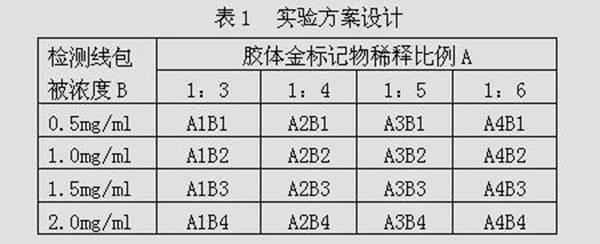
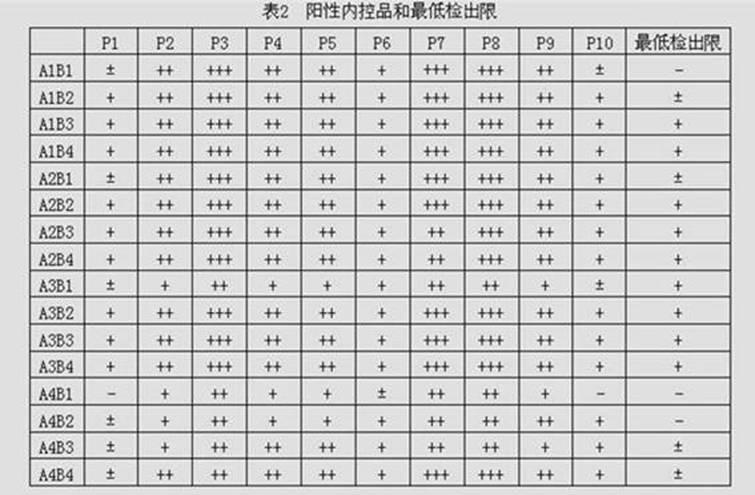
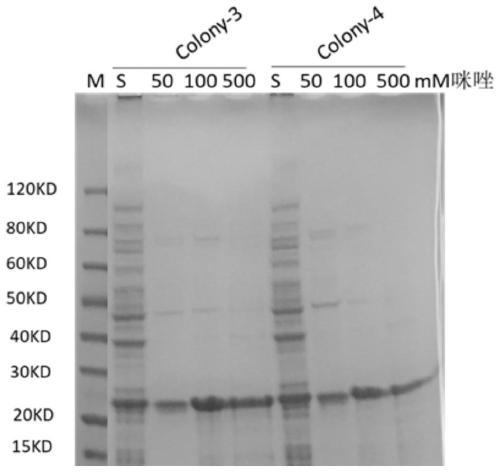
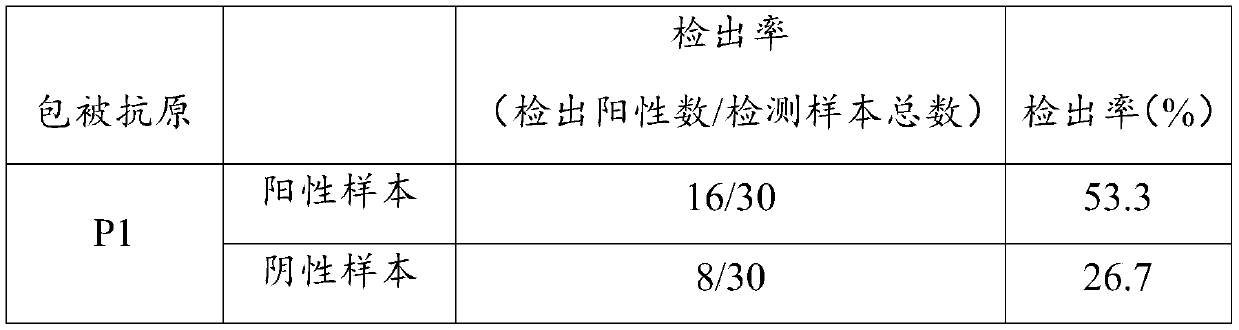
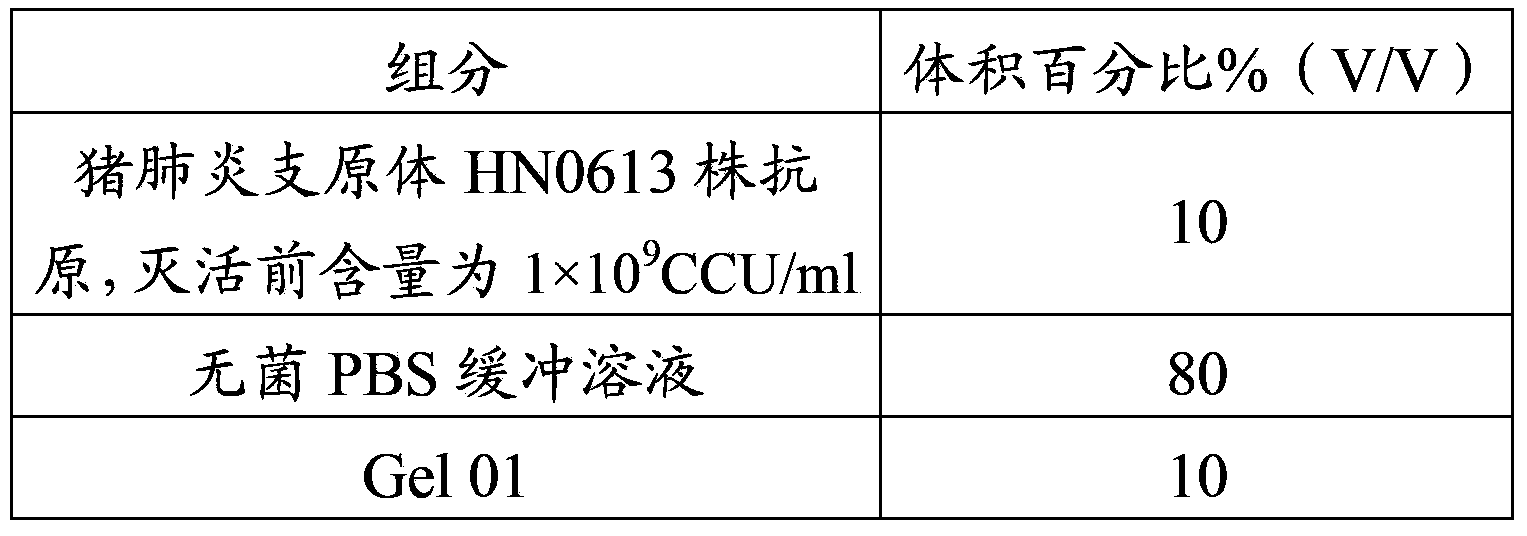
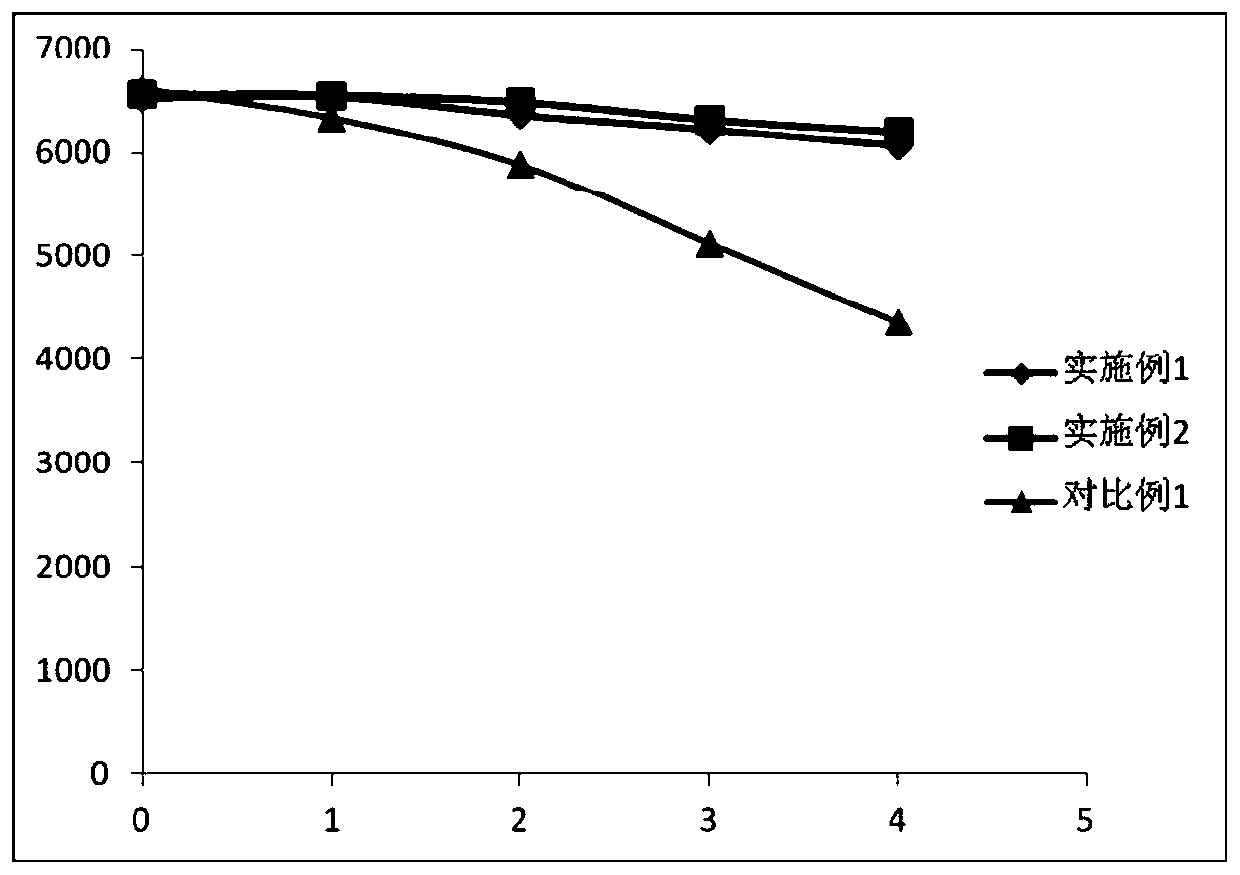
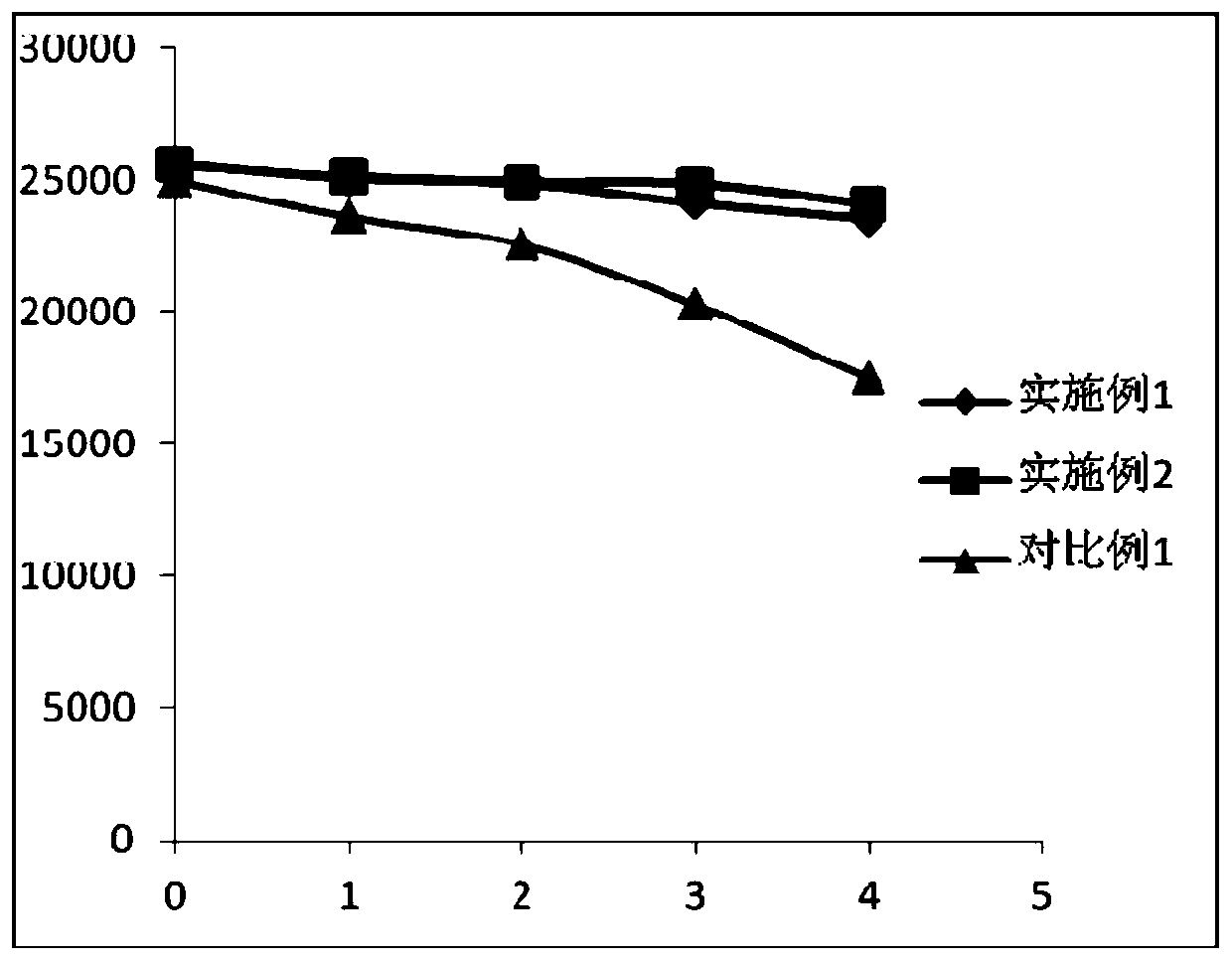
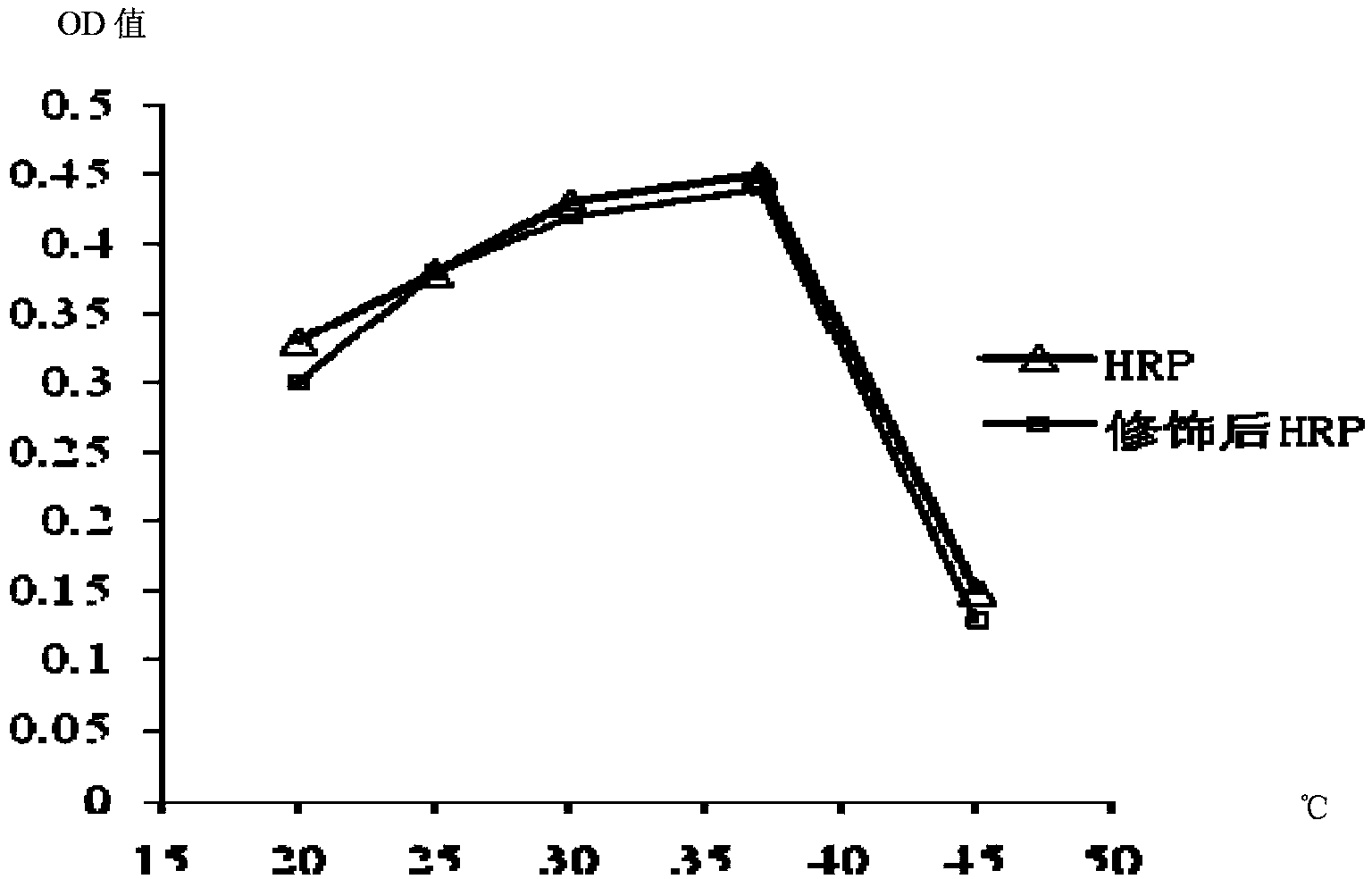
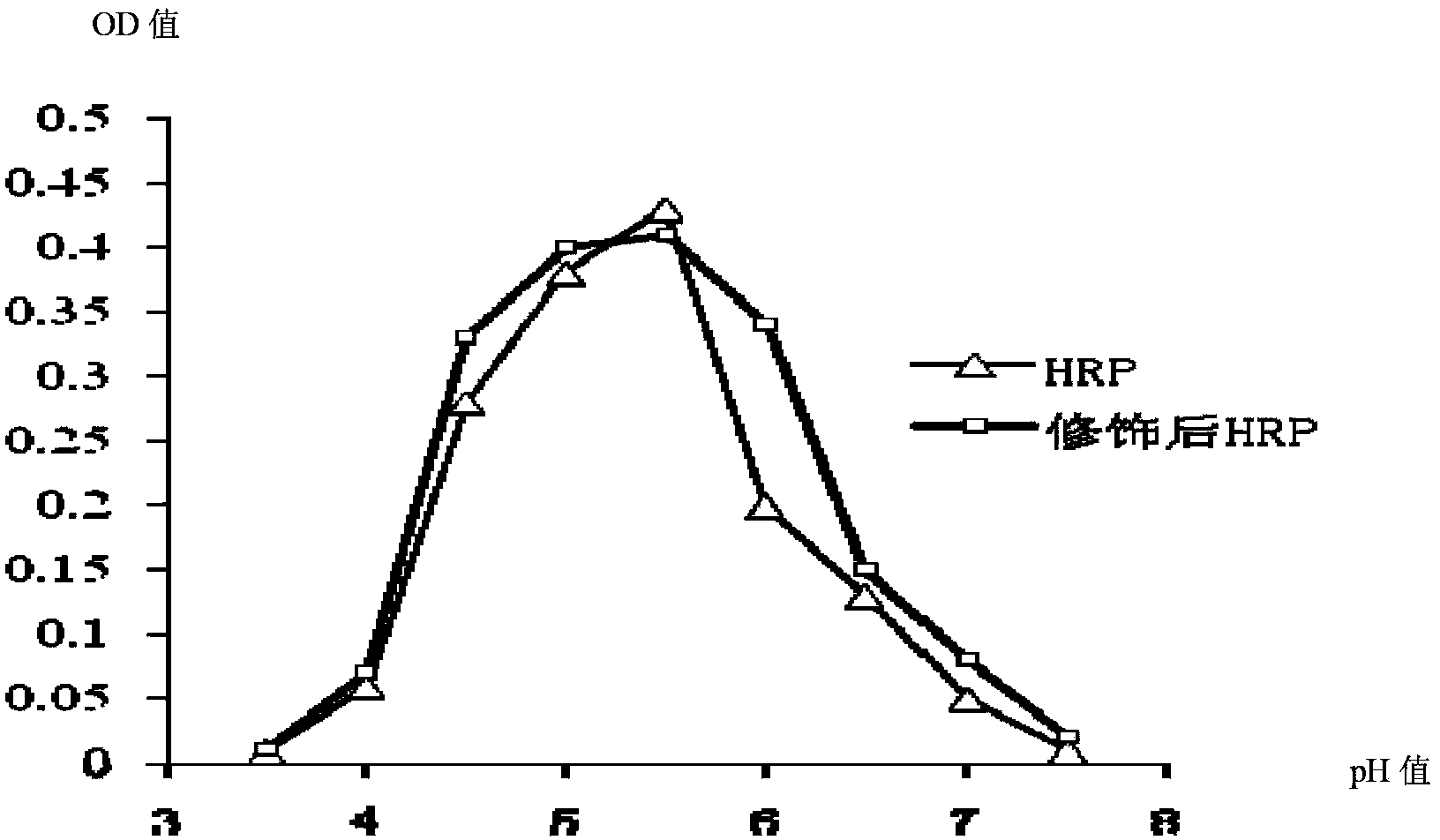
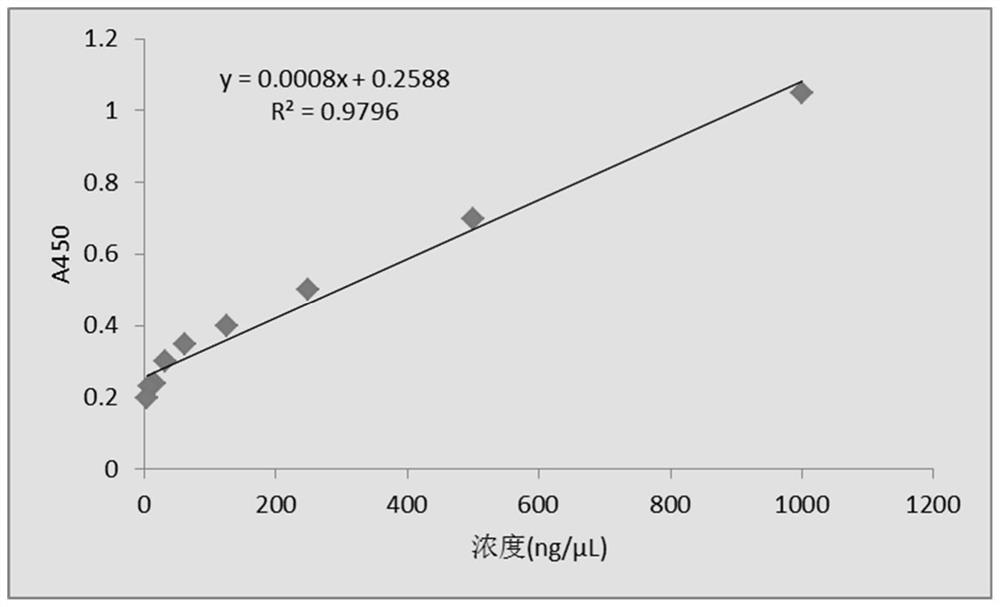
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
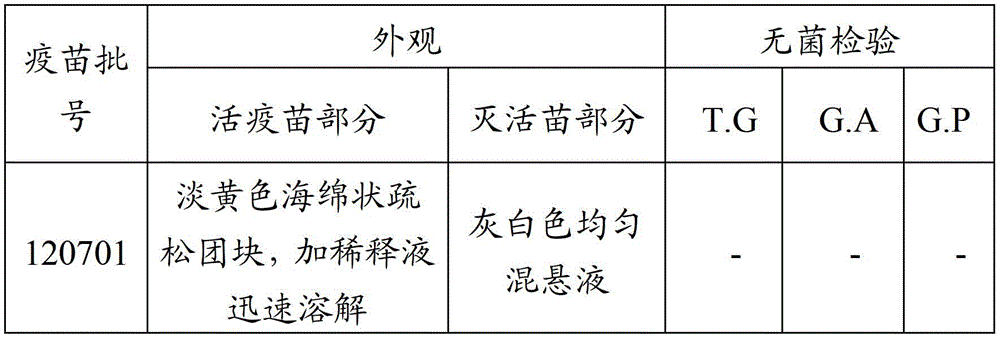
36 results about "Mycoplasma antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Native Antigen Company’s Mycoplasma pneumoniae antigen is produced from whole organisms that have been purified and detergent treated to enrich antigenic proteins and decrease background. Mycoplasma pneumoniae is recognized as one of the most common causes of community-acquired pneumonia.
Mycoplasma bovis monoclonal antibody, and preparation method and application thereof
ActiveCN103509756AStrong variabilityEasy to operateImmunoglobulins against bacteriaMicroorganism based processesMycoplasma bovis antigenMycoplasma antigen
The invention discloses a mycoplasma bovis monoclonal antibody, and a preparation method and an application thereof. The preparation method of the mycoplasma bovis monoclonal antibody comprises the following steps: 1, preparing a mycoplasma bovis antigen; 2, immunizing mice by the antigen; 3, preparing a hybridomas cell secreting the mycoplasma bovis monoclonal antibody and monoclonal antibody ascites; and 4, purifying the above obtained monoclonal antibody. In the invention, mice are immunized by a mycoplasma bovis geographical strain HB0801 antigen, a hybridomas cell strain 1C11, CCTCC NO:C201218, which can efficiently secrete the mycoplasma