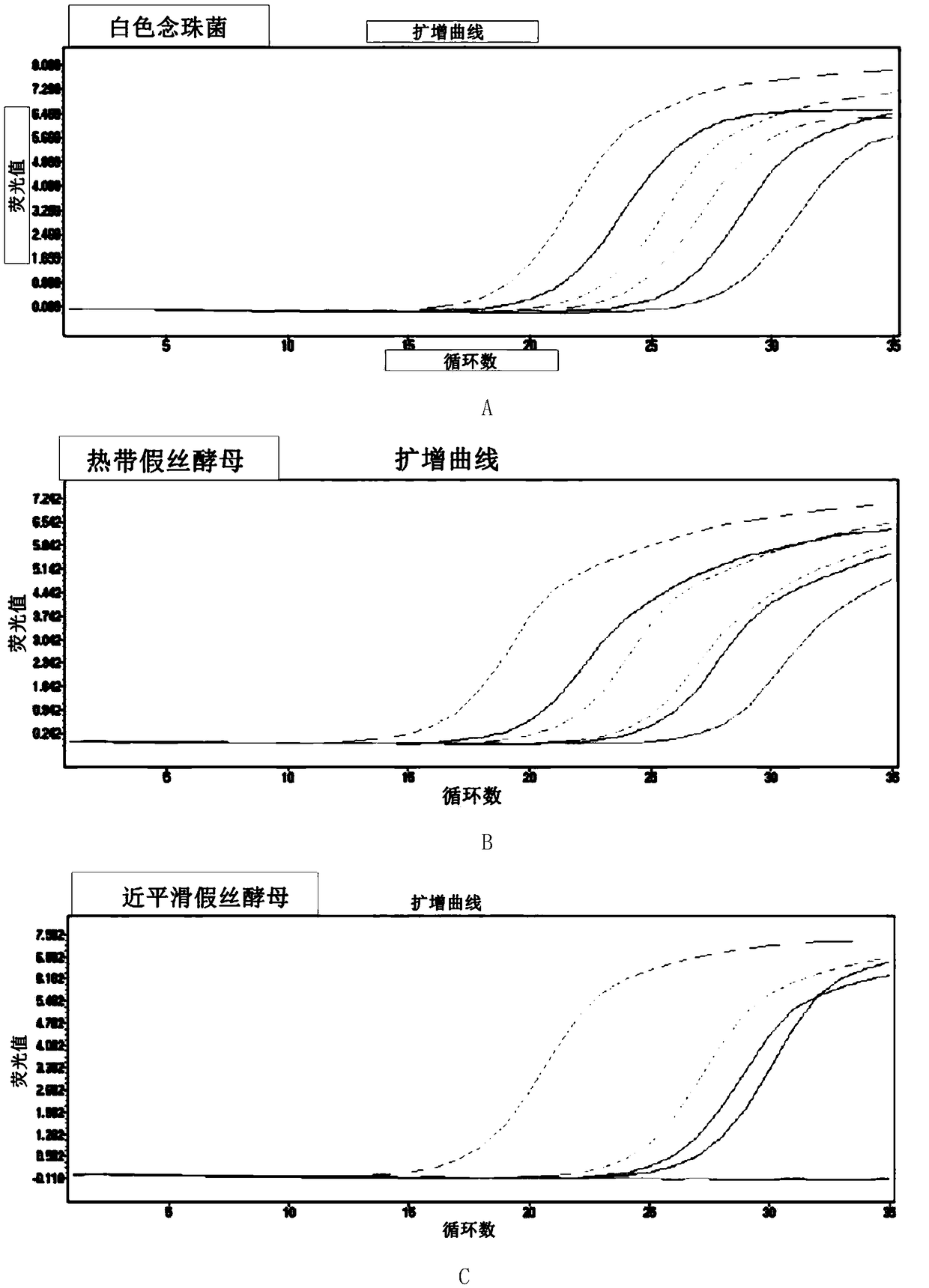
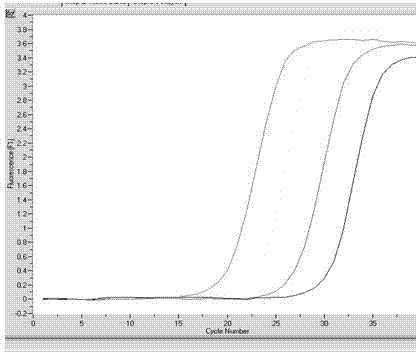
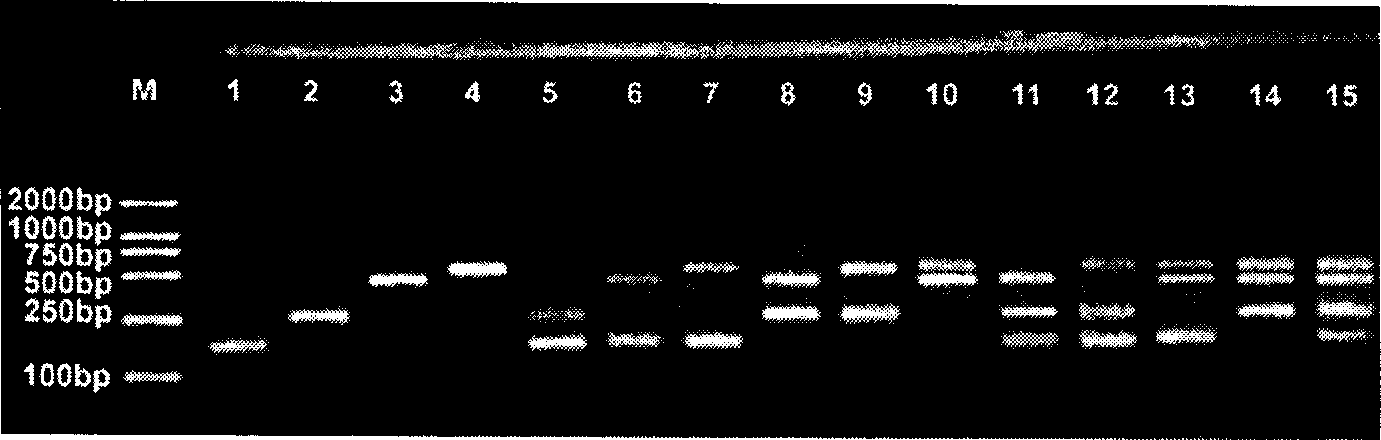
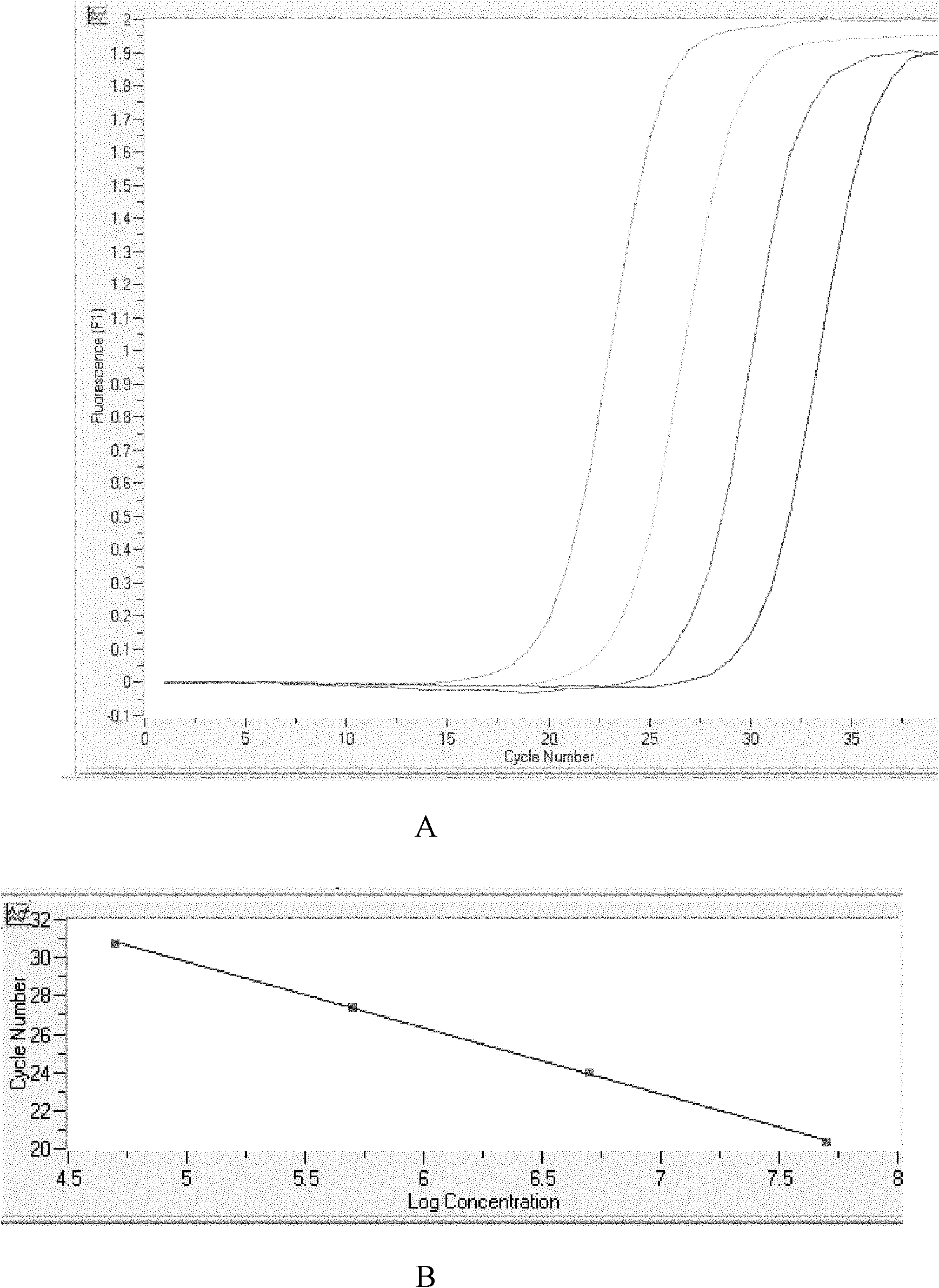
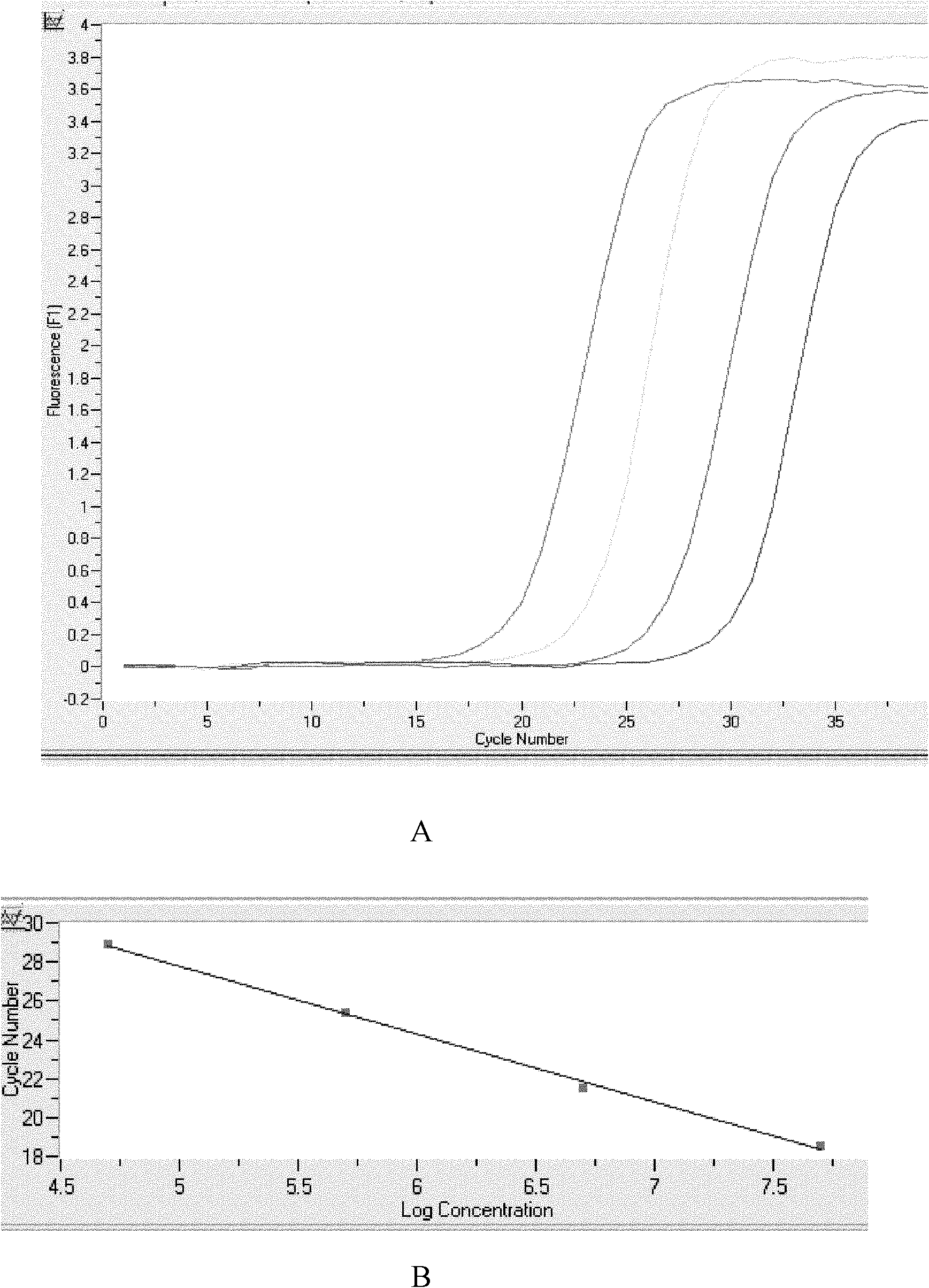
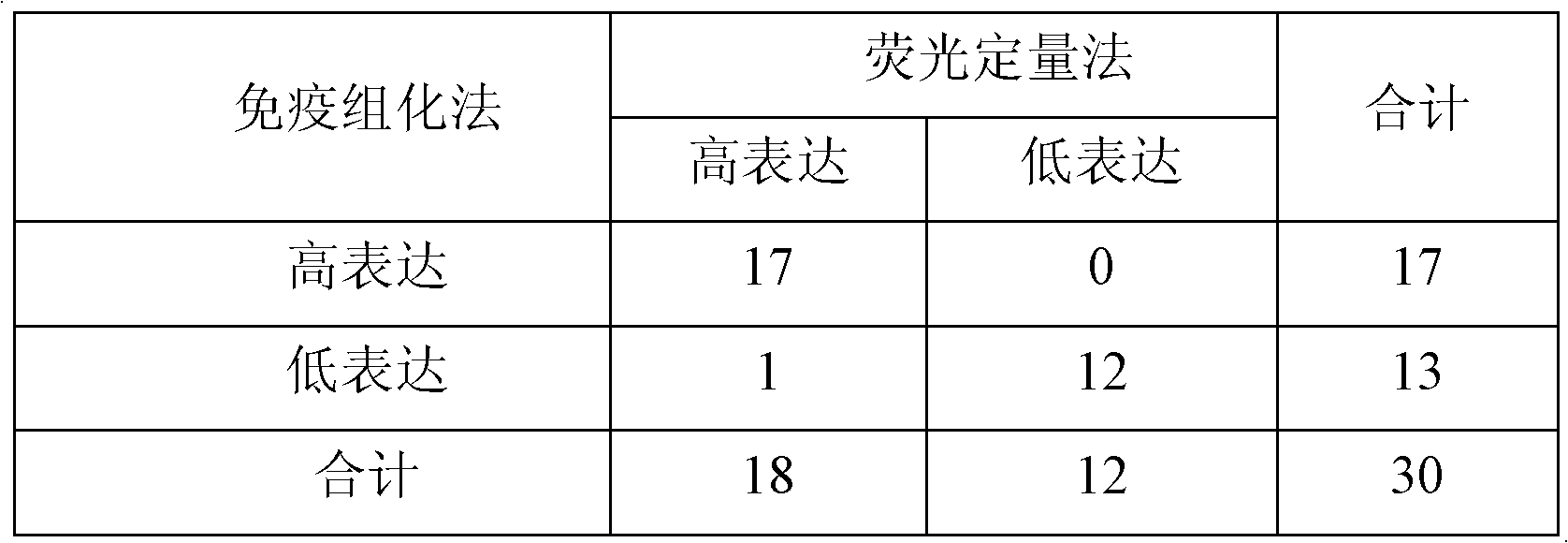
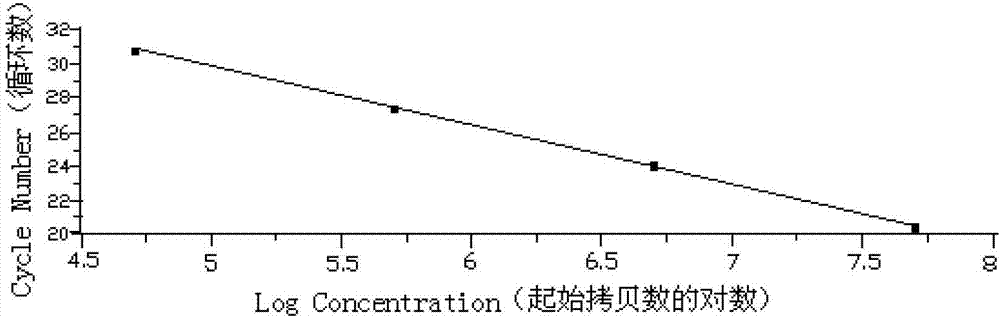
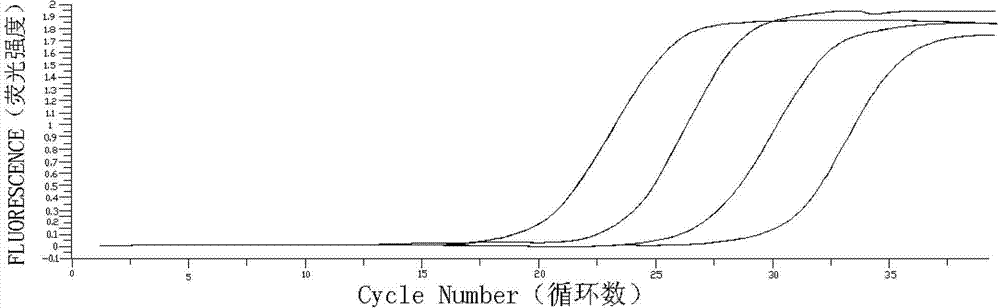
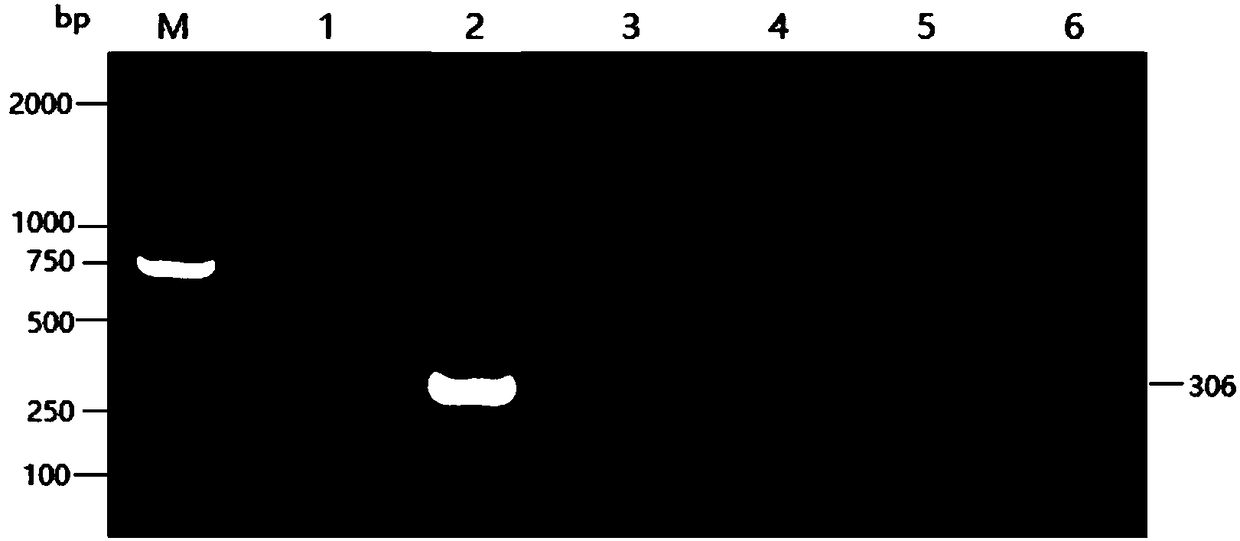
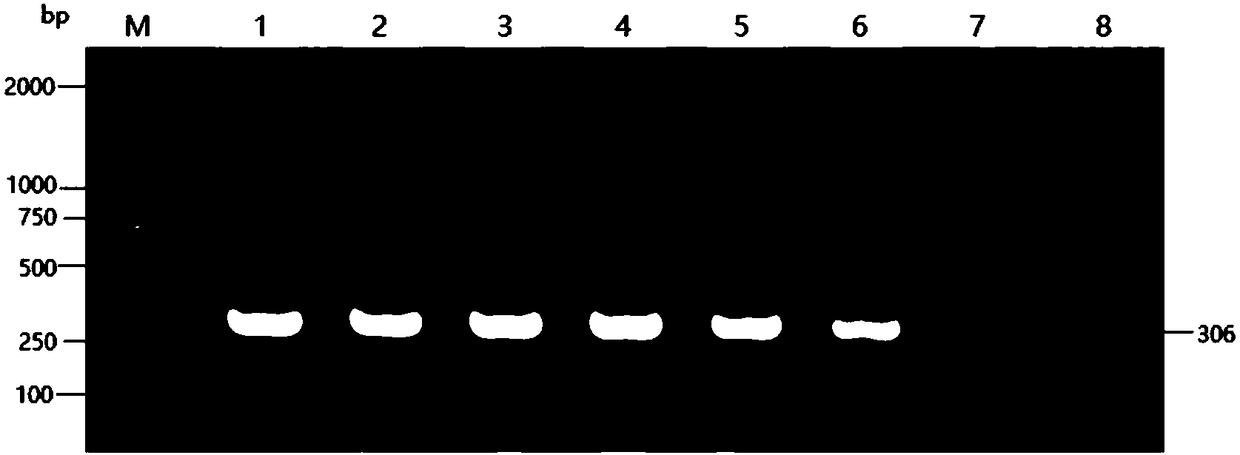
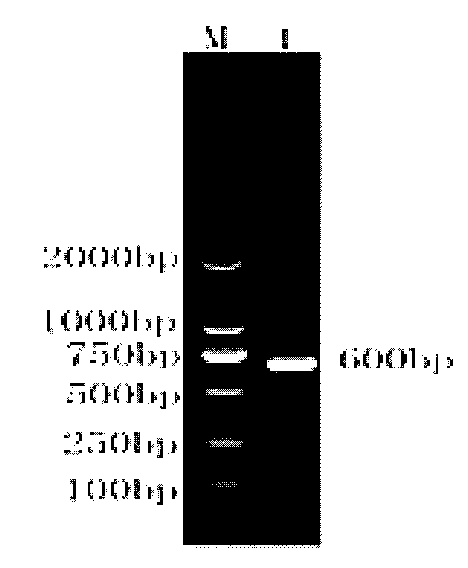Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Pcr diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
PCR tests diagnose calicivirus infections present in the sample by detecting the genetic sequence of certain strains of bacteria. This method of testing is considered more effective in comparison to antibody tests, as diagnosis doesn't depend on increased levels of antibodies in the blood.
High-throughput diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
InactiveUS7547512B2Rapid and reliable diagnostic assayHigh detection sensitivitySugar derivativesMicrobiological testing/measurementPresent methodSevere acute respiratory syndrome
The present invention relates to a high-throughput diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a high-throughput reverse transcription-PCR diagnostic test for SARS associated coronavirus (SARS-CoV). The present assay is a rapid, reliable assay which can be used for diagnosis and monitoring the spread of SARS and is based on the nucleotide sequences of the N (nucleocapsid)-gene of the hSARS virus. The present method eliminates false negative results and provides increased sensitivity for the assay. The invention also discloses the S (spike)-gene of the hSARS virus. The invention further relates to the deduced amino acid sequences of the N-gene and S-gene products of the hSARS virus and to the use of the N-gene and S-gene products in diagnostic methods. The invention further encompasses diagnostic assays and kits comprising antibodies generated against the N-gene or S-gene product.
