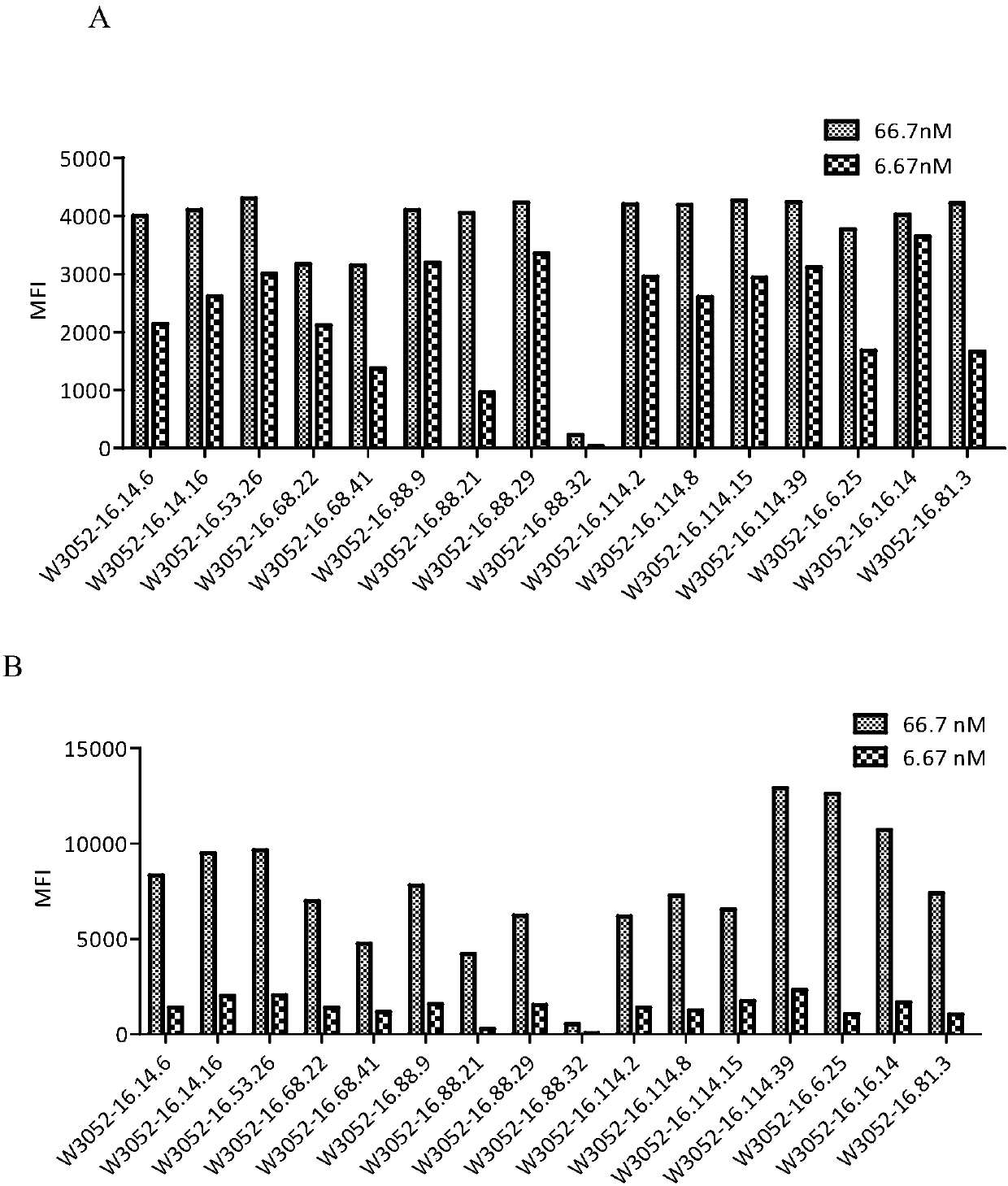
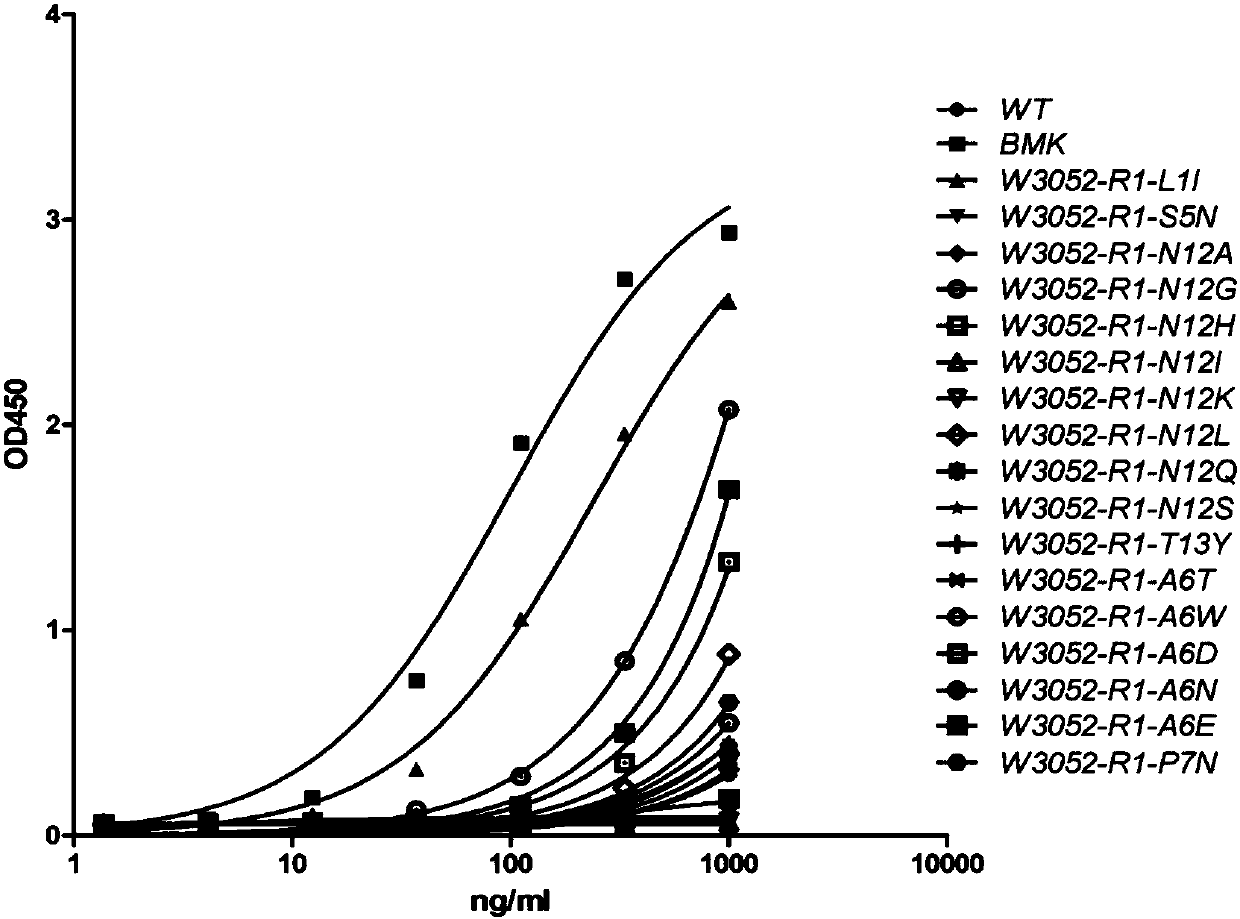
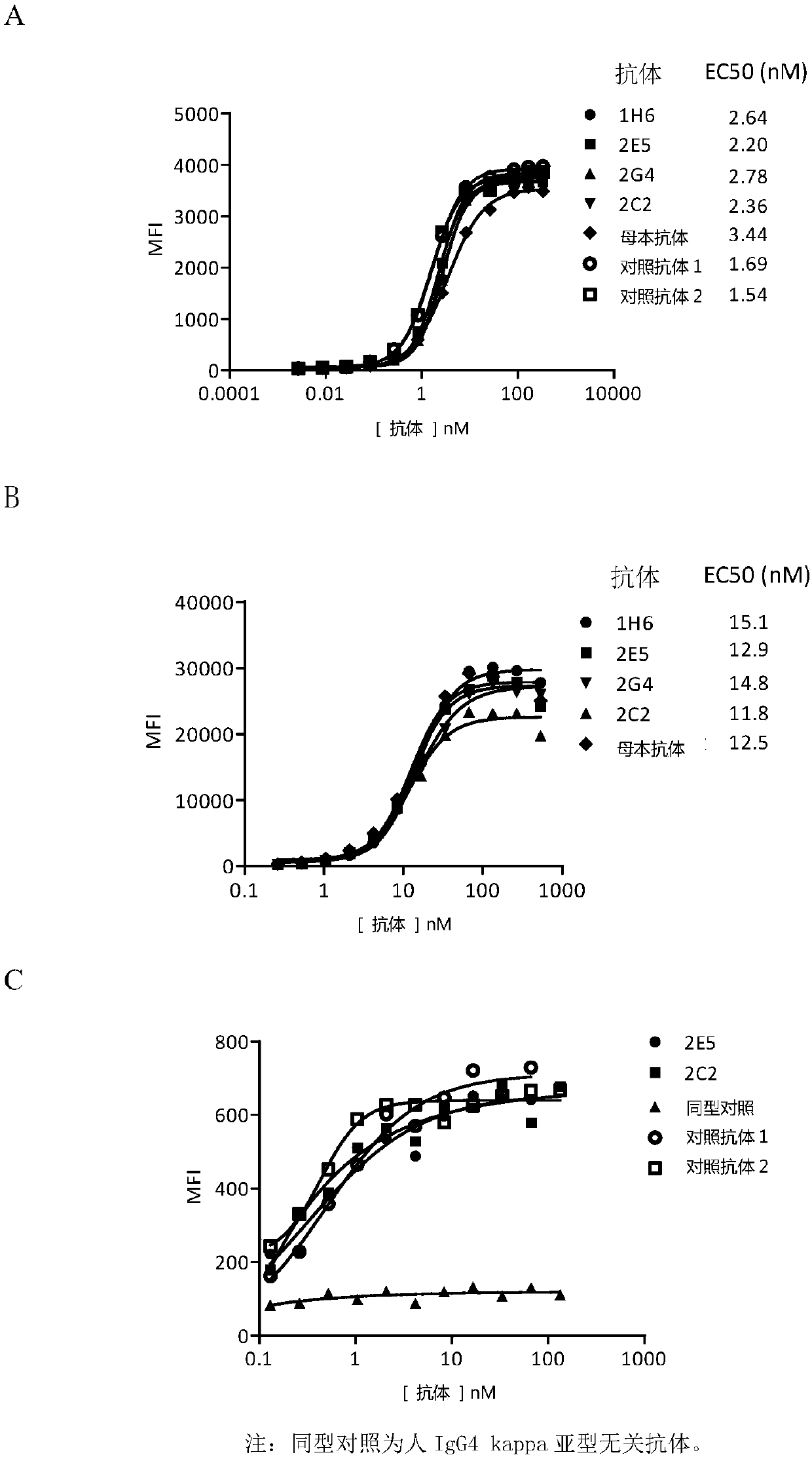
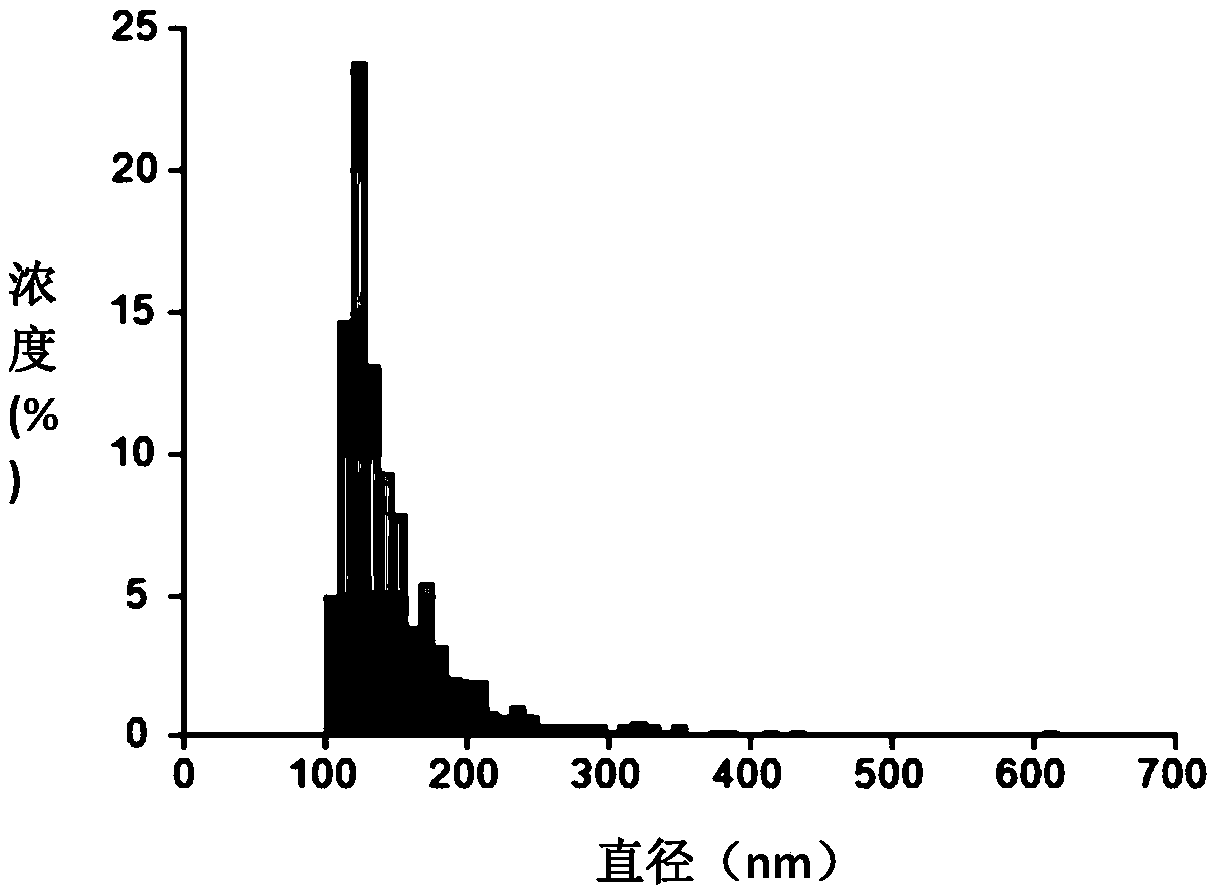
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1080results about "Urinary tract/kidney cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
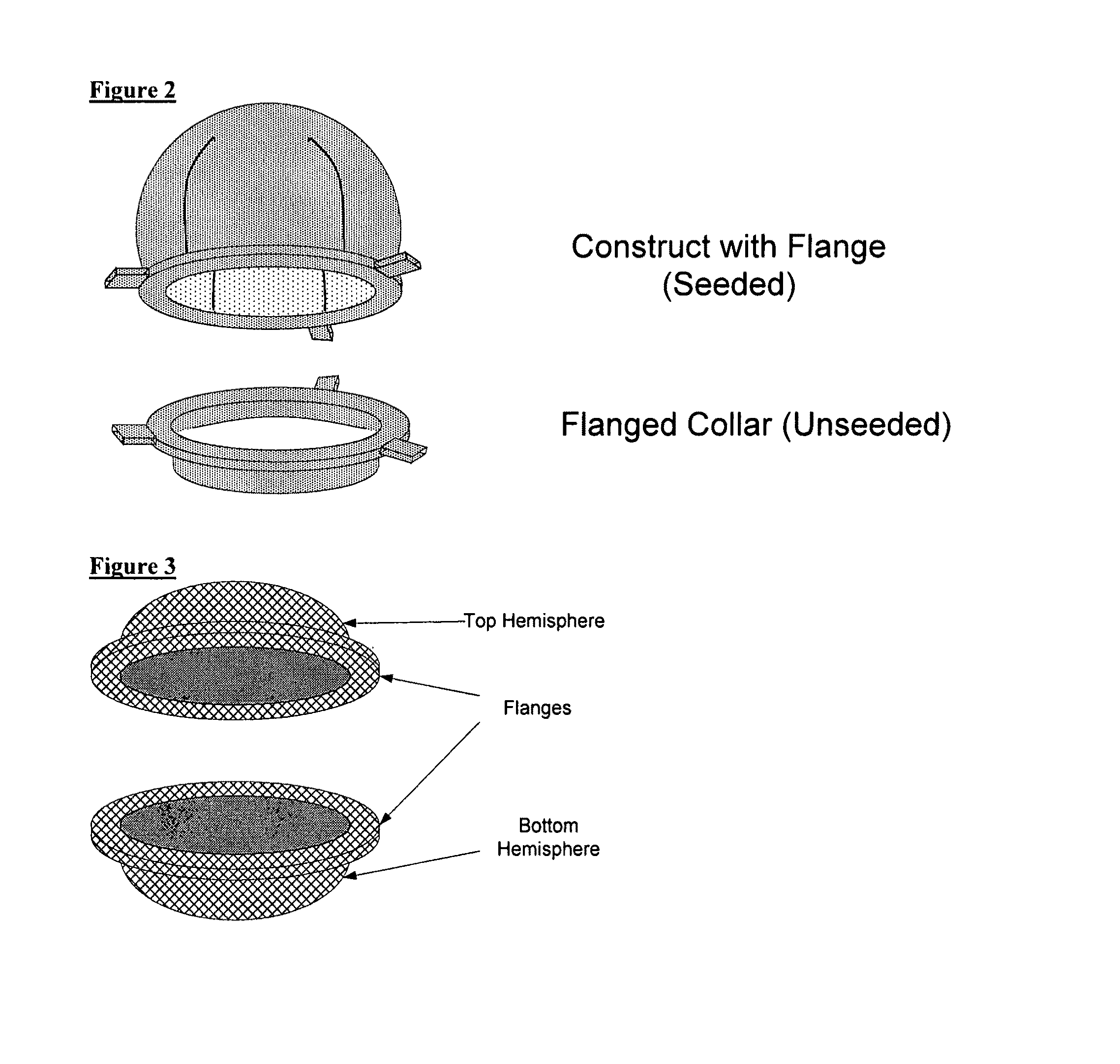
Scaffolds for organ reconstruction and augmentation
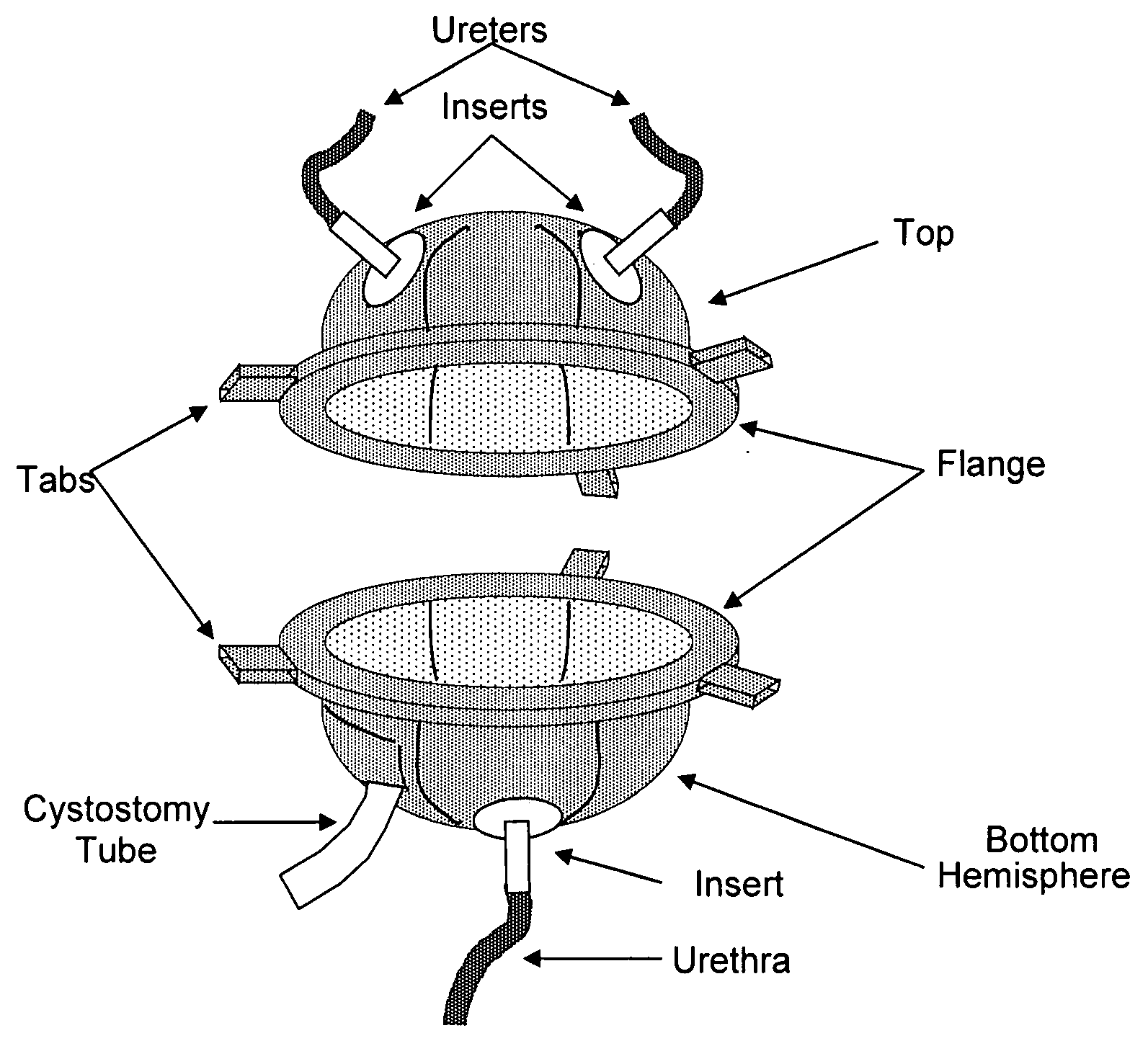
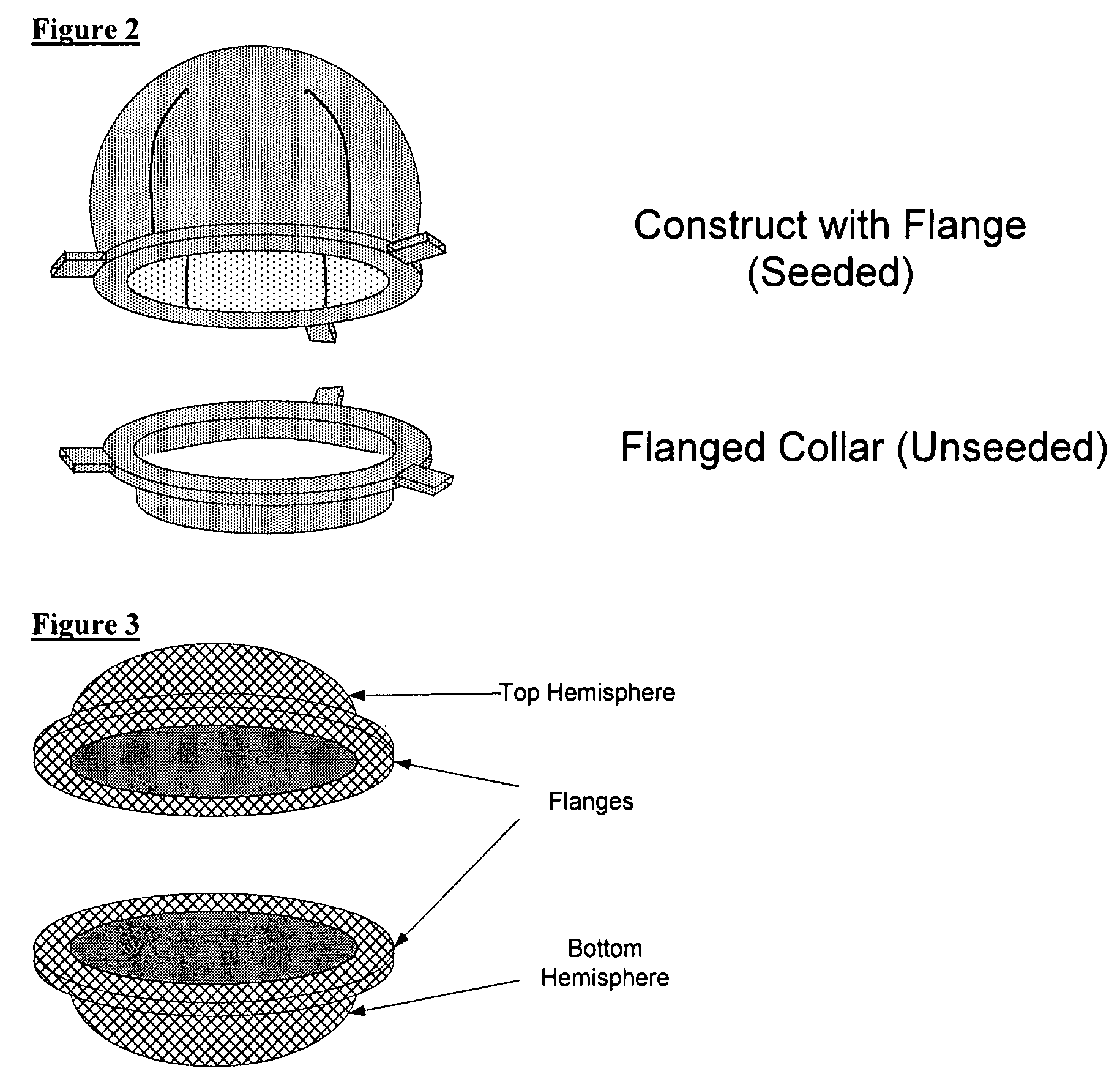
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
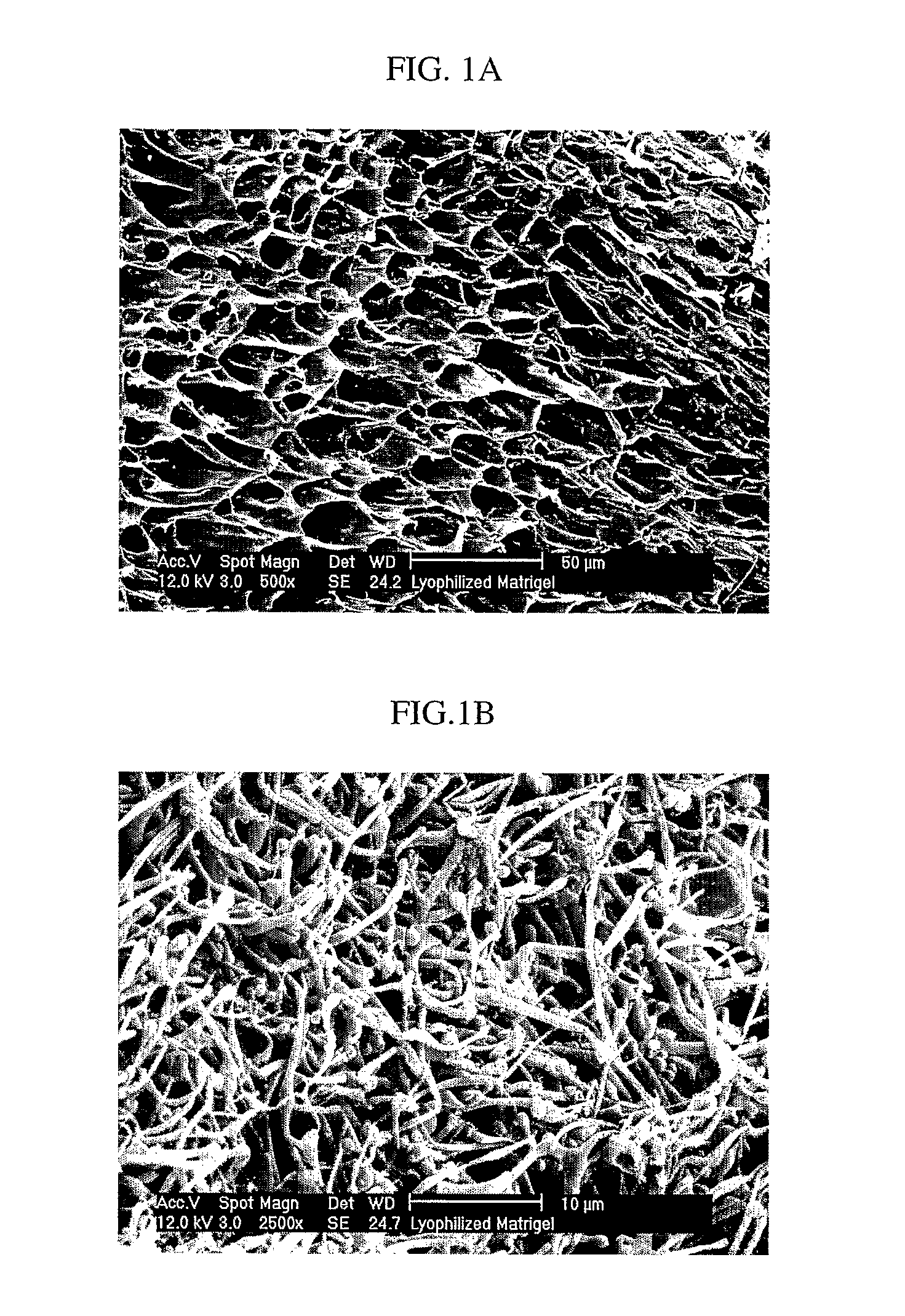
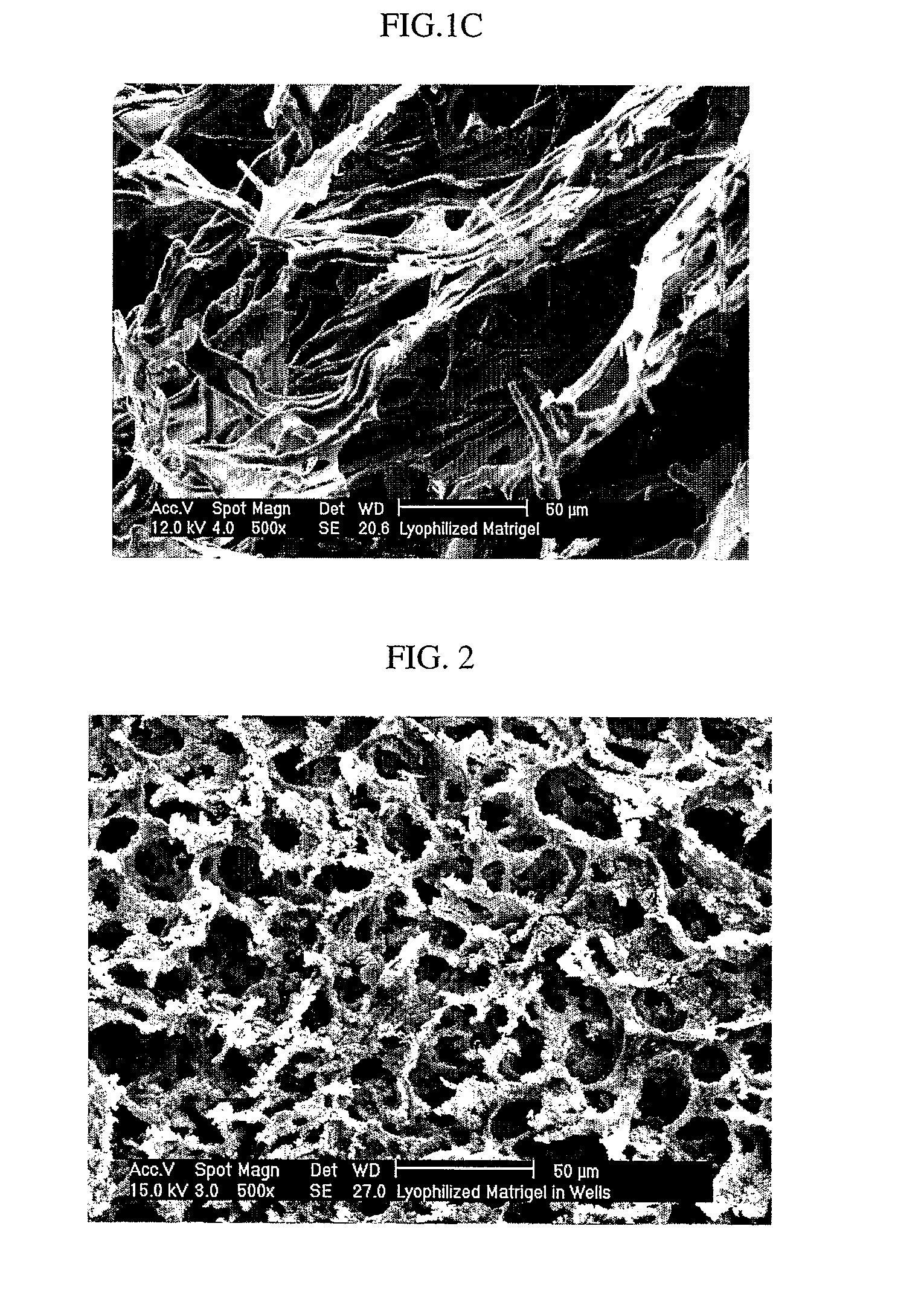
Three-Dimensional Scaffolds for Tissue Engineering Made by Processing Complex Extracts of Natural Extracellular Matrices
InactiveUS20080213389A1Avoiding immune complicationFacilitate cell penetrationPeptide/protein ingredientsMammal material medical ingredientsFiberPorosity
Methods of making a biologically active three-dimensional scaffold capable of supporting growth and differentiation of a cell are described. Biologically active three-dimensional scaffold made by the methods of the invention and an engineered tissue made from the scaffolds are described. Fibers of desired porosity can be obtained from non-structural ECM by lyophilization and / or electrospinning which can be useful for numerous tissue engineering applications requiring complex scaffolds, such as wound healing, artificial skin (burns), soft tissue replacement / repair and spinal cord injury.
Owner:DREXEL UNIV
Kidney derived stem cells and methods for their isolation, differentiation and use
InactiveUS20060177925A1Facilitate kidney regenerationPromote regenerationCulture processMammal material medical ingredientsRenal stem cellKidney
The invention relates generally to methods for isolation and culture of kidney stem cells, cells isolated by the methods, and therapeutic uses for those cells.
