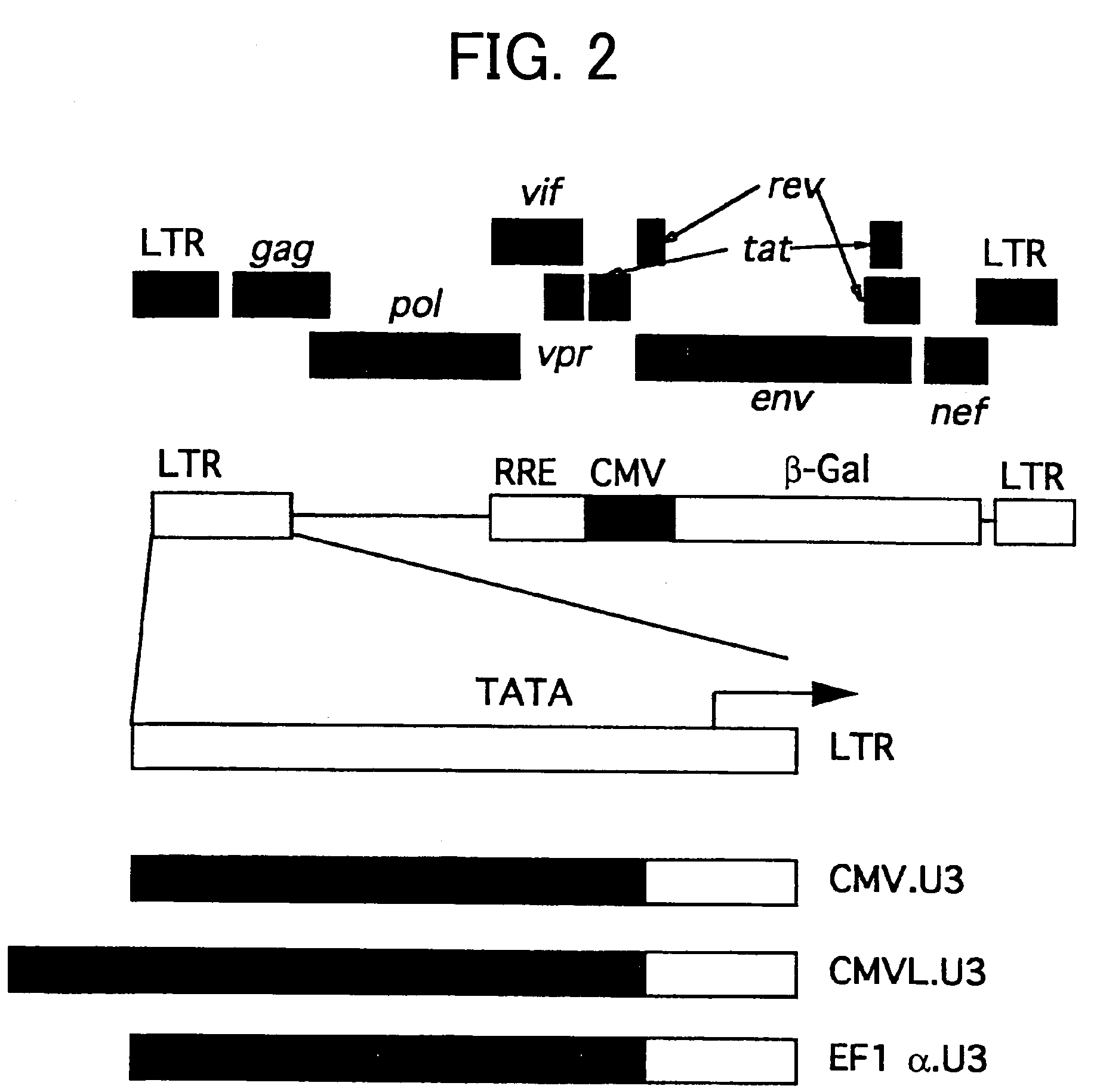
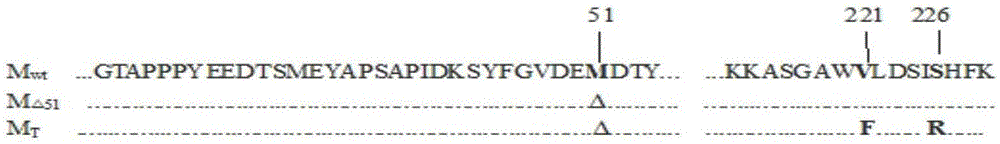Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
110 results about "Vesicular stomatitis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
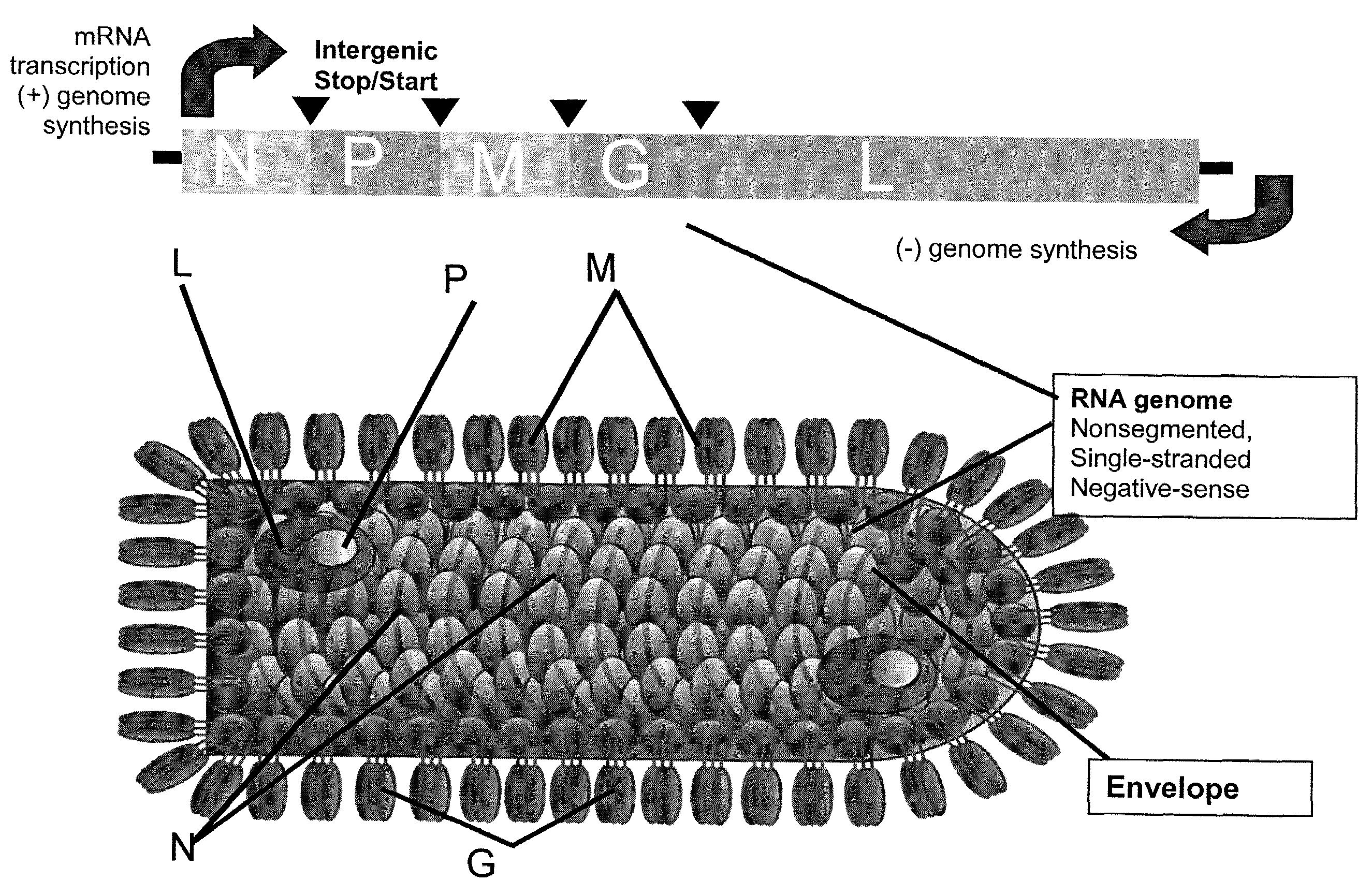
Indiana vesiculovirus, formerly Vesicular stomatitis Indiana virus (VSIV or VSV) is a virus in the family Rhabdoviridae; the well-known Rabies lyssavirus belongs to the same family. VSIV can infect insects, cattle, horses and pigs. It has particular importance to farmers in certain regions of the world where it infects cattle. This is because its clinical presentation is identical to the very important foot and mouth disease virus.
