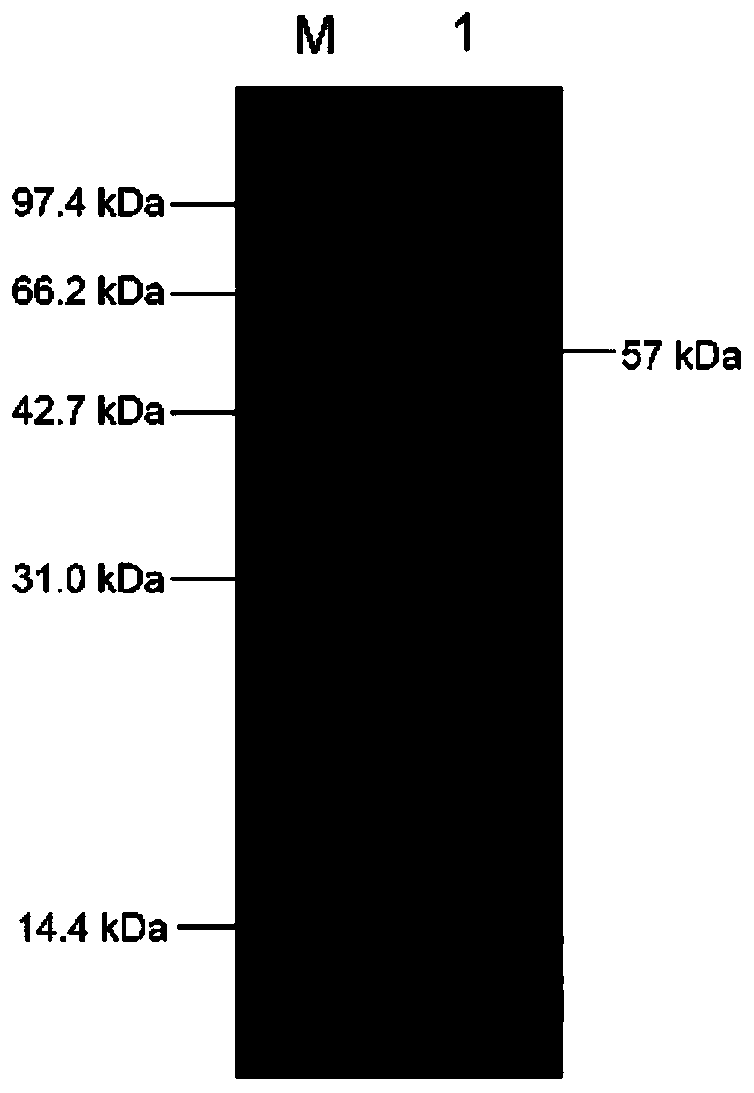
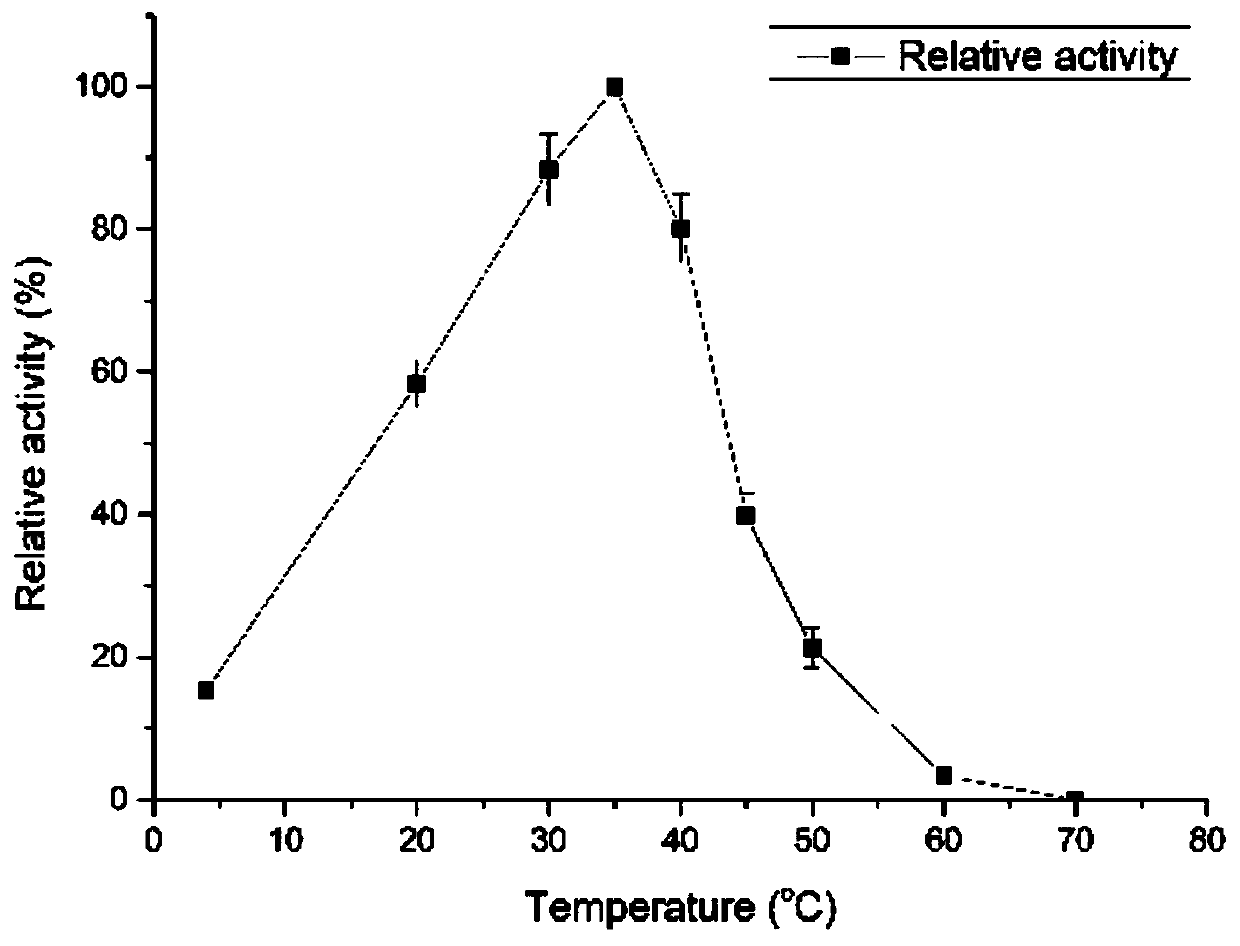
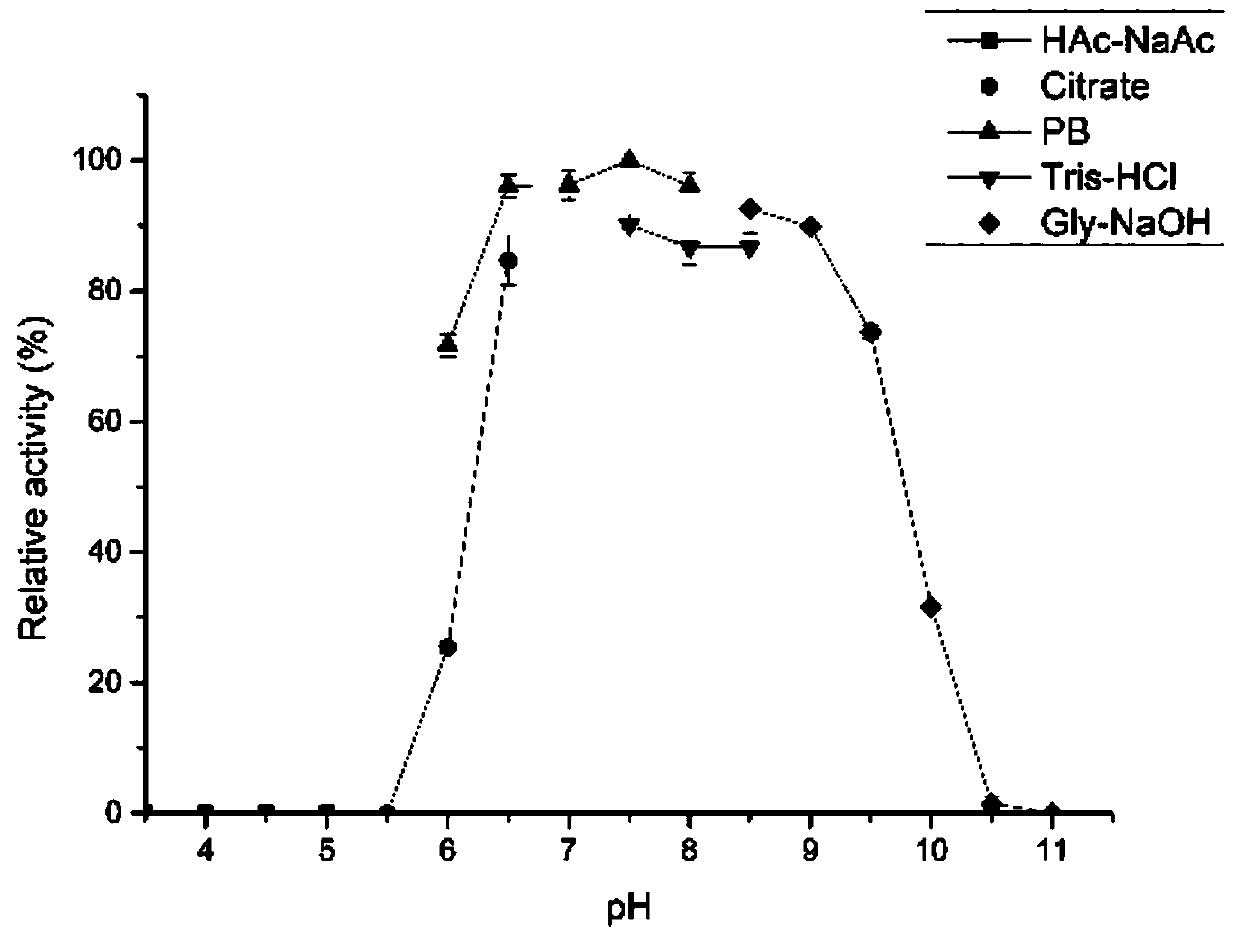
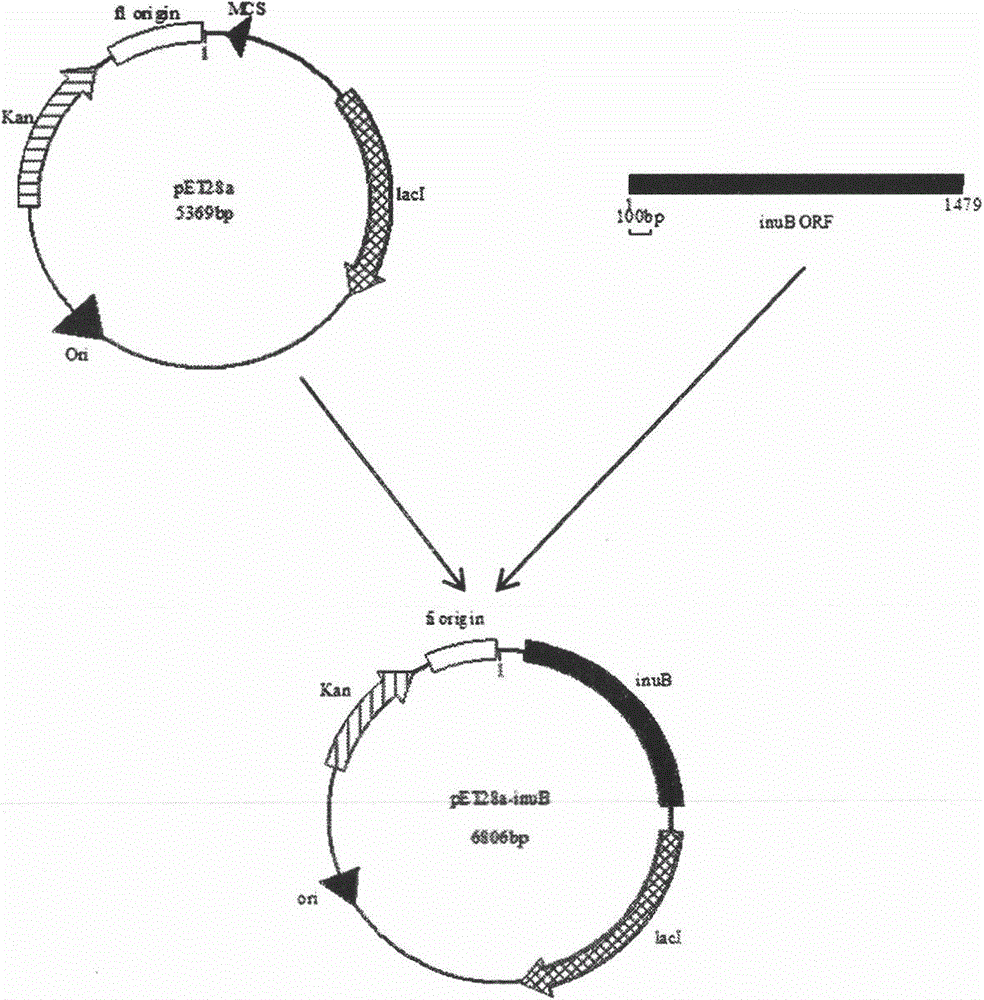
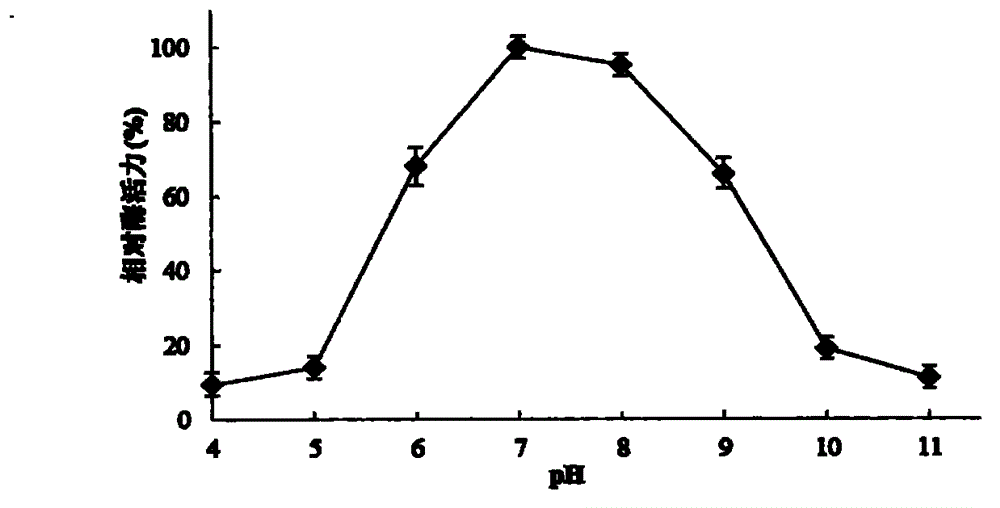
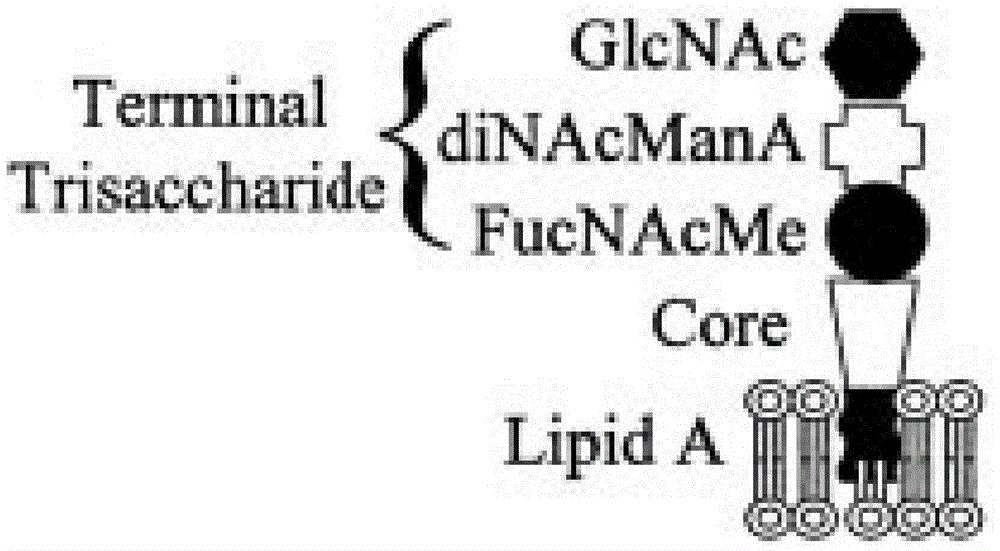
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
214 results about "Trisaccharide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trisaccharides are oligosaccharides composed of three monosaccharides with two glycosidic bonds connecting them. Similar to the disaccharides, each glycosidic bond can be formed between any hydroxyl group on the component monosaccharides. Even if all three component sugars are the same (e.g., glucose), different bond combinations (regiochemistry) and stereochemistry (alpha- or beta-) result in trisaccharides that are diastereoisomers with different chemical and physical properties.
