Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
235 results about "Flu immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
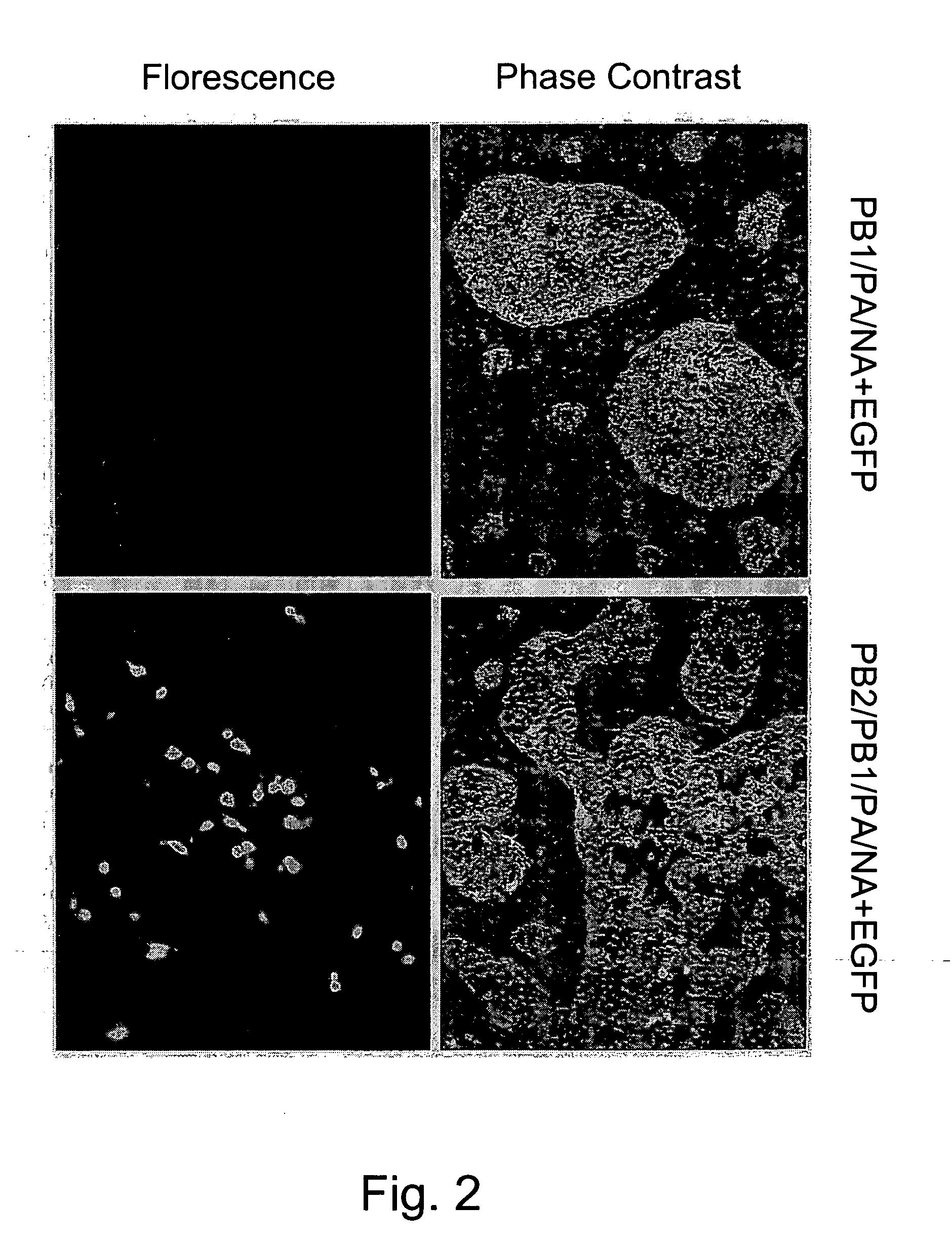
Multi plasmid system for the production of influenza virus
InactiveUS20050158342A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggEukaryotic plasmids
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. In addition, the present invention includes an improved method of rescue, wherein animal cells (e.g., SF Vero cells) are electroporated with plasmids and vectors of the invention.
Owner:MEDIMMUNE LLC
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Influenza vaccine
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:SMITHKLINE BEECHAM PHARMA GMBH +1
Peptide-based vaccine for influenza
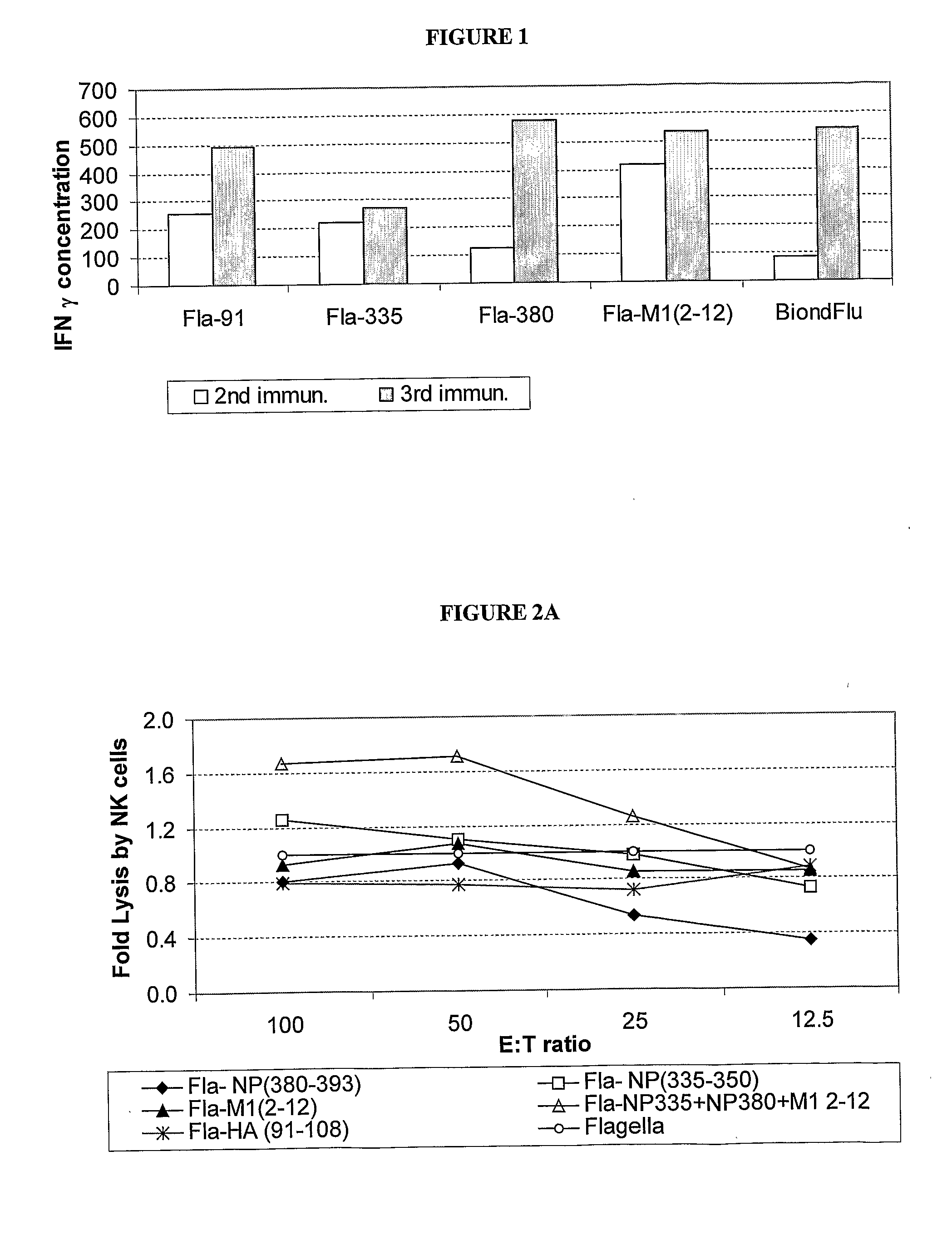
A human synthetic peptide-based influenza vaccine for intranasal administration comprises a mixture of flagella containing at least four epitopes of influenza virus reactive with human cells, each expressed individually in Salmonella flagellin, said influenza virus epitopes being selected from the group consisting of: (i) one B-cell hemagglutinin (HA) epitope; (ii) one T-helper hemagglutinin (HA) or nucleo-protein (NP) epitope that can bind to many HLA molecules; and (iii) at least two cytotoxic lymphocyte (CTL) nucleoprotein (NP) or matrix protein (M) epitopes that are restricted to the most prevalent HLA molecules in different human populations.