bovis monoclonal antibody, is obtained through a hybridomas cell technology, the generated monoclonal antibody can be specifically combined with mycoplasma bovis and mycoplasma agalactiae, and the monoclonal antibody is utilized to establish sandwich ELISA for detecting the mycoplasma bovis antigen. The method has the advantages of simple operation, short required time and high sensitivity.
Owner:HUAZHONG AGRI UNIV
Preparation method of chicken infectious rhinitis and mycoplasma gallisepticum bivalent lipid inactivated vaccine
InactiveCN102406925AReduce manufacturing costIncrease production costAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma gallisepticum antigen
The invention provides a preparation method of a chicken infectious rhinitis and mycoplasma gallisepticum bivalent lipid inactivated vaccine, belonging to the technical field of biological products for animals. The preparation method is implemented by the following steps of: preparing a type A haemophilus paragallinarum inactivated antigen bacterial liquid, a type C haemophilus paragallinarum inactivated antigen bacterial liquid and a mycoplasma gallisepticum antigen liquid; uniformly mixing the type A haemophilus paragallinarum inactivated antigen bacterial liquid with the type C haemophilus paragallinarum inactivated antigen bacterial liquid in the volume ratio of 1:1 to obtain a mixed haemophilus paragallinarum inactivated antigen bacterial liquid; uniformly mixing the mixed haemophilus paragallinarum inactivated antigen bacterial liquid with the mycoplasma gallisepticum antigen liquid in the ratio of 1:1-1:1.5, pouring into an emulsifying tank and stirring; slowly adding poplar bark lipid till the final volume percentage concentration of the poplar bark lipid in the mixture