Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Deoxyribonucleotide Triphosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nucleotide comprised of either a purine or pyrimidine nitrogenous base bound to a deoxyribose sugar esterified with three phosphate groups. These molecules are essential for DNA synthesis.
Nucleic acid sequencing methods, kits and reagents
ActiveUS8399196B2Highly controllable homogeneousOvercome limitationsSugar derivativesMicrobiological testing/measurementNucleic acid sequencingProcessing enzymes
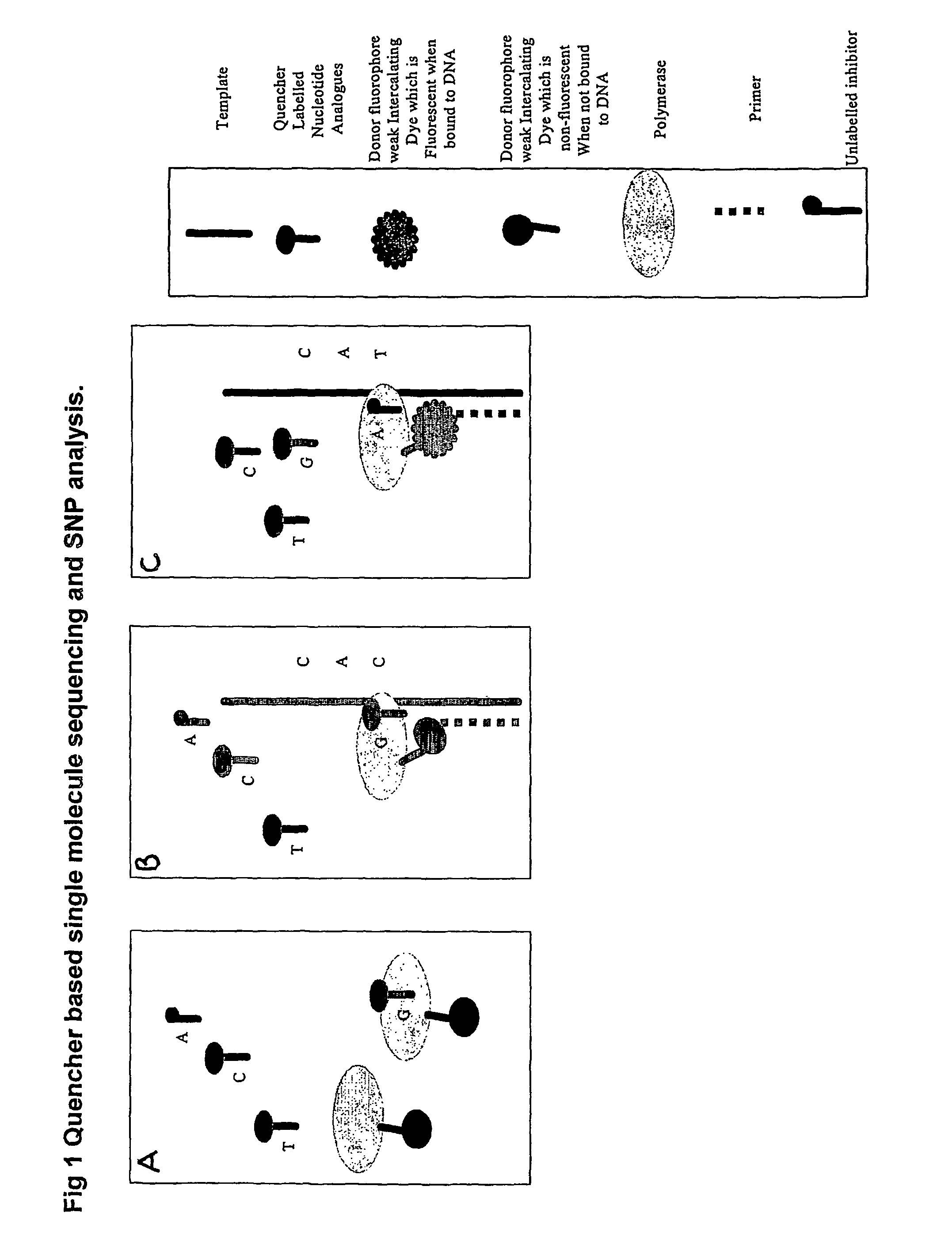
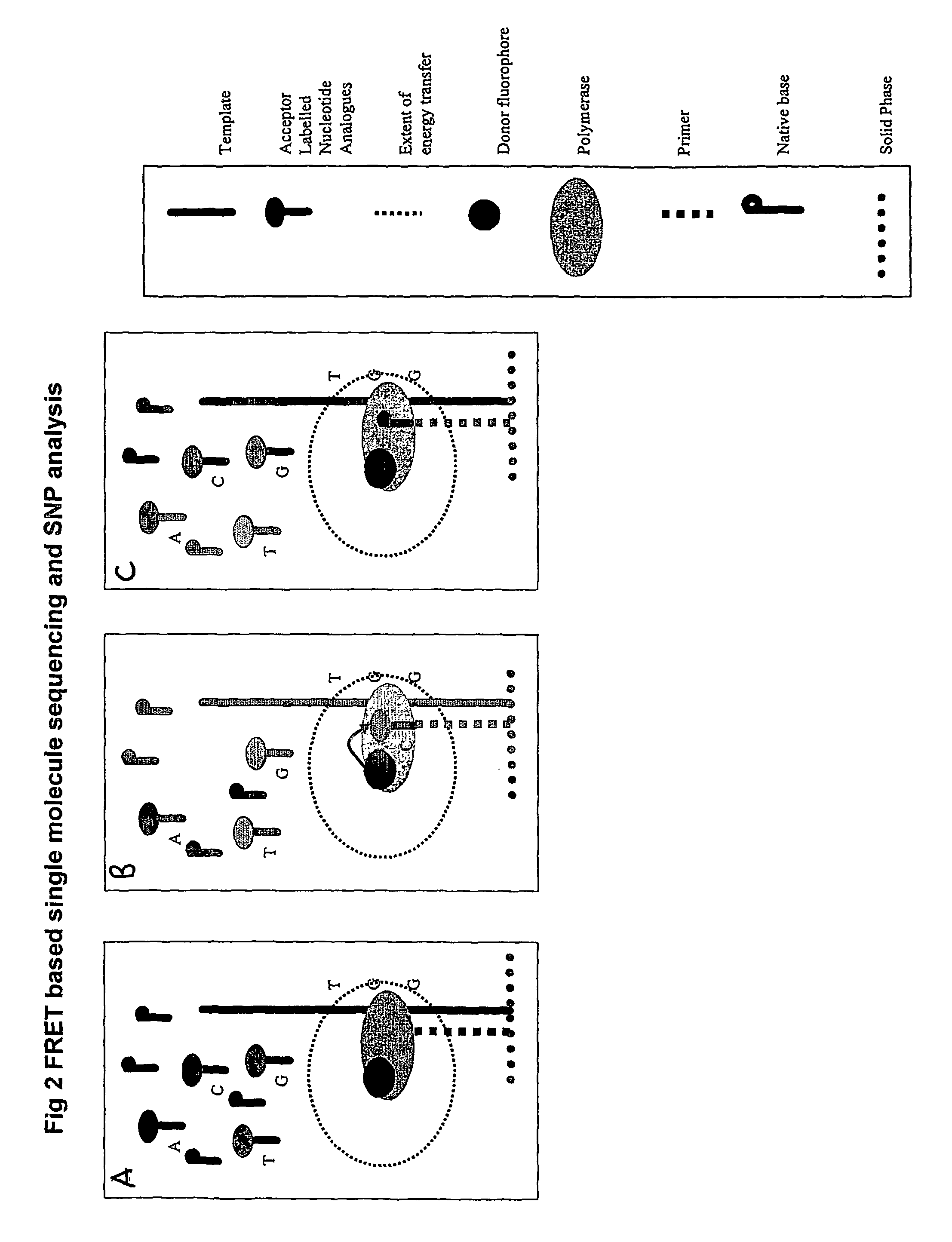
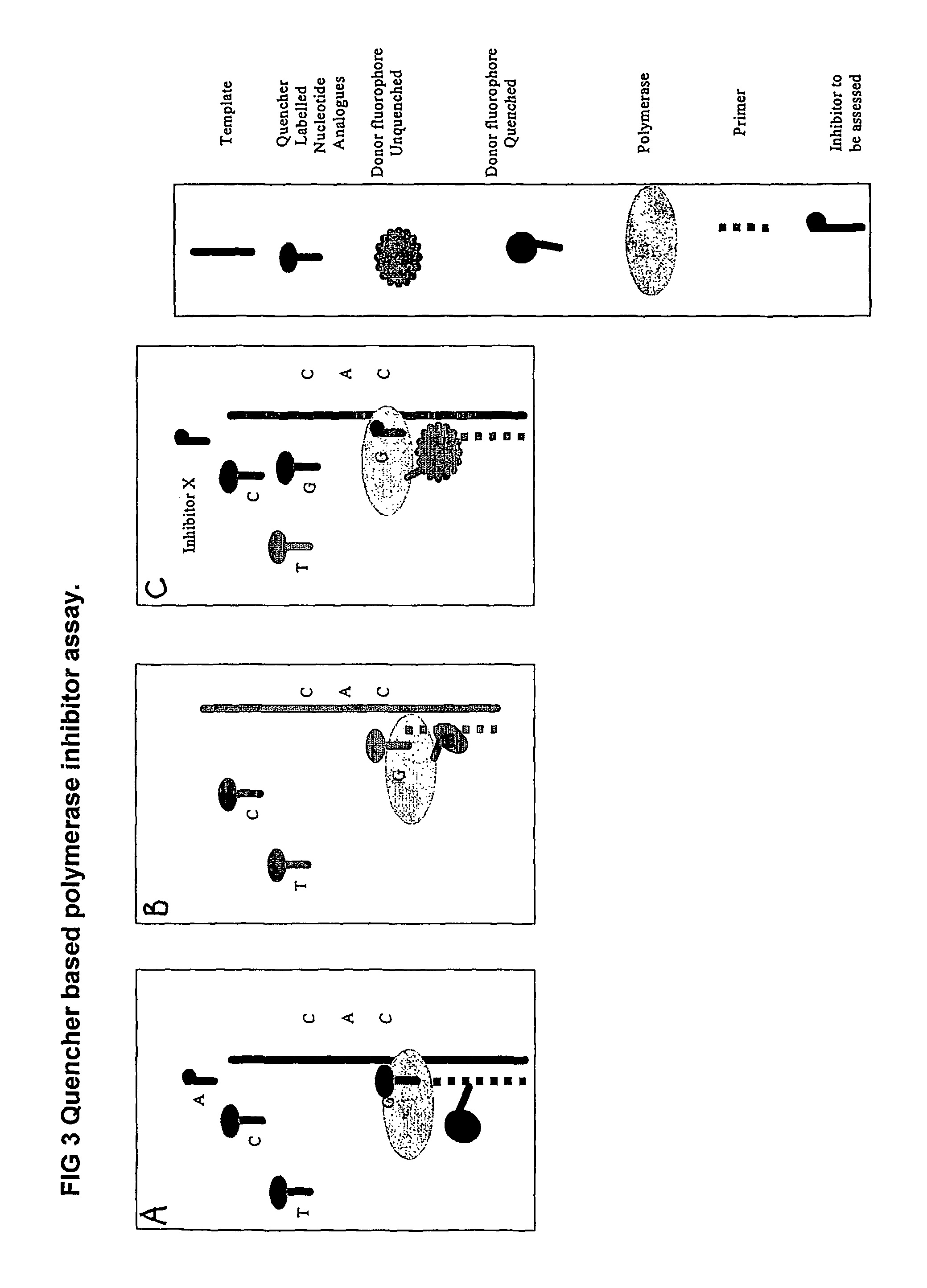
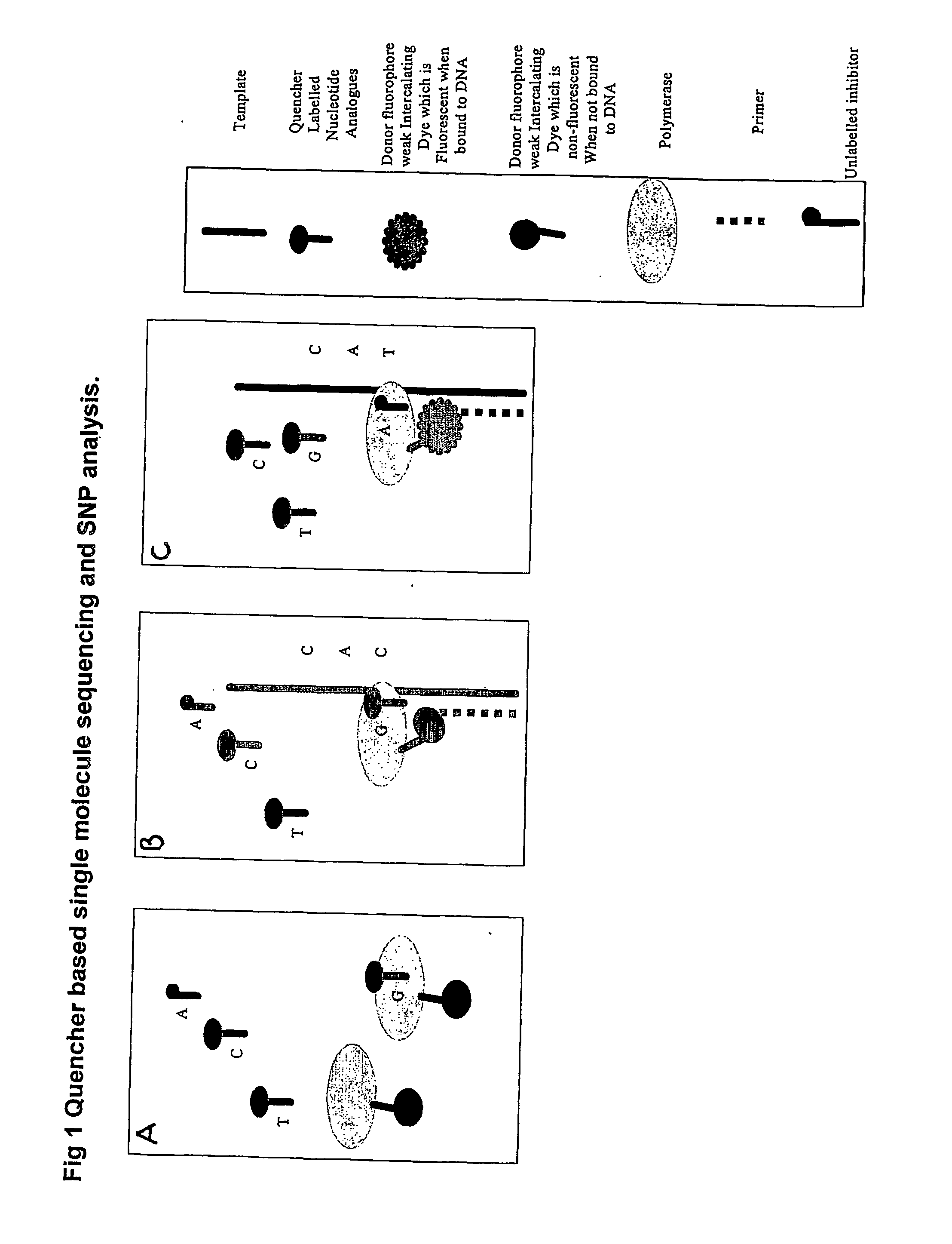
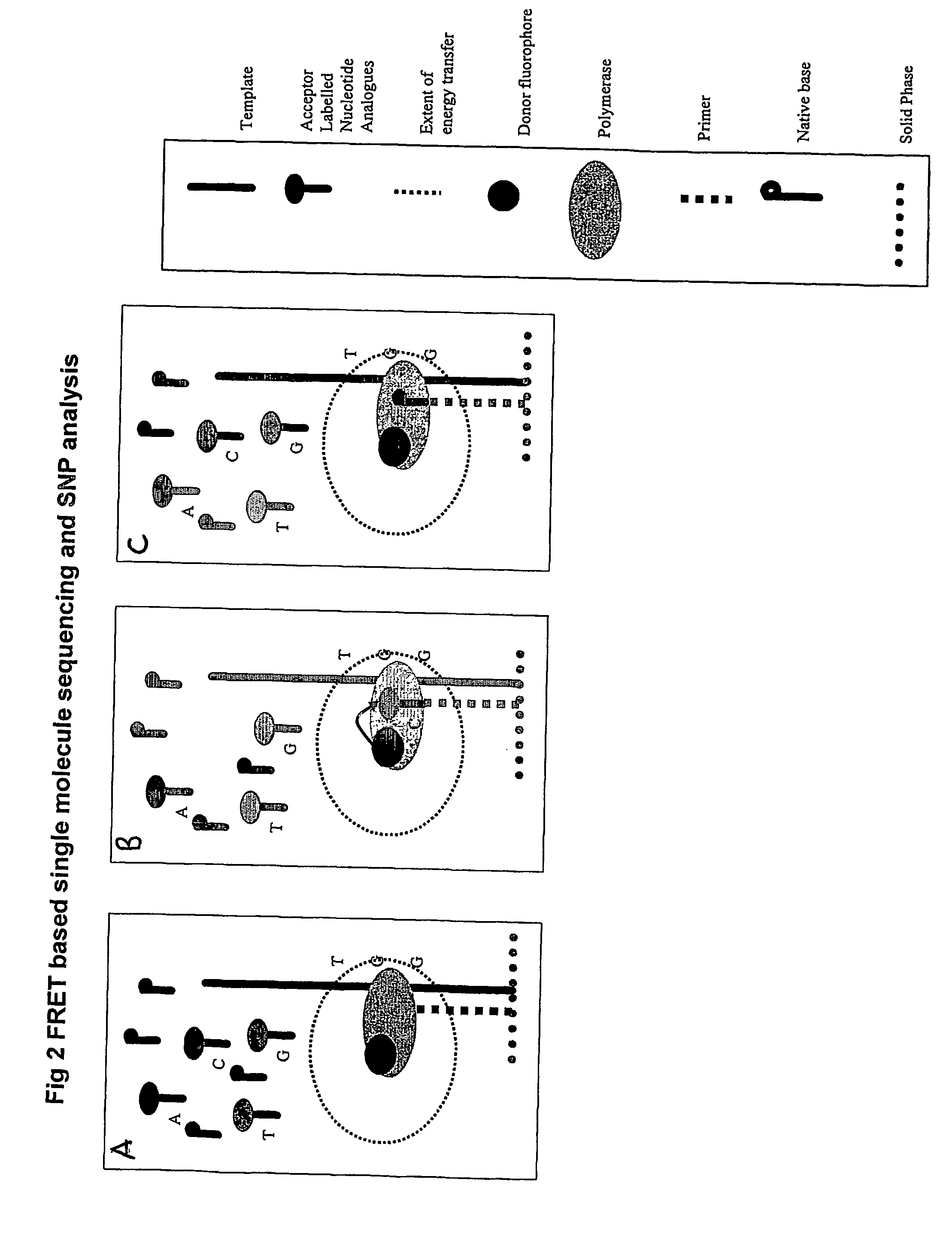
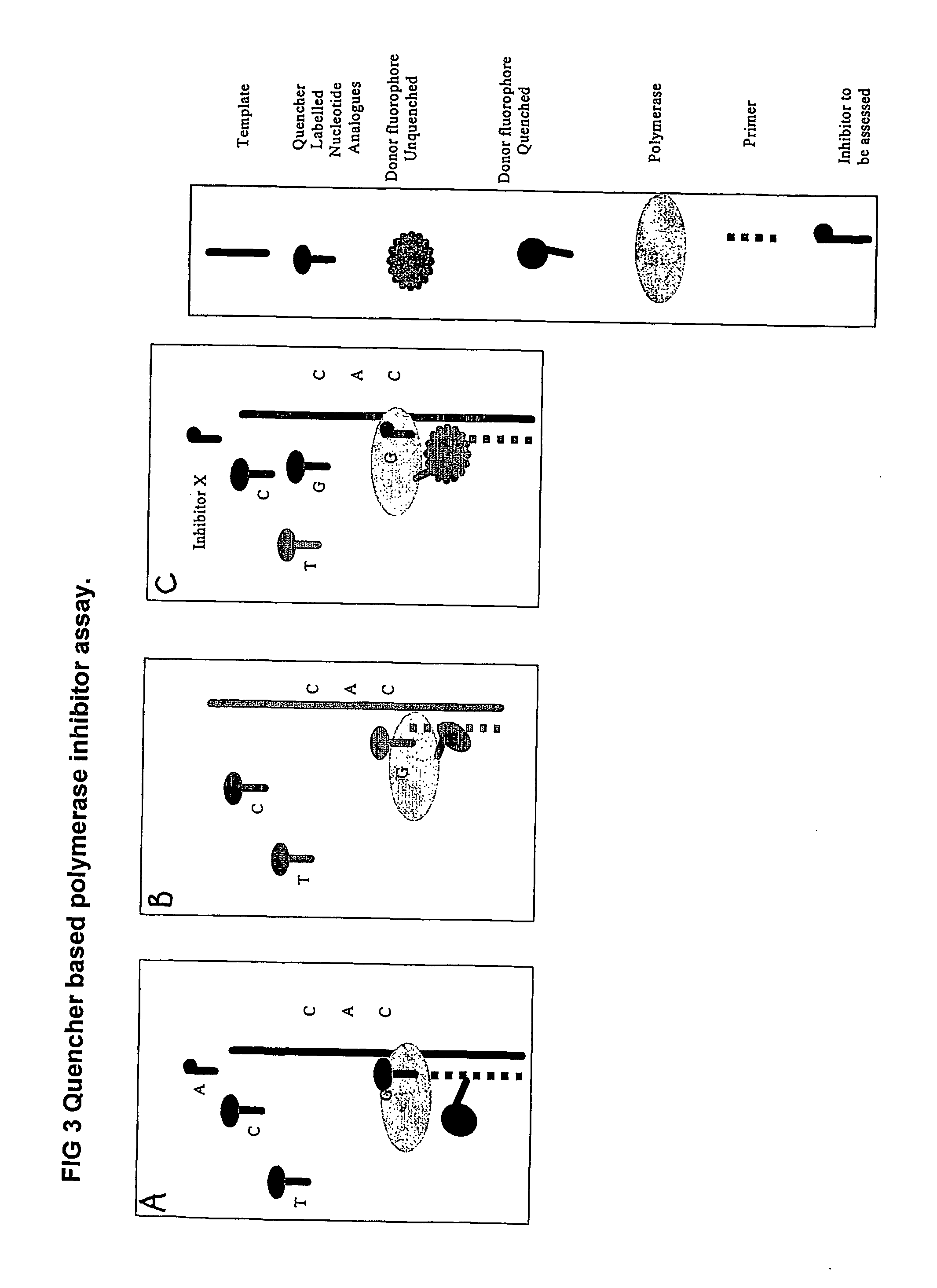
The present invention relates to nucleic acid sequencing methods, kits and reagents, and more particularly to methods of sequencing nucleic acid which employ a nucleic acid processing enzyme and one or more nucleotide analogues that are capable of binding to the active site of the enzyme and to complementary bases in the nucleic acid molecule being sequenced, but which are non-incorporable or inhibitors of the nucleic acid processing enzyme. In further aspects, the present invention relates to conjugates which comprise a deoxyribonucleotide triphosphates (DNTPs) or an analogue thereof linked to an intercalating dye.
Owner:GENEFORM TECH LTD
Isothermal chimeric primer nucleic acid amplification methods using blocking oglionucleotide
