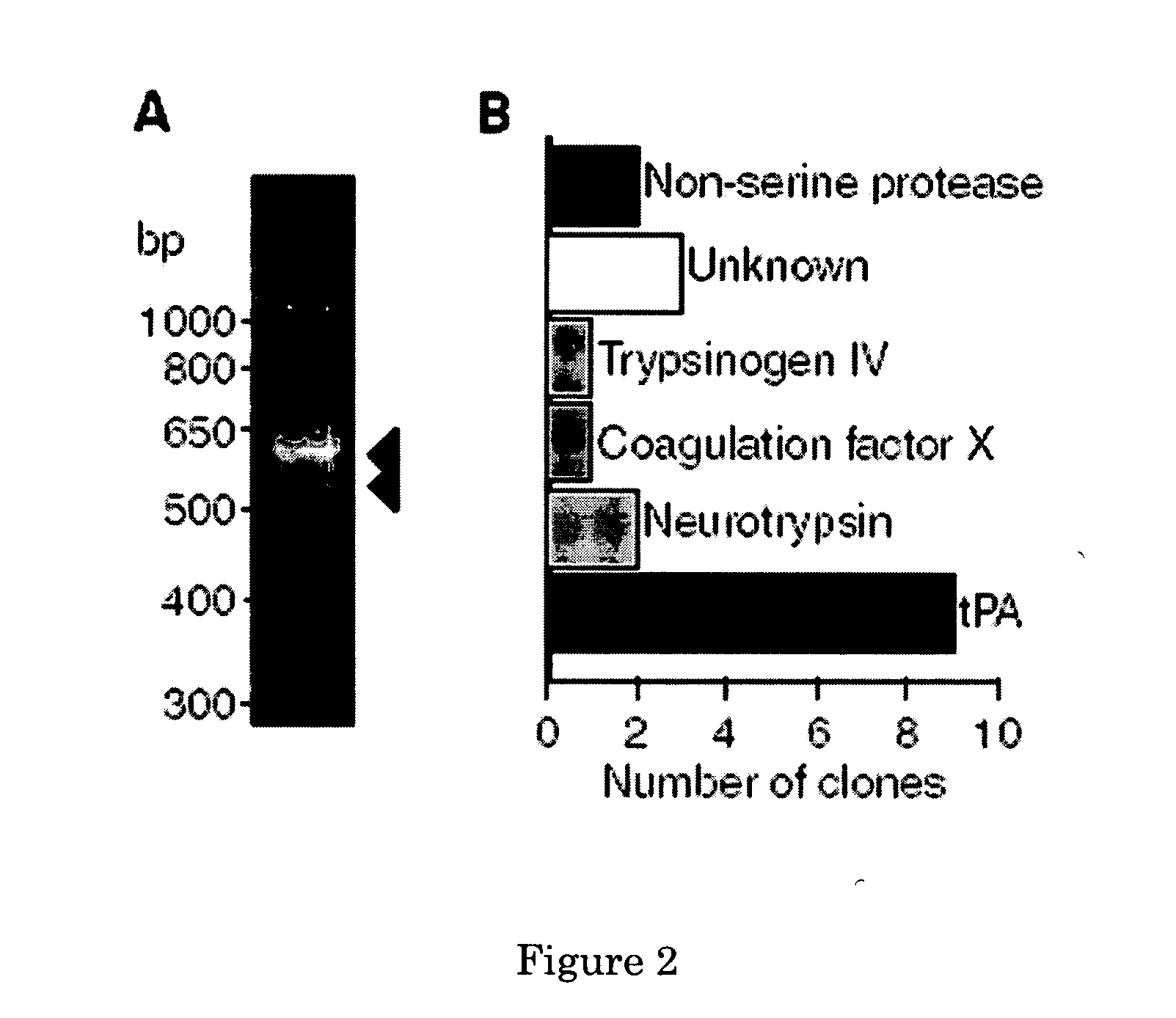
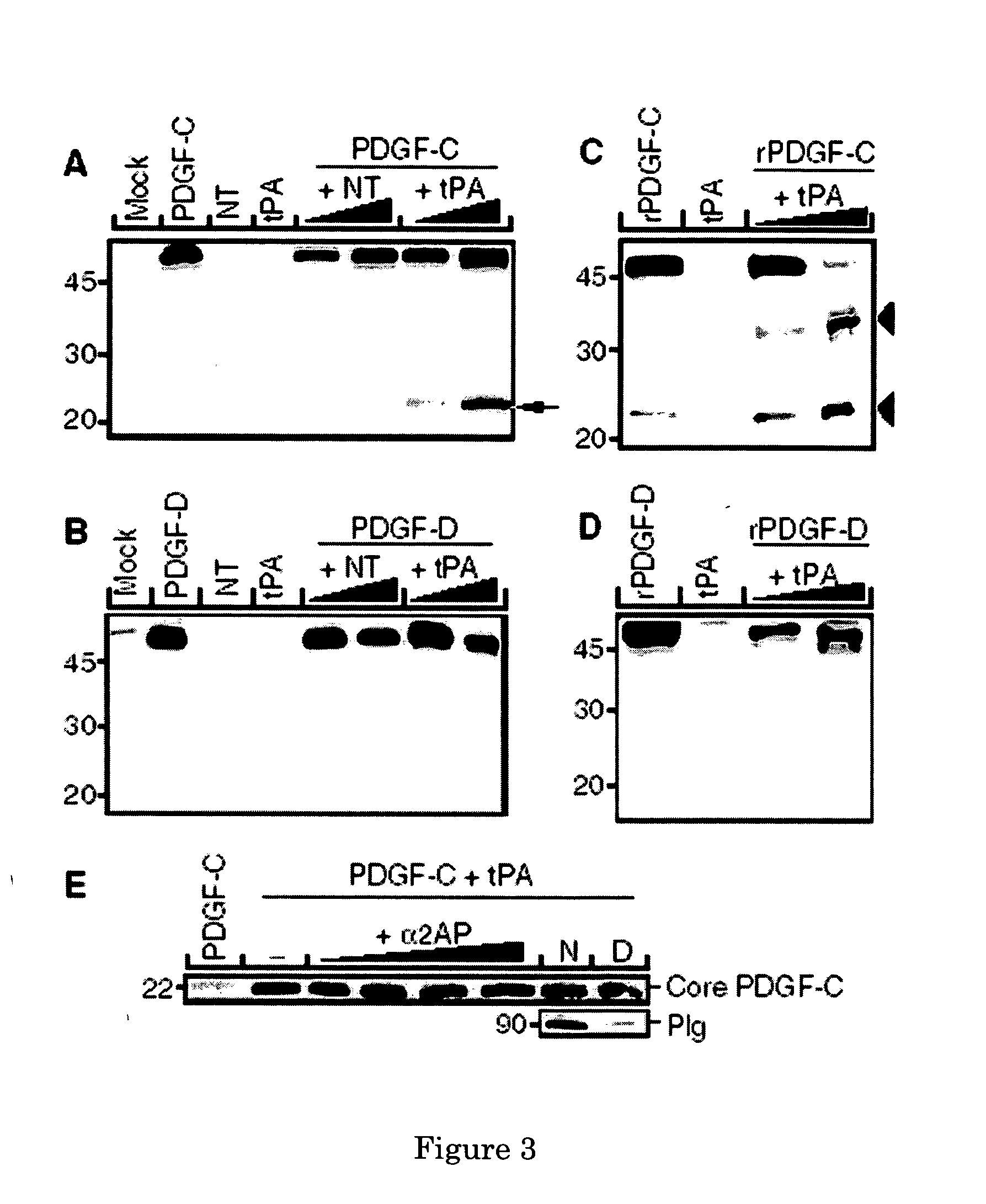
Methods for inhibiting angiogenesis comprising administering tissue-plasminogen activator (tPA) inhibitors, and pharmaceutical compositions suitable for the methods comprising the tPA inhibitors. Also provided are methods for stimulating angiogenesis comprising administering tPA to a patient in need thereof, and pharmaceutical compositions comprising an effective amount of tPA for the methods of stimulation. The present invention discloses that tPA is a specific PDGF-C activating protease, and that the CUB-domains in PDGF-CC directly interact with the protease, are required for efficient proteolysis, and released CUB-domains are tPA inhibitors. Preferably, the method and compositions of the present invention are used for simultaneously stimulating, or simultaneously inhibiting, thrombolysis and angiogenesis.
Owner:LUDWIG INST FOR CANCER RES
Combination treatment with t-PA variant and low molecular weight heparin
InactiveUS7084118B2Prolonged Circulatory Half-LifeRetained fibrin bindingData processing applicationsFibrinogenPLG - PlasminogenRegimen
The invention concerns an improved therapeutic regimen for the treatment of thrombolytic disorders, such as acute myocardial infarction (AMI). In particular, the present invention concerns the treatment of thrombolytic disorders, e.g. AMI, with a combination of a tissue plasminogen activator (t-PA) variant having improved fibrin specificity and extended plasma half-life when compared with wild-type human t-PA and a low molecular weight heparin.
