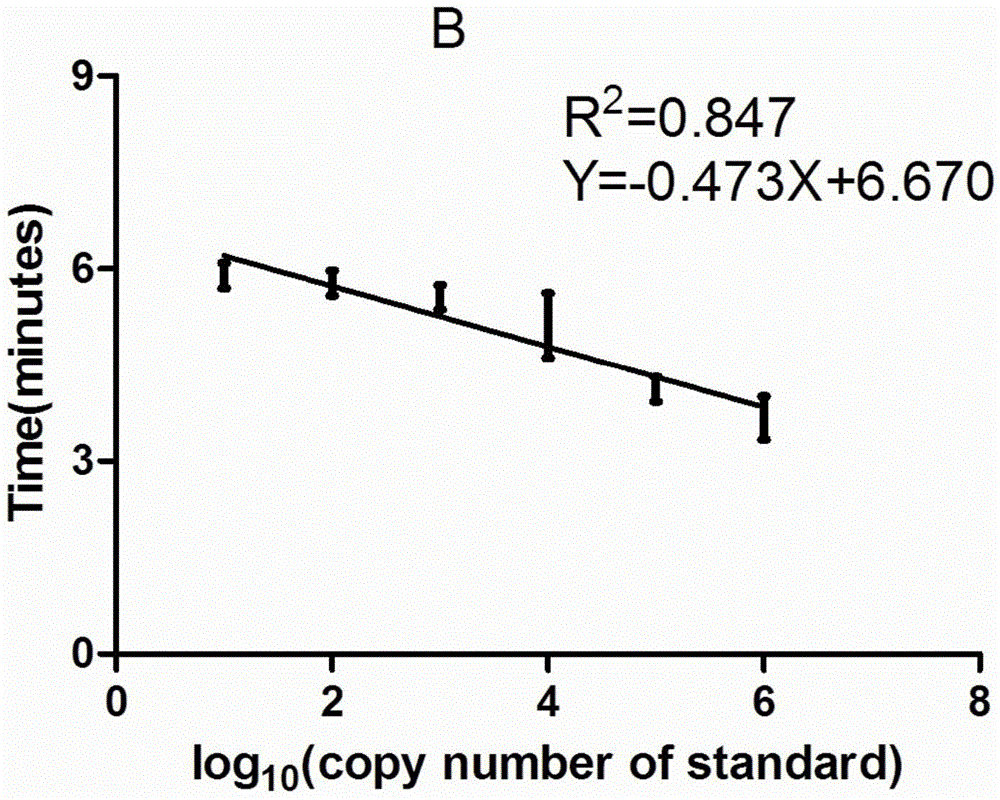
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
294 results about "Foot mouth disease virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
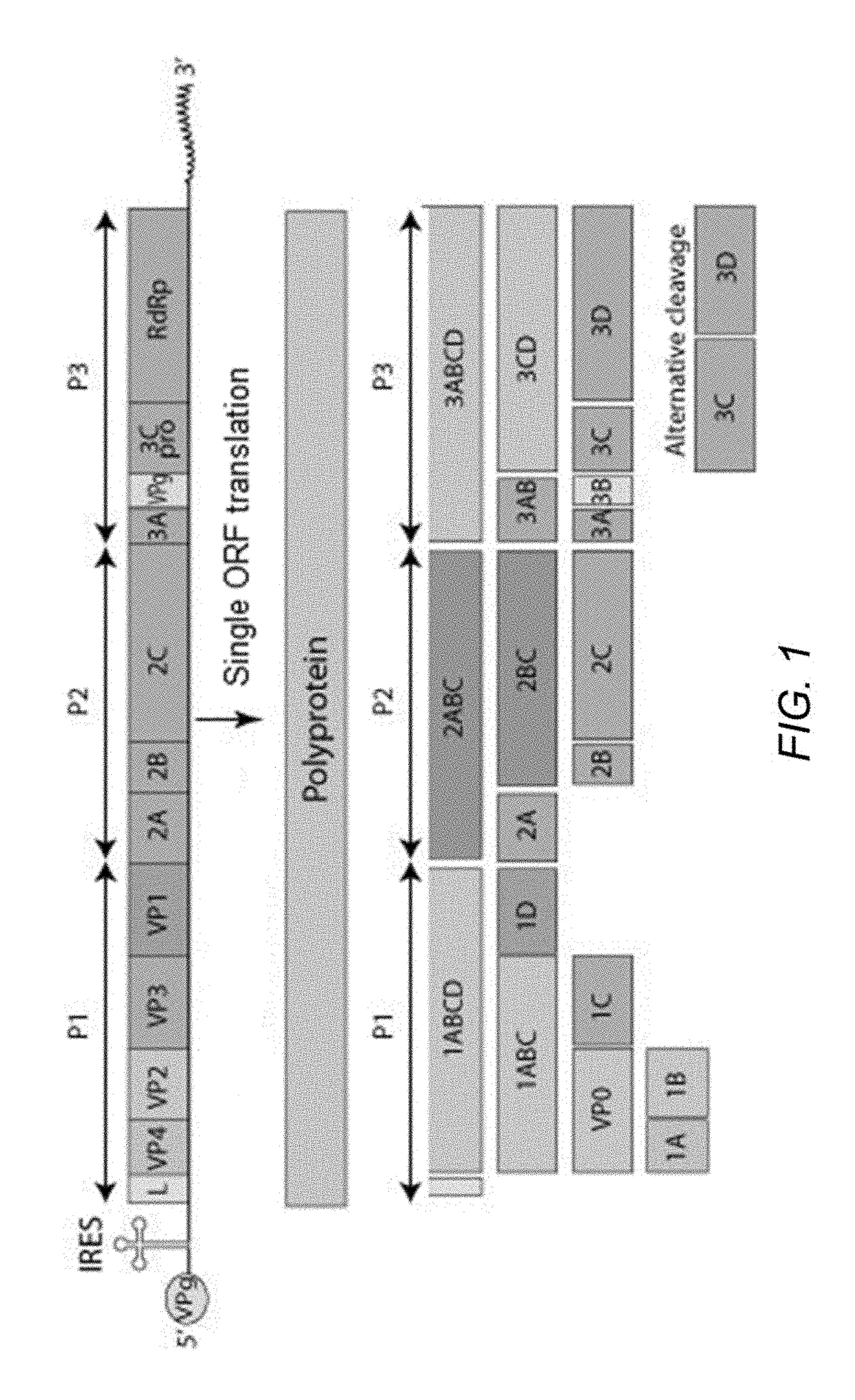
The foot-and-mouth disease virus (FMDV) is the pathogen that causes foot-and-mouth disease. It is a picornavirus, the prototypical member of the genus Aphthovirus.
Foot-and-mouth disease virus capsid protein tandem coexpressions and virus-like particle preparation method
ActiveCN104404074AHigh activityNatural binding activityBacteriaInactivation/attenuationEscherichia coliVirus-like particle
The invention relates to escherichia coli-derived single-plasmid-tandem soluble coexpression foot-and-mouth disease virus capsid proteins VP0 (which is a VP4 and VP2 fusion gene), VP1 and VP3, and a foot-and-mouth disease virus capsid protein virus-like particle preparation method. Foot-and-mouth disease virus capsid protein virus-like particles can be used for preparation of a foot-and-mouth disease vaccine. According to the method, a plurality of aspects of escherichia coli-derived soluble coexpression foot-and-mouth disease virus capsid protein are studied, by comprehensive use of tandem coexpression and SUMO(suggested upper merged ontology) technology with a tag for soluble coexpression of the foot-and-mouth disease virus capsid proteins VP0 (which is the VP4 and VP2 fusion gene), VP1 and VP3, the ultimate objective protein accounts for about 20% of total bacterial protein, and the foot-and-mouth disease virus capsid proteins obtained by purification can be successfully assembled into the virus like particles.
Owner:SA BIOTECH (SUZHOU) PTE LTD
A type foot-and-mouth disease recombinant vaccine strains and preparation method and application thereof
ActiveCN103266091AMultiple phenotype improvements and enhancedPhenotype Improvement and EnhancementMicroorganism based processesAntiviralsAntigenDisease
The invention relates to A type foot-and-mouth disease recombinant vaccine strains prepared by using a reverse genetic manipulation technology and a preparation method and application of the strains. One strain is an A type foot-and-mouth disease recombinant vaccine strain with high titer, antigen matching property and immune protection rate, and the other strain is an A type foot-and-mouth disease recombinant non-pathogenic vaccine strain with high titer, antigen matching property and immune protection rate and without pathogenicity for a host; an antigen nucleotide sequence of each of the vaccine strains is shown as SEQ ID NO: 1; eukaryotic plasmids of viruses can be saved by using a reverse genetic manipulation system; after pigs and cattle are immunized by using the inactivated vaccines prepared from the prepared recombinant vaccine strains, the bodies can be effectively stimulated to produce immune response, and an immune protection effect is provided for the bodies of the pigs and the cattle; through a 10,000-times cattle median infectious dose (BID50) challenge assay of A type AISA topological strains, the immune protection rate reaches 100 percent, and the median protective dose (PD50) is 10.81 to 13.59; and the recombinant vaccine strains can be applied to prevention and control of A type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Broad-spectrum antivirals against 3c or 3c-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
ActiveUS20140243341A1Preventing and inhibiting replicationBiocideSsRNA viruses positive-senseEnterovirusDisease
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfite salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Broad-spectrum antivirals against 3C or 3C-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfate salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Modified Foot-And-Mouth Disease Virus 3C Proteases, Compositions And Methods Thereof