Recombinant viral vectors
ActiveUS20130266611A1Escalate trimer abundanceImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsViral vectorImmunogenicity
The present relation relates to recombinant vesicular stomatitis virus for use as prophylactic and therapeutic vaccines for infectious diseases of AIDS. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Immunoselection of recombinant vesicular stomatitis virus expressing hiv-1 proteins by broadly neutralizing antibodies
InactiveUS20130189754A1Simplifies enrichmentIncrease exposureViral/bacteriophage medical ingredientsElectrical/wave energy microorganism treatmentImmunogenicityVesicular stomatitis virus
The present relation relates to recombinant vesicular stomatitis virus for use as prophylactic and therapeutic vaccines for infectious diseases of AIDS. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Synergistic Attenuation of Vesicular Stomatitis Virus, Vectors Thereof and Immunogenic Compositions Thereof
The present invention broadly relates to the synergistic attenuation of vesicular stomatitis virus (VSV). More particularly, the invention relates to the identification of combined mutation classes which synergistically attenuate the pathogenicity of VSV vectors in mammals and immunogenic compositions thereof.
Owner:WYETH LLC
Viromembrane protein with site-specific mutagenesis and site-specific decoration, preparation method and applications of viromembrane protein
ActiveCN102838663AAvoid dissociationSsRNA viruses negative-senseBacteriaViral Membrane ProteinsUltraviolet lights
The invention discloses viromembrane protein with site-specific mutagenesis and site-specific decoration, a preparation method and applications of the viromembrane protein. Amber codon TAG is introduced to the specific site of envelope protein G (VSV G) gene of vesicular stomatitis virus, unnatural amino acid DiZPK with photo-crosslinking property is introduced to the specific site of VSV G by utilizing the orthorhombic aminoacyl-tRNA synthetase-tRNA through site-specific mutagenesis. In the interaction process of virus and host cells, the DiZPK photo-crosslinking reaction is triggered by 365nm ultraviolet light, the covalent bond is formed between the VSV G and interacting protein, the interaction dissociation can be avoided, and the powerful measure is provided for the research of the interaction of the virus and protein of the host cells.
Owner:PEKING UNIV
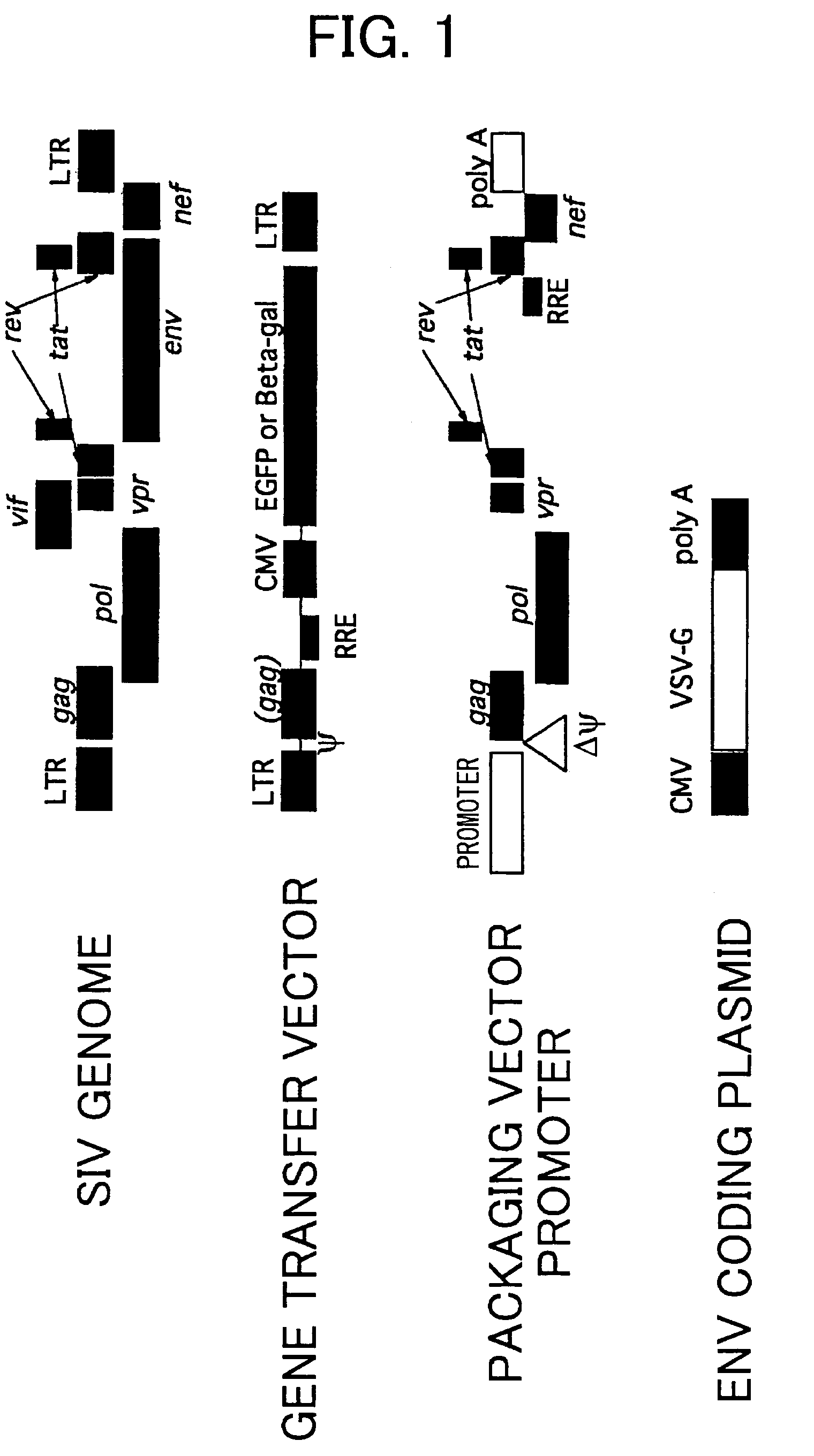
Gene transfer into primate embryonic stem cells using VSV-G pseudotyped simian immunodeficiency virus vectors
InactiveUS7323337B2Increase transcriptionEnhance mRNA transcriptionGenetic material ingredientsEmbryonic cellsDiseaseSimian immunodeficiency viruses SIV
Highly efficient gene transfer into primate-derived embryonic stem (ES) cells has successfully been achieved by using a simian immunodeficiency virus vector (SIV) pseudotyped with VSV-G protein, which is a surface glycoprotein of vesicular stomatitis virus (VSV) The present invention provides simian immunodeficiency virus vectors for gene transfer to primate ES cells. The method for gene transfer to primate ES cells using the vectors of the present invention is useful in, for example, research into embryology and disease, clinical applications, and experimental models for primates. The method is also useful in assaying and screening for genes and reagents able to enhance the specific differentiation of tissues or cells, and which are useful in preparing desired cells or tissues differentiated from ES cells.
Owner:DNAVEC RES +1
Multiplex PCR kit for simultaneously detecting four viruses carried by ruminants
ActiveCN104450966AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesDNA ContaminationMultiplex pcrs
The invention discloses a multiplex PCR kit for simultaneously detecting four viruses carried by ruminants. The kit comprises four pairs of specific primers. The experiment proves that bluetongue, foot-and-mouth disease, peste des petits ruminants and vesicular stomatitis virus nucleic acid in clinical samples of ruminants can be simultaneously detected. The kit has the characteristics of high sensitivity, high specificity and simplicity in operation, the detection time can be saved, and the condition that DNA contamination occurs in detection is reduced.
Owner:艾军 +2
Host targeted inhibitors of dengue virus and other viruses
ActiveUS9879003B2Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeOrganic chemistryHerpes simplex diseaseJunin virus
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Production process of fusion expression recombinant chicken interferon alpha
InactiveCN106399321AStrong antiviral activityEfficient expressionFermentationInterferonsEscherichia coliInclusion bodies
The invention discloses a production process of fusion expression recombinant chicken interferon alpha. The process includes the steps of: S1, according to the preference of Escherichia coli codon, conducting codon optimization on a chicken interferon alpha gene sequence published in Genebank, and artificially synthesizing the chicken interferon alpha gene; S2, according to the codon optimized chicken interferon alpha gene, designing three specific primers; S3, constructing recombinant chicken interferon alpha plasmid containing ProS2 dissolution promoting label; S4, transforming and identifying the recombinant expression plasmid; S5, conducting inducible expression of recombinant chicken interferon alpha; S6, extracting an expression product and conducting protein renaturation purification: S61, inclusion body extraction and treatment; S62, inclusion body denaturation; S63, denaturation solution renaturation; and S64, nickel column affinity purification. By means of cell cytopathic inhibition, the invention detects that the interferon has the activity of inhibiting vesicular stomatitis virus proliferation, and the activity unit reaches 7.32*10<7>UI / mg.
