Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
245 results about "In vitro proliferation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Induced malignant stem cells
InactiveUS20140137274A1High and low degree of methylationSugar derivativesPeptide/protein ingredientsMicrosatelliteSomatic cell
PROBLEMThere are provided induced malignant stem cells capable of in vitro proliferation that are useful in cancer research and drug discovery for cancer therapy, as well as processes for production thereof, cancer cells derived from these cells, and applications of these cells.MEANS FOR SOLVINGAn induced malignant stem cell capable of in vitro proliferation are characterized by satisfying the following two requirements:(1) having at least one aberration selected from among (a) an aberration of methylation (high or low degree of methylation) in a tumor suppressor gene or a cancer-related genetic region in endogenous genomic DNA, (b) a somatic mutation of a tumor suppressor gene or a somatic mutation of an endogenous cancer-related gene in endogenous genomic DNA, (c) abnormal expression (increased or reduced / lost expression) of an endogenous oncogene or an endogenous tumor suppressor gene, (d) abnormal expression (increased or reduced / lost expression) of a noncoding RNA such as an endogenous cancer-related microRNA, (e) abnormal expression of an endogenous cancer-related protein, (f) an aberration of endogenous cancer-related metabolism (hypermetabolism or hypometabolism), (g) an aberration of endogenous cancer-related sugar chain, (h) an aberration of copy number variations in endogenous genomic DNA, and (i) instability of microsatellites in endogenous genomic DNA in an induced malignant stem cell; and(2) expressing genes including POU5F1 gene, NANOG gene, SOX2 gene, and ZFP42 gene.
Owner:ISHIKAWA
Compositions and methods for culturing stem cells
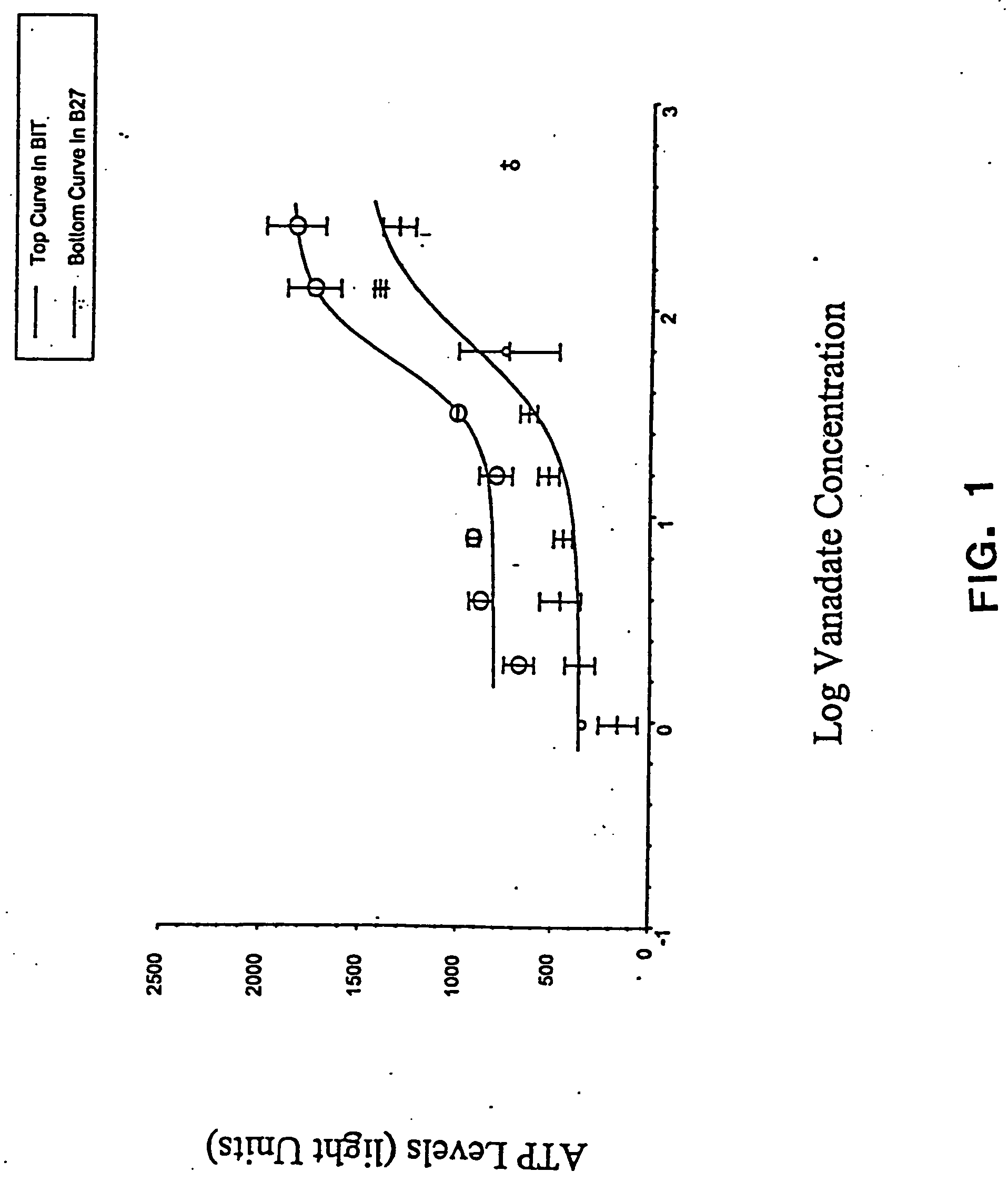
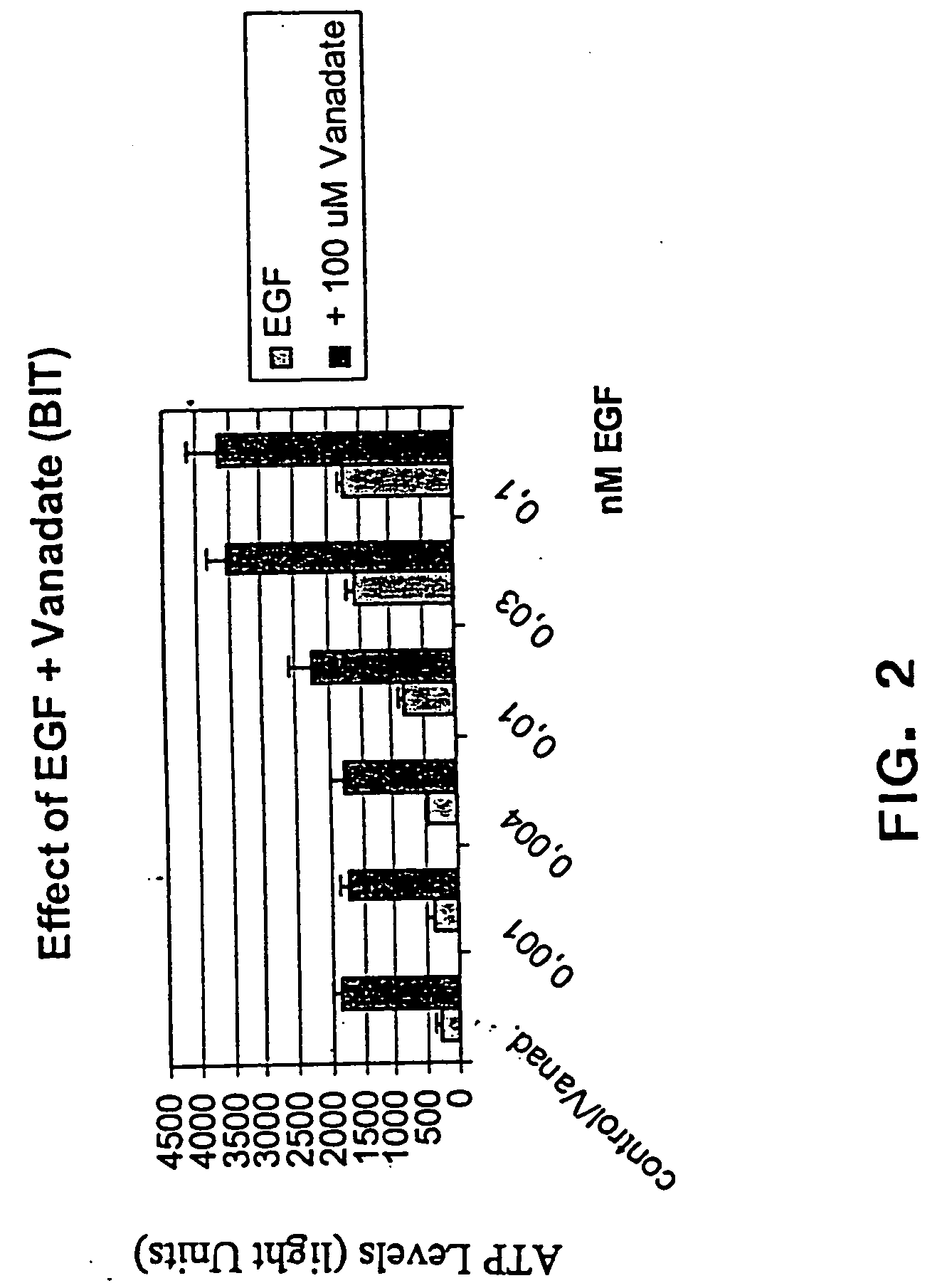
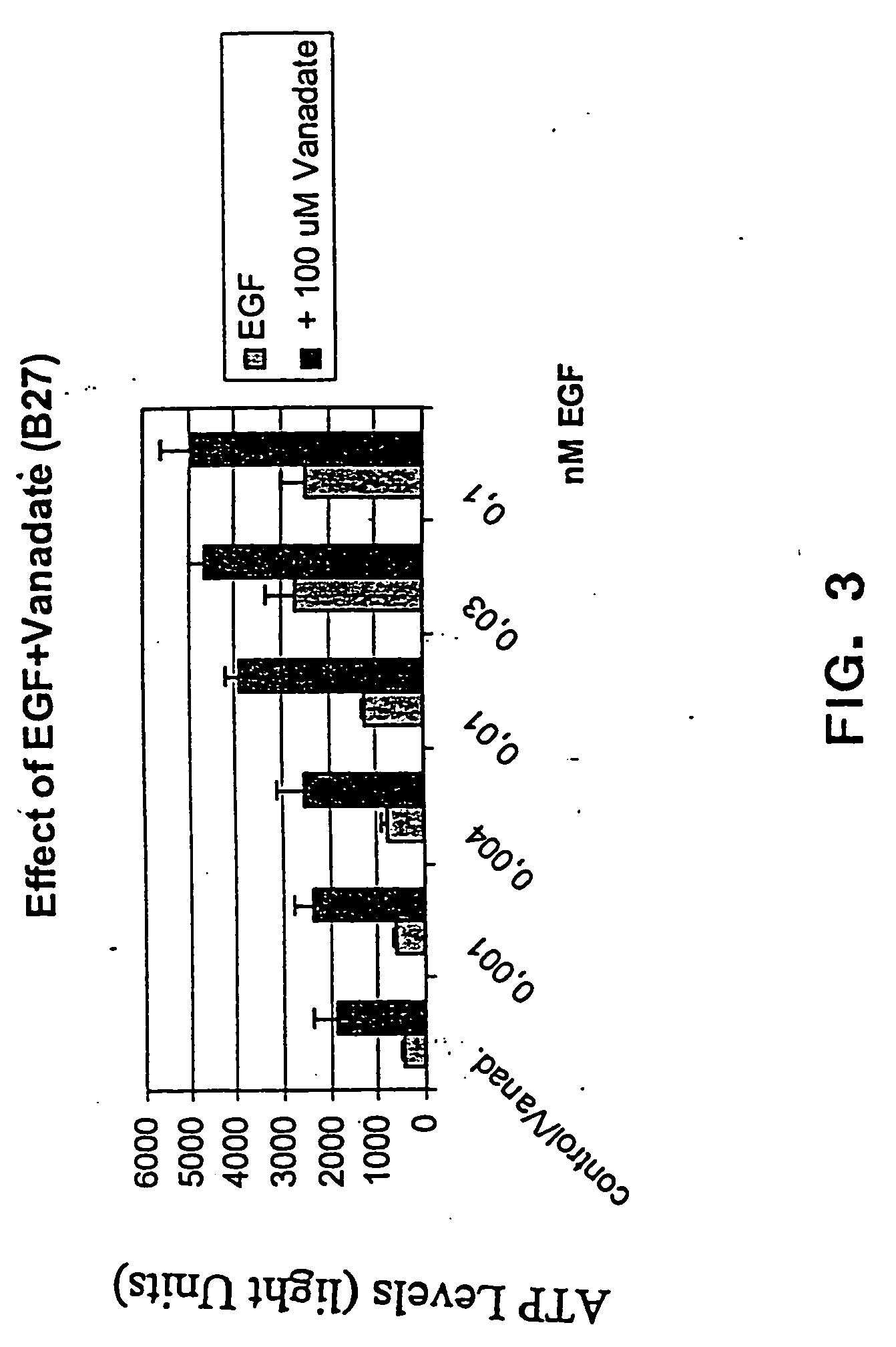
The present invention provides methods of culturing, propagating, treating, and maintaining stem cells in the presence of a phosphate mimic. In particular, the invention relates to the propagation of stem cells in vitro, the formation of neurospheres in vitro, and to tissue culture of stem cells in the presence of a phosphate mimic. The invention further relates to the treatment of neurodegenerative disorders.