is 2.0-3.8 percent; and continually stirring and emulsifying for 30-60 minutes. The vaccine has the advantages of easiness for absorbing, no toxic or side effect, short immunogenic time, long immune duration, good immune effect and capability of effectively preventing chicken infectious rhinitis and mycoplasma gallisepticum.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
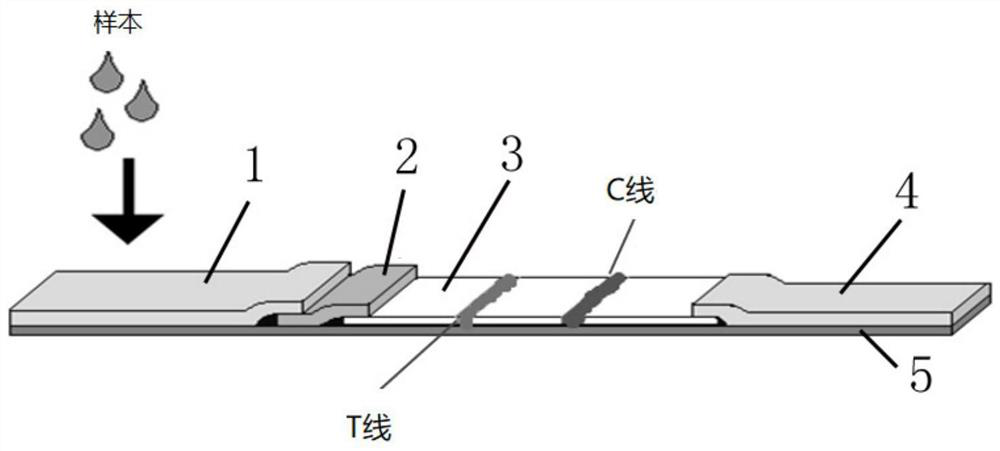
Multi-item respiratory tract antigen detection card and kit
ActiveCN112362869AAdequate responseHigh sensitivityBiological testingImmunoassaysAntibody conjugateAntigen testing
The invention relates to a multi-item respiratory tract antigen detection card which comprises an influenza A virus antigen test strip, an influenza B virus antigen test strip, a respiratory tract adenovirus antigen test strip, a respiratory tract syncytial virus antigen test strip and a mycoplasma pneumoniae antigen test strip, wherein each of the influenza A virus antigen test strip, the influenza B virus antigen test strip, the respiratory tract adenovirus antigen test strip, the respiratory tract syncytial virus antigen test strip and the mycoplasma pneumoniae antigen test strip comprisesa colloidal gold conjugate pad, and each colloidal gold conjugate pad comprises a streptavidin conjugate pad and a double-nanoparticle double-labeled antibody conjugate pad; the test strip in the detection card enables biological raw materials to react fully, improves the sensitivity of antigen detection, effectively reduces missing detection, and meanwhile, the blocking agent is added into the sample pad to improve the specificity, so that influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus and mycoplasma pneumoniae antigen can be detected at the same time.