Owner:AVENTIS PHARMA SA (US) +2
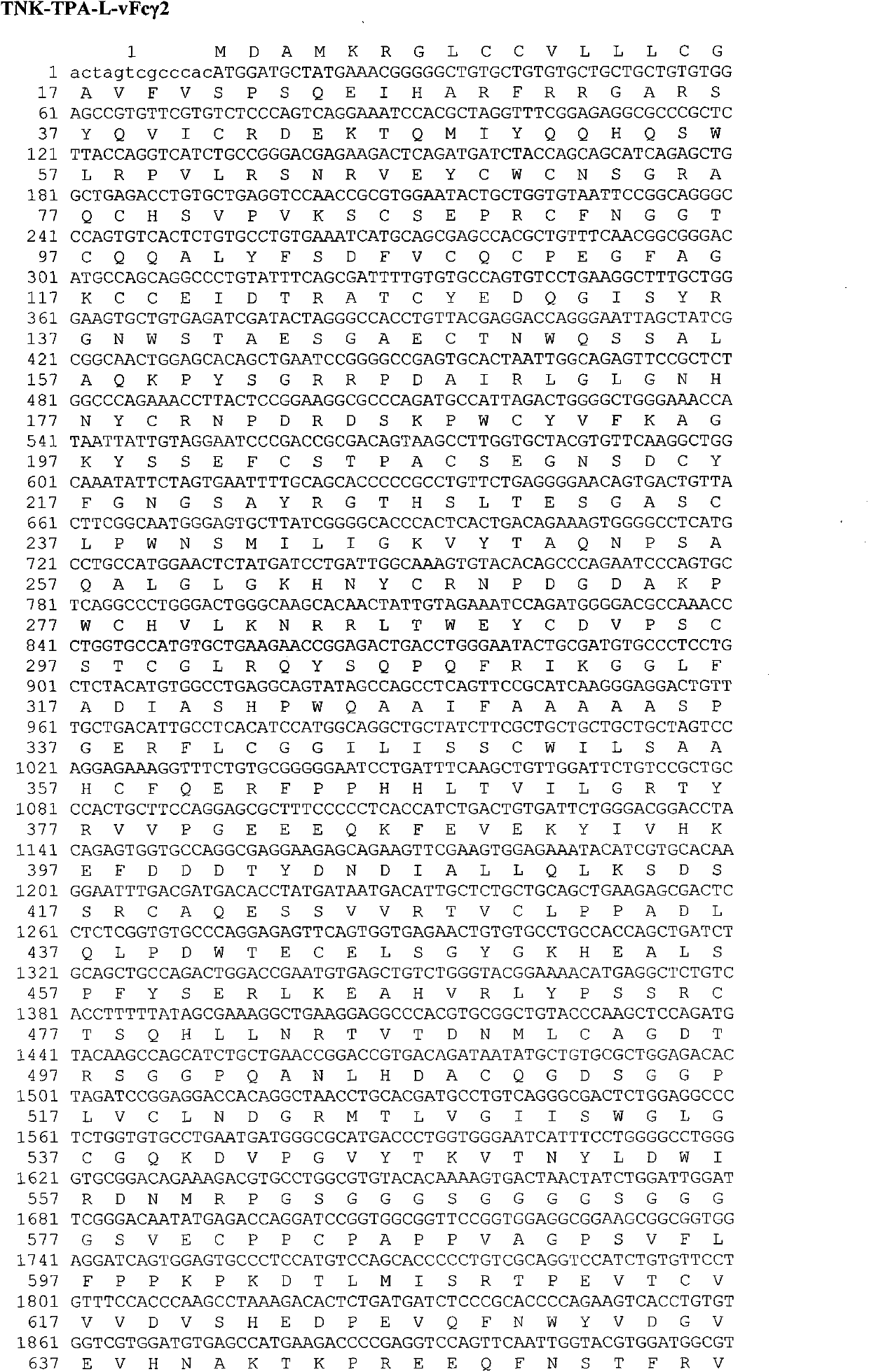
Recombinant tissue-type plasminogen activator, and preparation method and use thereof
ActiveCN103159860AHigh expressionImprove stabilityPeptide/protein ingredientsSurgeryHalf-lifeTissues types
The invention discloses a recombinant TNK-tPA-Fc fusion protein, and a preparation method and application thereof. The protein orderly contains human TNK-tPA, a flexible peptide joint and human IgG natural or variant Fc. The fusion protein has invitro and invivo biological activity similar to human TNK-tPA, and greatly prolonged plasma half-life.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Ligands binding the complex of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) that inhibit downstream uPAR interactions: identification and use in diagnosis or therapy
Antibodies or other ligands specific for the binary uPA-uPAR complexes, for ternary complexes comprising uPA-uPAR and for complexes of uPAR and proteins other than uPA such as integrins inhibit the interaction of uPA and uPAR with additional molecules with which the complexed interact. Such antibodies or other ligands are used in diagnostic and therapeutic methods, particularly against cancer.
Owner:ATTENUON LLC
Use of cross-linked, covalently bound urokinase plasminogen activator (scuPA)-urokinase plasminogen activator receptor (suPAR) complex as a fibrinolytic agent
InactiveUS6759042B2Peptide/protein ingredientsMammal material medical ingredientsCross-linkUrokinase Plasminogen Activator
The present invention relates to the cross-linked scuPA / suPAR complex and / or tcuPA / suPAR cross-linked complex, the process of preparation of the covalently bound single compound having fibrinolytic activity and use of the cross-linked scuPA / suPAR or tcuPA / suPAR complex in the prevention and / or treatment of thrombolytic disorders. The invention further relates to combination compositions and / or therapy regimens, comprising the cross-linked scuPA / suPAR complex or tcuPA / suPAR and one or more currently used plasminogen activators to achieve improved therapeutic efficacy and / or reduce side effects.
Owner:THROMBOTECH LTD (IL)
Method of purifying therapeutic proteins
InactiveUS20140154233A1Reduce concentrationLower Level RequirementsFactor VIIFibrinogenFactor VIII vWFTherapeutic protein
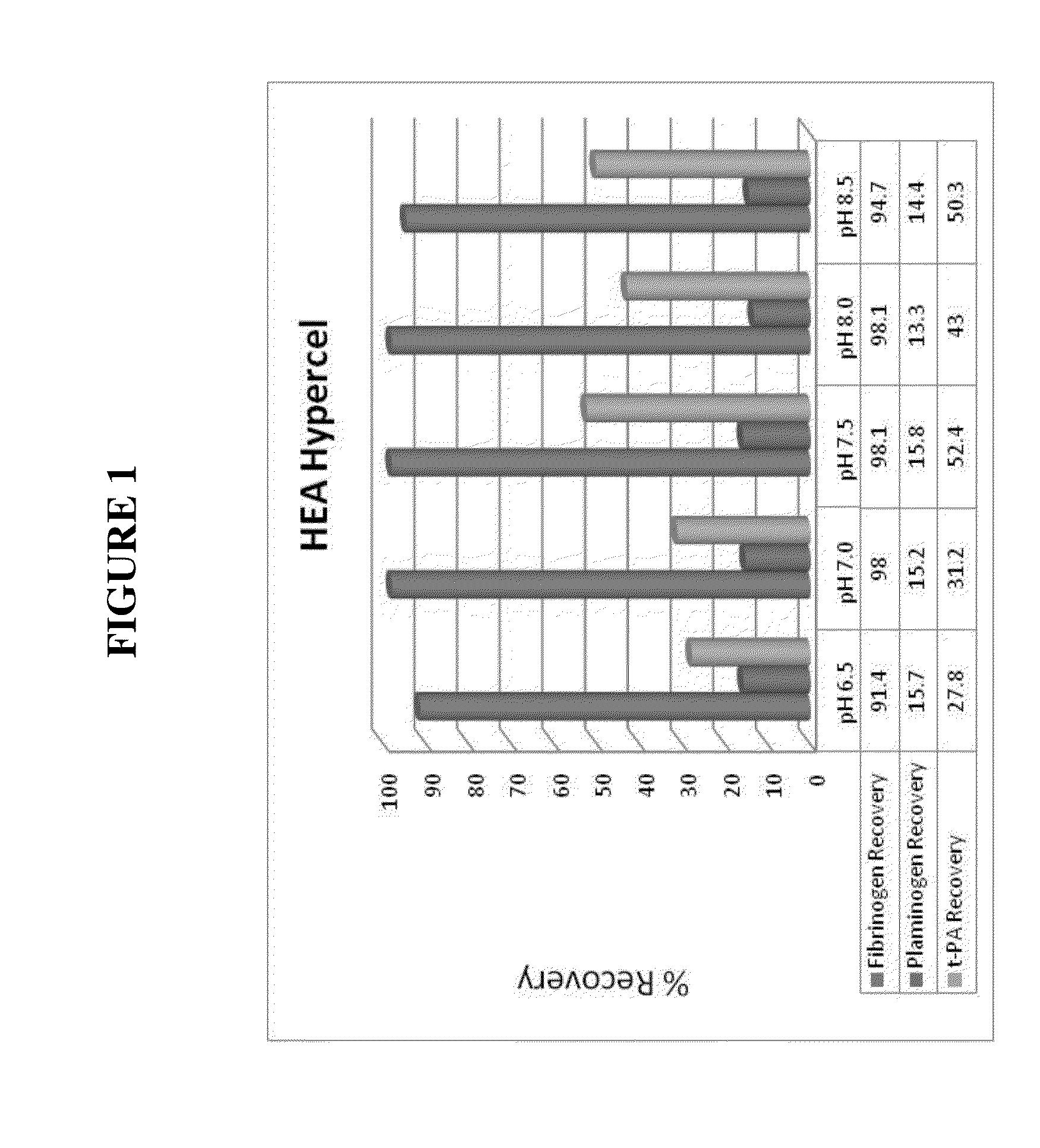
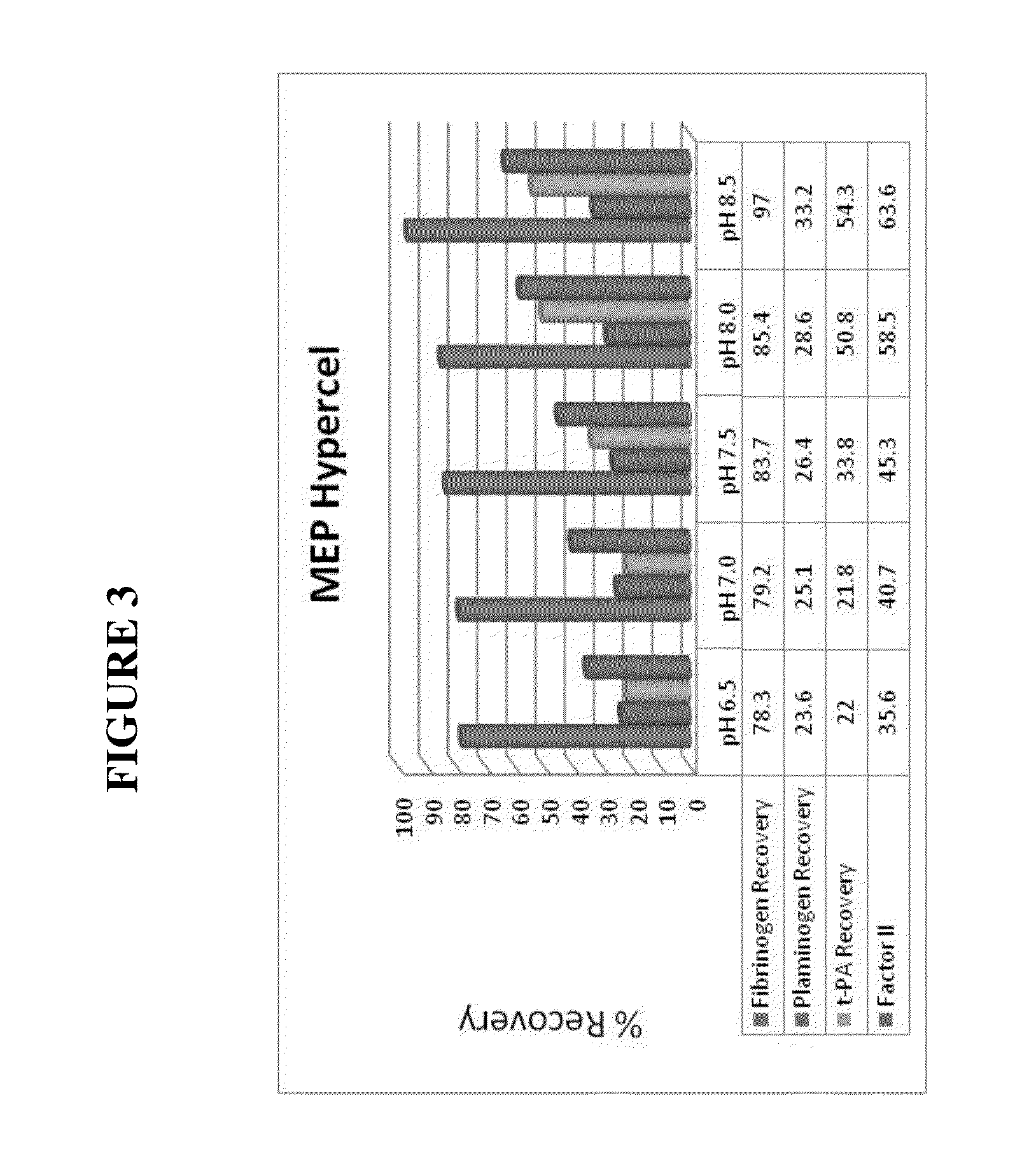
The present invention relates generally to a method of reducing the level of plasminogen and / or tissue plasminogen activator and / or other protease(s) in a solution comprising fibrinogen and / or Factor VIII and / or von Willebrand factor (VWF), the method comprising: (i) passing a feedstock comprising fibrinogen and / or Factor VIII and / or VWF through a hydrophobic charge-induction chromatographic resin under conditions selected such that the plasminogen and / or tissue plasminogen activator and / or other protease(s) is bound to the resin; and (ii) recovering the solution comprising fibrinogen and / or Factor VIII and / or VWF which passes through the resin; wherein the concentration of the plasminogen and / or tissue plasminogen activator and / or protease(s) in the recovered solution is reduced by at least 50% compared to the feedstock. Also provided are solutions and pharmaceutical formulations comprising the fibrinogen and / or Factor VIII and / or VWF recovered by such methods, and uses thereof.