Owner:NEURONOVA
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
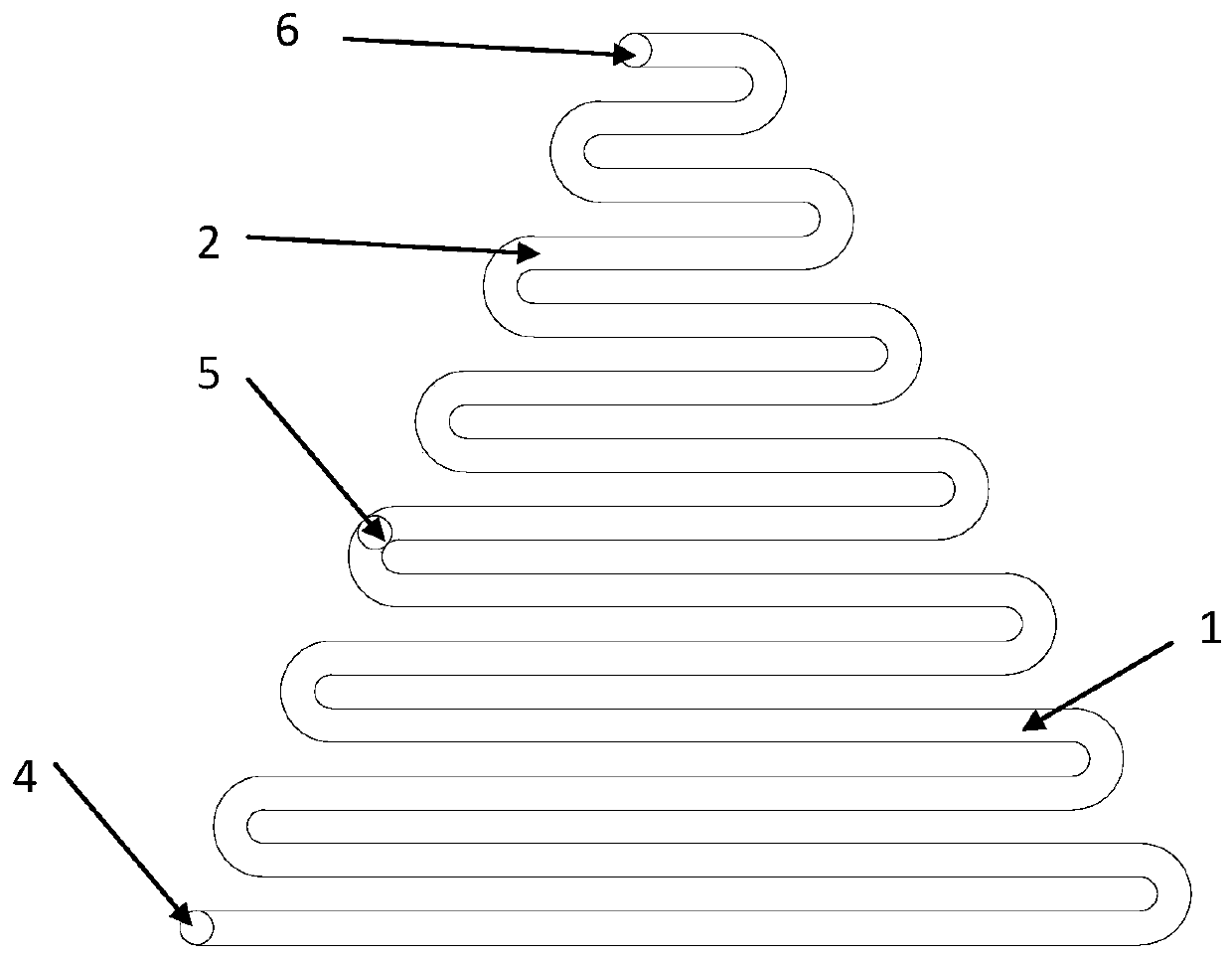
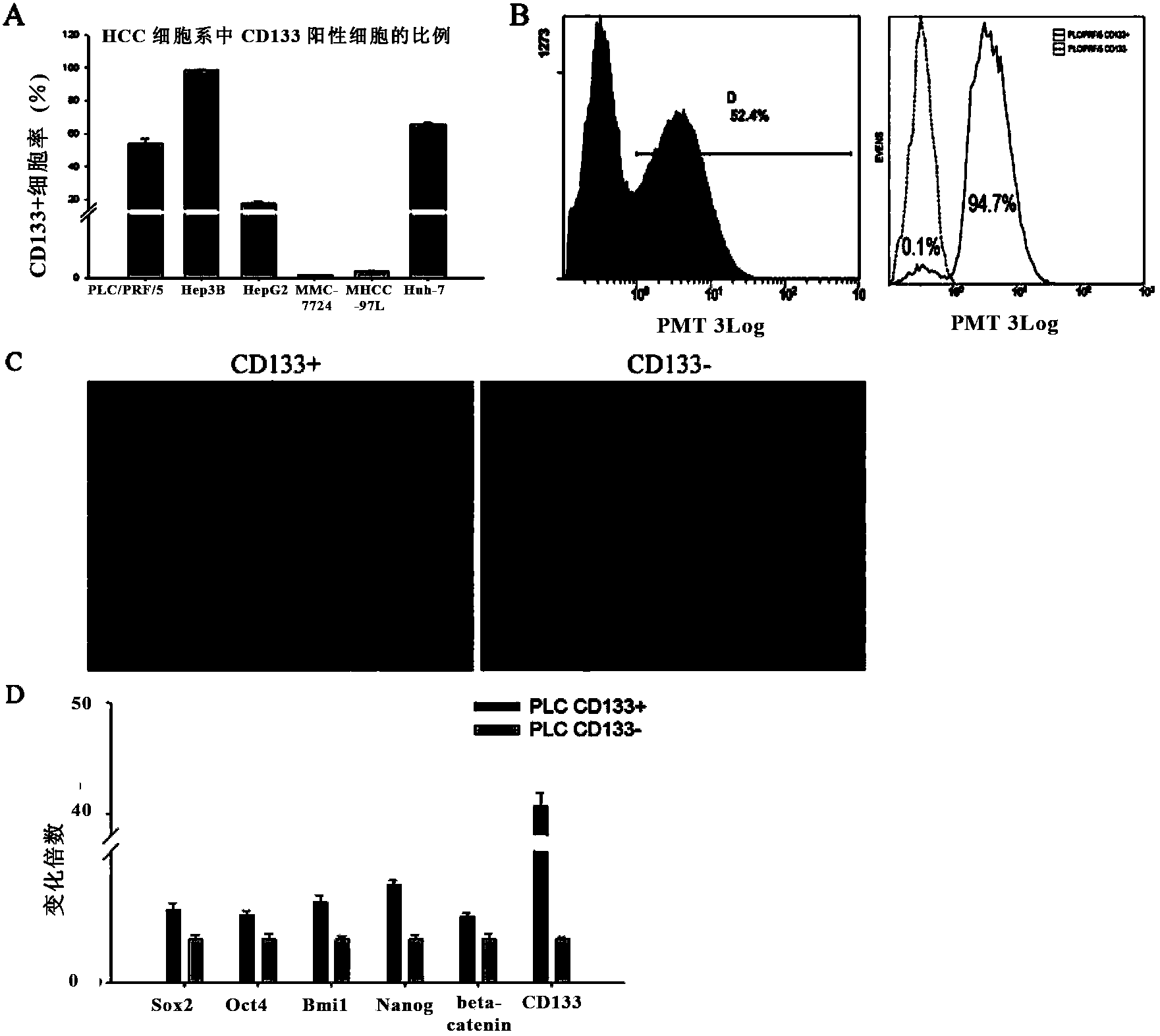
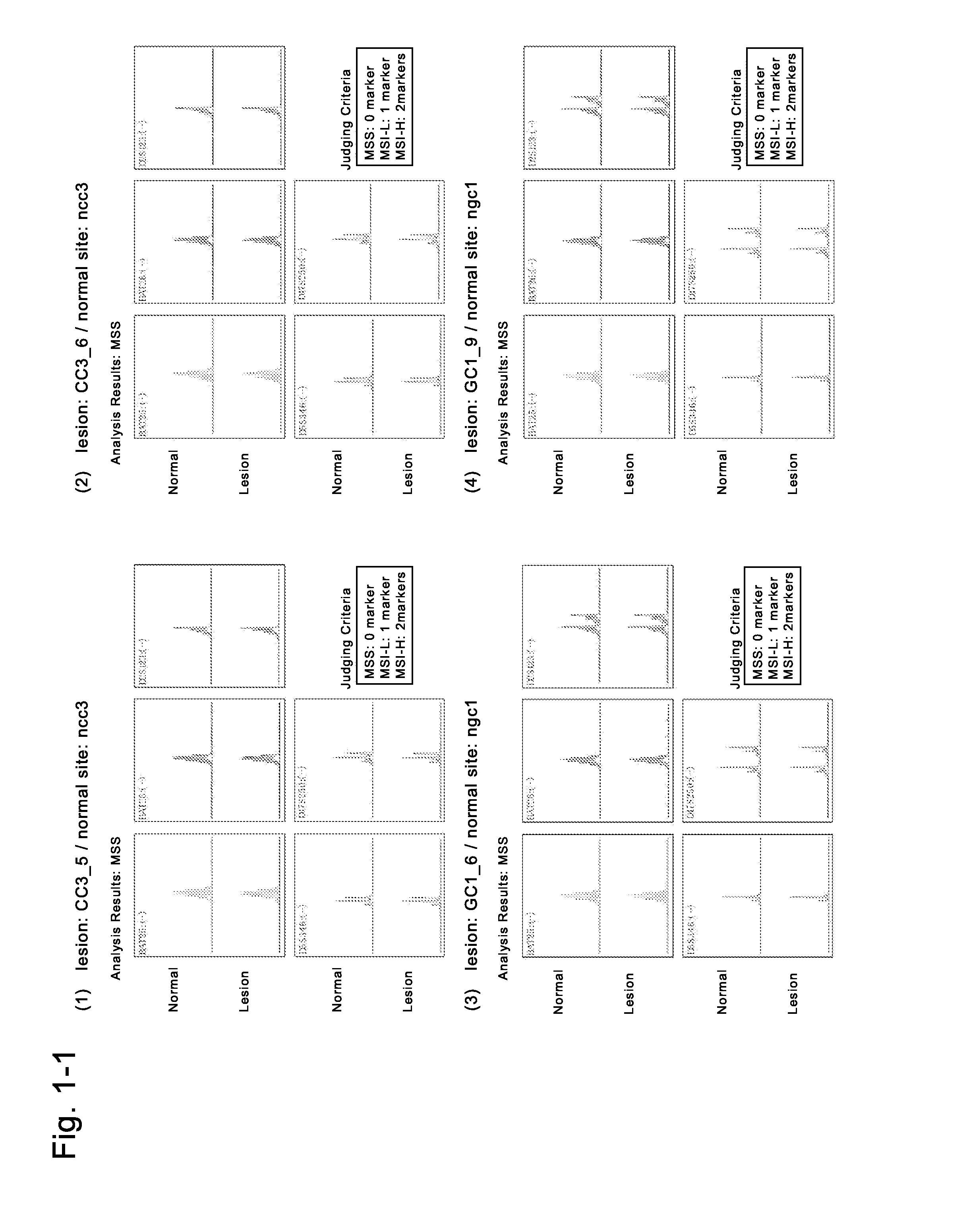
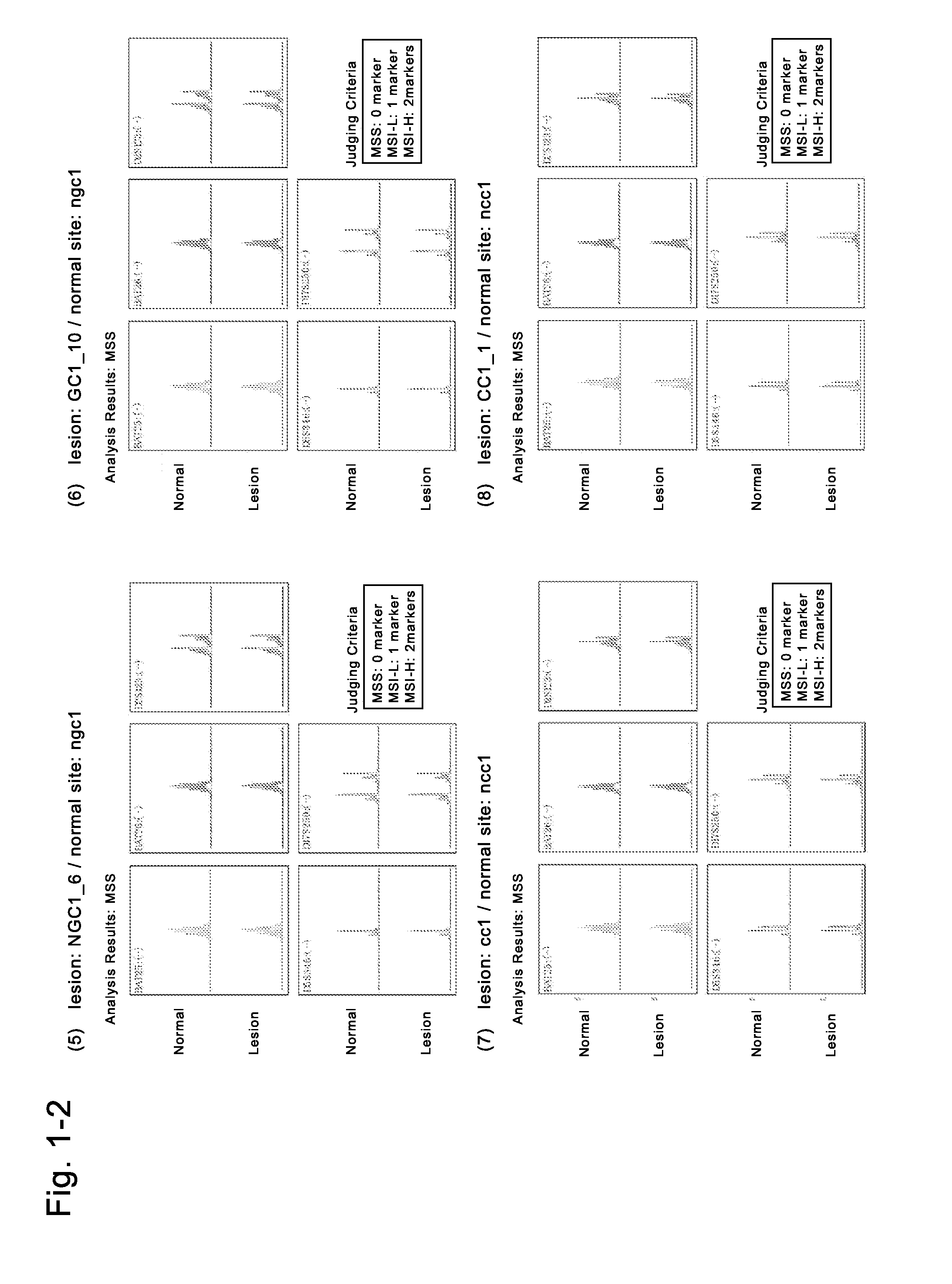
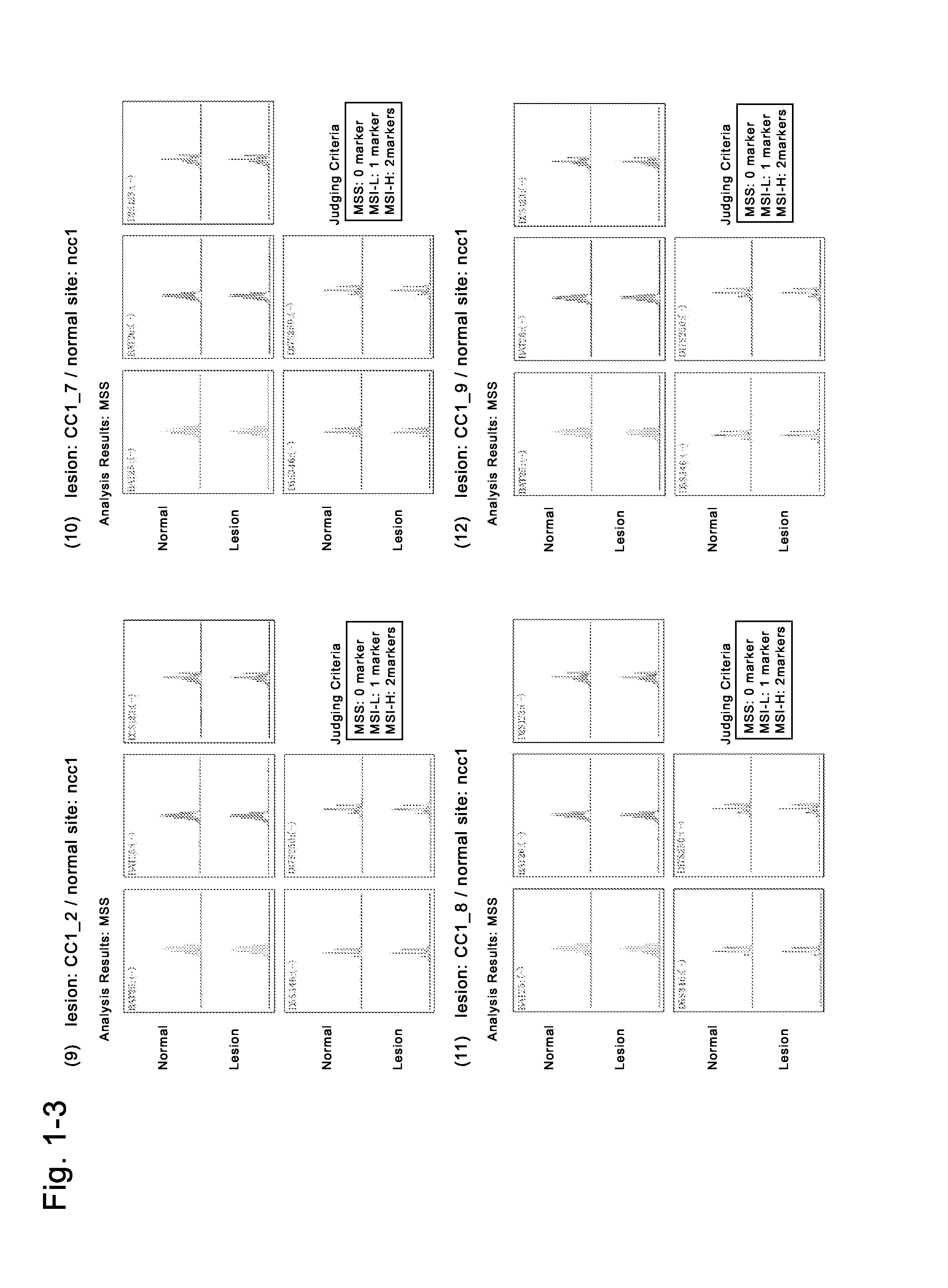
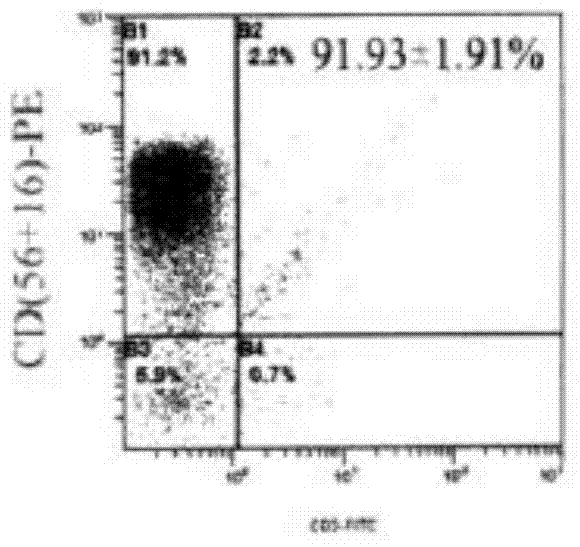
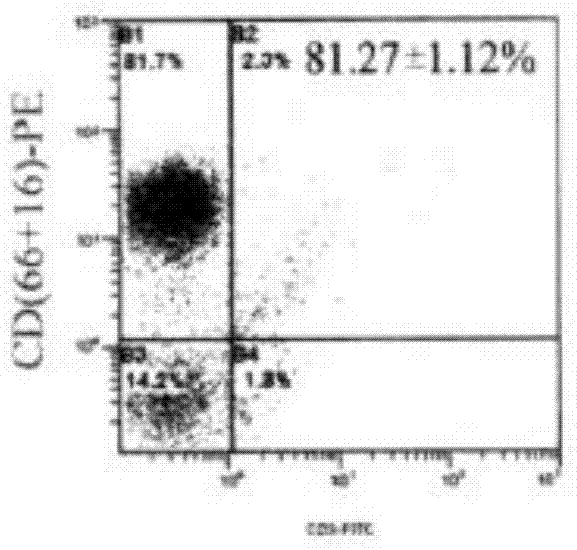
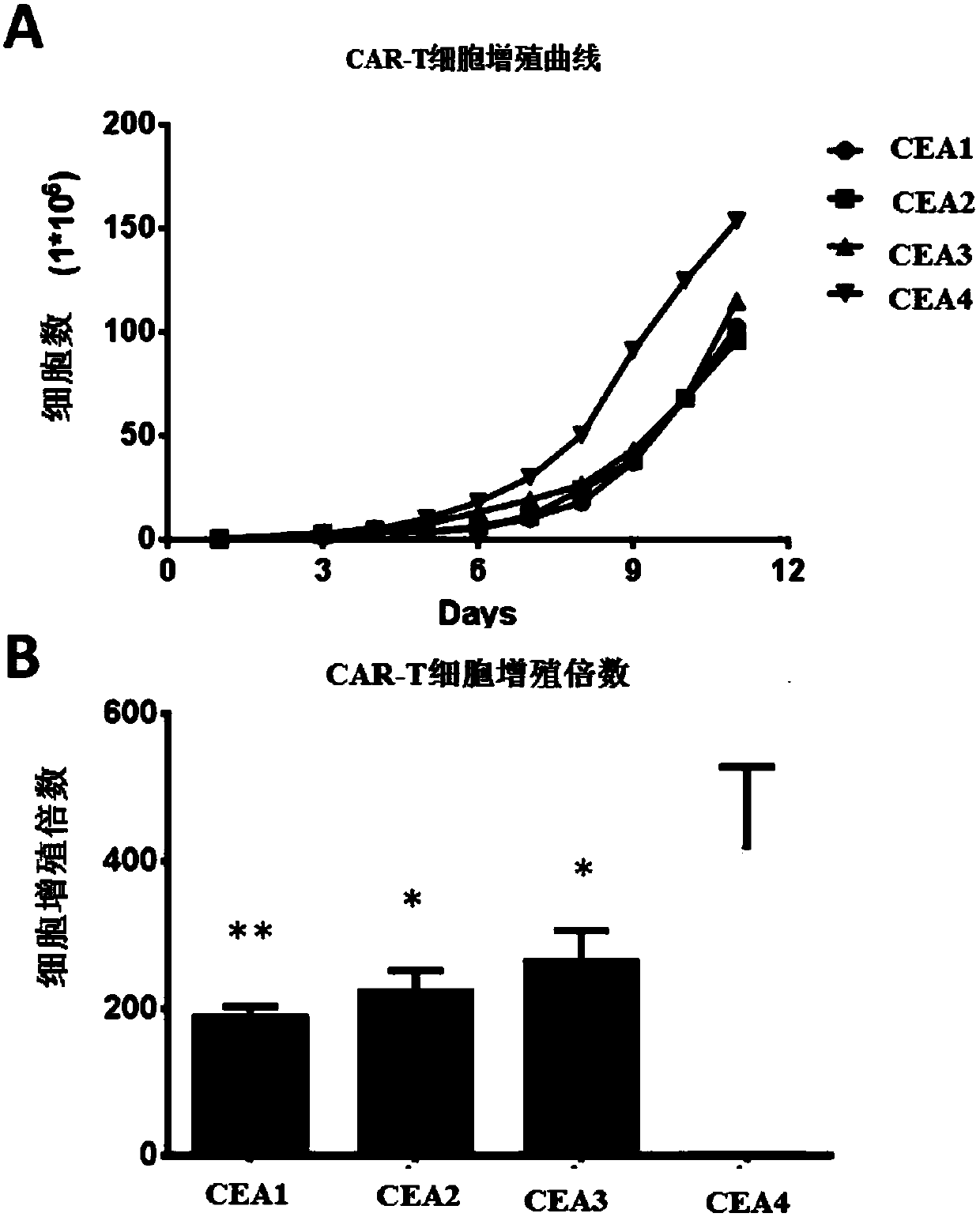
AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell
ActiveCN104087592AHigh activityImprove anti-tumor effectGenetic material ingredientsDigestive systemEpitopeIn vitro proliferation
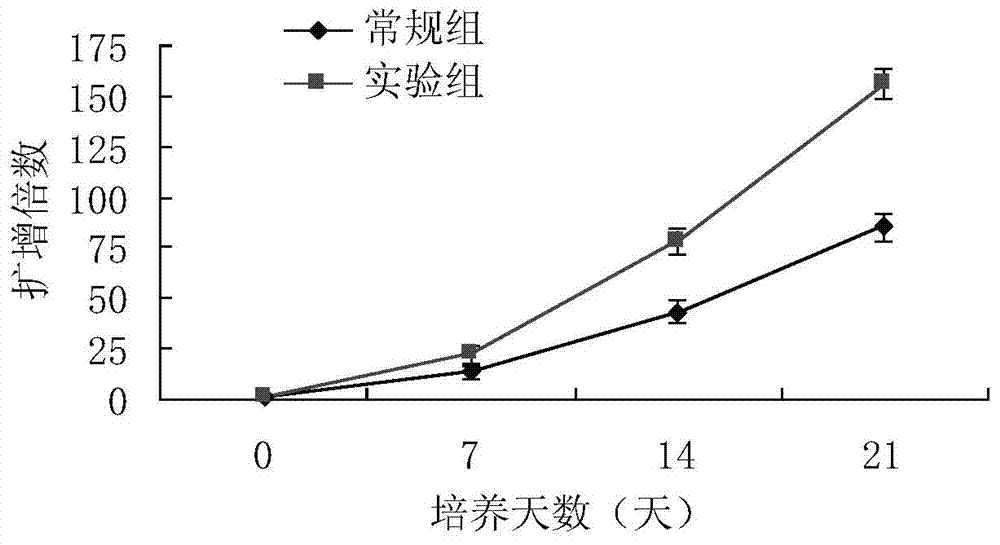
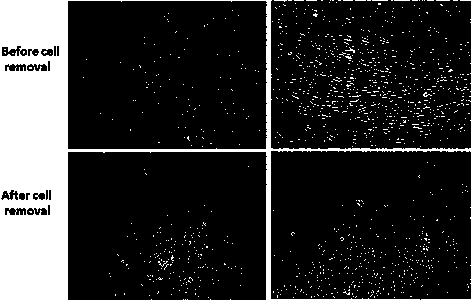
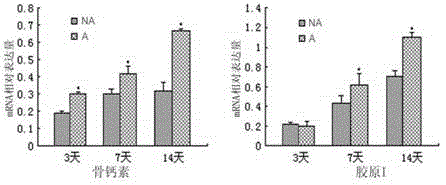
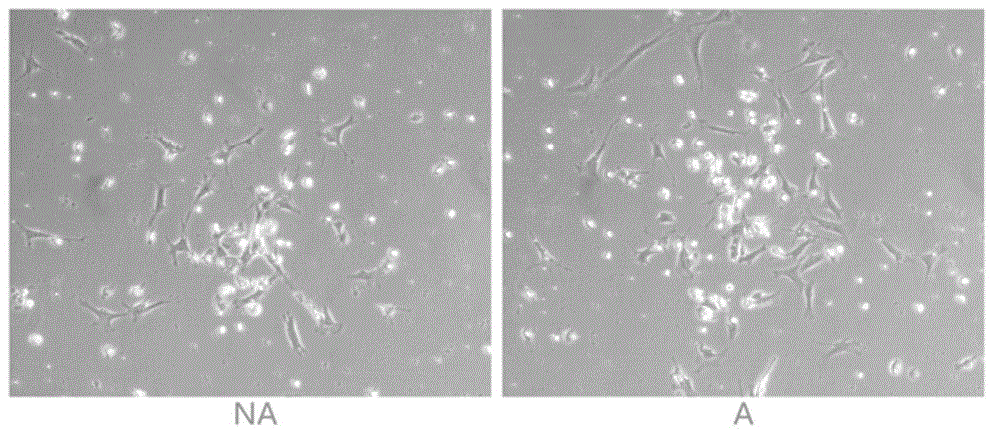
The invention discloses an AFP[158-166] specific TCR gene, its transgenic T cell, and an in-vitro proliferation method and a use of the transgenic T cell. The AFP[158-166] specificity TCR gene is represented by SEQ ID NO.1 in a sequence table. The transgenic T cell of the AFP[158-166] specific TCR gene has a specific killing effect on AFP positive liver cancer cells. Artificial antigen presentation cells can present AFP[158-166] epitope peptides through the MHCI type molecule of the cell surface when the artificial antigen presentation cells are secreted by IL-15 to induce the AFP specific immune reaction, and the stimulation of the AFP[158-166] specific TCR gene transgenic T cell by the AFP[158-166] specific TCR gene can improve the proportion of the transgenic T cell, enhances the specificity and enhances the T cell activity and the antitumor capability in an auxiliary manner through the secretion of IL-15.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Application of dehydrogenated silibinin diether in preparation of medicaments for preventing and treating leukemia
InactiveCN101785767ASimple methodShort stepsOrganic active ingredientsAntineoplastic agentsMyeloid leukemiaLeukemia
The invention provides application of dehydrogenated silibinin diether in the preparation of medicaments for preventing and treating leukemia and relates to medical application of lignin flavone silibinin to the prevention and treatment of chronic myeloid leukemia. Particularly, the invention relates to application of dehydrogenated silibinin diether, of which the seventh bit and the twentieth bit are substituted by 1-butylene, or pharmaceutically acceptable salts thereof in the preparation of medicaments for preventing and treating chronic myeloid leukemia. Natural products of the silibinin are treated by simple steps to synthesize the compound. The pharmacological tests prove that the compound can potently inhibit the in-vitro proliferation of human chronic myeloid leukemia cell strains(K562) and adriamycin drug-resistant strains (K562 / ADR), and the IC50 values are 11.9+ / -1.6 micromoles and 15.9+ / -1.2 micromoles respectively.
Owner:DALI UNIV
Chimeric antigen receptor containing CD27 intracellular domain, lentiviral vector and application thereof
ActiveCN105949325AHave the ability to bind antigenEfficient killingMammal material medical ingredientsNucleic acid vectorAntigenSignalling molecules
The invention discloses a chimeric antigen receptor containing a CD27 intracellular domain, a lentiviral vector and application thereof. The immune receptor tyrosine activation sequence motif of the chimeric antigen receptor containing CD27 intracellular domain contains CD27molecule intracellular signal domain and T cell costimulatory signal molecules. The CD27molecule intracellular signal domain is connected with costimulatory signals related to T cell activation, so that the in-vitro proliferation and apoptosis effect of T cells can be obviously enhanced, and the ratio of young sample Tscm cells (CD45RA+CD62L+) with in vivo therapeutic action and central memory cells (CD45RO+CD62L+) in CAR-T cells is increased; expression of IL-10 factors capable of restraining immunization is reduced, an obvious effect of improving the CAR-T (T cell chimeric antigen receptor) cellular therapy effect is achieved, and a new idea and a new selection are provided for the field of CAR-T cellular therapy.