Owner:VERSITECH LTD
Variant porcine reproductive and respiratory syndrome virus (PRRSV) TaqMan fluorescence quantitative RT-PCR detecting kit and application thereof
InactiveCN101736094AGuaranteed specificityStrong specificityMicrobiological testing/measurementMicroorganism based processesHighly pathogenicFluorescence
The invention discloses variant porcine reproductive and respiratory syndrome virus (PRRSV) TaqMan fluorescence quantitative RT-PCR detecting kit and application thereof. A primer and a TaqMan probe are designed and synthesized by referring to an NSP2 fragment gene sequence of the variant PRRSV and common PRRSV of a GenBank. By optimizing the reaction condition and constructing a standard plasmid product, a method for diagnosing the variant PRRSV by TaqMan fluorescence quantitative RT-PCR is established. A result indicates that the method has the advantages of strong specificity, high sensitivity, and the like and can detect the standard plasmid product with 264 copy numbers, and the virus quantity of 0.5623TICD50 is 10 times more sensitive than RT-PCR. By detecting 22 disease samples, 8 disease samples are positive, and the positive rate is 36.4 percent. Because the method has the advantages of quantification, high speed, accuracy, sensitivity, and the like, the invention is suitable for the diagnosis on the swinery infected variant PRRSV in the early stage, the medium stage and the later stage and plays an important role in effectively diagnosing, preventing and treating the highly pathogenic PRRSV.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +6
Preparation method for PCV-II Cap protein monoclonal antibody, antibody and application
InactiveCN101768218AAvoid distortionThe ability to secrete antibodies is strong and stableImmunoglobulins against virusesFluorescence/phosphorescenceBALB/cIndirect elisa
The invention discloses a preparation method for a PCV-II Cap protein monoclonal antibody, an antibody and application. The invention adopts ultracentrifuged and purified PCV-II as an immunogen to immunize a BALB / c mouse by the conventional method, takes spleen cells of the immunized BALB / c mouse to fuse with SP2 / 0 cells, obtains two strains of hybridoma cells secreting the PCV2-Cap protein monoclonal antibodies by indirect ELISA screening, respectively names the two strains of hybridoma cells as 8-60 and 10-48, identifies biological characteristics of the two strains 8-60 and 10-48, and usesthe two strains 8-60 and 10-48 as the first antibodies to establish an indirect immunofluorescence diagnostic method. The result of the indirect immunofluorescence diagnostic method is basically consistent with that of the PCR diagnostic method, and the positive and negative coincidence rates are respectively 93.75 percent and 100 percent so as to provide reference for preventing and treating theporcine circovirus disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +7
Detection method of invasive fungus infection, detection kit and application
ActiveCN109055502ASimplify detectionSimplify operabilityMicrobiological testing/measurementDNA/RNA fragmentationCell freeFluorescence
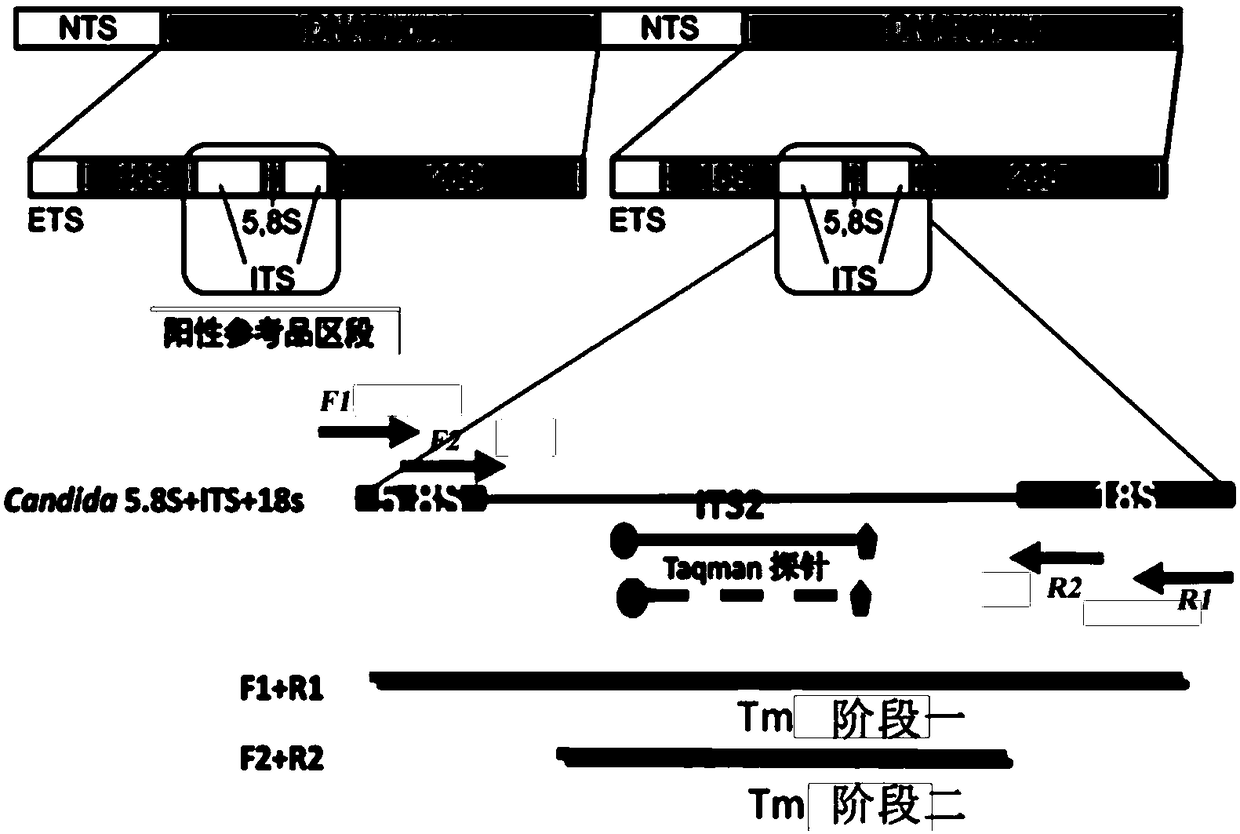
The invention provides a fast multiplex PCR identification diagnosis detection method of invasive fungus infection based on cfDNA (cell free DNA). The method can be used for identifying the invasive infection caused by clinic common and high-incidence Candida albicans, Candida tropicalis, Candida parapsilosis, Candida krusei, Candida glabrata and Aspergillus fumigtus. An amplification primer is designed according to characteristic genome segments of each fungus category and species; a detection fluorescence probe of a strain can be distinguished according to the amplification segment design; the real time PCR can be performed on a sample to be tested; high sensitivity of nested PCR and high specificity and multi-target performance advantages of multiple fluorescent hybrid probe PCR are integrated for identifying the fungus strain. The invention also provides a PCR diagnosis kit for the invasive fungus infection and application thereof.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
Hepatitis B virus (HBV) fluorescent quantificationally PCR detecting kit
InactiveCN101158634AMicrobiological testing/measurementMaterial analysis by optical meansHepatitis B virusTarget gene
The present invention relates to a real time fluorescence metered PCR test way of HBV and a reagent kit. The present way adopts alkali cracking to extract HBV DNA, gets and adds a portion of the trace lysate directly into PCR reaction liquid for augmentation after cracking procedure; the reaction liquid includes a pair of specificity primers and a probe marked with fluorescent dye, PCR method is applied to augment target genes, the intensity of the reaction system is real-time tested, and Ct value is calculated according to analysis software as well as the virus content value of specimen. Under the conditions that sensitivity and accuracy are guaranteed, the reagent kit of the present invention can be operated easily and fast, thus reducing the labor intensity of clinical test and the cost of clinical PCR diagnosis at the same time, and continuing the rapidness and easiness function of fluorescence metered PCR test way. The way which is suitable for the clinical HVB metered test can be used as an assistant diagnosis way of HBV infection and a monitor mean of clinical treatment effect in a clinical lab, has comparatively low requirements of test operator, and possesses practical clinical application value.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Kit for rapid detection of mRNA expression level of BRCA1 gene
InactiveCN102312002AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceBrca1 gene
The invention relates to a fluorescence quantitative PCR (Polymerase Chain Reaction) diagnostic kit for rapid detection of mRNA expression level of a BRCA1 gene. The kit comprises BRCA1gene primer, a reference gene GAPDH primer and a Taqman fluorescence probe. The BRCA1 is an important cancer suppressor gene, and proteins coded by the BRCA1 gene play an important role in DNA damage and repair. A fluorescence quantitative PCR with high sensitivity and specificity is employed to detect the mRNA expression level of BRCA1; and a detection result has substantially increased specificity and sensitivity. The kit provides a novel rapid simple gene diagnostic technique for whether a clinical malignant tumor patient should use platinum chemotherapeutics and antimicrotubular medicaments.
