Method of high efficiency expression tPA exogenic protein using methanol yeast system and its purification preparation technology of expressed product
A protein and protein technology, applied in recombinant DNA technology, fermentation, DNA/RNA fragments, etc., can solve the problems of research work without large-scale production and high expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
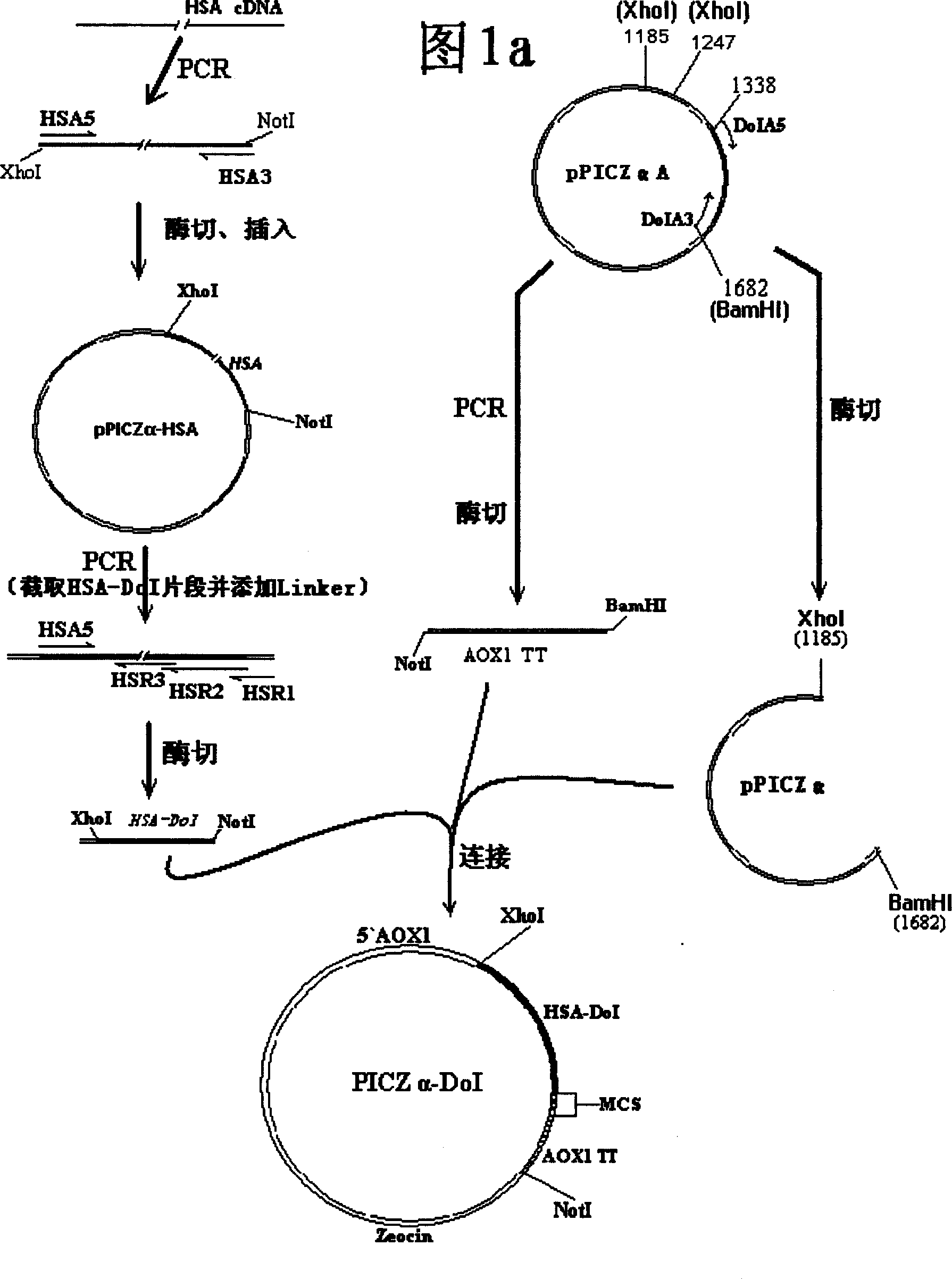
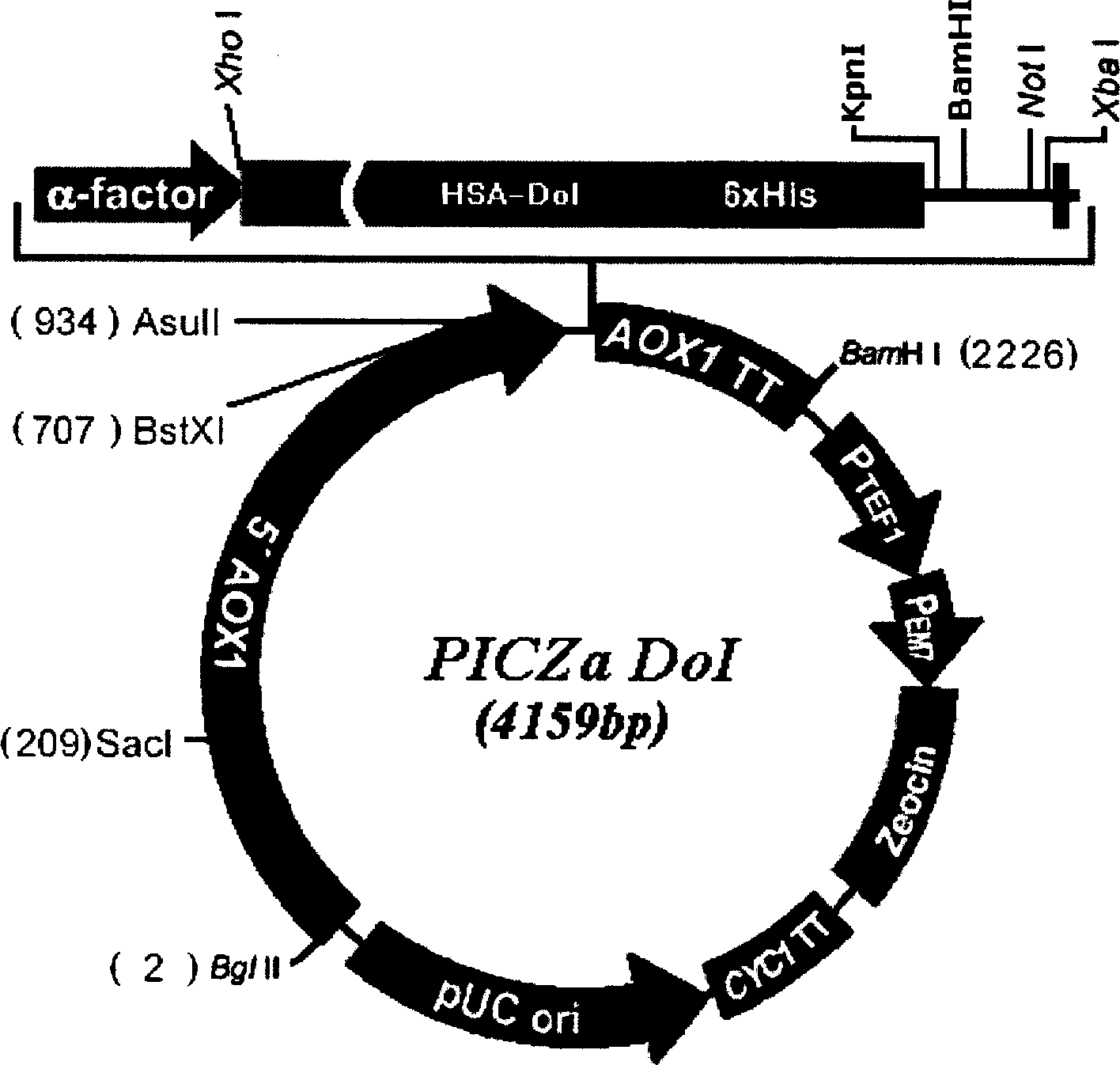
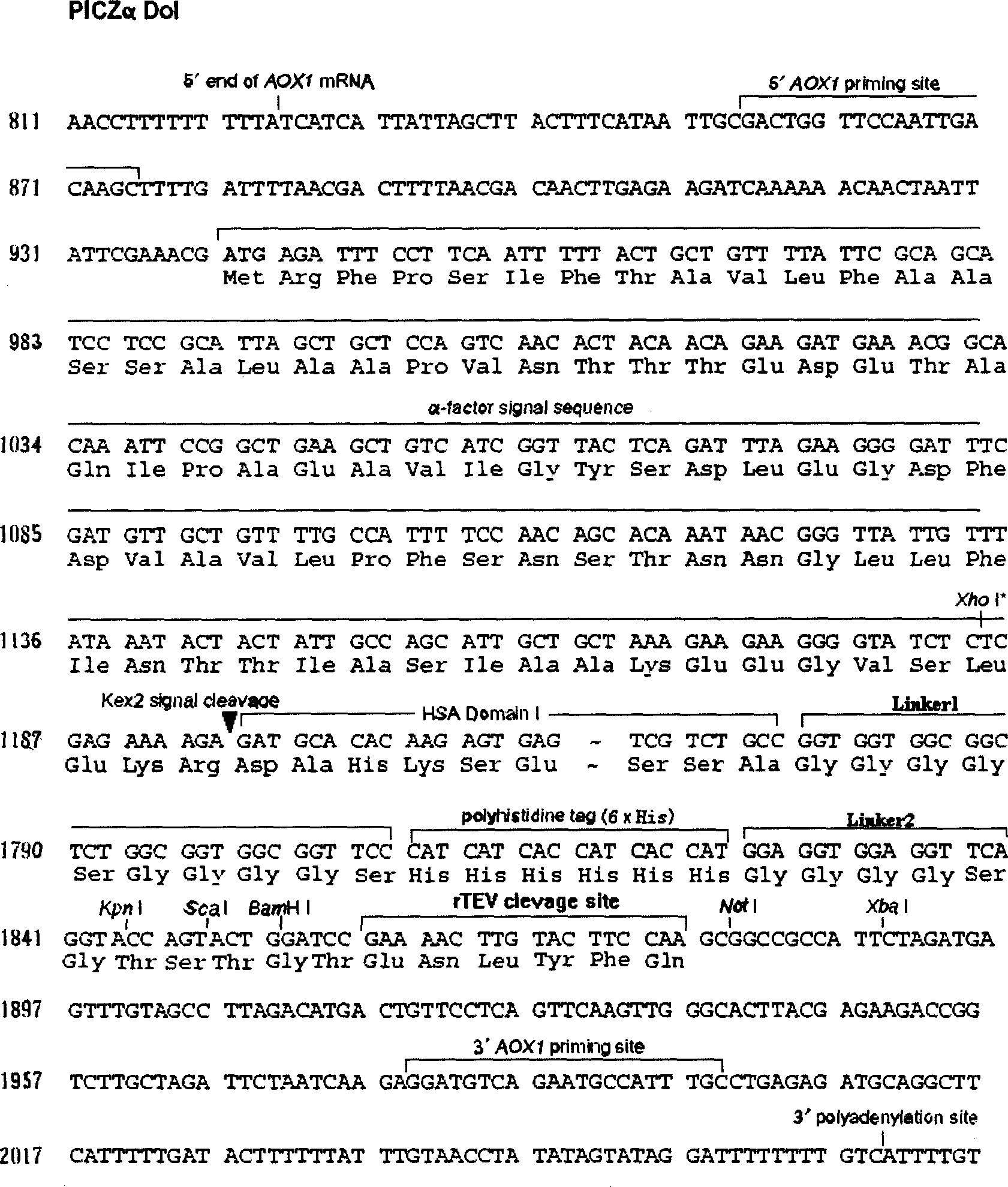
[0052] Construction of expression vector PICZa-DoI
[0053] This vector is transformed on the basis of Invitrogen's commercial secretory vector pPICZαA. The biggest difference between it and pPICZαA is that the domain I of HSA (HSA-DoI) is inserted after the α-signal peptide sequence, and pPICZα series vectors are eliminated. c-myc epitope and 6×His region after the multiple cloning site.
[0054] 1. pPICZαA vector
[0055] See Invitrogen's operating manual pPICZαA, B, and C-----Pichia expression vectors for selection on Zeocin TM and purification of secreted, recombinant proteins (Catalog no. V 195-20).
