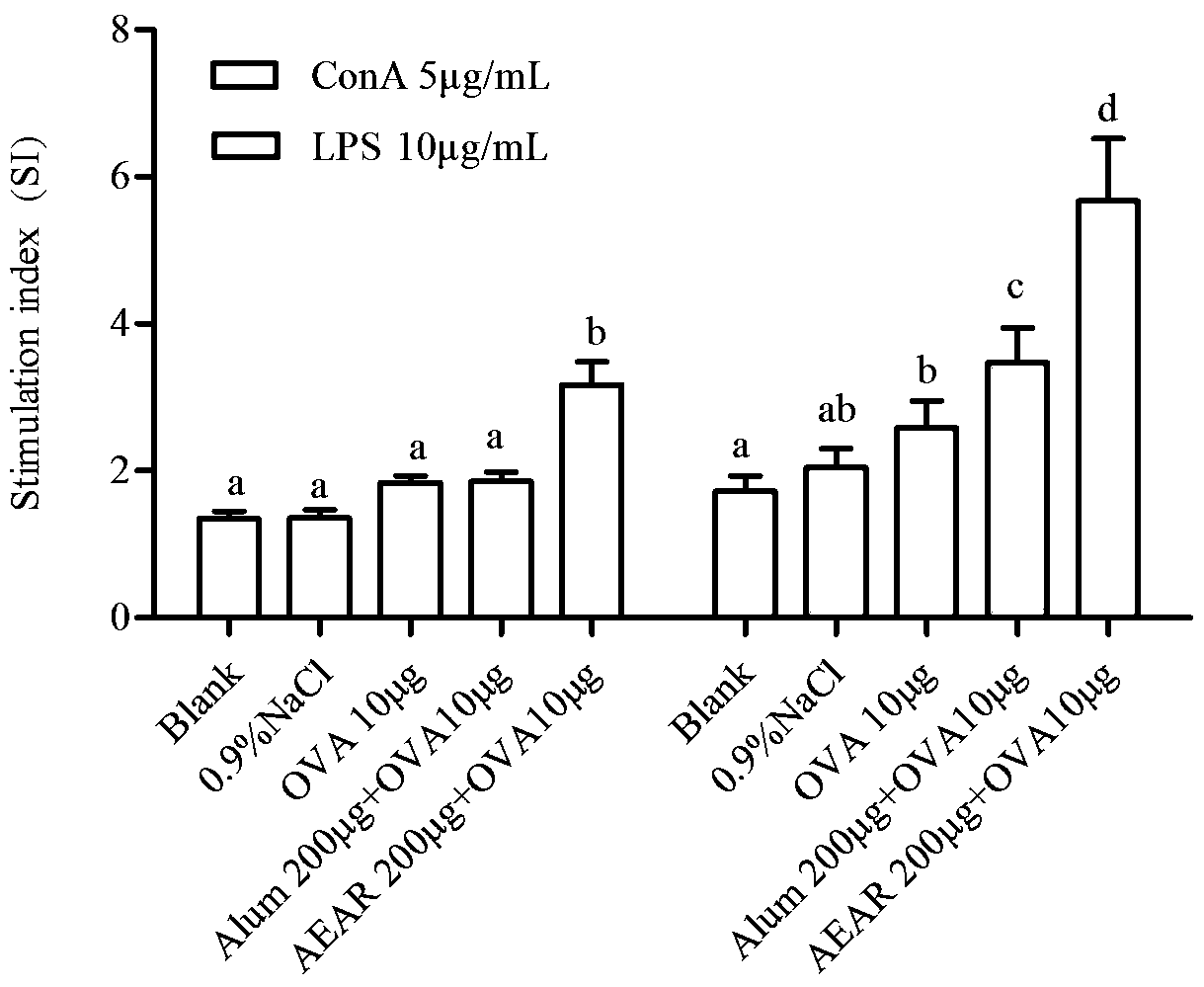
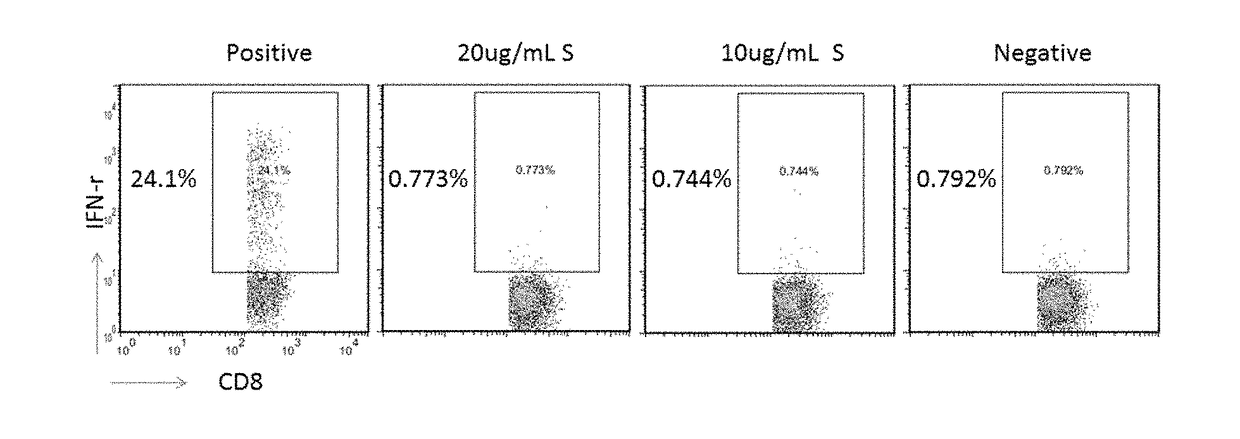
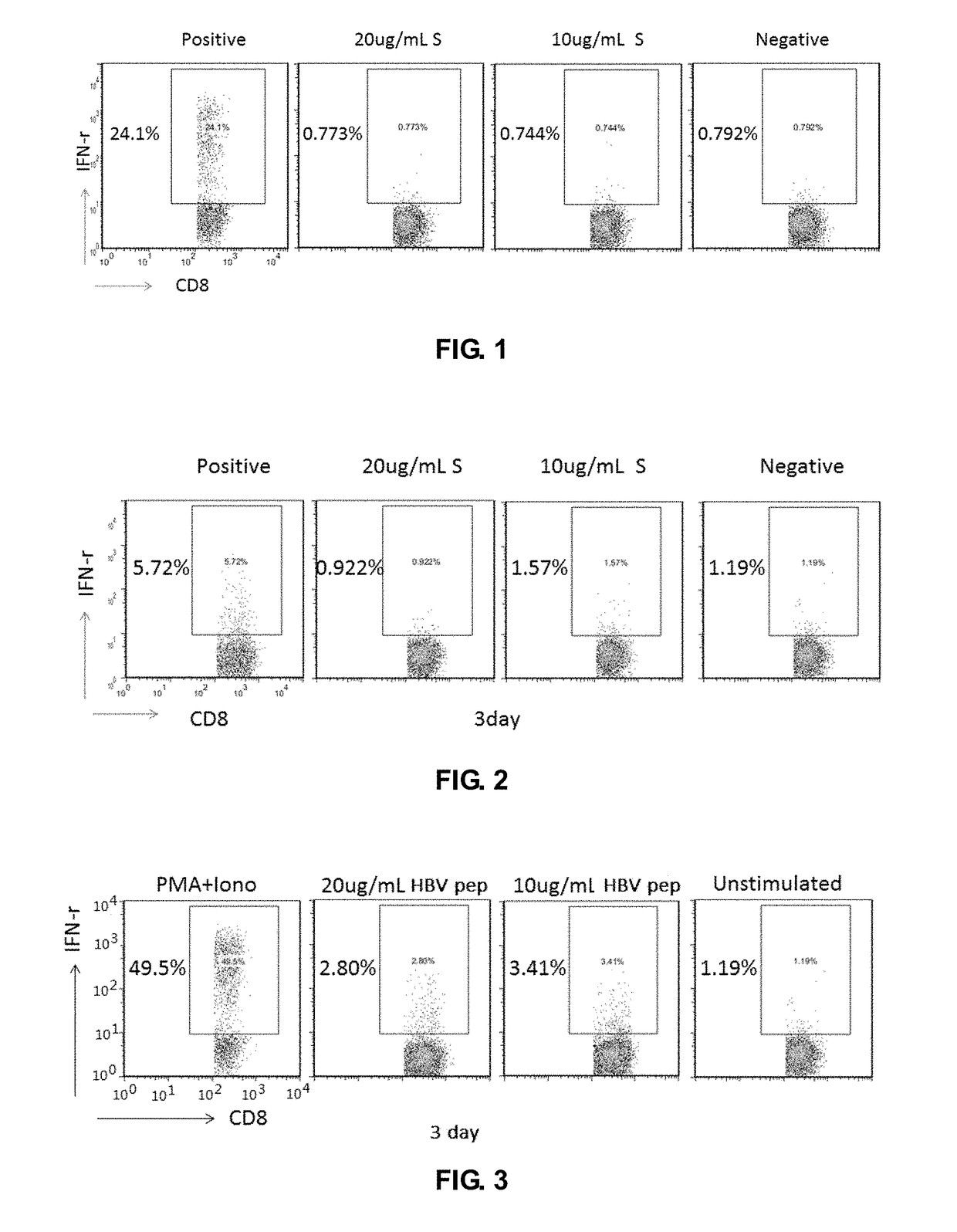
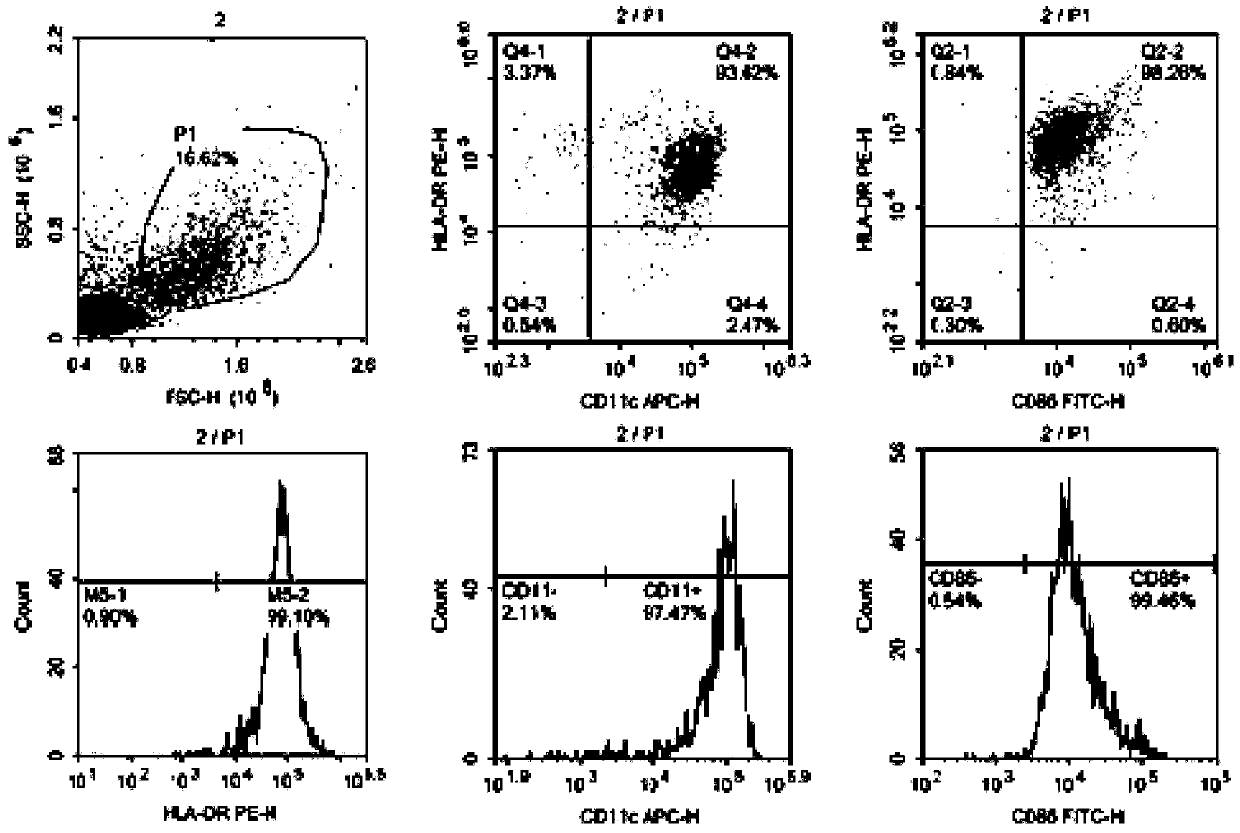
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Cellular Immunology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The study of those manifestations of the immune system which are mediated by antigen-sensitized T-lymphocytes.
Preparation method and application of tumour cell specific polyclonal T cells
InactiveCN104630146AHigh killing efficiencyApparent tumor cell specificityMammal material medical ingredientsBlood/immune system cellsCell specificPeripheral blood mononuclear cell
The invention relates to the technical fields of molecular biology and cellular immunology and in particular relates to a preparation method of tumour cell specific polyclonal T cells. The preparation method of the tumour cell specific polyclonal T cells comprises the following steps: (1) obtaining peripheral blood mononuclear cells; (2) mixing the obtained peripheral blood mononuclear cells with gene modifying tumour cells; and (3) resuspending the obtained mixed cells in a culture medium containing cell factors which can maintain cell growth, proliferation and differentiation. The prepared tumour cell specific polyclonal T cells are mainly applied to medicines which are sued for preventing or treating cancers. The preparation