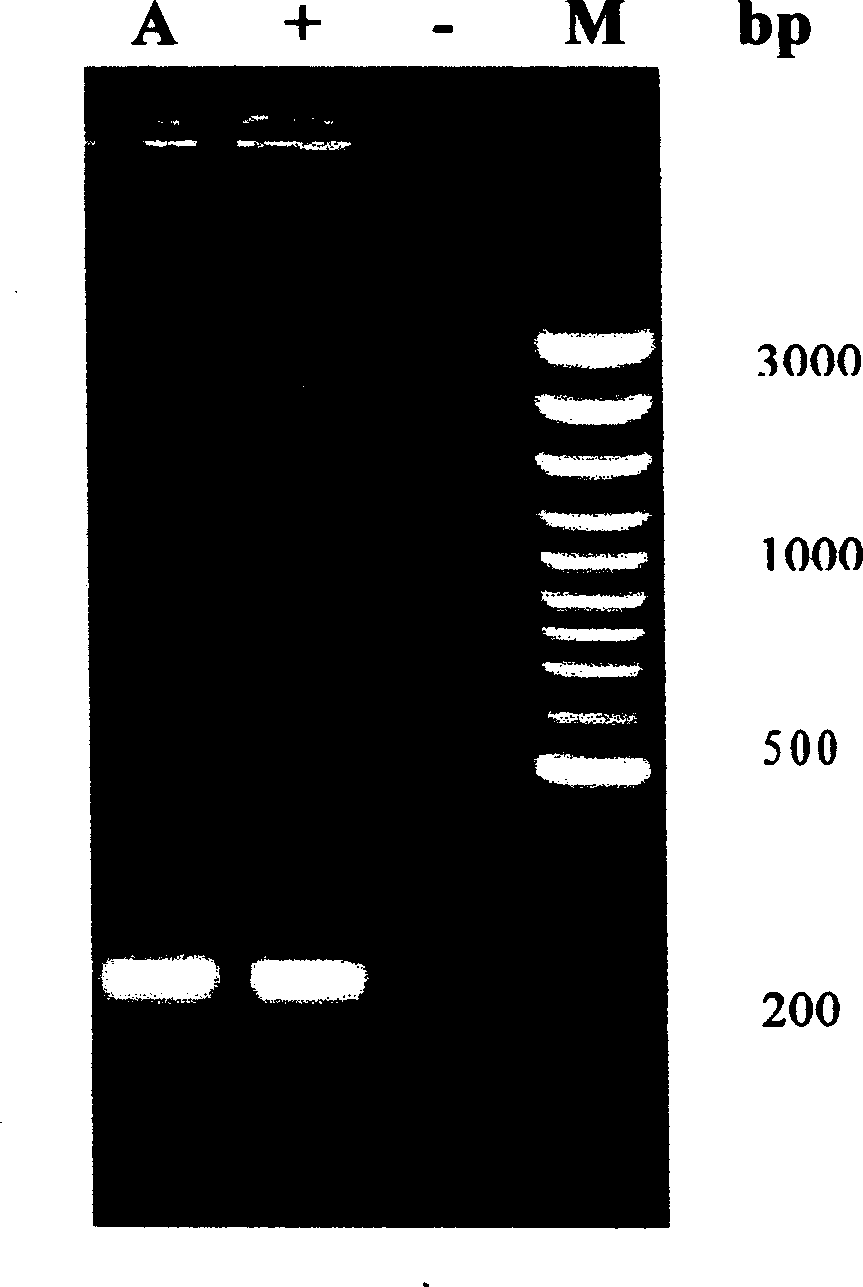
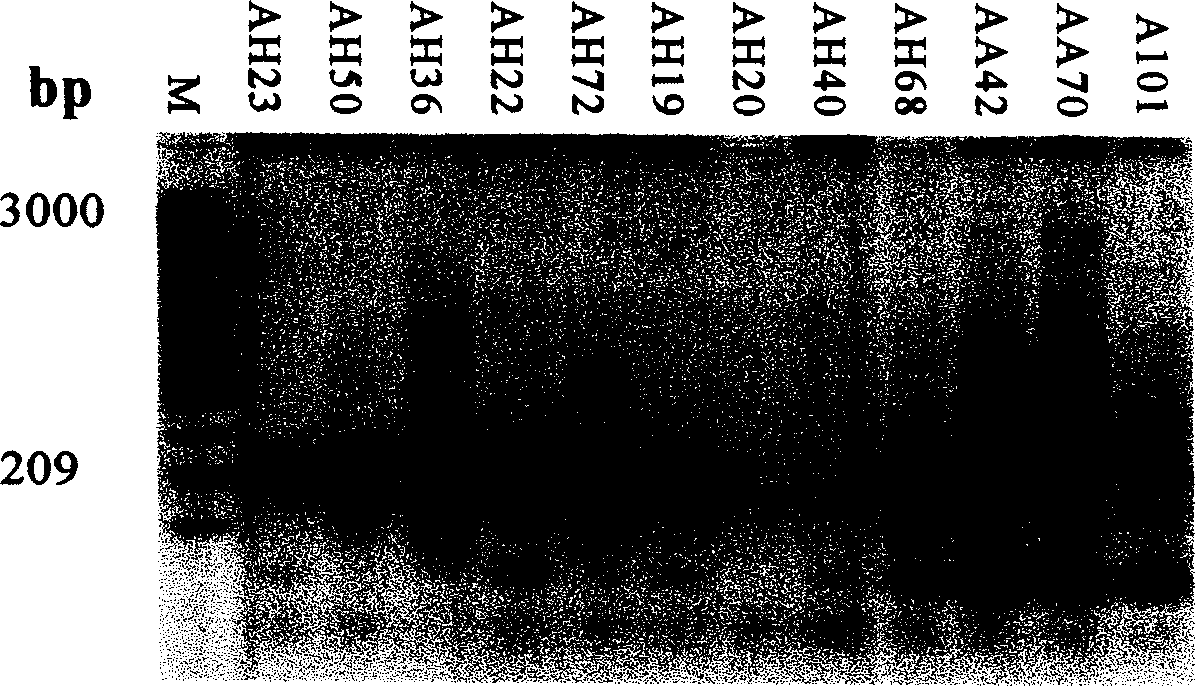
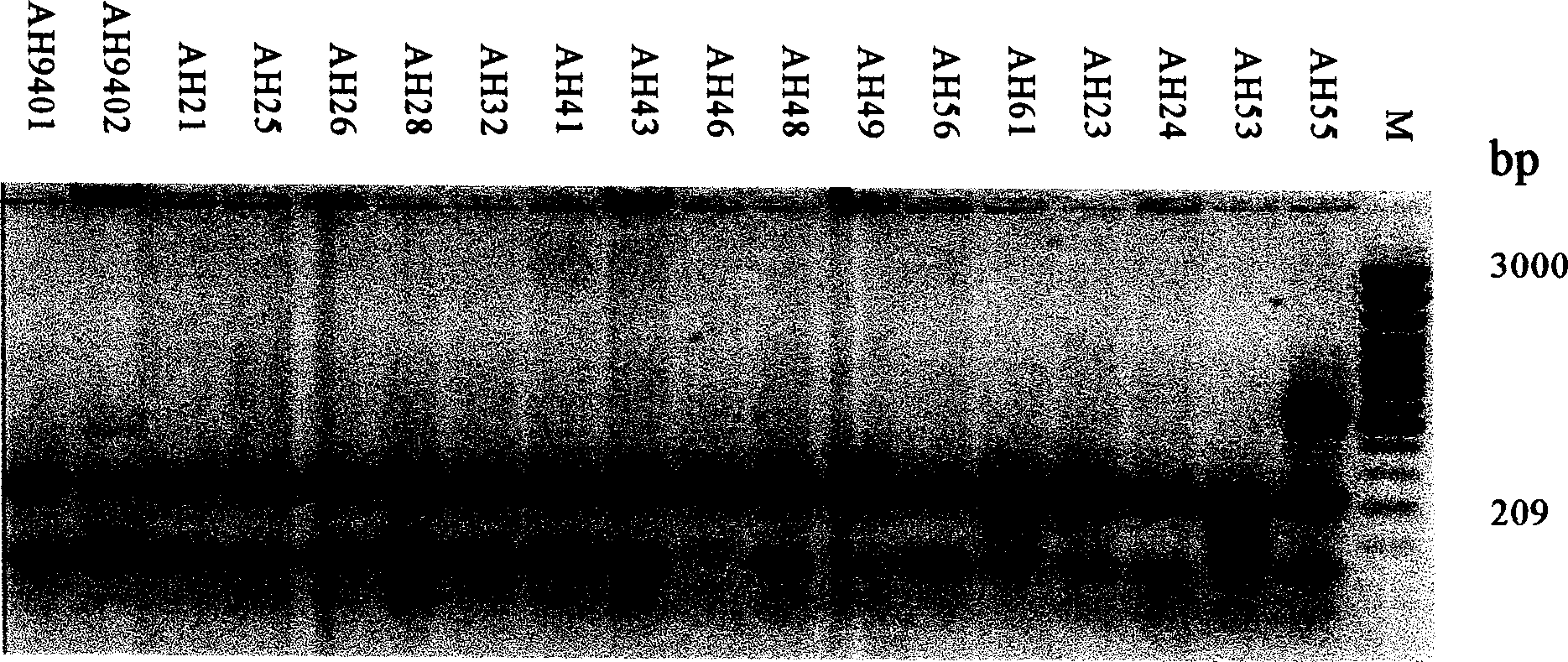
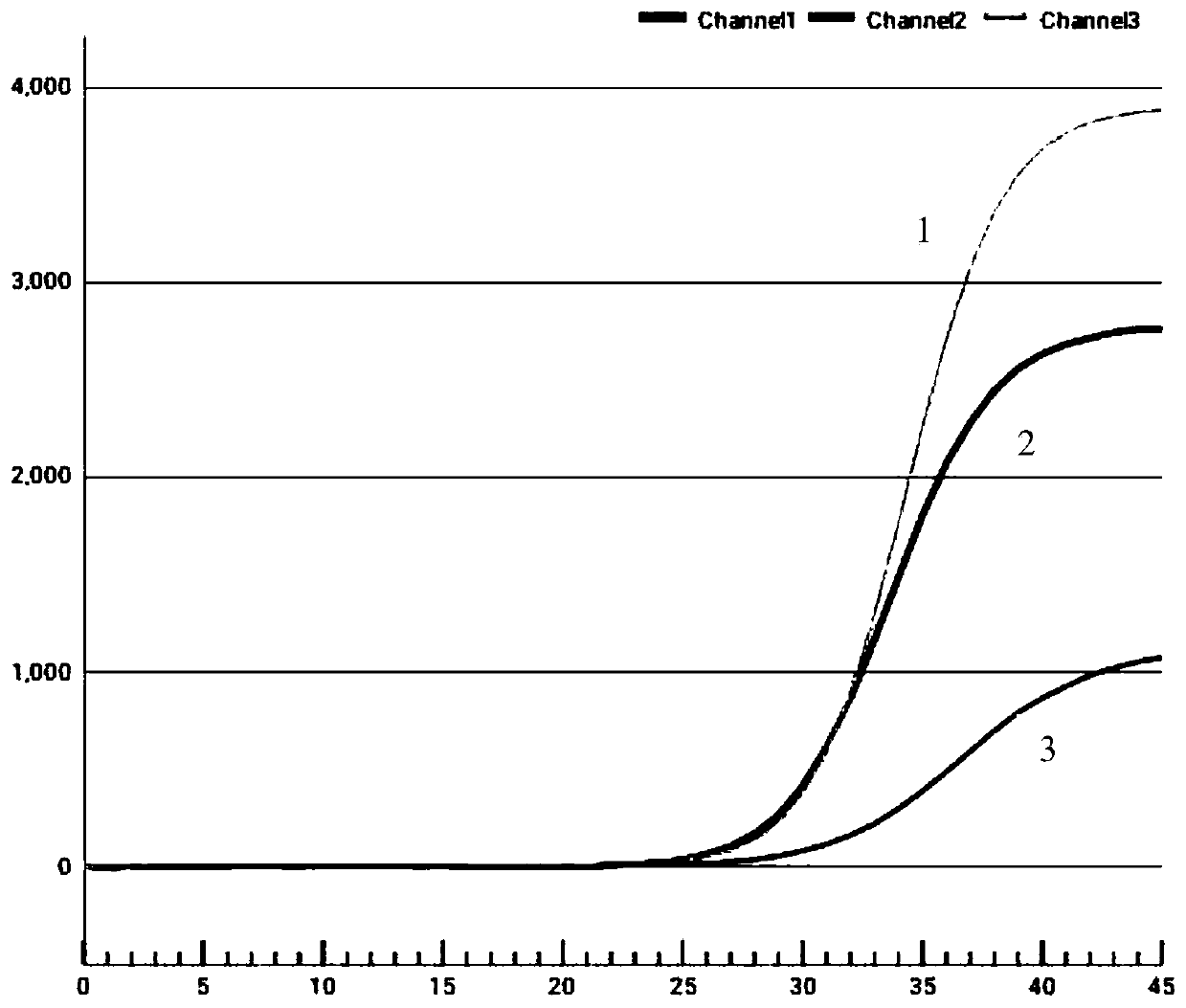
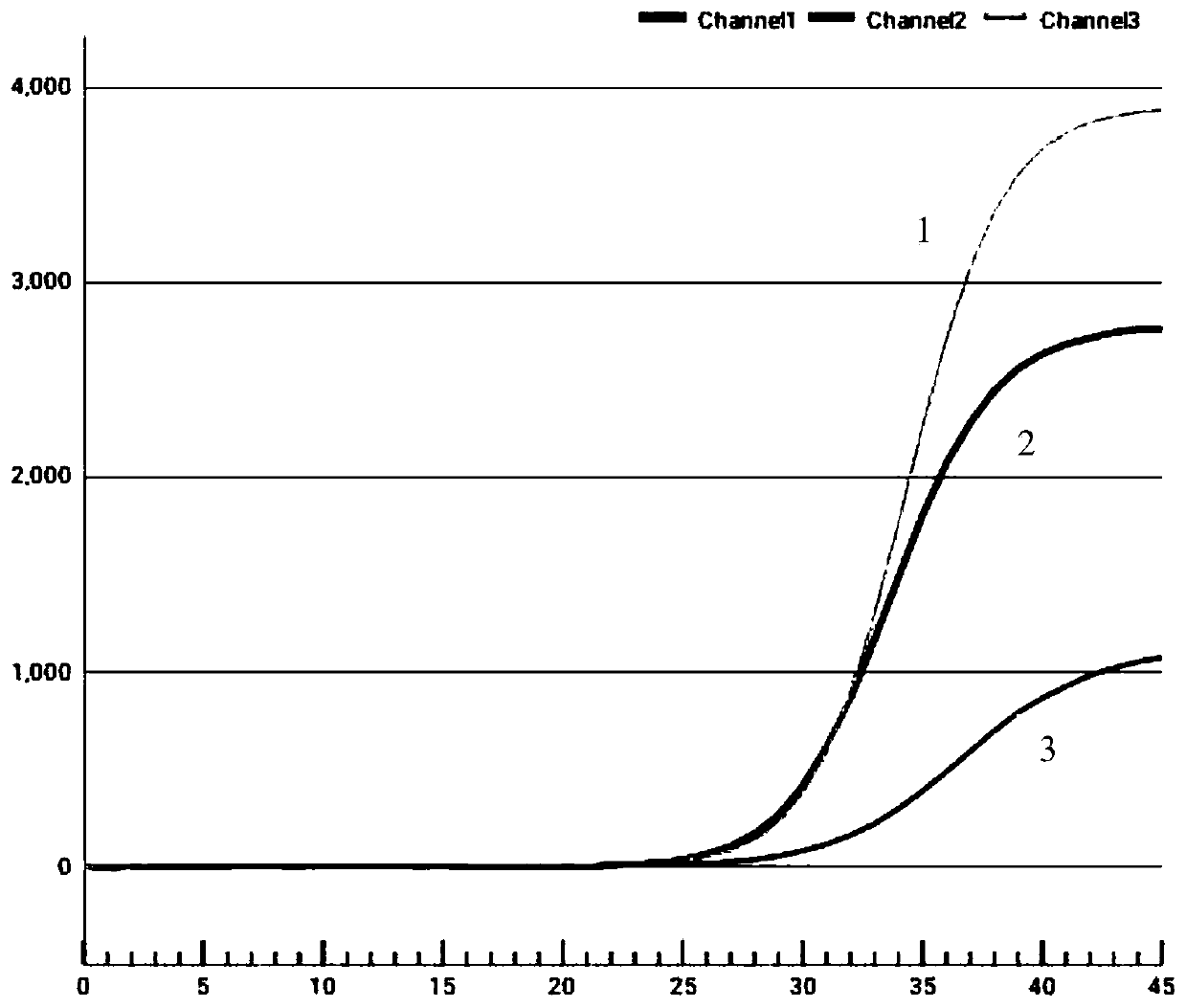
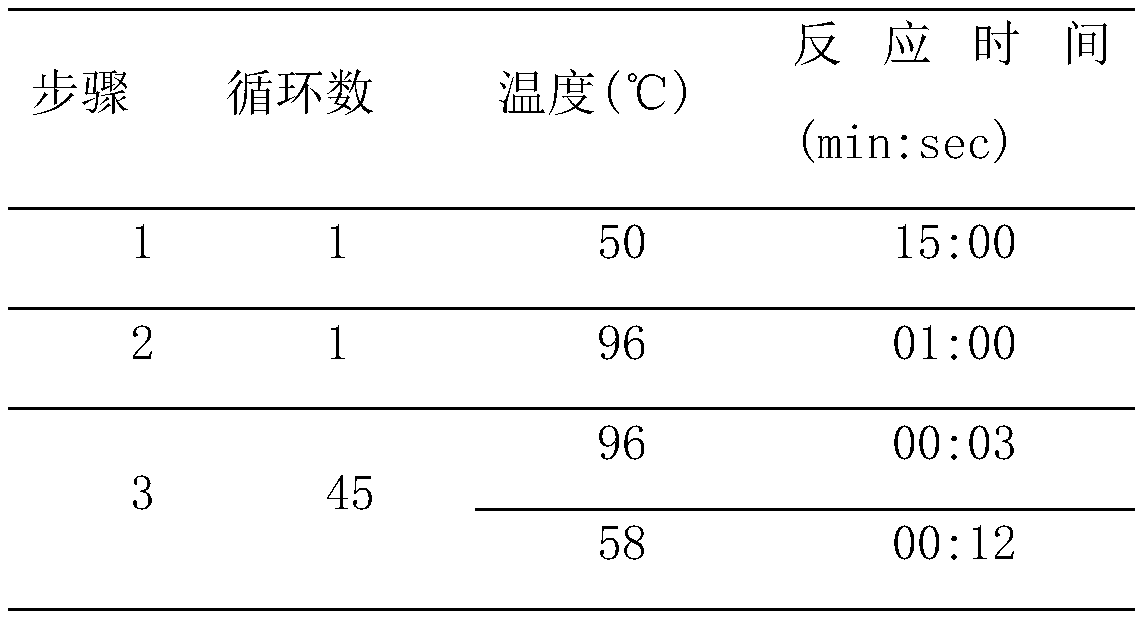
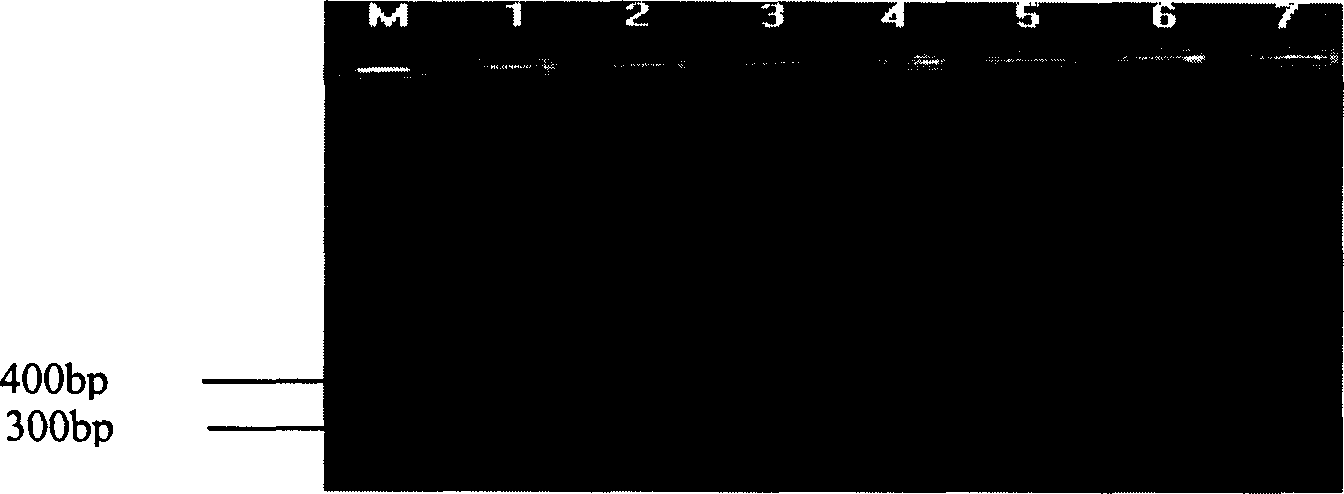
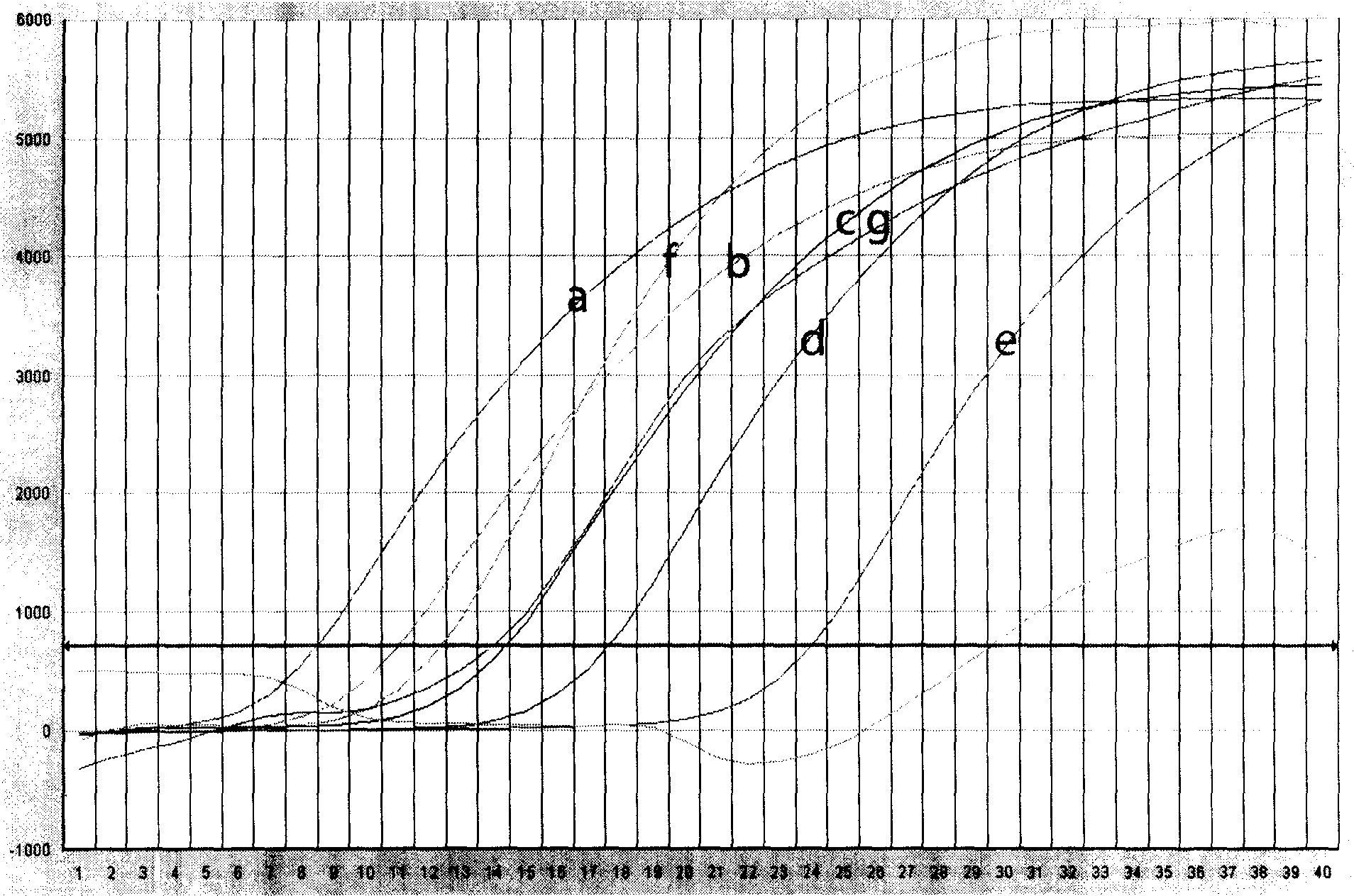
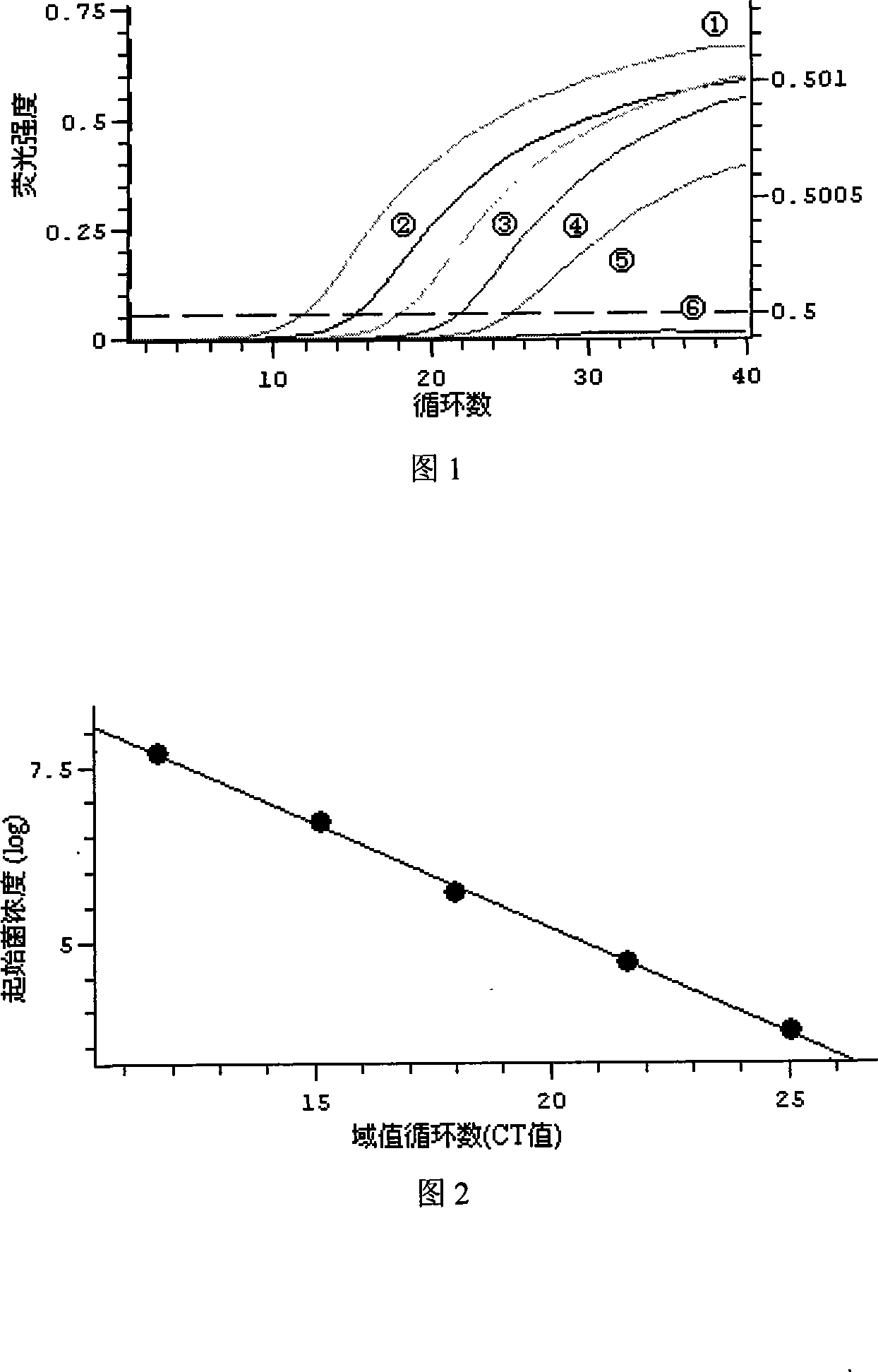
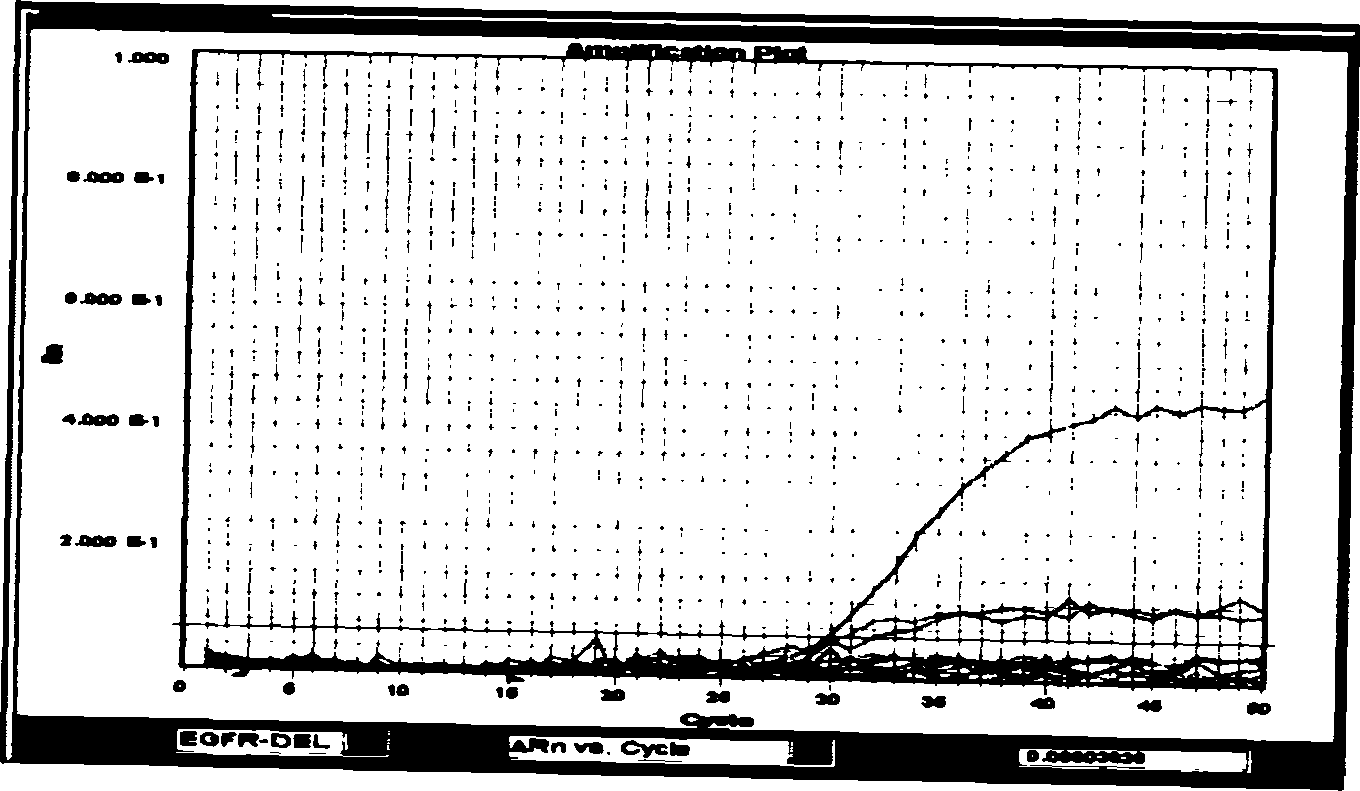
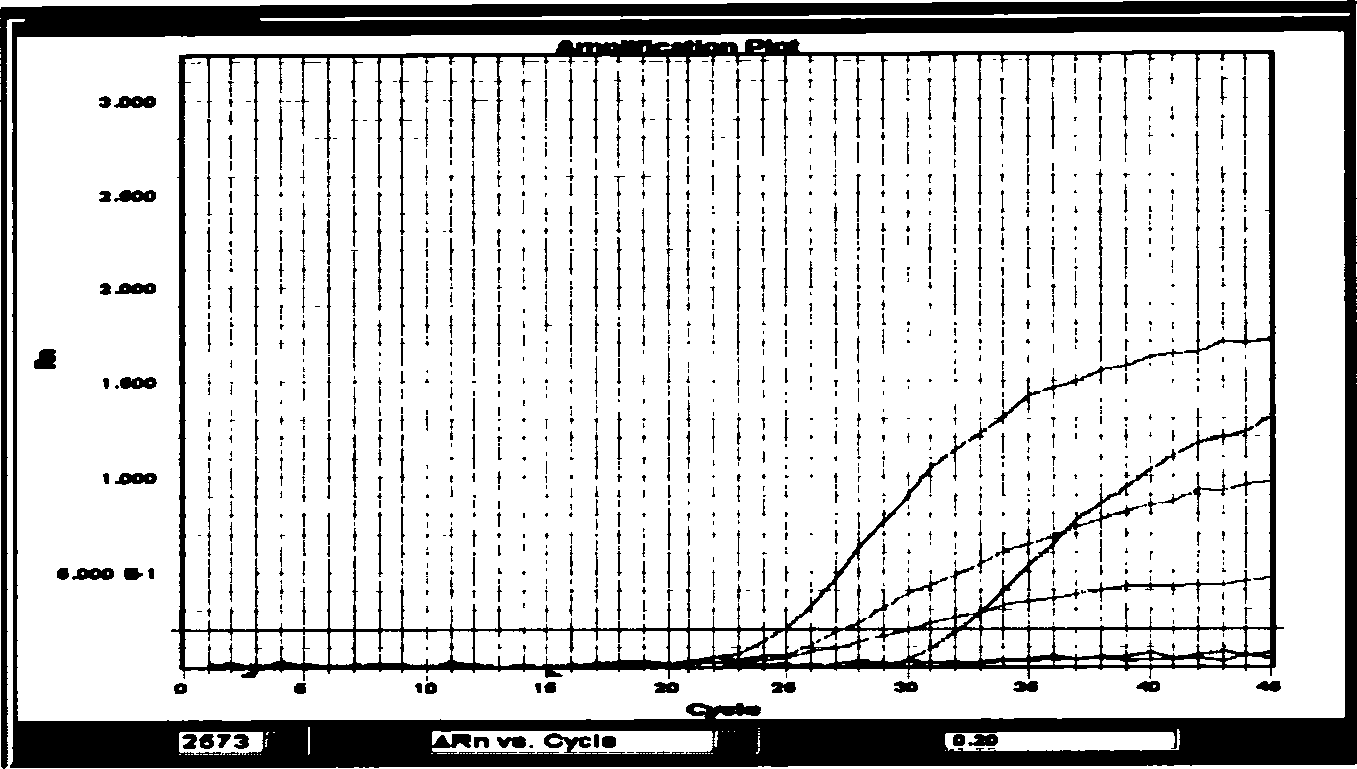
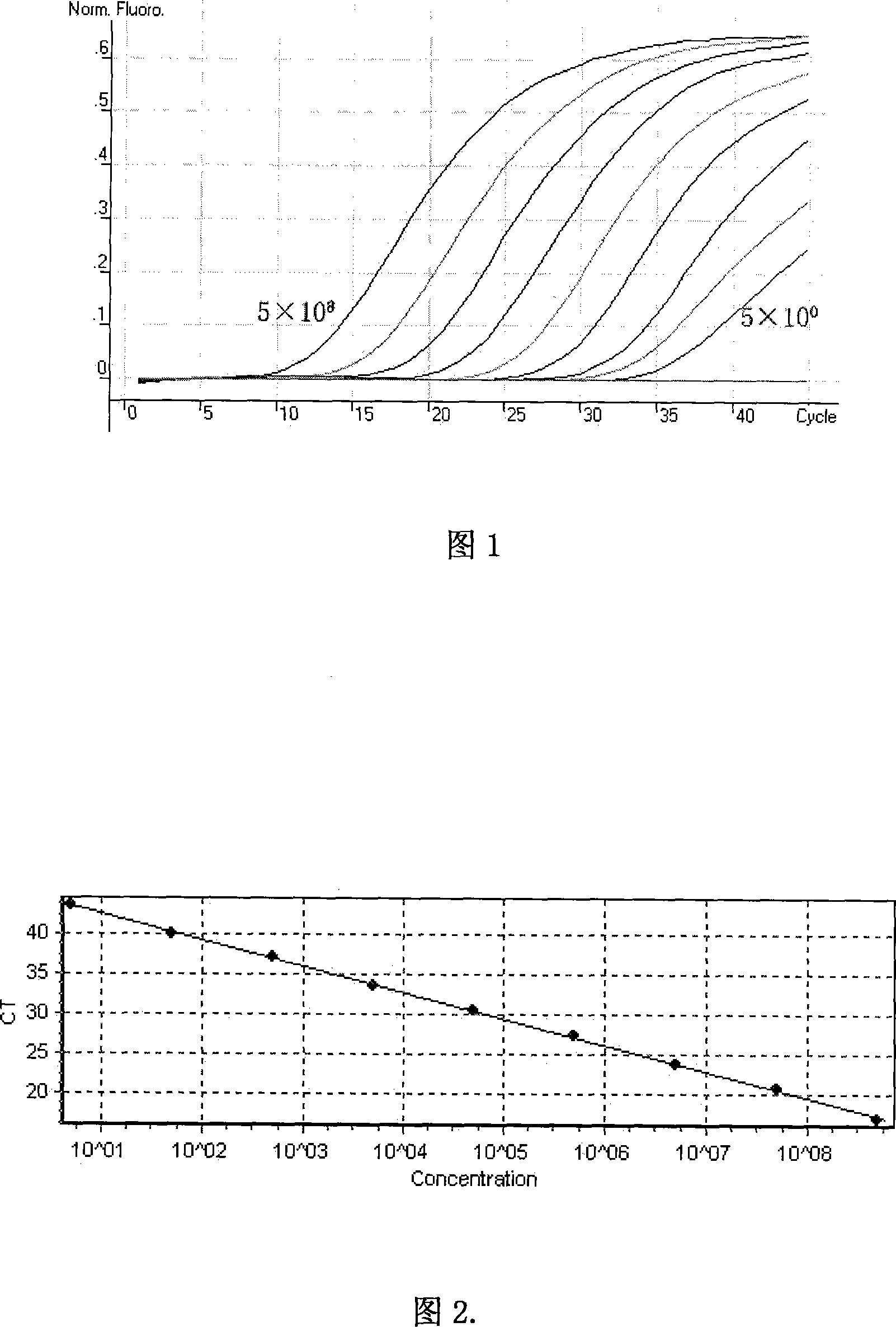
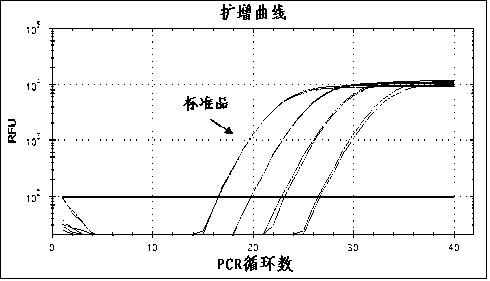
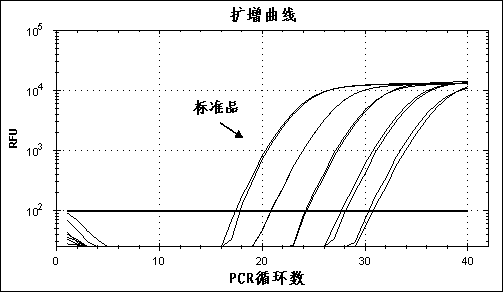
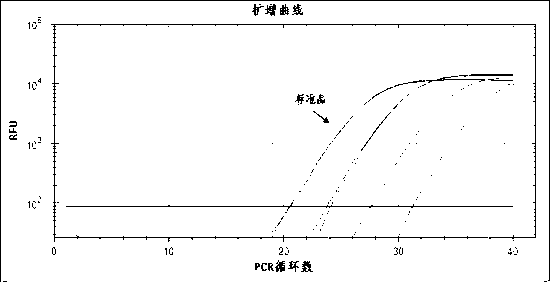
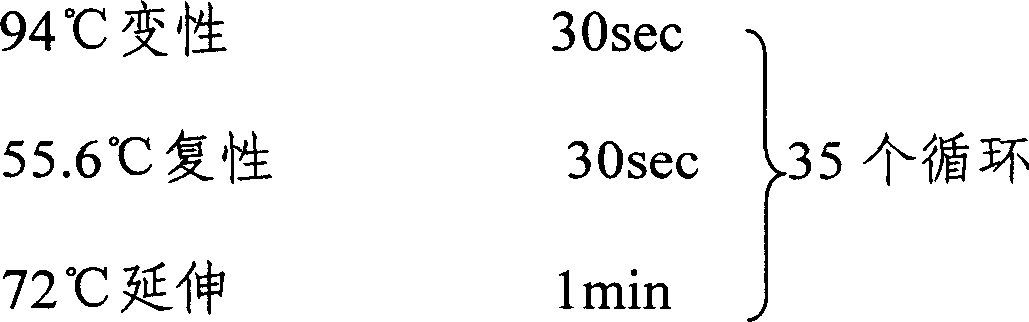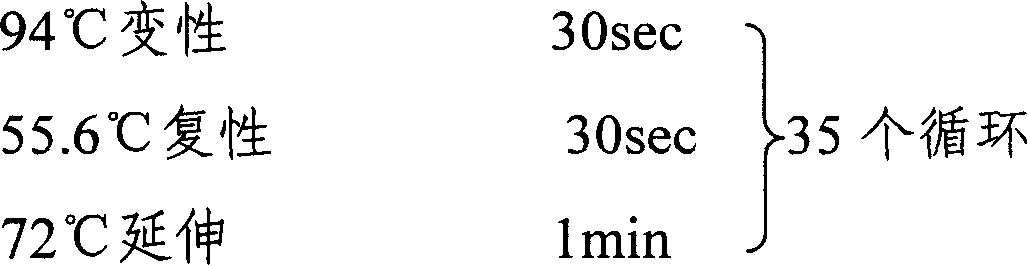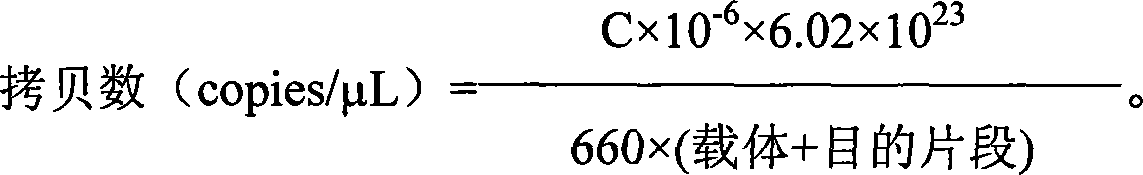Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Pcr test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
PCR stands for Polymerase Chain Reaction and is a common way of testing for a variety of different organisms. The overall process of extracting and amplifying the genetic material of an organism (in this case HIV) and then testing for it with a PCR test is called Nucleic-acid Amplification Testing or NAT.
Non-Invasive Fetal Genetic Screening by Digital Analysis
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Random access system and method for polymerase chain reaction testing
ActiveUS20100112567A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSingle vesselElution
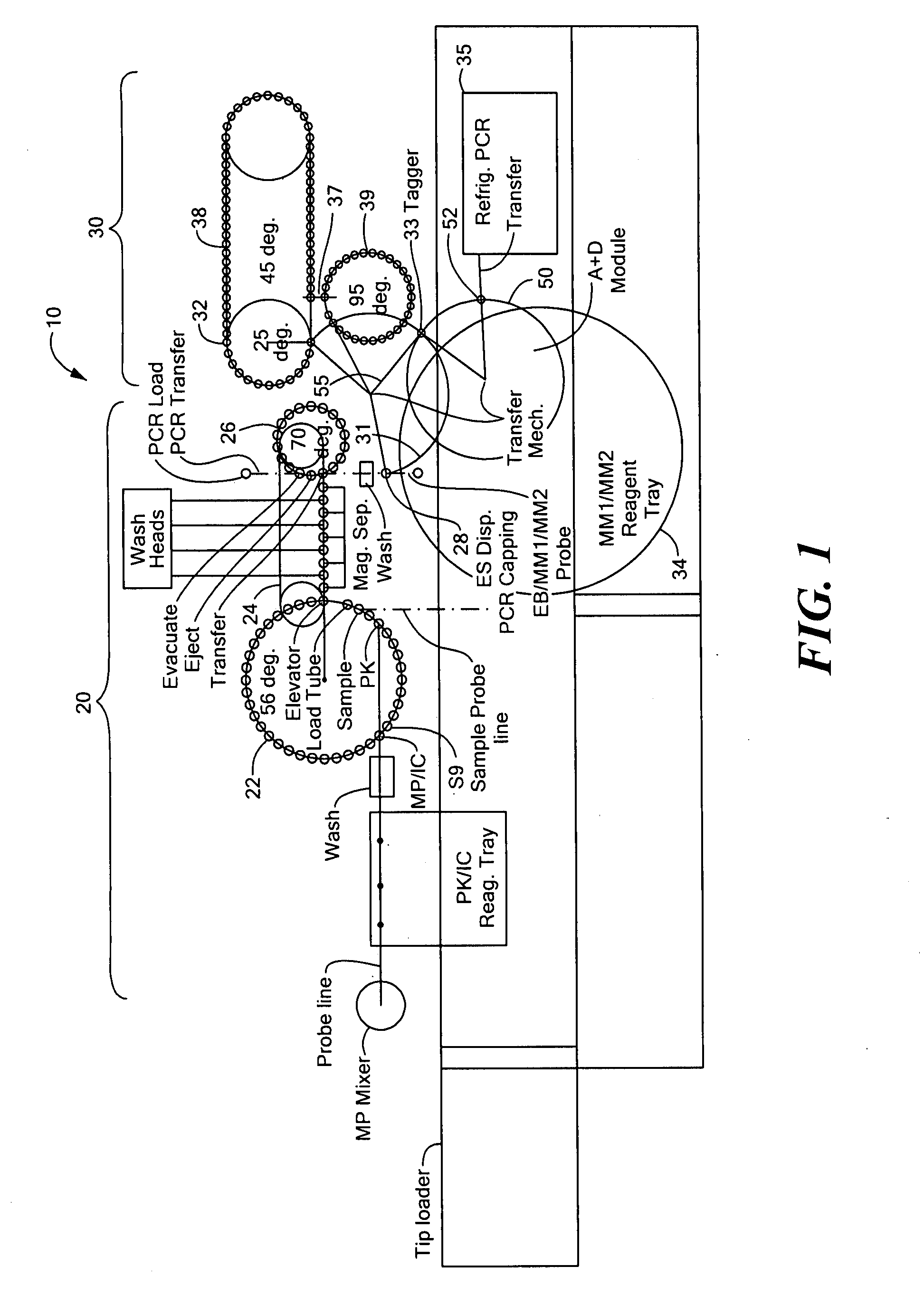
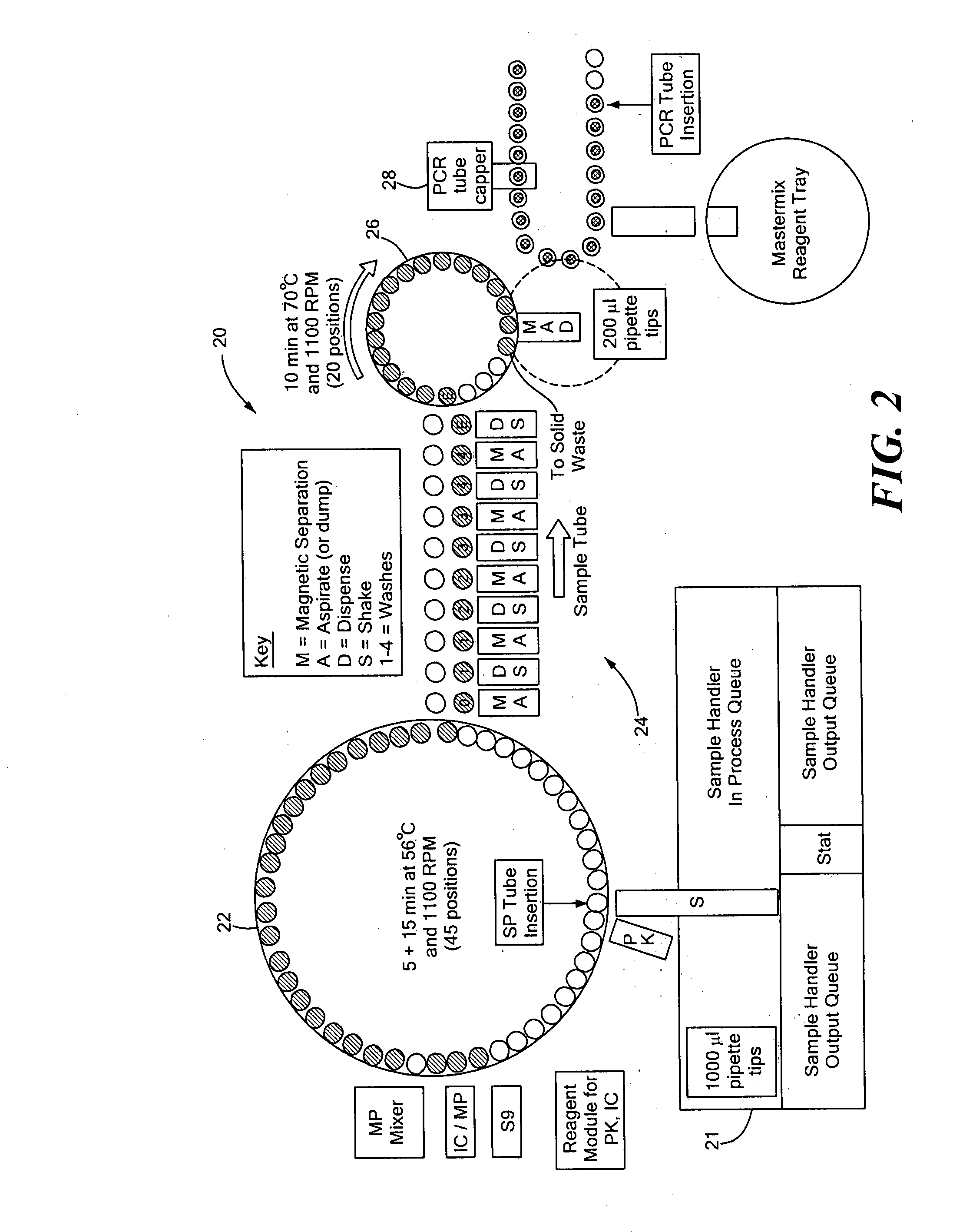
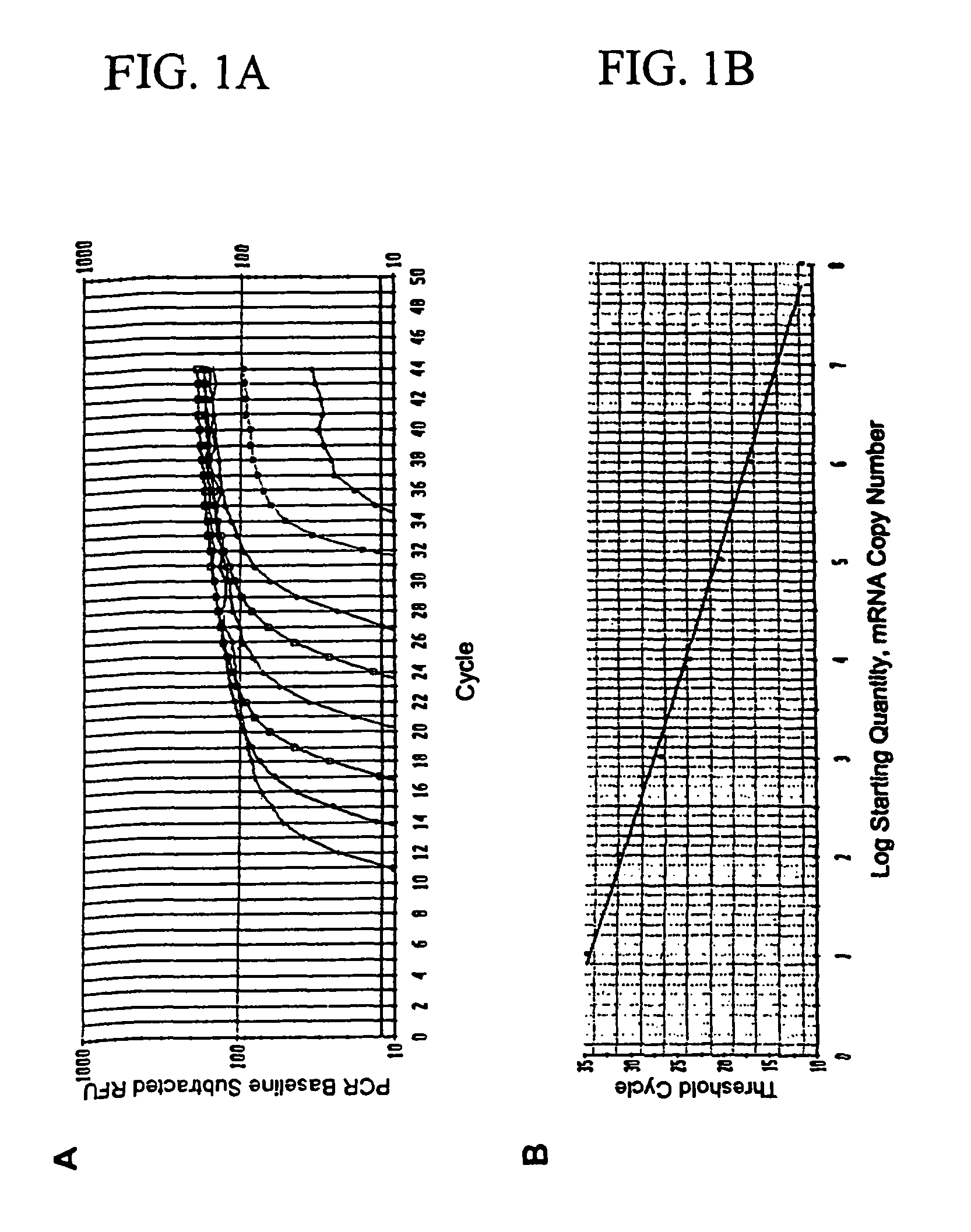
