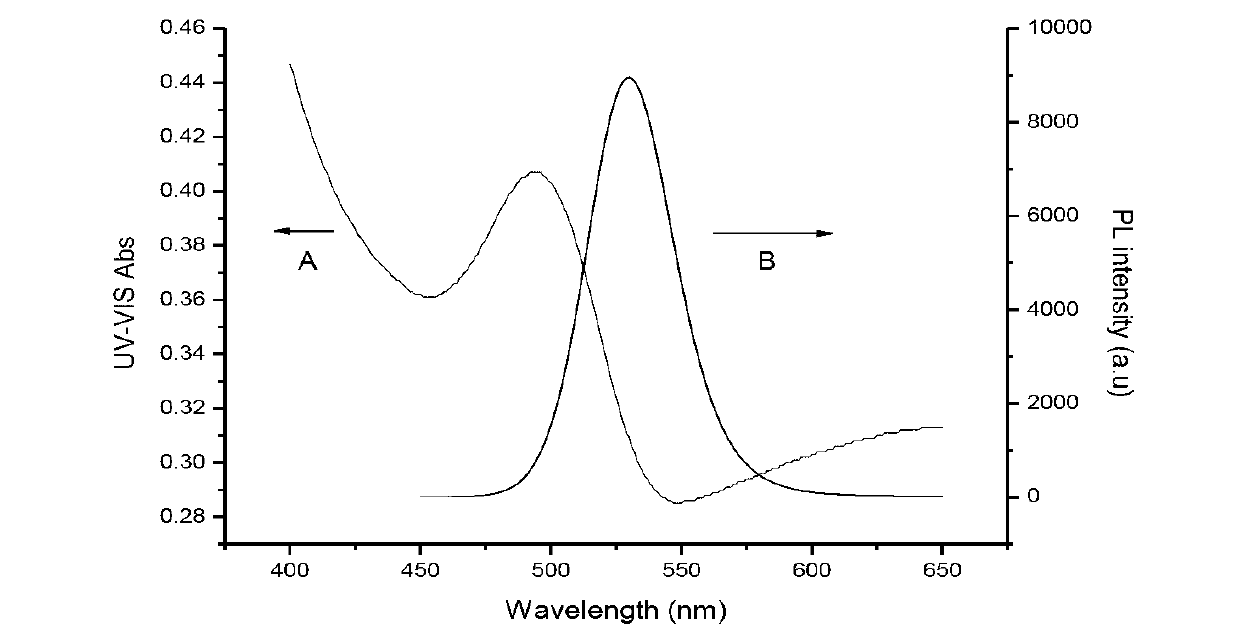
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Canine kidney" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
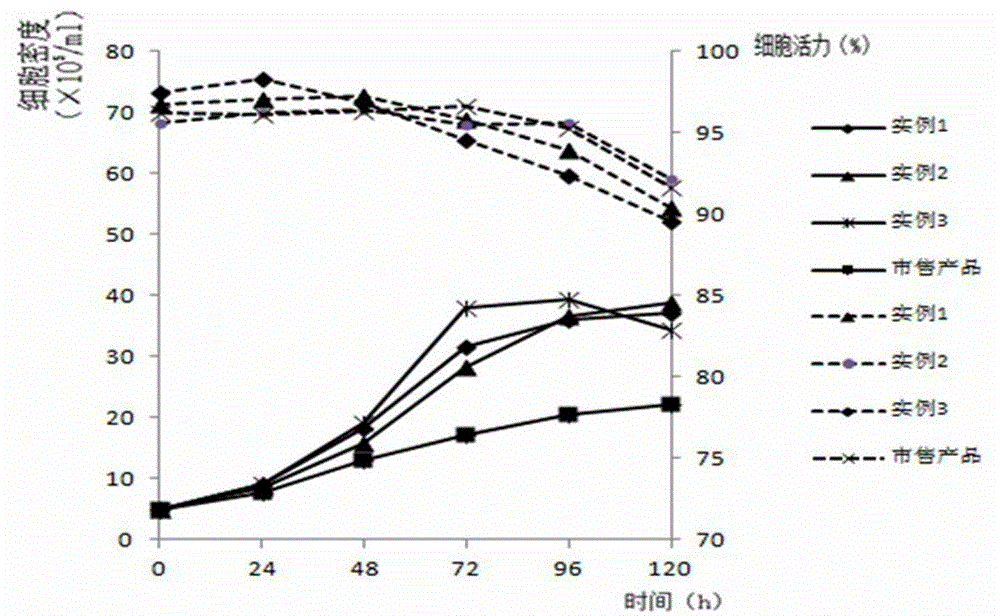
Serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture
ActiveCN103555659AHigh densityClear ingredientsVertebrate cellsArtificial cell constructsCanine kidneyFlu immunization
The invention discloses a serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture. The serum-free medium for MDCK cell full suspension culture comprises an amino acid part, a vitamin part, an inorganic salt part and other additive parts. The serum-free medium has the beneficial effects that the serum-free medium for MDCK cell full suspension culture, provided by the invention, is high in cell culture density and clear in composition and does not contain animal serum, a downstream product is purified, the product quality is improved, and the serum-free medium is convenient to prepare and use and suitable for large-scale production of influenza vaccines and avian influenza vaccines.
Owner:无锡市赛尔百灵生物技术有限公司
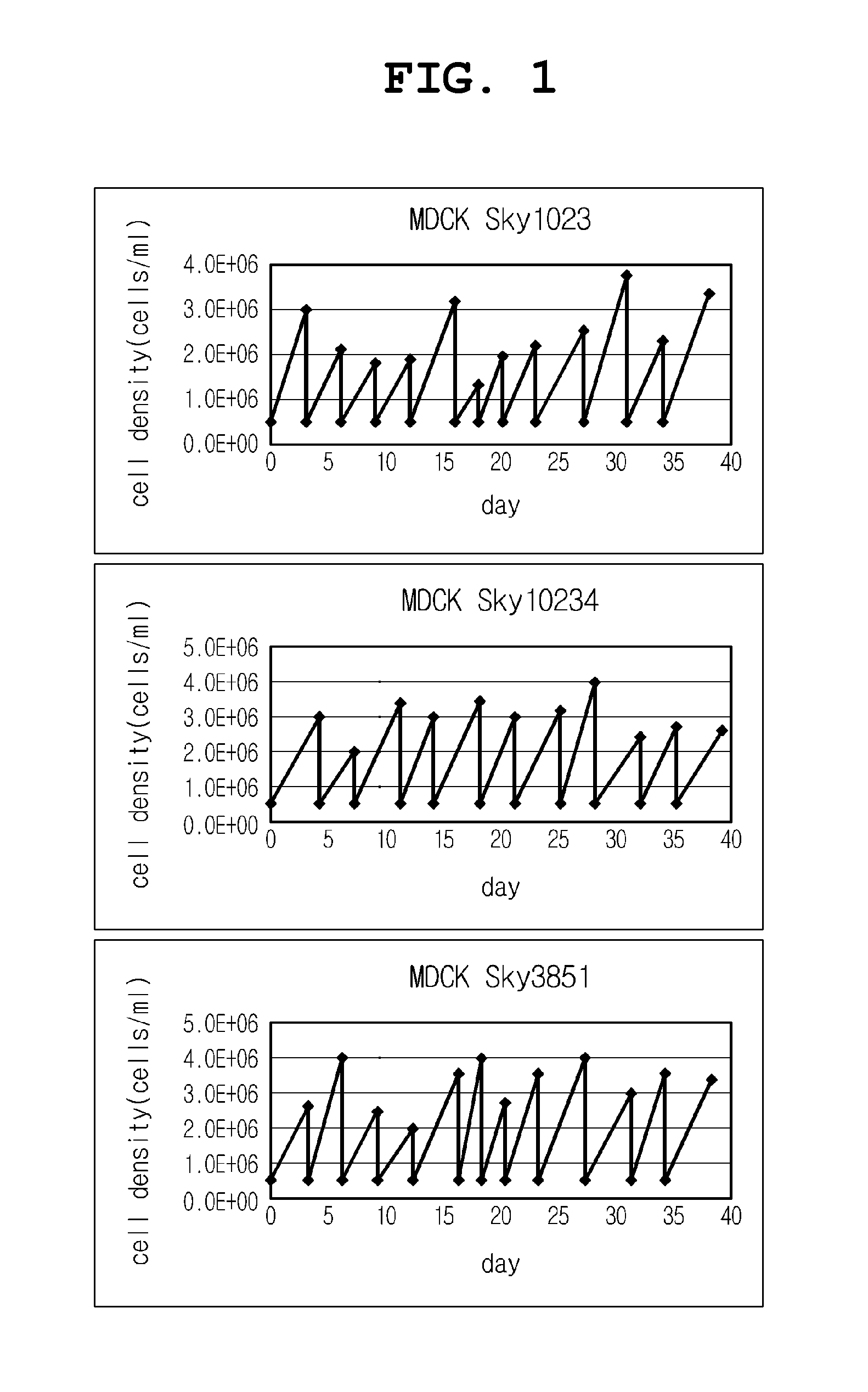
Mdck-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
InactiveUS20130183741A1Low and no tumorigenicityEasy to useSsRNA viruses negative-senseViral antigen ingredientsSerum igeSerum free
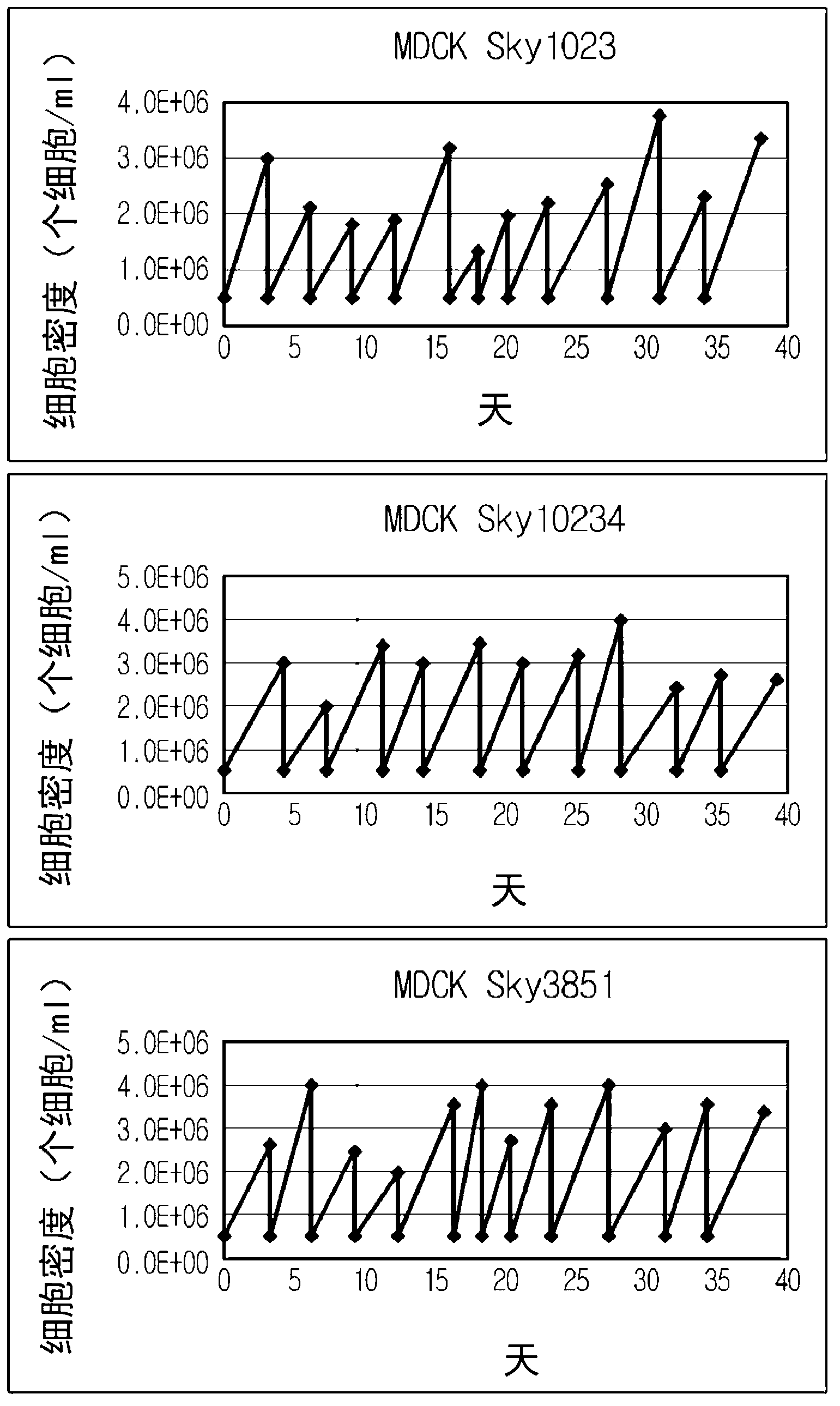
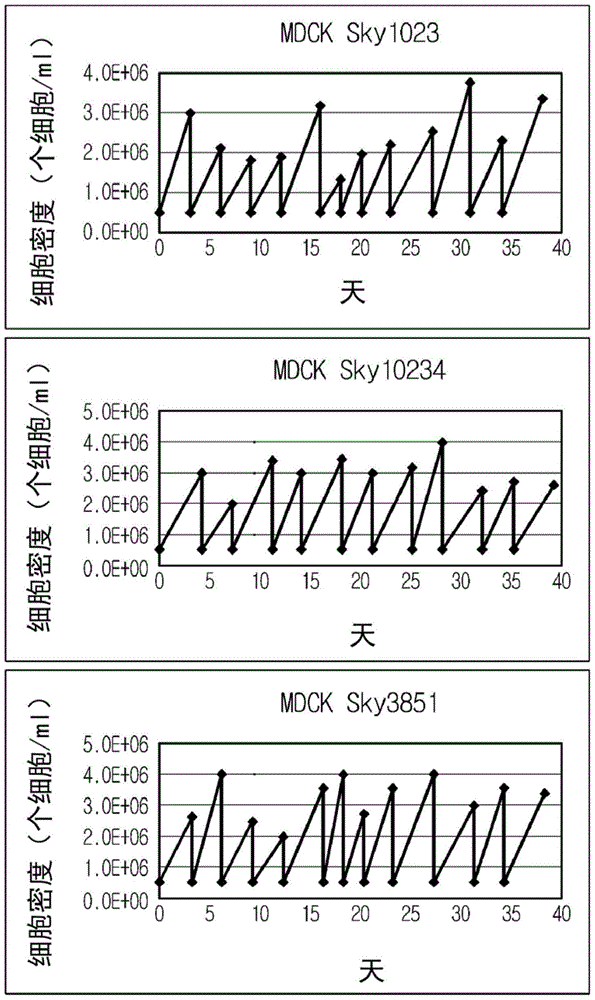
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK CHEM CO LTD
New application of ginkgolide B
InactiveCN102018702APromote apoptosisPromote growthOrganic active ingredientsUrinary disorderCanine kidneyCytotoxicity
The invention discloses application of ginkgolide B to preparation of medicament for preventing and / or treating autosomal dominant polycystic kidney disease. A madin-darby canine kidney (MDCK) vesicle model is used for screening to find that the ginkgolide B inhibits formation and growth of vesicles. An experimental result shows that: the ginkgolide B has an obvious inhibiting effect on the formation and growth of MDCK vesicles and the effect of the ginkgolide B is in dose response relationship; the ginkgolide B has no cytotoxicity to MDCK cells, so that the vesicle inhibiting effect of the ginkgolide B is independent of the cytotoxicity; the ginkgolide B does not obviously induce MDCK cell apoptosis, so that the vesicle inhibiting effect of the ginkgolide B is independent of cell apoptosis promotion of the ginkgolide B; the ginkgolide B can promote the MDCK cells or vesicles to form tubular structures; and the effect is in dose response relationship; and the ginkgolide B has an inhibiting effect on the growth of the embryonic kidney vesicles. The ginkgolide B provides experimental data for development of a specific medicament for preventing and / or treating autosomal dominant polycystic kidney disease.