Owner:李艳 +1
I, II herpes simplex virus fluorescence quantitative PCR detection method and kit thereof
InactiveCN101676409AHigh sensitivityNo amplification signalMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceType ii herpes simplex
The invention relates to an I, II herpes simplex virus fluorescence quantitative PCR detection method and kit thereof. Primers are designed direct towards DNA enzyme highly conservative region and then processed by PCR and a fluorescent probe is designed in the middle of the amplification sequence and then the annealing temperature of the reaction and the concentrations of the primers, the probe,the magnesium ion are optimized to establish the I, II herpes simplex virus real-time fluorescence PCR detection method. The kit ensures the sensitivity and accuracy and has features of simple and quick operation, reduced labor intensity of clinical detection, reduced cost of clinical PCR diagnosis, quick and simple performance of fluorescence quantitative PCR detection method. The method is usedfor quantitative detection of I, II herpes simplex virus with practical clinical application value.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Diagnosis of penaeus monodon-type baculovirus by PCR
InactiveUS6284455B1Sugar derivativesViral antigen ingredientsConserved sequenceNuclear Polyhedrosis Virus
This invention relates to the methods of detecting Penaeus monodon baculovirus (MBV). Two methods are established: the first one is a polymerase chain reaction (PCR) and the second one is an ELISA. For the PCR method, two sets of primers are designed. The first set of primers is designed from the conserved sequences of nuclear polyhedrosis viruses (NPVs) DNA polymerase genes. The second set of primers is designed from the genomic DNA of MBV. The antibody for ELISA is an antiserum against the occlusion bodies of MBV.
Owner:YA LI HSU
Multiple PCR detection reagent kit for pathogenic bacterium and its detecting method
InactiveCN1900306AStrong specificityWide applicabilityMicrobiological testing/measurementEscherichia coliStreptococcus lactarius
The present invention synthesizes four pairs of specific amplifying primers separately corresponding to the 16S-23S rRNA of main pathogenic bacteria of milk cow mammitis including staphylococcus aureus, agalactia streptococcus, delactation streptococcus and colibacillus, and detects pathogenic bacteria of milk cow mammitis with multiple PCR reaction system. The amplified segments lengths are 439 bp for staphylococcus aureus, 162 bp for agalactia streptococcus, 264 bp for delactation streptococcus and 544 bp for colibacillus. The multiple PCR detection reagent kit and detection method has powerful specificity, high sensitivity, capacity of detecting and identifying four kinds of pathogenic bacteria simultaneously, simple detection process and low detection cost.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Fluorescent quantitative PCR diagnostic kit for rapidly detecting HER-2 mRNA
InactiveCN102465176AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseFluorescence
The invention discloses a fluorescent quantitative PCR diagnostic kit for rapidly detecting HER-2 mRNA, and relates to an oncogene detection technology. The fluorescent quantitative PCR diagnostic kit is composed of a first chain cDNA synthesis kit, a PCR reaction solution and a lightcycler PCR instrument. The first chain cDNA synthesis kit comprises MgCl2, a reverse transcriptase buffer, dNTP, a RNA enzyme inhibitor, Oligo (dT) 15, AMV reverse transcriptase and DEPC water, and synthesis of HER-2, a reference gene primer and a Taqman fluorescent probe; the primer is divided into an upstream primer and a downstream primer. The invention has the advantages of good specificity, sensitivity and repeatability, accurate quantification, rapidity and convenience, is suitable for specifically qualitative and quantitative determination of HER-2 gene at early stage, and provides better reference for the chemotherapy and prognosis for the clinical breast cancer.
Owner:WUHAN KANGYUAN BIOLOGICAL MEDICAL TECH
Immune microsphere in use for detecting SARS antigen, preparation method and application
InactiveCN1661371ADrawbacks of Avoiding False PositivesImprove accuracyMaterial analysisAntigenSARS coronavirus
The present invention relates to a kind of immune microsphere in use for detecting SARS antigen, preparation method and application.This immune microsphere takes polystyrene microsphere which surface is modified with carboxyl as kernel,the carboxyl couples with the antibodyies which resists SARS coronavirus of S N M or E proteini through multi polylysine chemically.The immune microsphere can be used for detecting SARS antigen as detecing reagent.Comparing to the existing technology,the immune microsphere using for detecting SARS antigen possesses better sensitivity and specificity,higher accuracy,avoiding the disadvantage of false positive of PCR disgnosis kit.The process is fast.On the other hand,detection needs no instrument equipment,so this immune microsphere is suitable for the largeness base sanitary and anti-epidemic units to use.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Kit for detecting ERCC1 mRNA (Excision Repair Cross Complement Group 1 Messenger Ribonucleic Acid) expression by using fluorescence quantitative PCR (Polymerase Chain Reaction) technology
InactiveCN102154475AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceExcision repair cross-complementingFluorescence
The invention relates to the field of biotechnology, discloses a fluorescence quantitative PCR (Polymerase Chain Reaction) diagnostic kit for rapidly detecting ERCC1 mRNA (Excision Repair Cross Complement Group 1 Messenger Ribonucleic Acid), and aims to provide a kit capable of rapidly, conveniently, sensitively and specifically detecting ERCC1 mRNA and application thereof. An expression status of a DNA (Deoxyribonucleic Acid) repair gene can be used as a relatively ideal predictor for a chemotherapeutic effect. Nucleotide excision repair (NER) is in close relation with platinum drug resistance, wherein the excision repair cross complement group 1 (ERCC1) is an important factor which plays a role in a repair process and causes platinum drug resistance; the ERCC1 mRNA level is detected by using higher-sensitivity and higher-specificity fluorescence quantitative PCR; and the specificity and the sensitivity of a detection result are obviously improved. The kit provides a completely new rapid and convenient gene diagnosis technology for use of platinum type chemotherapeutic medicaments of clinical malignant tumor patients.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
HPV detection kit
ActiveCN104561384AHigh detection specificitySolve the characteristicsMicrobiological testing/measurementMicroorganism based processesConserved sequenceHpv detection
The invention discloses a qPCR primer for detecting HPVs. The qPCR primer comprises the following primer pairs of two types: the primer pairs of the first type are capable of amplifying conserved sequences in the HPV genomes of all the types; the primer pairs of the second type are capable of detecting the HPVs of at least two types in the same tubular qPCR system. The invention also discloses an HPV detection kit comprising the primer pairs. The HPV detection kit is high in HPV detection specificity and completely reaches the specificity level of qPCR by use of a probe method. Compared with traditional PCR diagnosis, the HPV detection kit is simpler and more reliable to operate, and meanwhile, applicable to high-throughput sample detection. The HPV detection kit further has the characteristics of high specificity, strong sensitivity and low cost by applying a double-stranded DNA combined fluorochrome qPCR detection method to the diagnosis of the HPVs; as a result, a quick and accurate molecular detection method is provided for general investigation of the HPVs and prevention and treatment of the cervical cancer, and meanwhile, the test cost is greatly reduced; in short, the HPV detection kit has an important popularization and application value.
