Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Mycoplasma ovipneumoniae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma ovipneumoniae is a species of Mycoplasma bacteria that most commonly inhabits and affects ovine animals. M. ovipneumoniae is a respiratory pathogen of domestic sheep, domestic goats, bighorn sheep, mountain goats, and other caprinae that can both cause primary atypical pneumonia and also predispose infected animals to secondary pneumonia with other agents, including Mannheimia haemolytica. Several mechanisms are involved in the pathogenicity of M. ovipneumoniae, including altering macrophage activity, adhering to the ruminants' ciliated epithelium via its polysaccharide capsule, inducing the production of autoantibodies to cilary antigens, and suppressive activity on lymphocytes, all of which are important factors that contribute to the disease in sheep and other small ruminants. The bacterium also has the ability to act as a prediposing factor for other bacterial and viral infections.
Preparation technology of mycoplasma ovipneumoniae inactivated vaccine
InactiveCN103110583ASimple preparation processInfection controlAntibacterial agentsBacterial antigen ingredientsTiterTGE VACCINE
The invention discloses a preparation technology of a mycoplasma ovipneumoniae inactivated vaccine, which is based on the improvement on the preparation technology of the mycoplasma ovipneumoniae inactivated vaccine, and provides a preparation method of the mycoplasma ovipneumoniae inactivated vaccine. Through the application of the method provided by the invention, an ideal efficient mycoplasma ovipneumoniae inactivated vaccine adjuvant is screened, the content of toxin in antigen is greatly reduced, and the vaccine safety is improved. After the vaccine is applied to sheep, the antibody is generated early, the titer is high, the duration is long, and the mycoplasma ovipneumoniae infection can be effectively prevented and controlled.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Kit for loop-mediated isothermal amplification detection of Mycoplasma ovipneumoniae and preparation and usage methods thereof
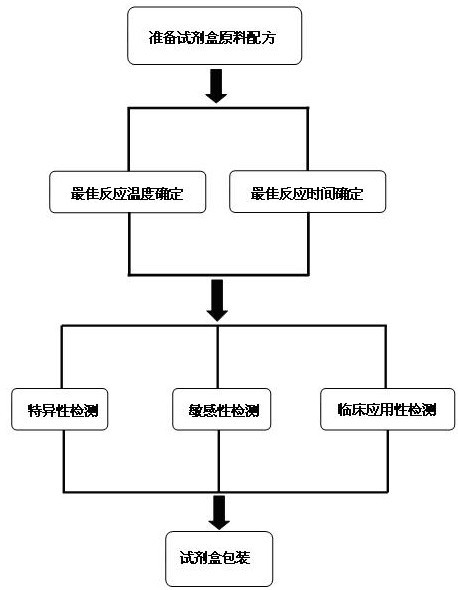
ActiveCN102634602AQuick checkReduce high costMicrobiological testing/measurementMicroorganism based processesPositive controlSpecific detection
The invention discloses a raw material composition of a kit for loop-mediated isothermal amplification (LAMP) detection of Mycoplasma ovipneumoniae and a preparation method and a usage method of the kit. The raw material composition comprises 1000-2000muL of reaction solution, 100-200muL of Bst (Bacillus stearothermophilus) DNA polymerase, 50-100muL of positive control, 50-100muL of negative control, 1-2mL of liquid paraffin and 1-2mL of ultrapure water. The reaction solution comprises an inner primer mixture solution, an outer primer mixture solution, an LAMP reaction buffer, Mg<2+> and dNTPs (deoxyribonucleotide triphosphates), wherein the volume ratio of the five liquids in the reaction solution is 2:2:2.5:2:1. The preparation method comprises the following steps: 1) determination of an optimum reaction temperature and an optimum reaction time; 2) specific detection, sensibility test and clinical application detection and 3) kit packaging. The kit disclosed by the invention has rapid, simple and accurate characteristics for pathogen detection of goat suspected cases of mycoplasma ovipneumoniae infection in goat farms.
Owner:GUIZHOU UNIV
RPA primer for detecting mycoplasma ovipneumoniae
InactiveCN108913793AStrong specificityThe test result is accurateMicrobiological testing/measurementMicroorganism based processesProduct analysisBiology
The invention discloses a RPA primer for detecting mycoplasma ovipneumoniae. The upstream primer P1 is 5'-CTTAATGATGAGCGTAGTAGATACCAAACCAC-3', and the downstream primer P2 is 5'-GCTGAGGTCGGATTTGGACTAACAATATTTG-3'. The RPA primer is high in specificity, high in sensitivity and accurate in detection result. The invention further provides a RPA method for detecting the mycoplasma ovipneumoniae. The RPA method comprises the steps of primer synthesis, extraction of DNA in to-be-detected samples, RPA amplification, amplification product analysis and the like. The detecting method is easy to operate,and the quick, convenient and specific diagnostic method is provided for effective detecting and identifying the mycoplasma ovipneumoniae by basic veterinary workers.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
PCR (Polymerase Chain Reaction) amplification primer for quickly detecting mycoplasma ovipneumoniae, and application thereof
ActiveCN107988340AHigh sensitivityThe test result is accurateMicrobiological testing/measurementMicroorganism based processesNucleotide sequencingRepeatability
The invention discloses a PCR (Polymerase Chain Reaction) amplification primer for quickly detecting mycoplasma ovipneumoniae, and application thereof, which belong to the field of animal bacteriologyand molecular biology. The PCR amplification primer is formed by an upstream primer with a nucleotide sequence as shown in an SEQ ID NO:1 and a downstream primer with a nucleotide sequence as shown in an SEQ ID NO:2; the primer is used for preparing a PCR amplification kit for detecting the mycoplasma ovipneumoniae. A PCR detection method provided by the kit has high specificity and sensibility,good repeatability, and high reliability, can specifically detect the mycoplasma ovipneumoniae and quickly and accurately obtain a detection result, is low in price, simple and convenient to operate,and suitable for grass roots at the same time, and can be used as a quick, accurate, simple and convenient detection tool for quick identification in a mycoplasma ovipneumoniae laboratory and large-scale epidemiological investigation.