Owner:SOUTH CHINA AGRI UNIV +1
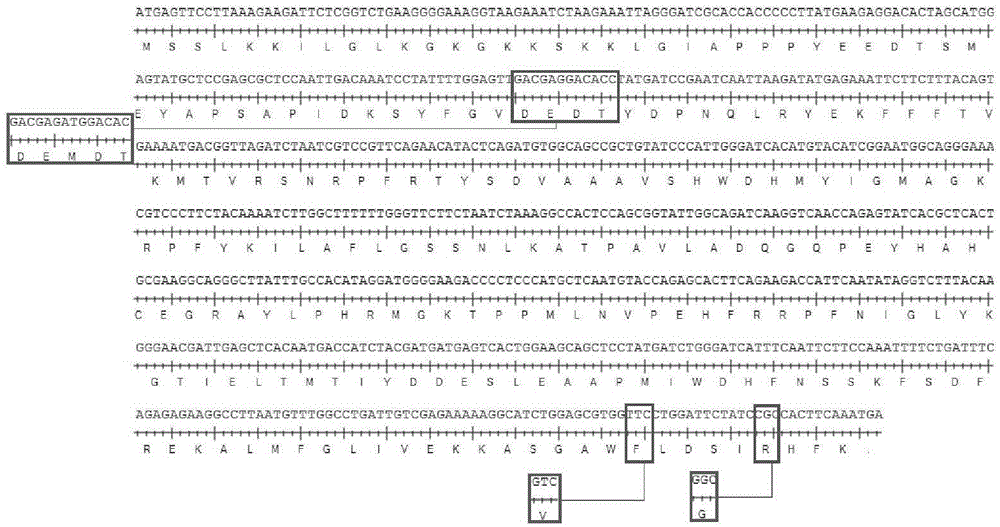
Building and application of M protein three-amino acid site-mutated vesicular stomatitis virus (VSV) carrier for pigs
InactiveCN105087645AComprehensive immune responseReduce pathogenicityAntiviralsAntibody medical ingredientsGlycineArginine
The invention provides building and application of an M protein three-amino acid site-mutated vesicular stomatitis virus (VSV) carrier for pigs. The VSV carrier is matrix protein (M) three-amino acid site-mutated recombinant virus VSV-MT, the 51st-site methionine of the virus M protein is knocked out, the 221st-site valine of the virus M protein is mutated into phenylalanine, and the 226th-site glycine of the virus M protein is mutated into arginine. The VSV carrier is extremely low in pathogenicity, high in safety, capable of effectively stimulate a body to produce protective immune response, applicable to vaccine or vaccine carrier preparation, and promising in application prospect.
Owner:SHANGHAI JIAO TONG UNIV
Recombinant vesicular stomatitis virus vaccines for viral hemorrhagic fevers
InactiveUS8012489B2SsRNA viruses negative-senseViral antigen ingredientsViral VaccineVesicular stomatitis virus
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Recombined chicken alpha interferon gene and recombinant vector thereof
InactiveCN101338314AStrong antiviral activityEase of mass productionFungiMicroorganism based processesHigh concentrationAnti virus
The invention relates to a novel gene sequence of a recombinant chicken Alpha interferon, constructs a recombinant expression vector thereof and belongs to a gene engineering biological product obtained by a molecular biology method. The gene sequence of a newly designed chicken Alpha interferon is recombined into a pPICZ Alpha-A vector and then is confirmed on the special position of a microzyme by an electric conversion mode. Besides, a pichia expression system is adopted to express a foreign gene, thus being beneficial to the commercial production of the chicken interferon. The protein expressed by a gene group after being diluted by 4365158.3 times can completely restrain the attraction of vesicular stomatitis virus of 100-1000TCID50. Compared with a natural chicken Alpha interferon, the novel gene sequence of a recombinant chicken Alpha interferon has a higher anti-virus effect; the anti-virus effect thereof is improved by 8 times. Test also detects that the protein expressed by the gene can restrain the proliferation of a newcastle disease virus and an avian influenza virus; besides, the effect of the interferon with high concentration is more remarkable.
Owner:NANJING AGRICULTURAL UNIVERSITY
Foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP (loop-mediated isothermal amplification) detection primer group, kit and application thereof
InactiveCN106893787ANot affected by amplification efficiencyIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBovine virusFluorescence
The invention belongs to the technical field of bovine virus detection and particularly relates to a foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP (loop-mediated isothermal amplification) detection primer group, a kit and application thereof. The foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP detection primer group comprises two groups of specific primers, wherein one group is FMDV-F3, FMDV-B3, FMDV-FIP (F1c-F2) and FMDV-BIP (B1c-B2), and the other group is VSV-F3, VSV-B3, VSV-FIP (F1c-F2) and VSV-BIP (B1c-B2), and sequences of the two groups are shown as SEQ ID NO.1 and SEQ ID NO.8 respectively. An established foot and mouth disease virus and vesicular stomatitis virus identifying duplex fluorescence RT-LAMP method has advantages of simplicity, convenience, quickness, specificity, sensitivity and the like and can be used for FMDV (foot and mouth disease virus) and VSV (vesicular stomatitis virus) clinical detection and epidemiological investigation. The FMDV and VSV duplex fluorescence RT-LAMP method is a simple, quick and low-cost diagnosis method and suitable for large-scale epidemiological investigation.
Owner:GUANGXI VETERINARY RES INST
Primer combination for identifying foot-and-mouth disease virus and vesicular stomatitis virus and application thereof
ActiveCN105671201ALow costMicrobiological testing/measurementMicroorganism based processesDiagnosis methodsQuarantine
The invention discloses a primer combination for identifying foot-and-mouth disease virus and vesicular stomatitis virus and application thereof. The primer combination is composed of a primer set I and a primer set II. The primer set I is composed of primers FMDV-F3, FMDV-B3, FMDV-FIP and FMDV-BIP which are sequentially disclosed as Sequence 1-4. The primer set II is composed of primers VSV-F3, VSV-B3, VSV-FIP and VSV-BIP which are sequentially disclosed as Sequence 5-8. The invention also discloses application of the primer combination in identifying foot-and-mouth disease virus and vesicular stomatitis virus, application in identifying whether a virus to be detected is foot-and-mouth disease virus or vesicular stomatitis virus, and application in identifying whether a sample to be detected is infected by foot-and-mouth disease virus and / or vesicular stomatitis virus. The duplex RT-LAMP (reverse transcription-loop-mediated isothermal amplification) method established by the invention is a simple quick low-cost diagnosis method, can be used for grass-root and field quarantine inspection under poor conditions, and is also suitable for large-scale epidemiological survey.
Owner:GUANGXI VETERINARY RES INST
Gene chip and detection method for detecting FMDV, VSV, SVDV, PPRV and BTV
InactiveCN104694668AStrong and stable hybridization signalStrong specificityNucleotide librariesMicrobiological testing/measurementSequence analysisMicroarray cgh
The invention discloses a gene chip and a detection method for detecting FMDV, VSV, SVDV, PPRV and BTV. The detection method comprises the step of detecting foot and mouth disease viruses (type A, Asian type I and type O), vesicular stomatitis virus, swine vesicular disease virus, Peste des petits ruminants virus and bluetongue virus. The method comprises the following specific steps: designing a PCR primer by virtue of sequence analysis of standard strain genome, and performing cloning and sequence analysis on target genes; designing a specific probe, and simultaneously detecting the foot and mouth disease viruses, vesicular stomatitis virus, swine vesicular disease virus, Peste des petits ruminants virus and bluetongue virus. The invention aims at establishing a method for detecting the foot and mouth disease viruses, vesicular stomatitis virus, swine vesicular disease virus, Peste des petits ruminants virus and bluetongue virus by adopting a microarray chip which is high in sensitivity and high in specificity and through which the time and labor are saved and the result is easily observed.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Genetically Modified Attenuated Vesicular Stomatitis Virus, Compositions and Methods of use Thereof
InactiveUS20090175906A1Improving consistency of manufactureIncrease productionSsRNA viruses negative-senseAntibacterial agentsImmunogenicityVesicular stomatitis virus
The present invention relates to methods for generating genetically modified and attenuated strains of vesicular stomatitis virus (VSV) for use in the preparation of immunogenic compositions. More particularly, the invention relates to the identification of particular genetic modifications of attenuated VSV that result in an increased yield of virus and an increase in stability of the attenuated strains for preparation of the immunogenic compositions. Methods for cell culture propagation and use in large scale production of VSV is also disclosed.