InactiveUS7056671B2Improve targeting efficiencyHigh detection sensitivityMicrobiological testing/measurementFermentationPolymerase LRibonucleotide synthesis
Methods of amplifying a target nucleic acid whereby the target nucleic acid is amplified in the presence of a deoxyribonucleotide triphosphate, a DNA polymerase having strand displacement activity, at least one chimeric oligonucleotide primer, at least one upstream block oligonucleotide and a RNase H; wherein the chimeric oligonucleotide primer contains a ribonucleotide positioned at the 3′-terminus; wherein the upstream block oligonucleotide is capable of annealing to a region 3′ to a portion in the nucleic acid as the template to which the chimeric oligonucleotide primer anneals; and compositions and kits thereof.
Owner:TAKARA HOLDINGS
Nucleic acid sequencing methods, kits and reagents
ActiveUS20070148645A1Conveniently preparedHighly controllable homogeneousSugar derivativesMicrobiological testing/measurementReactive siteNucleic acid sequencing
The present invention relates to nucleic acid sequencing methods, kits and reagents, and more particularly to methods of sequencing nucleic acid which employ a nucleic acid processing enzyme and one or more nucleotide analogues that are capable of binding to the active site of the enzyme and to complementary bases in the nucleic acid molecule being sequenced, but which are non-incorporable or inhibitors of the nucleic acid processing enzyme. In further aspects, the present invention relates to conjugates which comprise a deoxyribonucleotide triphosphates (DNTPs) or an analogue thereof linked to an intercalating dye.