ActiveUS20180066235A1Poor recombinant yieldLow toxicitySsRNA viruses positive-senseViral antigen ingredientsNucleotideVirus-like particle
This application is directed generally to foot-and-mouth disease virus (FMDV) 3C proteases that have been modified by mutating a polynucleotide sequence coding for the FMDV 3C protease. The modified FMDV proteases exhibit proteolytic activity on FMDV P1 precursor protein and exhibit a reduction in one or more toxic or inhibitory properties associated with an unmodified FMDV 3C protease on a host cell used to recombinantly produce it. Vectors carrying polynucleotides encoding modified FMDV 3C protease sequences can induce production of FMDV virus-like particles in a host cell when expressed in the host cell. The modified FMDV 3C proteases can generally be used to produce immunogenic FMDV preparations capable of inducing an immune response against FMDV.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF HOMELAND SECURITY
Multiplex PCR (polymerase chain reaction) primer, probe and gene chip for detecting bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus
InactiveCN103695566AImprove throughputShorten diagnostic timeMicrobiological testing/measurementDNA/RNA fragmentationForward primerMultiplex
The invention relates to a multiplex PCR (polymerase chain reaction) primer, a probe and a gene chip for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus. The multiplex PCR primer and probe have the nucleotide sequences shown by SEQ ID No.1 to SEQ ID and No.9. The gene chip comprises a solid-phase carrier, a sample application quality control probe, a positive hybrid quality control probe and a multiplex PCR primer for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus and the corresponding probe. In the invention, the forward primers of three viruses are marked with fluorescence, a gene chip detection technology carrying three viruses in animal fur is established based on multiplex RT-PCR (reverse transcription-polymerase chain reaction), and the RNA virus in the fur can be sensitively and specifically detected with high flux; the three viruses are screened at the same time in detection once, and the situation that a specific method is required for each virus before is changed, thereby saving the diagnosis time, meeting the needs for quick detection of mass imported / exported fur samples of the exit-entry inspection and quarantine departments and the fur import and export enterprises, and realizing relatively high application values.
Owner:徐超
RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN105567871AShorten test timeLow reaction temperatureMicrobiological testing/measurementMicroorganism based processesBiologyDifferential diagnosis
The invention discloses an RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting a high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof. The kit comprises a pair of primers and a probe, the sequences of the primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe is shown as SEQ ID NO.3. It is proved through experiments that the kit can detect adverse effects of the high-pathogenicity porcine reproductive and respiratory syndrome virus (HP-PRRSV), a hog cholera virus, a C-type porcine reproductive and respiratory syndrome virus, a porcine circovirus type II, a porcine pseudorabies virus and a foot and mouth disease virus in a specificity mode. It is proved through experiments that the kit can detect out templates of at least 70 copies at the temperature of 40 DEG C on the condition of 20 min amplification, and the conformity between the kit and RT-qPCR is high. This shows that the kit can detect HP-PRRSV fast, efficiently and sensitively and provides an effective technological means for differential diagnosis of HP-PRRSV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
