Human Fc<gamma>R II linear ligand binding epitope
An epitope and linear technology, applied in the field of Fc receptors, can solve the problem of low affinity, achieve the effect of small side effects, solve autoimmune diseases, and high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
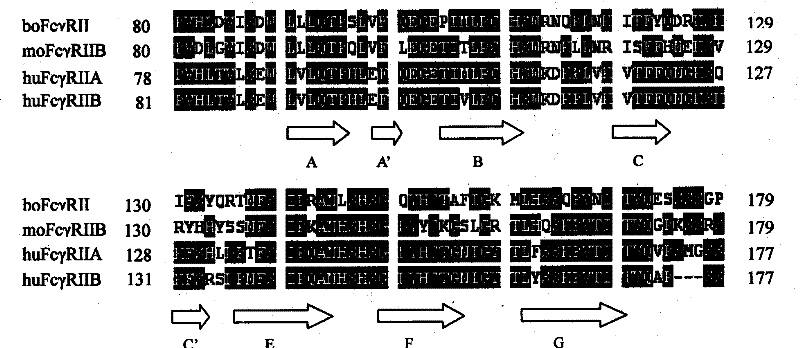
[0028] Example 1: Design of huFcγRII polypeptide
[0029] Using DNASIS (Ver.2.5, Hitachi) software, the amino acid sequences of huFcγRIIA (NP_067674) and boFcγRII (NP_776964), huFcγRIIB (NP_003992) and moFcγRIIB (NP_034317) were compared and analyzed ( figure 1 ), and referring to the crystal structures of huFcγRIIA (Maxwell et al., 1999; Sondermann et al., 2001) and huFcγRIIB (Sondermann et al., 1999), six huFcγRII polypeptides were designed and synthesized, covering A-B, B-C, C-C', C'-E, E-F and F-G loops, except for huRII2 and huRII6 polypeptides that contain Cys at the N-terminus, Cys is added to the N-terminus of the other four polypeptides for carrier protein coupling (see Table 1).
[0030] Table 1 Characteristics of huFcγRII polypeptides and reactivity with human IgG
[0031]
[0032] a For subsequent linking of the N-terminal brackets of the polypeptide sequence is an additional cysteine residue, b Dot-blot detection of the binding of each peptide to human IgG...
Embodiment 2
[0033]Example 2: Synthesis procedure and screening procedure of huFcγRII polypeptide
[0034] Using the Symphony12-channel peptide synthesizer (Protein Technologies Inc.) on Fmoc-Amino Acids attached to Wang Resin (Fmoc-Amino Acids attached to Wang Resin, Shanghai Jier) with solid-phase peptide synthesis (solid-phase peptide synthesis) to synthesize peptides, peptides Synthesis was performed according to standard operating procedures. The specific process is as follows:
[0035] Design and synthesize peptide sequence→peptide program analysis→design synthesis program→calculate and weigh Fmoc-AA-Wang-resin or Rink resin according to the substitution value of the resin→DMF swelling resin→ * Add 20% piperidine to remove Fmoc protection, stir for 6min→ * Add the next amino acid and HBTU for acylation reaction, N 2 Stir the reaction for 30min→ * Test the completion of the reaction by the Kaiser method or the TNBS method → * Wash 5 times with DMF, 1 min each time → repeat with ...
Embodiment 3
[0037] Example 3: Coupling of huFcγRII polypeptide
[0038] Application of heterobifunctional reagent Sulfo-SMCC (MW: 436.37, Spacer Arm Length: 11.6 , Pierce) by adding -NH on the carrier protein BSA 2 It is connected with the -SH of the N-terminal Cys of the polypeptide to form the artificial binding antigen polypeptide BSA-Pep. The coupling steps are:
[0039] (1) 4 mg of IgG-free bovine serum albumin (IgG-free BSA, Sigma-Aldrich) was weighed and dissolved in 0.5 ml of coupling buffer (0.1M PB pH7.2, 0.15M NaCl, 1 μM EDTA). (2) Dissolve 1 mg Sulfo-SMCC (MW: 436.37, Spacer arm length: 11.6 , Pierce), add BSA solution, mix thoroughly, react at room temperature for 1 h or 30 min at 37°C, and mix from time to time. (3) Dialyze the coupling buffer solution overnight at 4°C, and change the solution 3 times to remove excess coupling agent. The solution is SMCC-activated BSA carrier protein (SMCC-BSA), and the protein concentration is adjusted to 5 mg / ml with coupling buffer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com