Owner:YEDA RES & DEV CO LTD
Immunoprotective influenza antigen and its use in vaccination
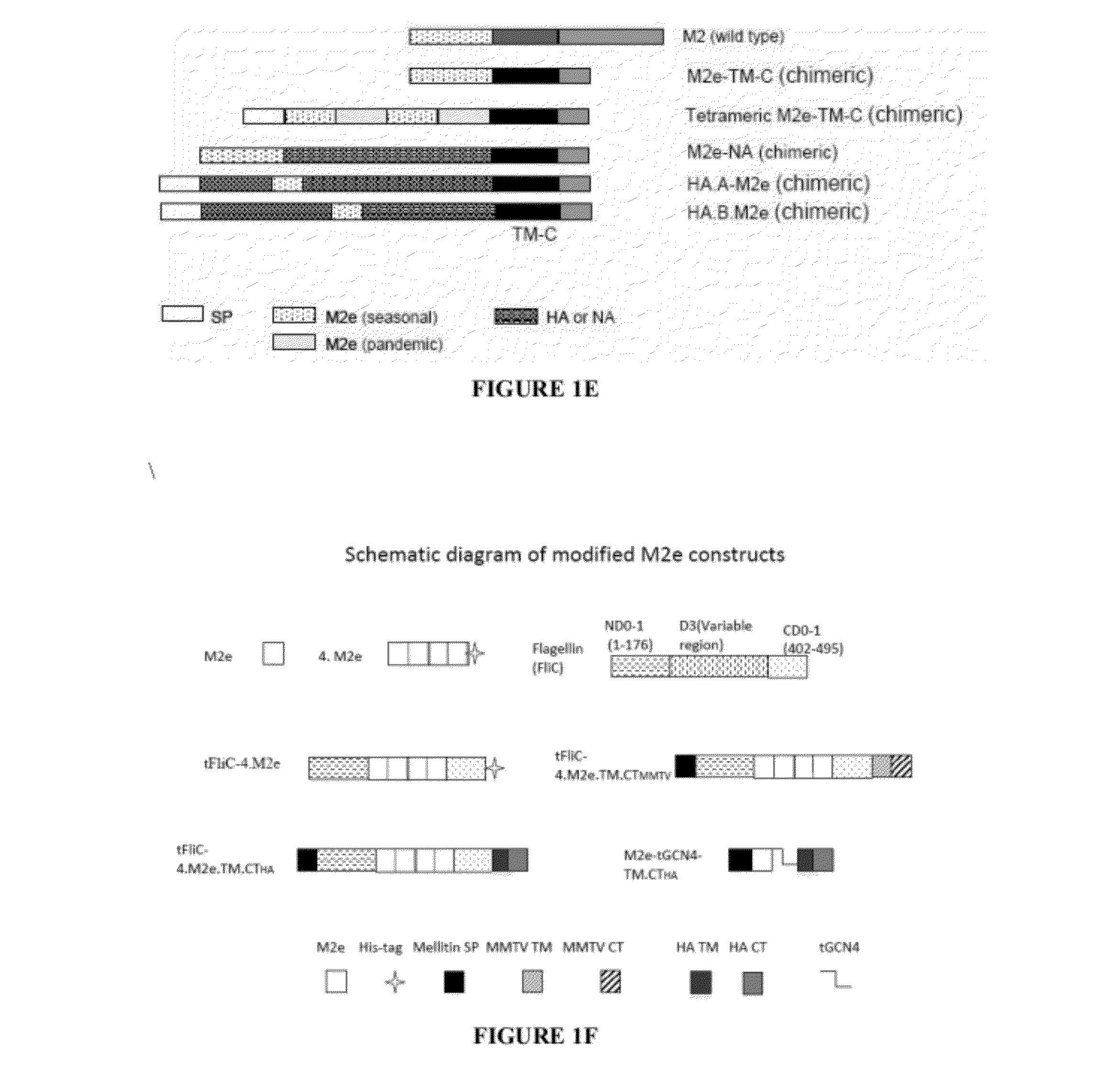
The present invention relates to an influenza antigen, comprising a fusion product of at least the extracellular part of a conserved influenza membrane protein or a functional fragment thereof and a presenting carrier, which may be a presenting (poly)peptide or a non-peptidic structure, such as glycans, peptide mimetics, synthetic polymers. The invention further relates to a vaccine against influenza, comprising at least an antigen of the invention, optionally in the presence of one or more excipients. The invention also relates to use of the antigen, a method for preparing the antigen and acceptor cells expressing the antigen.