Owner:GENEFORM TECH LTD
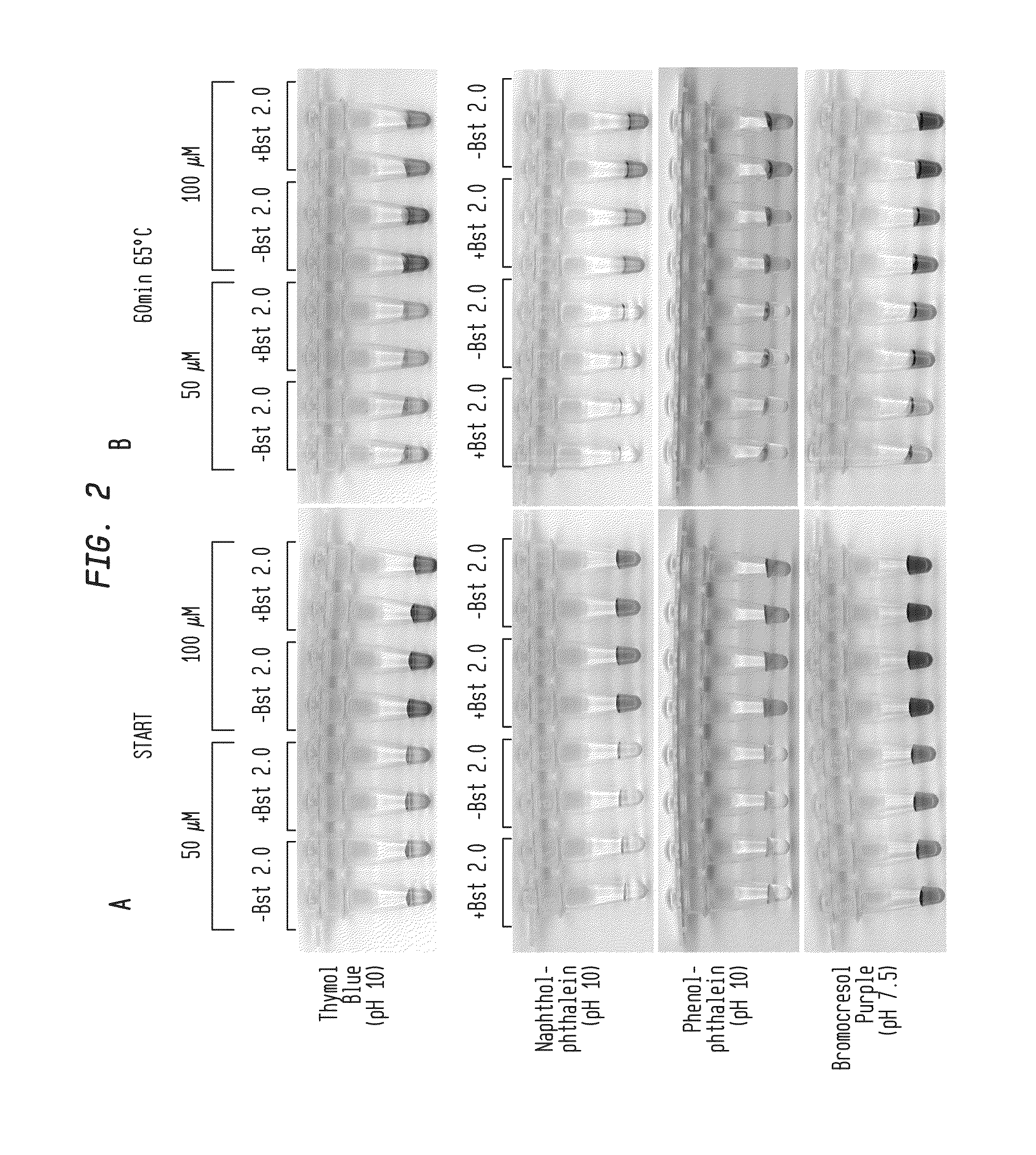
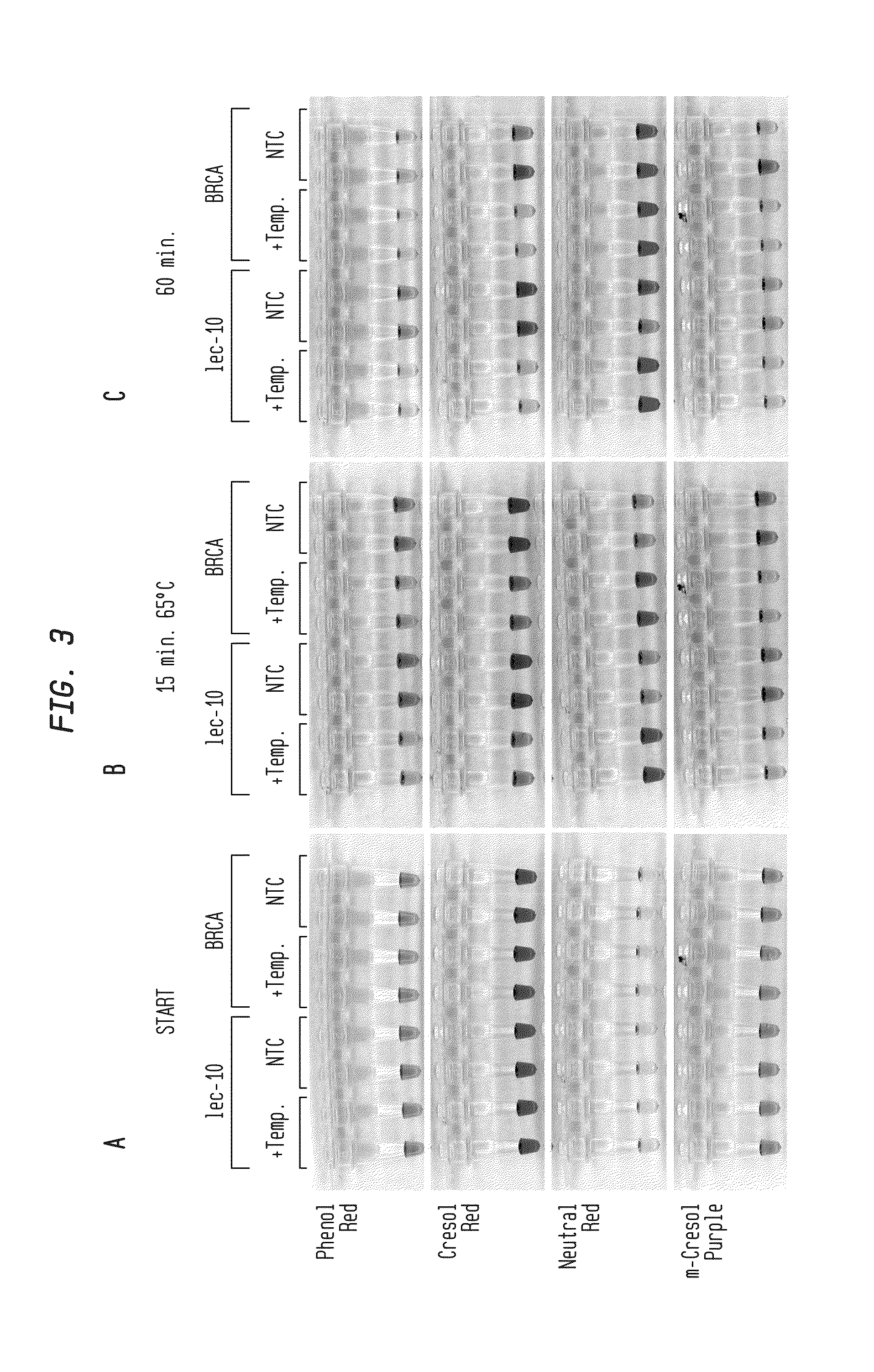
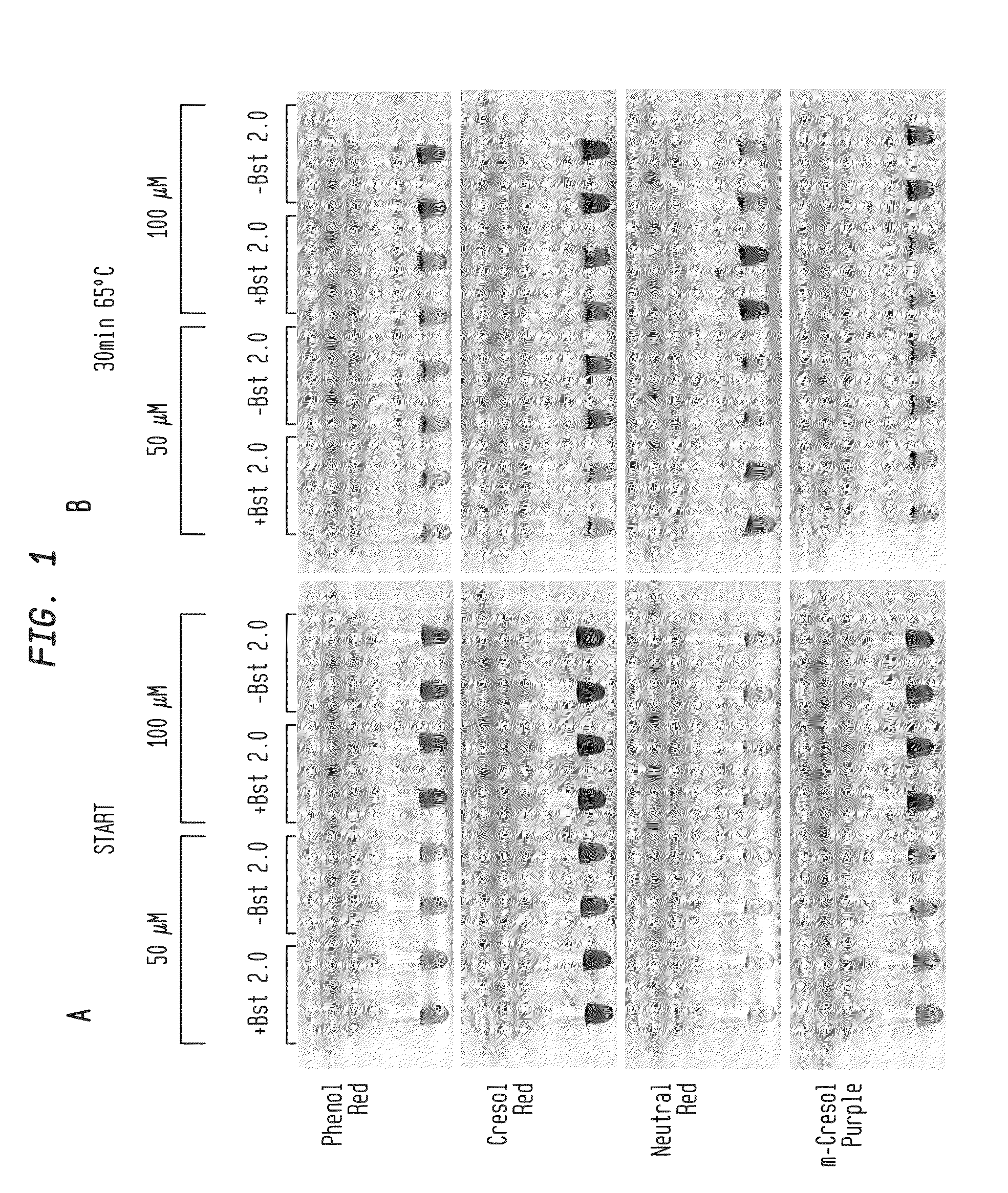
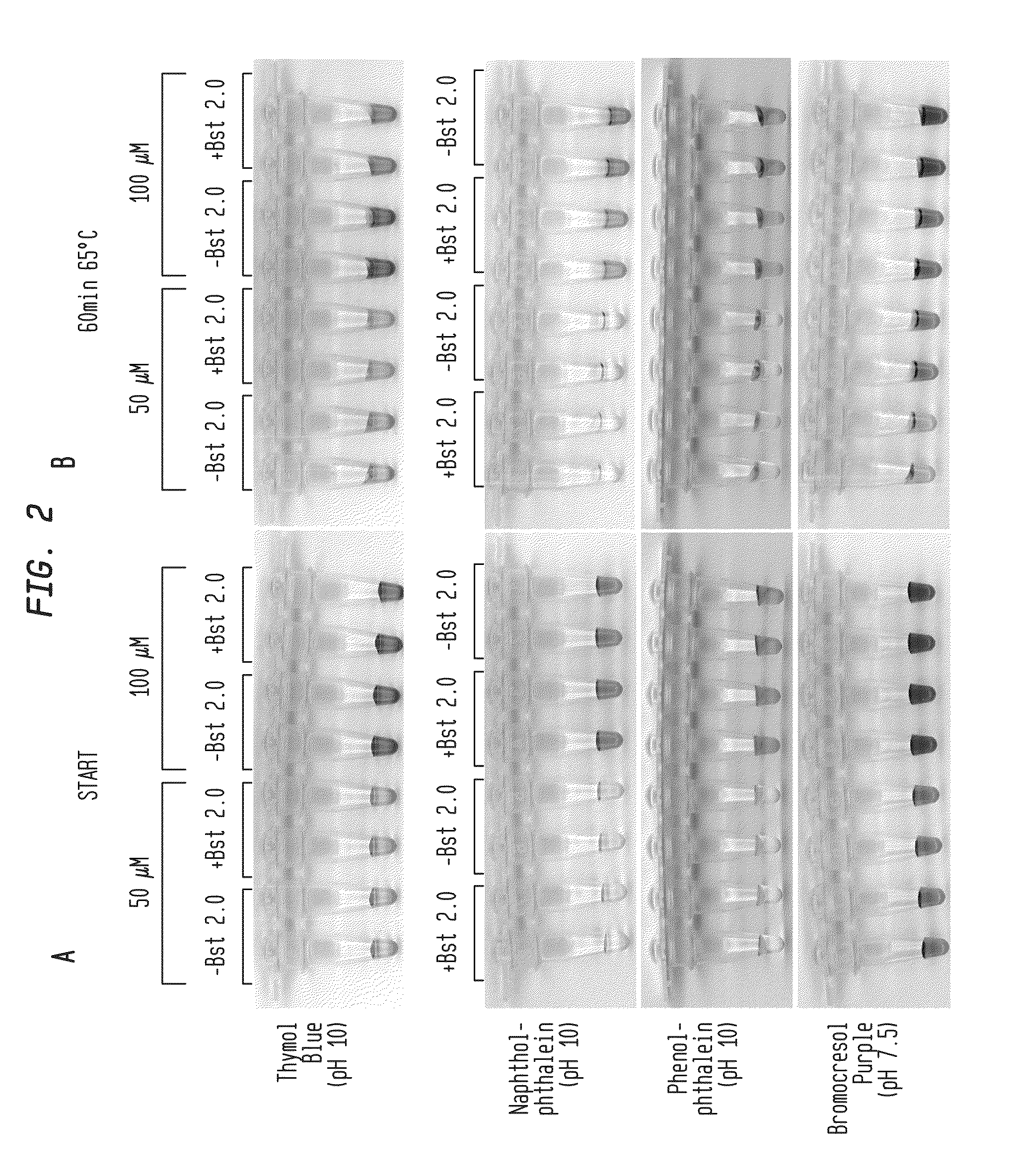
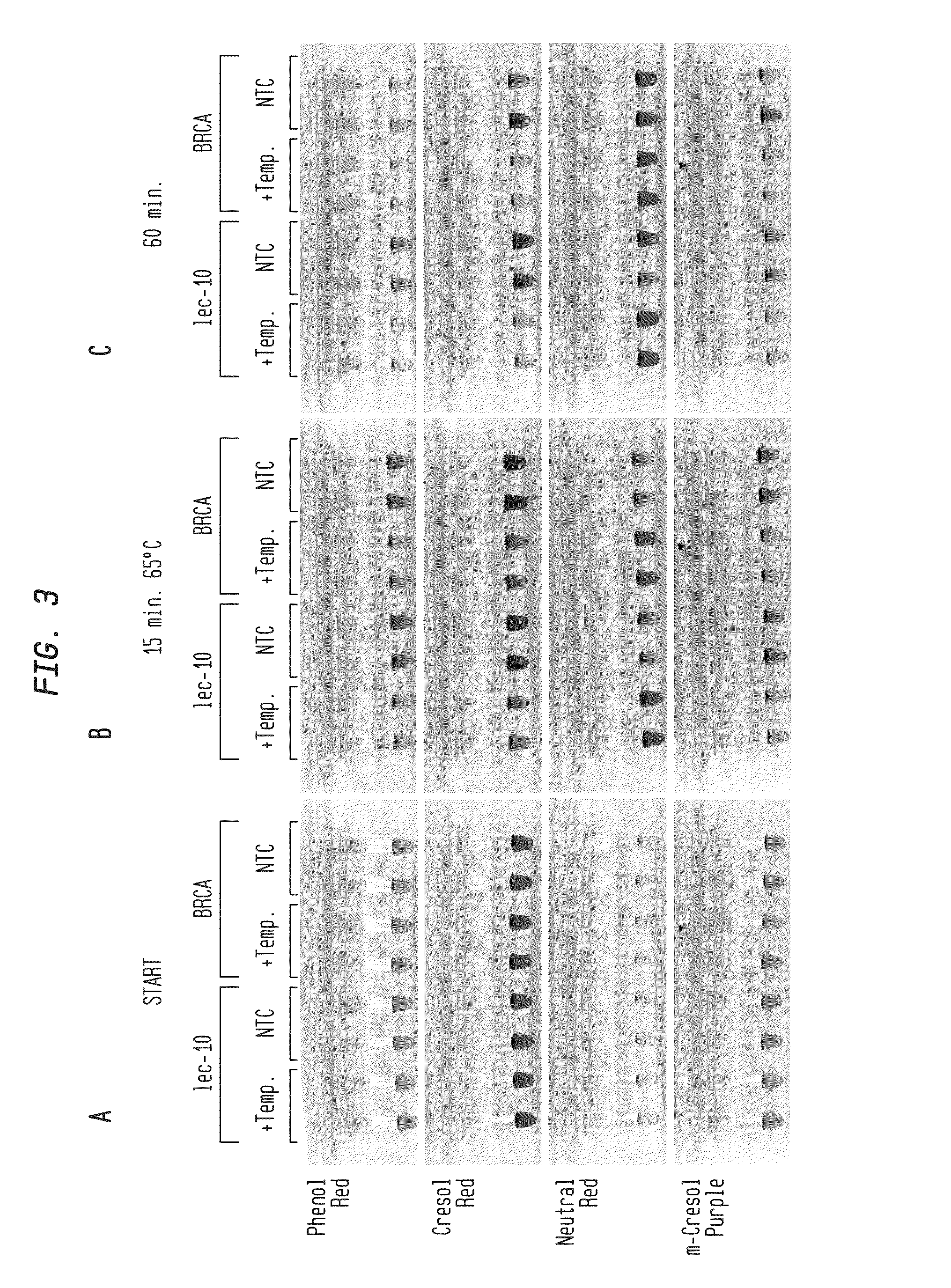
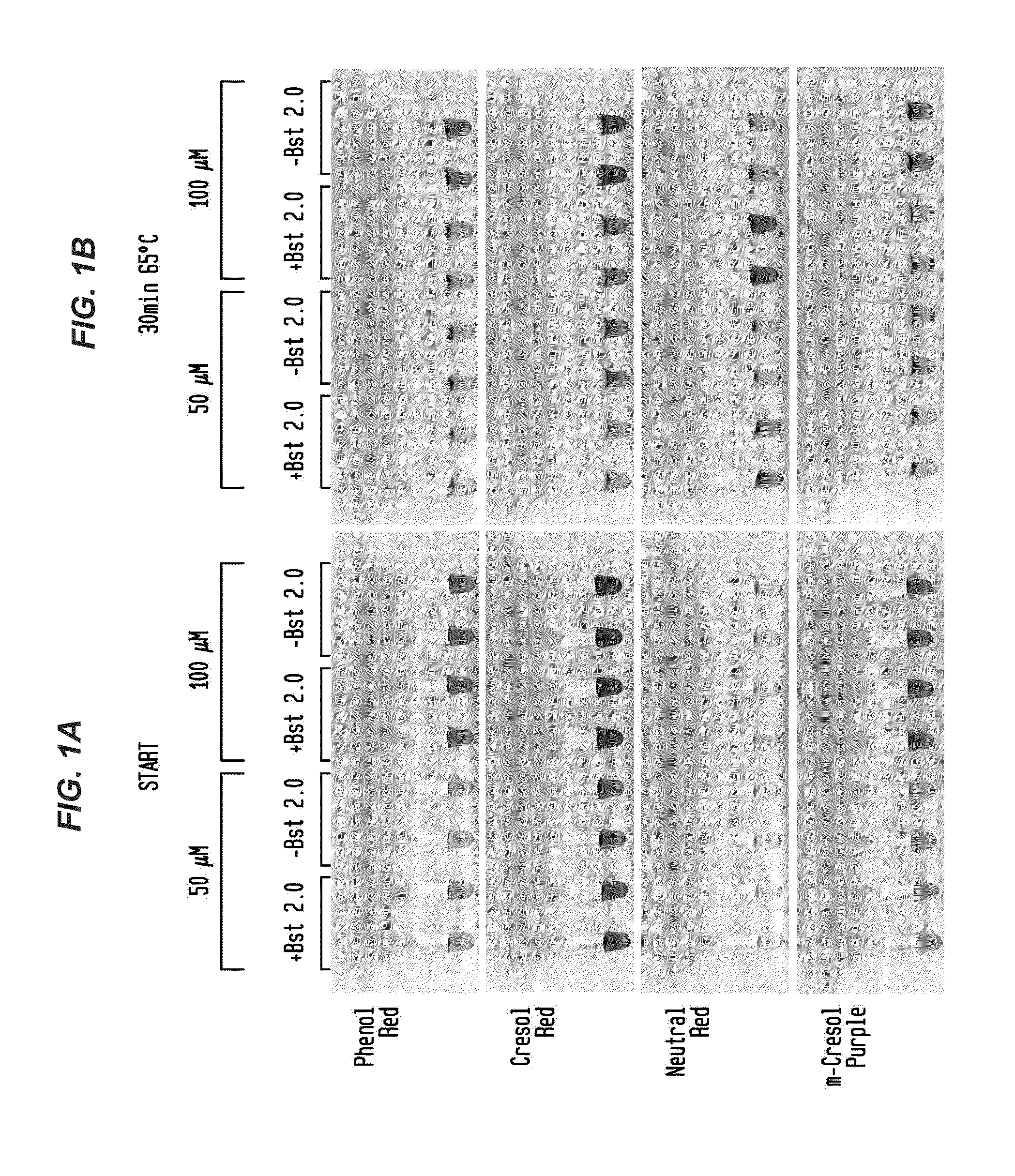
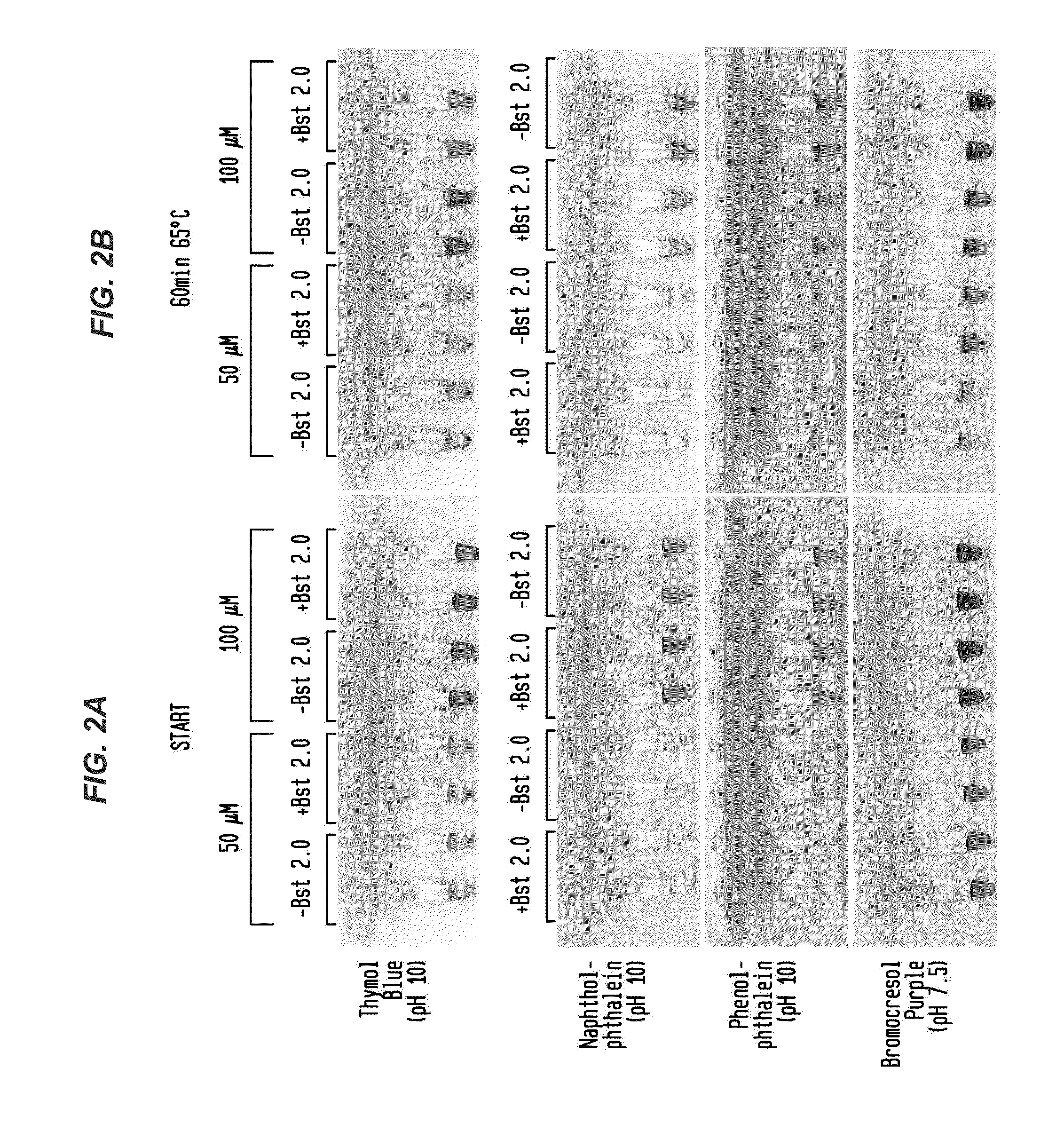
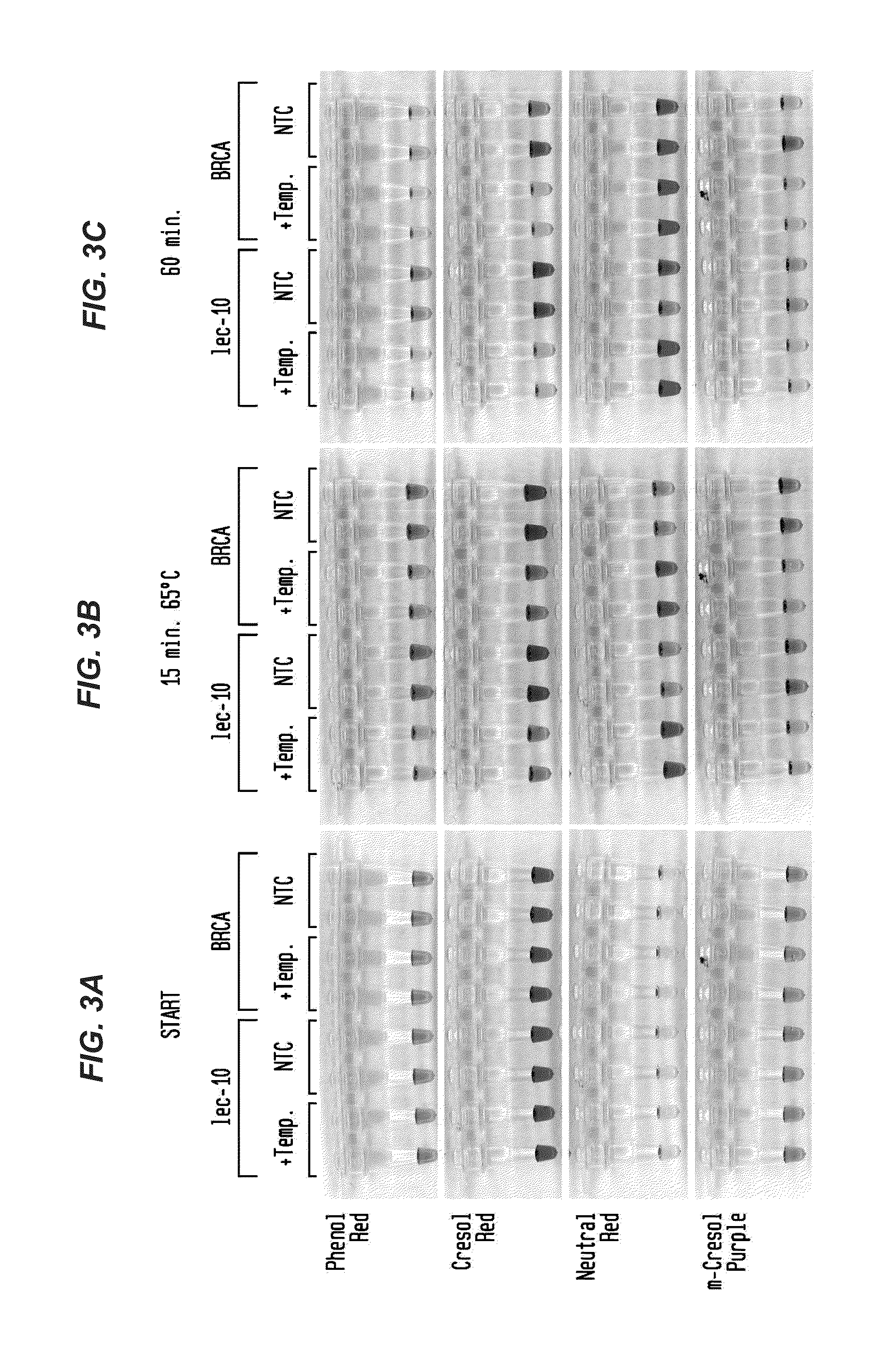
Detection of an Amplification Reaction Product Using pH-sensitive Dyes
ActiveUS20140057268A1Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementFluorescenceDna polymerasen