Owner:PEKING UNIV
Quantum-dot-based method for carrying out in-situ and real-time detection on heavy metal ions in cells
ActiveCN102998291ADoes not affect normal growthRealize real-time in-situ detectionFluorescence/phosphorescenceCanine kidneyFluorescence
The invention discloses a quantum-dot-based method for carrying out in-situ and real-time detection on heavy metal ions in cells. The method comprises the following steps of: A. preparing a carboxymethyl chitosan-CdTe quantum dot solution with certain concentration; B. digesting canine kidney cells overgrowing in a T25 cell culture flask bottle into a unicellular suspension, inoculating into a cell culture dish, and culturing; C. adding the carboxymethyl chitosan-CdTe quantum dot solution into the culture dish, and hatching for a period of time, thereby obtaining the canine kidney cells marked with fluorescence; and D. adding a DMEM (dulbecco's modified eagle medium) nutrient solution containing heavy metal ions with gradient concentration and unknown concentration respectively in washed cells in the culture dish, hatching together with the cells, detecting the average fluorescent intensity of the cells respectively by a flow cytometry, and drawing a standard curve through the changes of the cell fluorescent intensity, so that the concentration of the heavy metal ions in the cells can be calculated. The method is simple and convenient to operate; the fluorescence of marked cells can be quenched regularly as the increase of heavy metal ions, and the real-time and in-situ detection on the outer source heavy metal ions in the cells is realized.
Owner:武汉市疾病预防控制中心 +1
Process for the Production of an Influenza Vaccine
InactiveUS20080254067A1High potencySsRNA viruses negative-senseViral antigen ingredientsAntigenCanine kidney
The present invention relates to a commercial-scale process for the production of influenza virus or antigens for prophylactic, diagnostic, immunotherapeutic or therapeutic purposes. Particularly, the invention provides a Madin-Darby Canine Kidney (MDCK)-derived, cell line and a cell culture-based process for the production of an influenza vaccine and more particularly, a human vaccine comprising influenza types A and B.
