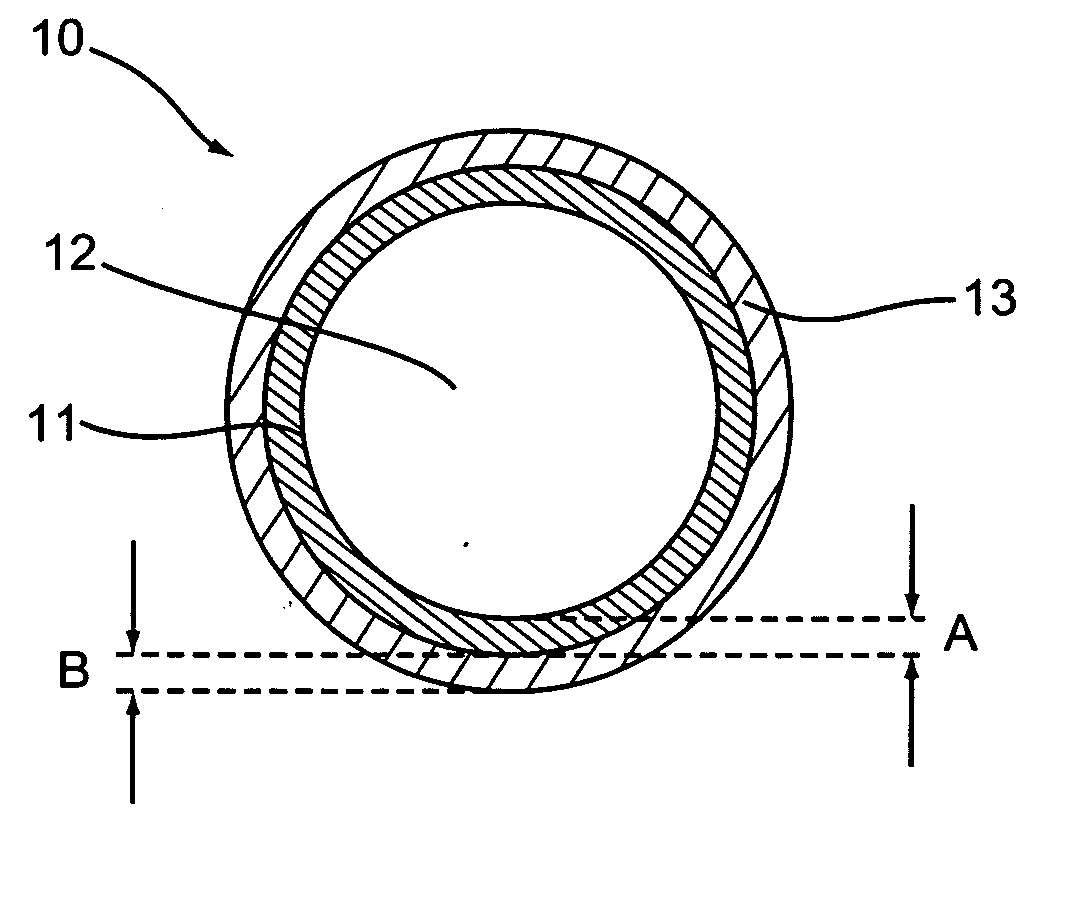
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
163results about "Ureters" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
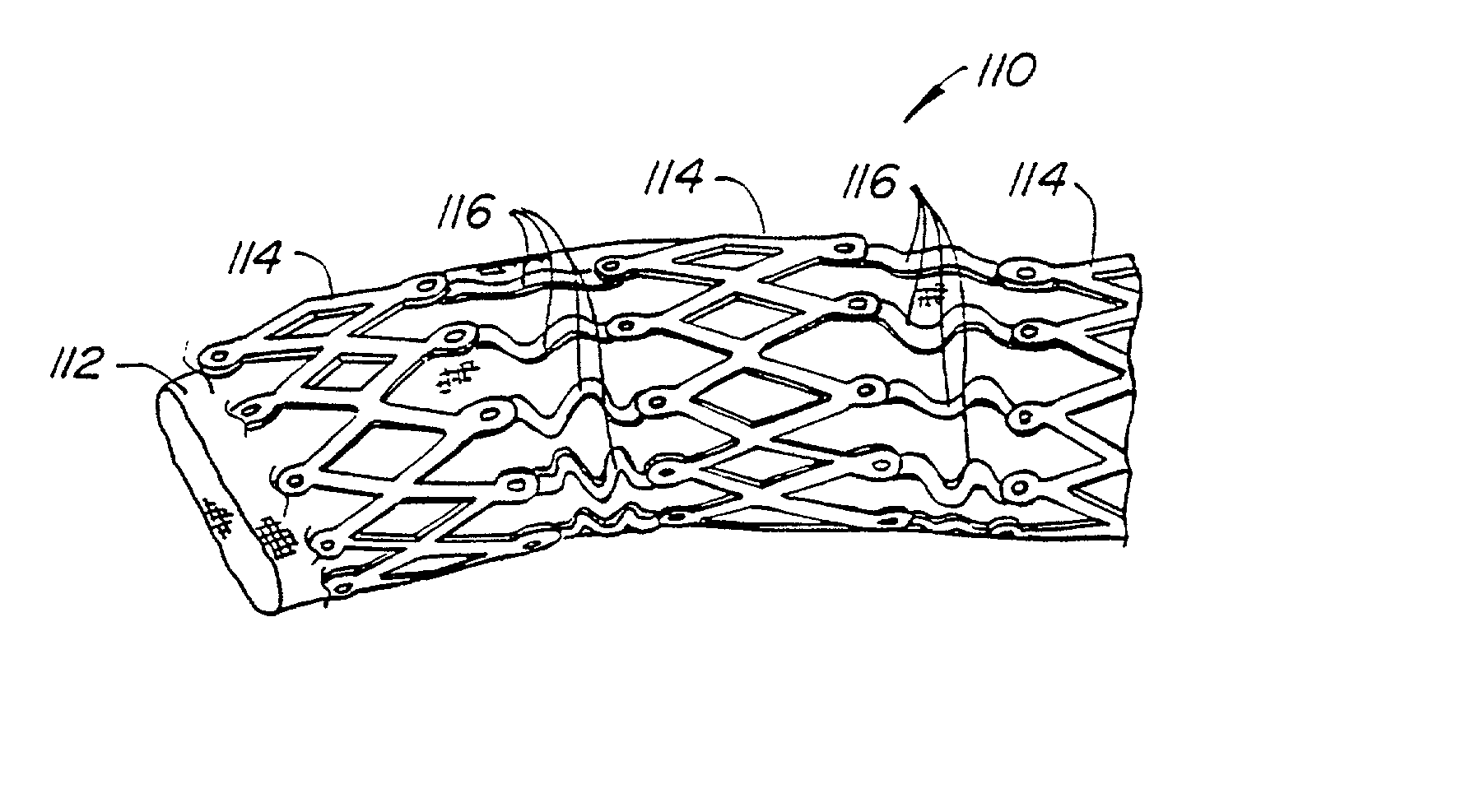
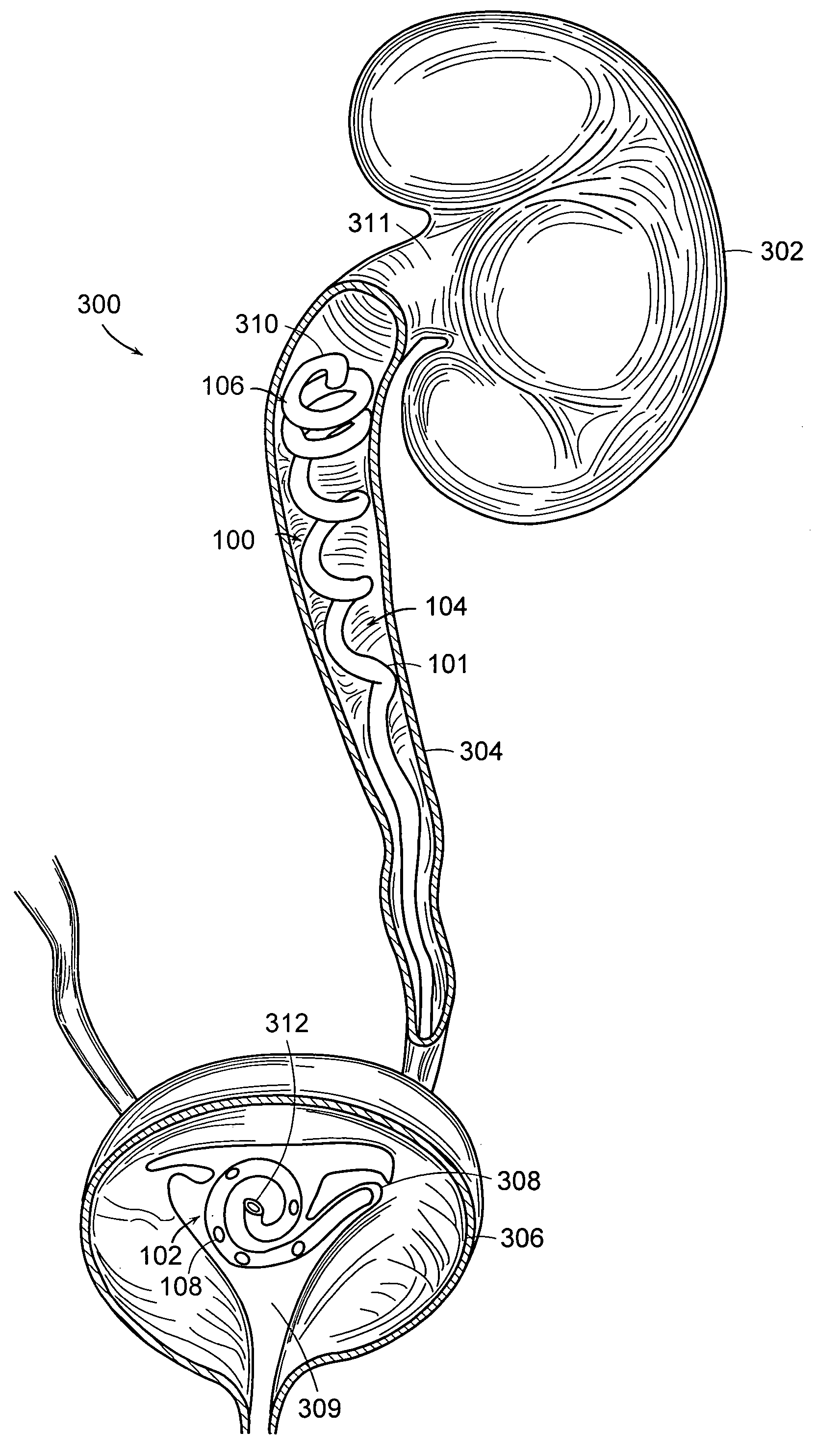
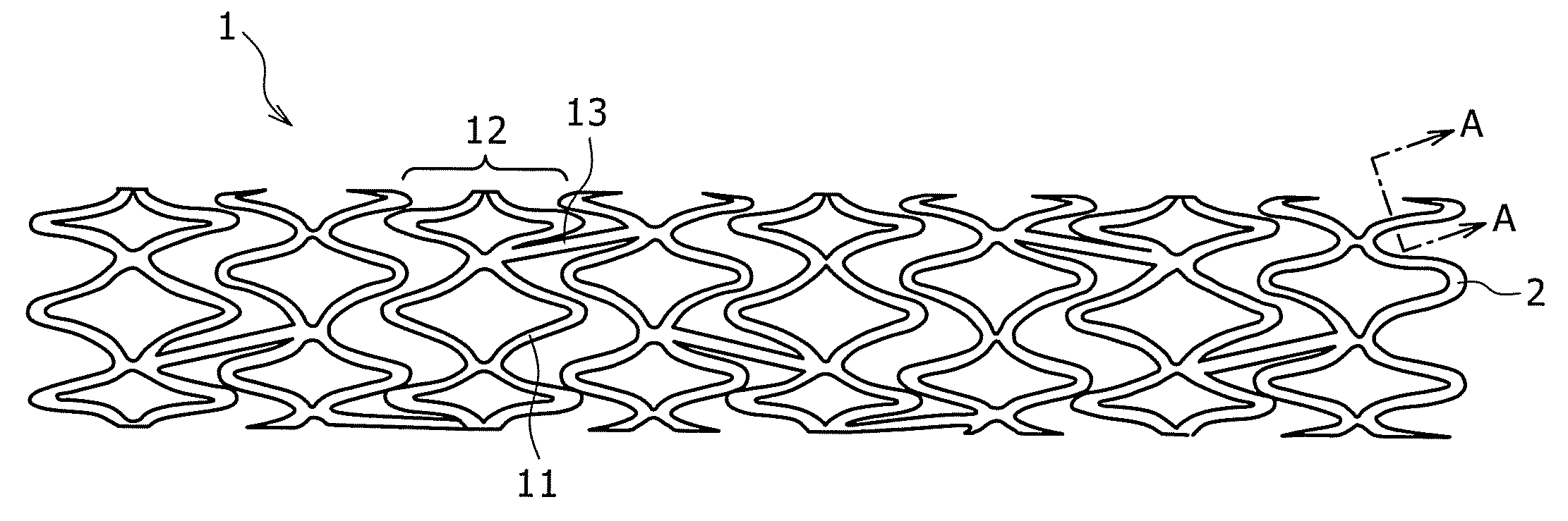
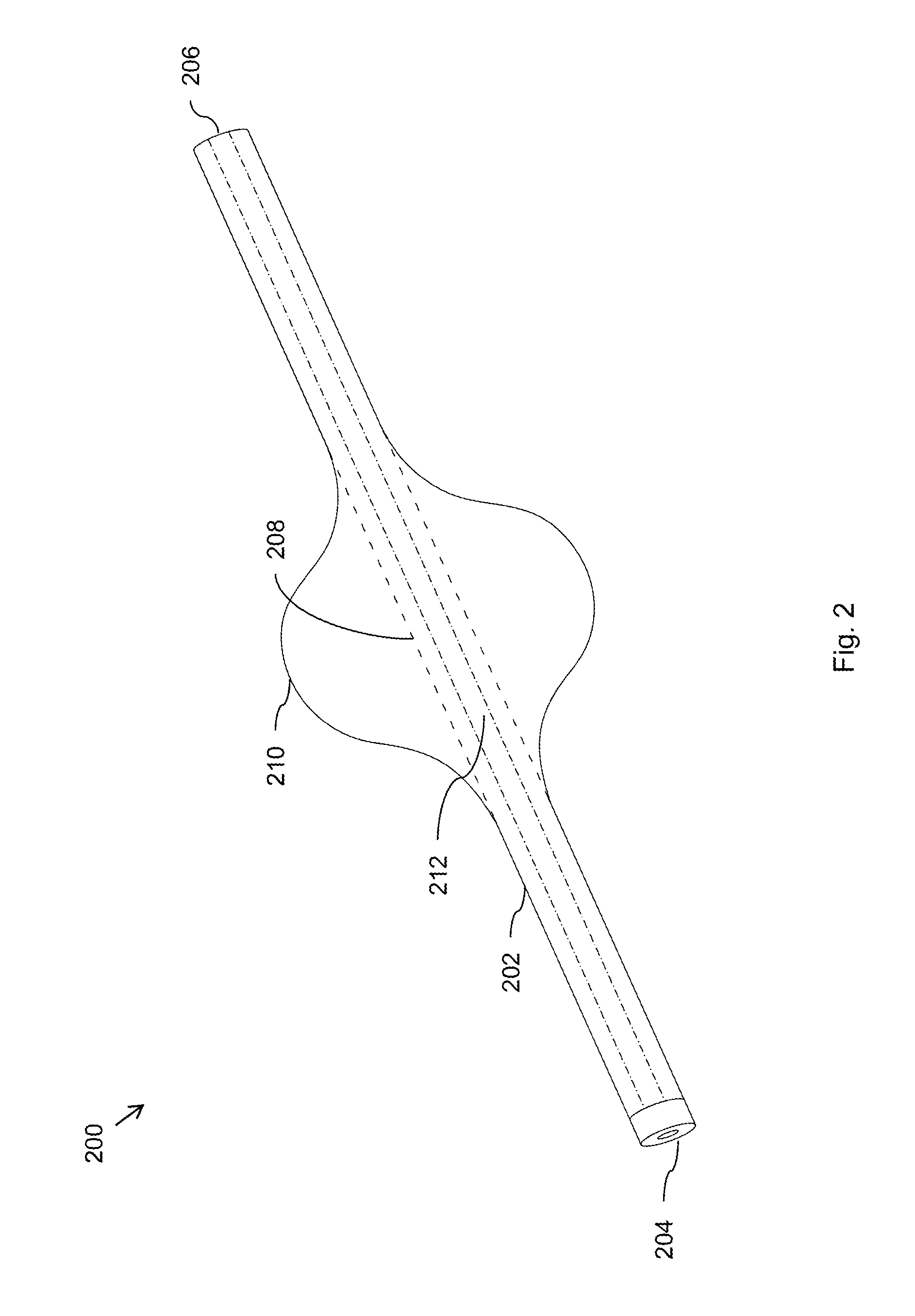
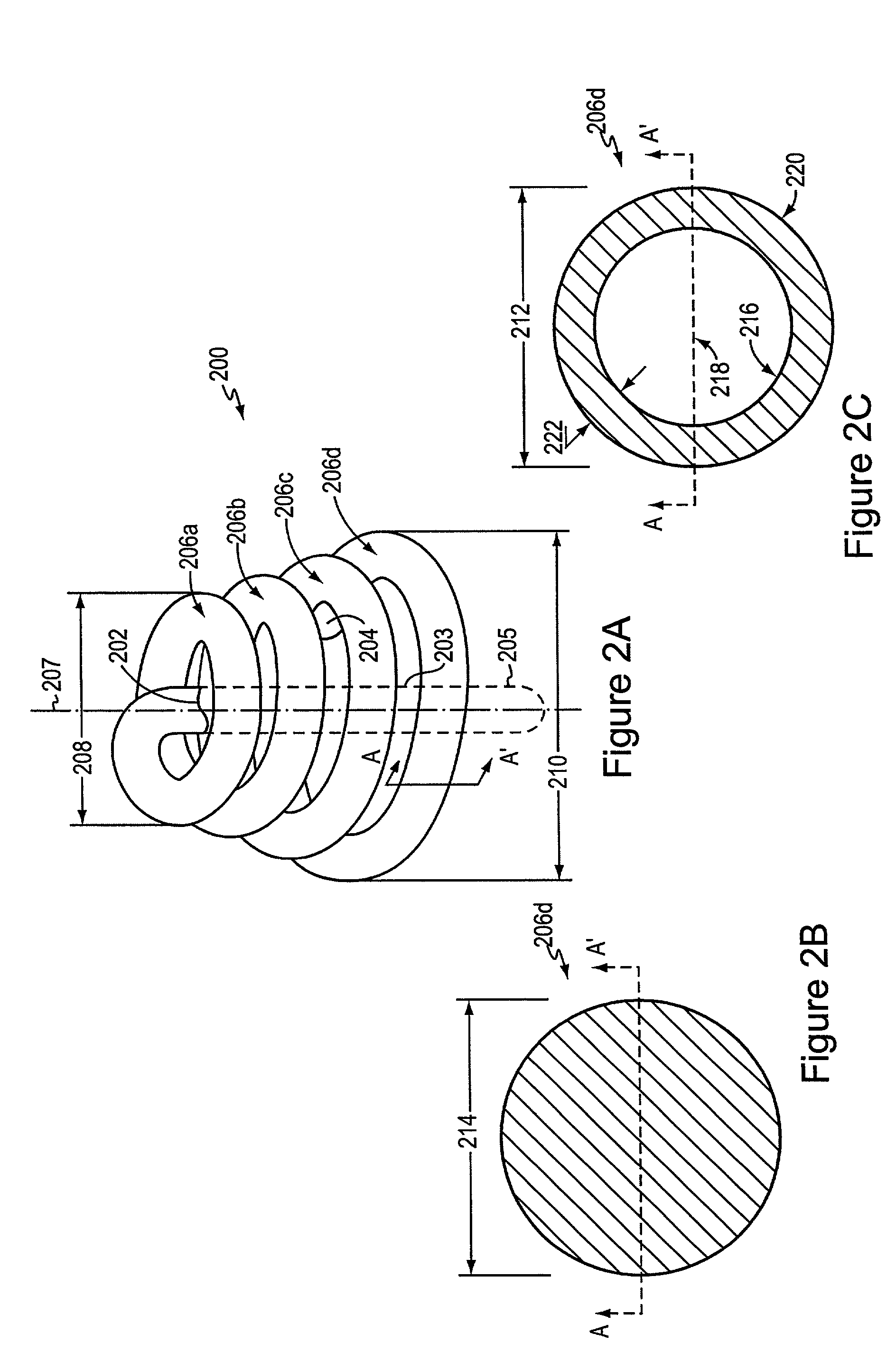
Endoluminal prostheses and therapies for highly variable body lumens
InactiveUS20020120327A1Maximize column strengthAvoid distortionStentsBile ductsY connectorProsthesis
The present invention provides a branching endoluminal prosthesis for use in branching body lumen systems which includes a trunk lumen and first and second branch lumens. The prostheses comprises a radially expandable tubular trunk portion having a prosthetic trunk lumen, and radially expandable tubular first and second branch portions with first and second prosthetic branch lumens, respectively. A radially expandable tubular Y-connector portion provides fluid communication between the prosthetic trunk lumen and the first and second prosthetic branch lumens. Although it is often considered desirable to maximize the column strength of endoluminal prostheses, and although the trunk portion will generally have a larger cross-section than much of the remainder of a branching endoluminal prostheses, the expanded trunk portion is more axially flexible than the expanded Y-connector portion, as insufficient flexibility along the trunk portion may result in leakage between the prosthesis and the trunk lumen of the body lumen system. In contrast, the Y-connector portion benefits form a less axially flexible structure to avoid distortion of the flow balance between the luminal branches.