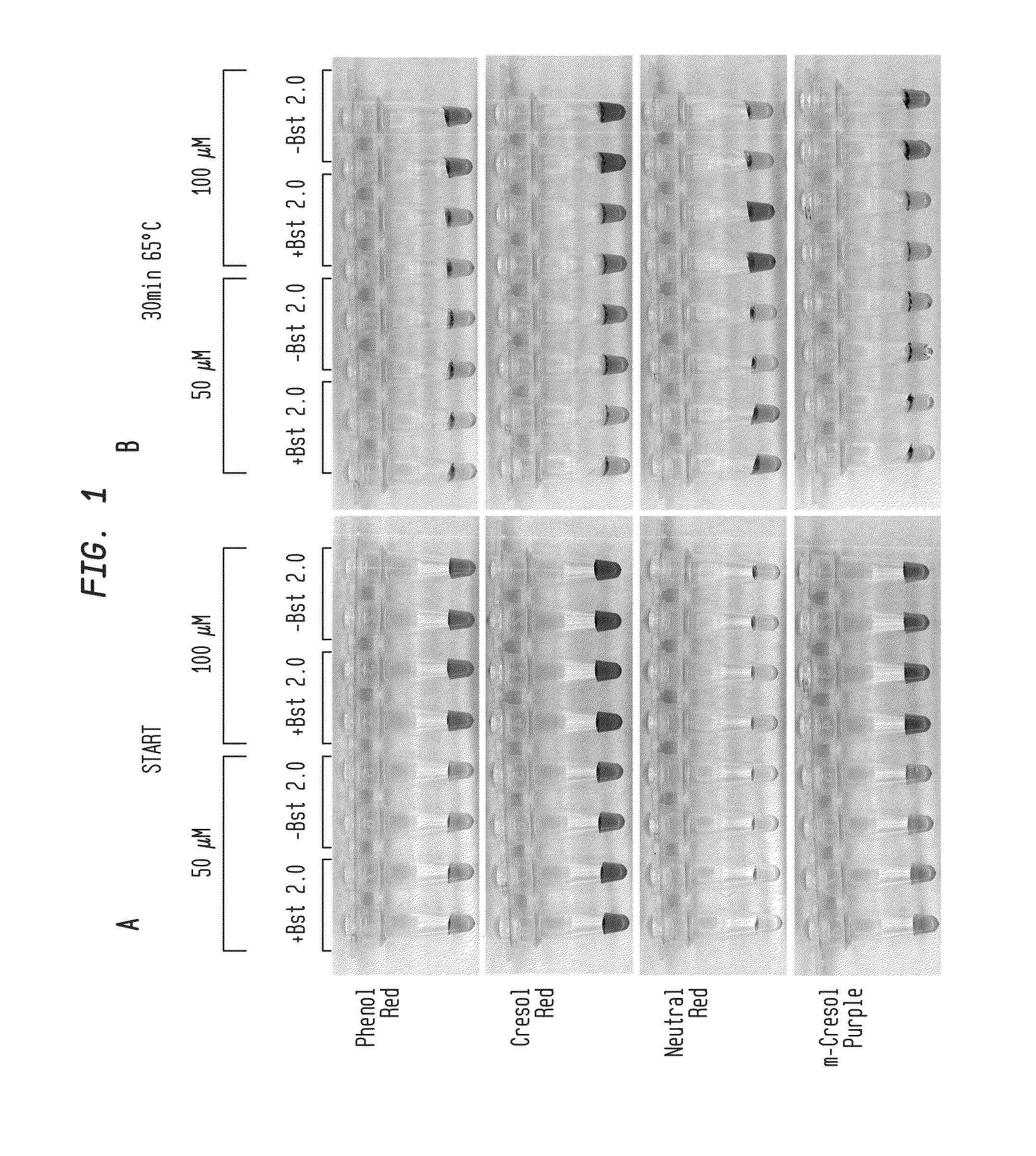
Methods are provided for a rapid, low cost approach to monitoring an amplification reaction. This includes monitoring the progress of isothermal or PCR amplification reactions to completion using pH-sensitive dyes that are either colored or fluorescent. Compositions are described that include a mixture of a DNA polymerase, deoxyribonucleotide triphosphate and a weak buffer of less than 1 mM Tris or equivalent or no buffer.
