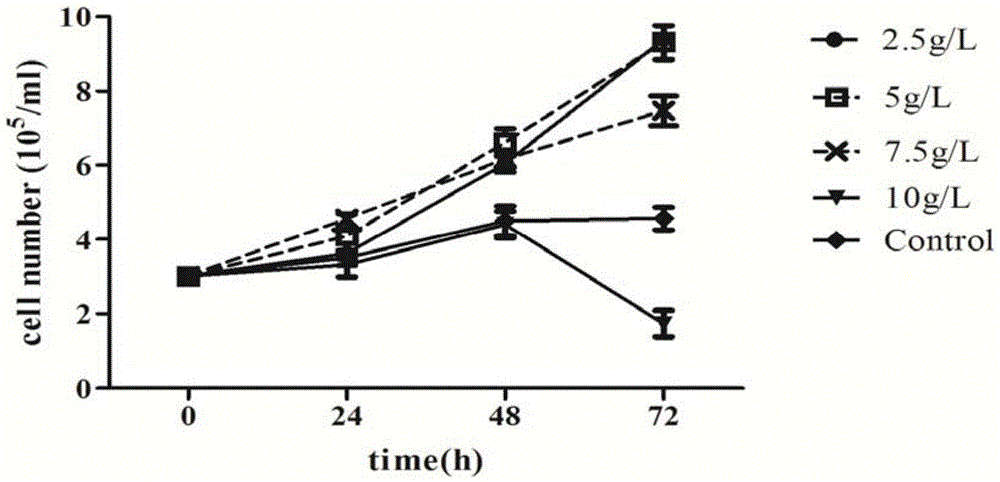
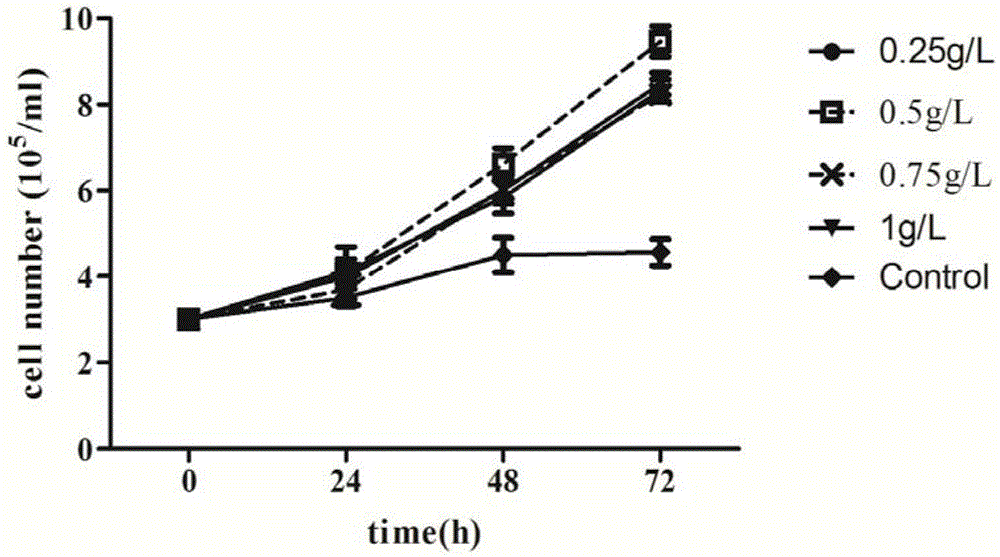
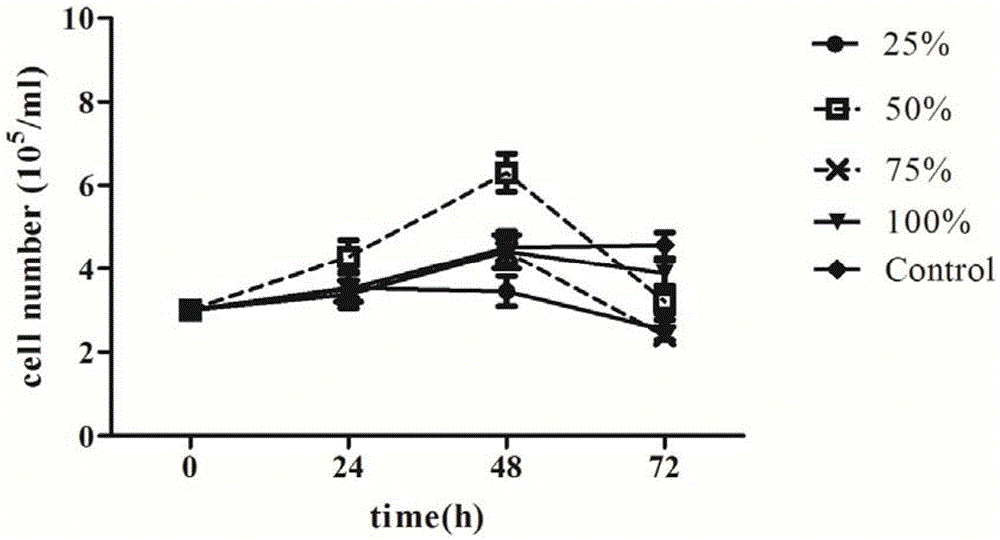
Serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells
A serum-free culture medium and culture medium technology, applied in animal cells, culture process, tissue culture, etc., can solve the problems of unclear serum composition and high requirements for culture conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] The serum-free medium for full suspension culture of MDCK cells of the present invention is prepared by adding the following components (in g / L) on the basis of the DMEM / F12 medium containing NaCl of 5g / L:
[0163] Trace elements:
[0164] Sodium stannate (Na 2 SeO 3 -5H 2 O) 0.000005
[0165] Growth Factors:
[0166] Recombinant human epidermal growth factor (EGF) 0.0001
[0167] Hydrocortisone 0.000036
[0168] Recombinant whole chain insulin 0.005
[0169] Prostaglandin E10.000025
[0170] Human transferrin 0.0047
[0171] Thyroxine (T3) 4.0×10 -10
[0172] Lipid complex:
[0173] Vitamin E0.00005
[0174] Cholesterol 0.00005
[0175] Ethanolamine 0.000925
[0176] β-mercaptoethanol 0.000975
[0177] Tween-800.00625 (density is 1.09g / ml, weighed as 1g / ml)
[0178] Plant Hydrolyzate:
[0179] HyPep15105
[0180] Recombinant Human Albumin:
[0181] ACF1
[0182] other:
[0184] Emulsification method: take 2.5ml Tween-80 to...
Embodiment 2
[0189] The difference between this embodiment and embodiment 1 is:
