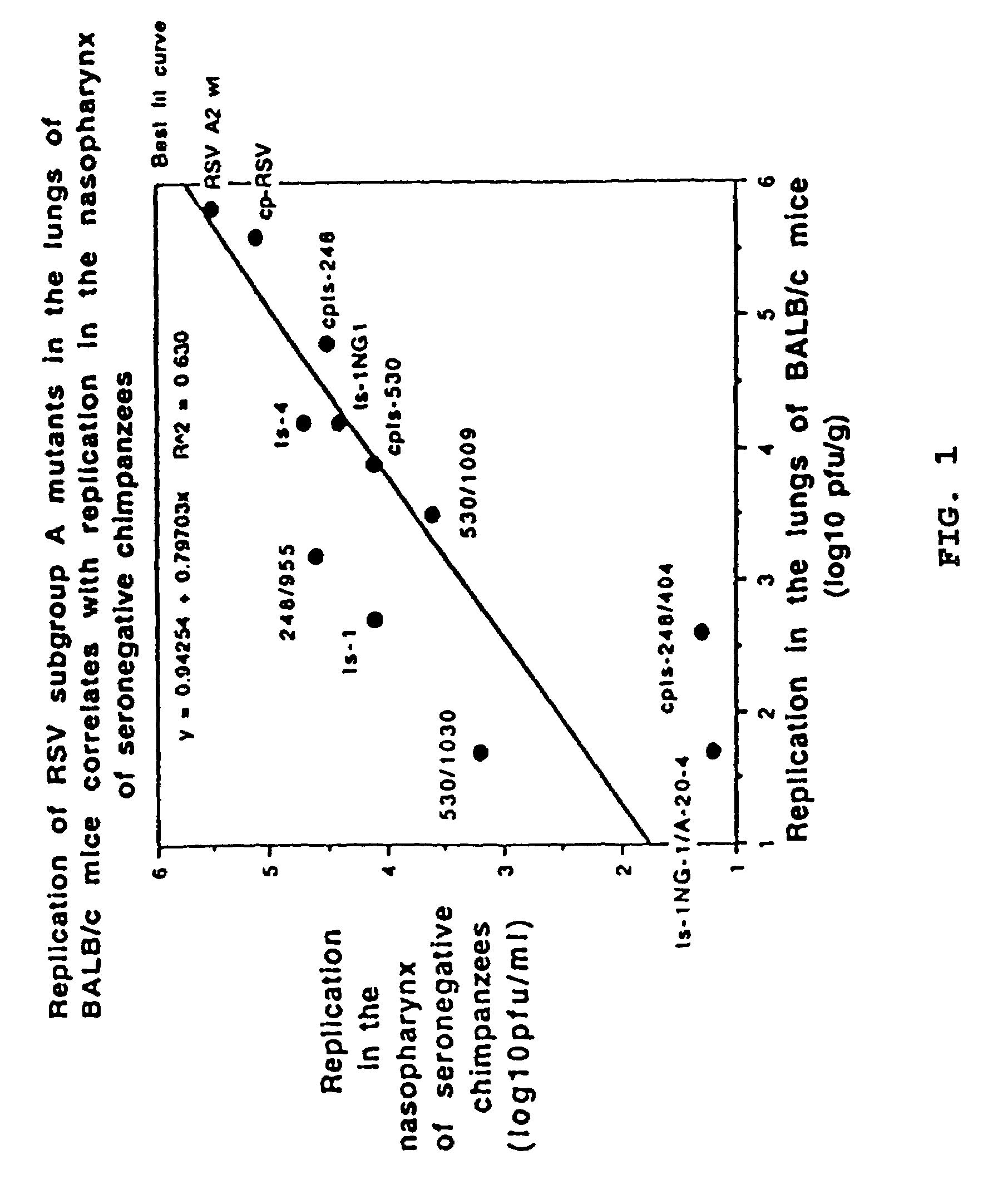
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
96 results about "Cold adapted" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
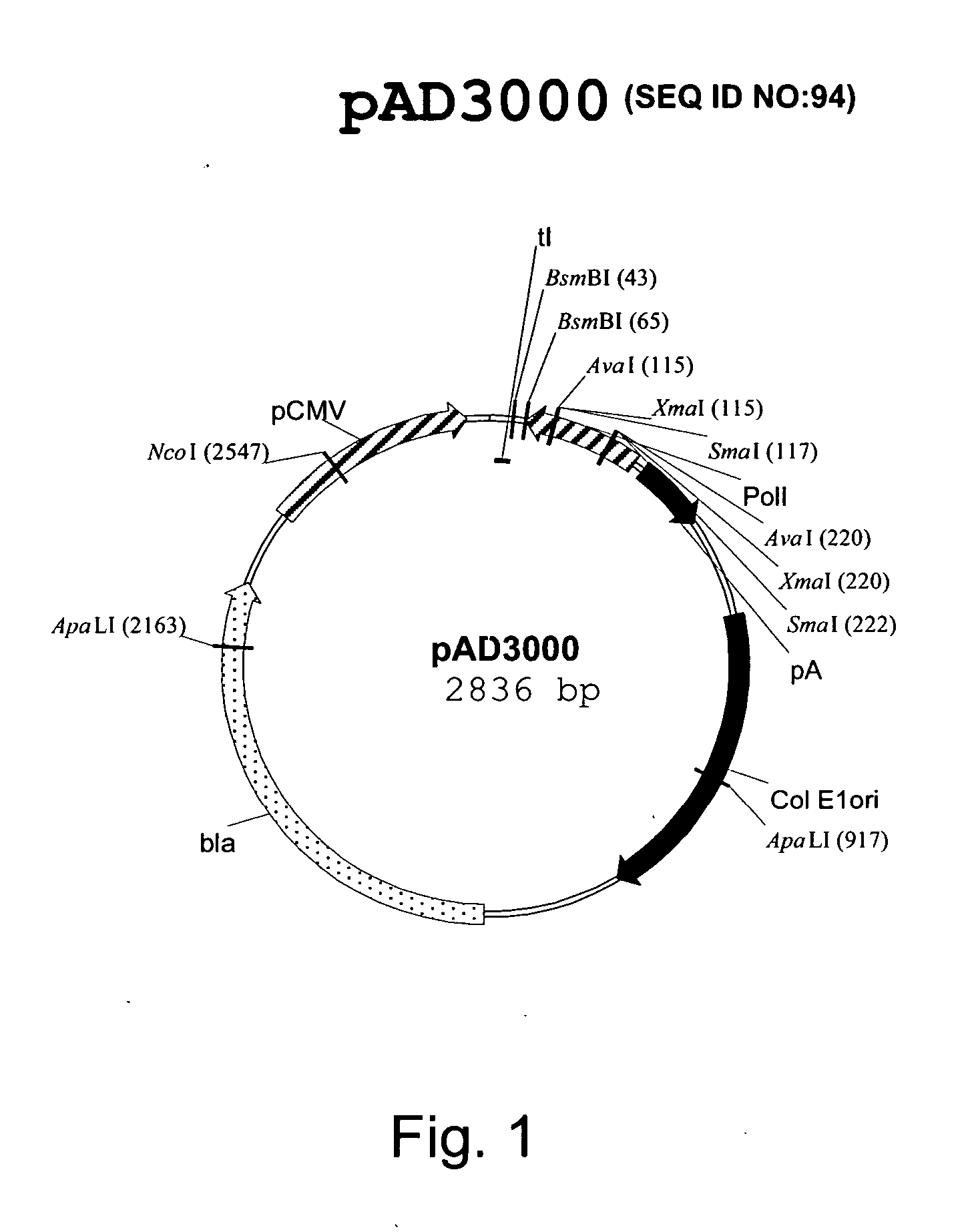
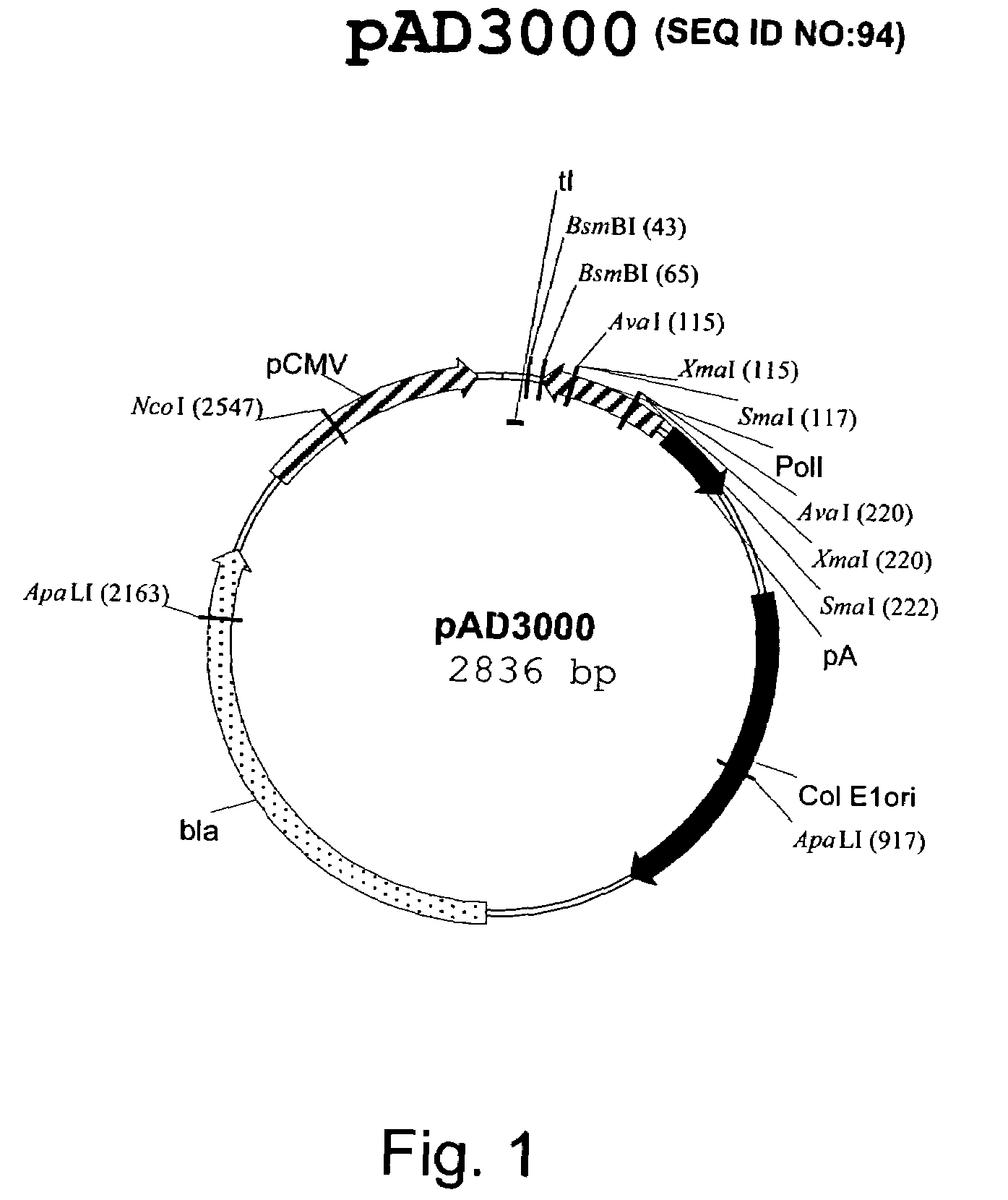
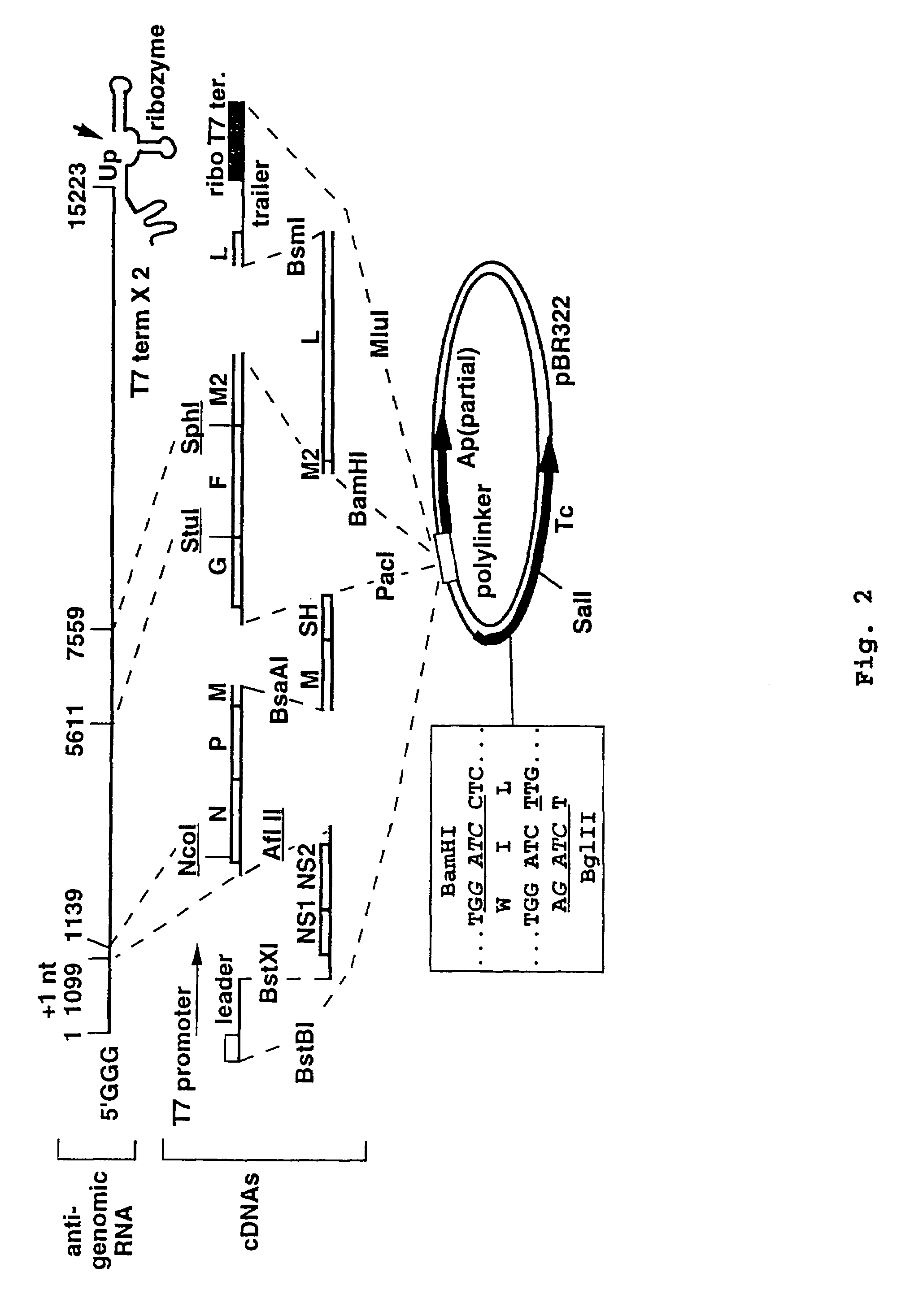
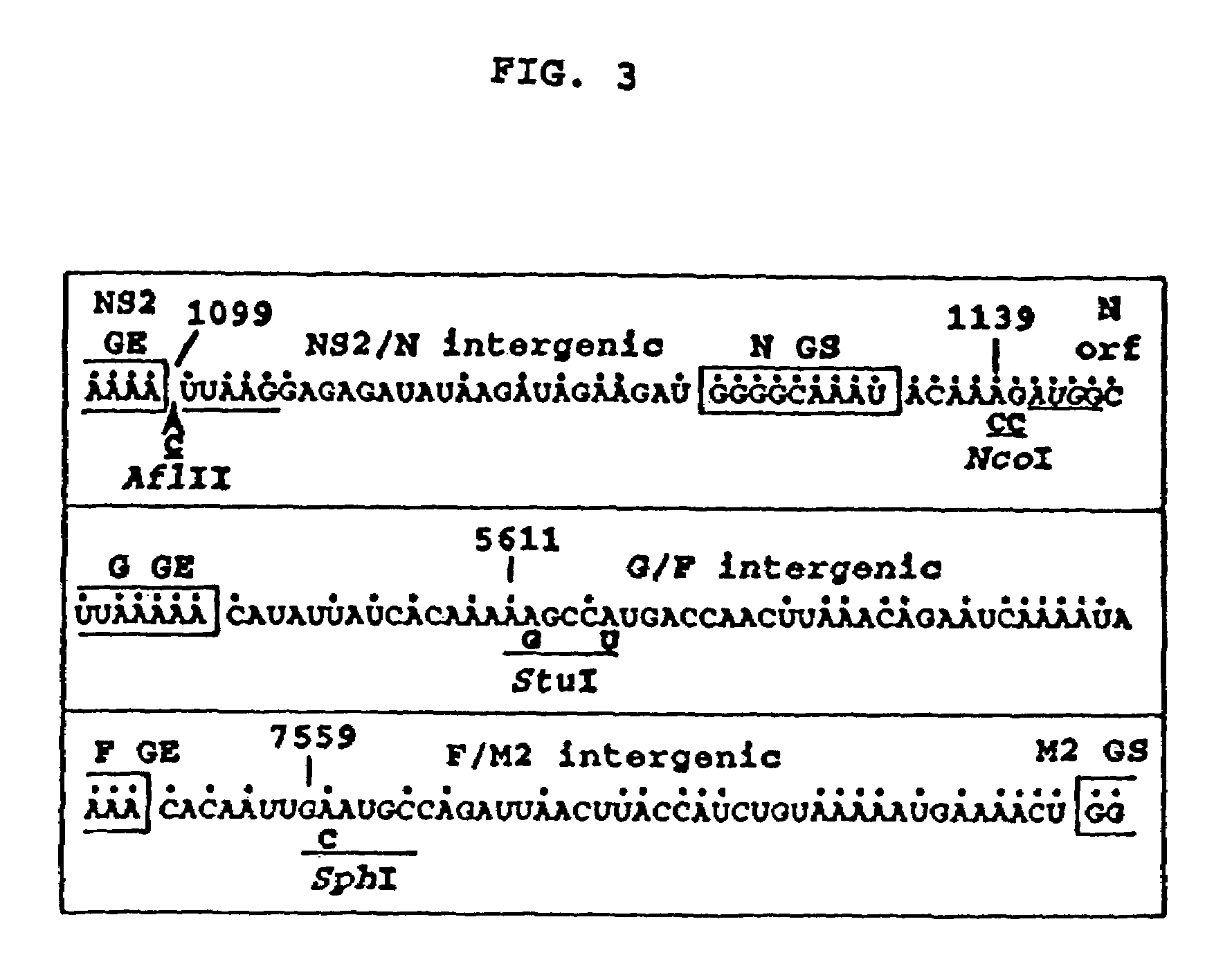
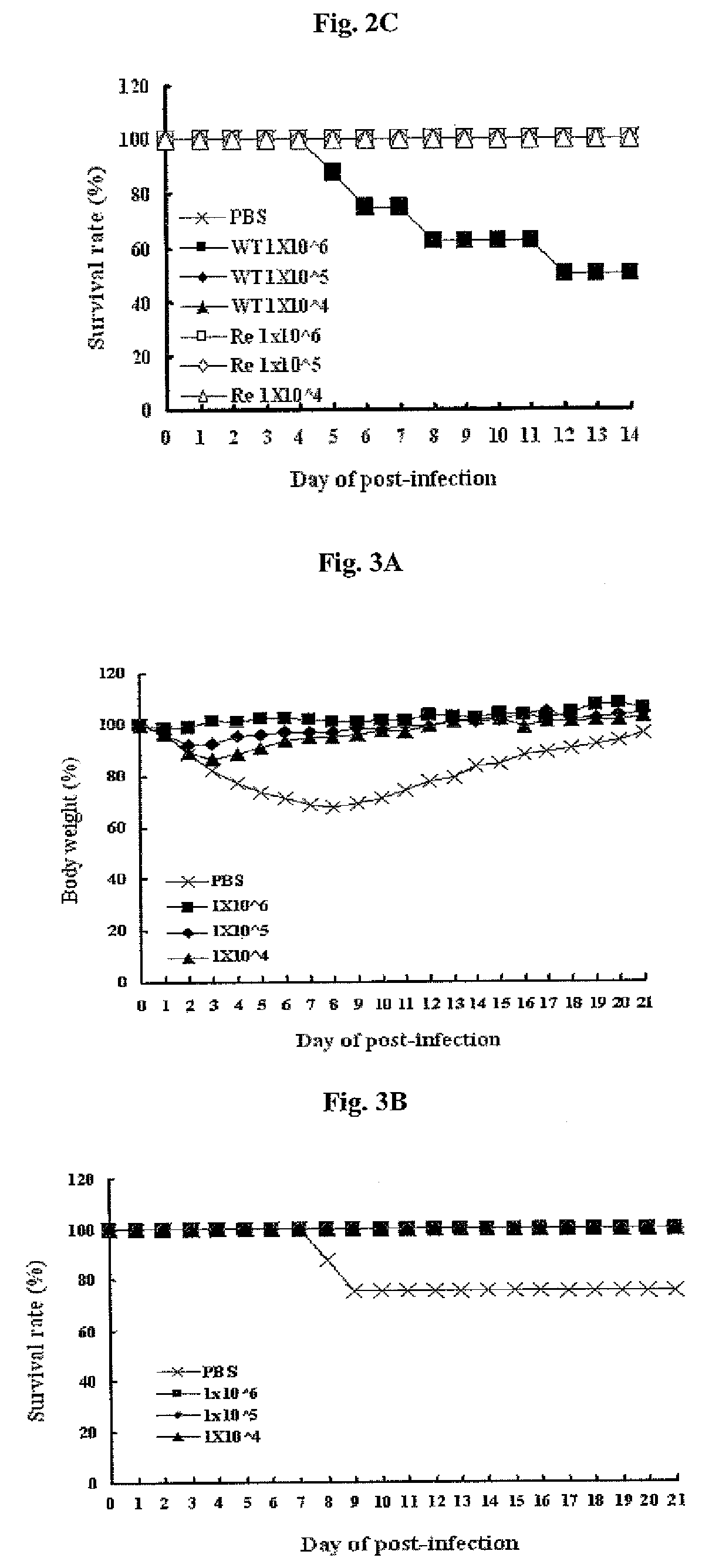
Multi plasmid system for the production of influenza virus
InactiveUS20050266026A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
Cold-Adapted Influenza Virus
InactiveUS20070172929A1Promote growthSsRNA viruses negative-senseViral antigen ingredientsCold adaptedWild type
The cold-adapted master strain A / Ann Arbor / 6 / 60 7PI (H2N2) and progenitor wild type E2(3) viral strains have been deposited and their genomic sequences identified. Seven nucleotide differences were found between the sequences identified herein and the previously published sequences for cold-adapted A / Ann Arbor / 6 / 60 genes. The cold-adapted live influenza virus of the present invention can be reasserted with a variety of epidemic wild type influenza viruses and used to produce vaccines to prophylactically and therapeutically treat influenza.