method of the tumour cell specific polyclonal T cells has the advantages that the prepared tumour cell specific polyclonal T cells have the characteristics of large quantity, high specificity and strong killing capability; besides, a technology is simple, and the preparation cost is obviously reduced compared with the prior art.
Owner:马飞
Special-purpose kit for preparing human dendritic cell vaccines
InactiveCN103638517AHigh selectivityAchieve the purpose of preventionAntibody medical ingredientsAntineoplastic agentsHigh risk populationsMicrobiology
The invention belongs to the technical field of cellular immunology and specifically discloses a special-purpose kit for preparing human dendritic cell vaccines capable of largely secreting IL(Interleukin)-12. The kit consists of a monocyte separating-obtaining culture medium, a culture medium for promoting DC (Dendritic Cell) to induce and differentiate, an agent for promoting the DC to mature and a tumor antigen. The DC prepared by the special-purpose kit disclosed by the invention is mainly applied to treating cancer patients or preventing cancer high-risk population. The special-purpose kit disclosed by the invention has the advantage that the prepared DC can secrete a great deal of IL-12, so that T cells are promoted to differentiate towards Th1 type immune response direction; and meanwhile, the special-purpose kit has the advantages of being simple in preparation process, low in cost, easy for large-scale production, and the like.
Owner:SHENZHEN HORNETCORN BIOTECH
Method for external amplification natural killer cell
InactiveCN101314764AAmplification realizedLow costBlood/immune system cellsMethyl-beta-cyclodextrinInterleukin II
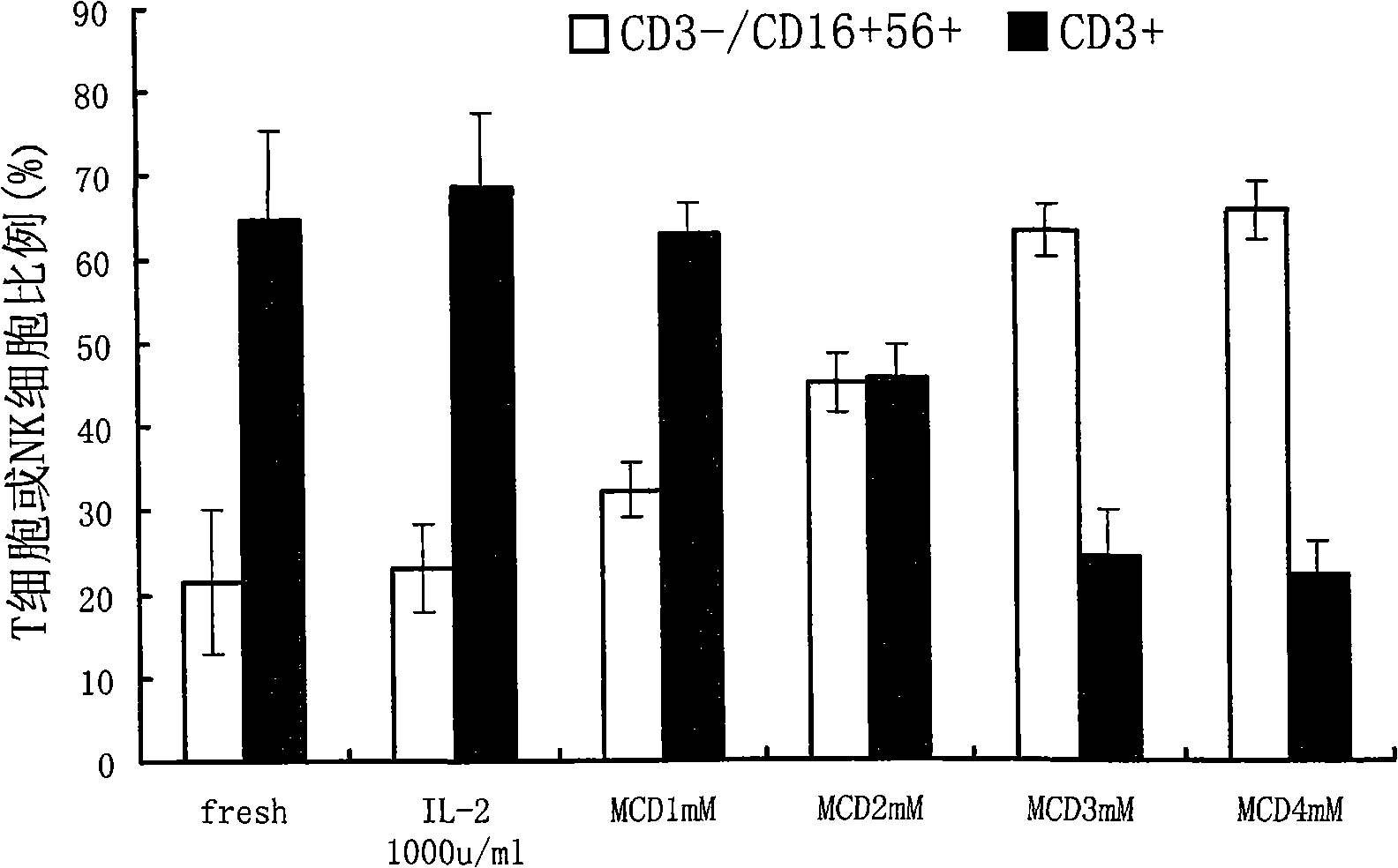
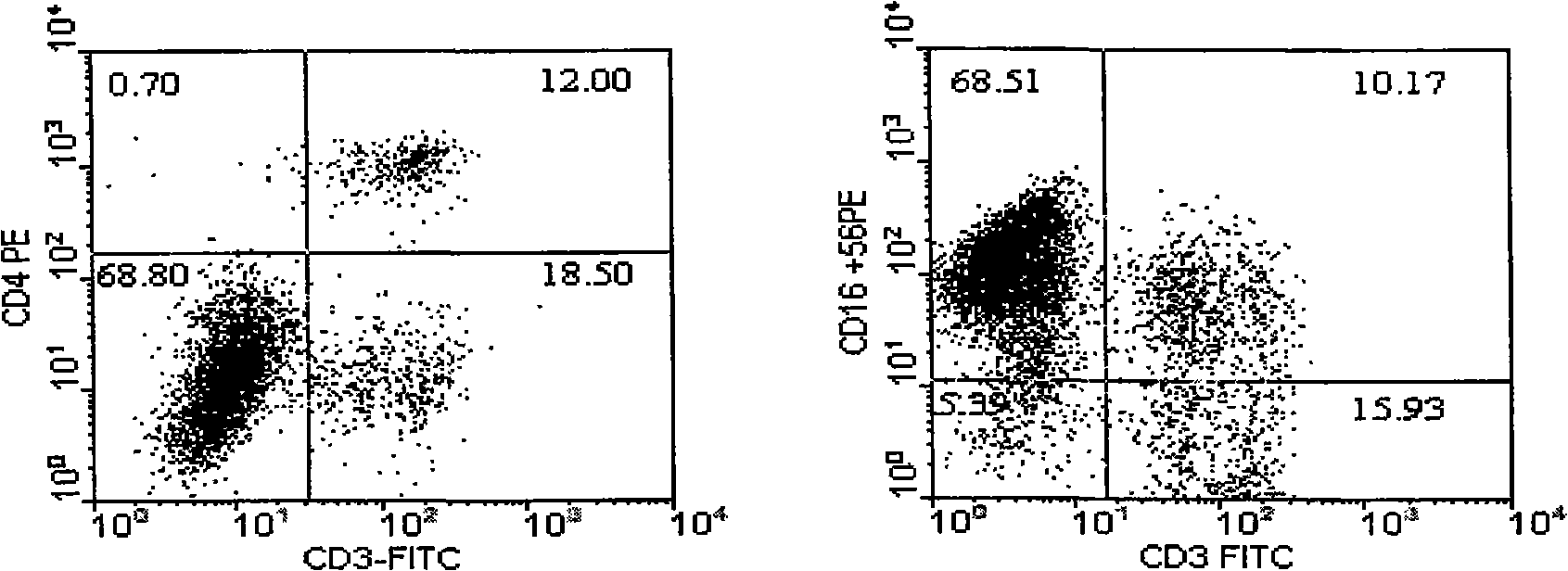
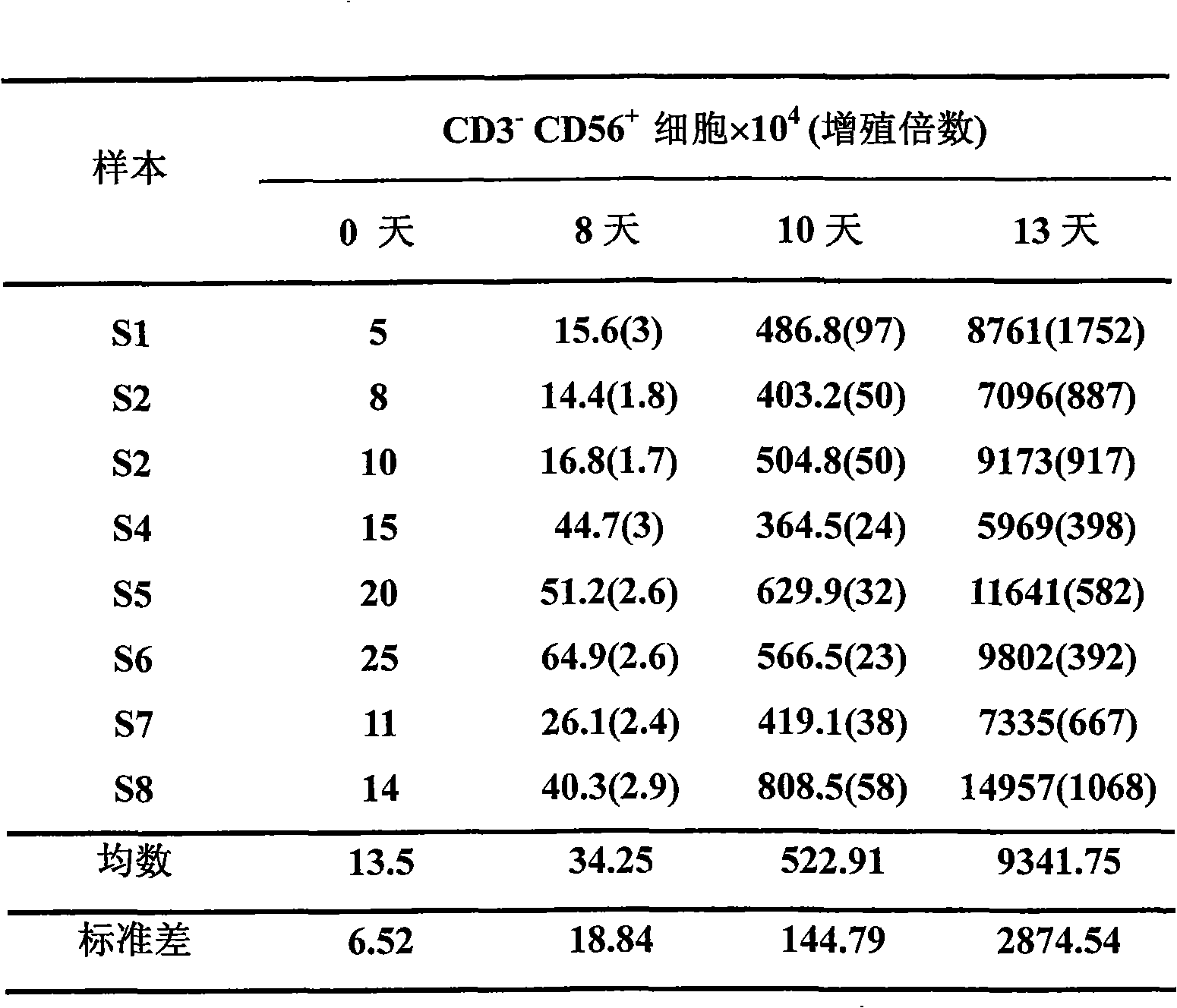
The invention belongs to the technology field of the cellular immunology, and particularly relates to a method for the dominant amplification of in vitro peripheral blood nature killer cells with a large amount. The method comprises the steps as follows: (1) separating single nucleus cell from human or animal peripheral blood; (2) suspending the single nucleus cell in an RPMI 1640 culture solution containing 5%-15% autologous serum and pretreating with methyl-Beta-cyclodextrin with an effective concentration of 1-4mmol / L for 36-60 hours; and (3) adding recombined interleukin 2, and amplification-culturing in an RPMI 1640 culture solution containing 5% of new-born calf serum and 5% of autologous serum for above 10 days. The method has the advantages that the blood sample amount is small; the cost is low; the operation is convenient; and the amplification multiple is high, and the nature killer cells can be amplified by 392-1752 times in a short term.
