Cellular immunological detection kit for evaluating curative effect of vaccine and storage method thereof
A detection kit, a technology of cellular immunity, applied in the field of kits for evaluating the efficacy of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Embodiment 1, the clinical sample cellular immunology evaluation of hepatitis B therapeutic vaccine B gram
[0132] 1. Materials and Reagents
[0133] 10ml EDTA anticoagulant blood collection tube (BD, catalog number: 367525), freezing tube (Corning, 430659), 15ml centrifuge tube (Corning, 430791), 12-well cell culture plate (Costar, 3513), 96-well U-bottom cell culture plate (Costar, 3799), 10ml pipette (Costar, 4488), sterile 1.5ml LEP tube, flow cytometry tube, and other consumables.
[0134] (1) Sterile phosphate buffer solution (phosphate buffer solution, PBS) was purchased from Gibco Company, the product number is 20012-027. (2) Human lymphocyte separation medium (LymphoprepTM) was purchased from Axis-Shield Company, the product number is 11114547. (3) 4% paraformaldehyde (paraformaldehyde, PFA, purchased from Sinopharm Chemical Reagent Co., Ltd.): Dissolve 8g of PFA in PBS, with a final volume of 200mL, heat and stir and add a few drops of concentrated NaOH...
Embodiment 2
[0196] Example 2: Long-term stability monitoring of Eke clinical sample cellular immunology detection kit
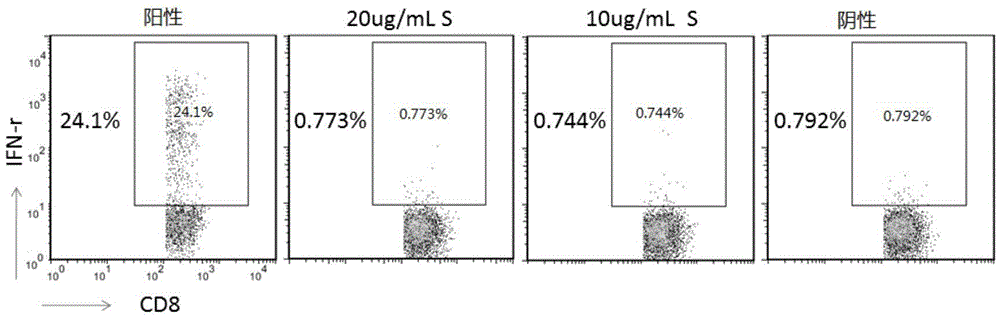
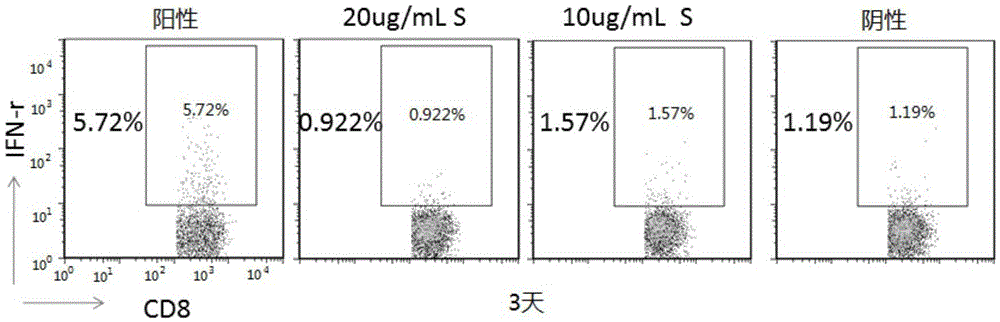
[0197] Materials and Reagents Reference Example 1, PBMCs are derived from chronic hepatitis B patients in the B-gram treatment group, and the detection index is mainly CD8 + Expression of T cell IFN-γ.
[0198] We monitored the stability of EK Clinical Sample Cellular Immunology Detection Kit for 1 month, 3 months, 6 months and 12 months. Store the 96-well pre-coated cell stimulation plate in the kit at -20°C, and store other reagents at 4°C.
[0199] Such as Figure 7A As shown, kits stored for 1 month, 3 months, 6 months, and 12 months compared with day 0 were tested to positively stimulate wells CD8 + The expression level of IFN-γ in T cells basically did not change, and negative stimulation wells were used as controls. Such as Figure 7B , assuming that the stability of the kit is 100% at day 0, after conversion, the stability of the kits stored for 1 month, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com