A random access, high-throughput system and method for preparing a biological sample for polymerase chain reaction (PCR) testing are disclosed. The system includes a nucleic acid isolation / purification apparatus and a PCR apparatus. The nucleic acid isolation / purification apparatus magnetically captures nucleic acid (NA) solids from the biological sample and then suspends the NA in elution buffer solution. The PCR testing apparatus provides multiple cycles of the denaturing, annealing, and elongating thermal cycles. More particularly, the PCR testing apparatus includes a multi-vessel thermal cycler array that has a plurality of single-vessel thermal cyclers that is each individually-thermally-controllable so that adjacent single-vessel thermal cyclers can be heated or cooled to different temperatures corresponding to the different thermal cycles of the respective PCR testing process.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
H5, H7, H9 subtype auian flu virus real-time fluo rescent quantixative PCR detecting method
InactiveCN1814805AStrong specificityGood linear relationshipMicrobiological testing/measurementFluorescenceBio engineering
This invention relates to a real time fluorescence quantitative PCR test method for H5, H7 and H9 sub-avian flu viruses including the following steps: set-up of standard molecules, the design of specific virus quantitative PCR primer and the probes, pick-up of virus sample RNA, the inverse transcription polyenzyme chain reaction to the pic-up sample RNA to get a sample cDNA, the optimization and set-up of the PCR quantitative test method for M genes.
Owner:SHANGHAI JIAO TONG UNIV