Owner:山东康华生物医疗科技股份有限公司
Fused protein, gene therefor, recombinant vector, recombinant virus, and its use
InactiveUS7348422B2Enhanced infection prevention activityEasy to identifyAntibody mimetics/scaffoldsVirus peptidesA-DNAMycoplasma gallisepticum
A DNA coding for a fusion protein comprising a polypeptide having the antigenicity of Mycoplasma gallisepticum and a polypeptide derived from Herpesvirus outer membrane protein, in which the polypeptide derived from the outer membrane protein has been ligated with the polypeptide having the antigenicity of Mycoplasma gallisepticum at the N terminus thereof, is prepared. The DNA is inserted into a region non-essential to growth of Avipox virus and the resulting recombinant Avipox virus is provided as a more potent recombinant virus as an anti-Mycoplasma vaccine.
Owner:ZEON CORP
Mycoplasma pneumoniae IgM antibody colloid gold method detecting kit and preparation method thereof
InactiveCN102305856AObvious detectabilityReduce the effectMaterial analysisPaper tapeMurine antibody
The invention relates to a mycoplasma pneumoniae IgM antibody colloid gold method detecting kit which comprises a nitrocellulose membrane detecting line coated recombinant antigen MP-Ag, a quality control line coated goat anti-mouse IgG antibody and a gold labeled pad coated mouse anti-human IgM monoclonal antibody which is marked by colloidal gold, wherein the recombinant mycoplasma pneumoniae antigen is colorless transparent liquid, has the concentration of larger than 2mg / ml and is detected by SDS-PAG; the goat anti-mouse IgG antibody is colorless transparent liquid and has the concentration of larger than 4mg / ml; the mouse anti-human IgM monoclonal antibody is colorless transparent liquid, has the concentration of larger than 2mg / ml and is detected by SDS-PAGE; and the sample loading amount uses two strips under the condition of 10 microliters. The mycoplasma pneumoniae IgM antibody colloid gold rapid detecting test paper tape uses multiepitope recombinant antigen as a raw materials, has the characteristics of simpler operation, low cost, high specificity and high sensitivity, can carry out single detection, is easy for popularization and has obvious detecting and control effect on the mycoplasma pneumoniae IgM antibody.
Owner:北京中检安泰诊断科技有限公司
Mycoplasma pneumoniae antigen as well as preparation method and application thereof
ActiveCN111253478AStrong specificityHigh sensitivityNucleic acid vectorDepsipeptidesMycoplasma antigenImmuno detection
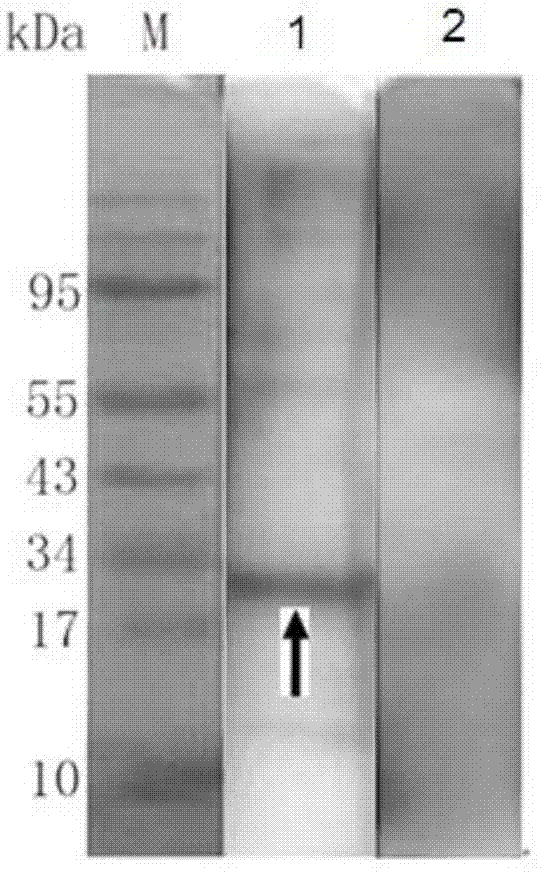
The invention relates to the field of in-vitro diagnostic immunoassay, and in particular provides a mycoplasma pneumoniae antigen as well as a preparation method and application thereof. The mycoplasma pneumoniae antigen provided by the invention is protein composed of an antigenic protein sequence P1M:residues 1340-1518, specifically an amino acid sequence shown in SEQ ID NO.1, or protein which is obtained by performing substitution and / or deletion and / or addition on the amino acid sequence shown in the SEQ ID NO.1 by one or more amino acid residues and has same function. The antigen is verified by an immunoserological detection technology to have strong specificity and high sensitivity, and the antigen is easy to culture and purify, is more conducive to industrial production and saves costs; and in addition, the antigen is suitable for preparation of MP antibody detection products, can be used for any forms of products in the field of in-vitro immunodiagnosis, and has broad market prospects.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Application of mycoplasma hyopneumoniae antigen in prevention and treatment of porcine respiratory disease complex
InactiveCN104338125APromote growthReduced feed efficiencyAntibacterial agentsBacterial antigen ingredientsDiseaseVaccination
The invention provides application of a mycoplasma hyopneumoniae antigen or an immunogenic composition containing the antigen in prevention and treatment of PRDC (porcine respiratory disease complex) and diseases with PRDC relevant clinical evidence. Specifically, the application includes immunization of pigs with an immune dosage of the mycoplasma hyopneumoniae antigen or the immunogenic composition containing the mycoplasma hyopneumoniae antigen. Vaccination with vaccines containing the mycoplasma hyopneumoniae antigen on animals can significantly alleviate and reduce the severity and duration of PRDC relevant clinical evidence.
Owner:PU LIKE BIO ENG
Coating liquid for improving stability of chlamydia pneumoniae antigen/mycoplasma antigen in immunochromatographic reagent and preparation method thereof
The invention relates to the field of biological reagents, in particular to coating liquid for improving the stability of chlamydia pneumoniae antigen / mycoplasma antigen in an immunochromatographic reagent and a preparation method thereof. The coating solution comprises a buffer solution, wherein the buffer solution comprises a first component for immobilizing a chlamydia antigen or a mycoplasma antigen on a nitrocellulose membrane, a stabilizer, an antioxidant and a preservative. The first component is beneficial to immobilization of the antigen on the nitrocellulose membrane. The stabilizercan improve the stability of the mycoplasma antigen and the chlamydia antigen. The antioxidant can prevent the mycoplasma antigen and the chlamydia antigen from being oxidized. The preservative can play a role in preventing mycoplasma antigens and chlamydia antigens from being corroded. All the components in the coating liquid cooperate with one another to protect the stability of the antigen in the immunochromatographic reagent.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Mycoplasma pneumoniae antigen and application thereof in simultaneous quantitative detection of Mycoplasma pneumoniae IgG and IgM content in peripheral blood
ActiveCN110964089AImprove filtering effectEnhance immune responseDepsipeptidesGenetic engineeringImmune profilingAssay
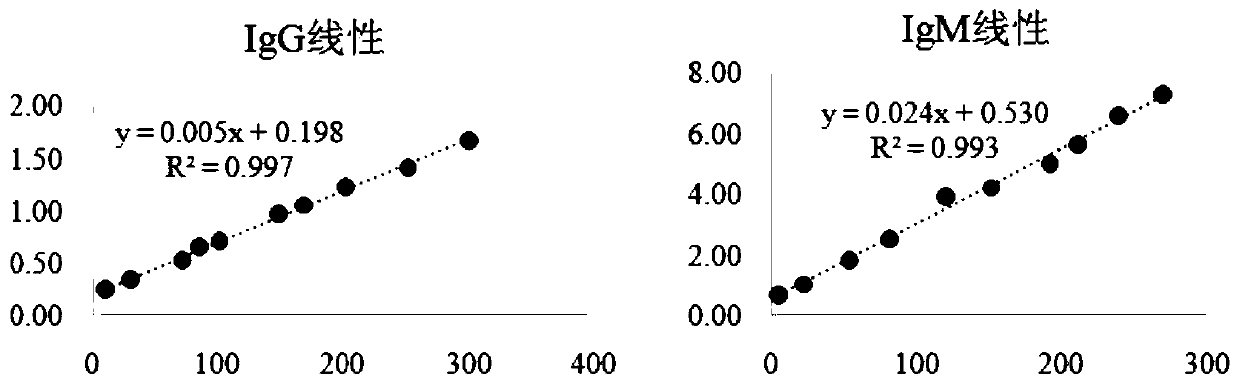
The invention discloses a Mycoplasma pneumoniae antigen and its application in simultaneous quantitative detection of Mycoplasma pneumoniae IgG and IgM content in peripheral blood. The amino acid sequence of the mutant Mycoplasma pneumoniae antigen P80 of the invention is as shown in SEQ ID NO.1. The immunoreactivity of the Mycoplasma pneumoniae antigen p80 and the antibody is about 34.6% higher than that of the natural antigen. By combining the Mycoplasma pneumoniae antigen P80 with the quantum dot immunochromatography technology, the shortcoming that existing detection methods of Mycoplasmapneumoniae antibody, such as immunochromatography assay (ICA) and enzyme-linked immunosorbent assay (ELISA), can only detect qualitatively or semi-quantitatively, and the MP-IgM and MP-IgG detection must be carried out twice is solved; and the detection of MP-IgM and MP-IgG can be realized at the same time, so as to distinguish the patients with recent infection or previous infection and reduce the misdiagnosis rate.