Owner:BENGBU MEDICAL COLLEGE
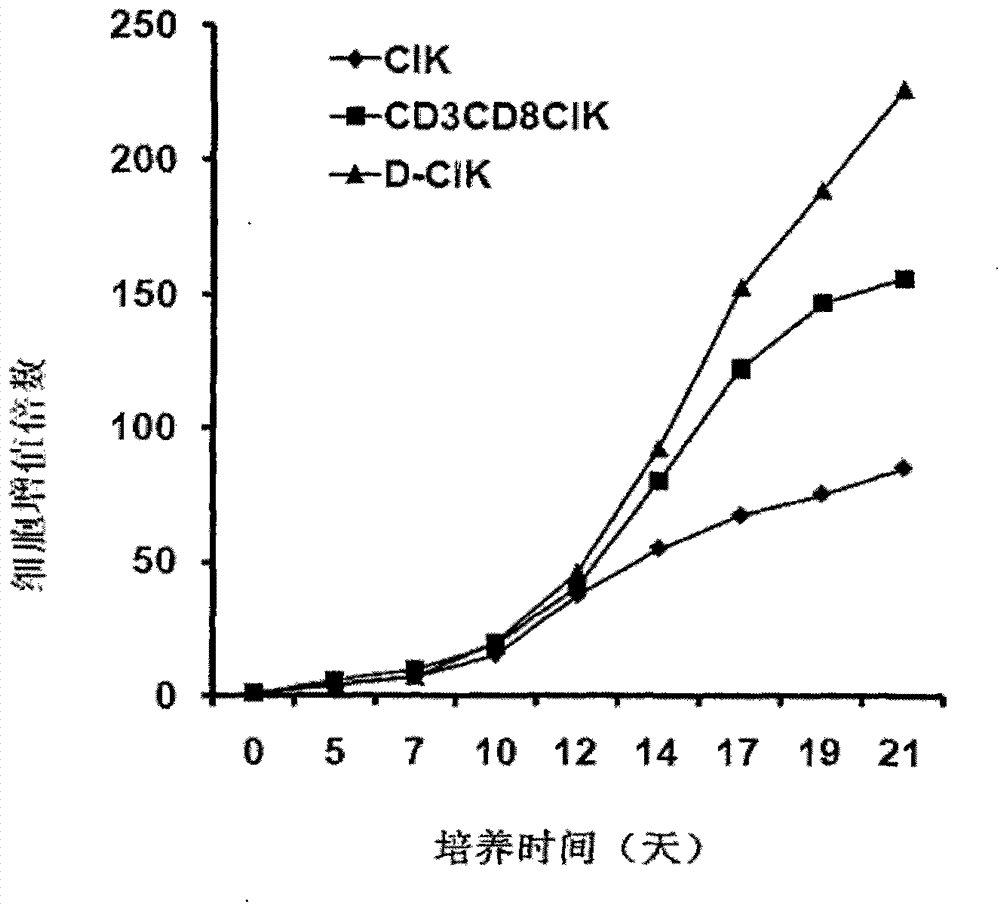
Preparation method of human D-CIK (dendritic cell activated and cytokine induced killer) cell with high toxicity and high value-adding capacity
InactiveCN103642754AHigh amplification efficiencyEnhance killing activityBlood/immune system cellsDendritic cellVenous blood
The invention belongs to the technical field of cell immunity, and particularly relates to a preparation method of a human D-CIK (dendritic cell activated and cytokine induced killer) cell with high toxicity and high value-adding capacity. The preparation method comprises the following steps of 1) collecting peripheral venous blood so as to obtain a single karyocyte; 2) further separating the single karyocyte so as to obtain a mononuclear cell and a suspension cell; 3) induced differentiating the mononuclear cell to a mature DC (dendritic cell); 4) induced culturing CD3+CD8+CIK cells; 5) induced culturing the D-CIK cells so as to obtain a novel heterogeneity cell mass, i.e. D-CIK cells. Compared with the CIK cell, the value-adding activity and the cell toxic activity of the prepared D-CIK cell are stronger, and the prepared D-CIK cell also has the advantages of simple preparation process, low cost, and the like, and mass production is easy to realize. The prepared D-CIK cell is mainly used for treating cancer patients or preventing the cancer high-risk group.
Owner:SHENZHEN HORNETCORN BIOTECH
Trachyostracous mussel extract capable of increasing immunity function
InactiveCN1682769AEnhance immune functionImprove immunityAnthropod material medical ingredientsImmunological disordersLymphocyteCell immunity
The present invention relates to immunological preparation, and a kind of trachyostracous mussel extract capable of raising immunity function. The trachyostracous mussel extract is the effective components extracted from the homogeneous slurry of soft trachyostracous mussel tissue. Animal test and cellular immunological experiment show that the trachyostracous mussel extract can increase the natural killer cell, macrophage and lymphocyte in immunity system, promote the formation of antibody and complement and strengthen the non-specific cell immunity function of body, so that it may be used in preparing immunity function raising preparation.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for preparing human dendritic cell vaccine
ActiveCN103599528ASimple processLow costBlood/immune system cellsAntibody medical ingredientsPeripheral blood mononuclear cellHigh-risk cancer
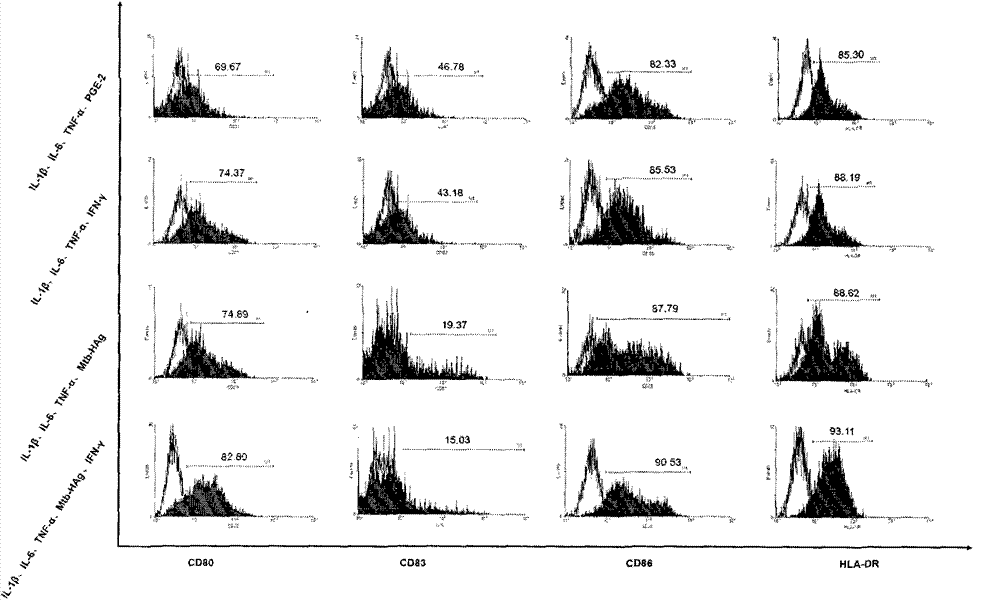
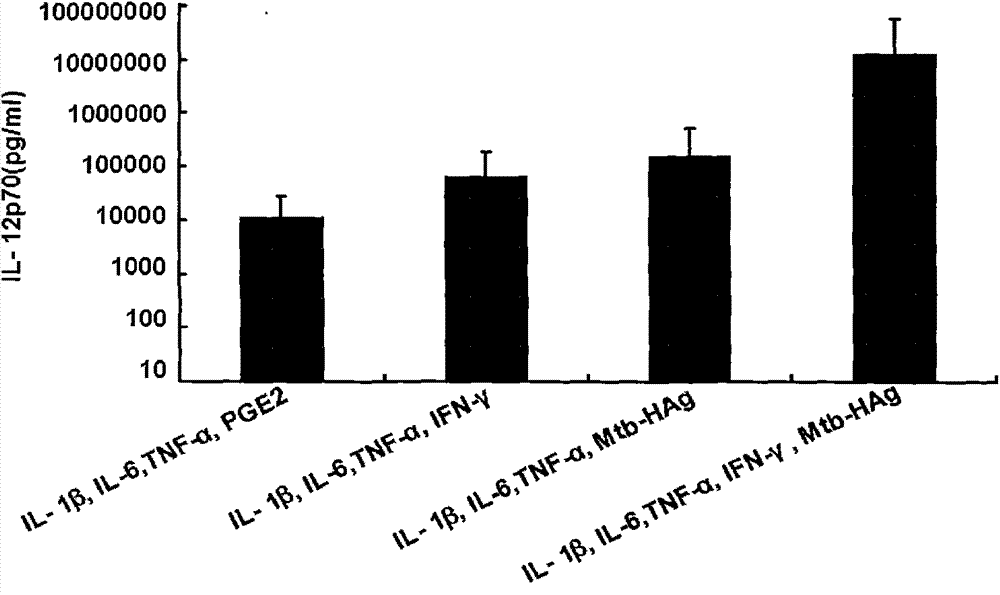
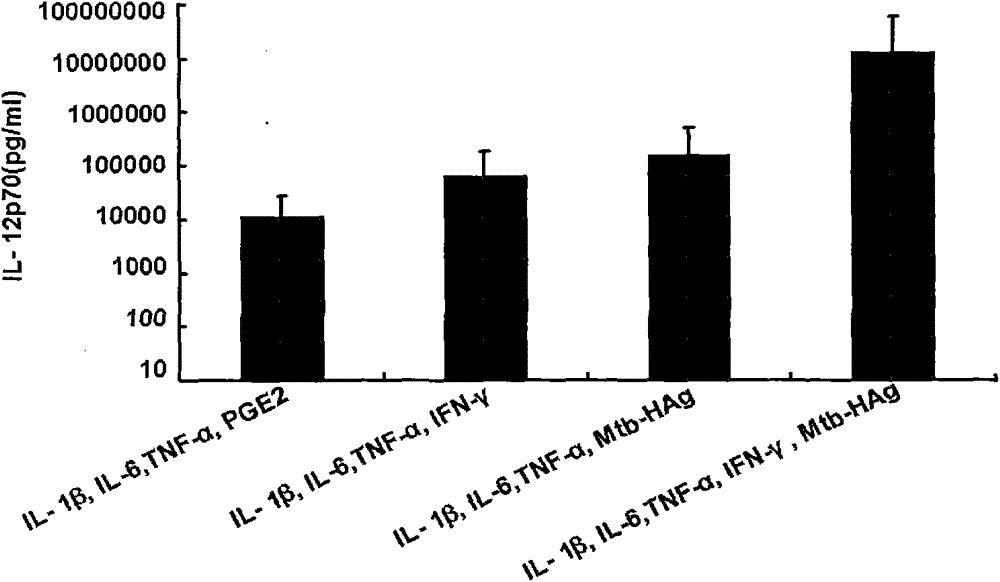
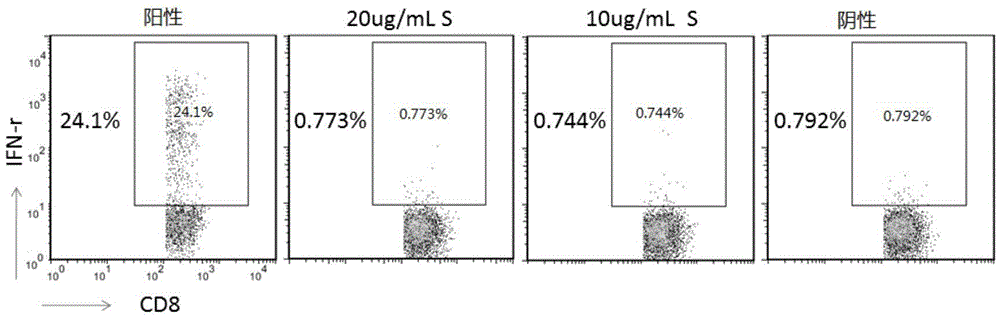
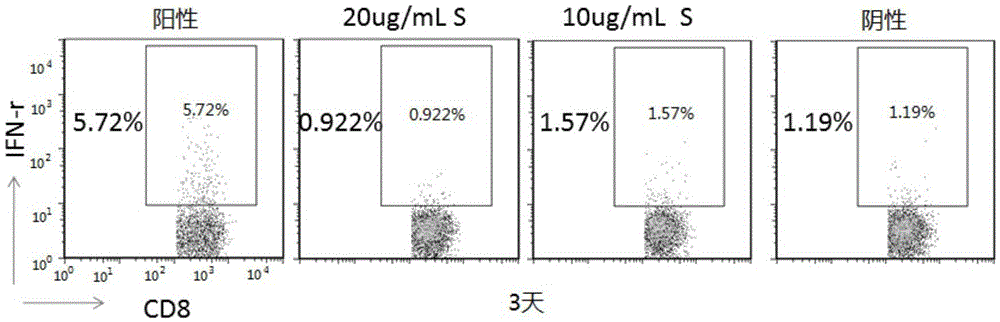
The invention belongs to the technical field of cellular immunology, and particularly relates to a method for preparing a human dendritic cell vaccine. The method comprises the following steps: (1) separating a monocyte from peripheral blood mononuclear cells by an adherent method; (2) inducing the monocyte to differentiate into DC by using a DC culture medium, and adding a tumor antigen on the third day; (3) on the fifth day, adding a combined type DC maturity promoting agent of IL-1beta, IL-6, TNF-alpha, IFN-gamma and Mtb-HAg; and (4) on the sixth-seventh day, detecting the maturity degree of the DC and the secretion amount of IL-12. The prepared DC is mainly applied in treatment of cancer patients or prevention of high-risk cancer groups. The method has the advantages that the prepared DC can secrete a large amount of IL-12, thereby prompting T cells to differentiate in the direction of Th1-type immune response; and moreover, the method has the superiorities of simple preparation technology, low cost, easy large-scale production and the like.
Owner:SHENZHEN HORNETCORN BIOTECH
Method for easily and efficiently preparing human gamma/deltaT cells
The invention belongs to the technical field of cellular immunology, in particular to a method for amplifying human peripheral blood gamma / deltaT cells massively and preferentially in vitro. The method comprises the following steps of: (1) preparing tubercle bacillus heat stable antigen by using the cultured tubercle bacillus; (2) stimulating to amplify the gamma / deltaT cells by combining the prepared tubercle bacillus heat stable antigen and cell factors rhIL-2 and rhIL-21; and (3) performing morphologic observation through a microscope, performing purity and immunophenotyping analysis by using a flow cytometry, and performing cytotoxicity detection through MTT assay. The method has the advantages that: culture conditions and operation are simple, a large number of gamma / deltaT cells with high purity and high cytotoxicity can be prepared in short time, and the cost is low.
Owner:SHENZHEN HORNETCORN BIOTECH
Method for diagnosing Lyme disease using a cellular immunological test
