Use Of An Influenza Virus, An Oil-In-Water Emulsion Adjuvant, To Induce Cd4 T-Cell And/Or Improved B-Memory Cell Response
a technology of oil-in-water emulsion and influenza virus, which is applied in the field of influenza vaccine formulations and vaccination regimes, can solve the problems of increased hospitalization or mortality, significant morbidity and even mortality, and increased incidence of pneumonia and lower respiratory disease, and achieves the effect of improving the cd4 respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Immunological Read-Out Methods
[0242]I.1. Mice Methods
[0243]I.1.1. Hemagglutination Inhibition Test
[0244]Test Procedure
[0245]Anti-Hemagglutinin antibody titers to the three influenza virus strains were determined using the hemagglutination inhibition test (HI). The principle of the HI test is based on the ability of specific anti-influenza antibodies to inhibit hemagglutination of chicken red blood cells (RBC) by influenza virus hemagglutinin (HA). Heat inactivated sera were previously treated by Kaolin and chicken RBC to remove non-specific inhibitors. After pretreatment, two-fold dilutions of sera were incubated with 4 hemagglutination units of each influenza strain. Chicken red blood cells were then added and the inhibition of agglutination was scored. The titers were expressed as the reciprocal of the highest dilution of serum that completely inhibited hemagglutination. As the first dilution of sera was 1:20, an undetectable level was scored as a titer equal to 10.
[0246]Statistic...
example ii
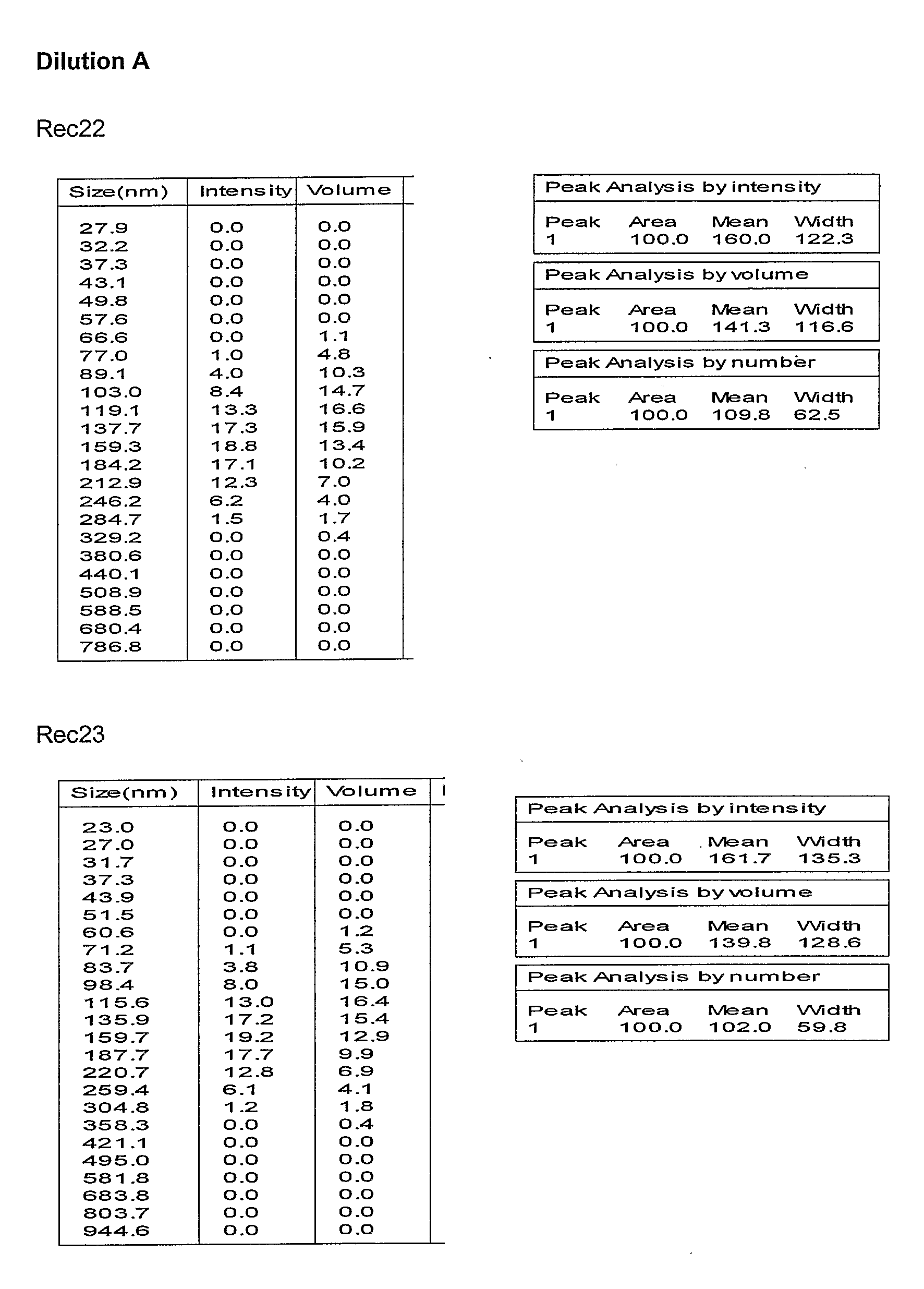
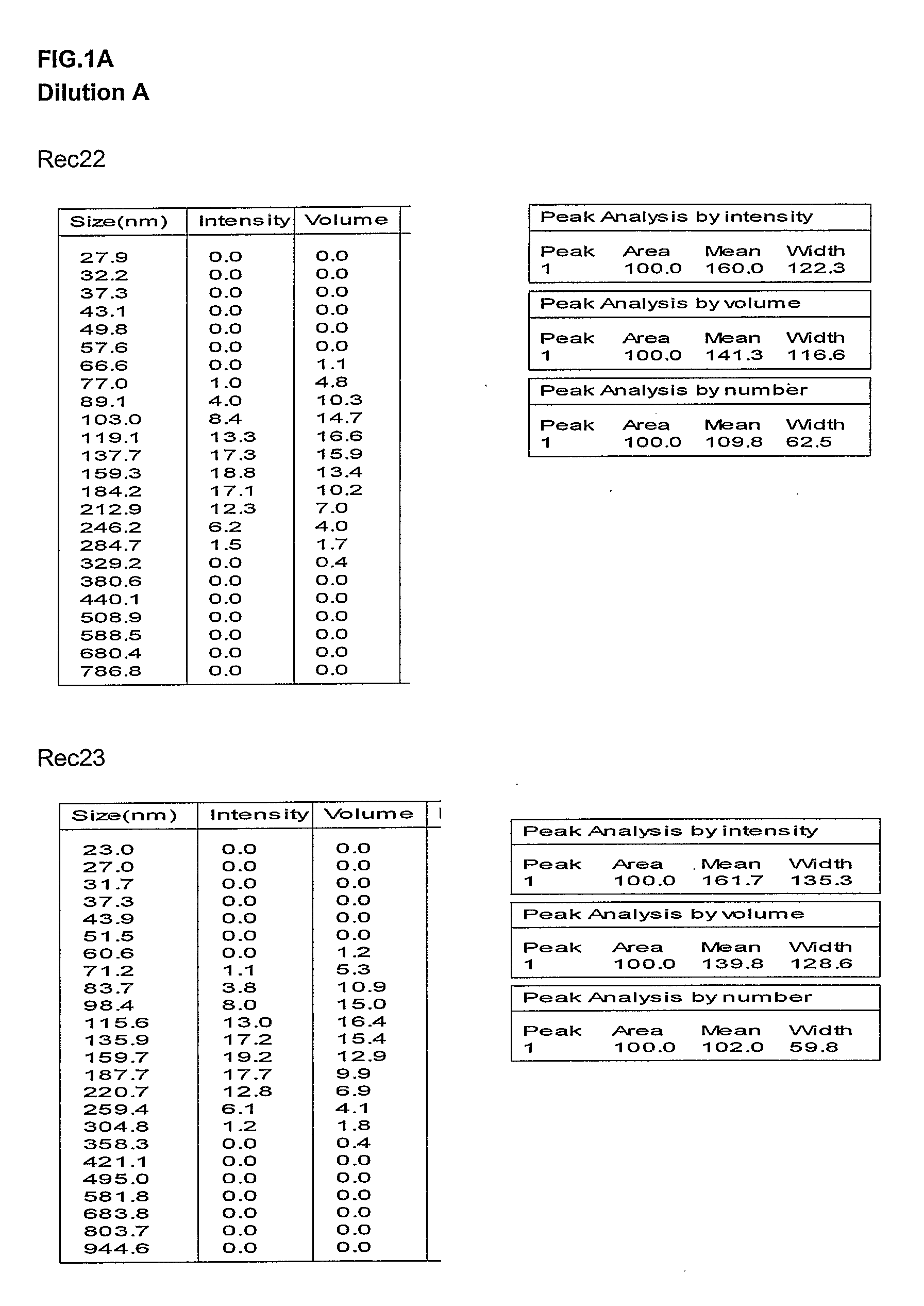
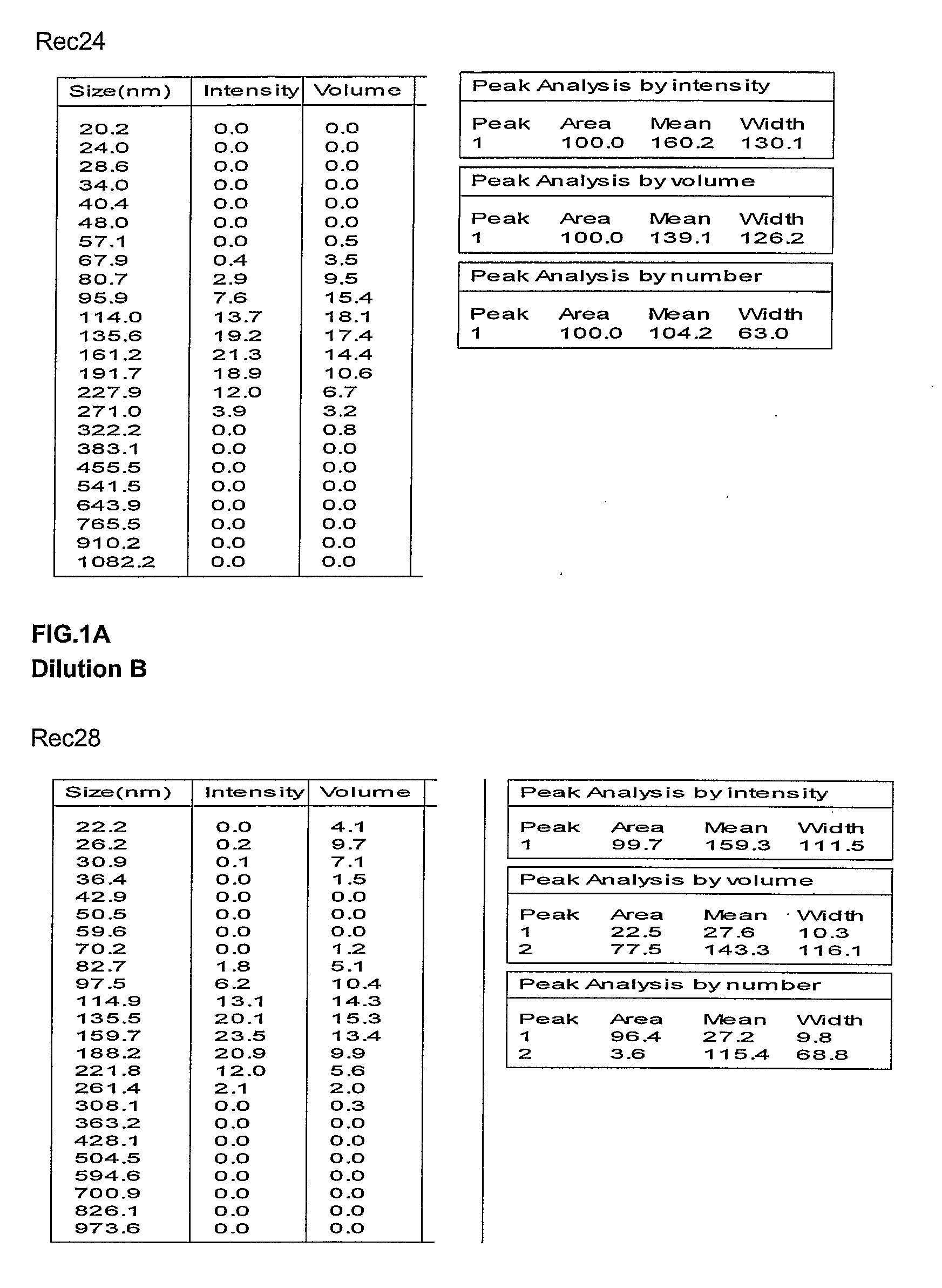
Preparation and Characterization of the Oil in Water Emulsion and Adjuvant Formulations
[0332]Unless otherwise stated, the oil / water emulsion used in the subsequent examples is composed an organic phase made of 2 oils (alpha-tocopherol and squalene), and an aqueous phase of PBS containing Tween 80 as emulsifying agent. Unless otherwise stated, the oil in water emulsion adjuvant formulations used in the subsequent examples were made comprising the following oil in water emulsion component (final concentrations given): 2.5% squalene (v / v), 2.5% alpha-tocopherol (v / v), 0.9% polyoxyethylene sorbitan monooleate (v / v) (Tween 80), see WO 95 / 17210. This emulsion, termed AS03 in the subsequent examples, was prepared as followed as a two-fold concentrate.
[0333]II.1. Preparation of Emulsion SB62
[0334]II.1.1. Lab-Scale Preparation
[0335]Tween 80 is dissolved in phosphate buffered saline (PBS) to give a 2% solution in the PBS. To provide 100 ml two-fold concentrate emulsion 5 g of DL alpha tocophe...
example iii
Clinical Trial in an Elderly Population Aged Over 65 Years with a Vaccine Containing a Split Influenza Antigen Preparation and AS03 Adjuvant (Explo-Flu-001)
[0405]A phase I, open, randomised study was conducted in an elderly population aged over 65 years in 2003 in order to evaluate the reactogenicity and the immunogenicity of GlaxoSmithKline Biologicals influenza candidate vaccine containing the adjuvant AS03. The humoral immune response (i.e. anti-hemagglutinin, neutralising and anti-neuraminidase antibody titres) and cell mediated immune response (CD4 and / or CD8 T cell responses) was measured 21 days after intramuscular administration of one dose of an AS03 adjuvanted vaccine or a WV vaccine. Fluarix™ was used as reference.
[0406]III.1. Study Design
[0407]Three groups of subjects in parallel received the following vaccine intramuscularly:[0408]one group of 50 subjects receiving one dose of the reconstituted and adjuvanted SV influenza vaccine (FluAS03)[0409]one group of 50 subjects ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com