Owner:CSL BEHRING GMBH
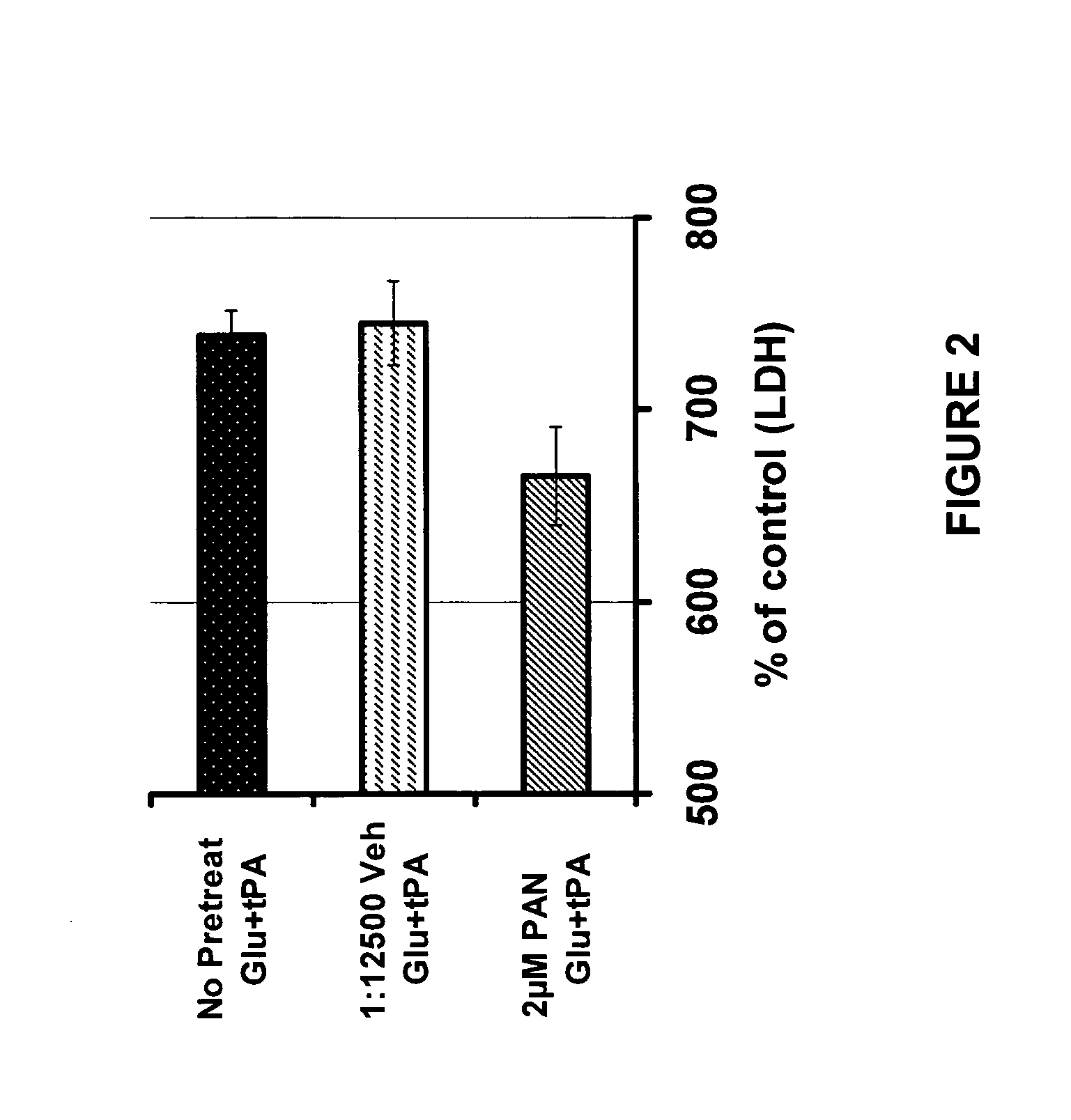
Composition and method to inhibit tissue plasminogen activator (tPA) - potentiated neurotoxicity
The present invention relates to methods of treating, preventing or ameliorating ischemia-related, neural cell degeneration in a subject being treated with a thrombolytic agent, by administering to the subject one or more neuroprotective thiosemicarbazone compounds. More particularly, the present invention relates to methods of preventing, treating or ameliorating the adverse neurological side effects of tissue plasminogen activator, which is used in the treatment of ischemic stroke, by co-administering one or more compounds of the present invention. One such neuroprotective compound is PAN-811. The invention also relates to compositions comprising PAN-811 or analogs thereof in admixture with a thrombolytic agent, such as tPA.
Owner:PANACEA PHARMA
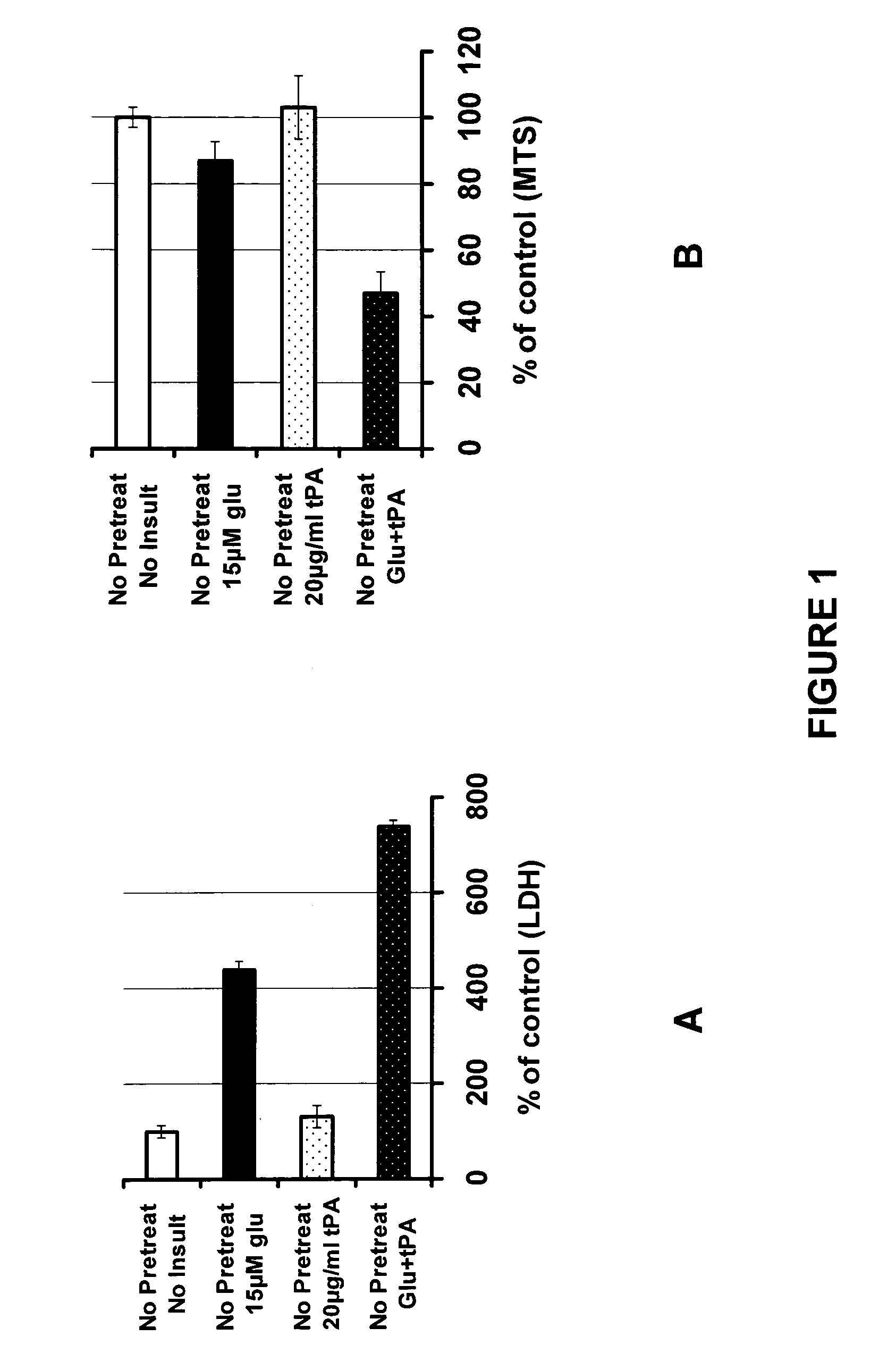
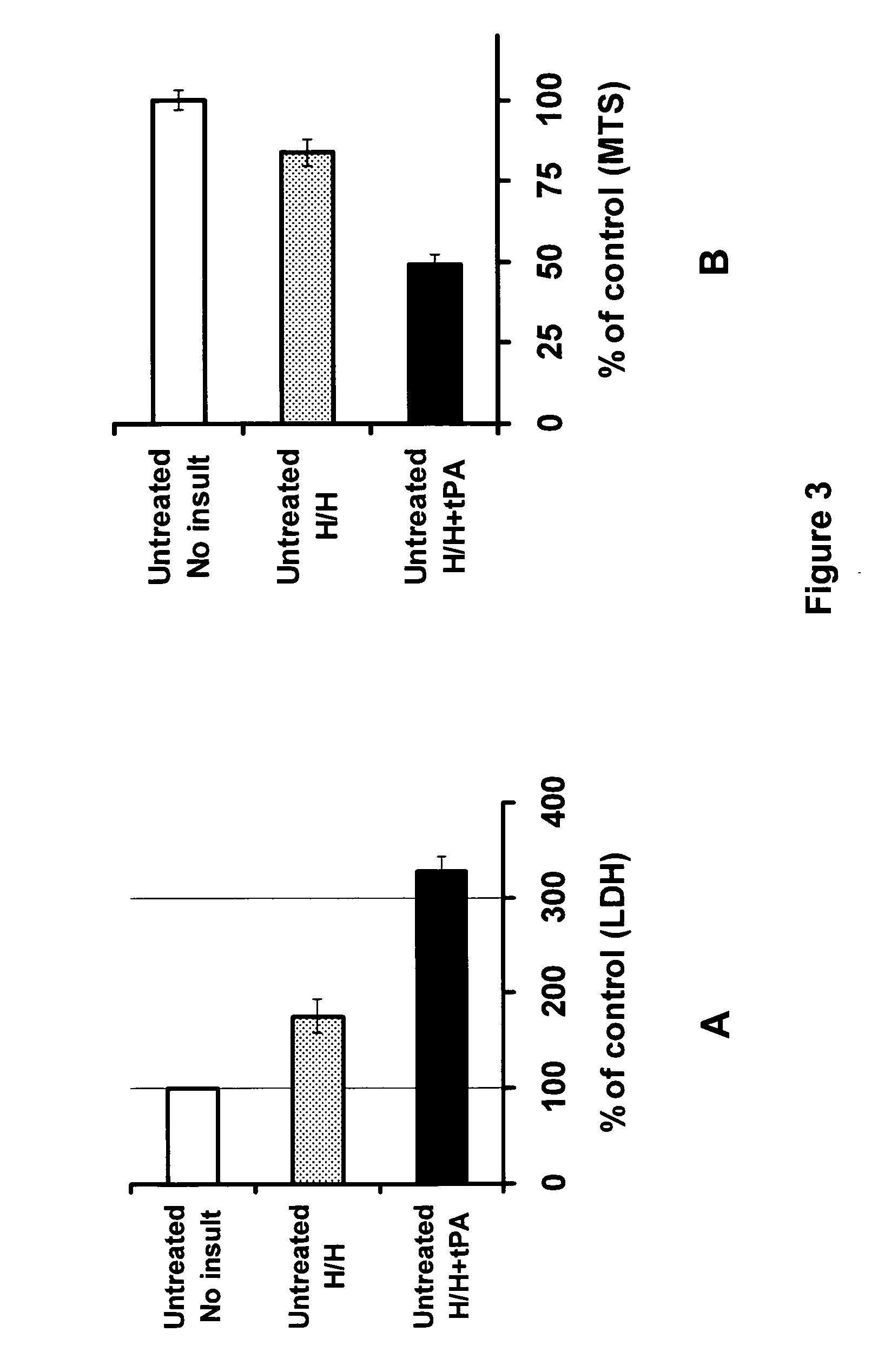
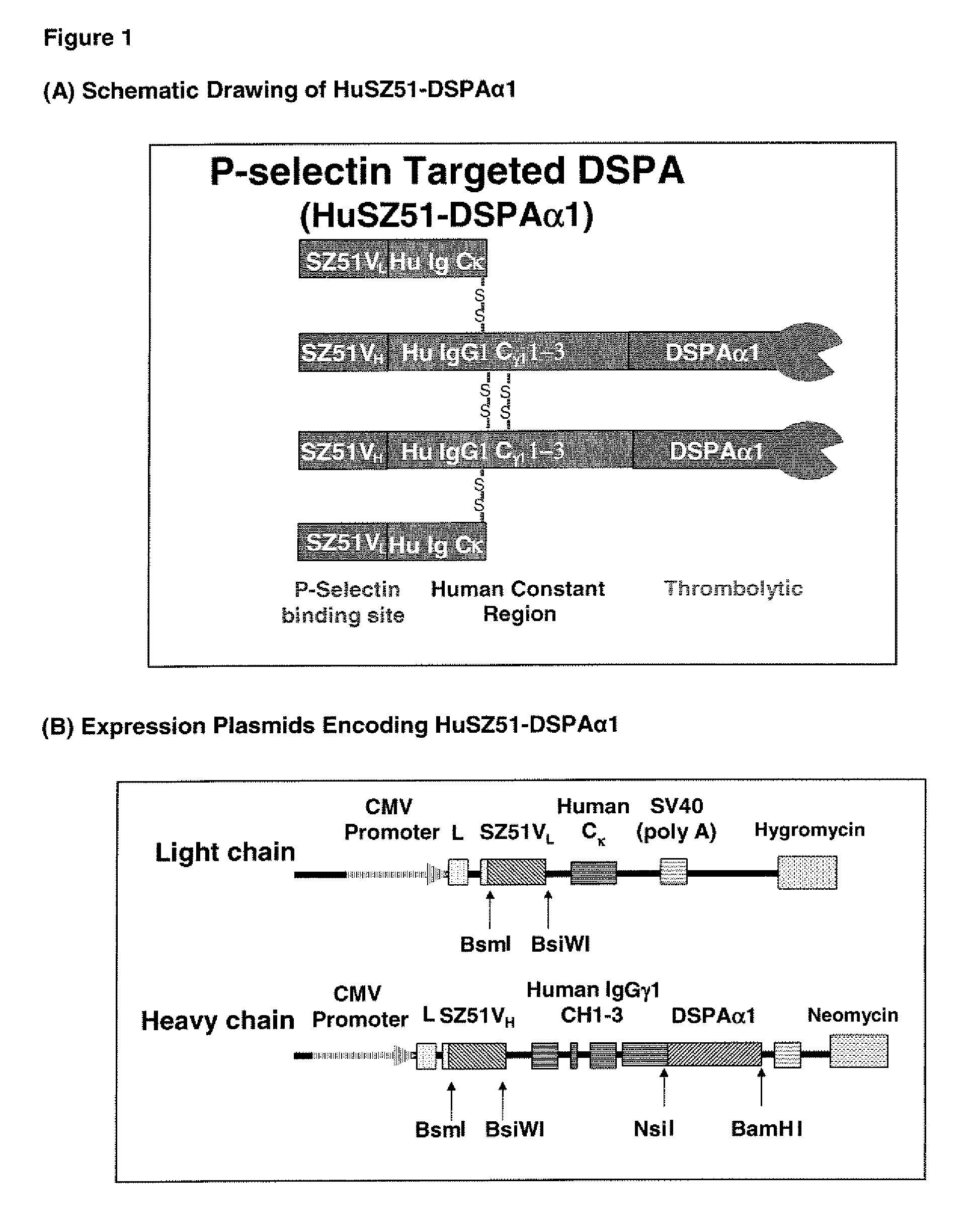
Targeted plasminogen activator fusion proteins as thromobolytic agents
InactiveUS20090286721A1Reduction in systemic doseMinimize impactPeptide/protein ingredientsGenetic material ingredientsP-selectinProtein target