Owner:VLAAMS INTERUNIVIR INST VOORS BIOTECH
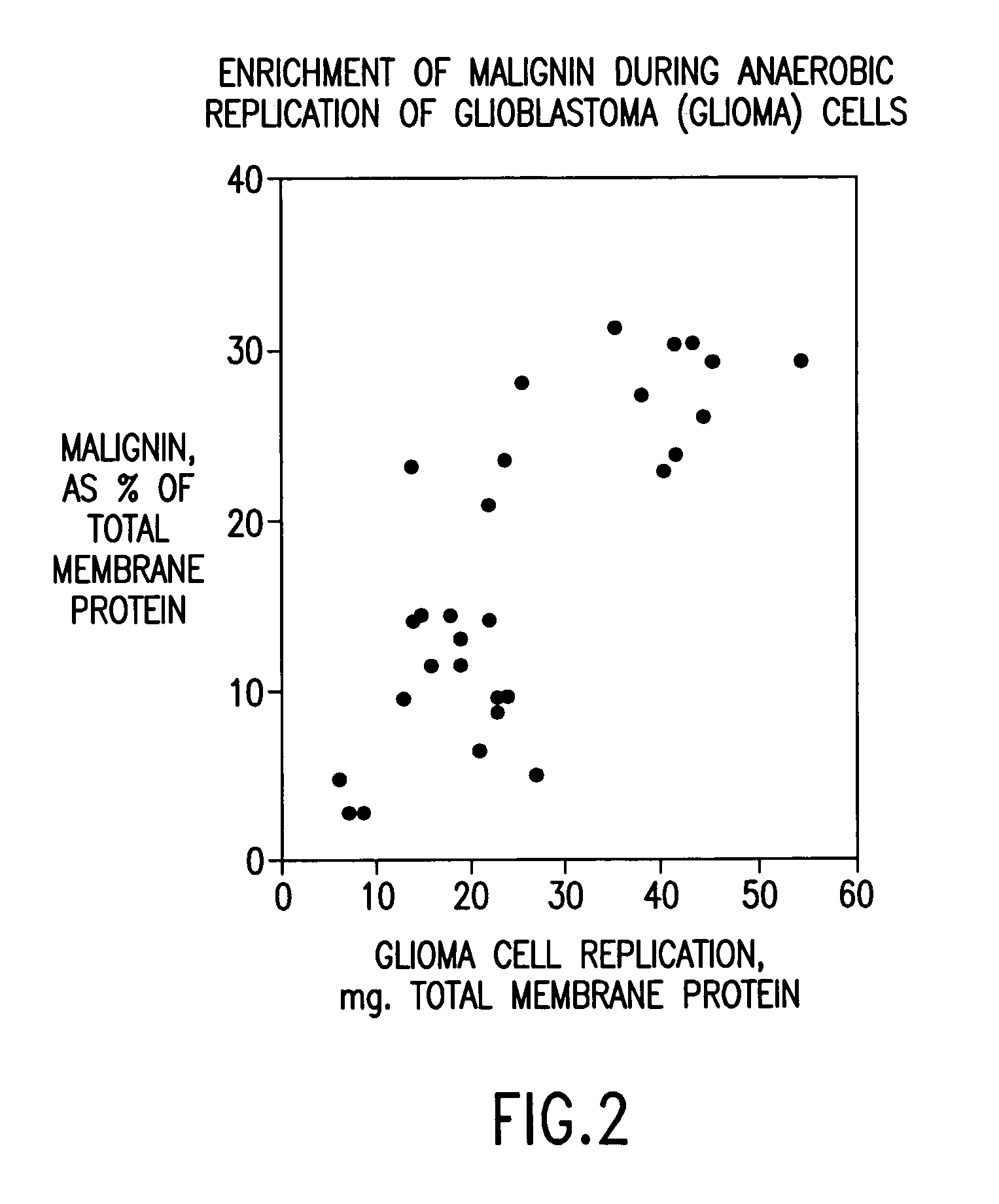
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Feline vaccines against avian influenza
InactiveUS20080107687A1Elicit immune responseSsRNA viruses negative-senseViral antigen ingredientsEpitopeViral Vaccine
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a recombinant poxvirus vaccine or an inactivated vaccine. The invention also encompasses recombinant poxvirus vectors encoding and expressing avian influenza antigens, epitopes or immunogens which can be used to protect animals, in particular felids, against avian influenza.
Owner:MERIAL LTD
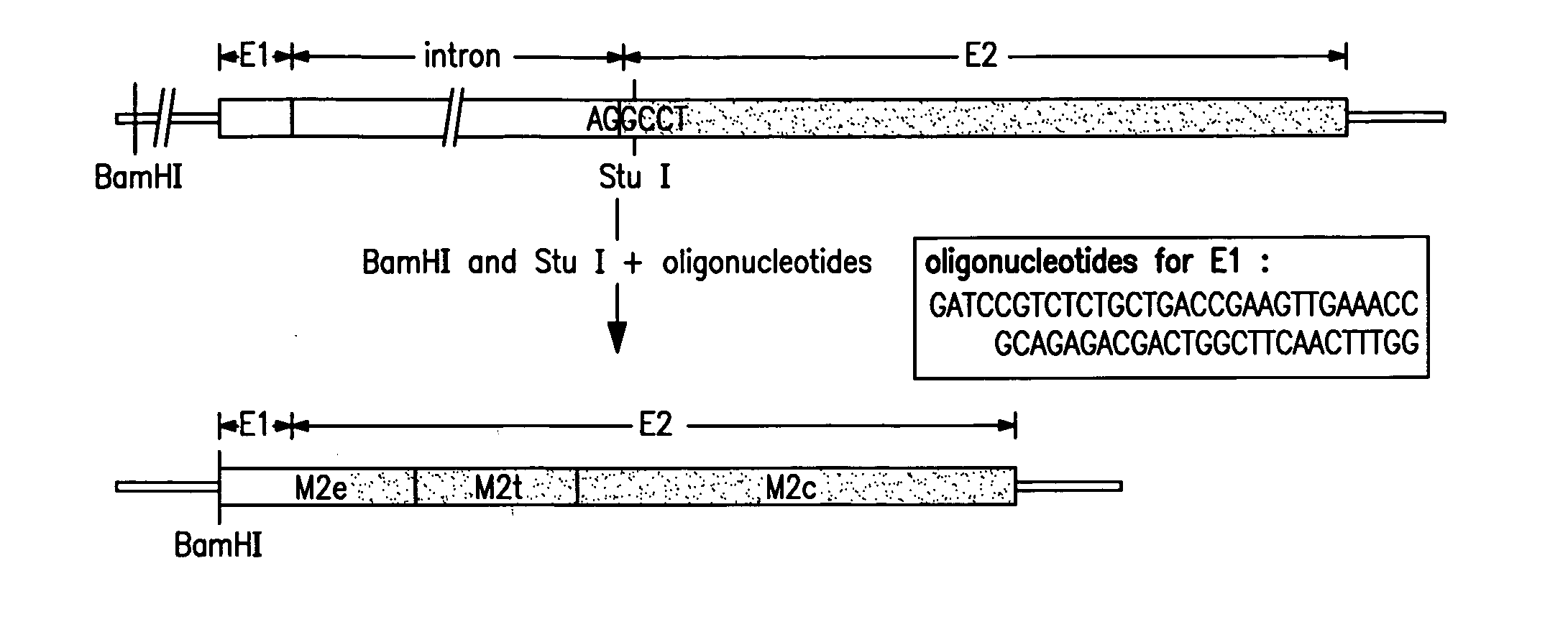
Cross-protective influenza vaccine
InactiveUS20120052082A1Broad and improved cross protectionSsRNA viruses negative-senseViral antigen ingredientsMultiple copyVirosome