Owner:CHONGQING PRECISION BIOTECH CO LTD
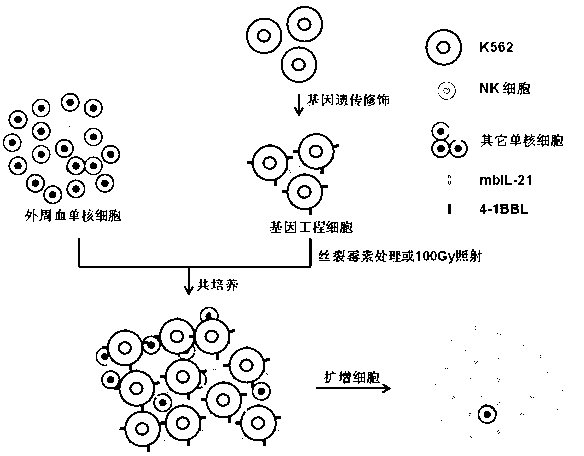
Gene engineering cell and application thereof in NK (Nature Killer) cell proliferation
InactiveCN102911918AStrong cytotoxicityHigh purityBlood/immune system cellsForeign genetic material cellsHigh cellCancer cell
The invention discloses a gene engineering cell and application thereof in NK (Nature Killer) cell proliferation, and provides an in vitro proliferation method of an NK cell with high efficiency and high cytotoxicity. A 4-1BB ligand (4-1BBL) and a membrane fixed interleukin 21 (mbIl-21) are stably expressed on the surface of a cell membrane by adopting a genetic engineering technology for constructing 4-1BBL-mbIl-21-gene engineering cells (4-1BBL-mbIl-21-Gene Engineering Cells, 4-1BBL-mbIl-21-GEC), such as 4-1BBL-mbIl-21-K562 cells, to be used as feeder cells for proliferation, and the NK cells are directly proliferated from peripheral blood monouclear cells, thus the operation is simpler and easier. Studies such as cell counting, flow cytometry and cytotoxicity tests indicate that high cell proliferation times, high NK cell purity, strong cytotoxicity and remarkable cancer cell killer effect are achieved.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
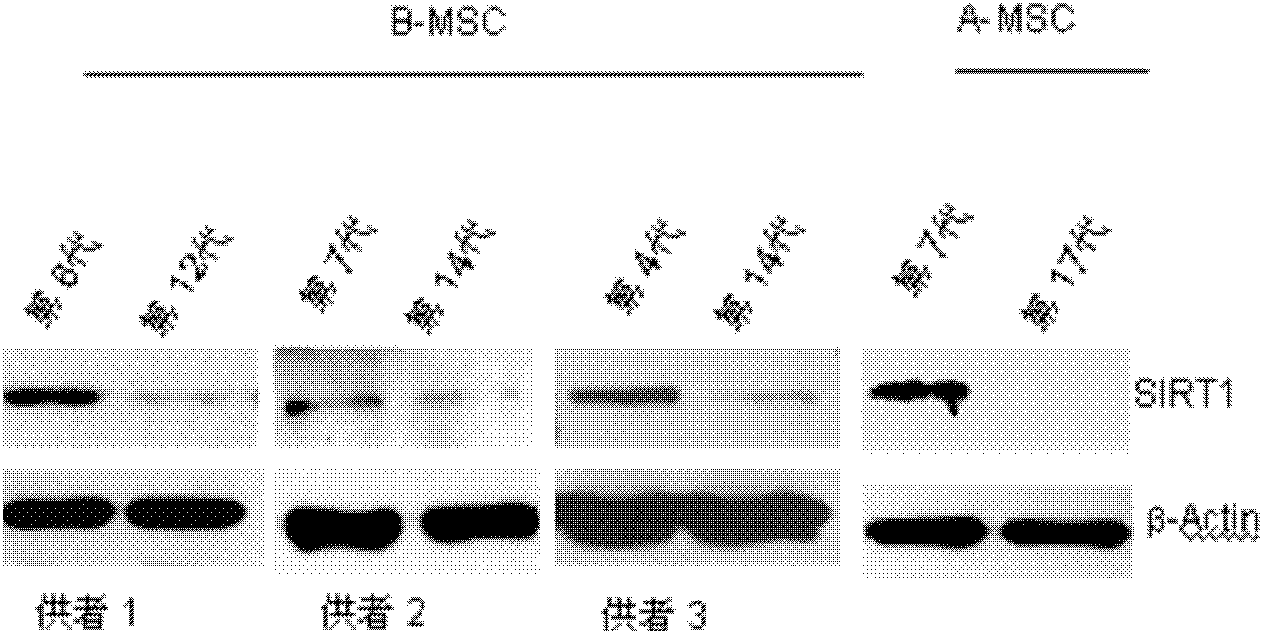
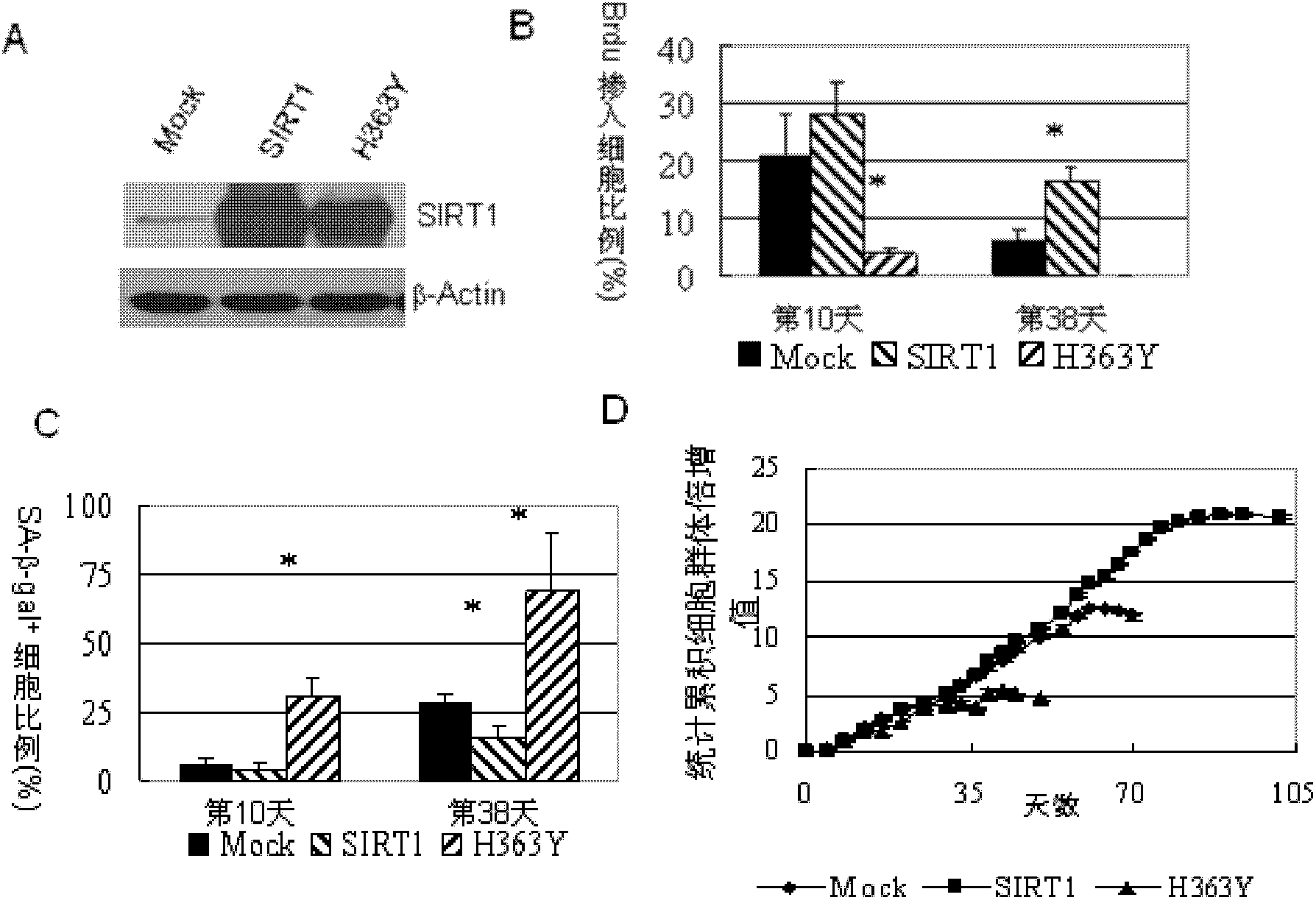
Application of protein acetylation enzyme SIRT1 (Silent Mating type Information Regulation 2Homolog1) in promoting proliferation and delaying senility of mesenchymal stem cells
ActiveCN102382801APromote proliferationReduce proliferationSkeletal/connective tissue cellsGenetic engineeringIn vitro proliferationMesenchymal stem cell proliferation
The invention discloses an application of protein acetylation enzyme SIRT1 (Silent Mating type Information Regulation 2Homolog1), namely promoting proliferation and delaying senility of mesenchymal stem cells. The experimental result shows that the SIRT1 is needed by the in vitro proliferation of the mescenchymal stem cells, and the condition is verified by MSC (mesenchymal stem cells from marrow) and MSC from fat, and the proliferation and the senility of the MSC can be regulated by regulating the expression level of SIRT1 in the MSC. The invention has very important significances on the determination of the regulation mechanism of the proliferation and the senility of the MSC, the prolonging of the service life of the MSC, and the histocyte substitution and repairing treatment based on the MSC, and is broad in application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
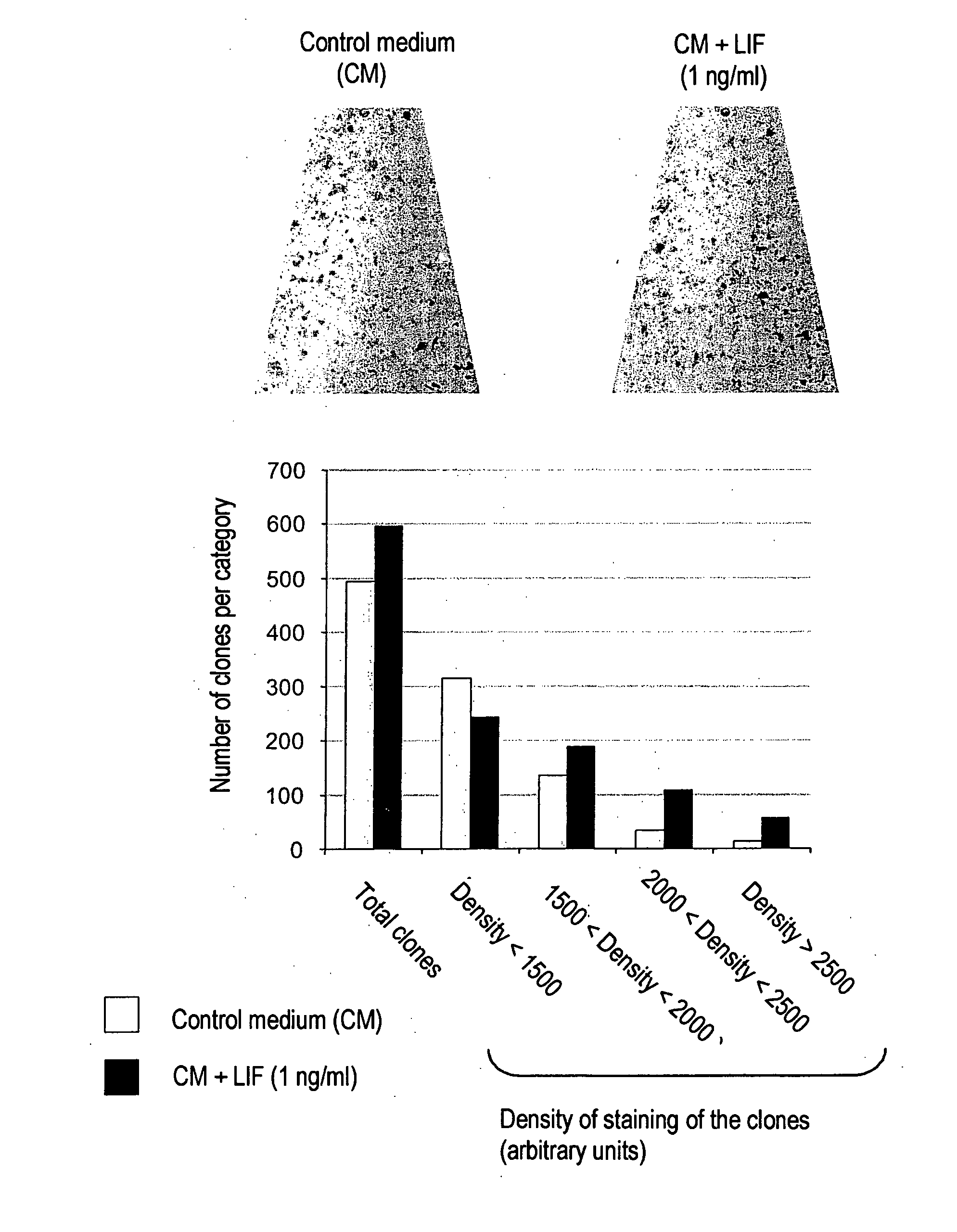
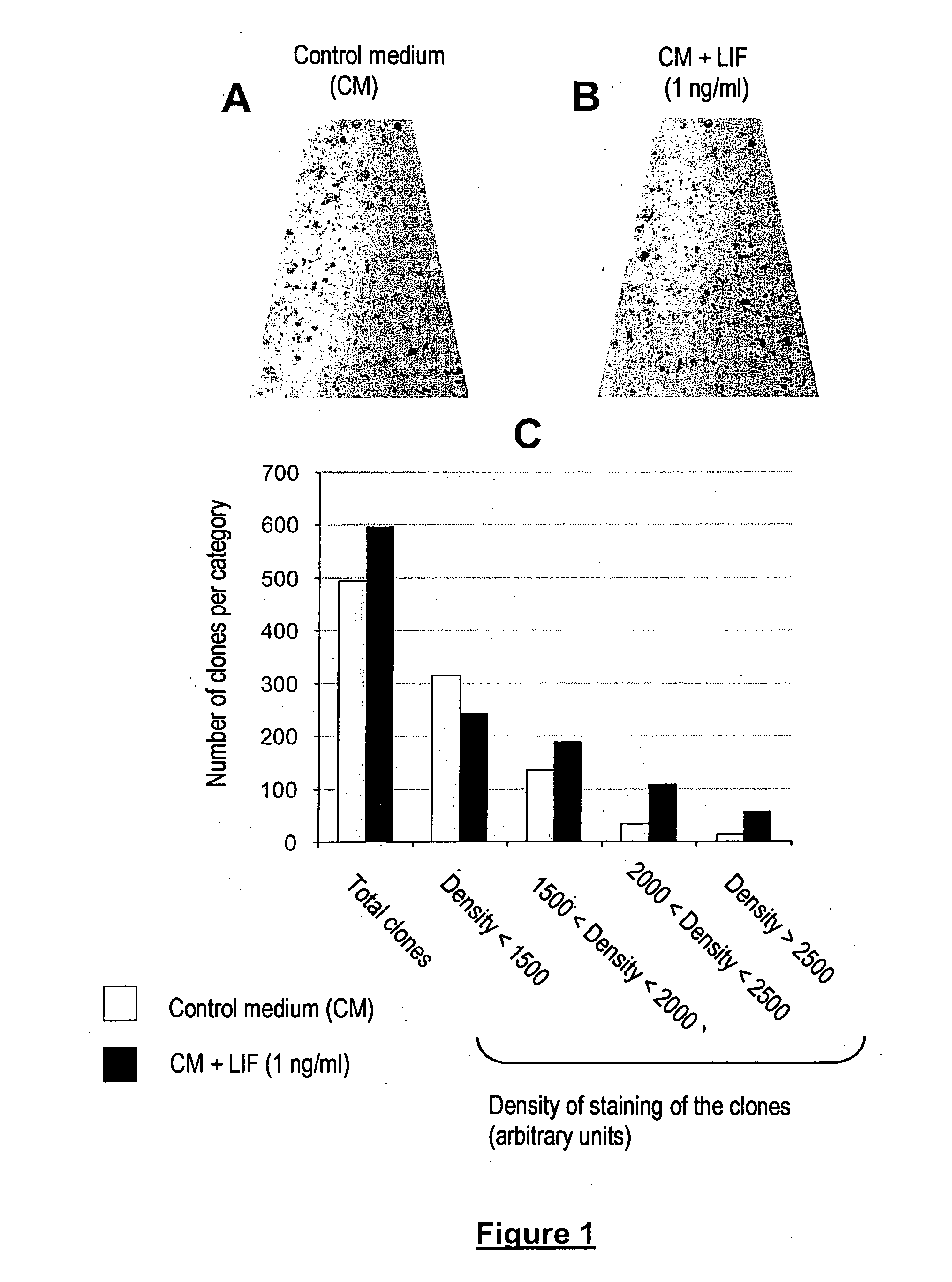
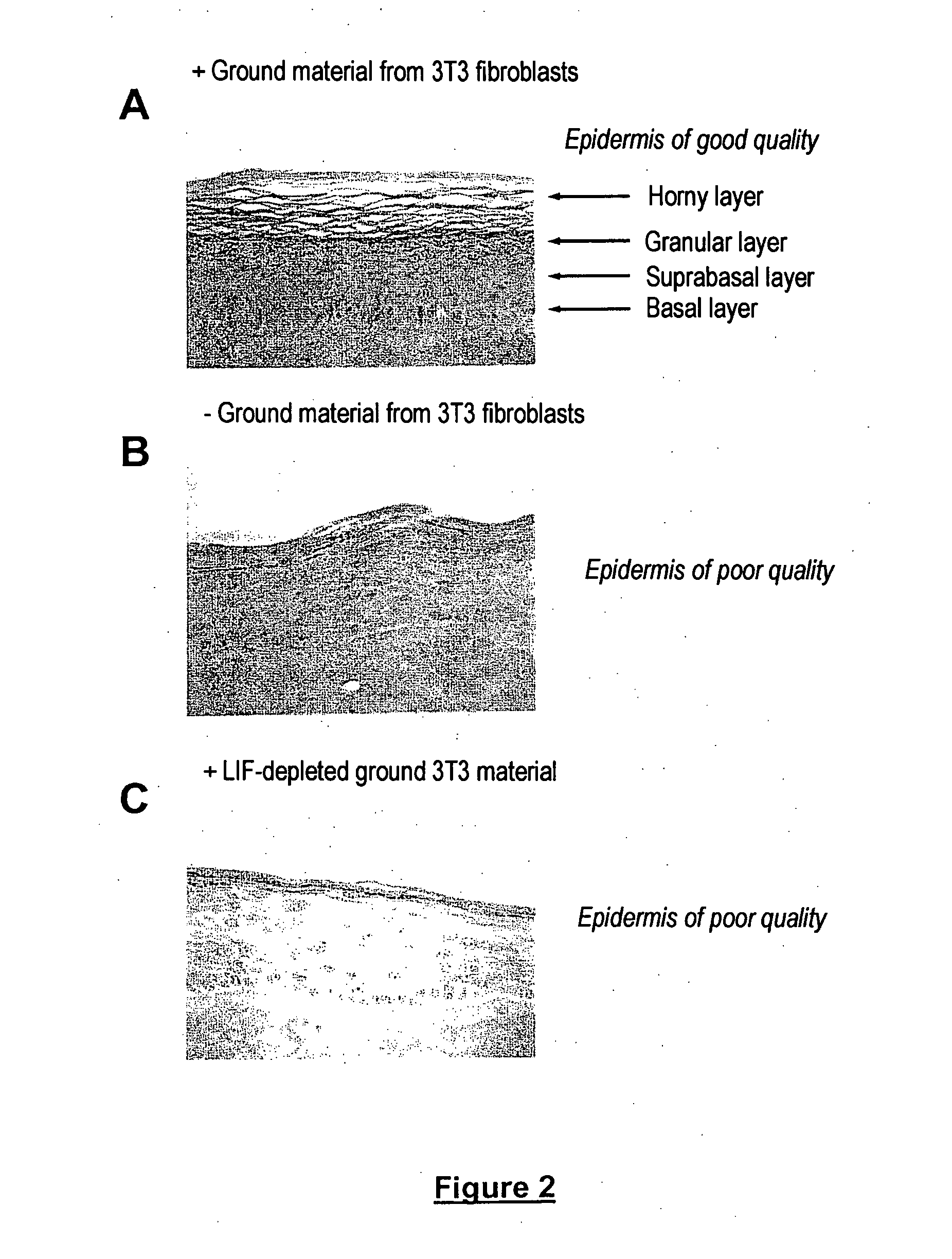
Cell and tissue engineering utilizing LIF
InactiveUS20060014282A1Promotes epidermal reconstitutionQuality improvementMicrobiological testing/measurementEpidermal cells/skin cellsEpitheliumProgenitor
Leukemia Inhibitory Factor (“LIF”), an LIF analogue, an LIF mimetic and a product capable of stimulating the expression of endogenous LIF, and mixtures thereof are useful, for (i) promoting the multiplication, in vitro, of a population of human skin stem cells and / or epidermal progenitors while maintaining them in an undifferentiated state, and / or for (ii) maintaining and / or increasing their capacity to generate a pluristratified epithelium, in particular a pluristratified epidermis and / or all or some of the skin appendages; also a library or a culture of human undifferentiated skin stem cells and / or epidermal progenitors is obtained in the presence of LIF, as are reconstructed epidermides and / or reconstructed skin, and kits for producing libraries of cells or reconstructed epidermides, and stem cells or reconstructed epidermides are prepared for the treatment of individuals exhibiting damaged skin (individuals suffering from third degree burns and individuals suffering from genetic diseases affecting the skin).
