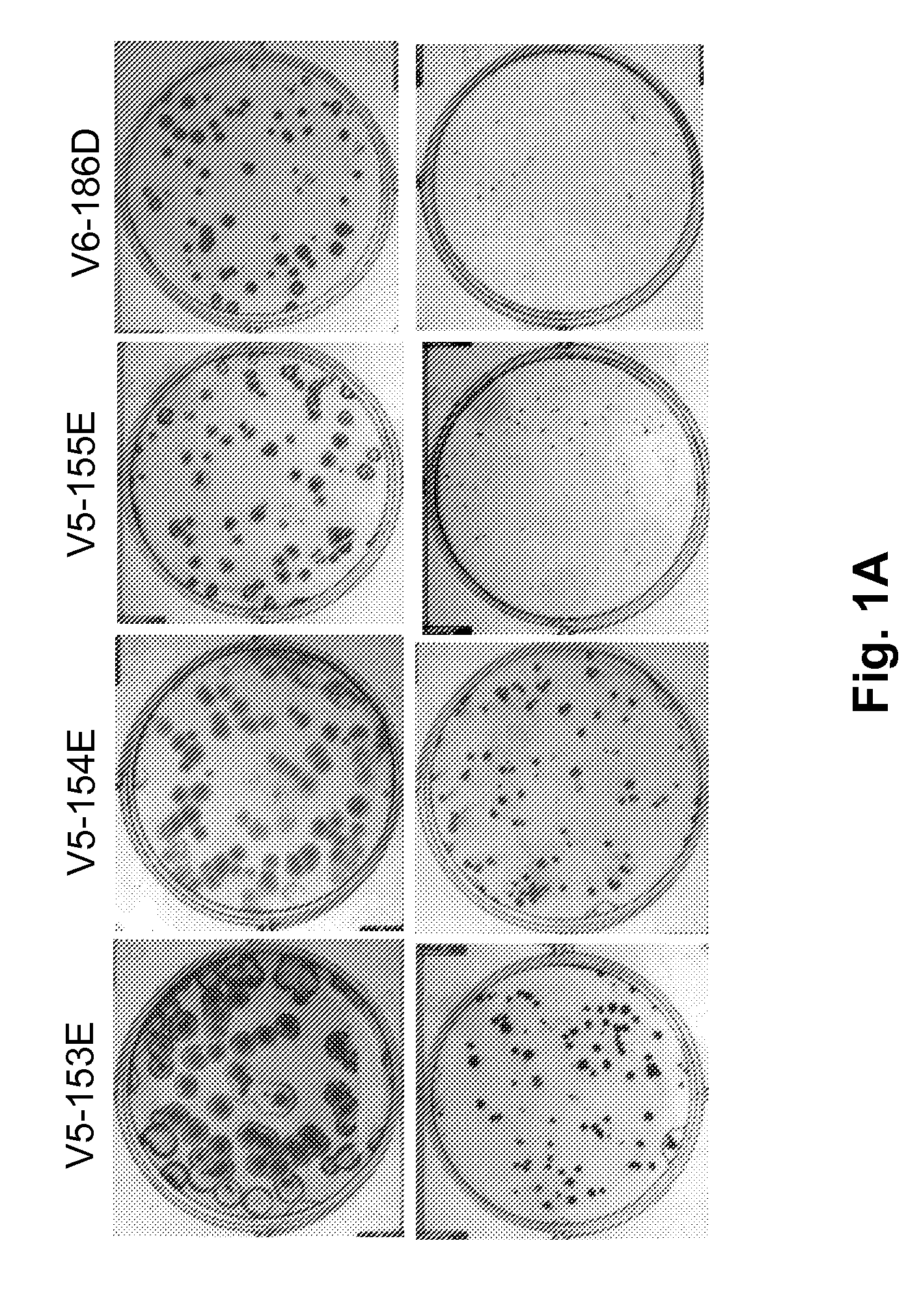
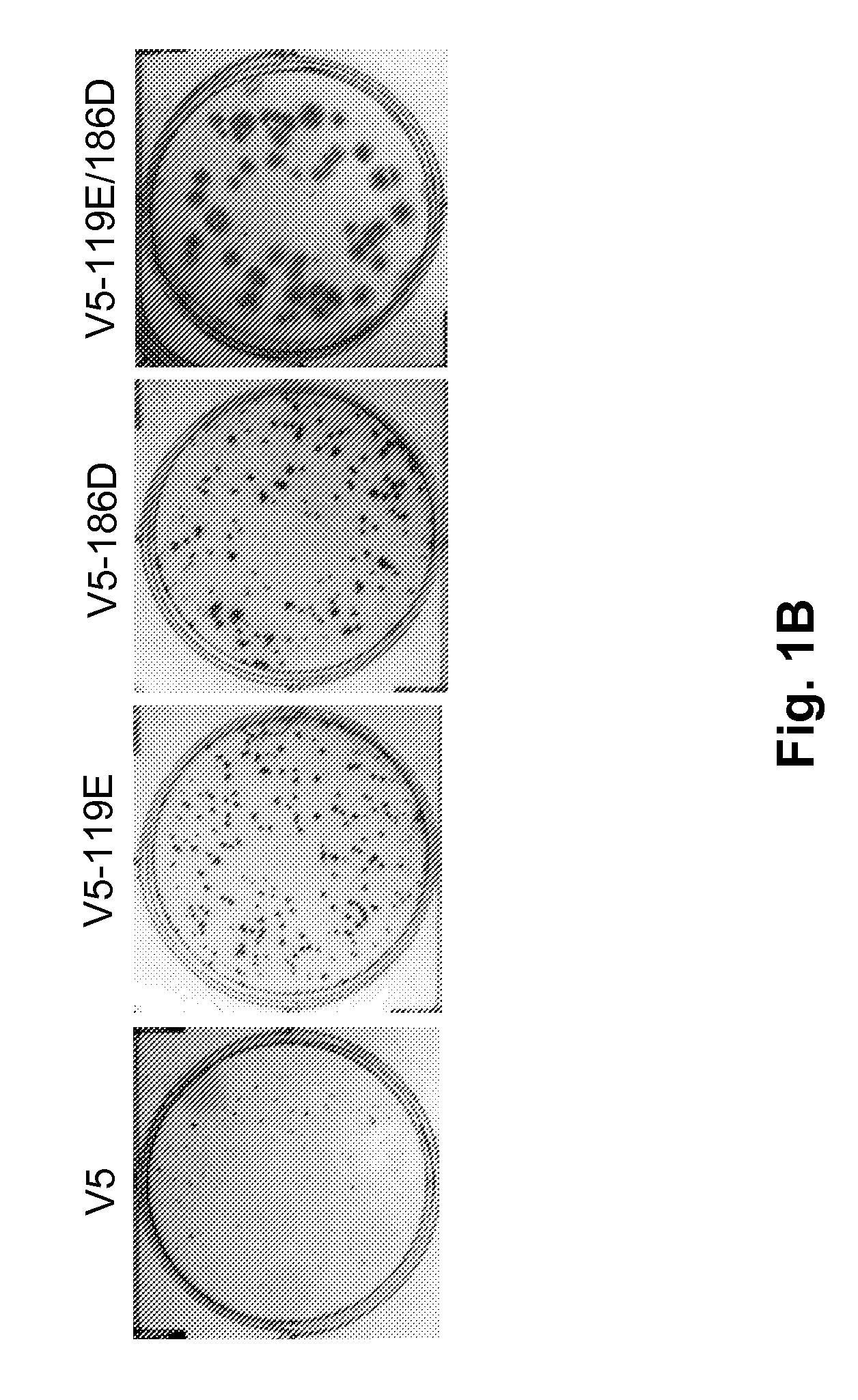
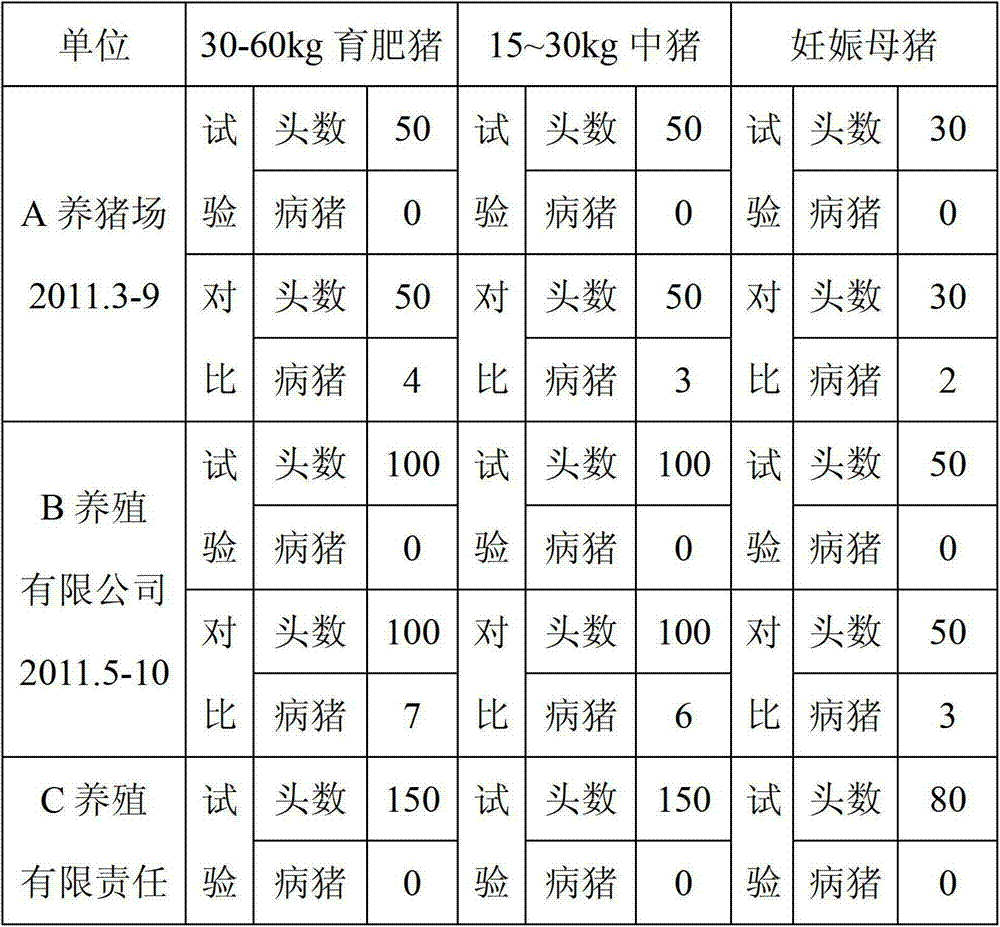
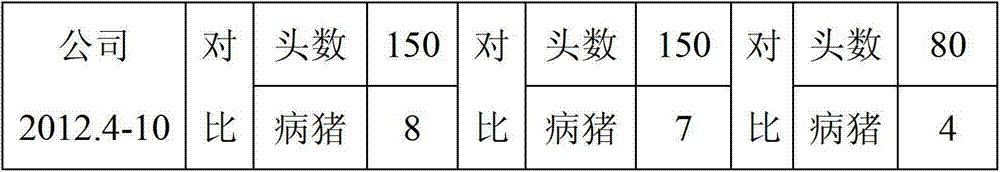
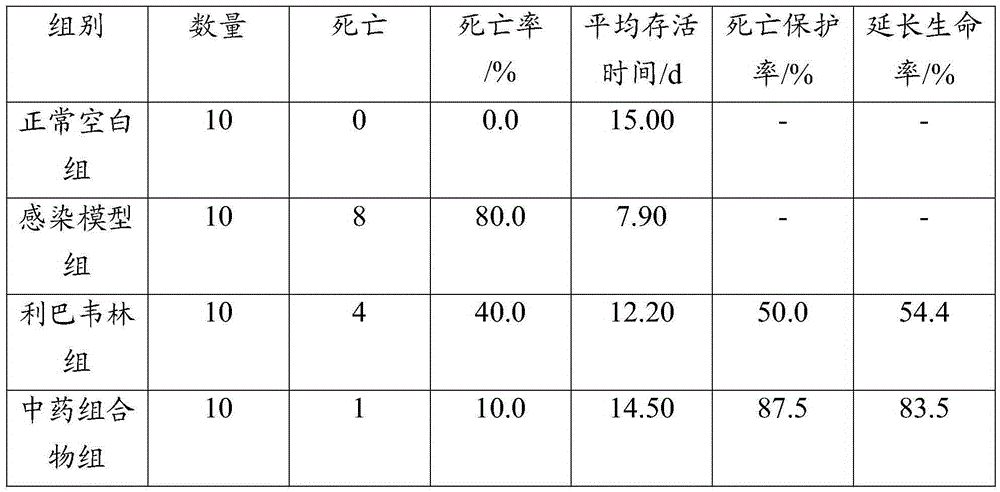
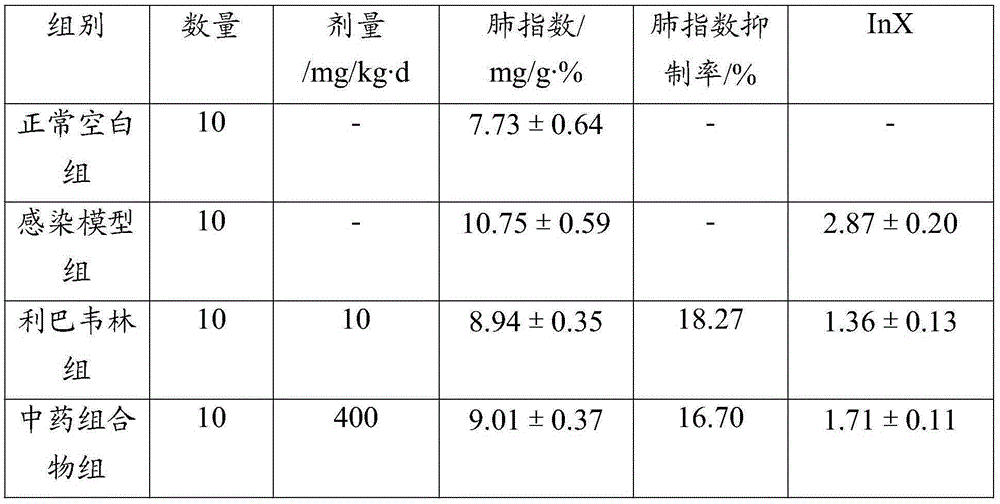
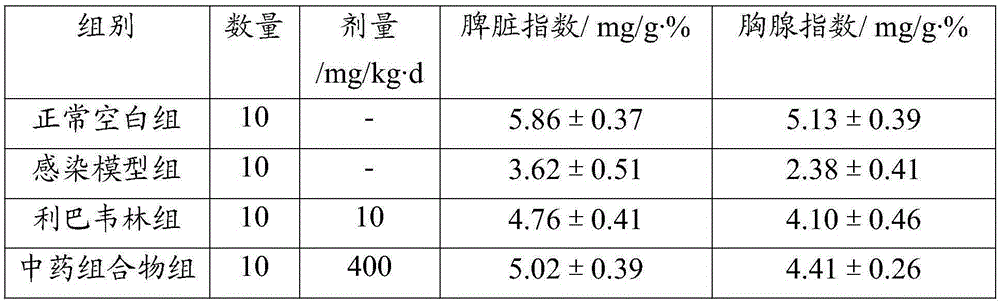
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Porcine influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prokaryotic expression protein of VP73 gene from African swine fever virus and preparation method thereof
InactiveCN102101889ANo risk of poisoningHigh detection sensitivityMicroorganism based processesPeptide preparation methodsAfrican swine fever virus AntibodySwine Fever Virus
The invention relates to a method for preparing a genetic engineering product, in particular to a prokaryotic expression protein of VP73 gene from African swine fever virus (ASFV) and a preparation method thereof. The preparation method comprises: artificially synthesizing the whole-length sequence of VP73 gene according to the sequence of the VP73 gene from ASFV in GenBank, constructing a recombinant expression vector pET32a-VP73, sequencing, verifying, transforming prokaryotic expression recipient bacteria E.coli BL21(DE3), and inducing expression by isopropyl-1-thio-beta-d-galactopyranoside (IPTG), wherein the molecular weight of the recombinant fusion protein is about 65KD. Protein purified by nickel column affinity chromatography can undergo a specific immune imprinting reaction with ASFV positive serum and avoid cross reaction with viruses such as swine fever virus, hog cholera virus, porcine circovirus, porcine reproductive and respiratory syndrome virus, swine influenza virus and pseudorabiesvirus. Experiments show the expressed protein has high detection sensitivity, and high specificity. When the antigen is used for detection, risk of spreading poison is avoided. And the antigen can be used as a detection antigen for use in an enzyme-linked immuno sorbent assay (ELISA) method for identifying an ASFV antibody.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