[0190] In the medium formula, the concentration of recombinant human serum albumin ACF is 0.5g / L.
[0191] All the other parts are the same as in Example 1.
[0192] Apply the culture medium of this example to suspend MDCK cells without adding serum, and the cell seeding density is: 3.0×10 5 / ml, culture temperature: 37°C, rotation speed: 120rpm. See results Figure 10 , Cell map of cultured for 72h.
Embodiment 3
[0194] The serum-free medium for full suspension culture of MDCK cells of the present invention is prepared by adding the following components (in g / L) on the basis of the DMEM / F12 medium containing NaCl of 5g / L:
[0195] Trace elements:
[0196] Sodium stannate (Na 2 SeO 3 -5H 2 O) 0.000001
[0197] Growth Factors:
[0198] Recombinant human epidermal growth factor (EGF) 0.00005
[0199] Hydrocortisone 0.000012
[0200] Recombinant whole chain insulin 0.001
[0201] Prostaglandin E10.00001
[0202] Human transferrin 0.002
[0203] Thyroxine (T3) 1.0×10 -10
[0204] Lipid complex:
[0205] Vitamin E0.0001
[0206] Cholesterol 0.0001
[0207] Ethanolamine 0.001
[0208] β-mercaptoethanol 0.001
[0209] Tween-800.01 (density is 1.09g / ml, weighed as 1g / ml)
[0210] Plant Hydrolyzate:
[0211] HyPep15101
[0212] Recombinant Human Albumin:
[0213] ACF0.5
[0214] other:
[0216] The preparation method of the medium in this example ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com