Fluorescence probe for hgih temperature polyase exonuclease activity in real time PCR test
ActiveCN1900312AImprove performanceEliminate non-specific signalsMicrobiological testing/measurementFluorescencePolymerase L
The present invention relates to fluorescent probe, and is especially fluorescent probe for real-time PCR detection and capable of resisting high temperature polyase exonuclease activity. The fluorescent probe has two-stage structure containing complementary sequences and including replacing probe, molecular beacon probe, scorpion primer, etc. The fluorescent probe includes a fluorescent probe such modified that it can resist the 5'-->3' exonuclease activity of high temperature polyase without generating non-specific signal, a fluorescent probe such modified that it can resist the 3'-->5' exonuclease activity of high temperature polyase without generating non-specific signal in real-time PCR detection, and a fluorescent probe such modified that it can resist both 5'-->3' exonuclease activity and 3'-->5' exonuclease activity of high temperature polyase without generating non-specific signal.
Owner:XIAMEN UNIV
Detection of micro metastasis of melanoma and breast cancer in paraffin-embedded tumor draining lymph nodes by multimarker quantitative RT-PCR
InactiveUS7910295B2High sensitivityStrong specificityMicrobiological testing/measurementBiological testingAbnormal tissue growthMetastatic melanoma
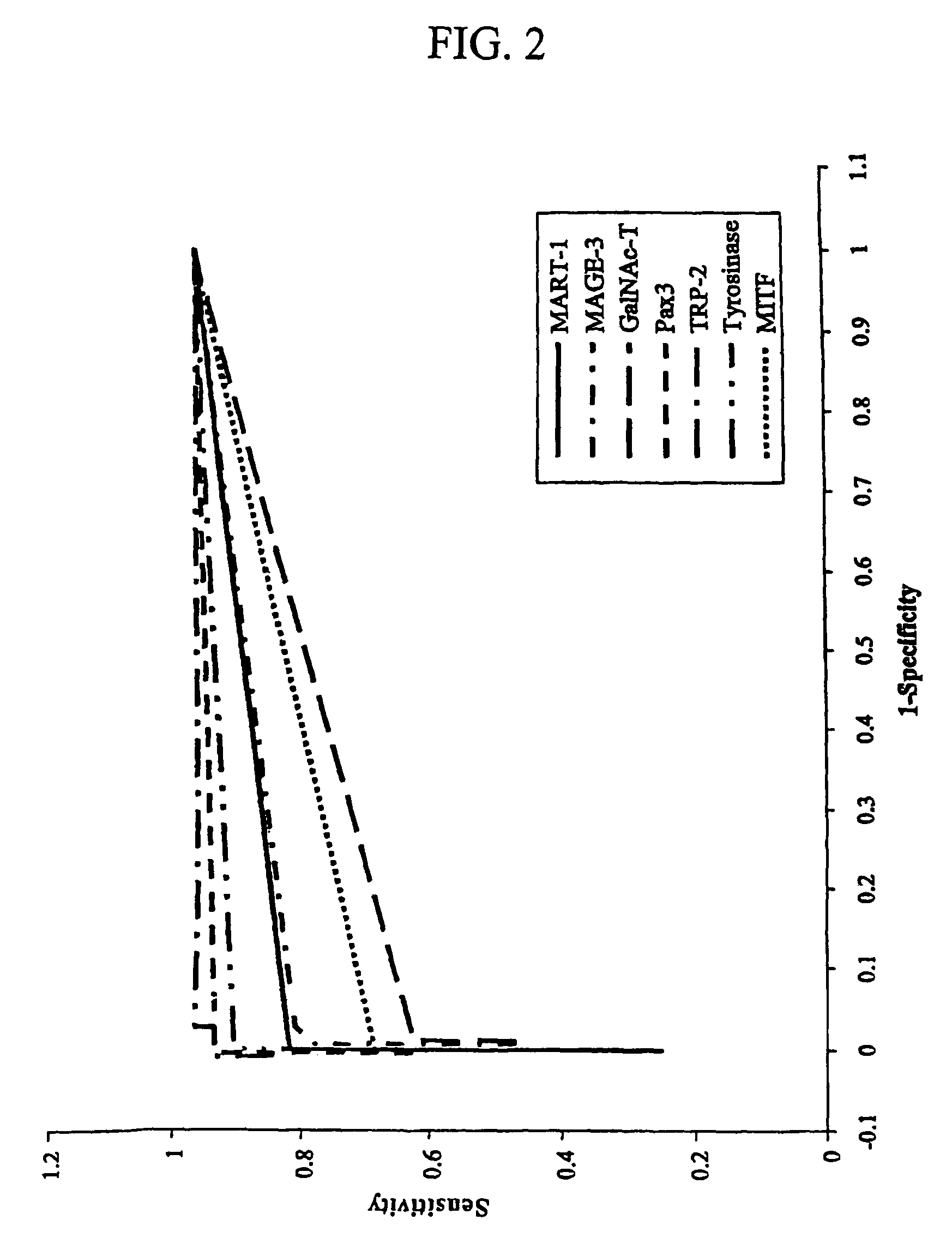
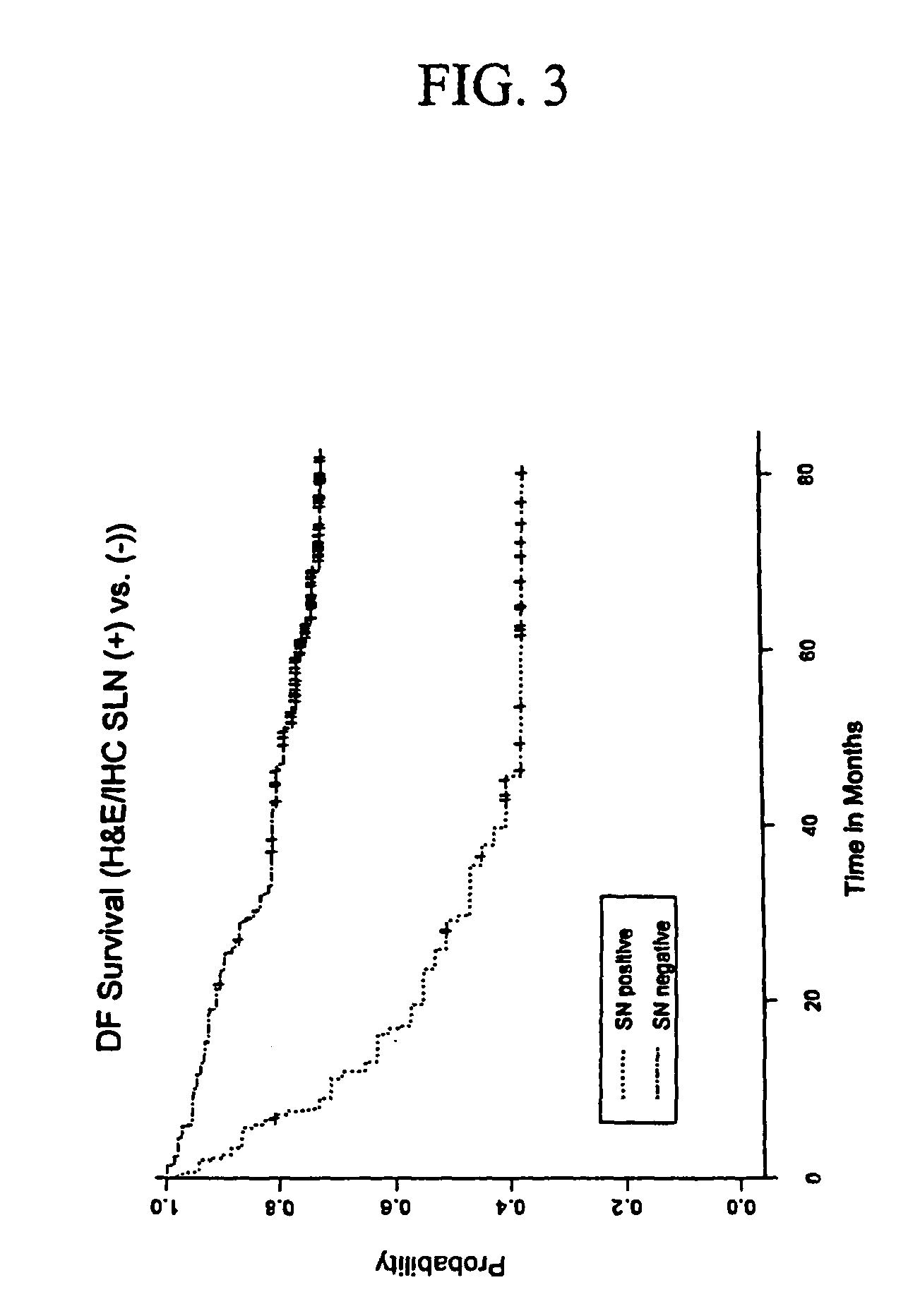