O type foot-and-mouth disease virus variant as well as coding gene and application thereof
The invention discloses an O type foot-and-mouth disease virus variant as well as a coding gene and application thereof. In the invention, an O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 is firstly separated out, the nucleotide sequence of the O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 SEQ ID NO: 1, and the amino acid sequence is SEQ ID NO: 2. In comparison with a VP1 amino acid sequence, the virus strain has 7 variable sites, five of which are centralized in a G-H ring. Mutation of the sites ensures that the virus variant has the capability of escaping from host immunity so as to have the superiority for becoming a popular virus strain. Therefore, the variant can be employed to prepare an inactivated vaccine for prevention and treatment of the variant and relevant strains, dominant antigen epitope of the variant can be employed to prepare a synthetic peptide vaccine, and the variant can be employed to develop novel O type foot-and-mouth disease virus vaccines such as VLP vaccine and the like. Therefore, the invention has important value in controlling the popularity of O / YS / CHA / 05 and relevant variable strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method of foot-and-mouth disease vaccine
ActiveCN106474466ASsRNA viruses positive-senseViral antigen ingredientsDiseaseFoot mouth disease virus
The invention relates to a preparation method of a foot-and-mouth disease vaccine. The method comprises the following steps: (i) obtaining a foot-and-mouth disease virus cell culture solution; (ii) performing separation and purification on the foot-and-mouth disease virus cell culture solution by a double-membrane combined-use integral filtering system; (iii) collecting concentrated liquid containing the foot-and-mouth disease virus, obtained in the step (ii). The invention also relates to the foot-and-mouth disease vaccine prepared by the method, and a purpose of using the foot-and-mouth disease vaccine to prepare medicines for preventing animal foot-and-mouth diseases. The invention also relates to a device used for preparing the foot-and-mouth disease vaccine. The device comprises the double-membrane combined-use integral filtering system.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
HRM detecting primers and method for distinguishing foot-mouth disease virus and Seneca Valley virus
ActiveCN105695628AStrong specificityGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceGene type
The invention belongs to the technical field of biological detection, and discloses HRM detecting primers and a method for distinguishing the foot-mouth disease virus (FMDV) and the Seneca Valley virus (SVV). The primers have the sequences shown in SEQ ID NO:1 and SEQ ID NO:2 and are high in specificity. By means of the primers, PCR amplification is conducted on the FMDV and the SVV, then fluorescent data is collected by monitoring the combination situation of double-chain DNA fluorescent dyes and PCR amplification products in the temperature rise process in real time, and the FMDV and the SVV are distinguished according to the difference of two dissolution curves; the two gene types can be distinguished after PCR amplification is conducted through the primers, it takes people only 3 hours for the whole operation process, no virus cell culture is needed, and the type distinguishing time is greatly shortened; expanses are low, no specific probe is needed, and fluorescent saturated dyes are low in price and easy to obtain; accuracy, specificity and repeatability are high, analysis can be accurately and rapidly conducted at high throughput, and the primers and method are easy to apply and popularize in clinical practices.