Owner:RGT UNIV OF MICHIGAN
Cold-adapted influenza virus
InactiveUS7344722B1Promote growthSsRNA viruses negative-senseSugar derivativesNucleotideCold adapted
The cold-adapted master strain A / Ann Arbor / 6 / 60 7PI (H2N2) and progenitor wild type E2(3) viral strains have been deposited and their genomic sequences identified. Seven nucleotide differences were found between the sequences identified herein and the previously published sequences for cold-adapted A / Ann Arbor / 6 / 60 genes. The cold-adapted live influenza virus of the present invention can be reassorted with a variety of epidemic wild type influenza viruses and used to produce vaccines to prophylactically and therapeutically treat influenza.
Owner:RGT UNIV OF MICHIGAN
Attenuated chimeric respiratory syncytial virus
InactiveUS7846455B2Reduce the possibilityDesired characteristicSsRNA viruses negative-senseViral antigen ingredientsHeterologousProtective antigen
Owner:UNITED STATES OF AMERICA
Cold-adapted equine influenza viruses
The present invention provides experimentally-generated cold-adapted equine influenza viruses, and reassortant influenza A viruses comprising at least one genome segment of such an equine influenza virus, wherein the equine influenza virus genome segment confers at least one identifying phenotype of the cold-adapted equine influenza virus, such as cold-adaptation, temperature sensitivity, dominant interference, or attenuation. Such viruses are formulated into therapeutic compositions to protect animals from diseases caused by influenza A viruses, and in particular, to protect horses from disease caused by equine influenza virus. The present invention also includes methods to protect animals from diseases caused by influenza A virus utilizing the claimed therapeutic compositions. Such methods include using a therapeutic composition as a vaccine to generate a protective immune response in an animal prior to exposure to a virulent virus, and using a therapeutic composition as a treatment for an animal that has been recently infected with a virulent virus, or is likely to be subsequently exposed to virulent virus in a few days whereby the therapeutic composition interferes with the growth of the virulent virus, even in the absence of immunity. The present invention also provides methods to produce cold-adapted equine influenza viruses, and reassortant influenza A viruses having at least one genome segment of an equine influenza virus generated by cold-adaption.
Owner:UNIVERSITY OF PITTSBURGH
Intranasal delivery system
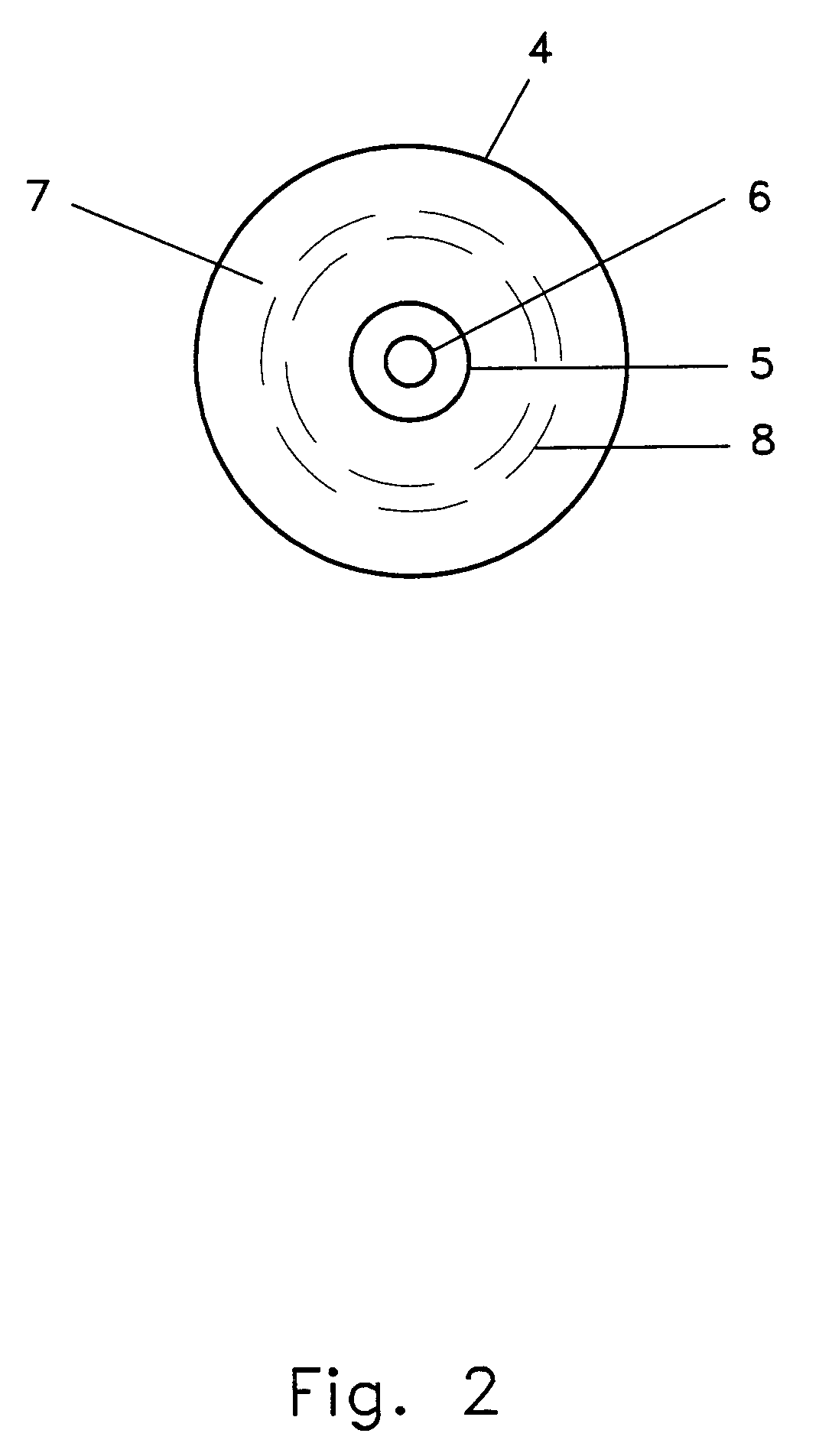
InactiveUS7204822B1Minimize cuttingMinimize scoringMedical devicesInhalatorsDose deliveryCold adapted
This invention relates to apparatus and methods of delivering various compositions including medicaments to a variety of targets. The invention includes a dose administrator (1) which may be used for intranasal delivery of compositions or medicaments, such as live virus vaccines, to both humans and animals. An axial collapse prevention element (2) to prevent excessive axial deflection of the dose administrator (1) or a dose-location coordinate indicator (3) to facilitate the delivery of a dose to the desired target location may be coupled to the dose administrator (1). An intranasal probe (4) having a force dissemination contact surface (7) may be responsive to a first end of the dose administrator (1). The dose may be delivered from a conformable dose sequestration element (10) through an aperture which penetrates the dose delivery aperture element (5) and the dose may be caused to stream by coupling a stream delivery element (6) to the dose delivery aperture element (5). The force application element (12) which acts upon the dose may be separated from the dose by a fluid dose propellent (13). While the invention may be used for numerous applications, it specifically addresses the difficulties of delivering cold-adapted live equine influenza viruses intranasally to equids.