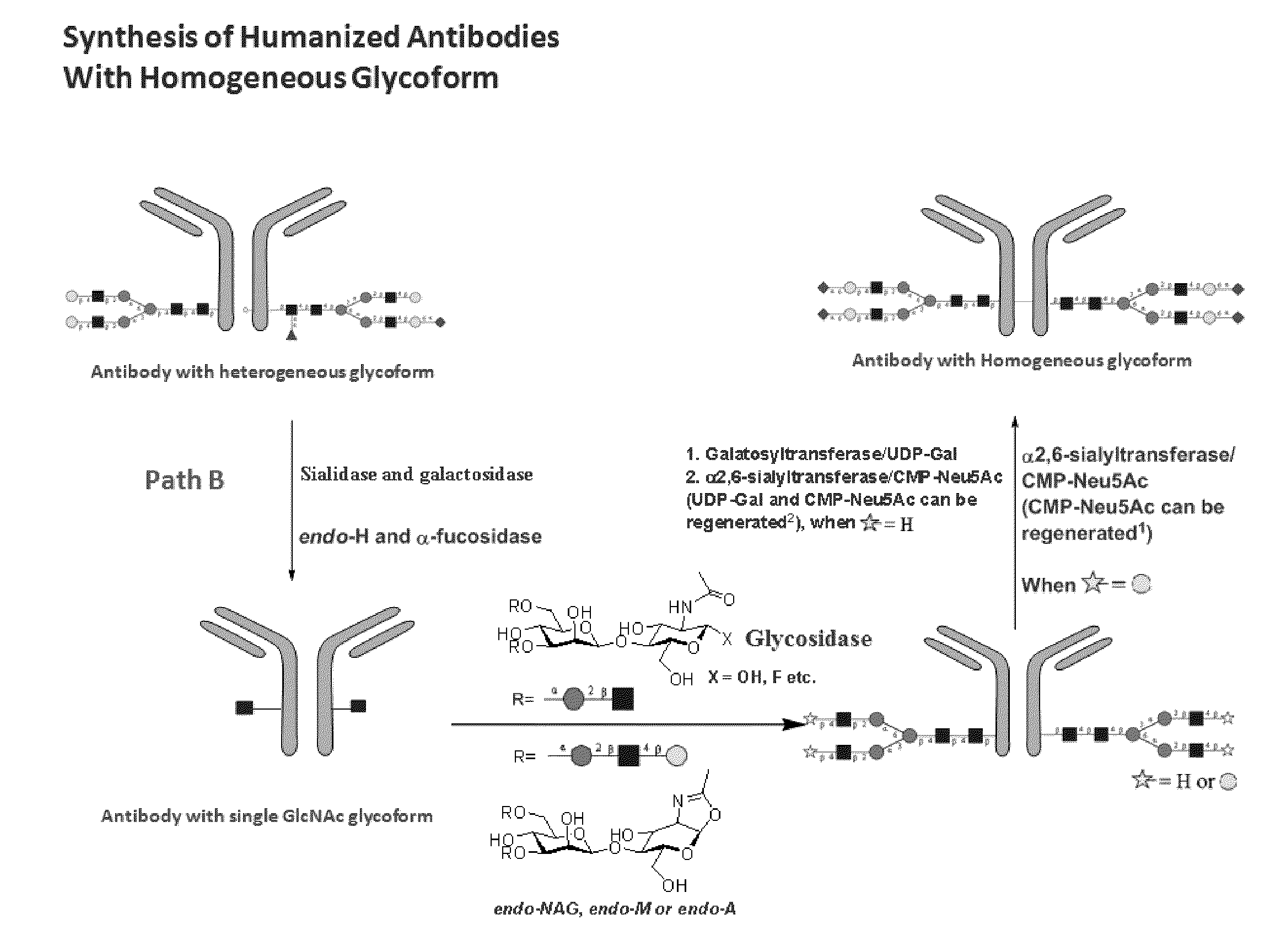
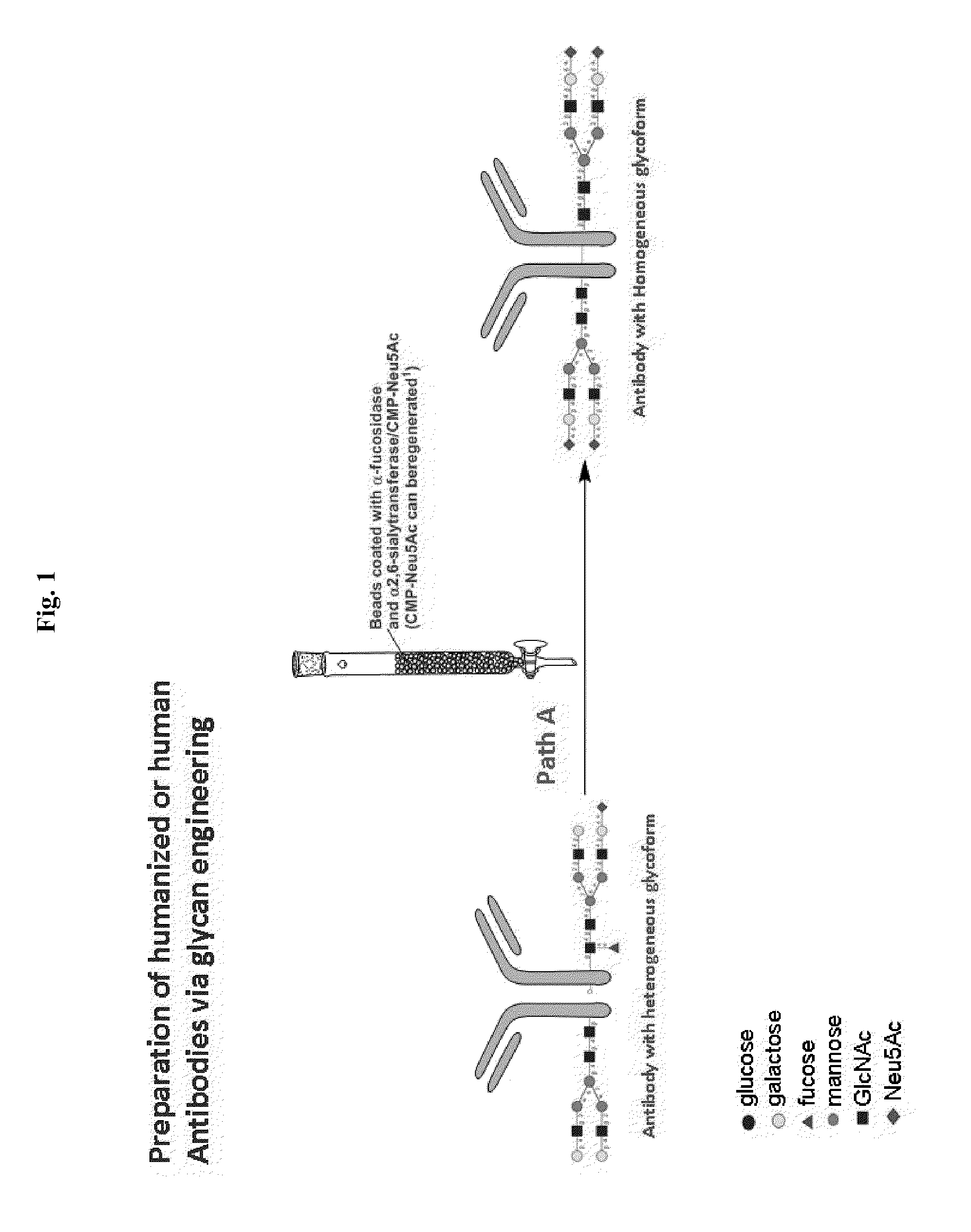
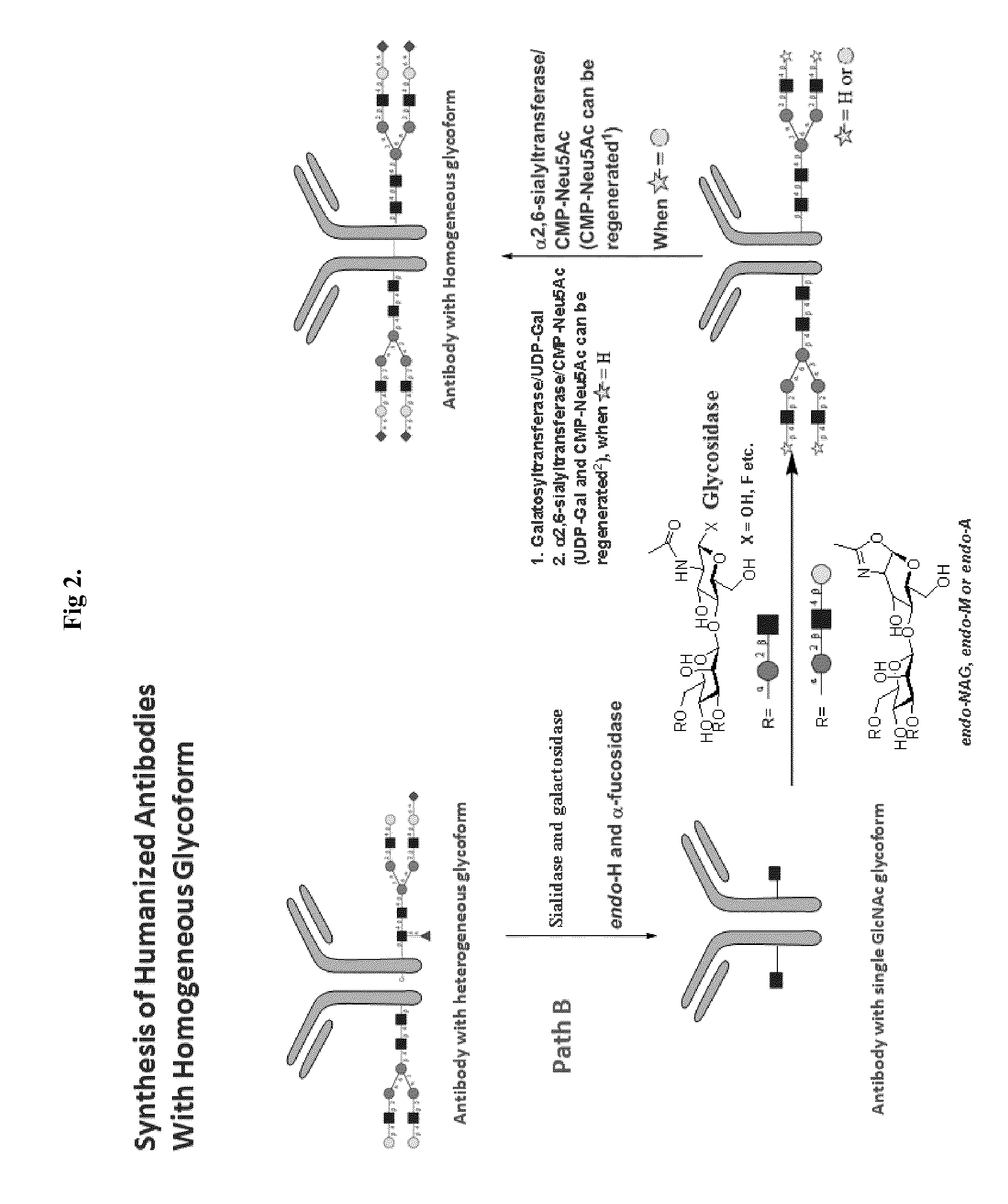
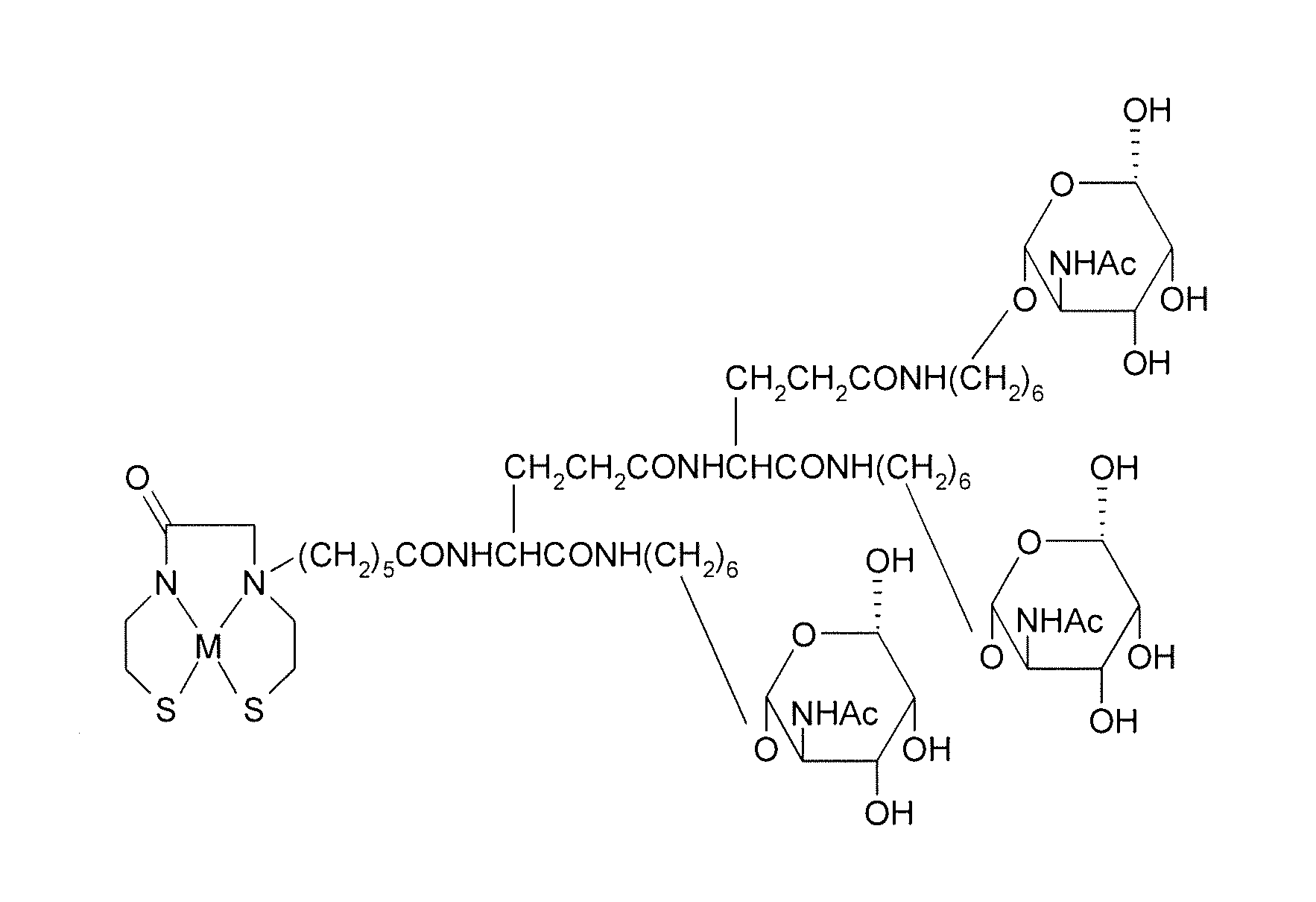
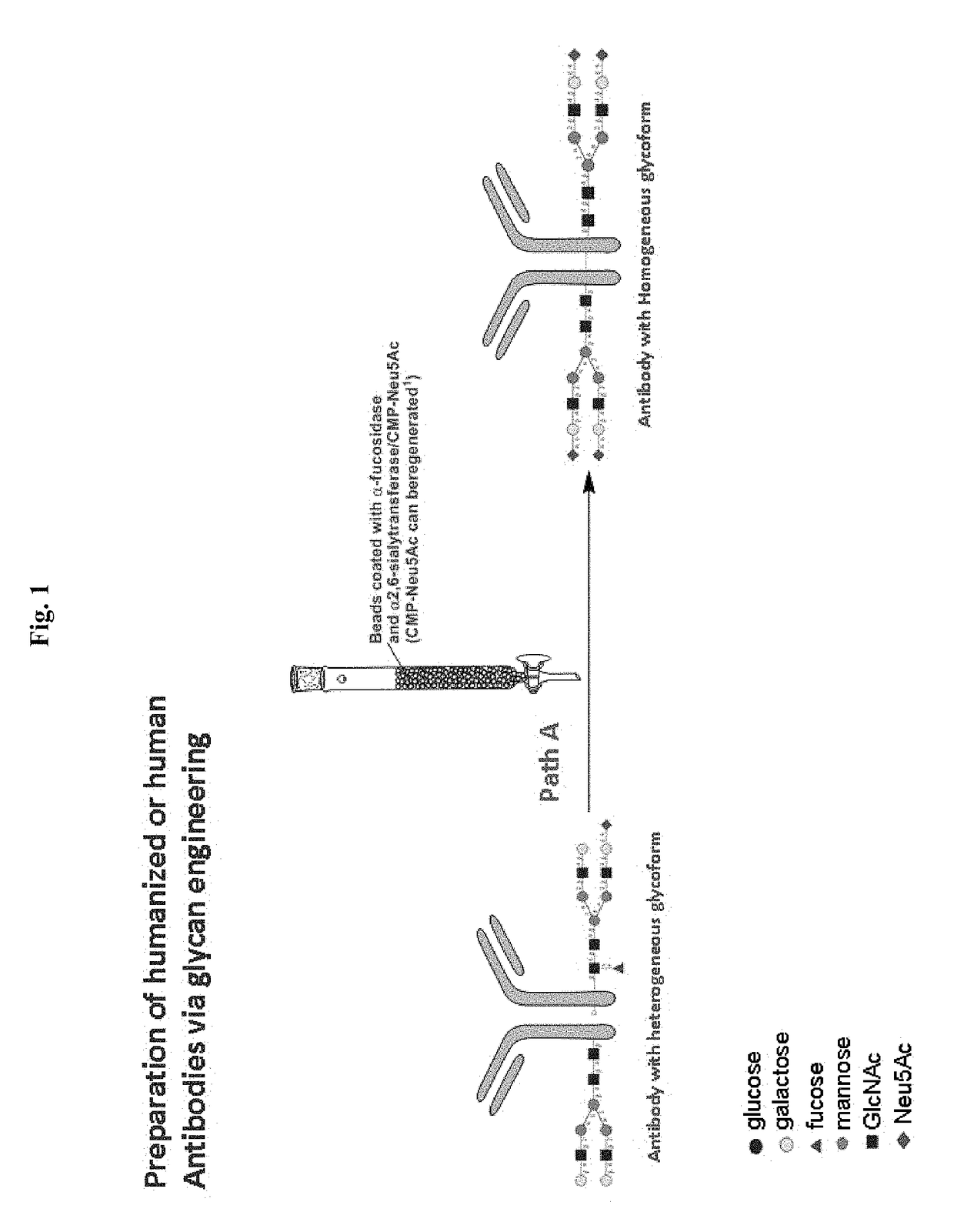
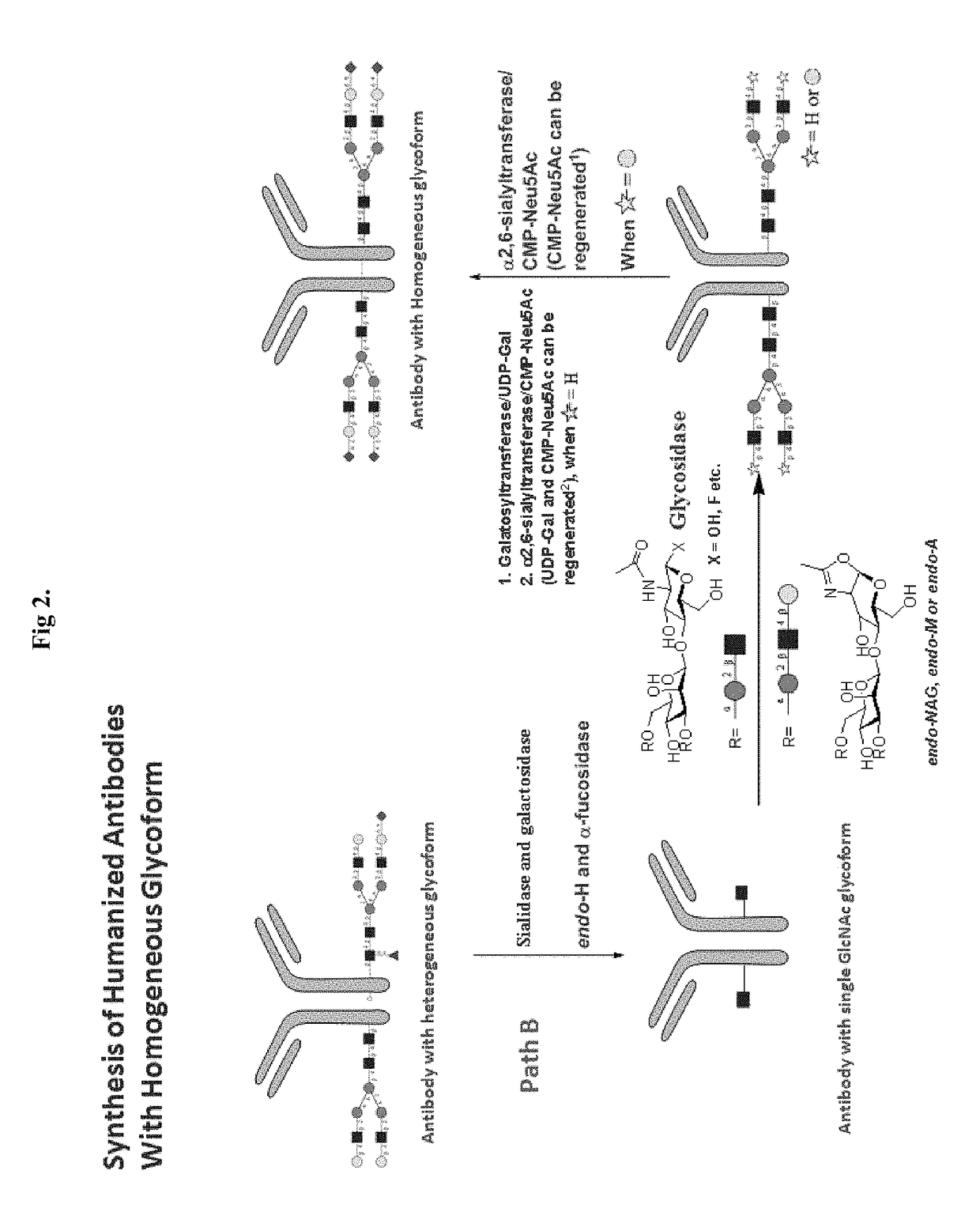
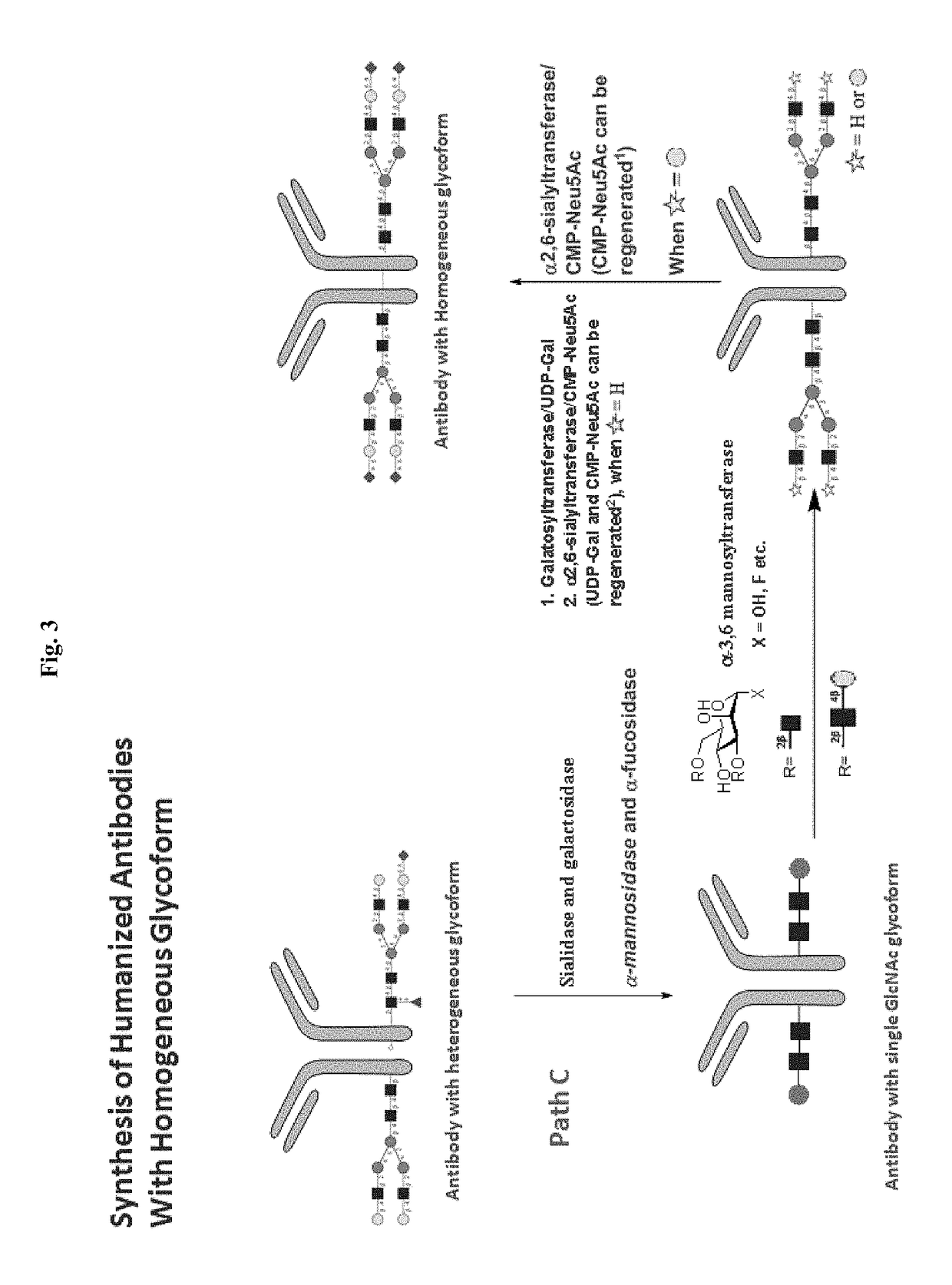
Methods for modifying human antibodies by glycan engineering
ActiveUS20110263828A1Improve efficacyImprove stabilityImmunoglobulinsFermentationGlycanAntibody fragments
Modified Fc regions of antibodies and antibody fragments, both human and humanized, and having enhanced stability and efficacy, are provided. Fc regions with core fucose residues removed, and attached to oligosaccharides comprising terminal sialyl residues, are provided. Antibodies comprising homogeneous glycosylation of Fc regions with specific oligosaccharides are provided. Fc regions conjugated with homogeneous glycoforms of monosaccharides and trisaccharides, are provided. Methods of preparing human antibodies with modified Fc using glycan engineering, are provided.