H1N1 subtype swine influenza virus and application thereof
The invention discloses an H1N1 subtype swine influenza virus A / Swine / Shanxi / D5 / 2011 (H1N1) with the preservation number of CGMCC No.5323 and also discloses a vaccine containing the virus. The H1N1 subtype swine influenza virus provided by the invention is not polluted by extraneous pathogeny, has higher multiplication capacity on both a chicken embryo and a cell, shows obvious clinical symptoms after infecting a BALB / c rat and a swine influenza negative piglet and has typical autopsy pathological changes and good immunogenicity, and an oil emulsion inactivated vaccine prepared by using the H1N1 subtype swine influenza virus has 100% of protective efficiency for the attack of a homologous virus after immunizing a pig and serves as a favorable candidate swine influenza vaccine strain.
Owner:兆丰华生物科技(南京)有限公司 +1
Vaccine strain of HIN1 swine influenza virus and application thereof
ActiveCN102899294AImprove protectionLong durationMicroorganism based processesAntiviralsEngineeringImmunogenicity
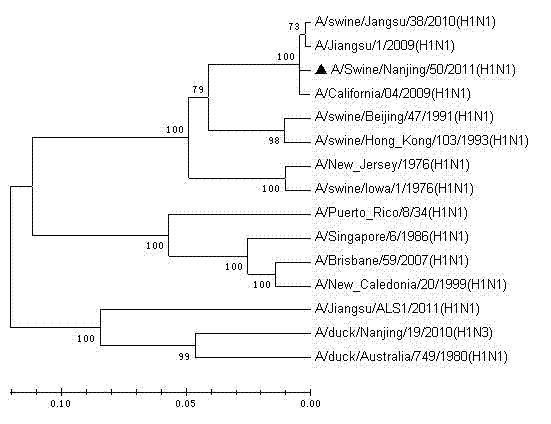
The invention relates to a vaccine strain of HIN1 swine influenza virus, wherein a classification naming is Swine influenza virus A / Swine / Nanjing / 50 / 2011 (H1N1), and a microbial accession number is CCTCCNO: V201218. According to the invention, isolation, identification and whole genome sequencing of the HIN1 swine influenza virus strain are carried out, and an inactivated vaccine against the swine flu is prepared by using the HIN1 swine influenza virus strain. The inactivated vaccine against the H1NI swine flu prepared from the virus strain can provide good protection for pigs attacked by homologous virulent strains, has good immunogenicity, and can effectively prevent H1NI swine flu with single vaccine or combined vaccines.