Owner:NEW ENGLAND BIOLABS
Detection of an amplification reaction product using pH-sensitive dyes
ActiveUS9034606B2Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementFluorescencePolymerase L
Methods are provided for a rapid, low cost approach to monitoring an amplification reaction. This includes monitoring the progress of isothermal or PCR amplification reactions to completion using pH-sensitive dyes that are either colored or fluorescent. Compositions are described that include a mixture of a DNA polymerase, deoxyribonucleotide triphosphate and a weak buffer of less than 1 mM Tris or equivalent or no buffer.
Owner:NEW ENGLAND BIOLABS
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay kit and detection method for grass carp reoviruses
InactiveCN102703608AEfficient detectionStrong specificityMicrobiological testing/measurementMicroorganism based processesWater bathsBetaine
The invention discloses a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay kit and a detection method for grass carp reoviruses (GCRV), and belongs to the technical field of the detection of fish viruses. The RT-LAMP assay kit for the grass carp reoviruses comprises the following ingredients: 1*ThermoPol Reaction Buffer, Bacillus stearothermophilus deoxyribonucleic acid (BstDNA) polymerase, deoxyribonucleotide triphosphate (dNTPs), AMV reverse transcriptase, outer primers (F3 and B3), inner primers (FIP and BIP), Betaine, MgCl2, dithiothreitol (DTT) and 1,000*SYBR Green I. The assay kit has the characteristics of simpleness, convenience, quickness and high specificity and sensitivity; GCRV-104 in a sample can be accurately detected within 2 hours only by using a water bath kettle or metal bath; and the assay kit can detect grass carp tissues infected by the GCRV-104 and cells (such as cytokine-induced killer (CIK) cells) infected by the GCRV-104, and is applicable to the on-site rapid detection of the GCRV-104.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Detection of an Amplification Reaction Product Using pH-sensitive Dyes
ActiveUS20150240293A1Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementFluorescencePolymerase L
Methods are provided for a rapid, low cost approach to monitoring an amplification reaction. This includes monitoring the progress of isothermal or PCR amplification reactions to completion using pH-sensitive dyes that are either colored or fluorescent. Compositions are described that include a mixture of a DNA polymerase, deoxyribonucleotide triphosphate and a weak buffer of less than 1 mM Tris or equivalent or no buffer.
Owner:NEW ENGLAND BIOLABS
Chicken Infectious Bronchitis Virus Rapid Detection Kit Based on Loop-Mediated Isothermal Amplification Technology and Its Application Method
InactiveCN102286633AQuick responseShorten detection reaction timeMicrobiological testing/measurementFluorescence/phosphorescencePositive controlManganese
The invention relates to an avian infectious bronchitis virus quick detection kit based on loop-mediated isothermal amplification technology and an application method thereof. The kit is composed of a (1) reaction solution, an (2) enzyme solution, a (3) primer solution and a (4) positive control solution, wherein the primer solution comprises three pairs of specific primers, i.e. inner primers, outer primers and loop primers. Before the reaction, a calcein-manganese complex is previously added, manganese is bonded with pyrophosphate ions precipitated by dNTP (deoxyribonucleotide triphosphate)to release calcein, and the result can be visually inspected and identified, wherein greenish yellow indicates a positive result, and light yellow indicates a negative result. The invention solves the problems of long period, low sensitivity, difficulty in field application and the like in the detection of avian infectious bronchitis virus in the prior art.
Owner:JIANGSU INST OF POULTRY SCI +2
Method for detecting variation of gene for non-diagnostic purpose based on fluorescence quenching and probe thereof
InactiveUS20140272978A1Low costStrong specificitySugar derivativesMicrobiological testing/measurementFluorescenceQuenching
A method for detecting variation of gene based on fluorescence quenching quantification comprises: performing single base extension at a specific site of a gene to be detected with marked probes and marked dideoxyribonucleotide triphosphate; detecting fluorescence quenching values, and determining SNP of the gene to be detected. The present invention also provides an oligonucleotide probe for the method.
Owner:SHANGHAI QY BIOTECH CO LTD
Method for determining DNA nucleotide sequence
PCT No. PCT / JP95 / 02254 Sec. 371 Date May 5, 1997 Sec. 102(e) Date May 5, 1997 PCT Filed Nov. 6, 1995 PCT Pub. No. WO96 / 14434 PCT Pub. Date May 17, 1996Disclosed is a method for determining a nucleotide sequence of DNA product amplified by polymerase chain reaction not requiring removal of primers and / or 2'-deoxyribonucleoside-5'-triphosphates and / or derivatives thereof, which comprises reacting ribonucleoside-5'-triphosphates comprising ATP, GTP, CTP, UTP and derivatives thereof and one or more of 3'-deoxyribonucleotide-5'-triphosphates comprising 3'-dATP, 3'-dGTP, 3'-dCTP, 3'-dUTP and derivatives thereof in the presence of an RNA polymerase and a DNA product which has been amplified by polymerase chain reaction and contains a promoter sequence for the RNA polymerase to afford a nucleic acid transcription product, separating the obtained nucleic acid transcription product and determining a nucleic acid sequence from the resluting separated fractions.
Owner:RIKEN
Method and kit for detecting KRAS gene mutations in human colon and rectum cancers
InactiveCN102115792ASignificant progressEasy to detectMicrobiological testing/measurementFluorescenceWild type
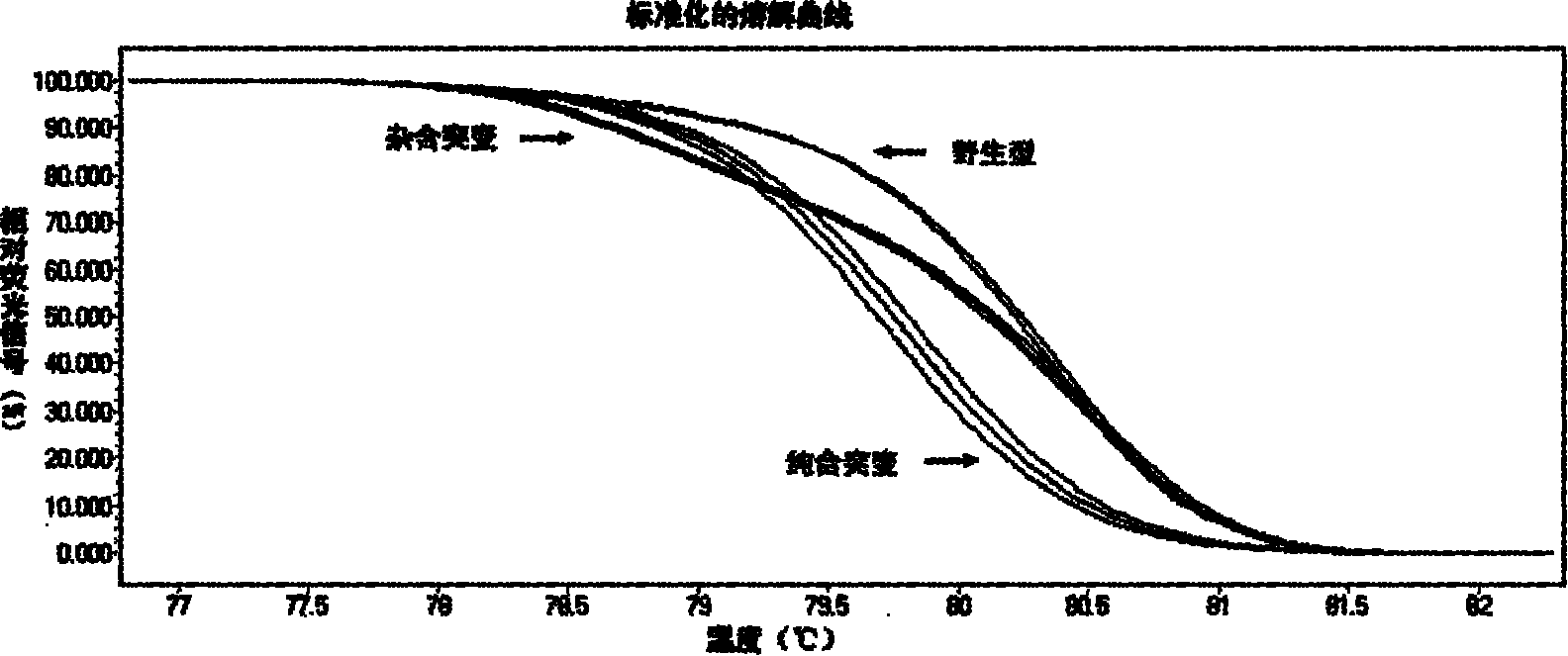
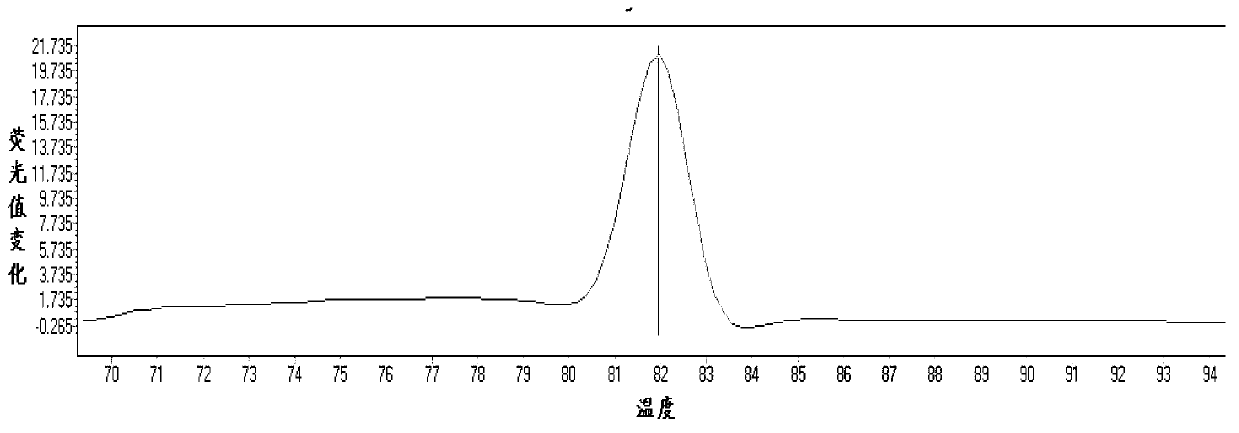
The invention relates to a method and kit for detecting gene mutations, particularly a method and kit for detecting KRAS gene 12, 13 codon mutations. The kit comprises a PCR (Polymerase Chain Reaction) buffer solution, a dNTP (deoxyribonucleotide triphosphate), a DNA (deoxyribonucleic acid) polymerase, a specific primer pair, fluorescent dye, water, a specific probe and a wild-type control. The kit is characterized in that the DNA polymerase is a HotStarTaq DNA polymerase, and the fluorescent dye is SYTO9 fluorescent dye. The method comprises the following steps: (1) acquiring a genome DNA to be analyzed according to a conventional method; (2) carrying out PCR amplification on the genome DNA to obtain a PCR amplified product; after the reaction finishes, carrying out denaturation and renaturation on the PCR product; and (3) carrying out melting curve analysis on the PCR amplified product, and comparing with a melting curve generated by the PCR amplified product of the wild-type genome DNA, wherein the melting curve generated by the PCR amplified product of the mutant genome DNA firstly descends.