Owner:HESKA
Multi plasmid system for the production of influenza virus
InactiveUS7465456B2Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
Immobilized cold-adapted microorganismbe carbonized sludge carrier filler for sewage treatment and application thereof
InactiveCN109019876ASave resourcesHigh densityRunoff/storm water treatmentTreatment with microorganism supports/carriersActivated sludgeCross-link
The invention relates to an immobilized cold-adapted microorganismmicrobe carbonized sludge carrier filler for sewage treatment and application thereof. According to the filler, carbonized sludge, polyvinyl alcohol, sodium alginate and concentrated carrageenan powder are optimally proportioned; mixed with the concentrated carrageenan powder, activated sludge biochar as an adsorbing carrier mixed with three strains of pyschrophiles, and with polyvinyl alcohol, sodium alginate and the concentrated carrageenan powder as embedding carriers and calcium chloride as a cross-linking agent, the carbonized sludge carrier filler with enriched immobilized cold-adapted microbes is produced. When the microorganismmicrobe filler disclosed by the invention is applied into the intensified treatment of winter sewage, the removal rates of ammonia nitrogen, TN, TP and COD in water can reach 80.09 to 85.27 percent, 76.24 to 83.67 percent, 79.72 to 85.05 percent and 85.01 to 90.52 percent, and the method disclosed by the invention is an energy-saving and high-efficiency biological intensified remedication technique.
Owner:QUFU NORMAL UNIV
Compound microbial agent, water-soluble fertilizer containing same and applications of compound microbial agent and water-soluble fertilizer
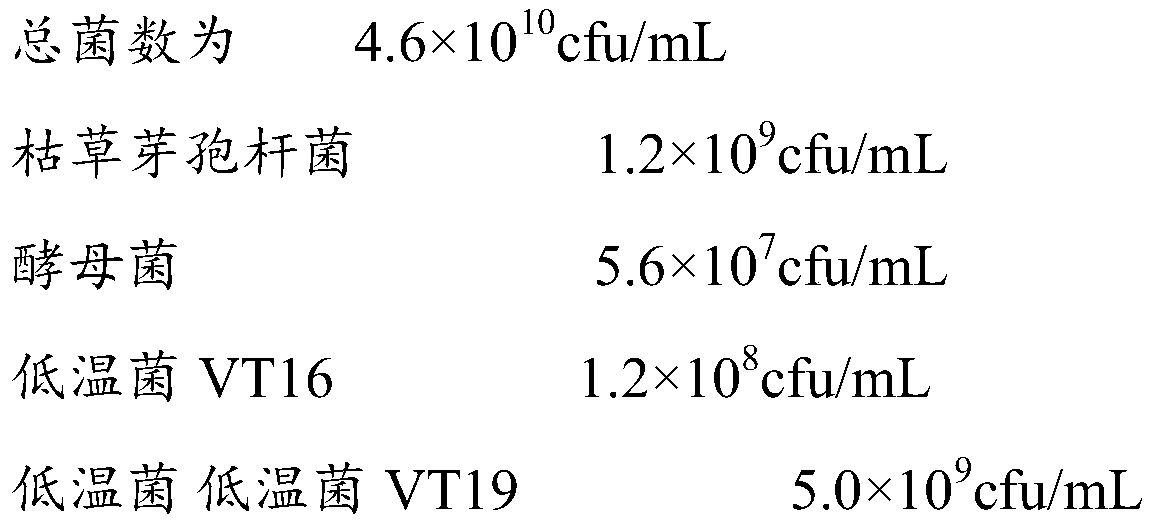
The invention discloses a compound microbial agent, a water-soluble fertilizer containing the same and applications of the compound microbial agent and the water-soluble fertilizer. The compound microbial agent is a mixture of high organic matter yeast fermentation liquid and traditional Chinese medicine extraction waste liquid, wherein the high organic matter yeast fermentation liquid is produced through microbial fermentation and is rich in amino acid. A preparation method is simple, dispenses with aseptic conditions and is low in cost. The compound microbial agent is prepared through mixed inoculated fermentation by taking bacillus subtilis, saccharomycetes, a cold-adapted bacterium VT16 and a cold-adapted bacterium VT19 as a composite strain and utilizing the high organic matter liquid produced through fermentation and rich in amino acid and the traditional Chinese medicine extraction waste liquid as raw materials, is comprehensive in nutrients and can not only provide nutrient elements required by crop growth but also inhibit reproduction of harmful microbes. The compound microbial agent is then combined with nutrients N, P and K essential to crops, wherein the added nutrients can meet the requirements of plant growth and have high absorptivity and good absorption effects; the added microbes can conduce to inhibiting growth of plant pathogens, preventing continuous cropping diseases, promoting growth of crops and inducing plants to become resistant.
Owner:北京沃土天地生物科技股份有限公司
Methods for purification of viruses
The present invention provides methods for the purification of cell-associated viruses from adherent cells (e.g., MDCK or Vero cells). In particular, the present invention provides purification methods for the production of immunogenic compositions comprising a live attenuated cell-associated virus (e.g., an attenuated respiratory syncytial virus (RSV) or cold-adapted, and / or temperature sensitive influenza virus) that result in levels of host cell DNA (HCD), host cell protein (HCP) and non-specific endonuclease (e.g., Benzonase), which are below the specifications required by regulatory agencies. The immunogenic compositions can be used to actively immunize subjects or to generate antibodies for a variety of uses, including passive immunization and diagnostic immunoassays.
Owner:MEDIMMUNE LLC
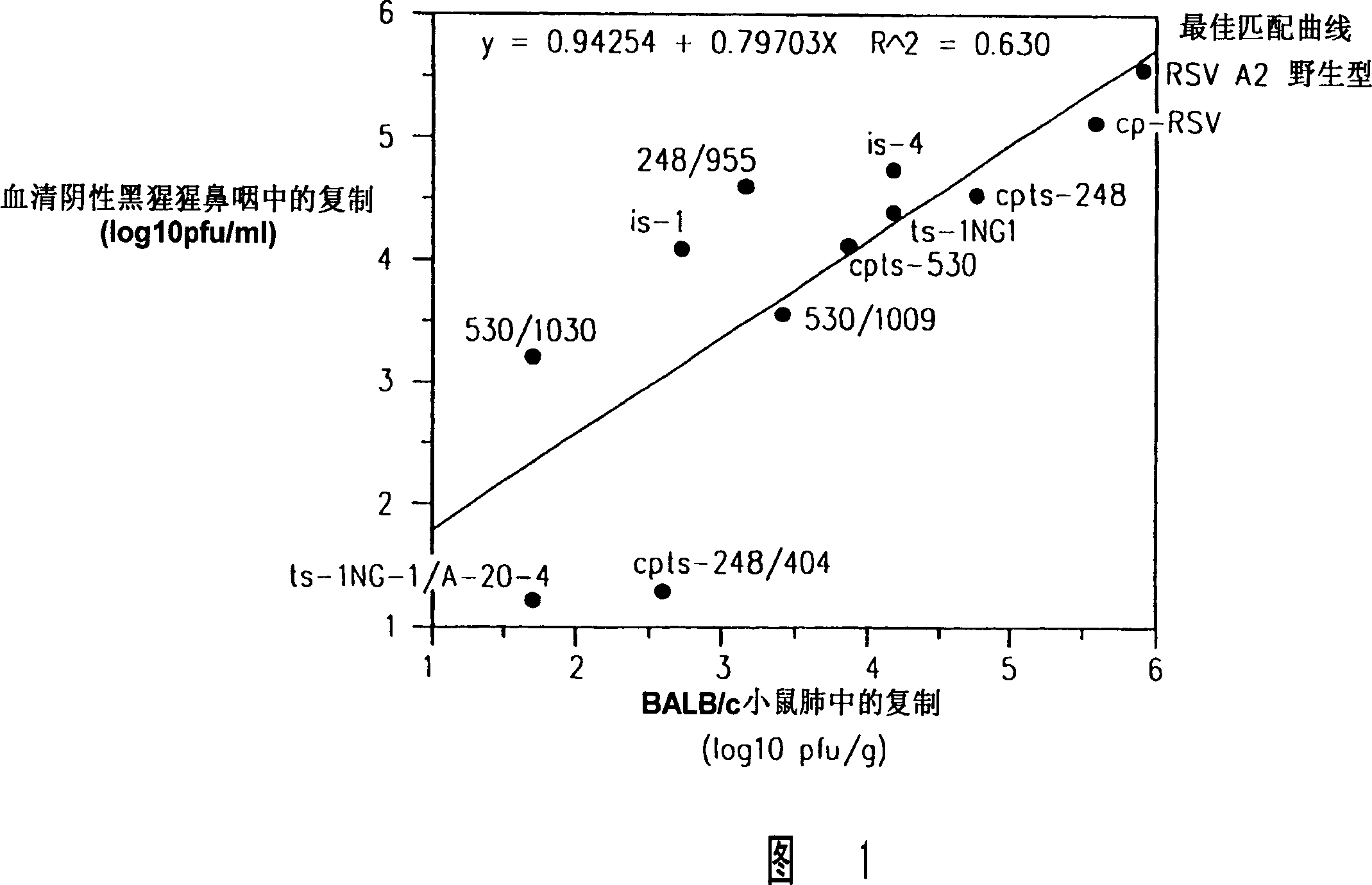
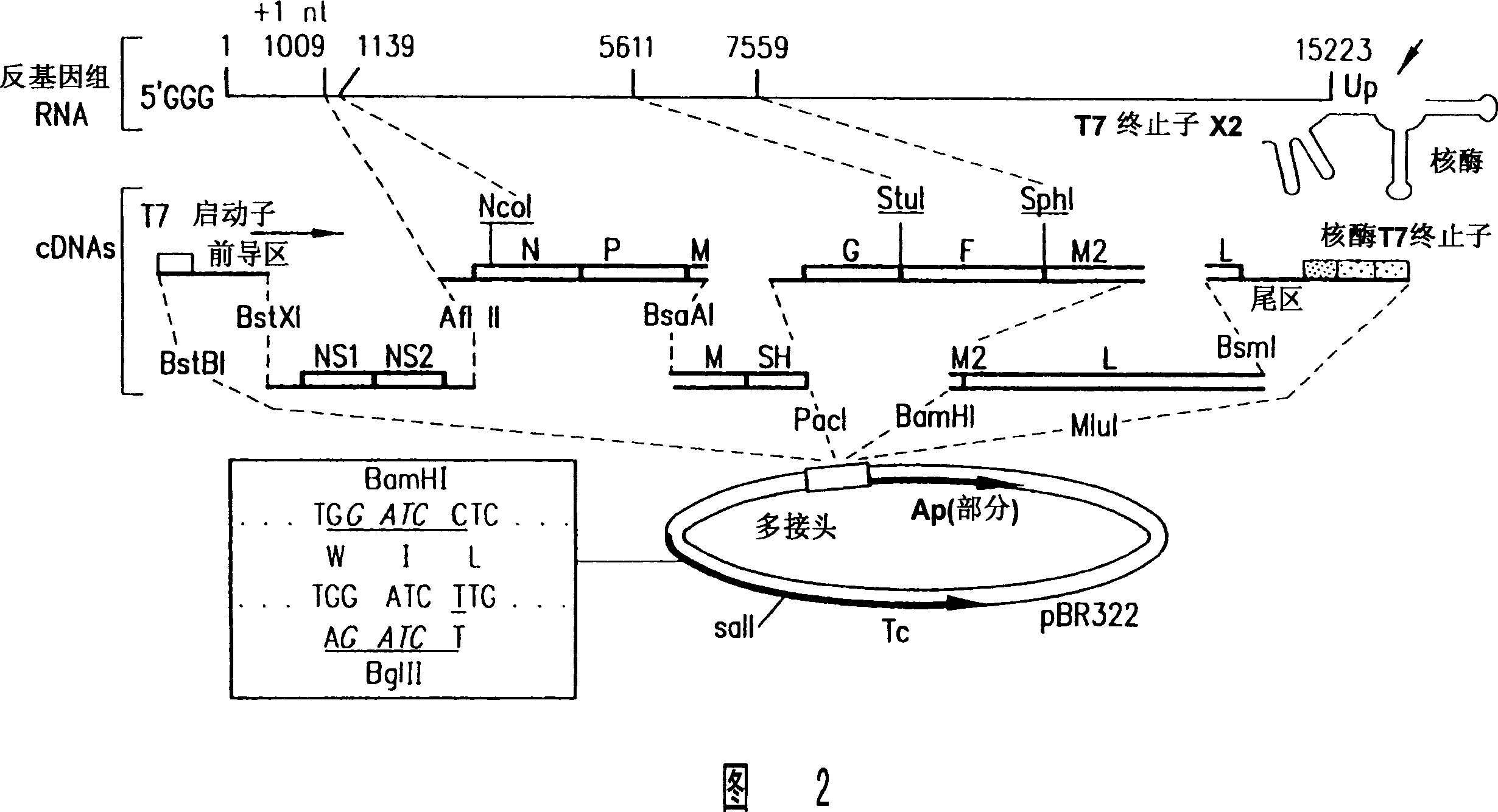
Production of attenuated chimeric respiratory syncytial virus vaccines from cloned nucleotide sequences
InactiveUS20050100557A1Satisfactory level of attenuationReduce the possibilitySsRNA viruses negative-senseViral antigen ingredientsHeterologousProtective antigen
Chimeric respiratory syncytial virus (RSV) and vaccine compositions thereof are produced by introducing one or more heterologous gene(s) or gene segment(s) from one RSV subgroup or strain into a recipient RSV backround of a different subgroup or strain. The resulting chimeric RSV virus or subviral particle is infectious and attenuated, preferably by introduction of selected mutations specifying attenuated phenotypes into a chimeric genome or antigenome to yield, for example, temperature sensitive (ts) and / or cold adapted (ca) vaccine strains. Alternatively, chimeric RSV and vaccine compositions thereof incorporate other mutations specifying desired structural and / or phenotypic characteristics in an infectious chimeric RSV. Such chimeric RSV incorporate desired mutations specified by insertion, deletion, substitution or rearrangement of one or more selected nucleotide sequence(s), gene(s), or gene segment(s) in a chimeric RSV clone. This provides a method for development of novel vaccines against diverse RSV strains by using a common attenuated backbone as a vector to express protective antigens of heterologous strains. The immune system of an individual is stimulated to induce protection against natural RSV infection, preferably in a multivalent manner to achieve protection against multiple RSV strains and / or subgroups.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Attenuated human rotavirus vaccine