Owner:JIANGSU ACAD OF AGRI SCI
Swine influenza A H1N1 virus and use thereof
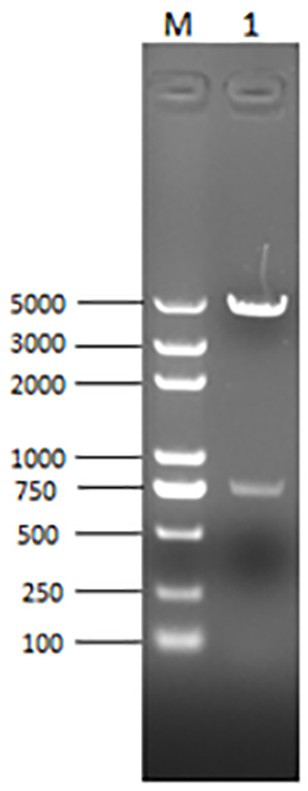
The invention belongs to the field of microbial virology and provides a swine influenza A H1N1 virus. The virus contains eight fragments, namely HA, NA, NP, PB1, PB2, PA, NS and M, wherein the nucleotide sequences of the eight fragments are shown by the sequences from No.1 to No.8 in a nucleotide sequence table in the description in turn. In the invention, by separate culture of a nasopharyngeal swab sample of a healthy pig, differential item functioning (DIF) and reverse transcription-polymerase chain reaction (RT-PCR) identification and the comparison of full sequences of 8 genes of the virus, a virus is obtained and verified to be a swine influenza A H1N1 virus strain. The swine influenza A H1N1 virus strain is named A / swine / Zhejiang / 26 / 2009(H1N1), with a collection number of CCTCC No.V201016. The strain obtained by the invention supplies valuable genetic information of swine influenza A virus in China, has a great significance for completing a system for monitoring the infection caused by the pathogen and can be used for preparing a reagent for quickly diagnosing infection with the influenza A H1N1 virus.
Owner:FUDAN UNIV
Recombinant swine influenza virus and uses thereof
InactiveUS20130189303A1Efficient growth processHinders its propagationSsRNA viruses negative-senseAntiviralsHemagglutininInfluenza A virus subtype H5N1
Recombinant, chimeric porcine influenza viruses are disclosed that include hemagglutinin segments from more than one influenza virus subtype. Also described are methods of producing the recombinant influenza viruses, immunogenic compositions comprising the recombinant influenza viruses, methods of stimulating an immune response against influenza virus, and methods of treating and preventing influenza virus infection.