Owner:苏州科贝生物技术有限公司
Cyprinidherpesvirus 2 (CyHV-2) nested PCR (polymerase chain reaction) detection kit
ActiveCN102851403AHigh detection sensitivityShorten detection timeMicrobiological testing/measurementMicroorganism based processesBiologyDeoxyribonucleotide Triphosphate
The invention discloses a Cyprinidherpesvirus 2 (CyHV-2) nested PCR (polymerase chain reaction) detection kit which is characterized in that six 1.5mL centrifuge tubes containing reagents are arranged on an inner pad (2) of a box body (1). The kit comprises a 10* reaction buffer, primers P1 and primers P2, wherein the 10* reaction buffer contains 100mM of Tris-HCL, 500mM of KCl, 15mM of MgCl2, 2mM of dNTPs (deoxyribonucleotide triphosphates) (3), and 1U / mu L Taq enzyme (4); the primers P1 contain 10mu M of CyHV-2 P1F and 10mu M of CyHV-2 P1R (5); and the primers P2 contain 10mu M of CyHV-2 P2F and 10mu M of CyHV-2 P2R (6), pure water (7) and 10mu g / mL positive DNA (deoxyribonucleic acid) (8). The sequences of the outer primers P1 are as follows: CyHV-2 P1F: TGAAATGTCAAAAGTGGATGG, and CyHV-2 P1R: TATTCCCAGACAGCCTTCAAA; and the sequences of the inner primers P2 are as follows: CyHV-2 P2F: GAACACCGCTGCTCATCATC, and CyHV-2 P2R: ACTCTTCGCAAGTCCTCACC. Two amplification processes are carried out by the inner and outer primers to finally obtain the DNA product of 357bp. The invention is simple to operate, is convenient and quick, and has the advantages of low detection limit, high sensitivity and accurate and reliable detection result.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Kit for loop-mediated isothermal amplification detection of Mycoplasma ovipneumoniae and preparation and usage methods thereof
ActiveCN102634602AQuick checkReduce high costMicrobiological testing/measurementMicroorganism based processesPositive controlSpecific detection
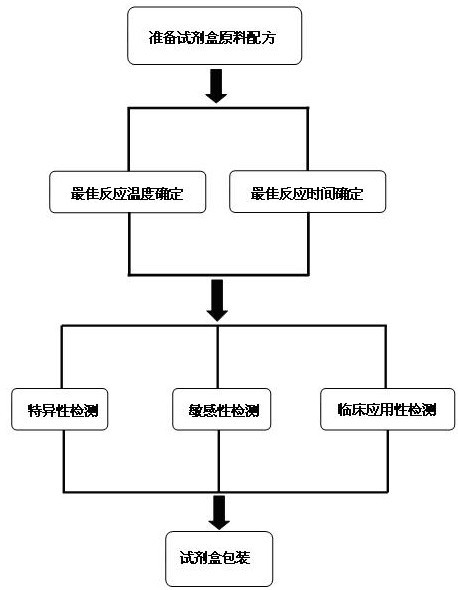
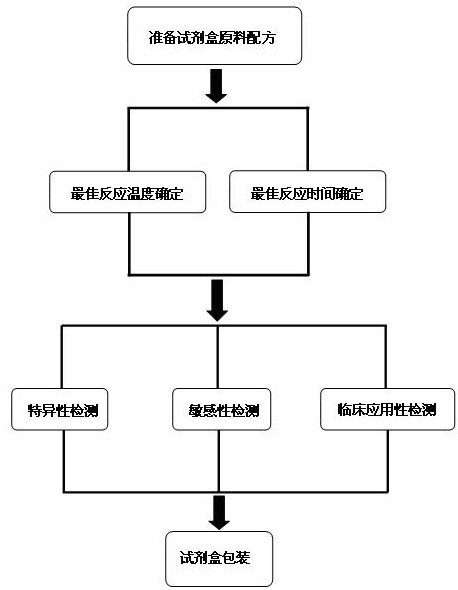
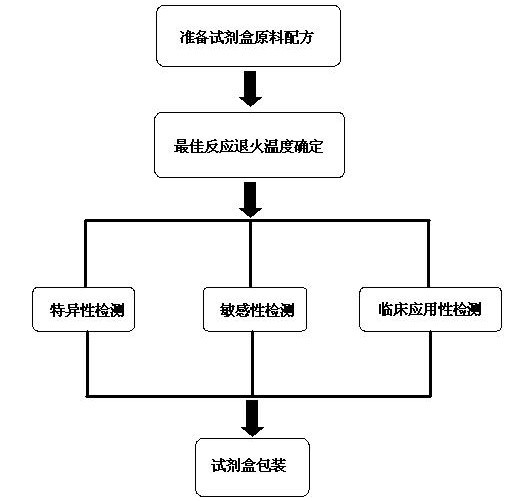
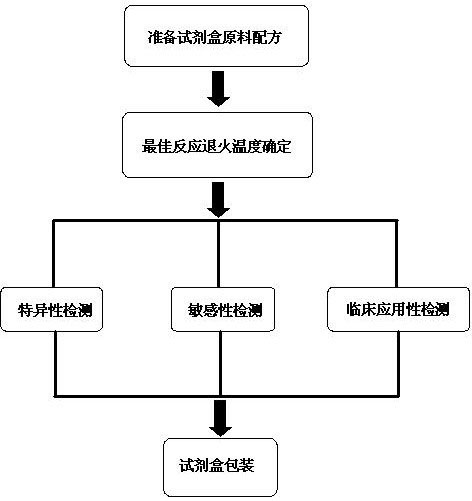
The invention discloses a raw material composition of a kit for loop-mediated isothermal amplification (LAMP) detection of Mycoplasma ovipneumoniae and a preparation method and a usage method of the kit. The raw material composition comprises 1000-2000muL of reaction solution, 100-200muL of Bst (Bacillus stearothermophilus) DNA polymerase, 50-100muL of positive control, 50-100muL of negative control, 1-2mL of liquid paraffin and 1-2mL of ultrapure water. The reaction solution comprises an inner primer mixture solution, an outer primer mixture solution, an LAMP reaction buffer, Mg<2+> and dNTPs (deoxyribonucleotide triphosphates), wherein the volume ratio of the five liquids in the reaction solution is 2:2:2.5:2:1. The preparation method comprises the following steps: 1) determination of an optimum reaction temperature and an optimum reaction time; 2) specific detection, sensibility test and clinical application detection and 3) kit packaging. The kit disclosed by the invention has rapid, simple and accurate characteristics for pathogen detection of goat suspected cases of mycoplasma ovipneumoniae infection in goat farms.
Owner:GUIZHOU UNIV
Kit and method for detecting aminoglycoside drug-induced deafness-sensitive gene
ActiveCN102925562ALong operating timeEasy to operateMicrobiological testing/measurementFluorescent pcrAminoglycoside Drugs
The invention relates to the field of gene detection, and particularly relates to a kit and method for detecting an aminoglycoside drug-induced deafness-sensitive gene through a fluorescent PCR (polymerase chain reaction)-melting curve method. The technical scheme of the invention provides a kit for detecting an aminoglycoside drug-induced deafness-sensitive gene. The kit comprises a PCR solution, a negative quality control sample and a positive quality control sample, wherein the PCR solution comprises primers, a PCR Buffer, a dNTP (deoxyribonucleotide triphosphate) mixed solution, saturated fluorescent dyes, a Taq enzyme and a UNG enzyme. In the invention, the specific PCR primers are designed according to SNP (single nucleotide polymorphism) sites of the aminoglycoside drug-induced deafness-sensitive gene, two aminoglycoside drug-induced deafness-sensitive sites can be detected in one PCR, the operation can be finished in one tube, and the detection is quick, accurate and wide in range.
Owner:亚能生物技术(深圳)有限公司
Primer, kit and method for quickly detecting HLA-B*5801 allele
ActiveCN104017898AImprove bindingAvoid it happening againMicrobiological testing/measurementDNA/RNA fragmentationHLA-BBiology
The invention belongs to the technical field of gene engineering, and discloses a primer for quickly detecting HLA-B*5801 allele, a kit containing the primers and a method for quickly detecting HLA-B*5801 allele by using the primers and kit. By using the HLA-B*5801 gene specific primer and adopting the multiplex primer combination design, the SNP (single-nucleotide polymorphism) site of the HLA-B*5801 gene is completely covered, the specific combination is enhanced, and the generation of the false positive is prevented more effectively. Besides, the specific primer, dNTPs (deoxyribonucleotide triphosphates), PCR (polymerase chain reaction) buffer solution and dyes are mixed previously, thereby greatly saving the operation time and workload; and the method is quick, simple, accurate and visual, and can the screening and typing experiment of the whole gene within 3 hours, thereby solving the problem of safe application instructions of the HLA-B*5801 gene in drugs for treating gout and the like.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
LAMP (loop-mediated isothermal amplification) kit for rapid detection of staphylococcus aureus
InactiveCN102864226AQuick checkThe detection process is fastMicrobiological testing/measurementMicroorganism based processesForward primerSerodiagnoses
The invention discloses an LAMP (loop-mediated isothermal amplification) kit for rapid detection of staphylococcus aureus. The LAMP kit for rapid detection of staphylococcus aureus comprises LAMP reaction fluid, a standard positive template and a negative quality control standard. The LAMP reaction fluid contains large fragments in Bst DNA (deoxyribonucleic acid) polymerase, primers, LAMP 10Xbuffer, dNTPs (deoxyribonucleotide triphosphates) solution, MgSO4 solution and betaine. The primers include forward primers and reverse primers. The LAMP kit for rapid detection of staphylococcus aureus has the advantages that the LAMP kit is high in specificity, high in sensitivity, high in speed, simple and high in reliability, results are recognizable to naked eyes, and the like. The LAMP kit is applicable to rapid qualitative detection of staphylococcus aureus in industrial foods, and can be a substitute for commonly used traditional culture methods and serodiagnosis methods.
Owner:WUHAN ZHENFU PHARMA CO LTD
Primers and probes for detecting genes associated with schizophrenia, bipolar affective disorders and major depression and kits and preparation methods thereof
InactiveCN102146479AMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologySchizophrenia
The invention discloses a kit for detecting genes associated with schizophrenia, bipolar affective disorders and major depression, which belongs to the field of biotechnology, and consists of a polymerase chain reaction (PCR) reagent group and a ligase detection reaction (LDR) reagent group, wherein the PCR reagent group comprises buffer solution, deoxyribonucleotide triphosphate (dNTP) mixed solution, Taq DNA polymerase, pure water, an upstream primer represented by SEQ ID No.1 and a downstream primer represented by SEQ ID No.2; and the LDR reagent group comprises buffer solution, dNTP mixed solution Taq DNA polymerase, pure water, a probe represented by SEQ ID No.3, a probe represented by SEQ ID No.4 and a probe represented by SEQ ID No.5. In the invention, the operation is simple and convenient, the cost is low, and the kit is developed for bipolar affective disorders. The result is reliable, the stability is high and the sensitivity is high.
Owner:SHANGHAI JIAO TONG UNIV +1
Detection of nucleic acid biomarkers using polymerization-based amplification
InactiveUS20090137405A1Easy to detectFast labelingMicrobiological testing/measurementLibrary member identificationSingle strandBiotin
The invention provides methods for highly-specific detection of hybridization of single stranded nucleic acids. The invention also provides methods for target identification which rely on this highly-specific hybridization detection. Targets suitable for detection include, but are not limited to, nucleic acid biomarkers. The methods of the invention can employ an on-chip, DNA polymerase-dependent labeling scheme termed primer extension (PEX) to couple biotinylated deoxyribonucleotide triphosphate (dNTP) molecules to nucleic acid hybrids bound to a solid substrate, allowing for subsequent recognition by biotin-binding-protein-labeled photoinitiators. Surface-initiated polymerization from these surface bound photoinitiators can lead to the formation of macroscale amounts of polymeric material, thereby amplifying the signal from the initial molecular recognition event.
Owner:UNIV OF COLORADO THE REGENTS OF
High throughput mutation screening methods and kits using a universalized approach - differential sequence fill-in (dsf)-enabled sequential adapter ligation and amplification
InactiveUS20090075276A1Facilitate simultaneous detectionProhibiting further ligationMicrobiological testing/measurementFermentationScreening methodSingle strand
This disclosure teaches high throughput mutation screening methods allowing simultaneous analysis of multiple genetic regions of interest and sensitive detection of very low frequency mutation(s) by the use of a universalized approach. Methods comprise treating RNA:DNA heteroduplexes of interest with a ribonuclease, sequence extension by an RNA-primed DNA polymerase, ligation with a blocking adapter, and differential sequence fill-in followed by single-strand-specific nuclease digestion to permit full-length sequence extension and subsequent ligation with a tagged reporter adapter solely in mutants filled in with a complementary deoxyribonucleotide triphosphate. By forming tagged mutant-dual adapter hybrids or mutant-triple adapter hybrids, the detection and / or quantification of mutants may be directed to the commonly shared tag(s) or flanking adapter sequences for signal detection / enhancement or sequence amplification in all different mutants regardless of the source or the number of mutations involved, thereby avoiding the tremendous effort of multiple target-specific sequence amplifications. Methods may be performed wholly or partially in solution, on solid phase media, in large scale, adapted for automated or semi-automated analysis, and any combinations thereof.