Owner:ID BIOMEDICAL
Method for preparing MDCK (Madin-Darby canine kidney) cell line adaptive to serum-free full-suspension culture and MDCK cell line
ActiveCN105861422AShort cycleLess generationsCulture processMicroorganism based processesCanine kidneySerum free
The invention discloses a method for preparing an MDCK (Madin-Darby canine kidney) cell line adaptive to serum-free full-suspension culture and the MDCK cell line. The method includes steps of 1), discarding culture media for cultivating MDCK cells, cleaning cell layers by the aid of pancreatin solution, then discarding the pancreatin solution, adding pancreatin solution into the MDCK cells again, digesting the MDCK cells, stopping digesting the MDCK cells after the cells are rounded, centrifuging cell suspension and then discarding supernatant to obtain cell clusters; 2), re-suspending the cell clusters obtained at the step 1) by the aid of serum-free culture media to obtain cell re-suspension; 3), arranging the cell re-suspension obtained at the step 2) in a shaking table, cultivating the cell re-suspension in culture tanks, diluting the cell re-suspension by the aid of the serum-free culture media and carrying out passage on the cell re-suspension to obtain the MDCK cell line. The method and the MDCK cell line have the advantages that the MDCK cell line is high in cell density and activity and uniform in size, individually grows in a dispersion manner, is plump in form and is suitable for serum-free suspension culture by the aid of bioreactors.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +2
Serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells
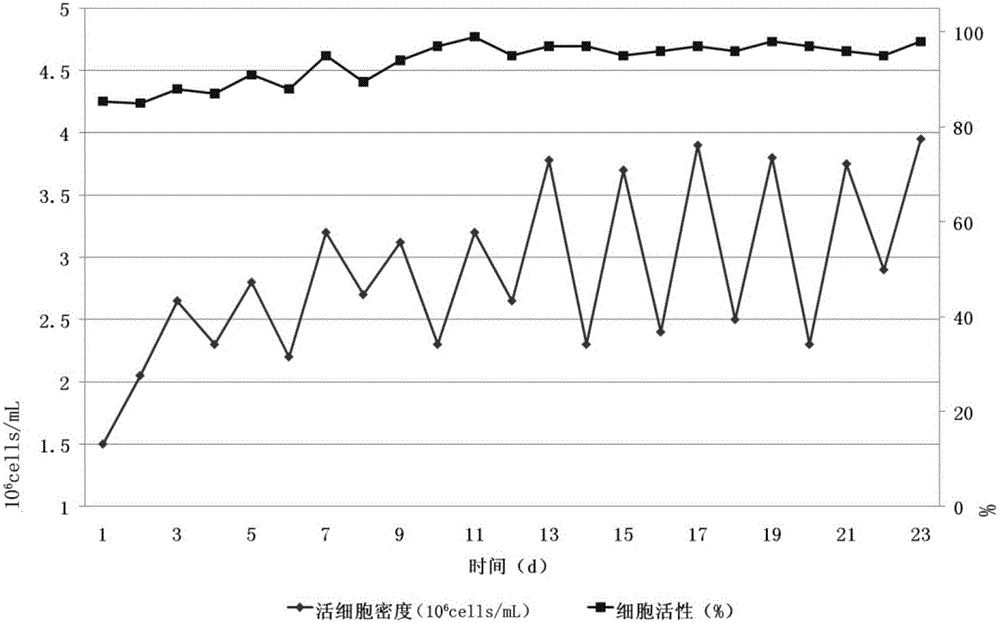
The invention provides a serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells. The culture medium is prepared from a DMEM / F12 culture medium containing 5g / L of NaCl and the following components: sodium stannite, recombinant human epidermal growth factors, hydrocortisone, recombinant full-chain insulin, prostaglandin E1, human transferrin, thyroxine (T3), vitamin E, cholesterol, ethanol amine, beta-mercaptoethanol, Tween-80, Hypep1510, recombinant