Owner:GUANGXI VETERINARY RES INST
Mycoplasma ovipneumoniae multi-epitope fusion antigen MO-meAg5 and preparing method and application thereof
InactiveCN105859845AAntibacterial agentsBacterial antigen ingredientsEscherichia coliExpression Library
The invention discloses a mycoplasma ovipneumoniae multi-epitope fusion antigen MO-meAg5 and a preparing method and application thereof. Antigen protein high in immunogenicity namely the mycoplasma ovipneumoniae multi-epitope fusion antigen MO-meAg5 can be obtained by extracting an MO genome, constructing and screening an expression library, constructing the MO multi-epitope fusion antigen and expressing the multi-epitope fusion antigen in escherichia coli, and a foundation is laid for further researching and developing a safe and efficient mycoplasma ovipneumoniae multi-epitope protein vaccine.
Owner:SHIHEZI UNIVERSITY
Primer and probe for detecting Mycoplasma ovipneumoniae
PendingCN107142331AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePcr method
The invention provides a primer and a probe for detecting Mycoplasma ovipneumoniae. A primer sequence comprises an upstream primer: 5'-CTTCGGGACTTATTGGAG-3', and a downstream primer: 5'-GATGCAAACTGATTTACTTG-3', and the probe sequence is as follows: 5'- AAGACCGATTGTCAGGCCGA-3'. The specific primer and the probe are designed according a p113 gene sequence of KR270152.1 registered in GenBank, a real-time fluorescence quantification PCR method is established aiming at the Mycoplasma ovipneumoniae p113 gene for the first time, the method has the advantages of good specificity, high sensitivity and good repeatability, and the primer and the probe can be used for detecting content of Mycoplasma ovipneumoniae in a clinic sample.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
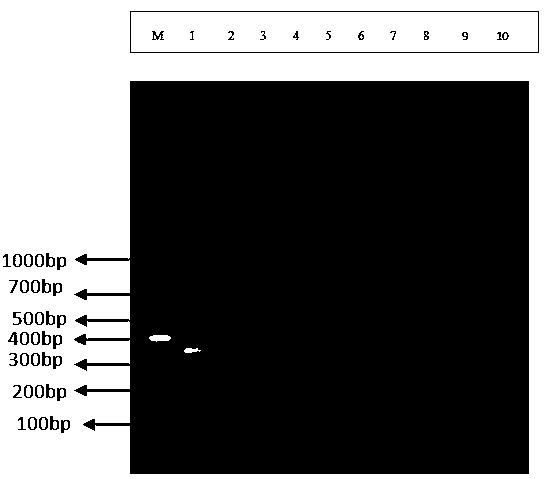
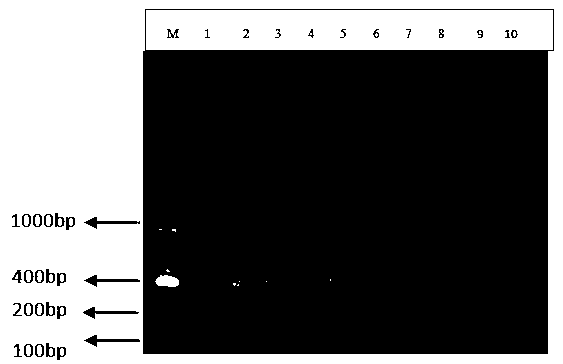
Special primer for double PCR of contagious pustular dermatitis virus and mycoplasma ovipneumoniae
ActiveCN106636470AQuick checkAccurate diagnosisMicrobiological testing/measurementMicroorganism based processesImpetigo contagiosaEpidemiologic survey
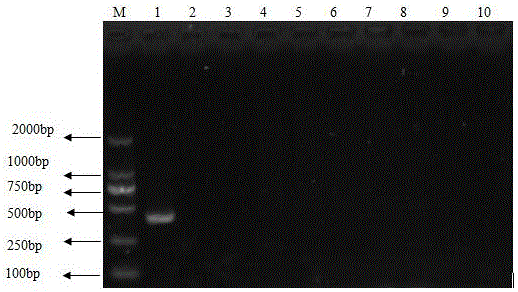
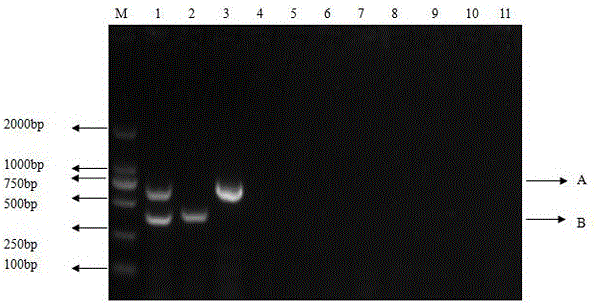
The invention provides a special primer for double PCR of a contagious pustular dermatitis virus and mycoplasma ovipneumoniae. According to the special primer, two pairs of specific primers capable of amplifying a B2L gene and a P80 gene are designed according to the B2L gene of the contagious pustular dermatitis virus and the P80 gene of the mycoplasma ovipneumoniae. Through optimization of the conditions of the primer concentration, the annealing temperature and the like, a double PCR method for fast detecting the contagious pustular dermatitis virus and the mycoplasma ovipneumoniae is built. A specific segment of 401bp appearing in an electrophoretogram has positive contagious pustular dermatitis virus, and the specific segment of 700bp has positive mycoplasma ovipneumoniae. Detection of the contagious pustular dermatitis virus and the mycoplasma ovipneumoniae in the same reaction system can be achieved, the special primer has the advantage that the pathogens are fast, specifically and accurately diagnosed, and a novel method is provided for differential diagnosis of infection of the two pathogens in production and epidemiological investigation.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Mycoplasma ovipneumoniae low-serum medium and preparation method thereof
ActiveCN106479936AAvoid pollutionLong storage timeBacteriaMicroorganism based processesMycoplasma culturePenicillin
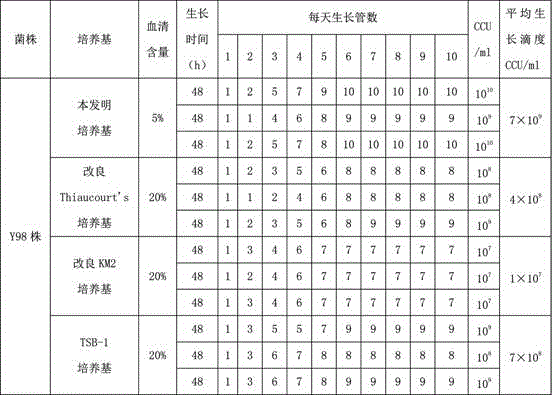