Owner:COX BRIAN +5
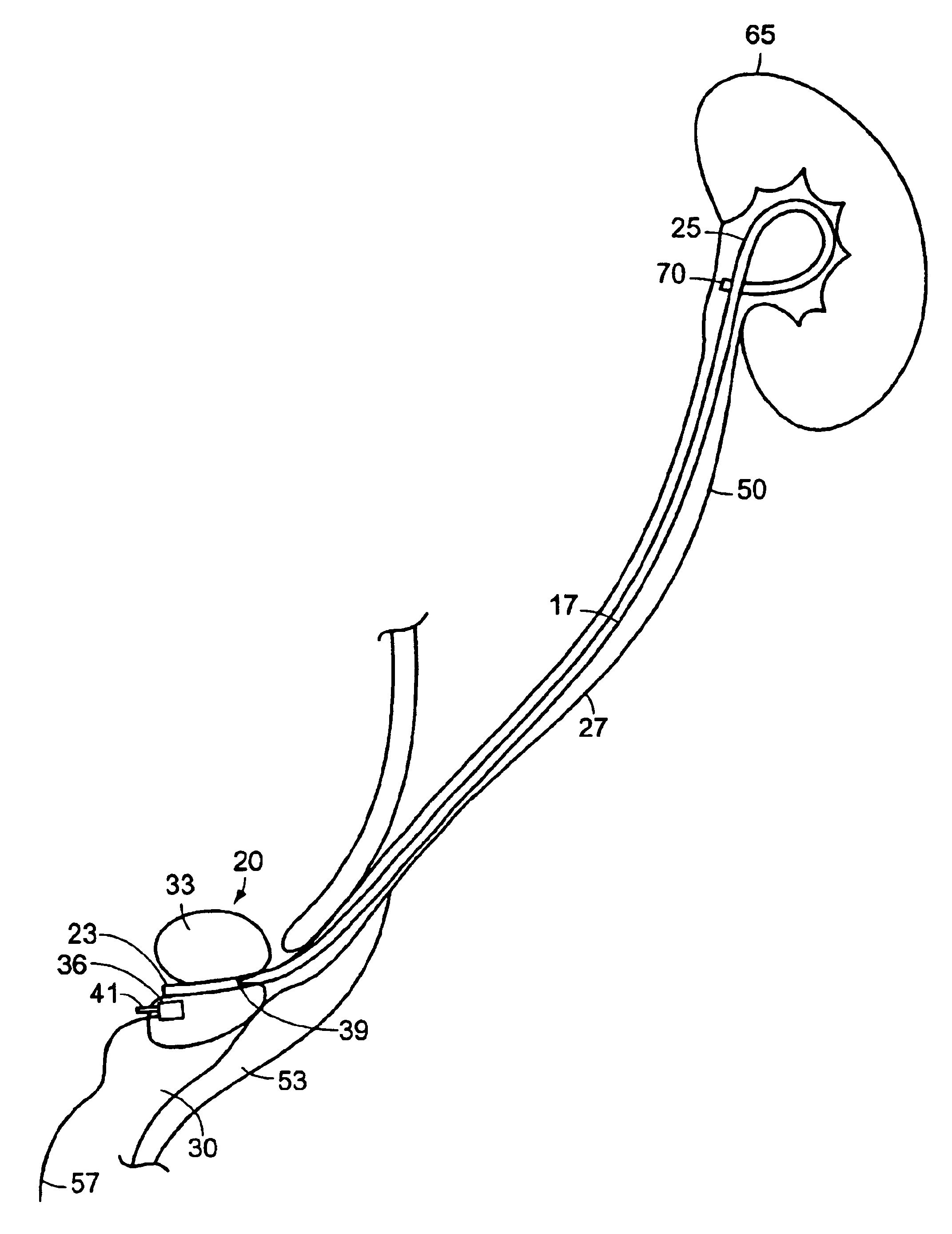
Ureteral stent system apparatus and method
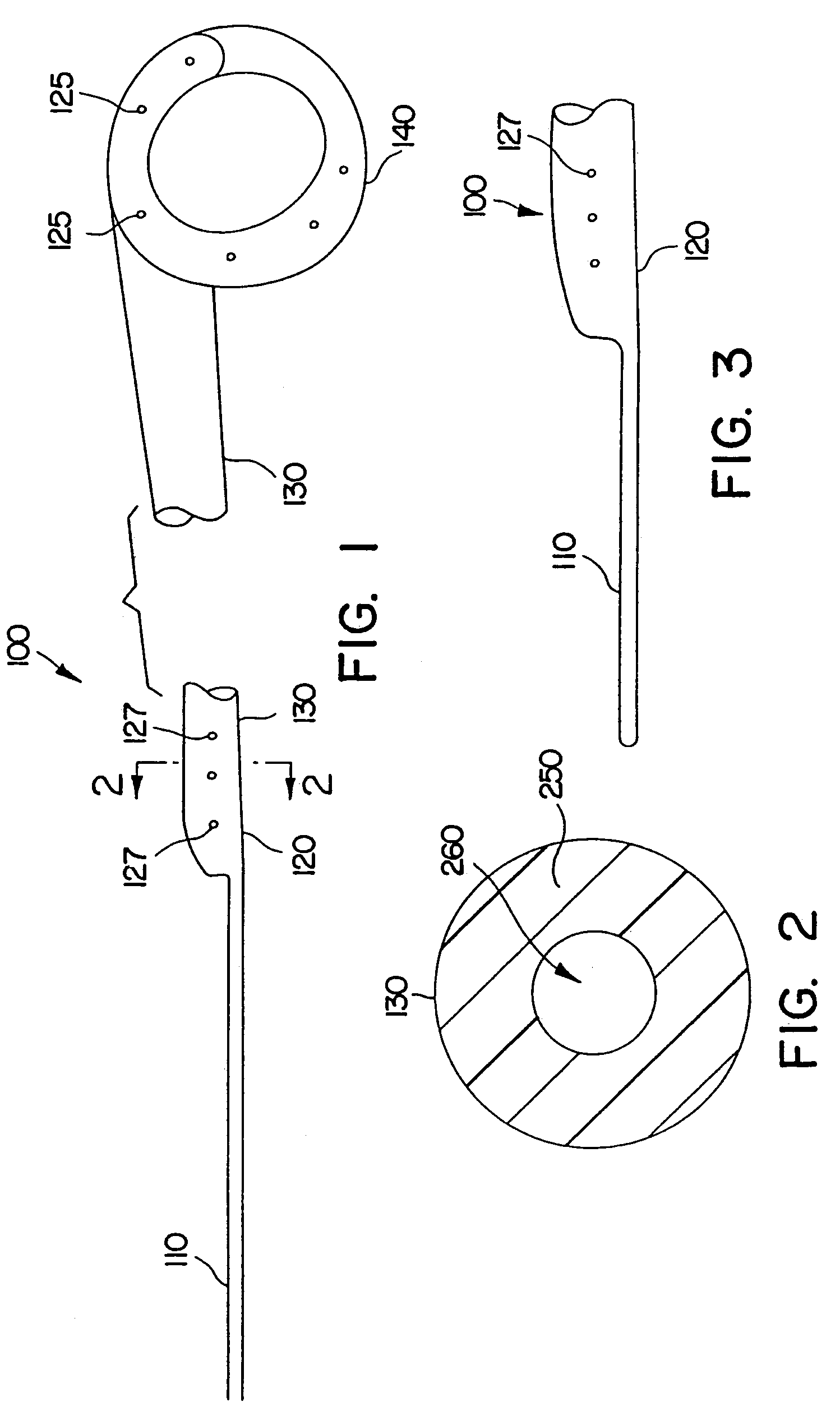
A stent having an elongate tubular configuration is formed of a plurality of elongate elements interwoven or braided to form a tubular configuration. The elements may be relatively strong and rigid, but movable relative to each other within the weave or braid in order to provide the stent with generally soft characteristics. The elements may be formed of different materials, such as an absorbent material permitting the stent to be doped with materials such as drugs and chemicals. Even the absorbency can be controlled and varied to provide a predetermined time-release of the absorbent.
Owner:APPL MEDICAL RESOURCES CORP
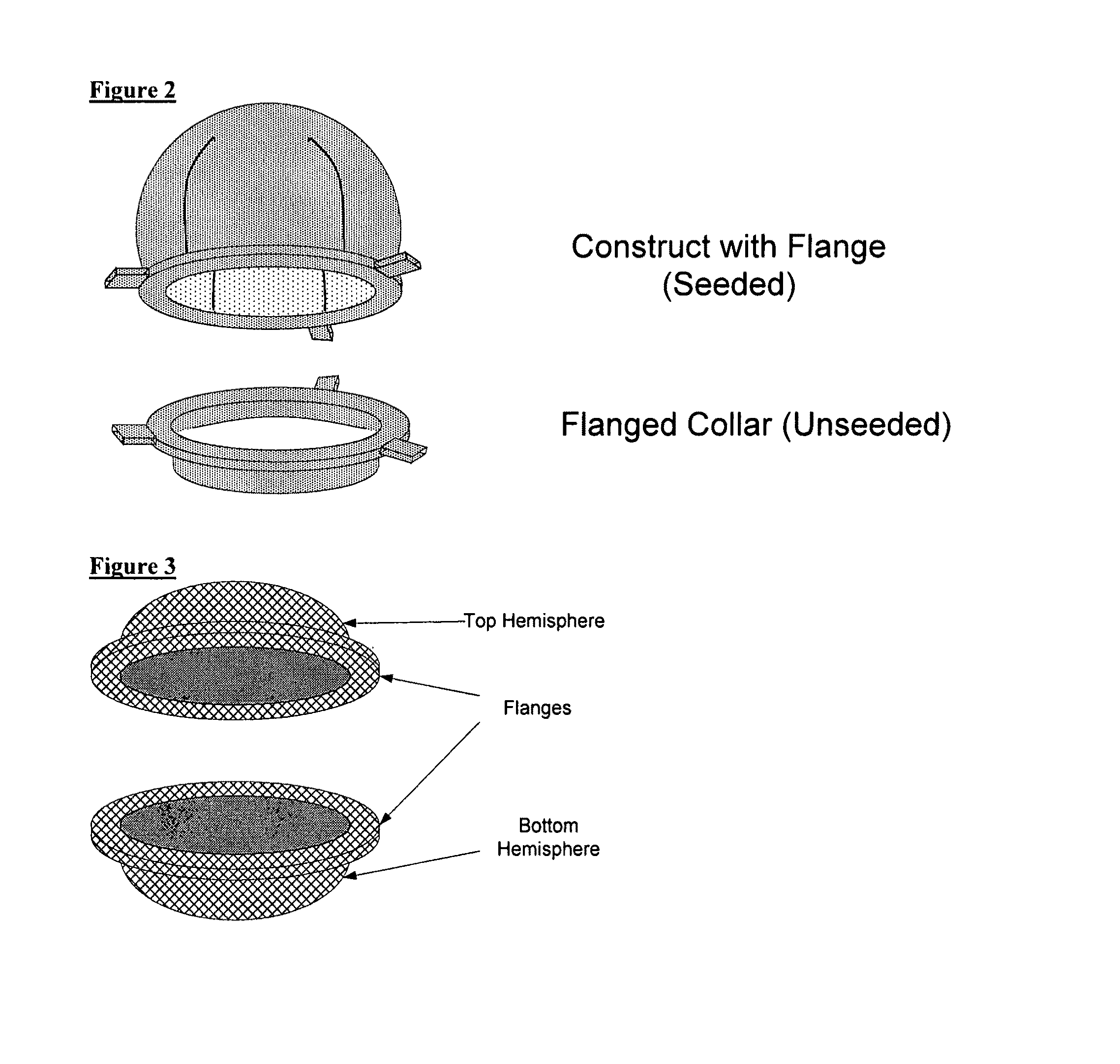
Scaffolds for organ reconstruction and augmentation
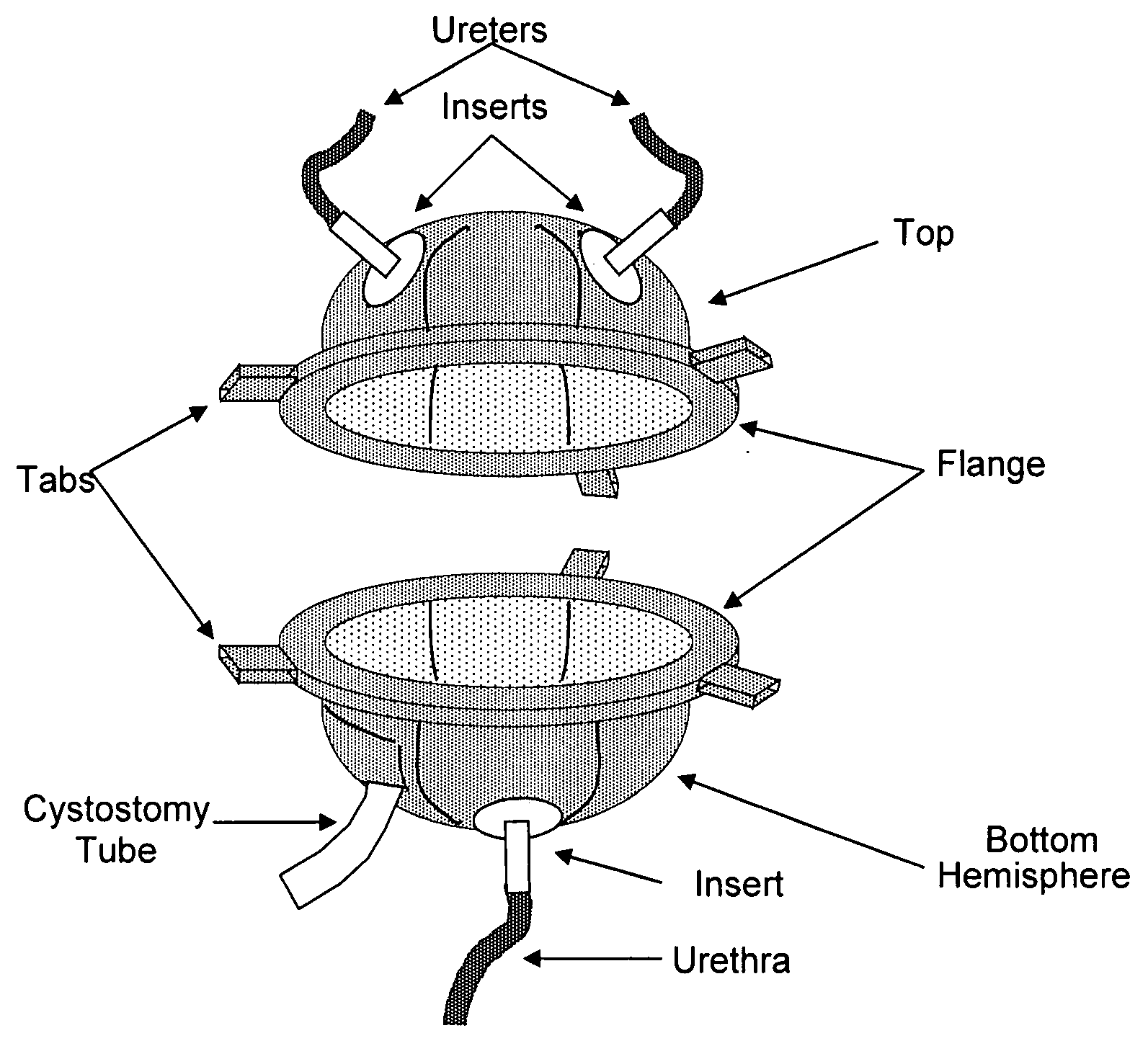
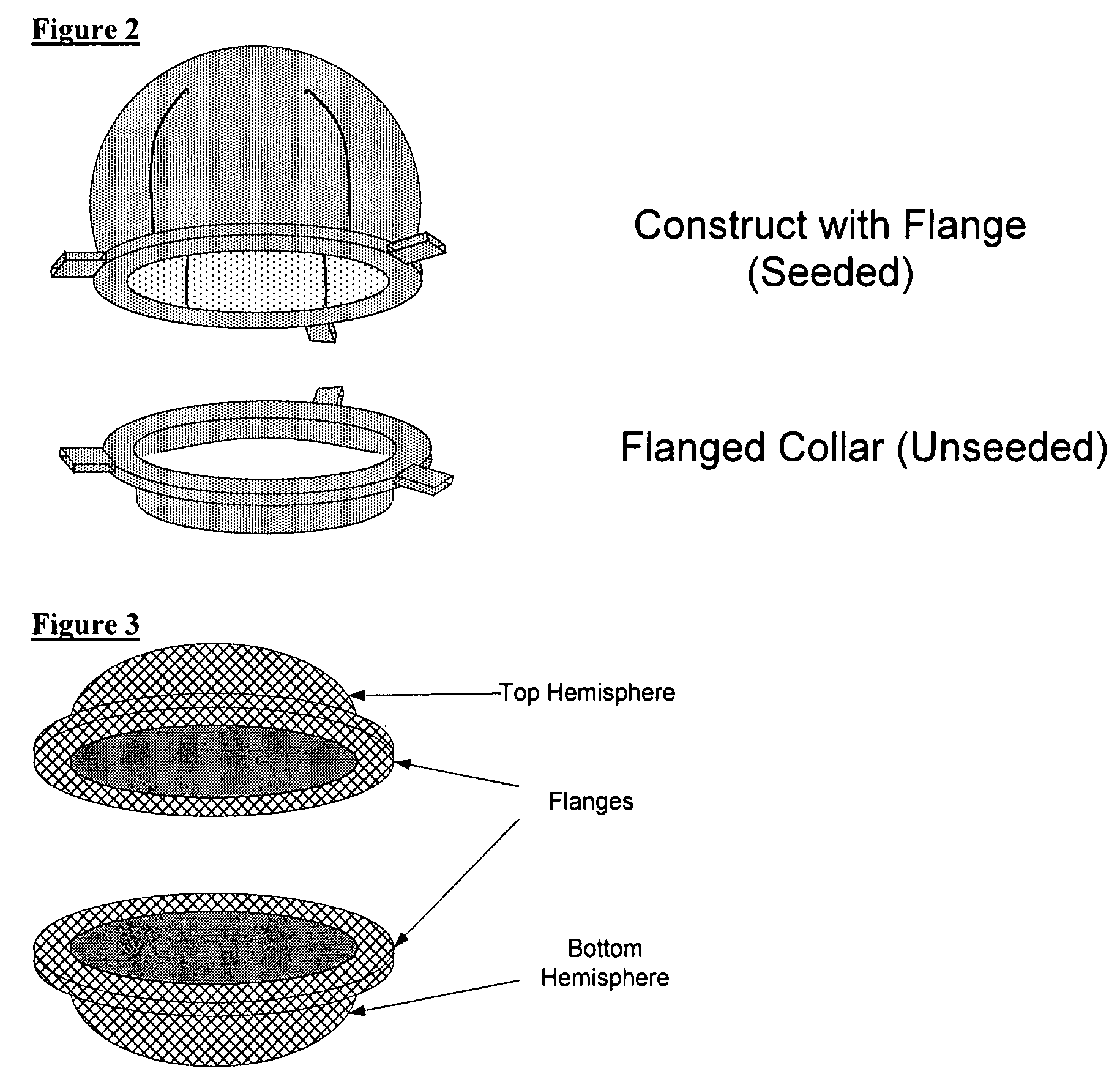
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
Ureteral stent configured for improved patient comfort and aftercare
A ureteral stent is configured for improved patient comfort and aftercare. The stent can have one or more of the following features: a distal portion with a somewhat flattened, non-circular cross-section that provides reduced irritation and elimination of urine reflux; a proximal portion with a helical coil shape that allows self-anchoring of the stent below the kidney; and a portion along the body of the stent having a coil shape that allows self-adjustment of the stent with ureteral movement.