The present invention relates to a method for diagnosing Lyme disease in a subject, the method comprising the steps of: (a) obtaining a sample from said subject, (b) contacting said sample with a source of Borrelia antigens and (c) determining the expression level of a pro-inflammatory cytokine in said sample at the end of step (b).
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
Application of cistanche deserticola polysaccharides as vaccine adjuvant
InactiveCN105079801ASmall immune boosting effectExcellent immune boosting effectImmunological disordersAntibody medical ingredientsSide effectSpecific antibody
The invention provides application of cistanche deserticola polysaccharides as a vaccine adjuvant. Through tests such as detecting OVA (ovalbumin) specific antibody titer and cellular immunological activity in mice sera by animal grouping and immunizing methods and ELISA (enzyme linked immunosorbent assay), results show that the cistanche deserticola polysaccharides can serve as the adjuvant of a protein vaccine, a nucleic acid vaccine, a polypeptide vaccine, an inactivated vaccine or a live attenuated vaccine, and are obviously superior to an aluminum adjuvant in immunoenhancement effect, little in side effect, wide in source and easy to produce, and a preparation process of the cistanche deserticola polysaccharides is simple, low in cost, high in yield and beneficial to large-scale production.
Owner:XINJIANG UNIVERSITY
Tumor specific target and application thereof in preparing preparation for cellular immunotherapy
ActiveCN104693276ASpecific killing effectEffective expansionPeptidesBlood/immune system cellsDendritic cellT lymphocyte
The invention relates to the technical field of biology, and discloses a tumor specific target and application thereof. The target amino acid sequence is SAKYGVRKF. The tumor specific target can implement extraneous effective amplification and activation of dendritic cells. The provided T lymphocyte has specific killing effects on tumors, and is a cellular immunotherapy technique which most conforms to the cellular immunology principle at present.
Owner:河北博海生物工程开发有限公司
Immunofluorescence microscopy observation method for marine bivalve meiosis device
InactiveCN101639441AEasy to operateOperation time savingBiological testingFluorescence/phosphorescenceCytochemistryFluorescence
The invention relates to cytochemistry and cellular immunology, which are particularly used for observing a marine bivalve meiosis device through immunofluorescence microscopy. A sample can be observed after pre-treatment of fixing and the like, dyeing and flaking. The sample is fixed by phosphate buffered saline (PBS) of paraformaldehyde at a mass percentage concentration of 2 to 6 percent; then,permeabilization treatment and sealing treatment are performed sequentially; immunofluorescence dyeing is performed on sample spindle bodies, and fluorescence dyeing is performed on chromosomes withthe phosphate buffered saline containing propidium iodide of which the concentration is 5 to 20 mg of per liter; and the sample is flaked after dyeing for observation through fluorescence microscopy.The cytological immunofluorescence observation method is suitable for observing the spindle bodies and the chromosomes of the marine bivalve meiosis device synchronously, has the advantages of simpleand direct operation, high definition and high study efficiency, and is a qualitative, positioning and quantitative cytological immunofluorescence observation method which inosculates forms and functions.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for detecting killing activity of human (gamma)(delta)T cells against K562 cell strain
InactiveCN103571913AIncrease the number ofHigh purityMicrobiological testing/measurementMicroorganism based processesVolumetric Mass DensityMicrobiology
The invention belongs to the technical field of cellular immunology, and particularly relates to a method for detecting the killing activity of human (gamma)(delta)T cells against a K562 cell strain, which is used for detecting the prepared human (gamma)(delta)T cells. The method comprises the following steps: preparing the human (gamma)(delta)T cells; fetching the K562 cell strains of the logarithmic phase as target cells, and adjusting the cell density to 1*10<5> / ml, 5*10<4> / ml and 2.5*10<4> / ml; paving the target cells in a 96-hole culture plate with 100mu l per hole, and culturing for 24 hours; fetching the prepared human (gamma)(delta)T cells, and adjusting the cell density to 1*10<6>ml; adding the human (gamma)(delta)T cells into the 96-hole culture plate, wherein the effect-target ratios are 10:1, 20:1 and 40:1 respectively; and setting 3 parallel holes for each density, setting blank controls respectively and calculating the killing activity. By adopting the method provided by the invention, the toxicity of the human (gamma)(delta)T cells is guaranteed.
