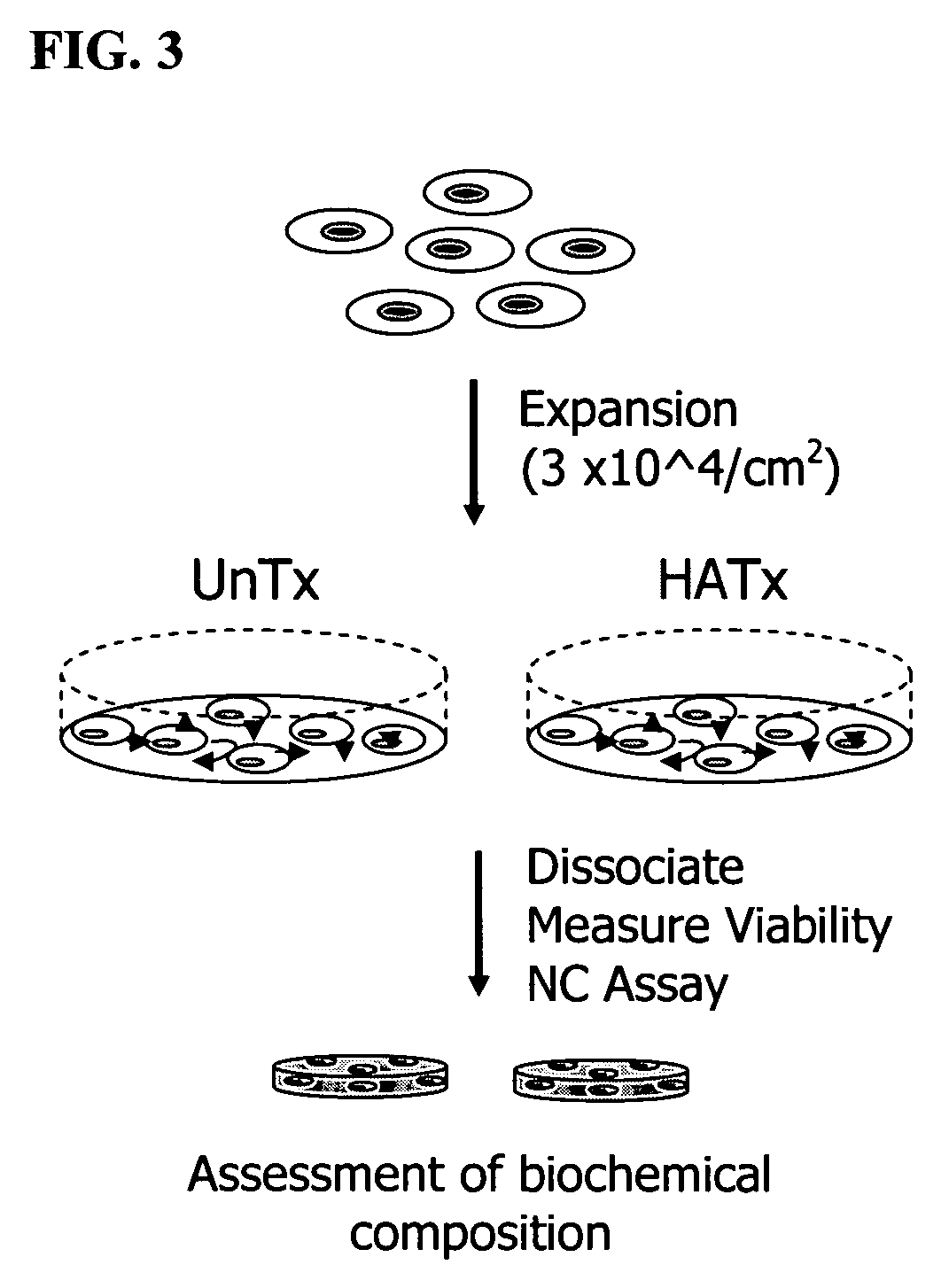
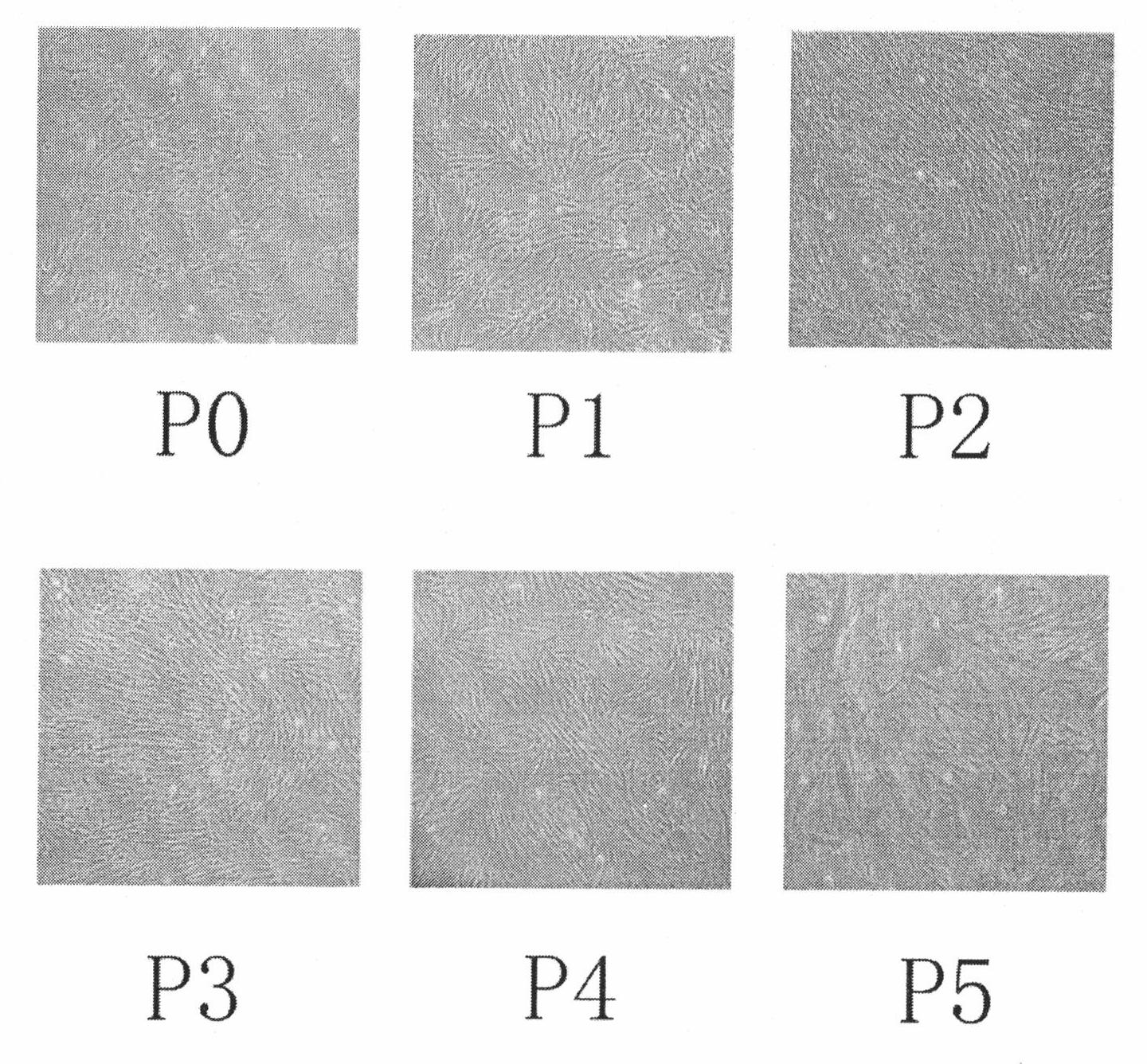
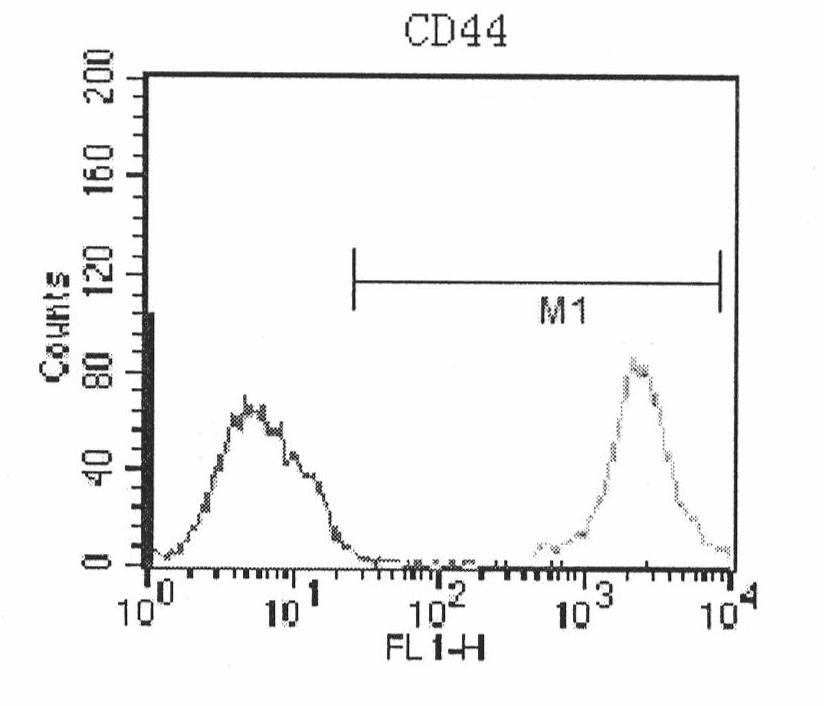
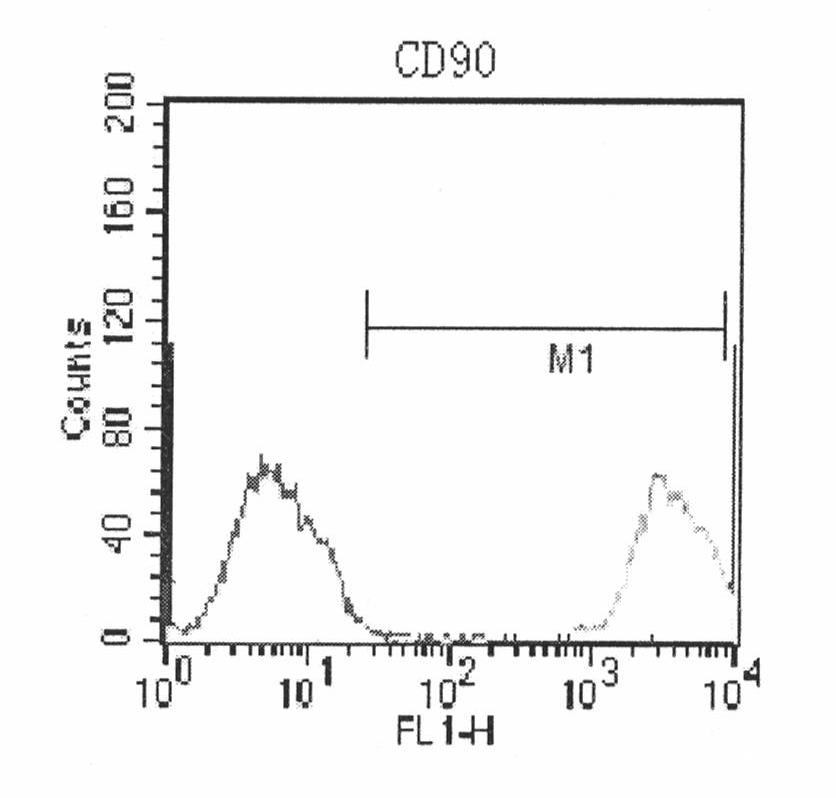
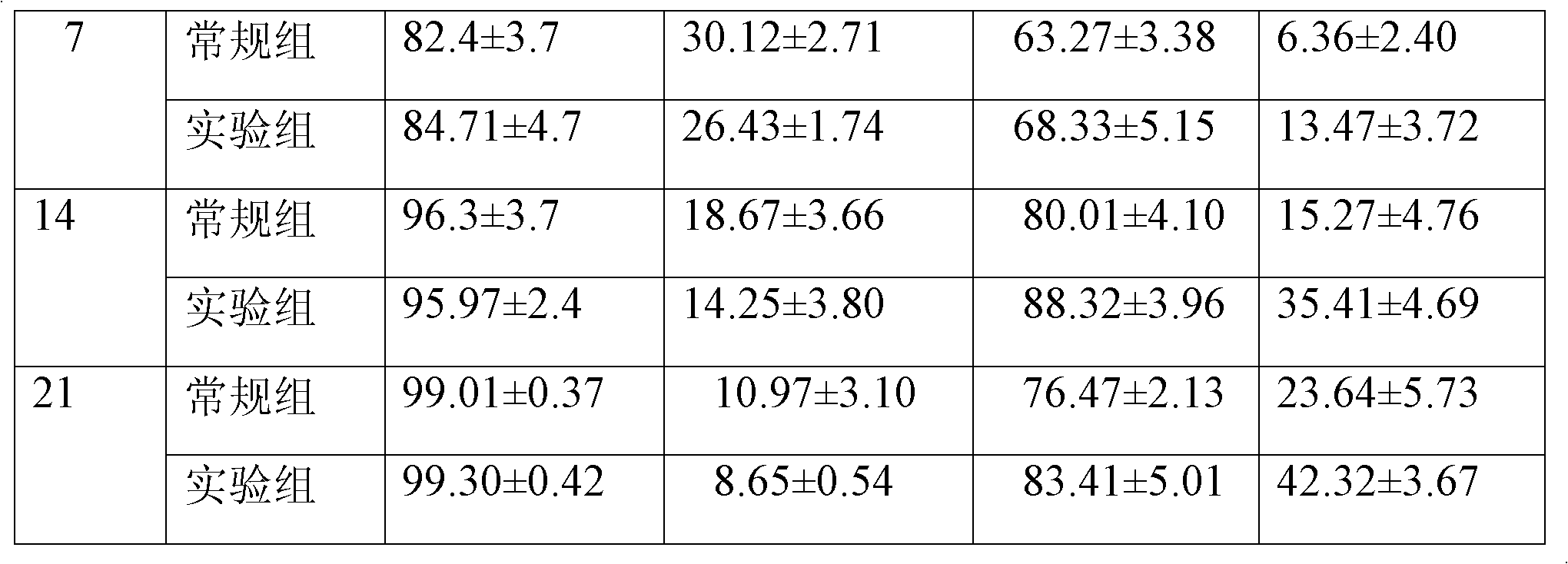
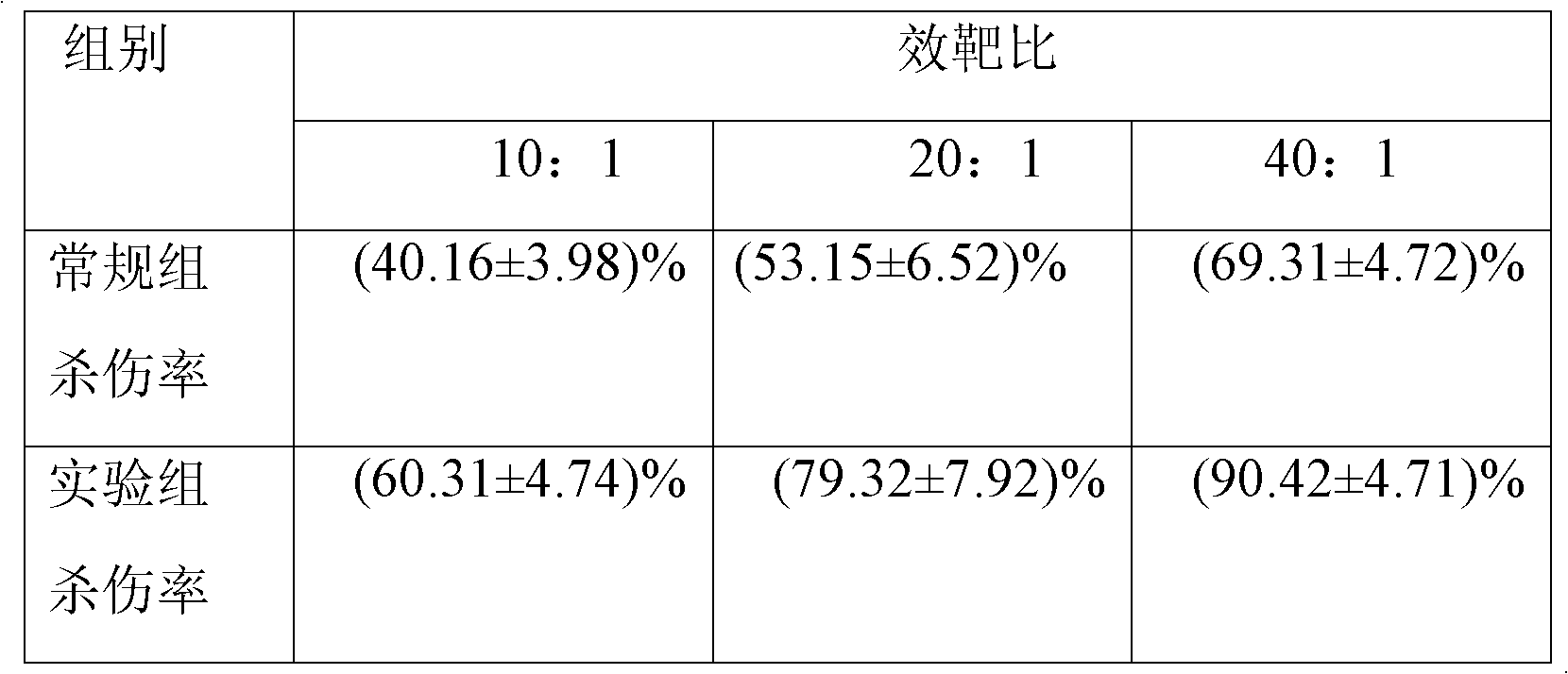
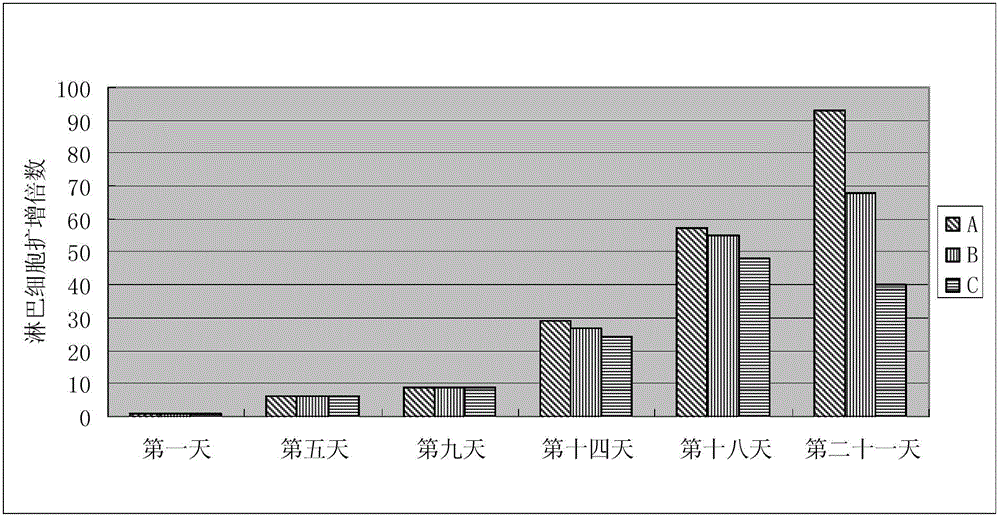
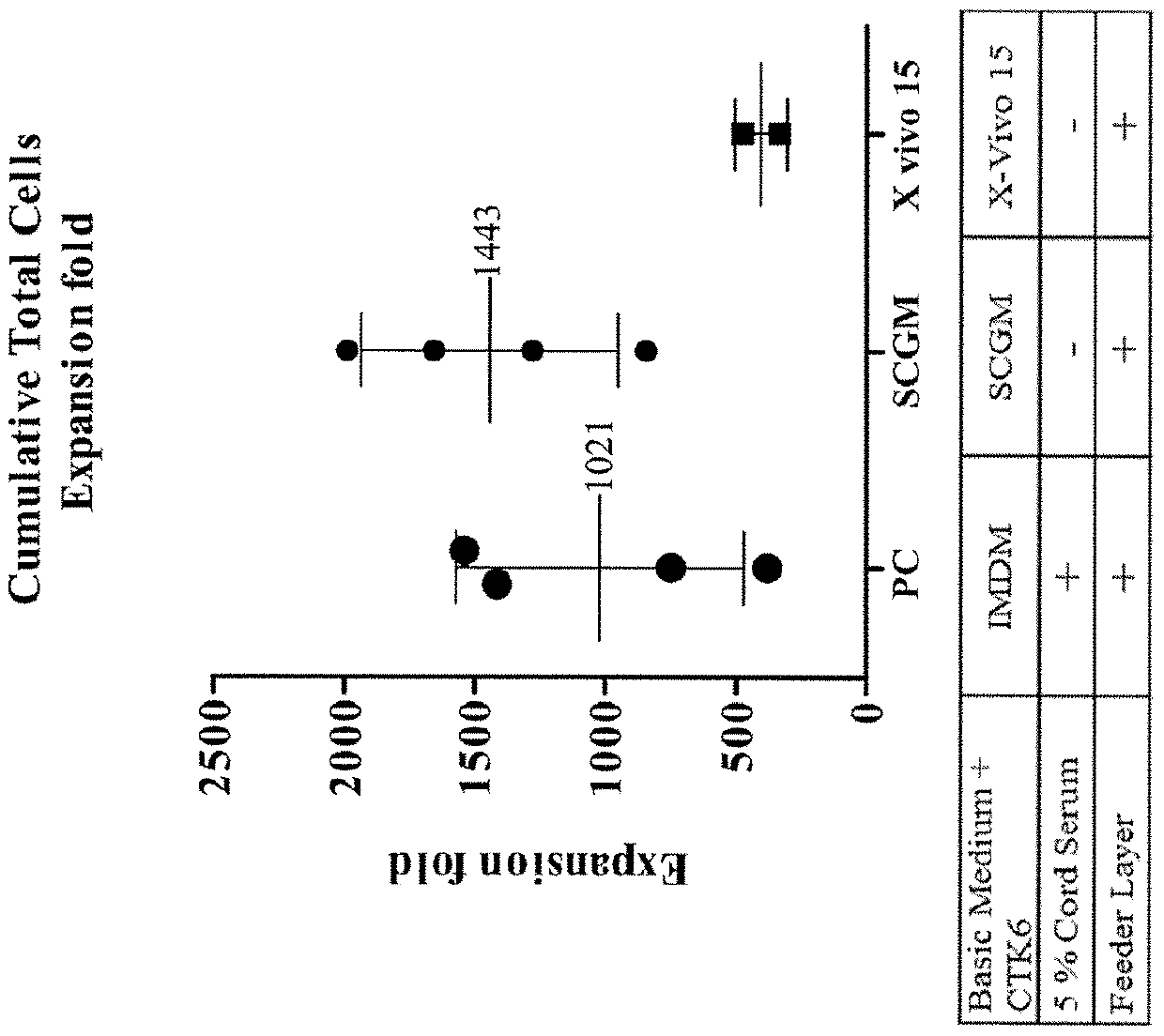
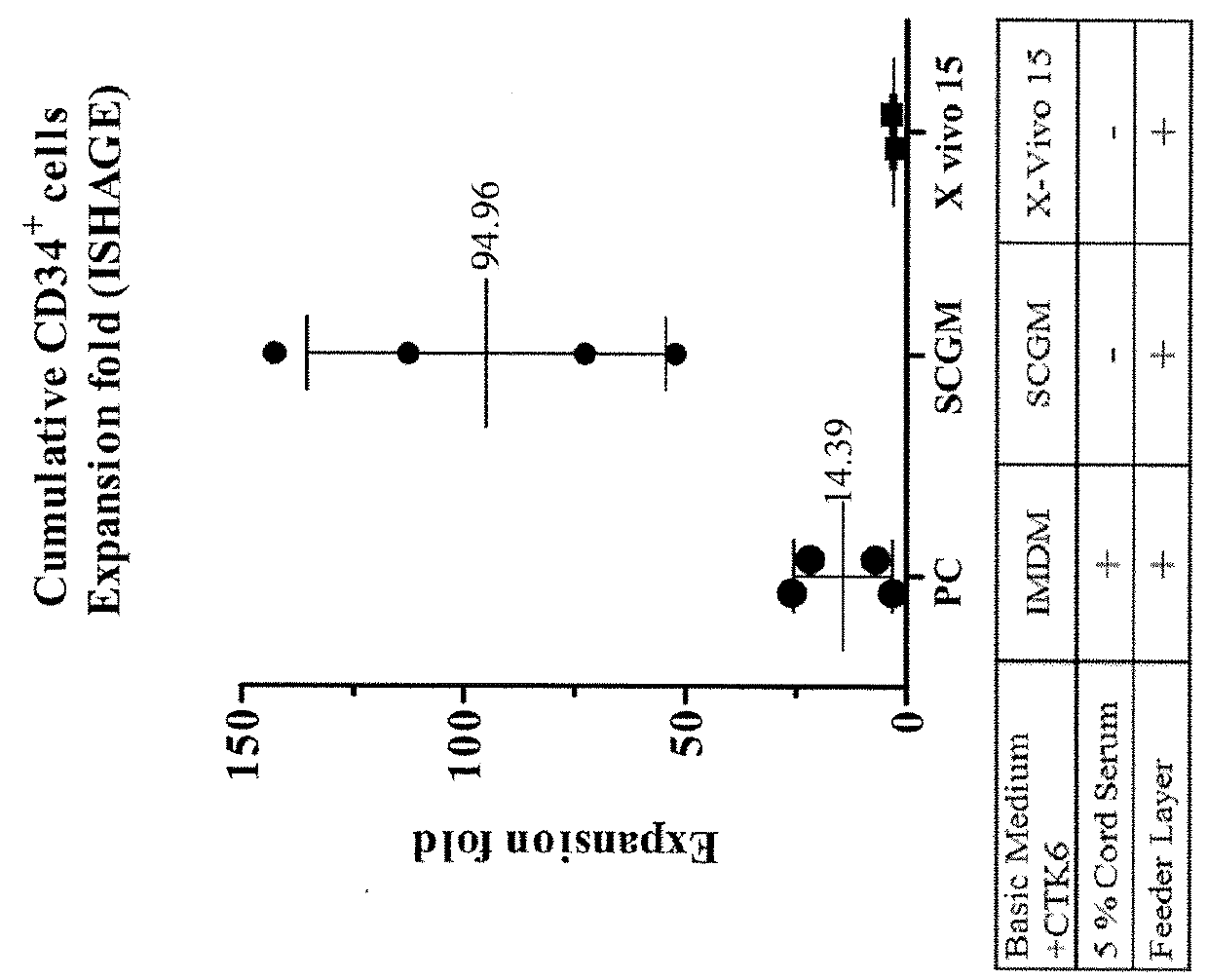
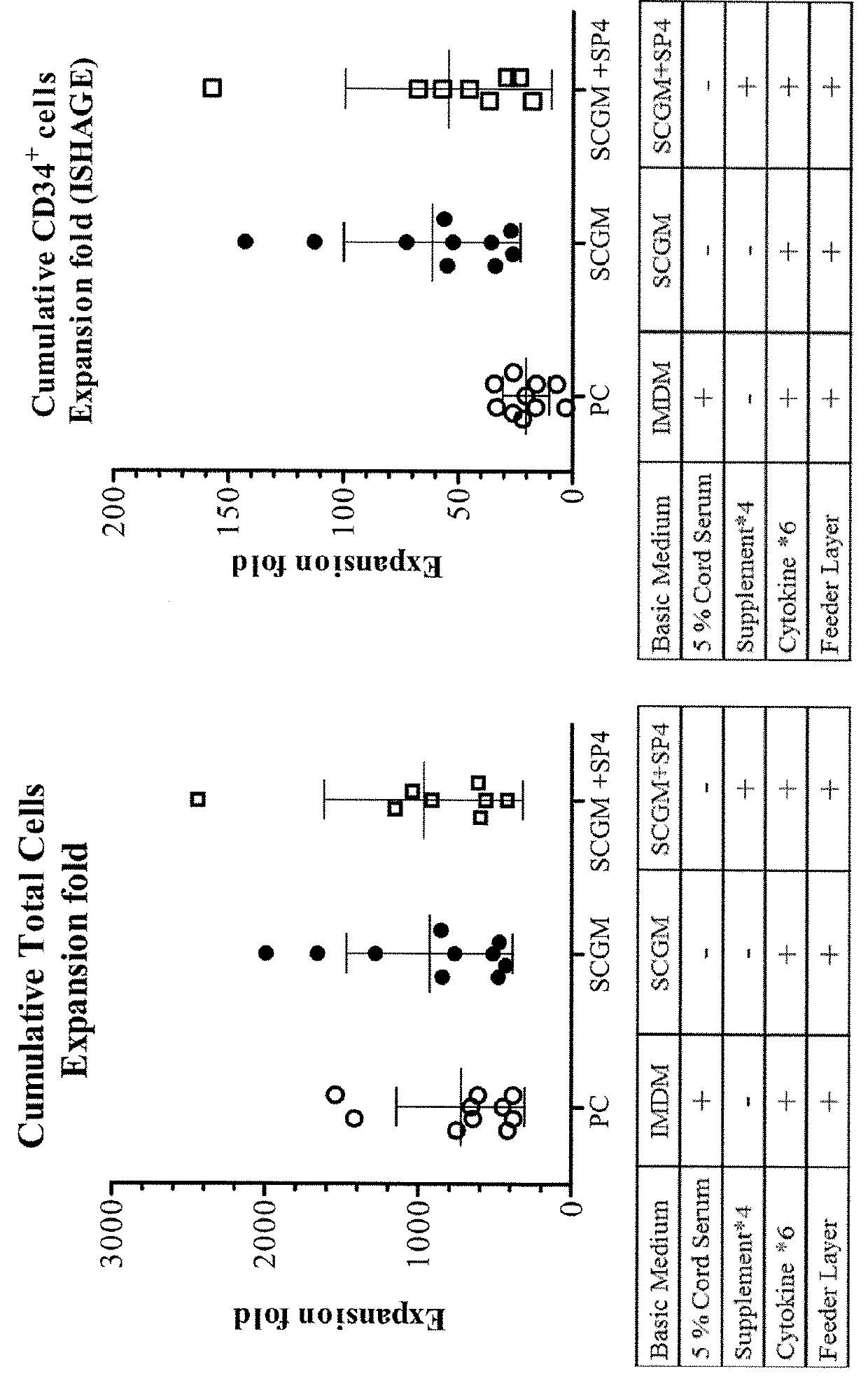
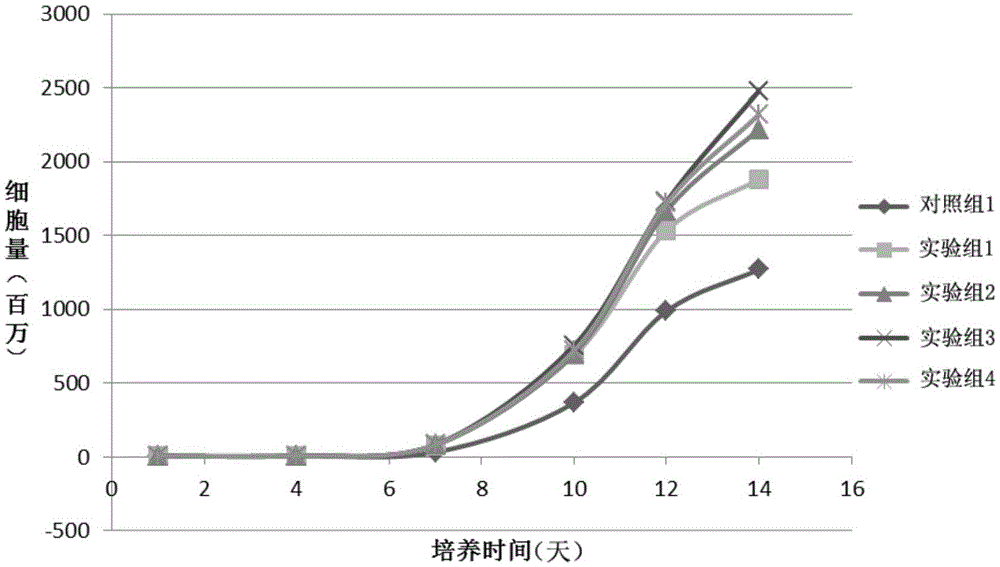
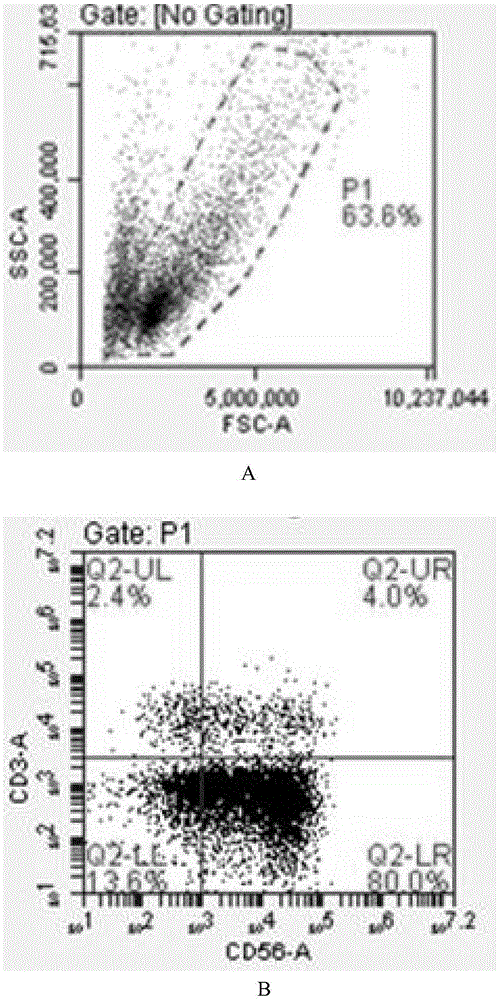
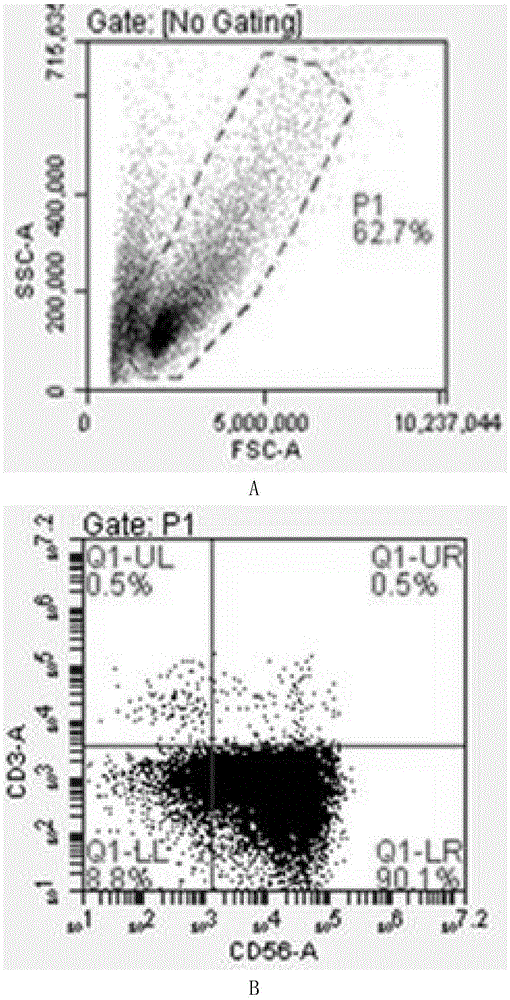
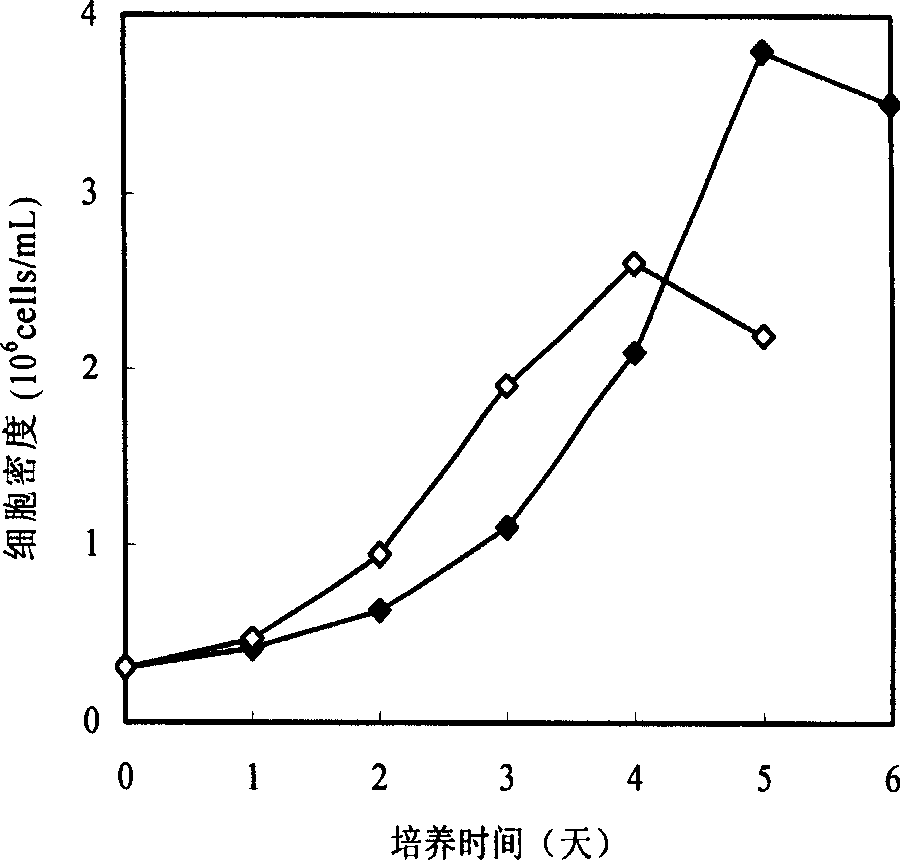
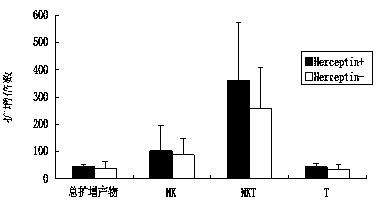Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
629 results about "Serum-Free Culture Media" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
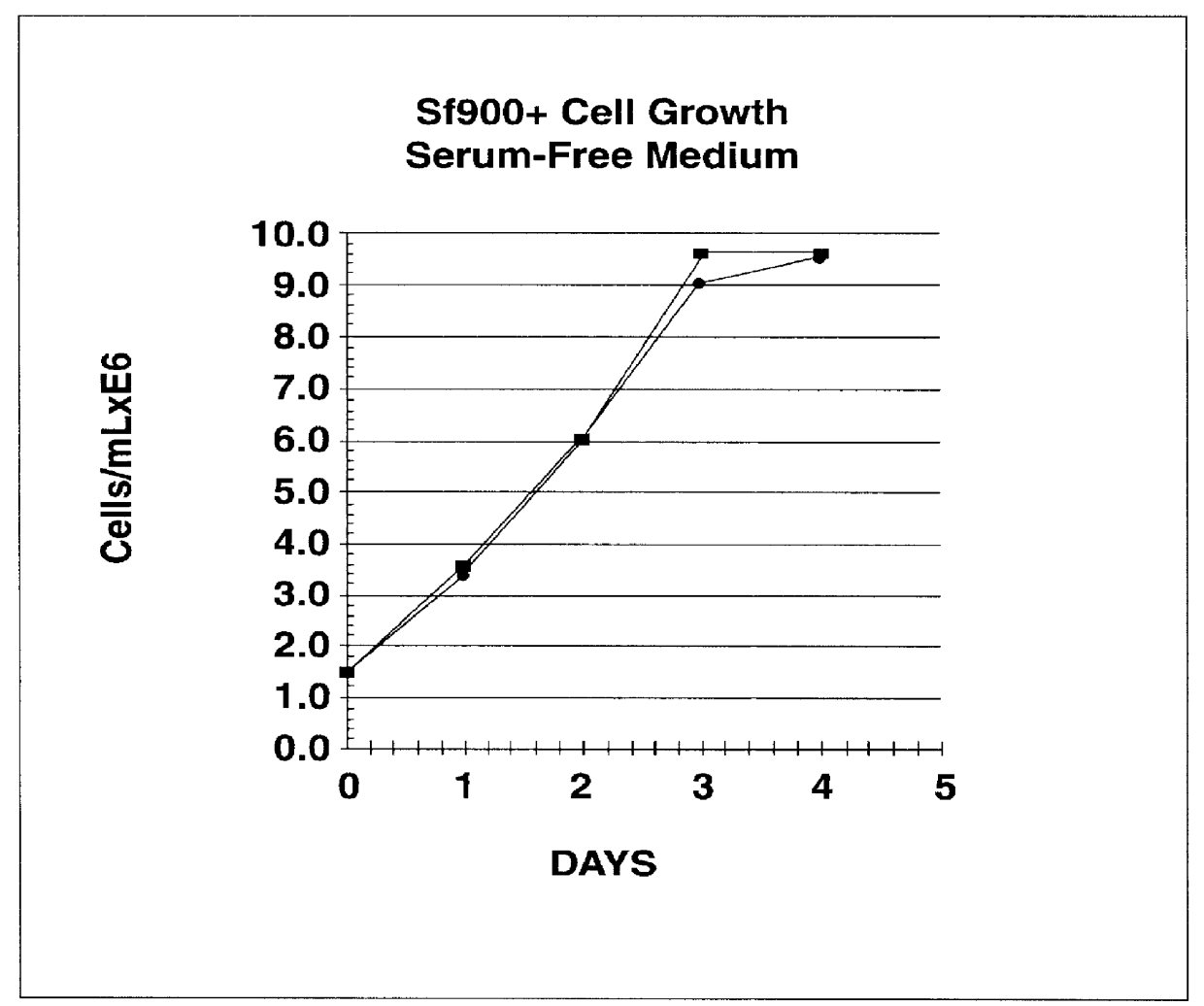
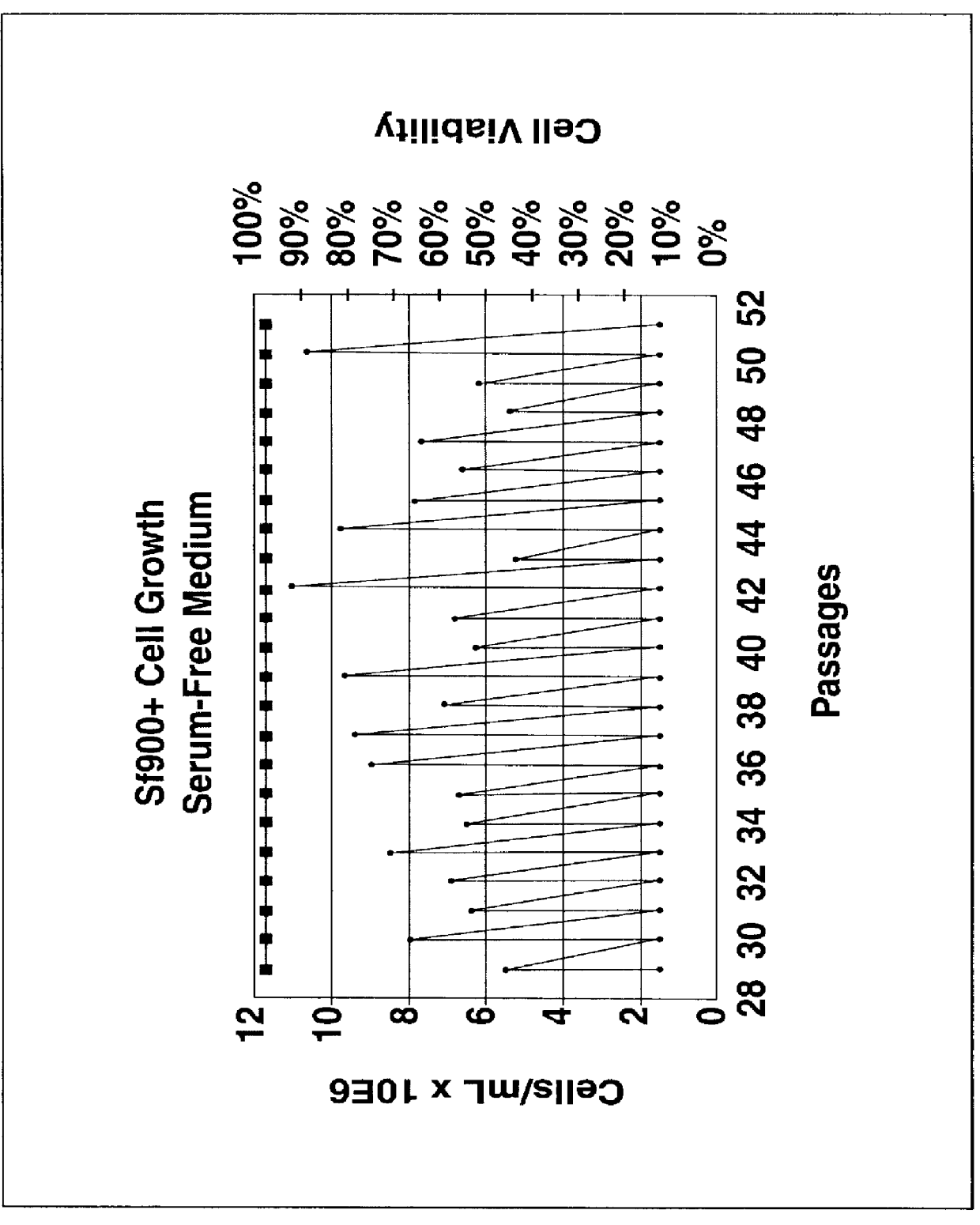
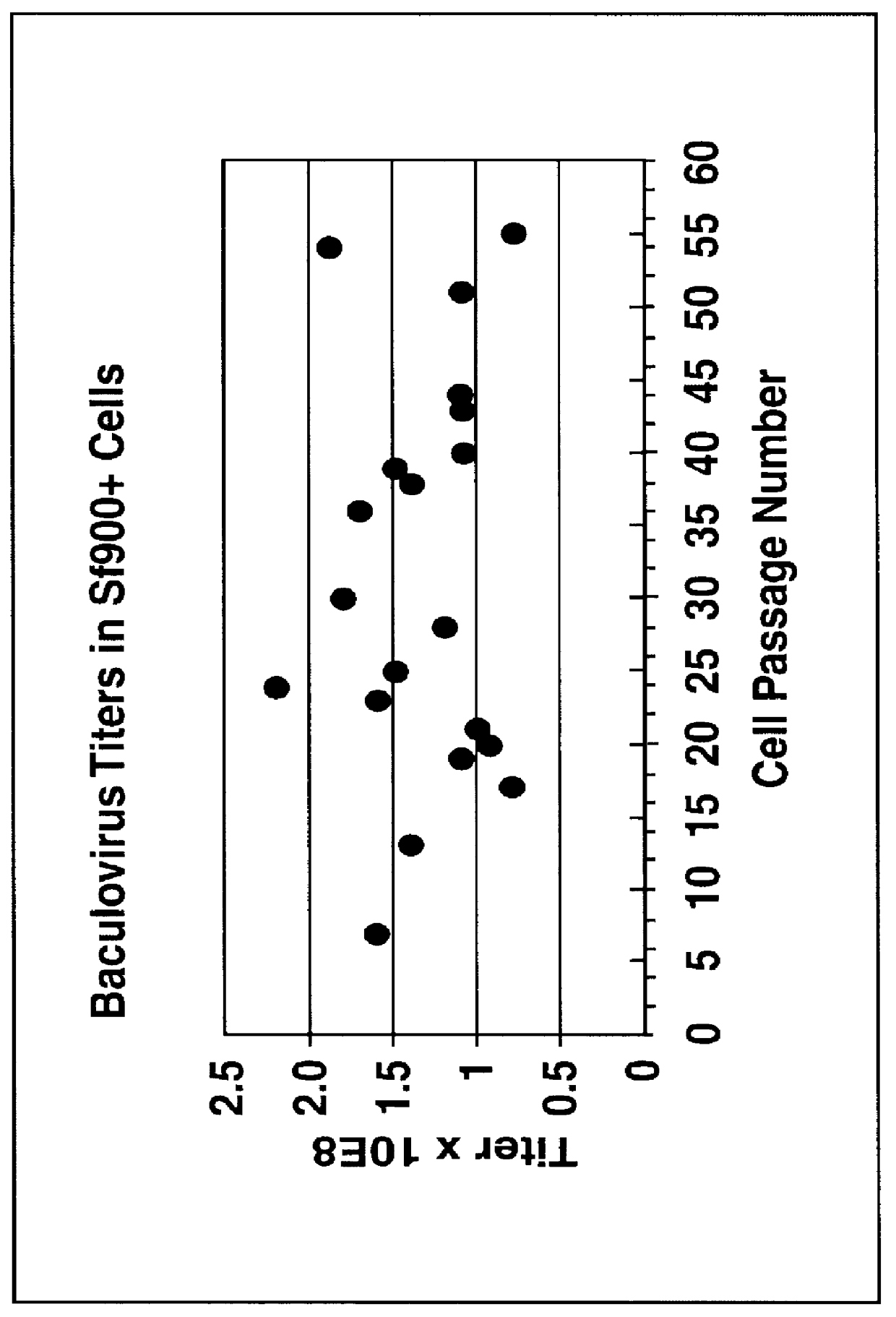
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Method for in vitro amplifying, and in 3D solid culturing nerve stem
InactiveCN101092606AUniform penetrationIncrease the cultivation areaNervous system cellsCuticleCell growth