Owner:BOSTON SCI SCIMED INC
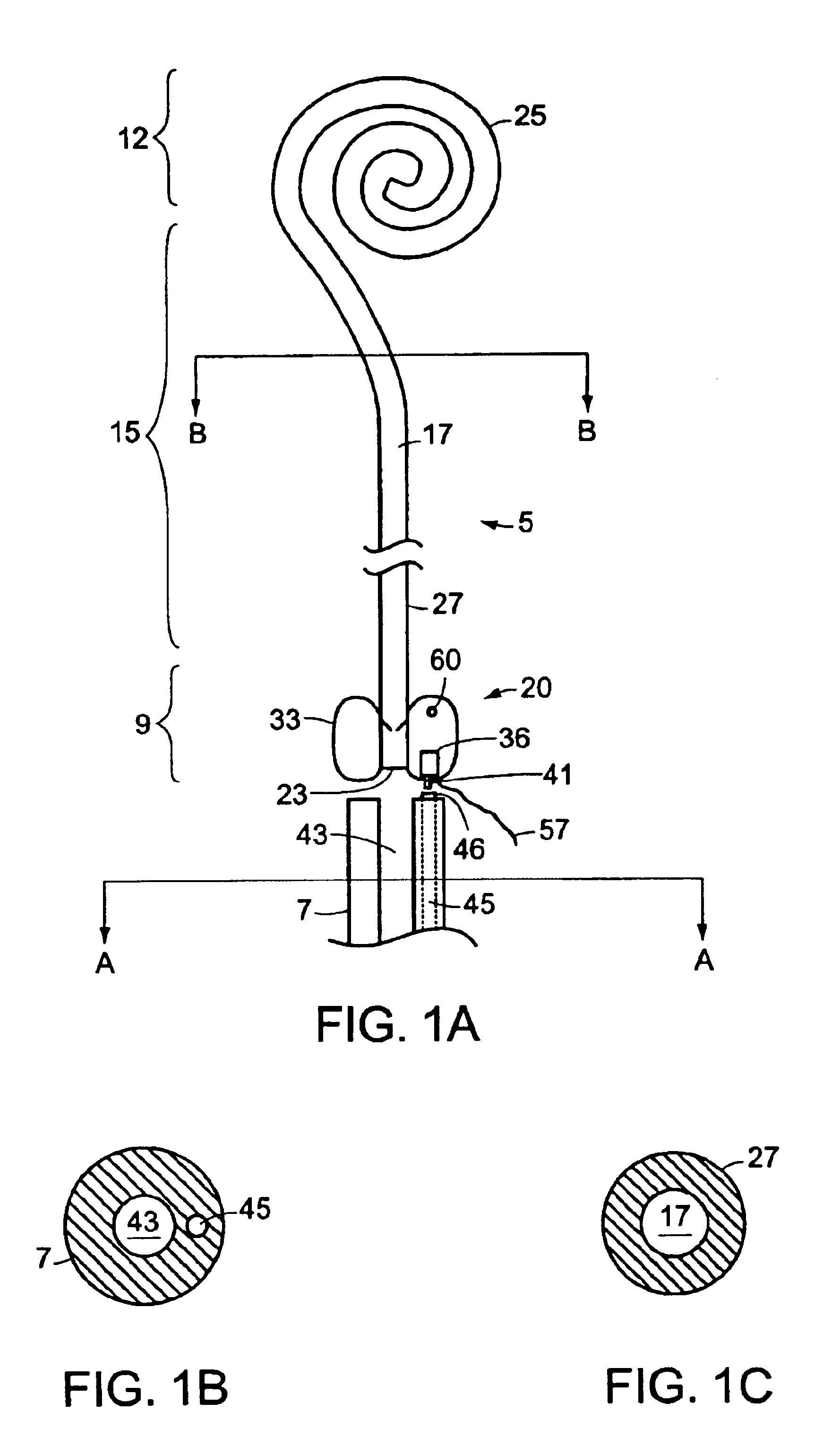
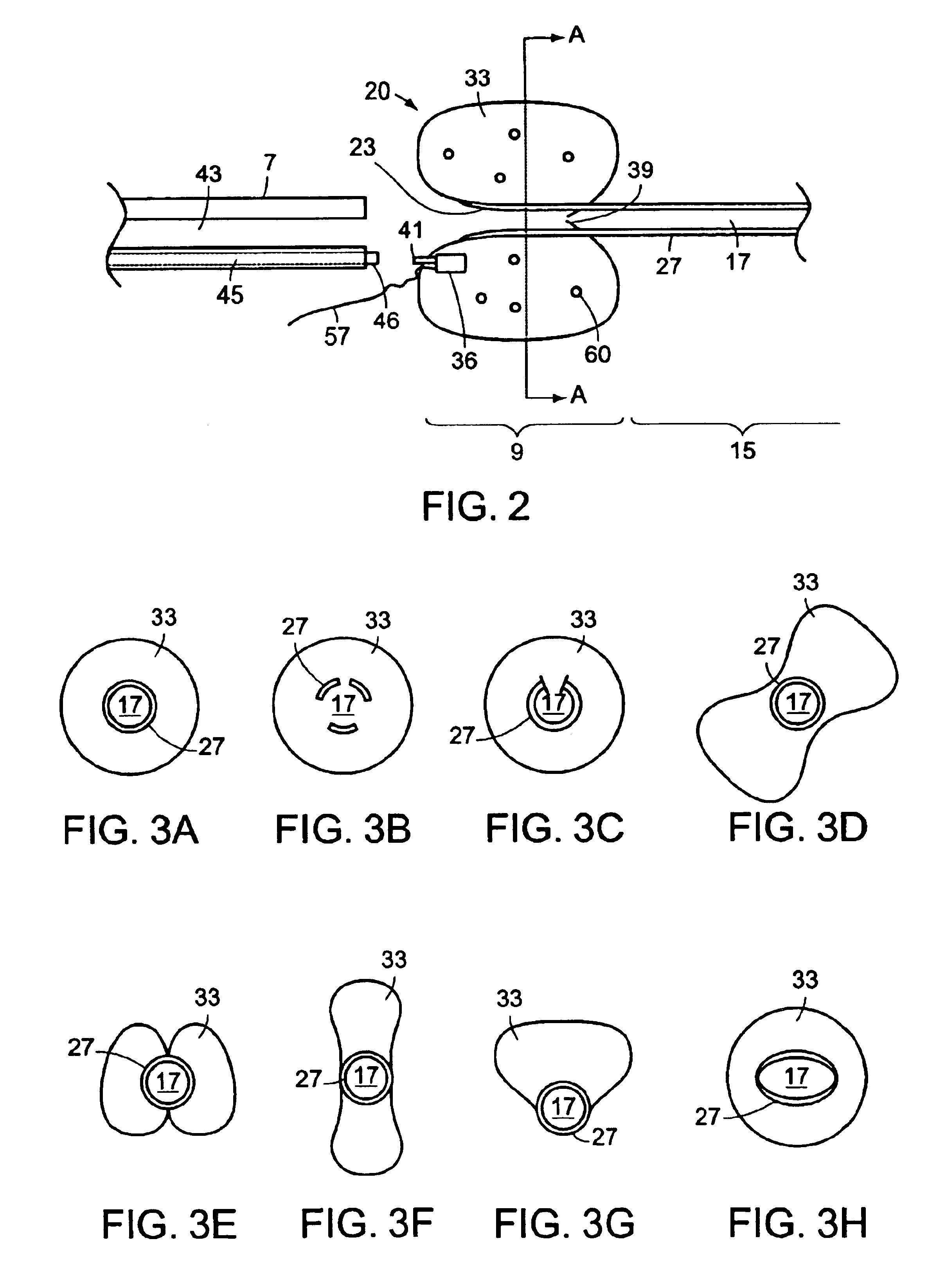
Ureteral stent with end-effector and related methods
InactiveUS6949125B2Decreased fluid retentionMinimizing patient discomfortBalloon catheterSurgeryUrethraControl release
A system and related methods for maintaining the patentcy of the ureter comprising a pusher tube having a pusher tube lumen and an inflate lumen disposed within a wall of the pusher tube and a urinary stent having a proximal and distal portions with an elongated body portion therebetween configured to fit the ureter of the patient and defining a lumen. The system further includes an end-effector that may comprise an inflatable balloon positioned at the proximal portion of the urinary stent for retaining the proximal portion in the urinary bladder. At the distal portion, a retention end-piece is positioned for retaining the distal portion of the stent in the renal pelvis. The end-effector and the retention end-piece of the stent maintain the elongated body portion in situ. The end-effector may also include an inflatable balloon and may contain pharmaceutical or biologic agents for controlled release into the bladder.
Owner:BOSTON SCI SCIMED INC
Device for maintaining a passage for urine through the prostate
PCT No. PCT / SE96 / 00607 Sec. 371 Date Nov. 12, 1997 Sec. 102(e) Date Nov. 12, 1997 PCT Filed May 9, 1996 PCT Pub. No. WO96 / 35395 PCT Pub. Date Nov. 14, 1996A device for maintaining a passage through the prostate gland (13) after treatment of a catheter (10) inserted through the urethra into the prostate gland, the catheter (10) being provided with means (11) for heating the prostate gland. A sleeve (12) is received over the catheter (10) so as to follow the catheter (10) during insertion into a desired position within the prostate gland, and the sleeve (12) is formed to remain in the desired position when the catheter is removed from the prostate gland, thereby maintaining a passage having a predetermined lower inner diameter through the prostate gland when tissue in the prostate gland swells.
Owner:PROSTALUND OPERATIONS
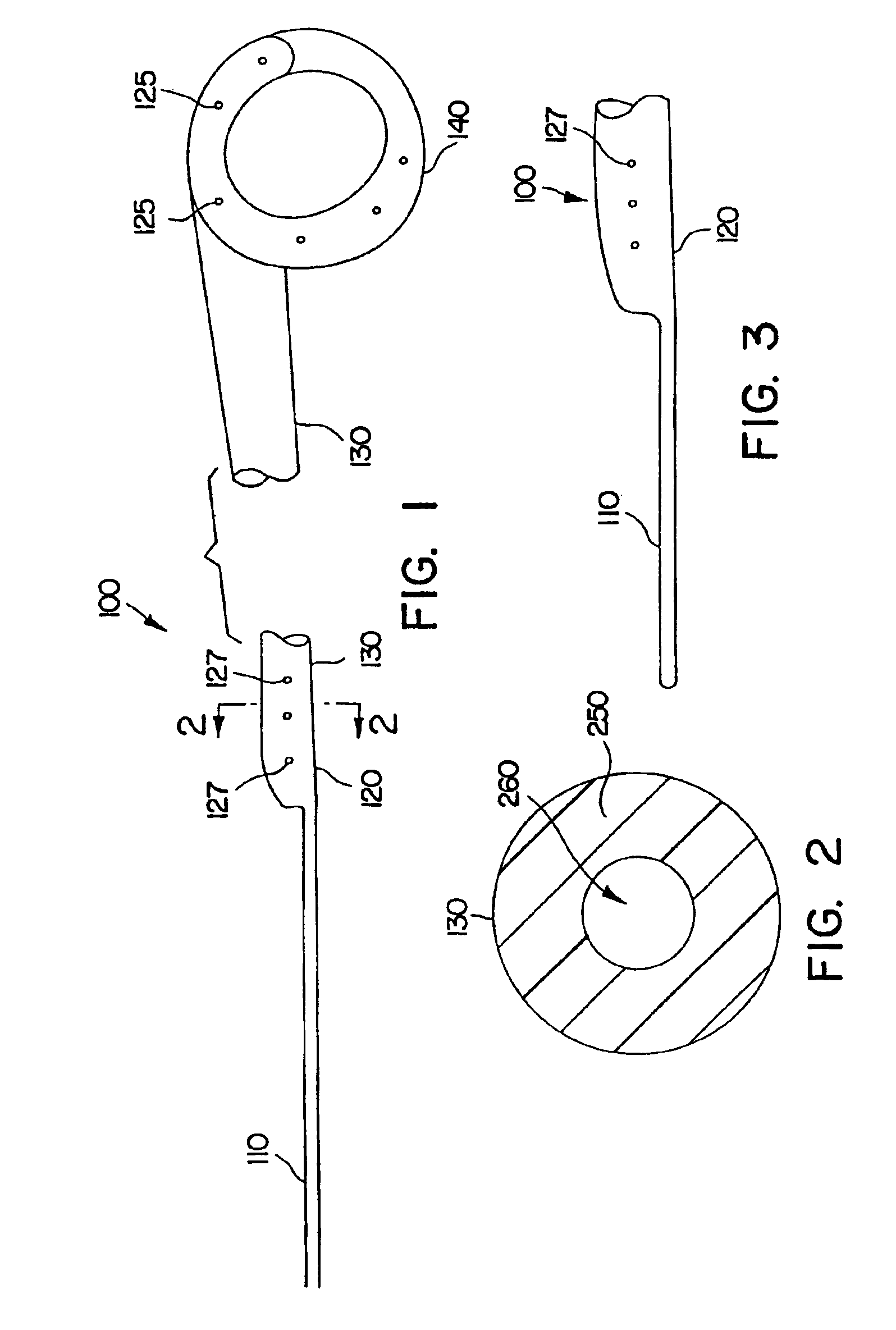
Intraluminally placeable textile catheter, drain and stent
InactiveUS6929626B2Great indwelling timeLow-profileStentsCeramic shaping apparatusYarnInsertion stent
An intraluminally placeable tubular device includes an elongate hollow tubular member having a luminal surface and an exterior surface defining a wall portion therebetween. The hollow tubular member has an open proximal end and an opposed distal end. The wall portion has sufficient self-supporting rigidity to permit the device to be advanced through a body lumen during intraluminal placement. The tubular member includes yarns interconnected in a pattern defining opposed interior and exterior textile surfaces. At least one of the textile surfaces is the body fluid-contacting luminal surface or the body lumen-contacting exterior surface. A textile infusion and / or a textile aspiration valve is also provided. A percutaneously or orificially placeable catheter with such textile valves is provided.