Owner:ACAD SINIC
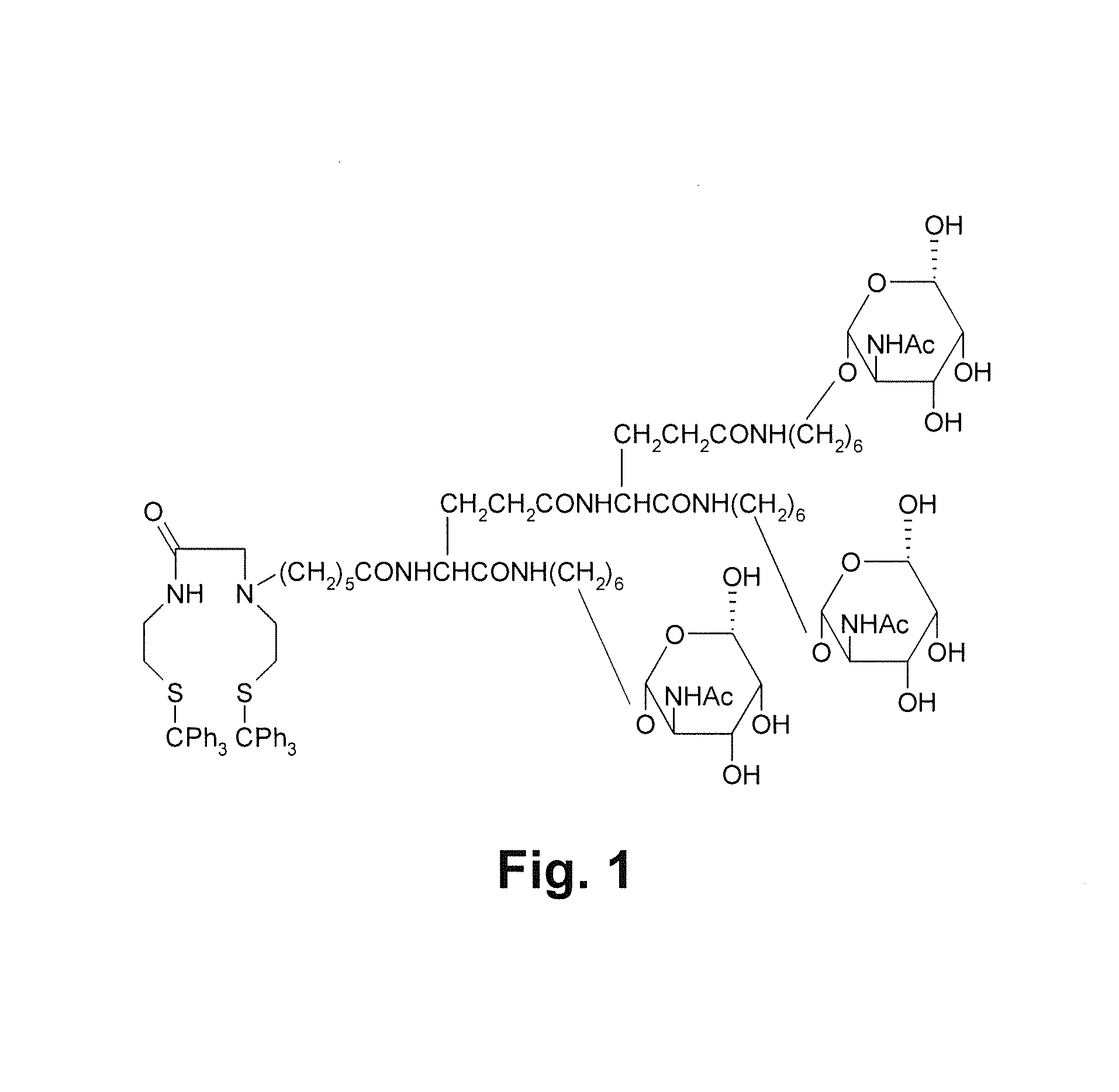
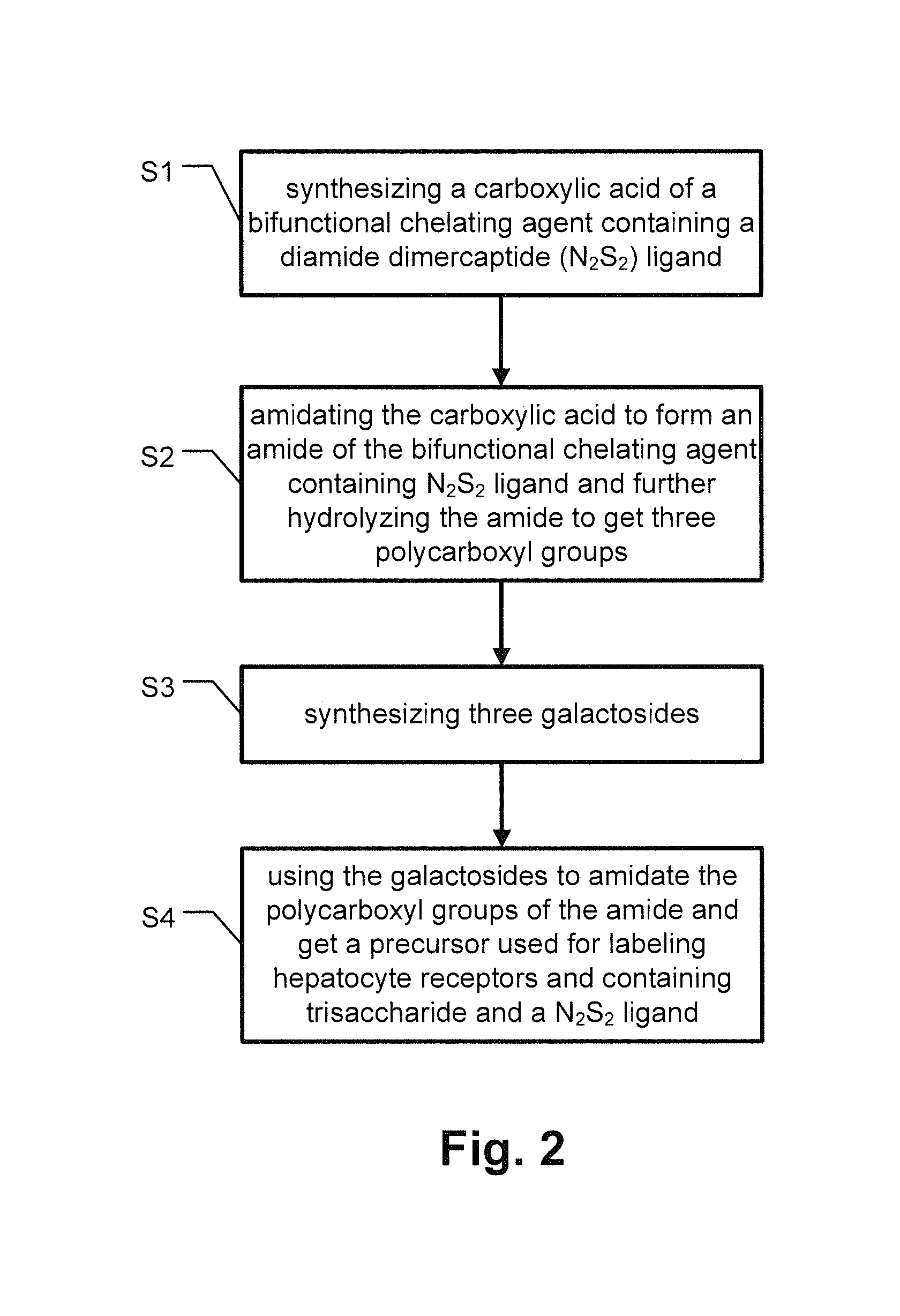
Precursor used for labeling hepatorcyte receptor and containing trisaccharide and diamide demercaptide ligand, method for preparing the same, radiotracer and pharmaceutical composition of the same
InactiveUS20140031533A1Easy to useFacilitated releaseSugar derivativesRadioactive preparation carriersRadioactive tracerThiol
A precursor used for labeling hepatocyte receptors and applied to radiotracers for imaging or pharmaceutical compositions for liver cancers is revealed. The precursor is a bifunctional compound. The bifunctional group includes a trisaccharide structure and a diamide dimercaptide (N2S2) ligand. The trisaccharide has high affinity to asialoglycoprotein receptors (ASGPR) on surfaces of hepatocytes while N2S2 ligand reacts with radioisotopes to form neutral complexes. Thus the precursor stays on surfaces of hepatocytes to provide radioisotope labeling or treatment effect of liver cancers.
Owner:INST NUCLEAR ENERGY RES ROCAEC
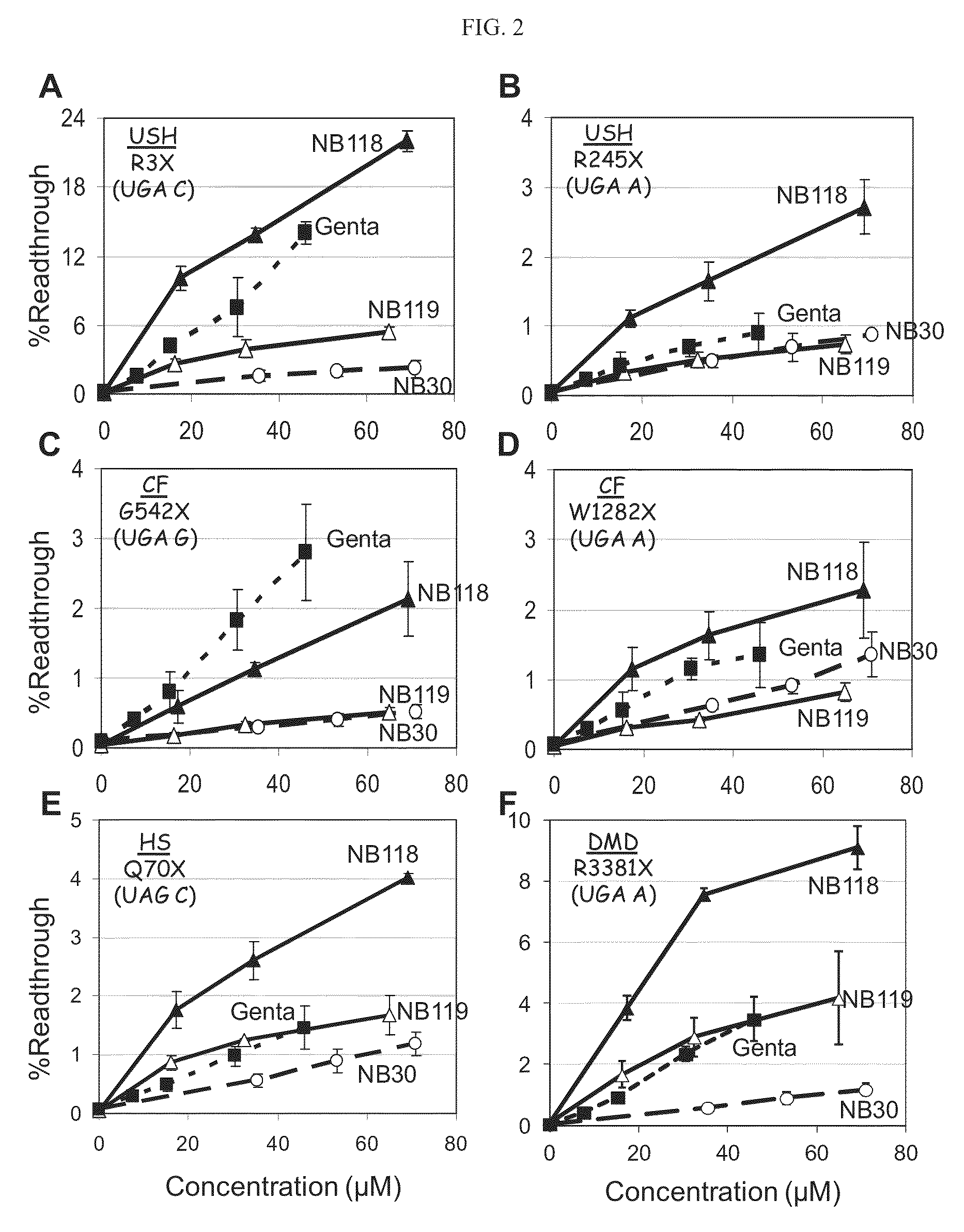
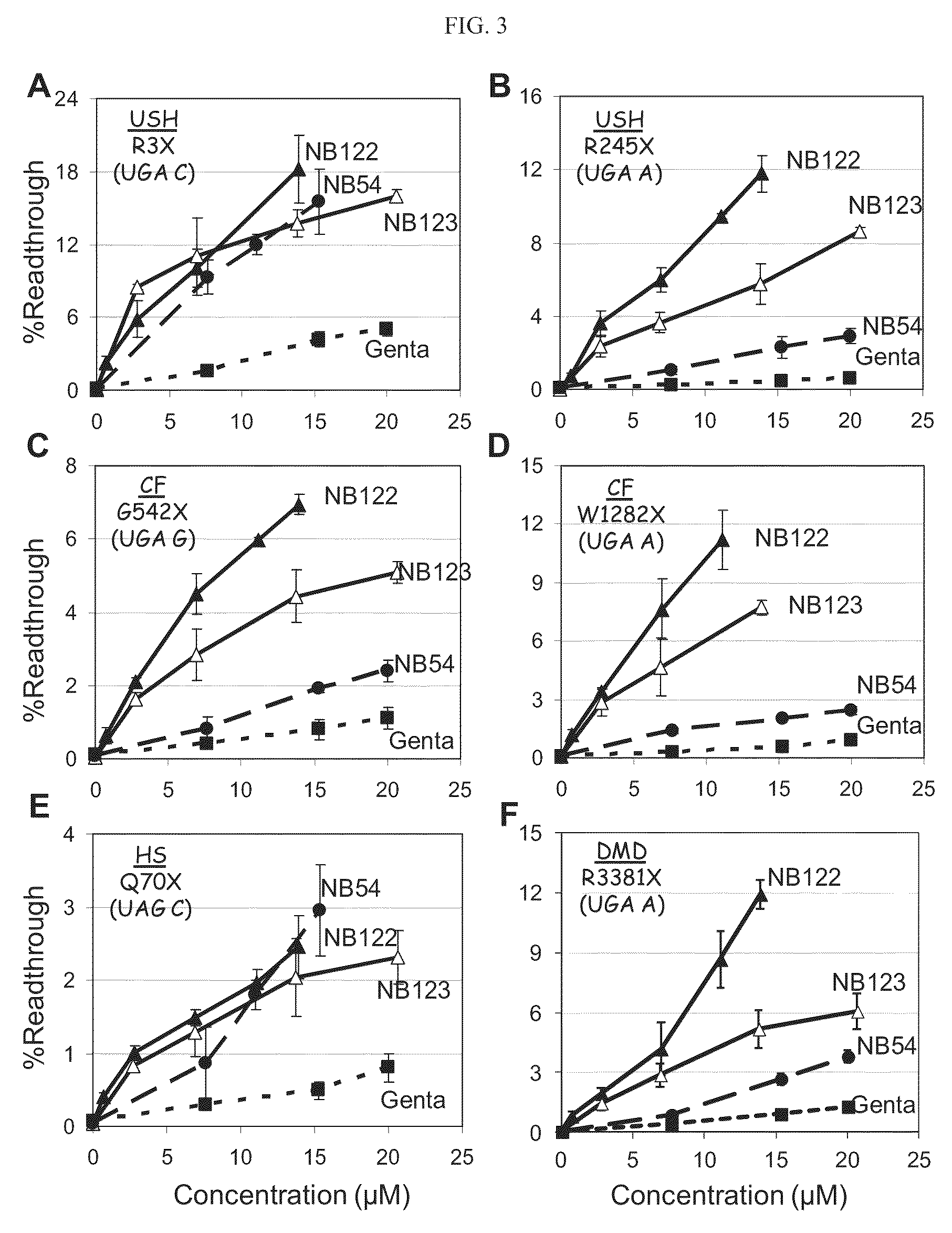
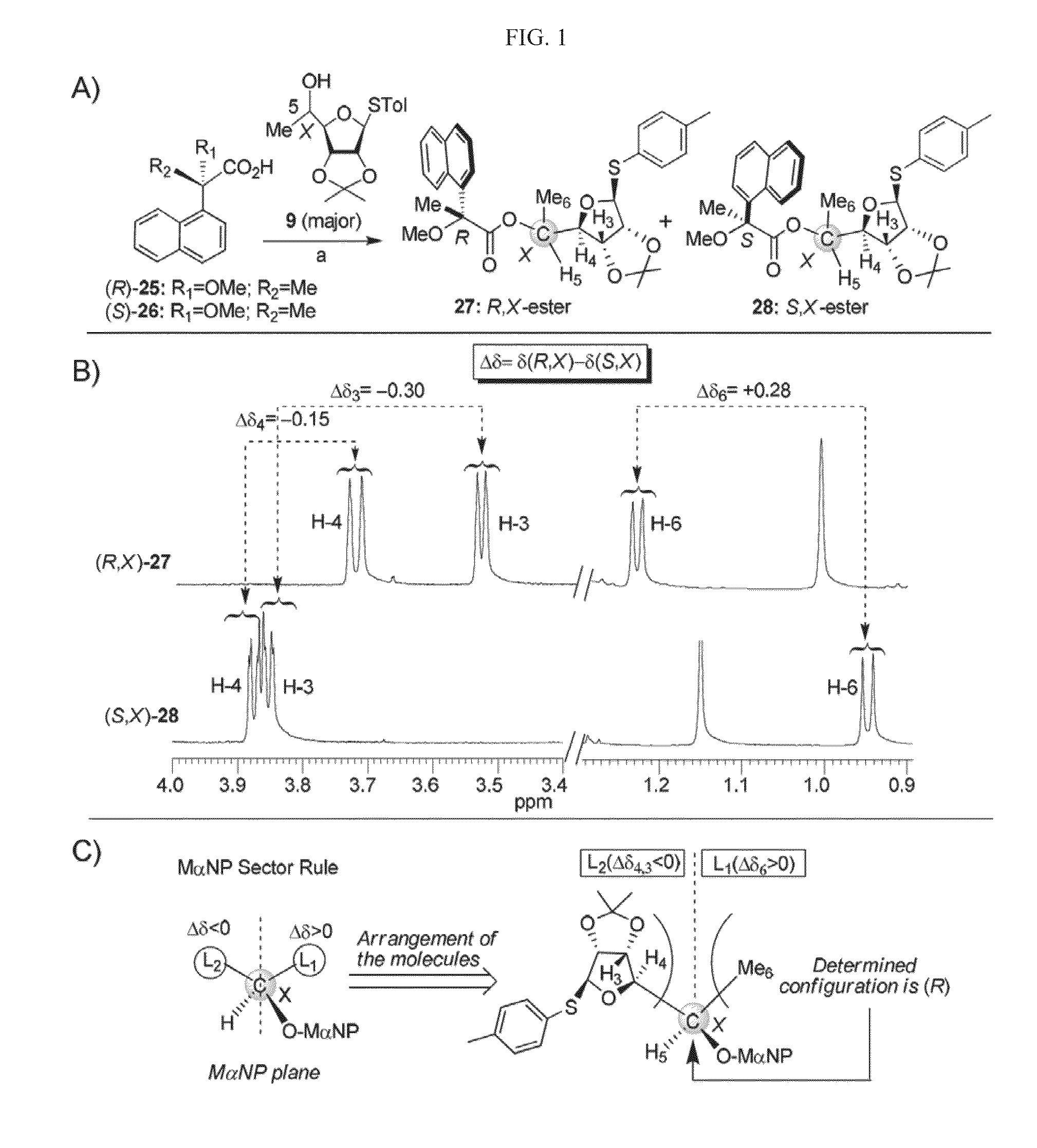
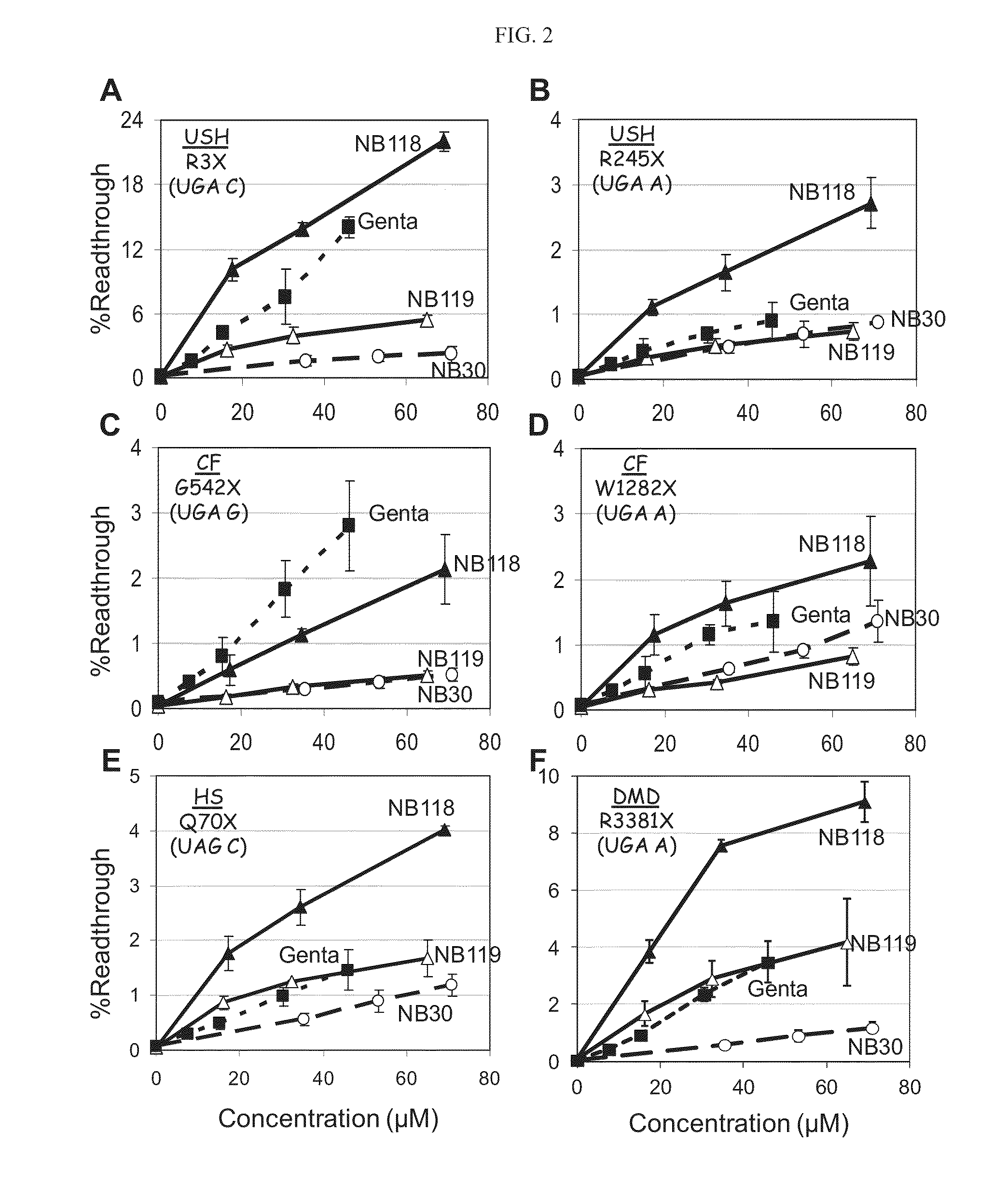
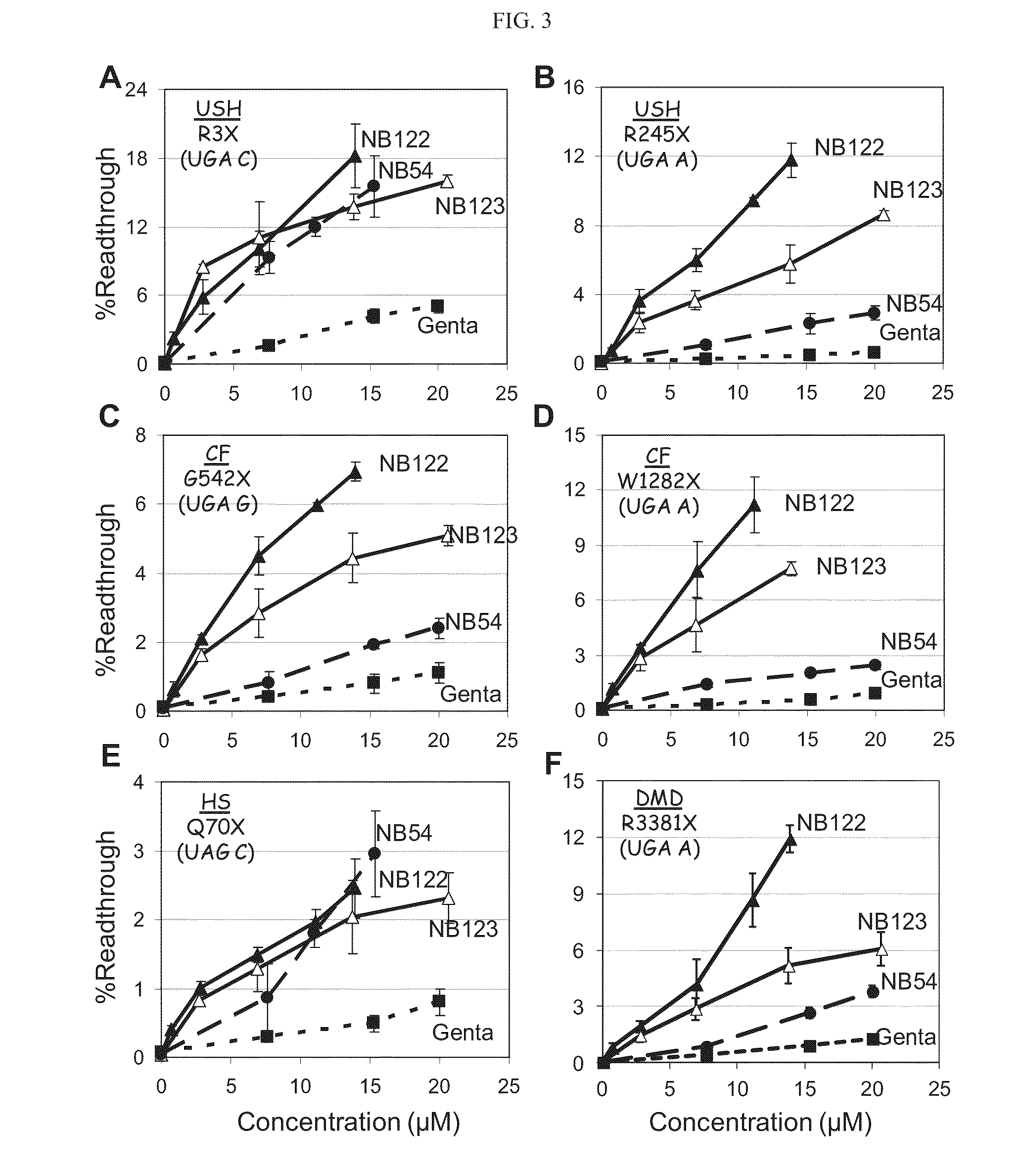
Aminoglycosides and uses thereof in treating genetic disorders
ActiveUS8895519B2High premature stop-codon mutations read-through activityLow toxicityBiocideMuscular disorderCytotoxicityHigh selectivity
A new class of pseudo-trisaccharide aminoglycosides having an alkyl group at the 5″ position, exhibiting efficient stop codon mutation readthrough activity, low cytotoxicity and high selectivity towards eukaryotic translation systems are provided. Also provided are pharmaceutical compositions containing the same, and uses thereof in the treatment of genetic disorders, as well as processes of preparing these aminoglycosides. The disclosed aminoglycosides can be represented by the general formula I:or a pharmaceutically acceptable salt thereof, wherein R1 is selected from the group consisting of alkyl, cycloalkyl and aryl; and all other variables and features are as described in the specification.