human serum albumin ACF and mannitol. With the adoption of the culture medium provided by the invention, the full-suspension culture of the MDCK cells can be carried out very well under the condition that serum is not added; after the MDCK cells are continuously cultured for 20 generations, the average proliferation concentration of the MDCK cells is 2.308*10<6> / mL, the average cell viability is 97.4 percent and the average doubling time is 34.48 hours, so that the serum-free culture medium provides technical supports for developing influenza vaccines of cell matrixes of mammals in China.
Owner:马忠仁 +2
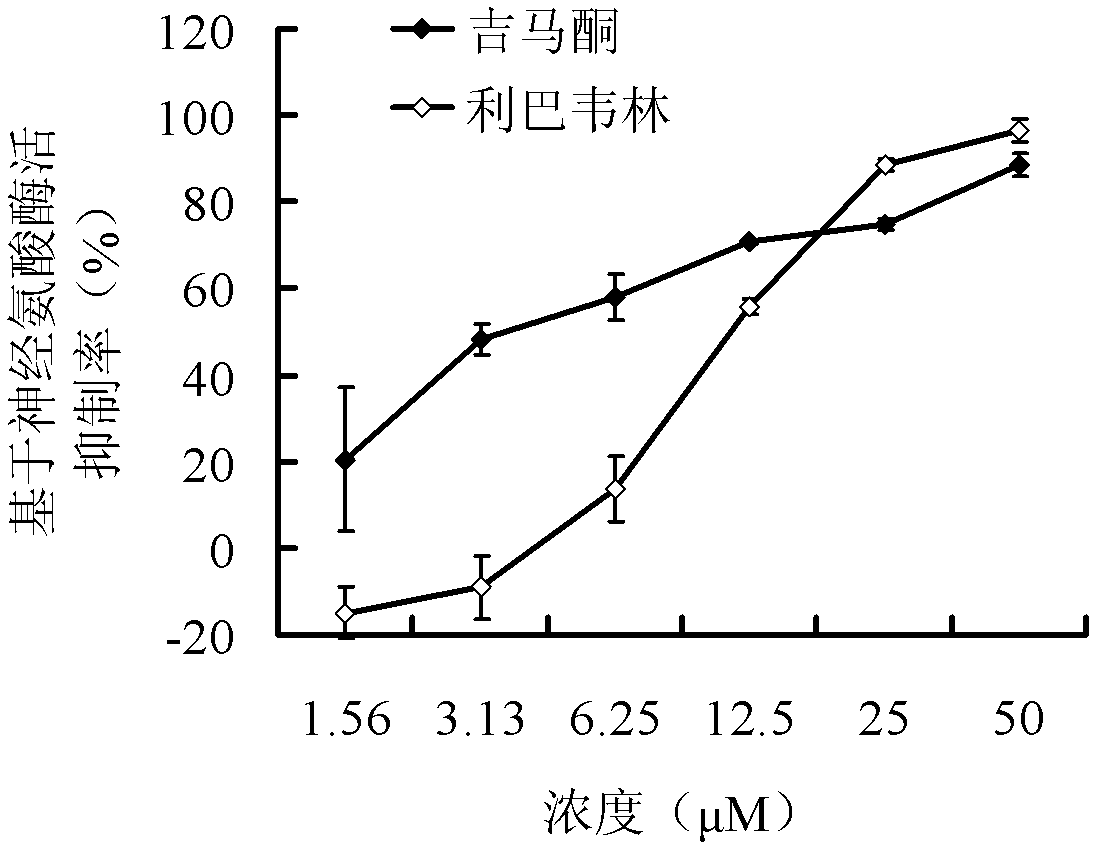
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409AAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
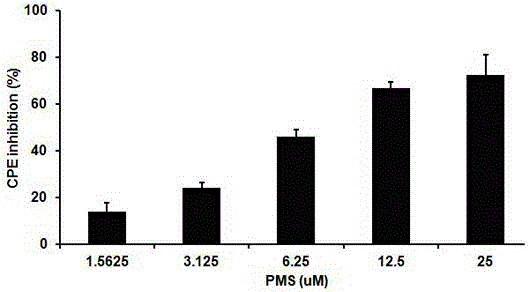
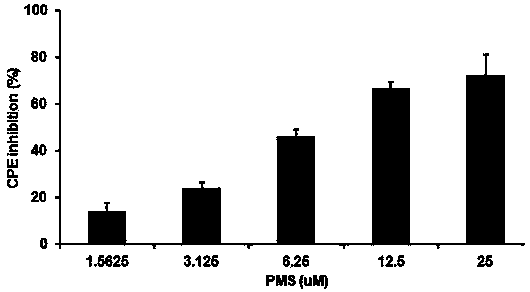
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
Method for producing influenza virus vaccine
ActiveCN101955915ASolve pollutionGuaranteed to be pureAntiviralsViruses/bacteriophagesCanine kidneyEquine influenza vaccine
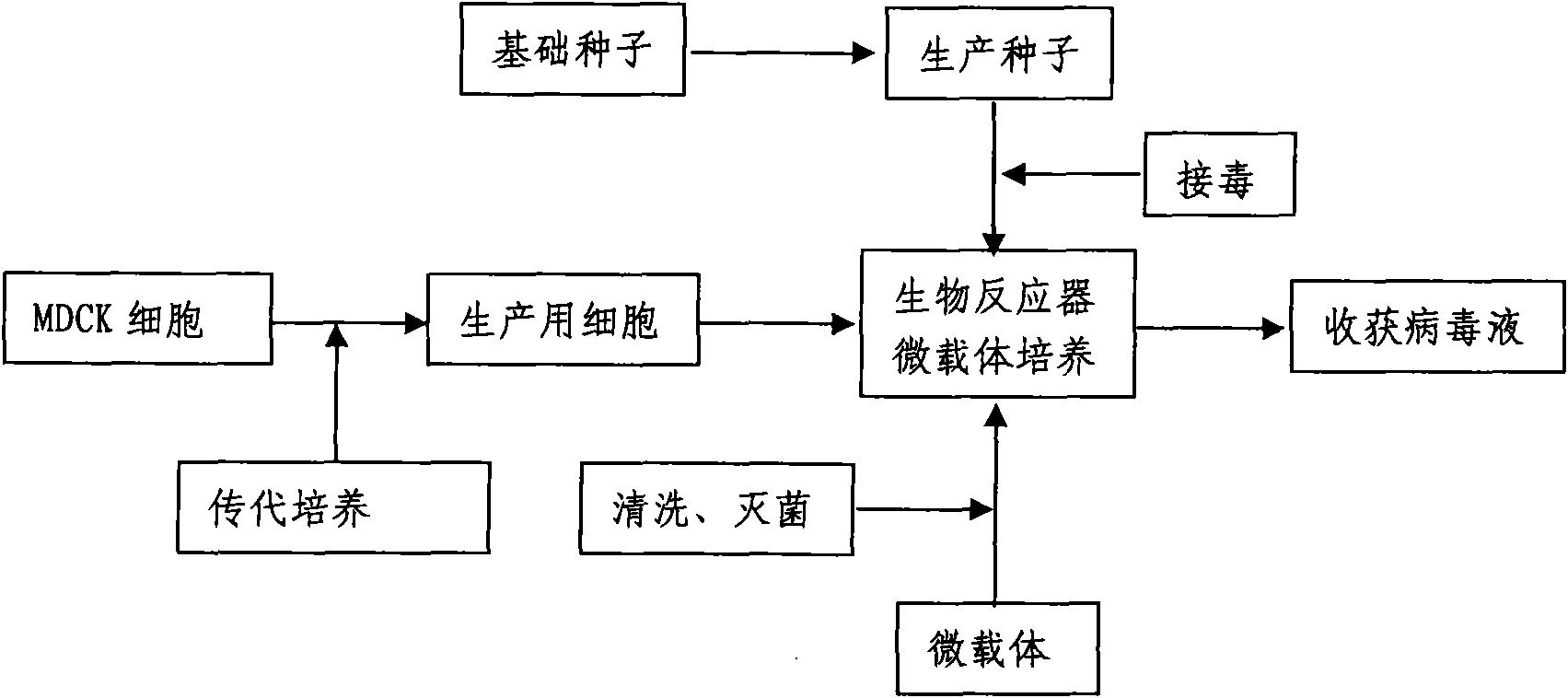
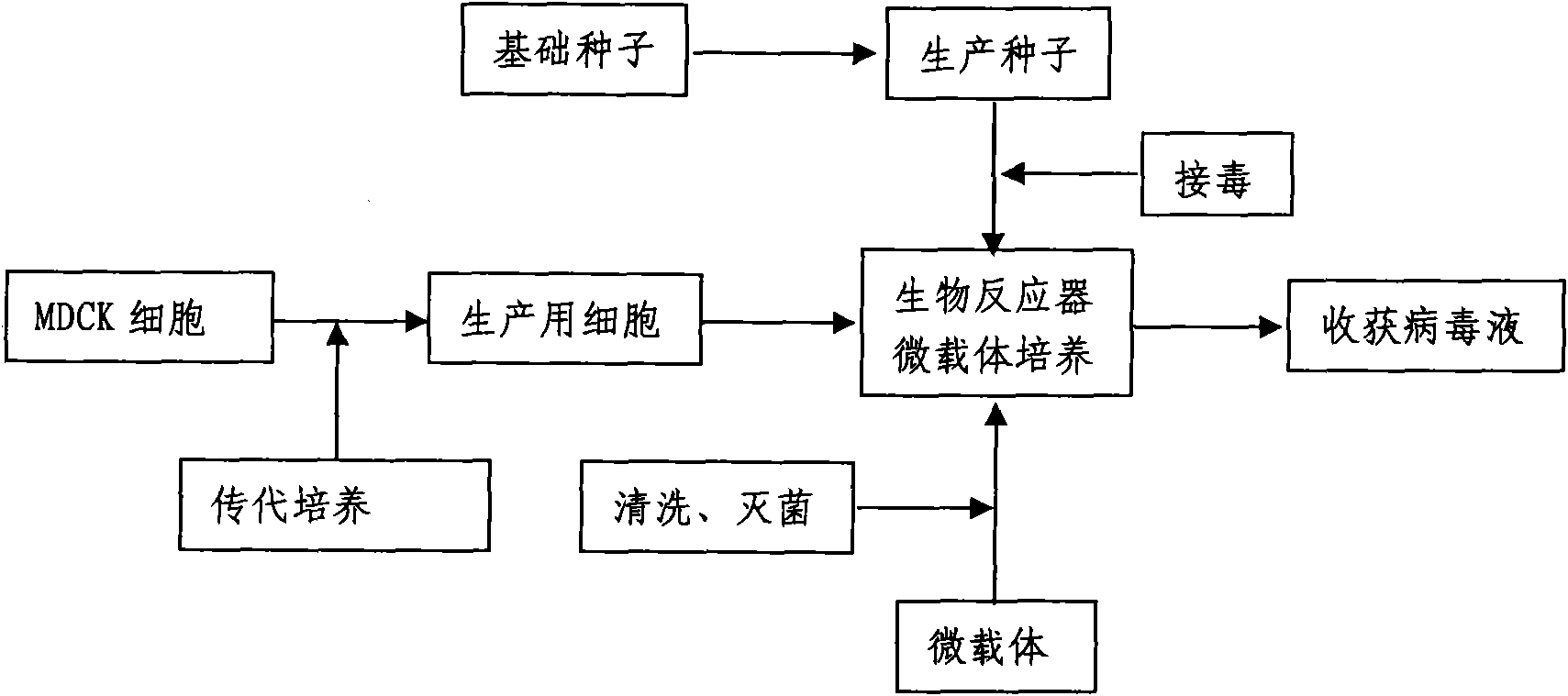
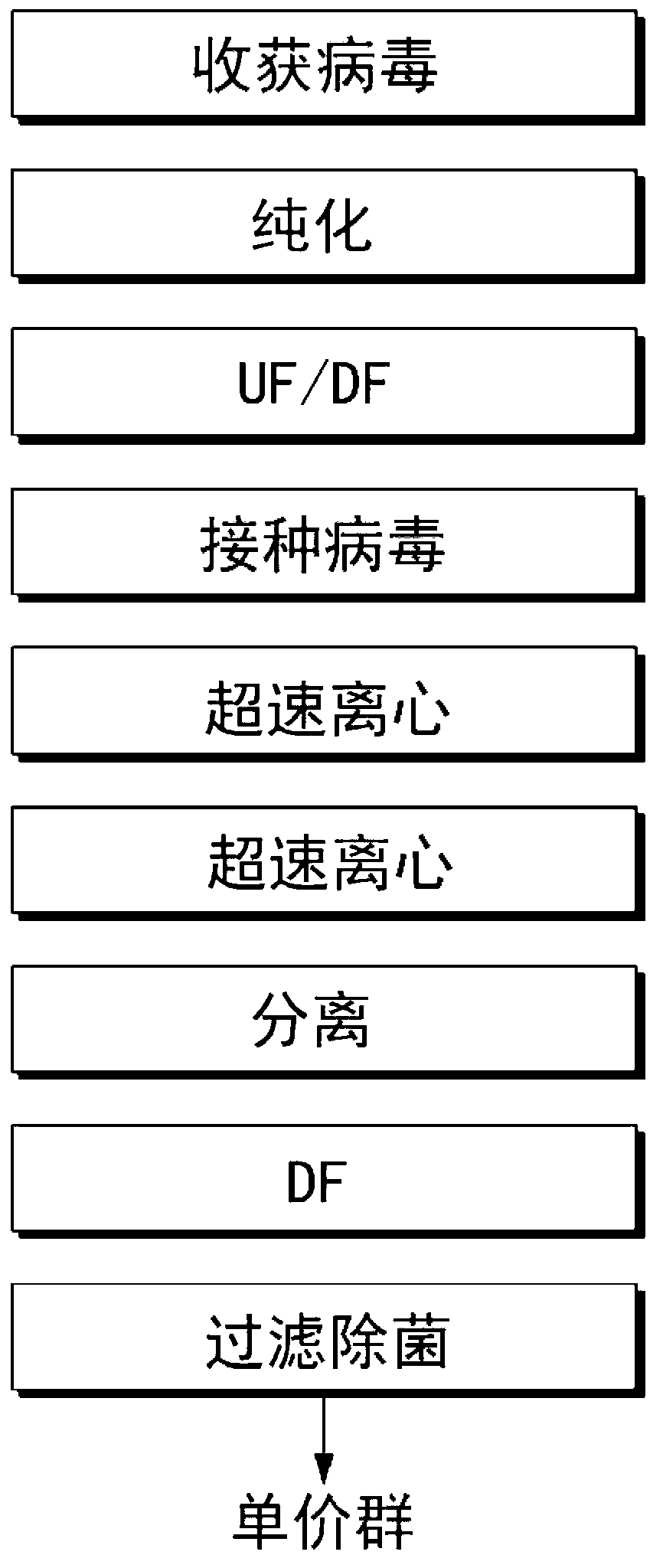
The invention discloses a method for producing a vaccine for avian influenza viruses and other influenza viruses such as a swine influenza virus, a canine influenza virus and an equine influenza virus. The method comprises the following steps of: subculturing cells for preparing the vaccine; (2) reproducing cytotoxic varieties; (3) performing microcarrier suspension culture on darby canine kidney (MDCK) cells in a bioreactor; (4) reproducing venom for preparing the vaccine; and (5) harvesting virus liquid. The method has the advantages of great reduction in production cost, short production period, no restriction to raw material supply, small occupied area, easy and quick expansion in production scale, low and readily treated environmental pollution, high degree of automation, few labors, easy equilibrium and stabilization in quality and obvious improvement on the yield and the quality of the vaccines.