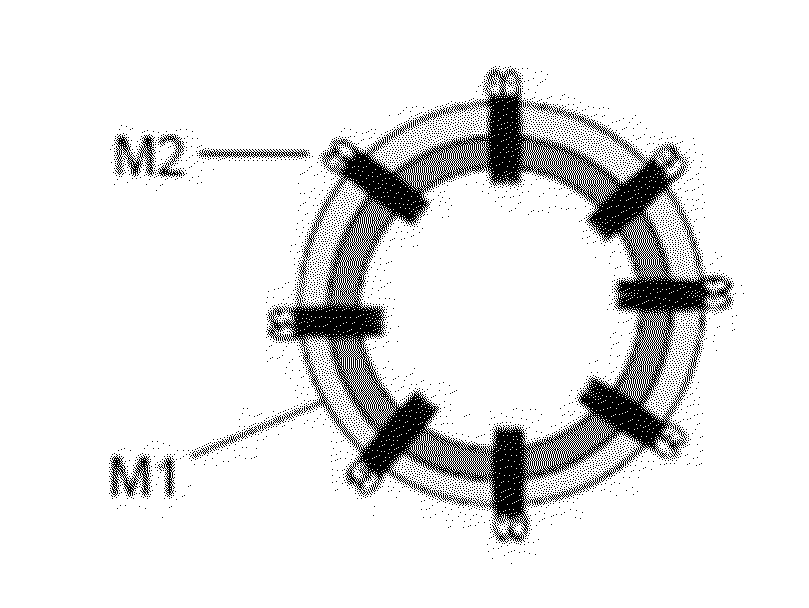
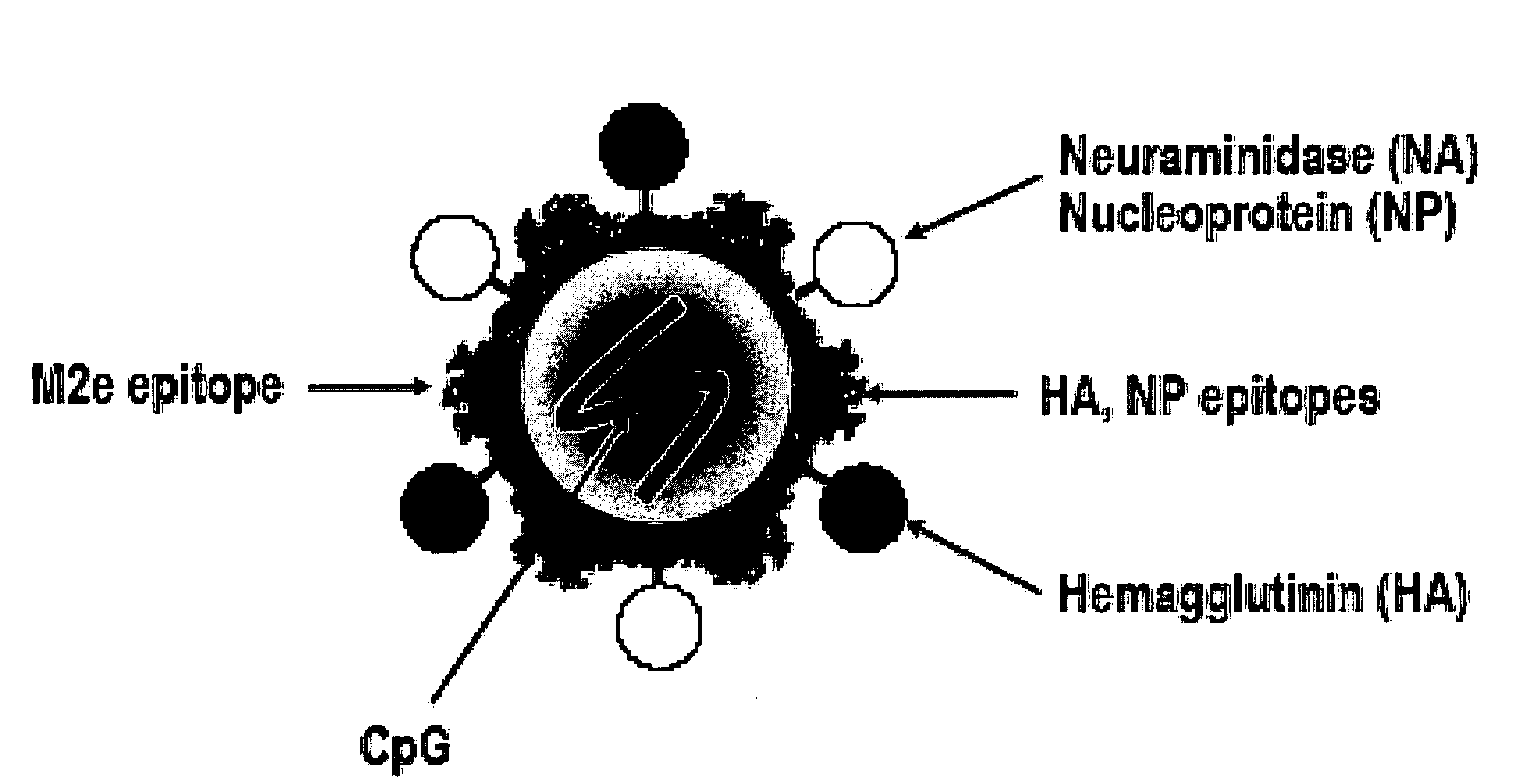
A cross-protective influenza virus vaccine has been designed based on the incorporation of the genetically engineered, highly conserved M2 influenza viral protein optionally in combination with an adjuvant such as a bacterial flagellin protein incorporated into the membrane of a virosome or virus-like particles. Immunogenicity and the breadth of cross protection efficacy are significantly enhanced using multiple copies of the influenza M2 protein as a membrane bound tetramer and / or in combination with a membrane bound adjuvant. A method for vaccinating a subject for influenza A has also been developed that results in broad and improved cross-protection against multiple subtypes of influenza A virus.