This invention relates to novel fusion proteins, comprising a targeting protein and a plasminogen activator, preferably an antibody that binds to P-selectin, operably linked to the plasminogen activator DSPAalpha1, or analogs, fragments, derivatives, or variants thereof, which are useful as thrombolytic agents. Pharmaceutical compositions containing these fusion proteins, methods of using these fusion proteins as thrombolytic agents, and processes for synthesizing these fusion proteins are also described herein.
Owner:PAN JUNLIANG +3
Compound and method for regulating plasminogen activation and cell migration
InactiveUS7919103B2Prevents and reduces capillary tube formationPeptide/protein ingredientsSnake antigen ingredientsDiseaseAngiogenesis growth factor
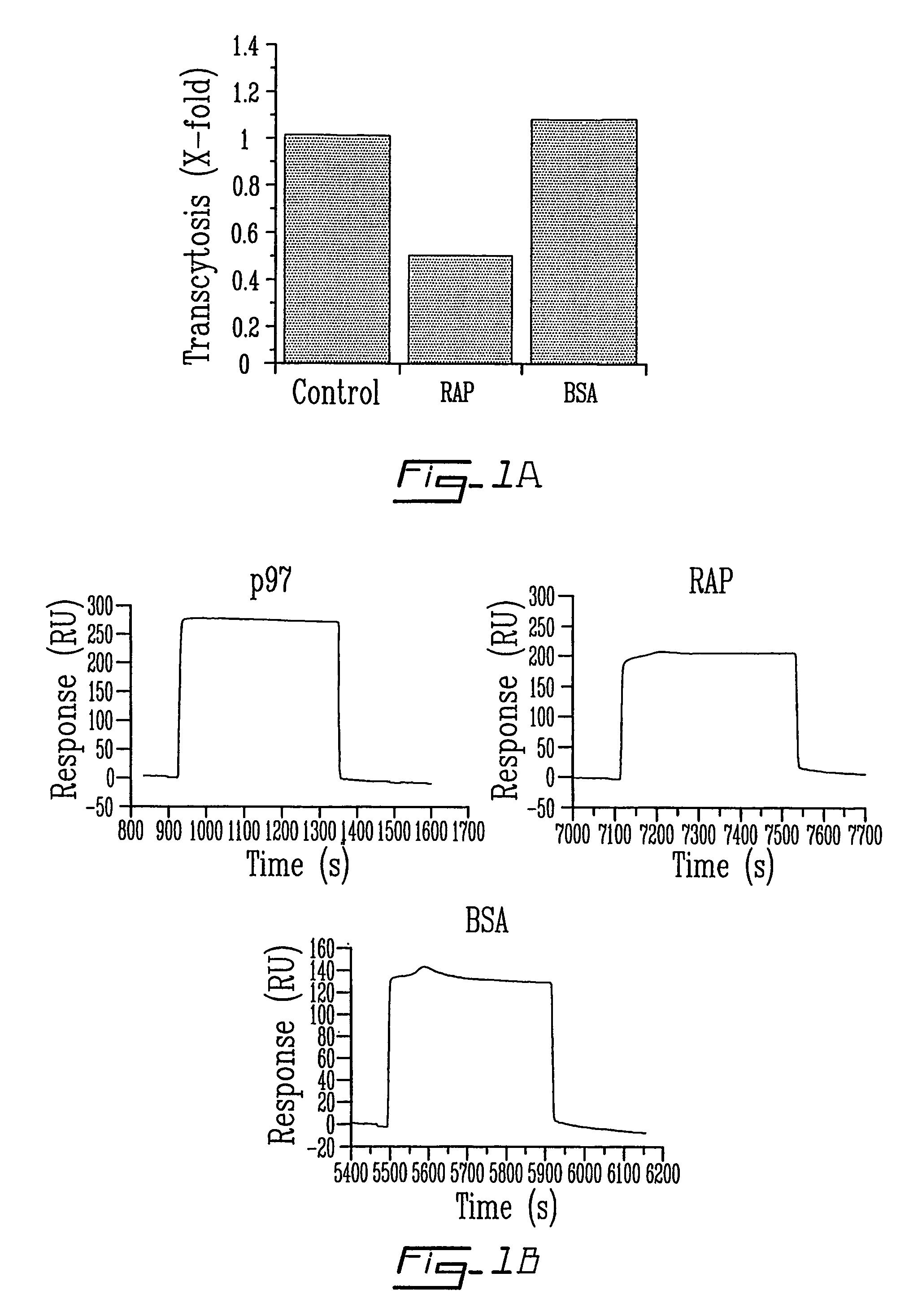
The invention relates to novel regulators of plasminogen activation and their use for regulating cell migration, plasminolysis, angiogenesis, fibrinolysis, for treating cancer and thrombo-embolic diseases such as heart stroke. Furthermore, the present invention relates to novel pharmaceutical compositions form regulating cell migration, plasminolysis, angiogenesis and for treating cancer. In particular, the present invention relates to a method of regulating the activation of plasminogen comprising contacting a solution of pro-urokinase (uPA) or tissue plasminogen activator (tPA) and plasminogen with melanotransferrin (p97) for a time sufficient to effect regulation thereof.
