Antithrombotic agents
a technology of antithrombotic agents and thrombolytic agents, which is applied in the field of monoclonal antibodies, can solve the problems of shunts and prostheses such as artificial heart valves, affecting the function of shunts and prostheses, so as to reduce inhibit thrombolytic agents, the effect of reducing the required dose of thrombolytic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
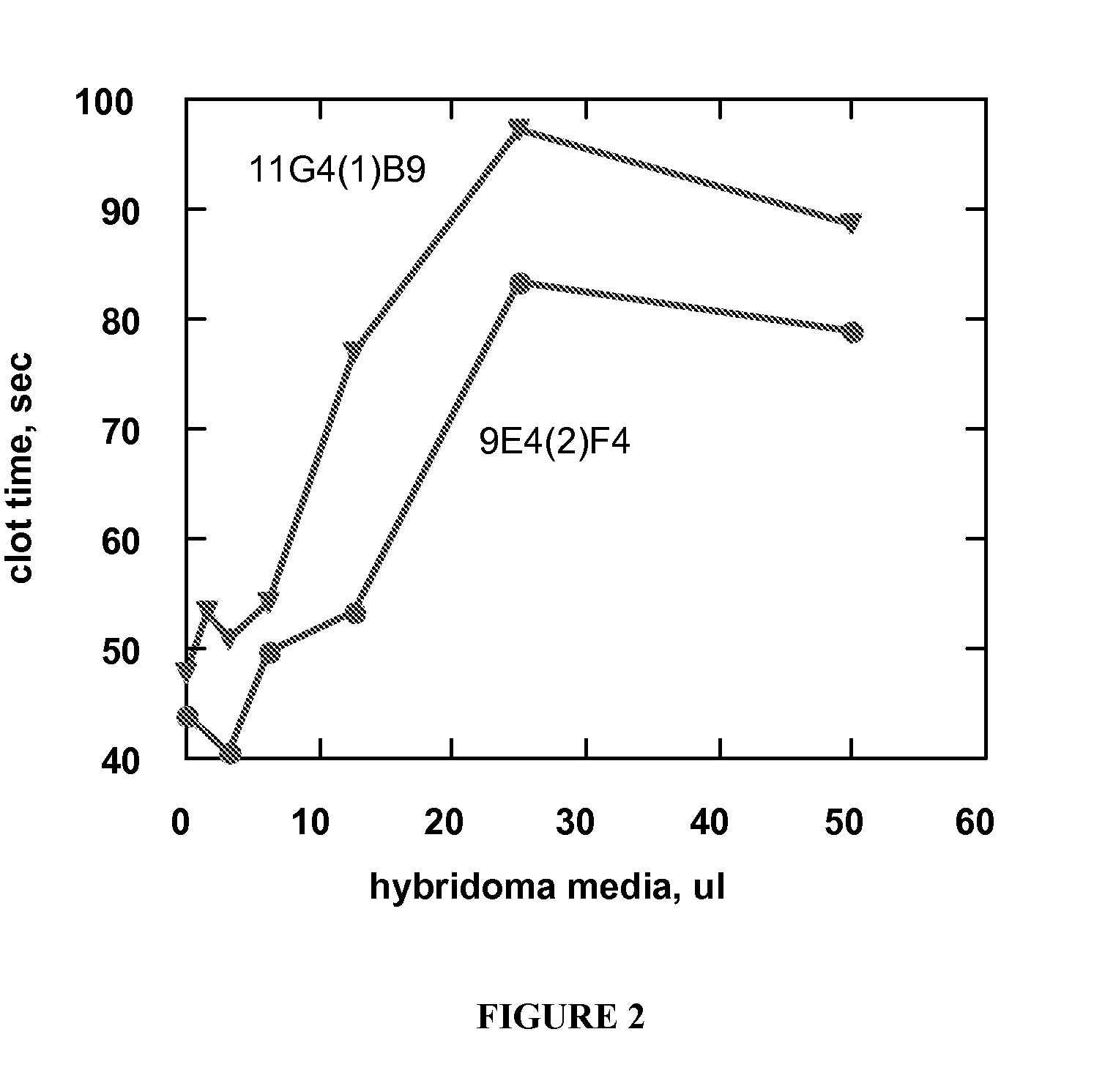
example 1
Preparation and Screening of Anti-Factor IX Monoclonal Antibodies
[0120] Female Balb / C mice were injected with human factor IX purified as described in Jenny, R. et al., Prep Biochem, 16, 227-245 (1986). Typically, each mouse received an initial injection of 100 ug protein dissolved in 0.15 mL phosphate-buffered saline (PBS) and mixed with 0.15 mL complete Freund's adjuvant. Booster immunizations of 50 ug protein in 0.15 mL PBS with 0.15 mL incomplete Freund's adjuvant were given approximately biweekly over a 2-3 month period. After the final boost, the mouse received 50 ug of Factor IX in PBS three days before spleen / myeloma cell fusions. Spleen cells were isolated from an immunized mouse and fused with NS-1 myeloma cells (Kohler, G. et al., Eur J Immunol, 6, 292-295 (1976)) using polyethylene glycol as described by Oi, V. T. et al. in “Selected Methods in Cellular Immunology,” Mishell, B. B. and Shigii, S. M., eds., Freeman Press, San Francisco. Following the fusion, the cells wer...