Owner:RGT UNIV OF MINNESOTA
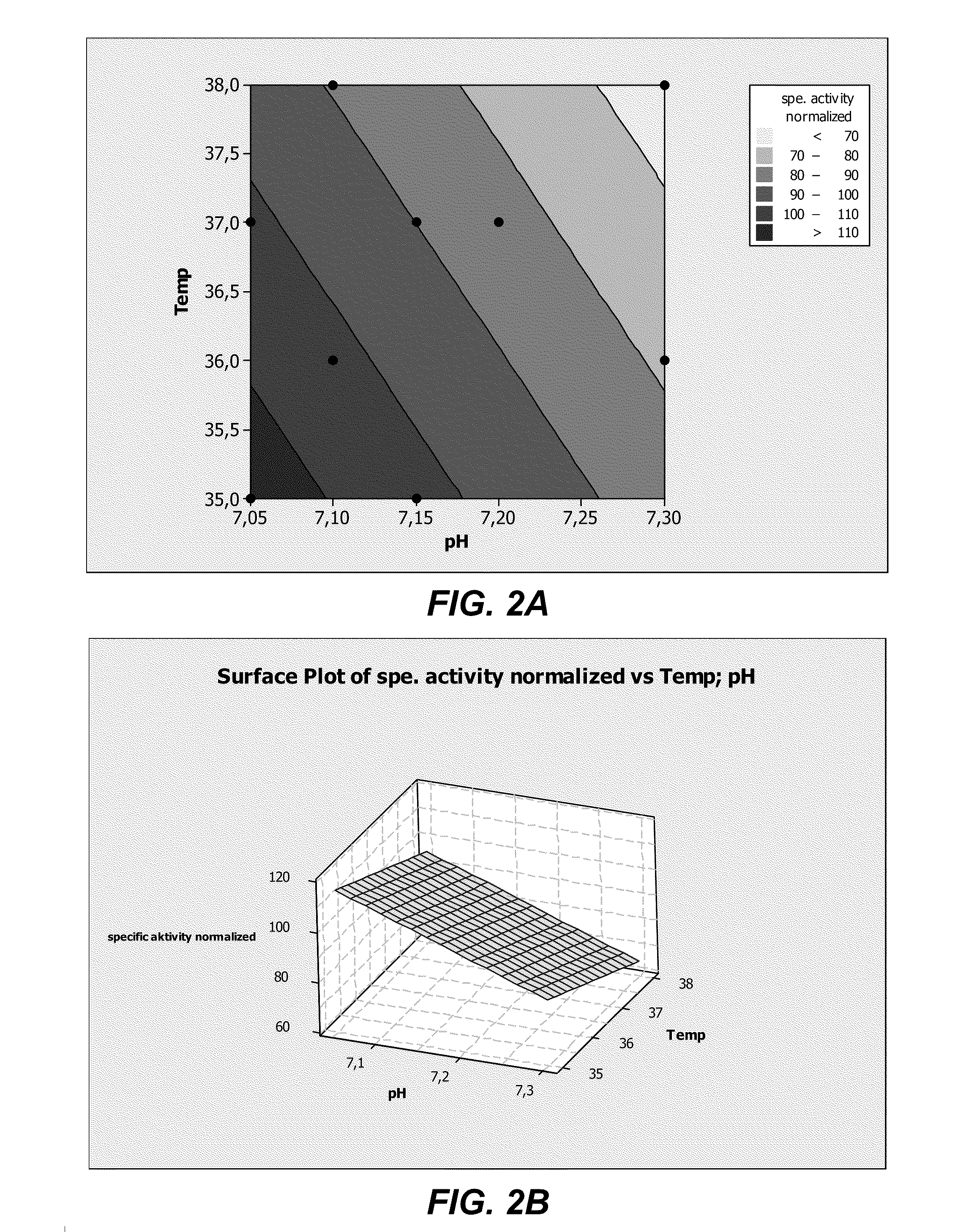
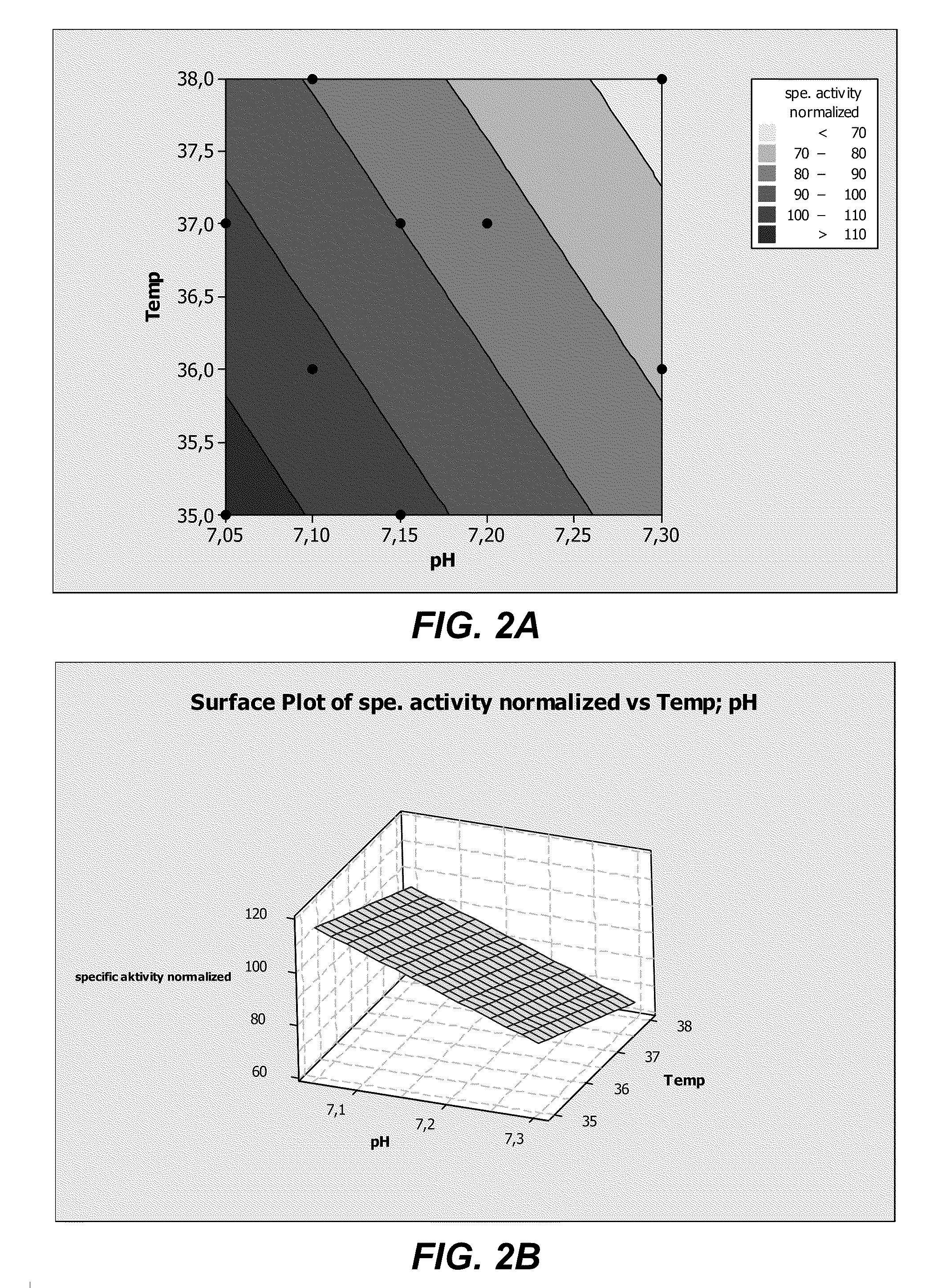
Cell Culture Medium For ADAMTS Protein Expression
ActiveUS20110086413A1Improve expression levelHigh activitySugar derivativesGenetically modified cellsProtein compositionADAMTS Proteins
The present invention provides culture mediums that are useful for the expression of ADAMTS proteins, such as ADAMTS13. Methods for the expression and purification of ADAMTS proteins are also provided. In some embodiments, the mediums and methods of the invention are useful for the expression of ADAMTS proteins having high specific activities. Also provided are ADAMTS, e.g., ADAMTS13, protein compositions with high specific activities, which are expressed and purified according to the methods provided herein.
Owner:TAKEDA PHARMA CO LTD
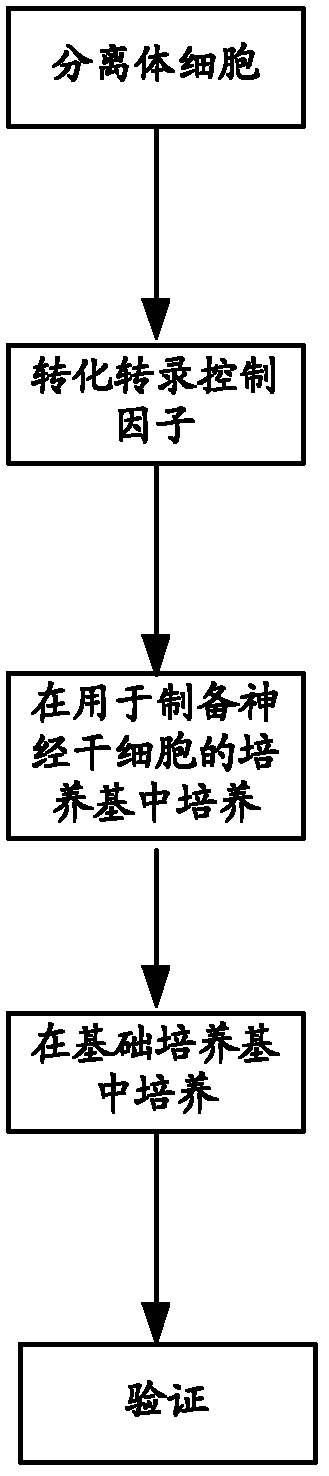
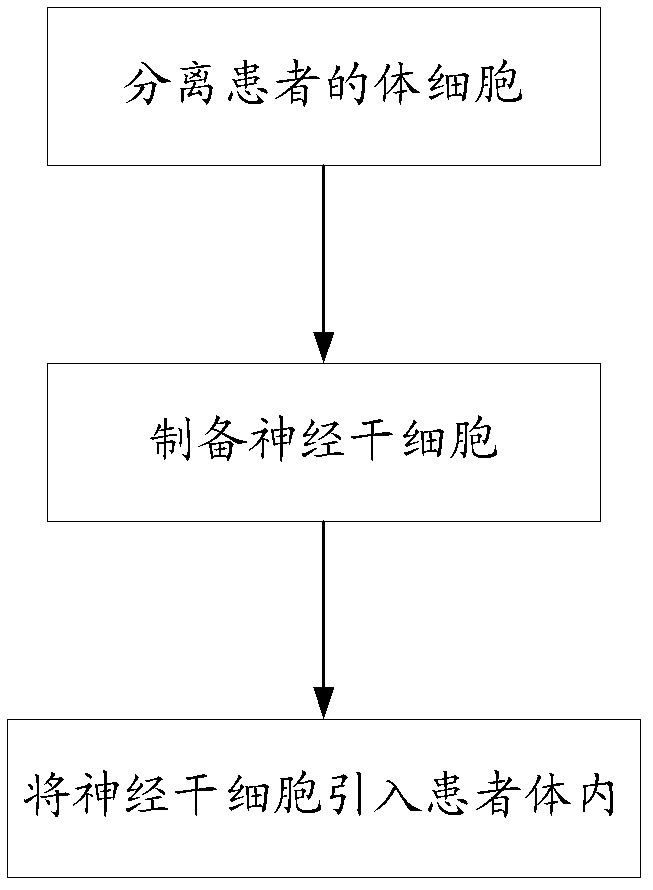
Culture medium for preparing neural stem cells and application thereof
ActiveCN102604894ABioreactor/fermenter combinationsNervous disorderTransdifferentiationTranscription regulator
The invention relates to a culture medium for preparing neural stem cells and an application of the culture medium. The culture medium for preparing the neural stem cells comprises a culture medium suitable for the growth of stem cells, and a cell signal pathway inhibitor which is selected from at least one of the following agents: a GSK (Glaxo Smith Klein) inhibitor, an MEK (Methyl Ethyl Ketone) inhibitor, a TGF (Transforming Growth Factor)-beta inhibitor, a ROCK inhibitor and a BMP inhibitor. The somatic cells are cultured by using the culture medium, especially the somatic cells for expressing the transcription regulator are cultured, so as to effectively transdifferentiate the somatic cells into neutral stem cells and the transdifferentiation time is greatly shortened.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Construction method of cell model for detecting pyrogens, cell model and pyrogen detection kit
ActiveCN106148286AImprove stabilityIncreased sensitivityCell receptors/surface-antigens/surface-determinantsCulture processWestern blotCytokine
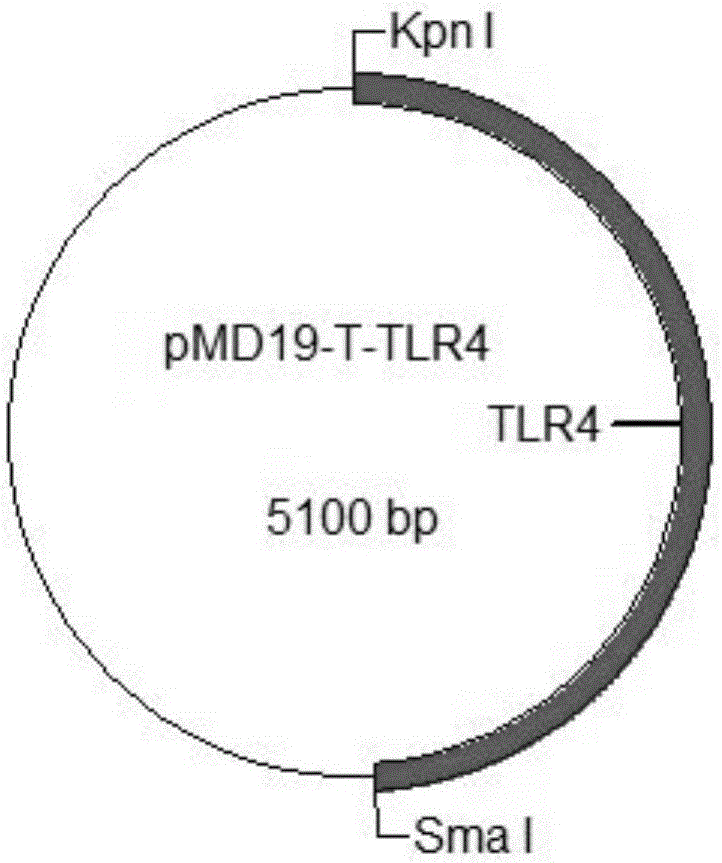
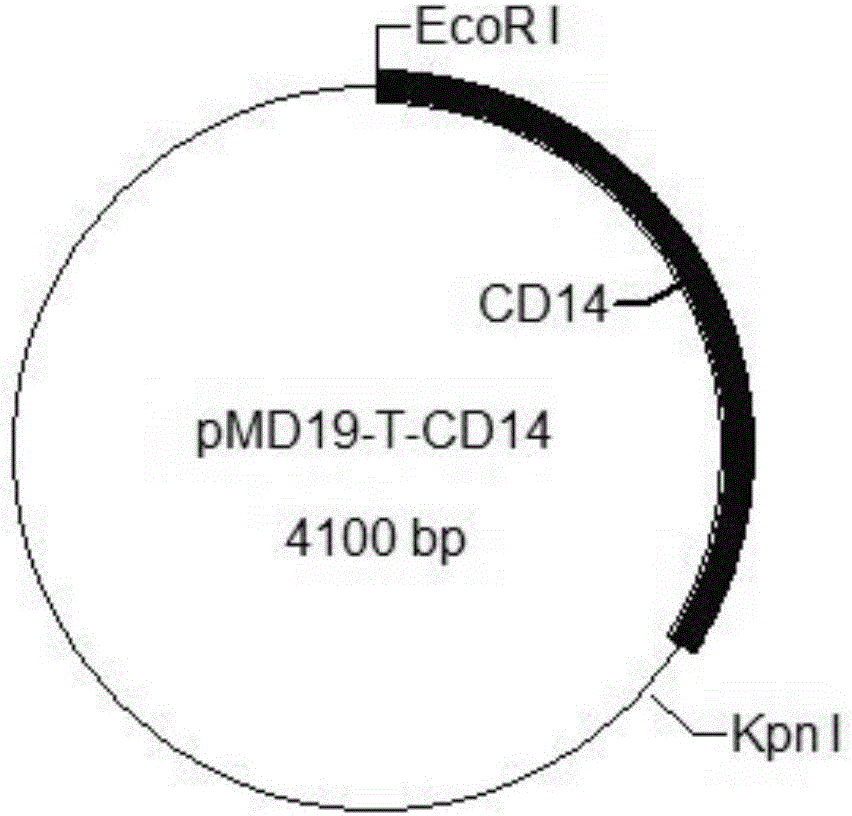
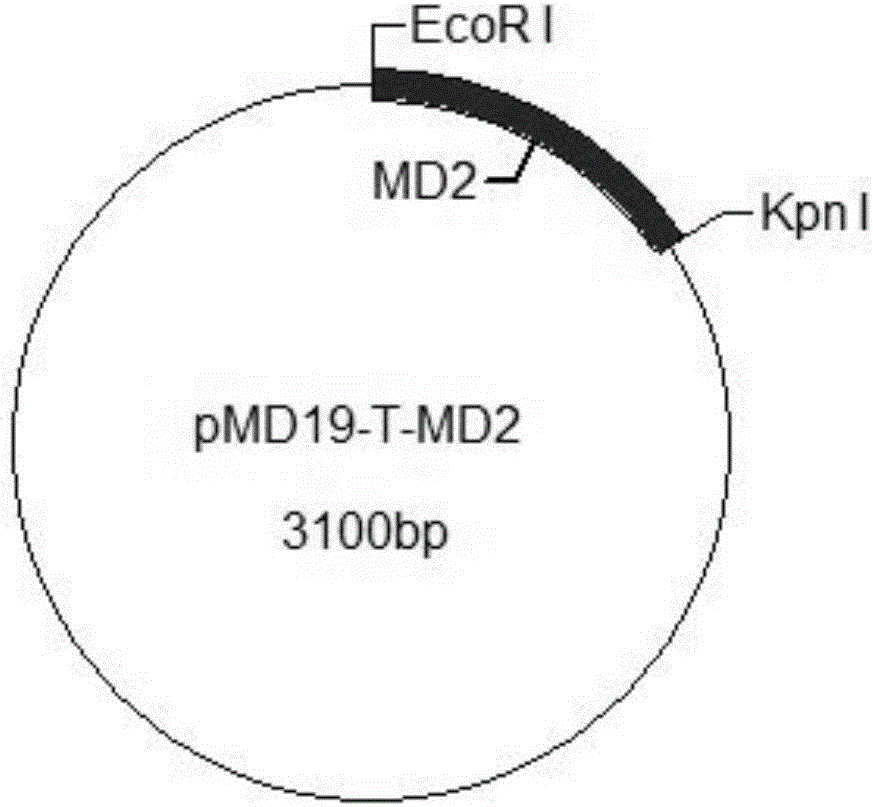
The invention provides a construction method of a cell model for detecting pyrogens, the cell model and a pyrogen detection kit. The cell model utilizes specific locations of CRISPR / CAS9 induced genomes to form double-bond fission, TLR4 and CD14-MD2 are knocked into two chromosomes of a cell line respectively by the aid of the homologous recombination repair principle, green fluorescence GFP and red fluorescence RFP are respectively used for tracing finally successfully constructed TLR4 / CD14 / MD2 fixed-point knocked-in fluorescent tracer cell models, and the LPS stimulating cell model can detect release of IL-6 and TNF-a cytokines by means of ELISA, Western Blot, mass spectrum and immunomagnetic beads. The cell model is good in stability and high in sensitivity, and the lowest detectable limit can reach 0.005EU / mL and is far lower than 0.025EU / mL of the Tachypleus Amebocyte Lysate method.
Owner:牛刚
Mixed cell diagnostic systems
InactiveUS6946291B2Readily discernableReadily distinguishableMicrobiological testing/measurementBiological material analysisDiagnostic microbiologyMixed cell
The present invention generally relates to the field of diagnostic microbiology, and, more particularly, to compositions and methods for detecting and differentiating one or more viruses or other intracellular parasites present in a specimen. The present invention also provides compositions and methods to evaluate the susceptibility of a organisms to antimicrobial agents.
