Quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, porcine influenza virus and haemophilus parasuis
A technology of mycoplasma hyopneumoniae and swine influenza virus, which is applied in the direction of virus antigen components, virus/bacteriophage, vaccines, etc., and can solve the problem of no porcine circovirus haemophilus parasuis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The construction of embodiment 1 PCV2 type virus recombinant baculovirus
[0079] Optimization and synthesis of target genes
[0080] Design the PCV2 type ORF2b gene nucleotide sequence with reference to the PCV2b subtype DBN-SX07 strain, and obtain the original nucleotide sequence of the PCV2 subtype strain ORF2b gene as shown in SEQ ID No.5 (702bp), and the PCV2b subtype strain Cap protein The original amino acid sequence is shown in SEQ ID No.6 (233aa).
[0081] In this embodiment, the optimization of the ORF2b gene of the PCV2b subtype strain is taken as an example, and its codon optimization process is described in detail, and the optimization of the ORF2a gene of the corresponding PCV2a subtype strain is carried out with reference to this process.
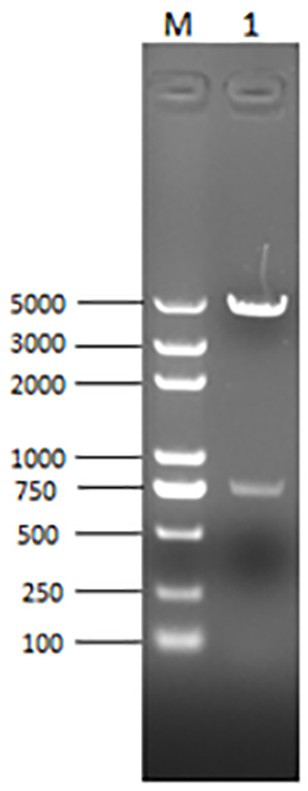
[0082] Under the premise of keeping the amino acid of PCV2b subtype strain ORF2b unchanged, the codons were transformed into eccentric codons in the expression system of insect cell baculovirus, EcoRI and XhoI restric...
Embodiment 2
[0103] Example 2 Insect cell bioreactor serum-free suspension culture expresses CAP protein
[0104] Preparation of poisonous seeds for production
[0105] The basic virus seeds were inoculated at MOI=0.01-5.0 and grew well, with a cell density of 2.0×10 6 -2.5×10 6 / ml of Sf9 cells, cultivated at 27°C for 72-96 hours, harvested the virus liquid, quantitatively dispensed, indicated the name, harvest date, virus generation, etc., and carried out virus content detection, specificity detection, and purity detection. Content ≥ 1.0×10 8 PFU / ml, store at 2-8°C for later use.
[0106] Serum-free Suspension Culture of SF9 Cells in Bioreactor and Quantification of CAP Protein Expression
[0107] Suspension cell culture method was used. Take a 2000ml shake flask to culture 800ml of SF9 cells, the cell viability is 95%, and the cell density reaches 2.0×10 6 -2.5×10 6 cells / ml, according to 0.5×10 6 Cells / ml were transferred to a 5L bioreactor and continued to culture for 3-4 days...
Embodiment 3
[0119] In this example, the PCV2 type ORF2a gene nucleotide sequence was designed with reference to the PCV2a subtype strain, and the original nucleotide sequence of the PCV2a subtype strain ORF2a gene was obtained as shown in SEQ ID No.7 (702bp), and the PCV2a subtype strain The original amino acid sequence of the Cap protein is shown in SEQ ID No.8 (233aa).
[0120] Similarly, the optimization process of the above-mentioned PCV2a subtype strain ORF2a gene is carried out with reference to the method of the above-mentioned embodiment 1-2, and the obtained PCV2b type CAP protein has an amino acid sequence as shown in SEQ ID No.2, and its coding gene has a sequence as shown in SEQ ID No. The nucleotide sequence shown in 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com