Duplex PCR diagnostic kit for detection of contagious caprine pleuropneumonia pathogen and preparation and use methods thereof
A diagnostic kit and technology for pleuropneumonia, applied in biochemical equipment and methods, methods based on microorganisms, measurement/inspection of microorganisms, etc., can solve the problems of not being able to be used as a diagnostic method for pathogens, high diagnostic costs, and long time consumption, and achieve Facilitate the deterministic diagnosis of pathogens, reduce tediousness and blindness, and have good convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
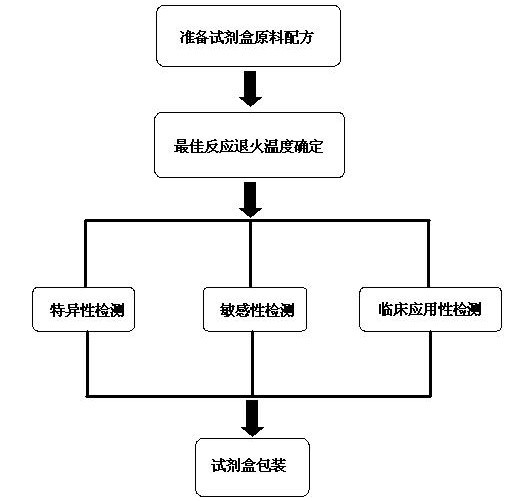
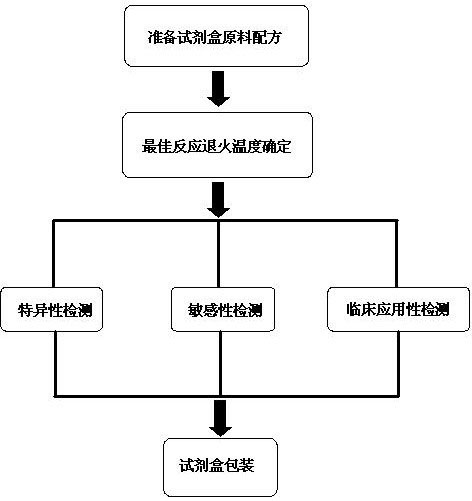
[0027] Embodiment of the present invention: goat infectious pleuropneumonia pathogen detection double PCR diagnostic kit raw material formula is:
[0028] 1000~2000μL PCR reaction solution, 100~200μL Taq DNA polymerase, 50~100μL positive control, 50~100μL negative control, 1mL-2 mL ultrapure water.
[0029] The PCR reaction liquid includes primer 1 mixed liquid, primer 2 mixed liquid, PCR reaction buffer and dNTP, and the volume ratio of the four liquids in the PCR reaction liquid is 2:2:5:1.
[0030] The primer 1 is the general primer Ps and Pa for Mycoplasma mycoides cluster 16S rRNA gene, the primer concentration is 10 μmol / L, and the primer sequence is shown in Table 1.
[0031] Table 1 Primer 1 (Ps and Pa) sequences
[0032] Primer Sequence (5'—3') PS GGGAGGCAGCAGTAGGGAAT Pa CAGCGTCAATAACAAGCCAGTAAG
[0033] The primer 2 is specific primers Ms and Ma for Mycoplasma ovipneumoniae 16S rRNA gene, the primer concentration is 10 μmol / L, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com