This invention relates to a method for amplifying neural stem cells in vitro by 3-dimensional culture. The method comprises: selecting microcarrier with 3-dimensional environment, pre-treating, coating the microcarrier with 40-60 ng / mL alkaline fibroblast growth factor, 40-60 ng / mL epidermal growth factor, and B27 DMEM / F12 neural stem cell serum-free culture medium, adding 1X105-1X106 neural stem cells into the culture bottle, taking out the microcarrier grown with neural stem cells, removing the microcarrier, and rinsing cells to obtain neural stem cells. The porous microcarrier can enlarge the culture area. The alkaline fibroblast growth factor and epidermal growth factor can promote cell multiple fission and improve cell microenvironment, which is advantageous for multiple fission of neural stem cells.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
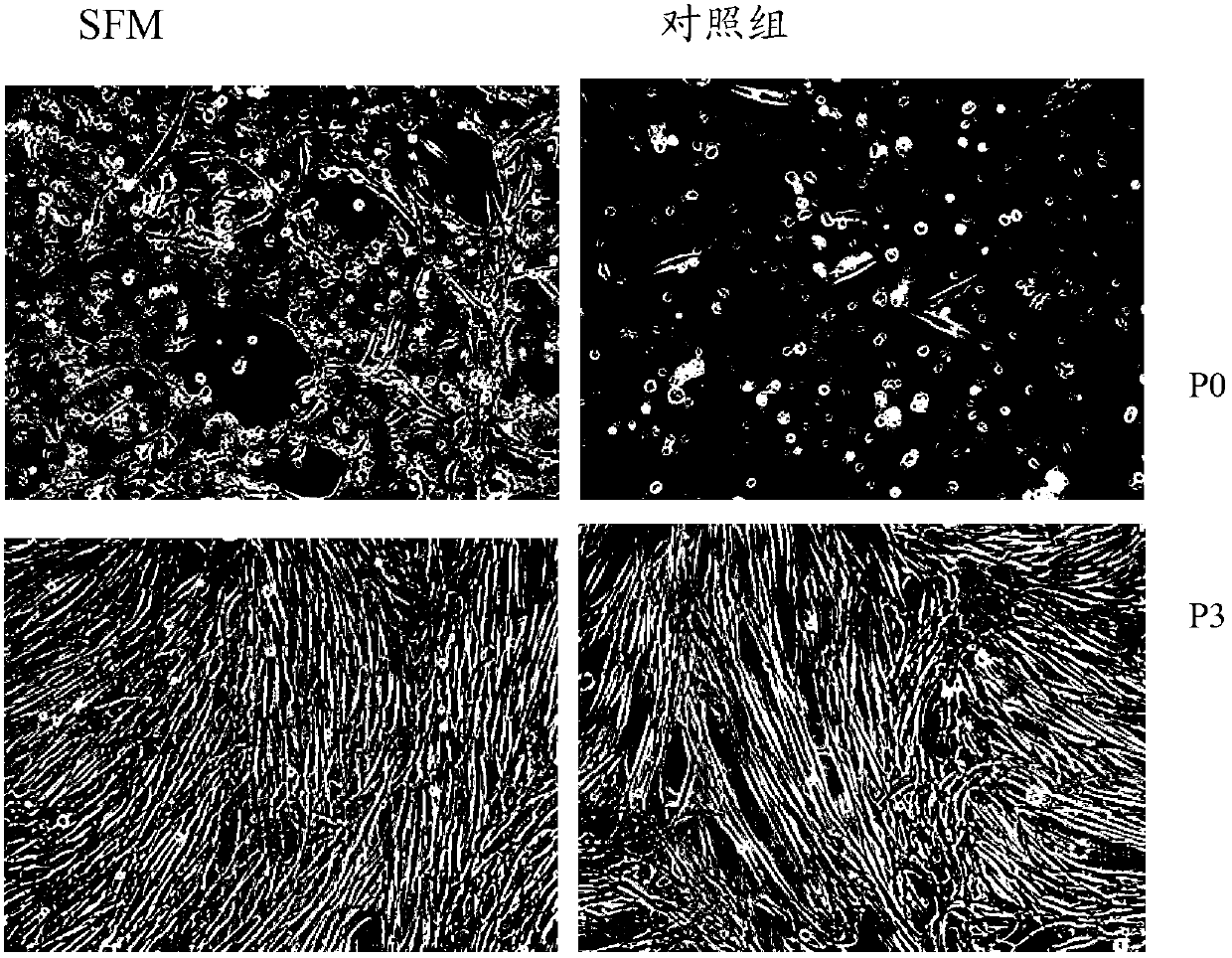
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
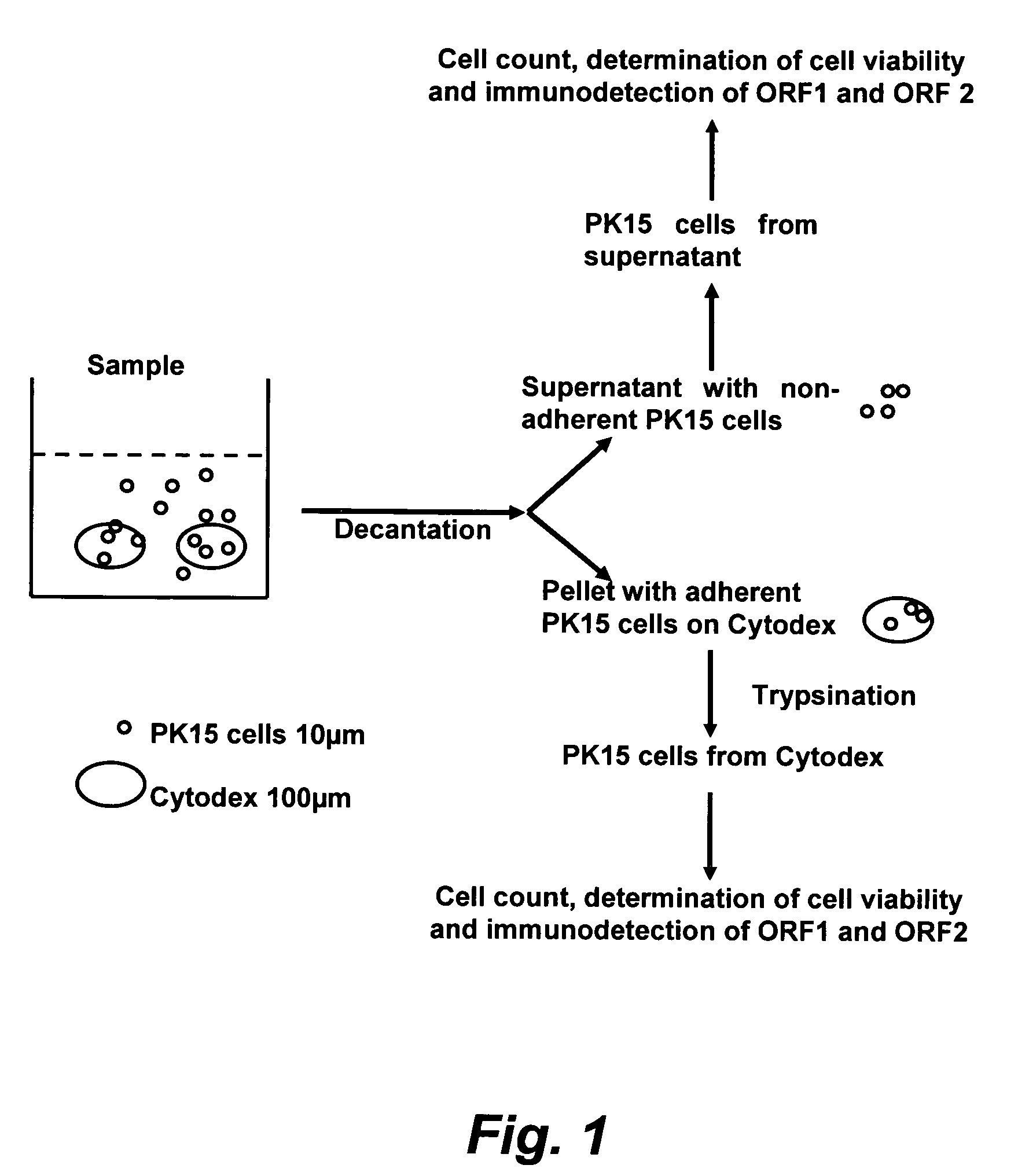
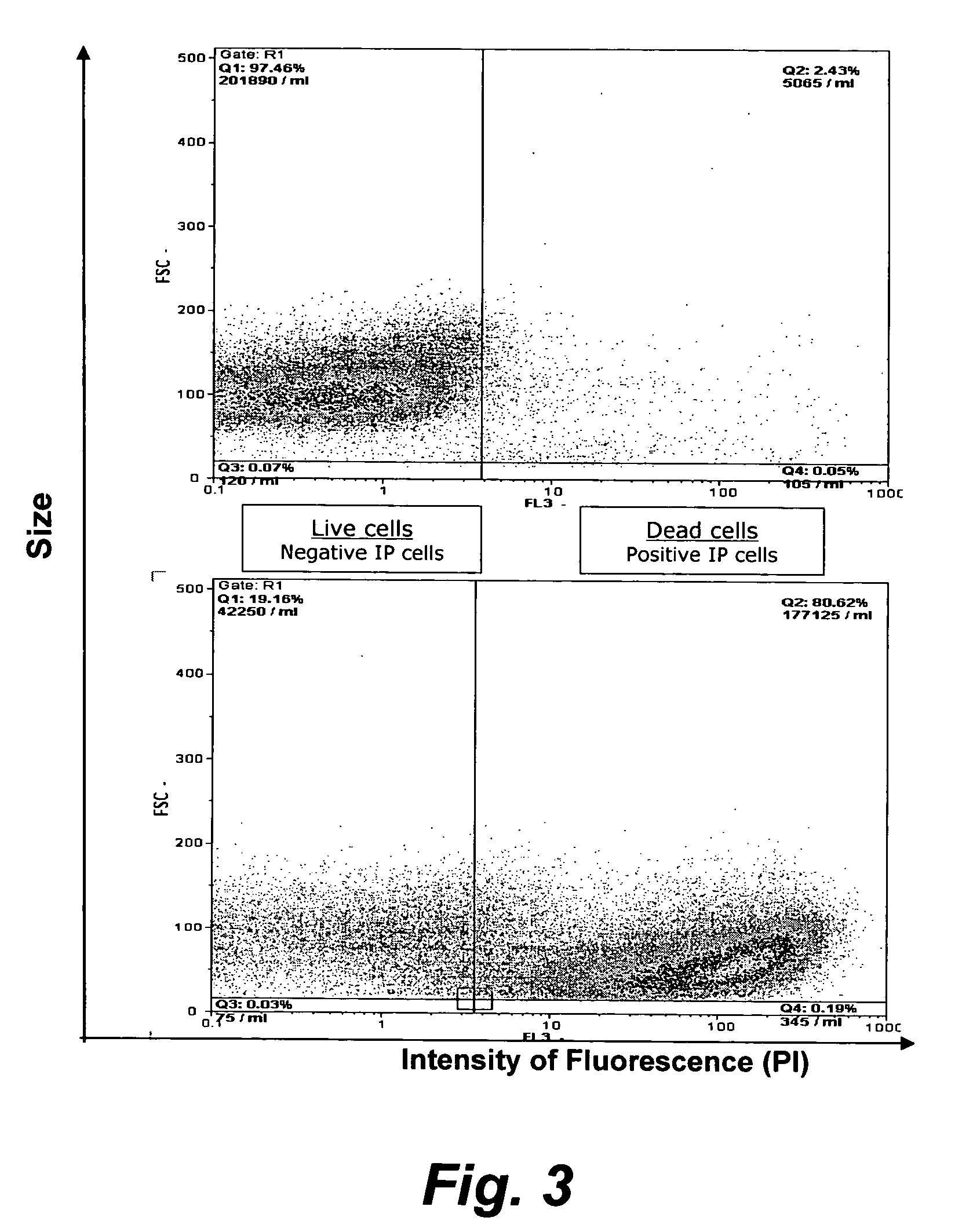
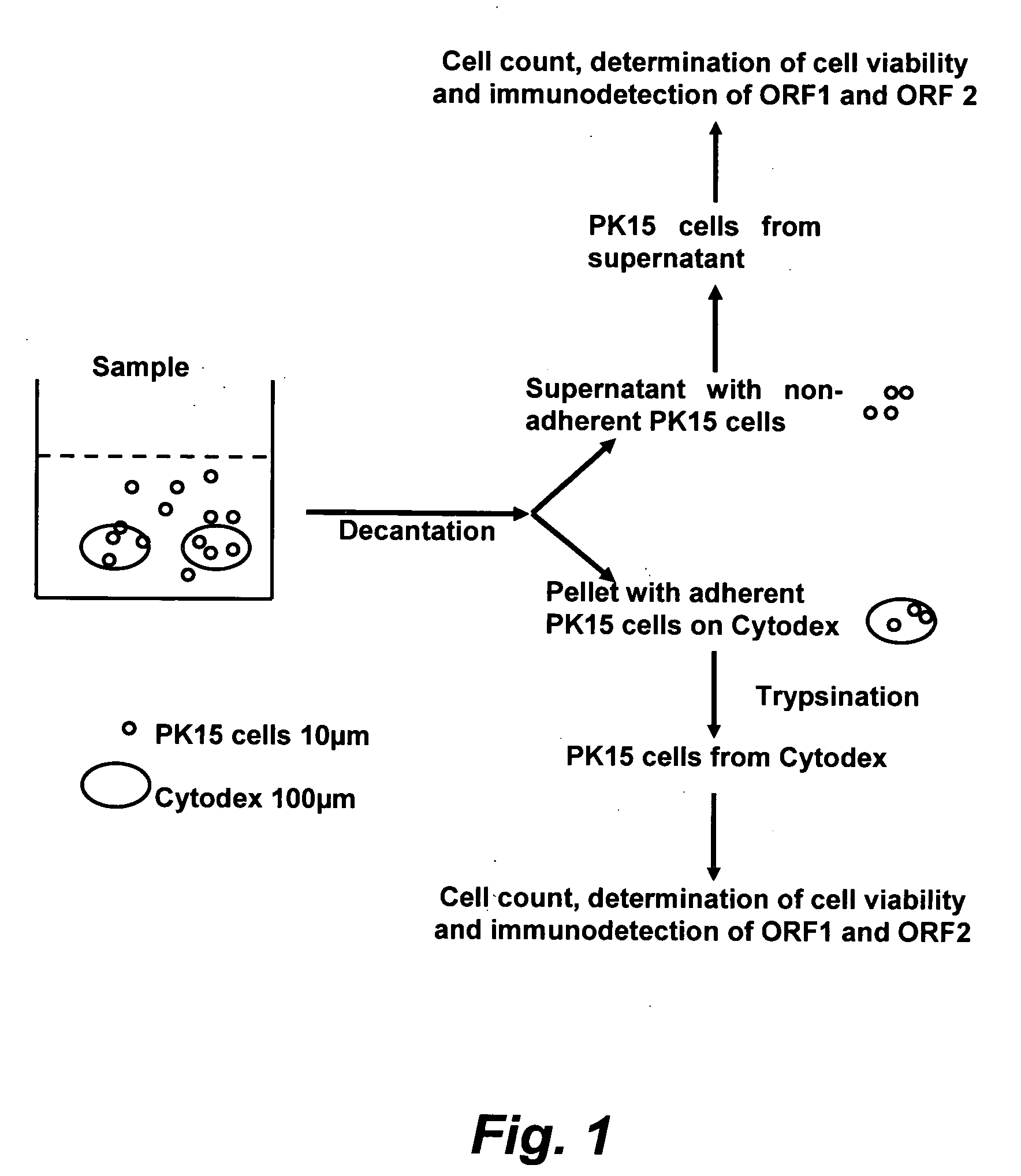