The invention provides a quantitative realtime RT-PCR assay for detection of metastatic breast, gastric, pancreas or colon cancer cells or metastatic melanoma. The assay allows to predict disease recurrence and survival in patients with AJCC stage I and II, and III disease using multimarker panels. The method for detecting metastatic melanoma cells utilizes panels of markers selected from a group consisting of MAGE-A3, GalNAcT, MART-1, PAX3, Mitf, TRP-2, and Tyrosinase. The method for detecting metastatic breast, gastric, pancreas or colon cancer cells in paraffin-embedded samples utilizes panels of markers selected from a group consisting of C-Met, MAGE-A3, Stanniocalcin-1, mammoglobin, HSP27, GalNAcT, CK20, and β-HCG.
Owner:JOHN WAYNE CANCER INST
PCR test kit for hygrophilous aeromonad and its test method
InactiveCN1506468AShort detection cycleDetection speedMicrobiological testing/measurementTest sampleElectrophoresis
The PCR test kit for detecting hygrophilous aeromonad includes primer, dNTP, buffering solution and DNA polymerase, and its upstream primer is 5-CCAAGGGGTCTGTGGCGACA-3í» and downstream primer is 5í»-TTTCACCGGTAACAGGATTG-3í». The PCR test method includes providing sample template; mixing in PCR thin-wall tube dNTP, MgCl2, buffer, primer, DNA, polymerase, tested sample and ddH2O; amplifying the mixture in PCR instrument; electrophoresing the amplified product in electrophoresis equipment while recording result; and analysis and judgment. The PCR test kit is easy to produce and the test method has high sensitivity, short test period, high test precision reaching 10 aeromonad or 1 ng template DNA, and relatively low test cost.