Owner:NANJING VAZYME MEDICAL TECH CO LTD
Immunoglobulin-binding human mycoplasma antigens and methods of use thereof
InactiveUS9593150B2Bacterial antigen ingredientsImmunoglobulins against bacteriaDiseaseMonoclonal gammopathy of undetermined significance
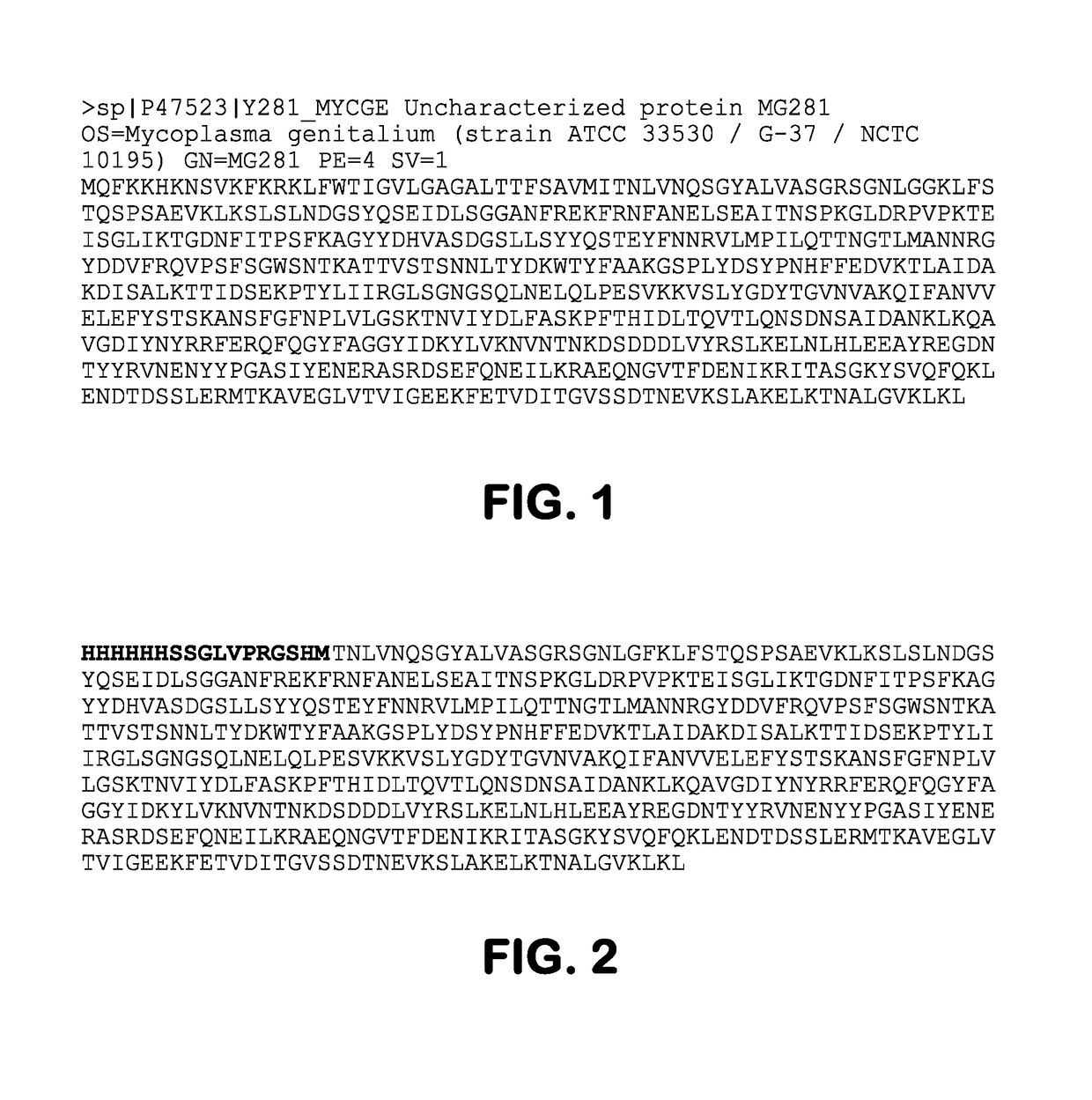
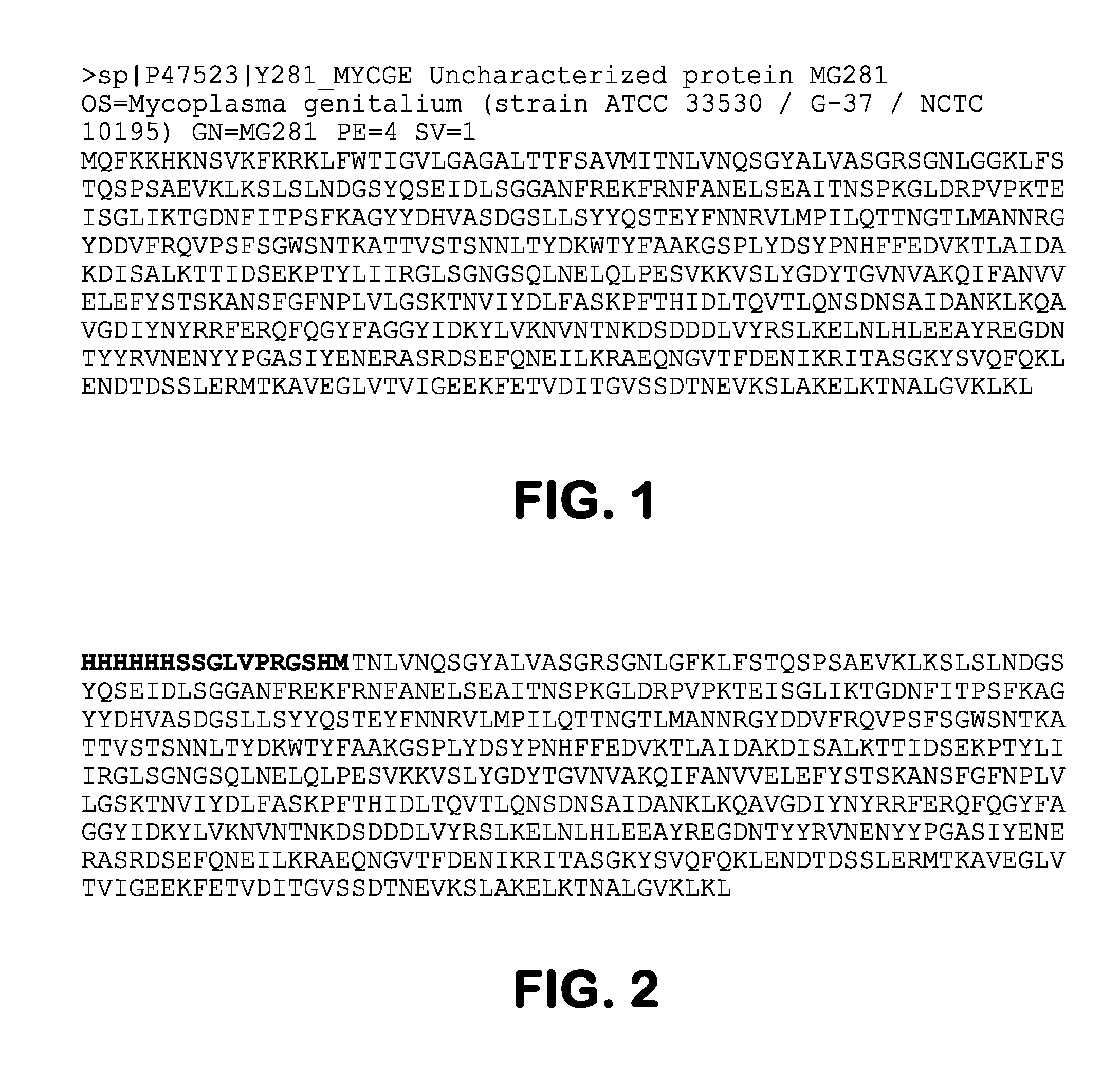
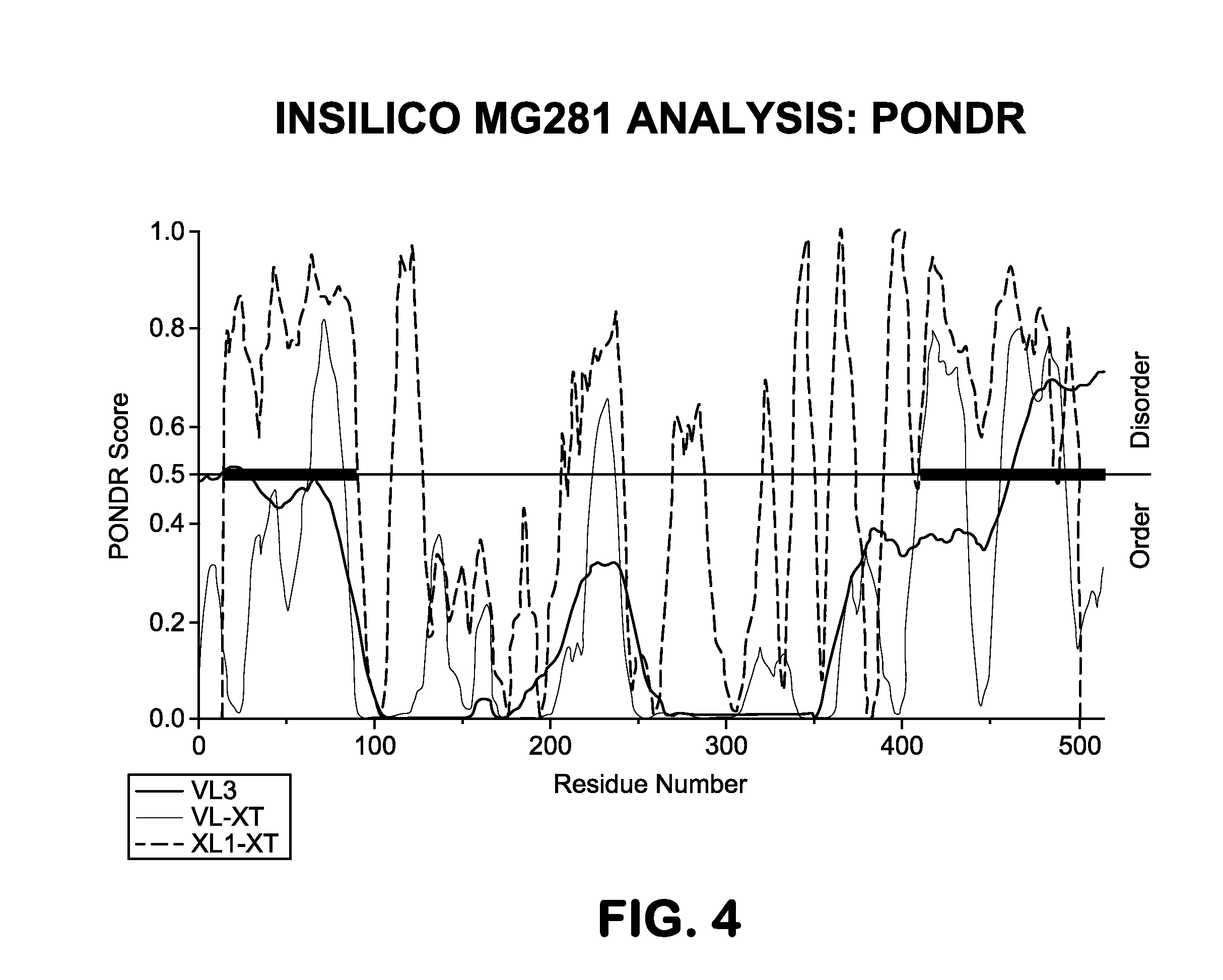
Provided herein methods of alleviating the symptoms of multiple myeloma in a patient or subject in need thereof, methods of treating multiple myeloma in a patient or subject, and methods of preventing development of multiple myeloma in a patient at risk thereof, such as a patient with monoclonal gammopathy of undetermined significance (MGUS), which methods include administering an agent effective to treat a Mycoplasma infection. The invention also relates to a new class of antigen, MG281 protein {also referenced as Protein M), that binds to various immunoglobulins with high affinity, and their uses in purifying immunoglobulins. The invention additionally relates to using MG281 protein and analog or derivative molecules for treating autoimmune diseases. Further provided are antigens and antibodies for use in the disclosed methods, and the identification of molecules that bind to MG281 protein.
Owner:THE SCRIPPS RES INST
Immunoglobuljin-binding human mycoplasma antigens and methods of use thereof
InactiveUS20150246953A1Bacterial antigen ingredientsImmunoglobulins against bacteriaAutoimmune diseaseMycoplasma antigen
Provided herein methods of alleviating the symptoms of multiple myeloma in a patient or subject in need thereof, methods of treating multiple myeloma in a patient or subject, and methods of preventing development of multiple myeloma in a patient at risk thereof, such as a patient with monoclonal gammopathy of undetermined significance (MGUS), which methods include administering an agent effective to treat a Mycoplasma infection. The invention also relates to a new class of antigen, MG281 protein {also referenced as Protein M), that binds to various immunoglobulins with high affinity, and their uses in purifying immunoglobulins. The invention additionally relates to using MG281 protein and analog or derivative molecules for treating autoimmune diseases. Further provided are antigens and antibodies for use in the disclosed methods, and the identification of molecules that bind to MG281 protein.
Owner:THE SCRIPPS RES INST
Combined device and detection method for synchronously detecting influenza A virus, influenza B virus, chlamydia pneumoniae IgM antibody and mycoplasma IgM antibody
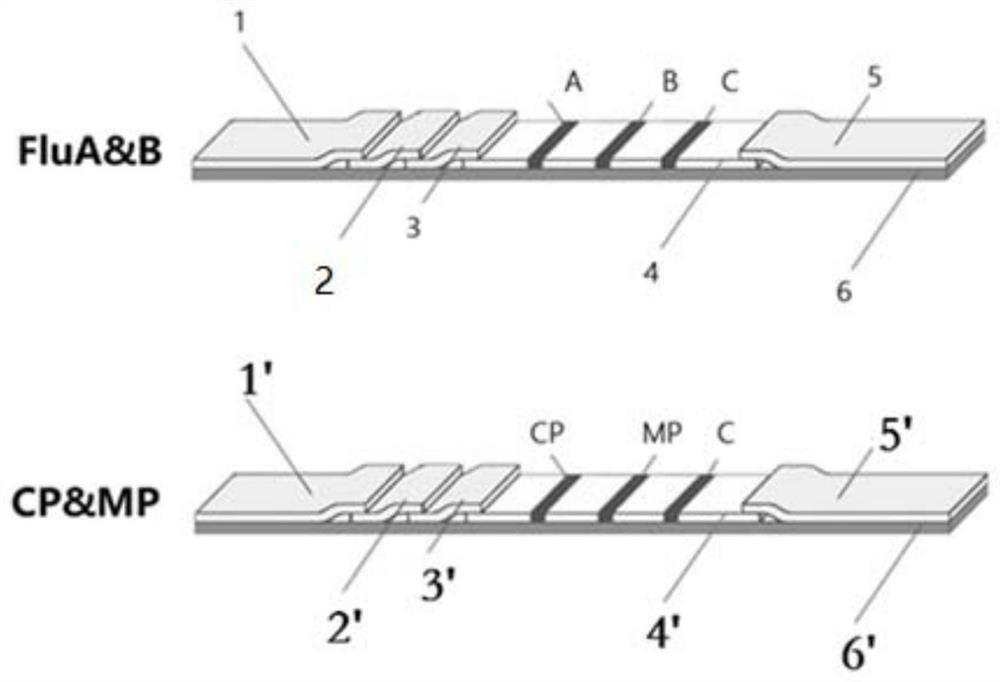
PendingCN111610329ASynchronize independent test resultsIndependent Testing ProcessMaterial analysisReceptorIgm antibody
The invention belongs to the field of medical detection equipment, and provides a combined device for synchronously detecting IgM antibodies of influenza A and B viruses and chlamydia pneumoniae and mycoplasma pneumoniae. The device comprises a double-channel clamping shell, a test strip FluA & B and a test strip CP & MP which are arranged in the double-channel clamping shell in parallel; influenza virus A detection lines arranged on a nitrocellulose membrane of the test strip FluA & B at intervals are coated with high-specificity influenza virus A antigens, influenza virus B detection linesare coated with high-specificity influenza virus B antigens, and first quality control lines are coated with quality control line coating receptors; chlamydia pneumoniae detection lines (CP) arranged on a nitrocellulose membrane of the test strip CP & MP at intervals are coated with high-specificity chlamydia pneumoniae antigens, mycoplasma pneumoniae detection lines (MP) are coated with high-specificity mycoplasma pneumoniae antigens, and second quality control lines are coated with quality control line coating receptors.