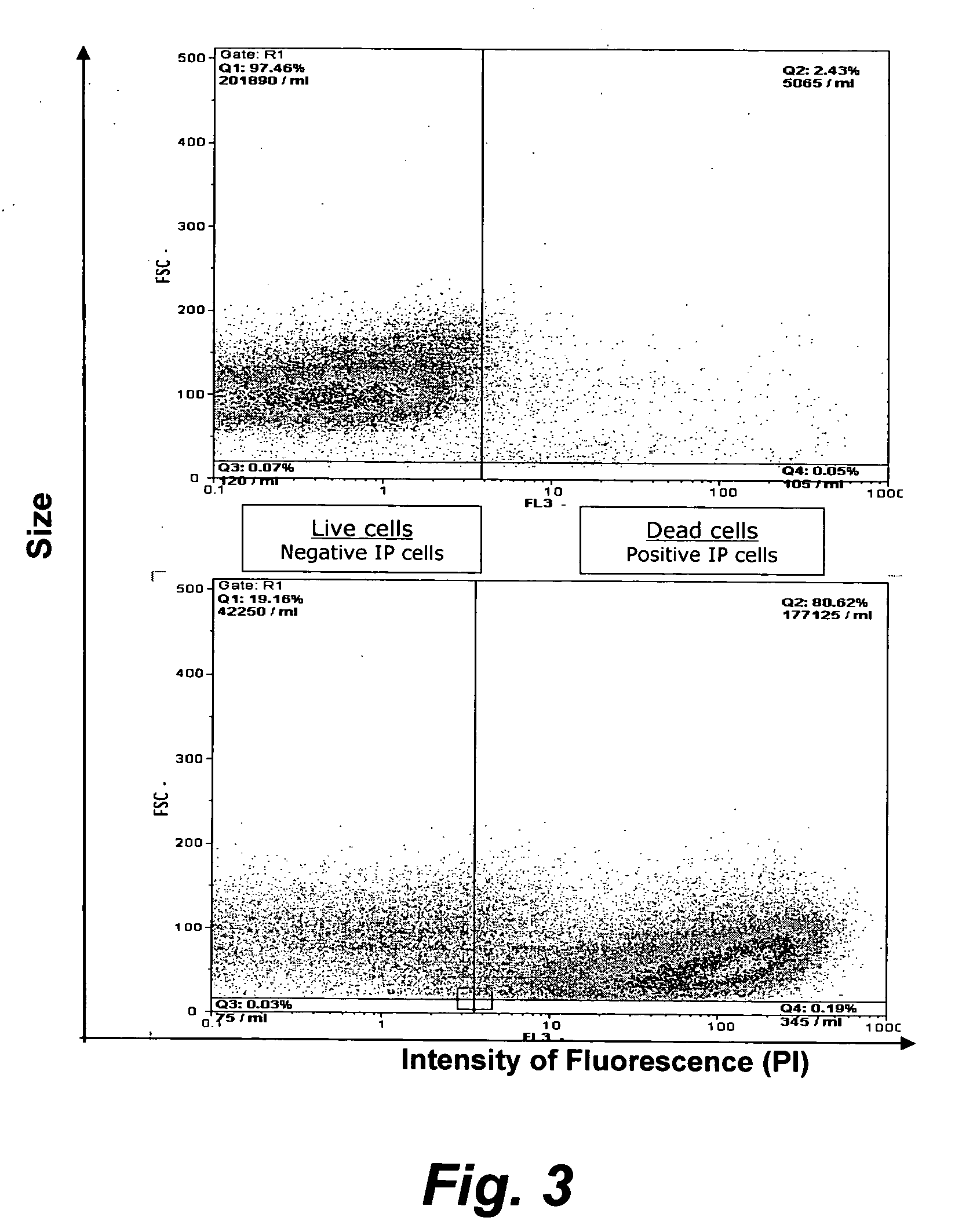
Assay for porcine circovirus production
ActiveUS7358075B2Microbiological testing/measurementBiological material analysisAssayCell culture media
The present invention provides methods for the determination of the viral titer of a culture of host animal host cells infected with a circovirus. The FACS-based methods of the invention may include determining the viability of the host cells in a cell culture medium supernatant and of those cells that remain adherent to a solid support. Detecting and measuring the percentage of cells that expressed the viral antigens ORF1 and ORF2 may determine the viral load of the cultured host cells. The yield of the virus may be established by the detection and measurement of both antigens in supernatant cells, for example 5 to 7 days from when the host cells are transferred to a serum free medium. The methods of the invention may yield rapid quantitative data. This allows the repeated in-process monitoring of the viral production throughout the incubation period, and ready selection of the most appropriate harvesting point.
Owner:MERIAL INC
Assay for porcine circovirus production
ActiveUS20060246425A1Microbiological testing/measurementBiological material analysisIncubation periodViral load
The present invention provides methods for the determination of the viral titer of a culture of host animal host cells infected with a circovirus. The FACS-based methods of the invention may include determining the viability of the host cells in a cell culture medium supernatant and of those cells that remain adherent to a solid support. Detecting and measuring the percentage of cells that expressed the viral antigens ORF1 and ORF2 may determine the viral load of the cultured host cells. The yield of the virus may be established by the detection and measurement of both antigens in supernatant cells, for example 5 to 7 days from when the host cells are transferred to a serum free medium. The methods of the invention may yield rapid quantitative data. This allows the repeated in-process monitoring of the viral production throughout the incubation period, and ready selection of the most appropriate harvesting point.