Owner:TRANSFERT PLUS
DTAT fusion toxin
InactiveUS6846484B2Peptide/protein ingredientsAntibody mimetics/scaffoldsBiologyTissue plasminogen activator
The invention provides fusion toxins that contain one or more regions of diphtheria toxin and a portion of urokinase-type plasminogen activator, as well as the nucleic acids that encode the fusion toxins and methods of using the fusion toxins.
Owner:RGT UNIV OF MINNESOTA
Construction and application of recombinant duck viral enteritis virus vaccine for expressing secretory duck Tembusu virus M/E protein
ActiveCN103667197AGenetic material ingredientsMicroorganism based processesTembusu virusSv40 promoter
The invention provides a recombinant duck viral enteritis virus vaccine for expressing secretory duck Tembusu virus M / E protein, and a construction method and application thereof. The collection number is CCTCC NO:V201215, and the name is rDEV-TME-tPAS. By using a recombinant clone technique, a gene segment rDEV-TME-tPAS comprising an SV40 promoter, duck Tembusu virus M and E proteins and a tPA (Tissue plasminogen activator) signal peptide sequence is inserted into a spacer between the US7 and US8 genes of the duck viral enteritis virus to construct a cosmid in which the rDEV-TME-tPAS expression frame is inserted between the US7 and US8 genes, thereby obtaining the recombinant duck viral enteritis virus vaccine CCTCC V201215 for expressing secretory duck Tembusu virus M / E protein. The invention also provides a method for constructing a recombinant duck viral enteritis virus vaccine strain and application of the recombinant duck viral enteritis virus vaccine strain in preparing a vaccine for preventing infectious diseases caused by duck viral enteritis virus and duck Tembusu virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Combination treatment with t-PA variant and low molecular weight heparin
The invention concerns an improved therapeutic regimen for the treatment of thrombolytic disorders, such as acute myocardial infarction (AMI). In particular, the present invention concerns the treatment of thrombolytic disorders, e.g. AMI, with a combination of a tissue plasminogen activator (t-PA) variant having improved fibrin specificity and extended plasma half-life when compared with wild-type human t-PA and a low molecular weight heparin.
Owner:AVENTIS PHARMA SA (US) +2
Glaucoma treatment
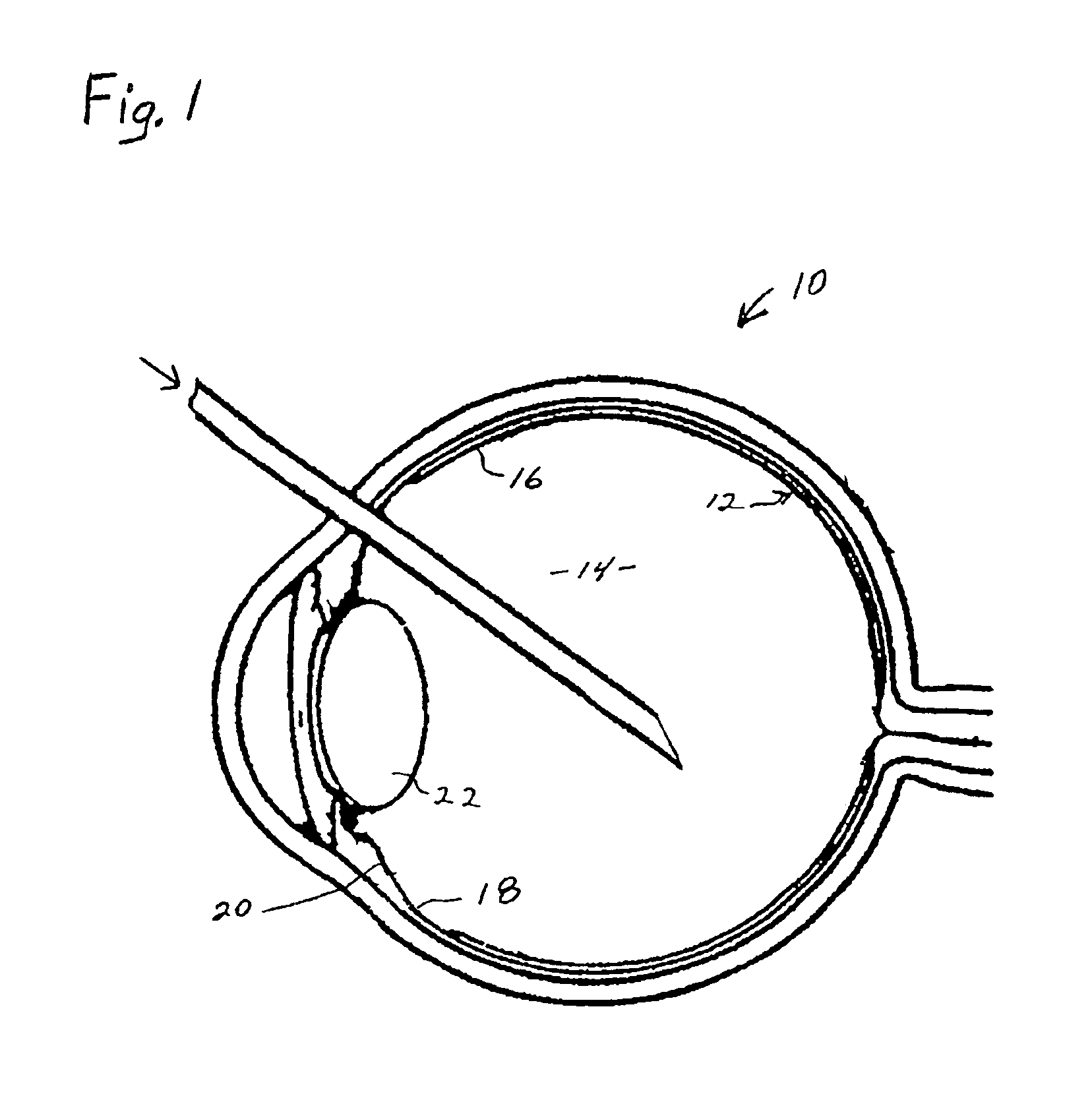
Disclosed herein are methods of treatment for an intraocular pressure (IOP)-associated condition in a subject, that include administering to the subject an effective amount of a tissue plasminogen activator (tPA) therapeutic agent. In one embodiment, the IOP-associated condition is glaucoma. The administration of a tPA therapeutic agent can be an extended administration intended to cause a reduction in IOP in the subject for a period of at least one day to a year or more, relative to IOP levels in the subject prior to administration of the tPA therapeutic agent. The tPA therapeutic agent can be, for example, tPA, a tPA derivative, a small molecule direct or indirect tPA agonist, or a gene therapy vector.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Biodegrading implantable ocular sustained release drug delivery system
An ocular implant is provided for an intraocular delivery of a therapeutic biologic agent. The implant may be used intracamerally or intravitreally. The implant may include a sustained-release biodegradable core and a biodegradable shell, wherein the shell has a longer biodegradable half-life than the core. The core may include a biodegradable gel medium, an active therapeutic biologic agent, and a biologic stabilizer. Upon insertion into the anterior chamber or vitreous body of an eye, the therapeutic biologic agent is released over an extended period, that may range from one day to one year. The therapeutic biologic agent may be, for example, tissue-plasminogen activator, an anti-VEGF agent, or another biopharmaceutical. The biodegradable implant may completely dissolve after implantation and need not be removed.
Owner:BIOHEALTHWAYS INC
Mimic peptide of human plasminogen activator and preparation thereof
InactiveCN1680430AWith anti-thrombolytic functionLow immunogenicityMicrobiological testing/measurementPeptidesSurface displayProtein target
Human plasminogen activator analog peptide and its production are disclosed. It is based on protein molecule interaction critical theory and uses solvent of streptokinase and glycosylzyme as mechanism. It is carried out by taking human plasminogen as target protein by surface displaying technology of phagicin random polypeptide base, biological elutriating, screening, obtaining polypeptide sequence with mu Pg high affinity, and solid-phase synthesizing the analog peptide. Its advantages include small molecular weight, low immunity, simple process and easy control of product output, purity and activity. It can be used to prepare oral preparation and prevent thrombogenesis.