Owner:SHENZHEN HORNETCORN BIOTECH
Morphology and purity and immunophenotyping detection method of human (gamma)(delta)T cells
ActiveCN103571791AIncrease the number ofHigh purityBlood/immune system cellsMaterial analysisAntigenPhosphate
The invention belongs to the technical field of cellular immunology, and particularly relates to a morphology and purity and immunophenotyping detection method of human (gamma)(delta)T cells, which is used for detecting the prepared human (gamma)(delta)T cells. The method comprises the following steps: preparing human (gamma)(delta)T cells; and performing morphological observation with a microscope and purity and immunophenotyping analysis with a flow cytometer. The method provided by the invention has the advantages that the (gamma)(delta)T cells obtained by the test have the characteristics of large quantity, high purity, strong cytotoxicity and the like so as to ensure that the prepared human (gamma)(delta)T cells can meet the clinical needs; and moreover, the effects that the culture cost is greatly reduced from the non-peptide phosphate antigens such as IPP and the irritants such as anti-TCR (gamma)(delta) antibody are realized and the problems of small quantity, low toxicity, high cost and the like of in-vitro culture of (gamma)(delta)T cells in the prior art are solved.
Owner:SHENZHEN HORNETCORN BIOTECH
Cellular immunological detection kit for evaluating curative effect of vaccine and storage method thereof
InactiveCN106610423AImprove stabilityViral antigen ingredientsBiological material analysisMHC restrictionCurative effect
The invention provides a cellular immunological detection kit for evaluating the curative effect of a vaccine and a storage method thereof. The kit comprises MHC-restricted antigen peptide. The cellular immunological detection kit for evaluating the curative effect of the vaccine can utilize a therapeutic vaccine research database and specimens in a clinic trial phase and employs flow cytometry for comprehensive detection of immune cells and cytokines secreted by the immune cells, so a cellular immunological curative effect evaluation system is established. The cellular immunological detection kit for evaluating the curative effect of the vaccine has good stability, and the stability can maintain 90% or above after for storage for one year or above.
Owner:FUDAN UNIV
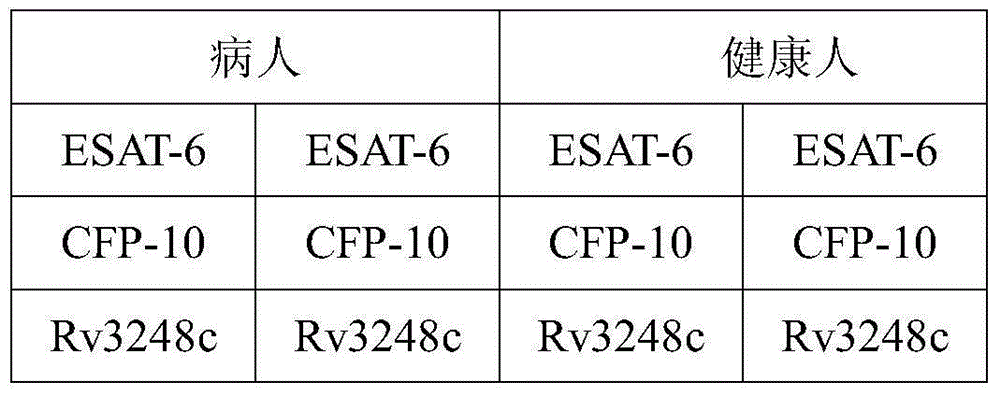
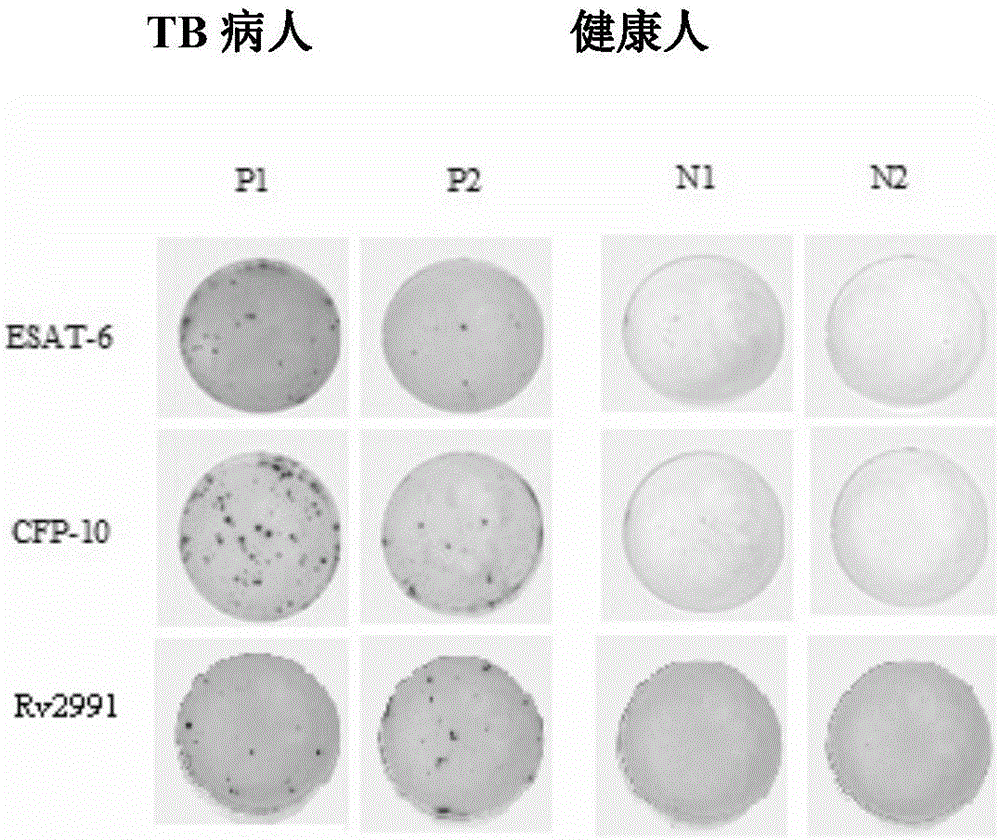
Mycobacterium tuberculosis Rv 3248 c recombinant protein, preparation method and application of mycobacterium tuberculosis Rv 3248 c recombinant protein
InactiveCN105131094AReduce manufacturing costImprove stabilityDepsipeptidesBiological testingSkin testImmunodominant Antigens
The invention discloses a mycobacterium tuberculosis Rv 3248 c recombinant protein, a preparation method and application of the mycobacterium tuberculosis Rv 3248 c recombinant protein. The recombinant protein disclosed by the invention has an amino acid sequence shown as SEQ ID NO. 1. The invention also provides the preparation method of a coded nucleotide sequence of the mycobacterium tuberculosis Rv 3248 c recombinant protein and the recombinant protein. Based on a research result of immunomics, a mycobacterium tuberculosis immunodominant antigen Rv 3248 c is reported for the first time. By applying the mycobacterium tuberculosis Rv 3248 c recombinant protein to a tuberculosis cellular immunology diagnosis, higher sensitivity is achieved, besides the recombinant protein has the advantages of higher speed, safety and reliability in comparison with a conventional skin test.
Owner:SHANGHAI JIAO TONG UNIV
Application of alpine yarrow herb polysaccharide as vaccine adjuvant
InactiveCN105497890AEasy to produceEasy to makeSsRNA viruses negative-senseSsRNA viruses positive-senseSerum igeSide effect
The invention provides application of alpine yarrow herb polysaccharide as a vaccine adjuvant. Through an animal grouping and immune method, ELISA testing of titer and cellular immunological activity of an anti-OVA specific antibody in mouse sera and other experiments, results prove that the alpine yarrow herb polysaccharide can serve as an adjuvant for protein vaccines or polypeptide vaccines or inactivated vaccines or attenuated live vaccines or nucleic acid vaccines, and the immunological enhancement effect of the alpine yarrow herb polysaccharide is obviously better than that of an aluminum adjuvant. The alpine yarrow herb polysaccharide has the advantages of being small in side effect, wide in source and easy to produce. Moreover, the preparation process of the alpine yarrow herb polysaccharide is simple, cost is low, yield is high, and large-scale production is facilitated.