Owner:SOUTH CHINA AGRI UNIV
Foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and identified epitope and application thereof
ActiveCN101724605AGood passive immunityImprove immunityVirus peptidesImmunoglobulins against virusesIn vivoAmino acid
The invention discloses a foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and an identified epitope and application thereof, and belongs to the field of prevention and control of the FMDV. The microbial collection number of a hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to Asia-1 FMDV, is CGMCC No.2692; and the microbial collection number of the hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to O-type FMDV, is CGMCC No.2691. The invention also discloses amino acid sequences of a conformational neutralizing epitope of the Asia-1 FMDV VP1 protein and a linear neutralizing epitope of the O-type FMDV VP1 protein which are identified by the two monoclonal antibodies respectively. In-vitro neutralization tests and in-vivo animal protection tests show that both the two monoclonal antibodies have excellent passive immunity effect, can be applied to emergency prevention of the FMDV and have excellent immunity effect on the passive immunity of the FMDV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Hybridoma cell line producing monoclonal antibody against foot-and-mouth disease virus, the monoclonal antibody therefrom, immunoassay reagent and kit, and immunoassay method
ActiveUS20110014639A1Useful for developmentImprove efficacyAnimal cellsMicrobiological testing/measurementMonoclonal antibody 14G2AStructural protein
Provided herein are a hybridoma cell line producing monoclonal antibody against foot-and-mouth disease virus (FMDV), the monoclonal antibody therefrom, reagent and kit for ELISA, and immunoassay method. The hybridoma cell line is produced by cell fusion of a parental cell and a myeloma cell line and has the same characteristics as the cell line whose strain designation is CmA40 and deposition number is ATCC (To be Provided). The parental cell is a splenocyte isolated from the spleen of a mouse immunized by an antigen derived from a 3ABC non-structural protein (NSP) of FMDV. The antigen used here is expressed by a prokaryotic cell. The monoclonal antibody produced by the hybridoma cell line can specifically recognize a 3ABC polypeptide and does not cross-react with an antiserum of swine vesicular disease virus.
Owner:NAT INST FOR ANIMAL HEALTH COUNCIL AGRI EXECUTIVE YUAN