Owner:WYETH LLC
Porcine alpha interferon and interleukin 2 chimeric gene, construction method and protein purification method thereof
InactiveCN101570757AGuaranteed dual activityGood antiviral activityMicroorganism based processesAntiviralsAdditive ingredientInterferon alpha
The invention belongs to the technical field of biological genetic engineering, and discloses technology for constructing and expressing porcine alpha interferon (PoIFN-alpha) and interleukin 2 (PoIL-2) chimeric gene and quickly renaturing and purifying expression protein. The technology constructs mature peptide genes of PoIFN-alpha and PoIL-2 into PoIFN-alpha-linker-PoIL-2 chimeric gene through a genetic flexible linker (linker) (G4S)3 and clones the chimeric gene into a pGEM-T Easy vector by adopting an overlap extension PCR method, and subclones the chimeric gene into a pQE-30 expression vector for prokaryotic expression. The recombinant fusion protein (rPoIFN-alpha-linker-PoIL-2) can be quickly renatured and purified through urea modification, renaturation by low concentration protein renaturing solution, PBS solution dialysis and other steps. The purified rPoIFN-alpha-linker-PoIL-2 fusion protein has proliferation activity for inhibiting vesicular stomatitis virus (VSV) on a cell, is used for prevention and treatment of porcine virosis as a main ingredient of an antivirus preparation, and has high efficiency, broad spectrum, safety and low price.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Recombinant human hepatitis B virus core protein fused protein
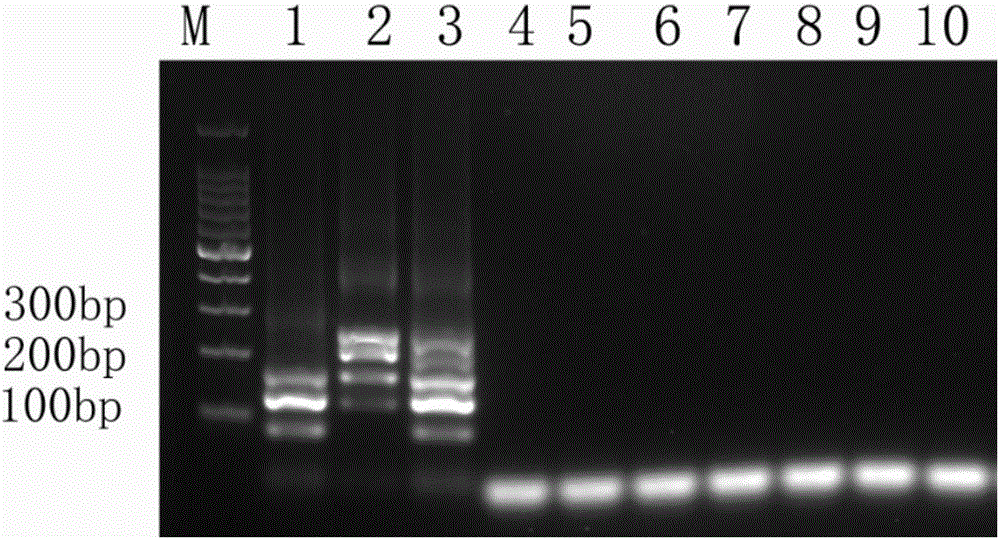
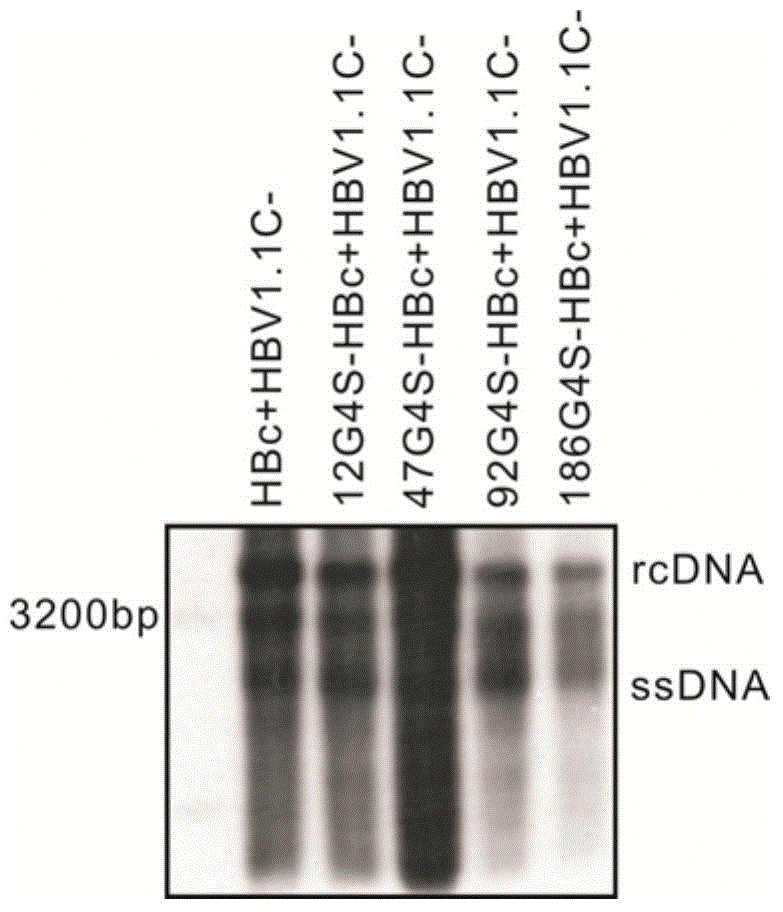
The invention discloses a recombinant human hepatitis B virus core protein fused protein. The fused protein comprises a protein (X), a linker peptide (L) and a hepatitis B virus core protein (HBc) from the end N to the end C in sequence; the linker peptide (L) has the amino acid sequence of Gly-Ser-(Gly-Gly-Gly-Gly-Ser)n, and n is an integer between 2 and 20 and is 9 or 18, particularly; the end C of the linker peptide (L) is connected with the end N of the hepatitis B virus core protein (HBc); the end C of the protein (X) is connected with the end N of the linker peptide (L); and the protein (X) is a red fluorescent protein or vesicular stomatitis virus G glycoprotein. The hepatitis B virus core protein (HBc) is connected with the functional protein, and the functions of the proteins on two ends of the linker peptide (L) can be both guranteed. The problem in the prior art that the functions of the hepatitis B virus core protein (HBc) and the functions of the functional protein can not be both guaranteed after the hepatitis B virus core protein (HBc) is fused with the functional protein is solved. The fused protein is of great importance to the research on the hepatitis B virus (HBV).