Owner:北京柏兆嘉业科技有限公司
Preparation method of serum-free mycoplasma ovipneumoniae antigen
PendingCN110452855AEasy to operateAntibacterial agentsBacterial antigen ingredientsSerum freeMycoplasma antigen
The invention provides a preparation method of serum-free mycoplasma ovipneumoniae antigen. The method comprises the following steps: (1) preparation of chicken embryos: preparing the healthy SPF chicken embryos at 7-8 days of age, the embryos are vigorous, clear in blood vessels, and cultured in an incubator; (2) preparation of mycoplasma ovipneumoniae inoculum; and (3) chicken embryo inoculation, culture and harvest: inoculating the healthy SPF chicken embryos of 7-8 days old in step (1) of the mycoplasma ovipneumoniae inoculum, 0.2ml of the inoculum is inoculated for each chicken embryo, After inoculation, the incubator is further cultured for 5 days, and the allantoic fluid of dead chicken embryos and / or the allantoic fluid of chicken embryos that do not died after 5 days are aseptically obtained to obtain the serum-free mycoplasma ovipneumoniae antigen. In the present invention, the mycoplasma ovipneumoniae is subcultured in chicken embryos, and the allantoic fluid of chicken embryos is collected to obtain the serum-free mycoplasma ovipneumoniae antigen. The method of the present invention does not use serum, and can avoid many problems in using serum.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Diagnostic kit for combined diagnosis of mycoplasma pneumonia and application of diagnostic kit
ActiveCN111505313AHigh sensitivityStrong specificityBiological testingImmunoassaysMurine antibodyMycoplasma antigen
The invention relates to a diagnostic kit, in particular to the diagnostic kit for the combined diagnosis of mycoplasma pneumonia. The kit comprises a single-side detection test strip and a double-side detection test strip, a mycoplasma pneumoniae antibody marked by time-resolved fluorescent microspheres is fixed on a combination pad of the single-side detection test strip, a mycoplasma pneumoniaeantibody is coated on a first detection line, and a goat-anti-mouse IgG antibody is coated on a first quality control line. A time-resolved fluorescent microsphere labeled mouse anti-human IgM monoclonal antibody and a mouse anti-human IgG antibody are fixed on a combination pad of the double-detection test strip, a second detection line and a third detection line are both coated with natural MP-Ag, and the second quality control line is coated with a goat anti-mouse IgG antibody. According to the invention, the time-resolved fluorescent microsphere is used as a chromogenic marker, the detection test strip for detecting the mycoplasma pneumoniae IgM and IgG three times is combined into a whole, and the human mycoplasma pneumoniae antigen and the mycoplasma pneumoniae IgM and IgG are simultaneously detected, so that the kit has higher sensitivity and stronger specificity compared with a colloidal gold method.