Owner:XINJIANG UNIVERSITY
Method for easily and efficiently preparing human gamma/deltaT cells
The invention belongs to the technical field of cellular immunology, in particular to a method for amplifying human peripheral blood gamma / deltaT cells massively and preferentially in vitro. The method comprises the following steps of: (1) preparing tubercle bacillus heat stable antigen by using the cultured tubercle bacillus; (2) stimulating to amplify the gamma / deltaT cells by combining the prepared tubercle bacillus heat stable antigen and cell factors rhIL-2 and rhIL-21; and (3) performing morphologic observation through a microscope, performing purity and immunophenotyping analysis by using a flow cytometry, and performing cytotoxicity detection through MTT assay. The method has the advantages that: culture conditions and operation are simple, a large number of gamma / deltaT cells with high purity and high cytotoxicity can be prepared in short time, and the cost is low.
Owner:SHENZHEN HORNETCORN BIOTECH
Morphology and purity and immunophenotyping detection method of human (gamma)(delta)T cells
ActiveCN103571791BIncrease the number ofHigh purityBlood/immune system cellsMaterial analysisAntigenPhosphate
Owner:SHENZHEN HORNETCORN BIOTECH
Immunological test kit for evaluating vaccine efficacy and storage method thereof
InactiveUS20180246105A1Improve stabilityViral antigen ingredientsBiological material analysisMHC restrictionClinical trial
Embodiments of the invention provide a cell immunological assay kit used for evaluating the efficacy of a vaccine on the aspect of cell immunology and a method for storing the cell immunological assay kit. Some embodiments of the invention comprise a MHC-restricted viral antigenic peptide. Further, in some embodiments, the kit may use a vaccine research database of clinical trials and samples which are utilized in the therapeutic and conduct comprehensive testing on immune cells secrete cytokines by flow cytometry to establish a cellular immunology evaluation system. The kit may have a good storage stability and, in particular embodiments, the kit may provide a stability that maintains more than 90% of a material stored therein over a year or more.
Owner:FUDAN UNIV
A virus-specific target and its application in the preparation of cellular immunotherapy preparations
ActiveCN104693275BSpecific killing effectEffective expansionAntiviralsPeptidesDendritic cellT lymphocyte
The invention relates to the field of biotechnology, and discloses a virus-specific target and application thereof. The hepatitis B virus specific target amino acid sequence of the present invention is FCWEWASAK. The present invention also provides the application of the target in the preparation of preparations for treating diseases related to hepatitis B. The target of the present invention can realize the effective expansion and activation of dendritic cells in vitro, and the presented T lymphocytes have a specific killing effect on the virus, which is currently the most cellular immunotherapy technology in line with the principle of cellular immunology, and has the advantages of Good application prospects.
Owner:河北博海生物工程开发有限公司
A tumor-specific target and its application in the preparation of cellular immunotherapy preparations
ActiveCN104693276BSpecific killing effectEffective expansionPeptidesBlood/immune system cellsDendritic cellT lymphocyte
The invention relates to the technical field of biology, and discloses a tumor specific target and application thereof. The target amino acid sequence is SAKYGVRKF. The tumor specific target can implement extraneous effective amplification and activation of dendritic cells. The provided T lymphocyte has specific killing effects on tumors, and is a cellular immunotherapy technique which most conforms to the cellular immunology principle at present.
Owner:河北博海生物工程开发有限公司
Novel Method for Diagnosing Lyme Disease Using a Cellular Immunological Test
The present invention relates to a method for diagnosing Lyme disease in a subject, the method comprising the steps of: (a) obtaining a sample from said subject, (b) contacting said sample with a source of Borrelia antigens and (c) determining the expression level of a pro-inflammatory cytokine in said sample at the end of step (b).
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
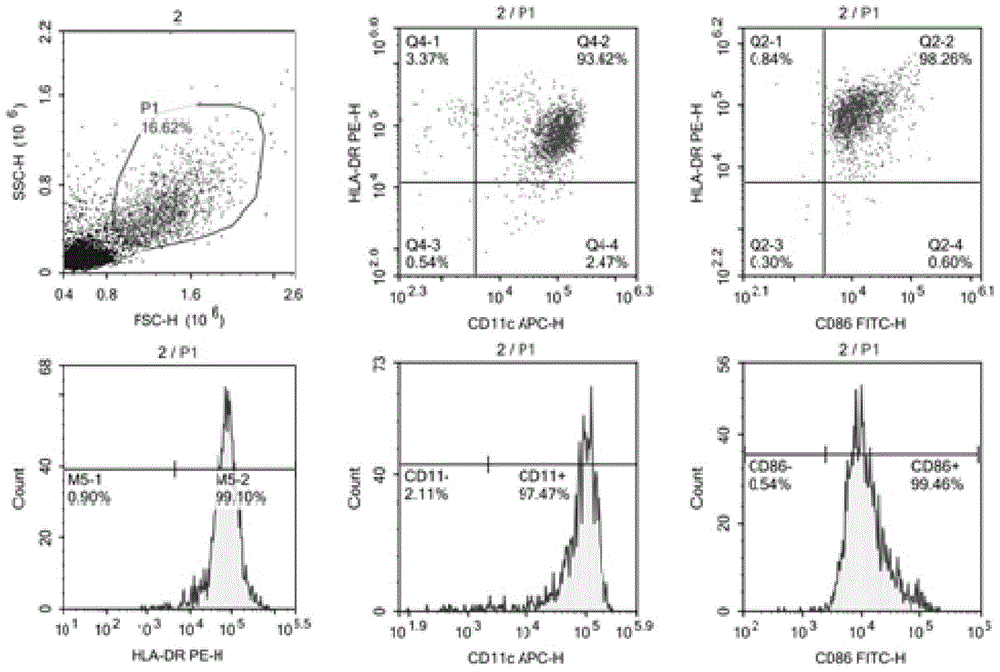
Mass preparation method of dendritic cells derived from umbilical cord blood CD34+ hematopoietic stem cells
ActiveCN104845934BGenerate a large number of DCStrong Antigen PhagocytosisBlood/immune system cellsDendritic cellMass generation
The invention belongs to the field of cellular immunology, and discloses a methodological study for inducing mass generation of dendritic cells (DCs) from cord blood CD34+ hematopoietic stem cells. The method comprises the following main steps: separating mononuclear cells from cord blood, separating CD34+ hematopoietic stem cells through an immunomagnetic bead positive screening method, continuously amplifying the CD34+ cells for 30 days by using an amplification culture medium (IMDM, FBS, GM-CFS, SCF), periodically collecting mass CD34-DC precursor cells during the amplification process, and inducing the precursor cells to be differentiated into immaturate DCs through a GM-CFS / IL-4 culture medium. The method disclosed by the invention is capable of obtaining the DCs with 109 orders of magnitudes; compared with the traditional DCs induced by peripheral blood mononuclear cells, the CD34-DCs have high antigen phagocytic capacity and the capacity of inducing T lymphocyte proliferation.
Owner:玥特农生物科技河北有限责任公司
Preparation method of human DC (dendritic cell) tumor vaccine
InactiveCN109468276AHigh purityPromote maturityCulture processCancer antigen ingredientsAgent CombinationDendritic cell
The invention belongs to the field of cellular immunology and particularly relates to a preparation method of a human DC (dendritic cell) tumor vaccine. Specifically, differentiated but not mature monocytes of DCs are induced by a DC maturity promoting agent to become mature DCs, wherein the DC maturity promoting agent is a DC maturity promoting agent combination of IL-1 beta, TNF-alpha, IFN-gammaand human recombinant protein gp100. DC purity and maturity of the human DC tumor vaccine prepared with the method are improved greatly, fusion of the DCs with a tumor antigen is promoted, and tumortreatment is facilitated; the preparation process is simple, and the cost is low.