Owner:CHONGQING MEDICAL UNIVERSITY
Heterologous prime-boost immunization regimen
InactiveUS20090092635A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsRegimenPrime boost
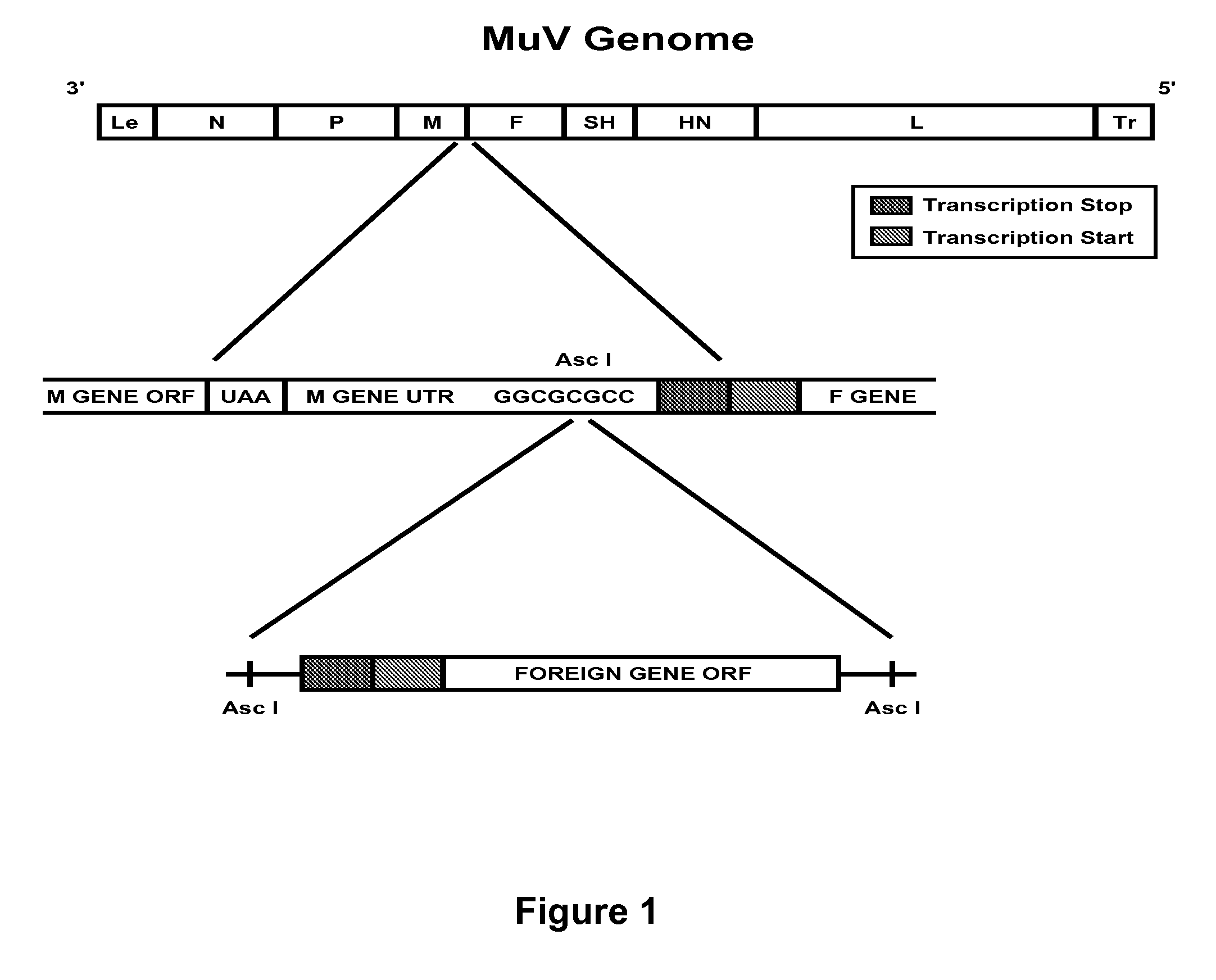
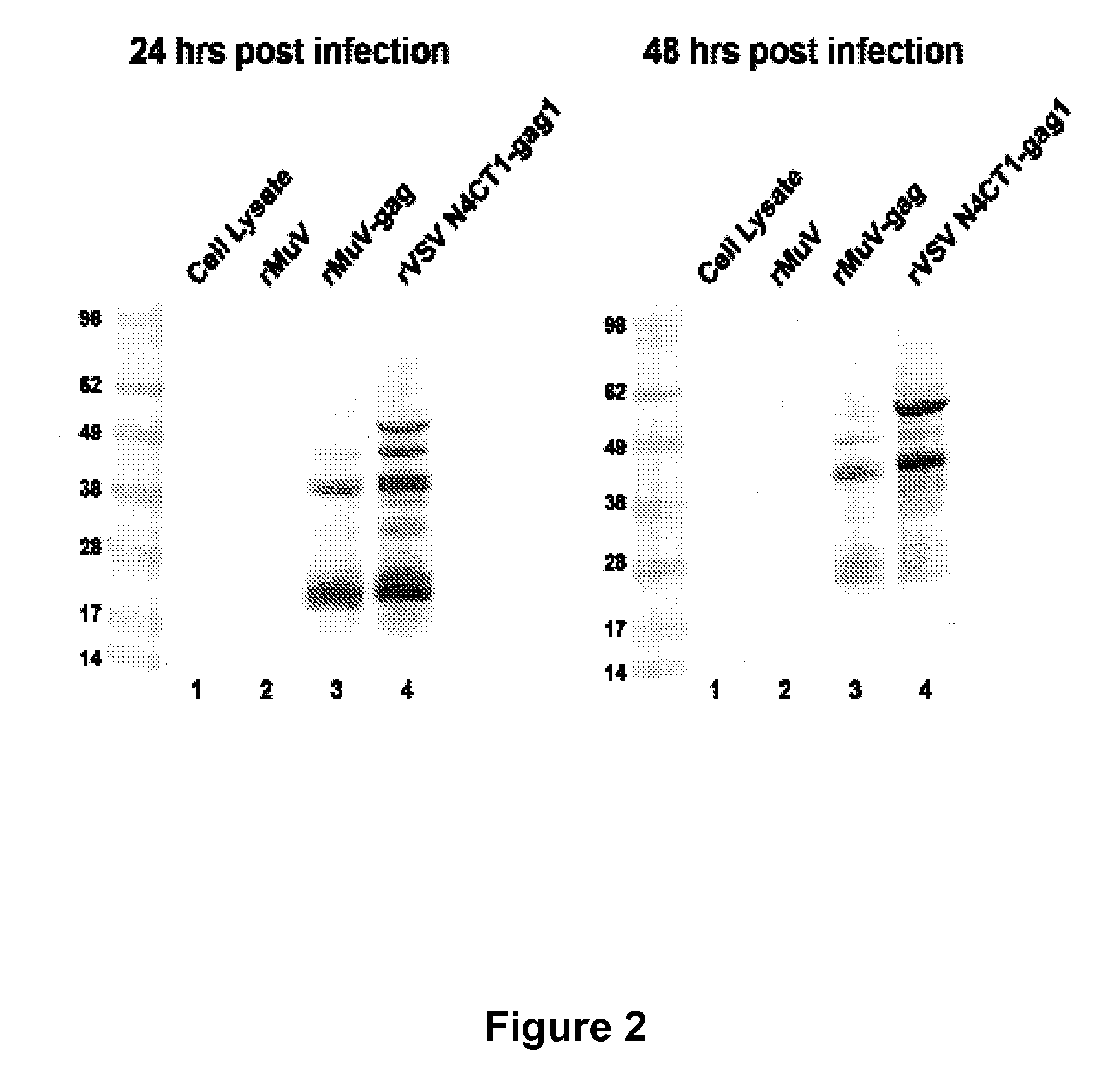
The present invention is directed to a method for generating an antigen-specific immune response in a subject in general and in particular to administering a priming dose of an immunogenic composition of a recombinant mumps virus (rMuV) that encodes an antigen followed by administering a boosting dose of recombinant vesicular stomatitis virus (rVSV) encoding an antigen.
Owner:WYETH
Recombinant porcine alpha interferon and application thereof in preparing medicines for treating Porcine cytomegalovirus (PCMV)
InactiveCN102796758APeptide/protein ingredientsMicroorganism based processesInfected cellProtein target
The invention discloses a recombinant porcine alpha interferon, prepared by the following methods: A1, synthesizing an artificially modified porcine alpha interferon; A2, constructing a recombinant eukaryotic expression vector pGAPZ alpha-IFN alpha; and A3, highly expressing IFN alpha by an eukaryotic cell yeast expression system. The invention is characterized by taking a supernatant to purifying the porcine alpha interferon which is cultured by a lot of engineering bacteria and expressed with a chromatography column, then collecting a target protein eluate, filtering through a 0.22 mum microfiltration membrane, then determining the activity by cytopathic inhibition, and calculating the potency unit according to 50% pathology; wherein the cell used for determination is Madin-Darby bovine kidney (MDBK), and vesicular stomatitis virus (VSV) is used for attacking the virus. According to the invention, the recombinant porcine alpha interferon obtained by purification is applied in controlling PCMV, and experiments prove that the recombinant porcine alpha interferon has obvious protection effect on PCMV infected cells. The route of administration of the porcine alpha interferon is injection or mucous membrane administration, and the dosage form comprises injection or nasal drops.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Pharmaceutical Compositions Comprising A Pancreatic Enzyme Preparation With Viral Infectivity Reduced Below A Significant Level And Methods Of Preparing And Using The Same
InactiveUS20140017223A1Reduce alkylation activitySure easySsRNA viruses positive-sensePeptide/protein ingredientsAmylasePorcine circovirus
The present invention provides for pharmaceutical compositions comprising pancreatic enzyme preparations (PEPs) with viral infectivity reduced below significant levels and having high enzymatic activity. The PEPs can comprise lipases, proteases, amylases, non-enveloped viruses (e.g., porcine parvovirus (PPV), porcine circovirus type 2 (PCV-2), porcine encephalomyocarditis virus (EMCV)), and enveloped viruses (e.g., vesicular stomatitis virus (VSV), and influenza A (IFA)). The present invention also includes methods of treating pancreatic insufficiency by administering these pharmaceutical compositions and methods of making the same by treating the PEP with beta-propiolactone (BPL) to reduce viral infectivity.