The invention discloses a mycoplasma ovipneumoniae low-serum medium, which consists of the following components: a PPLO broth, sodium pyruvate, phenol red, de-ionized water, lactose, tryptone, a fresh yeast extract, insulin, L-glutamine, L-cysteine, lactoalbumin hydrolysate, transferrin, penicillin and little horse serum; and the invention provides a preparation method of the mycoplasma ovipneumoniae low-serum medium. The mycoplasma ovipneumoniae medium provided by the invention has the following beneficial effects: a serum content is merely 5%, which is 1 / 4 of the serum content of a medium in the prior art, and the living bacterium titer of cultivated mycoplasma ovipneumoniae reaches 7*10<9> CCU / mL or above, which is significantly higher than that of the medium in the prior art. In comparison with the prior art, the mycoplasma ovipneumoniae low-serum medium provided by the invention has the characteristics of being low in serum content, rapid in growth and high in living bacterium titer.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Bivalent inactivated vaccine for goat mycoplasma pneumonia and chlamydia psittaci disease and preparation method thereof
ActiveCN111789941AAvoid infectionImprove securityAntibacterial agentsBacterial antigen ingredientsChlamydia psittaci infectionsMycoplasma pneumonia
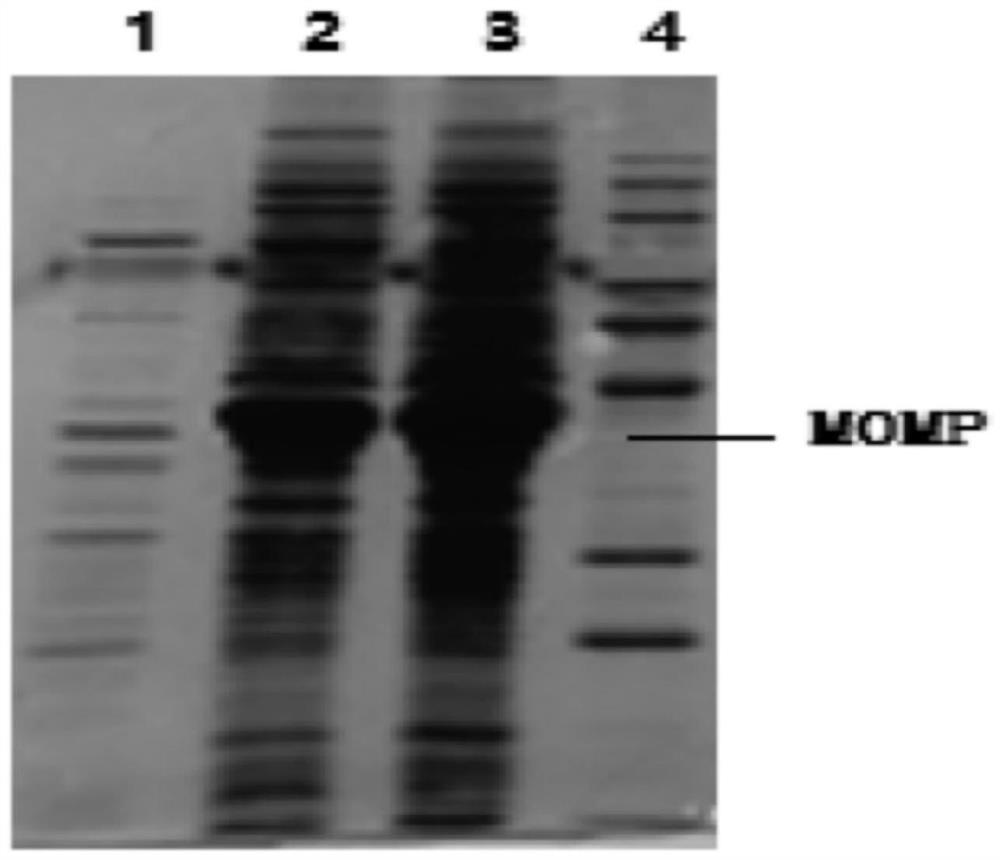
The invention discloses a bivalent inactivated vaccine for goat mycoplasma pneumonia and chlamydia psittaci and a preparation method thereof. The active ingredients of the bivalent inactivated vaccinecomprise inactivated Mycoplasma capricolum subsp. Capripneumoniae, inactivated mycoplasma ovipneumoniae and inactivated MOMP protein of chlamydia psittaci. The amino acid sequence of the MOMP proteinof the chlamydia psittaci is shown as a sequence 2 in a sequence table. The mycoplasma ovipneumoniae is mycoplasma ovipneumoniae SH-01 CGMCC No.16503. The Mycoplasma capricolum subsp. Capripneumoniaeis Mycoplasma capricolum subsp. Capripneumoniae GT-01 CGMCC No.16504. Experiments prove that the bivalent inactivated vaccine has good safety in immunizing experimental animals and target animals, and can effectively prevent goat mycoplasma pneumonia and chlamydia psittaci from infecting the experimental animals and the target animals. The vaccine has important significance for preventing and controlling prevalence and propagation of mycoplasma pneumonia and chlamydia psittaci of goats. The vaccine has important application value.
Owner:INNER MONGOLIA HUAXI BIOTECH
Mycoplasma ovipneumoniae vaccine strain, vaccine composition prepared from vaccine strain and application of vaccine composition
ActiveCN112481154AMake up for the shortcomings of limited protectionSafety test sampleAntibacterial agentsSsRNA viruses negative-senseDiseaseRespiratory tract disease
The invention discloses a mycoplasma ovipneumoniae strain, a vaccine prepared from the strain and an application of the vaccine. The mycoplasma ovipneumoniae strain is separated from diseased sheep, is named as a WM01 strain, is preserved in China General Microbiological Culture Collection Center, and has a strain preservation number of CGMCC No.19979. The mycoplasma ovipneumoniae WM01F6 strain obtained by separation is prepared into an inactivated vaccine, or is prepared into a bivalent inactivated vaccine together with a sheep parainfluenza virus type 3, and a safety test shows that all testsheep of a single vaccine and a bivalent vaccine have no local and systemic adverse reactions after being vaccinated. Efficacy test results show that after the vaccine immunizes a test animal, a goodprotection effect can be achieved on virulent virus attack, and the protection rate is at least 80%. Therefore, the invention provides an effective technical means for preventing the respiratory diseases of the sheep.