Owner:BOSTON SCI SCIMED INC
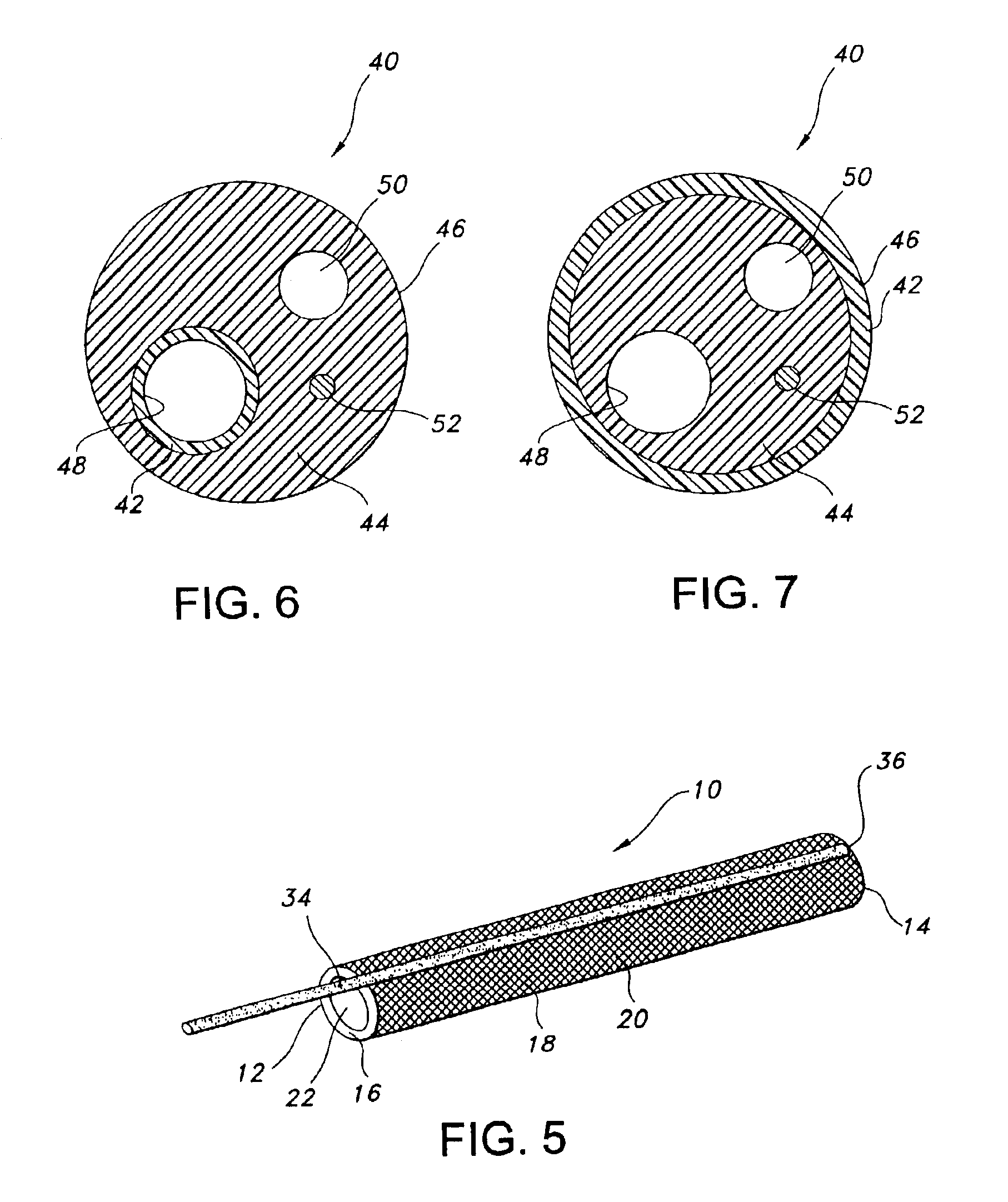
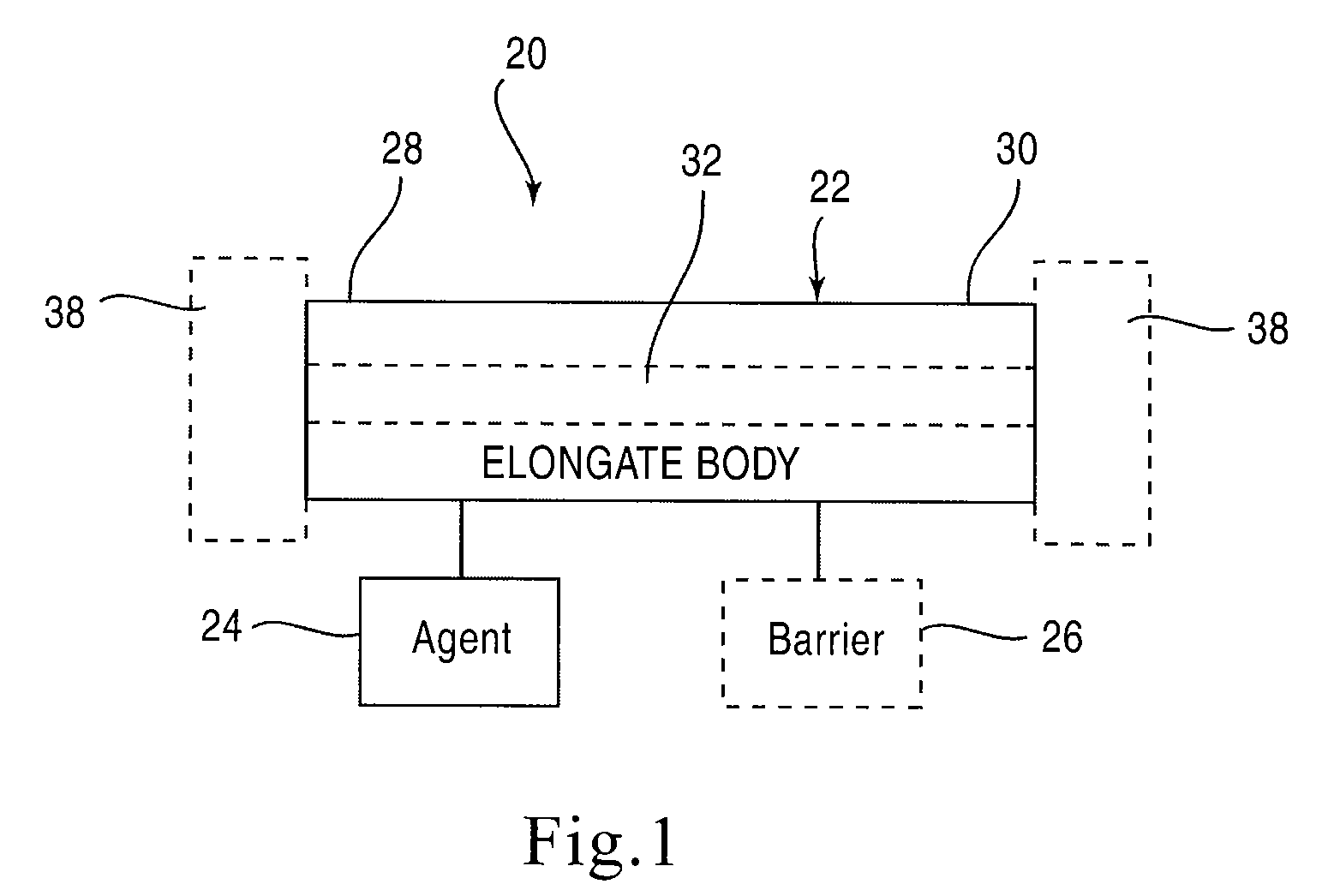
Stent Having Time-Release Indicator
A medical device includes an elongate body configured to be disposed within a urinary tract of a patient such that a first end portion of the elongate body is disposed at a first location of the urinary tract and a second end portion of the elongate body is disposed at a second, different location of the urinary tract. The elongate body defines a lumen that is configured to convey urine from the first location of the urinary tract to the second location of the urinary tract. An agent is formulated to visually alter urine of the patient, the agent is coupled to the elongate body and releasable into the patient's urine.
Owner:BOSTON SCI SCIMED INC
Medical implant
InactiveUS20090192595A1Limited decomposition rateHigh strengthUrinary bladderStentsBiomedical engineeringBiodegradable polymer
Disclosed herein is a medical implant including an implant body of which at least a part is comprised of a biodegradable metal, wherein the part comprised of the biodegradable metal has a crystal grain diameter of not more than 10 μm.
Owner:TERUMO KK
Balloon expandable stent
An apparatus is provided that includes an elongate member having a distal end portion, a proximal end portion, and a medial portion that is disposed between the distal end portion and the proximal end portion. The medial portion is configured to be disposed in a ureter of a patient. The apparatus further includes an expandable member that is coupled to the elongate member. The expandable member has an expanded configuration and a collapsed configuration. The expandable member is further configured to be inserted into the ureter of the patient and configured to contact the ureter to help retain the elongate member in place within the patient.
Owner:BOSTON SCI SCIMED INC
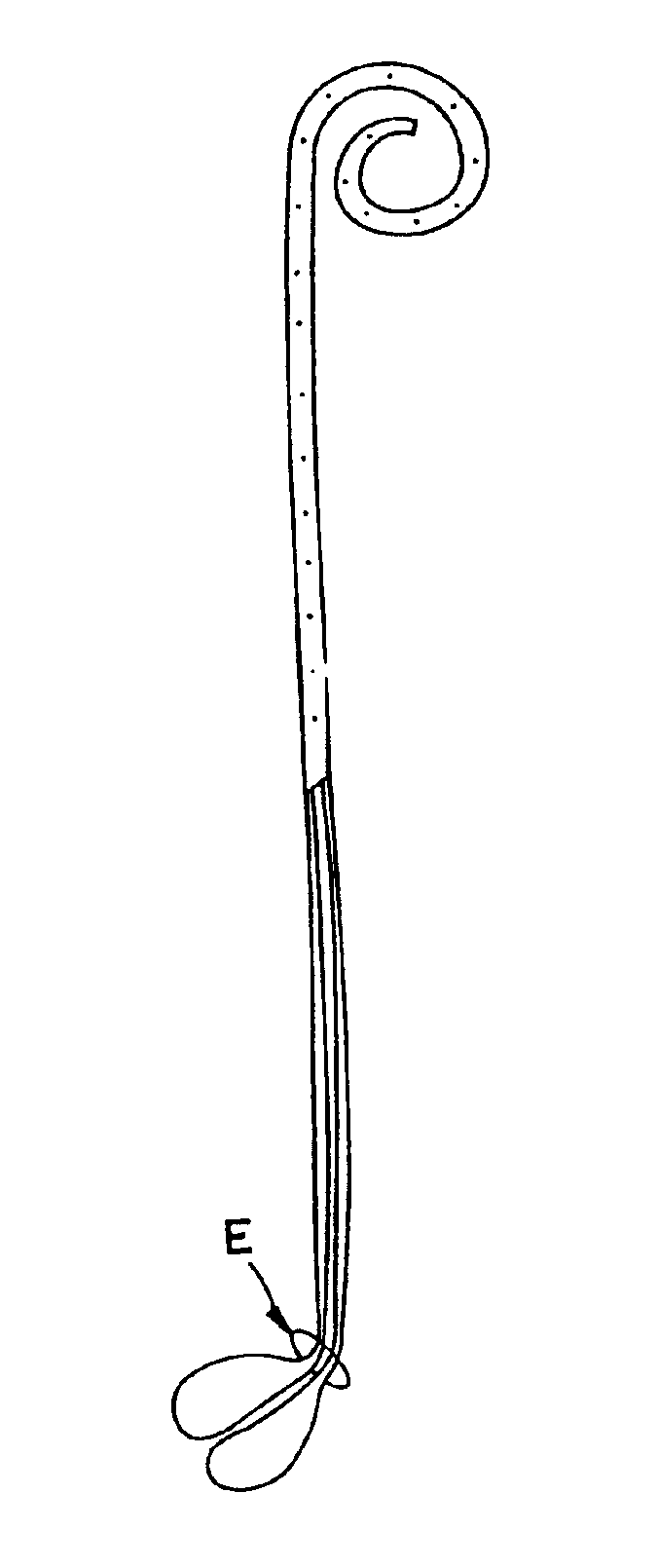
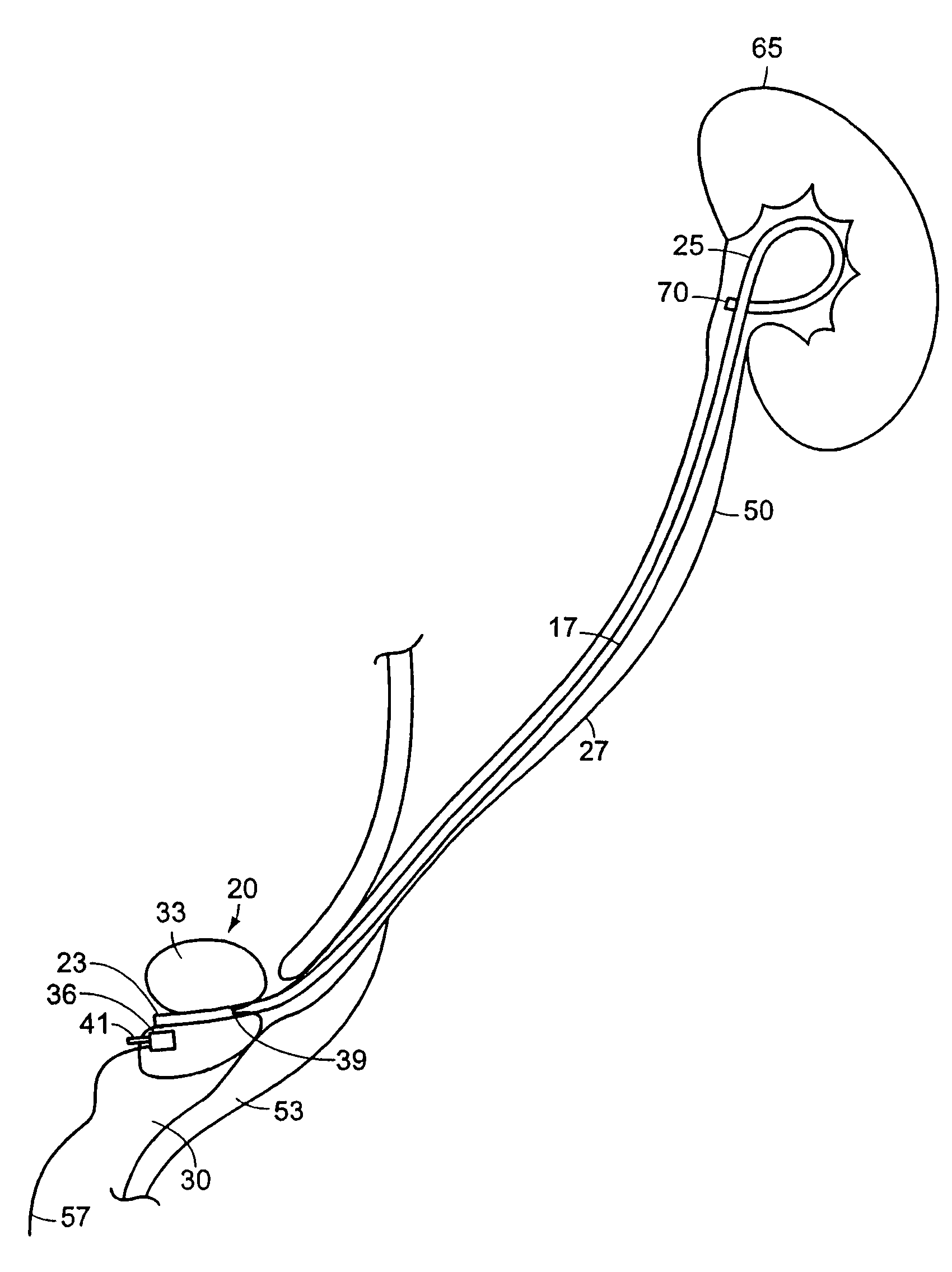
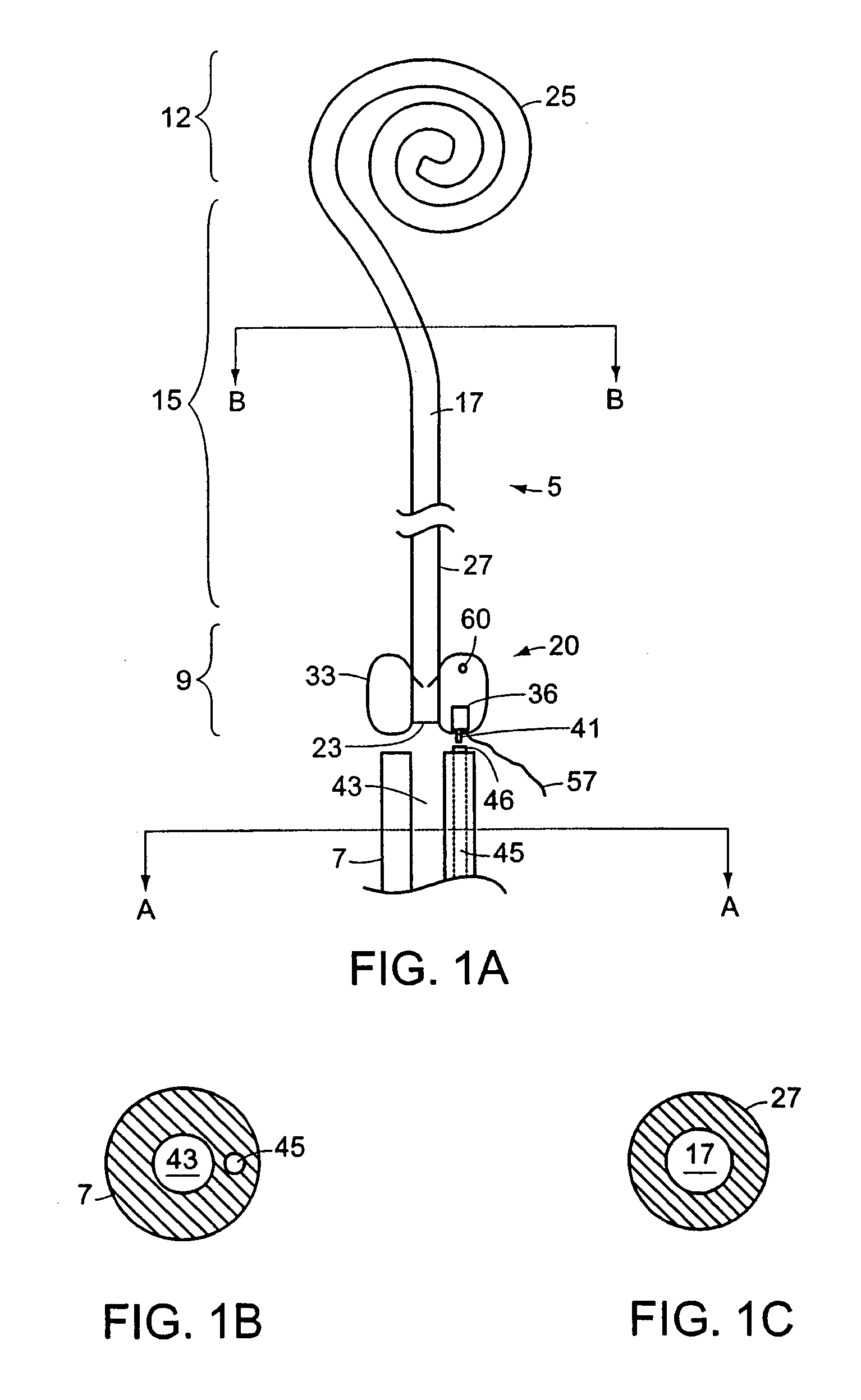
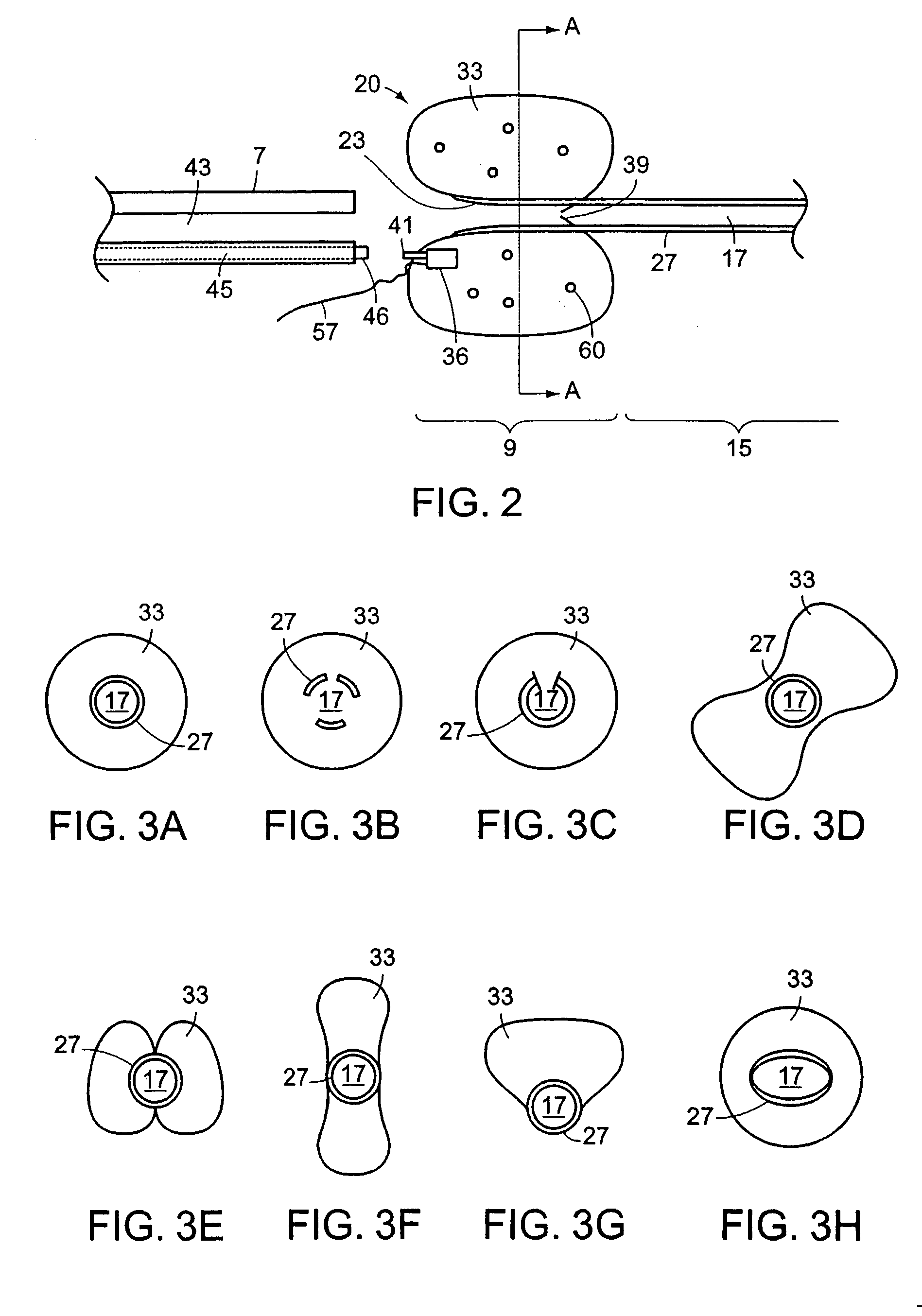
Ureteral stent with small bladder tail(s)
InactiveUS6945950B2Decreases patient discomfortInhibit migrationUrinary bladderStentsInsertion stentLeft ureter
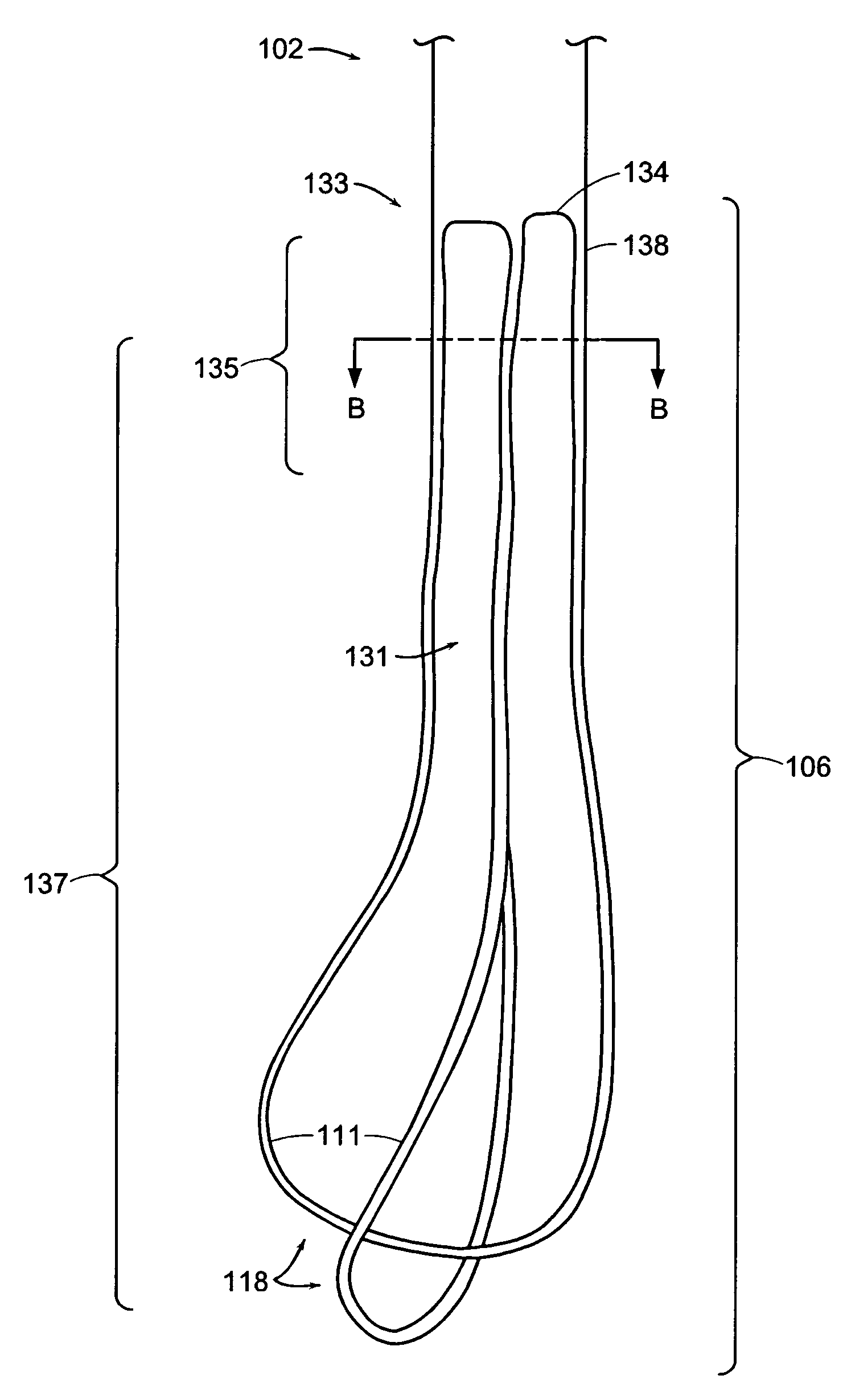
A ureteral stent for assisting movement of urine along a patient's ureter and into the patient's bladder. The stent includes an elongated tubular segment extending toward the bladder from a kidney end region for placement in the renal cavity to a bladder end region. A central lumen connects at least one opening at the first end region to at least one opening in the bladder end region. Thin flexible tail(s) are attached to the bladder end region of the tubular segment at a point outside the bladder so as to receive urine from the opening in the bladder end region and to transport urine from there across the ureter / bladder junction and into the bladder. The tails include an elongated external urine-transport surface configured to transport urine. The transporting surface(s) are configured to extend along at least part of the ureter, across the ureter / bladder junction, and into the bladder.