Owner:APTALIS PHARMA CANADA
Antiviral Activity of Bovine Type III Interferon Against Foot-and-Mouth Disease Virus
ActiveUS20120164171A1Reduce degreeReduce rateOrganic active ingredientsSugar derivativesVaccinationInterferon alpha
Interferons are the first line of defense against viral infections and administration of interferons as biotherapeutics has been demonstrated to be effective in controlling several viral infections. Here we report for the first time the identification and characterization of a member of the bovine type III IFN family, boIFN-λ3. We have expressed boIFN-λ3 using a recombinant replication defective human adenovirus type 5 (Ad5) and demonstrated antiviral activity against foot-and-mouth disease virus (FMDV) and vesicular stomatitis virus (VSV) in bovine cells in vitro. Furthermore, we have tested the efficacy of boIFN-λ3 against FMDV in vivo by inoculation of cattle with Ad5-boIFN-λ3 followed by intradermolingual or aerosol virus challenge. Our results demonstrate that the type III IFN family is conserved in bovines and that treatment of cattle with boIFN-λ3 alone or in combination with IFN-α is able to confer delayed and reduced severity of FMD. Furthermore inoculation with Ad5-boIFN-λ3 alone conferred full protection against aerosol challenge for at least 7 days after administration suggesting that type III IFN used in combination with FMD vaccines could fill one of the current gaps in emergency vaccination against FMDV.
Owner:US SEC AGRI
Reagent and detection method for detecting vesicular stomatitis virus, and applications
ActiveCN106350609AControl spreadLower requirementMicrobiological testing/measurementMicroorganism based processesForward primerPolymerase L
The invention discloses a reagent and a detection method for detecting the vesicular stomatitis virus, and applications. The reagent for detecting the vesicular stomatitis virus comprises a first primer probe combination set and a second primer probe combination set for detecting the amplification of vesicular stomatitis virus recombinant polymerase; the forward primer, reverse primer and probe of the first primer probe combination set are sequences shown by Seq IP N.1-3; and the forward primer, reverse primer and probe of the second primer probe combination set are sequences shown by Seq IP N.4-6. According to the reagent for detecting the vesicular stomatitis virus, the two sets of primer probe combinations are designed aiming at two subtypes respectively, thus providing a new detection scheme and approach for the detection of the vesicular stomatitis virus. A reagent-based vesicular stomatitis virus RPA detection method is rapid and accurate, is low in requirements on hardware, and applicable to on-site rapid detection, especially suitable for use by inspection and quarantine departments and the like.
Owner:SHENZHEN CUSTOMS ANIMAL & PLANT INSPECTION & QUARANTINE TECH CENT
Animal insect-borne disease multi-RT-PCR distinguishing and detecting reagent as well as preparation method and application
InactiveCN101724712AQuick checkEfficient detectionMicrobiological testing/measurementMicroorganism based processesAgricultural scienceEpizootic haemorrhagic disease virus
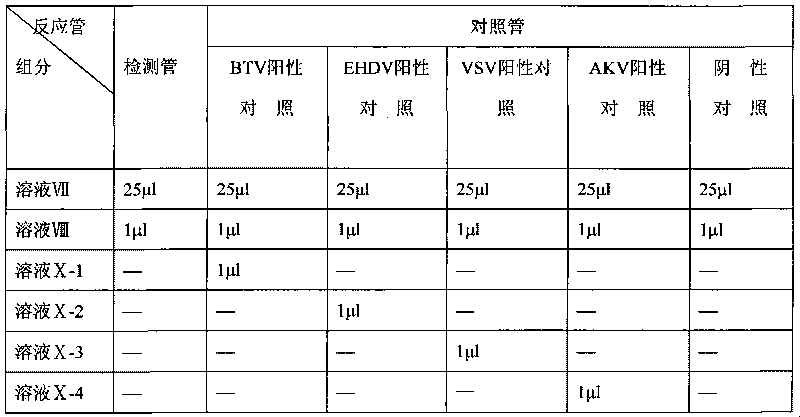
The invention relates to the technical field of biology, in particular to a reagent which can simultaneously distinguish and detect four animal insect-borne diseases as well as a preparation method and application of the reagent. The animal insect-borne disease multi-RT-PCR distinguishing and detecting reagent comprises four pairs of specific primers, and the respective amplification target fragment lengths of Bluetongue virus (BTV), epizootic haemorrhagic disease virus of deer (EHDV), Vesicular stomatitis virus (VSV) and Akabane virus (AKV) are 351bp, 536bp, 300bp and 250bp. The VP7 of the BTV, the EHDV, the VSV and the AKV and a conservative fragment of an N gene are respectively selected as targets, and the primer Express software and the primer prere 5.0 software are applied to deign and combine a primer. An optimal matching and screening test and a multi-RT-PCR test are carried out on a plurality of pairs of designed primers, and four pairs of primers which can carry out the distinguishing and detection on the four animal insect-borne diseases and have high amplification efficiency and good specificity can be obtained by a plurality of reaction condition optimization and comparison tests and verification tests. A kit formed by the reagent can obtain a qualitative distinguishing and detecting result in six hours after a sample is received. The multi-RT-PCR distinguishing and detecting reagent is a sensitive and reliable method for detecting the BTV, the EHDV, the VSV and the AKV in the clinical sample.
Owner:花群义
Fast test strip of vesicular stomatitis virus (VSV) and preparation method thereof
The invention relates to a fast test strip of vesicular stomatitis virus (VSV) and a preparation method thereof. The test strip comprises a base plate (1), a sample absorption pad (2) of a monoclonal antibody absorbed with VSV labelled with colloidal gold, a nitrocellulose membrane (3) provided with a detection line (4) and a control line (5) and an absorbent pad (6), and the absorption pad (2), the nitrocellulose membrane (3) and the absorbent pad (6) are sequentially arranged in the base plate (1) along with the length direction. The method comprises the steps of: a, preparing the VSV monoclonal antibody; b, burning colloidal gold and preparing a monoclonal antibody to be labelled; c, labelling the antibody with colloidal gold and purifying the antibody; d, absorbing the purified antibody labelled with colloidal gold on a multiporous fiber sample absorption pad; e, forming the detection line and the control line coated or sprayed by VSV and protein A; and f, arranging the sample absorption pad, the nitrocellulose membrane and the absorbent pad on the plate of the base plate sequentially and forming the test strip. Without special equipment and special technicians, the VSV can be directly and fast detected.
Owner:花群义
Methods for packaging propagation-defective vesicular stomatitis virus vectors using a stable cell line that expresses g protein
InactiveUS20090162321A1Improving the packaging of a propagation-defective Vesicular Stomatitis VirusSsRNA viruses negative-senseBiocideInfected cellStable cell line
A method of producing propagation-defective Vesicular Stomatitis Virus (VSV) is provided. The method involves providing a cell that includes an optimized VSV G gene, wherein expression of VSV G protein from the optimized VSV G gene is inducible; and inducing the cell to express VSV G protein from the optimized VSV G gene. The method also involves infecting the induced cell with an attenuated VSV; growing the infected cells in culture; and recovering attenuated VSV from the culture.
Owner:WYETH LLC
Preparation method of dressing skin
InactiveCN104940982AImprove securityImprove inactivation efficiencyAbsorbent padsBandagesNeutral proteaseCuticle
The invention discloses a preparation method of a dressing skin. The preparation method comprises the following steps of: removing hairs of xenoskin raw materials, and scraping to remove fat and a cuticle so as to obtain a xenoskin material with the thickness of 0.3-0.5 mm; sequentially immersing by using NaCl solution, cleaning by using sterile water, immersing by using neutral protease with the mass fraction of 0.2-0.5% at 37 DEG C, and cleaning by using sterile water so as to obtain a xenoskin; and inactivating the xenoskin so as to obtain the dressing skin, wherein the inactivating step comprises the steps of heating the xenoskin at 50-60 DEG C for 60-120 MIN and irradiating the xenoskin by using gamma rays. The preparation method of the dressing skin is simple to operate and low in cost; four kinds of viruses including PRV (Pseudorabies Virus), VSV (Vesicular Stomatitis Virus), PV1 (Poliovirus 1) and PPV (Porcine Parvovirus) can be completely inactivated; the virus inactivating efficiency is obviously increased; the problem that PV1 cannot be completely inactivated only by irradiating gamma rays can be solved; and therefore, the safety of the dressing skin is increased.