Owner:UNIVERSITY OF SASKATCHEWAN
Method for determining titer of swine flu inactivated vaccine
ActiveCN102735853AReduced measurement timeReduce operating errorsBiological testingRed blood cellEngineering
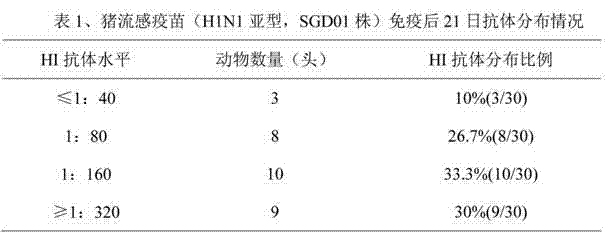
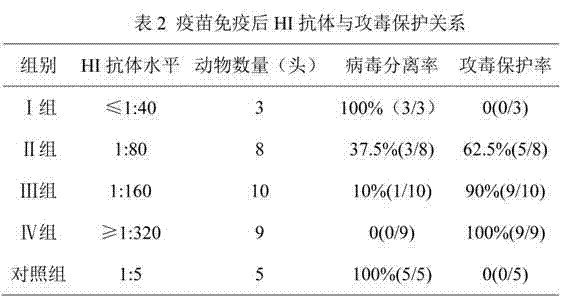
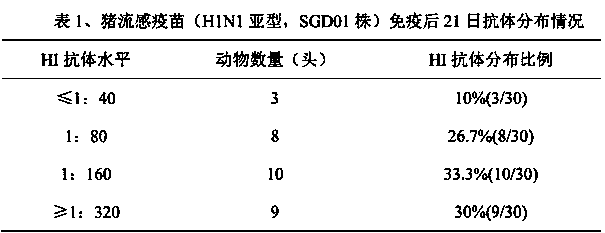
The invention discloses a method for determining titer of a swine flu inactivated vaccine. The method comprises the following steps: immunizing a pig with a swine flue inactivated vaccine, collecting blood 21-28 days after immunization and separating blood serum; removing non-specific components in the serum to obtain a serum to be tested; diluting the swine flue inactivated antigen, and preparing a unit swine flue antigen diluent with a concentration of 4HA; and diluting the serum to be tested by multiple proportions, successively adding the 4HA unit swine flue antigen diluent and 1% red cells to conduct hemagglutination inhibition test, so as to completely inhibit highest dilution of the 4HA unit swine flu antigen serum at HI titer. The method has advantages of greatly shortened measurement time, little operation error, strong controllability, and small inter-batch difference, and also reduces detection errors caused by different levels of experimental animals in an animal challenge protection experiment for testing the titer.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE +1
Veterinary compound hydrochloric acid injection and preparation method thereof
InactiveCN102151263AGood effectEasy to prepareAntibacterial agentsOrganic active ingredientsProtozoaDisease
The invention relates to veterinary compound hydrochloric acid injection and a preparation method thereof. The veterinary compound hydrochloric acid injection consists of oxytetracycline dihydrate injection, imidocarb and diclofenac sodium; the veterinary compound hydrochloric acid injection per 100 ml comprises the required raw materials of: 10-25g of oxytetracycline dihydrate injection, 1.5-5g of diclofenac sodium, 0.1-0.2g of imidocarb, 0.2-0.6g of sodium formaldehyde sulphoxylate, 5-15g of magnesium chloride, 5-13 ml of ethanolamine and 60-81 ml of organic solvents, wherein the allowance is water for injection. The veterinary compound hydrochloric acid injection has remarkable effect when being used for treating acute respiratory infection caused by eperythrozoon suis, babesiosis and other blood protozoa diseases, porcine respiratory disease complex (PRDC) and swine influenza virus (SIV), airway inflammation induced by porcine reproductive and respiratory syndrome (PRRS), haemophilus parasuis, pasteurella, pleuropneumonia and mycoplasma diseases; and the preparation method is simple and easy to operate, and is suitable for batch production.
Owner:XUCHANG TIANYUAN BIOLOGICAL TECH CO LTD
New swine influenza vaccine
ActiveUS20180080044A1Low costSimilar efficiencySsRNA viruses negative-senseViral antigen ingredientsNucleotideHerpes simplex virus DNA
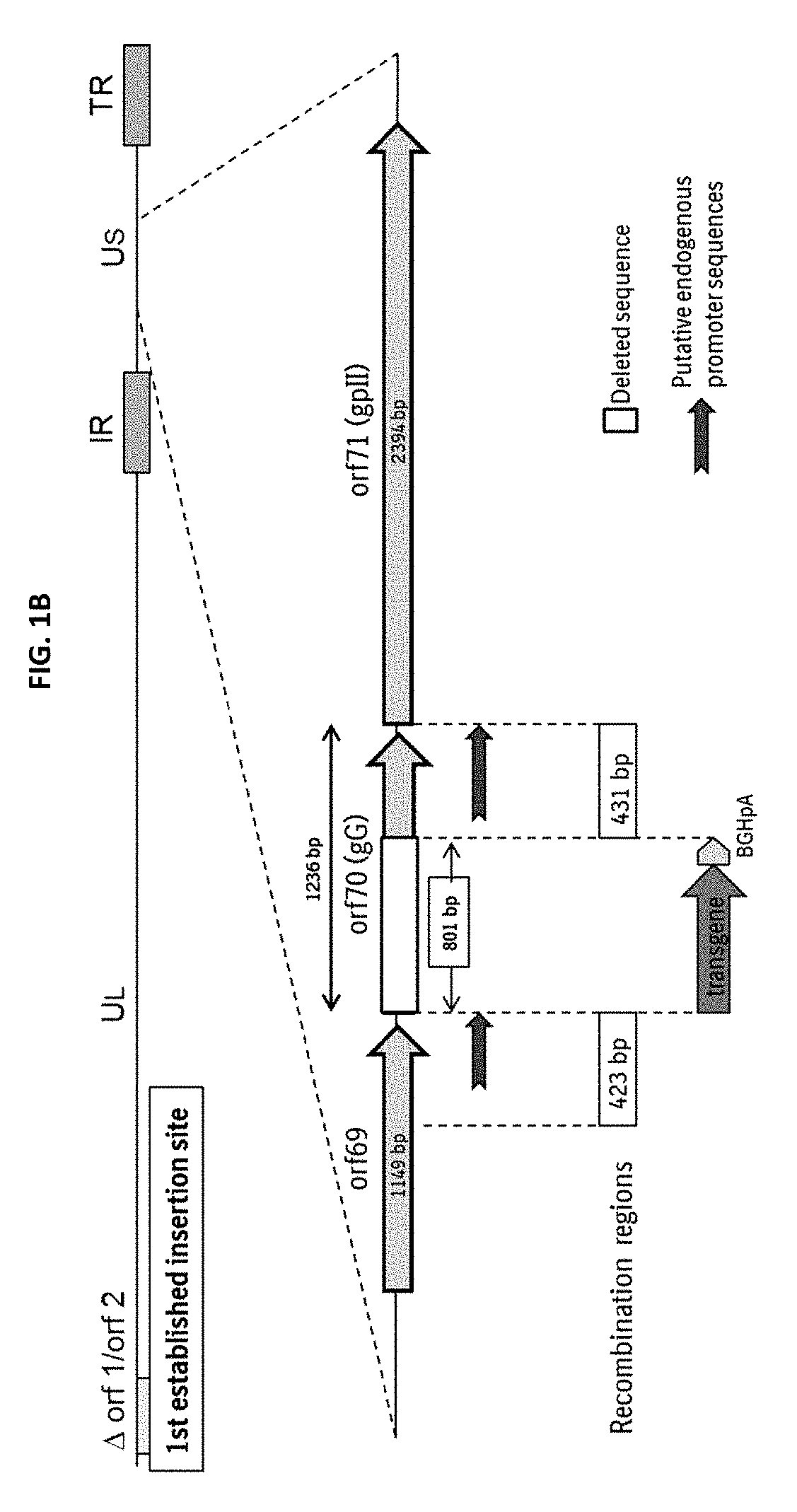
The present invention relates to Equine Herpes Virus (EHV) vectors comprising at least one exogenous antigen encoding sequence relating to a pathogen infecting food producing animals, wherein said exogenous antigen encoding sequence is inserted into an insertion site, preferably ORF70, and said exogenous antigen encoding sequence is operably linked to a promoter sequence, preferably the promoter sequence comprising 4pgG600 (SEQ ID NO:1) or 4pMCP600 (SEQ ID NO:2) or the complementary nucleotide sequences thereof or a functional fragment or a functional derivative thereof or the complementary nucleotide sequences thereof. Furthermore, the present invention relates to methods for immunizing a food producing animal comprising administering to such food producing animal an immunogenic composition comprising embodiments of the present invention. Moreover, the present invention relates to methods for the treatment or prophylaxis of clinical signs caused by swine influenza virus in a food producing animal.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Universal influenza vaccine and preparation method thereof
ActiveCN105148264AImproving immunogenicityInhibit productionAntiviralsAntibody medical ingredientsHemagglutinin proteinViral Vaccine
The invention relates to universal influenza vaccine and a preparation method thereof. After an influenza virus HA is subjected to gene transfection to eukaryocyte, influenza haemagglutinin (HA) protein subjected to eukaryotic expression modification is inserted into a host plasma membrane, then vesicles largely displaying influenza virus envelope glycoprotein is produced with a method that a surface active agent induces a cell to bud, and the vesicles are changed into controllable and nanometer mimicry virus vesicles with uniform dimensions after purification and re-adding of the surface active agent for treatment. At the same time, by the aid of establishment of stably expressing an HA cell strain and usage of a bioreactor, the method is also applicable to large scale influenza vaccine production. The method is simple and feasible, hemagglutinin of most influenza virus (including avian influenza and swine influenza) can be displayed, and the method has excellent universality, has a great potential and can be used for designing emerging highly lethal influenza virus vaccines.