Owner:LEE MING SHENG +2
Kit and method for detecting mutant alpha-Mediterranean anemia genes through HRM (high resolution melting) method
InactiveCN102925560AInhibitory complexEasy to detectMicrobiological testing/measurementFluorescence/phosphorescenceFluorescencePolymerase L
The invention relates to a disease gene detection technology, and particularly relates to a kit and method for detecting mutant alpha-Mediterranean anemia genes through an HRM (high resolution melting) method. The kit provided by the invention comprises two PCR (polymerase chain reaction) tubes, namely an alpha-PCR tube 1 and an alpha-PCR tube 2, wherein each PCR tube contains a PCR reagent; the PCR reagent comprises primers, a PCR Buffer, dNTPs (deoxyribonucleotide triphosphate), MgCl2, DNA (deoxyribonucleic acid) polymerase and saturated fluorescent dyes. By using the optimized PCR-HRM reaction conditions, 6 mutant alpha-Mediterranean anemia genes, including one of alpha-cod30, alpha-cod31, alpha-cod59, alpha-WM-cod122, alpha-QS-cod125 and alpha-CS-cod142 or a combination of more than one, can be simultaneously detected.
Owner:泰普生物科学(中国)有限公司
Taq DNA (deoxyribonucleic acid) polymerase cold start activity detection metho
InactiveCN102534036ASimple and fast operationHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceForward primerDeoxyribonucleotide Triphosphate
The invention relates to a Taq DNA (deoxyribonucleic acid) polymerase cold start activity detection method which comprises the following steps: designing primers and a false template according to a template, mixing the forward primer or reverse primer which is complementary withthe 5'terminal part of the false template, and carrying out denaturalizing annealing reaction to obtain the mixture;Adding the mixture and various constituents required by the PCR reaction, including template DNA, 5'terminal part, forward primer or reverse primer which is not complementary with the false template, PCR buffer, magnesium chloride, dNTP (deoxyribonucleotide triphosphate) mixture and Taq DNA polymerase to be measured, into a PCR tube to carry out PCR reaction; and judging whether the detected DNA polymerase has cold start activity according to the detection result of the PCR product. The method is simple to operate, has the characteristic of sensitivity, can effectively detect very weak cold start activity, and overcomes the defects of subjectivity and arbitrariness in the existing method.
Owner:HENAN UNIV OF SCI & TECH
Chip and method for real-time PCR (polymerase chain reaction) gene detection at polygenic mutation site
The invention discloses a chip and method for real-time PCR (polymerase chain reaction) gene detection at a polygenic mutation site. The gene detection chip comprises a porous PCR plate, wherein a PCR reaction system is arranged in each pore of the porous PCR plate; the PCR reaction system comprises a plurality of primer pairs for the specific amplification of target gene targeting sequences, fluorescent probes, PCR buffer solutions, dNTPs (deoxyribonucleotide triphosphates) and a GoldTaq enzyme; each primer pair contains a continuous nucleotide sequence composed of at least 15 continuous nucleotides in target gene exons; and fluorescent molecules of the fluorescent probes emit fluorescent signals in the PCR amplification process. The detection rate of the method on big genes can be up to 98% or above.
Owner:杨楠 +1
Kit and detection method for detecting vibrio harveyi
InactiveCN102108402AQuick checkGuaranteed reliabilityMicrobiological testing/measurementBetainePolymerase L
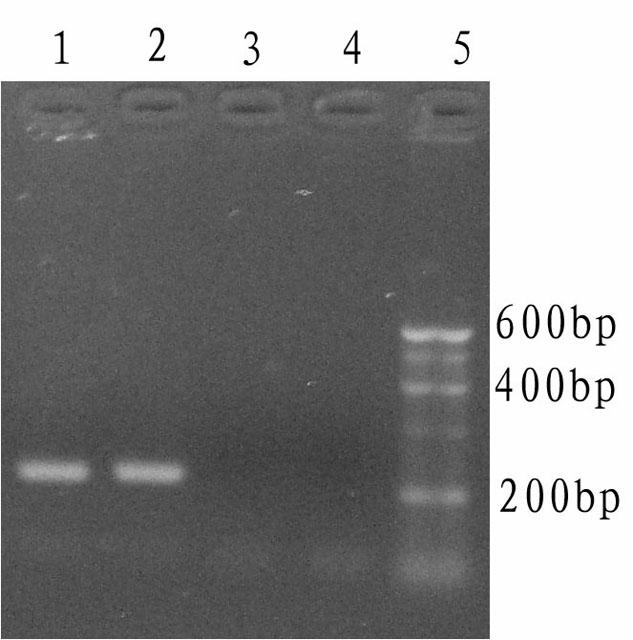
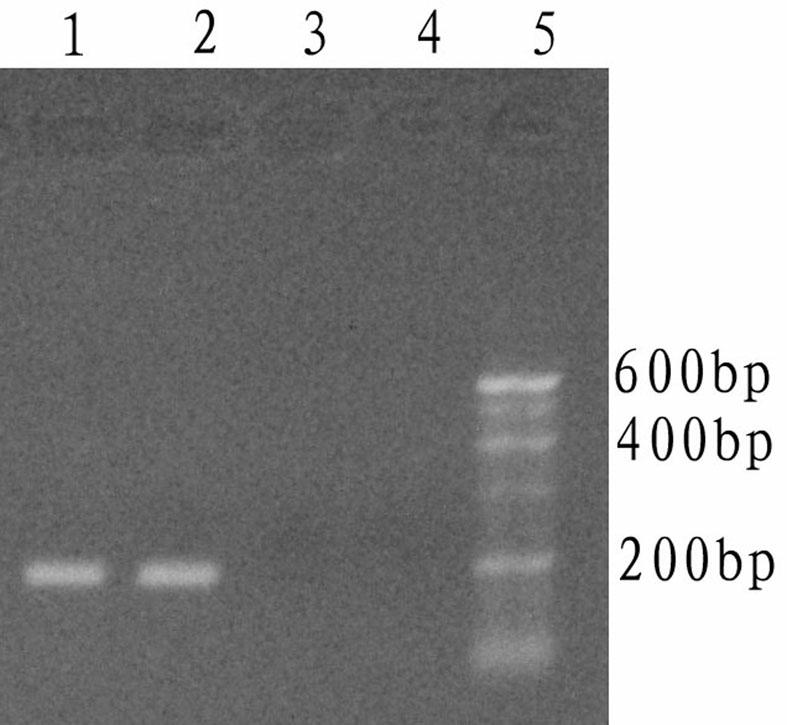
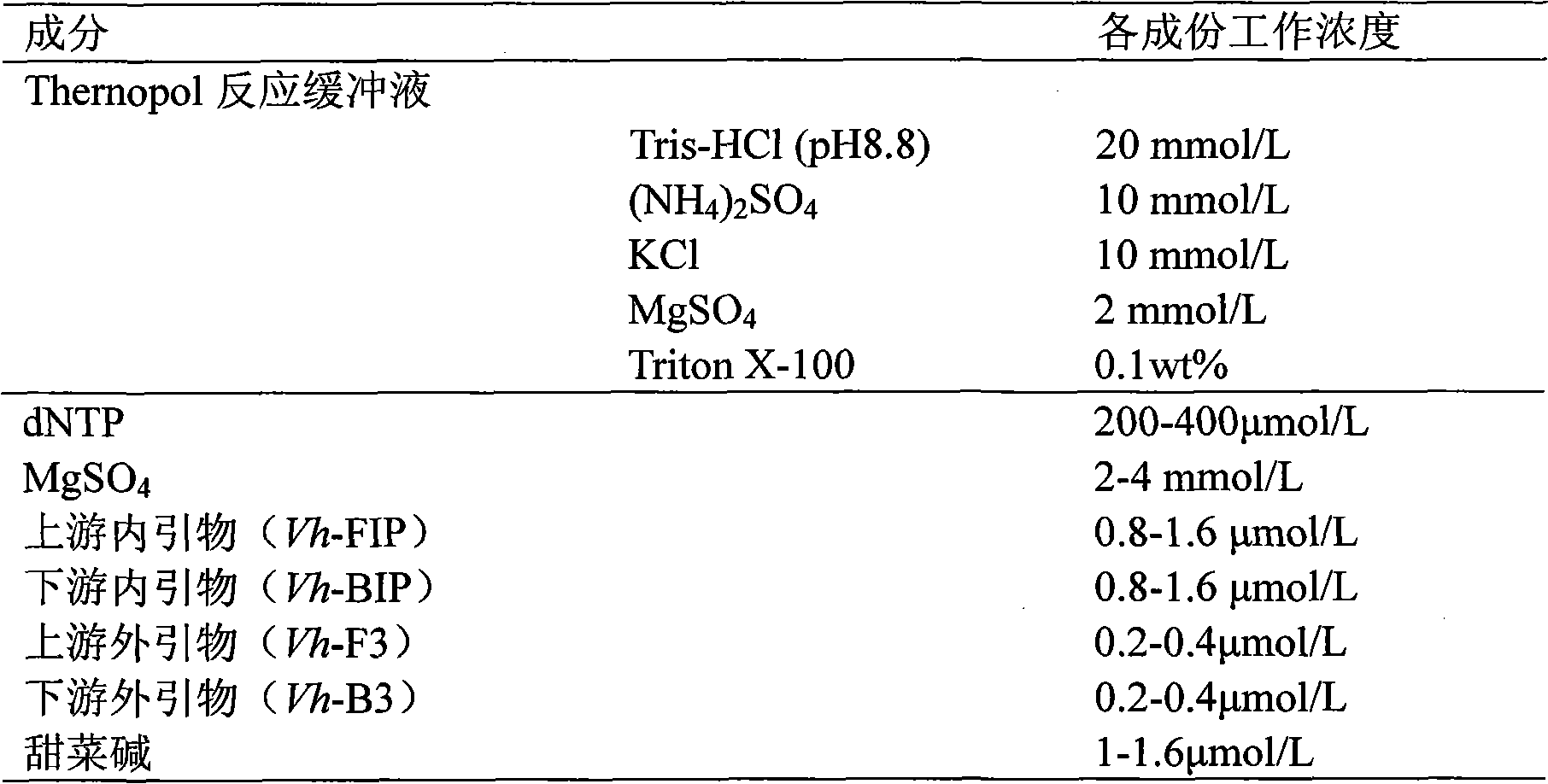
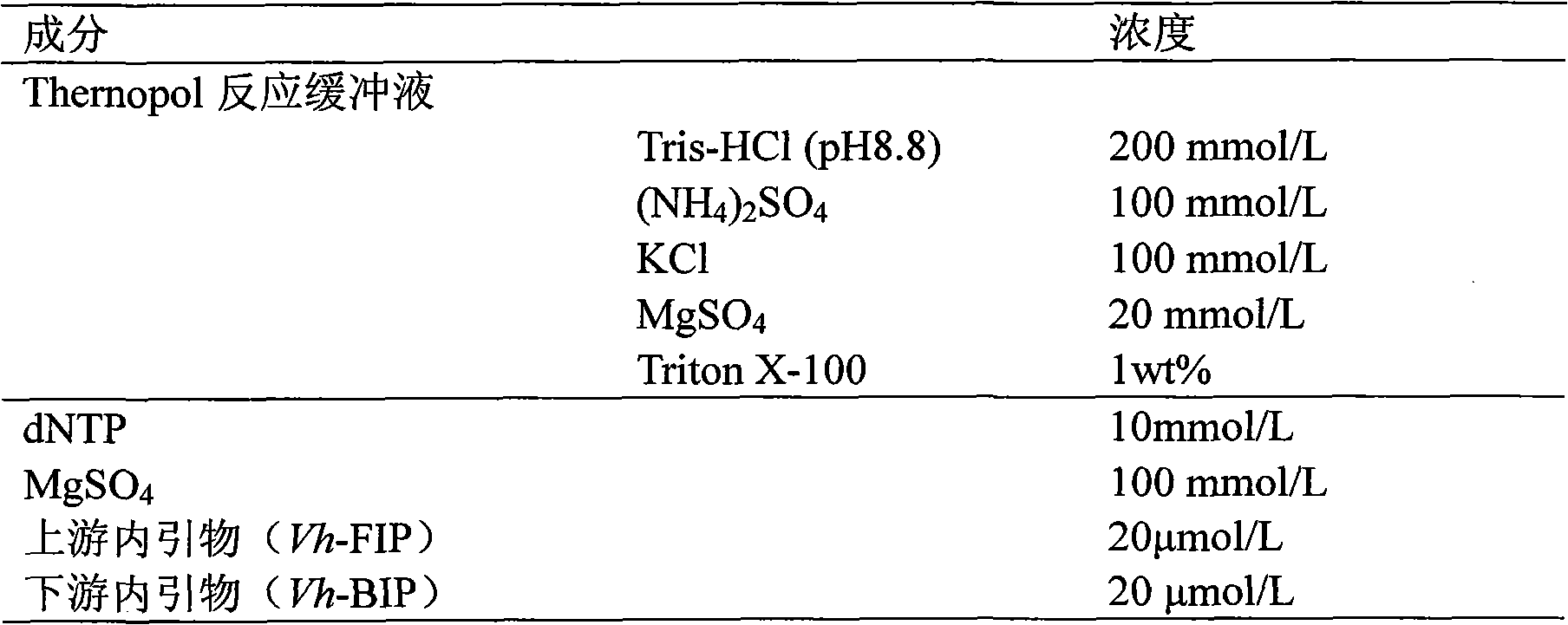
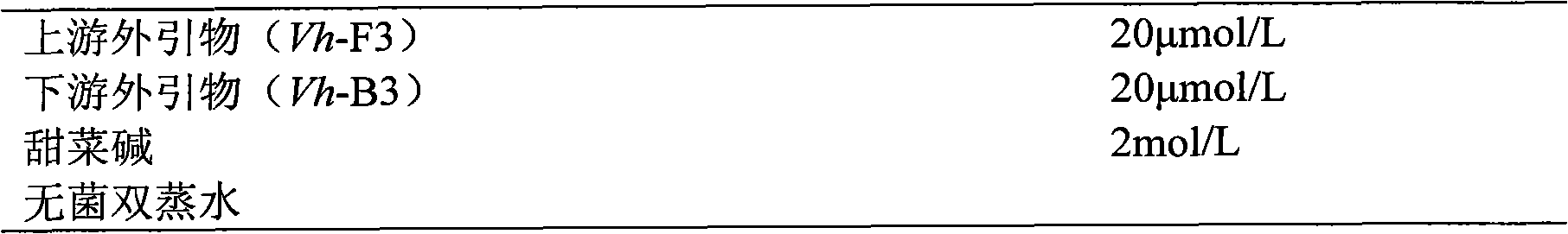
The invention relates to a kit and detection method for detecting vibrio harveyi by using the loop-mediated isothermal amplification technique. The kit comprises a loop-mediated isothermal amplification reaction solution, a Bst DNA (deoxyribonucleic acid) polymerase and a color-developing agent, wherein the reaction solution contains a reaction buffering solution Thernopol, dNTP (deoxyribonucleotide triphosphate), MgSO4, a forward inter primer (Vh-FIP) TTCGCTTTCGCGAGCCATCTGGTTACCAATTGATCGCCCG, a reverse inter primer (Vh-BIP) ACGCAGAATCAAGCAGTGTGCCGATTTATTCGCCACGACA, a forward outer primer (Vh-F3) CAAAACGGTTCCGAAACGC, a reverse outer primer (Vh-B3) TCGATTCCCCAAGTTTGGAG, a betaine, and sterile distilled water. The detection method comprises the following steps: extracting bacterium DNAs, carrying out loop-mediated isothermal amplification, carrying out color-developing detection and the like. By designing the primers according to the vibrio harveyi toxR gene conservation area and detecting by using the LAMP (loop-mediated isothermal amplification) technique, the invention achieves high specificity and high sensitivity; the kit has the advantages of high detection speed, high accuracy, excellent sensitivity, convenient on-site application and the like; and the defects of long cycle, low sensitivity, high cost, difficult on-site application and the like in the prior art are solved.