Owner:成都史纪生物制药有限公司
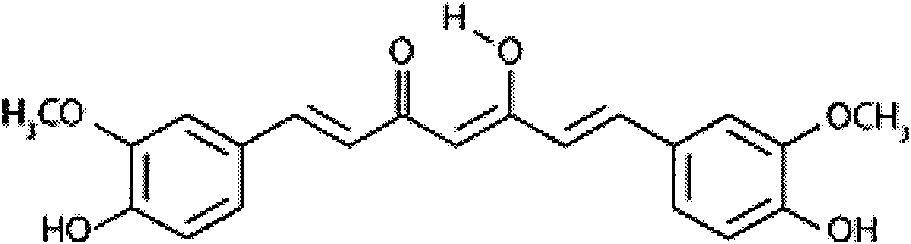
New application of curcumin
InactiveCN102018689ADoes not induce apoptosisPromote apoptosisKetone active ingredientsUrinary disorderCanine kidneyCytotoxicity
The invention discloses application of curcumin to preparation of medicaments for preventing and / or treating autosomal dominant polycystic kidney disease. A madin-darby canine kidney (MDCK) vesicle model is used for screening to obtain the curcumin which inhibits formation and growth of vesicles. An experimental result shows that: the curcumin has an obvious inhibiting effect on the formation and growth of MDCK vesicles and the effect of the curcumin is in dose response relationship; the curcumin has no cytotoxicity to MDCK cells, so that the vesicle inhibiting effect of the curcumin is independent of the cytotoxicity; the curcumin does not obviously induce MDCK cell apoptosis, so that the vesicle inhibiting effect of the curcumin is independent of cell apoptosis promotion of the curcumin; the curcumin can promote the MDCK cells or vesicles to form tubular structures; and the effect is in dose response relationship; and the curcumin has an inhibiting effect on the growth of the embryonic kidney vesicles. The curcumin is expected to be developed into a specific medicament for preventing and / or treating autosomal dominant polycystic kidney disease.
Owner:PEKING UNIV
Cryopreservation method for protecting cell junction
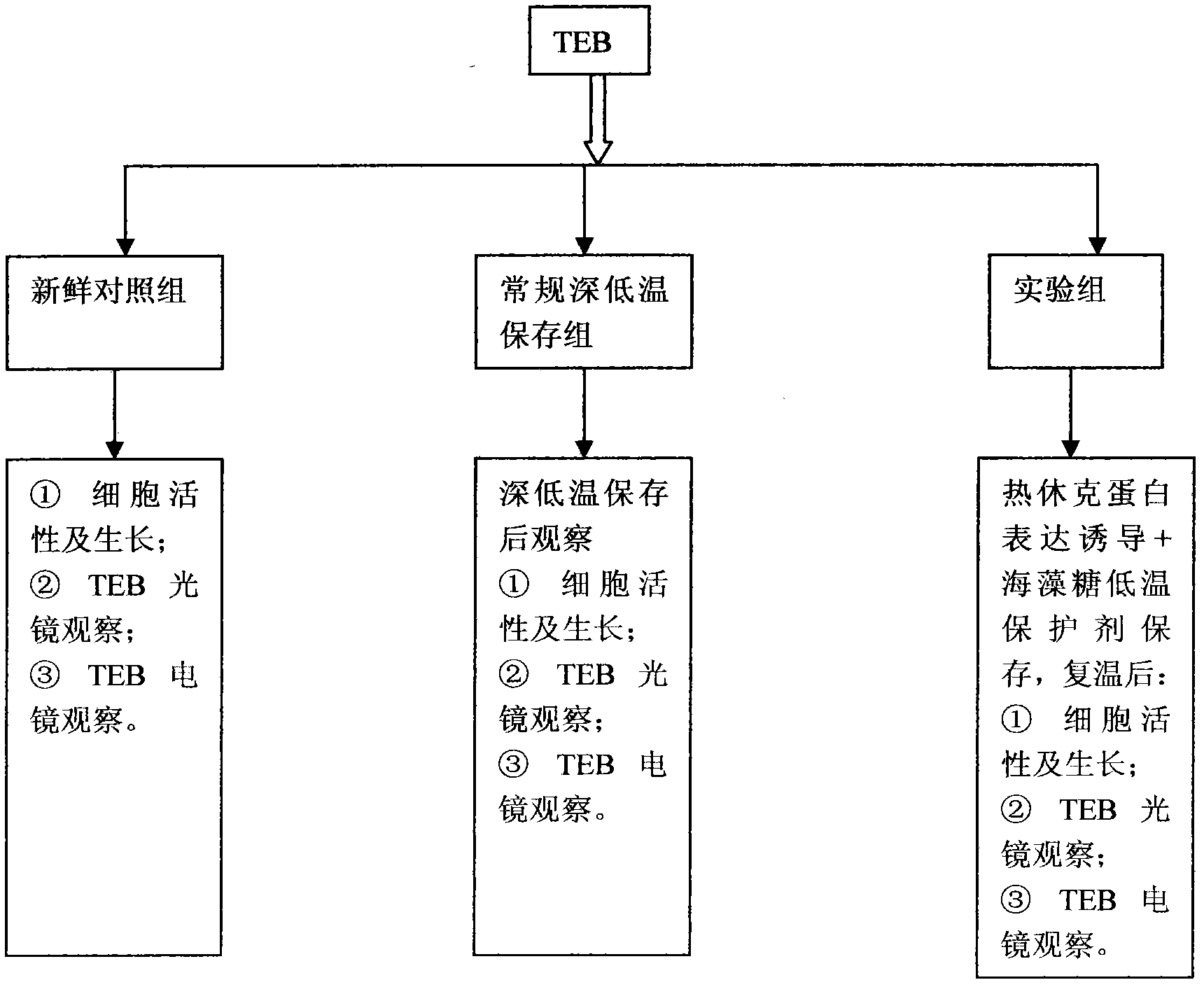
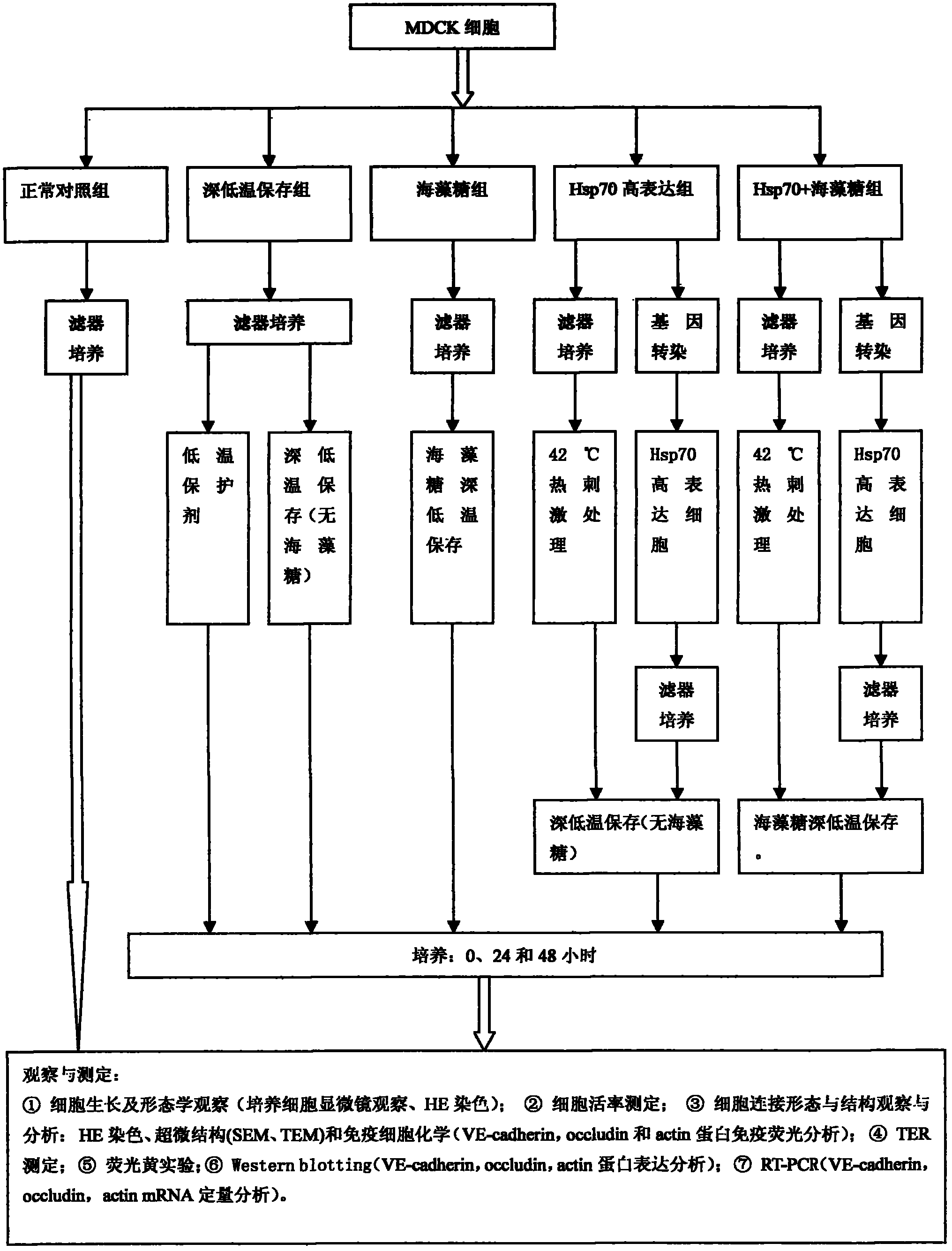
InactiveCN102487938AImprove toleranceFree from damageDead animal preservationCanine kidneyCultured cell
The invention discloses a cryopreservation method for protecting cell junction. The method comprises the following steps of: a) culturing Madin Darby canine kidney (MDCK) cells; b) dividing the MDCK cells in the step a) into a normal control group, a cryopreservation group, a trehalose group, an Hsp70 high expression group and an Hsp70+ trehalose group; c) performing filter culture on the normal control group, the cryopreservation group and the trehalose group respectively, performing filter culture and gene transfection on the Hsp70 high expression group, and performing filter culture and gene transfection on the Hsp70+ trehalose group; d) performing cryopreservation on the normal control group, the cryopreservation group, the trehalose group, the Hsp70 high expression group and the Hsp70+ trehalose group; e) growing cells and performing morphologic observation (the cultured cells are observed by a microscope and are subjected to HE staining); f) testing the survival rate of the cells; g) observing and analyzing the cell junction morphology and structure; and h) performing a fluorescein experiment. The method is simple, and convenient to use; and the structure and function of the cell junction after tissues and organs are subjected to cryopreservation can be kept complete, and the damage of the cell junction can be effectively avoided.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Urea transporter inhibitors, and preparation method and application thereof
InactiveCN102757447AGood selective diuretic effectIncrease urine outputOrganic chemistryBlood disorderErythrocyte membraneCanine kidney
The invention discloses a series of urea transporter inhibitors of which the structural formula is disclosed in the specification. An erythrocyte model is used for screening to obtain compounds for inhibiting urea transporters. The experimental result indicates that the compounds (such as Youti) can inhibit the permeation of urea transporter UT-B mediated erythrocyte membranes for urea, and the action forms a dosage dependency relationship; Youti within effective dose range has no cytotoxic action on the MDCK (Madin-Darby Canine Kidney) cells, which indicates that the action of Youti for inhibiting cell permeable urea is irrelevant to cytotoxicity; the inhibiting action of Youti on the urea transporter UT-B gradually increases; the inhibiting action of Youti on the urea transporter UT-B is reversible; and the in-vivo test result proves that Youti can obviously increase the uresis amount of a rat, lower the concentration of urea in the urine of the rat, and lower the osmotic pressure, which indicates that Youti has selective diuresis action on urea in vivo.