Owner:福建省农业科学院生物技术中心
Hepatitis B virus (HBV) fluorescent quantificationally PCR detecting kit
InactiveCN101158634AMicrobiological testing/measurementMaterial analysis by optical meansHepatitis B virusTarget gene
The present invention relates to a real time fluorescence metered PCR test way of HBV and a reagent kit. The present way adopts alkali cracking to extract HBV DNA, gets and adds a portion of the trace lysate directly into PCR reaction liquid for augmentation after cracking procedure; the reaction liquid includes a pair of specificity primers and a probe marked with fluorescent dye, PCR method is applied to augment target genes, the intensity of the reaction system is real-time tested, and Ct value is calculated according to analysis software as well as the virus content value of specimen. Under the conditions that sensitivity and accuracy are guaranteed, the reagent kit of the present invention can be operated easily and fast, thus reducing the labor intensity of clinical test and the cost of clinical PCR diagnosis at the same time, and continuing the rapidness and easiness function of fluorescence metered PCR test way. The way which is suitable for the clinical HVB metered test can be used as an assistant diagnosis way of HBV infection and a monitor mean of clinical treatment effect in a clinical lab, has comparatively low requirements of test operator, and possesses practical clinical application value.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Application of GeXP multiple gene expression heredity analysis system in detection of nine encephalitis related viruses
InactiveCN102952893AMicrobiological testing/measurementMicroorganism based processesPathogenEpidemiology
The invention belongs to the biotechnological application filed, and relates to simultaneous detection and typing of the infection of nine encephalitis viruses (comprising banna viruses, GI encephalitis B viruses, GIII encephalitis B viruses, tick-borne encephalitis viruses, Tahyna viruses, Liaoning viruses, Kyasanur forest fever viruses, Sindbis viruses and Yunnan orbiviruses) of viral encephalitis patient specimens in disease prevention control mechanisms at all levels, sentinel point hospitals and the like. The nucleotide sequences of nine encephalitis related virus strains are downloaded from NCBI, the pathogen relative conservation region is determined through literature consulting and multiple sequence alignment, and multiple specific primers are designed. The single tube 13-plex multiplex PCR test is carried out to detect nine encephalitis virus conservation regions, and the consumption time of the whole reaction is less than 2h. According to the invention, the non-typing disadvantage of routine single-tube multiple fluorescent qualitative PCR detection is overcome, the disadvantages comprising complex operation, long time, high cost and the like of routine chip detection methods are overcome, a new thought is provided for an encephalitis virus typing technology, the characteristics comprising high specificity, high sensitivity and rapidness of the GeXP multiple gene expression heredity analysis system provide a strong technological support for the rapid and accurate screening and typing of the encephalitis viruses, and the GeXP multiple gene expression heredity analysis system is of important significance to the encephalitis syndrome patient infection pathogen spectrum and molecular epidemiology investigation in China.
Owner:中国疾病预防控制中心病毒病预防控制所
Extraction-free SARS-CoV-2 nucleic acid detection method and kit
InactiveCN111172327AShorten detection timeEasy to operateMicrobiological testing/measurementMicroorganism based processesDisease monitoringRespiratory secretion
The invention provides an extraction-free method and a kit for qualitatively detecting SARS-CoV-2 by fluorescence polymerase chain reaction (fluorescence PCR), and belongs to the field of biotechnology and medicine. The method integrates sample nucleic acid release and amplification. The kit comprises a lysis solution and a rapid enzyme, can directly lyse and amplify respiratory secretions of a patient, make a whole detection process fast and simple, reduce chance of mutual contamination, obtain accurate and reliable PCR test results, can diagnose presence of SARS-CoV-2 in patient secretions as soon as possible, and has important application value in the fields of disease monitoring and clinical diagnosis.
Owner:杭州丹威生物科技有限公司
Rotavirus RT PCR detecting kit and its detecting method
InactiveCN1814790AHigh sensitivityHigh speedMicrobiological testing/measurementRotavirus RNATest sample
Thisa invention relates to a semi-set RT-PCR test reagent box for testing and verifying wheel-like virus including a MLV inverse transcriptase, Rnasin, DNA poly-enzyme, primers, dNTP, MgCl2 and 10 x buffer solution, in which, the upstream primer P1 is 5'-GTA TGG TAT TGA ATA TAC CAC-3', the downstream primer P2 is 5'-GAT CCT GTT GGC CAT CC-3. The RT-PCR test method includes: picking up a due test sample template, adding MLV inverse transcriptases, Rnasin, DNA poly-enzymes, primers, dNTP, MgCl2, 10 x buffer solution, the due test sample template and DECP water to be mixed uniformly to be enlarged on a PRC instrument, then the product is electrophoresis-enlarged in a device to get a result, which is recorded, analyzed and judged.
Owner:GUANGDONG INST OF MICROORGANISM +1
Green smut bug real-time fluorescent quantitative PCR test kit and its use
InactiveCN1851448AOvercoming time consumingOvercome the resultMicrobiological testing/measurementFluorescence/phosphorescenceFarming environmentAgricultural science
The invention relates to a green smut germ real time fluorescence fix quantity PCR agent box and the application. The box uses Taqman and positive primer, negative primer and masculine standard product to build up real time fluorescence PCR reacting system that could rapidly, and quantified testing green smut germ. It is suited for farm environment. It is also could be used to testing the germ testing for paddy seed and the multiplication speed in different paddy kinds.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
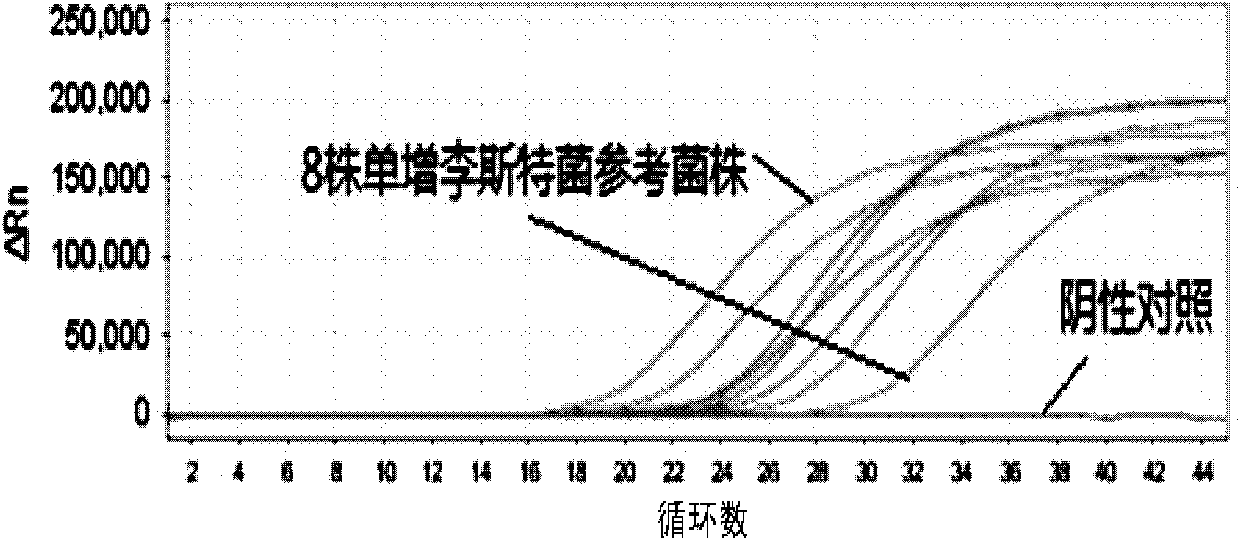
Listeria monocytogenes fluorescence quantitative PCR (Polymerase Chain Reaction) test kit and test method
InactiveCN101805799AHigh detection specificityHigh detection sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceA-DNA
The invention relates to a Listeria monocytogenes fluorescence quantitative PCR (Polymerase Chain Reaction) test kit and a test method, belonging to the technical field of food safety. The test kit comprises a fluorescence quantitative PCR reaction liquid, a fluorescent probe, a warm start Taq DNA polymerase, a standard positive templet and a negative quality control standard product. The method comprises the following steps of: extracting a DNA of a sample to be tested with a common method; using the obtained DNA as a template; carrying out amplification to PCR by using the Listeria monocytogenes fluorescence quantitative PCR test kit to obtain reaction products; then placing the reaction products in a quantitative PCR instrument for fluorescence test; and carrying out a qualitative test or a quantitative test on the Listeria monocytogenes in the sample. The test kit of the invention has the advantages of high test specificity, high test sensitivity, accurate quantification and high test speed.
Owner:SHANGHAI JIAO TONG UNIV
Method for detecting vibrio parahaemolyticus PCR added with amplification internal sign
InactiveCN101109019AImprove accuracyAvoid misjudgment of resultsMicrobiological testing/measurementAgainst vector-borne diseasesFood safetyVibrio parahemolyticus
The invention relates to a PCR test method of a vibrio parahaemolyticus with internal amplification control in the food safety technology filed, which comprises steps as follows: firstly the construction and synthesis of the internal amplification control sequence artificially and design of the specific primers; secondly the PCR reaction system is laid in the best reaction condition; thirdly PRC is amplified; fourthly DNA of the different-serotype vibrio parahaemolyticus and different-species non vibrio parahaemolyticus is extracted for PRC test to identify the specific primers and confirm whether the internal amplification control can be normally amplified in the non vibrio parahaemolyticus sample test; finally the evaluation of the built PCR reaction through the artificial and natural pollution samples tests. The invention shows the occurrence of the false negative in the testing process and improves the accuracy rate. The invention provides efficient and reliable technical means to satisfy the urgent need of the foodborne patheogen investigation and test in the quarantine law enforcement.
Owner:SHANGHAI JIAO TONG UNIV
Method for manufacturing multiple amplification internal mark for four-bacteria PCR test
InactiveCN101220393AImprove accuracyReduce testing costsMicrobiological testing/measurementVector-based foreign material introductionStaphylococcus cohniiNucleotide
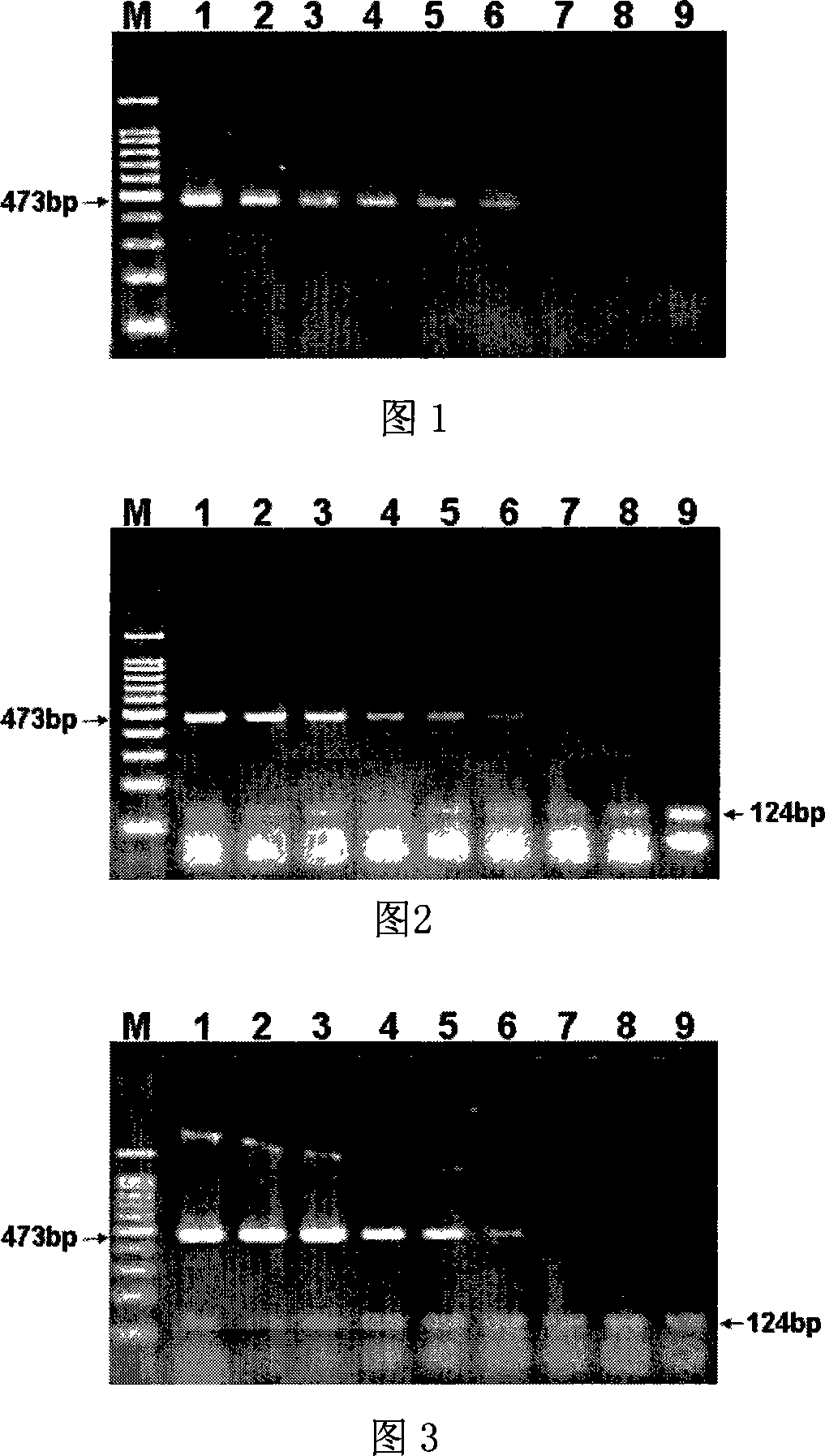
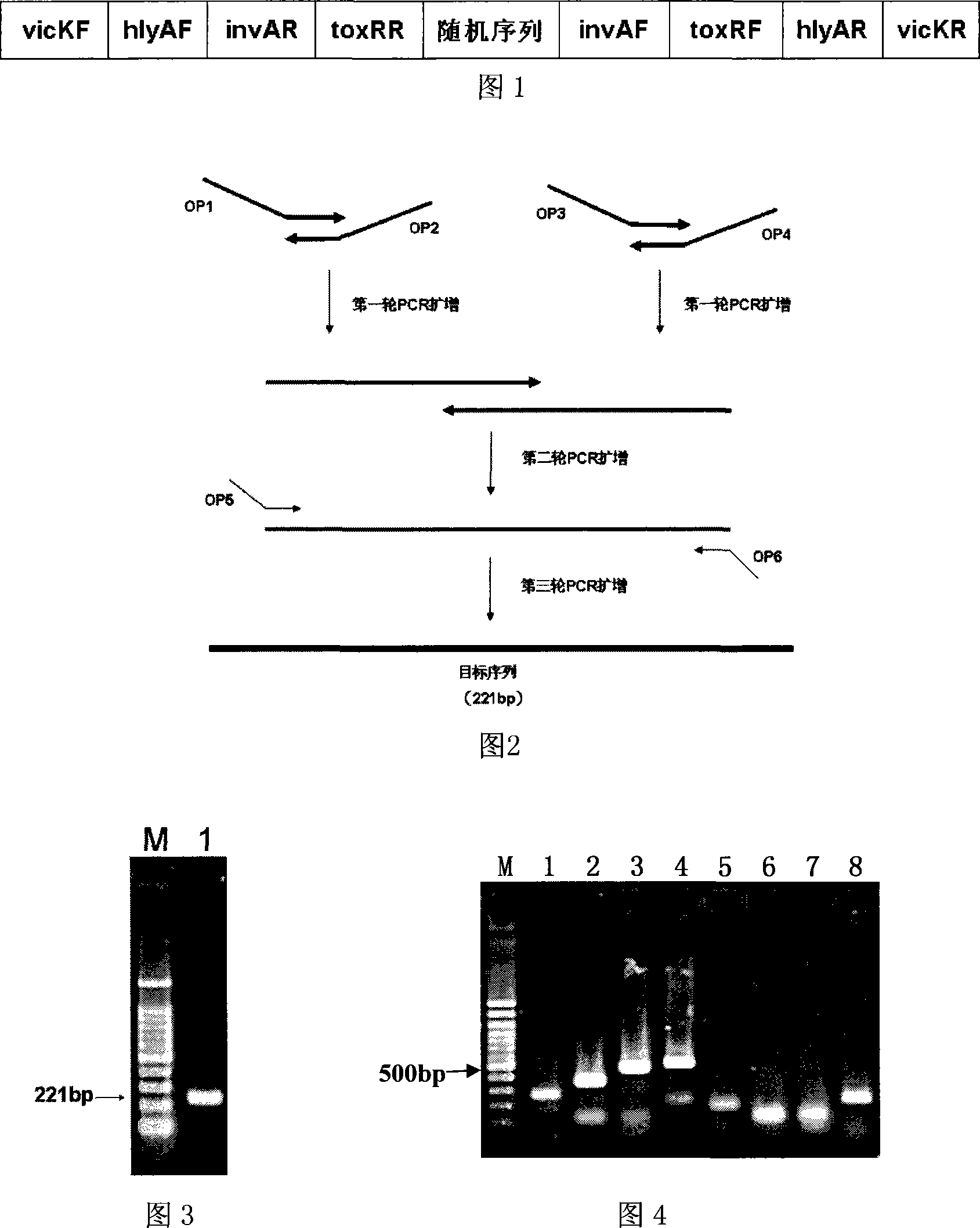
The invention relates to a preparation method of a multiple amplification internal standard used for the PCR detection of four bacteria of the technical field of food safety, the steps are that: a specific detection primer is designed according to a staphylococcus aureus vicK gene, a salmonella invA gene, a listeria monocytogene hlyA gene and vibrio parahaemolyticus toxR gene; the invention adopts an overlap PCR technology for assembling the sequence of the specific detection primer of the staphylococcus aureus vicK gene, the salmonella invA gene, the listeria monocytogene hlyA gene and the vibrio parahaemolyticus toxR gene into a nucleotide segment so as to construct the multiple amplification internal standard; the artificial sequence is cloned to a plasmid vector pMD-18T to constitute a plasmid pMD-mIAC which comprises the sequence of the multiple amplification internal standard; the multiple amplification internal standard is respectively verified in the detections of staphylococcus aureus, salmonella, listeria monocytogenes and vibrio parahaemolyticus. The invention can greatly reduce the detection cost and ensure the accuracy and the high efficiency of the detection at the same time.