InactiveUS7150984B2Enhance immune responseMaximum efficacyViral antigen ingredientsInactivation/attenuationRotavirus RNACold adapted
The present invention provides vaccine compositions of attenuated human rotavirus. More particularly, the attenuated human rotavirus is produced by cold passage and thus contains attenuating mutations which produce virus having a cold-adapted (ca) and temperature sensitive (ts) phenotype. The attenuated strains are used in methods for stimulating the immune system of an individual to induce protection against human rotavirus by administration of the ca attenuated rotavirus.
Owner:GOVERNMENT UNITED STATES OF AMERICA THE
Cold-adapted equine influenza viruses
InactiveUS20060121521A1Antibacterial agentsSsRNA viruses negative-senseVirus influenzaRespiratory disease
The present invention provides experimentally-generated cold-adapted equine influenza viruses, and reassortant influenza A viruses comprising at least one genome segment of such an equine influenza virus, wherein the equine influenza virus genome segment confers at least one identifying phenotype of the cold-adapted equine influenza virus, such as cold-adaptation, temperature sensitivity, dominant interference, or attenuation. Such viruses are formulated into therapeutic compositions to protect animals from diseases caused by influenza A viruses, and in particular, to protect horses from disease caused by equine influenza virus. The present invention also includes methods to protect animals from diseases caused by influenza A virus or other infectious agents utilizing the claimed therapeutic compositions. Such methods include using a therapeutic composition as a vaccine to generate a protective immune response in an animal prior to exposure to an infectious agent, as well as using a therapeutic composition as a treatment for an animal that has been recently infected with an infectious agent leading to respiratory disease, or is likely to be subsequently exposed to such an agent in a few days whereby the therapeutic composition reduces such respiratory disease, even in the absence of antibody-mediated immunity. The present invention also provides methods to produce cold-adapted equine influenza viruses, and reassortant influenza A viruses having at least one genome segment of an equine influenza virus generated by cold-adaptation.
Owner:DOWLING PATRICIA W +1
Production of attenuated respiratory syncytial virus vaccines from cloned nucleotide sequences
InactiveUS7709007B2Reduce the possibilityStabilizing mutationSsRNA viruses negative-senseFungiNucleotideWild type
Attenuated respiratory syncytial virus (RSV) and vaccine compositions thereof are produced by introducing specific mutations associated with attenuating phenotypes into wild-type or RSV which is incompletely attenuated by cold-passage or introduction of mutations which produce virus having a temperature sensitive (ts) or cold adapted (ca) phenotype. Alternatively, recombinant RSV and vaccine compositions thereof incorporate attenuating and other mutations specifying desired structural and or phenotypic characteristics in an infectious RSV. Recombinant RSV incorporate desired mutations specified by insertion, deletion, substitution or rearrangement of a selected nucleotide sequence, gene, or gene segment in an infectious RSV clone. The immune system of an individual is stimulated to induce protection against natural RSV infection, or multivalently against infection by RSV and another pathogen, such as PIV, by administration of attenuated, biologically derived or recombinant RSV.
Owner:UNITED STATES OF AMERICA
Antarctic cold-adapted microbial extracellular polysaccharide capable of improving body immunity
ActiveCN102174614AImprove immunityPromote proliferationOrganic active ingredientsMicroorganism based processesBiotechnologyCold adapted
The invention relates to an Antarctic cold-adapted microbial extracellular polysaccharide capable of improving body immunity, and belongs to the technical field of medicaments and health-care food. The cold-adapted microbial extracellular polysaccharide is prepared by culturing, fermenting, separating and purifying Antarctic cold-adapted bacteria (Pseudoaltermonas sp.) S-5, the strain collection number of which is CGMCC NO.3433. The molecular weight of the polysaccharide is 39,7046Da, and the polysaccharide consists of mannose, dextrose and galactose in a molar ratio of 4.8: 50.9: 44.3. The prepared Antarctic cold-adapted microbial extracellular polysaccharide capable of improving the body immunity can promote the proliferation of in vitro spleen lymphocytes, enhance the activity of macrophages and improve the body immunity.
Owner:博德生物技术(德州)有限公司
LDL-T strain as attenuated vaccine strain for avian infectious bronchitis and application thereof
ActiveCN106947746AGood immune protectionImprove securitySsRNA viruses positive-senseViral antigen ingredientsInfectious bronchitisCold adapted
The invention discloses an LDL-T strain as an attenuated vaccine strain for avian infectious bronchitis and application thereof, which belong to the field of prevention and treatment of the avian infectious bronchitis. The attenuated live vaccine strain CGMCC No.12549 for the avian infectious bronchitis provided by the invention has good safety and immune protection, the immune protection rate reaches 80 percent or more, and the LDL-T strain has good protective efficacy on TW I type avian infectious bronchitis viruses, and can effectively protect chickens against the attack of the TW I type infectious bronchitis viruses with strong virulent; moreover, the LDL-T strain is safe for chickens and laying breeders, side reaction does not exist, and the safety is good. The cold-adapted attenuated vaccine strain disclosed by the invention can be applied to the preparation of single vaccines, mixed vaccines and the like for preventing and treating the avian infectious bronchitis viruses.