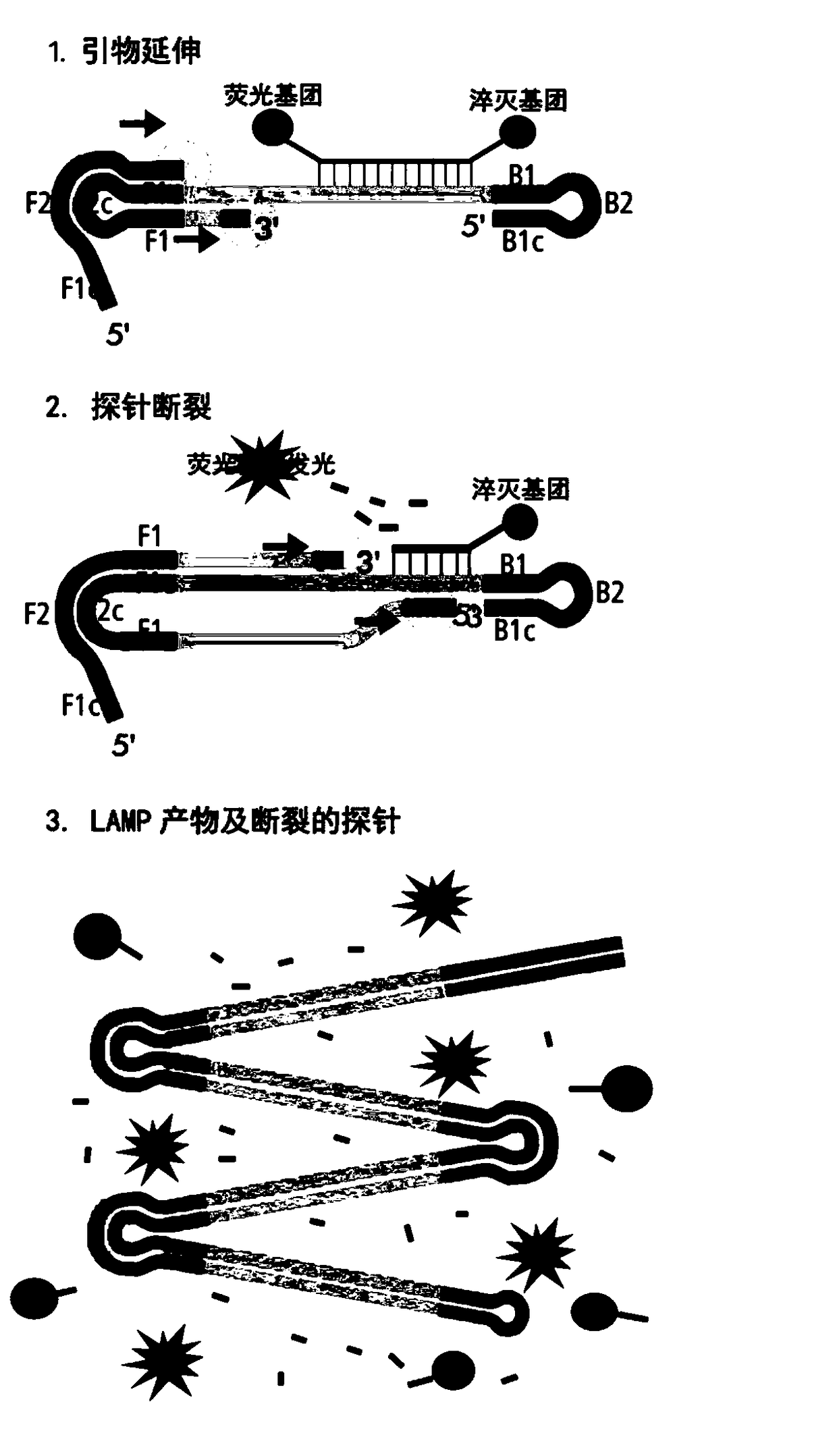
Duplex fluorescent RT-LAMP detection group and kit for visually identifying foot-and-mouth disease virus and Bluetongue virus and application of detection group and kit
ActiveCN108796131AExtended reaction timeStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceGenetics
The invention discloses a duplex fluorescent RT-LAMP detection group and kit for visually identifying foot-and-mouth disease virus and Bluetongue virus and an application of the detection group and the kit. The duplex fluorescent RT-LAMP detection group comprises two groups of specific primers and probes, the first group of specific primers and probes comprise FMDV-F3, FMDV-B3, FMDV-FIB(F1c-F2), FMDV-BIP(B1c-B2) and FMDV-probes, the second group of specific primers and probes comprise BTV-F3, BTV-B3, BTV-FIB(F1c-F2), BTV-BIP(B1c-B2) and BTV-probes, and the two groups of specific primers and probes are as shown in a sequence table SEQ ID No.1 to SEQ ID No.10. The duplex fluorescent RT-LAMP detection group can identify and diagnose the foot-and-mouth disease virus and the Bluetongue virus inthe same reaction tube and has the advantages of good specificity, high sensibility, less pollution, convenience, rapidness and the like, a detection result can be directly observed by naked eyes andis judged according to colors of reaction products, and the detection group can be used for basic-level quarantine with poor conditions.
Owner:GUANGXI VETERINARY RES INST
Foot and mouth disease virus recombinant virus sample particle as well as preparation method and application thereof
The invention discloses a foot and mouth disease virus recombinant virus sample particle as well as a preparation method and application thereof. The recombinant virus sample particle is commonly assembled and expressed by components in a DNA molecule composition. The DNA molecule composition contains an O-type foot and mouth disease VP0 gene, an O-type foot and mouth disease VP1 gene and an O-type foot and mouth disease VP3 gene and further contains a green fluorescent protein gene. By virtue of the property that FMDV VLPs is self-assembled through VP0, VP1 and VP3, a construction method of a baculovirus recombinant vector is improved, a green fluorescent protein label is added into the vector, and FMDV VLPs is successfully prepared by virtue of a pFBDM Bac-to-Bac system, so that a theoretical foundation is laid for the further development of safe and efficient O-type FMDV genetic engineering vaccines.