Owner:ZETRA BIOLOGICALS
Serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture
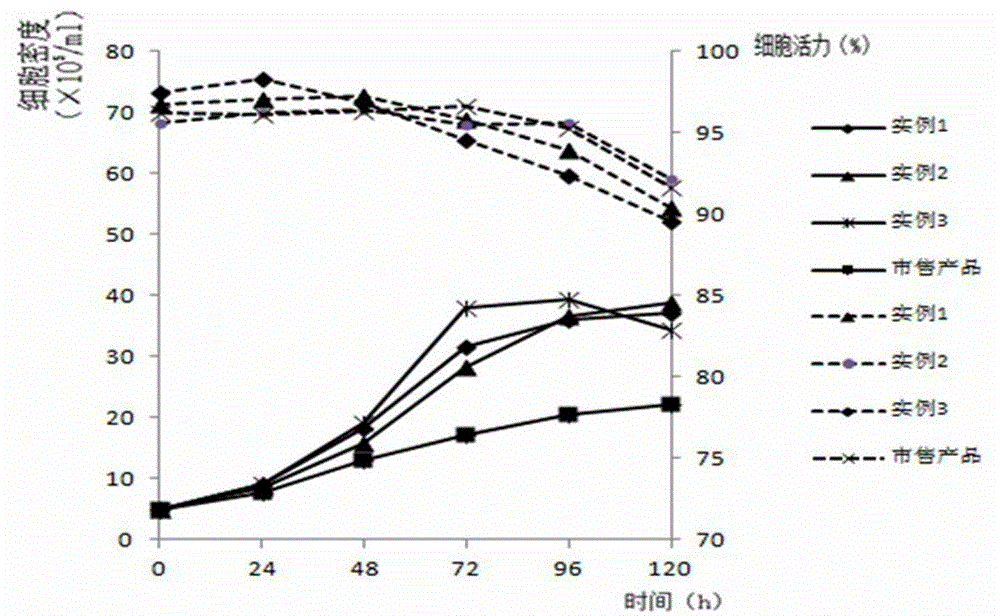
ActiveCN103555659AHigh densityClear ingredientsVertebrate cellsArtificial cell constructsCanine kidneyFlu immunization
The invention discloses a serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture. The serum-free medium for MDCK cell full suspension culture comprises an amino acid part, a vitamin part, an inorganic salt part and other additive parts. The serum-free medium has the beneficial effects that the serum-free medium for MDCK cell full suspension culture, provided by the invention, is high in cell culture density and clear in composition and does not contain animal serum, a downstream product is purified, the product quality is improved, and the serum-free medium is convenient to prepare and use and suitable for large-scale production of influenza vaccines and avian influenza vaccines.
Owner:无锡市赛尔百灵生物技术有限公司
Influenza vaccine
InactiveUS20090304730A1Overcomes drawbackSsRNA viruses negative-senseViral antigen ingredientsEpitopeInfluenza vaccine
The present invention relates to influenza vaccines for human and veterinary use. In particular, the present invention provides a vaccine able to effect long term and cross-strain protection by including at least two influenza virus epitopes expressed as a chimeric polypeptide wherein at least one epitope is influenza A virus matrix protein epitope and the second epitope is a haemagglutinin peptide epitope.
Owner:YEDA RES & DEV CO LTD
Proteosome influenza vaccine
InactiveUS7399840B2Straightforward to produceSsRNA viruses negative-senseAerosol deliveryProteasomeInfluenza vaccine
Improved forms of vaccines which comprise proteosomes and protein antigens are described. Vaccines which contain influenza HA as the antigen are used for illustration as to demonstrate efficacy. Improvements in the preparation of the vaccines themselves and the proteosome component are also included.
Owner:ID BIOMEDICAL CORP LAVAL
Chimeric Influenza Virus-Like Particles Comprising Hemagglutinin
ActiveUS20120189658A1Easy to captureStrong immune responseSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininVirus-like particle
A method for synthesizing chimeric influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of chimeric influenza HA in a plant or a portion of a plant. The invention is also directed towards a VLP comprising chimeric influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding chimeric influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Influenza vaccines including combinations of particulate adjuvants and immunopotentiators
InactiveUS20090304739A1Eliminate needAvoid the needSsRNA viruses negative-senseViral antigen ingredientsParticulatesTh2 response
Influenza vaccines containing insoluble particulate adjuvants have been found to elicit an IgG response that is primarily a TH2 response (IgG1). This response can be shifted towards a TH1 response (IgG2a) by including immunopotentiators in the compositions. Thus the invention provides an immunogenic composition comprising: (i) an influenza virus antigen; (ii) an insoluble particulate adjuvant; and (iii) a immunopotentiator.
Owner:CHIRON CORP
Gene encoding hemagglutinin protein of H5 avian influenza virus and its application
ActiveCN1632124AHigh level of immune responseImproving immunogenicityViral antigen ingredientsAntibody ingredientsHemagglutininHighly pathogenic
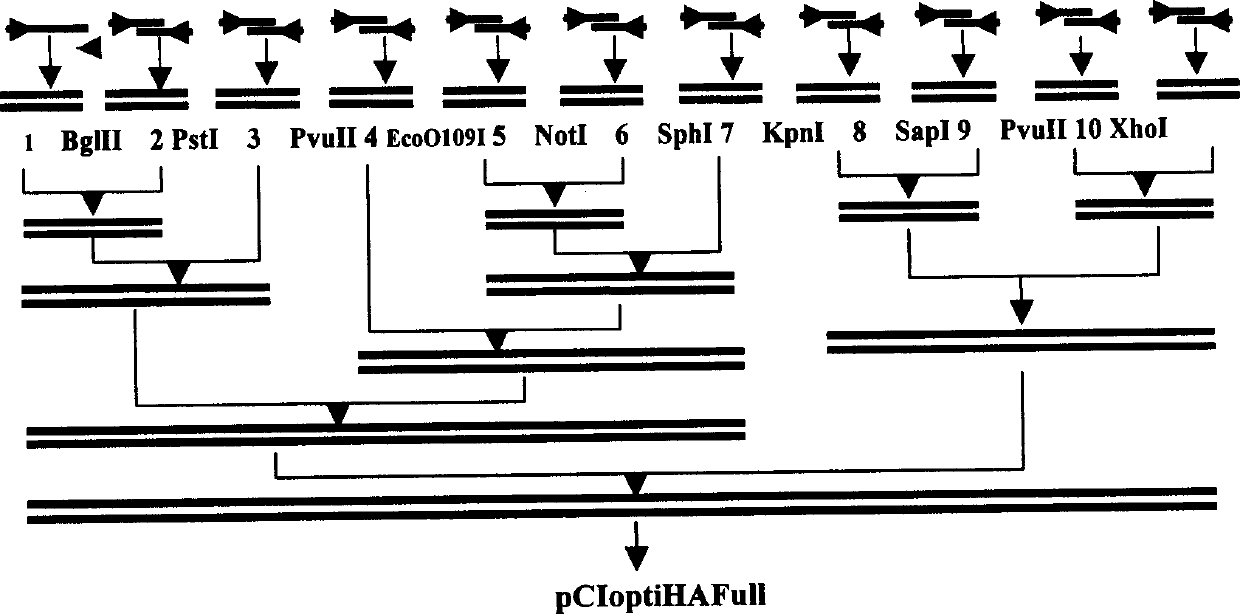