Owner:镇江爱必梦生物科技有限公司
PCR diagnostic kit for porcine infectious pleuropneumonia
InactiveCN101724709AThe test result is accurateThe detection method is simpleMicrobiological testing/measurementMicroorganism based processesPositive controlTE buffer
The invention discloses a PCR diagnostic kit for porcine infectious pleuropneumonia, which comprises 400mu L of proteinase K with the concentration of 20mg / mL, 1000mu L of cracking solution, 1500mu L of TE buffer solution, 250mu L of PCR enzyme, 170mu L of ultra-pure water, 50mu L of MarkerDL2000, 40mu L of primer P1 and primer P2 which are mixed in the same volume and have the same concentration of 20mu M, 20mu L of negative control and 20mu L of positive control. By optimizing the PCR reaction conditions, the invention develops a PCR kit for detecting the actinobacillus of the porcine infectious pleuropneumonia; and by comparing the PCR detection result with the negative control and the positive control, detection conclusions can be obtained, thereby achieving the aim of quickly detecting whether a sample has porcine infectious pleuropneumonia or not. The invention has accurate detection result, quick and sensitive detection process, simple detection mode and good using effect.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
Method for rapidly diagnosing shrunken-fruitsclerotiniose of mulberry
InactiveCN104513860AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseaseConserved sequence
The invention discloses a method for rapidly diagnosing shrunken-fruitsclerotiniose of mulberry. Through the discovered pathogen scleromitrula shiraiana specificity conserved DNA sequence, a specificity SCAR-PCR primer is obtained and a shrunken-fruitsclerotiniose of mulberry SCAR-PCR diagnosis and detection method is established. The method is high in sensitivity (the detection sensitivity is 1pg / [mu]L, high in specificity, convenient and rapid to use and accurate and reliable in detection result, can be used for the field diagnosis of the shrunken-fruitsclerotiniose of mulberry and the detection of the mulberry bacteria carrying condition, and also can be used for the diagnosis of the disease incubation period (the mulberries are infected but the symptoms do not appear) so as to forecasting the diseases in an early stage, take necessary control measures in time, safely and effectively prevent and control the disease and lighten or avoid the economic loss. According to the method, the product such as a detection kit for the shrunken-fruitsclerotiniose of mulberry can be researched and developed; the method is widely applied to the scientific research and production practice of the industries related to mulberries.
Owner:SOUTHWEST UNIVERSITY
Immunization microsphere in use for detecting SARS antibody, preparation method and application
InactiveCN1661372ADrawbacks of Avoiding False PositivesImprove accuracyMaterial analysisAntigenSARS coronavirus
The present invention relates to a kind of immunization microsphere in use for detecting SARS antibody, preparation method and application.This immune microsphere takes polystyrene microsphere which surface is modified with carboxyl as kernel,the carboxyl couples with the antibodyies which resists SARS coronavirus of S N M or E proteini through multi polylysine chemically.The immune microsphere can be used for detecting SARS antigen as detecing reagent.Comparing to the existing technology,the immune microsphere using for detecting SARS antigen possesses better sensitivity and specificity,higher accuracy,avoiding the disadvantage of false positive of PCR disgnosis kit.the process is fast.on the other hand,detection needs no instrument equipment,so this immune microsphere is suitable for the largeness base sanitary and anti-epidemic units to use.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Multiplex RT-PCR detection method of porcine delta coronavirus, porcine epidemic diarrhea virus and porcine sapelovirus, and applications thereof
InactiveCN110904270AIncrease concentrationOptimal annealing temperatureMicrobiological testing/measurementMicroorganism based processesMultiplexEpidemic diarrhea
The invention belongs to the field of virus detection, and particularly relates to a multiplex RT-PCR detection method of porcine delta coronavirus, porcine epidemic diarrhea virus and porcine sapelovirus, and applications thereof. Three pairs of specific primers for synthesizing conserved sequences of PEDV M gene, PDCoV N gene and PSV 2C gene are designed; then, RT-PCR reaction conditions are optimized, the multiplex RT-PCR diagnosis method capable of simultaneously detecting porcine delta coronavirus, porcine epidemic diarrhea virus and porcine sapelovirus is established, and specificity, sensitivity and repeatability analysis is performed on the established method. It is shown by results the method disclosed by the invention has very good specificity and relatively high sensitivity; theminimum detection limit of PDCoV is 140 copies / <mu>L, the minimum detection limit of PEDV is 153 copies / <mu>L, and the minimum detection limit of PSV is 1570 copies / <mu>L.
Owner:HENAN AGRICULTURAL UNIVERSITY
Multiple PCR reaction kit and detecting process thereof
InactiveCN1861802AIncreased sensitivityStrong specificityMicrobiological testing/measurementMultiplex pcrsBiology
The invention discloses a PCR diagnostic reagent box for detecting the milk cattle foot rot. It has the advantage of quick, high sensibility, strong speciality and low cost.
Owner:吕占军 +1
Duplex PCR diagnostic kit for detection of contagious caprine pleuropneumonia pathogen and preparation and use methods thereof
InactiveCN102634601ADefinitive diagnosisrapidReduce complexityMicrobiological testing/measurementMicroorganism based processesDuplex pcrPositive control
The invention discloses a raw material composition of a duplex PCR (polymerase chain reaction) diagnostic kit for detection of contagious caprine pleuropneumonia pathogen and a preparation method and a use method of the kit. The raw material composition comprises 1000-2000muL of PCR reaction liquid, 100-200muL of TaqDNA polymerase, 50-100muL of positive control, 50-100muL of negative control and 1-2mL of ultrapure water. The PCR reaction solution comprises a primer 1 mixture solution, a primer 2 mixture solution, a PCR reaction buffer and dNTPs (deoxyribonucleotide triphosphates), wherein the volume ratio of the four liquids in the PCR reaction solution is 2:2:5:1. The preparation method comprises the steps of determination of an optimum reaction annealing temperature, specific detection, sensibility test, clinical application detection and kit packaging. An effective method for rapid deterministic diagnosis of suspected goat pathogen and suspected cases of contagious caprine pleuropneumonia in goat farms is provided by the invention, providing technical support for the prevention and control of contagious caprine pleuropneumonia.
Owner:GUIZHOU UNIV
Multiple PCR detection method of swine pathogens
InactiveCN109439775AStrong specificitySimple and fast operationMicrobiological testing/measurementMicroorganism based processesDiseaseElectrophoresis
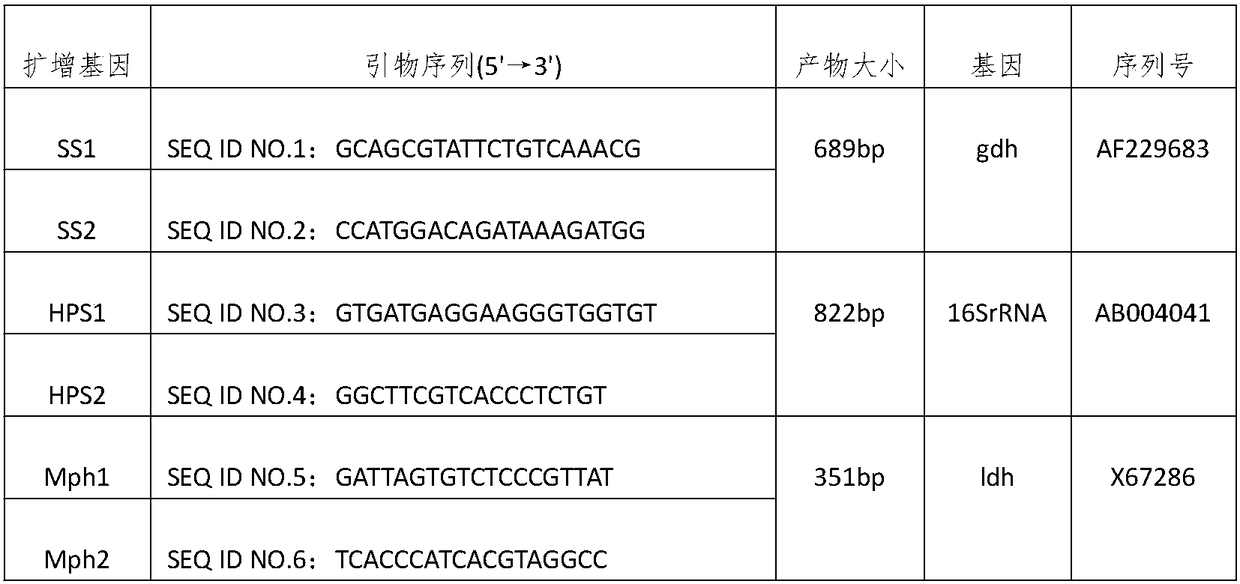
The invention discloses a multiple PCR detection method of swine pathogens. The method includes steps of to-be-detected sample DNA extraction, PCR, PCR amplification, amplification product electrophoresis detection and electrophoresis detection result analysis. A system of PCR comprises 2.5uL of Buffer, 1.5uL of Mg2+, 2uL of dNTP, 0.5uL of SS upstream primer, 0.5uL of downstream primer, 0.5uL of HPS upstream primer, 0.5uL of HPS downstream primer, 0.5uL of Mph upstream primer, 0.5uL of Mph downstream primer, 0.25uL of Taq enzyme, 2uL of template and the balance of sterile deionized water up to25uL. Conventional detection methods of the swine pathogens are relatively time-consuming and labor-consuming, thereby being bad for efficient detection; on the basis of an objective of quickly and accurately diagnosing mixed infectious pathogens, various advantages of multiple PCR detection are utilized to establish a quick, specific and sensitive multiple PCR diagnosis method of the swine pathogens, and quick, accurate and sensitive detection and diagnosis of several diseases are realized.
