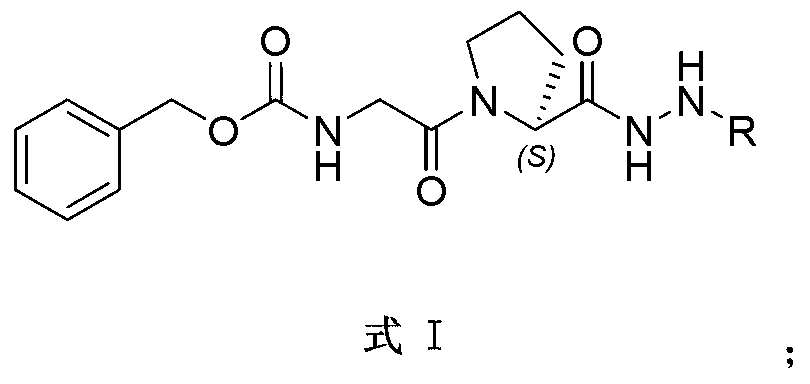
Indole alkaloid adduct, and preparation method and application thereof in preparing anti-tumor drug
A technology of indole alkaloids and indole alkaloid hydrazides, which is applied in the application field of indole alkaloid adducts and their preparation, and preparation of antitumor drugs, can solve problems such as limited applications, and achieve reduced toxicity and in vivo Toxicity, mild reaction conditions, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation and separation and purification of indole alkaloid adducts
[0041]1.1.1 Preparation of the hydrazine hydrolysis product of vinblastine: Weigh 182 mg (0.2 mmol) of vinblastine sulfate into a 35 ml thick-walled pressure-resistant tube, add 8 ml methanol and 0.9 ml 80% hydrazine hydrate (23 mmol), ultrasonicate for 5 minutes, and degas Remove the air contained in the solution, then plug the stopper tightly, cover it with tin foil to avoid light, put it in a 60°C oil bath and stir for 24 hours. After the reaction is completed, add water to dilute, extract with dichloromethane (DCM) several times, and combine organic phase, washed successively with water, saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The resulting mixture was separated and purified by RP-HPLC (reversed phase preparative high performance liquid chromatography) (mobile phase was MeOH:H 2 O:Et 3 N=70:30:0.005, V / V / V), t...
Embodiment 2
[0061] Example 2 In vitro cytotoxicity test of indole alkaloids and their derivatives
[0062] Experimental method: Cells in the logarithmic growth phase (A549 (human non-small cell lung cancer cells), Lovo (human colon cancer cells), CNE-2 (human nasopharyngeal carcinoma cells), HepG2 (human liver cancer cells), Hela (human cervical cancer cells), MCF-7 (human breast cancer cells), MDA-MB-231 (human breast cancer cells), NCI-N87 (human gastric cancer cells), PC-3 (human prostate cancer cells), DU145 (human Prostate cancer cells), K562 (human leukemia cells), A375 (human melanoma cells), SH-SY5H (human neuroblastoma cells), human promyelocytic leukemia cells (HL-60), BEL-7402 / 5- Fu (fluorouracil-resistant strain of human liver cancer), HepG2 / ADM (doxorubicin-resistant strain of human liver cancer), MCF-7 / ADR (doxorubicin-resistant Bovine serum 10%, penicillin 100U / mL), adjust cell concentration to 5×10 5 cells / mL, inoculated in a 96-well culture plate, and inoculated 100 μL ...
Embodiment 3
[0072] Example 3 In vitro toxicity test of indole alkaloids and their derivatives to normal cells
[0073] Experimental method: detect the effect of indole alkaloids, hydrazinolysis products and indole alkaloid adducts on human normal liver cancer cell LO by the method of embodiment 2 2 and in vitro cytotoxicity of human umbilical vein endothelial cells HUVEC.
[0074] Experimental results: hydrazinolysis products and adducts on LO 2 and HUVEC were much less cytotoxic than the corresponding indole alkaloids (see Table 3). It shows that the cytotoxicity of the compound after targeted modification is significantly reduced.
[0075] Table 3. MTT method to determine the toxic effect of indole alkaloids and their derivatives on normal cells
[0076]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com