Owner:SHANGHAI JIAO TONG UNIV
Wild ginseng and cultivated ginseng multiple polymerase chain reaction (PCR) test kit and identification method
InactiveCN102191308ASpecificity Accurate Simultaneous DistinctionThe identification method is simpleMicrobiological testing/measurementRibonucleosideOligonucleotide primers
The invention discloses a wild ginseng and cultivated ginseng multiple PCR test kit, which is characterized by containing: buffer solution, 12.5mM of deoxy-ribonucleoside triphosphate (dNTP), 0.1mM of one of a primer 1 and a primer 2, Taq DNA polymerase, a sample DNA to be tested and a double-distilled water identification reaction system; or the buffer solution, 12.5 mM of dNTP, 0.1 mM of one of the primer 1 and the primer 2, Taq DNA polymerase, wild ginseng and cultivated ginseng DNA 1:1 mixture and a double-distilled water positive reference reaction system; or the buffer solution, 12.5 mM of dNTP, 0.1 mM of one of the primer 1 and the primer 2, Taq DNA polymerase, araliaceae congeneric DNA 1:1 mixture, and a double-distilled water negative reference reaction system. The detection and identification method comprises the steps of designing two pairs of specific oligonucleotide primers of wild ginseng and cultivated ginseng mitochondrion DNAs, designing two pairs of specific oligonucleotide primers of synthetic wild ginseng and cultivated ginseng mitochondrion DNAs, determining a reaction process, determining result and the like. The method can accurately determine the specificity of both the wild ginseng and the cultivated ginseng, and the detection result is reliable.
Owner:BEIHUA UNIV
Watermelon anthrax bacteria detecting kit and its detecting method
InactiveCN1814804AHigh sensitivityPracticalMicrobiological testing/measurementSpecific testMicrobiology
This invention provides a primer combination of melon anthracins used in a specific test sample and a PCR test reagent box containing said primer combination and a method for testing melon anthracins.
Owner:EAST CHINA UNIV OF SCI & TECH
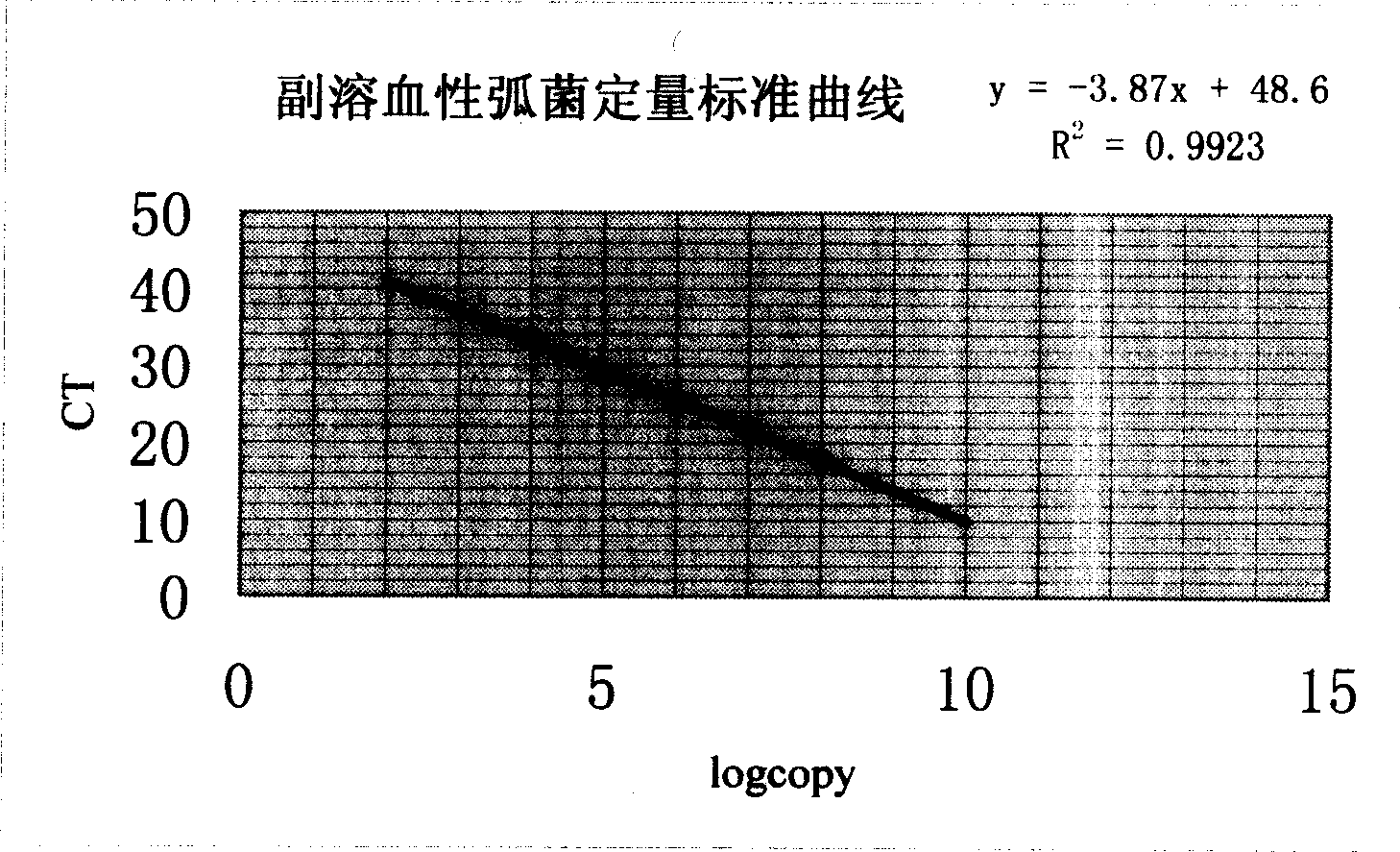
Quantitative PCR detecting kit and method for detecting vibrio parahaemolyticus thereof
InactiveCN1676613AOvercoming pollutionOvercoming technical issues with false positivesMicrobiological testing/measurementAgainst vector-borne diseasesMethod testFluorescence
This invention relates to a fluorescence quantitative PCR reagent box and a method testing vibro parahaemolyticus. The reagent box is composed of PCR reacting agent tube, the tube of sample treatment, the tube of positive comparison, the tube of negative comparison, the tube of standard product, the tube of Taq DNA polyose and the tube of dehydronium. The testing procedures are: first collect and treat the pending tested sample, then implement molecular quantitative PCR test, according the CT values of positive and negative sample comparisons and the CT of tested samples to judge whether the sample is positive or negative, then record the fluorescence value and CT value of each sample, and get the standard curve from the computer to calculate the copy's number of vibro parahaemolyticus of each sample. This invention has sealed-loop operation, avoids the fake positive, and the reaction and hybridization is synchronous, and the testing time is short.
Owner:SOUTHERN MEDICAL UNIVERSITY
PCR kit for testing banana apical tuft virus and its test method
The present invention relates to microbe detecting composition and method, and is especially PCR kit and test method of testing banana apical tuft virus. The PCR test kit includes primer, dNTPs, buffering solution and DNA polymerase, and its upstream primer is 5-CTC GTC ATG TGC AAG GTT ATG TCG-3' and downstream primer is 5'-GAA GTT CTC CAG CTA TTC ATC GCC-3'. The PCR test kit is easy to produce and the test method of testing banana apical tuft virus has high sensitivity and high repeatability and is simple and practical. In detecting coarsely purified virus liquid, the method can detect banana apical tuft virus equivalent to the amount of 1 ng of banana tissue in high test precision and relatively low test cost.
Owner:福建省农业科学院生物技术中心
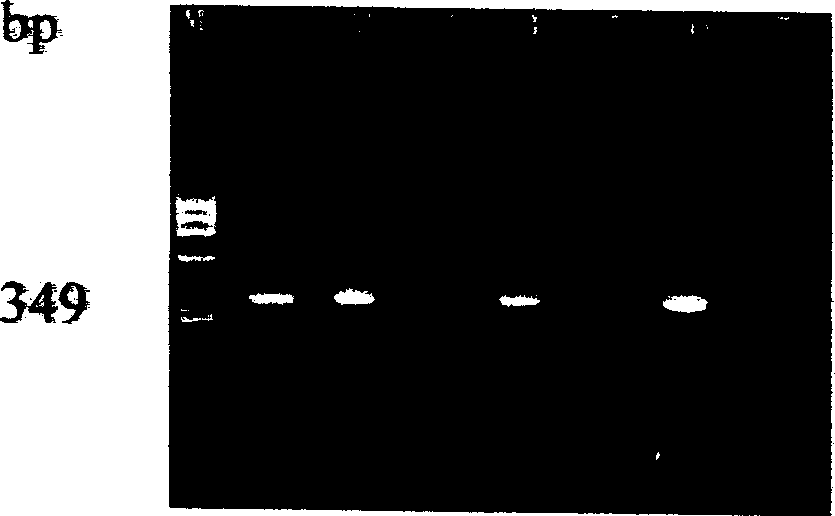
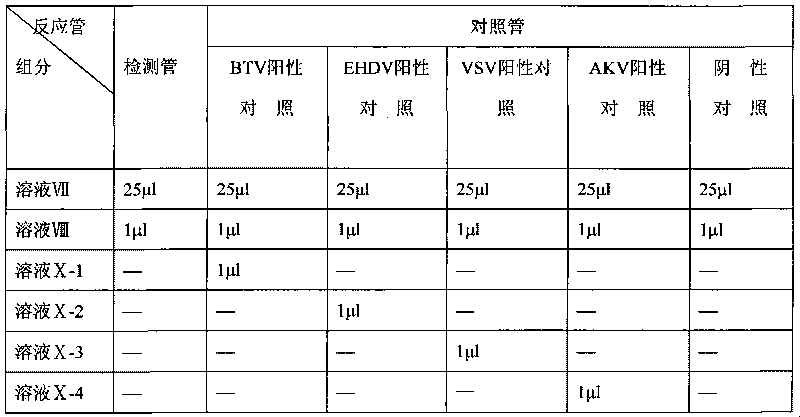
Animal insect-borne disease multi-RT-PCR distinguishing and detecting reagent as well as preparation method and application
InactiveCN101724712AQuick checkEfficient detectionMicrobiological testing/measurementMicroorganism based processesAgricultural scienceEpizootic haemorrhagic disease virus
The invention relates to the technical field of biology, in particular to a reagent which can simultaneously distinguish and detect four animal insect-borne diseases as well as a preparation method and application of the reagent. The animal insect-borne disease multi-RT-PCR distinguishing and detecting reagent comprises four pairs of specific primers, and the respective amplification target fragment lengths of Bluetongue virus (BTV), epizootic haemorrhagic disease virus of deer (EHDV), Vesicular stomatitis virus (VSV) and Akabane virus (AKV) are 351bp, 536bp, 300bp and 250bp. The VP7 of the BTV, the EHDV, the VSV and the AKV and a conservative fragment of an N gene are respectively selected as targets, and the primer Express software and the primer prere 5.0 software are applied to deign and combine a primer. An optimal matching and screening test and a multi-RT-PCR test are carried out on a plurality of pairs of designed primers, and four pairs of primers which can carry out the distinguishing and detection on the four animal insect-borne diseases and have high amplification efficiency and good specificity can be obtained by a plurality of reaction condition optimization and comparison tests and verification tests. A kit formed by the reagent can obtain a qualitative distinguishing and detecting result in six hours after a sample is received. The multi-RT-PCR distinguishing and detecting reagent is a sensitive and reliable method for detecting the BTV, the EHDV, the VSV and the AKV in the clinical sample.