Owner:XIAMEN UNIV
Nucleic-acid sequence-based amplification (NASBA) method for detecting swine influenza virus (SIV)
InactiveCN102534052AMicrobiological testing/measurementMicroorganism based processesQuarantineSwine influenzavirus
The invention discloses a nucleic-acid sequence-based amplification (NASBA) method for detecting a swine influenza virus (SIV). The NASBA method mainly comprises the following steps of: extracting the ribonucleic acid (RNA) from the SIV, preparing an NASBA system, preparing an NASBA program, carrying out identification on an NASBA product and carrying out PCR (Polymerase Chain Reaction) amplification. According to the NASBA method, the NASBA rapid detection method is established by taking the NP gene of the SIV as a target gene and is used for the diagnosis of the SIV. The method has higher specificity and sensitivity so as to detect a virus solution with the dilution factor of 10<-5>, so that the method can be used for the rapid detection of clinically-suspected SIV samples, meanwhile, the technical level of China in the diagnosis, epidemic surveillance, inspection and quarantine of swine influenza can be improved so as to ensure the healthful and rapid development of swine industry.
Owner:天津市宁河原种猪场有限责任公司
Anti-porcine pseudorabies and swine flu vaccine composition and application thereof
ActiveCN103893750AImprove immunityNo interferenceAntiviralsAntibody medical ingredientsDiseaseAntigen
The invention relates to an anti-porcine pseudorabies (PRV) and swine flu (SIV) vaccine composition. Besides porcine pseudorabies and swine flu, the composition can additionally prevent clinical manifestations of porcine respiratory disease complex (PRDC) caused by infection of other pathogenic causes. Furthermore, the invention provides application of the vaccine composition for preparing a medicine in preventing and / or controlling immunized pig PRDC disease. The composition not only avoids interference between antigens in a process of using but also achieves a surprising preventive effect on the porcine respiratory disease complex. Therefore, the vaccine composition disclosed by the invention can simplify an immune procedure and achieve an effect of preventing two diseases by one dosage. The anti-porcine pseudorabies and swine flu vaccine composition, after immunizing pigs, can effectively prevent spread of the PRDC.
Owner:PU LIKE BIO ENG
Mixed virus-like particles of swine influenza virus and foot and mouth disease virus, preparation method and application thereof
ActiveCN102370976AStrong immunityLong durationMicroorganism based processesAntiviralsAdjuvantFoot-and-mouth disease virus
The invention provides a mixed virus-like particles, containing stromatin M1 of influenza virus, surface antigen erythrohemagglutinin HA of influenza virus and a nexus protein containing main antigenic epitope contained capsid protein of foot and mouth disease virus and a transmembrane zone and an inner membrane zone of the erythrohemagglutinin HA of influenza virus. The main antigenic epitope contained capsid protein of foot and mouth disease virus substitutes a basically same length outer membrane zone of a 5' terminal of the erythrohemagglutinin HA of influenza virus. Surface antigen erythrohemagglutinin HA of influenza virus and the main antigenic epitope contained capsid protein of foot and mouth disease virus are expressed simultaneously on surfaces of the mixed virus-like particles. The invention also provides a divalent vaccine of swine influenza and foot and mouth disease; and the vaccine contains the mixed virus-like particles and adjuvants. The invention also provides a method for preparing the mixed virus-like particles.
Owner:SUN YAT SEN UNIV
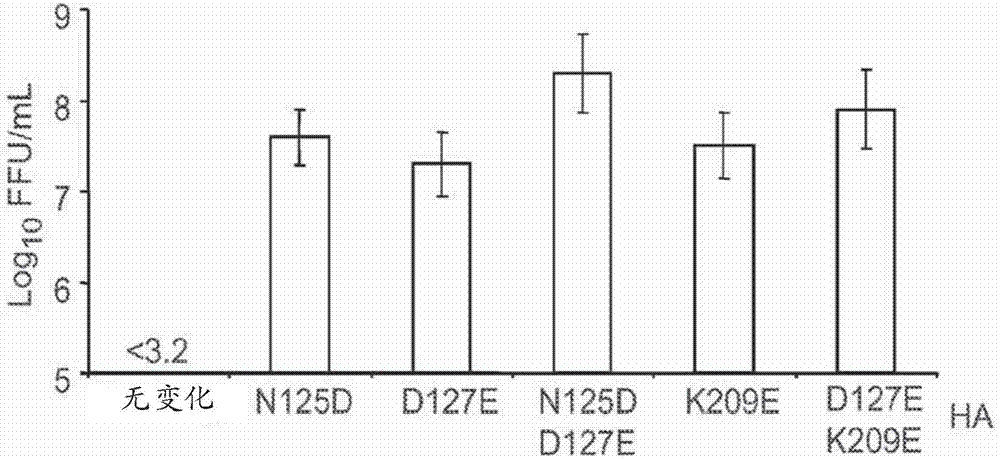
Swine influenza hemagglutinin variants
InactiveUS20110123559A1Easy to copyIncreased egg growthSsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Amino acid nano-silver liquid as well as preparation method and application thereof in disease-free safe raising of pigs
ActiveCN103109978AGood bactericidal effectPrevent swine fluAnimal feeding stuffChemicalsSolventBactericidal effect
The invention discloses an amino acid nano-silver liquid as well as a preparation method and an application thereof in disease-free safe raising of pigs. The amino acid nano-silver liquid is prepared by mainly compounding silver sulfate, a cosolvent, an amino acid primary ligand, an amino hetero-ligand, a dispersing agent, a thickening agent, a complexing stabilizer, a pH conditioning agent and deionized water. The amino acid nano-silver liquid has the advantages of having a broad spectrum, high-efficiency and a bactericidal effect, being safe and reliable, being capable of being used for preparation of sterile pig feeds, preventing from serious diseases such as swine influenza and swine fever and disinfecting environments, and being beneficial to realization of the disease-free safe raising of the pigs.