Owner:FUDAN UNIV
Process for purifying recombinanat tissue plasminogen activator (TPA)
InactiveUS20090130714A1Reduce lossesImprove conductivityFermentationPeptidasesEscherichia coliInclusion bodies
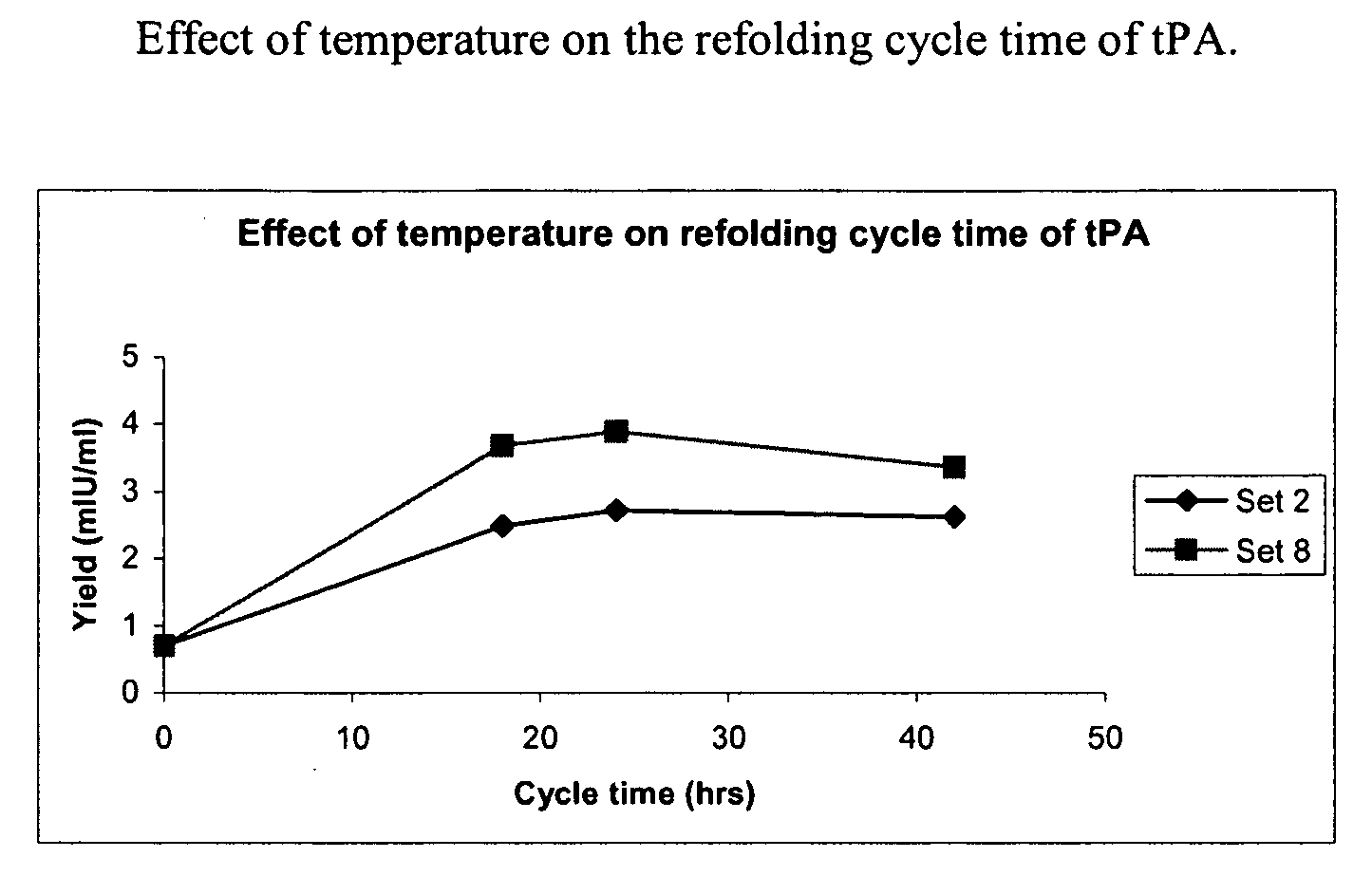
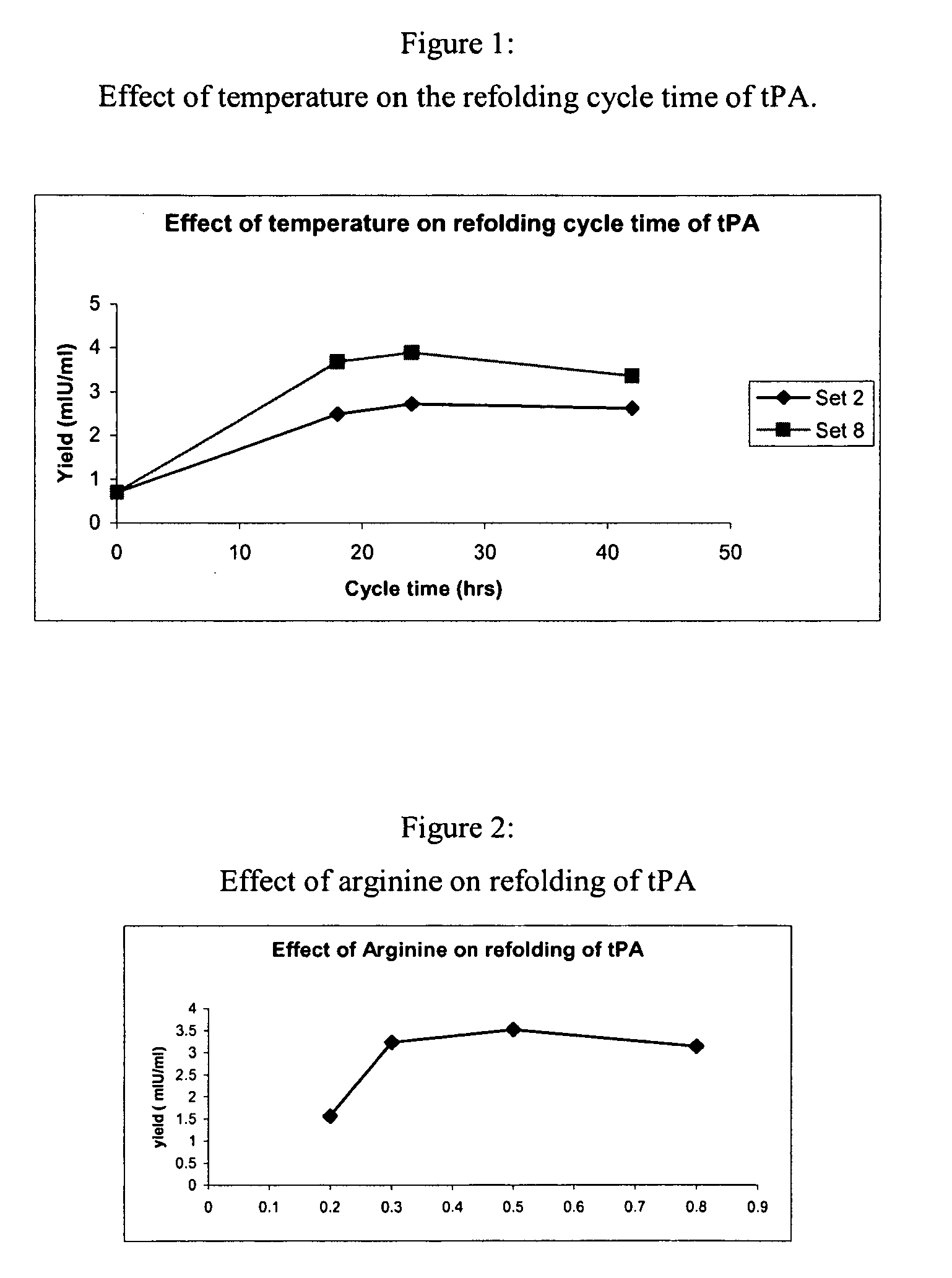
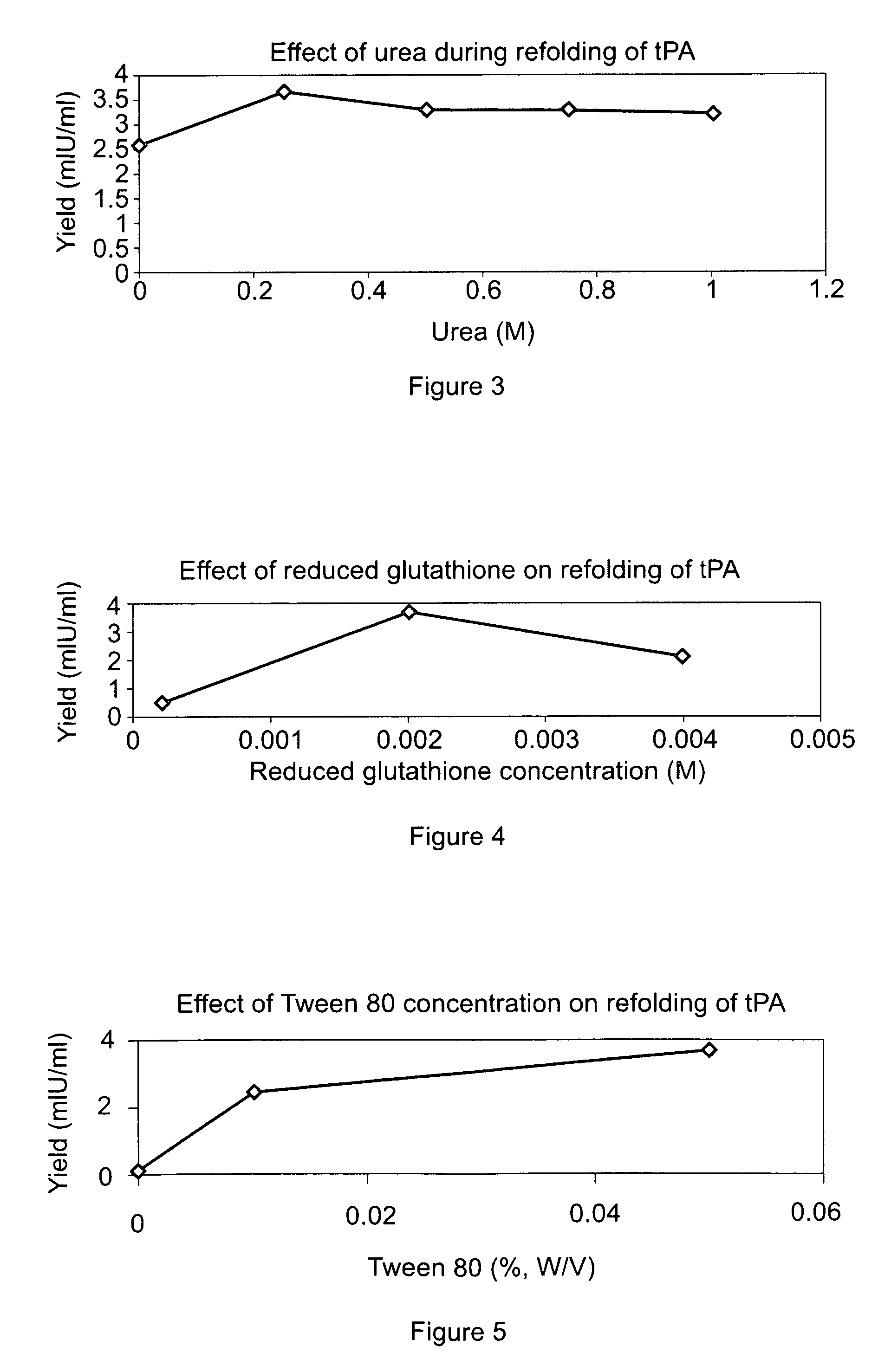
The present invention relates to an efficient and improved process for purifying a recombinant protein. The invention relates to the purification of tissue plasminogen activator (tPA), such as truncated human tPA, recombinantly produced in bacteria, for example in E. coli. The present invention provides a process that requires less refolding volume after solubilization of inclusion bodies isolated from cells expressing the recombinant tPA, without affecting the yield and purity of the tPA protein. The invention also provides optimum arginine concentrations for use during protein refolding and during ion exchange chromatography.
Owner:RELIANCE LIFE SCI PVT
Antithrombotic agents
InactiveUS20070224198A1Immunoglobulins against blood coagulation factorsOrganic active ingredientsAntithrombotic AgentMedicine
Monoclonal antibodies directed against coagulation factors and their use in inhibiting thrombosis in combination with plasminogen activators are disclosed.
Owner:UNIVERSITY OF VERMONT
Preparation method of osteoarthritis animal model
InactiveCN104056260AConvenient researchEasy to get materialsPeptide/protein ingredientsArticular cavityIndividual animal
The invention relates to a preparation method of an osteoarthritis animal model. The method comprises the following steps: blending a matrix metalloproteinase-3 with normal saline so as to obtain a 5-10nmol / L matrix metalloproteinase-3 solution; blending a urokinase-type plasminogen activator with the normal saline so as to obtain a 2-4nmol / L urokinase-type plasminogen activator solution; then, blending the two solutions in proportion of 3:1 or 2:1 so as to obtain a mixed solution; injecting 0.5mL / kg of mixed solution into an articular cavity of an experimental animal. According to the method, the matrix metalloproteinase-3 and the urokinase-type plasminogen activator which are of conventional chemical reagents in a laboratory are taken as raw materials; the mixture is injected into the articular cavity of the animal, so that the rapid degeneration of cartilage tissues is generated. Thus, the pathogenesis process of osteoarthritis is simulated, thereby bringing great convenience for the research of an osteoarthritis intervention treatment technology. The method has the characteristics of being simple and convenient to operate, rapid in modelling, economical, practical, convenient in material obtainment, and the like.