Owner:CHANGSHA DARUIQI IND
Pharmaceutical composition containing interferon and application
InactiveCN103341160AReduce dependenceEliminate side effectsPeptide/protein ingredientsAerosol deliveryPatient complianceInterferon alpha
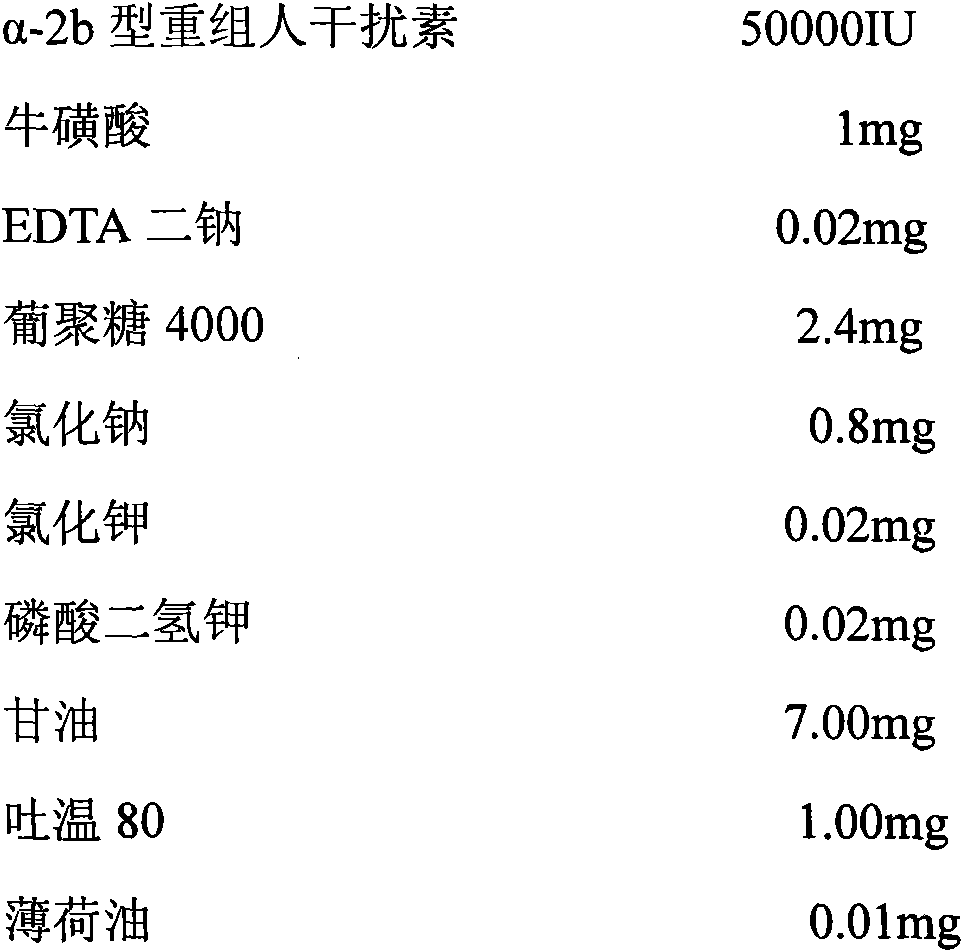
The invention discloses a pharmaceutical composition containing interferon and an application. Pharmacologically active ingredients of the pharmaceutical composition are the interferon and taurine, wherein an activity unit content of the interferon is 10 to 1x10<6> IU / mL, and a content of the taurine is 0.05-0.5 mg / ml. The pharmaceutical composition containing the interferon provided by the invention has effects of resisting activity of vesicular stomatitis virus and murine encephalomyocarditis virus, and protecting cells. Especially, each of nasal spray agent provided by the invention only has 50000 IU of alpha-2b recombinant human interferon and 1 mg of the taurine, but has a specific anti-viral activity of 9.15x10<5> to 2.8x10<6> IU, and shows substantial synergistic effect, thereby being significant for improving patient compliance and reducing medication costs, and meeting clinical needs of interferon medications.
Owner:澳蒲生物科技(上海)有限公司 +1
Oncolytic virus
InactiveUS20070166287A1Reduce riskReduced effectivenessSsRNA viruses negative-senseBiocideNormal cellVesicular stomatitis virus
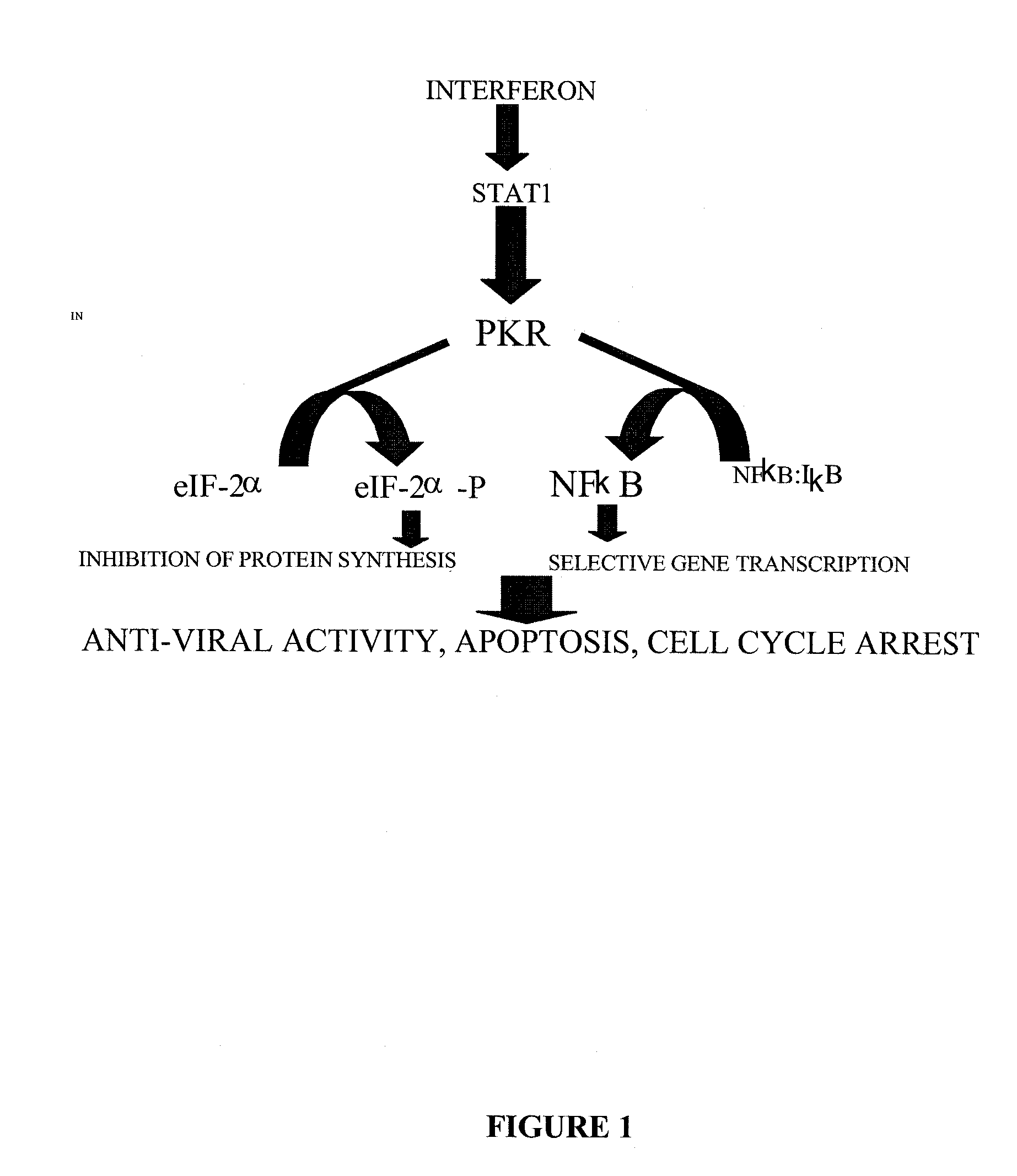
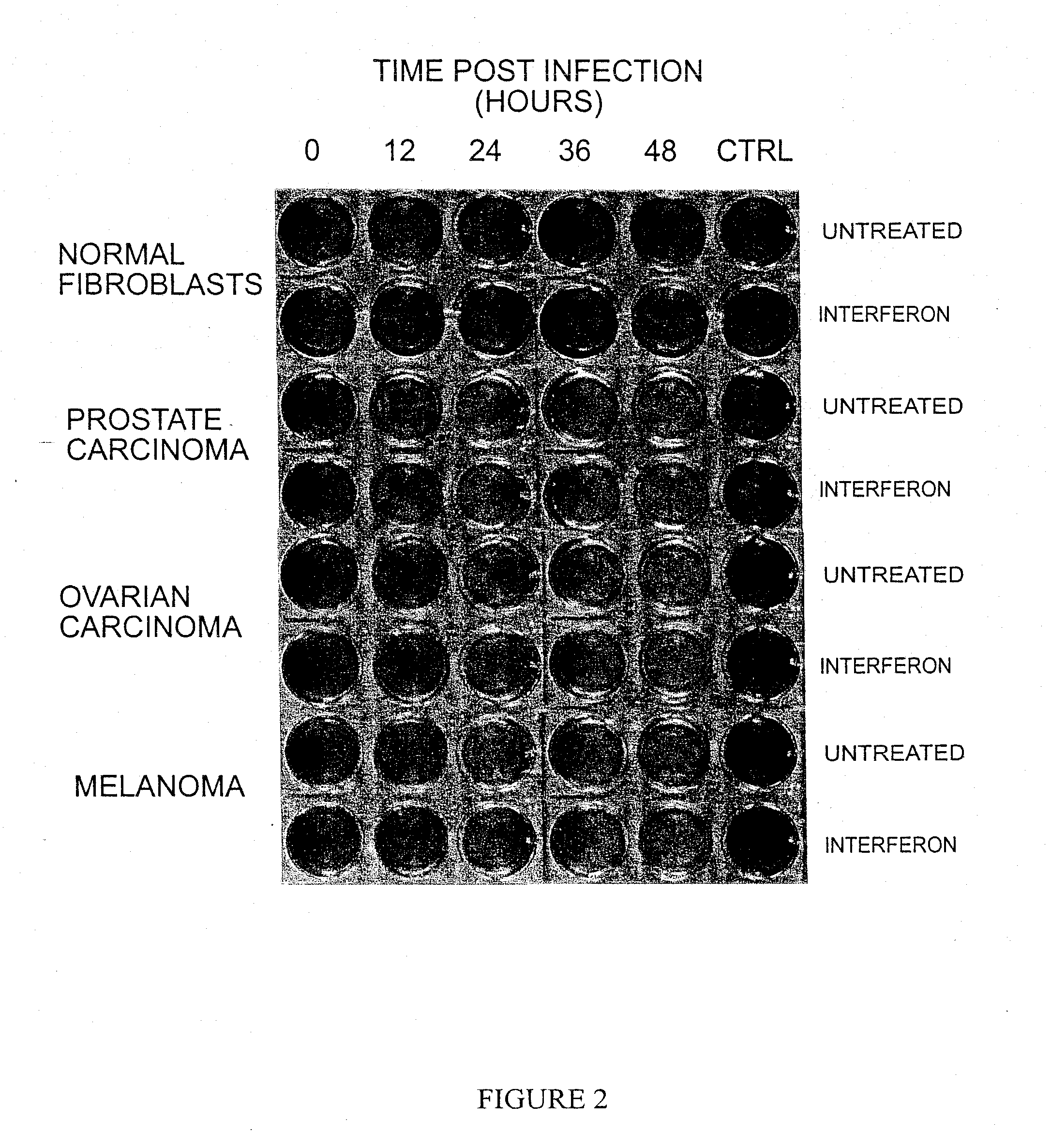
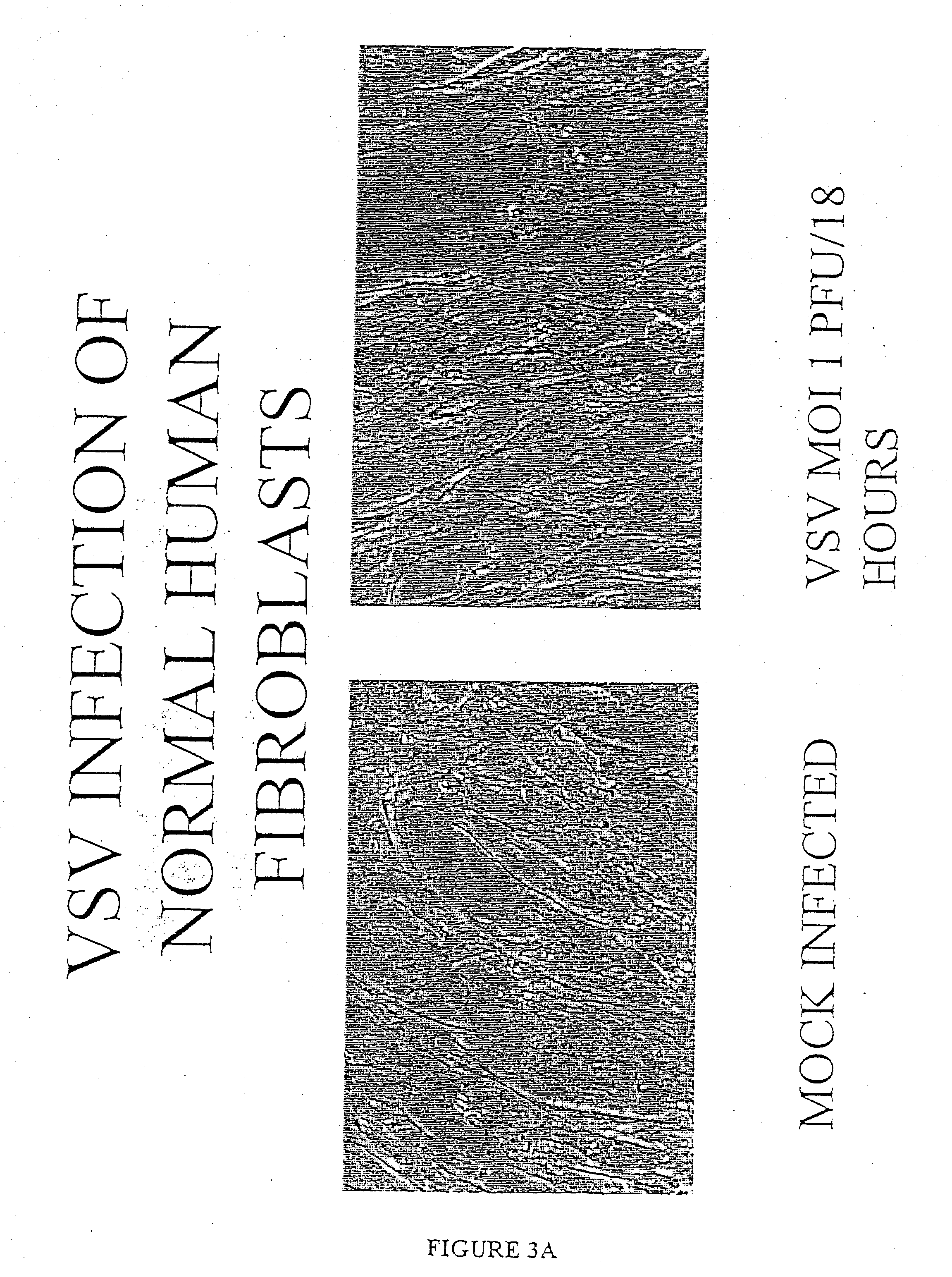