Owner:BOSTON SCI SCIMED INC +1
Stent
A stent is deployed within a body passageway. The stent is configured to curl a varying degree upon itself as the stent is inserted into the passageway depending on the size of the passageway. In this way a single size stent may be employed in different size passageways. The stent may have a generally rectangular shape or other shape. The stent may be made of one or more materials. For example, a spine of the stent may be made of a material different than a material used for the outer edges of the stent. The edges of the stent curl towards the spine during insertion into the passageway without any further manipulation of the stent or by application of any additional mechanical means. The stent may have a varying thickness and / or width along the longitudinal axis of the stent.
Owner:CR BARD INC
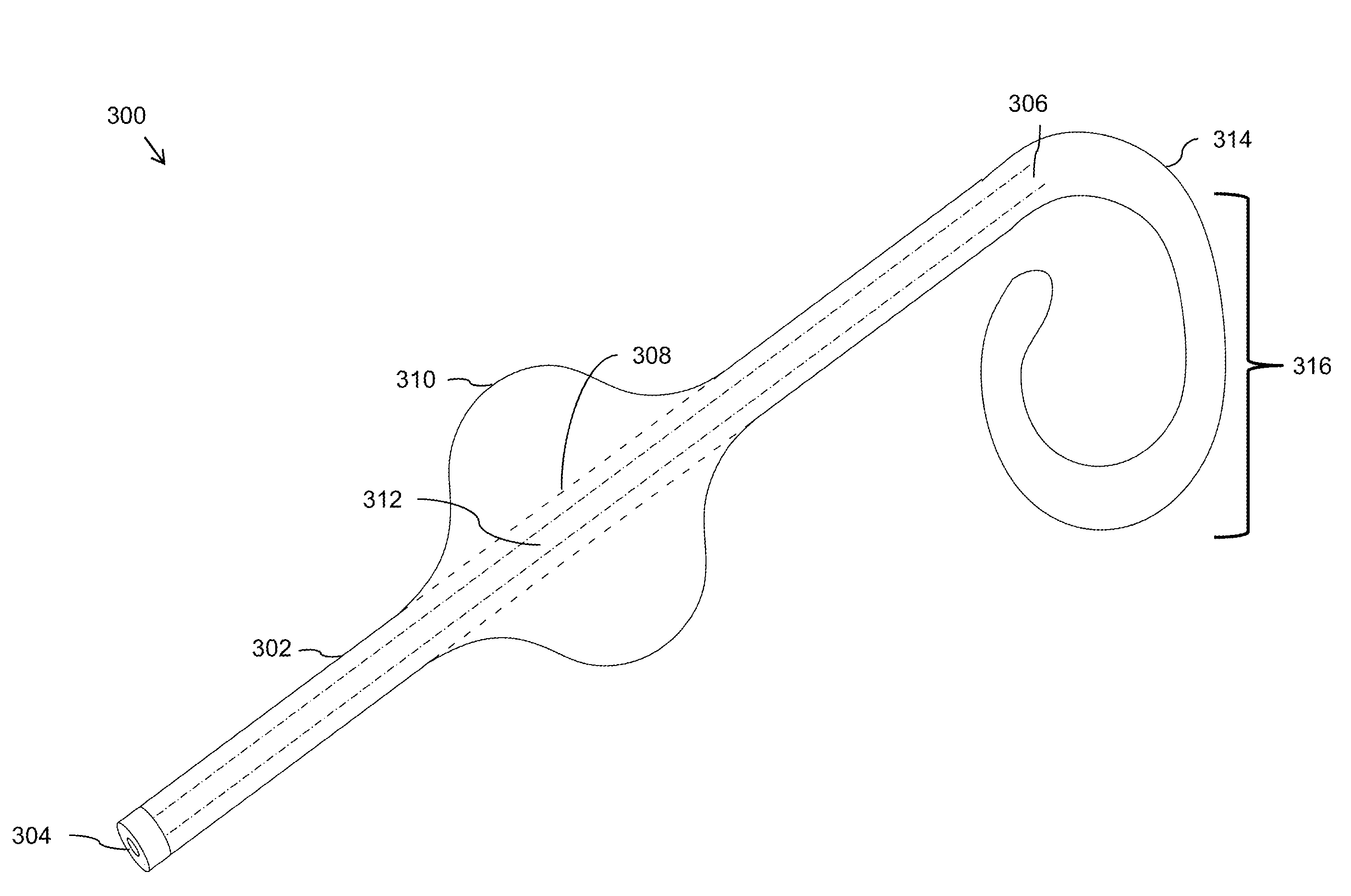
Stent retention element and related methods
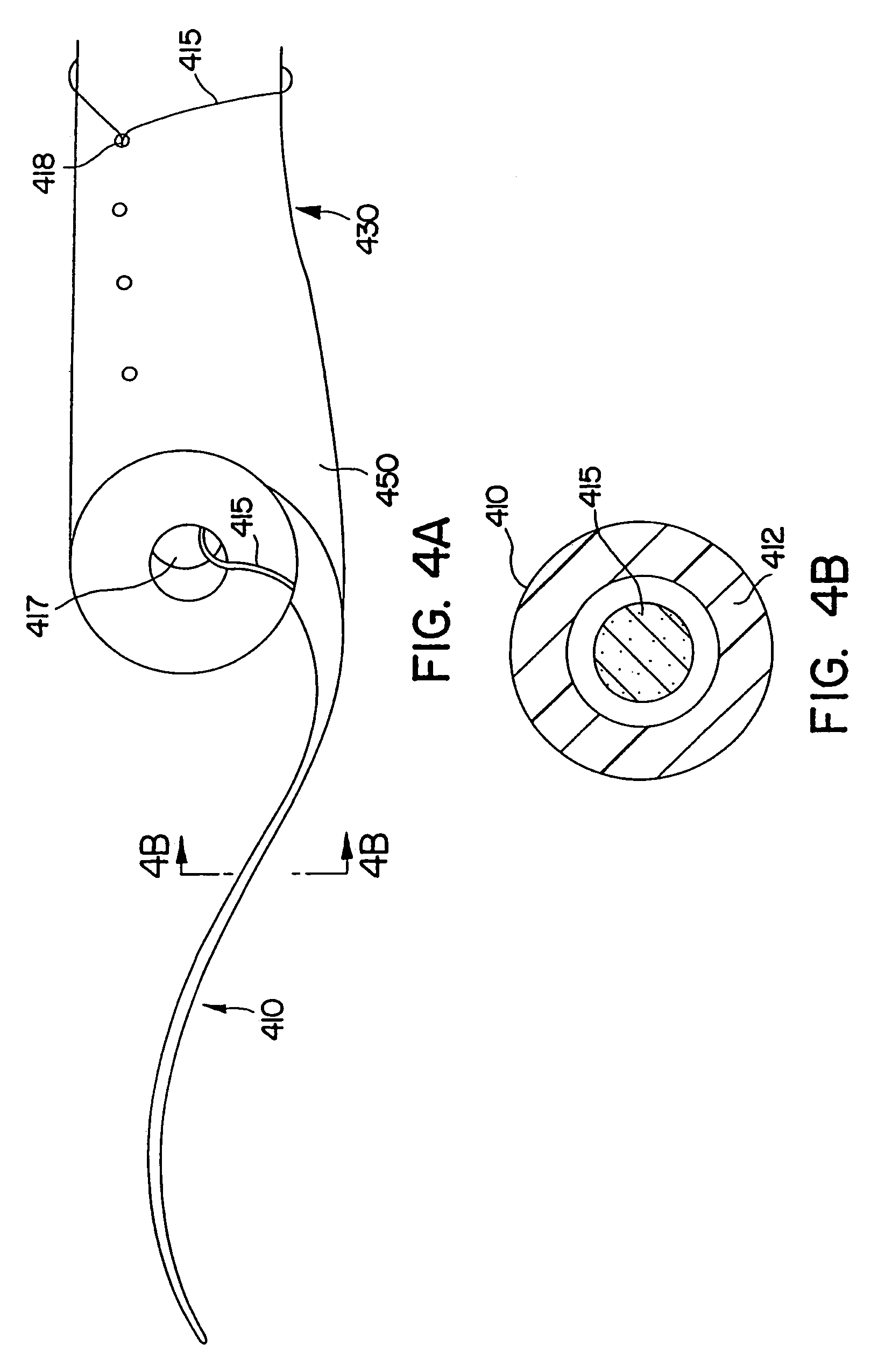
In one embodiment, the invention is directed to a stent retention element having an elastic member adapted to be incorporated with a first end of an elongate stent and to coil toward a second end of the elongate stent to anchor the elongate stent at an anatomical site. According to one feature, the stent retention element expands and compresses to effectively lengthen and shorten, respectively, the stent to accommodate ureter lengthening and shortening.
Owner:BOSTON SCI SCIMED INC
Scaffolds for organ reconstruction and augmentation
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
A connectable catheter
There are provided a connectable catheter system, device and methods of use thereof. The connectable catheter system, comprising: an intermediary catheter comprising an external section and a tip section, the tip section is configured to be inserted into a body of a subject; and a reconnectable indwelling stent comprising a connecting section and a target section, the target section being configured to be located within a body of the subject, wherein the connecting section of the reconnectable indwelling stent is configured to reversibly connect, within the subject body, to the tip section of the intermediary catheter to form a continuous conduit between the intermediary catheter and the reconnectable indwelling stent.
Owner:UROGEN PHARMA LTD
Pre-Positioned Anastomosis Device and Related Methods of Use
Embodiments of a medical device and related methods of use are provided in the disclosure. The medical device includes an elongate member having a first end defining a first opening, a second end defining a second opening, and a tapered lumen extending between the first and second openings. The diameter of the first end may be smaller than that of the second end. The medical device also may include a securing mechanism protruding from one of the first or second ends to penetrate tissue.
Owner:BOSTON SCI SCIMED INC
Ureteral stent for improved patient comfort
A ureteral stent for assisting the movement of urine along a patient's ureter and into the patient's bladder. The stent includes an elongated tubular segment extending toward the bladder from a kidney end region for placement in the renal cavity to a bladder end region. A central lumen connects at least one opening at the first end region to at least one opening in the bladder end region. Thin flexible tail(s) are attached to the bladder end region of the tubular segment at a point outside the bladder so as to receive urine from the opening in the bladder end region of the tubular segment and to transport urine from there across the ureter / bladder junction and into the bladder. The tails include an elongated external urine-transport surface sized and configured to transport urine along the ureter. The urine transporting surface(s) are sized and configured to extend along at least part of the ureter, across the ureter / bladder junction, and from there into the bladder. In some embodiments, the distal region includes a tubular body with a lumen in fluid communication with an interstitial area defined by one or more flexible filaments of the proximal region forming at least one loop.