The present invention relates to an artificially synthesized gene optiHA containing codons for chicken partial tropism. Its reading frame contains 1707 bp nucleotides and encodes a total of 568 amino acids. The gene is compatible with H5 subtype highly pathogenic avian influenza virus A / Goose / GuangDong / 1 / 96(H5N1)[GD / 1 / 96(H5N1)]hemagglutinin (HA) gene has a nucleotide homology rate of 70%, an amino acid homology rate of 100%, and encodes the H5 subtype Hemagglutinin (HA) protein of avian influenza virus GD / 1 / 96 (H5N1). The invention also relates to the application of the gene as an immunogenic gene of H5 subtype influenza DNA vaccine and other genetic engineering vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Use Of An Influenza Virus, An Oil-In-Water Emulsion Adjuvant, To Induce Cd4 T-Cell And/Or Improved B-Memory Cell Response
InactiveUS20080181911A1Enhance immune responseImprove responseSsRNA viruses negative-senseViral antigen ingredientsAntigenDisease
The present invention relates to influenza vaccine formulations and vaccination regimes for immunising against influenza disease. In particular the invention relates to vaccine formulations comprising an oil-in-water emulsion adjuvant and optionally 3D-MPL, their use in medicine, in particular their use in augmenting immune responses to influenza antigens, and to methods of preparation, wherein the oil in water emulsion comprises a sterol, a metabolisable oil and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Multi plasmid system for the production of influenza virus
InactiveUS7465456B2Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
Serum-free medium suitable for large-scale production of influenza vaccines
ActiveCN103045533AEasy to separateEasy to purifyArtificial cell constructsVertebrate cellsBiotechnologyNutrition
The invention relates to the technical field of culture medium development and research of biotechnology and discloses a serum-free medium suitable for large-scale production of influenza vaccines. The serum-free medium comprises 23 basic metabolism nutrients, two nucleic acid compounds, 6 vitamins, 9 inorganic salt compounds, a shear force protective agent, two acidity and alkalinity buffer agents, an acidity and alkalinity indicator, 10 virus reproduction promoters and three additives. The conventional preparation method of the serum-free medium comprises the following steps of: dissolving the components in ultrapure water without a heat source so as to prepare the serum-free medium. The using method is a conventional method. The serum-free medium has the beneficial effects that the serum is not contained, the total protein content is lower than 10mg per liter, separation and purification of the product are promoted, and the serum-free medium is suitable for production of influenza vaccines, supports the normal growth and long-term continuous cell culture of animal cells and can be used without adaption; and moreover, because the components are clear, the serum-free medium is convenient to prepare, controllable in cost and suitable for large-scale production of the influenza vaccines.
Owner:EAST CHINA UNIV OF SCI & TECH
Recombinant flu vaccines
InactiveUS20090117144A1Sufficient immunogenicityReduce loadSsRNA viruses negative-senseSsRNA viruses positive-senseInfluenza virus vaccinePlant virus
The present invention provides compositions for use as vaccines against the influenza virus, and rapid methods of producing such compositions. The composition include i) at least one peptide derived from an influenza virus, wherein the peptide is fused to a capsid protein derived from a plant virus forming a recombinant capsid fusion peptide and ii) at least one isolated antigenic protein or protein fragment derived from a human or avian influenza virus. The isolated antigenic protein or protein fragment derived from the human or avian influenza virus can be conjugated to the surface of the recombinant capsid fusion peptide.
Owner:PFENEX
Preparation of nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and application thereof
InactiveCN101732711ANo side effectsAntiviralsViruses/bacteriophagesVirus-like particleMultivalent Vaccine