Owner:CLEVELAND UNIV HOSPITALS OF +1
Enhanced production of recombinant proteins by transient transfection of suspension-growing mammalian cells
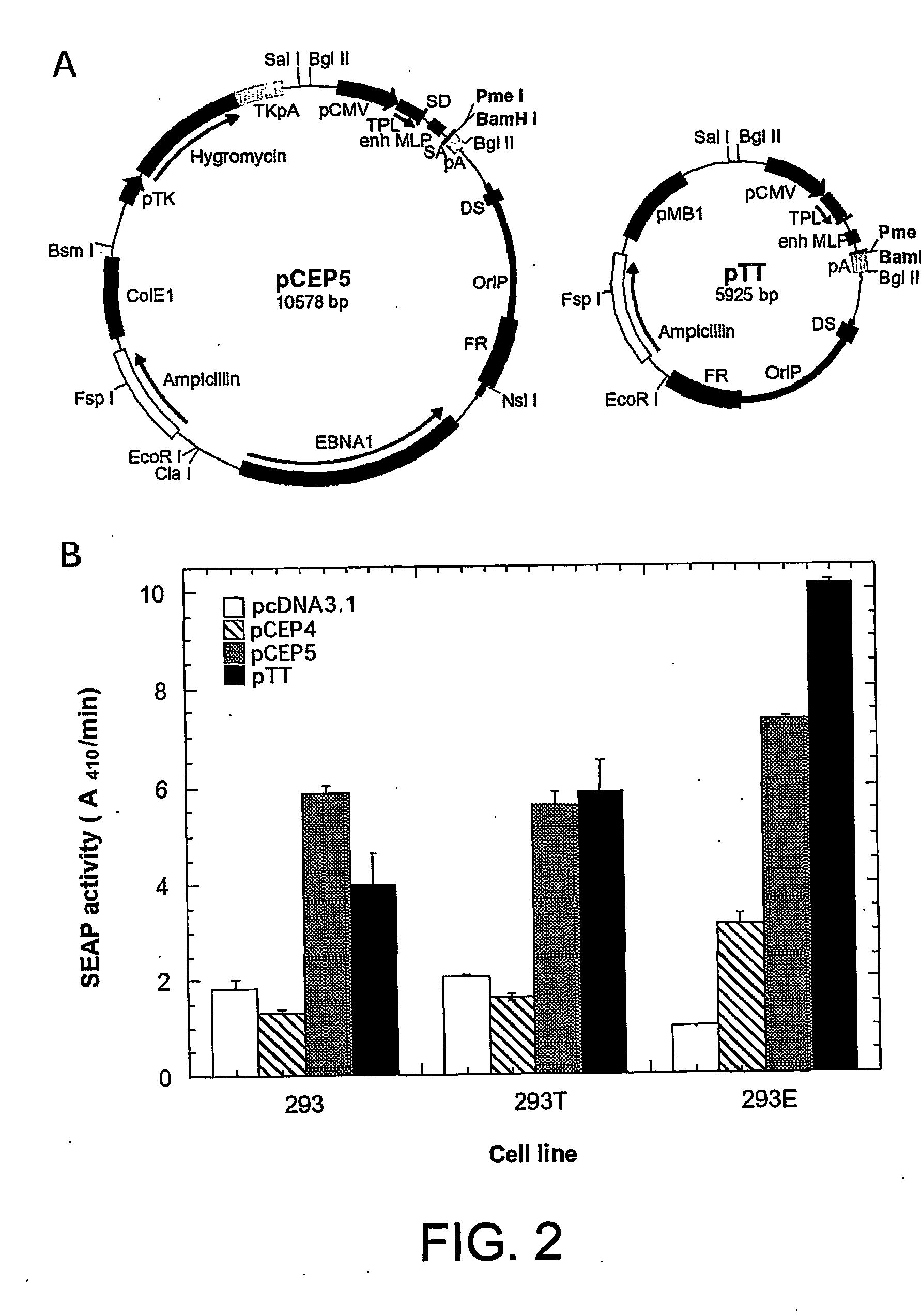
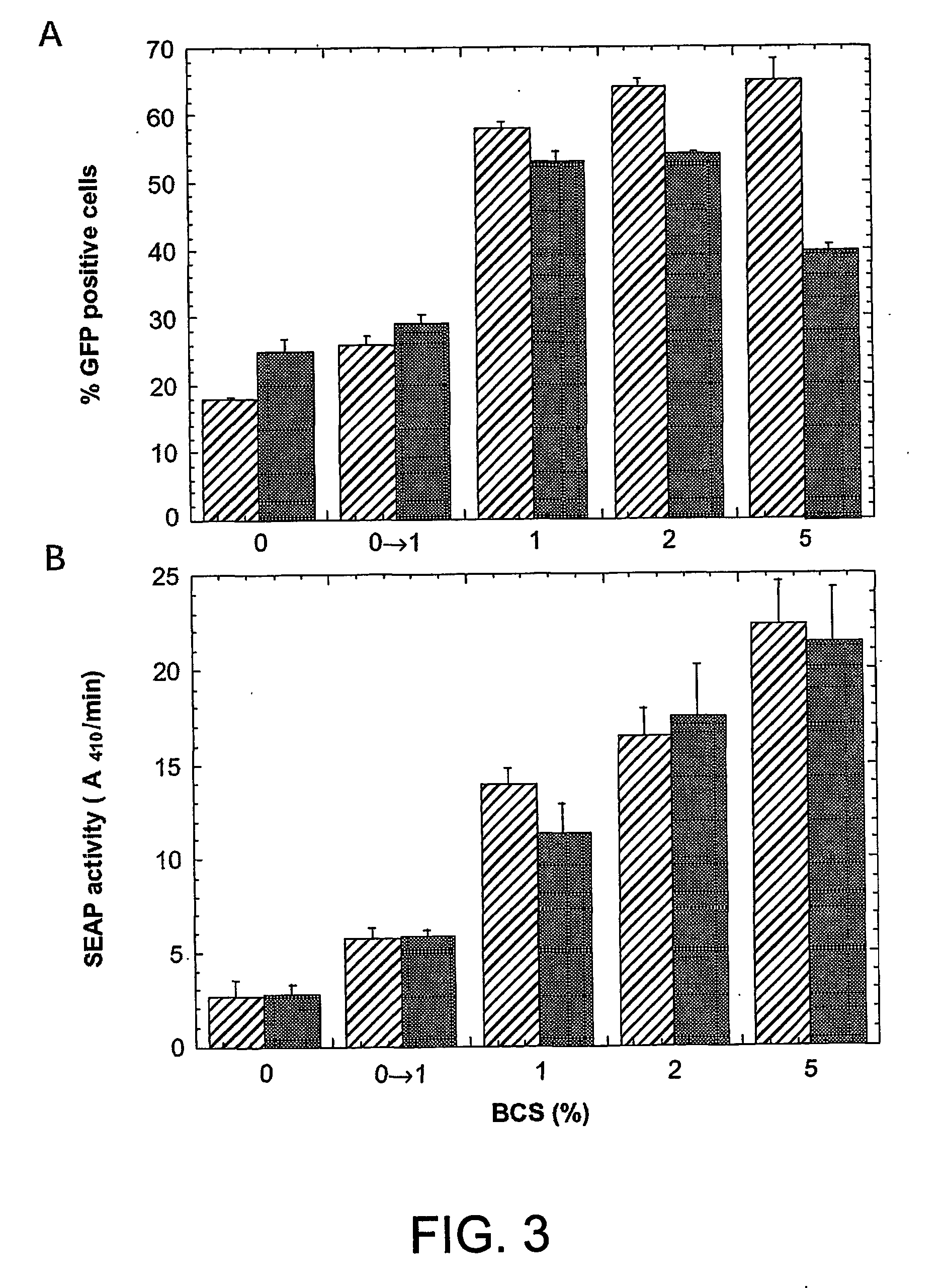
ActiveUS20050170450A1Increase transcriptional activityIncreasing nuclear importGenetically modified cellsVirus peptidesEpstein–Barr virusGene Variant
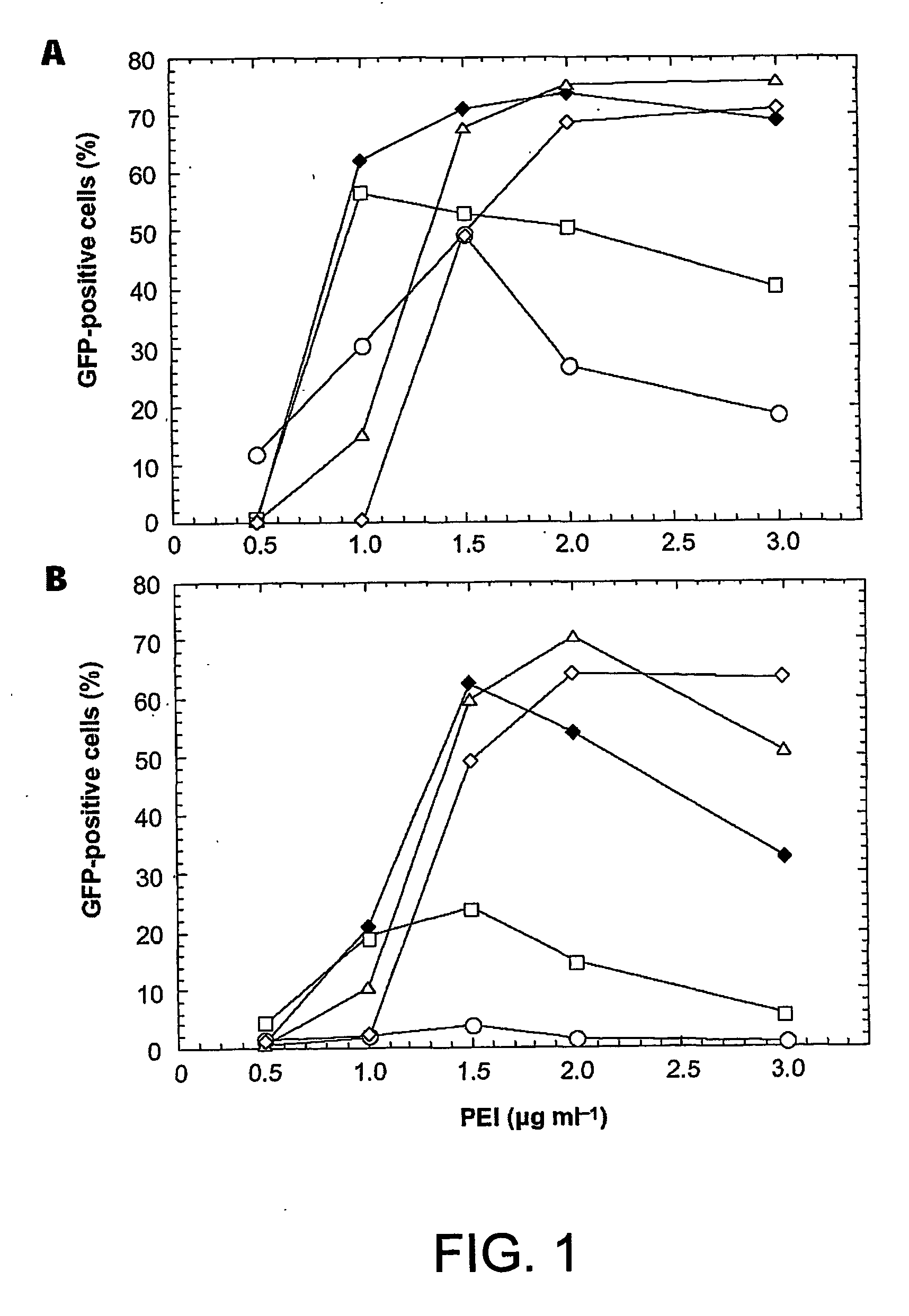
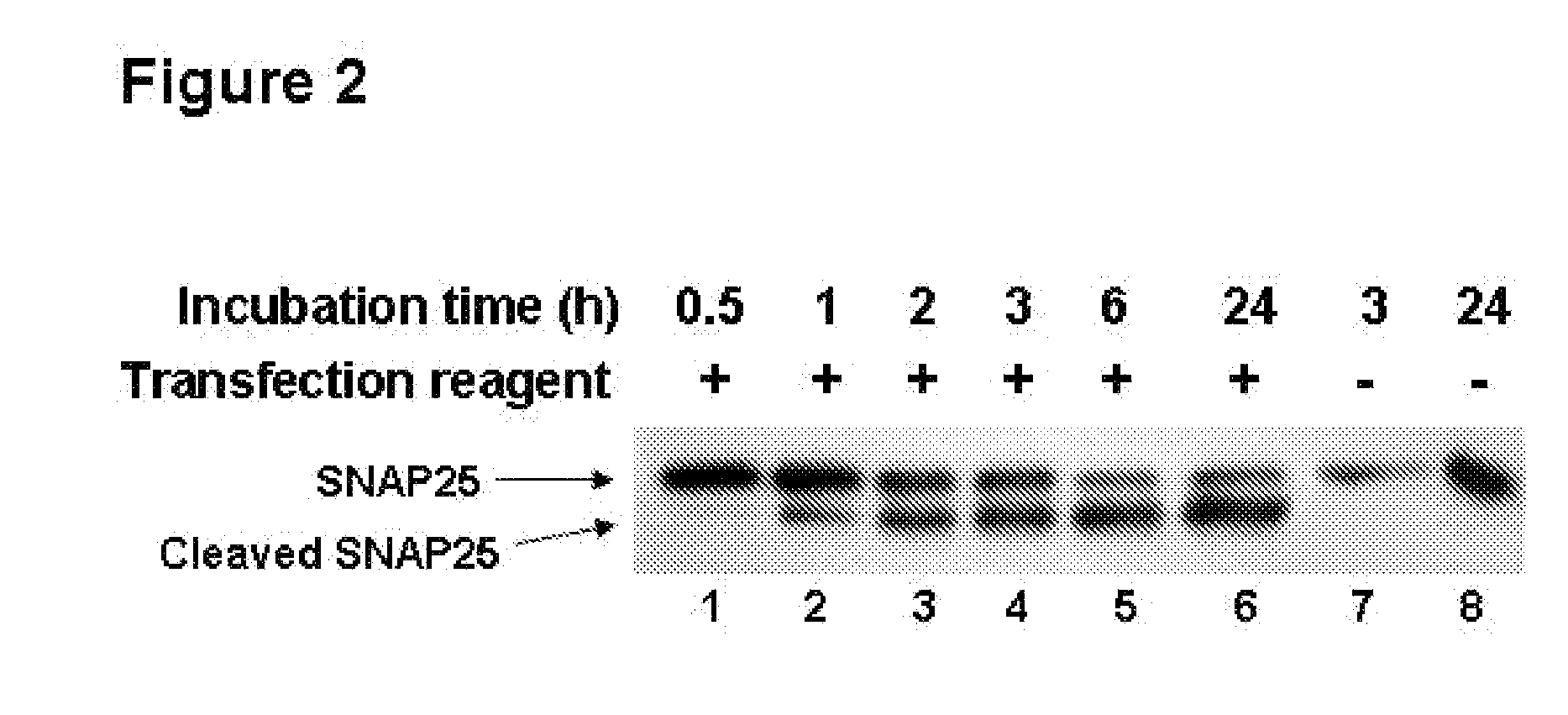
Disclosed is a new process for the production of recombinant proteins, by transient transfection of suspension-grown human embryonic kidney cells (293 cell line and its genetic variants) with an expression vector, using polyethylenimine (PEI) as a transfection reagent. In a preferred embodiment, the process uses 293E cells expressing the Epstein-Barr virus (EBV) EBNA 1 protein, in combination with an oriP-based episomal expression vector having an improved cytomegalovirus expression cassette comprising the CMV5 promoter. The process combines in a single step the cell growth, transfection and protein expression, is carried out without changing the culture medium, and allows to achieve high expression levels in a short period of time. The process may be carried out in a serum-free, low-protein culture medium, is easily scalable, compatible with continuous production processes, and fully adapted to high-throughput production of milligram quantities of recombinant proteins.
Owner:NAT RES COUNCIL OF CANADA
Mixed cell diagnostic systems
InactiveUS6875600B2Microbiological testing/measurementArtificial cell constructsMixed cellDiagnostic microbiology
The present invention generally relates to the field of diagnostic microbiology, and, more particularly, to compositions and methods for detecting and differentiating one or more viruses or other intracellular parasites present in a specimen. The present invention also provides compositions and methods to evaluate the susceptibility of a organisms to antimicrobial agents.
Owner:DIAGNOSTIC HYBRIDS +1
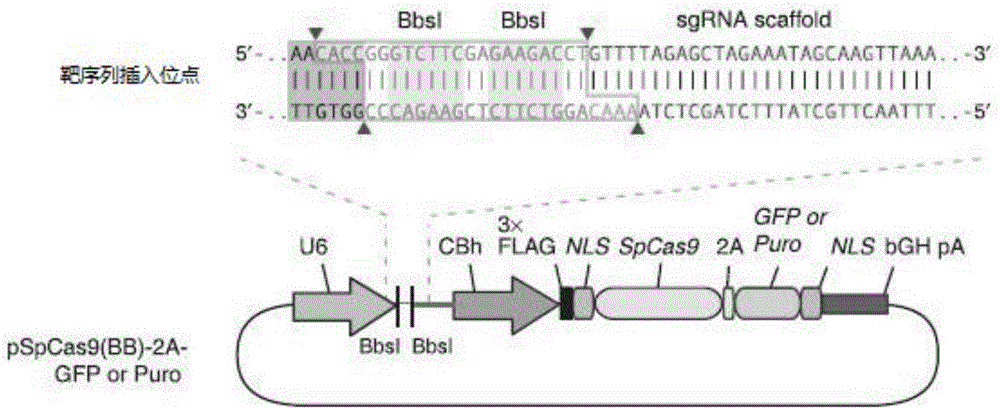
sgRNA sequence for knocking out human CYP2E1, construction method of deficiency cell strain of CYP2E1 and application thereof
ActiveCN106191057AAchieve silencingImproved silence is not completeGenetically modified cellsEnzymesHuman bodyDisease
The present invention provides an sgRNA sequence for knocking out the human CYP2E1 gene. The target DNA sequence of the sgRNA is at least one of the sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2. The present invention also provides a method for knocking out CYP2E1 gene of human embryonic kidney cells, which is adapted to transform CYP2E1 gene in human embryonic kidney cells by using CRISPR / Cas system. The invention also provides a CYP2E1 knockout cell strain. CYP2E1 involves in the important metabolism function of a human body. The CYP2E1 gene knock-out cell strain provided by the invention provides an effective platform for studying the metabolism function of exogenous chemical or exogenous poison in the body, and a powerful tool for studying chronic diseases (such as alcoholic liver disease and diabetes) and tumor-related diseases.
Owner:SUN YAT SEN UNIV
Methods and compositions of bioartificial kidney suitable for use in vivo or ex vivo
A novel cell seeded hollow fiber bioreactor is described as a potential bioartificial kidney. Endothelial cells along with pericyte, vascular smooth muscle, and / or mesangial cells or any mesenchymally derived support cells are seeded along a hollow fiber in a perfused bioreactor to reproduce the ultrafiltration function and transport function of the kidney. Maintenance of tissue specific function and ultrastructure suggest that this bioreactor provides an economical device for treating renal failure.