Owner:HARBIN WEIKE BIOTECH DEV
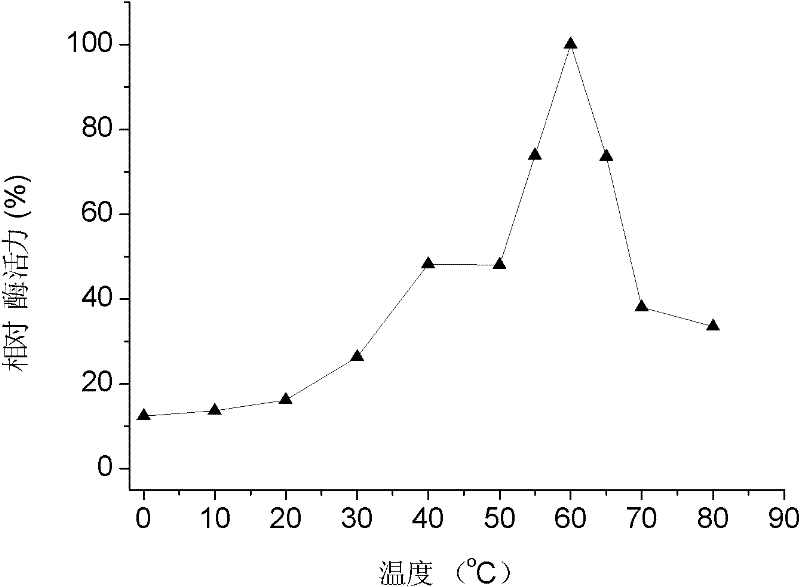
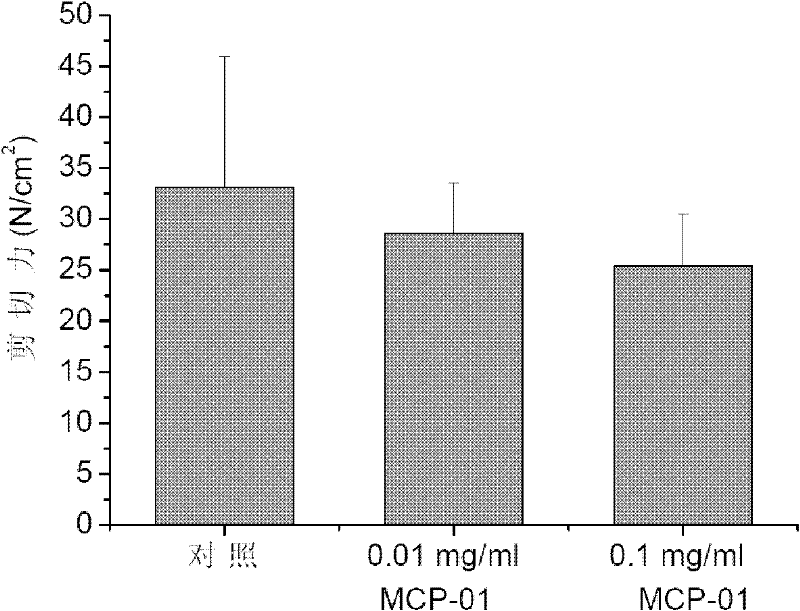
Application of cold-adapted protease MCP-01 for tenderizing meat
InactiveCN102224938AImprove tendernessGreat application potentialFood preparationProteinase activityCold adapted
The invention relates to an application of cold-adapted protease MCP-01 for tenderizing meat, which belongs to the technical field of food biological technology. The present invention has the following technical schemes for tenderizing meat: (1) preparing cold-adapted protease MCP-01 to a cold-adapted protease MCP-01 solution with the concentration of 5 - 20 micrograms / ml; (2) washing the meat required to be tenderized, and immersing into the cold-adapted protease MCP-01 solution obtained in step (1), storing at the temperature of 0 - 8 DEG C for 18 - 30 hours. The protease is capable of specifically degrading collagen in connective tissue of meat under the temperature of 0 - 4 DEG C, which has good potential application in the process of tenderizing meat.
Owner:SHANDONG UNIV
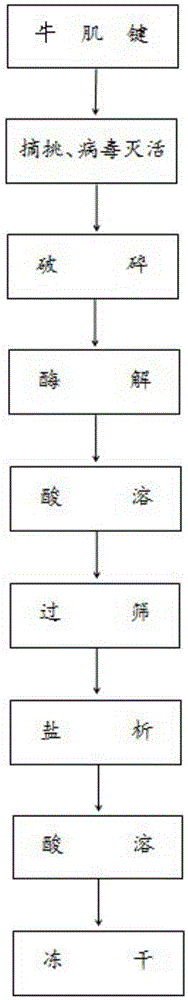
Extraction method of bovine tendon collagen
InactiveCN105524965AStrong characteristic absorption peakImprove extraction efficiencyConnective tissue peptidesPeptide preparation methodsFiberFiltration
The invention provides an extraction method of bovine tendon collagen. The extraction method has the advantages of high yield, short production period and high product purity, and comprises the following steps of: preprocessing bovine tendon, and freezing and crushing the bovine tendon; putting the crushed bovine tendon into a Tris-HCL buffer solution; then, adding cold-adapted protease MCP-01 accounting for 0.1 percent of the weight of the bovine tendon into the buffer solution; performing enzymolysis at 40 DEG C; then, obtaining an enzymolysis solution; centrifuging the enzymolysis solution; taking precipitates; dissolving the precipitates by 0.1-percent acetic acid; performing filtration to obtain filter liquid; salting out the filter liquid to obtain a salting-out solution; performing centrifugation twice; adding water for dissolution; then, adding 0.1-percent acetic acid for continuous dissolution; and performing freeze drying to obtain the bovine tendon collagen. The extraction method has the beneficial effects that the cold-adapted protease MCP-01 is used for hydrolyzing the bovine tendon in a buffer liquid system, so that collagen helix molecules of collagen micro fiber are exposed; collagen filaments expand and loose, so that the hydrolysis is fast and sufficient; and the yield and the product purity are further improved.
Owner:FUJIAN HUAGENE BIOSCI CO LTD
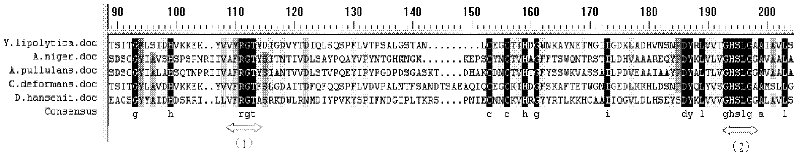
Cold-adapted marine yeast BoHai Sea-9145 low-temperature alkaline lipase gene, amino acid sequence and recombinant lipase
ActiveCN102363786AImprove featuresImprove temperature stabilityHydrolasesFermentationSequence signalRapid amplification of cDNA ends