Owner:胡克矩 +1
Quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, porcine influenza virus and haemophilus parasuis
InactiveCN112386685AProlonged occurrenceExtension of timeSsRNA viruses negative-senseAntibacterial agentsAntigenAdjuvant
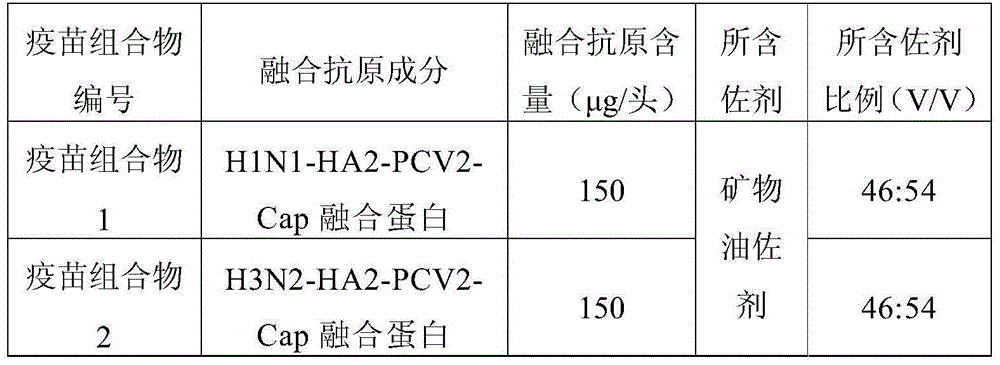
The invention belongs to the field of veterinary vaccines, particularly relates to a quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, a porcine influenza virus and haemophilus parasuis and further discloses a preparation method for the quad inactivated vaccine and application of the quad inactivated vaccine. The quad inactivated vaccine disclosed by the inventioncontains inactivated protein Cap expressed by the PCV2-type baculovirus, inactivated swine mycoplasma hyopneumoniae, an inactivated porcine influenza virus H1N1subtype virus, inactivated haemophilus parasuis types 4, 5 and 13 and an adjuvant of the vaccine; four kinds of antigens are free of interference to one another, four kinds of protection can be achieved by one injection, and four kinds of epidemic diseases can be prevented through one-time immunization; and meanwhile, in view of toxic substance counteracting protection and serum antibody level, the immunization effect reaches or exceedsthat of each single commodity vaccine, the immunization duration is long, the efficacy is durable, and the quad inactivated vaccine has the advantages that the safety is good, the preparation methodis simple, the immunization is convenient, the immunization cost is reduced, and the like.
Owner:BEIJING KEMUFENG BIOLOGICAL PHARMA +3
shRNA transgenic recombinant plasmid for inhibiting swine influenza viruses and use thereof
InactiveCN105002179AInhibition of replicationGenetic material ingredientsAntiviralsLentivirusIntramuscular injection
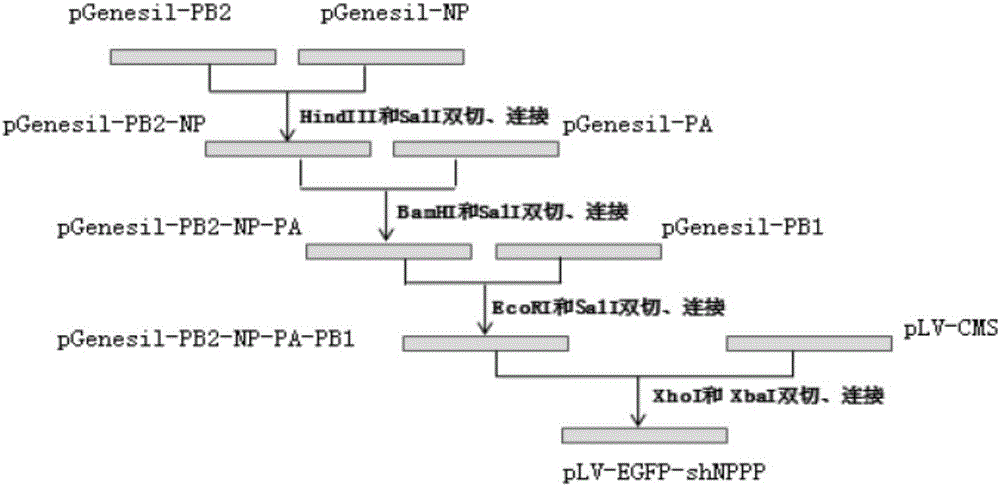
The invention provides a shRNA transgenic recombinant plasmid for inhibiting swine influenza viruses. The plasmid contains four shRNA expression genes for simultaneously coding four specific shRNAs capable of respectively targeting influenza virus Pa, PB1, PB2 and NP gene conserved regions. The plasmid is suitable for a plurality of transgenic technologies. After the plasmid is transferred into a MDCK cell cultivated in vitro by a lentivirus carrier, a challenge assay proves that the screened transgenic cell line has a function of resisting infection caused by different subtypes of influenza viruses. The plasmid is injected into a pigling with 4 week old by intravenous injection and intramuscular injection and then the pigling is infected by H3N2 subtype of pig influenza viruses, and the test result shows that an influenza virus replication inhibition function of the blood injection group is better. RNAi is used for pig influenza virus research and pig influenza prevention and treatment so that necessary experiment data is accumulated and thus a base is established for researching influenza resistance of a transgenic pig.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Vaccine composition for porcine circovirus and swine influenza and its preparation method and use
ActiveCN105709220AImproving immunogenicityEasy to breedBacteriaViral antigen ingredientsAntigenDisease
The invention relates to a vaccine composition for preventing and / or treating porcine circovirus diseases and swine influenza and its preparation method and use. The vaccine composition comprises i, a porcine circovirus subunit antigen or a nucleic acid which is used for coding the porcine circovirus subunit antigen and can be expressed in a pig, and ii, a swine influenza virus subunit antigen or a nucleic acid which is used for coding the swine influenza virus subunit antigen and can be expressed in a pig. The invention also relates to a nucleic acid molecule, a recombinant plasmid and a host cell for treating and / or preventing two types of pig diseases.
Owner:PU LIKE BIO ENG
Method for producing a permeable material that filters out harmful particles and products created therefrom
A permeable material that allows air to easily pass through the interstices in any direction. The permeable material may be a cloth, a mesh, latex, rubber, or any polymer. The material is saturated with a water and / or alcohol based solution containing either a cationic or anionic agent, a disinfecting agent, and a biocide. The solvent on the permeable material is permitted to evaporate. What remains on the permeable material is the cationic or anionic agent and the biocide. This coated material is now surrounded by an electrostatic field. The electrostatically charged material attracts oppositely charged particles and repels similarly charged particles. The particles that contain living organisms are inactivated by the biocide. The formulations have been shown to have efficacy against rhinovirus causing colds, influenza and corona viruses causing diseases such as swine flu and COVID-19, as well as other harmful airborne microscopic and sub-microscopic particles. The material may be formed into products such as face masks, unitary filters, and filtration devices. Users of these masks might want to periodically spray or otherwise apply the solution to the mask to restore its disinfecting, filtration, and biocidic properties. This would also render normally disposable cloth or surgical face masks reusable.