Owner:CHINA ANIMAL HUSBANDRY IND
Type O foot-and-mouth disease virus mutant and preparation method and application thereof
The invention provides a type O foot-and-mouth disease virus mutant and a preparation method and application thereof, and belongs to the technical field of vaccine candidate strains. According to thetype O foot-and-mouth disease virus mutant disclosed by the invention, an rHN virus strain is used as a female parent virus strain, and the following amino acids in a G-H ring of VP3 protein are mutated: the 173rd aspartic acid is mutated into asparagine, the 174th valine is mutated into glutamic acid and the 179th asparagine is mutated into cysteine. The virus mutant has heredity stability and obtains the capacity of caveolin for performing mediated infestation on CHO-K1cells. The result of detecting the cross neutralization capacity of immune positive serum indicates that compared with a female parent virus strain, the virus mutant has the advantages that the cross protection capacity of the virus mutant for inducing organisms to produce foot-and-mouth disease virus neutralizing antibodies is notably improved, and the virus mutant shows excellent antigen broad spectrum properties. Vaccines prepared through inactivation of the virus mutant can be used for preventing infection with type O foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Sheep aphthovirus Asial type multi-epitope recombinant vaccine and preparation method thereof
ActiveCN101864434AComprehensive immune efficiencyComprehensive evaluation of immune efficiencyGenetic material ingredientsVirus peptidesAdjuvantAdditive ingredient
The invention discloses a sheep aphthovirus Asial type multi-epitope recombinant vaccine and a preparation method thereof. The recombinant vaccine comprises the following ingredients: a protein encoded by aphthovirus multi-epitope genes and carrier protein fusion genes, and an aphthovirus 3D protein. The preparation method comprises the following steps: respectively diluting the protein encoded by the aphthovirus multi-epitope genes and the carrier protein fusion genes and the aphthovirus 3D protein; then, uniformly mixing the diluted proteins; adding auxiliary agents into the mixture; carrying out emulsification; and obtaining the sheep aphthovirus Asia1 type multi-epitope recombinant vaccine. Animal model and animal immune efficiency experiments show that the sheep Asial epitope recombinant vaccine of the invention can generate overall immunoprotection reaction, injected immune sheep can be induced to generate high-level neutralizing antibodies, and can also induce the cellullar immunologic response. The recombinant vaccine of the invention can effectively protect animals from the virulent strain attack of the aphthovirus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Development and application of multiple fluorescence RT-PCR detection method for foot-and-mouth disease, vesicular stomatitis and swine vesicular disease
InactiveCN103602757AMicrobiological testing/measurementMicroorganism based processesSwine vesicular diseaseAnimal virus
Based on a highly conserved domain of a foot-and-mouth disease virus 3D protein coding gene, a vesicular stomatitis virus N protein coding gene and a swine vesicular disease virus VP1 protein coding gene, the invention designs a specific primer and a probe and develops a multiple fluorescence RT-PCR detection method used for simultaneously detecting the three animal viruses. The detection method can detect 102 copied plasmids containing target amplification sequences in 1 h. The method has high sensitivity and good specificity, and can achieve rapid high-throughput fluorescence RT-PCR detection for single-virus infection or mixed infection by a plurality of viruses.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Foot-and-mouth disease virus detecting test paper tape and its preparation method and using method
ActiveCN1877331ASimple and fast operationIntuitive and easy to judgeBiological testingMedicinePaper tape
Disclosed are a method for preparing and virus type definition test paper for foot and mouth disease and the using method. The test paper comprises O, A, Asia l, C four kinds of serum detecting test paper. The test paper comprises a PVC substrate plate, nitrocellulose membrane, an absorbing pad, a golden mark pad and a sample pad. The PVC substrate plate is installed at the most bottom layer; the nitrocellulose membrane is set on the middle part of the substrate plate; the absorbing pad is stuck on the left end of the nitrocellulose membrane, and the golden mark pad is set on the right end of the nitrocellulose membrane; the sample pad is set on the right upper end of the golden pad. The invention can identify the foot and mouth virus O, A, Asia l and C four serum type.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
O-type foot-and-mouth disease virus antibody chemiluminescence detection kit
InactiveCN106226519AStrong specificityCarcinogenicity is smallChemiluminescene/bioluminescenceAntigenViral antibody
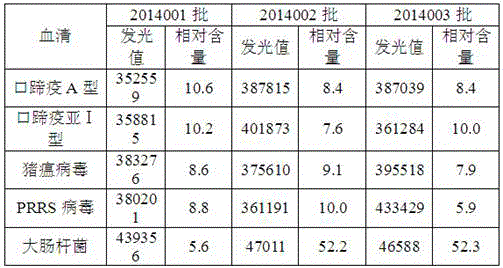
The invention relates to an O-type foot-and-mouth disease virus antibody chemiluminescence detection kit. The kit includes an polystyrene board coated by antigen, a monoclonal antibody, a standard substance, a luminescence substrate solution A and a luminescence substrate solution B. The polystyrene board coated by antigen is an opaque polystyrene plate coated by an O-type foot-and-mouth disease virus RE2 recombinant protein; the monoclonal antibody is a horseradish peroxidase labeled O-type foot-and-mouth virus monoclonal antibody; the luminescence substrate solution A comprises a Lumino, hydroxycoumarin, gallic acid, a Tris-Hcl buffer and water; the standard substance is foot-and-mouth virus O-type antibody respectively diluted buy a calibration diluent, wherein the dilution is 0NU / mL, 2NU / mL, 5NU / mL, 10NU / mL, 30NU / mL and 60NU / mL respectively. The kit of the invention can be used for detecting O-type foot-and-mouth disease virus antibody, has the advantages of high sensitivity and wide detection range, and realizes the quantitative detection of foot-and-mouth disease antibody.