Owner:BOSTON SCI SCIMED INC
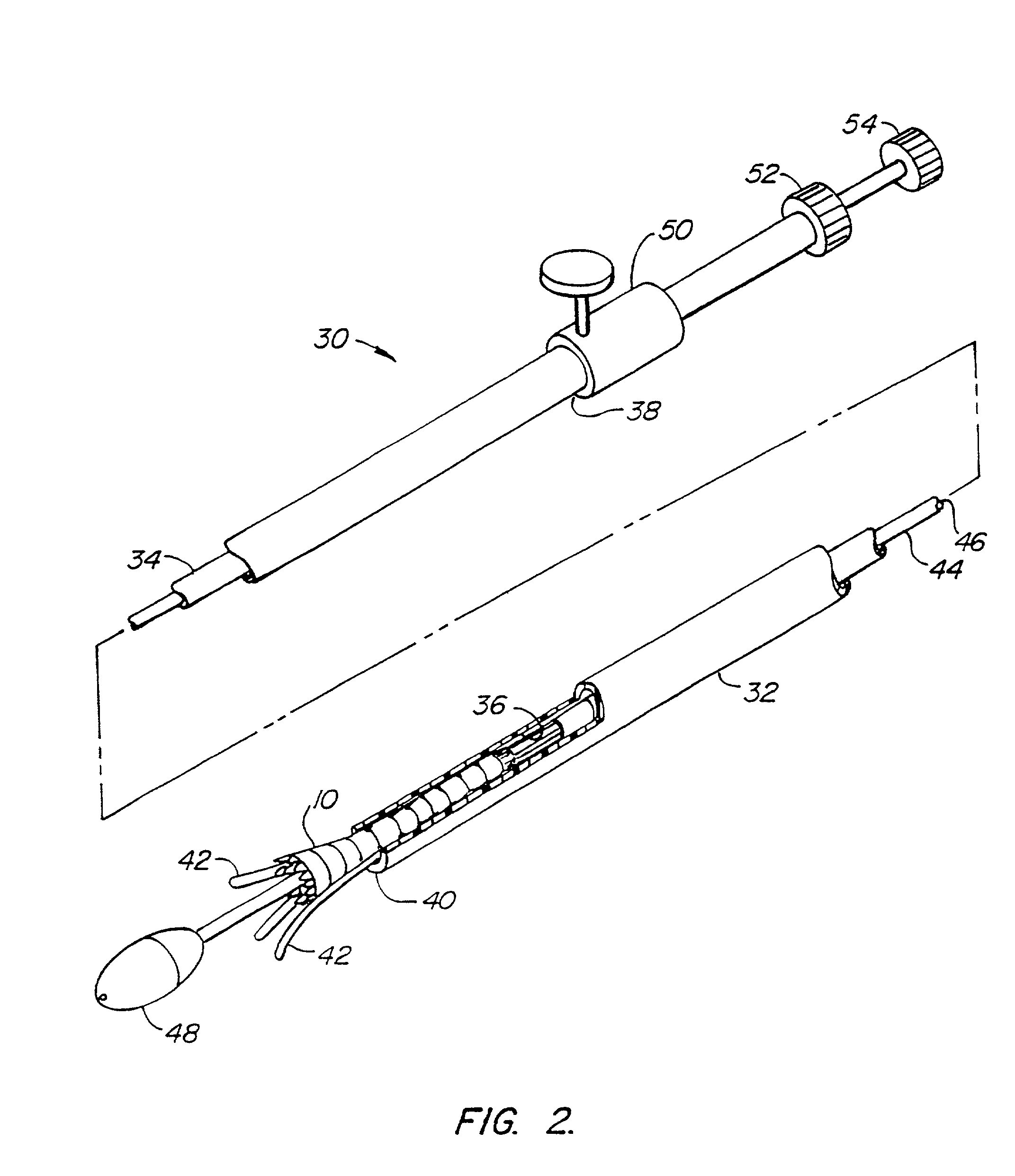
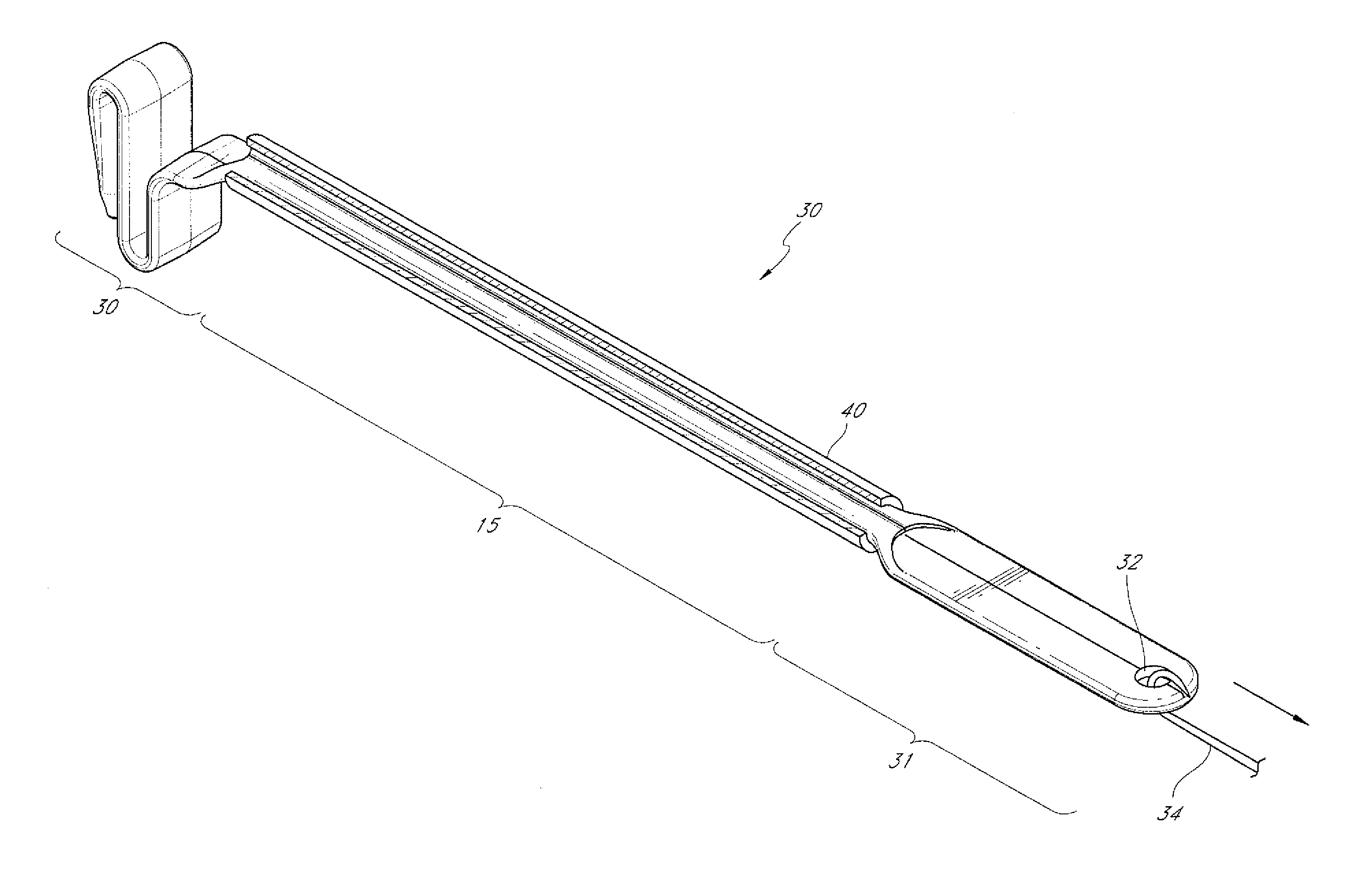
Catheter delivery system
A method for delivering catheters, and stents composed of soft, compliant polymers through anatomical passages. These devices have a bulbous anchorage end with a diameter greater than the rest of the catheter. To facilitate implant and delivery a pusher catheter or sheath with an internal lumen larger than the outer diameter of the catheter but smaller than the outer diameter of the bulbous anchorage end. The distal end of pusher catheter or the sheath physically engages the proximal end of the bulbous anchorage end and applies an axial force to coaxially advance the catheter over a guidewire though anatomical passages. This method allows a physician to move the catheter to an anatomical site without the device exhibiting buckling due to axial force applied. Similarly, this delivery method will allow more force to be applied to the distal end of the catheter diminishing the likelihood of buckling.
Owner:EPSTEIN SCOTT
Methods and devices for urethral treatment
InactiveUS20120010645A1Preventing clinically significant compressionPrevents recompression of the urethraDilatorsCatheterUrethraInsertion stent
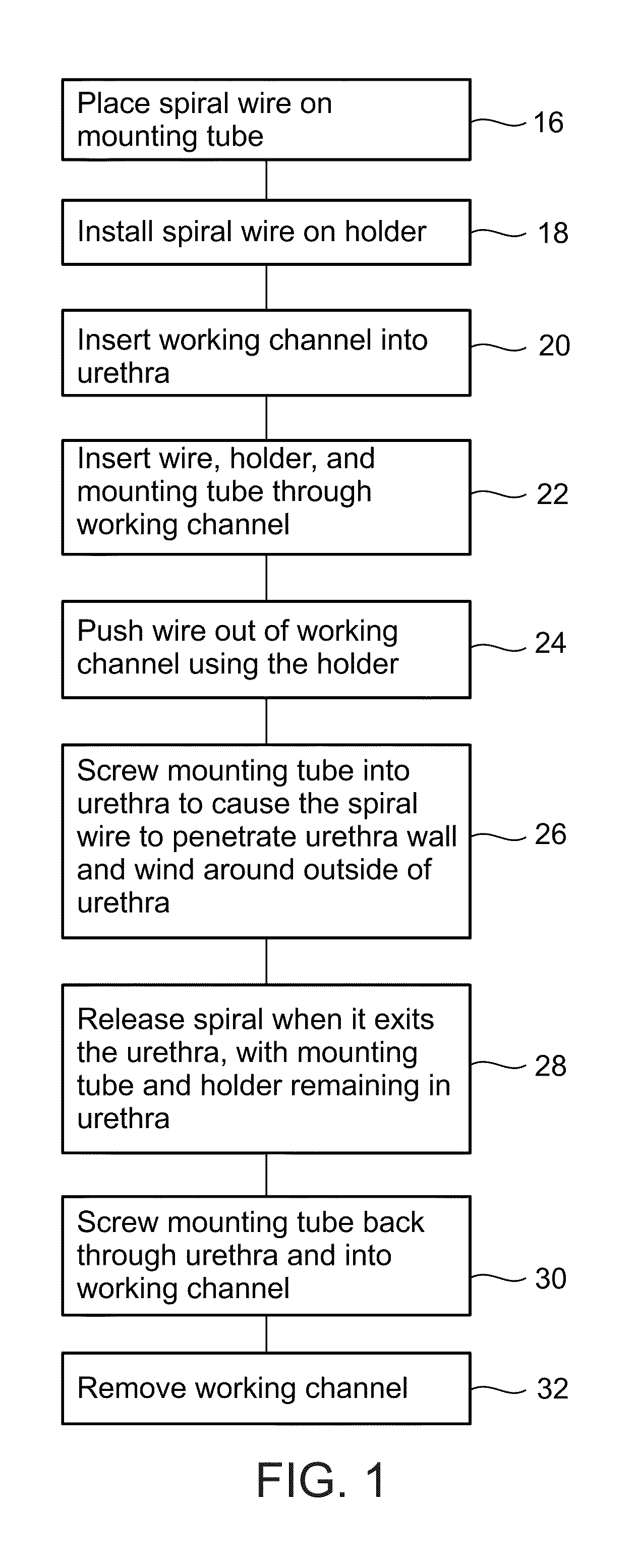
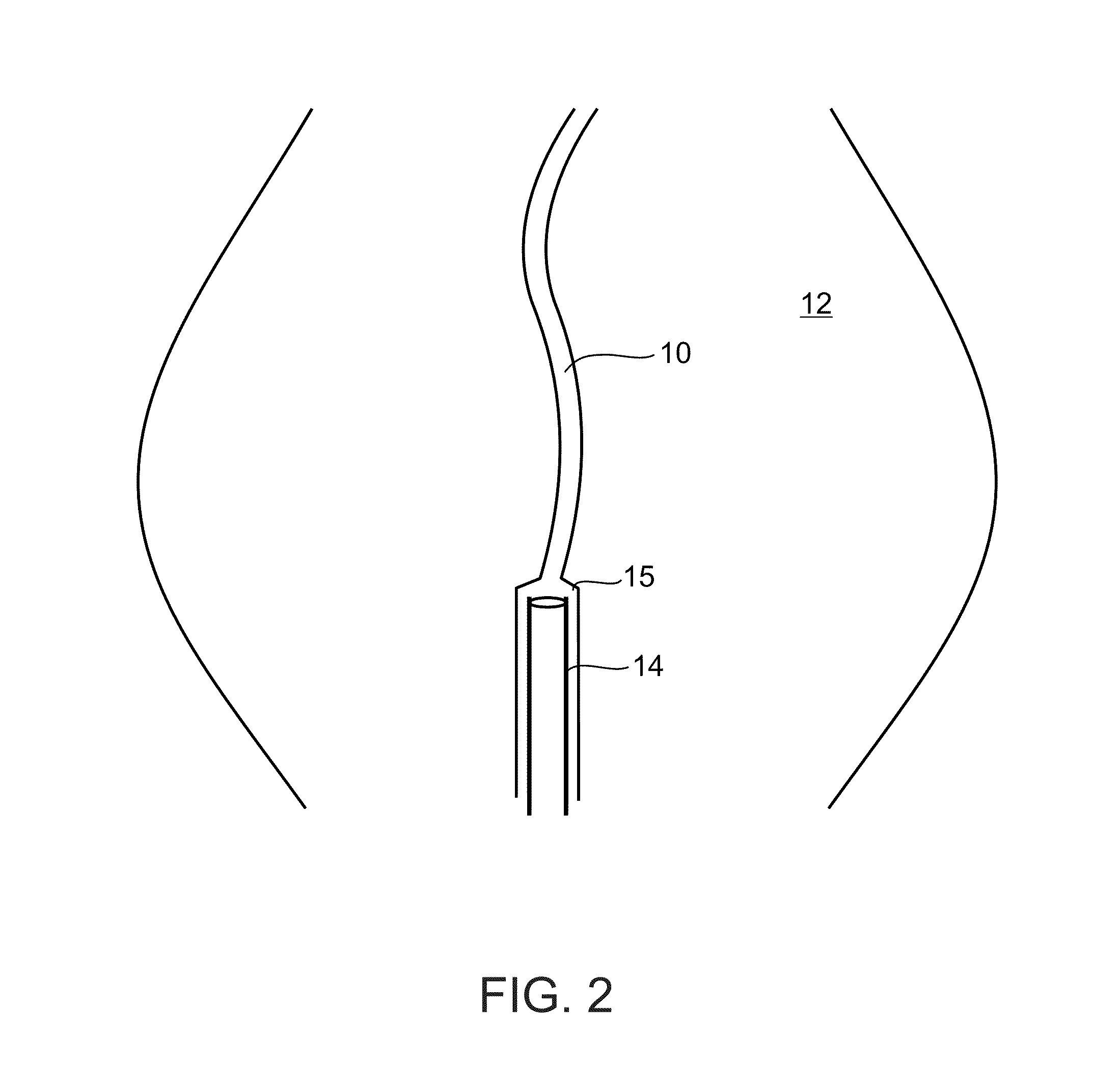
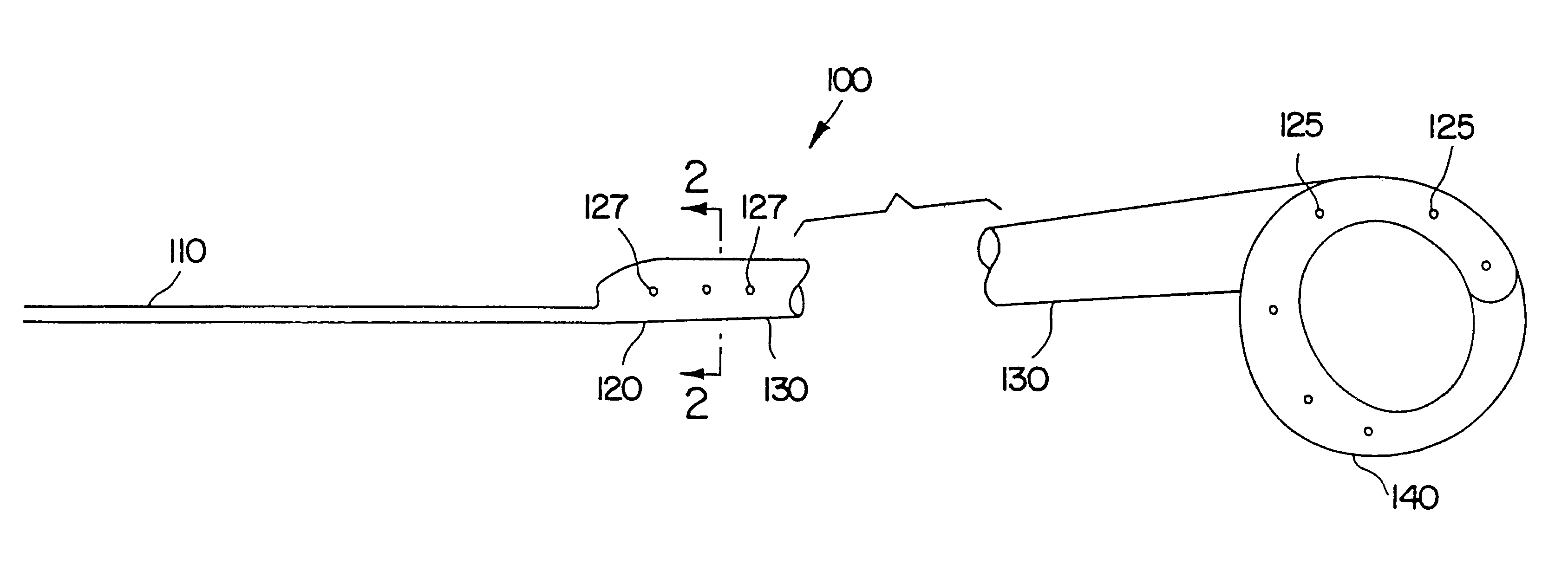
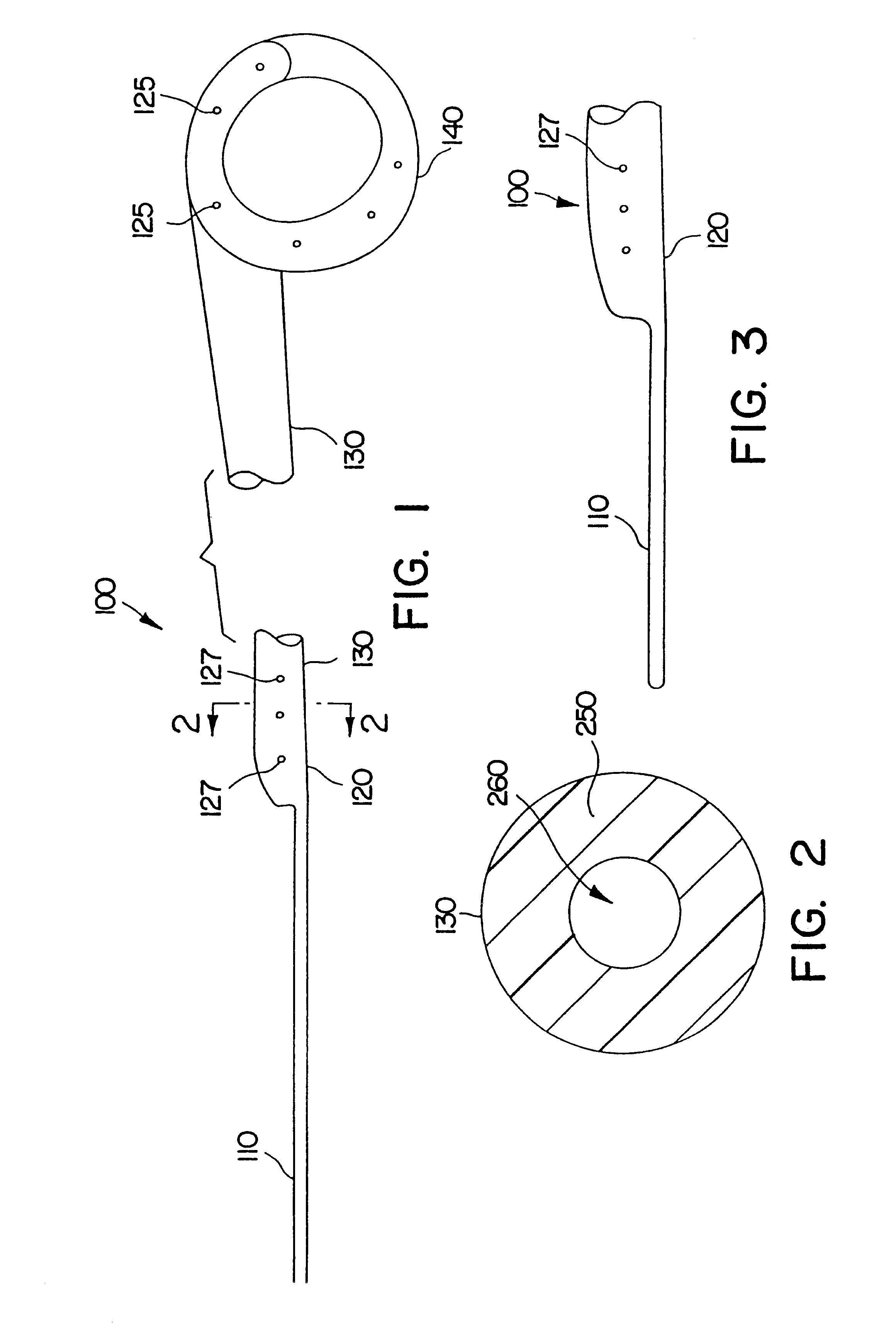
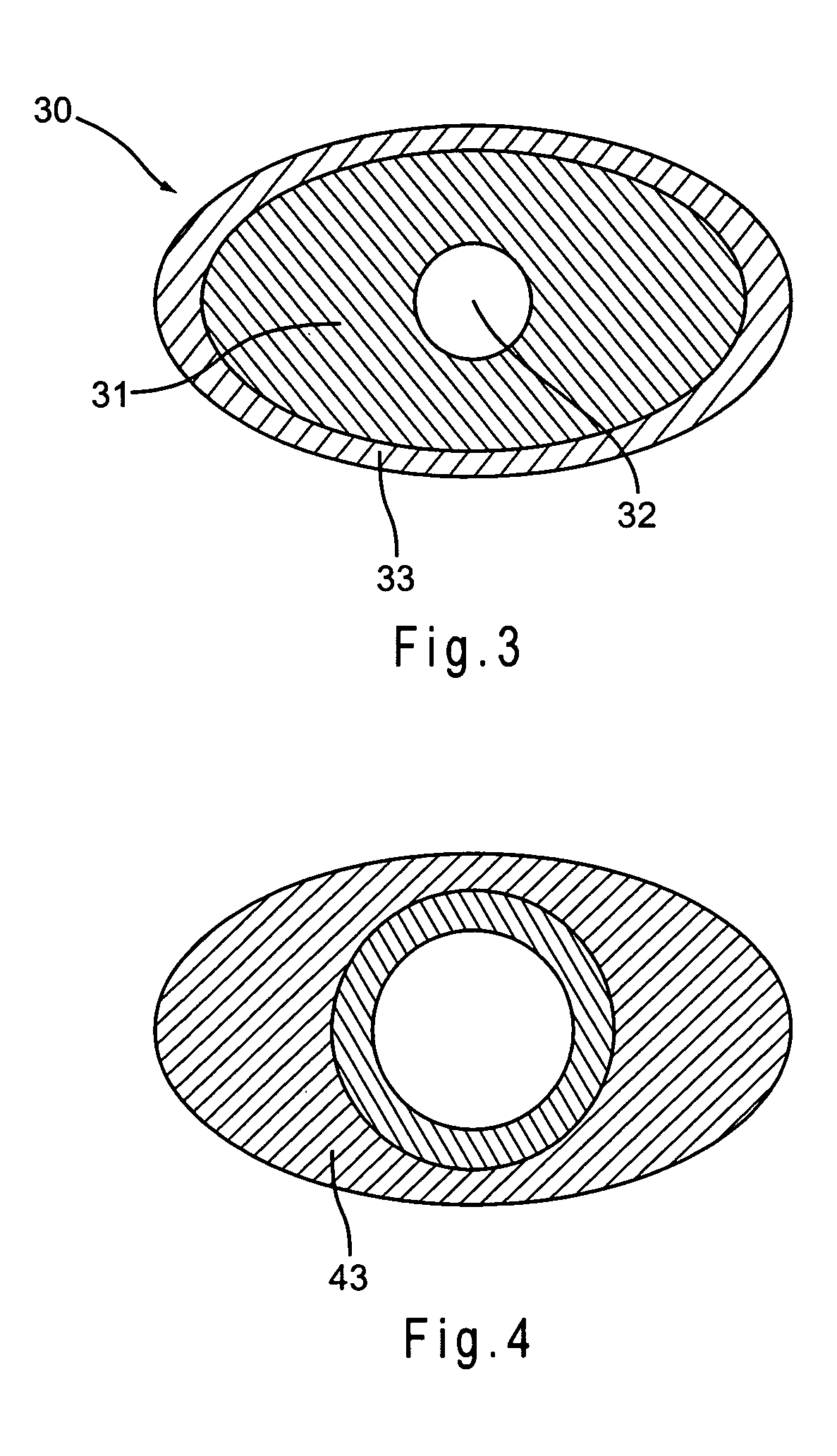
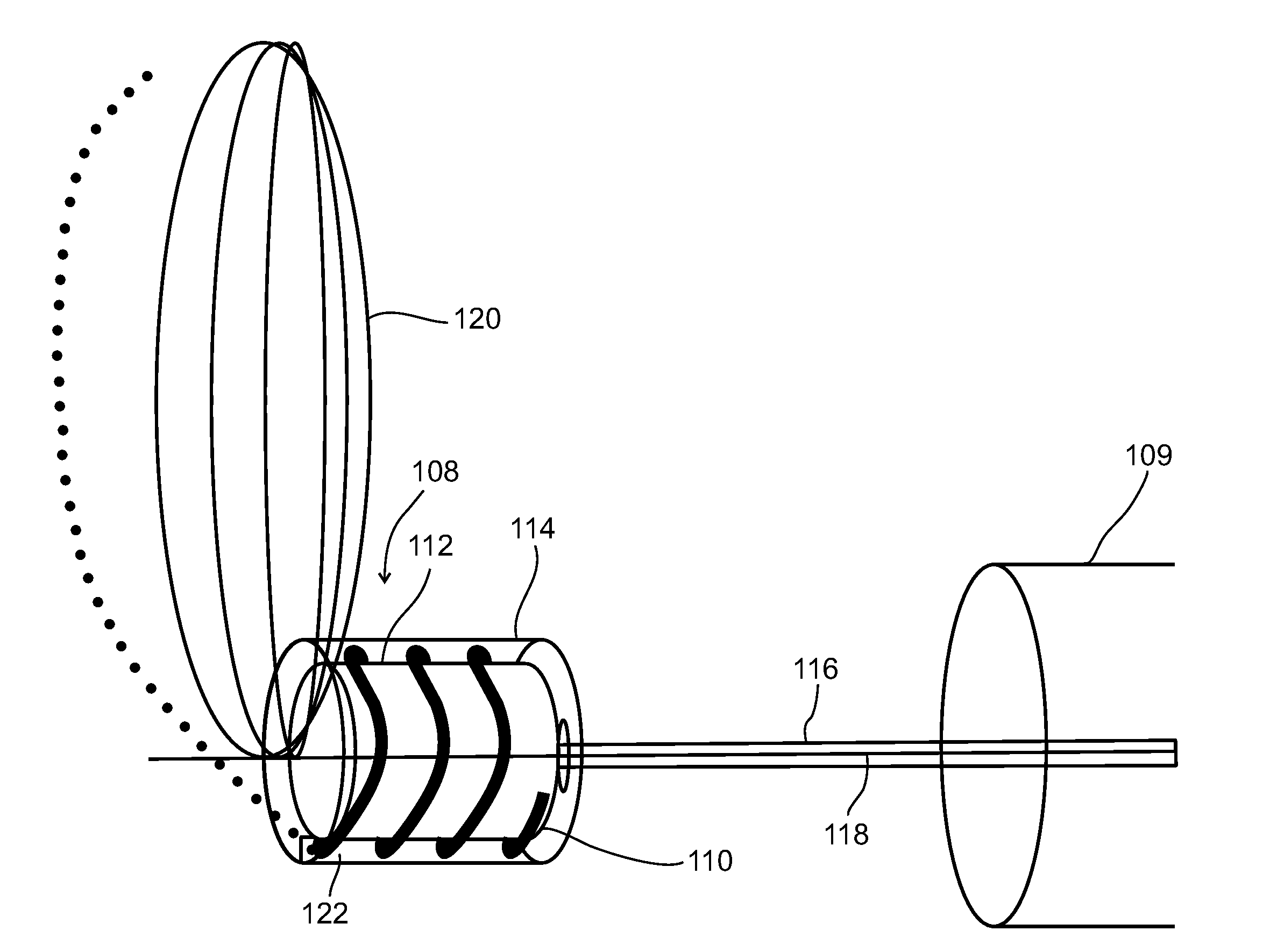
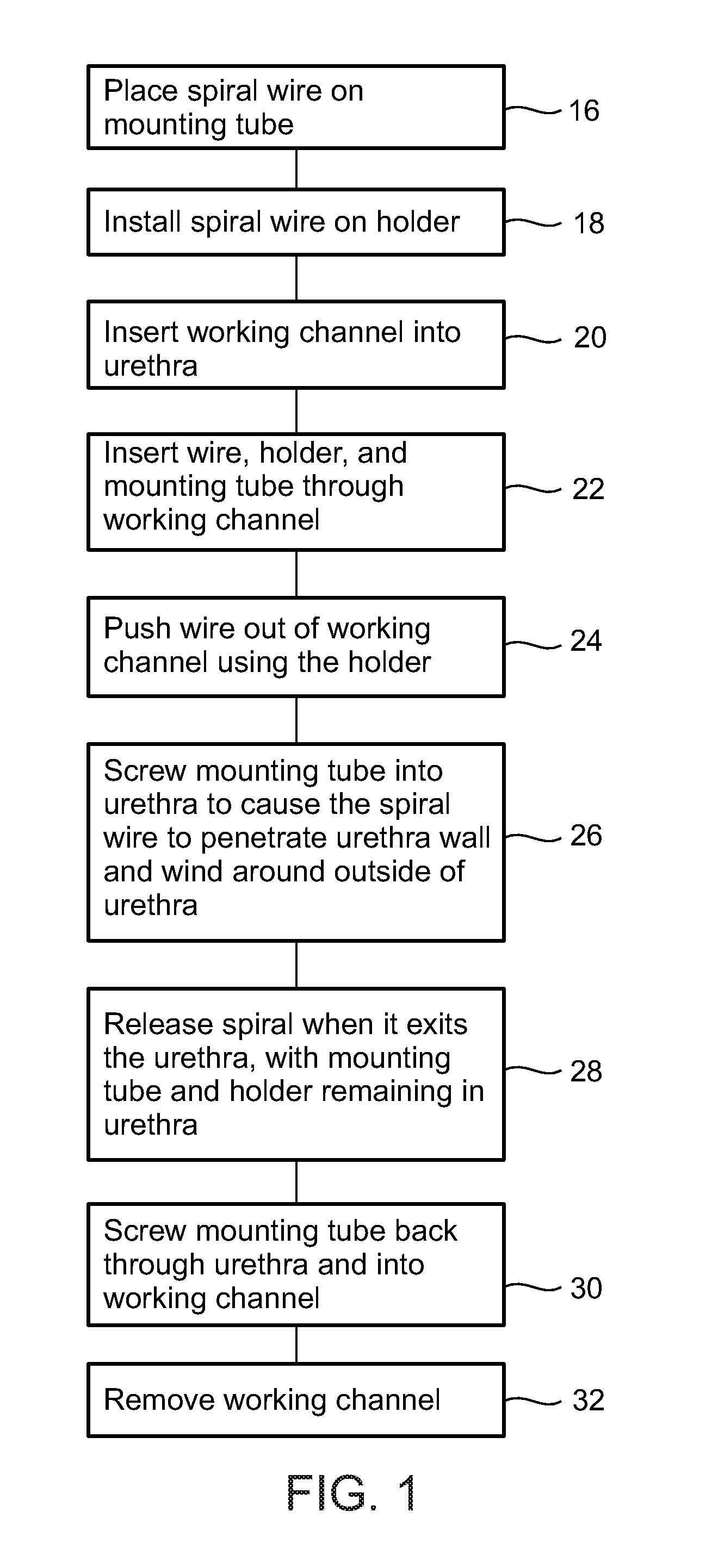
A dilation device for an intrabody lumen, for example, a urethra partially occluded by an enlarged prostate in the form of a curved element configured to be implanted around the lumen and add resilience to the lumen, and a method for dilating such a lumen by implanting a dilation device around the outside of the lumen with at least part of the dilation device embedded in surrounding, thereby preventing clinically significant compression of the lumen. Also, an implantation device for such a dilation device in the form of a mounting device configured to carry the dilation device; and a releasably attached movable holder, with the mounting device and holder configured to deliver the dilation device though a working channel to a desired position in a lumen, and to implant the dilation device through a wall of the lumen to a position surrounding the lumen.
Owner:PROARC MEDICAL
Medical device with tail(s) for assisting flow of urine
InactiveUS6849069B1Decrease patient discomfortDecreases patient discomfortUrinary bladderStentsInsertion stentLeft ureter
A ureteral stent for assisting movement of urine along a patient's ureter and into the patient's bladder. The stent includes an elongated tubular segment extending toward the bladder from a kidney end region for placement in the renal cavity to a bladder end region. A central lumen connects at least one opening at the first end region to at least one opening in the bladder end region. Thin flexible tail(s) are attached to the bladder end region of the tubular segment at a point outside the bladder so as to receive urine from the opening in the bladder end region of the tubular segment and to transport urine from there across the ureter / bladder junction and into the bladder. The tails include an elongated external urine-transport surface sized and configured to transport urine along the ureter. The urine transporting surface(s) are sized and configured to extend along at least part of the ureter, across the ureter / bladder junction, and from there into the bladder.
Owner:BOSTON SCI CORP
Reconstruction of urological structures with polymeric matrices
A method for repairing defects and reconstructing urothelial structures in vivo has been developed using a fibrous, open synthetic, biodegradable polymeric matrix which is configured to provide the desired corrective structure. The matrix is shaped to correct the defect, then implanted surgically to form a scaffolding for the patient's own cells to grow onto and into. The implantation of the matrix initiates an inflammatory reaction, resulting in urothelial cells, endothelial cells and mesenchymal cells, to migrate into the matrix. The polymer forming the matrix is selected to be biocompatible and degradable in a controlled manner over a period of one to six months, in the preferred embodiment. A preferred material is a poly(lactic acid-glycolic acid) in a fibrous form, such as a woven or non-woven mesh. Examples demonstrate the repair of defects in bladder in rabbits.