Owner:INNER MONGOLIA UNIVERSITY
Combined strain for preparing mycoplasma ovipneumoniae vaccine, mycoplasma ovipneumoniae trivalent inactivated vaccine and preparation method of inactivated vaccine
ActiveCN111690554AHigh immune protection rateImprove protectionAntibacterial agentsBacterial antigen ingredientsBiotechnologyStructural protein
The invention provides a combined strain for preparing a mycoplasma ovipneumoniae vaccine, a mycoplasma ovipneumoniae trivalent inactivated vaccine and a preparation method of the inactivated vaccine,and belongs to the technical field of veterinary biological products. The combined strain comprises mycoplasma ovipneumoniae strains MO_NM01, MO_NM02 and MO_NM03, the preservation number of the mycoplasma ovipneumoniae strain MO_NM01 is CGMCC No.19698, the preservation number of the mycoplasma ovipneumoniae strain MO_NM02 is CGMCC No.19699, and the preservation number of the mycoplasma ovipneumoniae strain MO_NM02 is CGMCC No.19700. Housekeeping genes, structural proteins and polyclonal antibodies of the strains in the combined strain are different, the trivalent inactivated vaccine preparedfrom the combined strain can play a good role in protecting different strains, and has high immune protection rate.
Owner:INNER MONGOLIA AUTONOMOUS REGION ACAD OF AGRI & ANIMAL HUSBANDRY SCI
Lower-serum and efficient culture medium of mycoplasma ovipneumoniae and preparation method of lower-serum and efficient culture medium
ActiveCN106635895AAvoid pollutionLong storage timeAntibacterial agentsBacterial antigen ingredientsHeterologousMycoplasma culture
The invention discloses a lower-serum and efficient culture medium of mycoplasma ovipneumoniae. The lower-serum and efficient culture medium is prepared from the following components: MEM, sodium pyruvate, glucose, Hank's liquid, fresh yeast leaching liquid, L-glutamine, L-cysteine, lactalbumin hydrolysate, calf thymus DNA (Deoxyribonucleic Acid), insulin, transferrin, penicillin, horse serum, phenol red and de-ionized water; the invention provides a preparation method of the lower-serum and efficient culture medium. The lower-serum and efficient culture medium of the mycoplasma ovipneumoniae, disclosed by the invention, has the beneficial effects that the content of the serum is only 5 percent and is 1 / 4 of the content of the serum in a culture medium in the prior art; the titer of mycoplasma ovipneumoniae semi-finished-product bacterium liquid prepared by the method reaches 10<10>CCU / ml and is obviously higher than that of the culture medium in the prior art. According to the lower-serum and efficient culture medium of the mycoplasma ovipneumoniae, disclosed by the invention, the sensitive stress response to a goat body, caused by heterologous serum, is alleviated, and the biosafety is improved; the titer of bacterium liquid of living bacteria is also improved and the production cost is reduced.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
In-vitro culture medium of mycoplasma ovipneumoniae and preparation method thereof
InactiveCN103992974ASimple compositionLow costBacteriaMicroorganism based processesHigh pressurePorcine serum
The invention discloses an in-vitro culture medium of mycoplasma ovipneumoniae and a preparation method thereof. The preparation method comprises the steps of mixing tryptone, soy peptone, sodium chloride, lactose, a yeast leaching agent and phenol red, then sterilizing under high pressure, cooling the mixture to below 37 DEG C, then adding 100-200mL / L of deactivated healthy porcine serum and 200 thousand U / L of penicillin dissolved by sterilizing water into the mixture, and regulating the pH value to be 7.4-7.8 with NaOH, so as to obtain the culture medium. Being cultivated by the culture medium, the mycoplasma ovipneumoniae can grow only in 24 hours, the growing titer can reach 10<-10>ccu, and compared with the prior art, the culture medium has simpler composition and lower cost.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Construction method of sheep mycoplasmal pneumonia bivalent nucleic acid vaccine containing adjuvant gene
PendingCN113444743AEffective against weak immunity and other problemsSolve many problems faced by R&DAntibacterial agentsAntibody mimetics/scaffoldsMycoplasmal pneumoniaTGE VACCINE
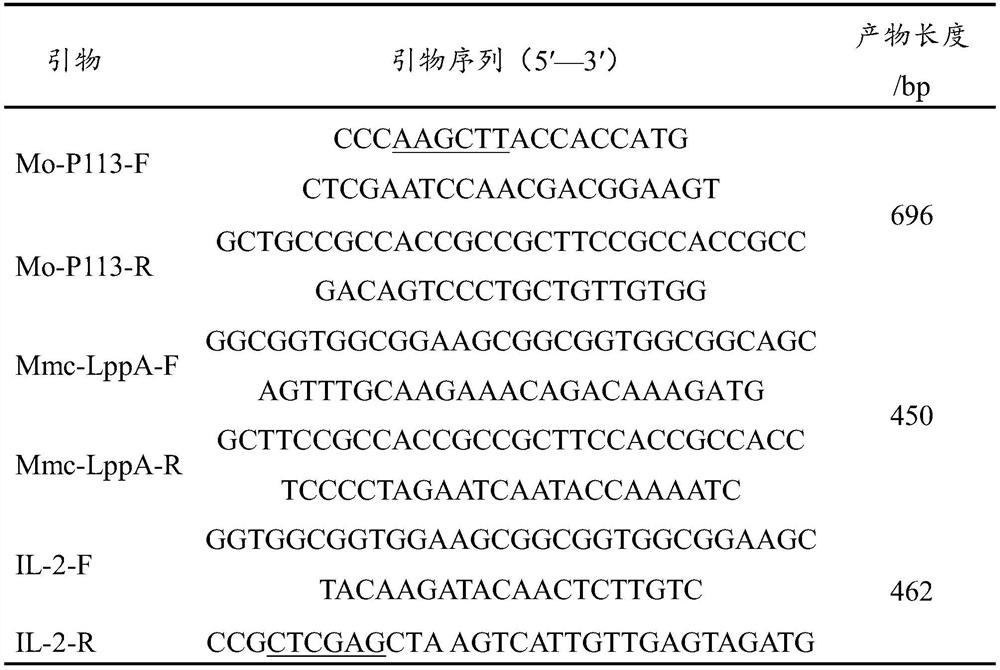
The invention discloses a construction method of a sheep mycoplasmal pneumonia bivalent nucleic acid vaccine containing an adjuvant gene. The construction method comprises the following specific steps of: step 1, extraction of a Mo / Mmc genome: S1, recovery and culture of a Mo / Mmc Guizhou strain, and S2, extraction of the DNA of the Mo / Mmc Guizhou strain; step 2, amplifying a P113-LppA-IL-2 fusion gene: S1, amplification of a mucosal immunologic adjuvant gene and mycoplasma ovipneumoniae and Mycoplasma mycoides subsp. Capri antigen gene, S2, the amplification of the P113-LppA fusion gene, and S3, the amplification of the P113-LppA-IL-2 fusion gene; and step 3, preparing the sheep mycoplasmal pneumonia bivalent nucleic acid vaccine containing the mucosal immunologic adjuvant gene. According to the construction method disclosed by the invention, the nucleic acid vaccine is developed by selecting two different antigen genes and mucosal immunologic adjuvant genes, so that the problems of a weak immune effect and the like of the sheep mycoplasmal pneumonia DNA vaccine can be effectively solved, and many problems confronted by current vaccine research and development are solved.