Owner:LOREAL SA
Preparation method of specific extracellular matrix ECM of periodontium and application thereof
ActiveCN103966158APromote in vitro proliferationPromote directed differentiationArtificial cell constructsArtificially induced pluripotent cellsCell-Extracellular MatrixPeriodontal ligament stem cells
The invention relates to a preparation method of a specific extracellular matrix ECM of periodontium. The preparation method comprises the following steps: 1) separating and culturing a human periodontal ligament cell; and 2) preparing the specific extracellular matrix ECM of periodontium; applying the specific extracellular matrix ECM of periodontium and applying the specific extracellular matrix ECM of periodontium as a human periodontal ligament stem cell in in-vitro amplification; and promoting directional differentiation of the ECM. The preparation method is simple and can remarkably promote in-vitro amplification and directional differentiation of the periodontal ligament stem cell.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Bioactive polypeptide PMIGVNQELAY, and preparation method and application thereof
ActiveCN107236031AGood antioxidant activityGood for regulating the body's immune systemCosmetic preparationsPeptide/protein ingredientsDrugChemistry
The invention relates to the field of protein, in particular to a bioactive polypeptide PMIGVNQELAY, and a preparation method and application thereof. The amino acid sequence of the bioactive polypeptide PMIGVNQELAY is Pro-Met-Ile-Gly-Val-Asn-Gln-Glu-Leu-Ala-Tyr. In vitro antioxidant experiments and in vitro immune function promoting experiments prove that the polypeptide PMIGVNQELAY has better antioxidant biological activity and immune modulating functions; on one hand, good antioxidant activity is realized; free radicals in the body can be cleared away; the life quality is improved; on the other hand, the bioactive polypeptide PMIGVNQELAY can enhance the in vitro multiplication capacity of lymphocytes and macrophages; the capability of the macrophages for devouring toluylene red is promoted; the capability of the body for resisting the external pathogen infection is improved; the body morbidity is reduced; very important significance is realized for developing food, health care products and medicines with the immune modulating function and the antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Expansion in vitro purification culture method of mesenchymal stem cells and culture medium
InactiveCN102978156AFacilitate early purificationDoes not affect vitalitySkeletal/connective tissue cellsPenicillinCell culture media
The invention provides an expansion in vitro purification culture method of mesenchymal stem cells and a cell culture medium used in a culture process. According to the method, mesenchymal stem cells differentiate to osteogenic stem cells. The cell culture medium comprises the following components: 8-12mu g / L of bFGF (Basic Fibroblast Growth Factor), 8-12mu g / L of EGF (Epidermal Growth Factor), 8-12mu g / L of PDGF-BB (Platelet Derived Growth Factor-BB), 10 percent of fetal calf serum, 100U / mL of penicillin and 100mg of L of streptomycin; a solvent is a basic culture medium; and the basic culture medium is a DMEM (Dulbecco Modified Eagle Medium) culture solution, an F12 culture solution or a mixture of the DMEM culture solution and the F12 culture solution. The mesenchymal stem cells obtained by adopting a method comprising the steps of early replacement of the cell culture medium, cell adherence passage and combined addition of cell factors (bFGF, EGF and PDGF-BB) in the culture solution are high in purity and strong in vitro proliferation capacity, and have good capability of differentiation to osteogenic stem cells.