Owner:TRUTEK CORP
Swine influenza hemagglutinin and neuraminidase variants
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
H9N2 swine influenza virus and its kit
InactiveCN103320392ASignificant technological progressMicrobiological testing/measurementMicroorganism based processesGenotypeBiology
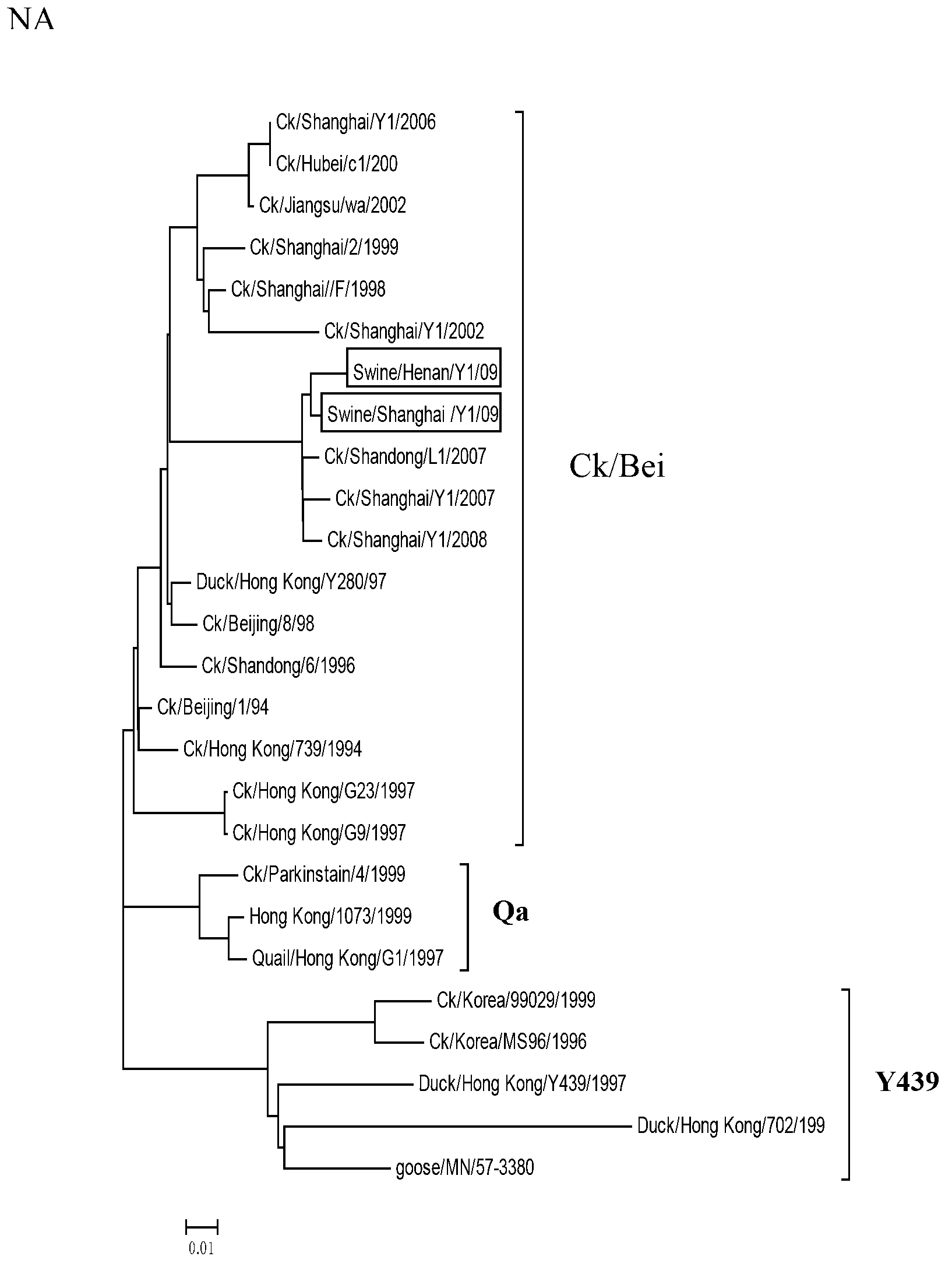
The invention belongs to the field of biotechnology and relates to a novel separated strain swine influenza virus. The invention discloses two separated H9N2 swine influenza viruses. According to the invention, it firstly proves that the two strains both belong to Ck / Bei series. The invention also discloses a kit for detecting the H9N2 swine influenza viruses. Through the kit provided by the invention, whether swine flu is caused by the genotype of H9N2 swine influenza viruses can be rapidly detected or eliminated so as to control the swine flu epidemic situation as soon as possible. In addition, by the use of the kit, bioinformation for a swine influenza virus monitoring system can be provided.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Pathogenic bacteria killing and environment control breeding house applied to epidemic prevention of swine influenza
ActiveCN112470952AEasy to sterilizeEasy to classifyAnimal housingMedical waste disposalAnimal scienceWindow shutter
The invention discloses a pathogenic bacteria killing and environment control breeding house applied to an epidemic prevention of swine influenza. The pathogenic bacteria killing and environment control breeding house comprises a breeding house body, shutter bodies and a device box, a house door is hinged and fixed to an outer side of the breeding house body, positioning columns are welded and fixed to a top part of the breeding house body, outer sides of the shutter bodies are connected with the breeding house body in an embedded mode, flexible solar panels are bonded and fixed to the outer side of the breeding house body, a waste collecting box is welded and fixed to a bottom plate of the breeding house, chassis supporting piles are welded and fixed to two sides of the waste collecting box, and pollution discharge pipelines are welded and fixed to a bottom plate of the waste collecting box. The pathogenic bacteria killing and environment control breeding house applied to an epidemicprevention of swine influenza is provided with first eccentric wheels and bevel gears, the first eccentric wheels are used for driving spraying supports to move left and right, a moving range betweenthe spraying supports is adjusted according to a width of the breeding house, and an interior of the breeding house can be conveniently and automatically sterilized.
Owner:衡阳市绿院农牧有限公司
Attenuated swine influenza vaccines AMD methods of making and use thereof
ActiveUS20180147276A1Safe and effective and broad protective-includingSsRNA viruses negative-senseViral antigen ingredientsInfluenza vaccineMethods of production
This disclosure provides attenuated swine influenza strains, particularly those produced via a reverse genetics approach, compositions comprising same, and methods of production and use thereof.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Vaccine composition for preventing and treating porcine respiratory syndrome, and preparation method and application thereof
ActiveCN103961695ALow costReduce the number of vaccinationsAntibacterial agentsAntiviralsHaemophilusMedicine
A provided vaccine composition for preventing and treating porcine respiratory syndrome comprises immunization amount of porcine influenza virus antigen, immunization amount of porcine pneumonia mycoplasma antigen, immunization amount of haemophilus parasuis antigen, and an adjuvant. The vaccine composition is capable of effectively preventing and controlling porcine respiratory syndrome caused by mixed infection of the three kinds of pathogeny, and also is capable of reducing vaccine inoculating frequency and avoiding unavailable full-access immunization caused by missed inoculation.