Owner:GUIZHOU UNIV
Mycoplasma ovipneumoniae, A-type sheep pasteurella multocida and D-type sheep pasteurella multocida triple inactivated vaccine
ActiveCN111514285AReduce stressLow costAntibacterial agentsBacterial antigen ingredientsP. multocidaTGE VACCINE
The invention provides a mycoplasma ovipneumoniae, A-type pasteurella multocida and D-type pasteurella multocida triple inactivated vaccine, and belongs to the technical field of vaccines. The tripleinactivated vaccine comprises inactivated bacterium liquid of mycoplasma ovipneumoniae MO_NM01, inactivated bacterium liquid of A-type sheep pasteurella multocida PM-NM1, inactivated bacterium liquidof D-type sheep pasteurella multocida P<-NM2 and an immunologic adjuvant. Compared with commercially available mycoplasma ovipneumoniae inactivated vaccines, the mycoplasma ovipneumoniae, A-type pasteurella multocida and D-type pasteurella multocida triple inactivated vaccine provided by the invention has basically equivalent immune protection force against corresponding pathogens, but can achievethe purposes of multiple prevention by one injection, cost reduction and sheep stress reduction.
Owner:INNER MONGOLIA AUTONOMOUS REGION ACAD OF AGRI & ANIMAL HUSBANDRY SCI
Mycoplasma ovipneumoniae Hsp70 (DnaK) C terminal gene recombinant plasmid
InactiveCN102242140AMicroorganism based processesVector-based foreign material introductionProtein targetHsp70
The invention discloses a mycoplasma ovipneumoniae Hsp70 (DnaK) C terminal gene recombinant plasmid. The production method is that: first, the following primers are designed and synthesized: P2: GGGGTCGACTTAATTTTGTTTGATTTC; P3: CAGGGATCCACTCCTTTAACTTTAGG; then a plasmid T-Hsp70 is adopted as a model, and PCR (polymerase chain reaction) amplification is carried out with the primers P2 and P3, such that a Hsp70C terminal gene cDNA segment is obtained, wherein a size of the segment is 700bp; the segment is connected to pET28a(+), such that a recombinant plasmid pET28a(+)-Hsp70C is obtained; the recombinant plasmid is further transformed into BL21(DE3), and SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) is carried out upon an expression product, such that a target protein with a size of approximately 29kDa is obtained.
Owner:NINGXIA UNIVERSITY
Combined inactivated vaccine for goat mycoplasma pneumonia and preparation method of combined inactivated vaccine
PendingCN112972667AToxicGood antigenicityAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureAdjuvant
The invention discloses a combined inactivated vaccine for goat mycoplasma pneumonia and a preparation method of the combined inactivated vaccine. The vaccine contains mycoplasma ovipneumoniae, mycoplasma mycoides subsp.capri and a propolis adjuvant. The vaccine is prepared through the following method, which comprises the following steps of 1, preparing a mycoplasma culture medium; 2, carrying out multiplication culture on mycoplasma; 3, inactivating a mycoplasma culture; and 4, mixing the inactivated mycoplasma with the propolis adjuvant to prepare the vaccine. The vaccine can be used for simultaneously preventing diseases caused by the mycoplasma mycoides subsp.capri and the mycoplasma ovipneumoniae, and avoiding the defects of single effect of a vaccine prepared through a single pathogen, is safe to goats with different physiological conditions and ages, and is suitable for goats with different varieties and various ages.
Owner:GUANGXI VETERINARY RES INST
Method for separating mycoplasma ovipneumoniae
PendingCN110452854AImprove separation rateImprove cleanlinessBacteriaMicroorganism based processesMycoplasma cultureChick embryos
The invention provides a method for separating mycoplasma ovipneumoniae, which comprises the following steps: (1) collecting a sample and treating the sample to obtain a sample inoculation solution; (2) inoculating chick embryos; (3) separating the mycoplasma ovipneumoniae: collecting dead chick embryos after inoculation and / or non-dead chick embryos after 9 days, taking chick embryo allantoic fluid to carry out viable bacteria detection on the mycoplasma ovipneumoniae, and if the chick embryo allantoic fluid is collected to be positive, storing the material, if the chick embryo allantoic fluid of the first generation is negative, the collected chick embryo allantoic fluid is blindly transmitted to the chick embryo by the same method, and if the chick embryo allantoic fluid is still negative after three generations of blind transmission, determining the sample to be negative for mycoplasma ovipneumoniae, and discharging the material. The method disclosed by the invention is simple to operate, does not need to prepare a mycoplasma ovipneumoniae culture medium with complex components, and avoids the problem that in the prior art, high-pressure sterilization or a high-capacity filteris often needed in preparation of an isolation culture medium; and according to the method, the mycoplasma ovipneumoniae without animal serum can be obtained.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation method of serum-free mycoplasma ovipneumoniae antigen