example 2
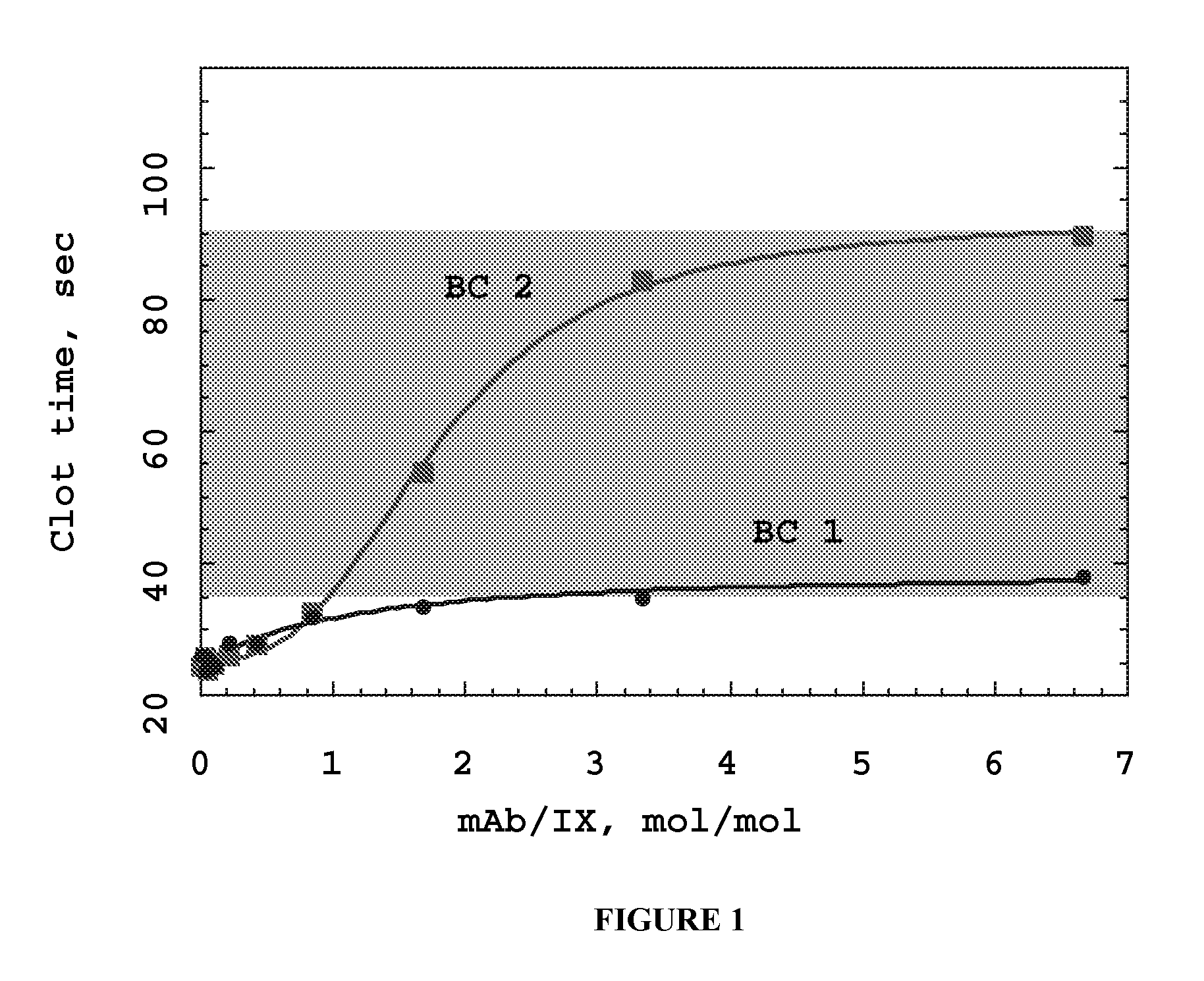
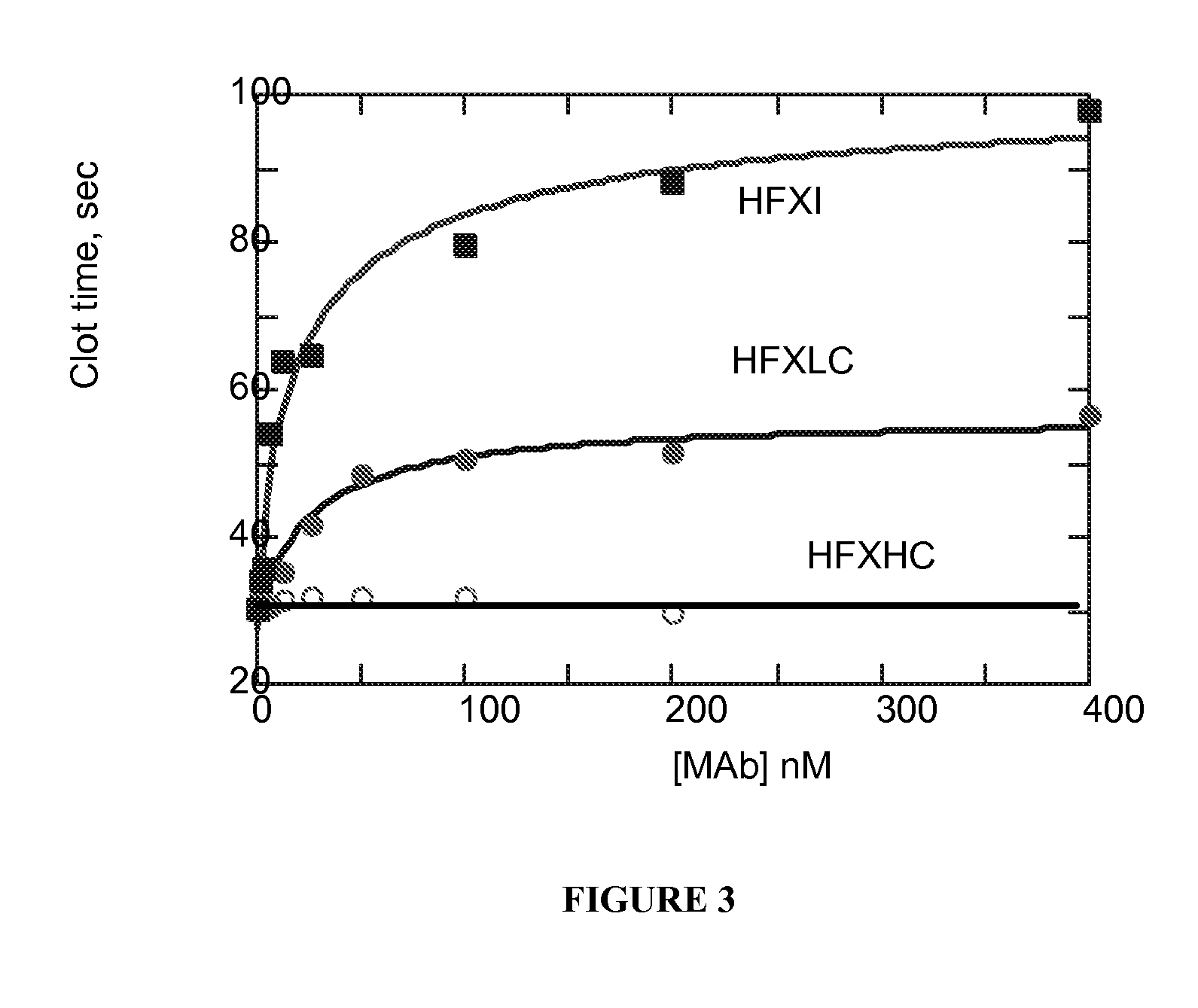
Self-Limiting Effect of Anti-Coagulation Factor Antibodies in Coagulation
[0123] The effect of increasing concentrations of anti-coagulation factor antibodies on activated partial thromboplastin time (aPTT) of human plasma was determined in a fibrometer (Becton-Dickinson Microbiology Systems, Cockeysville, Md.) using Baxter reference procedure LIB0293-J, 3 / 93 revision (Baxter Scientific, Edison, N.J.).
[0124] Prior to the start of the experiment, 2 to 3 mL of 0.02 M CaCl2 in a 5 mL tube were placed into the heating chamber of the fibrometer. Human plasma samples were either freshly drawn and kept on ice or reconstituted per the manufacturer's recommendation from Hemostasis Reference Plasma (American Diagnostics, Greenwich, Conn.).
[0125] Unfractionated heparin from porcine intestinal mucosa (Sigma Chemical, St. Louis, Mo.), low molecular weight heparin from porcine intestinal mucosa (Lovenox®, enoxaparin sodium, Rhone-Poulenc Rorer Pharmaceuticals, Collegeville, Pa.) or mAb anticoag...
example 3
Efficacy of murine Factor IX mAbs in Rat Thrombus Model
[0133] In order to evaluate the efficacy of anti-Factor IX antibodies in prevention of arterial thrombosis, the rat carotid artery thrombosis model as reported by Schumacher et al. in J Cardio Pharm, 22, 526-533 (1993) was adapted. This model consists of segmental injury to the carotid endothelium by oxygen radicals generated by FeCl3 solution applied on the surface of the carotid artery.
[0134] In brief, rats were anesthetized with pentobarbitone sodium, the jugular vein cannulated for intravenous injections and the left femoral artery cannulated for blood pressure and heart rate monitoring. The carotid artery was isolated by aseptic technique via a surgical incision in the neck and equipped with a magnetic flow probe for blood flow measurement. After a period of stabilization, baseline parameters were established for the following variables: carotid blood flow, arterial pressure, heart rate, activated partial thromboplastin t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| activated partial thromboplastin time | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com