Owner:花群义
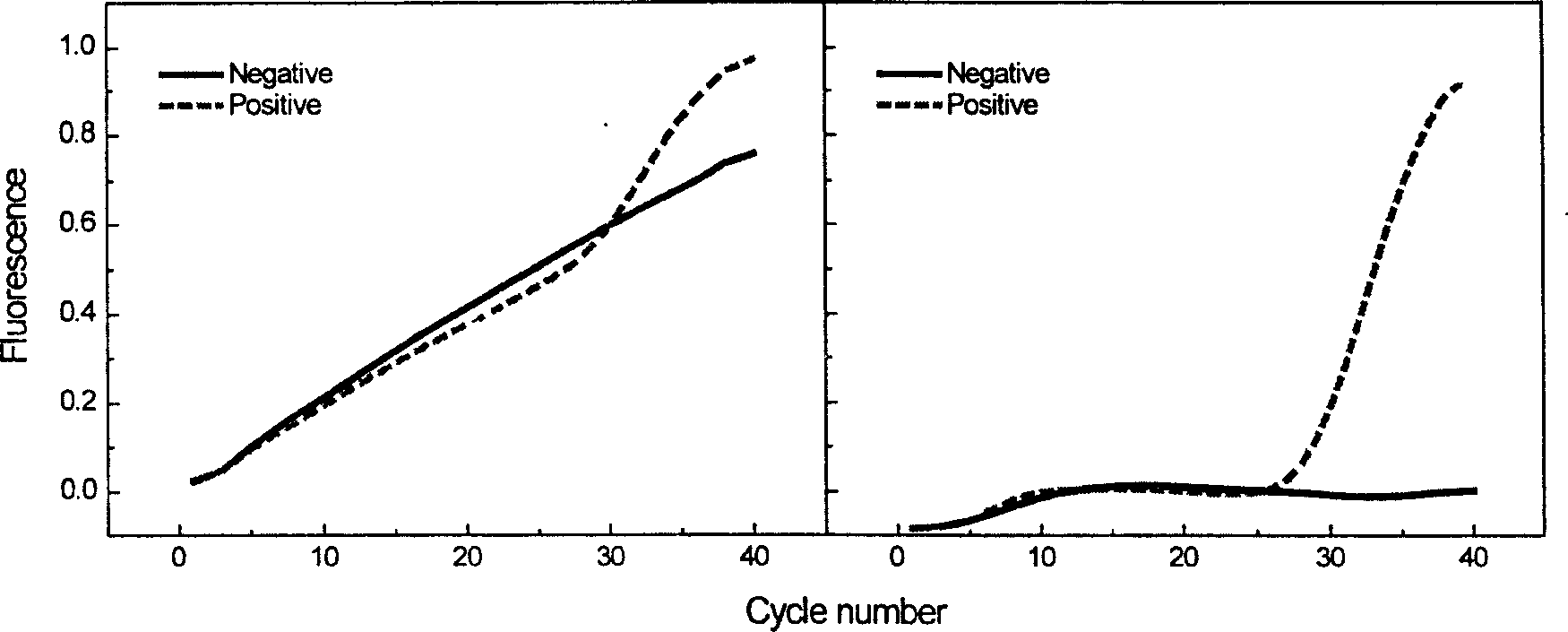
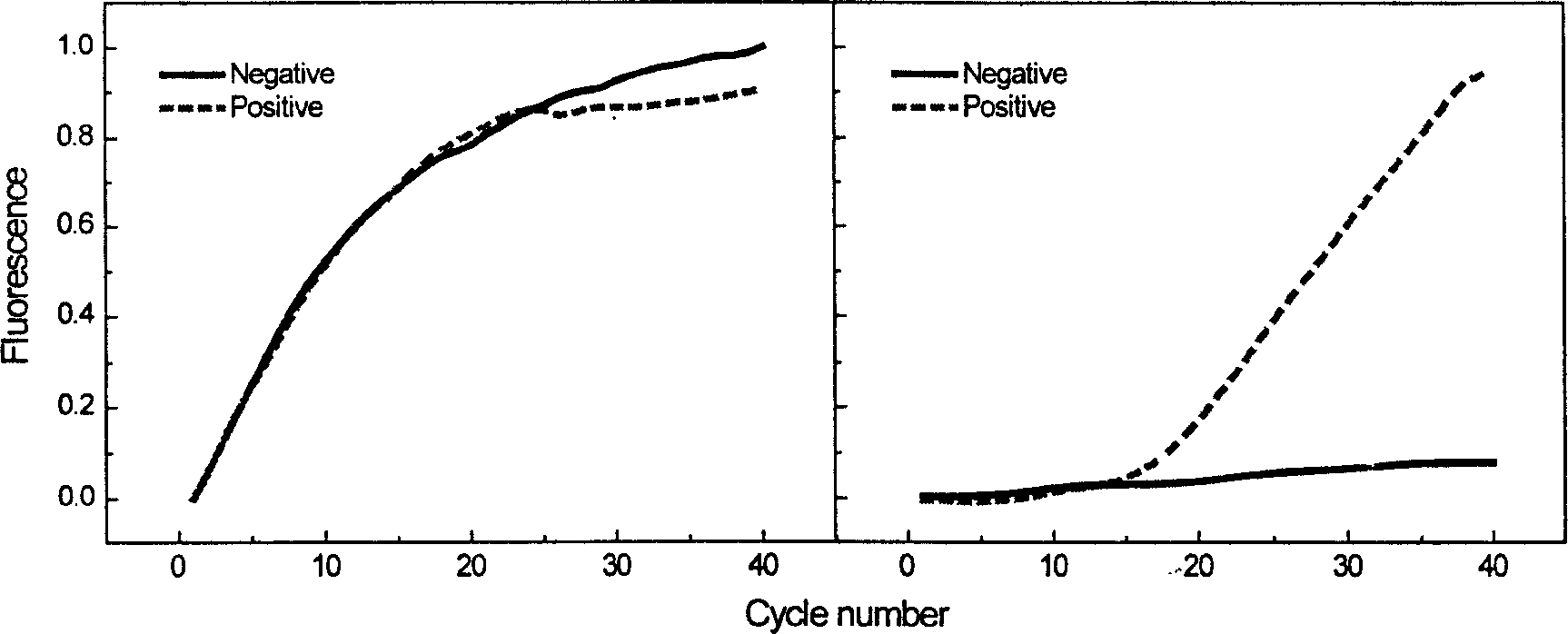
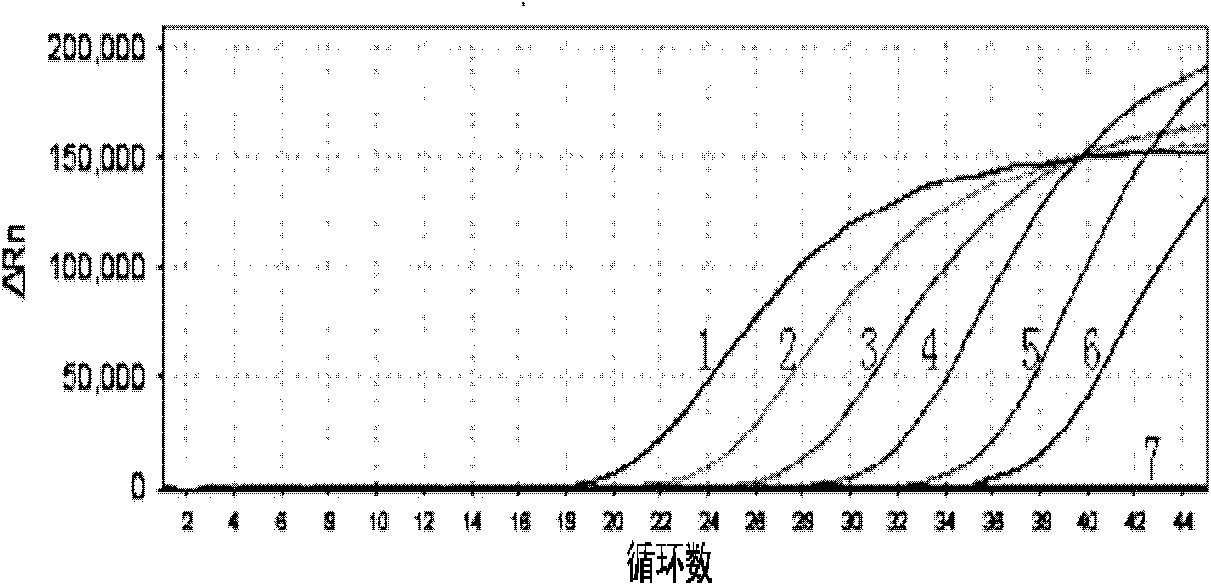
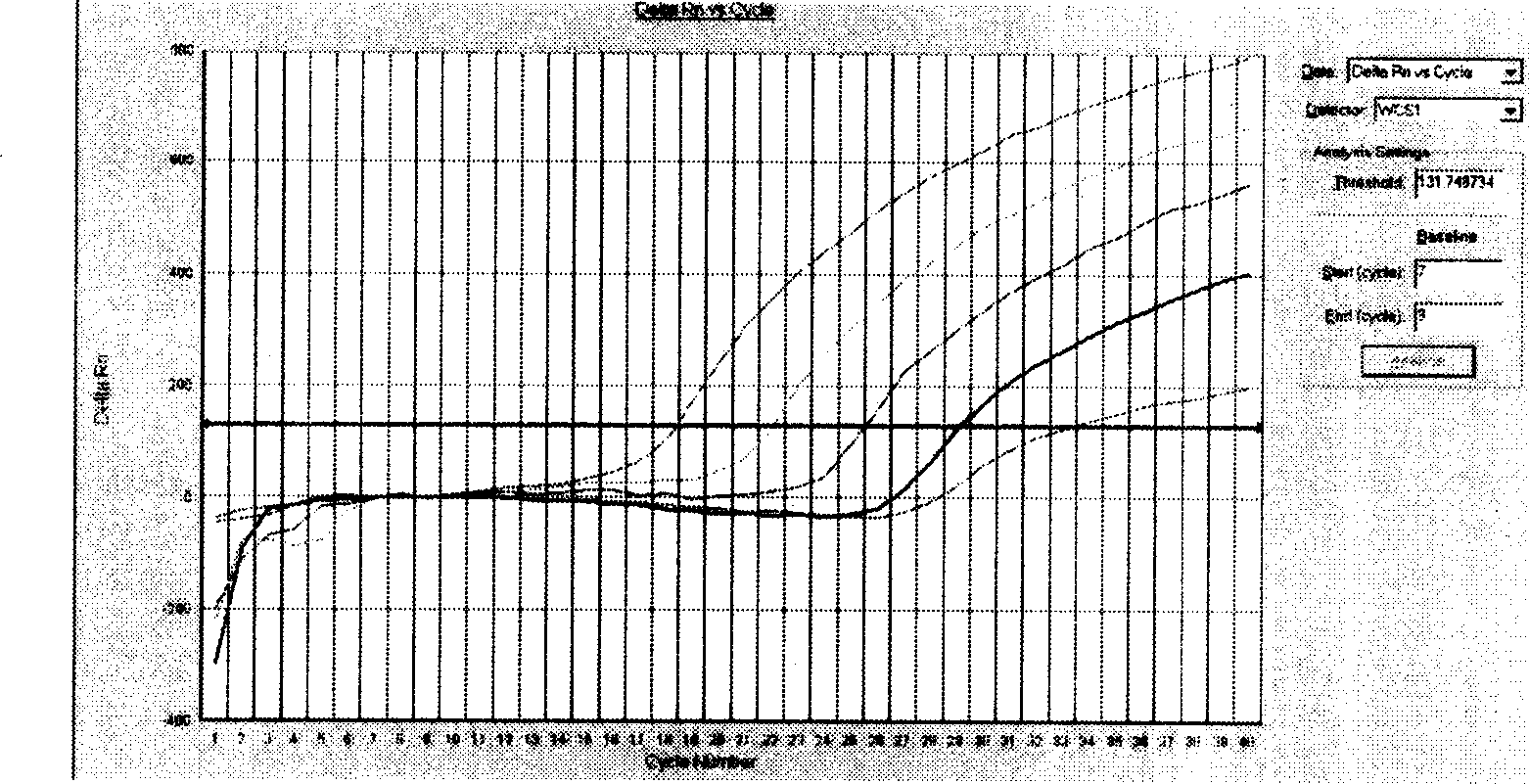
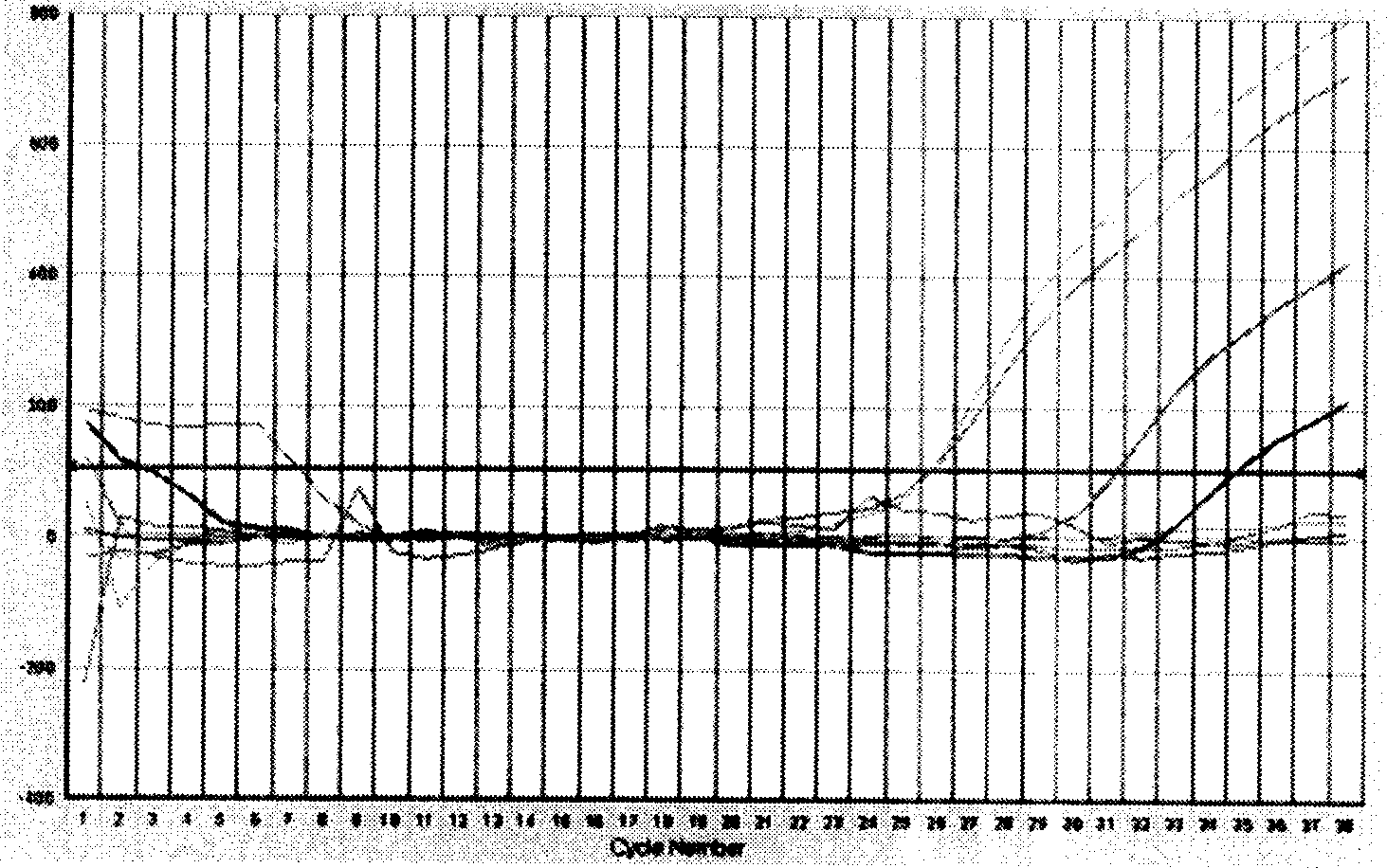
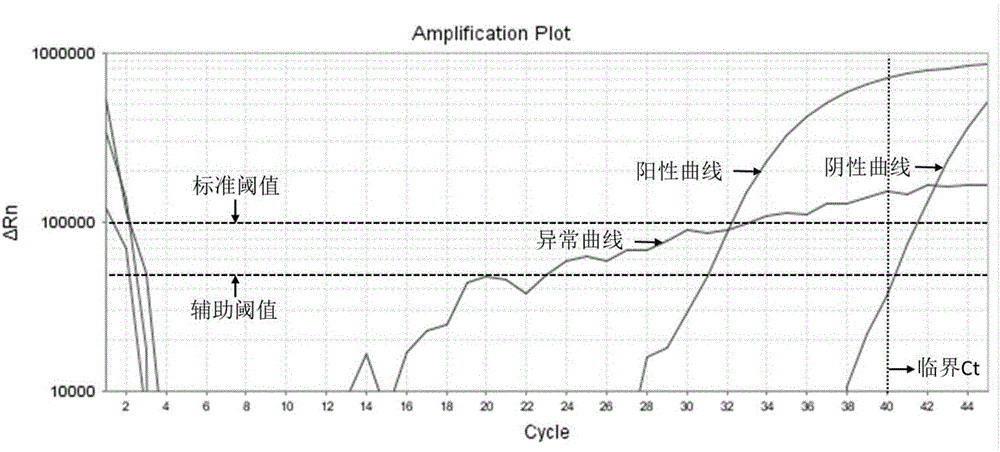
Method for automatically and quickly judging fluorescent quantitative PCR (Polymerase Chain Reaction) result
InactiveCN105755159AQuick DiscriminationAccurate discriminationMicrobiological testing/measurementComputer scienceBaselining
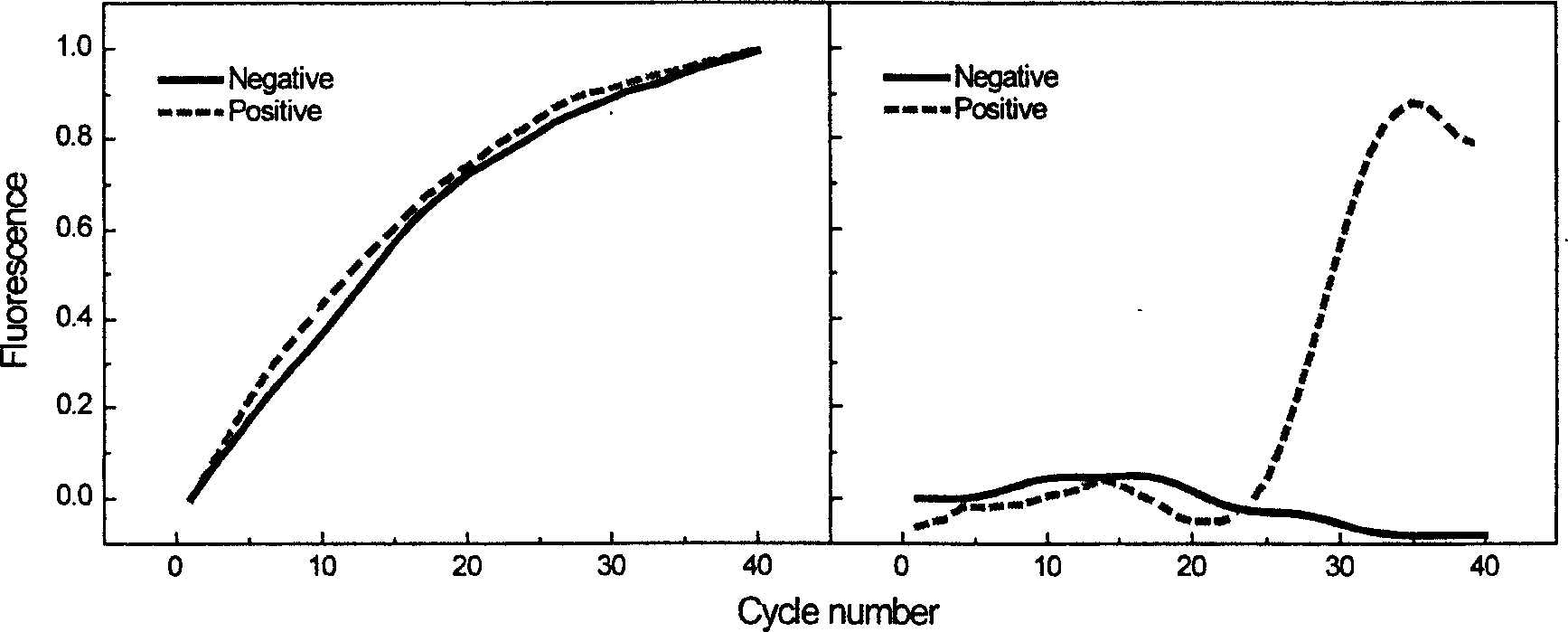
The invention relates to a method for automatically and quickly judging a fluorescent quantitative PCR (Polymerase Chain Reaction) result. The method comprises the steps of extracting a data curve of a fluorescent quantitative PCR; performing baseline setting on the data curve; setting a standard threshold of the fluorescence intensity during an exponential rise period of the data curve, wherein a cycle number corresponding to the standard threshold is Ct1; setting an auxiliary threshold of the fluorescence intensity during the exponential rise period of the data curve, wherein a cycle number corresponding to the auxiliary threshold is Ct2 and the standard threshold is not equal to the auxiliary threshold; establishing a scoring formula z (z=(Ct1-Ct2) / (log(the standard threshold, 2)-log(the auxiliary threshold, 2)); and if z is more than 0 and less than (1 / E)+1, judging that the data curve is a normal curve, or otherwise, judging that the data curve is an abnormal curve, wherein E is a fluorescent quantitative PCR efficiency value. By designing a brand-new identification method, fluorescent quantitative PCR test data can be quickly and accurately judged, so that the judgment speed of detection data is increased and the judgment accuracy of the detection data is improved.