PendingCN110452855AEasy to operateAntibacterial agentsBacterial antigen ingredientsSerum freeMycoplasma antigen
The invention provides a preparation method of serum-free mycoplasma ovipneumoniae antigen. The method comprises the following steps: (1) preparation of chicken embryos: preparing the healthy SPF chicken embryos at 7-8 days of age, the embryos are vigorous, clear in blood vessels, and cultured in an incubator; (2) preparation of mycoplasma ovipneumoniae inoculum; and (3) chicken embryo inoculation, culture and harvest: inoculating the healthy SPF chicken embryos of 7-8 days old in step (1) of the mycoplasma ovipneumoniae inoculum, 0.2ml of the inoculum is inoculated for each chicken embryo, After inoculation, the incubator is further cultured for 5 days, and the allantoic fluid of dead chicken embryos and / or the allantoic fluid of chicken embryos that do not died after 5 days are aseptically obtained to obtain the serum-free mycoplasma ovipneumoniae antigen. In the present invention, the mycoplasma ovipneumoniae is subcultured in chicken embryos, and the allantoic fluid of chicken embryos is collected to obtain the serum-free mycoplasma ovipneumoniae antigen. The method of the present invention does not use serum, and can avoid many problems in using serum.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A kind of low serum culture medium of ovine mycoplasma pneumoniae and preparation method thereof
ActiveCN106479936BAvoid pollutionLong storage timeBacteriaMicroorganism based processesMycoplasma culturePenicillin
The invention discloses a mycoplasma ovipneumoniae low-serum medium, which consists of the following components: a PPLO broth, sodium pyruvate, phenol red, de-ionized water, lactose, tryptone, a fresh yeast extract, insulin, L-glutamine, L-cysteine, lactoalbumin hydrolysate, transferrin, penicillin and little horse serum; and the invention provides a preparation method of the mycoplasma ovipneumoniae low-serum medium. The mycoplasma ovipneumoniae medium provided by the invention has the following beneficial effects: a serum content is merely 5%, which is 1 / 4 of the serum content of a medium in the prior art, and the living bacterium titer of cultivated mycoplasma ovipneumoniae reaches 7*10<9> CCU / mL or above, which is significantly higher than that of the medium in the prior art. In comparison with the prior art, the mycoplasma ovipneumoniae low-serum medium provided by the invention has the characteristics of being low in serum content, rapid in growth and high in living bacterium titer.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Bivalent inactivated vaccine for sheep mycoplasma pneumonia and chlamydia psittaci disease and preparation method thereof
ActiveCN111789942AAvoid infectionImprove securityAntibacterial agentsBacterial antigen ingredientsDiseaseBeagle
The invention discloses a bivalent inactivated vaccine for sheep mycoplasma pneumonia and chlamydia psittaci and a preparation method thereof. Active ingredients of the bivalent inactivated vaccine comprise inactivated mycoplasma ovipneumoniae and inactivated MOMP protein of chlamydia psittaci. The amino acid sequence of the MOMP protein of the chlamydia psittaci is shown as a sequence 2 in a sequence table. The mycoplasma ovipneumoniae is (Mycoplasma ovipneumoniae) SH-01 CGMCC No.16503. Experiments prove that the bivalent inactivated vaccine has good safety for immune experimental animals (such as guinea pigs, rabbits and Beagle dogs) and target animals (such as sheep), and can effectively prevent mycoplasma ovipneumoniae and chlamydia psittaci from infecting the experimental animals andthe target animals. The vaccine has important practical significance for preventing and controlling prevalence and propagation of mycoplasma pneumonia and chlamydia psittaci of sheep. The vaccine hasimportant application value.
Owner:INNER MONGOLIA HUAXI BIOTECH
IELISA method for screening mycoplasma ovipneumoniae and mycoplasma capricolum subsp. capripneumoniae serological negative sheep