[0056] 2. Obtaining the domain I (DoI) gene-joint region DNA sequence of HSA and constructing the PICZa-DoI vector. Purchase the commercialized human fetal liver cDNA library (Lot: A604235) from Biochain, and synthesize primers:
[0057] HSA5:
[0058] 5`-cat ctc gag aaa aga gat gca cac aag agt gag gtt gct cat cgg ttt aag-3`
[0059] HSA3:
[0060] 5`-cat gcg gcc...
Embodiment 2
[0078] Expression, assay, purification and preparation of mutant tPA
[0079] 1. Acquisition of mutant tPA gene
[0080] According to the literature report of the study, the whole gene sequence of the tPA active fragment was artificially synthesized, and the 184th and 448th Asn of the original tPA were mutated to Gln during the synthesis, so as to avoid possible occurrence in the tPA protein molecule during yeast secretion and expression. N-glycosylation modification. The synthetic whole gene sequence is shown in SEQ-2, and the corresponding amino acid sequence is shown in SEQ-3.
[0081] 2. Activity determination of expression products
[0082] The construction of expression vectors and the screening of engineered strains all adopt the conventional methods in the Invitrogen manual. The tPA gene fragment was inserted between KpnI and NotI, and the DNA sequence corresponding to the recognition sequence Glu-Asn-Leu-Tyr-Phe-Gln-Ser of rTEV protease was recreated behind the Kpn...
Embodiment 3
[0093] Expression of Human Annexin V-Human Urokinase Active Fragment Fusion Protein (Anx-scUK)
[0094] Two primers were synthesized, and the whole gene of human annexin V (AnnexinV) was obtained from the cDNA library of human placenta tissue by PCR method, and the active fragment of human urokinase (scUK for short) was obtained according to the ProUK amino acid sequence 144-411, a total of 268 For the amino acid sequence, the gene fragment is synthesized by the whole gene synthesis method, and its gene sequence is described in SEQ-4.
[0095] 1. Acquisition of Human Annexin V Gene
[0096] The human annexin V gene is a gene fragment obtained by PCR from the commercialized cDNA library of Biochiain Company (Lot: A700109) through the following two primers AnxP5 and AnxP3, and inserted into the pUC18 vector. Primer AnxP5 introduces a restriction endonuclease KpnI cutting site for the 5'-end of the Annexin V gene, and introduces the enterokinase recognition site Asp-Asp-Asp-Asp-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com