Owner:TECHNION RES & DEV FOUND LTD
Liquid Concentrated Formula
The invention pertains to a liquid complete nutritional composition suitable for feeding cachectic patients, having an energy density of at least 1.45 kcal / ml. The composition comprises a protein fraction in an amount of 7.8-12 g per 100 ml, a carbohydrate fraction, and a lipid fraction. At least 70 wt. % of the protein fraction can be obtained by demineralising milk, e.g. by ultrafiltration. The carbohydrate fraction (17-27 g per 100 ml) can comprise 0-35 wt. % of sucrose, 15-45 wt. % of other non-reducing mono-, di- and / or tri-saccharides, especially trehalose, 5-50 wt. % of other mono-and disaccharides and 5-40 wt. % of tri- and higher saccharides. The product is suitably packaged in small volumes (10-150 ml).
Owner:NUTRICIA
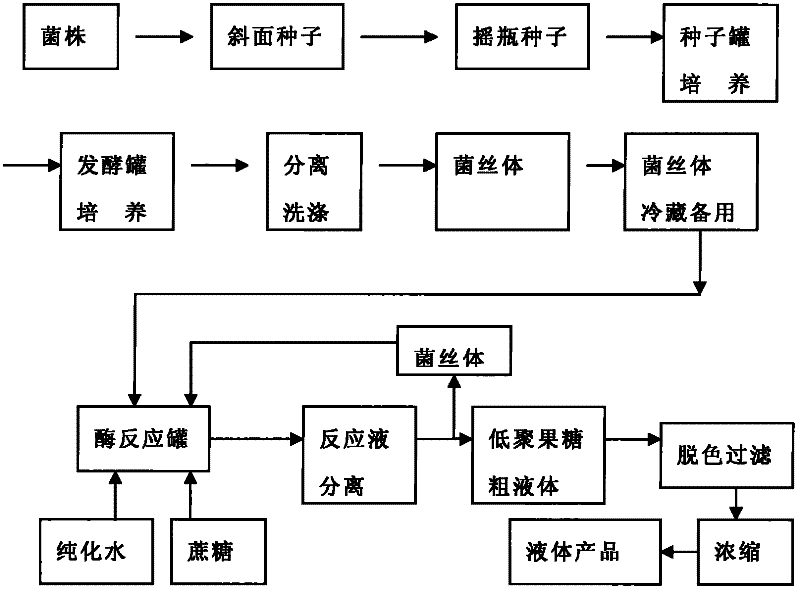
Method for producing fructooligosaccharide by using transfructosylase
InactiveCN102127574AImprove conversion rateHigh fructosyltransferase activityFermentationSucroseTrisaccharide
The invention relates to the technical field of food biotechnology, particularly a method for producing fructooligosaccharide by using transfructosylase, which comprises preparation of transfructosylase and industrial production of fructooligosaccharide by using the enzyme preparation. The method is characterized by comprising the following steps: selecting a good strain containing high-activity transfructosylase, culturing the strain in an appropriate culture medium, separating to obtain large-scale cultured mycelia containing high-activity transfructosylase, and storing the mycelia for later use; and converting sucrose into fructooligosaccharide by a batch process by using the mycelia containing high-activity transfructosylase as a biocatalyst. The method provided by the invention has the advantages of short bioconversion time, and high conversion rate of fructooligosaccharide; the mycelia can be repeatedly used; and the content of fructooligosaccharide (trisaccharide) prepared by using the transfructosylase is up to 57-62%, which is 3-5% higher than that of the fructooligosaccharide prepared by using Aspergillus niger.