Owner:PEKING UNIV
Culture medium for preparing influenza vaccine through MDCK cells and application method thereof
The invention provides a culture medium suitable for large-scale high-density cultivation of MDCK (Madin Darby Canine Kidney) cells for preparation of influenza vaccine.
Owner:杭州国牧生物科技有限公司
MDCK-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
InactiveCN103154238AEffective proliferationNon-tumorigenicSsRNA viruses negative-senseViral antigen ingredientsCanine kidneySerum free
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK CHEM CO LTD
Process for the production of an influenza vaccine
The present invention relates to a commercial-scale process for the production of influenza virus or antigens for prophylactic, diagnostic, immunotherapeutic or therapeutic purposes. Particularly, the invention provides a Madin-Darby Canine Kidney (MDCK)-derived, cell line and a cell culture-based process for the production of an influenza vaccine and more particularly, a human vaccine comprising influenza types A and B.
Owner:ID BIOMEDICAL
Method for proliferating influenza A viruses
InactiveCN102796708ASolve pollutionGuaranteed purityMicrobiological testing/measurementMicroorganism based processesCanine kidneyTiter
The invention belongs to the technical field of animal infectious diseases, and in particular relates to a method for separating and identifying influenza A viruses H1 and stably proliferating the viruses on Madin-Darby canine kidney (MDCK) cells. An influenza A viruses H1 strain A-Influ / JML-F9 is obtained by separation and identification, and the collection number of the strain is CCTCC NO: V201105. The influenza A viruses H1 are stably proliferated on the MDCK cells by an optimal method, an optimal culture medium and optical culture conditions, so that the average blood congeal titer of the viruses reaches 7log2 by large-scale proliferation, and the highest blood congeal titer of the viruses reaches 9log2.
Owner:HUAZHONG AGRI UNIV +1
Mdck-derived Cell Lines Adapted To Serum-free Culture And Suspension Culture And Method For Preparing Vaccine Virus Using The Cells
ActiveCN104862267AEffective proliferationNon-tumorigenicSsRNA viruses negative-senseViral antigen ingredientsCanine kidneySerum free
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK BIOSCI CO LTD
Canine recombinant interferon alpha and preparation method
InactiveCN102321169AMicroorganism based processesPeptide preparation methodsCanine kidneyEscherichia coli
The invention discloses canine recombinant interferon alpha, and also provides a preparation method of the canine recombinant interferon alpha. Recombinant genes of canine interferon alpha are expressed in escherichia coli, and canine interferon alpha with a purity of more than 98% is obtained through processes of renaturation and purification. The biological activity is determined by a MDCK (canine kidney cell)-VSV (vesicular stomatitis virus) virus system, and the specific activity reaches 2-3*107 IU / mg.
Owner:CHANGCHUN UNIV OF TECH
MDCK (Madin-Darby canine kidney) cell line capable of stably expressing human-derived TIGAR (TP53-induced glycolysis and apoptosis regulator) gene
ActiveCN106479983AImprove anti-apoptotic abilityReduce adhesionSsRNA viruses negative-senseCompound screeningCanine kidneyBiology
The invention belongs to the technical field of biology, and particularly relates to an MDCK (Madin-Darby canine kidney) cell line capable of stably expressing a human-derived TIGAR (TP53-induced glycolysis and apoptosis regulator) gene. The cell line is an MDCK cell line TG-418-E5 capable of stably expressing the human-derived TIGAR gene and contains a TIGAR coding gene sequence, and the preservation number of the cell line is CGMCC NO:12983. Through stable expression of the TIGAR gene in MDCK cells, anti-apoptosis capacity of the cells is improved, and the survival time of recombinant cells is prolonged. The method not only lays the foundation for research of application of MDCK serum-free fully-suspended culture in mass production of cell culture vaccines, but also increases breeding titer of avian influenza vaccine strains and saves the cost of a production process. Besides, the cell line TG-418-E5 has better application prospect in aspects of screening of anti-avian influenza virus drugs, screening of vaccine strains and production of the cell culture vaccines.
Owner:ZHONGCHONG XINNUO BIOTECH TAIZHOU CO LTD
Traditional Chinese medicine for treating rheumatic lumbocrural pain
InactiveCN102600345ASimple treatmentHeal fastHeavy metal active ingredientsAnthropod material medical ingredientsMonkshoodsCanine kidney
The invention discloses a traditional Chinese medicine for treating rheumatic lumbocrural pain, comprising the following bulk drugs in parts by weight: 400 parts of rehmannia glutinosa, 200 parts of Chinese yam, 150 parts of poria cocos, 200 parts of common macrocarpium fruit, 150 parts of tree peony bark, 150 parts of oriental waterplantain rhizome, 50 parts of cinnamon, 50 parts of monkshood, 100 parts of cortex eucommiae, 100 parts of radix achyranthis bidentatae, 100 parts of astragalus membranaceus, 20 parts of ephedra root, 50 parts of male silk moth, a couple of geckoes, 350 parts of pizzle, 600 parts of canine kidney, 50 parts of cornu cervi pantotrichum, 50 parts of palmleaf raspberry fruit, 20 parts of cinnabar, 100 parts of cynomorium songaricum, 50 parts of tuner onion seed, 50 parts of fossil fragments, 50 parts of oyster shell, 50 parts of pericarpium citri reticulatae, 100 parts of medicinal Indian mulberry, 100 parts of actinolite, 100 parts of epimedium, 50 parts of leech and the like. The medicine can dispel wind and eliminate dampness, reinforce kidney to strengthen yang, clear liver and improve vision, improve blood circulation, diminish inflammation and relieve pain and has obvious efficacies of treating diseases such as limb weakness and night sweat caused by lumbocrural pain, kidney coldness, waist pain and joint inflammation; the medicine is short in course of treatment and rapid in healing, and has no toxic and side effects; and by using the medicine, the diseases can be thoroughly and radically treated, and can not be relapsed.
Owner:姜喜
Method for marking canine kidney cells through chitosan-quantum dot fluorescent probe
InactiveCN101980005AGood biocompatibilityHigh fluorescence intensityFluorescence/phosphorescenceCanine kidneyFluorescence
The invention discloses a method for marking canine kidney cells through a chitosan-quantum dot fluorescent probe. The method comprises the following steps of: A, preparing solution of a carboxymethyl chitosan-CdTe quantum dot; transferring the solution of the carboxymethyl chitosan-CdTe quantum dot to a sterilized volumetric flask by using a pipette according to the concentration of the carboxymethyl chitosan-CdTe quantum dot, and adding high-purity water for constant volume; B, digesting the canine kidney cells overgrown in a T25 cell culture flask by using ethylene diamine tetraacetic acid (EDTA)-trypsin so as to form unicellular suspension, counting cells, inoculating the canine kidney cells to a cell culture dish, and adding nutrient solution for culturing; and C, after the canine kidney cells grow adherently, adding the chitosan-quantum dot fluorescent probe into the culture dish, and incubating the probe and the canine kidney cells together to obtain canine kidney cells marked with fluorescence. The method is easy and convenient to operate, has high fluorescent efficiency, good biocompatibility and long stable time, and can be used for observing the cells for a long time; and the fluorescent probe does not have cytotoxicity.
Owner:武汉市疾病预防控制中心 +1
Herbal medicine health wine
InactiveCN101675801ABalance yin and yangRelieve fatigueAlcoholic beverage preparationFood preparationCanine kidneyMedicinal herbs
The invention relates to herbal medicine health wine, which is prepared by grinding and wine steeping the following raw materials in parts by weight: the fruit of Chinese wolfberry, dried longan pulp,yam, flos caryophyllata, cortex cinnamomi, Chinese date, fructus amomi, dark plum fruit, poria cocos, canine kidney, crystal sugar and pink grapefruit. The herbal medicine health wine belongs to pureChinese traditional secret recipes, which takes traditional medicinal materials and high-quality liquor as raw materials and is prepared through infusing by adopting a scientific proportioning, thusbeing applicable to the majority of the middle-aged and aged persons, and being capable of regulating yin and yang, soothing fatigue, protecting health and nourishing. The herbal medicine health wineis also characterized by pure naturalness and no toxic and side effects.