Owner:HUZHOU CENT HOSPITAL
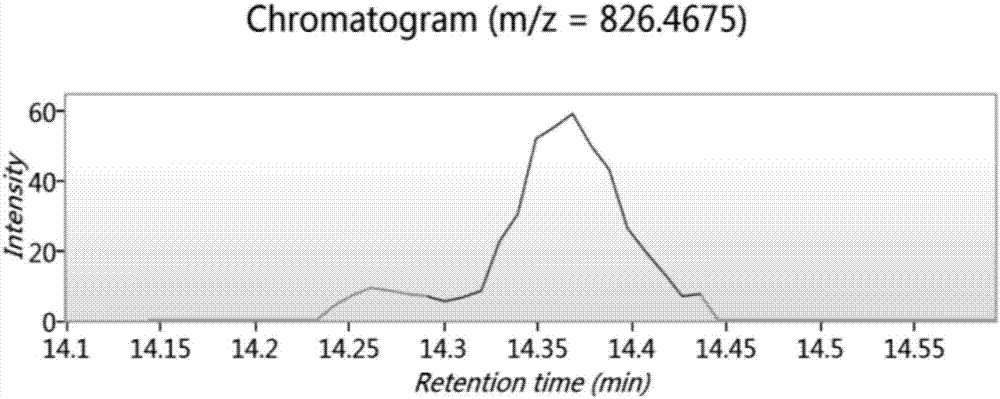
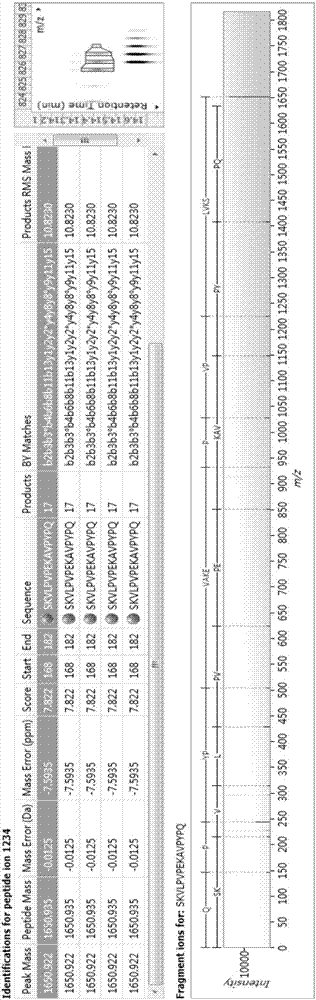
Bioactive peptide SKVLPVPEKAVPYPQ as well as preparation method and application thereof
ActiveCN107176995ARegulate immunityModulate immune activityCosmetic preparationsPeptide/protein ingredientsLife qualityVal-Leu-Pro-Val-Pro
The invention relates to the field of protein, in particular to a bioactive peptide SKVLPVPEKAVPYPQ as well as a preparation method and an application thereof. An amino acid sequence of the bioactive peptide SKVLPVPEKAVPYPQ is Ser-Lys-Val-Leu-Pro-Val-Pro-Glu-Lys-Ala-Val-Pro-Tyr-Pro-Gln. An in-vitro anti-oxidation experiment and an in-vitro immunity function promoting experiment prove that the bioactive peptide SKVLPVPEKAVPYPQ has better anti-oxidation biological activity and immunity regulation function, on one hand, the bioactive peptide SKVLPVPEKAVPYPQ has better anti-oxidation activity, can clear free radicals in an organism and improves life quality; on the other hand, by the aid of the bioactive peptide SKVLPVPEKAVPYPQ, in-vitro proliferation capacity of lymphocytes and macrophages can be enhanced, the phagocytosis capacity of the macrophages for neutral red is improved, the organism's capacity of resisting outside pathogen infection is improved, morbidity of the organism is reduced, and therefore, the bioactive peptide SKVLPVPEKAVPYPQ has quite great significance in development of food, healthcare products and drugs with an immune regulation function and an anti-oxidation function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Compositions and methods for biodegradable microspheres as carriers of bioactive substances
InactiveUS6962716B1Cost-effective and scalablePowder deliveryLiquid surface applicatorsActive matterPolymer solution
A cost-effective, scalable technique for producing microspheres loaded with biologically active solid proteins is provided. The microspheres degrade over time and release biologically active VEGF, as demonstrated by the proliferation of HUVECs in vitro compared to negative controls. A defined concentration of microspheres can deliver a quantifiable level of VEGF with known release kinetics. The invention can be used with other growth factors and applied to tissue engineering applications such as the regeneration of peripheral nerve, bone, adipose tissue, and solid organs. The method of the invention includes the steps of dissolving a polymer with an organic solvent to produce a polymer solution; adding a biologically effective amount of a bioactive substance to the solution to produce a mixture of the polymer and the bioactive substance; vibrating the mixture to produce a bioactive substance-polymer complex; emulsifying the mixture to produce an emulsion comprising the bioactive substance-polymer complex; and extracting the organic solvent from the emulsion to produce microspheres comprising the polymer-bioactive substance complex, wherein the bioactivity of the bioactive substance is usefully preserved.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
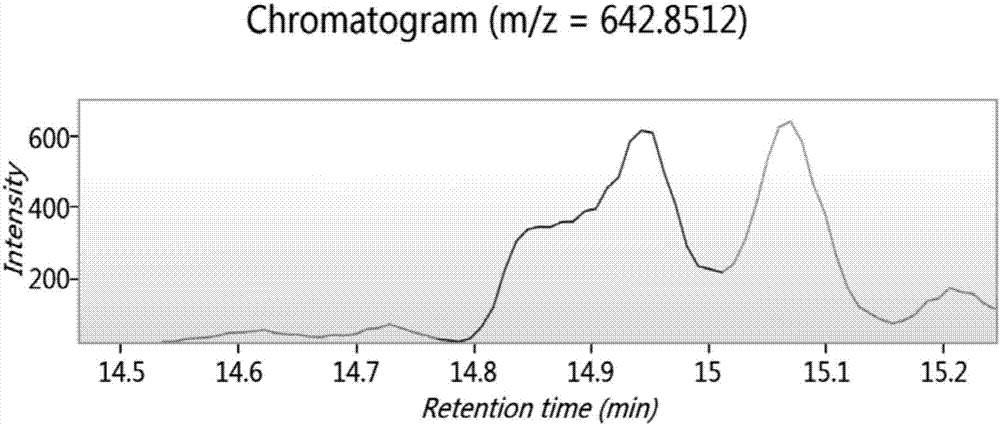
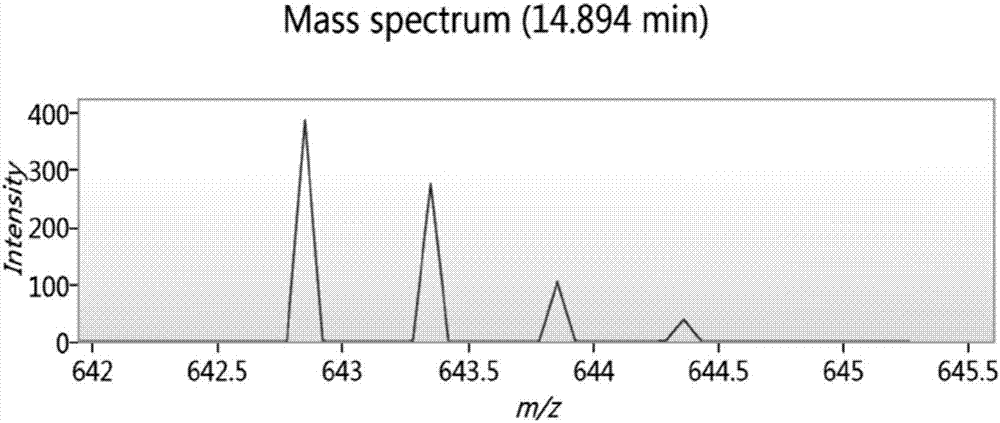
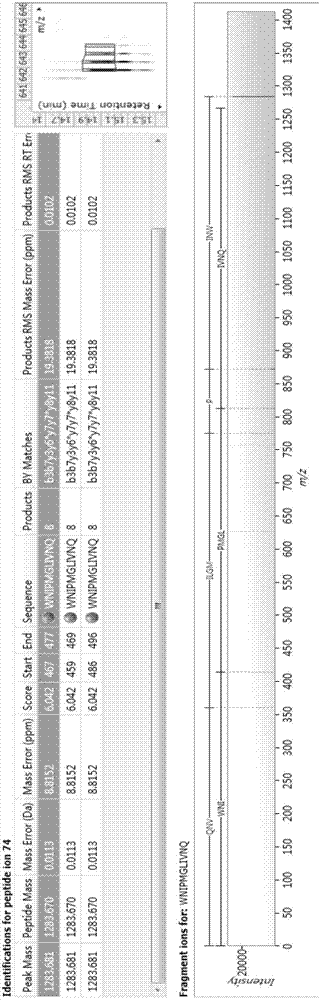
Biologically active polypeptide WNIPMGLIVNQ and preparation method and application thereof
ActiveCN107163136AReduce harmImproves antioxidant activityCosmetic preparationsPeptide/protein ingredientsDiseaseNitric oxide
The present invention relates to the field of protein, and in particular relates to biologically active polypeptide WNIPMGLIVNQ and a preparation method and the application thereof, and the amino acid sequence of the biologically active polypeptide WNIPMGLIVNQ is Trp-Asn-Ile-Pro-Met-Gly-Leu-Ile-Val-Asn-Gln. In vitro antioxidant experiments and in vitro immune function promotion experiments verify the biologically active polypeptide WNIPMGLIVNQ has good antioxidant biological activity and immunity regulating function, on the one hand, the biologically active polypeptide WNIPMGLIVNQ has good antioxidant activity to remove free radicals in the body and improve the quality of life; on the other hand, the biologically active polypeptide WNIPMGLIVNQ can enhance the in-vitro proliferation ability of lymphocytes and macrophages to promote the increase of macrophage induced nitric oxide, the biologically active polypeptide WNIPMGLIVNQ can improve the external pathogen infection resistance of the body and reduce the disease incidence of the body, and has very important significance for development of food, health care products and drugs with immune function regulating and antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