Owner:RGT UNIV OF MICHIGAN
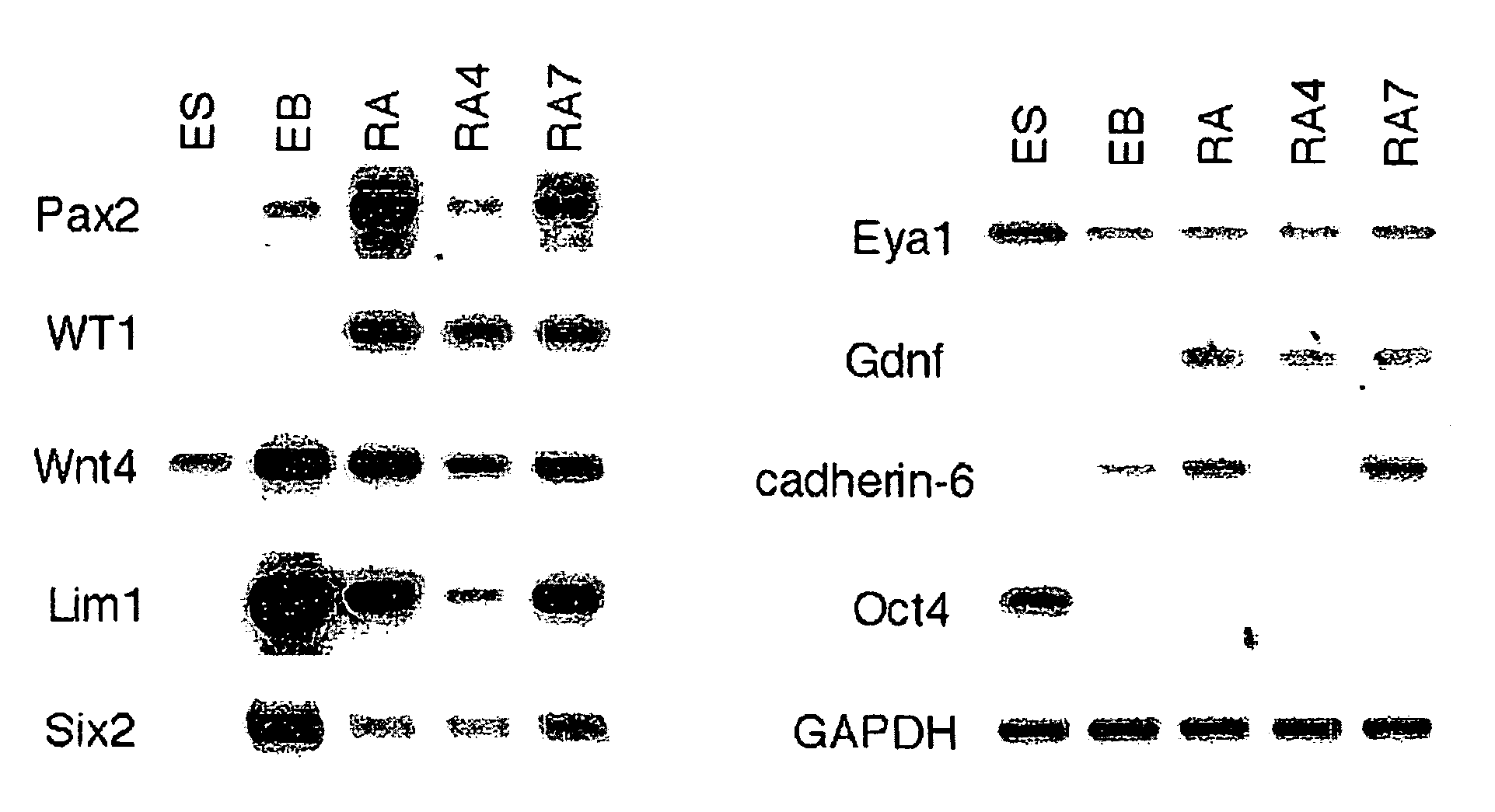
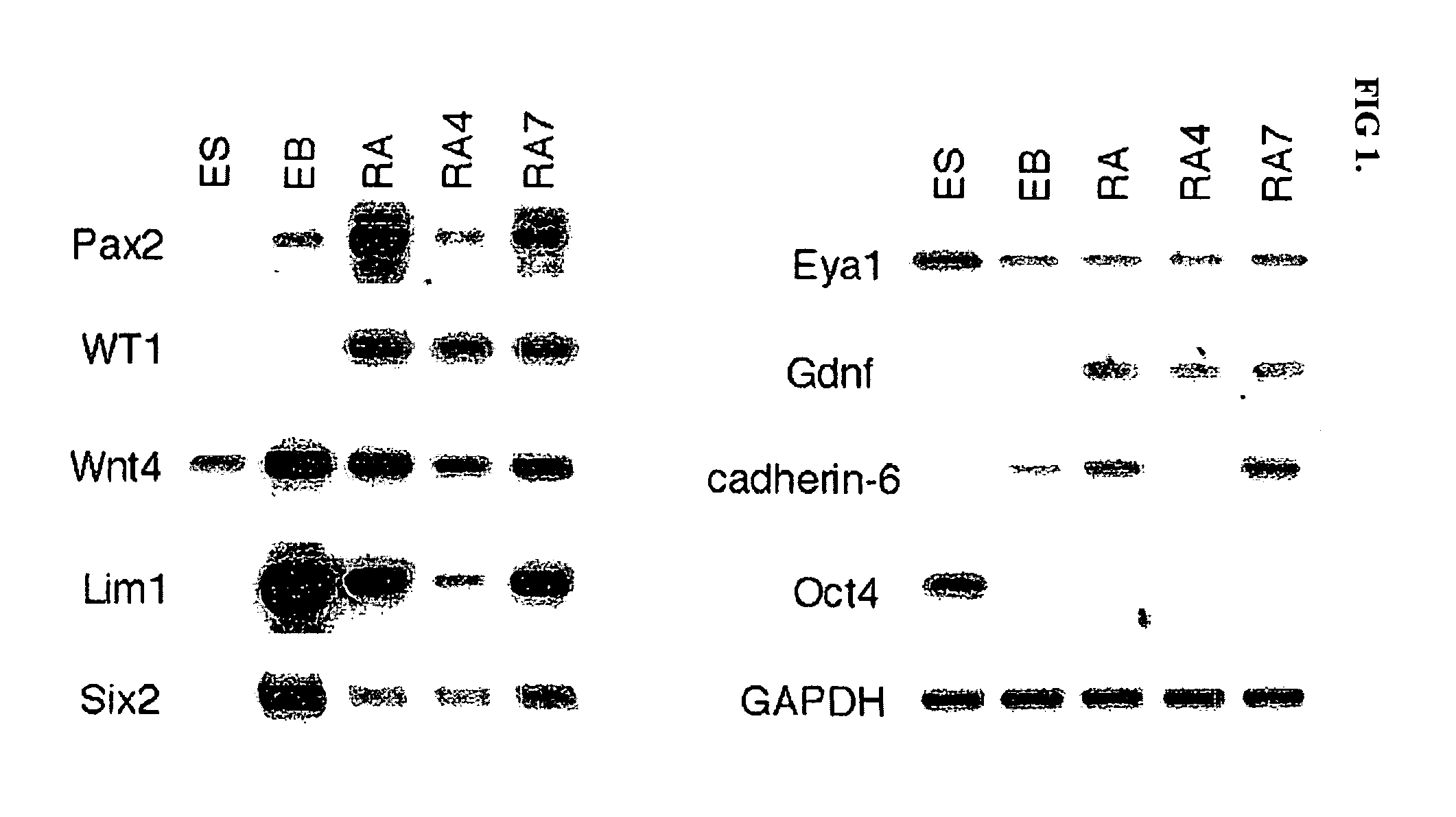
Renal progenitor cells from embryonic stem cells
The present invention relates to compositions and methods for inducing the differentiation of stem cells into renal progenitor cells. In particular, the present invention provides compositions containing activin-a, retinoic acid, and bmp-7, and variants thereof, for differentiating stem cells into renal cells containing tubular epithelia. In certain embodiments, the present invention provides stem cells cultured with compositions used to treat renal disease.
Owner:RGT UNIV OF MICHIGAN
Scaffolds for organ reconstruction and augmentation
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
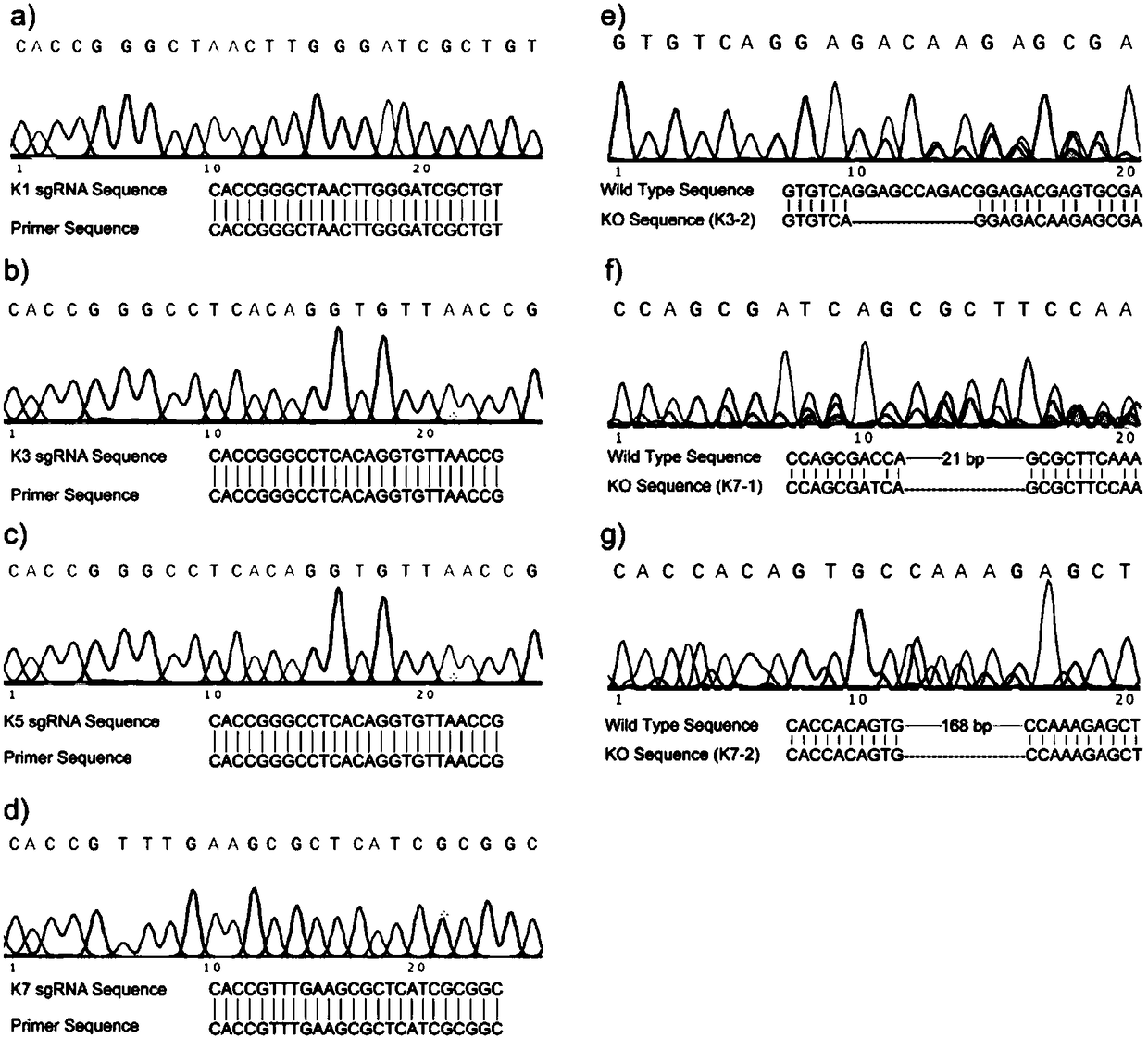
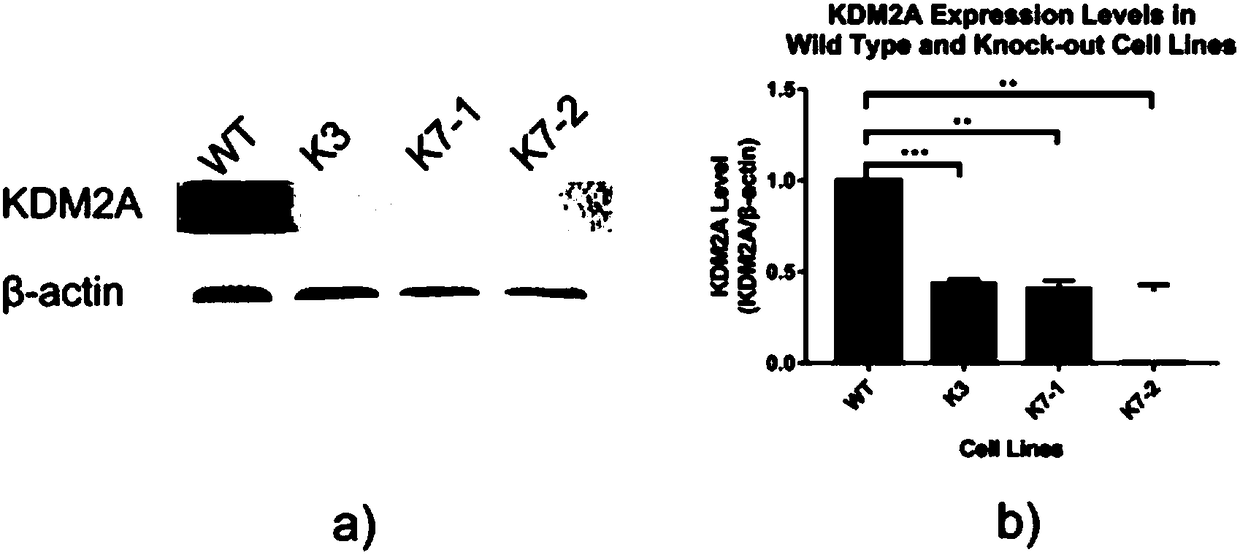
Method for knocking out KDM2A gene of HEK293T cells with CRISPR-CAS9 technology
InactiveCN108504657AConducive to research proliferationGood for studying the effects of apoptosisHydrolasesGenetically modified cellsT cellProtein level
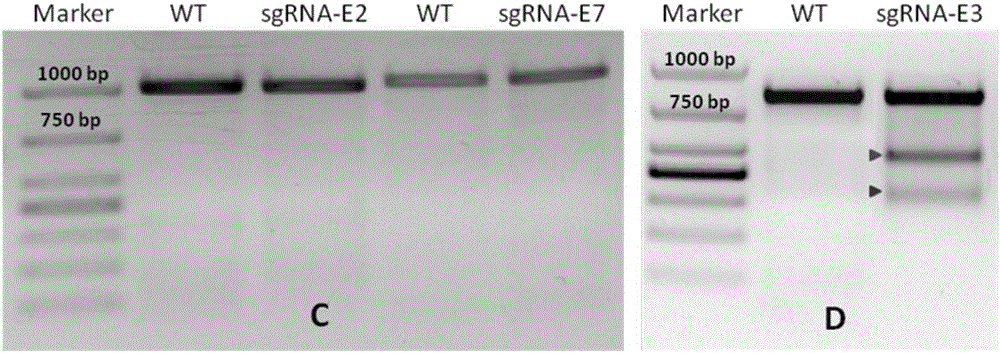
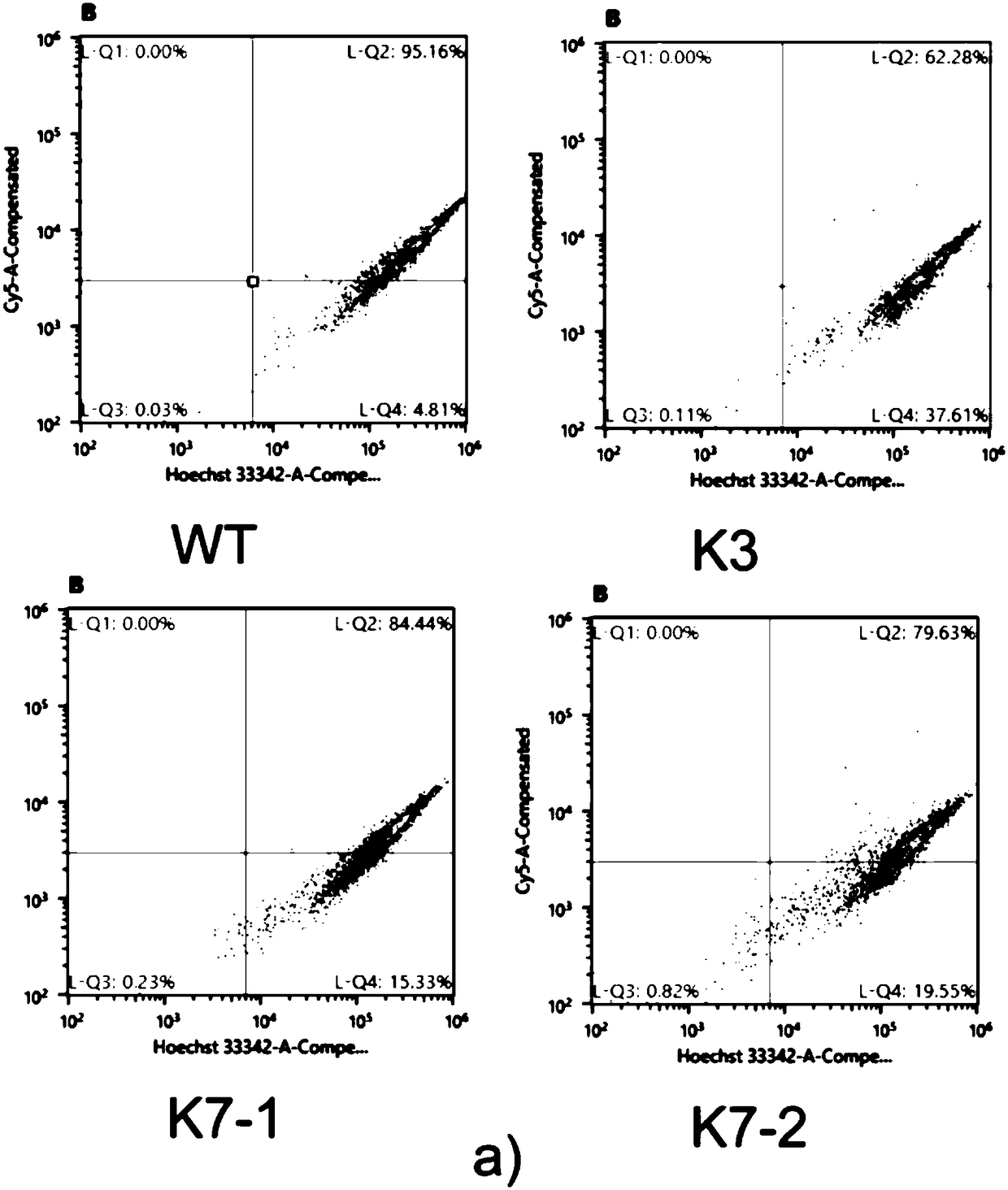
The invention provides a method for knocking out a KDM2A gene of HEK293T cells with a CRISPR-CAS9 technology. The method comprises the steps as follows: (1), cell transfection; (2), cell genome extraction; (3), PCR identification; (4), gene level verification; (5), protein level verification: whether K3, K7-1 and K7-2 are KDM2A gene-deficient is further verified by Western blotting after a mutantcell line is verified at the gene level. The invention further provides a KDM2A knockout HEK293T cell line prepared with method and an application of the KDM2A knockout HEK293T cell line as a cell model in study of cell proliferation and apoptosis signaling pathways involved in KDM2A methylase.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for establishing luciferase knock-in cell line based on CRISPR-targeted genome modification technology
InactiveCN108559760AAccurate and sensitive reflectionHydrolasesGenetically modified cellsLuciferasesBiological activation
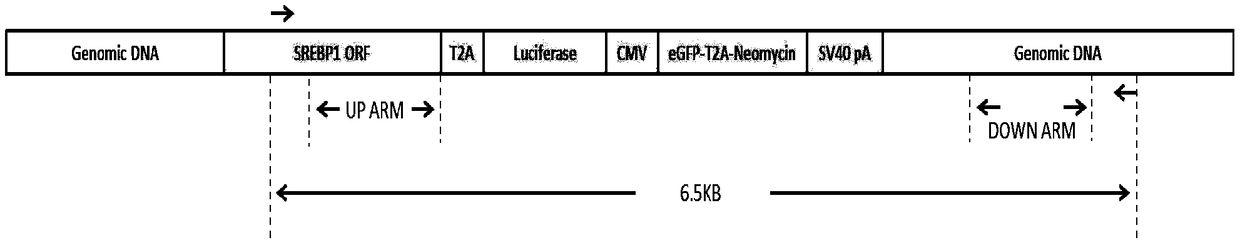
The invention relates to a method for establishing luciferase knock-in cell line based on CRISPR-targeted genome modification technology. The knock-in cell line of SREBP1- T2A-Luciferase was established by in situ integration of the 3'end of SREBP1 gene into the T2A-Luciferase reporter gene using CRISPR / Cas9 technique, the fixed point of the Chinese and foreign source genes of the cell line on thegenome is verified. The transcriptional activation of the SREBP1-T2A-Lucifasse cell line was performed using the reported transcription factor LXR Alpha, which has an active effect on the SREBP1. Theresults show that the expression level of the Luciferase in the SREBP1-T2A-Lucifasse cell line can accurately and sensitively reflect the expression level of the SREBP1 in the cell line. The establishment of the cell line will help to study the function of the SREBP1 gene and to screen the small molecular chemical drugs affecting the expression of SREBP1, and provide a new experimental thought and solution for lipid metabolism and related research.