Owner:厦门万渤生物技术有限公司
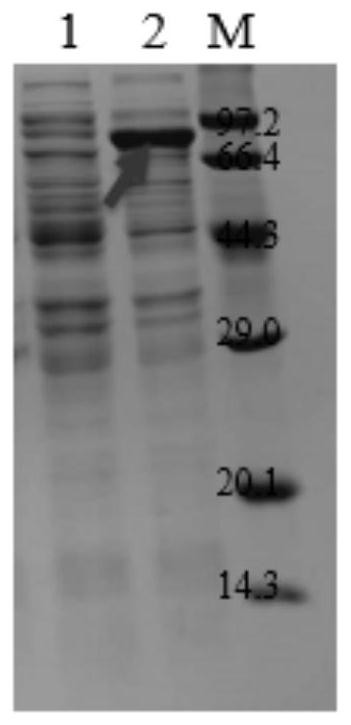
Preparation method of mycoplasma bovis immunomagnetic beads
InactiveCN111471695AImprove coupling efficiencyImprove featuresBiological material analysisImmunoglobulins against bacteriaMicrobiologyPolyclonal antibodies
The invention discloses a mycoplasma bovis P48 gene. The base sequence of the mycoplasma bovis P48 gene is shown as SEQ ID NO. 1 in a sequence table; a mycoplasma bovis P48 antigen protein is a protein expressed by the mycoplasma bovis P48 gene; an anti-mycoplasma bovis polyclonal antibody is a polyclonal antibody prepared by taking the mycoplasma bovis P48 antigen protein as an antigen; and a preparation method of an anti-mycoplasma bovis immunomagnetic bead is disclosed. Results show that 1) the coupling efficiency of NHS magnetic microspheres is highest when a coupling buffer solution is 2-morpholineethanesulfonic acid (MES) and the antibody concentration is 200 [mu] g / mL; and 2) the prepared immunomagnetic beads can be enriched into mycoplasma bovis within 15 minutes, the lowest enrichment concentrations are that the bacterium concentration is 10<-3>CCU / mL, and the immunomagnetic beads have good specificity and sensitivity.
Owner:JILIN AGRICULTURAL UNIV
Porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine
PendingCN112957460AReduce the chance of side effectsHigh antigen contentAntibacterial agentsBacterial antigen ingredientsUltrafiltrationVirus Protein
The invention discloses a porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine which comprises an antigen and a vaccine adjuvant, the antigen is composed of a porcine circovirus type 2 antigen, a porcine pseudorabies virus antigen and a mycoplasma antigen, the porcine circovirus type 2 antigen is a purified, concentrated and inactivated porcine circovirus type 2 protein antigen solution, and the content of Cap protein is more than or equal to 160 [mu]g / ml; the porcine pseudorabies virus antigen is a purified, concentrated and inactivated porcine pseudorabies virus protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; the mycoplasma antigen is an inactivated mycoplasma protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; and the vaccine adjuvant is composed of a water-based high-molecular polymer adjuvant and a composite polysaccharide immunopotentiator. Foreign protein is removed through clarification filtration and ultrafiltration concentration, and the side reaction probability of the vaccine is greatly reduced; and three-proofing can be achieved through one needle, so that the number of immunization times and stress are reduced. The method is economical and practical, the immunization procedure is simplified, and the epidemic prevention cost is reduced.
Owner:JIANGXI ZHENGBANG TECHNOLOGY CO LTD +1
Immunogenic composition comprising mycoplasma antigens
ActiveUS20180125957A1Reduction and lack of signFast recovery timeAntibacterial agentsBacterial antigen ingredientsBacilliMycoplasma antigen
The present invention relates to an immunogenic composition comprising: a) one or more antigen of M. hyorhinis and one or more antigens of M. hyosynoviae; and b) a pharmaceutically acceptable carrier. Furthermore, the present invention relates to an immunogenic composition that comprises a) one or more mycoplasma antigens of mycoplasma bacteria selected from the group consisting of M. hyorhinis, M. hyopneumoniae and M. hyosynoviae; and b) one or more components of a eukaryotic cell system. Moreover, the present invention also provides an immunogenic composition obtained by a method comprising a) cultivation of a mycoplasma bacteria selected from the group consisting of M. hyorhinis, M. hyopneumoniae and M. hyosynoviae in a serum-reduced, eukaryotic cell system; b) obtaining an antigen of such mycoplasma bacteria; and c) addition of a pharmaceutically acceptable carrier.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Mycoplasma media formulations
PendingUS20220235313A1Preventing and reducing and ameliorating diseaseAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureBovine brain
The present invention relates to media formulations free of swine serum and animal (primarily bovine brain and spinal cord) origin ingredients for Mycoplasma growth. Media formulations are rationally designed to preserve Mycoplasma antigenicity. Mycoplasma grown in these media formulations are useful in vaccines, particularly multivalent swine vaccines.
Owner:ELANCO US INC
Mycoplasma bovis monoclonal antibody, and preparation method and application thereof
ActiveCN103509756BStrong variabilityEasy to operateImmunoglobulins against bacteriaMicroorganism based processesMycoplasma bovis antigenMycoplasma antigen
Owner:HUAZHONG AGRI UNIV
Vaccine composition for preventing and treating porcine respiratory syndrome, and preparation method and application thereof
ActiveCN103961695ALow costReduce the number of vaccinationsAntibacterial agentsAntiviralsHaemophilusMedicine
A provided vaccine composition for preventing and treating porcine respiratory syndrome comprises immunization amount of porcine influenza virus antigen, immunization amount of porcine pneumonia mycoplasma antigen, immunization amount of haemophilus parasuis antigen, and an adjuvant. The vaccine composition is capable of effectively preventing and controlling porcine respiratory syndrome caused by mixed infection of the three kinds of pathogeny, and also is capable of reducing vaccine inoculating frequency and avoiding unavailable full-access immunization caused by missed inoculation.