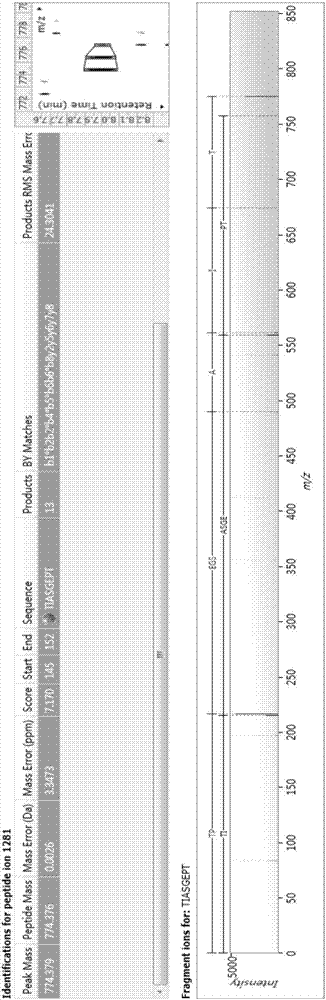
Bioactive polypeptide TIASGEPT, and preparation method and application thereof
ActiveCN107226857AReduce harmRegulate immunityCosmetic preparationsPeptide/protein ingredientsNitric oxideDrug biological activity
The invention relates to the field of protein, and in particular to a bioactive polypeptide TIASGEPT, and a preparation method and application thereof. An amino acid sequence of the bioactive polypeptide TIASGEPT is Thr-Ile-Ala-Ser-Gly-Glu-Pro-Thr. An in-vitro antioxidant experiment and an in-vitro immunological function promoting experiment verify that the bioactive polypeptide TIASGEPT has better antioxidant biological activity and a better immune modulating function; on one hand, the bioactive polypeptide TIASGEPT has better antioxidant activity and is capable of removing free radicals in a body and increasing the quality of life; on the other hand, the bioactive polypeptide TIASGEPT is capable of enhancing in-vitro proliferation ability of lymphocytes and macrophages, promoting the increment of nitric oxide induction amount of the macrophages, increasing the ability of defending external pathogen infection by the body and reducing the morbidity of the body, and has a very important significance in developing food, healthcare products and drugs, which have an immune modulating function and an antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
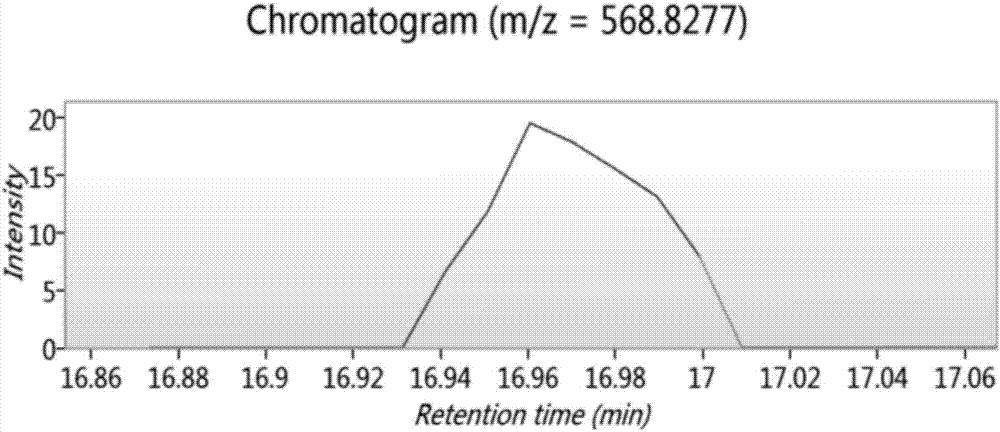
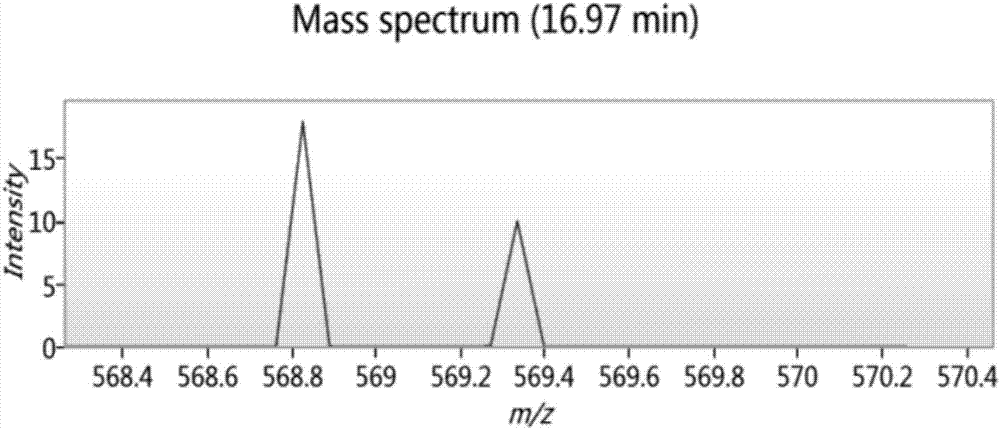
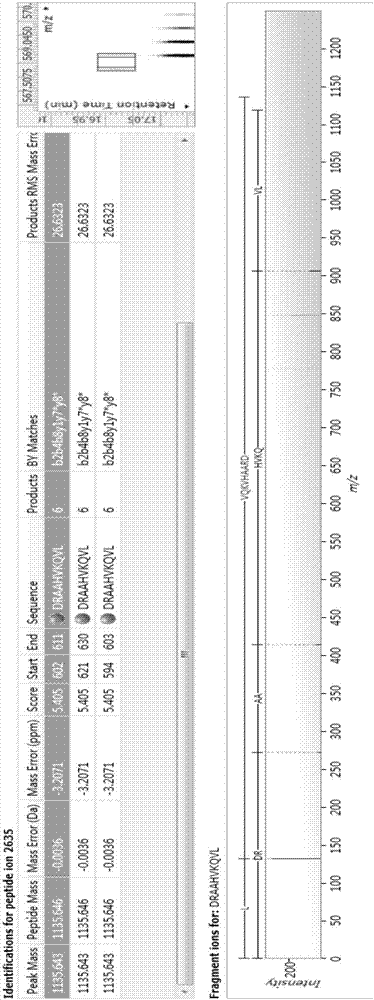
Bioactive peptide DRAAHVKQVL as well as preparation method and application thereof
ActiveCN107177000AReduce harmImproves antioxidant activityCosmetic preparationsPeptide/protein ingredientsLife qualityCytokine
The invention relates to the field of protein, in particular to a bioactive peptide DRAAHVKQVL as well as a preparation method and an application thereof. An amino acid sequence of the bioactive peptide DRAAHVKQVL is Asp-Arg-Ala-Ala-His-Vla-Lys-Gln-Val-Leu. An in-vitro anti-oxidation experiment and an in-vitro immunity function promoting experiment prove that the bioactive peptide DRAAHVKQVL has better anti-oxidation biological activity and immunity regulation function, on one hand, the bioactive peptide DRAAHVKQVL has better anti-oxidation activity, can clear free radicals in an organism and improves life quality; on the other hand, by the aid of the bioactive peptide DRAAHVKQVL, in-vitro proliferation capacity of lymphocytes and macrophages can be enhanced, the macrophages are promoted to secrete cytokines, the organism's capacity of resisting outside pathogen infection is improved, morbidity of the organism is reduced, and therefore, the bioactive peptide DRAAHVKQVL has quite great significance in development of food, healthcare products and drugs with an immune regulation function and an anti-oxidation function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
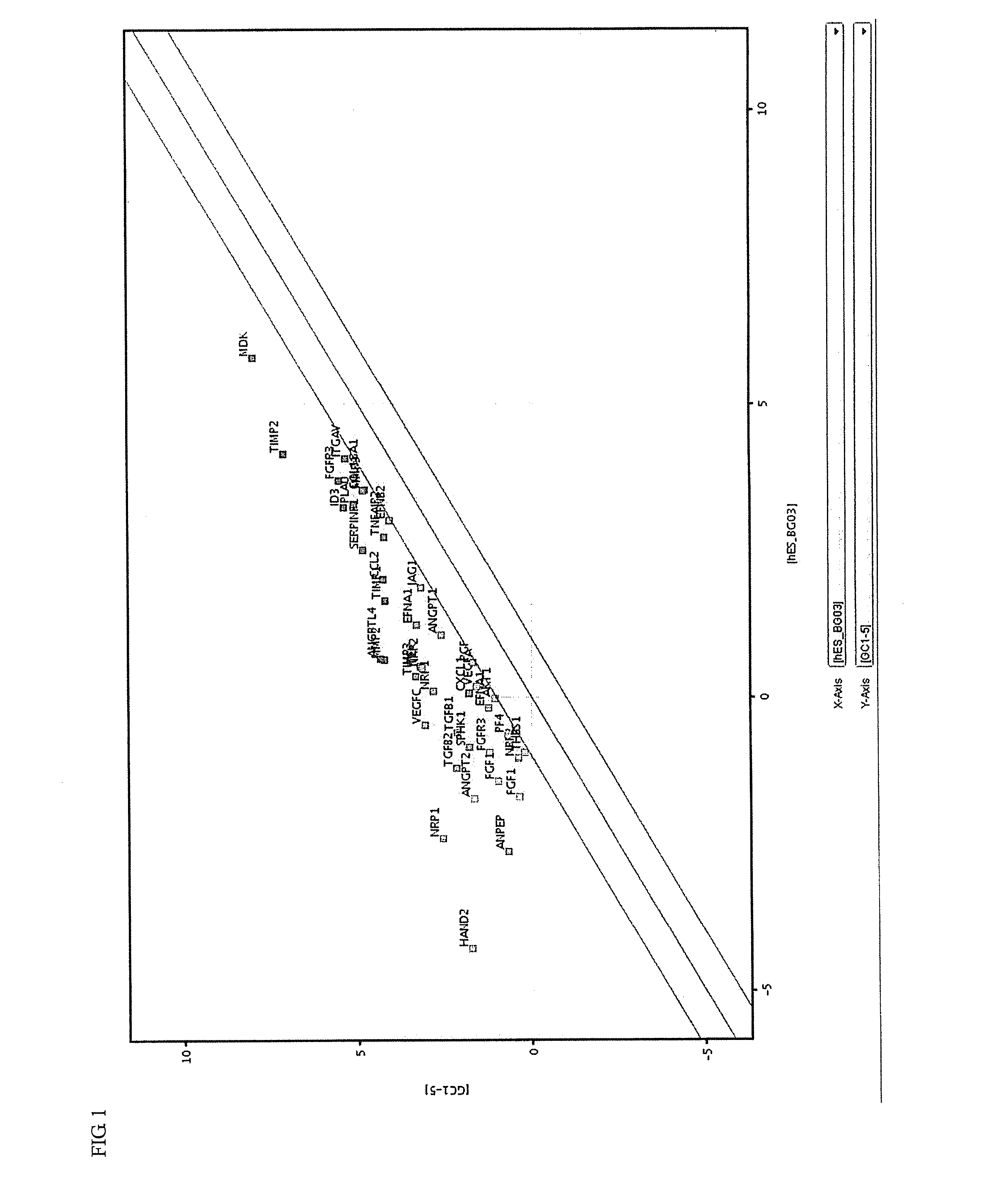
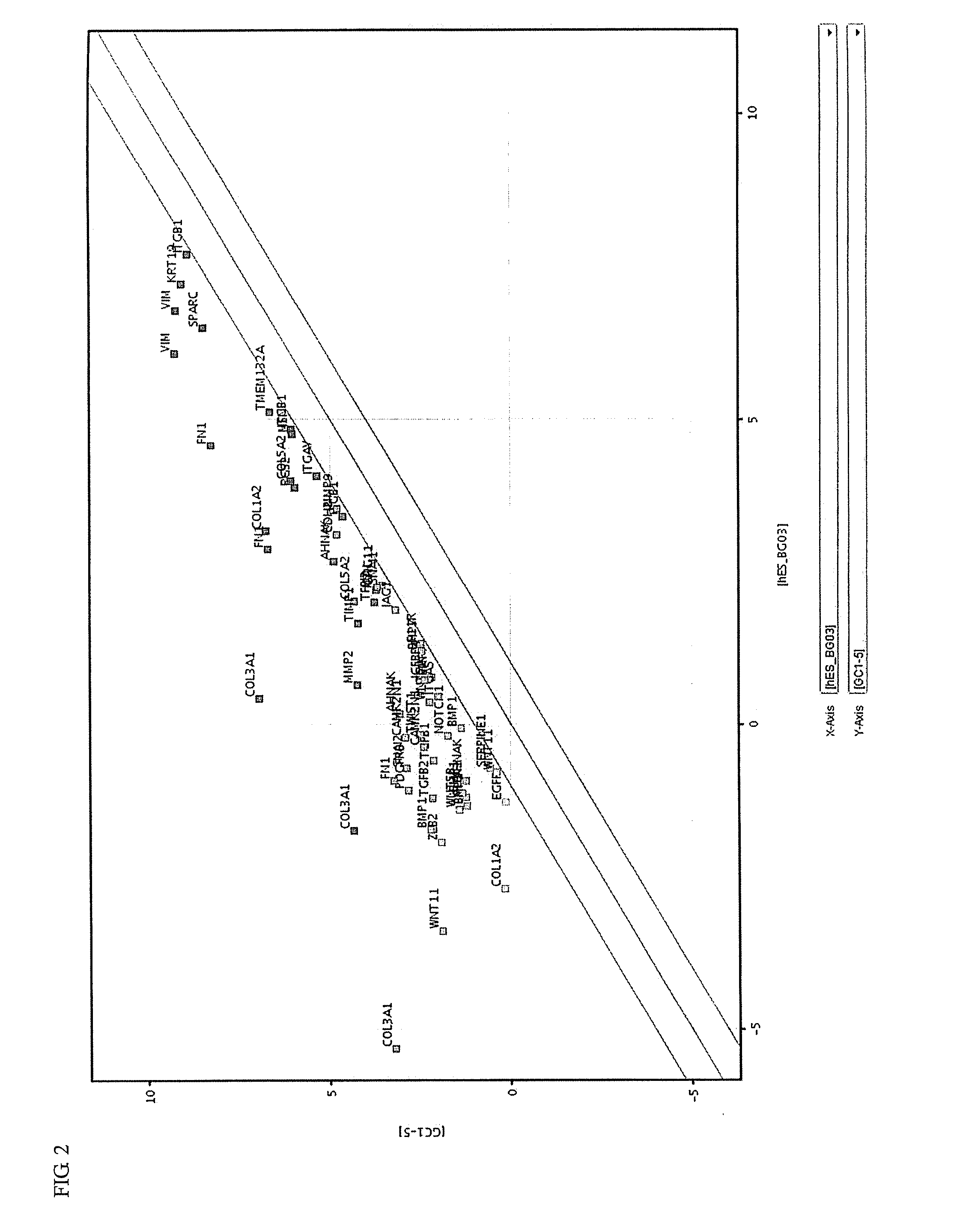
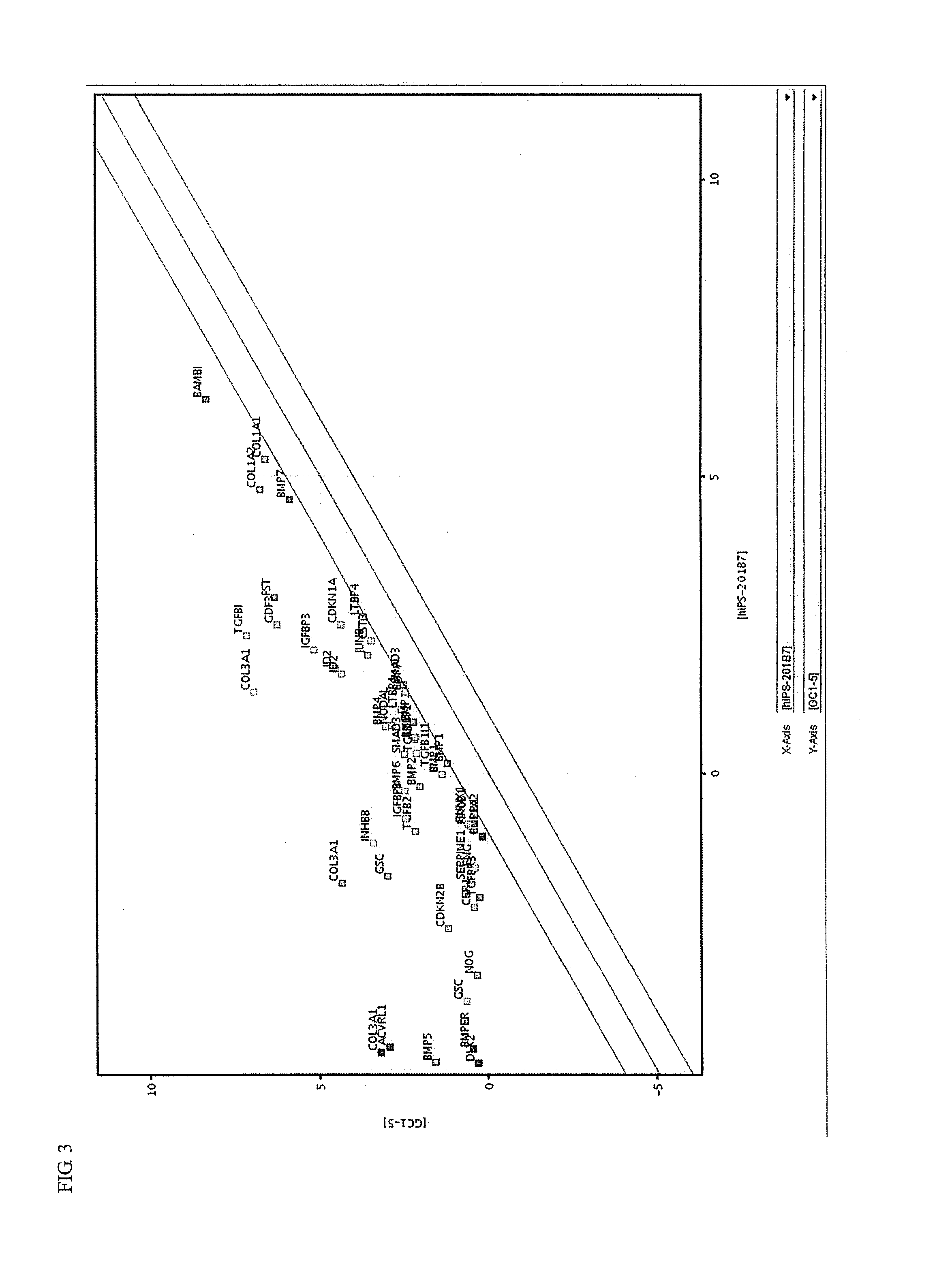
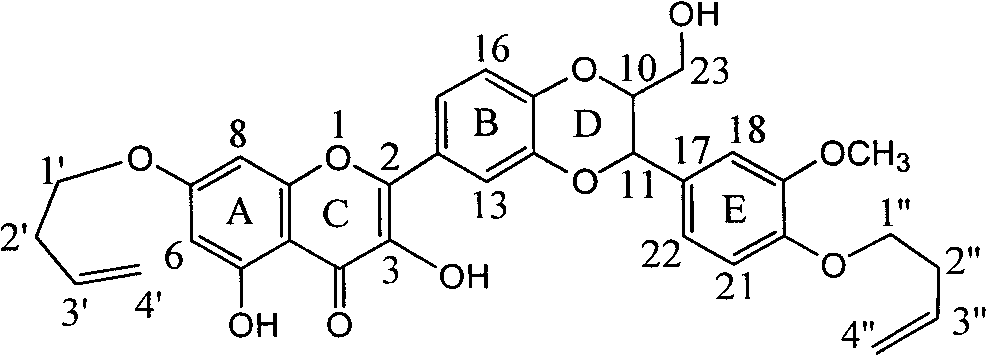
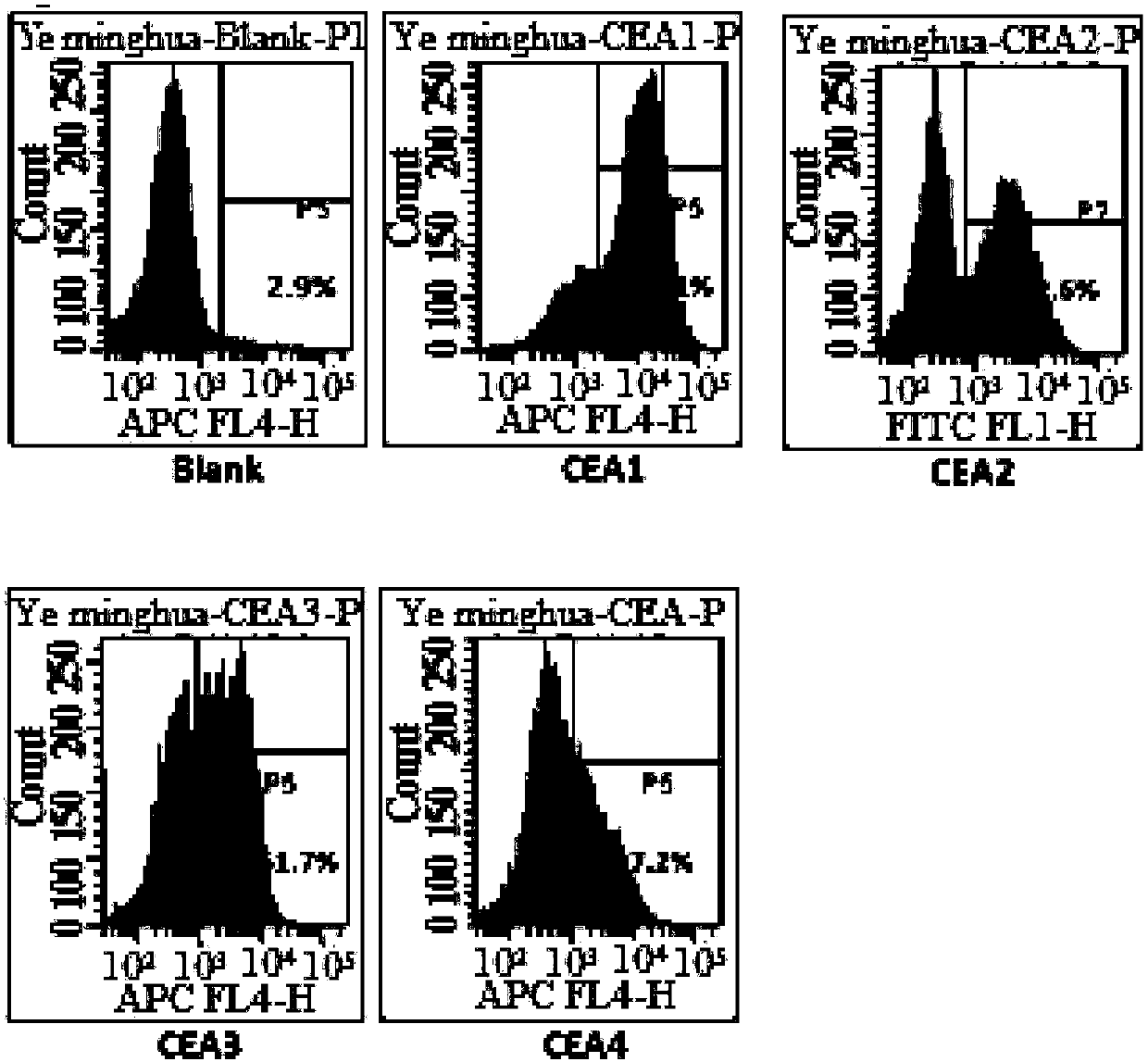
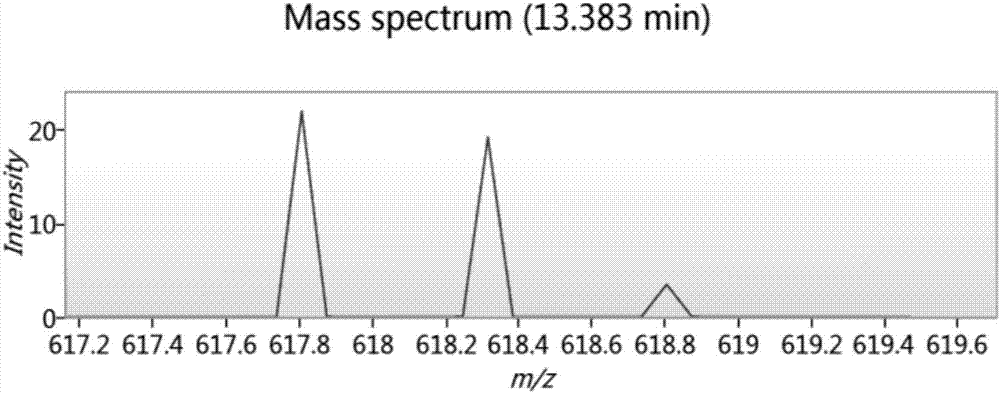
Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound
InactiveCN106432249AGood choiceApparently irreversible inhibitionOrganic chemistryAntipyreticSynthesis methodsProliferation activity
The invention discloses a synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound. The compound belongs to a novel-structure compound. The synthesis method is high in operating safety, mild in reaction condition and suitable for industrial production. The activity and the selectivity of BTK kinase and the in-vitro proliferation activity of a leukemia cell line are test, it is verified that the compound has the selective and irreversible inhibition effect on the BTK kinase, and has the different-degree inhibition effect on leukemia cells. According to measurement, the compound also has the good anti-arthritic activity on a collagen-induced arthritis (rCIA) model. The pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound can be used for preparing medicine for treating arthritis and leukemia.