Owner:GWP BIOTECHNOLOGIES INC
PCR detection kit for fruit tree main virus and using method
InactiveCN101016571AMicrobiological testing/measurementDNA/RNA fragmentationFruit treeRNA extraction
The invention discloses a PCR check agent case of fruit tree main virus and using method in biological technical domain, which is characterized by the following: composing by RNA extracting agent and PCR checking agent; utilizing PCR know-why; basing on agent constitution of scientific research optimism; integrating; getting the product; using to PCR test of apple chlorosis leaf speckle virus, apple micropteres virus, apple mosaic virus, plum genus necrosis ring speckle virus, grape leaf folding virus and plum dwarf virus; fitting-in PCR device and high speed refrigerated centrifuge. This invention possesses merits of high sensibility, simple operation and short time, which can be adopted by inspection department, high school and scientific research institute.
Owner:DALIAN UNIV
C. andersoni PCR detecting kit
This invention provides a PCR test reagent box for diagnosing and testing An- masked sporidiosis, which is prepared by designing specific primers according to the ITS-1 gene sequence of the masked sporidiosis, setting up PCR test method and optimizing the PCR reaction condition. This invention also provides a method for using the box.
Owner:SOUTH CHINA AGRI UNIV
Method for agrobacterium mediated gene conversion of grass sorghum
InactiveCN101319226ASolve the problem of not being able to obtain resistant callusPromote differentiationVector-based foreign material introductionPlant genotype modificationTransformation efficiencyAntibiotic Y
The invention relates to a method for transforming Sudan grass through an agrobacterium mediating gene, which belongs to the biotechnology field. When the agrobacterium mediating method is used to transforming exogenous gene of Sudan grass, a faint yellow granular callus which is obtained from the in-vitro culture and induction of a Sudan grass young ear is taken as acceptor material; after the infection and co-culturing of the agrobacterium , the acceptor material undergoes 10 to 20mg / L kanamycin resistance screening to obtain a resistance callus in a subculture medium of carbenicillin with attached antibiotics of 500mg / L; the resistance callus is translated into a differentiation culture medium of the carbenicillin with the attached antibiotics of 500mg / L to obtain a regenerated plantlet; the plantlet is cultured to have sound seedling and root and survives after transplantation; and a transgenic plant is obtained after the plant passes a PCR test. The Sudan grass genetic transformation method established by the invention has a transformation rate of more than 3 percent, thereby laying the foundation for carrying out the transgenic breeding of the multi-purpose Sudan grass through the agrobacterium mediating method.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
H5, H7, H9 subtype auian flu virus real-time fluo rescent quantixative PCR detecting method
InactiveCN100378230CStrong specificityGood linear relationshipMicrobiological testing/measurementFluorescenceBio engineering
This invention relates to a real time fluorescence quantitative PCR test method for H5, H7 and H9 sub-avian flu viruses including the following steps: set-up of standard molecules, the design of specific virus quantitative PCR primer and the probes, pick-up of virus sample RNA, the inverse transcription polyenzyme chain reaction to the pic-up sample RNA to get a sample cDNA, the optimization and set-up of the PCR quantitative test method for M genes.
Owner:SHANGHAI JIAOTONG UNIV
Primer and fluorescent probe for detecting sulfate reduction bacterial
InactiveCN101089195AStrong specificityReduce false positivesMicrobiological testing/measurementBiological testingMicroorganismFluorescence
The upstream pimer for testing sulfate reducing bacterium is 5-CGCTGAAATGACCATGATGG-3, and its downstream primer is 5-CGCGCATTTCACGAAGC-3. The fluorescent probe is composed of reporter fluorescent group, quenching fluorescent group and probe gene sequence, which being of 5-CTGAAGCCTTACGAAGATCG-3. Said 5 end of probe gene sequence labels that reporter fluorescent group is FAM; and said 3 end of probe gene sequence labels that quenching fluorescent group is TAMRA. This invented primers and fluorescent probe have advantages: high specificity; short-term testing of sulfate bacterium for fluorescent quantitative PCR test, and needing only 6 Hrs. after DNA extraction and fluorescent quantitative PCR test.
Owner:HARBIN INST OF TECH
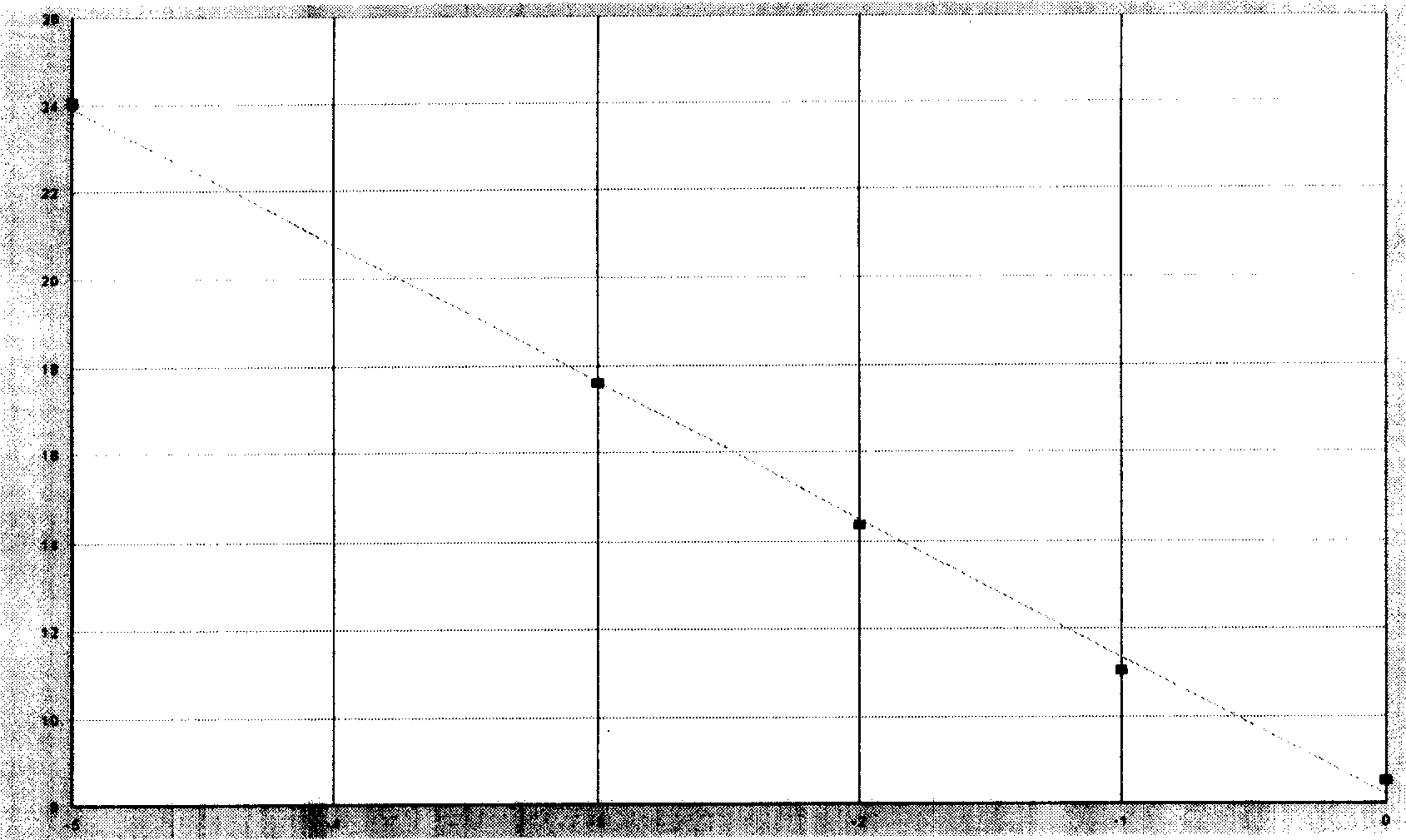
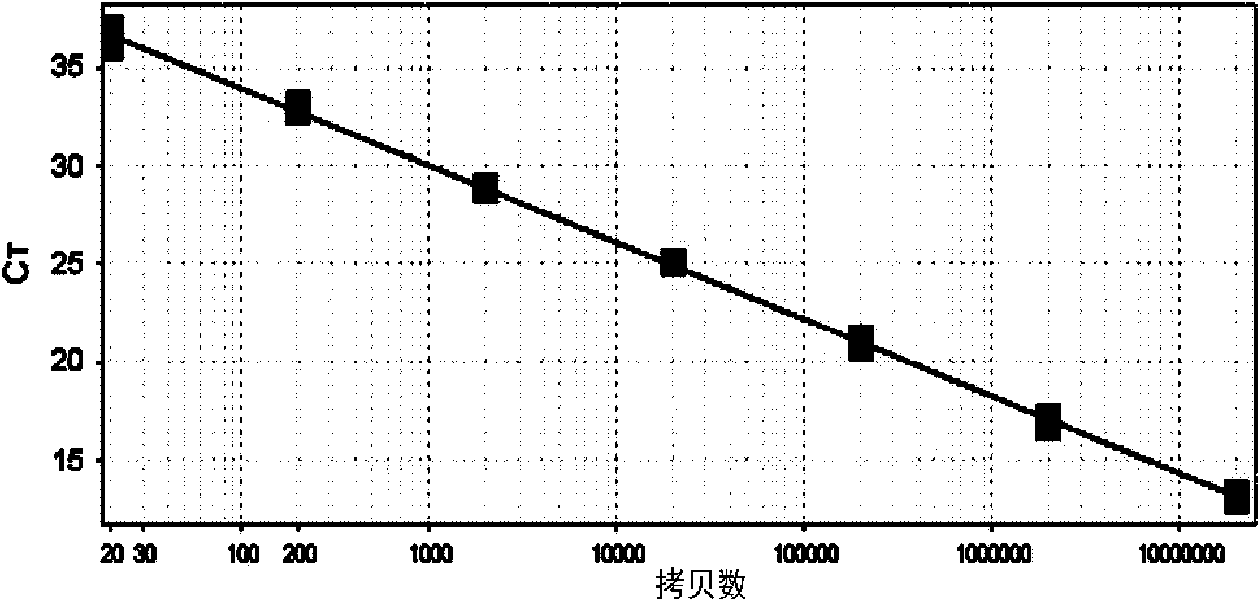
Real-time quantitative fluorescence PCR test method based on double external references of RNA and DNA and application thereof
ActiveCN101538611AIntegration and applicationImprove experimental designMicrobiological testing/measurementFluorescence/phosphorescenceExternal referenceFluorescent pcr
The invention relates to the field of bioinformatics, in particular to a real-time quantitative fluorescence PCR test method based on double external references of RNA and DNA and an application thereof, comprising the following steps: selecting external reference genes, preparing mRNA and DNA of the external reference genes, preparing premix of the mRNA and DNA of the external reference genes; adding the premix while extracting the samples, collecting the general mRNA and DNA part in the samples; carrying out real-time quantitative fluorescence PCR and calculating copy number of genes to be tested and mRNA and DNA of the external reference genes; carrying out data processing and normalization. The invention also discloses the application of the method in the technology that gene chips are used to analyze gene expression. The invention improves experimental design work on testing of genetic transcription level, which results in obtaining the true genetic transcription level of a certain gene instead of the relative level thereof, thus providing new concepts and frameworks for integration of gene expression data and gene copy data.
Owner:上海市生物医药技术研究院
Fluorescent quantitative PCR reagent kit for detecting epidermal growth factor receptor gene point mutation
InactiveCN1884580ASimple qualitative testShort detection timeMicrobiological testing/measurementBiological testingFluorescenceBiology
This invention belongs to the moleculal biology field. It involves a kind of fluorescent quantitive PCR test kit of detection gene mutations. The test kit includes fluorescent quantitive PCR premixed liquid, fluorescent quantitive reaction liquid A, luorescent quantitive reaction liquid B (the feature is that it contains primer and anti-primer and fluorescence probes), positive comparison sample A, positive comparison sample B, positive comparison sample C. This invention features that: short detection time, easy operation, low quality requirement of sample DNA, the result reading is clear, direct with high sensitivity.
Owner:邵建永
Hepatitis E virus fluorescent quantitative PCR detection method
InactiveCN101122563AHigh sensitivityStrong specificityMicrobiological testing/measurementMaterial analysis by optical meansFluorescenceTiter
The invention relates to a test method in the bioengineering field, in particular to a fluorescence quantitative PCR test method used for quantitatively test the titer of Hepatitis E virus (HEV) in a clinical specimen. The steps of invention include the design of virus specific quantitative PCR primers and probes, the construction of standard molecular, the extraction of a virus sample RNA, the cDNA synthesis of the sample RNA and the establishment and optimization of the fluorescence quantitative PCR test method. The testing sensitivity of the invention can reach to 10 degrees magnitude virus copy. The invention is equipped with good stability and specificity, which can be used for mass samples test and is capable of precisely measuring the virus load of the sample.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Quantitative fluorescent multiplex PCR test kit for Epstein-Barr virus, and application thereof in nasopharyngeal carcinoma screening
InactiveCN102839222ATo achieve the purpose of high-throughput screening NPCAccurate detectionMicrobiological testing/measurementFluorescence/phosphorescenceMultiplexPromoter
The invention relates to a quantitative fluorescent multiplex PCR test kit for Epstein-Barr virus, and the kit comprises the following reagents: a, specific primer pairs for amplifying mutations of key target genes in the Epstein-Barr virus; and b, specific probes for the mutations of key target genes in the Epstein-Barr virus, wherein the mutations are P-thr, V-val and V-leu in the coding region of EBNA-1 gene, promoters Cp, Fp and Zp of the EBNA-1 gene, and XhoI-loss, 30bp Deletion and Ser 366 Thr of LMP1 gene. The test kit is advantageous in that: the aim of precise prediction of the risk of nasopharyngeal carcinoma in high risk population can be achieved; the main carcinogenic mutations can be well known for the combination of population screening and population intervention for nasopharyngeal carcinoma; and the kit has the advantages of high in sensitivity, fast in test, and low in cost, having broad market prospect.
Owner:广州市第十二人民医院
Inverse probe for visually detecting single-nucleotide polymorphism site in gene sequence, kit and detection method thereof
ActiveCN106520948ASimple resultEasy to synthesizeMicrobiological testing/measurementDNA/RNA fragmentationStatistical analysisTesting tubes
The invention discloses an inverse probe for visually detecting a single-nucleotide polymorphism site in a gene sequence, a kit and a detection method thereof. The inverse probe comprises a sequence H1, a sequence H2, a sequence P1 and a G-four strobile structure; the sequence H1 is an inverse complementary sequence at the end of a sequence SNP site 5' to be detected; the sequence H2 is an inverse complementary sequence at the end of the sequence SNP site 3' to be detected; the sequence P1 is an inverse complementary sequence of a universal primer of the sequence to be detected; and the G-four strobile structure is an inverse complementary sequence of a G-four strobile gene of the sequence to be detected. According to the inverse probe disclosed by the invention, the result is simple and synthesis is convenient; the detection of the SNP site can be completed in a single PCR test tube; a detection result shows that the result can be judged via color observation with eyes; according to the detection method, the operation is easy, the detection is convenient, the use cost is low and the detection method is suitable for outdoor, field and site direct detection and species identification, so that statistic analysis of population heredity is realized.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com