The invention discloses a nose-spraying flu immunization pentavalent or multivalent inactivated vaccine and preparation method thereof. The vaccine is inactivated vaccine antigen of totivirus, lytic virus, viron or virus-like particles, flue multivalent vaccine antigen is flue pentavalent, namely H1N1, H3N2, B, H5N1 and A (H1N1) or multivalent vaccine antigen combined on the basis at will, or flue multivalent vaccine antigen obtained by containing all the combination of the HA selecting from H1, H2, H3, H4, H5, H6, H7, H8, H9, H10, H11, H12, H13, H14, H15 and H16 and the NA selecting from N1, N2, N3, N4, N5, N6, N7, N8 and N9 subtypes on the basis. The content of flu multivalent inactivated vaccine antigen HA in the vaccine of the invention is 1.0-15.0 Mug / 0.2ml / per person, and the vaccine of the invention can effectively prevent routine human flue, high pathogenicity H5N1 avian-human flu, influenza A (H1N1) and infection of other subtype influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
H9N2 avian influenza virus vaccine strain and application of H9N2 avian influenza virus vaccine strain in immune protection
The present invention relates to the field of animal virology, and provides a recombinant chicken-origin H9N2 avian influenza virus vaccine strain and a method for isolation, identification and purification of the strain. The invention further relates to a research of biological characteristics of the strain, especially to a research of characteristics of the strain adopted as the vaccine strain,and an evaluation of immune effects of the strain on SPF chickens. The preservation number of the strain is CCTCCNO:V201030. According to the present invention, the antigen variation conditions of the virus strain and other isolated virus strains are represented from the molecular level; after the virus strain is prepared into the vaccine, the prepared vaccine is adopted to immunize the 4 week old SPF chickens, with the protection effect analysis of the homologous H9 influenza wild virus strain and the heterologous H9 influenza wild virus strain, the results show that the influenza virus strain can be adopted as the spare vaccine strain of H9 subtype avian influenza. With the present invention, the spare vaccine strain is provided for prevention of the avian influenza outbreak by using the vaccine, the molecular biology technology program is provided for screen of the avian influenza virus vaccine strain, the molecular biology background is provided for study of the mechanism of animal infection by the avian influenza, and the important public health significance is provided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Rescue of influenza virus
The invention relates to the field of influenza vaccine production. Influenza vaccines have been produced in embryonated hens' eggs for over 50 years, but recently there have been considerable efforts to develop cell culture systems for vaccine production. The invention provides a nucleic acid comprising an influenza gene segment and a bacteriophage polymerase promotor or a complementary strand of said nucleic acid, and a cell comprising such a nucleic acid capable of producing desired influenza virus. Furthermore, the invention provides a composition comprising a cell or material derived from a cell according to the invention and a virus or material derived from a viral particle according to the invention.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC +1
Novel influenza m2 vaccines
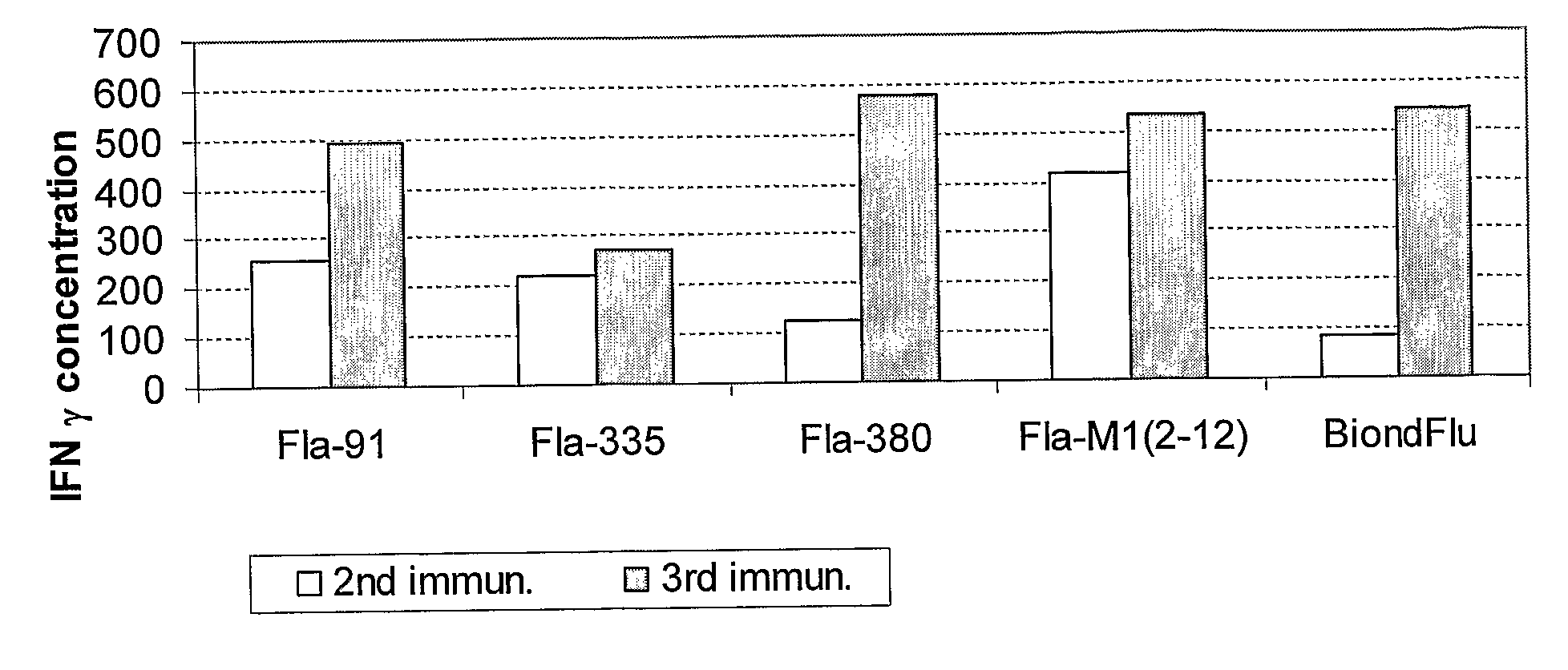
The present invention includes novel influenza antigenic formulations and vaccines that comprise influenza M2 peptide and VLPs comprising influenza M2 protein. The invention also includes methods of making and administering the novel antigenic formulation and vaccine. The invention also include methods of inducing immunity to ameliorate and / or prevent influenza infections in a subject.