Owner:王维山
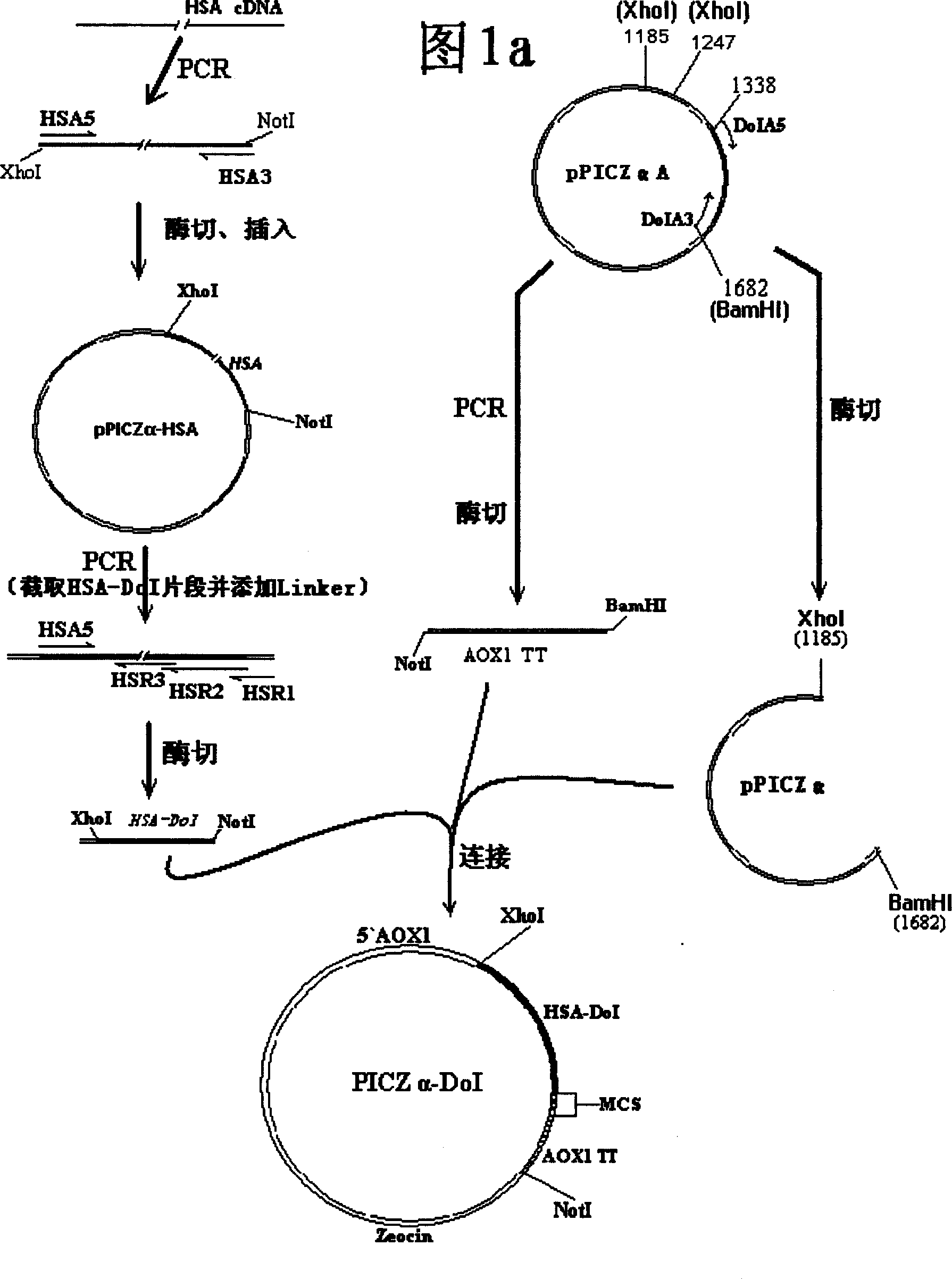
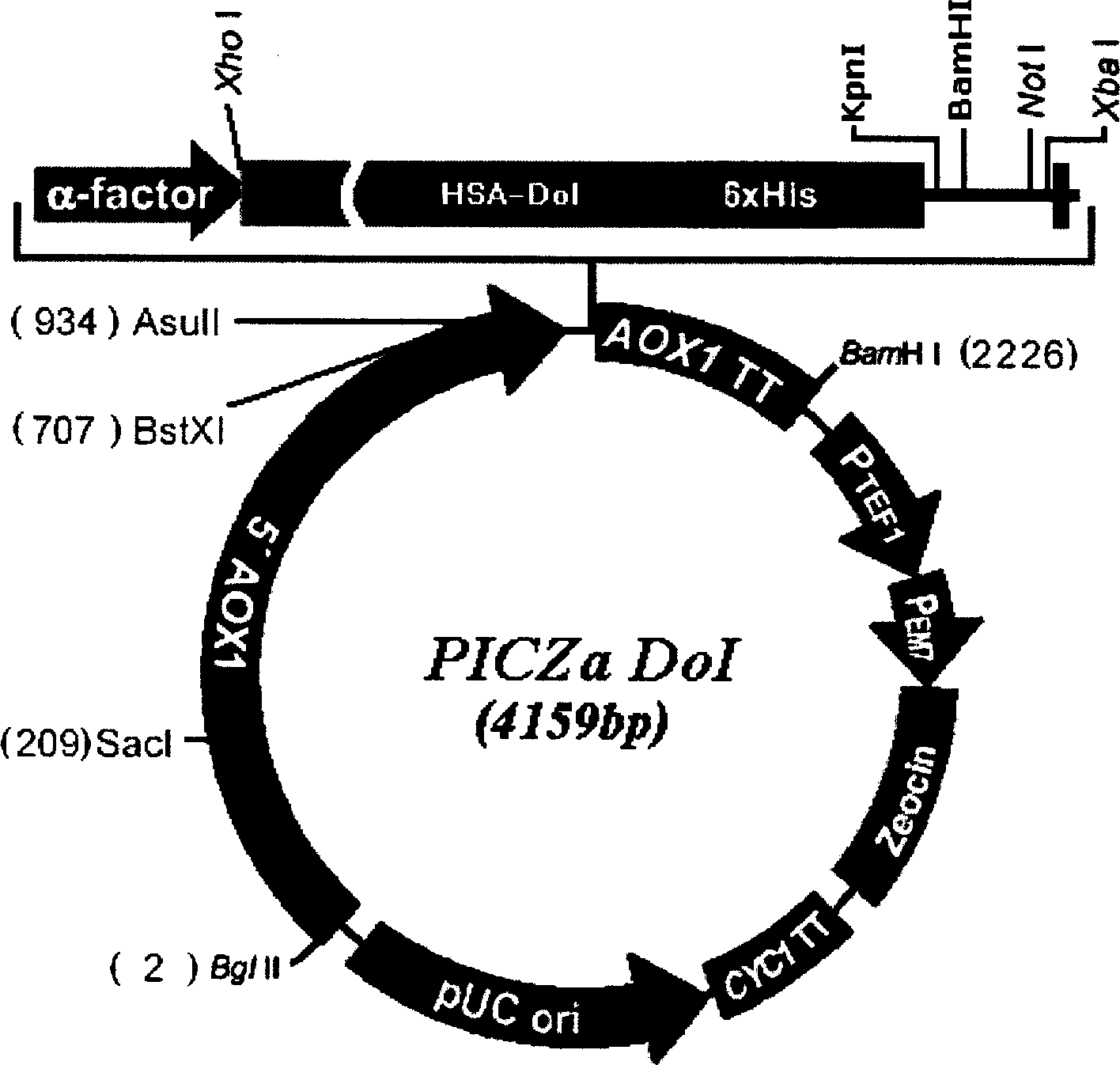
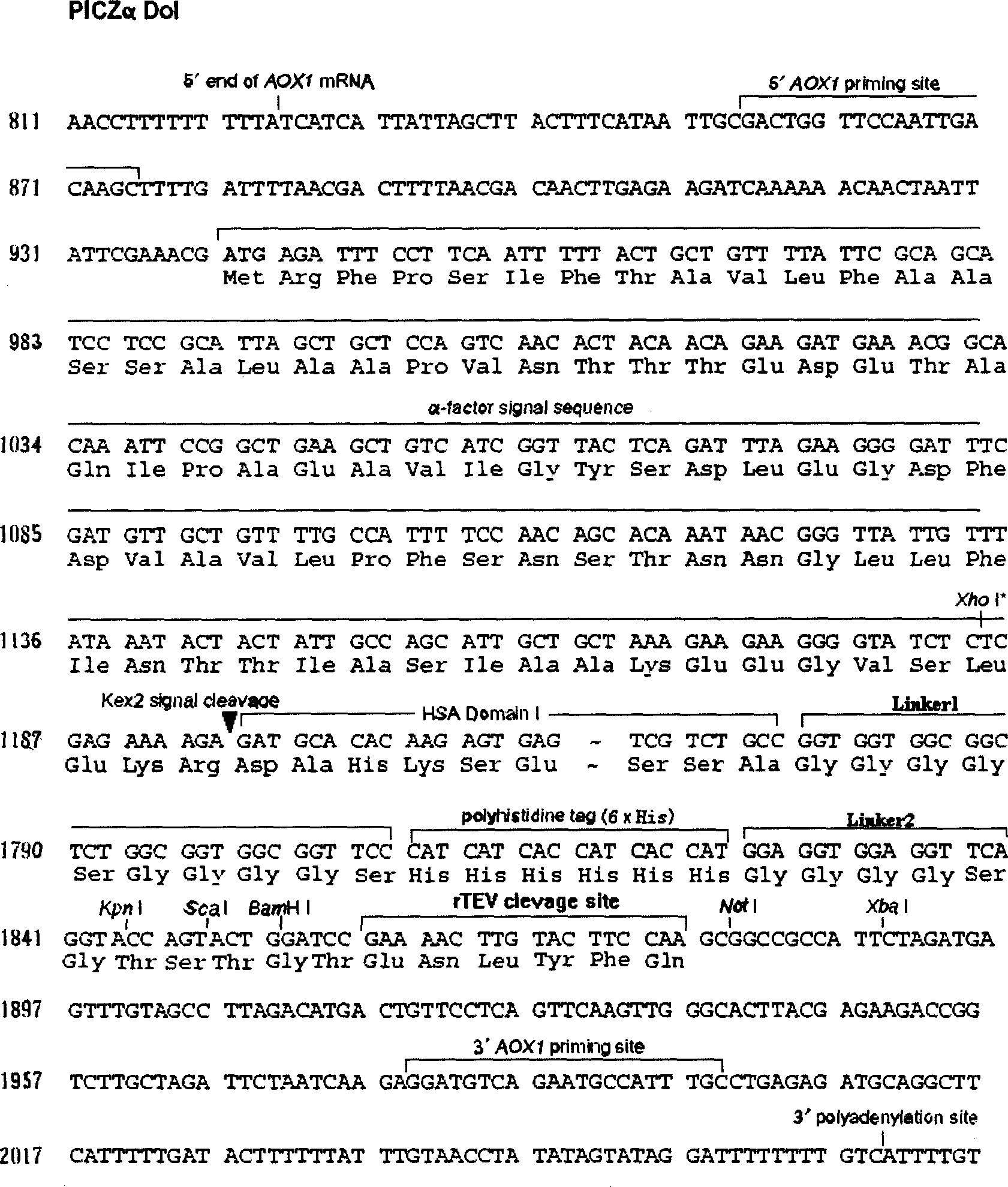
Method of high efficiency expression tPA exogenic protein using methanol yeast system and its purification preparation technology of expressed product
InactiveCN1757745AConducive to specific bindingTime did not increaseFermentationDNA/RNA fragmentationUrokinase Plasminogen ActivatorHigh level expression
A process for configuring the universal expression carrier PICZa-DoI of methanol yeast in disclosed. It can be used to produce the exogenous recombinant protein in the form of fusion protein which can not be highly expressed by conventional method. The mutant human tissue plsminogen activator (rPA), human annexin V-urokinase action fragment fusion protein (Ank-scUK) and mutant human nerve growth factor (mut-hNGF) has been successfully expressed by it. Said fusion protein can be used to prepared high-purity medical protein through direct capturing by chelating sepharose medium from the fermented liquid, desalting, enzyme (or chemical) severing, and chromatography for purifying.