Owner:山东文远生物技术有限公司
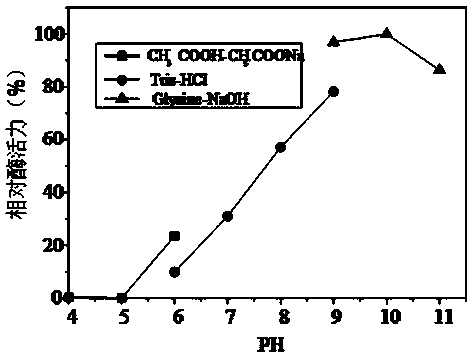
Novel alginate lyase, preparation method and application thereof
ActiveCN109295043AHigh activityStrong toleranceFermentationVector-based foreign material introductionPolymannuronic acidEscherichia coli
The invention discloses an alginate lyase (Alg509) derived from marine bacteria and a gene thereof, and also disclosed a method for recombinant expression and preparation of the alginate lyase. According to the method, the alg509 gene is cloned into an E. coli expression vector, and the vector is transformed into an E. coli host strain to obtain a recombinant engineering strain which can heterologously express the enzyme. The alginate lyase Alg509 disclosed in the invention has high enzyme activity, the specific enzyme activity can reach up to 48000 U / mg and above, the optimum reaction pH is 10, the optimum reaction temperature is 55 DEG C, and the enzyme activity has no dependence on various metal ions. The enzyme is active to sodium alginate, poly-guluronic acid (polyG) and ploymannuronic acid (polyM), and can completely degrade sodium alginate to produce alginate oligomers such as alginate disaccharide, alginate trisaccharide, alginate tetrasccharide, etc. The enzyme exhibits strongbasophilia, has certain tolerance to high pH, has certain potential of industrial applications, and can be widely applied in the fields of agriculture, food, feed additive, medicine and the like.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
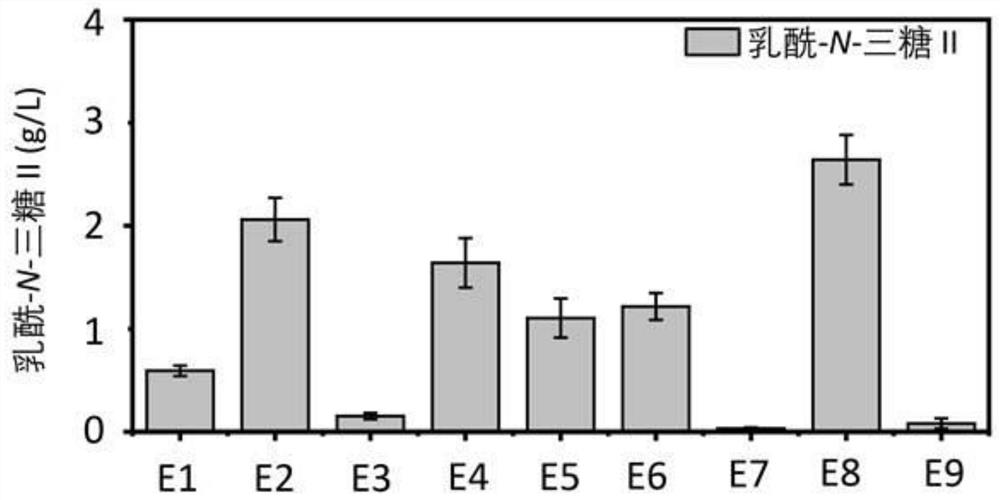
Genetically engineered bacteria capable of increasing yield of lactoyl-N-trisaccharide II and production method for genetically engineered bacteria
ActiveCN111979168AIncrease productionPrecise regulation of carbon fluxBacteriaMicroorganism based processesBiotechnologyEscherichia coli
The invention discloses genetically engineered bacteria capable of increasing the yield of lactoyl-N-trisaccharide II and a production method, and belongs to the field of microbial genetic engineering. According to the invention, the expression of glmM, glmU, glmS and lgtA in a lactoyl-N-trisaccharide II synthetic pathway is regulated and controlled in a combined manner, so the carbon flux of a metabolic pathway is accurately regulated and controlled, and the metabolic pressure is relieved; the expression of wecB, nagB and lacZ in an escherichia coli host, namely the lactoyl-N-trisaccharide IIsynthetic pathway is knocked out, so the yield of the lactoyl-N-trisaccharide II is further increased; in a flask shaking experiment, the capacity of escherichia coli for producing the lactoyl-N-trisaccharide II is increased to 4.82 g / L from 0.53 g / L; in a fermentation tank with a volume of 3 L, the yield of the lactoyl-N-trisaccharide II reaches 46.2 g / L; and thus, thegenetically engineered bacteria have industrial application prospect.
Owner:JIANGNAN UNIV
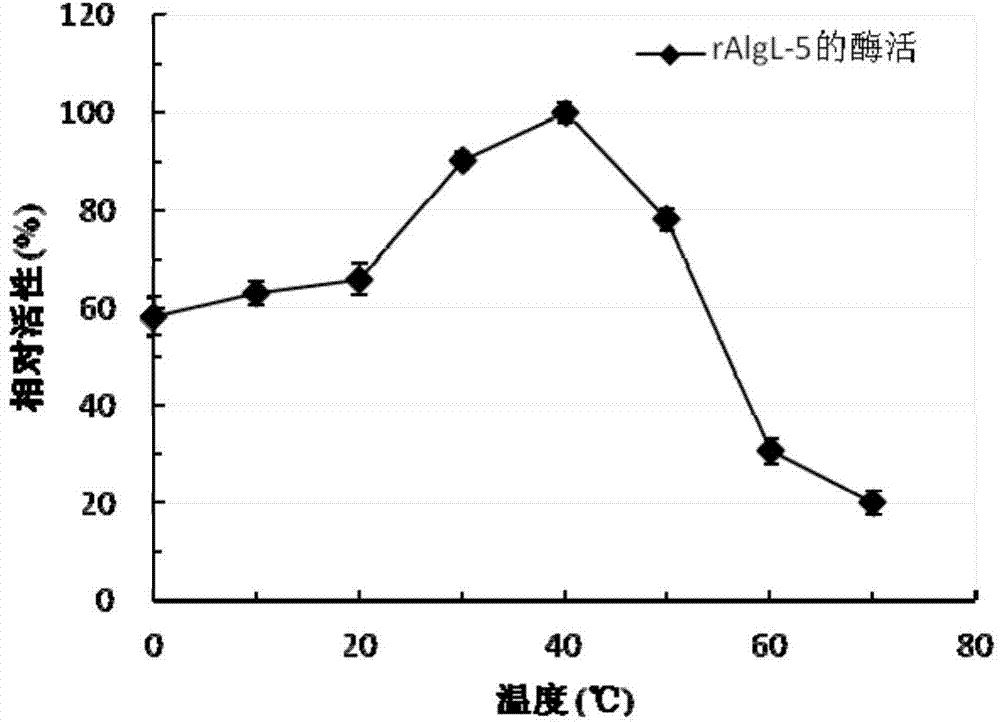
Incision-type sodium alginate lyase as well as encoding gene and application thereof
ActiveCN104293754AStable in naturePotential for industrial applicationsBacteriaFermentationTrisaccharideLyase
The invention relates to incision-type sodium alginate lyase as well as an encoding gene and application thereof. The amino acid sequence of the incision-type sodium alginate lyase AlgL-5 is as shown in SEQ ID NO.2. A gene for encoding the incision-type sodium alginate lyase AlgL-5 is as shown in SEQ ID NO.1. Main oligosaccharide products generated in a process of degrading sodium alginate by using the incision-type sodium alginate lyase AlgL-5 include unsaturated disaccharide, unsaturated trisaccharide, unsaturated tetrasccharide and unsaturated pentasaccharide. Pentasaccharide is the smallest unsaturated oligosaccharide substrate of lyase, and disaccharide is the smallest unsaturated oligosaccharide product of lyase. The recombinant enzyme is stable in property and has certain industrial application potential.
Owner:SHANDONG UNIV
Room temperature stable aqueous liquid pharmaceutical composition
InactiveUS20060013834A1Pleasant tasteUseful in masking bitternessDispersion deliveryPhosphorous compound active ingredientsSucrosePolyol
A liquid pharmaceutical composition is contemplated that comprises a pharmaceutically effective amount of a drug dissolved or dispersed in an aqueous medium. The aqueous medium consists essentially of water, about 3% to about 10% w / v polyvinylpyrrolidone, about 60% to about 75% w / v of C3-C6 polyol that includes more than 55% w / v of a non-reducing disaccharide, trisaccharide or tetrasaccharide such as sucrose, optionally about 0.01% to about 0.5% w / v of a glycyrrhetic acid, glycyrrhizinate derivative or salt thereof, and one or more flavorants, and preferably includes one or more preservatives. The liquid composition is room temperature stable, and may have a pleasant taste.
Owner:SHIONOGI PHARMA
Emulsified composition
ActiveUS20120108661A1High steric regularityImprove water retentionCosmetic preparationsBiocideAlcohol sugarsMethyl group
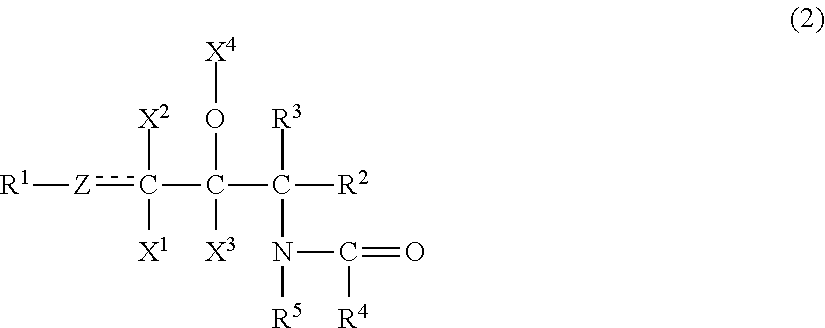
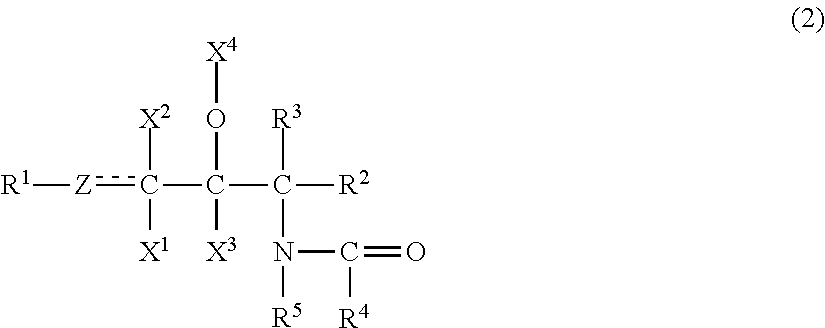
The emulsion composition of the present invention contains(A) 0.001 to 10 wt. % of an organic compound having two or more hydroxyl groups, an inorganic value of 220 to 450, and an organic value of 300 to 1,000;(B) 0.001 to 10 wt. % of an organic compound having one hydroxyl group, an inorganic value of 100 to 200, and an organic value of 280 to 700;(C) 0.001 to 10 wt. % of a compound represented by formula (2):wherein R1 is a C4 to C30 hydrocarbon group; Z is a methylene group, a methine group, or an oxygen atom; X1, X2, X3 is a hydrogen atom, a hydroxyl group, or an acetoxy group; X4 is a hydrogen atom, an acetyl group, or a glyceryl group; each of R2 and R3 a hydrogen atom, a hydroxyl group, a hydroxymethyl group, or an acetoxymethyl group; R4 is a C5 to C60 hydrocarbon group; and R5 is a hydrogen atom or a hydrocarbon group containing 1 to 30 carbon atoms in total;(D) 0.00012 to 10 wt. % of at least one compound selected from the group consisting of a nonionic surfactant having a polyoxyethylene group and an HLB of 10 or higher, an ionic surfactant, and a sphingosine salt;(E) 0.003 to 15 wt. % of at least one compound selected from the group consisting of a sugar alcohol selected from the group consisting of erythritol, threitol, xylitol, and mannitol, a disaccharide, and a trisaccharide; and(F) water.
Owner:KAO CORP
Preparation method of high-purity 95 isomaltose hypgather
ActiveCN103667392AHigh purityLow viscosityFermentationChromatographic separationIsomaltooligosaccharide
The invention provides a preparation method of high-purity 95 isomaltose hypgather. The preparation method comprises the following steps: performing jet liquefying on starch or starch milk used as a raw material; performing saccharification transglycosidation through maltase (fungus alpha-amylase or beta-amylase) and alpha-galctosyl-hydroxylysyl glucosyl transferase to obtain 50 type coarse isomaltose hypgather liquor; decomposing non-trisaccharide (isomaltose, panose and isomaltotriose hereinafter referred to as 'trisaccharide') ingredients by use of efficient liquid saccharifying enzyme to obtain glucose; separating by use of a chromatographic separation purification technology to remove glucose; decolorizing, desalting and refining to obtain 95 isomaltose hypgather (the 'trisaccharide' content is not less than 95%). The high-purity 95 isomaltose hypgather has the advantages that the effective ingredients 'trisaccharide' are high in purity reaching more than 95%, low in syrup viscosity and high in sweetness; the physiology functions are high; the bifidobacterium cultivation effect is three times higher than that of the common 90 type isomaltose hypgather.
Owner:SHANDONG BAILONG CHUANGYUAN BIO TECH
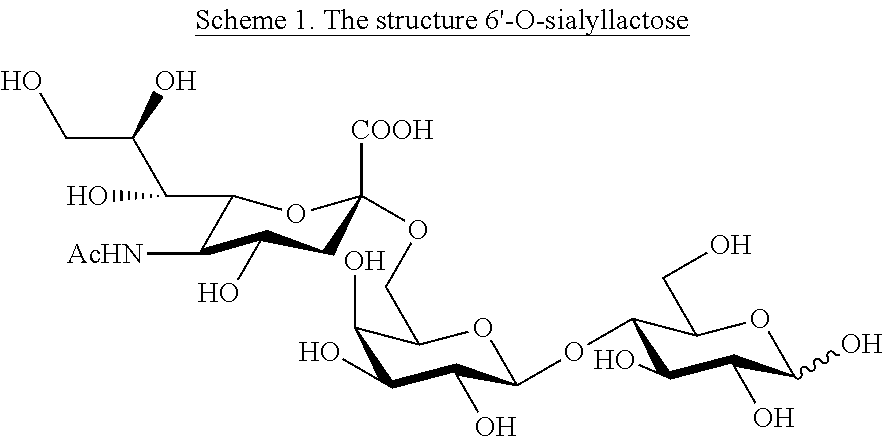
Production of 6'-o-sialyllactose and intermediates
InactiveUS20130035481A1Antibacterial agentsOrganic active ingredientsTrisaccharideMedicinal chemistry
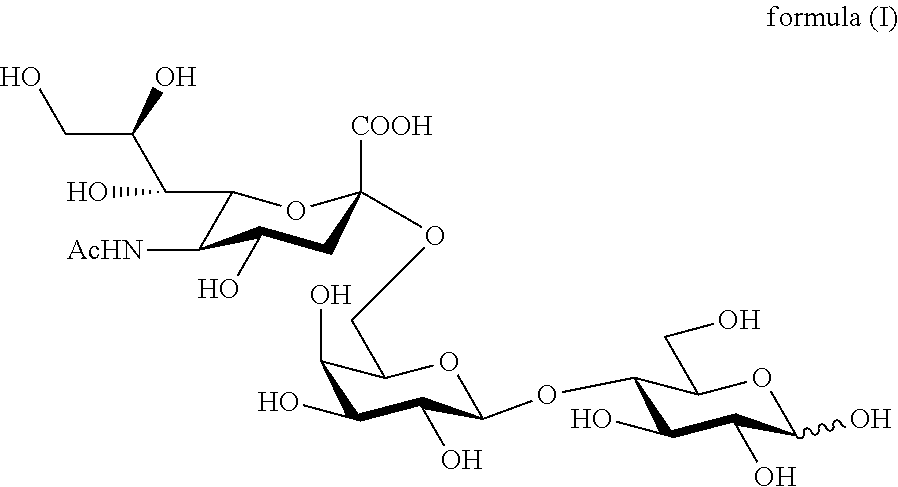
The present invention relates to a method for preparation of the trisaccharide 6′-0-sialyllactose (formula (I)) or salts thereof as well as intermediates in the synthesis and for the use of 6′-0-sialyllactose salts in pharmaceutical or nutritional compositions.