Owner:TONGREN POLYTECHNIC COLLEGE
Preparation method of swine fever virus nucleic acid standard substance
InactiveCN101906487ANo genetic information will be disclosedStrong specificityMicrobiological testing/measurementMicroorganism based processesSwine Fever VirusQuality control
The invention relates to a preparation method of a swine fever virus nucleic acid standard substance. The swine fever virus nucleic acid standard substance is prepared through the following technical steps: (1) selecting a gene conservation area as an amplification target area; (2) designing and synthesizing a primer; (3) carrying out sequence amplification; (4) carrying out cloning and identification; (5) carrying out in vitro transcription; (6) sub-packaging; (7) determining RNA copy number; (8) inspecting; (9) carrying out collaborative calibration; and (10) setting value. The swine fever virus nucleic acid standard substance prepared by utilizing the preparation method has no infection, a wide range of applications, good uniformity, high stability and accurate set value of the copy number, and can be used for quality control, contrast between scientific research and laboratory diagnosis and capacity contrast among laboratories of RT-PCR or fluorescent quantitative PCR diagnostic reagents.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
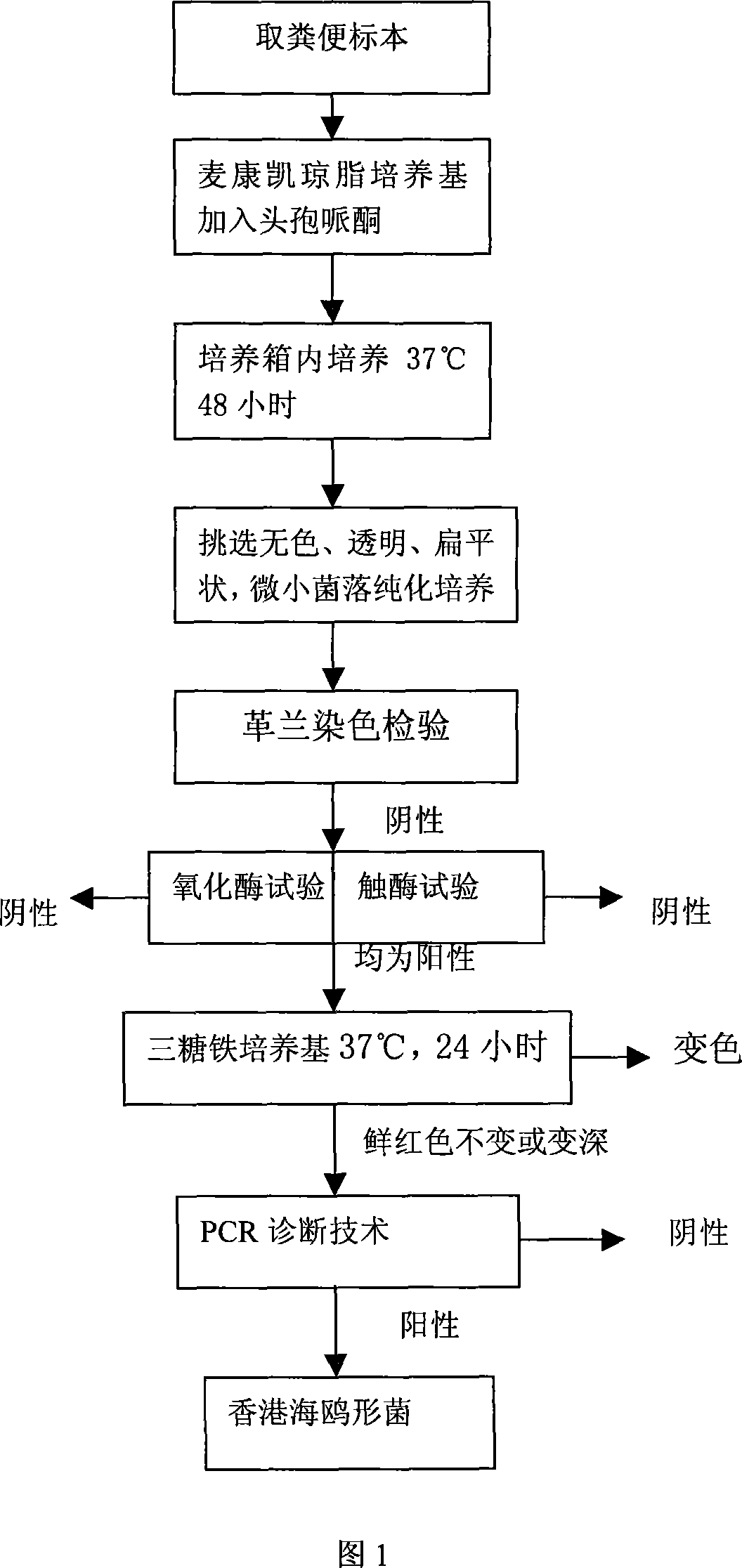
Hongkong sea-gull shape bacterium inspection technology
InactiveCN101063164AShorten the timeSave human effortMicrobiological testing/measurementGramClinical therapy
The invention discloses a testing technique of HongKong sea-gull shape bacteria, which comprises the following steps: fetching faecal specimen; seeding on MaiKangKai medium with cefoperazone; putting into 37 deg. c box for 48 h; taking-up; sorting colourless, transparent and flat dwarf colony with diameter at 0. 5-1. 0mm; proceeding purified culture; testing as negative bacillus brevis or vibrio with Gram's stain; checking as positive with oxidase and catalase; seeding in triose metal medium tube; placing in 37 deg. c box; culturing for 24 h; taking-up; keeping the colour of fresh red medium; or changing more red; diagnosing fitting result bacteria with PCR technique; diagnosing as HongKong sea-gull shape bacteria when PCR result as positive. This invention provides criterion for prevention and cure or clinical therapy of HongKong sea-gull shape bacteria disease, which is convenient, practical and rapid.