Owner:MERIAL INC
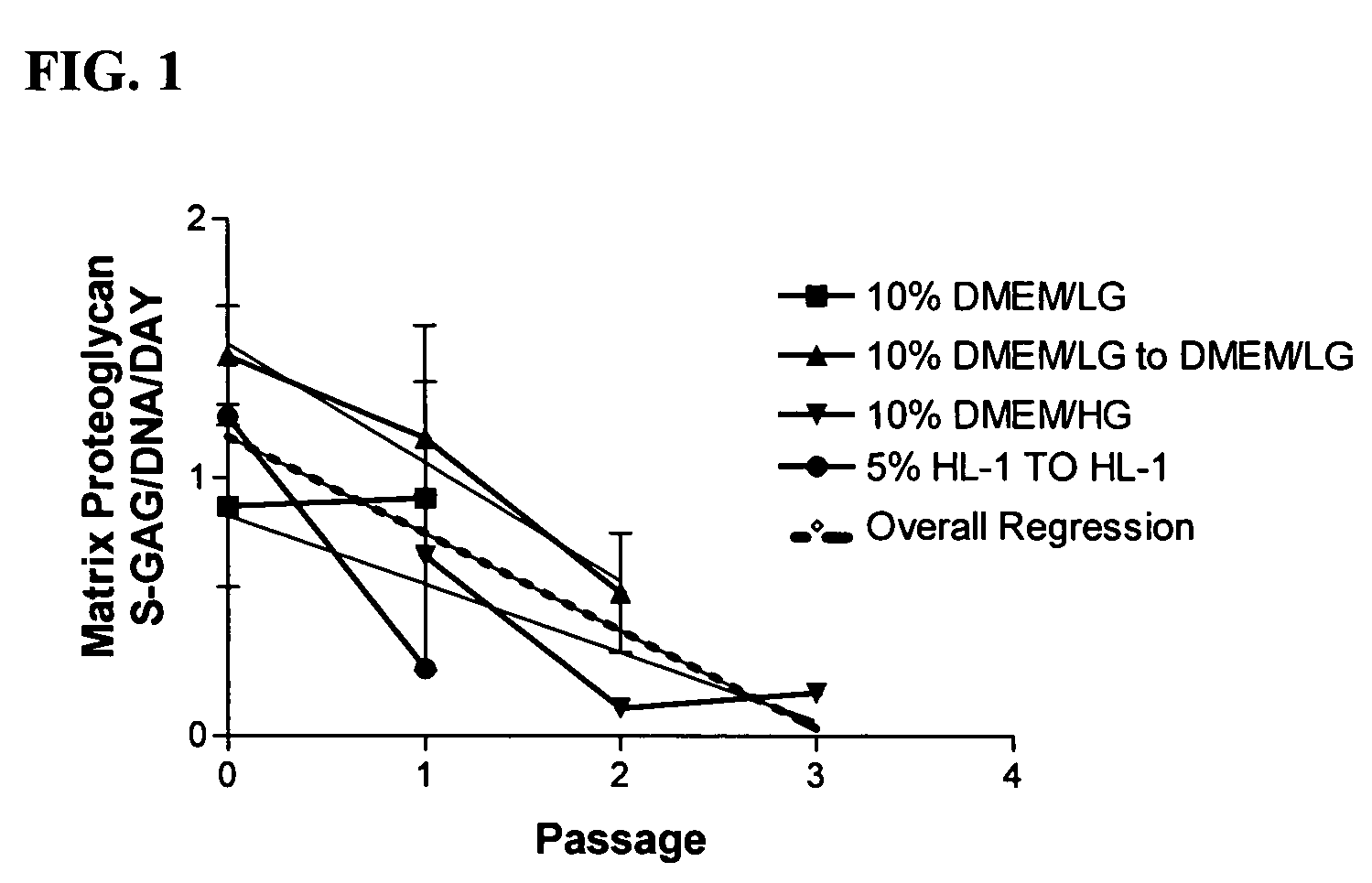
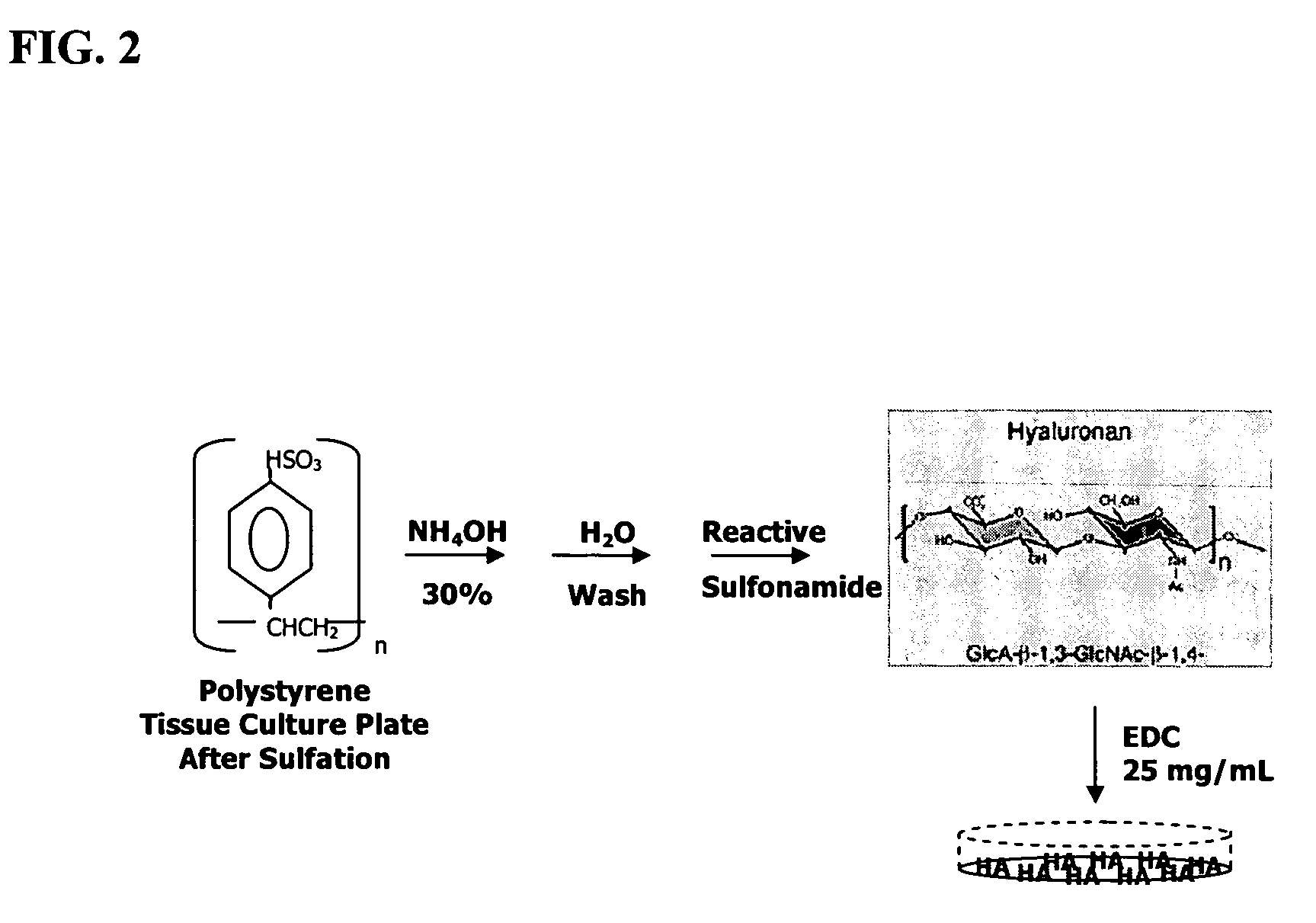
Method for chondrocyte expansion with phenotype retention
The present invention provides a method that maintains chondrocyte phenotype during serial expansion by culturing a population of chondrocytes in a defined serum-free culture medium containing cytokines and on a substrate that is modified by covalent attachment of hyaluronic acid. The underlying principle is to maintain native chondrocyte phenotype by growing the dissociated chondrocytes on a substrate modified by covalent attachment of hyaluronic acid to retain native chondrocyte morphology and function. Chondrocyte expanded in this manner can be used in various medical applications to repair cartilaginous tissues that have been injured by trauma or disease. This substratum provides a microenvironment that more closely mimics that of native articular cartilage, thereby promoting chondrogenesis in a predictable manner.
Owner:ZIMMER INC +1
Method of Inducing the Differentiation of Embryonic Stem Cells Into Nerve by Serum-Free Suspension Culture
ActiveUS20080044901A1Effectively lead to differentiationEfficient inductionNervous system cellsEmbryonic cellsSerum free mediaNervous system
The present invention provides a clinically applicable method of inducing differentiation of embryonic stem cells, particularly a method of inducing differentiation of embryonic stem cells into forebrain neurons. More specifically, the present invention provides a method of inducing differentiation of embryonic stem cells, comprising culturing the embryonic stem cells as a floating aggregate in a serum-free medium, particularly a method of inducing differentiation of the embryonic stem cells into nervous system cells such as forebrain neurons and cerebellar neurons and sensory organ cells; a floating aggregate of embryonic stem cells obtained by culturing the embryonic stem cells as a floating aggregate in a serum-free medium; and cells derived from a floating aggregate of embryonic stem cells, particularly nervous system cells such as forebrain neurons and cerebellar neuron, sensory organ cells such as retinal precursor cells, and the like.
Owner:RIKEN
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
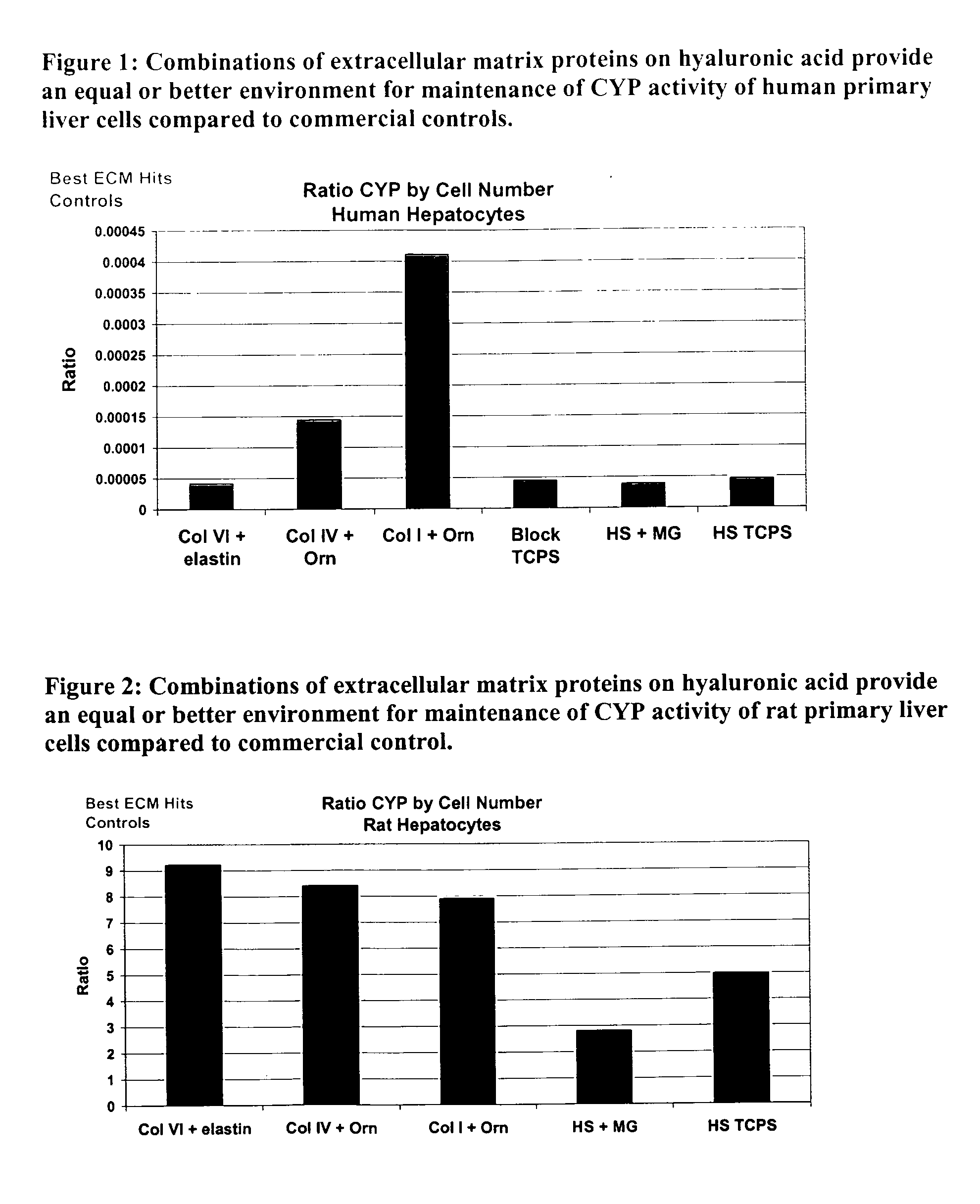
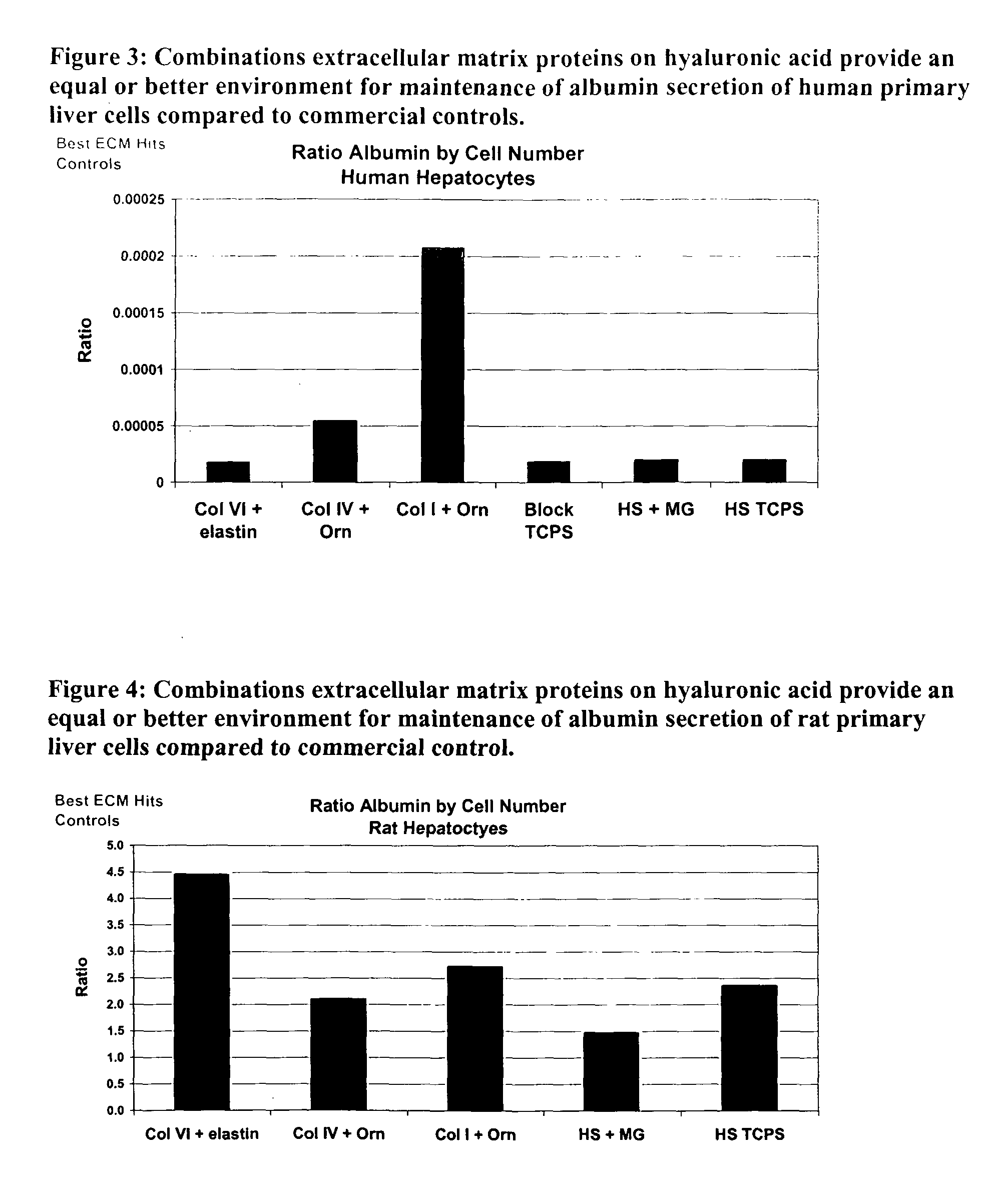
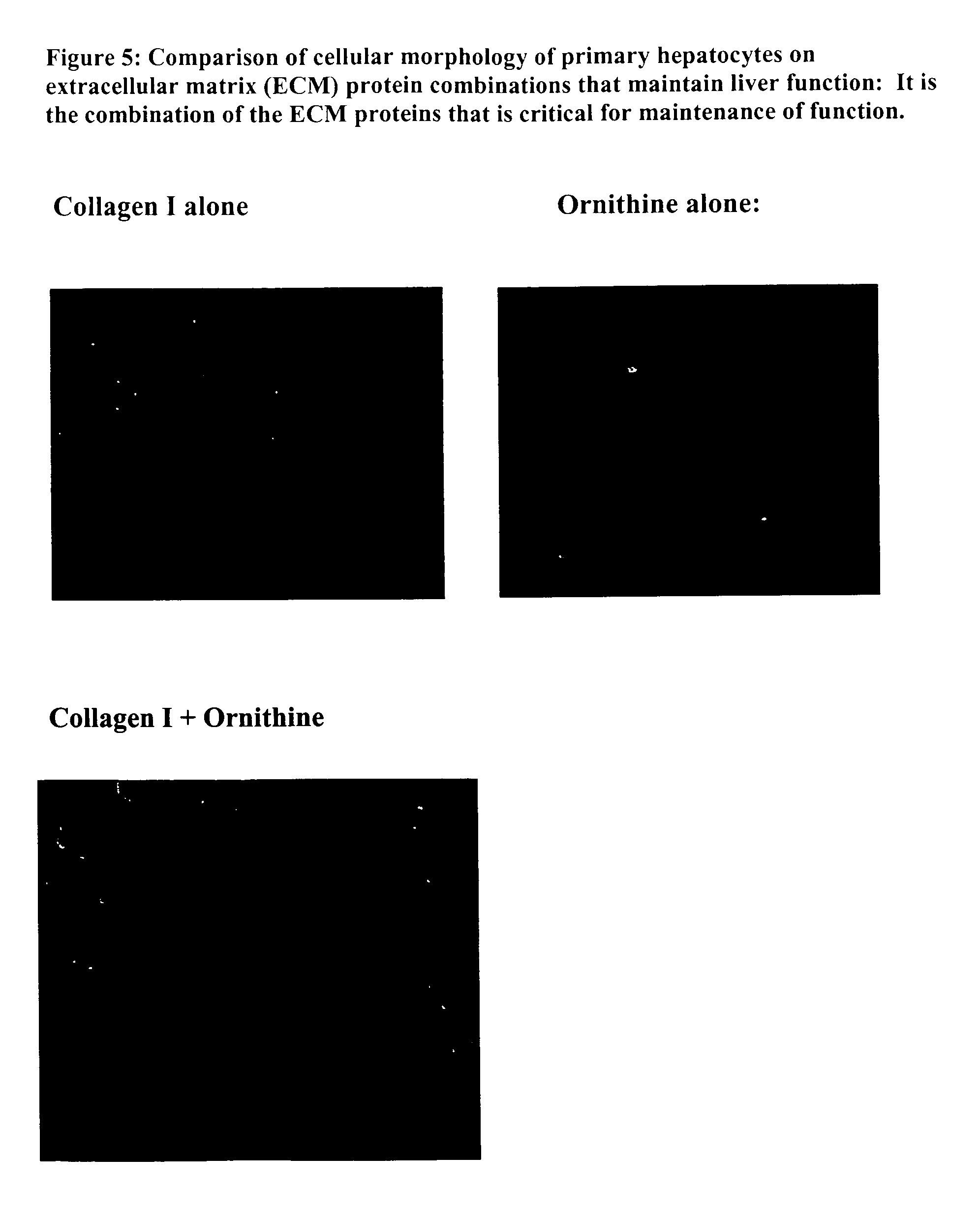
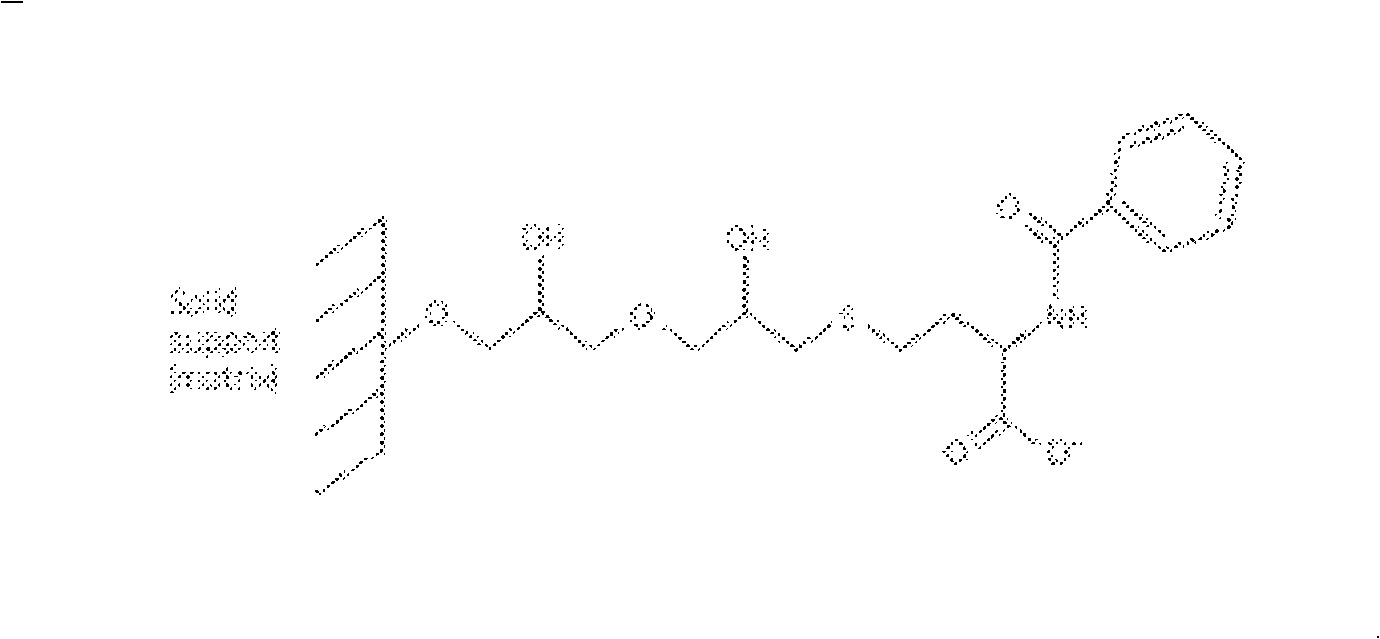
Environments that maintain function of primary liver cells
InactiveUS20050059150A1Good adhesionEasy maintenanceHepatocytesArtificial cell constructsECM ProteinL-Ornithine
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, an ECM protein, or a biologically active fragment or variant thereof such as elastin, fibronectin, vitronectin, laminin, collagen I, collagen III, collagen IV, and collagen VI. Also, optionally present on the surface is an active factor, preferably a polycationic polymer or a biologically active fragment or variant thereof, such as polyethyleneimine (PEI), poly-D-lysine (PDL), poly-L-lysine (PLL), poly-D-ornithine (PDO) or poly-L-ornithine (PLO). This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of primary liver cells. The invention also relates to methods utilizing this surface, such as methods for attachment, survival, and / or proliferation of cells. Further disclosed is the use of the surface in cell culture with serum-free medium. Methods of screening using the surface of the invention are also disclosed.