Owner:CHILDRENS MEDICAL CENT CORP
Methods and devices for urethral treatment
A system and method for treating a constricted bodily lumen, for example, a urethra constricted due to BPH. The method includes a planning stage, during which an area requiring treatment is visually identified, and an execution stage during which the lumen is expanded, a cut is formed in the inner surface of the tissue defining the lumen, and an implant is deployed in the cut. The implant can be an open C-shaped ring, and can be formed of a biodegradable material, or can be removed at a selected time after implantation. The system is formed of a planning device anchorable in the lumen and constructed to facilitate location of an area to be treated, and execution device constructed to deliver an implant, to expand the lumen, to form a cut in the inner surface of tissue defining the lumen, and to release the implant for deployment into the cut.
Owner:PROARC MEDICAL
Tissue-Engineered Constructs
InactiveUS20130013083A1Improve degradation rateImprove wettabilityUrethraeCell culture supports/coatingCell-Extracellular MatrixVein graft
Constructs including a tubular biodegradable polyglycolic acid scaffold may be coated with extracellular matrix proteins and are substantially acellular. The constructs can be utilized as an arteriovenous graft, a coronary graft, an arterial graft, a venous graft, a duct graft, a skin graft, or a urinary graft or conduit.
Owner:HUMACYTE INC
Implantable medical device with pharmacologically active ingredient
An implantable medical device comprising an inner region and an outer region positioned over at least a portion of the inner region and in contact with a surface of the inner region. The durometer of the inner region is greater than the durometer of the outer region and a pharmacologically active ingredient is present in at least a portion of the inner region or the outer region.
Owner:VANCE PROD INC D B A COOK UROLOGICAL INC
Methods and devices for urethral treatment
ActiveUS20160096009A1Preventing clinically significant compressionPrevents recompression of the urethraDilatorsCatheterUrethraInsertion stent
A dilation device for an intrabody lumen, for example, a urethra partially occluded by an enlarged prostate in the form of a curved element configured to be implanted around the lumen and add resilience to the lumen, and a method for dilating such a lumen by implanting a dilation device around the outside of the lumen with at least part of the dilation device embedded in surrounding, thereby preventing clinically significant compression of the lumen. Also, an implantation device for such a dilation device in the form of a mounting device configured to carry the dilation device; and a releasably attached movable holder, with the mounting device and holder configured to deliver the dilation device though a working channel to a desired position in a lumen, and to implant the dilation device through a wall of the lumen to a position surrounding the lume.
Owner:PROARC MEDICAL
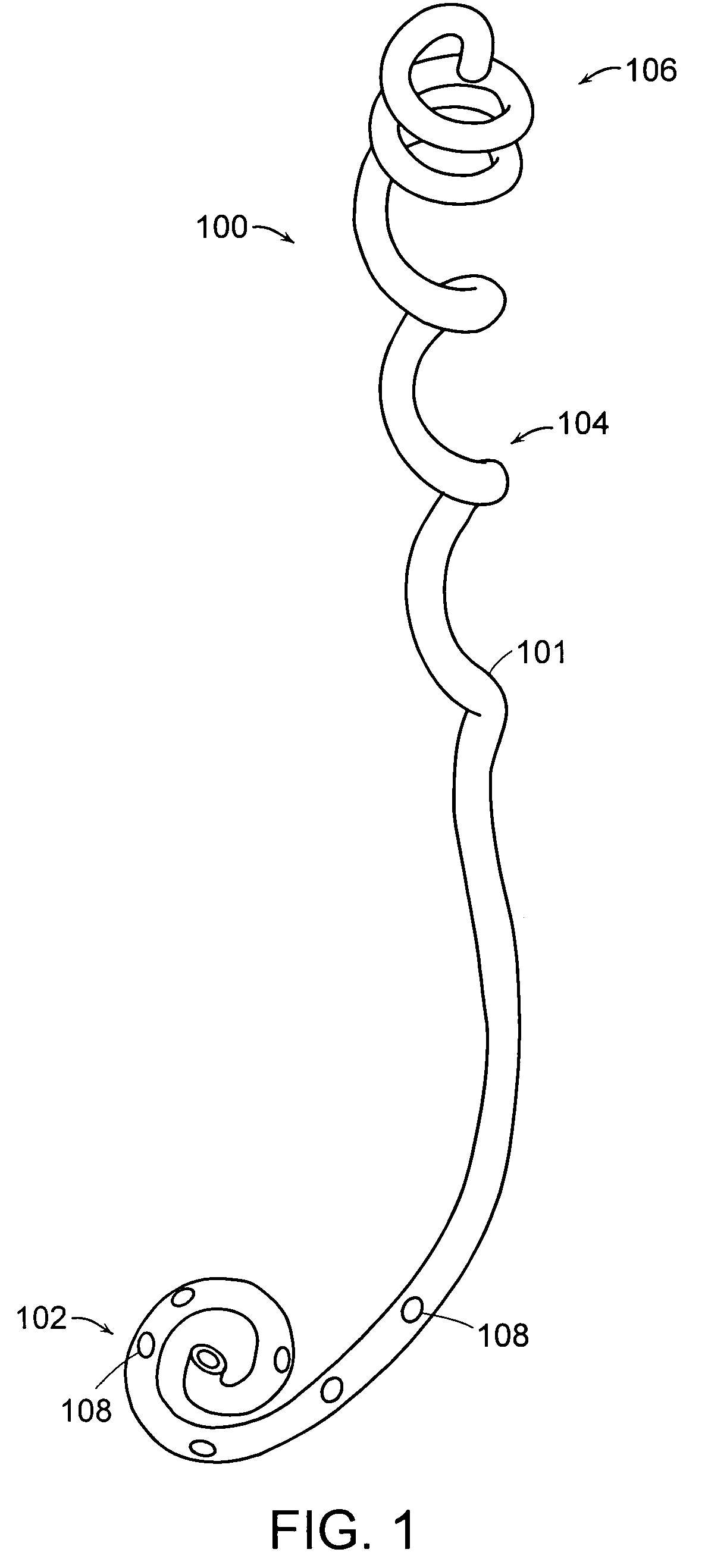
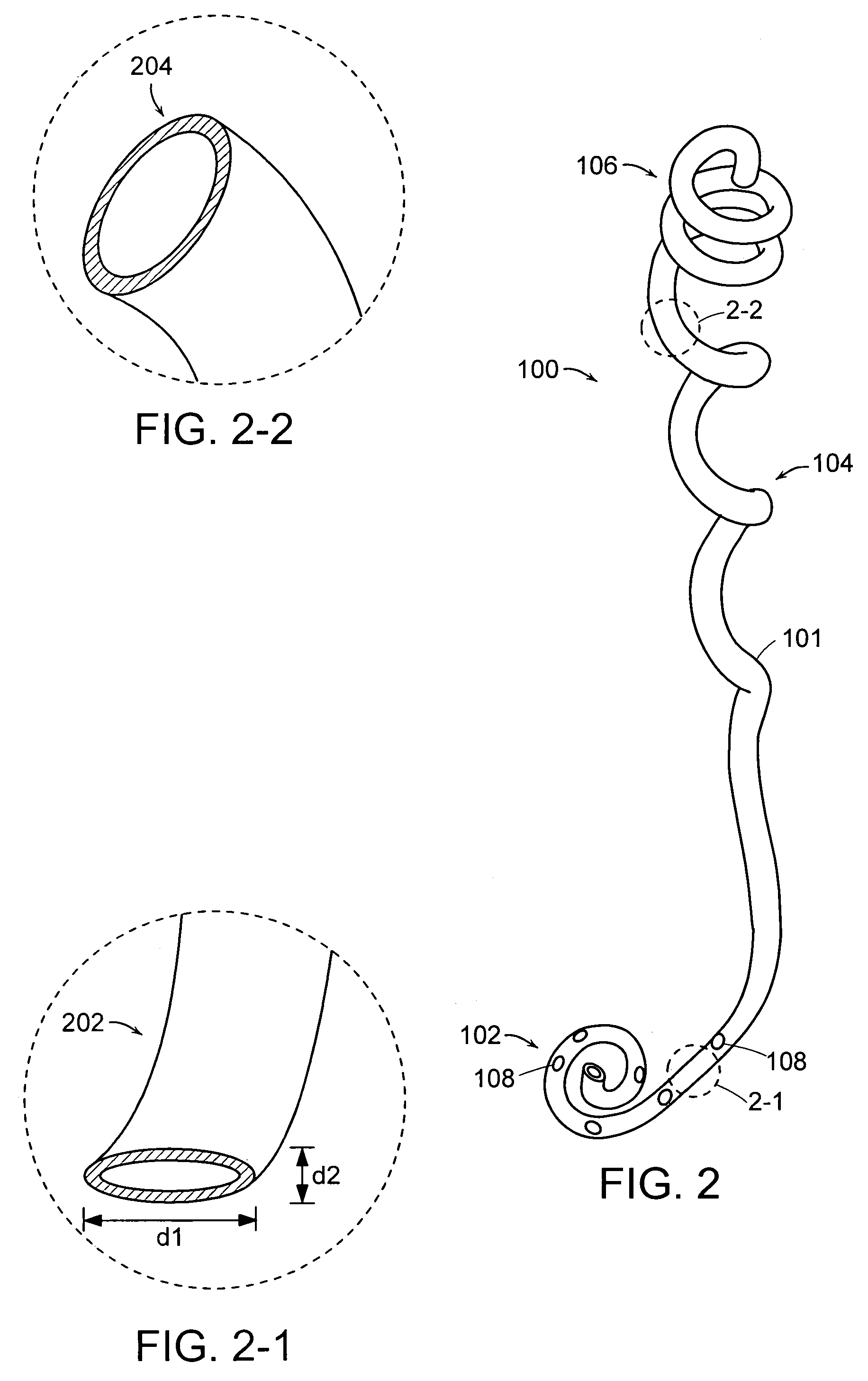
Ureteral Stent
A ureteral stent comprising a short renal coil made of a pliable material and a wick portion made of a material having a hydrophilicity or hydrophobicity different from that of the renal coil and extending from a ureteropelvic junction to a bladder so as to assist in the transfer of urine out of a kidney and into the bladder and to improve patient comfort. Due to increased hydrophilicity or hydrophobicity, wick may be significantly smaller in diameter than renal coil, resulting in less reflux of urine into the kidney and further decreasing patient discomfort. The stent may further comprise one or more couplers between the renal coil and wick portion, such as clamps, couplers, sutures, adhesives or receiving ends of the renal coil, and the wick portion may further comprise a sheath surrounding an elastic core to prevent kinking and enhance the ability of the wick portion to move with the patient. A novel method of ureteral stent placement is also disclosed.