Owner:杭州市疾病预防控制中心 +1
Multiplex PCR diagnosis kit for three pathogenic bacteria in milk
PendingCN111518931ARealize detectionNo obvious mutual interferenceMicrobiological testing/measurementMicroorganism based processesMultiplexStaphyloccocus aureus
The invention discloses a multiplex PCR diagnosis kit for three pathogenic bacteria in milk. Primers of the kit are specific primers in a staphylococcus aureus femB gene sequence, an escherichia coliphoA gene sequence and a salmonella invA gene sequence. Through optimization of multiplex PCR reaction and an optimization experiment, the optimal amplification procedure for multiplex PCR of the kitis determined to be 5 min at 95 DEG C, 30 s at 95 DEG C, 35 s at 50-60 DEG C, 45 s at 72 DEG C, amplification for 30 cycles, and final extension at 72 DEG C for 10 min. The multiplex kit disclosed bythe invention can rapidly and accurately detect and identify separate or mixed infection of staphylococcus aureus, salmonella and escherichia coli in a clinical milk sample. The experimental result proves that the kit is short in detection time, good in sensitivity and high in specificity and has a broad application prospect.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
A kit for rapid microscopic examination diagnosis of swine eperythrozoonosis and a preparing method thereof
InactiveCN107589117AImprove detection accuracyEasy to preparePreparing sample for investigationMaterial analysis by optical meansGiemsa stainMedicine
A kit for rapid microscopic examination diagnosis of swine eperythrozoonosis and a preparing method thereof are provided. The kit includes detection reagents, vessels and instruments. The reagents include a Wright's stain, a Giemsa stain, glycerol, Tween-20 and methanol. The vessels and instruments include reagent bottles and droppers. The kit is scientific and reasonable in matching. A detectionmethod is simple, and detection accuracy is as high as 98% and is equivalent to accuracy of a swine eperythrozoonosis PCR diagnosis kit.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
One-step RT-PCR diagnostic kit for identifying porcine epidemic diarrhea virus vaccine attenuated strain and/or prevalent strain
PendingCN106834538AAvoid degradationEasy to operateMicrobiological testing/measurementMicroorganism based processesPig farmsRotavirus RNA
The invention provides a one-step RT-PCR diagnostic kit for identifying a porcine epidemic diarrhea virus vaccine attenuated strain and / or prevalent strain. The one-step RT-PCR diagnostic kit comprises 20muL of RT-PCR one-step enzyme, 250muL of a enzyme buffer solution, 300muL of RNase Free dH2O, 40muL of a mixed primer, 20muL of positive control, 20muL of negative control, 80muL of DL2000 and 25muL of 6*Loading Buffer. Compared with the prior art, the kit has the advantages that the operation is simple, inverse transcription and PCR amplification are completed in one step, the reaction time is shortened, degradation of a template RNA is avoided and the detection sensitivity is improved. The amplification results of the primer on porcine transmissible gastroenteritis virus, group A swine rotavirus, classical swine fever virus, porcine reproductive and respiratory syndrome virus and porcine teschovirus are all negative. According to the method, 1pg porcine epidemic diarrhea virus vaccine attenuated strain and 1pg prevalent strain can be detected to the minimum extent; the kit can be widely applied to basic-level detection mechanisms and is of great significance in early differential diagnosis, control and purification of epidemic diarrhea virus diseases on a pig farm.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Enterotoxigenic escherichia coli heat stable enterotoxin gene SYBR Green I fluorescent quantitative PCR diagnostic kit
InactiveCN108179211AMicrobiological testing/measurementMicroorganism based processesEscherichia coliPositive control
The invention an enterotoxigenic escherichia coli heat stable enterotoxin gene SYBR Green I fluorescent quantitative PCR diagnostic kit, and relates to the technical field of kits. The enterotoxigenicescherichia coli heat stable enterotoxin gene SYBR Green I fluorescent quantitative PCR diagnostic kit comprises 40 mu L of specific primers, 250 mu L of fluorescent quantitative PCR reaction mixed liquor (Premix EX Taq), 20 mu L of ROX Reference Dye, 20 mu L of negative control, 20 mu L of positive control and 500 mu L of ultrapure water. A pair of special primers is designed according to pig source enterotoxigenic escherichia coli (ETEC) heat stable enterotoxin gene sequence (STII) in GenBank to perform a test, and the test method has sensitivity of 1.0*10<1> copies / mu L, is good in repeatability; and PCR product sequencing results are compared with the ST gene sequence in GenBank, and similarity is 98.2%-100%. The kit has the characteristics of being high in detecting speed and high indetecting rate.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
Pigeon variola, pigeon chlamydia and pigeon herpes virus triple PCR diagnostic kit and detection method thereof
InactiveCN108118101AEasy to operateThe detection method is convenient and fastMicrobiological testing/measurementMicroorganism based processesPositive controlHerpes simplex virus DNA
The invention provides a pigeon variola, pigeon chlamydia and pigeon herpes virus triple PCR diagnostic kit. The kit comprises a lysate, an amplifying reaction mixed liquid, negative control and positive control, wherein the component of the lysate is DNAiso Reagent; the amplifying reaction mixed liquid comprises sterilizing tri-distilled water, PCR Buffer, dNTP, a POX-F upstream primer, a POX-R downstream primer, a CPS-F upstream primer, a CPS-R downstream primer, a PiHV-F upstream primer, a PiHV-R downstream primer and rTaq DNA polymerase; negative control is sterilizing tri-distilled water;positive control is a mixture of pigeon variola, pigeon chlamydia and pigeon herpes virus positive plasmids. The kit is high in sensitivity, good in specificity, high in stability and intuitive in result, and a detection method of the kit is easily operated, can shorten the detection time greatly, thereby providing a powerful technical means for clinically controlling infection of pigeon variola,pigeon chlamydia and pigeon herpes virus.
Owner:XIANYANG VOCATIONAL TECHN COLLEGE
Kit for detecting mRNA expression quantity of M BCR fusion gene
InactiveCN102965433AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceReference genesPositive control
The invention relates to a fluorescent quantitative PCR (Polymerase Chain Reaction) diagnosis kit for detecting mRNA of an M BCR fusion gene, belonging to the field of biotechnology. The kit comprises a detection primer, a fluorescent probe, a cDNA first-chain synthesis reagent, a fluorescent quantitative PCR mixed liquor, negative control and positive control, wherein the detection primer and the fluorescent probe comprise an M BCR gene primer, a reference gene ABL primer and a Taqman fluorescent probe. The kit can effectively detect the M BCR fusion gene forms, such as e14a3, e13a3, e14a2 and e13a2. The M BCR fusion gene is a gene mark of malignant clone of pluripotential hemopoietic stem cells, is a typical molecular marker of chronic granulocytic leukemia and is related to inhibition of the apoptosis of the leukemic cell. The fluorescent quantitative PCR technology with higher sensitivity and specificity is used for detecting the mRNA level of the M BCR gene, and the specificity and the sensitivity of the detection result both are improved remarkably. The kit provided by the invention provides a brand-new rapid, simple and convenient gene diagnosis technology for making a therapeutic regimen for the patients with chronic granulocytic leukemia, and predicting prognosis.
Owner:李艳 +1
Multiple PCR detection reagent kit for pathogenic bacterium and its detecting method
InactiveCN100560735CStrong specificityWide applicabilityMicrobiological testing/measurementEscherichia coliMastitis
The present invention aims at the 16S-23S rRNA interval of the main pathogenic bacteria Staphylococcus aureus, Streptococcus agalactiae, Streptococcus dysgalactiae and Escherichia coli of dairy cow mastitis, synthesizes 4 pairs of corresponding specific amplification primers, and applies a multiplex PCR reaction system to the cow udder detection of pathogenic bacteria. The lengths of the amplified fragments were 162bp for Streptococcus agalactiae, 264bp for Streptococcus dysgalactiae, 439bp for Staphylococcus aureus and 544bp for Escherichia coli. The multiplex PCR detection kit and detection method have strong specificity and sensitivity. It can detect and identify four kinds of pathogenic bacteria at the same time, so as to achieve the purpose of reducing the workload and cost of PCR diagnosis.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Bird polyoma virus PCR (polymerase chain reaction) diagnostic kit and detection method thereof
InactiveCN108315492AStrong specificityThe test results are consistentMicrobiological testing/measurementMicroorganism based processesPositive controlPolyomavirus Infections
The invention provides a bird polyoma virus PCR (polymerase chain reaction) diagnostic kit and a detection method thereof. The bird polyoma virus PCR diagnostic kit comprises a lysate, an amplified reaction mixture, a negative control and a positive control, wherein the lysate is prepared from a DNAiso Reagent; the amplified reaction mixture is prepared from sterilized tri-distilled water, PCR buffer, dNTP, an APV-F upstream primer, an APV-R downstream primer and rTaq DNA polymerase; the negative control is the sterilized tri-distilled water; the positive control is bird polyoma virus positiveplasmids. The kit is high in sensitivity, good in specificity, high in stability, and intuitive in result; the detection method is easy to operate, convenient, fast, and capable of greatly reducing the detection time, provides a forceful technological means for clinically controlling bird polyoma virus infection, and particularly has a significance on rapid diagnosis of parrot bird polyoma virusinfection.
Owner:XIANYANG VOCATIONAL TECHN COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com