Owner:BECTON DICKINSON & CO
Technique for separating and purifying recombination human serum albumin and fusion protein thereof
ActiveCN101260145ASimplified purification stepsUniquePeptide preparation methodsHybrid peptidesProtein targetIon exchange
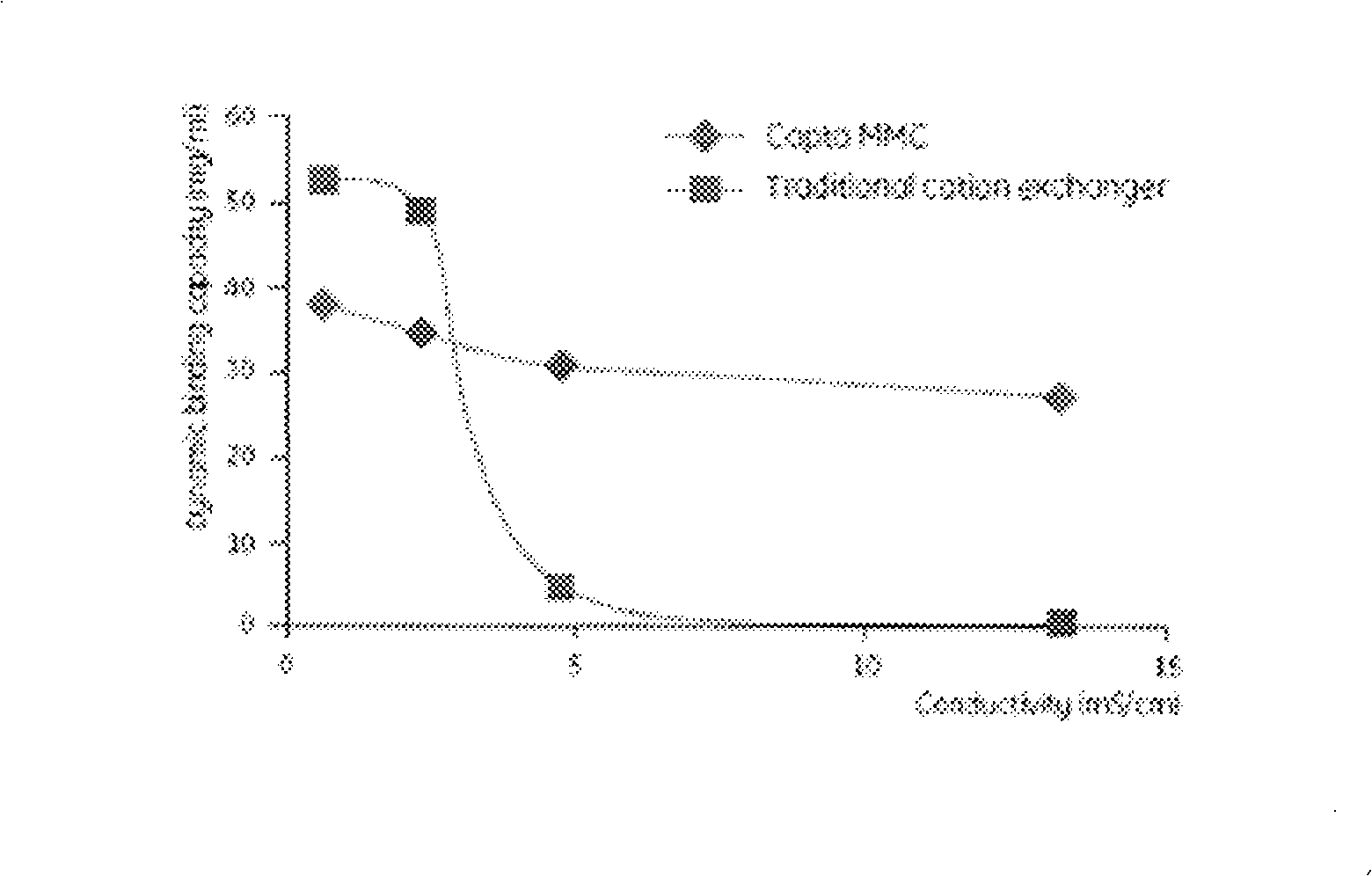
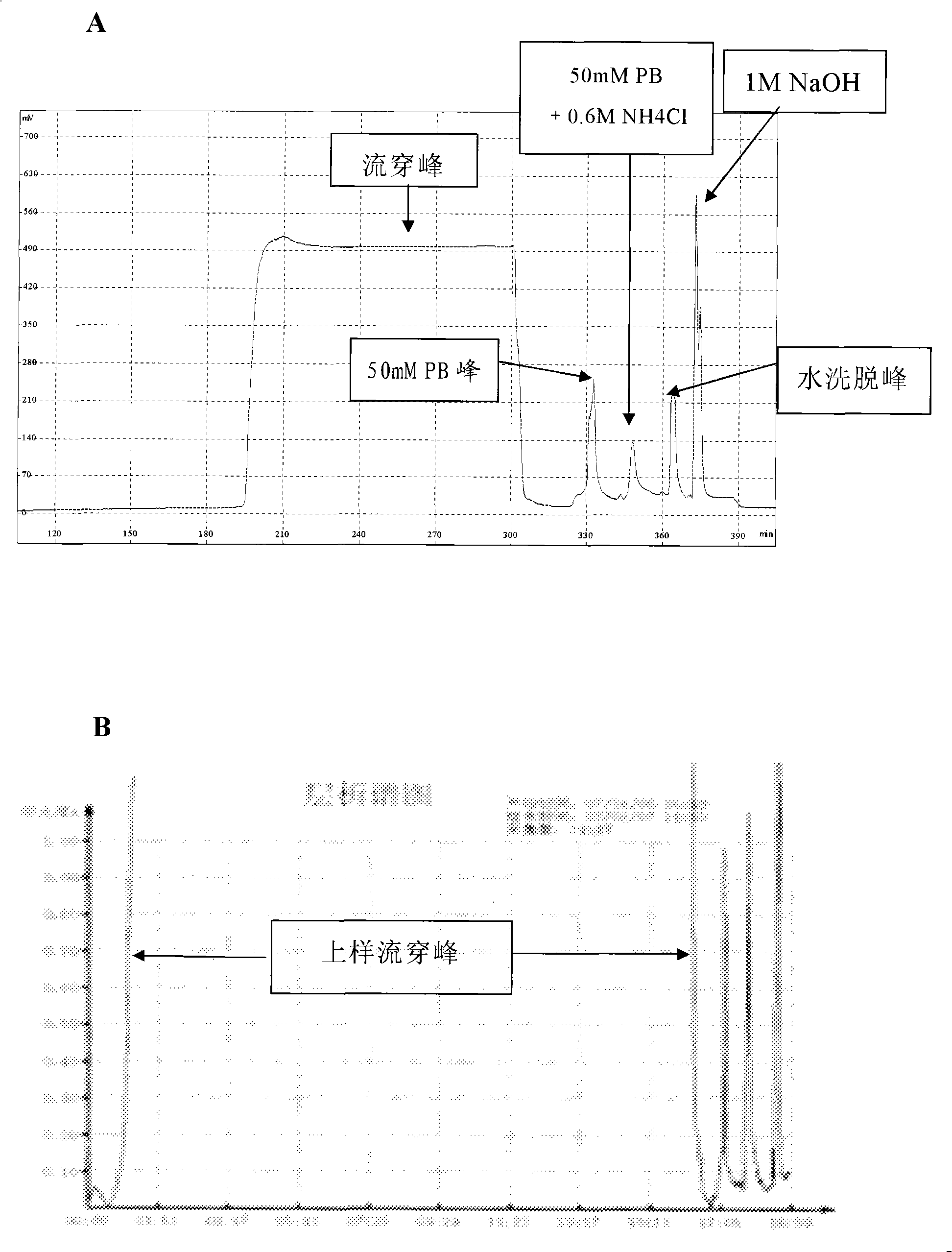
The invention provides a technological flow with versatility and high efficiency for reforming human serum albumins and fusion proteins of human serum albumins by separating and purifying target proteins. The steps of the method are as follows: the inorganic salt culture medium is used to acquire the genetic engineering microzyme fermented supernatant containing target proteins, and the serum-free culture medium is used to acquire a culture solution of reforming vertebrate cells, and ion exchange type affinity column chromatography medium Capto MMC fillers with higher salinity resistance and high capacity are directly used for separation and purification. The technological flow of the invention has the advantages of simple purification program step, uniqueness, low costs and convenient industrialization.
Owner:TIANJIN SINOBIOTECH
Novel serum-free culture medium for inducing fast and efficient production of pluripotent stem cells and use method thereof
The invention relates to a serum-free culture medium capable of fast and effectively inducing somatic cells to be reprogrammed to produce pluripotent stem cells and a method for inducing stem cells to be reprogrammed by using the serum-free culture medium without feeder, wherein the speed and efficiency of induced reprogramming are greatly increased. Moreover, the invention further relates to the use of the culture medium for inducing pluripotent stem cells and a method for screening of compounds, in particular for the high-throughput screening of compounds.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture
ActiveCN103555659AHigh densityClear ingredientsVertebrate cellsArtificial cell constructsCanine kidneyFlu immunization
The invention discloses a serum-free medium for Madin-Darby canine kidney (MDCK) cell full suspension culture. The serum-free medium for MDCK cell full suspension culture comprises an amino acid part, a vitamin part, an inorganic salt part and other additive parts. The serum-free medium has the beneficial effects that the serum-free medium for MDCK cell full suspension culture, provided by the invention, is high in cell culture density and clear in composition and does not contain animal serum, a downstream product is purified, the product quality is improved, and the serum-free medium is convenient to prepare and use and suitable for large-scale production of influenza vaccines and avian influenza vaccines.
Owner:无锡市赛尔百灵生物技术有限公司
Method for separating human adipose-derived stem cells from human adipose tissues
ActiveCN104357387AHigh purityReduced differentiation inductionSkeletal/connective tissue cellsParenchymaBrown adipose tissue
The invention relates to the field of cell engineering and particularly relates to a method for separating human adipose-derived stem cells from human adipose tissues. According to the method, by adding a small amount of low-molecular-weight heparin calcium into normal saline, a mixed solution is obtained and used as a cleaning fluid to clean the human adipose tissues, residual impurities such as red blood cells in the human adipose tissues can be well removed and thus human adipose-derived stem cells with relatively higher purity can be separated, the content of the parenchyma cell is significantly reduced, the differentiation induction of the human adipose-derived stem cells is facilitated, the consumption amounts of collagenase I, trypsase and a serum-free culture medium can be reduced and the cost of the process is also decreased. In addition, by centrifuging and digesting the human adipose tissues for 1 hour at a speed of 150r / min, the human adipose-derived stem cells with significantly better activity can be obtained.
Owner:深圳市赛欧细胞技术有限公司
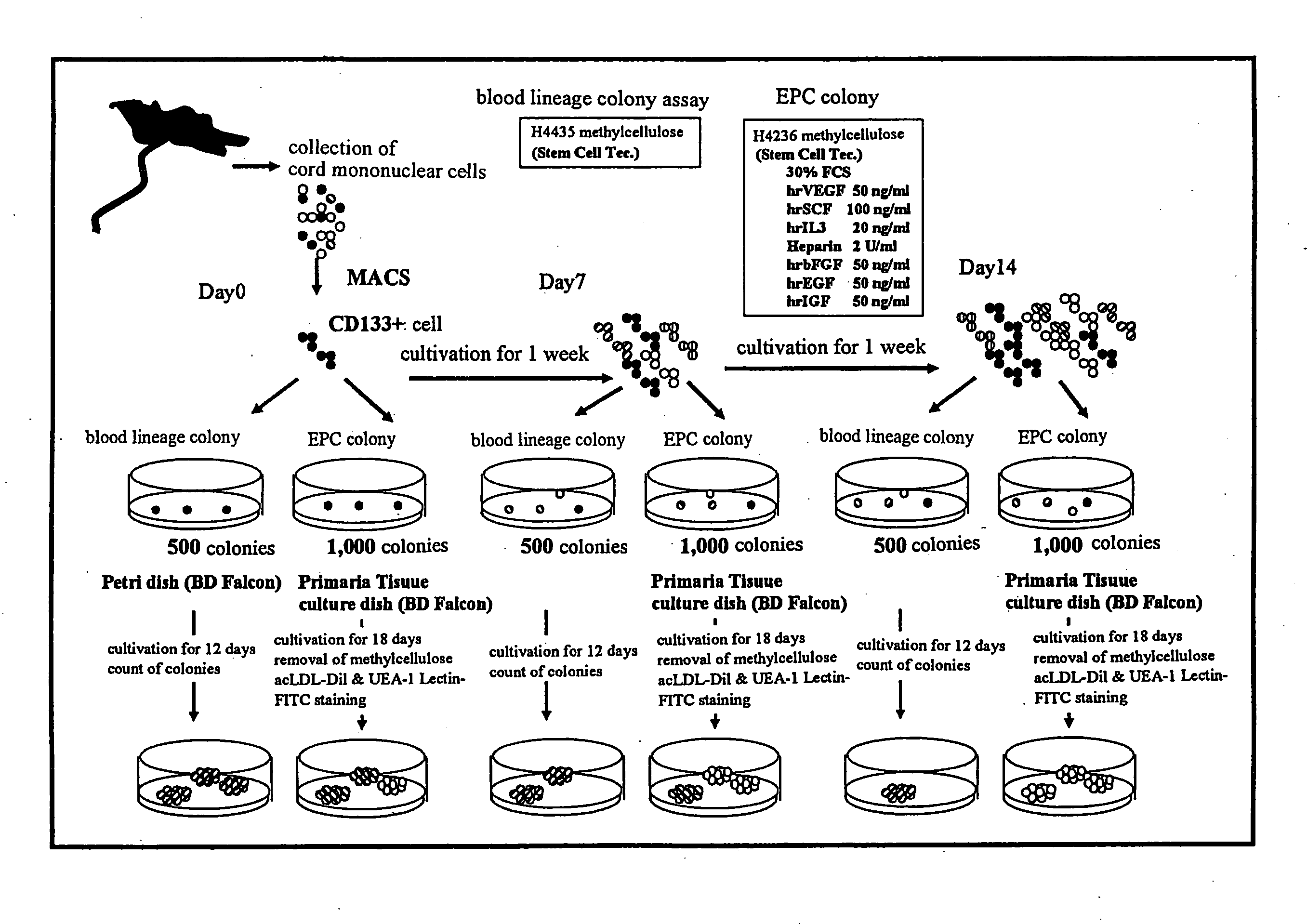
Method for Amplification of Endothelial Progenitor Cell in Vitro
ActiveUS20080166327A1Function increaseImprove heart functionBiocideMammal material medical ingredientsInterleukin 6Fms-Like Tyrosine Kinase 3
The present invention provides a method for expanding an endothelial progenitor cell in vitro. More particularly, the present invention provides a method for culturing a hemangioblast comprising incubating a hemangioblast in a serum-free culture medium containing one or more factors selected from the group consisting of stem cell growth factor, interleukin-6, FMS-like tyrosine kinase 3 and thrombopoietin, and a vascular endothelial cell produced by the method; and a serum-free culture medium containing one or more factors selected from the group consisting of stem cell growth factor, interleukin-6, FMS-like tyrosine kinase 3 and thrombopoietin, and a kit for the preparation of the serum-free culture medium and the like.
Owner:STEMMED +1
Human-derived adipose tissue-derived stem cell factor, preparation method and application thereof
ActiveCN106701672AIncrease productionEfficient removalCosmetic preparationsToilet preparationsSkin InjuryBrown adipose tissue
The invention discloses a human-derived adipose tissue-derived stem cell factor, a preparation method and application thereof, and particularly discloses a method for culturing a human-derived adipose tissue-derived stem cell factor. The method comprises the following steps: culturing human adipose tissue-derived stem cells in a high-coenzyme serum-free culture medium until the cell confluence is 75-85 percent, and collecting supernatant. The invention further discloses a method for preparing human-derived adipose tissue-derived stem cell factor concentrated solution, the concentrated solution prepared by the method, further application of the concentrated solution, a cell factor concentrate prepared by the method, cosmetics and medicines prepared from the cell factor concentrate. The concentrated solution prepared by the method has high content of adipose tissue-derived stem cell factors and stable quality, and a product prepared by combining the concentrated solution with other materials in a specific ratio has a good effect of repairing skin injury; and the method is simple and feasible, is suitable for large-scale production, and has excellent market prospect.