Owner:SHAANXI NORMAL UNIV
Mdck-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
InactiveUS20130183741A1Low and no tumorigenicityEasy to useSsRNA viruses negative-senseViral antigen ingredientsSerum igeSerum free
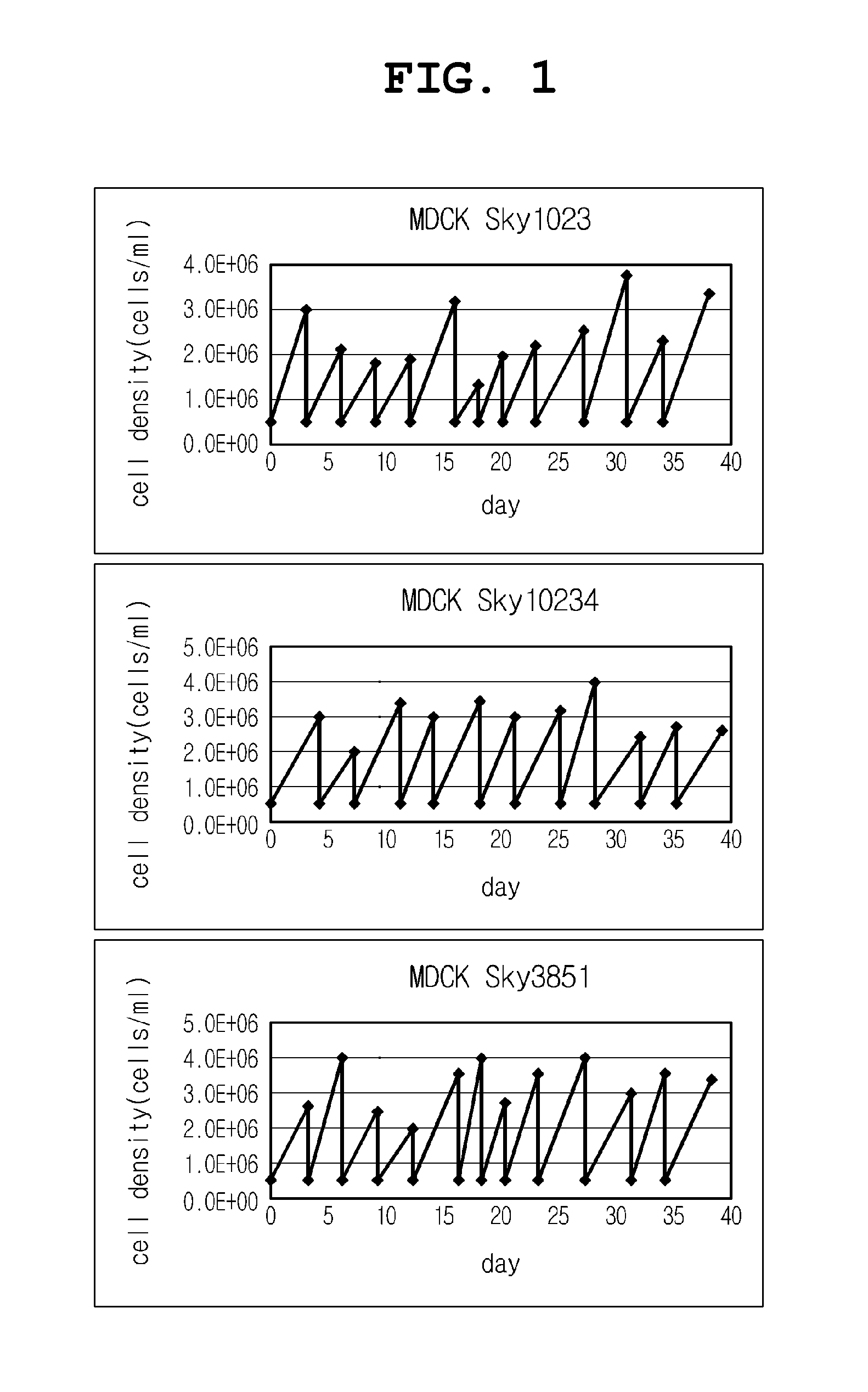
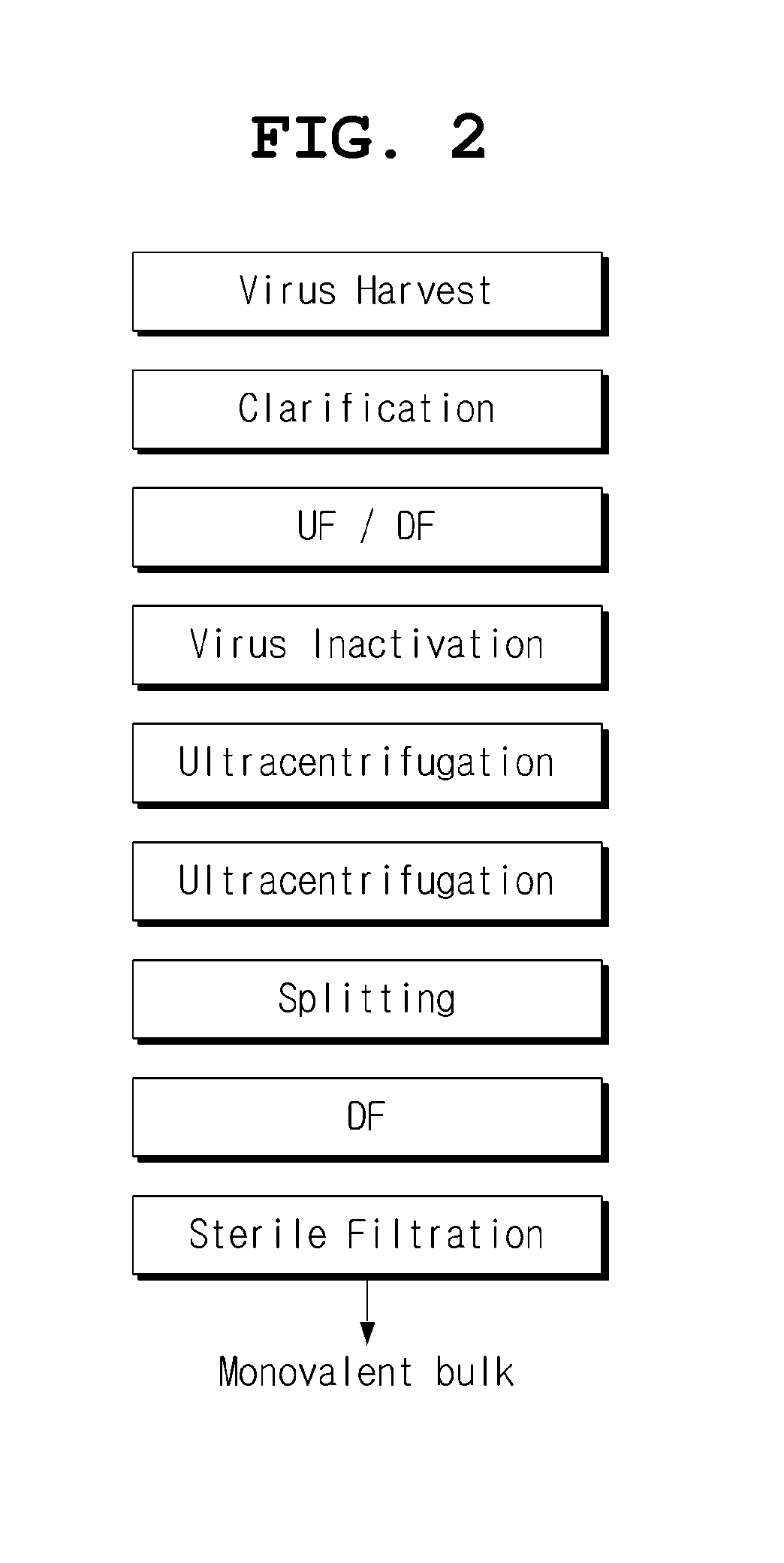
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK CHEM CO LTD
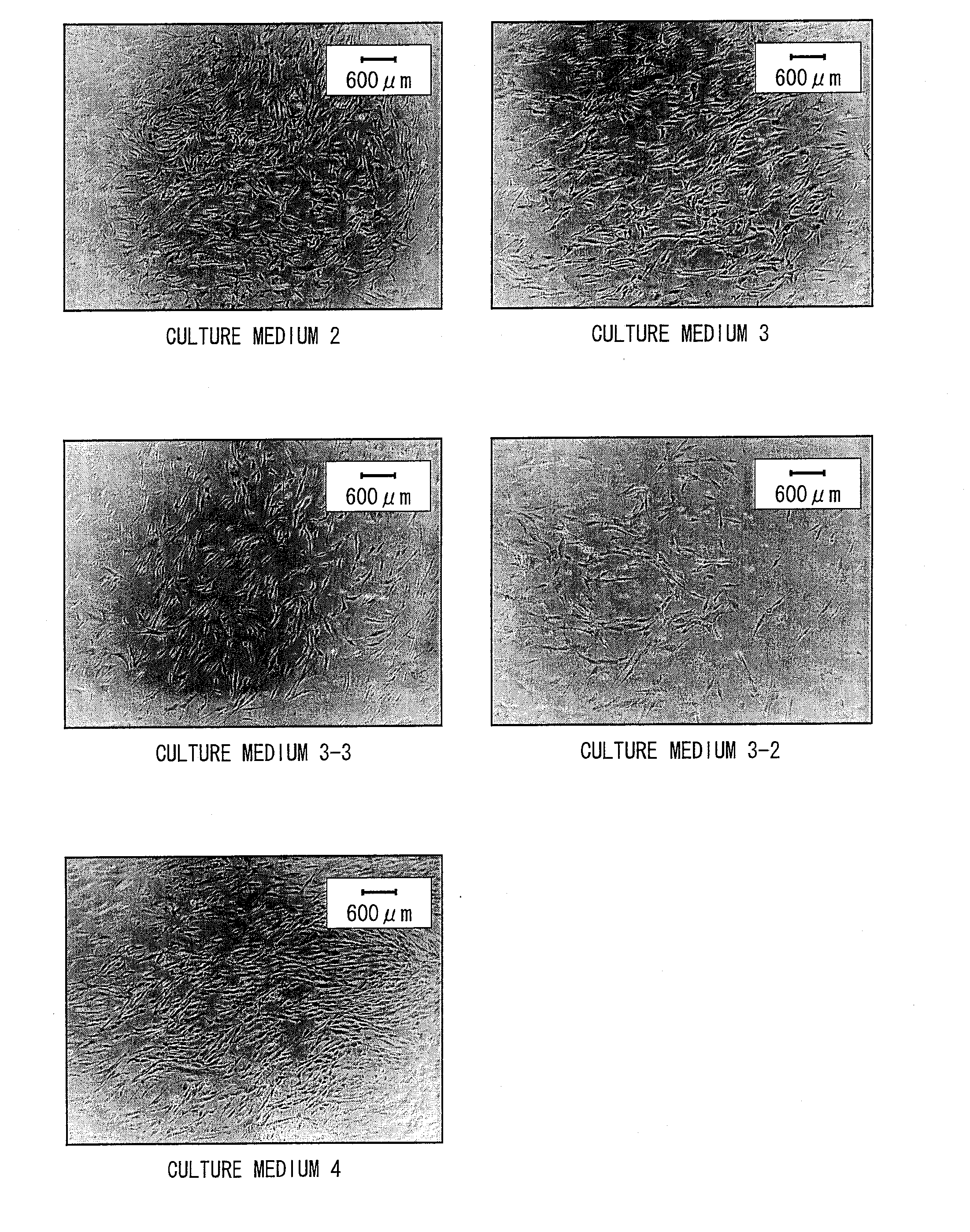
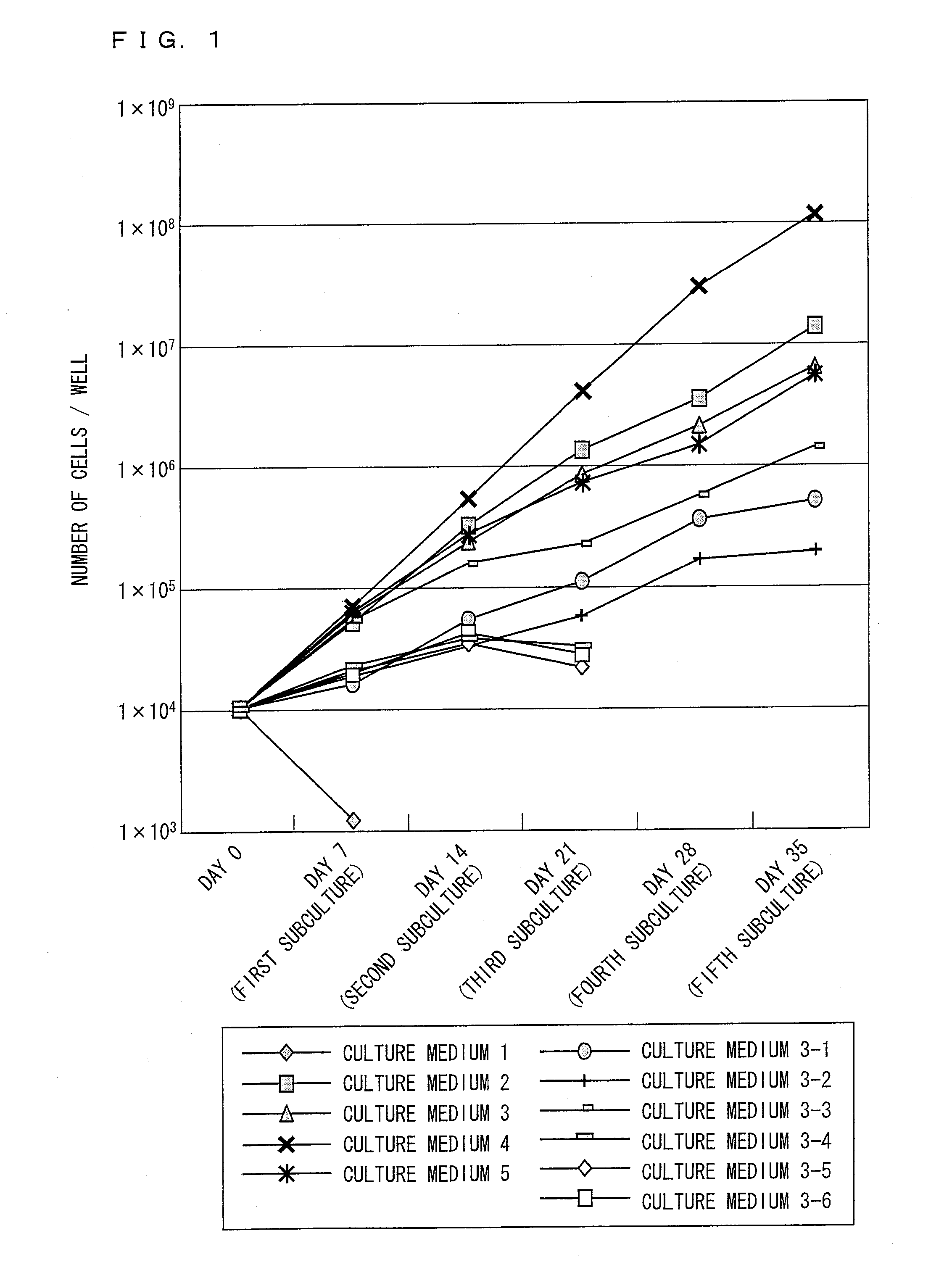
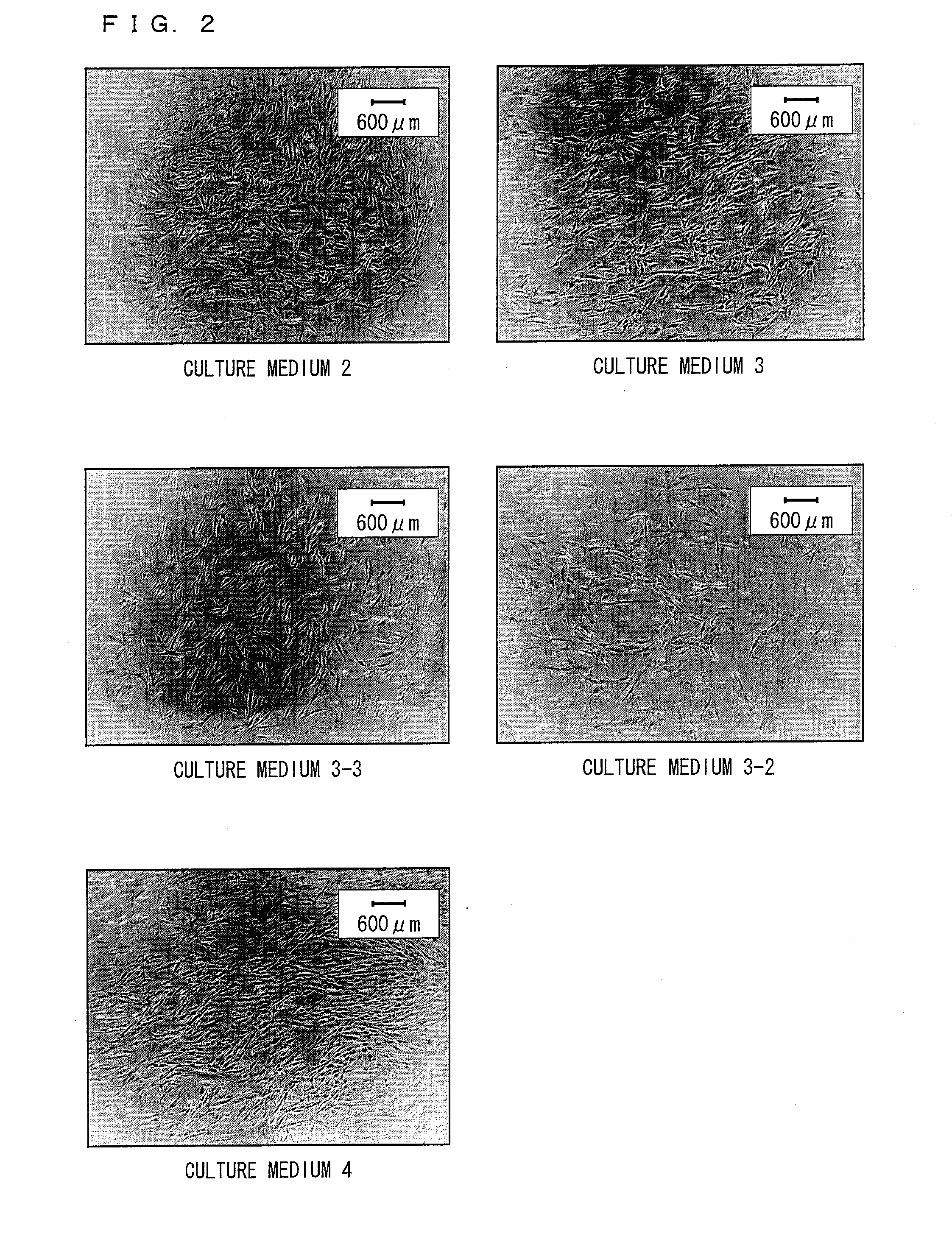
Composition and method for culturing potentially regenerative cells and functional tissue-organs in vitro
Compositions and methods are provided for culturing in vitro potentially regenerative cells (PRCs) from which functional tissue-organs are regenerated. In one aspect of the invention, a tissue culture medium is provided which comprises at least 50% of water and a sterol compound that is dissolved in a fatty acid-containing oil at a concentration at least 0.1% by weight based on the weight of the oil and added to the water. The culture medium can be used to culture PRCs that are isolated from the body of a mammal to generate functional tissue-organs in vitro with substantially the same physiological structure and function as the corresponding ones existing in vivo and in situ. The cultured PRCs, tissues, and tissue-organs can serve as valuable models for scientific investigation in life sciences, nutraceutical discovery, drug screening, pharmacokinetic studies, medical devices and tissue / organ transplantation.