Owner:GLYCOM AS
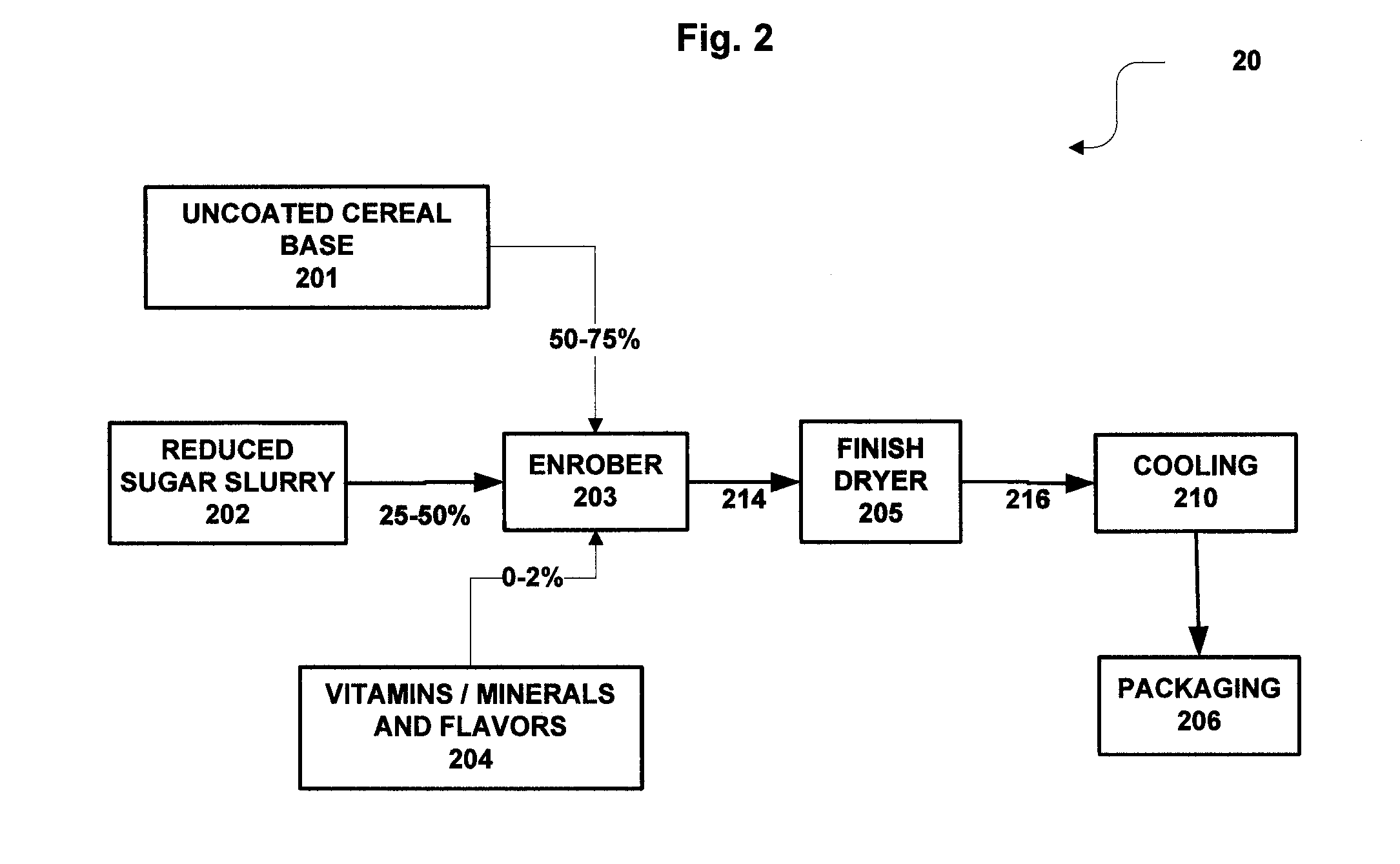
Reduced sugar pre-sweetened breakfast cereals comprising tri- and tetra saccharides and methods of preparation
InactiveUS20100173051A1Improve performanceReduced sugar coatingSugar food ingredientsConfectioneryReady to eatTrisaccharide
A reduced sugar presweetened ready to eat breakfast cereal is prepared by coating dried cereal base pieces or food pieces with a reduced-sugar composition comprising maltotriose, maltotetrose in full or partial substitution for sucrose, and a high potency sweetener. The reduced-sugar coating can have a sucrose content of less than 70%, yet provides taste, texture, appearance, and bowl life that mimics presweetened R-T-E cereals having a coating with more sucrose.
Owner:GENERAL MILLS INC
Pleasant-tasting aqueous liquid composition of prednisolone sodium phosphate
A liquid pharmaceutical composition is contemplated that comprises a pharmaceutically effective amount of prednisolone sodium phosphate (PSP) dissolved or dispersed in an aqueous medium that is free of ethanol. The aqueous medium consists essentially of water, about 3 to about 10 weight percent polyvinylpyrrolidone, about 60 to about 75 weight percent of a C3-C6 polyol that includes more than 55 weight percent non-reducing disaccharide or trisaccharide such as sucrose, about 0.01 to about 0.5 weight percent ammonium glycyrrhizinate and one or more flavorants, and preferably includes one or more preservatives. The liquid composition is transparent and has a pleasant taste.
Owner:ASCENT PEDIATRICS
Method for producing high-functional-trisaccharide-content isomaltooligosaccharide by using immobilized cells
ActiveCN103484512AThe effect of low enzyme activityStable and efficient enzyme production environmentMicroorganism based processesOn/in organic carrierChromatographic separationIsomaltooligosaccharide
The invention relates to a method for producing high-functional-trisaccharide-content isomaltooligosaccharide by using immobilized cells. The method comprises the following steps: liquefying and saccharifying the raw material starch to obtain malt syrup; and preparing the high-functional-trisaccharide-content isomaltooligosaccharide by combining an immobilized Aspergillus niger cell technique and a simulated moving bed chromatographic separation technique. After the saccharified liquid is subjected to glycoside transformation by the immobilized cells, the total amount of the three functional components (isomaltose IG2, panose P and isomaltotriose IG3) is up to higher than 60%, the transformation ratio of the immobilized cell substrate is higher than that of the immobilized enzyme, and the indexes of the product are much higher than those of like products at home and abroad; after the chromatographic separation, the total amount of the three functional components can reach higher than 90%; and meanwhile, the method greatly shortens the reaction time, and is convenient to operate and suitable for large-scale continuous production.
Owner:BAOLINGBAO BIOLOGY
Preparation method of reducible resistant dextrin
ActiveCN109182417ADelay and inhibit digestion and absorptionIncreased sensitivityFermentationBiotechnologyAmylase
The invention relates to a preparation method of reducible resistant dextrin, which comprises the following steps: (1) adding water into starch to regulate starch milk; (2) regulating pH of starch milk to add high-temperature resistant alpha; amylase, reacting to obtain liquefied liquid; (3) adjusting pH of liquefied liquid, adding fungus alpha-Amylase and pullulanase, concentrating to obtain a mixed solution after reaction; 4) adding phosphoric acid into that mixed solution, reacting under the conditions of high temperature and negative pressure to obtain a resistant dextrin crude solution; 5) adjusting the pH of the crude solution of the resistant dextrin and adding a catalyst Raney nickel to obtain a reaction solution aft the reaction; and 6, decoloring the reaction solution, filtering,ion exchange, concentrating, and drying to obtain reducible resistant dextrin. The reduced resistant dextrin prepared by the invention hardly contains small molecular sugar such as glucose, maltose and trisaccharide, has lower water activity value, better moisture retention and bacteriostatic performance, and is favorable for improving the shelf life of food and prolonging the shelf life of food.
Owner:SHANDONG BAILONG CHUANGYUAN BIO TECH
Methods for modifying human antibodies by glycan engineering
Modified Fc regions of antibodies and antibody fragments, both human and humanized, and having enhanced stability and efficacy, are provided. Fc regions with core fucose residues removed, and attached to oligosaccharides comprising terminal sialyl residues, are provided. Antibodies comprising homogeneous glycosylation of Fc regions with specific oligosaccharides are provided. Fc regions conjugated with homogeneous glycoforms of monosaccharides and trisaccharides, are provided. Methods of preparing human antibodies with modified Fc using glycan engineering, are provided.
Owner:ACAD SINIC
Preparation of monosaccharides, disaccharides, trisaccharides, and pentasaccharides of heparinoids
The present invention provides preparations of monosaccharides, disaccharides, trisaccharides, and pentasaccharides of heparinoids. The present invention also provides novel monosaccharides, disaccharides, trisaccharides and pentasaccharides for use in the preparation of heparinoids.
Owner:FORMOSA LAB
Method for producing low-sugar low-fat lotus paste through enzymolysis processing
The invention relates to a method for producing low-sugar low-fat lotus paste through enzymolysis processing. The method comprises the following specific steps of: raw material soaking, pectinase addition for peeling, thorough cooking, pulp grinding, enzyme addition for liquidation, burdening, grinding by a colloid grinder, frying, stuffing, packaging and sterilization, and finished product obtaining. The method is characterized in that the peeling by using pectinase is used for replacing conventional subtraction peeling, and after cooked lotus seeds are ground into pulp, one half of pulp is liquefied by using middle-temperature starch, so that the starch in lotus seeds is liquefied into oligosaccharide such as dextrin, trisaccharide and disaccharide; and the other half of ground lotus seeds, sugar, vegetable oil and other ingredients are added into the liquefied lotus seed pulp, and the low-sugar low-fat lotus paste is obtained by using the colloid grinder for grinding the mixture and frying stuffing. Compared with conventional produced lotus paste, the low-sugar low-fat lotus paste produced by the method has the advantages that the addition amounts of white sugar and oil are reduced by about 50%.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Chitosanase and application thereof
ActiveCN111235131AEfficient degradationGood biocatalytic activityFermentationGlycosylasesNucleotideGlucoside
The invention discloses chitosanase having an amino acid sequence as shown in SEQ ID NO.1. A gene encoding the chitosanase has a nucleotide sequence as shown in SEQ ID NO.2. The chitosanase is derivedfrom the GH5 family of glucoside hydrolase, can degrade chitosan to generate GlcN-(GlcN)4 and can be used for preparing an enzymatic COS mixture of a new component. The chitosanase has excellent biocatalytic activity, can hydrolyze chitosan to generate glucosamine, chitobiose, chitotriose and chitotetraose hydrochloride, and is relatively high in product purity; and when the pH is 10 and temperature is 50 DEG C, the chitosanase has relatively enzymatic activity of 898.6U / mg which is higher than those of most other known chitosanase, can effectively degrade chitosan, has mild reaction condition, easy control, high efficiency, environmental friendliness. The chitosanase has an industrial application value for producing novel chitosanase mixture.
Owner:OCEAN UNIV OF CHINA
Method for preparing kestose and nystose through yacon
InactiveCN102408457ASimple extraction methodSugar derivativesOligosaccharidesSucroseColumn temperature
The invention discloses a method for preparing kestose and nystose through yacon. Method of water extraction by alcohol sedimentation is adopted and supernate thereof is taken, concentrated, frozen and dried to obtain faint yellow yacon oligosaccharide. The yacon oligosaccharide is analyzed through high pressure liquid chromatography with a chromatography condition as follows: a chromatographic column is APS-2HYPERSIL, a column temperature is 30 DEG C, a moving phase is acetonitrile-water (75 to 25, V / V), a flow velocity is 0.8ml / min, a sample size is 20 Mul and a differential detector is arranged. The yacon oligosaccharide comprises fructose, glucose, saccharose, kestose, nystose and 1F-Fructofuranosyl nystose with contents respectively as 38,30 percent, 16.44 percent, 14.58 percent, 12.29 percent, 12.17 percent and 6.2 percent. Silicagel column chromatography is implemented on a prepared oligosaccharide sample; glacial acetic acid, chloroform, absolute ethyl alcohol and water with a proportion of 3 to 11 to 11 to 1 are used as an eluant for elution; the eluant is collected in sequence and then is concentrated, frozen and dried to obtain the kestose and nystose. The purity of the kestose and nystose prepared through high pressure liquid chromatography can be more than 90 percent.
Owner:TIANJIN UNIV OF SCI & TECH
Aminoglycosides and uses thereof in treating genetic disorders
ActiveUS20130237489A1Beneficially usedLow toxicityBiocideMuscular disorderCytotoxicityHigh selectivity
A new class of pseudo-trisaccharide aminoglycosides having an alkyl group at the 5″ position, exhibiting efficient stop codon mutation readthrough activity, low cytotoxicity and high selectivity towards eukaryotic translation systems are provided. Also provided are pharmaceutical compositions containing the same, and uses thereof in the treatment of genetic disorders, as well as processes of preparing these aminoglycosides. The disclosed aminoglycosides can be represented by the general formula I:or a pharmaceutically acceptable salt thereof, wherein R1 is selected from the group consisting of alkyl, cycloalkyl and aryl; and all other variables and features are as described in the specification.
Owner:TECHNION RES & DEV FOUND LTD
Method for controlling molecular weight distribution of starch sugar
InactiveCN1557839ALower glucose levelsIncreased content of three to five sugarsFermentationFood preparationTrisaccharideSlurry
The present invention discloses one method of controlling the ultralow DE value during reaction to control the molecular weight distribution of starch. The method consists of the following steps: mixing water and starch to form starch slurry and adding CaCl2 in the amount of 0.01-0.03 wt% of dry starch via stirring; regulating pH value of starch slurry; adding to the starch slurry heat resistant alpha-amylase in the amount of 0.03-0.08 wt% of dry starch via stirring; and reaction under the controlled conditions. The present invention is mainly improvement of available trisaccharide and pentasaccharide producing process via adopting available production process and increasing some apparatus.