Owner:洛阳现代生物技术研究院有限公司
Primer combination for identifying foot-and-mouth disease virus and vesicular stomatitis virus and application thereof
ActiveCN105671201ALow costMicrobiological testing/measurementMicroorganism based processesDiagnosis methodsQuarantine
The invention discloses a primer combination for identifying foot-and-mouth disease virus and vesicular stomatitis virus and application thereof. The primer combination is composed of a primer set I and a primer set II. The primer set I is composed of primers FMDV-F3, FMDV-B3, FMDV-FIP and FMDV-BIP which are sequentially disclosed as Sequence 1-4. The primer set II is composed of primers VSV-F3, VSV-B3, VSV-FIP and VSV-BIP which are sequentially disclosed as Sequence 5-8. The invention also discloses application of the primer combination in identifying foot-and-mouth disease virus and vesicular stomatitis virus, application in identifying whether a virus to be detected is foot-and-mouth disease virus or vesicular stomatitis virus, and application in identifying whether a sample to be detected is infected by foot-and-mouth disease virus and / or vesicular stomatitis virus. The duplex RT-LAMP (reverse transcription-loop-mediated isothermal amplification) method established by the invention is a simple quick low-cost diagnosis method, can be used for grass-root and field quarantine inspection under poor conditions, and is also suitable for large-scale epidemiological survey.
Owner:GUANGXI VETERINARY RES INST
Foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP (loop-mediated isothermal amplification) detection primer group, kit and application thereof
InactiveCN106893787ANot affected by amplification efficiencyIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBovine virusFluorescence
The invention belongs to the technical field of bovine virus detection and particularly relates to a foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP (loop-mediated isothermal amplification) detection primer group, a kit and application thereof. The foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP detection primer group comprises two groups of specific primers, wherein one group is FMDV-F3, FMDV-B3, FMDV-FIP (F1c-F2) and FMDV-BIP (B1c-B2), and the other group is VSV-F3, VSV-B3, VSV-FIP (F1c-F2) and VSV-BIP (B1c-B2), and sequences of the two groups are shown as SEQ ID NO.1 and SEQ ID NO.8 respectively. An established foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP method has advantages of simplicity, convenience, quickness, specificity, sensitivity and the like and can be used for FMDV (foot and mouth disease virus) and VSV (vesicular stomatitis virus) clinical detection and epidemiological investigation. The FMDV and VSV duplex fluorescence RT-LAMP method is a simple, quick and low-cost diagnosis method and suitable for large-scale epidemiological investigation.
Owner:GUANGXI VETERINARY RES INST
Foot and mouth disease virus antigen polypeptide, fusion antigen polypeptide and vaccine
InactiveCN102180952AImproving immunogenicityImprove securityVirus peptidesAntiviralsVariant strainImmunogenicity
The invention provides a foot and mouth disease virus antigen polypeptide, a foot and mouth disease virus fusion antigen polypeptide and a foot and mouth disease virus vaccine containing the antigen polypeptide and / or the fusion antigen polypeptide. The invention further provides application of the antigen polypeptide, the fusion antigen polypeptide and the vaccine in prevention and control of foot and mouth disease virus infection. The foot and mouth disease virus antigen polypeptide, the fusion antigen polypeptide and the vaccine have broad-spectrum immunogenicity, and can generate good immunogenicity on different foot and mouth disease viruses and variant strains thereof.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Gene chip and detection method for detecting FMDV, VSV, SVDV, PPRV and BTV
InactiveCN104694668AStrong and stable hybridization signalStrong specificityNucleotide librariesMicrobiological testing/measurementSequence analysisMicroarray cgh
The invention discloses a gene chip and a detection method for detecting FMDV, VSV, SVDV, PPRV and BTV. The detection method comprises the step of detecting foot and mouth disease viruses (type A, Asian type I and type O), vesicular stomatitis virus, swine vesicular disease virus, Peste des petits ruminants virus and bluetongue virus. The method comprises the following specific steps: designing a PCR primer by virtue of sequence analysis of standard strain genome, and performing cloning and sequence analysis on target genes; designing a specific probe, and simultaneously detecting the foot and mouth disease viruses, vesicular stomatitis virus, swine vesicular disease virus, Peste des petits ruminants virus and bluetongue virus. The invention aims at establishing a method for detecting the foot and mouth disease viruses, vesicular stomatitis virus, swine vesicular disease virus, Peste des petits ruminants virus and bluetongue virus by adopting a microarray chip which is high in sensitivity and high in specificity and through which the time and labor are saved and the result is easily observed.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Reagent for testing foot and mouth disese virus by fluorescent quantity RI-PCR and its preparation process