Owner:黄秀东 +1
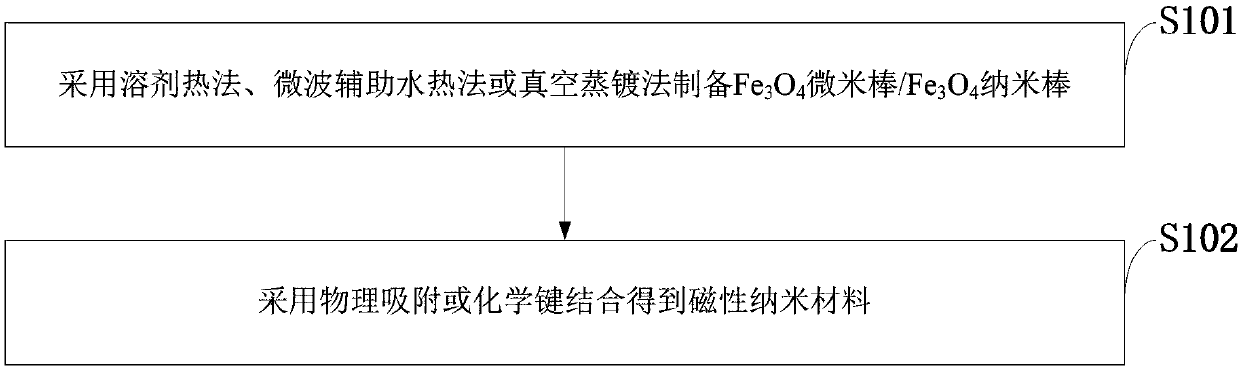
Magnetic nano material capable of improving intravenous thrombolysis treating efficiency and preparation method thereof
InactiveCN107753955AShort half-lifeImproved prognosisPeptide/protein ingredientsEnergy modified materialsMedicineTissue plasminogen activator
The invention belongs to the technical field of magnetic nano materials, and discloses a magnetic nano material capable of improving intravenous thrombolysis treating efficiency and a preparation method thereof. The method comprises the following steps: performing surface modifying on a Fe3O4 magnetic nano rod / Fe3O4 nano rod, and covalently binding with a tissue plasminogen activator; binging themagnetic nano road and the tissue plasminogen activator molecules; and realizing targeted therapy under the guide of an outside magnet; the magnetic nano material comprises the tissue plasminogen activator and the Fe3O4 nano rod. Compared with other magnetic materials, the prepared Fe3O4 magnetic nano bar has the advantages of being nontoxic and porous in structure, and can be covalently bound with tPA after surface modification; the tPA-nano rod is capable of effectively releasing the carried tPA in the outside rotating magnetic field, thus accelerating thrombolysis.
Owner:赵奕平 +1
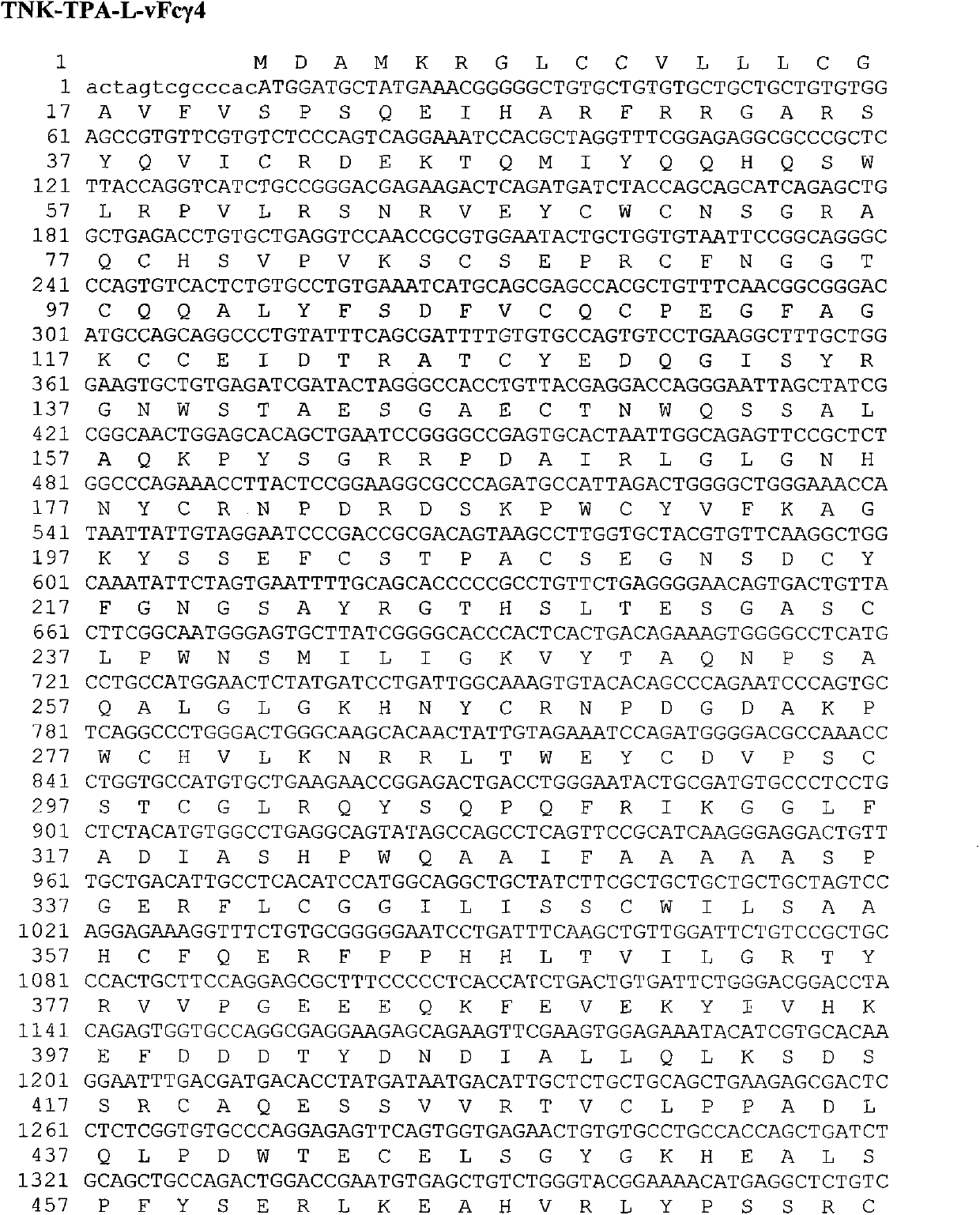
Pharmaceutical composition containing recombinant tissue plasminogen activator
ActiveCN103623396AHigh expressionImprove stabilityPeptide/protein ingredientsSurgeryRecombinant tissue plasminogen activatorHalf-life
The invention discloses a pharmaceutical composition containing a recombinant tissue plasminogen activator. The pharmaceutical composition consists of a pharmaceutically acceptable carrier, excipient or thinner, as well as an effective amount of recombinant TNK-tPA-L-Fc fusion protein; from N terminal to C terminal, the protein sequentially includes TNK-tPA, a flexible peptide joint and human IgG natural or variant Fc. The fusion protein is similar to human TNK-tPA in both in vitro and in vivo bioactivities, and can greatly prolong plasma half-life.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
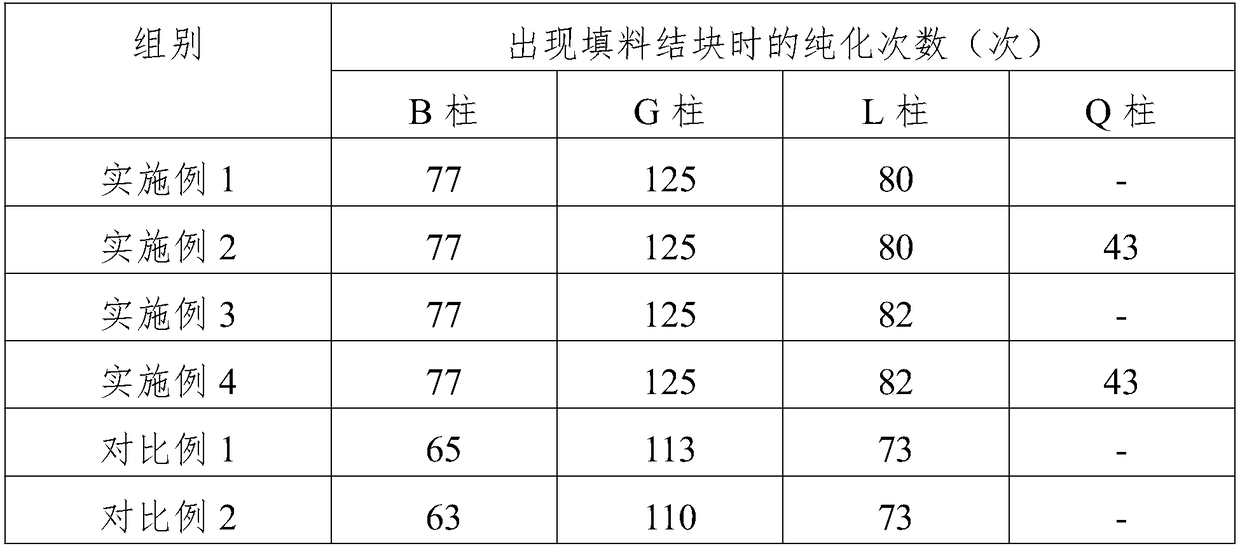
Purification method of rhTNK-tPA (recombinant human TNK tissue-type plasminogen activator for injection) cell harvesting fluid
The invention belongs to the technical field of protein separation and purification and particularly relates to a purification method of an rhTNK-tPA (recombinant human TNK tissue-type plasminogen activator for injection) cell harvesting fluid. The key point is that the rhTNK-tPA cell harvesting fluid is subjected to deep filtration and sterilizing filtration before column chromatography, so thatthe problem that the conventional filler for column chromatography after filtration is prone to caking can be solved effectively, clarity of a filtrate is high (smaller than or equal to 0.3 NTU), filter loss is small (smaller than or equal to 5%), and significant progress is made as compared with the prior art.
Owner:石药集团明复乐药业(广州)有限公司
Renaturation separation purification method of tissue plasminogen activator mutant
InactiveCN1556206AHigh yieldReduce manufacturing costPeptide preparation methodsEnzymesEscherichia coliPurification methods
A process for renaturating, separating and purifying the mutant of tissue plasminogen activator features that the imidazole and urea are used to make the tissue plasminogen activator 'Ruitipu enzyme' be correctly folded in order to have activity and make it be renaturated, separated and purified simultaneously.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com