Owner:NOVAVAX
Decreasing potential iatrogenic risks associated with influenza vaccines
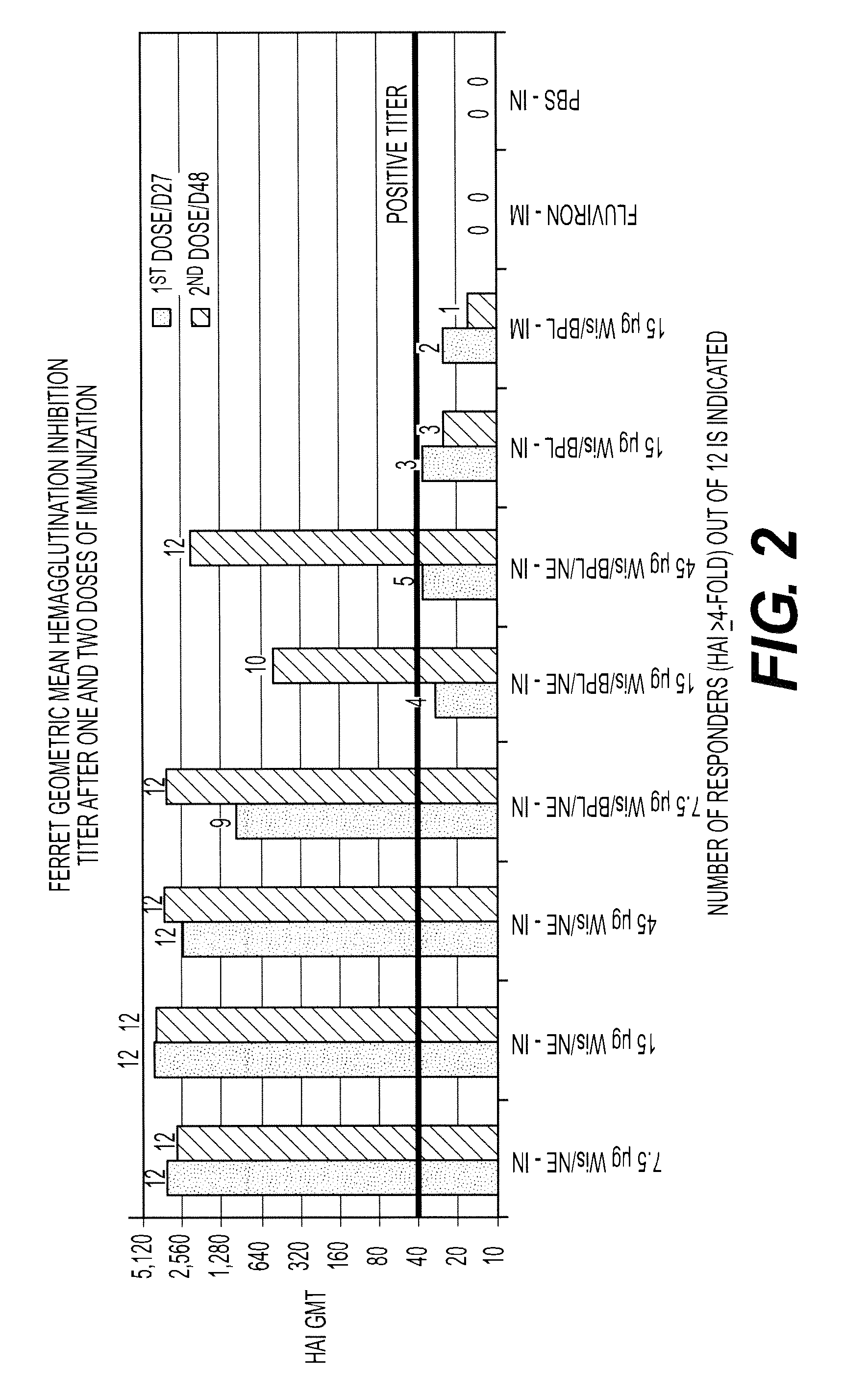
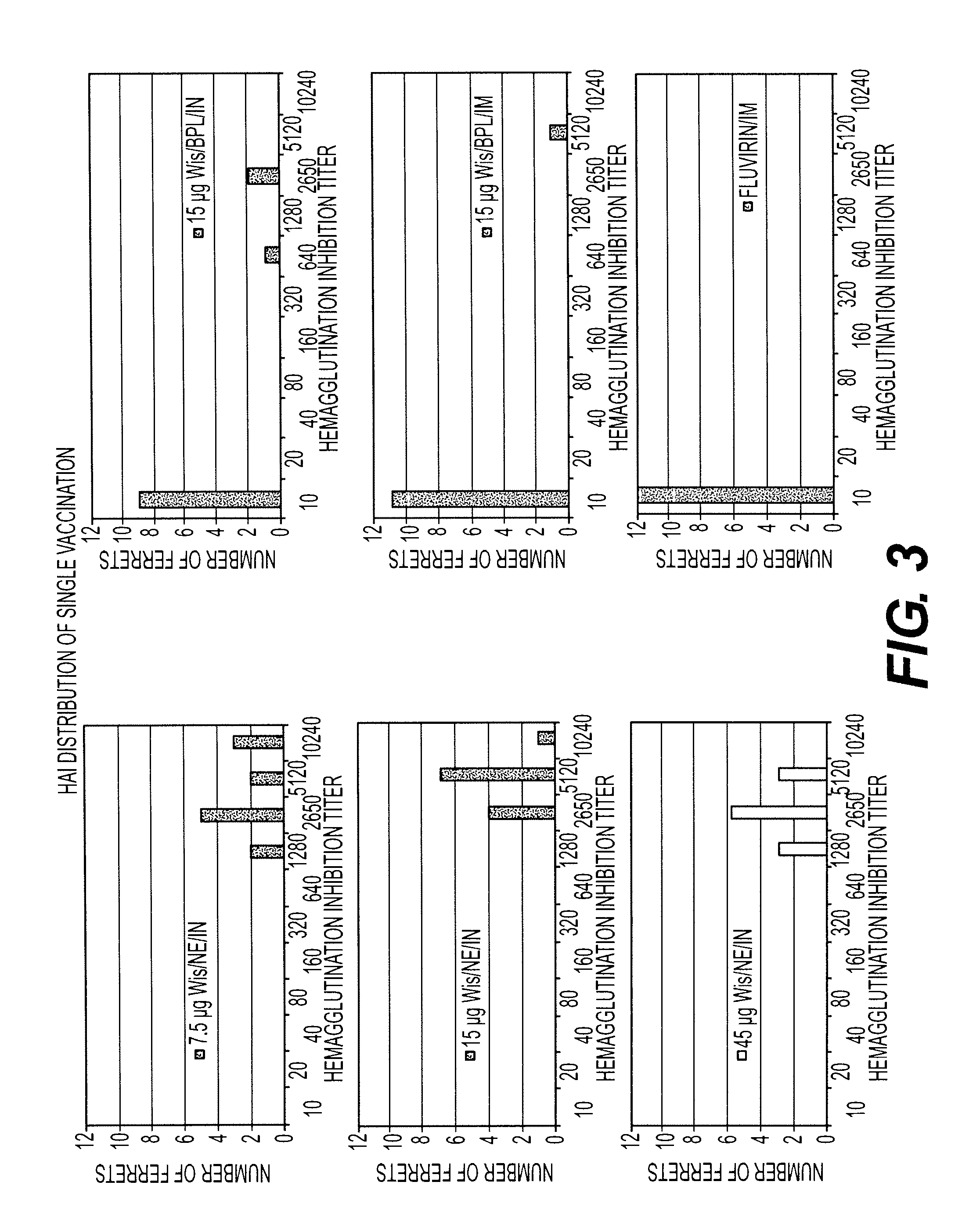
InactiveUS20120034600A1Increased risk of contaminationSsRNA viruses negative-senseViral antigen ingredientsFlu immunizationInfluenza virus culture
Influenza viruses for use in preparing human vaccines have traditionally been grown on embryonated hen eggs, although more modern techniques grow the virus in mammalian cell culture e.g. on Vero, MDCK or PER.C6 cell lines. The inventor has realised that the conditions used for influenza virus culture can increase the risk that pathogens other than influenza virus may grow in the cell lines and have identified specific contamination risks. Suitable tests can thus be performed during manufacture in order to ensure safety and avoid iatrogenic infections.
Owner:NOVARTIS AG
Multi plasmid system for the production of influenza virus
ActiveUS20090208527A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggElectroporation
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. In addition, the present invention includes an improved method of rescue, wherein animal cells (e.g., SF Vero cells) are electroporated with plasmids and vectors of the invention.
Owner:MEDIMMUNE LLC
Broadly reactive mosaic peptide for influenza vaccine
InactiveUS20140286981A1Enhance survivalLarge coverageSsRNA viruses negative-senseSugar derivativesPeptideInfluenza vaccine
Owner:WISCONSIN ALUMNI RES FOUND
Antibodies against influenza virus and methods of use thereof
The invention provides human scFv antibodies and monoclonal antibodies that neutralize influenza virus. Also provided are methods of treating and / or preventing a influenza related disease or disorder such bird flu The invention also provides methods of vaccinating a patient against influenza. Also provided are methods of diagnosing influenza-related diseases or disorders and methods of detecting the presence of a influenza in a sample.
Owner:BURNHAM INST FOR MEDICAL RES +1
Influenza vaccines with reduced amount of emulsion adjuvant
InactiveUS20090304742A1Eliminate needAvoid the needSsRNA viruses negative-senseViral antigen ingredientsHemagglutininInfluenza vaccine
Influenza vaccines with oil-in-water emulsion adjuvants are known. The amount of emulsion adjuvant required for an influenza vaccine can be reduced, thereby allowing more vaccines to be made from a given amount of emulsion, and / or minimizing the amount of emulsion that has to be produced for a given number of vaccine doses. These vaccines can conveniently be made by mixing (i) an oil-in-water emulsion and (ii) an aqueous preparation of an influenza virus antigen. In one aspect, substantially equal volumes of components (i) and (ii) are used; in another aspect, an excess volume of component (ii) is used. When using substantially equal volumes, component (ii) has a hemagglutinin concentration of more than 60 μg influenza virus strain per ml. Components (i) and (ii) can be presented in kit form.
Owner:NOVARTIS AG
Peptide-Based Influenza Vaccine Formulation
InactiveUS20090104216A1Improve responseBroad protectionSsRNA viruses negative-sensePeptide/protein ingredientsEpitopeInfluenza vaccine
Peptide-based anti-influenza formulations against influenza A and B are disclosed. The peptides are derived from influenza-based epitopes. The formulations are based on peptide mixtures which may be formulated so that variability is present at particular residues. The formulations can be used to prepare vaccines for preventing influenza in human, avian, murine or equine animals.
Owner:VARIATION BIOTECHNOLOGIES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com