InactiveCN1560279AThe sample has a wide range of applicationsNo biological safety hazardMicrobiological testing/measurementFluorescenceCell culture
The invention relates to a biopreparate designed and synthesized by applying primer Express and primer prere 5.0 softwares and using the gene fragment of foot and mouth disease virus as a target, especially a reagent able to detect the foot and mouth disease virus and a method of preparing the reagent. The fluorescent quantitative RT-PCR detecting reagent of foot and mouth disease virus contains a pair of specific primers and a specific fluorescent probe. It uses the most conservative fragment of the gene sequence in FMDV 3D region for the first time to design and synthesize the primers and probe, and builds the fluorescent quantitative RT-PCR and prepares a detecting reagent box, and can rapidly detect FMDV in cell culture substance, hydatid fluid, hydatid skin and secretion, blood, and meat products. It creates a fluorescent quantitative RT-PCR of FMDV.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR
Recombinant vaccine strain for foot-and-mouth disease type A as well as preparation method thereof and application thereof
InactiveCN102757942AIncrease production capacityHigh titerMicroorganism based processesAntiviralsAntigenDisease
The invention relates to a recombinant vaccine strain for foot-and-mouth disease type A, which is constructed by a gene recombination technology and featured with high valence, high antigen compatibility and high immune protective rate, as well as a preparation method thereof and an application thereof. An antigen nucleotide sequence of the vaccine strain is shown by SEQ ID NO. 1; a rescuing system is an efficient eukaryotic plasmid, which is manually constructed and can express an accurate foot-and-mouth disease virus genome RNA; therefore, a foot-and-mouth disease recombinant virus can be constructed and prepared; through the adoption of the plasmid, the vaccine strain with high titer and good antigen compatibility can be prepared so as to prepare inactivated vaccines; after pigs and cattle are immunized, bodies can be effectively stimulated to generate immune responses and immune protective effects on the pig and cattle bodies can be provided; an A type AISA lineage of strain is challenged with 10000 times of 50% cattle infective dose (BID50), so that the immune protective rate reaches 100% and 50% protective dose (PD50) is 10.81-13.59; and the vaccine can be used for preventing and controlling foot-and-mouth diseases type A in China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Cattle food-and-mouth disease virus A type synthetic peptide and preparation and application thereof
ActiveCN103193869AImprove the efficacy of immune protectionGood protective effectVirus peptidesAntiviralsAntigenFoot mouth disease virus
The invention discloses a cattle food-and-mouth disease virus A type synthetic peptide. The cattle food-and-mouth disease virus A type synthetic peptide has the following amino acid sequence: acetyl-YDLDF EALKP HFKSL GQTIT PADKS PPS VYNGT CKYSA PATRR GDLGS LAARL AACLP ASFNY GAIRA T-amide. The cattle food-and-mouth disease virus A type synthetic peptide can be used as an effective novel vaccine for the A type food-and-mouth disease in production practice; simultaneously, the realization of the test provides a certain foundation for further perfecting the construction of a food-and-mouth disease virus A type synthetic peptide vaccine and the construction of other food-and-mouth disease subtype synthetic peptide vaccines; and the new ideal proposed in the research of the synthetic peptide vaccine and the construction of a new method of the synthetic peptide vaccine provide theoretical foundation and technical support for further perfecting the development and research of the multiple antigenic peptide, multivalent peptides and joint peptide vaccines in future.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Real-time fluorescence PCR primer and probe for Asia-Europe type foot-and-mouth disease virus detection
InactiveCN101724711AGood scalabilityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceFoot-and-mouth disease virus
The invention provides a real-time fluorescence PCR primer and probe for Asia-Europe type foot-and-mouth disease virus detection, wherein the primer pair can specifically amplify the conservative region of foot-and-mouth disease virus gene group 5'-UTR sequence, the specificity of the probe directs at the GC high content region in the amplification region of the primer pair, the 5' end of the probe is provided with a report fluorescence dye mark, and the 3' end is provided with a quenching dye mark. The invention makes the best of high efficiency amplification of the fluorescence PCR technology, favourable specificity of the nucleotide hybridization and rapid sensitivity of the fluorescence detection technology, and whether the tissue sample to be detected contains Asia-Europe type foot-and-mouth disease virus is judged according to amplification curve after reaction is finished. The gene group region directed by PCR amplification is Asia-Europe type foot-and-mouth disease virus specified sequence and has no cross reaction with South Africa type foot-and-mouth disease virus, and universality is stronger while favourable specificity is obtained.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com