The invention relates a gene for coding low-temperature alkaline lipase (LipY). The gene is obtained by cloning cold-adapted marine yeast (BoHai Sea-9145), which can secrete low-temperature alkaline lipase and is screened from sea mud of Bohai, by a method of combining degenerate primer polymerase chain reaction (PCR) and rapid amplification of cDNA ends (RACE). The gene contains a 1206bp open reading frame (ORF), codes 401 amino acids and contains a signal peptide with 28 amino acids at the amino acid end. Each coded amino acid contains a conserved sequence (G-X-S-X-G) of the lipase and a potential N-glycosylation site. The lipase gene is cloned on an eukaryotic expression vector (pIc9K) and performs heterologous expression in Pichia pastoris (Gs115). High-activity recombinant lipase is obtained from supernate 96 hours after fermentation, wherein methanol serves as an inducer. An electrophoretic pure lipase sample is obtained by purifying the recombinant lipase by a nickel ion affinity column. The recombinant lipase has a series of advantages of high low-temperature catalytic activity, stable performance under the alkaline condition and the like, and has wide application prospect in the low-temperature catalytic fields of daily chemicals, textile, food processing and the like.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
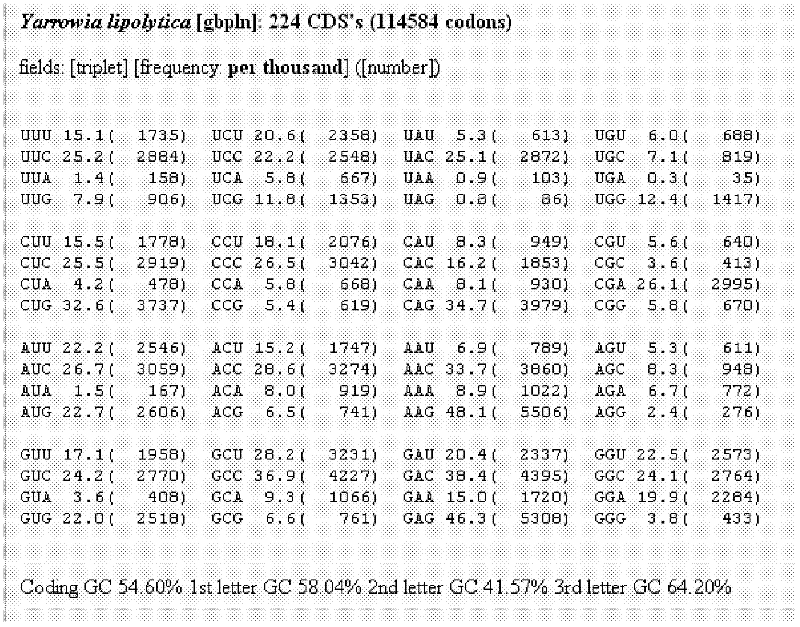
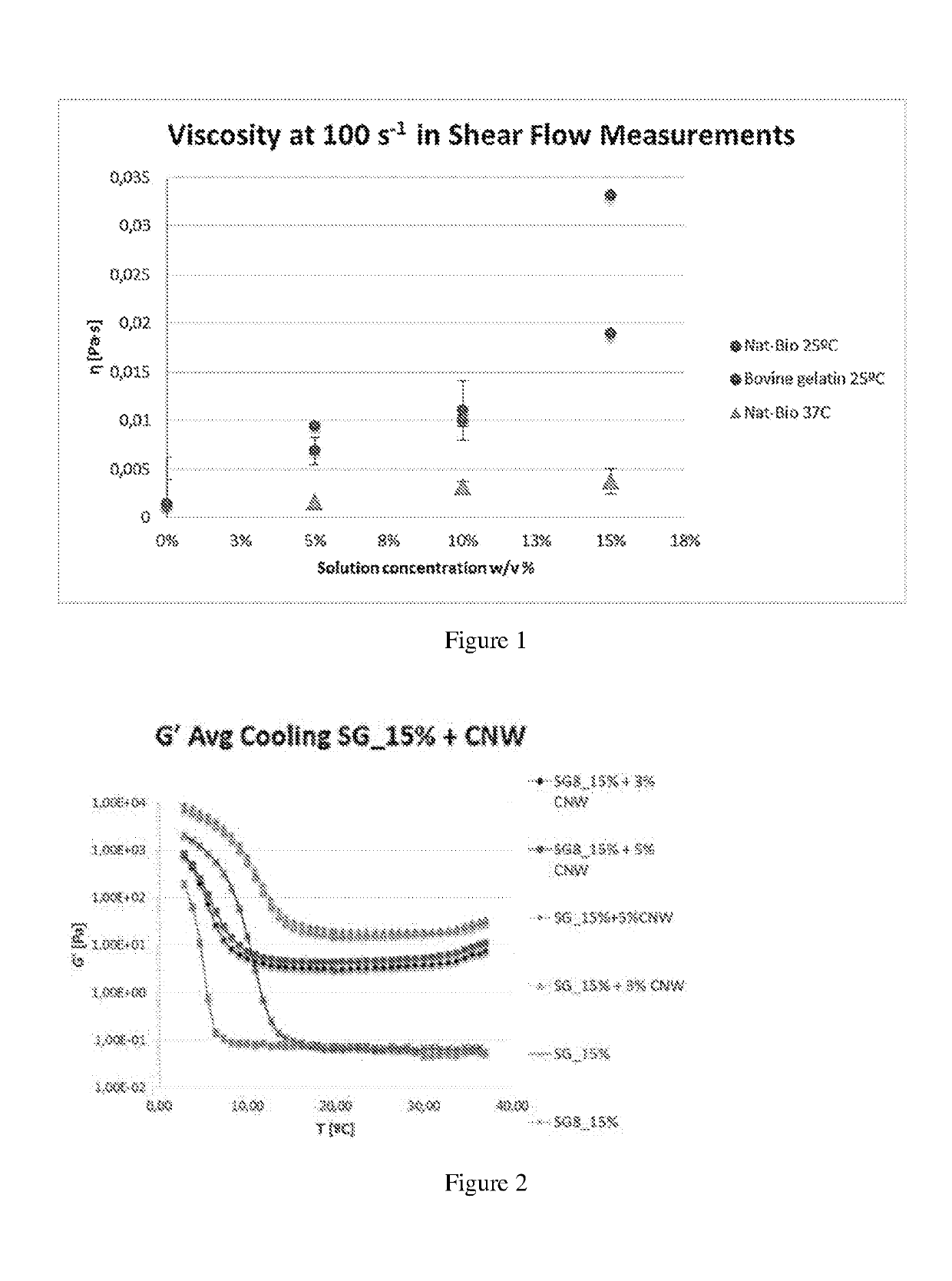
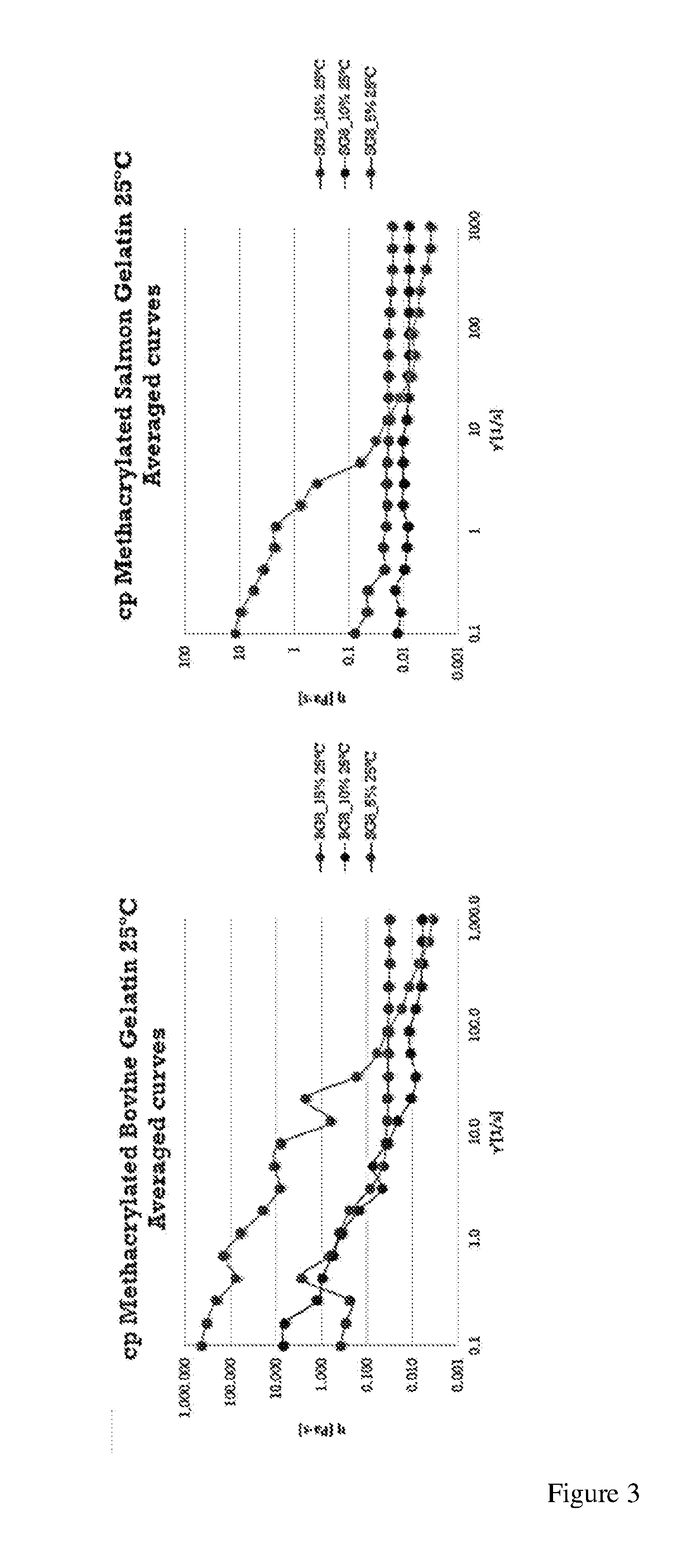
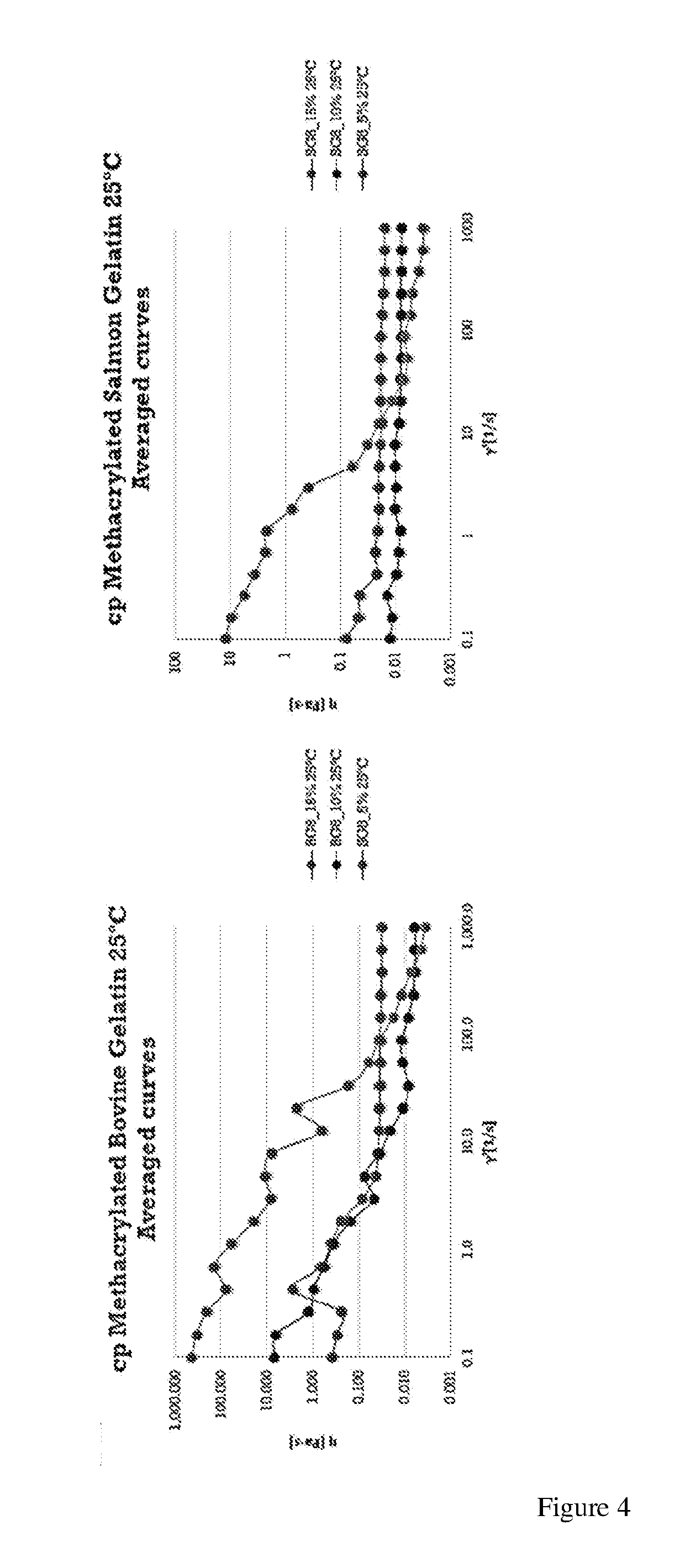
Gelatin polymer derived from natural sources of cold-adapted marine species and uses thereof
ActiveUS20190194460A1Low viscosityHigh catalytic efficiencyConnective tissue peptidesAdditive manufacturing apparatusNatural sourcePolymerization
The present invention refers to a composition comprising a solution which in turn comprises an amino acidic chain gelatin polymer derived from natural sources of cold-adapted marine species, at a concentration from 1% to 20% (w / v), which optionally further comprises a polymerizing initiator such as a photoinitiator, and is chemically functionalized to become reactive to polymerization or crosslinking in presence of free radicals. This composition is especially useful for 3D printing, extrusion systems (additive fabrication), spray systems, casting, micro- and nano fibers fabrication systems (electrospinning, solution blow spinning) or microfluidics.
Owner:UNIV DE LOS ANDES +1
Methods for cultivating cells, propagating and purifying viruses
InactiveUS8202726B2Increase cell densityHigh viral titersSsRNA viruses negative-senseViral antigen ingredientsPassive ImmunizationsPurification methods
The present invention provides novel serum-free cell culture medium and methods for cultivating MDCK cells. In particular, non-tumorigenic MDCK cells. The present invention also provides methods for producing influenza viruses (e.g., particularly cold-adapted, and / or temperature sensitive, and / or attenuated influenza viruses) that eliminate the need for a cell culture medium exchange step. The novel medium and methods are useful to grow influenza viruses, in cell culture to high titer. The present invention further provides purification methods for purifying influenza viruses with high overall recovery of live virus and result in levels of host cell DNA (HCD), host cell protein (HCP) and non-specific endonuclease (e.g., Benzonase), which are below the specifications required by regulatory agencies. The immunogenic compositions can be used to actively immunize subjects or to generate antibodies for a variety of uses, including passive immunization and diagnostic immunoassays.
