Flow electroporation chamber and method
a flow electroporation chamber and flow chamber technology, applied in the field of flow electroporation chamber and method, can solve the problems of significant hemolysis of red blood cells following treatment, poor reproducibility of ihp concentrations incorporated in red blood cells, and commercially impractical procedures on a scale suitable for commercial use, so as to reduce the affinity of hemoglobin for oxygen and enhance the delivery of oxygen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
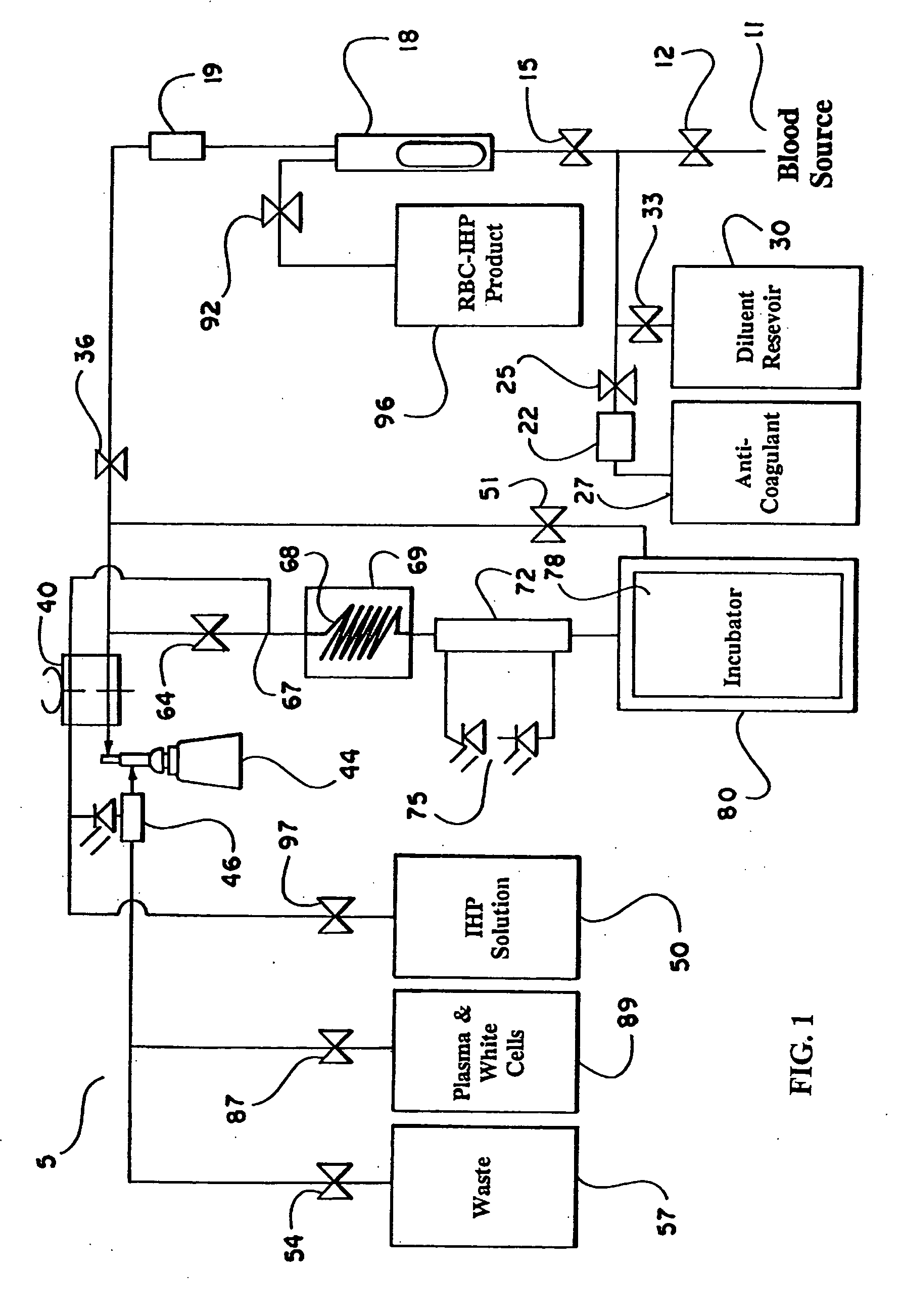
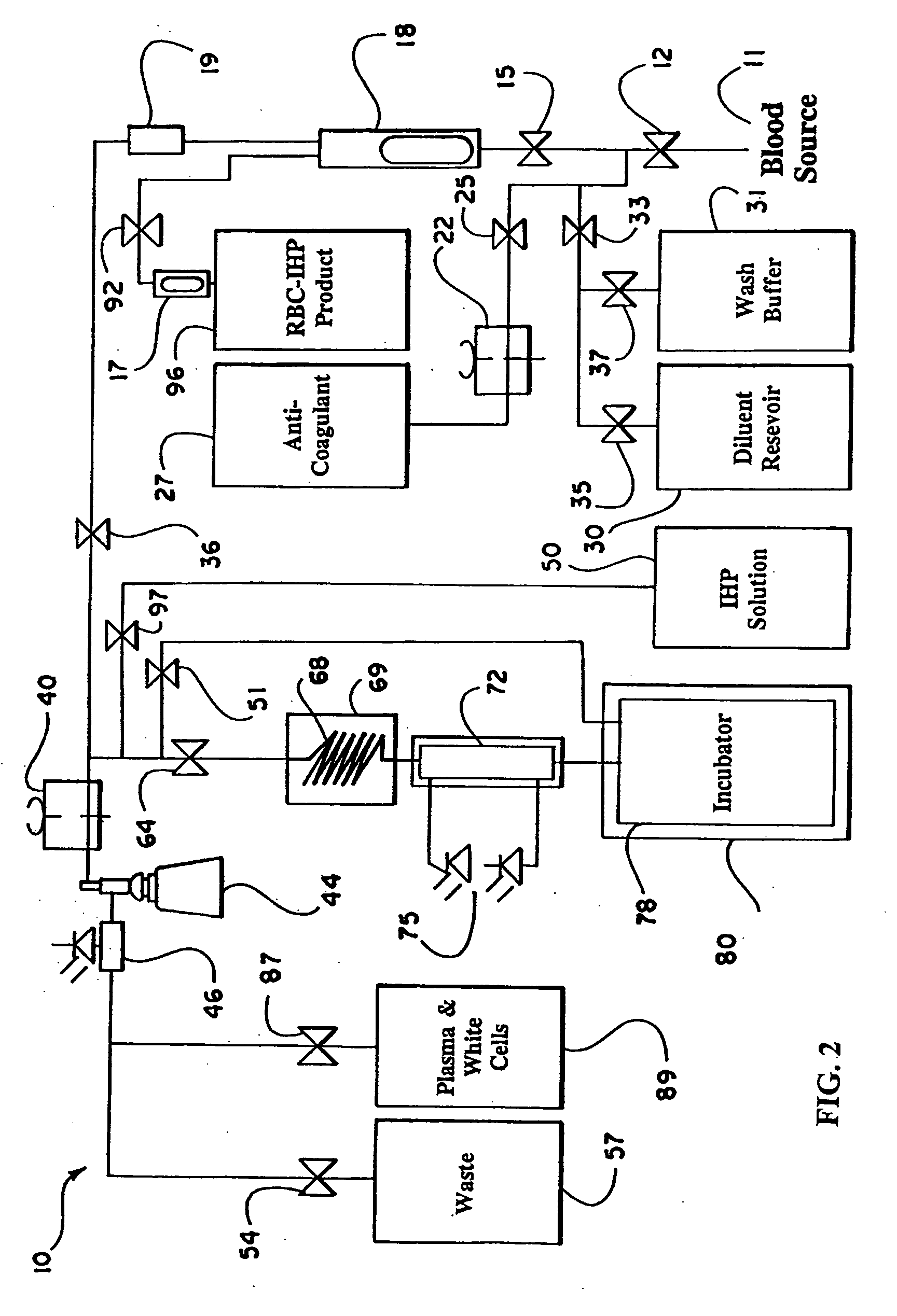
the present invention is described with reference to FIG. 1, which schematically illustrates the structure of the continuous flow encapsulation apparatus of the present invention.