Owner:NINGBO WESDON POWDER PHARMA COATINGS CO LTD
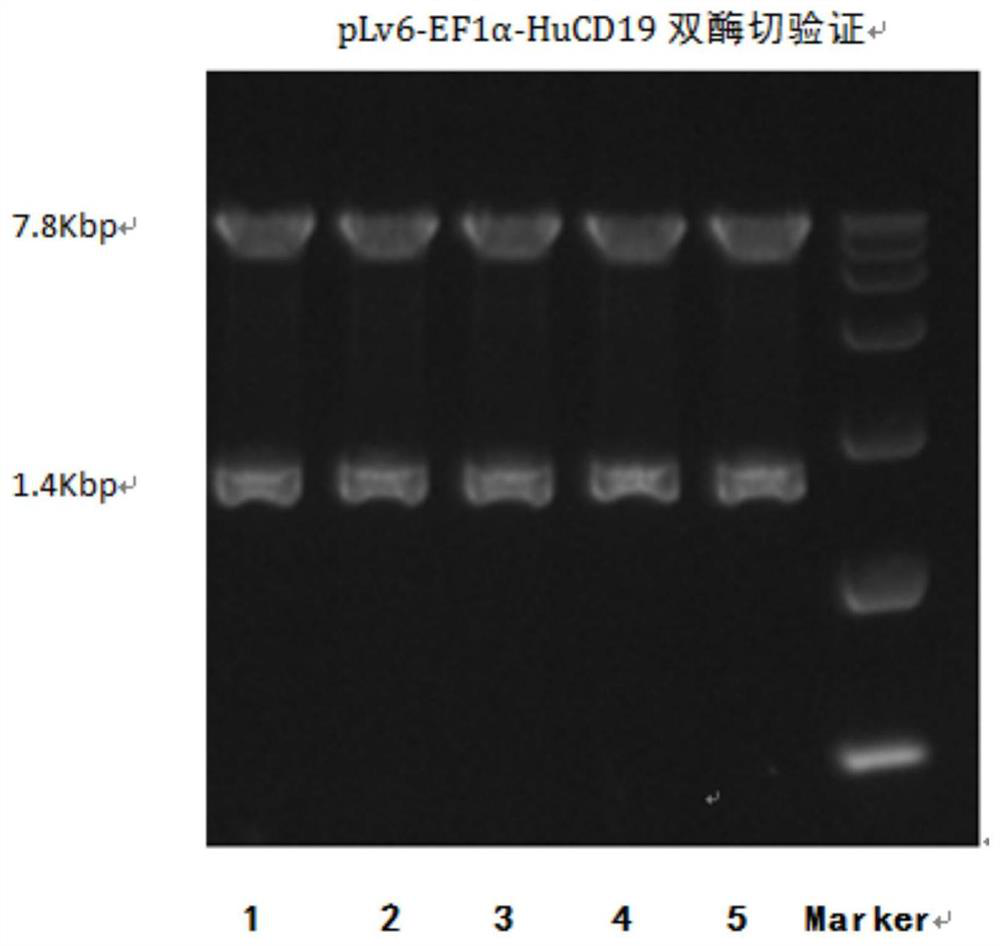
Preparation and application of CD19-targeting humanized chimeric antigen receptor
PendingCN111733186AIncrease lethalityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCell phenotypeCell kill
The invention relates to preparation and application of a CD19-targeting humanized chimeric antigen receptor, and concretely relates to construction of a human CD19-antigen-targeting humanized lentiviral vector expression plasmid. The human CD19-antigen-targeting humanized lentiviral vector expression plasmid is applied to the preparation of chimeric antigen receptor T cells; and then, the effectsare verified through the chimeric antigen receptor T cells in in-vitro proliferation tests, cell phenotype tests and cell killing tests. A CAR sequence of the invention consists of a leader peptide sequence, a human CD19-antigen-targeting single-chain scFv sequence, a hinge region, a transmembrane region structure domain and an intracellular signal conduction structure domain sequence. The chimeric antigen receptor is built into a lentivirus system expression vector, and immune cells are infected; the human CD19-antigen-targeting chimeric antigen receptor can be expressed on the surface of the immune cells; the effects of killing tumor cells can be achieved by recognizing the CD19 antigen expressed on tumor tissues; the goal of tumor treatment is achieved; and a novel measure is providedfor cancer treatment.
Owner:天津英科赛奥科技有限公司
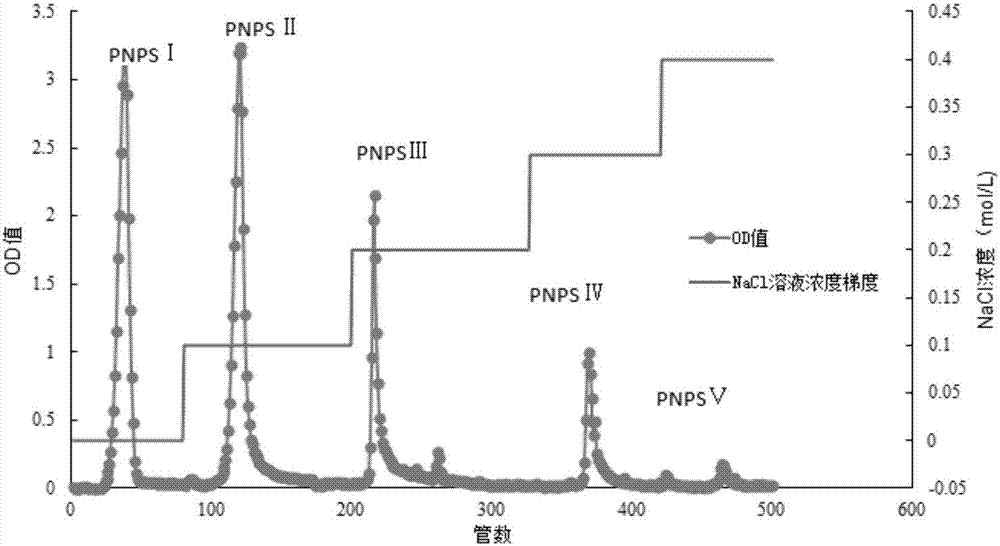
Method for extracting and purifying neutral pseudo-ginseng polysaccharide, research and application for pharmacological activity for promoting cell proliferation
ActiveCN107286265APromotes significant proliferationStrong value-added effectOrganic active ingredientsCosmetic preparationsOsteoblastPeriodontal ligament stem cells
The invention relates to a method for extracting and purifying neutral pseudo-ginseng polysaccharide, a research and an application for pharmacological activity for promoting cell proliferation. The pseudo-ginseng waste residue of the notoginsenoside extracted in the production process is taken as a raw material, a water extract and alcohol precipitation method is adopted for acquiring crude pseudo-ginseng polysaccharide and the DEAE Sepharose Fast Flow anion exchange chromatography is adopted for purifying the crude pseudo-ginseng polysaccharide so as to acquire five components: neutral pseudo-ginseng polysaccharide PNPS I and acidic pseudo-ginseng polysaccharide PNPS II, PNPS III, PNPS IV and PNPS V five samples. The research on the influence of the pseudo-ginseng polysaccharide on human periodontal ligament stem cell, mice osteoblast and human skin epidermis cell in vitro proliferation proves that the neutral pseudo-ginseng polysaccharide PNPS I is effective in boosting the periodontal ligament stem cell, mice osteoblast and human skin epidermis cell in vitro proliferation and the neutral pseudo-ginseng polysaccharide PNPS I can be used as an important drug intermediate for developing a new drug and a natural raw material for a functional healthcare food and also can be used as an active raw material of the household chemicals.
Owner:云南多糖生物科技有限公司
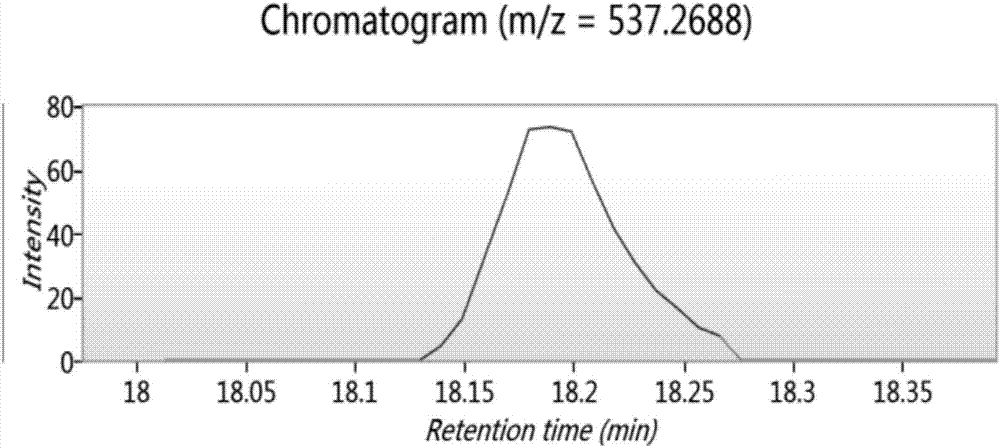
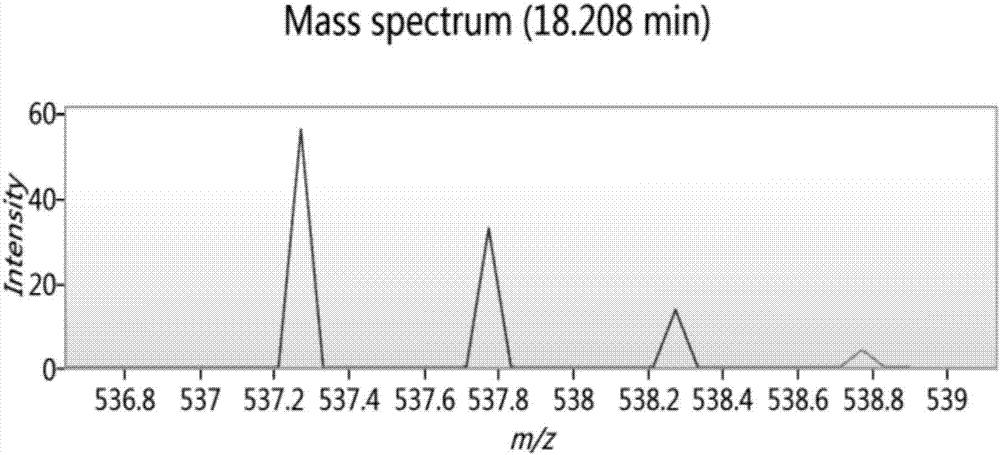
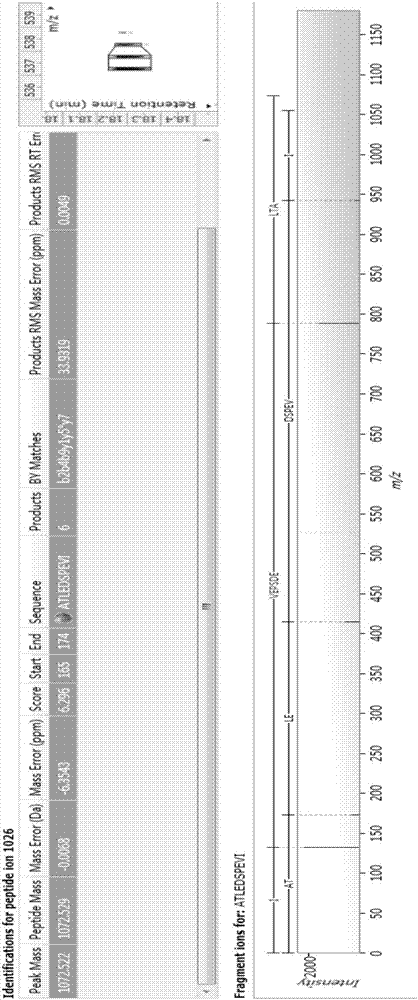
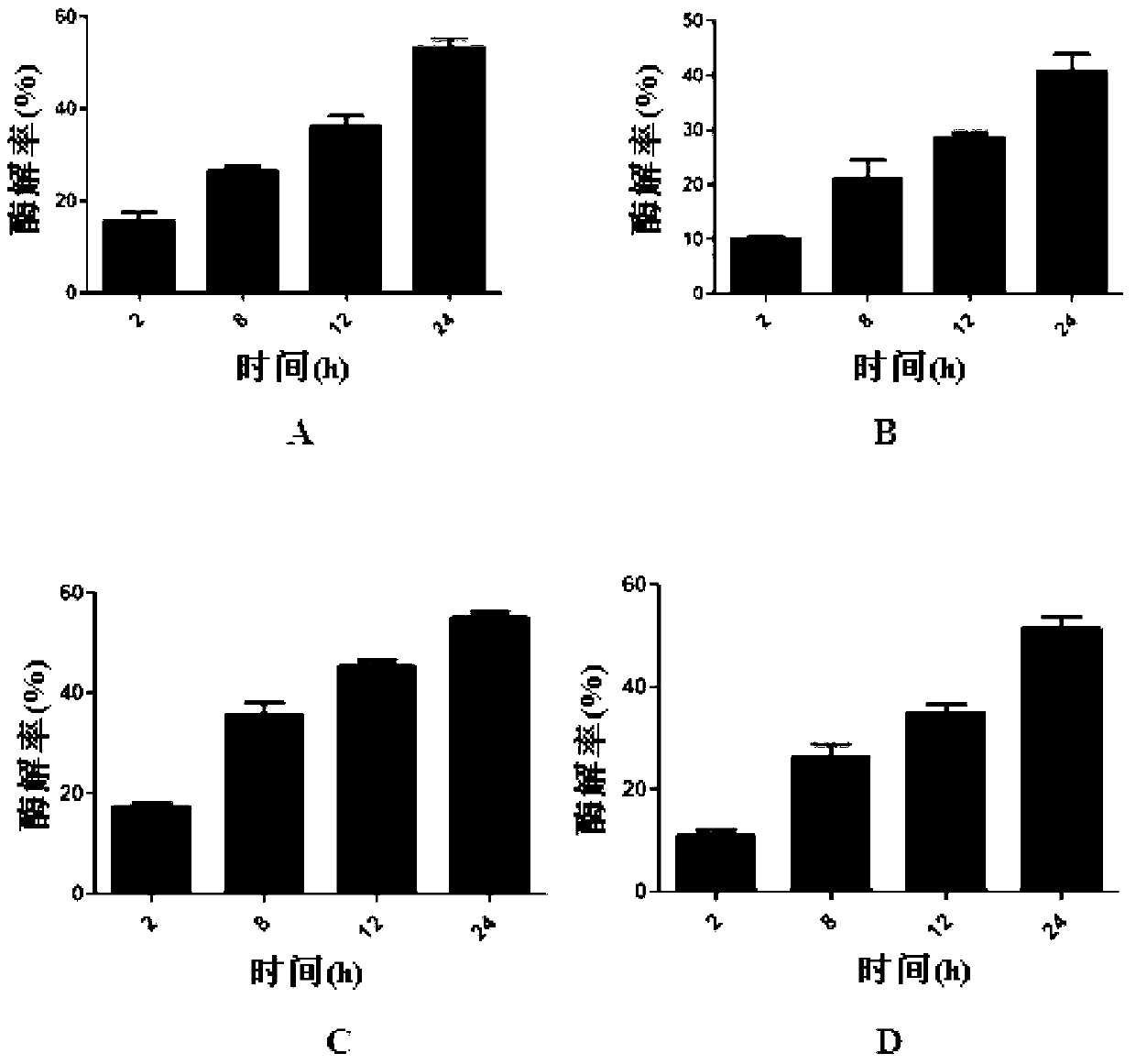
Bioactive polypeptide ATLEDSPEVI as well as preparation method and application thereof
ActiveCN107141346AReduce harmImproves antioxidant activityCosmetic preparationsPeptide/protein ingredientsQuality of lifeLymphocyte
The invention relates to the field of proteins and particularly relates to bioactive polypeptide ATLEDSPEVI as well as a preparation method and application thereof. The amino acid sequence of the bioactive polypeptide ATLEDSPEVI is Ala-Thr-Leu-Glu-Asp-Ser-Pro-Glu-Val-Ile. In-vitro anti-oxidation experiments and in-vitro immune function promotion experiments verify that the polypeptide ATLEDSPEVI has better antioxidant biological activity and immune modulating function, so that on one hand, the polypeptide ATLEDSPEVI has better antioxidant activity, so that free radicals in a body can be removed, and the quality of life is improved; on the other hand, the polypeptide ATLEDSPEVI can enhance the in-vitro multiplication capacity of lymphocytes and macrophages, the macrophages are promoted to secrete cytokines, the capability of the body in defense for external pathogen infection is enhanced, and the incidence rate of the body is reduced. Therefore, the polypeptide ATLEDSPEVI has great significance in development of food, health-care products and drugs with immune modulating function and antioxidant function.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Indole alkaloid adduct, and preparation method and application thereof in preparing anti-tumor drug
ActiveCN103275106ALow toxicityReduce toxicity in the bodyOrganic chemistryAntineoplastic agentsNormal cellIn vitro proliferation
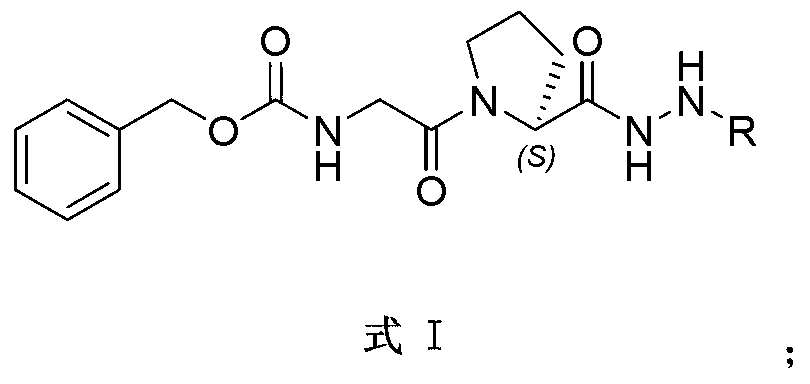
The invention relates to the field of anti-tumor drugs, and specifically discloses an indole alkaloid adduct, and a preparation method and an application thereof in preparing an anti-tumor drug. The indole alkaloid adduct has a structure shown as a formula I, wherein R is formed in the way that an indole alkaloid is subjected to hydrazinolysis and the hydrazinolysis product is bonded with hydrazine group. The indole alkaloid adduct can significantly reduce toxicity to normal cells and in-vivo toxicity and inhibit in-vitro proliferation of various tumor cell lines and the growth of tumor in the nude mouse bearing the tumor.