Owner:刘桂荣
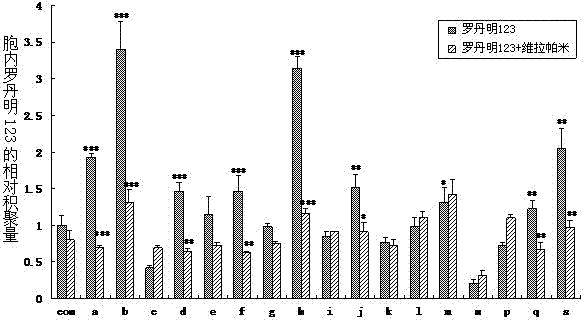
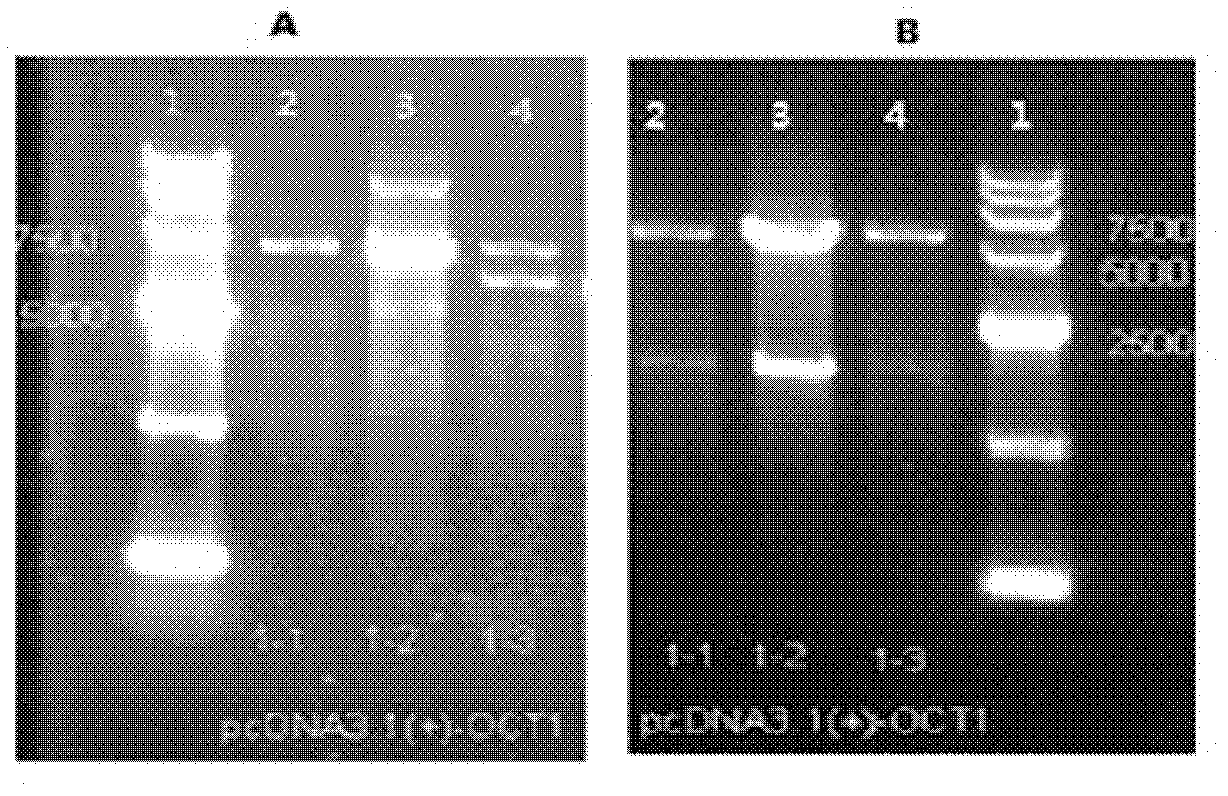
Construction and application of cell model for cotransfection of drug metabolic enzyme and transporter
InactiveCN102586191AShort training periodEasy to trainMicrobiological testing/measurementVector-based foreign material introductionCanine kidneyMetabolic enzymes
The invention provides construction of a cell model for cotransfection of CYP3A4 and MDR1. The method comprises the following steps of: cloning P-gp gene to pcDNA3.1(+), transfecting Madin-Darby canine kidney (MDCK) cells, and screening monoclonal cells by using G418, thus obtaining MDCK-MDR1 cell strains; and cloning CYP3A4 to a carrier pcDNA3.1(+) / Hygro which is provided with a hygromycin B selection marker, transfecting MDCK-MDR1 cells, and obtaining the cell model for cotransfection of CYP3A4 and MDR1 through hygromycin B selection. The MDCK-MDR1 / CYP3A4 was collected in China Center for Type Culture Collection (CCTCC) on Dec.15, 2011, with the collection number of CCTCC2011119. The model constructed can be used for simulating the absorption and metabolic process of a medicine in the intestinal tract so as to screen the common substrate of P-gp and CYP3A4.
Owner:ZHEJIANG UNIV
Construction and applications of cell model expressing human organic cation transporter-1
ActiveCN102181449ATransshipment predictionPredict interactionMicrobiological testing/measurementFluorescence/phosphorescenceCanine kidneyHuman body
The invention provides a construction method and applications of a cell model expressing human organic cation transporter-1. A hOCT1 wild-type gene segment is obtained from a hepatic tissue; two mutant gene segments P341L and M420del can be obtained by specific point mutation; the two segments are connected with a plasmid vector; darby canine kidney cells (MDCK) are transfected; G418 resistance screening is carried out to obtain wild-type cells expressing hOCT1 and cells of two mutants; mRNA level verification is carried out on the cells; and functional verification is carried out by utilizing a hOCT1 substrate and an inhibitor. The cell model provided by the invention can be used for screening the hOCT1 classic substrate and the inhibitor, and predicting the transportation of medicament in human bodies and medicament interaction which possibly happen; and by utilizing the cell model, the influence of gene polymorphism of the transporter on the medicament transportation function can be predicted; and the cell model provides a standard for clinical reasonable medicament administration and individualized medicament administration, and has reasonable design and good repetitiveness.
Owner:ZHEJIANG UNIV
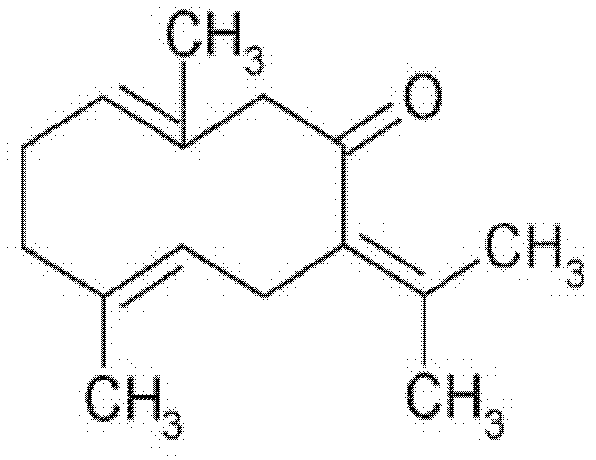
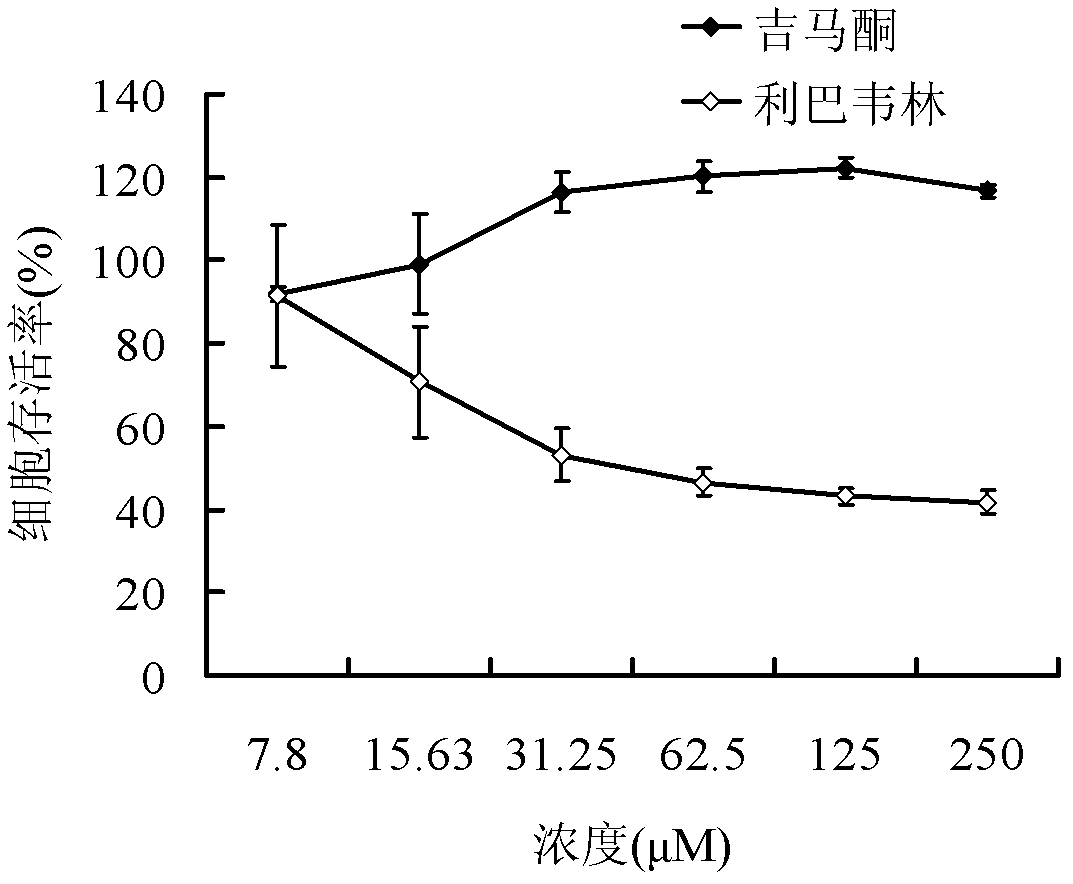
Application of germacrone in preparation of medicament for treating or preventing influenza viruses
InactiveCN102274206BNon-cytotoxicAvoid infectionOrganic active ingredientsAntiviralsCanine kidneyResistant strain
The invention discloses application of germacrone in preparation of a medicament for treating or preventing influenza viruses. The cell toxicity of the germacrone on madin-darby canine kidney (MDCK) cells is detected, and the result shows that CC50 is more than 250 microns. The influence of the germacrone on the expression of three antigens and the influence of germacrone on virus genome reproduction are detected, and the result shows that the germacrone formulation inhibits the expression of the virus antigens and the virus genome reproduction with dependency. The virus activity resistant mechanism of the germacrone is preliminarily researched. The virus activity resistance stage of the germacrone in a single reproduction period of the influenza viruses is researched by administration experiments of different time, and the result shows that the germacrone has inhibiting effect on adsorption, entry and early reproduction of the viruses. Moreover, the virus activity resistance of the germacrone to amantadine medicament resistant strains, B-type influenza virus strains and A-type influenza virus H3N2 subtype strains is detected, and the result shows that the germacrone has virus activity resistance to the three kinds of virus strains. The germacrone has the effects of relieving cough, relieving asthma and the like.