Owner:PU LIKE BIO ENG
Vaccine composition for porcine circovirus and swine influenza, preparation method and application
ActiveCN105709220BImproving immunogenicityEasy to breedBacteriaViral antigen ingredientsAntigenDisease
The present invention relates to a vaccine composition for preventing and / or treating porcine circovirus disease and swine influenza and its preparation method and application. Circular virus subunit antigen and nucleic acid capable of being expressed in pigs, and ii) swine influenza virus subunit antigen or nucleic acid encoding swine influenza virus subunit antigen and capable of being expressed in pigs. The present invention also relates to nucleic acid molecules, recombinant plasmids and host cells for simultaneous treatment and / or prevention of two porcine diseases.
Owner:PU LIKE BIO ENG
Attenuated swine influenza vaccines and methods of making and use thereof
ActiveUS10548967B2Safe and effective and broad protective-includingSsRNA viruses negative-senseViral antigen ingredientsInfluenza vaccineFlu immunization
This disclosure provides attenuated swine influenza strains, particularly those produced via a reverse genetics approach, compositions comprising same, and methods of production and use thereof.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Application of porcine β-defensin 2 gene in anti-swine influenza
ActiveCN108866069BReduce the severity of the diseaseLower titerFermentationCationic antimicrobial peptidesInfectious DisorderFeed additive
The invention belongs to the technical fields of zoonosis and animal transgenes, and particularly relates application of a PBD (porcine beta-defensins 2) gene in resisting of swine influenza. The application has the advantages that the segments of the PBD-2 gene are excessively expressed in a swine body, so as to obviously improve the resistance ability of the transgenic swine on the swine influenza, namely that the excessively expressed PBD-2 can obviously reduce the virus titer of swine influenza virus in lung tissues of the infected swine and the lesion degree of swine lung due to infectionby the swine influenza virus; by synthesizing or recombining the PBD-2 gene, the duplication of the swine influenza virus can be effectively inhibited; the polypeptide medicines or feed additives canbe prepared by in-vitro expression or synthesizing of the PBD-2 gene.
Owner:HUAZHONG AGRI UNIV
Feed for the prevention and treatment of porcine influenza
ActiveCN104082538BRich sources of medicineReduce residual toxicityFood processingAnimal feeding stuffDiseaseSide effect
Provided feed for controlling pig influenza comprises the raw materials: corn, soybean meal, edible salt, fish meal, soybean oil, zinc methionine, copper lysine, tryptophan, vitamin A, vitamin E, ethoxyquin and a traditional Chinese medicine composition feed additive for controlling pig influenza. The traditional Chinese medicine composition feed additive is abundant in drug source, relatively low in residual toxicity or free of residual toxicity, small in side effect and not easy for generating of drug resistance, is capable of regulating body immune function, inhibiting virus replication and improving disease symptoms, and has substantial effect on controlling pig influenza.
Owner:齐全农牧集团股份有限公司
Mixed virus-like particles of swine influenza virus and foot and mouth disease virus, preparation method and application thereof
ActiveCN102370976BImprove immunityLong durationMicroorganism based processesAntiviralsAdjuvantFoot mouth disease virus
The invention provides a mixed virus-like particles, containing stromatin M1 of influenza virus, surface antigen erythrohemagglutinin HA of influenza virus and a nexus protein containing main antigenic epitope contained capsid protein of foot and mouth disease virus and a transmembrane zone and an inner membrane zone of the erythrohemagglutinin HA of influenza virus. The main antigenic epitope contained capsid protein of foot and mouth disease virus substitutes a basically same length outer membrane zone of a 5' terminal of the erythrohemagglutinin HA of influenza virus. Surface antigen erythrohemagglutinin HA of influenza virus and the main antigenic epitope contained capsid protein of foot and mouth disease virus are expressed simultaneously on surfaces of the mixed virus-like particles. The invention also provides a divalent vaccine of swine influenza and foot and mouth disease; and the vaccine contains the mixed virus-like particles and adjuvants. The invention also provides a method for preparing the mixed virus-like particles.
Owner:SUN YAT SEN UNIV
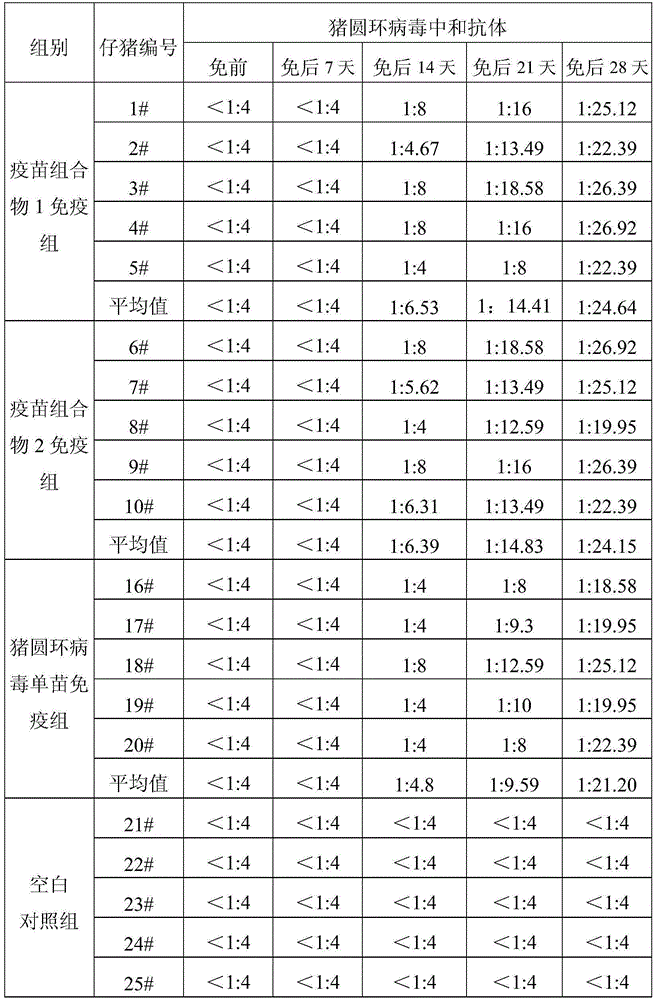
Method for determining titer of swine flu inactivated vaccine
ActiveCN102735853BReduced measurement timeReduce operating errorsBiological testingRed blood cellPorcine influenza
The invention discloses a method for determining titer of a swine flu inactivated vaccine. The method comprises the following steps: immunizing a pig with a swine flue inactivated vaccine, collecting blood 21-28 days after immunization and separating blood serum; removing non-specific components in the serum to obtain a serum to be tested; diluting the swine flue inactivated antigen, and preparing a unit swine flue antigen diluent with a concentration of 4HA; and diluting the serum to be tested by multiple proportions, successively adding the 4HA unit swine flue antigen diluent and 1% red cells to conduct hemagglutination inhibition test, so as to completely inhibit highest dilution of the 4HA unit swine flu antigen serum at HI titer. The method has advantages of greatly shortened measurement time, little operation error, strong controllability, and small inter-batch difference, and also reduces detection errors caused by different levels of experimental animals in an animal challenge protection experiment for testing the titer.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE +1
Compositions Useful in Both Homologous And Heterologous Vaccine Regimens
PendingUS20220023412A1SsRNA viruses negative-senseViral antigen ingredientsHeterologous vaccineFlu immunization
Owner:EPIVAX +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com