Owner:陈吉刚
Giant salamander iridescent virus taqman real-time fluorescent quantitative PCR kit and its application
ActiveCN102286642AAccurate determination of starting copy numberMeeting the requirements for rapid differential diagnosis of giant salamander iridescent virusMicrobiological testing/measurementFluorescence/phosphorescenceForward primerEscherichia coli
The invention discloses a giant salamander iridovirus TaqMan real-time fluorescent quantitative PCR (polymerase chain reaction) kit and application thereof. The kit comprises sterile double distilled water, a 10*Taq reaction buffer, 2.5mmol / L dNTPs (deoxyribonucleotide triphosphates) and a fluorescent quantitative reaction liquid. The kit is characterized in that the fluorescent quantitative reaction liquid contains primers and a fluorescent probe, wherein the primers are a forward primer and a reverse primer, the forward primer is 5'-GCGGTTCTCACACGCAGTC-3', the reverse primer is 5'-ACGGGAGTGACGCAGGTGT-3', and the fluorescent probe sequence is 5'-AGCCGACGGAAGGGTGTGTGAC-3'; the 5' terminal marker of the fluorescent probe is FAM, and the 3' terminal marker is ROX; 5U / mu L Taq DNA (deoxyribonucleic acid) polymerase and standard positive template pMCP are a pMD19-T vector composed of 1392 nucleotide segments containing giant salamander iridovirus MCP gene; and the vector proliferates in colibacillus E.coli DH5a. The invention also discloses application of the kit in detection of giant salamander iridovirus pathogen. The kit is quantitative and accurate, and has the advantage of high detection speed (the detection only takes 1 hour, and totally takes 2-3 hours plus the extraction and preparation of the DNA); the method is easy to implement and simple to operate; and the invention can be used for simultaneously detecting high-flux samples, and the accuracy is up to 98%.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Duplex PCR diagnostic kit for detection of contagious caprine pleuropneumonia pathogen and preparation and use methods thereof
InactiveCN102634601ADefinitive diagnosisrapidReduce complexityMicrobiological testing/measurementMicroorganism based processesDuplex pcrPositive control
The invention discloses a raw material composition of a duplex PCR (polymerase chain reaction) diagnostic kit for detection of contagious caprine pleuropneumonia pathogen and a preparation method and a use method of the kit. The raw material composition comprises 1000-2000muL of PCR reaction liquid, 100-200muL of TaqDNA polymerase, 50-100muL of positive control, 50-100muL of negative control and 1-2mL of ultrapure water. The PCR reaction solution comprises a primer 1 mixture solution, a primer 2 mixture solution, a PCR reaction buffer and dNTPs (deoxyribonucleotide triphosphates), wherein the volume ratio of the four liquids in the PCR reaction solution is 2:2:5:1. The preparation method comprises the steps of determination of an optimum reaction annealing temperature, specific detection, sensibility test, clinical application detection and kit packaging. An effective method for rapid deterministic diagnosis of suspected goat pathogen and suspected cases of contagious caprine pleuropneumonia in goat farms is provided by the invention, providing technical support for the prevention and control of contagious caprine pleuropneumonia.
Owner:GUIZHOU UNIV
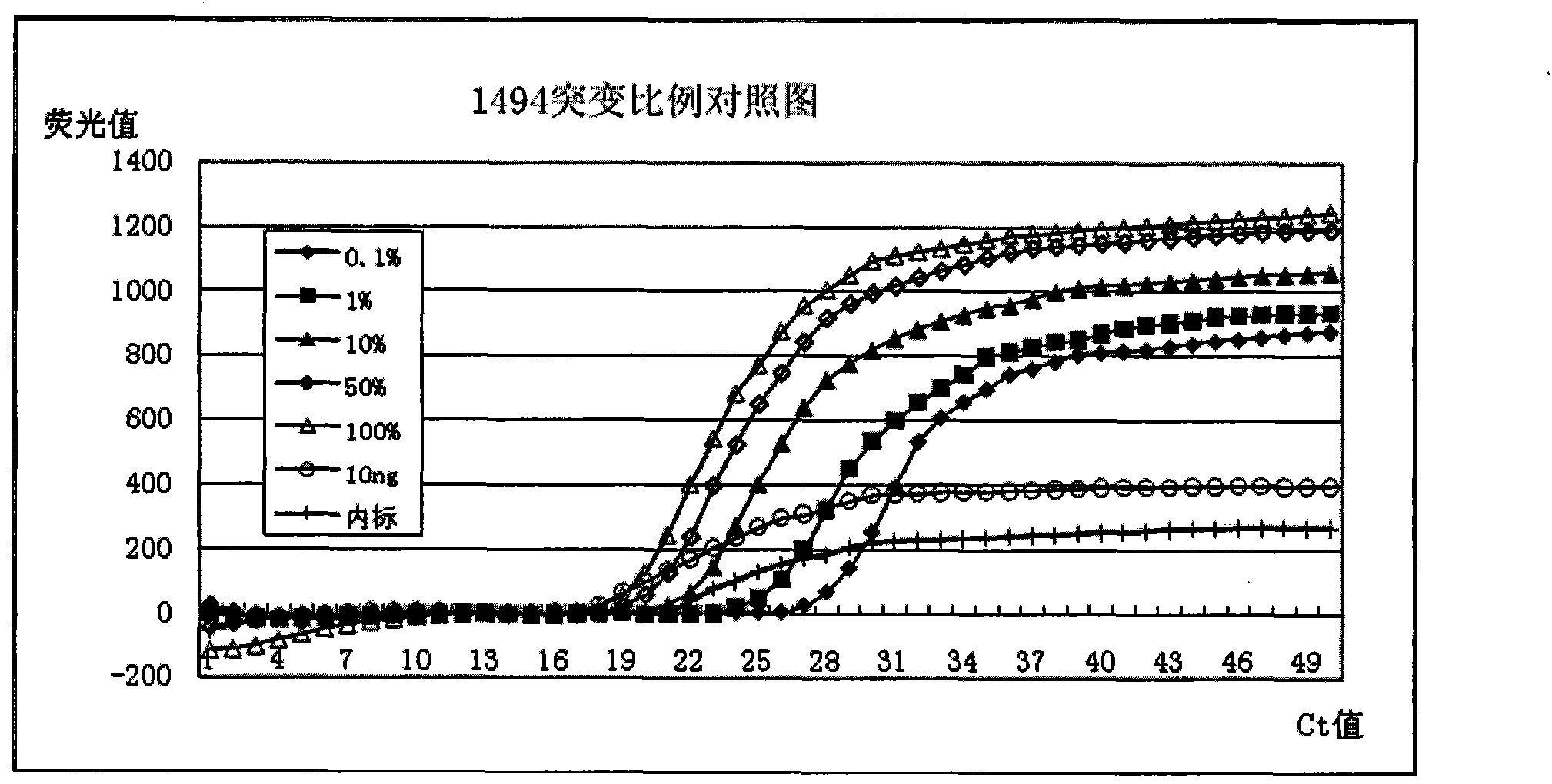
Deafness susceptible gene mitochondrion 12SrDNA 1555A>G and 1494C>T mutant ratio detection kit
InactiveCN103451302AIncrease the Tm valueShorten the lengthMicrobiological testing/measurementFluorescenceMutant
The invention discloses a deafness susceptible gene mitochondrion 12SrDNA 1555A>G and 1494C>T mutant ratio detection kit which comprises amplification reagents and a series of standard substances, wherein the amplification reagents comprise a reaction mixture of a PCR (polymerase chain reaction) buffer solution, MgCl2 and dNTPs (deoxyribonucleotide triphosphates), Taq enzyme, ultrapure water, and a high-specificity amplified mitochondrion 2SrDNA:1494C>T and 1555A>G primer mixture; and the series of standard substances comprise a 1494C>T mutant ratio standard substance and a 1555A>G mutant ratio standard substance. By using the 2 deafness susceptible gene sites as the detection objects, the deafness susceptible gene sites are subjected to amplification and fluorescence quantitative detection and are compared with the mutant ratio standard substances to screen out individuals containing the site mutants and determine various mutant ratios. The kit has important meanings for screening deafness susceptible genes and especially newborn deafness genes.
Owner:步迅 +1
PCR (polymerase chain reaction) reaction solution and kit, and PCR method
InactiveCN104328170AImprove efficiencySave configuration timeMicrobiological testing/measurementGlycerolBovine serum albumin
The invention relates to the technical field of biology, particularly a PCR (polymerase chain reaction) reaction solution and kit, and a PCR method. The PCR reaction solution is prepared from dimethyl sulfoxide (DMSO), bovine serum albumin (BSA), dNTP (deoxyribonucleotide triphosphate), 10*PCR buffer, bromphenol blue solution, glycerol solution with the solute volume percent of 30%, and rTaq enzyme. The PCR reaction solution for PCR experiments is optimized; especially a certain proportion of glycerol solution is added, and the combination of the BSA and DMSO is added to ensure the stability and activity of the Tag enzyme, reduce the formation of the primer dimer and shorten the PCR reaction solution configuration time; and in the PCR product detection process by agarose gel electrophoresis, the detection can be performed only by adding the nucleic acid dye, thereby saving abundant time for detecting the PCR product and enhancing the PCR amplification efficiency.
Owner:FUJIAN NORMAL UNIV
Method and reagent for gene sequence analysis
InactiveUS20120270210A1Easy to analyzeEliminate the effects ofSugar derivativesMicrobiological testing/measurementNucleotidePurine
Provided is a nucleic acid substrate which has nucleic acid substrate characteristics equivalent to those of dATP, has a low substrate specificity for luciferase, exerts no negative effect on enzymatic reactions such as a complementary-strand synthesis, and therefore is particularly suitable for the pyrosequencing method. As a nucleic acid substrate complementary to nucleotide T, a 7-substituted deoxyribonucleotide triphosphate whose 7-position of a purine group is modified by a substituent is used as a substitute for a nucleotide α-thiotriphosphate analog.
Owner:HITACHI LTD +1
Salmonella Indiana PCR (polymerase chain reaction) detection kit and non-diagnosis detection method thereof
ActiveCN107988405AStrong specificityQuick checkMicrobiological testing/measurementMicroorganism based processesTyping methodsPolymerase L
The invention discloses a salmonella Indiana PCR (polymerase chain reaction) detection kit and a non-diagnosis detection method thereof. The kit comprises a 10*PRC buffer solution, 2.5U / mul of Taq DNA(deoxyribonucleic acid) polymerase, 10mM dNTPs (deoxyribonucleotide triphosphates), a specific PCR detection primer of salmonella Indiana, a positive reference substance and a negative reference substance, wherein the positive reference substance is DNA of salmonella Indiana ATCC51959 genome; the negative reference substance is sterilized double distilled water. The invention also discloses a PRCmethod using the kit to detect the salmonella Indiana. The method has the advantages that the operation is quick and simple, the specificity is strong, and the sensitivity is high; the salmonella Indiana can be detected without using salmonella diagnosis serum; compared with the traditional salmonella serological typing method, more advantages are realized in aspects of ssdetection time and detection cost, and the method is suitable of batched detection.
Owner:JIANGSU INST OF POULTRY SCI
Kit for in-vitro single base damage repair detection through real-time quantitative PCR (Polymerase Chain Reaction)
InactiveCN102181528AGuaranteed specificityAchieving absolute quantificationMicrobiological testing/measurementLysisDamage repair
The invention discloses a kit for in-vitro single base damage repair detection through real-time quantitative PCR (Polymerase Chain Reaction). The kit comprises a 10X detection buffer solution, a 5X low-salt cell lysis solution, a 2X cell nucleoprotein or mitochondrion protein extract, a 20X detection substrate and a 2XqPCR damage repair buffer solution, wherein the repair buffer solution comprises taq enzyme, dNTP (Deoxyribonucleotide Triphosphate), probes and primers. In the kit, primers of an amplification internal reference and damage base qPCR (quantitative PCR) templates are the same, the amount of damage base repair can be calculated according to the amount of the internal reference. Since a complementary template only has 14 bases which are complementary with the primer 2, annealing of the complementary template and the primer 2 is not produced in a qPCR process, and specificity of damage repair detection is guaranteed. A 3' tail end of the complementary template is dideoxyoligonucleotide, so that extension of the template per se is avoided. The probe for detecting the damage template and the probe for detecting the internal reference template are marked with different fluorescent groups, so that absolute quantification of repair of the damage template is realized through detection in the same qPCR system.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com