Owner:陈绪林
Spinner-flask culture method for H9N2 subtype of avian influenza virus
InactiveCN104073470APromote rapid proliferationSignificant technological progressMicroorganism based processesViruses/bacteriophagesImmune effectsCanine kidney
The invention discloses a spinner-flask culture method for H9N2 subtype of avian influenza virus. The spinner-flask culture method comprises a step of culturing MDCK (Madin-Darby canine kidney)-HA cells, namely culturing the MDCK-HA cells in a spinner flask; a step of inoculating the MDCK-HA cells with the H9N2 subtype of avian influenza virus; particularly, in the inoculating step, when monolayer cells in the cells growing in the spinner flask are more than 90%, the H9N2 subtype of avian influenza virus is inoculated and incubated for 1-2 hours in a cell incubator, and 50ml of 2% NCS (new-born calf serum) DMEM (Dulbecco modified eagle medium) maintenance solution is added after the incubation is ended, and the cells inoculated with the H9N2 subtype of avian influenza virus are cultured in the cell incubator, and finally cell supernatant is collected. According to the spinner-flask culture method disclosed by the invention, the MDCK-HA cell system is adopted, and the conditions of spinner-flask culture are optimized, thus the H9N2 subtype of avian influenza virus is rapidly proliferated to reach a high virus titer and obtain a good immune effect.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
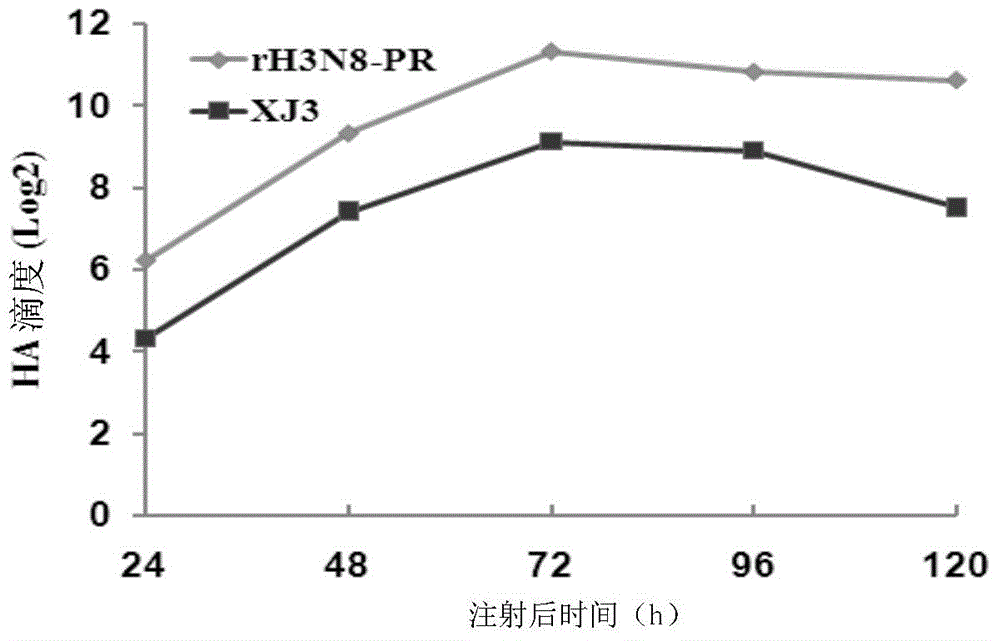
Recombinant equine influenza virus strain, preparation method thereof and vaccine prepared from recombinant equine influenza virus strain
InactiveCN103602638AImproving immunogenicityViral/bacteriophage medical ingredientsMicroorganism based processesCanine kidneyChick embryos
The invention discloses a recombinant equine influenza virus strain, a preparation method thereof and a vaccine prepared from the recombinant equine influenza virus strain. The recombinant influenza virus strain contains genes HA and NA of an equine influenza virus A / equine / xinjiang / 3 / 07 (H3N8) strain and six internal genes PB2, PB1, PA, NP, M and NS of an influenza virus A / Puerto Rico / 8 / 34 / Mount Sinai (H1N1) or A / PR / 8 / 34 (H1N1 short for PR8 virus). The recombinant equine influenza virus strain disclosed by the invention is named as rH3N8-PR and is preserved with the number of CGMCC NO.8161. The invention also discloses a preparation method of the recombinant equine influenza virus strain and a vaccine prepared from the recombinant equine influenza virus strain. Compared with a parental strain, the recombinant equine influenza virus strain disclosed by the invention can generate very high virus titer and blood clotting titer on both chick embryos and MDCK (Madin Darby Canine Kidney) cells, and the pathogenicity of the recombinant equine influenza virus strain to mice is remarkably reduced; experiments prove that the vaccine prepared from the recombinant equine influenza virus strain disclosed by the invention has favorable immunogenicity and protective effect.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of poly mannuronic acid propyl ester sulfate in preparing anti- H1N1 influenza A virus medication
InactiveCN102743409BAvoid infectionPrevent proliferationOrganic active ingredientsAntiviralsCanine kidneyPolymannuronic acid
The invention provides applications of poly mannuronic acid propyl ester sulfate (PMS) in preparing anti- H1N1 influenza A virus medication. Experiments of the invention prove that PMS not only has great inhibition effect on the neuraminidase of the influenza A virus, but also has relatively good protection effect on canine kidney epithelial cells infected with the H1N1 influenza A virus, and can reduce the replication of the H1N1 virus with an effect that equal to the positive control medication ribavirin. Additionally, the PMS can effectively reduce the death rate of mice infected with the H1N1 influenza A virus and the survival time of the mice are prolonged. The poly mannuronic acid propyl ester sulfate provided by the invention has significant activity of inhibiting the neuraminidase of the H1N1 influenza A virus, and is proved to have good anti- H1N1 influenza A virus activity both in vivo and in vitro experiments, which shows the wide application prospect of the poly mannuronic acid propyl ester sulfate in preparing anti-H1N1 influenza A virus medication.
Owner:OCEAN UNIV OF CHINA
Method for culturing MDCK (medin-darby canine kidney) cell proliferation recombination H5N1 subtype avian influenza virus
ActiveCN104531624AIncrease the number ofRapid expansionMicroorganism based processesArtificial cell constructsProduction lineCanine kidney
The invention provides a method for culturing MDCK (medin-darby canine kidney) cell proliferation recombinant H5N1 subtype avian influenza virus comprises the following steps: preparing a chip carrier, pretreating a paper scrap carrier, sterilizing a bioreactor, carrying out preculture, inoculating cells, adsorbing on the paper scrap carrier, continuously culturing cells, inoculating avian influenza virus and harvesting influenza virus bulk. The method for culturing the MDCK cell proliferation recombinant H5N1 subtype avian influenza virus has the beneficial effects that carrier cost is low, hematic coagulating rate of obtained influenza virus bulk is high, the paper scrap carrier is adopted for carrying out bioreactor large-scale culture of MDCK cell proliferation recombinant H5N1 subtype avian influenza virus, large-scale culture can be realized more easily, occupying space of a production line is small, production efficiency is high, and the adopted equipment has the advantages of strong stability, good repeatability and the like.
Owner:山东信得动物疫苗有限公司
Hybridoma cell strain 2C1 capable of secreting CAV-2 monoclonal antibodies, monoclonal antibodies secreted by cell strain and application
The invention discloses a hybridoma cell strain 2C1 capable of secreting CAV-2 monoclonal antibodies, the monoclonal antibodies secreted by the cell strain and application. The preservation number of the hybridoma cell strain 2C1 is CGMCC No. 10878. An indirect immunofluorescence result shows that the monoclonal antibodies secreted by the hybridoma cell strain 2C1 can specifically recognize CAV-2 viruses and do not react with MDCK (madin-darby canine kidney) cells. A result of a superposition experiment of the hybridoma cell strain 2C1 and two monoclonal antibodies secreted by another hybridoma cell strain shows that the two monoclonal antibodies are used for different antigenic epitopes of the CAV-2 viruses. The monoclonal antibody prepared by the invention can react with CAV-2 well and can be used for identifying a clinic isolated strain. The 2C1 monoclonal antibody and the 7D7 monoclonal antibody which are prepared by the invention are used for recognizing two different antigen determinants of the CAV-2, so that the monoclonal antibodies can be used for detecting preparation of CAV-2 pathogenic colloidal gold and establishment of a sandwiched ELISA (enzyme-linked immuno sorbent assay) method.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com