Owner:CHANGZHOU NO 2 PEOPLES HOSPITAL
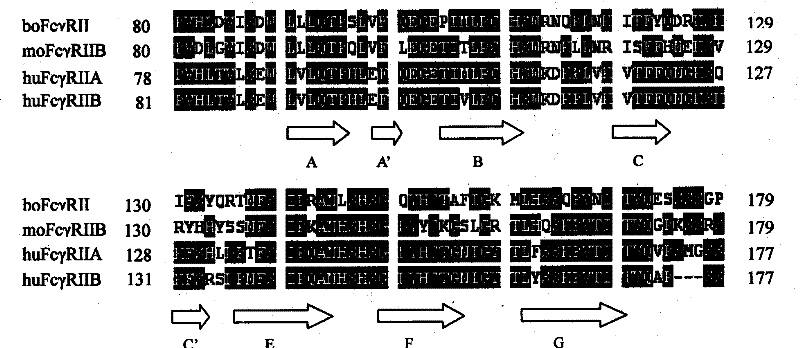
Human Fc<gamma>R II linear ligand binding epitope
InactiveCN101367868BEnhanced inhibitory effectAutoimmune disease resolutionPeptidesFermentationChemical synthesisLinear epitope
The present invention relates to a linear ligand-binding epitope of human Fc Gamma RII, the amino acid sequence of which is Cys-Thr-Gly-Asn-Ile-Gly-Tyr-Thr-Leu-Phe-Ser-Ser-Lys-Pro-Val-Thr-Ile-Thr-Val. The present invention utilizes technologies, such as bioInformatics, polypeptide synthesis, cellular immunology and molecular biology, to chemically synthesize a linear ligand-binding epitope polypeptide of human Fc Gamma RII and conducts analysis and identification. An experiment conducted on the inhibition effect of the polypeptide on human IgG-soluble receptor binding and human IgG-cell surface receptor binding indicates that the linear epitope polypeptide can show good inhibition effect on human Fc Gamma RII and human IgG, so the linear epitope polypeptide is significant for the in-depth understanding of the molecular foundation of Fc Gamma R-IgG interaction and provides a new approach for the molecular design of human FcR target drugs.
Owner:HENAN ACAD OF AGRI SCI
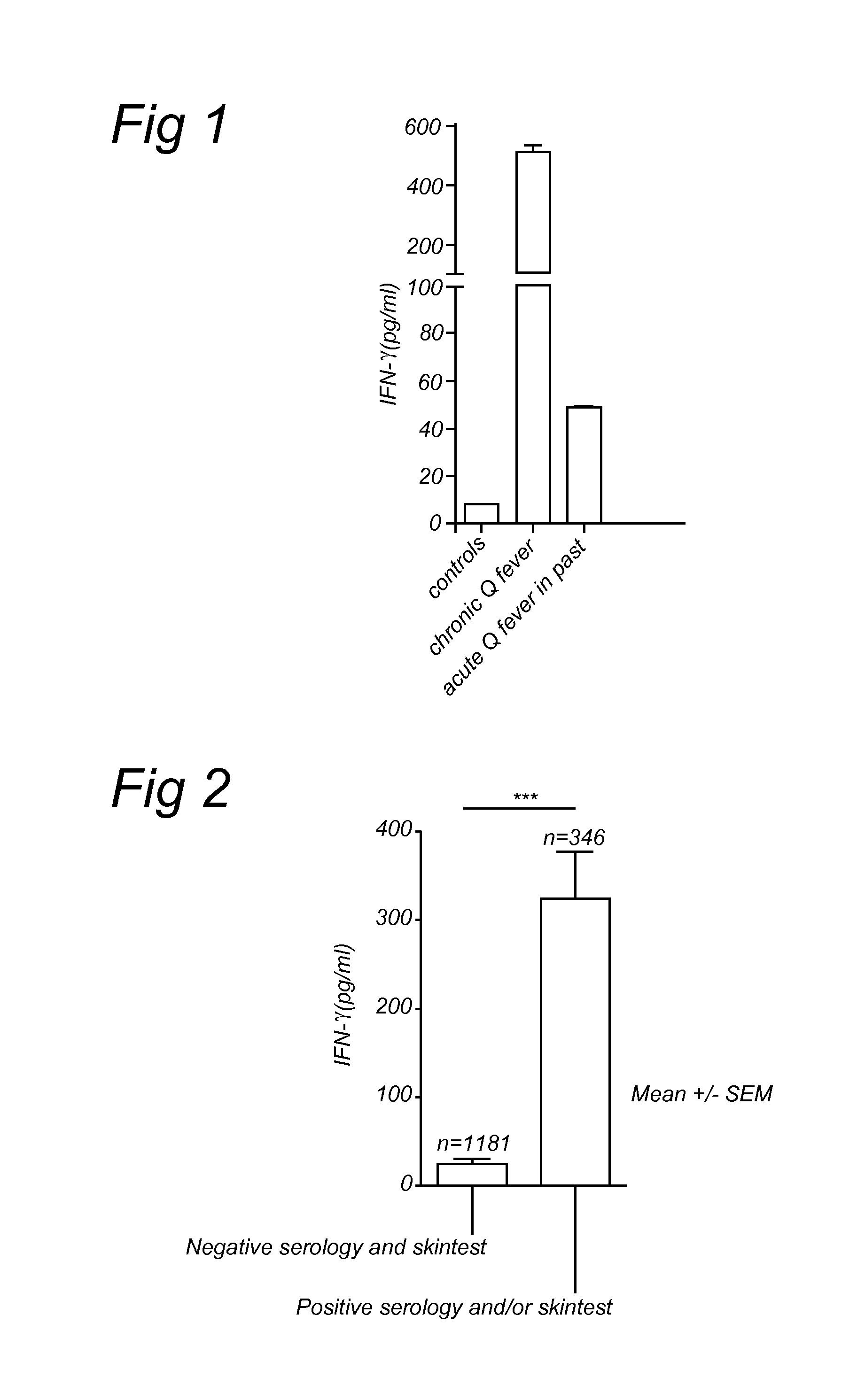
Method for diagnosing Q-fever using a cellular immunological test
The present invention relates to a method for diagnosing Q-fever in a subject, the method comprising the steps of: (a) obtaining a sample from said subject, (b) contacting said sample with a source of a Coxiella burnetii antigen and (c) determining the expression level of a pro-inflammatory cytokine such as IFN-γ in said sample at the end of step (b).
Owner:PRRIL HLDG BV
Method for detecting killing activity of human (gamma)(delta)T cells against K562 cell strain
InactiveCN103571913BIncrease the number ofHigh purityMicrobiological testing/measurementMicroorganism based processesVolumetric Mass DensityMicrobiology
The invention belongs to the technical field of cellular immunology, and particularly relates to a method for detecting the killing activity of human (gamma)(delta)T cells against a K562 cell strain, which is used for detecting the prepared human (gamma)(delta)T cells. The method comprises the following steps: preparing the human (gamma)(delta)T cells; fetching the K562 cell strains of the logarithmic phase as target cells, and adjusting the cell density to 1*10<5> / ml, 5*10<4> / ml and 2.5*10<4> / ml; paving the target cells in a 96-hole culture plate with 100mu l per hole, and culturing for 24 hours; fetching the prepared human (gamma)(delta)T cells, and adjusting the cell density to 1*10<6>ml; adding the human (gamma)(delta)T cells into the 96-hole culture plate, wherein the effect-target ratios are 10:1, 20:1 and 40:1 respectively; and setting 3 parallel holes for each density, setting blank controls respectively and calculating the killing activity. By adopting the method provided by the invention, the toxicity of the human (gamma)(delta)T cells is guaranteed.
Owner:SHENZHEN HORNETCORN BIOTECH
Novel Method for Diagnosing Q-fever Using a Cellular Immunological Test
The present invention relates to a method for diagnosing Q-fever in a subject, the method comprising the steps of: (a) obtaining a sample from said subject, (b) contacting said sample with a source of a Coxiella burnetii antigen and (c) determining the expression level of a pro-inflammatory cytokine such as IFN-γ in said sample at the end of step (b).
Owner:PRRIL HLDG BV
Mycobacterium tuberculosis Rv 2991 recombinant protein, preparation method and application of mycobacterium tuberculosis Rv 2991 recombinant protein
InactiveCN105131095AImprove stabilityReduce manufacturing costBiological material analysisDepsipeptidesSkin testImmunodominant Antigens
The invention discloses a mycobacterium tuberculosis Rv 2991 recombinant protein, a preparation method and application of the mycobacterium tuberculosis Rv 2991 recombinant protein. The mycobacterium tuberculosis Rv 2991 recombinant protein disclosed by the invention has an amino acid sequence shown as SEQ ID NO. 1. The invention also provides the preparation method of a coded nucleotide sequence of the mycobacterium tuberculosis Rv 2991 recombinant protein and the recombinant protein. Based on a research result of immunomics, a mycobacterium tuberculosis immunodominant antigen Rv 2991 is reported for the first time. By applying the mycobacterium tuberculosis Rv 2991 recombinant protein to a tuberculosis cellular immunology diagnosis, higher sensitivity is achieved, and the recombinant protein has the advantages of higher speed, safety and reliability in comparison with a conventional skin test.
Owner:SHANGHAI JIAO TONG UNIV
Virus specific target and application thereof in preparation of cellular immunotherapy preparation
ActiveCN104693275ASpecific killing effectEffective expansionPeptidesAntiviralsDendritic cellT lymphocyte
The invention relates to the field of biotechnology, and discloses a virus specific target and an application thereof. The amino acid sequence of the hepatitis B virus specific target is FCWEWASAK. The invention further provides an application of the target in preparation of a preparation for treating hepatitis B related diseases. The target disclosed by the invention can realize in vitro effective amplification and activation of dendritic cells, the submitted T lymphocytes have specific killing effect to the virus, and the virus specific target is the cellular immunotherapy meeting the cellular immunology principle furthest, so the virus specific target has excellent application prospect.
Owner:河北博海生物工程开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com