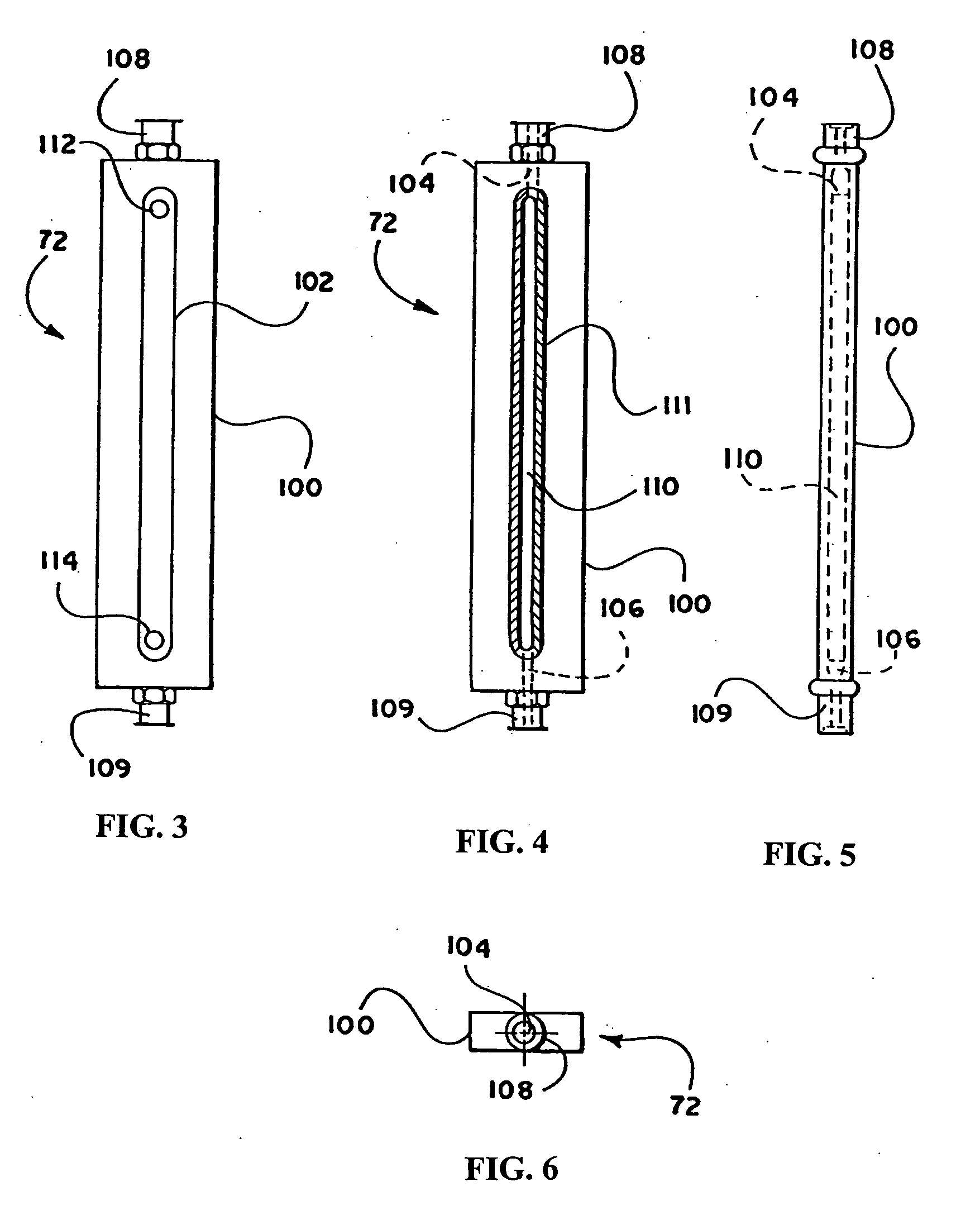
In accordance with the present invention, a volume of whole blood is admitted into the electroporation system 5 at input 11. The blood sample may optionally be drawn directly from a patient into the electroporation system 5 or the blood may be drawn at an earlier time and stored prior to introduction into the system 5. Valve 12 is opened to admit the sample into the system 5. Simultaneously, valve 25 is opened and pump 22 is engaged to admit an anti-coagulant from the anti-coagulant reservoir 27. A suitable anticoagulant is heparin, although other anticoagulants can be used. The preferred anticoagulant is ACD. Valves 15 and 36 are also opened and pump 40 is engaged. The admixture of anticoagulant and whole blood passes through a filter 18 and a pressure evaluation system 19 that monitors the flow through t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com