ActiveCN111323602AEasy to operateLarge amount of sample processingBiological testingPathogenMycoplasma ovipneumoniae
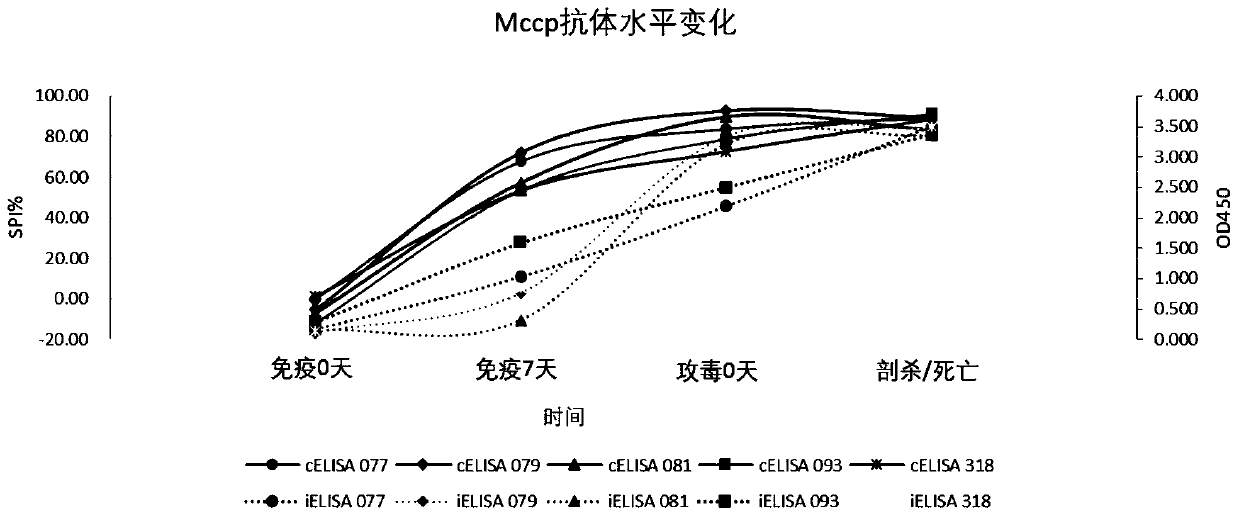
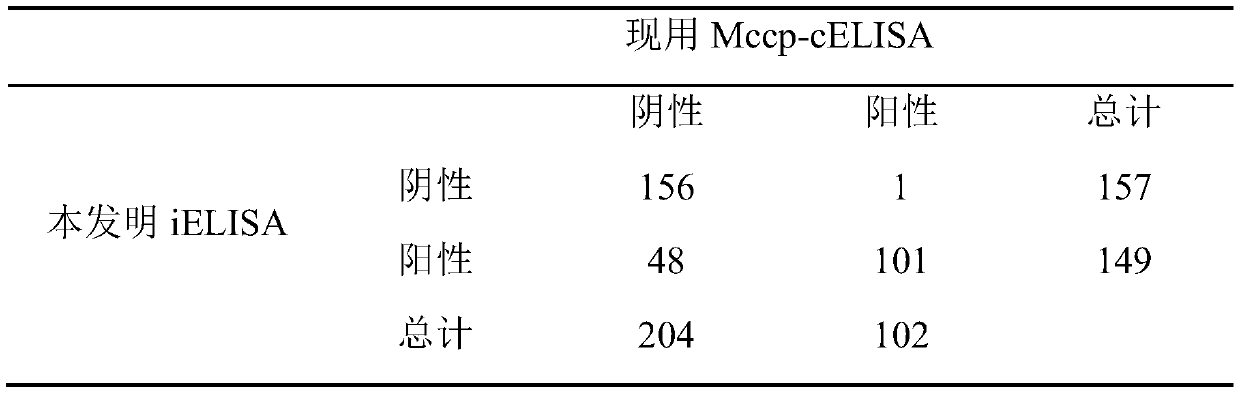
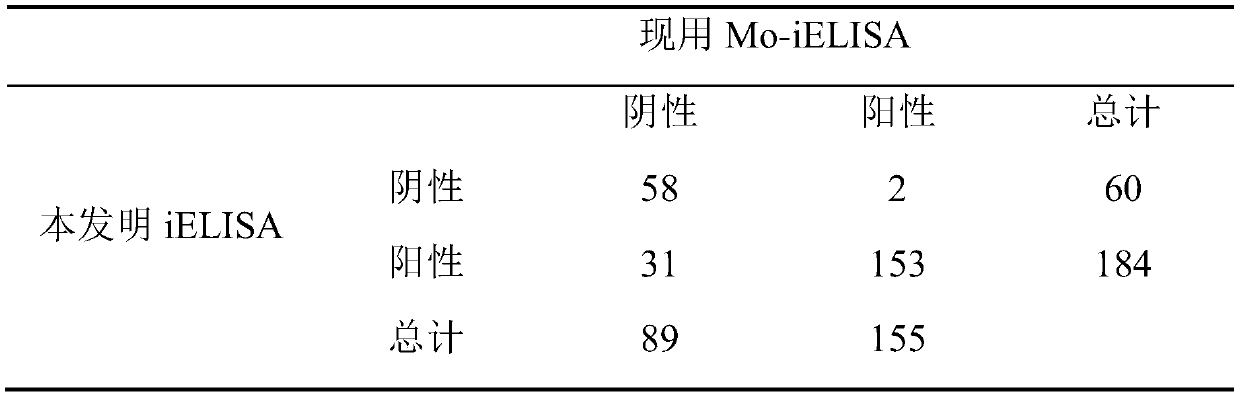
The invention discloses an iELISA method for screening mycoplasma ovipneumoniae and mycoplasma capricolum subsp. capripneumoniaeserological negative sheep. The method comprises the following steps: (1) inoculating a mycoplasma capricolum subsp. capripneumoniae1801 strain into an MTB culture medium for expanding culture, centrifuging and sterilizing a bacterial solution, performing PBS ultrasonic resuspension, and separating to obtain an antigen; (2) coating an elisa plate with the antigen, and staying overnight at 4 DEG C after 1 hour in an incubator at 37 DEG C; adding a confining liquid, andsealing at 37 DEG C for 2 hours; adding a serum sample diluted by 200 times, and incubating at 37 DEG C for 1 hour; adding a rabbit anti-goat secondary antibody which is diluted by 5,000 times by using a 5% skim milk powder, and incubating at 37 DEG C for 1 hour; and (3) washing the plate with PBST, adding a TMB color developing solution, and incubating at 37 DEG C for 10 minutes; adding a stop solution to stop areaction; and reading an OD450nm value by a microplate reader. The iELISA method has advantages that sensitivity can reach 98%, operation is simple, a sample treatment amount is large, the method can be used for screening Mccp and Mo pathogen serological negative sheep, and the method can also be used for monitoring a Mccp antibody level in experiments.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A dual inactivated vaccine for Mycoplasma ovine pneumonia and Chlamydia psittacosis and preparation method thereof
ActiveCN111789942BAvoid infectionImprove securityAntibacterial agentsBacterial antigen ingredientsBeagleChlamydia psittaci infections
The invention discloses a dual inactivated vaccine for ovine mycoplasma pneumonia and chlamydia psittaci and a preparation method thereof. The active components of the dual inactivated vaccine include inactivated Ovis Mycoplasma pneumoniae and inactivated MOMP protein of Chlamydia psittaci. The amino acid sequence of the MOMP protein of Chlamydia psittaci is shown as sequence 2 in the sequence listing. Mycoplasma ovis pneumoniae is Mycoplasma ovis pneumoniae (Mycoplasma ovipneumoniae) SH‑01CGMCC No.16503. Experiments have proved that the dual inactivated vaccine immunization experimental animals (such as guinea pigs, rabbits, Beagle dogs) and target animals (such as sheep) have good safety, and can effectively prevent Mycoplasma ovis pneumoniae and Chlamydia psittaci infection. animals and target animals. It is of great practical significance to prevent and control the prevalence and spread of mycoplasma pneumonia and chlamydia psittaci. The invention has important application value.
Owner:INNER MONGOLIA HUAXI BIOTECH
Mycoplasma ovipneumoniae HSP70-P113 fusion protein with immunogenicity
The invention discloses a mycoplasma ovipneumoniae HSP70-P113 fusion protein with immunogenicity, which is encoded by a fusion gene formed by splicing a mycoplasma ovipneumoniae heat shock protein gene HSP70 and a mycoplasma ovipneumoniae adhesin gene P113, and the amino acid sequence of the mycoplasma ovipneumoniae HSP70-P113 fusion protein is a sequence shown as SQE ID NO.2. A Western Blot test proves that the mycoplasma ovipneumoniae HSP70-P113 fusion protein has immunogenicity and can be used for preparing a mycoplasma ovipneumoniae subunit vaccine.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Mycoplasma ovipneumoniae in-vitro culture medium and preparation method thereof
The invention discloses a mycoplasma in-vitro culture medium and a preparation method thereof. The preparation method of the mycoplasma in-vitro culture medium comprises the following steps: mixing PPLO (poly-p-phenylene oxide) Both, glucose, sodium pyruvate, a 25% yeast leaching solution and phenol red according to a certain ratio, and then carrying out autoclaving; after the temperature is stabilized at 37 or below, adding inactivated healthy horse serum and 50 mg / mL ampicillin dissolved by autoclaving water; and then adjusting the pH value to 7.6-7.8 by using 1 mol / L NaOH to obtain the culture medium. The mycoplasma in-vitro culture medium disclosed by the invention can be used for shortening the time required by culture and obviously improving the viable bacteria titer of mycoplasma ovipneumoniae, thereby laying a foundation for the development of high-quality vaccines of mycoplasma ovipneumoniae.