Owner:MEDIMMUNE LLC
Production of attenuated chimeric respiratory syncytial virus vaccines from cloned nucleotide sequences
InactiveCN101012454ARecycling Mutations EffectiveEasy to operateSsRNA viruses negative-senseFungiWild typeRSV Infections
Attenuated respiratory syncytial virus (RSV) and vaccine compositions thereof are produced by introducing specific mutations associated with attenuating phenotypes into wild-type or RSV which is incompletely attenuated by cold-passage or introduction of mutations which produce virus having a temperature sensitive (ts) or cold adapted (ca) phenotype. Alternatively, recombinant RSV and vaccine compositions thereof incorporate attenuating and other mutations specifying desired structural and or phenotypic characteristics in an infectious RSV. Recombinant RSV incorporate desired mutations specified by insertion, deletion, substitution or rearrangement of a selected nucleotide sequence, gene, or gene segment in an infectious RSV clone. The immune system of an individual is stimulated to induce protection against natural RSV infection, or multivalently against infection by RSV and another pathogen, such as PIV, by administration of attenuated, biologically derived or recombinant RSV.
Owner:UNITED STATES OF AMERICA
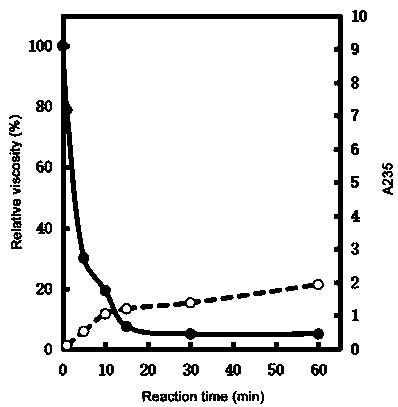
Cold-adapted alginate lyase AlgA5 and application thereof
InactiveCN109022405AHigh similarityIncrease vitalityFermentationVector-based foreign material introductionThree levelTrisaccharide
The invention relates to alginate lyase with cold-adapted characteristics and an application thereof. The alginate lyase is a novel alginate lyase AlgA5, and an amino acid sequence is as shown by SEQID NO.1. In the sequence, a section of F5-F8-Type C structural domain (Gglu160-Ggly220) is designed, and the combinational structural domain can form a section of stable three-level structure, therebyinfluencing the cold-adapted performance of the lyase. The identity between the amino acid sequence of the alginate lyase AlgA5 and the sequence of the existing alginate lyase reaches up to 75 percent. The alginate lyase AlgA5 provided byof the invention has the cold-adapted characteristics, an acting way is an endonuclease way, a main product of the degradation of the cold-adapted alginate lyaseis unsaturatedalginate disaccharide and unsaturated alginate trisaccharide. The alginate lyase provided byof the invention is high in yield, stable in performance and high in industrialized application potential.
Owner:王存良
Protein and nucleic acid sequence encoding a krill-derived cold adapted trypsin-like activity enzyme
The present invention provides nucleic acid and corresponding amino acid sequences of two isoforms of cold adapted trypsin-like activity protein, insolated from antarctic marine origin, preferably from antarctic krill (Euphausia superba) that can be used in a variety of industrial contexts and commercial purposes including laundry detergents, food processing, drugs and skin care products. The invention also relates to nucleic acid constructs, vectors, and host cells comprising the nucleic acid sequences as well as methods for producing and using the cold adapted trypsin-like protein.
Owner:UNIVERSITY OF CHILE
Attenuated influenza virus and a live vaccine comprising the same
The present invention relates to an isolated attenuated influenza virus strain and a live vaccine comprising the same. The isolated attenuated influenza virus strain is prepared by cold-adaptation of a mother strain which carries 6 internal genomes of A / PR / 8 / 34(H1N1) and two surface antigens HA and NA of A / Aichi / 2 / 68(H3N2). The attenuated influenza virus strain and the live vaccine of the present invention are useful for prevention of seasonal influenza episodes and sudden outbreak of influenza pandemics of predicted or unknown identity, since they have safety, efficacy, high production yield, immediate protection against variety of influenza subtypes and prolonged protection against specific influenza subtype.
Owner:RAPAVAX INC
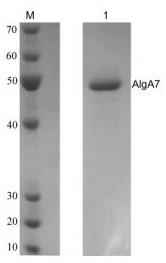
Novel cold-adapted alginate lyase AlgA7 and application thereof
InactiveCN109022404AReduce consumptionFermentationVector-based foreign material introductionCold adaptedLyase
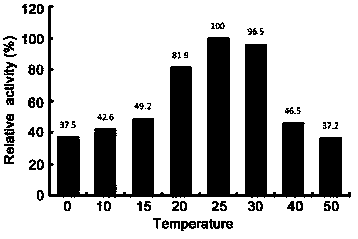
The invention relates to novel alginate lyase with cold-adapted characteristics and an application thereof. The alginate lyase is artificially-designed novel alginate lyase AlgA7y, an amino acid sequence is as shown by SEQ ID NO.1, an N-end N comprises a section of carbohydrate combination domain (Met1-Pro176), an C-end C comprises a section of alginate lyase preserved catalytic area (Ser177-Asp526), and the identity between the amino acid sequence of the alginate lyase in provided bythe invention and the alginate lyase with known functions is only 71 percent. The alginate lyase AlgA7 of provided by the invention has cold-adapted characteristics, the most appropriate reaction temperature is 25 DEG C, and the reaction activity at 20 to and 10 DEG C can reach 81.9 percent to and42.6 percentof the reaction activity at the most appropriate temperature. The alginate lyase can control the enzymatic reaction to be performed at a low temperature, so that the energy consumption in the industrialized application process can be saved, and the cost can be savedreduced.
Owner:王存良
Externally tangent type algin lyase VsAly7D and recombinant strain and application thereof
ActiveCN110951716AIncreased degradation rateGood protein expressionBacteriaMicroorganism based processesNucleotideCold adapted
The invention discloses externally tangent type algin lyase VsAly7D and a recombinant strain and application thereof. The amino acid sequence of the externally tangent type algin lyase disclosed by the invention is as shown in SEQ ID No.6, and the nucleotide sequence of the coding amino acid sequence is as shown in SEQ ID No.5. The suitable reaction temperature of the externally tangent type alginlyase is 20-35 DEG C, and the externally tangent type algin lyase has high stability at 0-30 DEG C, so that the enzyme can effectively degrade substrate at low temperature and belongs to cold-adaptedalgin lyase; the externally tangent type algin lyase has degradation capacity on algin, polyG and polyM, a degradation manner is an externally tangent type, and a final degraded product is unsaturated monose and has NaCl dependence. The invention further constructs a recombinant carrier and recombinant strain containing externally tangent type algin lyase VsAly7D coded genes, and a favorable basecan be provided for industrialized application and production of biologic ethanol.
Owner:OCEAN UNIV OF CHINA
Cold-adapted equine influenza viruses
InactiveUS20050175985A1SsRNA viruses negative-senseVirus peptidesGenomic SegmentUltrasound attenuation
The present invention provides experimentally-generated cold-adapted equine influenza viruses, and reassortant influenza A viruses comprising at least one genome segment of such an equine influenza virus, wherein the equine influenza virus genome segment confers at least one identifying phenotype of the cold-adapted equine influenza virus, such as cold-adaptation, temperature sensitivity, dominant interference, or attenuation. Such viruses are formulated into therapeutic compositions to protect animals from diseases caused by influenza A viruses, and in particular, to protect horses from disease caused by equine influenza virus. The present invention also includes methods to protect animals from diseases caused by influenza A virus utilizing the claimed therapeutic compositions. Such methods include using a therapeutic composition as a vaccine to generate a protective immune response in an animal prior to exposure to a virulent virus, and using a therapeutic composition as a treatment for an animal that has been recently infected with a virulent virus, or is likely to be subsequently exposed to virulent viruses in a few days whereby the therapeutic composition interferes with the growth of the virulent virus, even in the absence of immunity. The present invention also provides methods to produce cold-adapted equine influenza viruses, and reassortant influenza A viruses having at least one genome segment of an equine influenza virus generated by cold-adaptation.
Owner:UNIVERSITY OF PITTSBURGH
Deep-sea cold-adapted and salt-tolerant collagenase as well as encoding gene myr02 and application of same
The invention relates to a deep-sea cold-adapted and salt-tolerant collagenase as well as an encoding gene myr02 and application of the same, belonging to the field of biotechnology. The nucleotide sequence of a deep-sea cold-adapted and salt-tolerant collagenase gene myr02 is as shown in SEQ ID NO.1, and the nucleotide sequence of the collagenase myr02 encoded by the collagenase gene myr02 is as shown in SEQ ID NO.12. The collagenase myr02 can be used for degrading insoluble I type collagens, also has a higher activity for soluble substrates such as gelatins and the like, and has better cold adaptability and salt tolerance.
Owner:SHANDONG UNIV
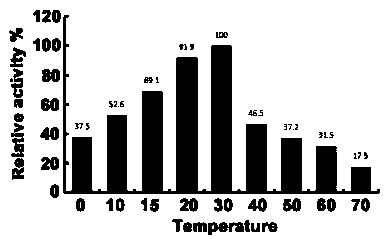
Cold-adapted cellulase-producing bacillus subtilis and screening method thereof
ActiveCN110551650AImprove aggregate structurePromote formationBacteriaMicrobiological testing/measurementScreening methodCulture mediums
The invention relates to cold-adapted cellulase-producing bacillus subtilis and a screening method thereof. The cold-adapted cellulase-producing bacillus subtilis comprises bacillus subtilis with a preservation mechanism being General Microbiological Culture Center for China Committee for Culture Collection of Microorganisms, a preservation number being CGMCC No. 18001 and a preservation date being June 19, 2019. For the cold-adapted cellulase-producing bacillus subtilis and the screening method, efficient cold-adapted cellulase bacillus subtilis is separated and screened from soil in different low-temperature areas, and the biological characteristics of the bacillus subtilis are as follows: a bacterial colony on an LB culture medium is relatively small, round, wet, smooth and sticky, is easy to pick and has typical bacterial colony characteristics; and in addition, the influences of the conditions, including temperature, carbon sources, nitrogen sources and a pH value, of the bacterial colony on cellulase are studied. By applying the cold-adapted cellulase-producing bacillus subtilis to straw returning in northeast low-temperature cold areas, the influence of straw incineration inthe northeast areas on environment is reduced, and a solid foundation is laid for sustainable development of agriculture.
Owner:LIAONING ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com