A virus-specific target and its application in the preparation of cellular immunotherapy preparations
A cellular immune and specific technology, applied in the field of virus-specific targets and its applications, can solve problems such as retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Specimen collection
[0020] The patient's blood count is required to be within the normal range (WBC: 4-10×10 9 , LYM%: 20%-40%), the fluctuation does not exceed 5%, the circulation volume of peripheral blood by machine sampling is 1000-4000ml, the number of mononuclear cells: more than 1×10 9 . Blood drawing method 50-100ml, the number of mononuclear cells: more than 1×10 6 .
[0021] The preferred anticoagulant is heparin, followed by sodium citrate, and EDTA is prohibited.
[0022] 2. Preparation before operation
[0023] Wipe the collection bag containing PBMC suspension from top to bottom with 75% alcohol, and then put it into the transfer window. Wipe it from top to bottom with a lint-free cloth of % alcohol, and then put it into the operating biological safety cabinet.
[0024] 3. Isolation of mononuclear cells
[0025] Transfer the PBMC cell suspension sample: Use sterilized scissors to cut the plastic tube, and carefully pour the PBMC cell suspension...
Embodiment 2
[0062] Example 2: Test method for detection of cell positive rate by target monoclonal antibody
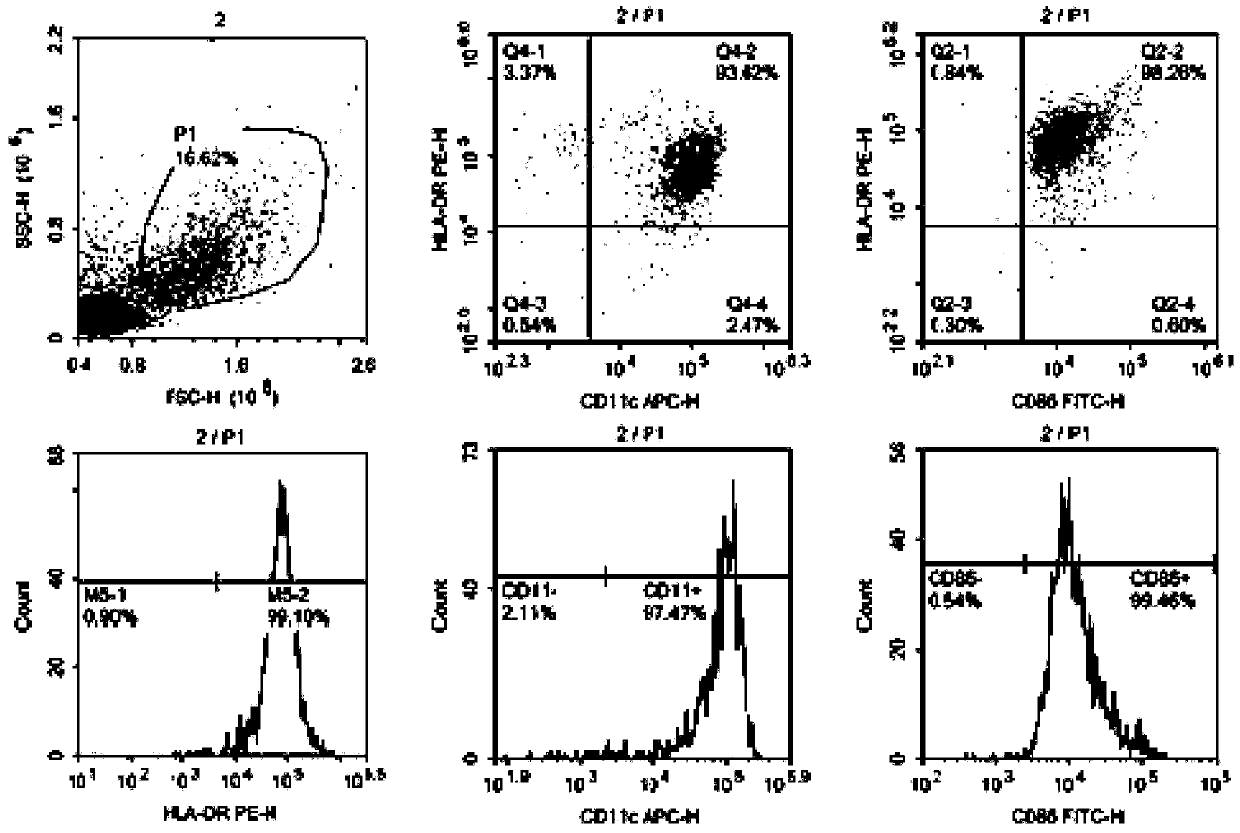
[0063] Positive detection steps of indirect immunofluorescence on the surface of DC cells in peripheral blood of patients with hepatitis B
[0064] 1. Take the peripheral blood DC cells (1×10 7 / ml) 200 μl, divided into two tubes, one tube for each experimental control (100 μl / tube).
[0065] 2. Centrifuge and wash once with PBA,
[0066] 3. Add 20 μl each of FITC-CD86, PE-HLA-DR, and APC-CD11c to the experimental tube, and add 20 μl each of FITC-mouse IgG1, PE-mouse IgG1, and APC-mouse IgG1 to the control tube.
[0067] 4. Gently blow and beat to mix well. After each tube is fully mixed, incubate in the dark for 30 minutes.
[0068] 5. After washing with PBS, perform flow cytometry detection.
[0069] See the test results figure 1 As shown, the results show that co-culture of patient DC cells with hepatitis B DC targets in vitro can make the targets bind to MHC in DC cells a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com