Owner:中科领康(广州)医疗有限公司
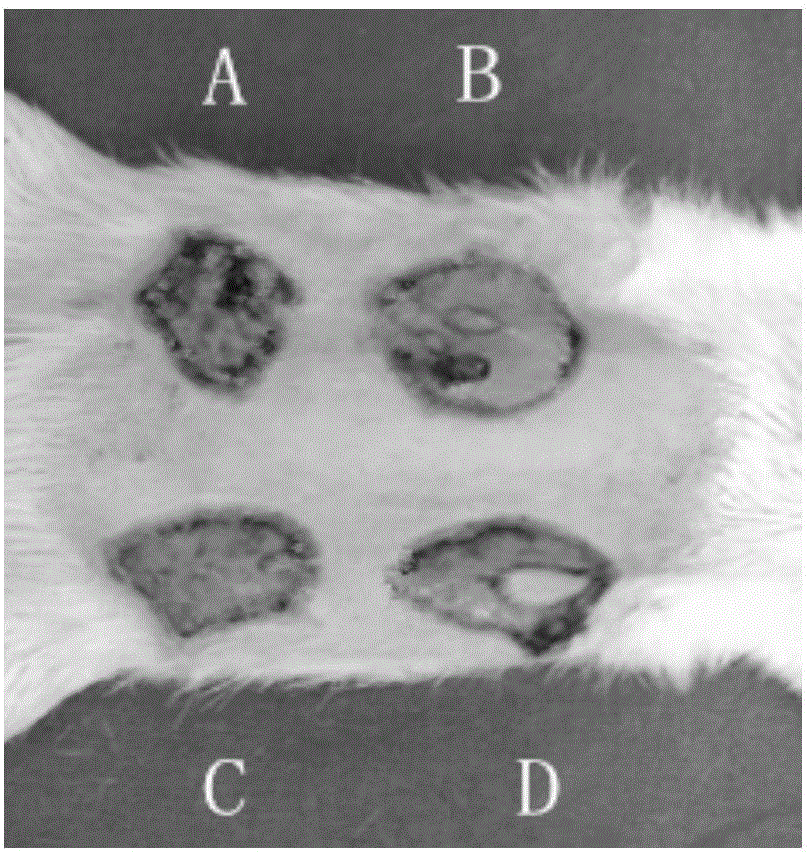
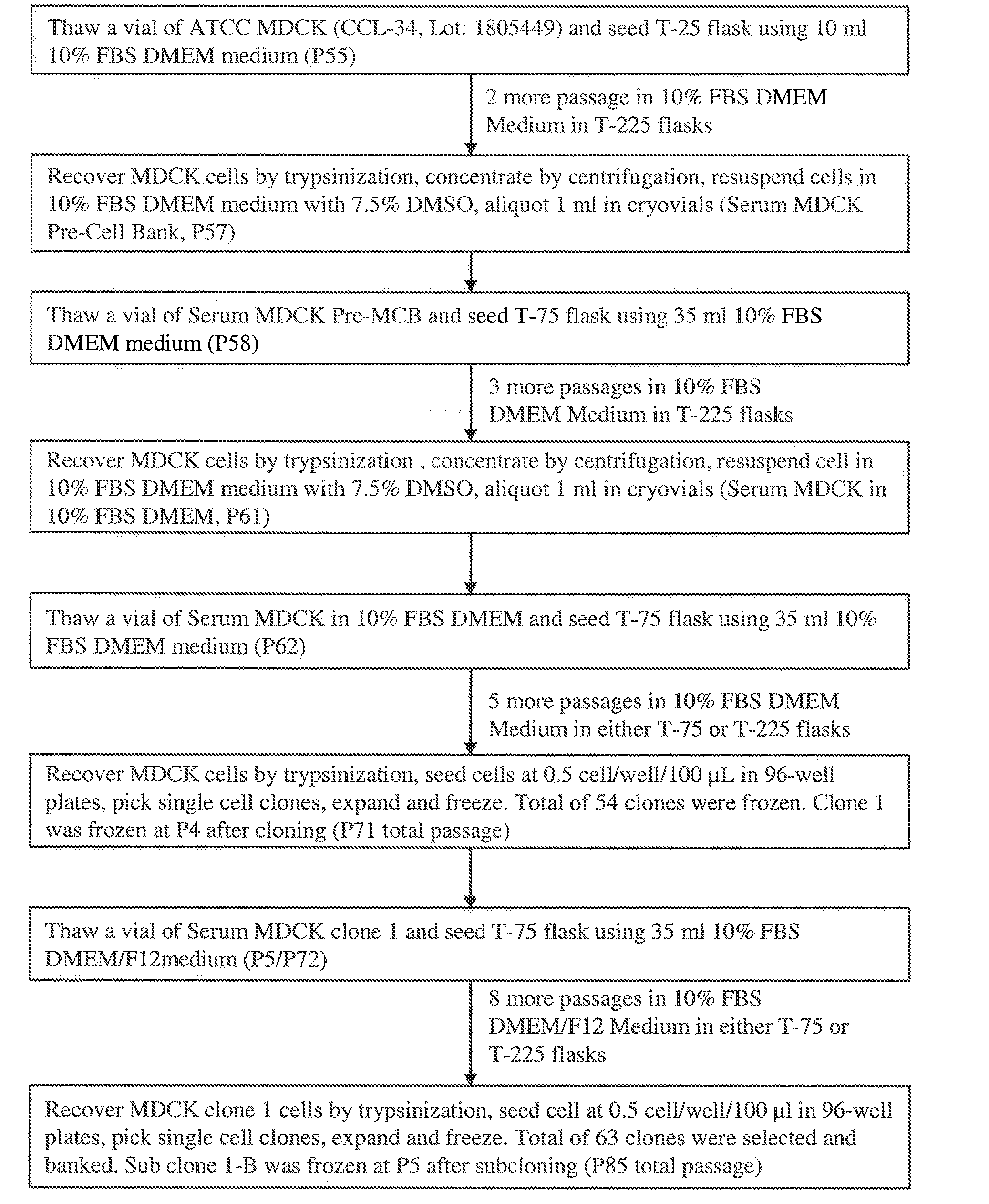
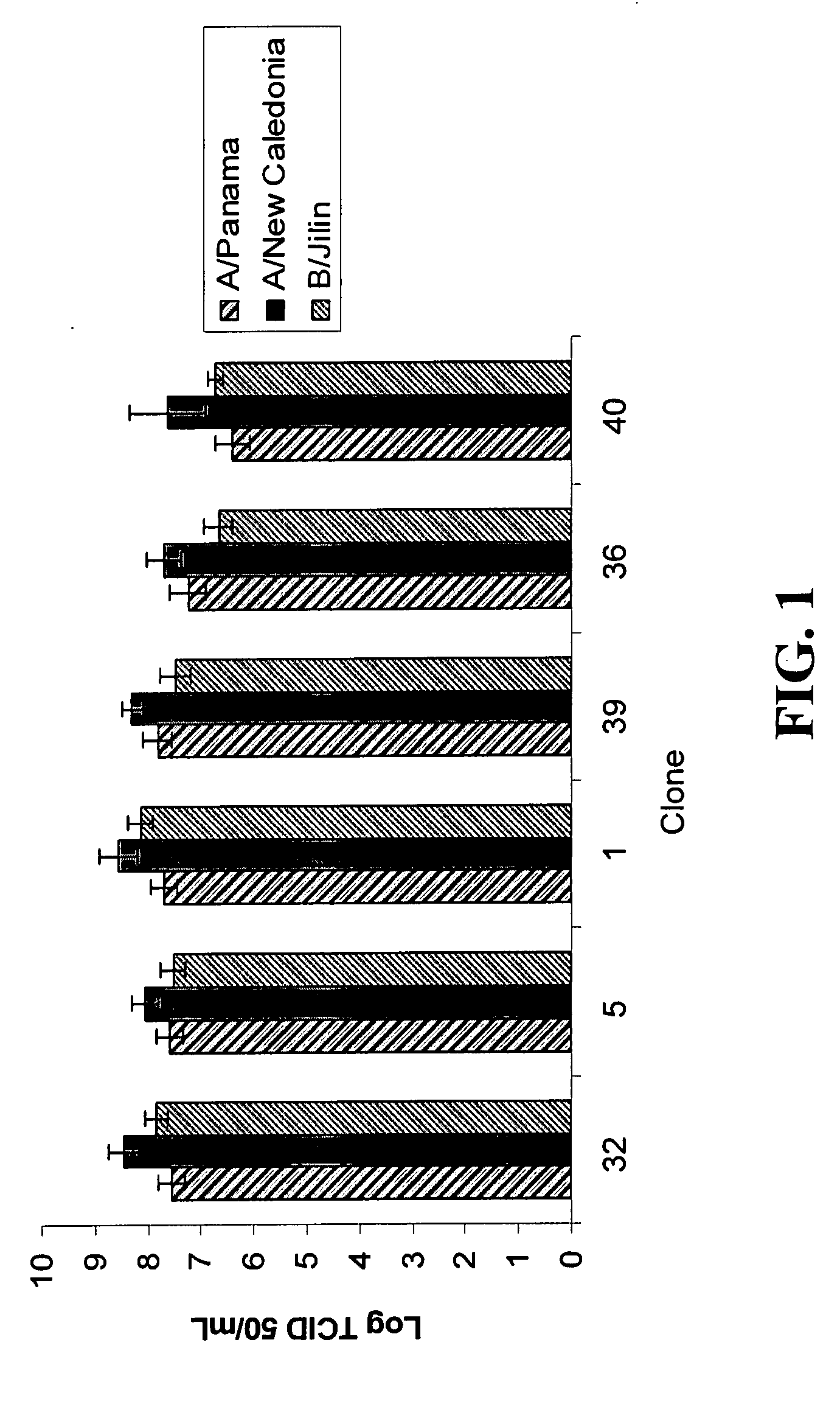
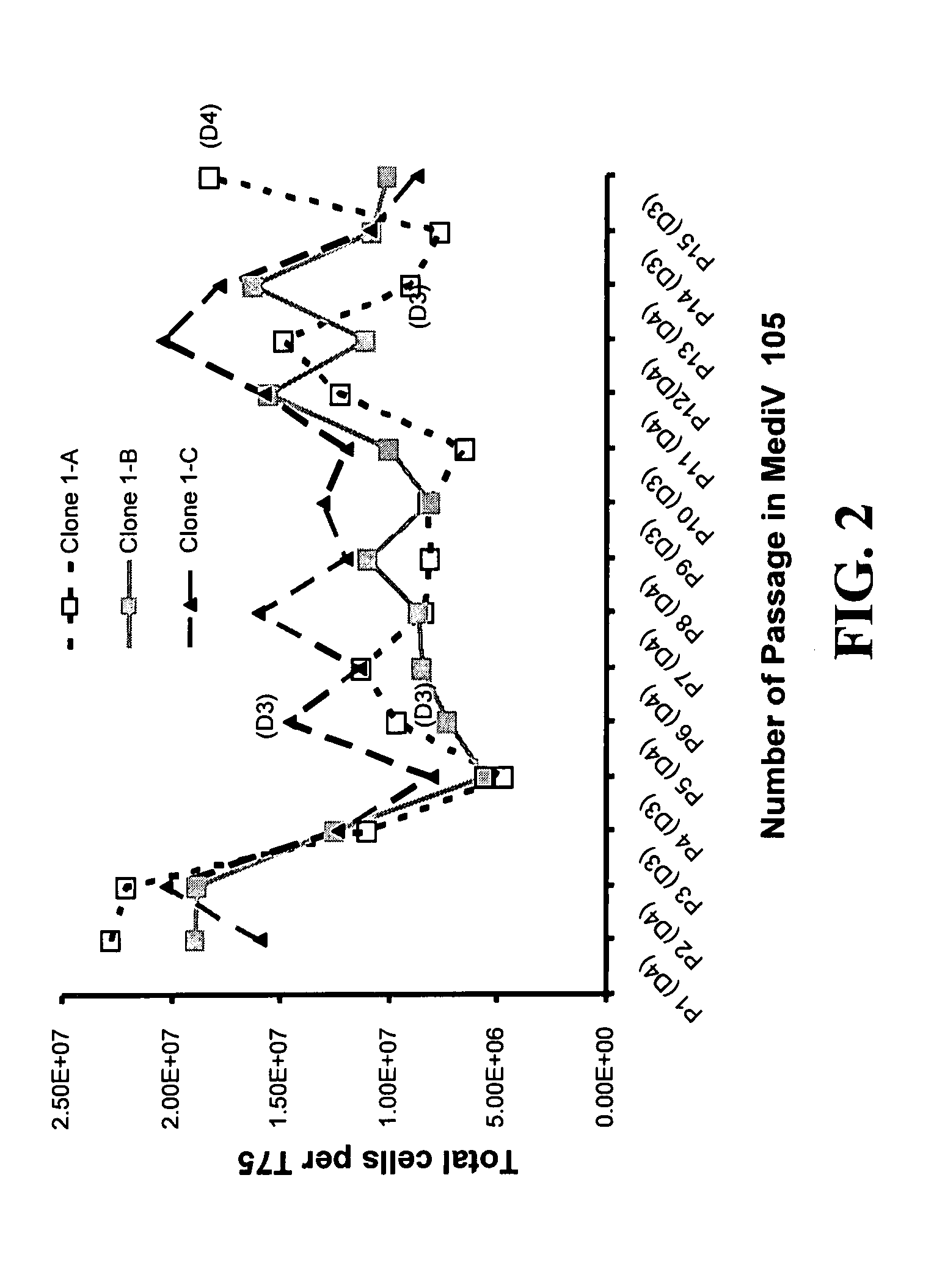
Mdck cell lines supporting viral growth to high titers and bioreactor process using the same
The present invention relates to novel MDCK cells which can be to grow viruses, e.g., influenza viruses, in cell culture to higher titer than previously possible. The MDCK cells can be adapted to serum-free culture medium. The present invention further relates to cell culture compositions comprising the MDCK cells and cultivation methods for growing the MDCK cells. The present invention further relates to methods for producing influenza viruses in cell culture using the MDCK cells of the invention.