Owner:GUANGZHOU FOBIBER BIOLOGICAL IND CO LTD
Incision difunctional alginate lyase Aly2 generating various monosaccharide products, encoding gene of Aly2 and application of Aly2
ActiveCN108048435AStable physical and chemical propertiesHigh degradation activityGenetic engineeringFermentationPolymannuronic acidNucleotide
The invention relates to incision difunctional alginate lyase Aly2 generating various monosaccharide products, an encoding gene of the Aly2 and an application of the Aly2. The amino acid sequence of the Aly2 is as shown in SEQ ID NO. 2, and the nucleotide sequence of the encoded Aly2 is as shown in SEQ ID NO. 1. The specific activity of prepared gene engineering recombinant protein rAly2 for alginate, polyguluronic acid and polymannuronic acid is 2025U / mg, 3672U / mg and 1324U / mg, so that the gene engineering recombinant protein rAly2 is a difunctional enzyme. The enzyme is an incision enzyme, and final main products are unsaturated disaccharide and unsaturated trisaccharide when an alginate polysaccharide substrate is thoroughly degraded. When different oligosaccharide substrates are degraded, various monosaccharide products such as saturated M and G or unsaturated delta can be generated. Besides, the rAly2 is stable in biochemical property, is a potential tool enzyme, can be applied tothe field of medicines, food and the like and has a wide application prospect.
Owner:SHANDONG UNIV
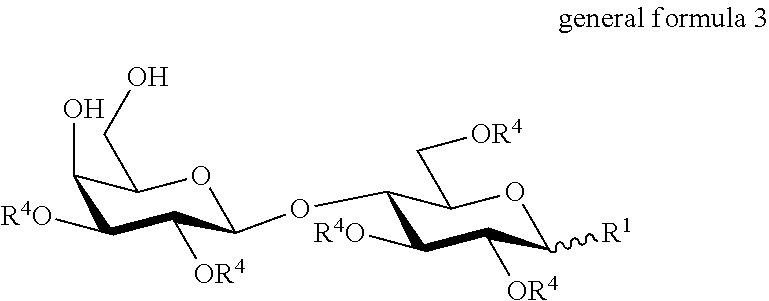
Derivatised carbohydrates and their use in solid delivery systems
InactiveUS7220731B2Easy to prepareHigh glass transition temperatureEsterified saccharide compoundsPowder deliveryTrisaccharideEster bond
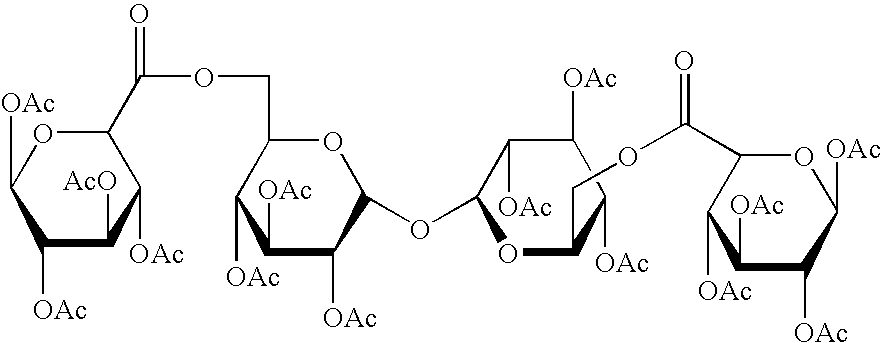
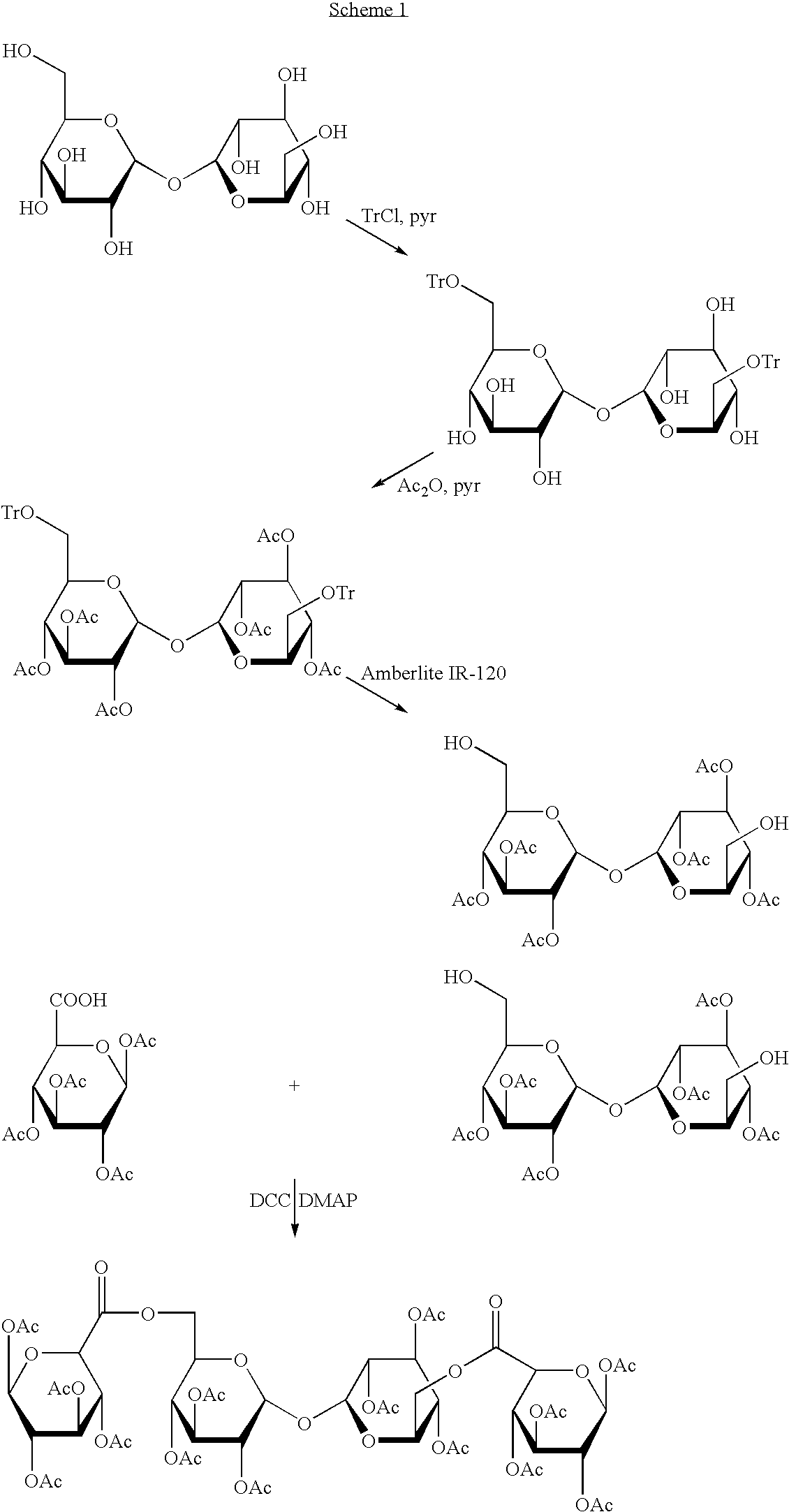
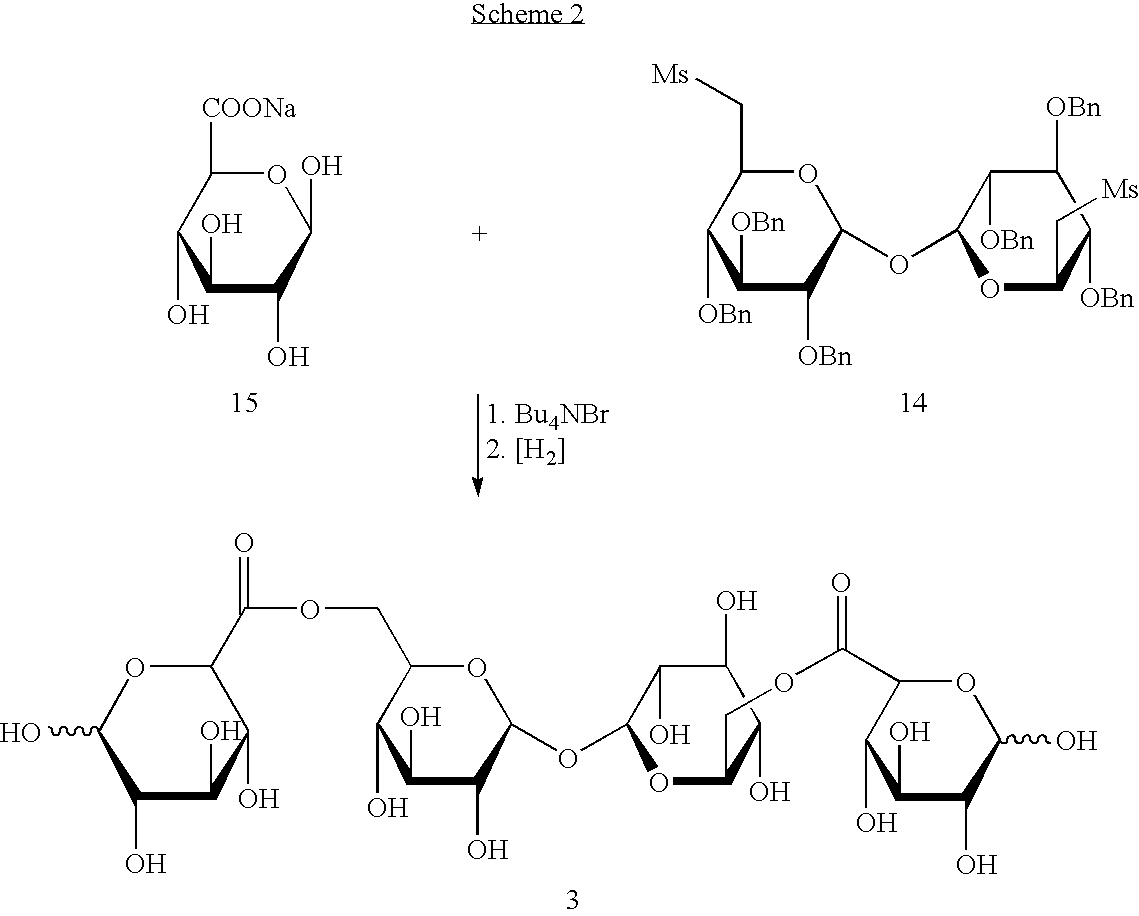
In a composition comprising a therapeutic agent and a compound which is a trisaccharide or higher polysaccharide, that compound has the formula X[—Y-Z]n wherein X and Z are each saccharide molecules in which none, some or all OH groups are derivatised; Y is an ester linkage to an exocyclic C atom in X; and n in an integer.
Owner:QUADRANT DRUG DELIVERY
Alginate lyase encoding gene
ActiveCN110257410AHigh degradation activityStable in natureBacteriaBiofuelsTrisaccharideAlginate lyase
The invention discloses an alginate lyase encoding gene, and belongs to the technical field of biology. Alginate lyase is high in degrading activity and stable in property, enzyme activity reaches 65U / mg, 98% or more of initial enzyme activity is still kept after the alginate lyase is stored for 18 months at the temperature of 4 DEG C, the alginate lyase has high product specificity, and brown algae oligosaccharide trisaccharide can be specifically produced. The alginate lyase has important industrial application values and scientific research values.
Owner:JIANGNAN UNIV
Heat-resisting alkali-resisting and salt stable inulase exonuclease, and coding gene and application thereof
The invention discloses an amino acid sequence and a nucleotide sequence of a heat-resisting alkali-resisting and salt stable inulase exonuclease, and construction and application of an inulase exonuclease recombinant expression vector. Results of enzymatic property research show that the optimum reaction conditions of the inulase exonuclease are as below: pH 7.0, 50 DEG C and 10% NaCl. The enzyme can catalyze the hydrolysis of inulase, levan, sucrose, raffinose, kestose, nystose and Kestopentaose. In a catalysis process using inulase as a substrate, only generation of fructose is detected rather than oligosaccharide or other carbohydrates. The inulase exonuclease provided by the invention can be applied to the field of food industry and biological energy source as a novel biocatalyst for fructose production.
Owner:QINGDAO AGRI UNIV
Pertussis vaccine preparation and combined vaccine thereof
InactiveCN104906569AAntibacterial agentsBacterial antigen ingredientsChemical synthesisCarrier protein
The invention relates to a pertussis vaccine preparation and combined vaccine thereof. Pertussis lipooligosaccharide is obtained through bacterial culture purification or chemical synthesis, wherein the pertussis lipooligosaccharide obtained through purification is hydrolyzed to remove the endotoxin activity, the pertussis lipooligosaccharide obtained through chemical synthesis comprises a pertussis lipooligosaccharide tail end trisaccharide structure, and the lipooligosaccharide and carrier protein are coupled to prepare conjugate, namely the pertussis lipooligosaccharide conjugate with immunogenicity. The pertussis toxin is detoxicated pertussis toxin obtained through chemical detoxication or genetic engineering detoxication, and the weight proportion of the pertussis lipooligosaccharide conjugate to the pertussis toxin is 5-20 [mu]g:15-30 [mu]g.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Probiotics-containing soybean oligosaccharide product and preparation thereof
Provided is a soybean oligosaccharide product containing acidic soluble saccharides of soybean and probiotics, which at least include fructose, glucose, sucrose, raffinose, and stachyose, with a percentage of the combined weight of raffinose and stachyose being at least 46%, a weight percentage of fructose being not greater than 8.5%, and a weight percentage of glucose being not greater than 1.0%, based on the total weight of fructose, glucose, sucrose, raffinose, and stachyose. The soybean oligosaccharide product is prepared by extracting a soybean raw material with water under a pH of 3-6 and at a temperature of 50-70° C. to obtain an extract containing acidic soluble saccharides of soybean, and inoculating and fermenting the extract with probiotics that are able to decompose monosaccharides and disaccharides, but substantially not able to decompose trisaccharides or tetrasaccharides.
Owner:FOOD IND RES & DEV INST
Aspergillus niger and application in production of isomaltose hypgather
ActiveCN104877911ASimple nutritional requirementsThe cultivation method is simpleFungiMicroorganism based processesAlgluceraseIsomaltooligosaccharide
The invention discloses an aspergillus niger which is classified and named aspergillus niger M1 with the preservation number being CCTCC NO: M2014421. The preservation date is September 23nd, 2014, and the preservation department is China Center for Type Culture Collection. Alpha-glucosidase produced by the aspergillus niger M1 strain is endoenzyme, and the aspergillus niger M1 strain can produce isomaltose hypgather containing more than 65% of active trisaccharide (IG+P+IG3) under the condition of high temperature. The required reaction time of preparing IMO-500 type isomaltose hypgather containing 35% of active trisaccharide is only half of the reaction time of preparing the isomaltose hypgather containing more than 65% of active trisaccharide. The alpha-glucosidase produced by the strain has advantages of excellent temperature characteristic, short glucoside conversion time, high content of the active trisaccharide in the product and the like.
Owner:GUANGXI NANNING ZHITIAN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com