Owner:RONGXIANG XU
Methods for expressing ADAMTS proteins in cell culture medium supplemented with zinc
ActiveUS8313926B2Improve expression levelHigh activitySugar derivativesGenetically modified cellsProtein compositionCell culture media
The present invention provides culture mediums that are useful for the expression of ADAMTS proteins, such as ADAMTS13. Methods for the expression and purification of ADAMTS proteins are also provided. In some embodiments, the mediums and methods of the invention are useful for the expression of ADAMTS proteins having high specific activities. Also provided are ADAMTS, e.g., ADAMTS13, protein compositions with high specific activities, which are expressed and purified according to the methods provided herein.
Owner:TAKEDA PHARMA CO LTD
Human Renal Stem Cells
InactiveUS20070065942A1Reduce mutationEasy to controlBiocideGenetic material ingredientsRenal stem cellNeuron
The present disclosure provides human renal stem cells. Also described are human renal stem cells isolated from the papillary region of the human kidney and methods of isolating the same. Also described are methods for culturing, characterizing, and differentiating the same, including methods for identifying human renal stem cells that are positive for Nestin and CD133, and methods for allowing the cells to differentiate into neurons.
Owner:STC UNM
Culture medium additive for use in serum-free culturing of animal cell, kit and use thereof
ActiveUS20100279412A1Facilitate animal cell proliferationCulture processCell culture mediaSerum freePhospholipid
Disclosed are: a culture medium containing a specific growth factor and at least one phospholipid; a composition for preparation of the culture medium; a kit; and a method. A technique can be provided which uses a serum-free or low-serum culture medium and has a promoting effect on the proliferation of an animal cell comparable to the promoting effect obtained by the culture in a serum-containing culture medium.
Owner:TWO CELLS
Remedies for kidney diseases and method for screening the same
InactiveUS6794154B1Useful in therapyConducive to screeningCompound screeningApoptosis detectionDiseaseRenal Tubular Epithelial Cells
The present invention provides a method for screening or identifying therapeutic or prophylactic agents for renal diseases, which comprises assaying a test substance for the activity of up-regulating the expression of fatty acid-binding protein (FABP), and novel mouse proximal renal tubular epithelial cell lines useful therein. The present invention also provides therapeutic or prophylactic agents for renal diseases comprising, as an active ingredient, an agent having activity of up-regulating FABP expression; agents for up-regulating the expression of FABP, and for treating or preventing renal diseases, which comprise a compound having activity of peroxisome proliferator-activated receptor (PPAR) agonist or carnitine palmitoyltransferase (CPT) inhibitor or the like.
Owner:CMIC HLDG
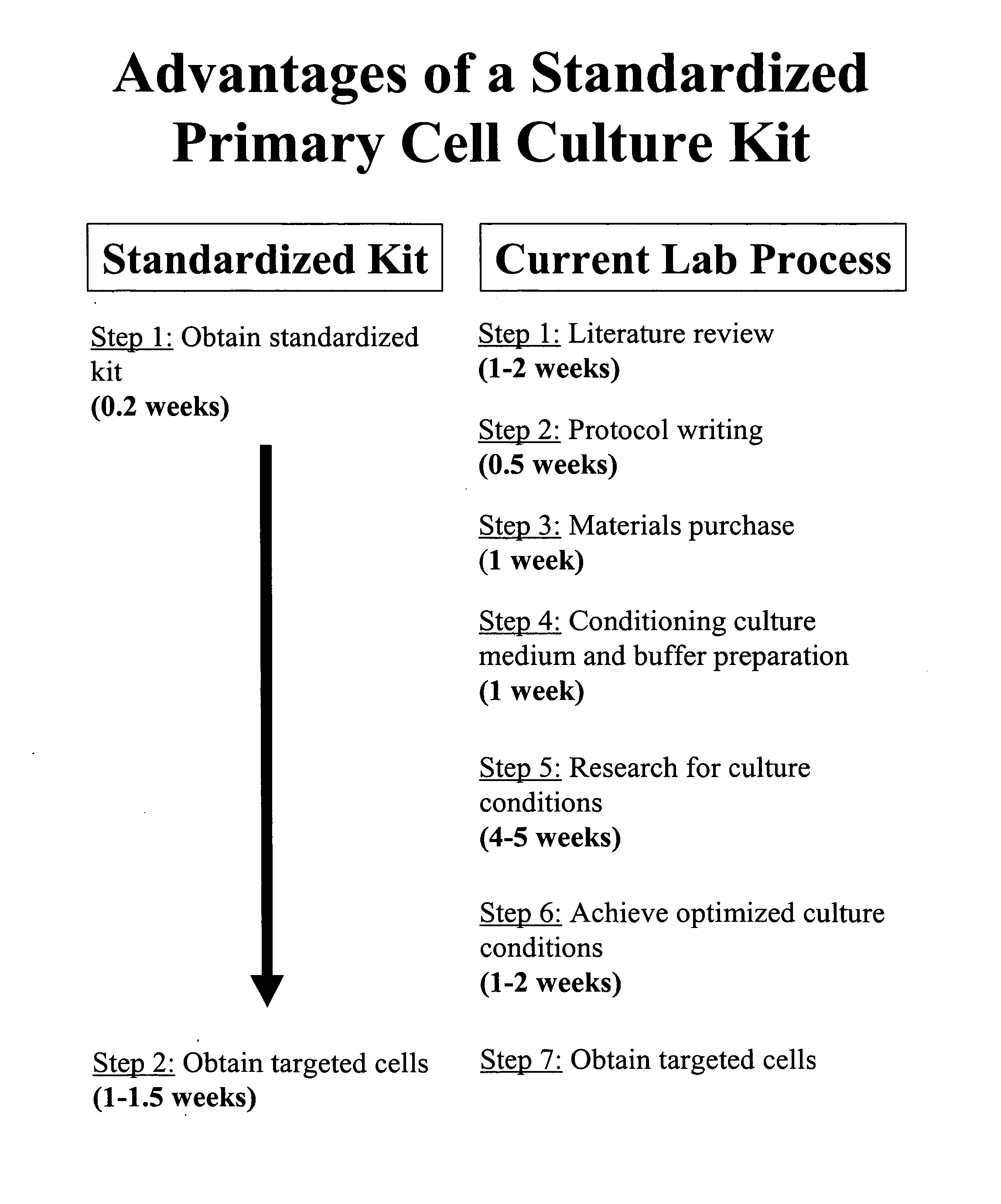
Standardization of processes for culturing primary cells
InactiveUS20070238175A1Promotes target cell enrichmentPromote growthCell dissociation methodsGastrointestinal cellsCell specificPrimary cell
The present invention provides a standardized tissue-specific and cell-specific kit and methods for promoting the enrichment and expansion of primary cells in culture while reducing the contamination of unwanted cell types. The present invention further provides the compositions for optimized tissue-specific and cell-type specific dissociation of tissues and inhibition of contaminating cell-types in primary cultures.
Owner:CHI SCI
Methods For The Delivery Of Toxins Or Enzymatically Active Portions Thereof
InactiveUS20100209955A1Increase the number of cellsIncrease rangeMicrobiological testing/measurementBiological material analysisCell based assaysToxin
The present invention relates to methods, systems, and kits for intoxicating cells, neuronal and non-neuronal cells, with a toxin or fragment thereof. This is done by subjecting toxin substrate and a lipid or polymeric carrier (e.g., DNA uptake facilitating agent) to one or more cells for use in cell based assays. In an aspect, the methods of the present invention allow for high throughput assays and, as such, for the evaluation of drug candidates.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Microfluidic Continuous Flow Device
ActiveUS20110256574A1Bioreactor/fermenter combinationsBiological substance pretreatmentsContinuous flowEngineering
A microfluidic continuous flow device comprising a channel which comprises a first and a second area wherein the first area of the channel is a compartment which is defined by partitioning elements and the second area of the channel is a space outside the compartment; wherein through passages which are formed between the partitioning elements are dimensioned such as to retain a biological material and optionally a sustained release composition which can be comprised in the compartment within the compartment; wherein the channel has a first inlet to the compartment through which biological material can be introduced into the compartment; a second inlet for introducing a cultivation medium into a space of the channel arranged outside of the compartment, and an outlet. The present invention further refers to methods of using the devices of the present invention and kits comprising the microfluidic continuous flow devices of the present invention.
Owner:AGENCY FOR SCI TECH & RES
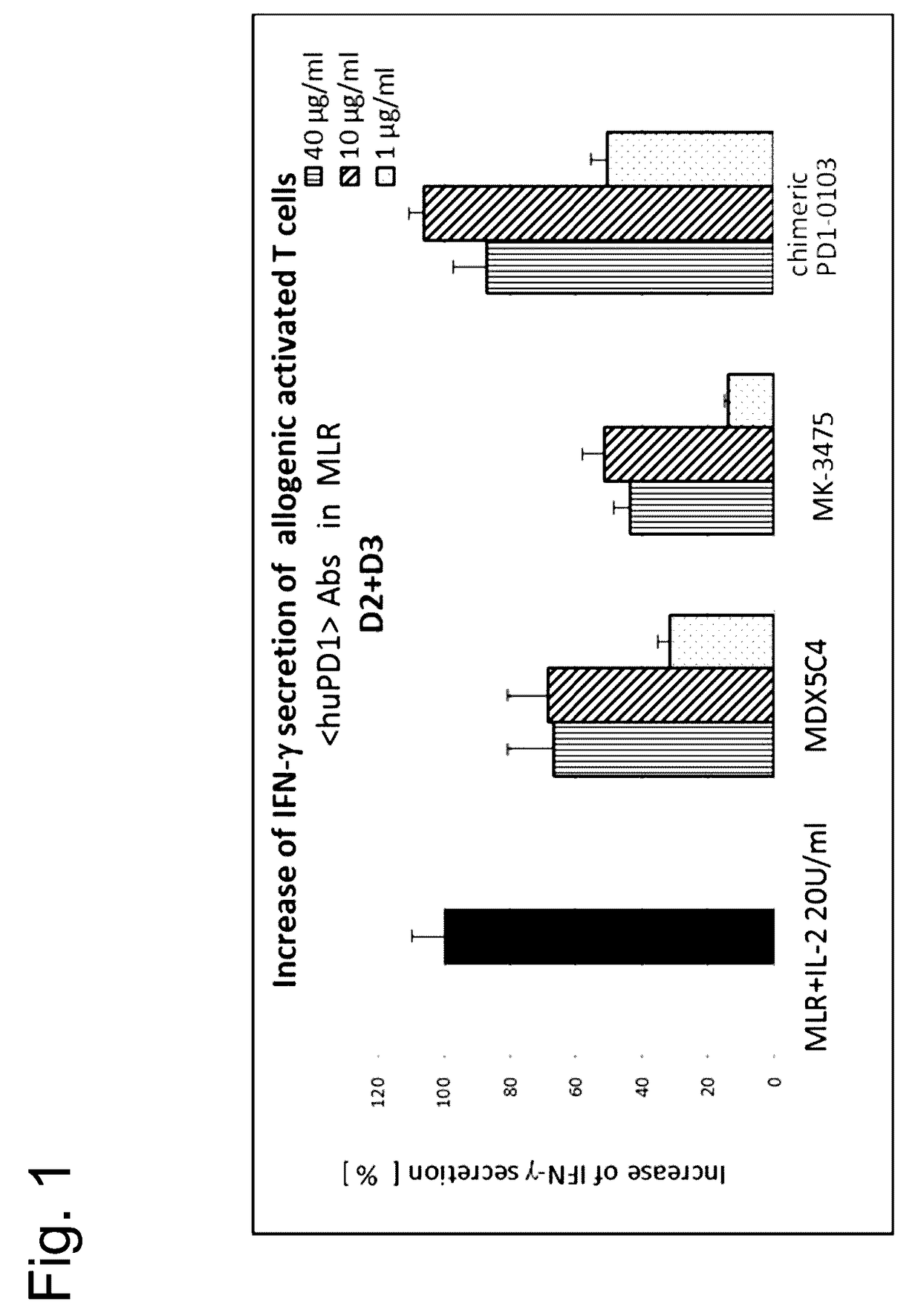
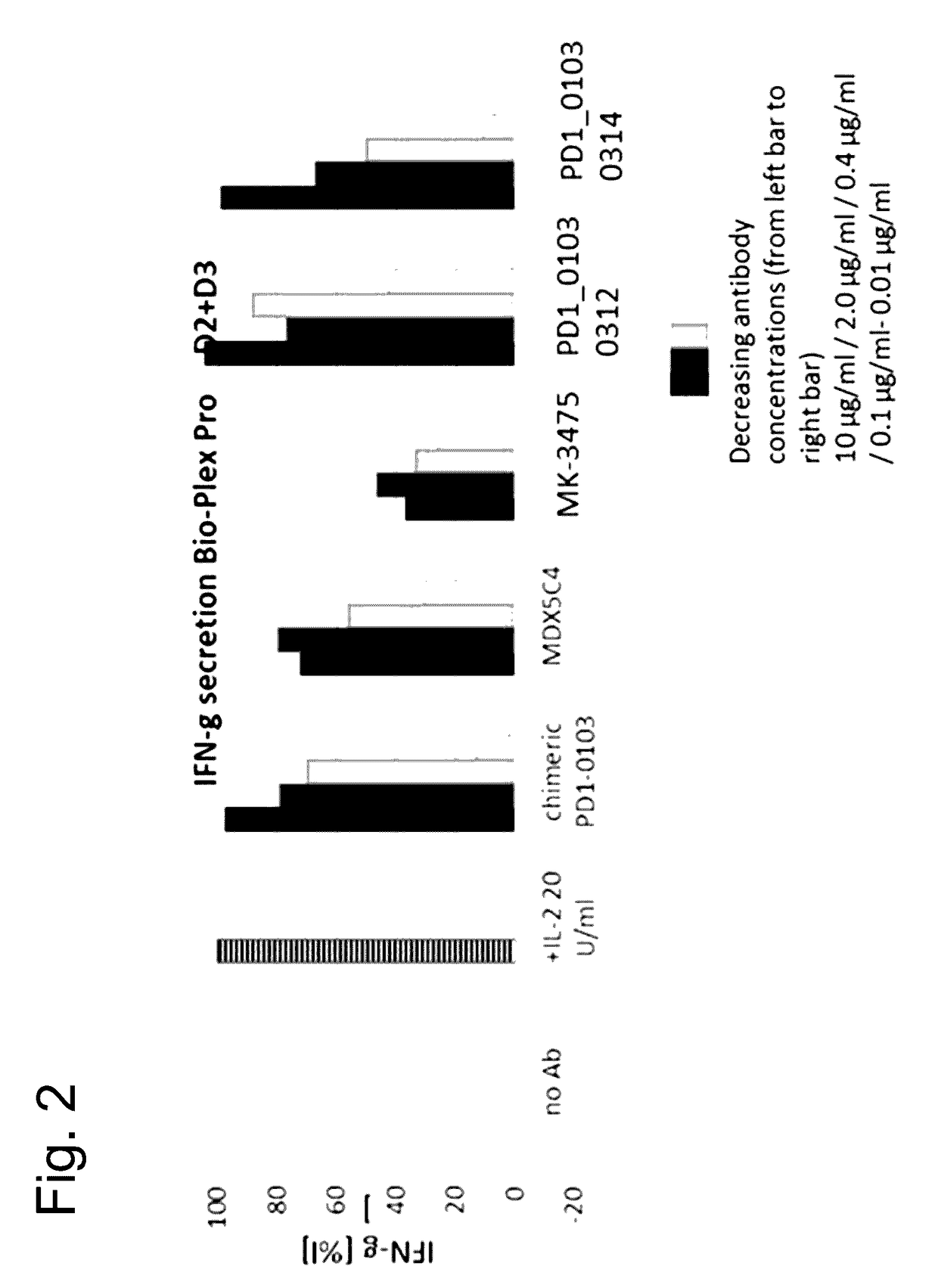
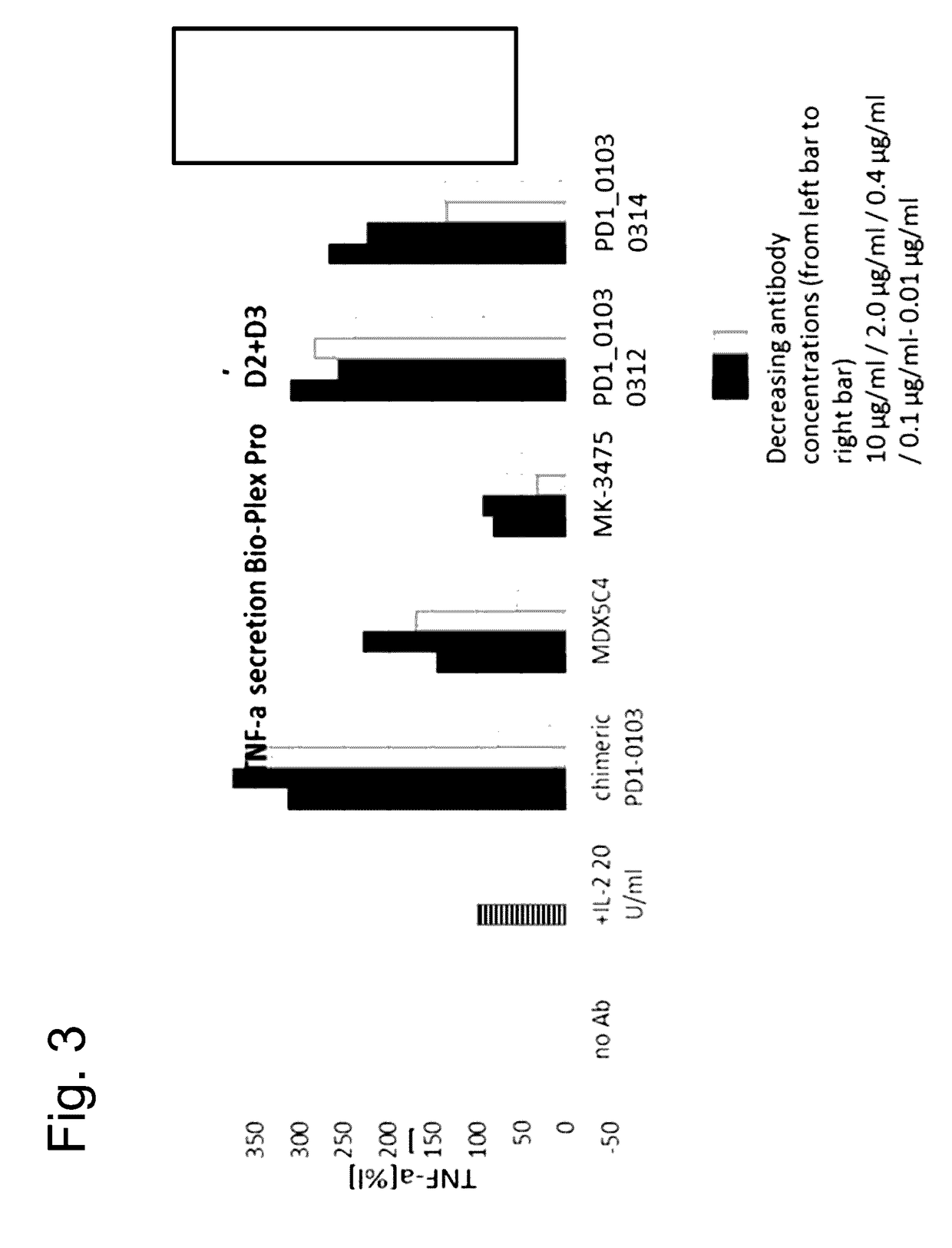
Novel monoclonal antibody against PD-1
ActiveCN107840887AHigh binding affinityPromote proliferationGenetically modified cellsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainNucleic acid sequencing
The invention provides a monoclonal antibody against PD-1, especially a human monoclonal antibody against PD-1. The antibody specifically binds to PD-1 in virtue of high affinity and comprises a heavychain and a light chain. The invention also provides a nucleic acid sequence coding the antibody, a cloning or expression vector, a host cell, a method for expressing or isolating the antibody, and an immunoconjugate and therapeutic composition containing the antibody. The invention further provides application of the anti-PD-1 antibody to treatment of various cancers.