Owner:MEDIMMUNE LLC
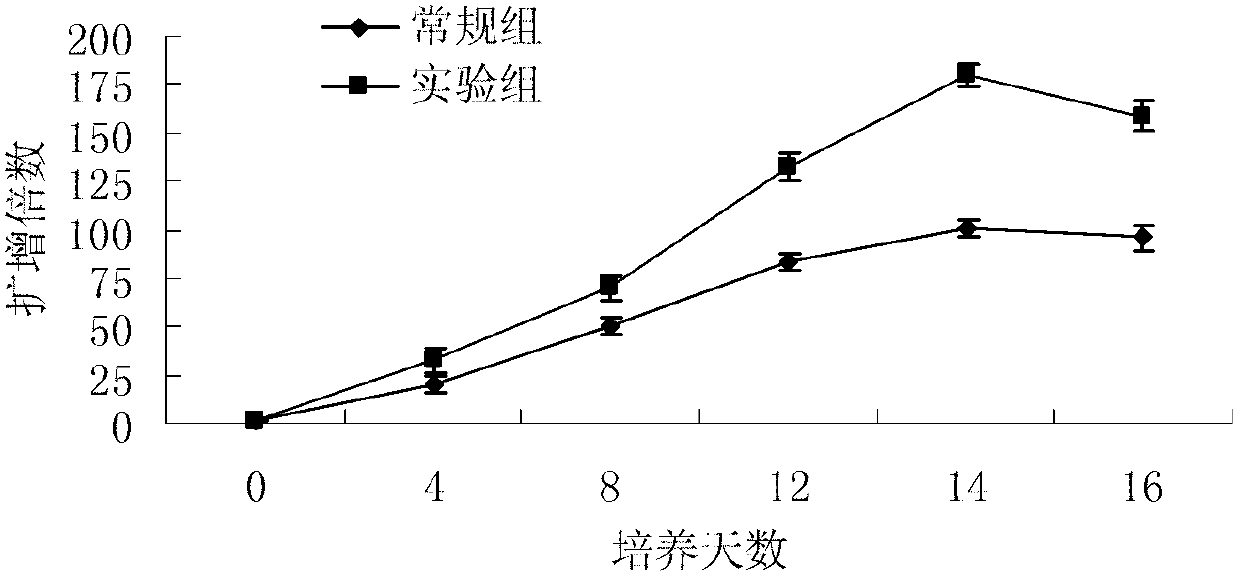
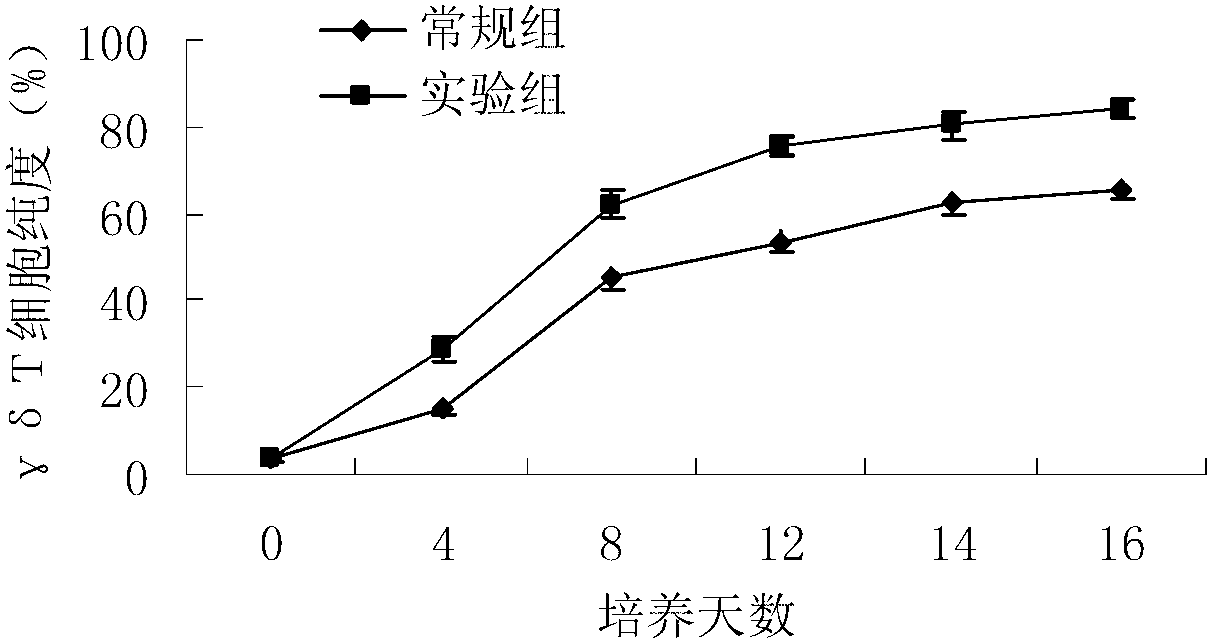
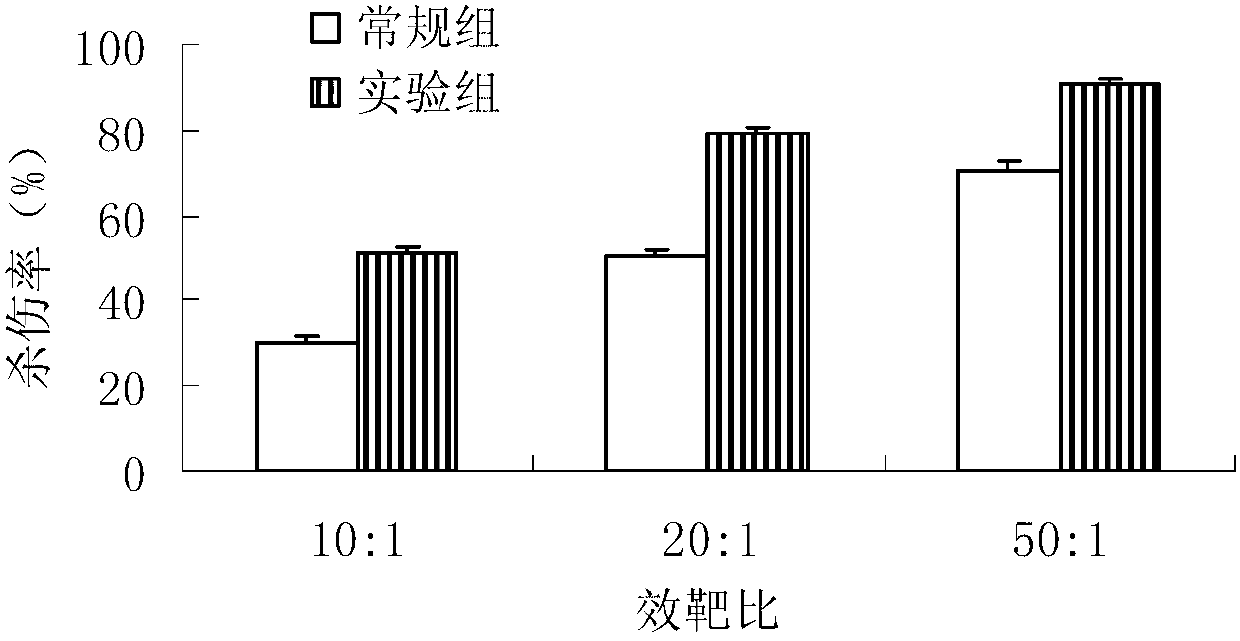
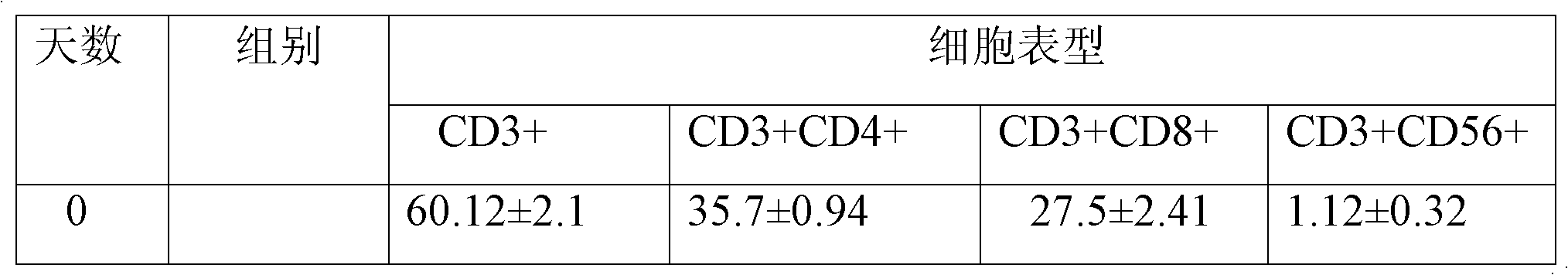
Method for in-vitro amplification of gamma-delta-T cells
InactiveCN102994448AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
The invention relates to a method for culturing gamma-delta-T cells, and in particular relates to a method for in-vitro amplification of gamma-delta-T cells, wherein the method comprises the following operating steps of: pre-coating a T75 culture bottle by a TCR-gamma-delta resisting antibody and CD28McAb for later use use; isolating the peripheral blood mononuclear cell (PBMC) of a patient; regulating the PBMC concentration to 1*10<6> 6 / ml by a serum-free culture medium which contains 5% of autologous plasma, and transferring PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; depending on growth situation of the cell, changing the culture medium every 2-3days, to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; and continuously culturing for 12-16days, to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
Method for amplifying cytokine induced kill cells (CIK) and CIK cell preparation
ActiveCN102352342AStrong mitogenic effectIncrease the level of amplificationMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellCell separation
The invention relates to a method for amplifying cytokine induced kill (CIK) cells and a CIK cell preparation, which belong to the field of in-vitro culture of immune cells. The method concretely adopts the following procedures that: a, lymphocyte cell separation liquid is used for separating out peripheral blood mononuclear cells (PBMC), a culture bag is covered by CD3mAb and CD137mAb in advance, the concentration of the PBMC obtained through separation is regulated to 1*10<6> / ml by a serum-free culture medium, in addition, IFN-gamma is added to obtain the final concentration being 1000 mu / ml, and the materials are transferred to the culture bag to be cultured; b, CD3mAb, CD28mAb and CD137mAb are added after the culture for 24h, in addition, the prepared serum-free culture medium is added, IL-1alpha, IL-2, IL-12 and IL-15 are added into the prepared serum-free culture medium, and obtained CIK cells are collected through centrifugation after the continuous culture for 7 to 21 days; and c, in the culture process of the step b, the cells in the culture bag are counted every three days, in addition, the culture medium is supplemented according to the concentration of the cells, and the CD3mAb, the CD28mAb and the CD137mAb are added to the corresponding concentration every six days, so the CIK cell generative cell times and the cytotoxin activeness are improved.
Owner:SHANGHAI CLAISON BIOTECH
Baby hamster kidney (BHK)-21 cells obtained by high-density suspension culture in low-serum and serum-free culture medium and preparation method thereof
ActiveCN102154197AReduce investmentConform to energy savingArtificial cell constructsVertebrate cellsCulture fluidHamster
The invention provides BHK-21 cells obtained by high-density suspension culture in a low-serum and serum-free culture medium and a culture method thereof. The culture method comprises the following steps: 1) resuscitating frozen BHK-21 cells and performing adherent culture to obtain adherently cultured BHK-21 cells; 2) subculturing the adherently cultured BHK-21 cells in culture solution to obtain suspension-cultured BHK-21 cells and freezing the BHK-21 cells; 3) performing resuscitation culture of the suspension-cultured BHK-21 cells and performing biological property identification; and 4) performing bioreactor adaptive culture of the suspension-cultured BHK-21 cells identified to be qualified. The invention has the advantages that: a cell domesticating method provided by the invention and the obtained BHK-21 cells which can be cultured in a suspended manner save culture solution and bovine serum, are in accordance of current energy-saving, environment-protection and low-carbon concepts for the application of suspended-cultured cells into actual production saves floor area and labor investment greatly, and create considerable economic benefit and social benefit.
Owner:马忠仁
Histoengineering bone and its making process
The present invention relates to histoengineering bone comprising porous histoengineering bone rack material, composite heterogene seed cell and / or bioactive factor. The histoengineering bone is constituted through treating heterogene cancellous bone via hypotonic solution and ultrasonic cleaning, complete or partial decalcification, defatting, antigen eliminating, etc to obtain high porosity rack material; compounding mesenchyme stem cell, osteoblast and other seed cell and / or bone morphogonetic protein of the heterogene, vascular endothelial growth factor, antibacterial medicine and other bioactive factors; and applying human serum or no serum culture medium following culturing in ox serum culture medium to reduce heterologous serum residue so as to constitute histoengineering bone product ultimately. The histoengineering bone has high performance and is used as bone repairing material clinically.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Natural killer cell culture medium and natural killer cell amplification culture method
ActiveCN104593324AImprove efficiencyHigh purityBlood/immune system cellsMicrobiologyNatural killer cell
The invention relates to the cell culture technical field, and particularly relates to a natural killer cell culture medium and a natural killer cell amplification culture method. The invention provides the culture medium used for amplification culture of natural killer cells and containing a serum-free culture medium, human plasma, IL-2, IL-21, IL-15 and OKT-3. The culture medium provided by the invention is used for amplification culture of the natural killer cells, can avoid risks caused by exogenous serum, has high amplification efficiency and allows the obtained natural killer cells to have high purity. With adopting the method for amplification of the natural killer cells, amplification of the natural killer cells can be maintained at a logarithmic phase for a longer period of time. Moreover, the cultured natural killer cells have good killing activity on tumor cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Culture medium and method for NK cell expansion in vitro
InactiveCN105567634AClinical diagnostic safetyAchieve explosive proliferationBlood/immune system cellsCell culture active agentsLymphocyteQuality control
The invention relates to a culture medium and method for NK cell expansion in vitro. The culture medium is prepared from, by volume, 100% of a lymphocyte serum-free culture medium or the mixture of 1-90% of an RPMI1640 culture medium and 10-99% of a lymphocyte serum-free culture medium, a serum substitute and interleukin 2, wherein the concentration of the serum substitute is 0.5-4g / L, and the concentration of the interleukin 2 is 50-500 micrograms / mL. According to the culture medium and the preparation method, explosive expansion of NK cells in a short time can be achieved, the cost is low, the expanded cells are extremely high in destruction, and the requirement for application of three kinds of medical technologies and the requirement for somatic cell treatment medicine quality control are met.
Owner:上海润泉生物技术有限公司
In-vitro culture method of NK (natural killer) cells
InactiveCN103627672AEnhance killing activityPromote growthBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsBottle
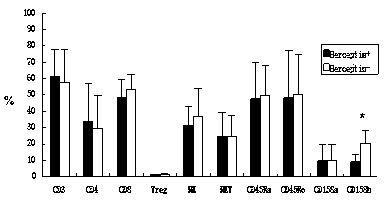
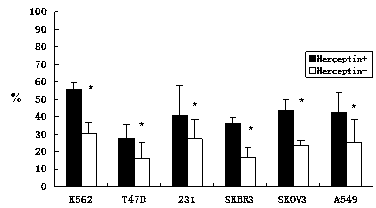
The invention discloses an in-vitro culture method of NK (natural killer) cells and belongs to culture of human cells. The in-vitro culture method disclosed by the invention comprises the following steps: merging herceptin diluted by PBS (phosphate buffered saline) and human immunoglobulin diluted by the PBS, then uniformly and fully spreading at the bottom of a culture bottle and standing overnight; additionally taking peripheral blood, performing density gradient centrifugation, sucking a single nuclear cell, adding into a serum-free culture medium, and adjusting the concentration of the cells to 1.0*10<6> / ml-3.0*10<6> / ml; and then adding cell factors IL-2 and IL-15, adding into the culture bottle coated by the herceptin and culturing in an incubator. Therefore, on the basis of ensuring the amplification multiple of various cell subgroups, the growth and the proliferation of the NK cells are promoted, the killing activity of lymphocytes is enhanced, the serum-free culture medium can replace a serum-containing complete culture medium, the number of obtained culture products is equivalent to the activity of the cells, the in-vitro large-scale culture of the NK cells is realized, the in-vitro culture method is used for clinical biological treatment of the NK cells, and the safety in clinical application can be increased by using the in-vitro culture method.
Owner:TIANJIN MEDICAL UNIV CANCER HOSPITAL
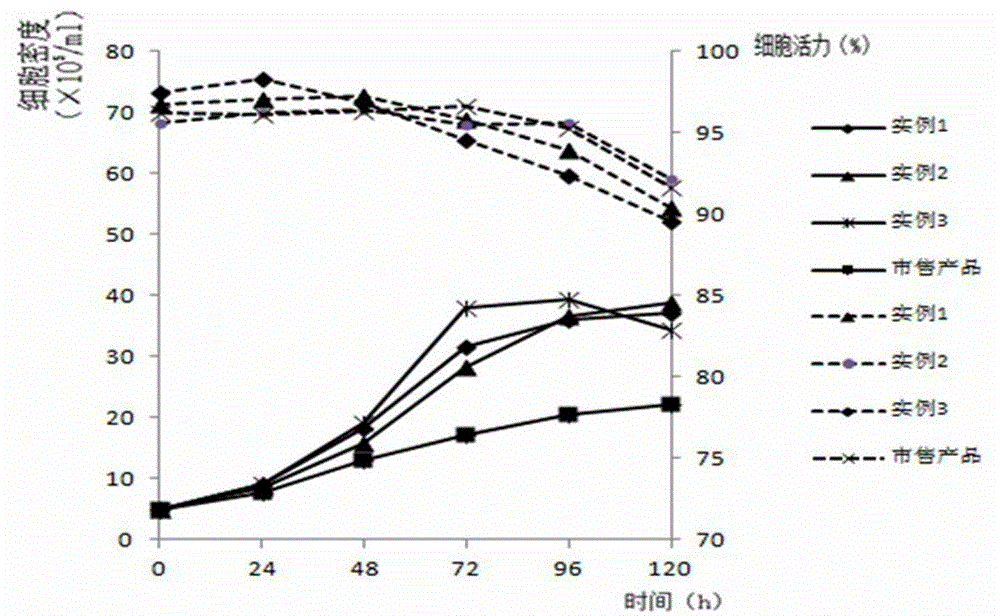
Cell culture process
InactiveUS20050069979A1High degree of sialylationHigh degreePeptide/protein ingredientsImmunoglobulinsBiotechnologyLipid formation
The invention provides a method for producing a recombinant polypeptide of interest which method comprises: (a) providing a host cell which comprises a nucleotide sequence which encodes the recombinant polypeptide of interest and which directs expression of the recombinant polypeptide of interest in the host cell; (b) providing a serum-free culture medium which comprises (i) water, a plant-derived peptone, an osmolality regulator, a buffer, an energy source, at least one amino acid, a lipid source or precursor, a source of iron, non-ferrous metal ions and optionally one or more vitamins and cofactors; and (ii) does not contain any full-length polypeptides; and (c) culturing the host cell in the culture medium under conditions that allow for expression of the recombinant polypeptide of interest.
Owner:ZENG STEFFEN +4
Animal source-free and serum-free culture medium of lymphocyte
InactiveCN103146648AGood amplification factorEnhance cell viabilityBlood/immune system cellsCell phenotypeSodium bicarbonate
The invention relates to the biological field and discloses an animal source-free and serum-free culture medium of lymphocyte. The culture medium disclosed by the invention essentially consists of IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, human transferrin, human serum albumin, 2-mercaptoethanol, N-acetyl-cysteine, lipid, amino acid, vitamin, microelement, ferric citrate, hydrocortisone, cholamine and non-essential amino acid. The serum-free culture medium disclosed by the invention has the advantages of clear chemical components, no animal source, no serum, safe and ideal culture cells; the instability caused by the doping of animal components and batches is avoided; the result of culturing lymphocyte shows that the total number of the cells and the cell phenotypes are normal; and the serum-free culture medium disclosed by the invention has a good industrial application prospect.
Owner:BEIJING JING MENG STEM CELL TECH
Pluripotent cells from rat and other species
InactiveCN101657535ALow costStable storageCompounds screening/testingCell culture active agentsSerum igeMEK inhibitor
Pluripotent cells are derived and maintained in a self-renewing state in serum-free culture medium comprising a MEK inhibitor, a GSK3 inhibitor and an antagonist of an FGF receptor.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
Serum-free culture medium and method for expanding hematopoietic stem cells
InactiveUS20180163177A1Culture processSkeletal/connective tissue cellsVitamin CHematopoietic growth factor
A serum-free culture medium for hematopoietic stem cell (HSC) expansion is provided. The serum-free culture medium includes a serum-free base medium, cytokines, an umbilical cord mesenchymal stem cell conditioned medium and supplemental components. The cytokines comprise stem cell factor, thrombopoietin and hematopoietic growth factor Flt3 ligand. The umbilical cord mesenchymal stern cell conditioned medium is derived from culturing human umbilical cord mesenchymal stem cells. The supplemental components comprise vitamin C, vitamin E or a combination of vitamin C and vitamin E.
Owner:HEALTHBANKS BIOTECH
Serum-free culture medium without animal origin components for culturing Vero cell micro-carrier
InactiveCN101864393APromote growthSame densityVertebrate cellsArtificial cell constructsSerotoninVitamin C
The invention discloses a serum-free culture medium without animal origin components for culturing a Vero cell micro-carrier. The serum-free culture medium consists of a DMEM / F12 (1:1) culture medium and culture medium additive components such as epidermal growth factors, insulin, serotonin, aurin tricar-boxylic acid, biotin, vitamin C, amino acid, fructose, trehalose, trace elements, hydroxypropyl-beta-cyclodextrin and the like. The serum-free culture medium has the following advantages that: (1) the attachment growth of Vero cells in a tissue culture vessel and on the surface of the micro-carrier can be supported, and the cells can be transferred from a serum culture medium to the serum-free culture medium without a course of adaptation; (2) the serum-free culture medium contains no animal origin components, and has basically definite chemical components, and a low cost; and (3) the cells grow well, and the cellular morphology, the density and the vitality are basically the same as those of the serum culture medium.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Culture method and serum-free culture medium composition of NK cells
ActiveCN105462924AThe total number of cells is excellentExcellent growth rateBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsMicrobiology
The invention discloses a culture method and serum-free culture medium composition of NK cells. The serum-free culture medium composition of the NK cells comprises an induction culture medium, a proliferation culture medium and an activation culture medium; the induction culture medium comprises a basal culture medium and an induction component, the proliferation culture medium comprises a basal culture medium and a proliferation culture component, and the activation culture medium comprises a basal culture medium and an activation component. The culture method of the NK cells comprises the step of performing cell culture by sequentially using the induction culture medium, the proliferation culture medium and the activation culture medium in stages. The serum-free culture medium composition of the NK cells comprises the induction culture medium, the proliferation culture medium and the activation culture medium which are designed for the different influence requirements of the induction, proliferation and activation periods of the NK cells, and the cultured NK cells are superior to those cultured through an existing technology on the aspects such as the total cell number, the proliferation speed, the amplification times and the killing activity on tumor cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Serum-free culture medium of human embryo kidney (HEK) 293 cell
the invention discloses a non-serum substratum for cell of HEK 293, which is composed of transferring, insulin, Primatone, lipoid compound, amino acid, inorganic salt and vitamin based on basic substratum.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com