Owner:TARIM UNIV
Composition for detecting mycoplasma ovipneumoniae by taking transketolase gene as target kit and method thereof
ActiveCN110564877AGood technical effectOptimize the reaction systemMicrobiological testing/measurementMicroorganism based processesTransketolaseFluorescence
The invention discloses a composition for detecting mycoplasma ovipneumoniae by taking a transketolase gene as a target, a kit and a method thereof. A detection target of the composition is the transketolase gene of mycoplasma ovipneumoniae; various different fragments in the gene can be targeted, the experiments prove that the composition disclosed by the invention can be used for designing various specific combination situations so as to be applied to various amplification methods such as traditional PCR, basic RPA, nfo RPA and exo RPA; in addition, the experimental results can be observed by means of agarose gel electrophoresis, a lateral flow chromatography test strip and a constant-temperature fluorescence amplification instrument. The optimal reaction time and the optimal reaction temperature in the detection process and the specificity, the sensitivity, the repeatability and the stability of detection are explored; the optimal reaction condition is found, the mycoplasma ovipneumoniae rapid detection method which is good in specificity, high in sensitivity and capable of being stably repeated is established, and the composition has the advantages of being easy to operate, convenient to implement, and capable of saving time and free of large experimental instruments and equipment.
Owner:NINGXIA UNIVERSITY
Genetic engineering subunit vaccine of goat contagious pleuropneumonia as well as preparation method and application thereof
InactiveCN113481178AImproving immunogenicityGood cross protectionAntibacterial agentsBacterial antigen ingredientsImmunogenicityHumoral immune reaction
The invention discloses a genetic engineering subunit vaccine of goat contagious pleuropneumonia as well as a preparation method and application thereof. The vaccine comprises a first recombinant protein, a second recombinant protein and a pharmaceutically acceptable carrier, wherein the first recombinant protein and the second recombinant protein respectively have sequences as shown in SEQ ID NO: 2 and SEQ ID NO: 6. The vaccine provided by the invention is free of any toxicity, high in safety and good in immunogenicity, and can generate relatively strong humoral immunity in a sheep body, antigens expressed by the first recombinant protein and the second recombinant protein have a very good cross protection effect, which can simultaneously resist attacks of mycoplasma capricolum subsp.capripneumoniae, mycoplasma capricomlusubsp.capricolum and mycoplasma ovipneumoniae, the immunized sheep can resist virulent virus attack, and the vaccine can be prepared through large-scale serum-free suspension culture by using a bioreactor, and has advantages of easy quality control, batch-to-batch stability, low production cost and the like.
Owner:SUZHOU MIDI BIOTECH CO LTD
Mycoplasma ovipneumoniae antibody indirect ELISA detection kit
The invention relates to the technical field of veterinary biological product detection, in particular to a mycoplasma ovipneumoniae antibody indirect ELISA detection kit. Wherein the coating antigenis mycoplasma ovipneumoniae adhesin gene P113 recombinant protein rP113 (C); the primer sequence of the primer is as follows: P113 (c) F: 5 to CGCGGATCCGAAGGTGCTCAAGACCAAGGTA-3, and P113 (c) R: 5 to CCGCTCCGTTGTTGTTGTTGAGGTGGTGTATCAGGT-3. The invention provides the indirect ELSIA detection kit for the mycoplasma ovipneumoniae antibody, which is established by taking purified P113 recombinant protein rP113 (C) as a coating antigen, and a powerful tool is provided for epidemiological investigation, disease diagnosis and vaccine immune effect evaluation.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Mycoplasma ovipneumoniae strain and application thereof in screening of anti-mycoplasma ovipneumoniae preparation
ActiveCN113025531AHigh infection rateEasy to trainBacteriaMicrobiological testing/measurementBiotechnologyMycoplasma pneumoniae
The invention discloses a mycoplasma ovipneumoniae strain and application thereof in screening of an anti-mycoplasma ovipneumoniae preparation. The classification name of the mycoplasma ovipneumoniae strain is Mycoplasma ovipneumoniae NJ01 strain, the mycoplasma ovipneumoniae strain is preserved in the China Center for Type Culture Collection, the preservation number is CCTCC NO: M 2020907, and the preservation date is December 14, 2020. The strain NJ01 provided by the invention is high in culture growth speed and high in titer which can reach 10<10> CCU / mL, and the color can be changed in 10-72 hours after the strain NJ01 is subjected to recovery subculture in a specific culture medium, so that time is saved for determination of an anti-mycoplasma ovipneumoniae preparation, the efficiency is improved, and the strain NJ01 can be used for rapid screening of the anti-mycoplasma ovipneumoniae preparation and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
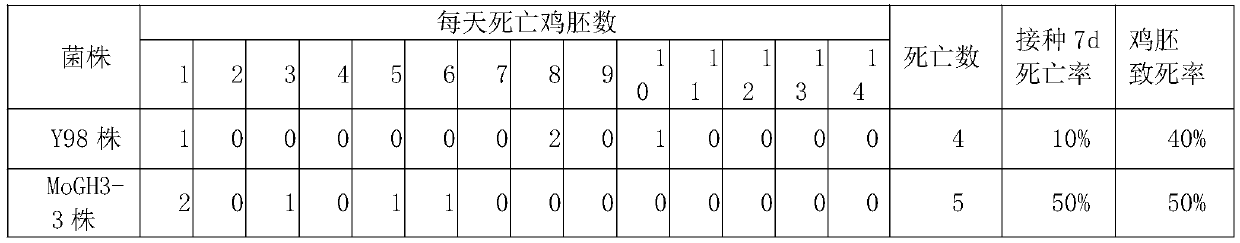
Method for comparative analysis of sheep mycoplasma ovipneumoniae virulence
ActiveCN110846374AGood repeatabilityEasy to getCompound screeningApoptosis detectionAnimal scienceAllantois
The invention discloses a method for comparative analysis of virulence of different sheep mycoplasma ovipneumoniae strains. The method includes: respectively inoculating resurgent sheep mycoplasma tobe analyzed comparatively onto different chicken embryos, putting the chicken embryos after being inoculated into an incubator for culture, regularly detecting state and death conditions of the chicken embryos, recording chicken embryo lethality rate and inoculation 7d death rate of each strain, and combining identifying results of dead chicken embryos allantoic fluid sheep mycoplasma ovipneumoniae for comparative judgment. The method has the advantages of low cost, easiness in getting experimental animal SPF chicken embryos, simplicity and convenience in operation, short experiment period andhigh repeatability and overcomes the defects of high price of experimental animals, high requirements on the experimental animals, troublesome animal feeding, long experiment period and low repeatability in the prior art.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com