The present invention is directed to a method of reducing the viability of a tumor cell involving administering a virus that is not a common human pathogen to the tumor cell. Preferably, the virus exhibits differential susceptibility, in that normal cells are not affected by the virus. This differential susceptibility is more pronounced in the presence of interferon. The tumor cell is characterized by having low levels, or no, PKR activity, or as being PKR− / −, STAT1− / − or both PKR− / − and STAT1− / −. The virus is selected from the group consisting of Rhabdovirus and picomavirus, and preferably is vesicular stomatitis virus (VSV) or a derivative thereof.
Owner:WELLSTAT BIOLOGICS CORP
Immunogenic composition and methods
InactiveUS20070134200A1Improve the level ofSsRNA viruses negative-senseAntibacterial agentsSpecific immunityEukaryotic plasmids
A method of inducing an antigen-specific immune response in a mammalian subject includes the steps of administering to the subject an effective amount of a first composition comprising a DNA plasmid comprising a DNA sequence encoding an antigen under the control of regulatory sequences directing expression thereof in a mammalian or vertebrate cell. The method also includes administering to the subject an effective amount of a second composition comprising a recombinant vesicular stomatitis virus (rVSV) comprising a nucleic acid sequence encoding the antigen under the control of regulatory sequences directing expression thereof in the mammalian or vertebrate cell. The rVSV is in one embodiment replication competent. Kits for use in immunizations and therapeutic treatments of disease include the components and instructions for practice of this method.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com