Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
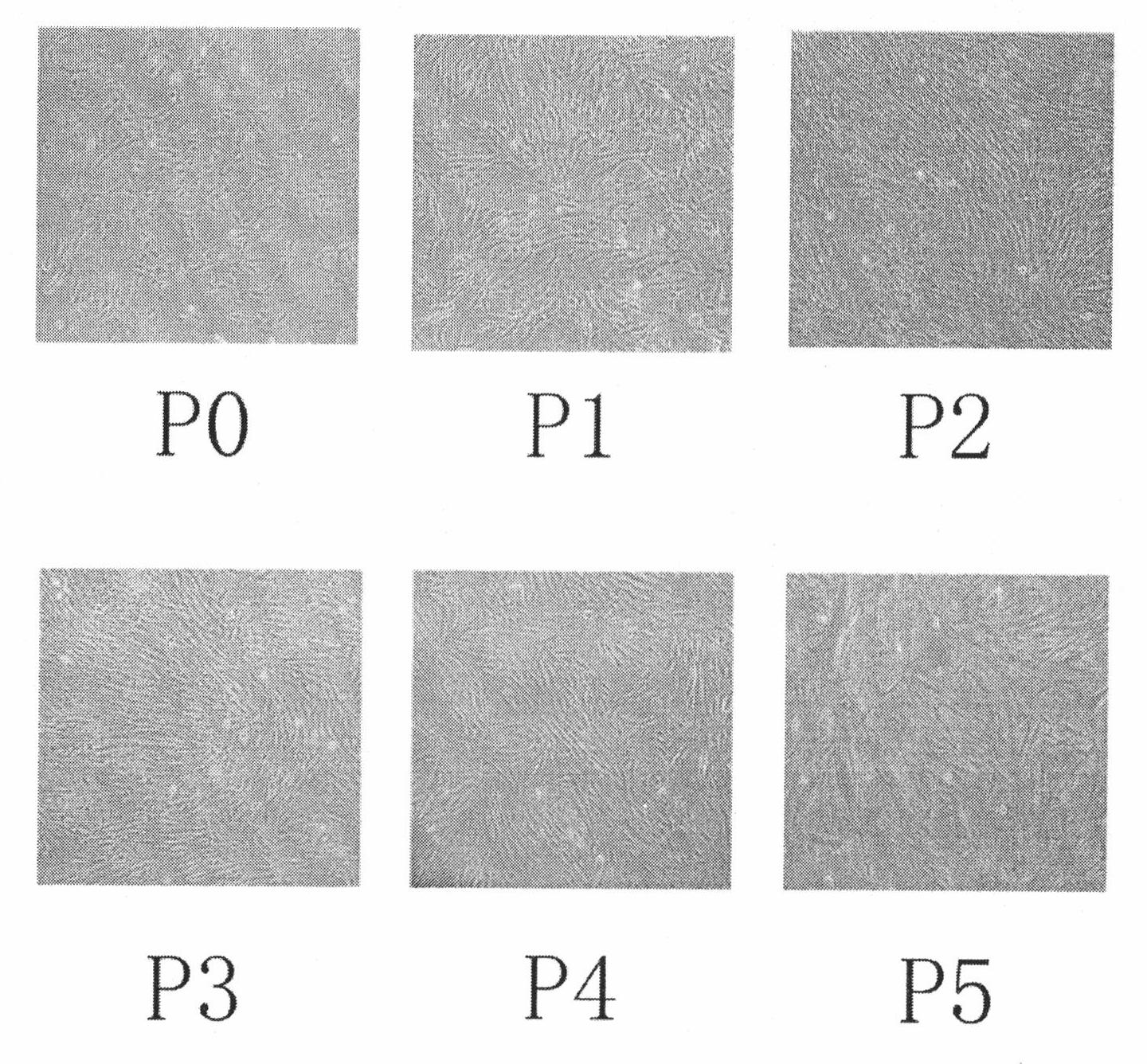
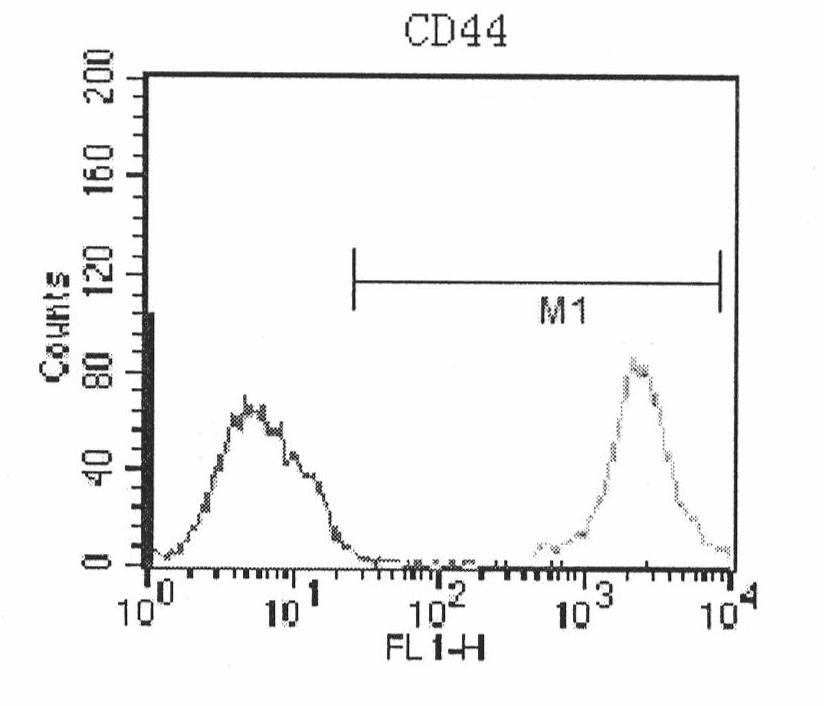
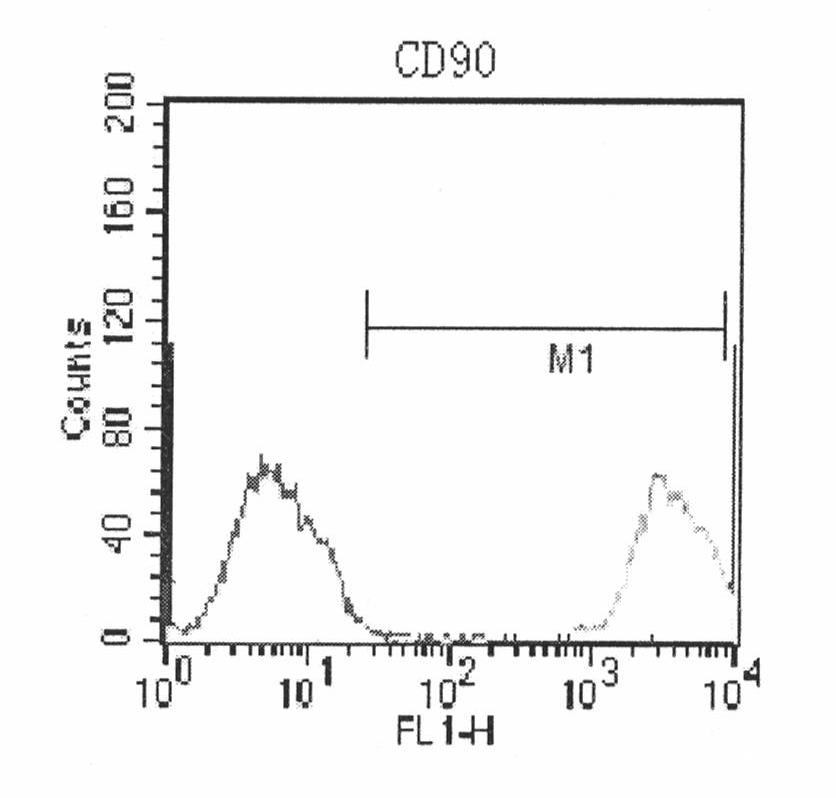
Image
Examples
Embodiment 1
[0032] A serum-free medium for human amniotic mesenchymal stem cells of the present invention, comprising:
[0033] Basal medium DMEM / F12 15.6g / L
[0034] (According to the volume of 1:1, take 15.6g and dissolve in 1000ml water, supplier GIBCO)
[0035] Human serum albumin 8.0×10 -1 %
[0036] Human transferrin 5.0×10 -1 g / L
[0037] Human insulin 8.0×10 -1 g / L
[0038] Sodium selenite 6.0×10 -6 g / L
[0039] made into an aqueous solution.
Embodiment 2
[0041] A serum-free culture method for human amniotic mesenchymal stem cells of the present invention comprises the following steps:
[0042] 1. Isolation of human amniotic mesenchymal stem cells and preparation of single cell suspension
[0043] Take the human placenta of normal full-term caesarean section fetus (male) under sterile conditions; detect hepatitis A antibody, hepatitis B virus surface antigen, hepatitis B virus surface antibody, hepatitis B virus e antigen, hepatitis B virus e Antibody, hepatitis B core antibody IgM, hepatitis C antibody, hepatitis E antibody, HIV antibody, Treponema pallidum antibody and other related infectious indicators were all negative; Amniotic membrane 5×5cm 2 , fully rinsed with PH7.2 phosphate buffer solution (PBS), put the amniotic membrane in physiological saline containing 0.1 million U / ml gentamicin and 2.5ug / ml amphotericin B, and soak for 20 minutes; Cut into pieces, add 5ml of trypsin at a final concentration of 2.5g / L per gra...
Embodiment 3
[0054] A method for the separation and serum-free culture of human amniotic mesenchymal stem cells of the present invention, comprising the following steps:
[0055] The 1st step of the present invention can also be: trypsin final concentration is 2.5g / L, and digestion time is 30-60 minutes, and number of times is 2-4 times; Score can be 5.0×10 -1 %; When digested with collagenase IV and deoxyribonuclease I, V DMEM :V F12 =The concentration of collagenase IV in the DMEM / F12 culture fluid of 1:1 adopts 0.5-1.5g / L, deoxyribonuclease 0.05-0.15g / L, and digestion time is 1-2 hour; All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com