Owner:JINAN UNIVERSITY
Molecular marker LncRNA DANCR for diagnosing and treating bladder cancer and application thereof
ActiveCN109371131APrevent proliferationInhibit migrationMicrobiological testing/measurementAntineoplastic agentsIn vivoCell migration
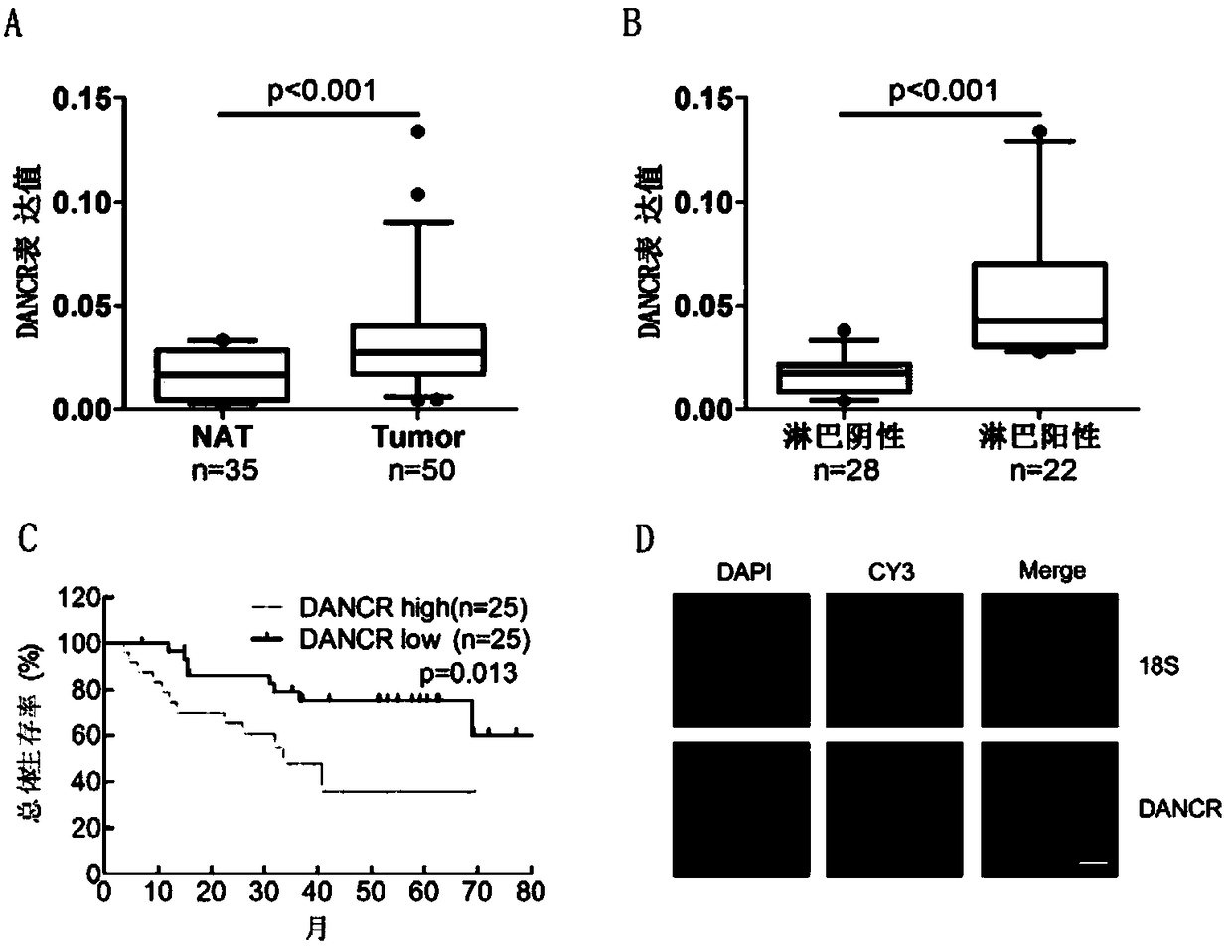
The invention discloses a molecular marker for diagnosing and treating the bladder cancer, namely LncRNA DANCR, and also discloses application of the molecular marker to screening or preparation of reagents used for diagnosing the bladder cancer diagnosing, bladder cancer lymphatic metastasis, bladder cancer prognosis relapse or bladder cancer clinical stages and application of the molecular marker to screening or preparation of drugs used for treating the bladder cancer. It is found that the LncRNA DANCR has high expression in bladder cancer cases with lymphatic metastasis, has negative correlation with overall survival prognosis of a patient and is an independent indicator for diagnosing the bladder cancer and judging bladder cancer development and survival prognosis; silencing of the LncRNA DANCR can restrain in-vitro proliferation, cell migration and invasion and in-vivo tumor formation and lymphatic metastasis of bladder cancer cells. It is verified that the LncRNA DANCR is an important cancerigenic factor of the bladder cancer for the first time, and can be used as a molecular marker for bladder cancer prognosis diagnosis and a new target spot for treatment.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Micro-fluidic chip for quickly capturing or detecting cells and method
PendingCN110093254AAchieve enrichmentEasy to captureCell dissociation methodsBioreactor/fermenter combinationsFood borneA-DNA
The invention relates to the technical field of biological detection, and discloses a micro-fluidic chip for quickly capturing or detecting cells. The structure of the micro-fluidic chip comprises oneor more groups of micro-fluidic units, wherein the structure of each micro-fluidic unit comprises a channel A and a channel B which are mutually connected; a plurality of groups of herring bone shaped structure units are arranged in each channel A; a microcavity structure is arranged in each channel B; and a DNA tetrahedron is modified in each channel A, and a specific aptamer sequence which canrecognize / capture target cells is connected to the DNA tetrahedron through avidin. According to the micro-fluidic chip disclosed by the invention, the herring bone shaped structure is combined with the cylinder microcavity structure, so that the high-efficient low-cost specific capturing and enriching and in vitro proliferation efficiency of target cells such as food-borne pathogenic bacteria or tumor circulating cells is improved, the captured cells are enriched to the small-volume microcavity structures, and the survival rate of in vitro culturing is further increased.
Owner:SHANGHAI OCEAN UNIV
Induced malignant stem cells or pre-induction cancer stem cells capable of selfreplication outside of an organism, production method for same, and practical application for same
InactiveUS20130198876A1High expressionIncreasing endogenous expressionFermentationAntineoplastic agentsCancer cellLIN28
The present invention provides an induced cancer cell capable of self-replication in vitro which is useful in cancer therapy research and the research for cancer-related drug discovery, processes for production thereof, cancer cells induced by the malignant cells, and applications of these cells.The present invention provides an induced cancer stem cell capable of proliferation (self-replication) in vitro, wherein the induced cancer stem cell has the following two characteristics:(1) expressing the six genes (self-renewal related genes) POU5F1, NANOG, SOX2, ZFP42, LIN28, and TERT selected from a certain group of genes; and(2) having an aberration which is either (a) a mutation in an endogenous tumor suppressor gene or (b) increased expression of an endogenous cancer-related gene.
Owner:NAT CANCER CENT
Method for transfecting bovine somatic cells into inducted pluripotent stem cells by adopting transcription factors
InactiveCN101705247ANormal growth characteristicsGood pluripotencyGenetic engineeringFermentationFeeder LayerEmbryo
The invention discloses a method for transfecting bovine somatic cells into inducted pluripotent stem cells (iPSCs) by adopting transcription factors, concretely comprising the following steps: 1) isolation and culture of bovine dermal fibroblasts; 2) cloning of bovine transcription factor genes maintaining pluripotency and construction of retroviral vectors of the transcription factor genes; 3) retrovirus packaging and preparation; and 4) infection of bovine dermal fibroblasts by retrovirus and acquisition of bovine inducted pluripotent stem cells. The invention first adopts the acellular human amniotic membranes as support to replace the mouse embryonic fibroblasts (MEFs) to maintain in vitro proliferation of the bovine iPSCs and keep the bovine iPSCs in undifferentiated pluripotent states for long time. The method which adopts gene inducing to generate the PSCs not only solves the technical problem that the PSCs of the animals such as cattle, sheep, pigs and other livestock are difficult to obtain but also avoids the problem of heterogeneous animal cell pollution caused by the feeder layer which applies the MEFs as the stem cells.
Owner:NORTHWEST A & F UNIV
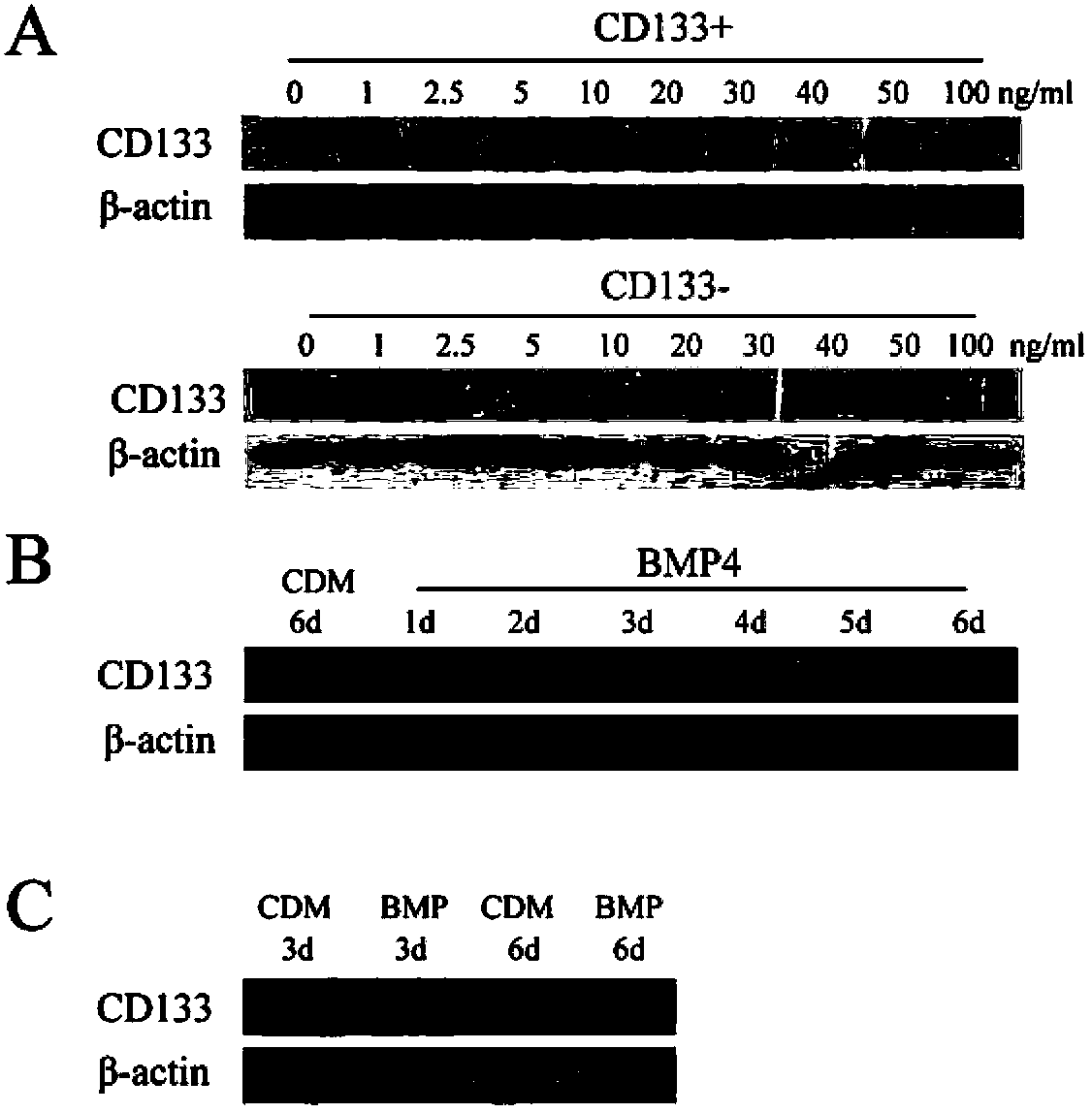
Application of bone morphogenetic protein 4 in cancer inhibition
The present invention relates to an application of bone morphogenetic protein 4 in cancer inhibition. Specifically bone morphogenetic protein (BMP) has a liver cancer stem cell differentiation induction effect, wherein CD133 protein expression of liver cancer cell lines can be down-regulated and liver cancer cell line CD133+ liver cancer stem cell differentiation can be promoted with BMP4, and BMP4 can further be provided for inhibiting in vitro proliferation and self-renewal of liver cancer cell lines and inhibiting tumorigenicity of immune-deficient mice. The present invention further provides BMP4-induced liver cancer stem cell differentiation action mechanism.
Owner:SHANGHAI INST OF ONCOLOGY
Hormone-secreting cells maintained in long-term culture
InactiveUS6372493B1Increased insulin secretionHigh glucose concentrationPeptide/protein ingredientsDiagnosticsCulture mediumsHuman chorionic gonadotropin
Methods are provided for the establishment and maintenance in long term culture of hormone secreting cells. Cells are derived from tumorous or non-tumorous animal or human tissues, including ovary, endometrium, trophoblast, pituitary, thyroid, and pancreas. The cells secrete into the culture medium hormones such as estrogens, progestins, follicle-stimulating hormone, luteinizing hormone, human chorionic gonadotrophin, thyroxin, glucagon, and insulin, depending on the tissue of origin of individual cell cultures. Contact with an appropriate secretogogue causes the cells to respond with increased hormone secretion. For instance, ovarian follicular cells respond to follicle-stimulating hormone with increased estrogen and progesterone secretion. Pancreatic cells respond to elevated glucose with increased insulin secretion. The cells proliferate in in vitro for up to one year or longer, during which time they retain their hormone-secretion profile. The cells may be frozen for storage, and retain their hormone-secretion profile after thawing. The cell cultures are useful for the production of human hormones, for the bio-assay of drugs such as therapeutic gonadotrophin, for the testing of drug efficacy and design, and for toxicity testing of drugs and chemicals. The cells may also be implanted in an individual to replace deficient hormone secretion. For instance, insulin secreting pancreatic cells may be implanted in a diabetic individual as an adjunct or replacement therapy for exogenously administered insulin.
Owner:PACIFIC BIOMEDICAL RES INC
Culture medium and culture method for constructing liver tumor scaffoldless organoid
InactiveCN111411084AGrow fastStable growthCell dissociation methodsCulture processChemo therapyAcetylcysteine
The invention discloses a culture medium and a culture method for constructing a liver tumor scaffoldless organoid. The culture medium comprises the following components: Advanced DMEM / F12, FBS, diabody, N-2, Noggin, B-27, EGF, FGF-10, Y-27632, A83-01, SB202190, R-Spondin, N-acetylcysteine, Nicotinamide, Wnt3A, HGF and PrimocinTM. The culture method comprises the steps of adding primary tumor cells separated and obtained into the above-mentioned special culture medium for resuspension, inoculating into an ultra-low adsorption U-shaped well plate after counting, and centrifugally culturing until organoids are formed. The culture medium and the culture method can realize in-vitro proliferation of primary liver cancer cells, can quickly construct the liver tumor scaffoldless organoids, and can maintain long-term stable growth of the liver tumor organoids. The tumor organoids formed by the culture medium and the culture method retain heterogeneity of tumor tissues of patients, and are suitable for drug sensitivity detection, tumor generation and development related research of in-vitro liver cancer chemotherapeutic drugs.
Owner:江苏信安佳医疗科技有限公司
Culture solution for delaying aging of mesenchymal stem cells and preparation method thereof
InactiveCN108676773AAnti agingImprove playbackCulture processSkeletal/connective tissue cellsCell phenotypeVitamin C
The embodiment of the invention provides a culture solution for delaying aging of mesenchymal stem cells and a preparation method thereof, and relates to the technical field of biomedical materials. The culture solution reduces the aging rate of the mesenchymal stem cells as much as possible, maintains cell phenotypes and functions of the stem cells and improves the utilization efficiency of the stem cells while in-vitro proliferation of the mesenchymal stem cells is increased, so that requirements of scientific research and clinical application are met. According to the culture solution for delaying aging of the mesenchymal stem cells and the preparation method thereof, the culture solution comprises a basal culture solution and exogenous additives, wherein the exogenous additives include5-15g / L of glucan, 0.1-0.5mL / L of a lipid-based concentrating agent, 0.05-5[mu]g / L of hydrocortisone, 0.1-10[mu]g / L of dexamethasone, 1-5[mu]g / L of sodium selenite, 10-50[mu]M of beta-mercaptoethanol, 10-100mg / L of vitamin C (VC), 10-50[mu]g / L of vitamin E (VE), 1-20mg / L of transferrin, 5-50[mu]g / L of insulin, 10-50[mu]g / L of growth hormone (GH), 10-50[mu]g / mL of glutathione and 1% of non-essential amino acids.
Owner:广东芙金干细胞再生医学有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/132f86aa-16b8-4790-9684-0ec8e558111e/HDA0000504551810000011.png)
![AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/132f86aa-16b8-4790-9684-0ec8e558111e/HDA0000504551810000012.png)
![AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell AFP[158-166] specific TCR gene, its transgenic T cell, and in-vitro proliferation method and use of transgenic T cell](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/132f86aa-16b8-4790-9684-0ec8e558111e/HDA0000504551810000013.png)












![Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ea4c1d28-2148-4b87-a68d-452b0c5666ca/160929161714.png)
![Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ea4c1d28-2148-4b87-a68d-452b0c5666ca/160929161717.png)
![Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound Synthesis and medical application of pyrrolo-[2,1-f] [1,2,4] triazine mother nucleus compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ea4c1d28-2148-4b87-a68d-452b0c5666ca/160929161720.png)