Owner:PU LIKE BIO ENG
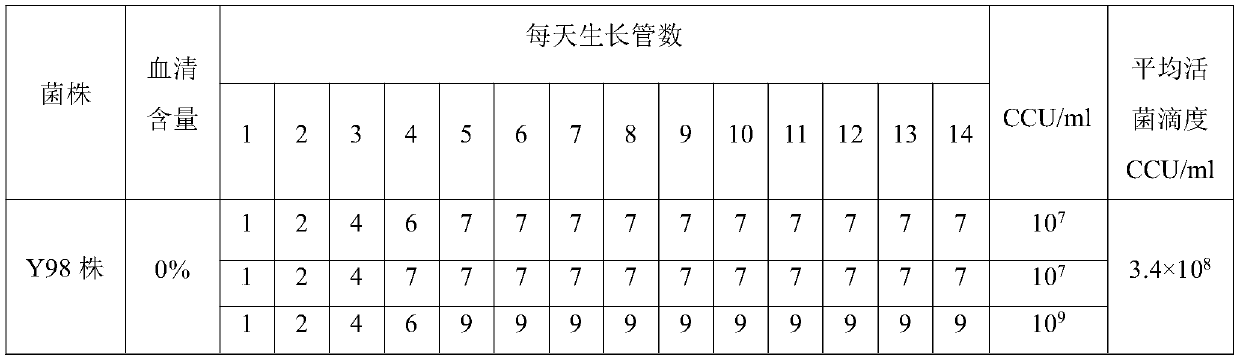
Method for preparing serum-free mycoplasma bovis antigen
PendingCN110551653AStable sourceEasy accessAntibacterial agentsBacterial antigen ingredientsSerum igeSerum free
The invention provides a method for preparing a serum-free mycoplasma bovis antigen. The method for preparing the serum-free mycoplasma bovis antigen comprises the following steps: (1) hatching freshSPF breeding eggs, carrying out culturing for 7-8 days, and selecting healthy SPF chicken embryos; (2) preparing a mycoplasma bovis inoculum; (3) inoculating the selected healthy SPF chicken embryos,which are 7-8 days old, with the mycoplasma bovis inoculum, continuing culturing after the inoculation in an incubator, observing status of the SPF chicken embryos, collecting dead inoculated SPF chicken embryos, and carrying out storing at 2-8 DEG C for 12-24 hours; and (4) aseptically collecting allantoic fluid of the dead SPF chicken embryos, thereby obtaining the serum-free mycoplasma bovis antigen. The method for preparing the serum-free mycoplasma bovis antigen is capable of realizing serum-free production of mycoplasma bovis antige, thereby avoiding multiple problems caused by use of animal serum; and moreover, the cultured mycoplasma bovis antigen is high in live bacterium titer.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A kind of vaccine composition and its preparation method and application
ActiveCN104096222BNo mutual interferenceSatisfied with the immune effectBiological testingRespiratory disorderHaemophilusRespiratory syndrome virus
Owner:PULIKE BIOLOGICAL ENG INC
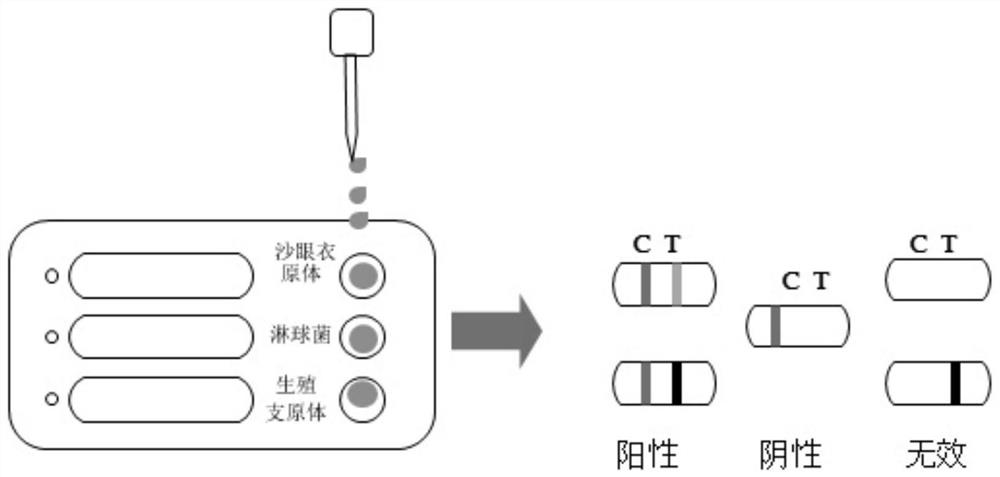
Chlamydia trachomatis/gonococcus/mycoplasma genitalium antigen combined detection kit and preparation method thereof
PendingCN114414798ARapid qualitative detection and judgmentStrong specificityImmunoassaysMycoplasma antibodyCellulose
The invention discloses a chlamydia trachomatis / gonococcus / mycoplasma genitalium antigen combined detection kit and a preparation method thereof, and relates to the field of detection kits. Comprising a detection card, the detection card comprises a bottom plate, a sample adding plate, a latex combination pad, a nitrocellulose membrane and absorbent paper, and the sample adding plate, the latex combination pad, the nitrocellulose membrane and the absorbent paper are sequentially connected end to end and are fixed on the bottom plate; a first latex microsphere-labeled chlamydia trachomatis / gonococcus / mycoplasma genitalium specific antibody and a second latex microsphere-labeled streptavidin are fixed on the latex combination pad, and the nitrocellulose membrane is provided with a detection line coated with a chlamydia trachomatis / gonococcus / mycoplasma genitalium antibody and a quality control line coated with biotin-BSA (Bovine Serum Albumin). The detection kit provided by the invention can simultaneously complete antigen detection of chlamydia trachomatis / gonococcus / mycoplasma genitalium under the condition of only one-time sampling and sample treatment process, and is high in efficiency, strong in specificity and high in sensitivity.
Owner:北京泰格科信生物科技有限公司
Mycoplasma pneumoniae antigen
ActiveCN110964089BImprove filtering effectEnhance immune responseDepsipeptidesFermentationImmune profilingMycoplasma pneumoniae IgG
The invention discloses a mycoplasma pneumoniae antigen and its application in the simultaneous quantitative detection of mycoplasma pneumoniae IgG and IgM contents in peripheral blood. The mutated mycoplasma pneumoniae antigen P80 of the invention has an amino acid sequence as shown in SEQ ID NO.1. The immunoreactivity between the mycoplasma pneumoniae antigen P80 and the antibody is about 34.6% higher than that of the natural antigen. The Mycoplasma pneumoniae antigen P80 of the present invention combines quantum dot immunochromatography technology, overcomes existing Mycoplasma pneumoniae antibody detection methods such as immunochromatography assay (ICA) and enzyme-linked immunoassay (ELISA) technology etc. can only qualitative or semi-quantitative detection , and the detection of MP-IgM and MP-IgG must be carried out twice, and the detection of MP-IgM and MP-IgG can be realized at the same time, so as to distinguish whether the patient has recent infection or past infection, and reduces the misdiagnosis rate.
Owner:NANJING VAZYME MEDICAL TECH CO LTD
Porcine circovirus type 2/mycoplasma hyorhinis combined vaccine
PendingCN110124026AAntibacterial agentsBacterial antigen ingredientsPorcine circovirusPorcine circovirus associated disease
The invention relates to a multivalent immunogenicity composition which comprises a porcine circovirus type 2 (PCV2) antigen and a mycoplasma hyorhinis antigen. The composition can resist the attack of PCV2 and mycoplasma hyorhinis, and the immunized group and the healthy control do not have evident difference in terms of clinical pathology and anatomy pathology while evident clinical pathology and anatomy pathology appears in the challenge control. In addition, the mycoplasma hyorhinis can promote the titer of the PCV2 antibody, and the comprehensive control of diseases related to porcine circovirus can be promoted.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Natural antigen P1 for detecting anti-mycoplasma pneumoniae antibody as well as preparation method and application of natural antigen P1
The invention belongs to the technical field of biological detection, and particularly relates to a natural antigen P1 for detecting an anti-mycoplasma pneumoniae antibody as well as a preparation method and application of the natural antigen P1. The preparation method comprises the following steps of taking an inactivated human mycoplasma pneumoniae antigen culture solution, centrifuging to obtain mycoplasma pneumoniae thalli, washing with a mycoplasma washing solution, centrifuging, and discarding supernatant and precipitate to obtain mycoplasma pneumoniae; putting into a lysis solution, carrying out water bath incubation at 37 DEG C, carrying out cryopreservation at -20 DEG C, then carrying out re-melting, carrying out resuspension on the centrifuged precipitate by using a mycoplasma washing solution, and carrying out centrifugation to leave the precipitate; resuspending the precipitate in the lysis solution, carrying out ultrasonic treatment, and centrifuging to obtain a supernatant which is an antigen crude product; and carrying out centrifugal ultrafiltration on the supernatant obtained by filtering the crude product, and collecting the protein on the membrane, namely the natural antigen P1. The method is simple in step and suitable for popularization and mass production, and the prepared natural antigen P1 has good specificity and sensitivity to the mycoplasma pneumoniae antibody, so that the clinical detection sensitivity and specificity of the mycoplasma pneumoniae antibody in disease diagnosis are further improved.
Owner:山东硕景生物科技有限公司 +1
A vaccine composition for preventing and treating porcine respiratory syndrome, its preparation method and application
ActiveCN103961695BLow costReduce the number of vaccinationsAntibacterial agentsAntiviralsHaemophilusMycoplasma antigen
A provided vaccine composition for preventing and treating porcine respiratory syndrome comprises immunization amount of porcine influenza virus antigen, immunization amount of porcine pneumonia mycoplasma antigen, immunization amount of haemophilus parasuis antigen, and an adjuvant. The vaccine composition is capable of effectively preventing and controlling porcine respiratory syndrome caused by mixed infection of the three kinds of pathogeny, and also is capable of reducing vaccine inoculating frequency and avoiding unavailable full-access immunization caused by missed inoculation.
Owner:PULIKE BIOLOGICAL ENG INC
Method for detecting mycoplasma pneumoniae antibody, kit for detection and preparation method of kit
ActiveCN103033615BRapid Simultaneous DetectionNot easy to interfereMaterial analysisMycoplasma antibodySerum ige
Owner:QINGDAO HIGHTOP BIOTECH
Monoclonal antibody blocking agent for novel crown antigen detection
ActiveCN114751980AHigh affinityInhibit bindingImmunoglobulins against virusesImmunoassaysVirus ProteinAntigen testing
The invention relates to a specific monoclonal antibody blocking agent aiming at a new coronavirus S protein RBD protein. The obtained monoclonal antibody blocking agent has high affinity, and can effectively and competitively inhibit the combination of the new coronavirus S protein RBD and ACE2. Pseudovirus neutralization experiments show that the antibody has good neutralization activity on new coronal pseudovirus and mutant strain (Detla) pseudovirus of the new coronal pseudovirus. The lower detection limit of the monoclonal antibody blocking agent is 3.9 ng / mL, the linear range is 1000 ng / mL to 7.8 ng / mL, the monoclonal antibody blocking agent does not have cross reaction with new coronavirus N protein, influenza virus, mycoplasma pneumoniae antigen, BSA, Vero cells and the like, and false positive of the reagent can be greatly reduced, so that the accuracy of the detection reagent is improved.
Owner:厦门博昂生物技术有限公司
A multiple respiratory antigen detection card and kit
ActiveCN112362869BAdequate responseHigh sensitivityBiological testingImmunoassaysAntigen testingRespiratory syncytial virus antigen
The invention relates to a multi-respiratory tract antigen detection card, which includes influenza A virus antigen test strips, influenza B virus antigen test strips, respiratory adenovirus antigen test strips, respiratory syncytial virus antigen test strips and Mycoplasma pneumoniae antigen test strips, Influenza A virus antigen test strips, Influenza B virus antigen test strips, Respiratory adenovirus antigen test strips, Respiratory syncytial virus antigen test strips and Mycoplasma pneumoniae antigen test strips all include Colloidal gold conjugate pads, colloidal gold conjugate pads include streptavidin conjugate pads and double-nanoparticle double-labeled antibody conjugate pads; the test strips in this test card can fully react biological raw materials and improve the sensitivity of antigen detection , effectively reducing missed detection, and adding blocking agents to the sample pad to improve specificity, and can simultaneously detect influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus, and Mycoplasma pneumoniae antigens.
Owner:山东康华生物医疗科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com