Owner:CSTONE PHARM (SUZHOU) CO LTD +2
Acquisition method for exosomes derived from human urinary cells and application
The invention provides an acquisition method for exosomes derived from human urinary cells. The acquisition method includes the steps that firstly, human urine source cells are separately cultured, a culture medium is collected, the culture medium of the human urine source cells is filtered through a 0.22-micrometer filter membrane, and then large cell fragments and other impurities are removed; then an organelle is centrifugally removed, and supernatant is reserved; a membrane capable of intercepting 100KD molecular weight is used, the exosomes in the supernatant are centrifugally intercepted, after interception, PBS is used for eluting the membrane, and an exosome concentrated solution is obtained. The exosomes can be used for preparing medicine with the effects of resisting apoptosis, promoting angiogenesis, restoring ischemia damage and promoting cell growth and used for treating skin defects, skin ulcers, pressure sores, bone defects, bone ununion, femoral head necrosis, renal injury, ischemic injury, spinal cord injury, islet damage, diabetes, complications of diabetes, Alzheimer's diseases and the like.
Owner:上海艾棵颂生物科技有限公司 +1
Bispecific antibodies specific for pd1 and tim3
ActiveUS20170114135A1Reduce the binding forceGrowth inhibitionGenetically modified cellsImmunoglobulins against cell receptors/antigens/surface-determinantsBinding siteBispecific antibody
The invention relates to bispecific antibodies comprising a first antigen-binding site that specifically binds to PD1 and a second antigen-binding site that specifically binds to TIM3, in particular to bispecific antibodies, wherein the bispecific antibody binds to TIM3 with a lower binding affinity when compared to the binding to PD1. The invention further relates to methods of producing these molecules and to methods of using the same.
Owner:F HOFFMANN LA ROCHE INC
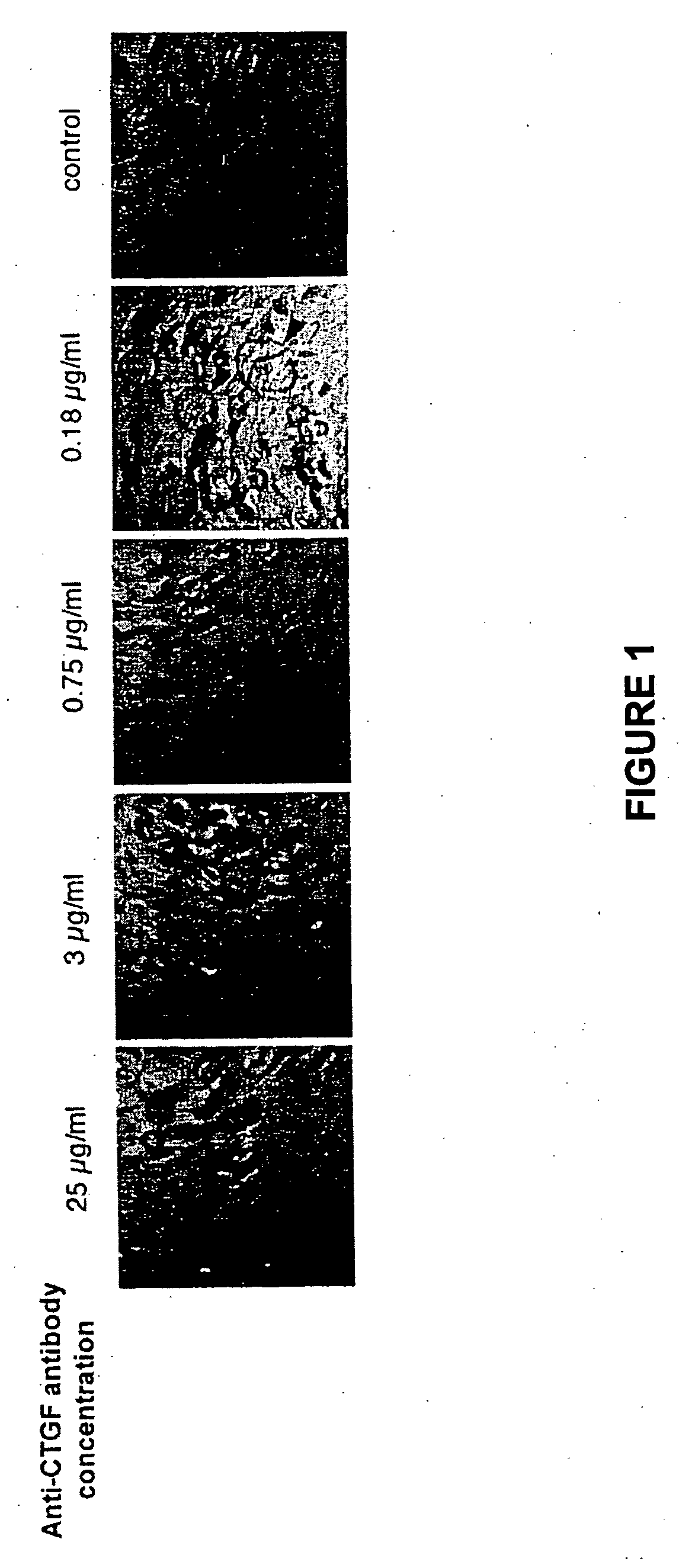
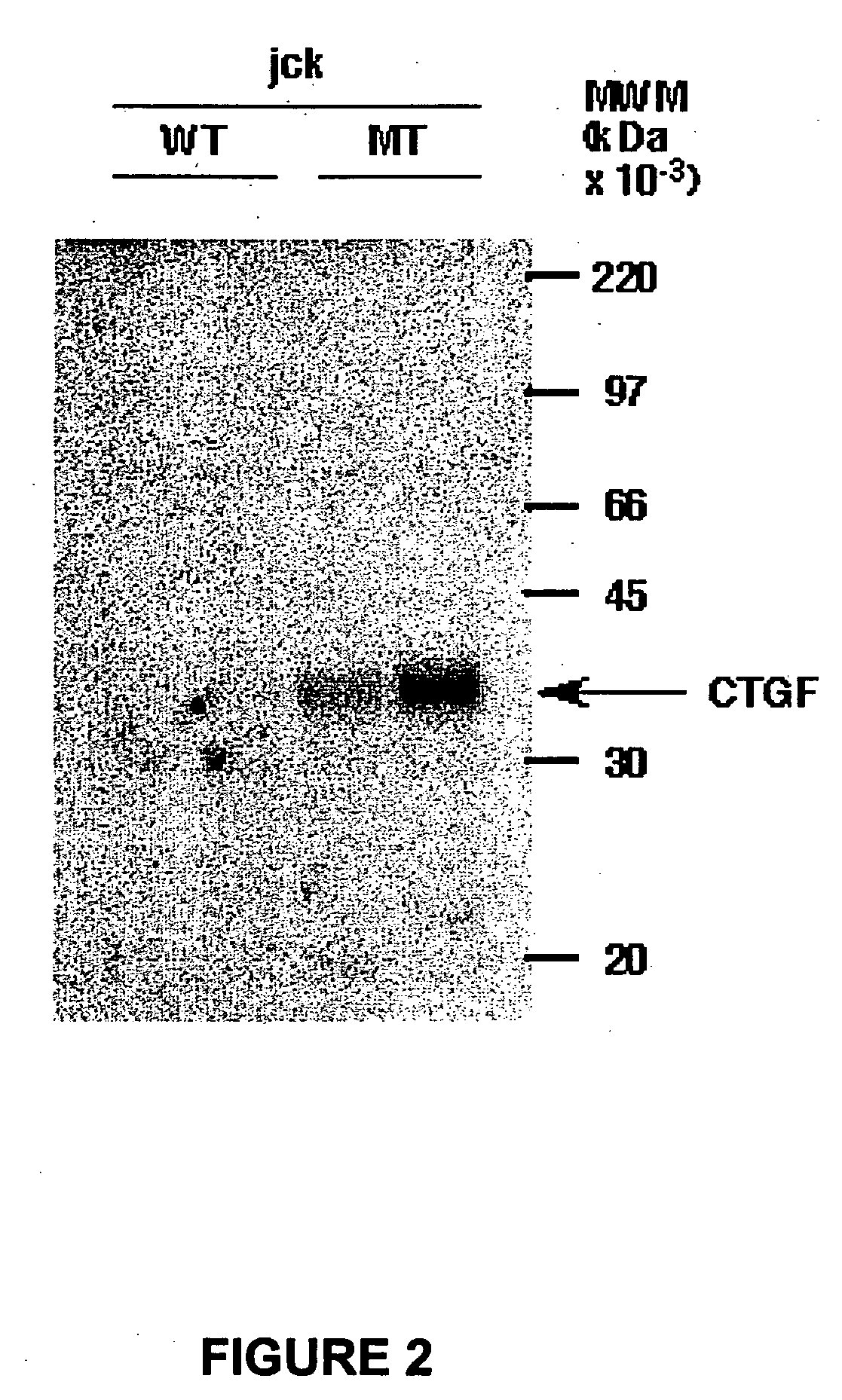
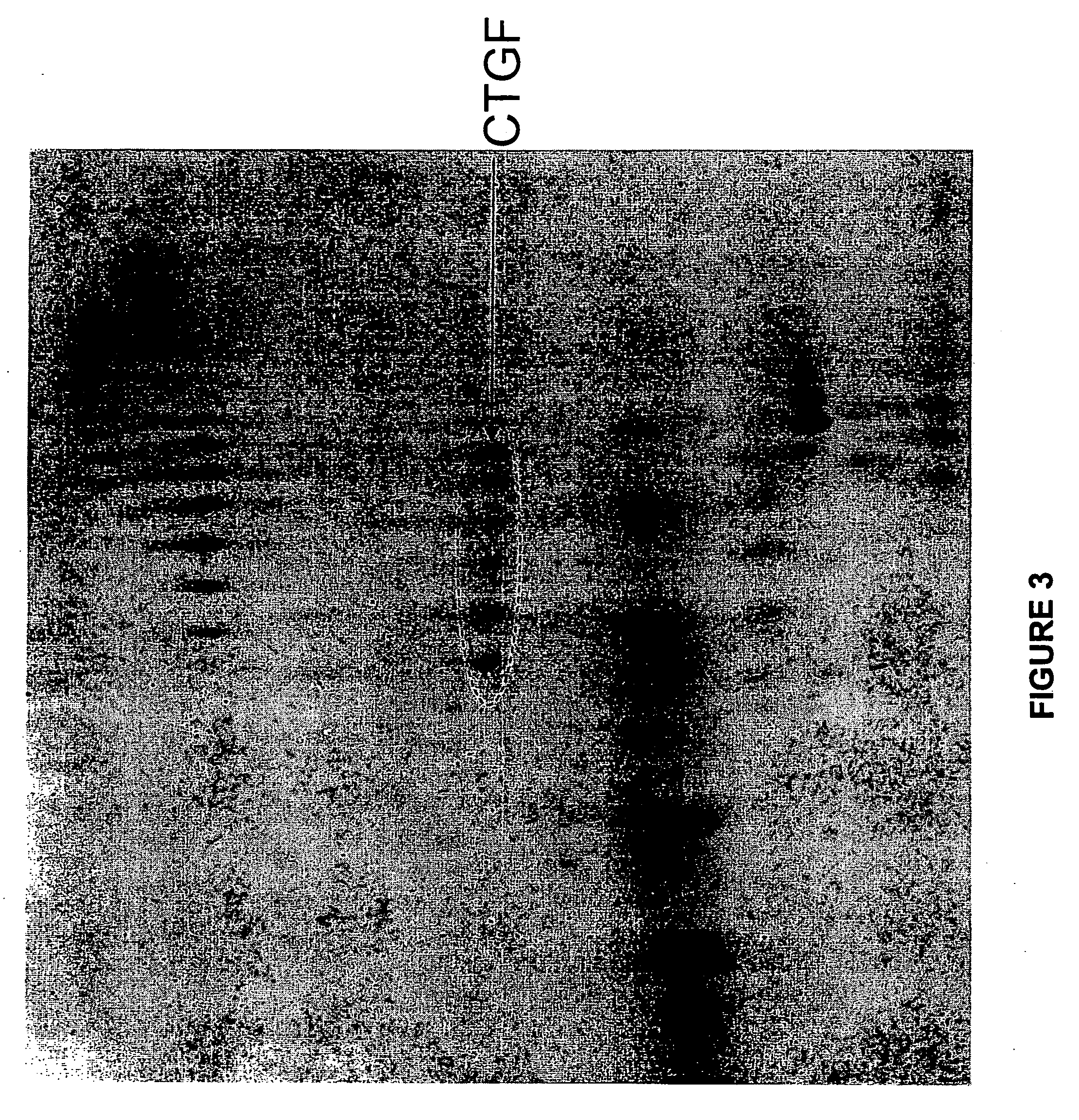
Methods and compositions for the treatment of polycystic diseases
InactiveUS20080193443A1DiagnosisEarly diagnosisGenetic material ingredientsImmunoglobulins against growth factorsDiseaseCTGF
This invention provides compositions and methods to diagnose and treat polycystic disorders by inhibiting the biological activity of a gene now correlated with appearance of this disorder. By way of illustrative only, the Connective Tissue Growth Factor (CTGF) gene is an example of such a gene. Also provided by this invention are compositions and methods to treat or ameliorate abnormal cystic lesions and diseases associated with the formation of cysts in tissue. The methods and compositions treat and ameliorate pathological cyst formation in tissue by inhibiting or augmenting gene expression or the biological activity of its gene expression product or its receptor.
Owner:GENZYME CORP
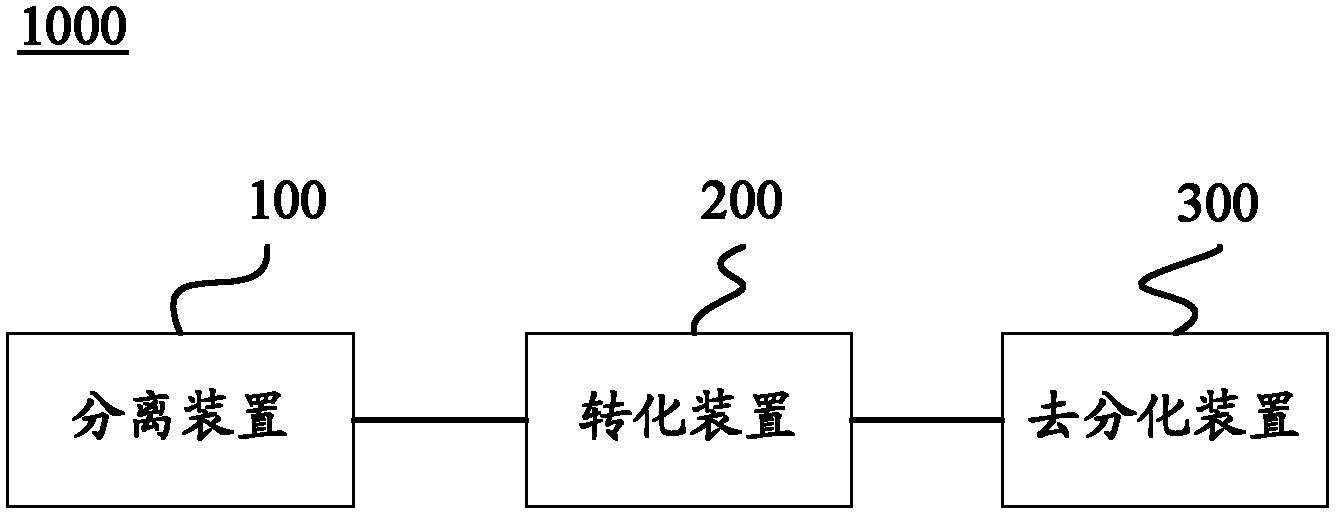
Device for the identification, separation and / or cell type-specific manipulation of at least one cell of a cellular system
InactiveUS20160060615A1Fast and convenient separation and identificationPrecise positioningBioreactor/fermenter combinationsBiological substance pretreatmentsCell type specificBiology
Owner:WALTHER THOMAS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com