Owner:BOSTON SCI SCIMED INC
Ureteral stent with drug-releasing structure
According to one aspect of the present disclosure, ureteral stents are provided that comprise an elongated stent body, at least one deployable retention structure, and at least one sleeve and / or sheet of drug-releasing material. In various embodiments, at least one sleeve and / or sheet of drug-releasing material is deployed concurrently with the deployment of at least one deployable retention structure. The ureteral stents of the present disclosure are adapted to release the urologically beneficial drug into a subject.
Owner:BOSTON SCI SCIMED INC
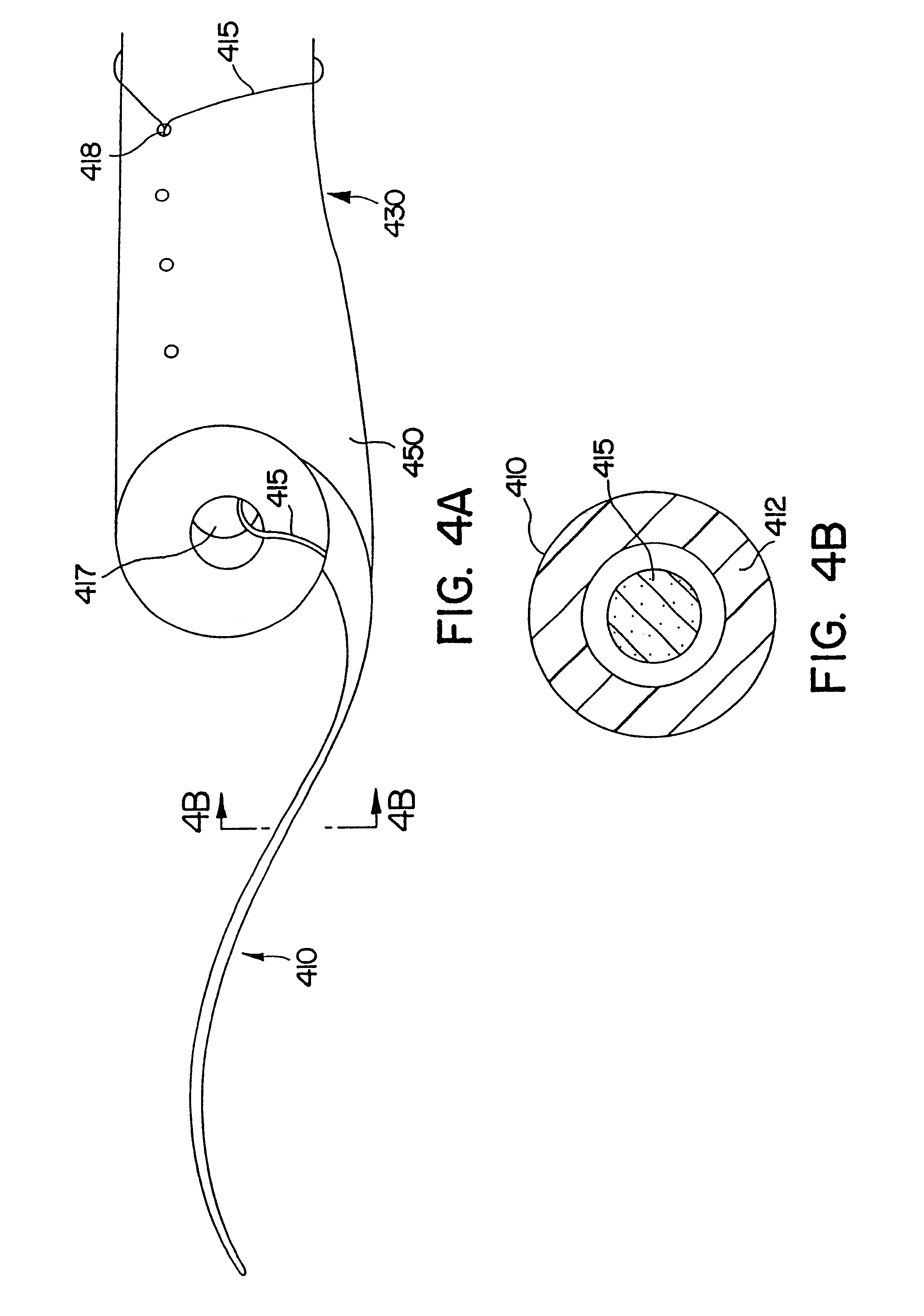
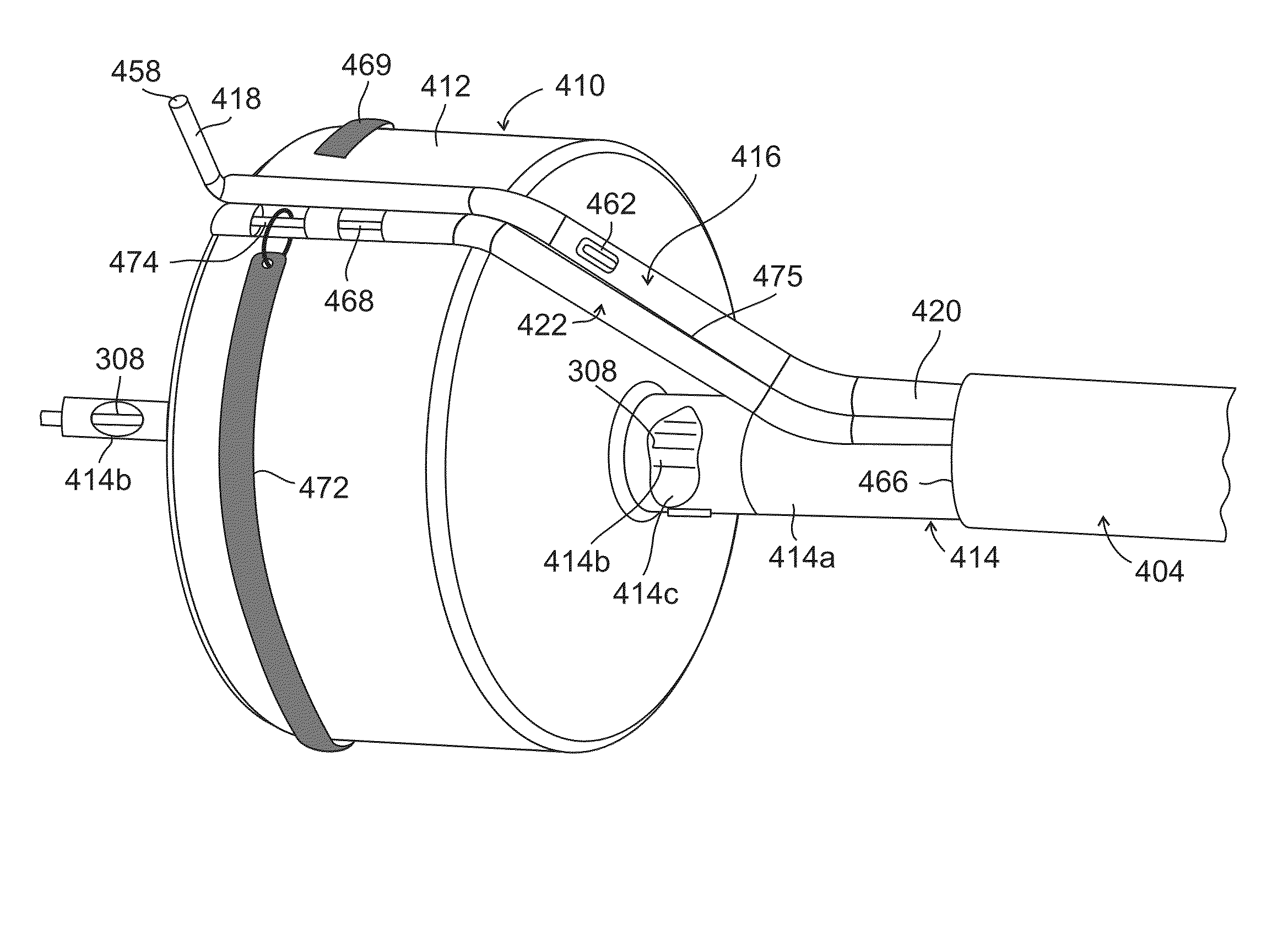
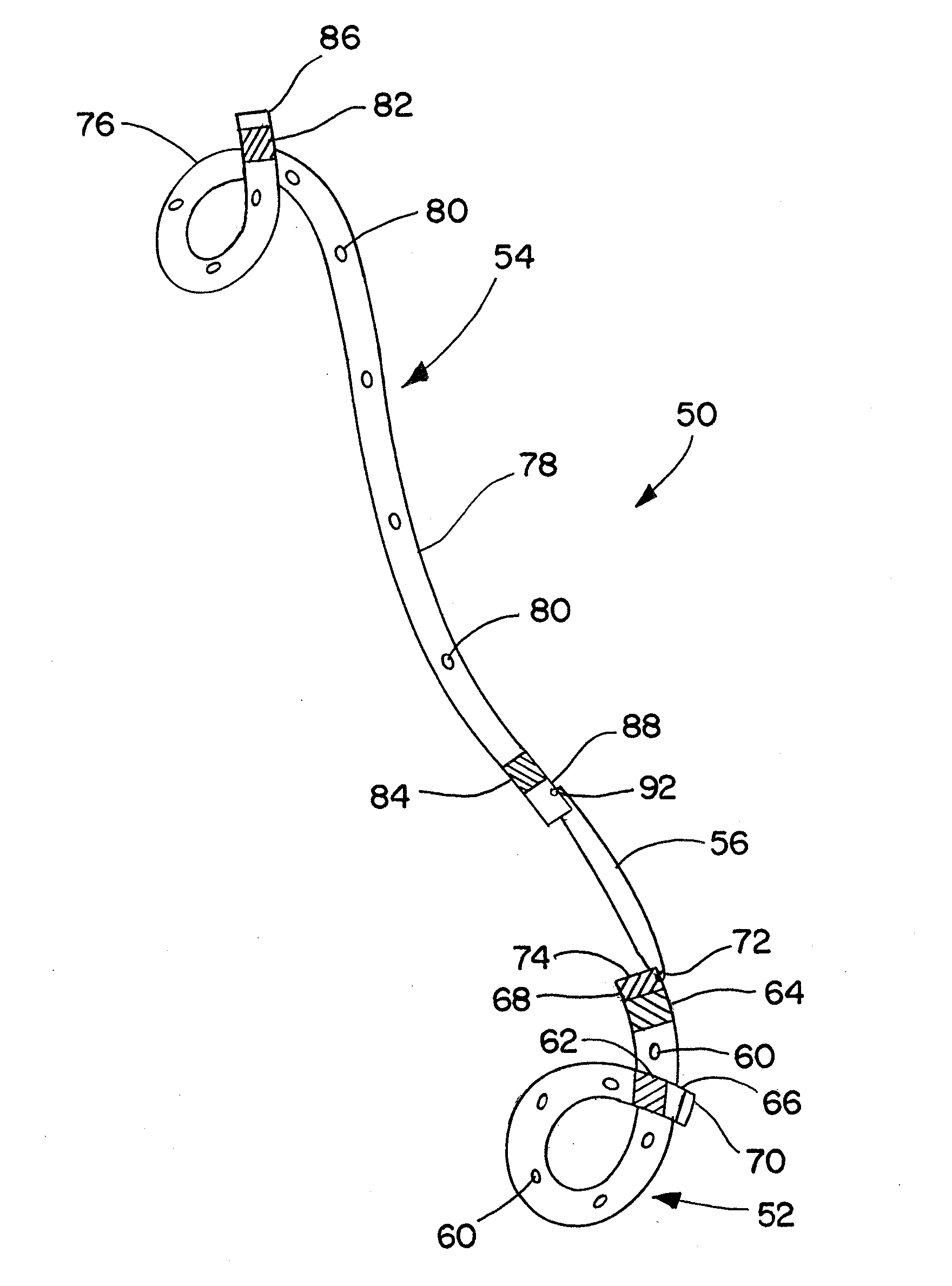
Ureteral stent for placement in a kidney and bladder
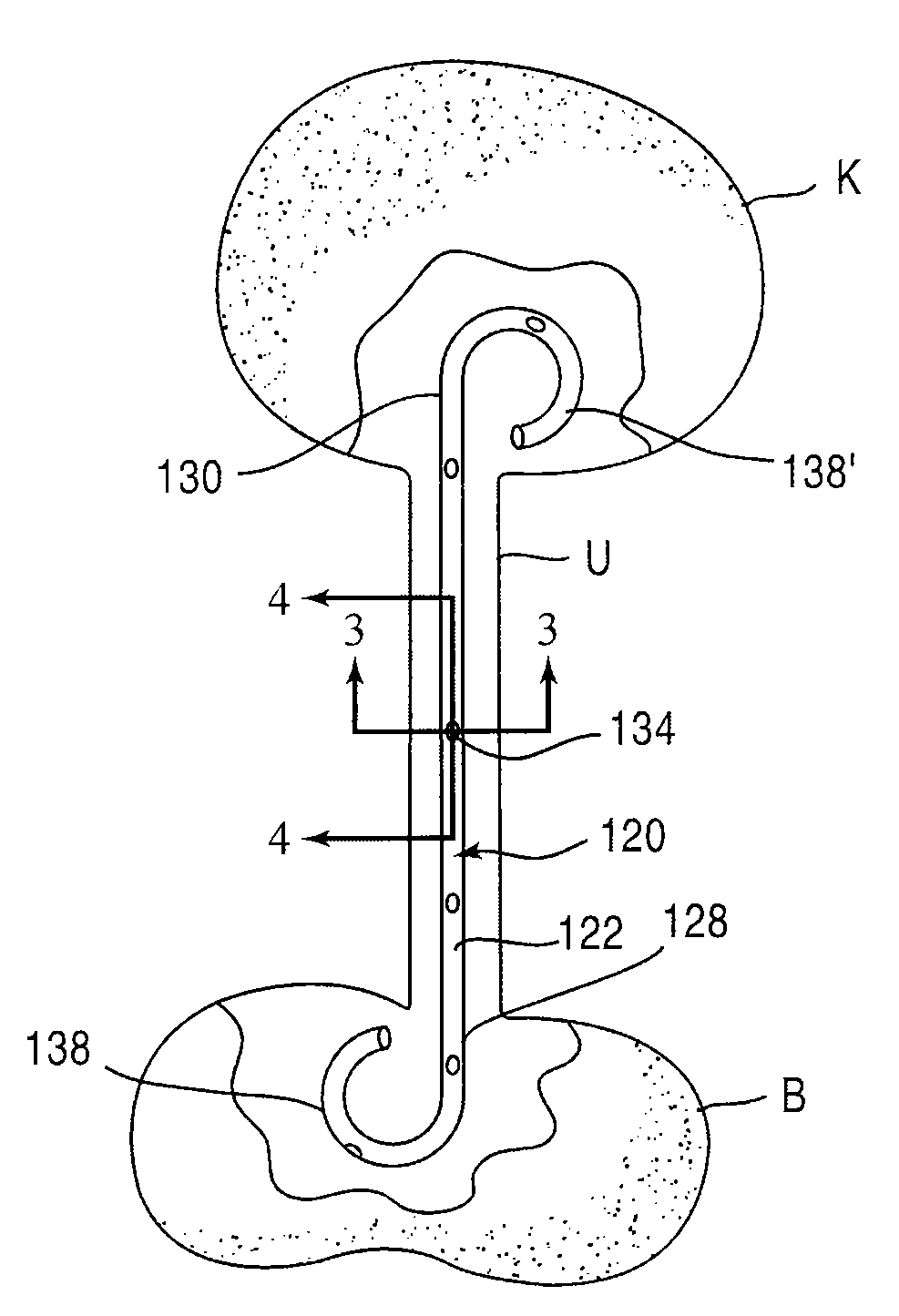
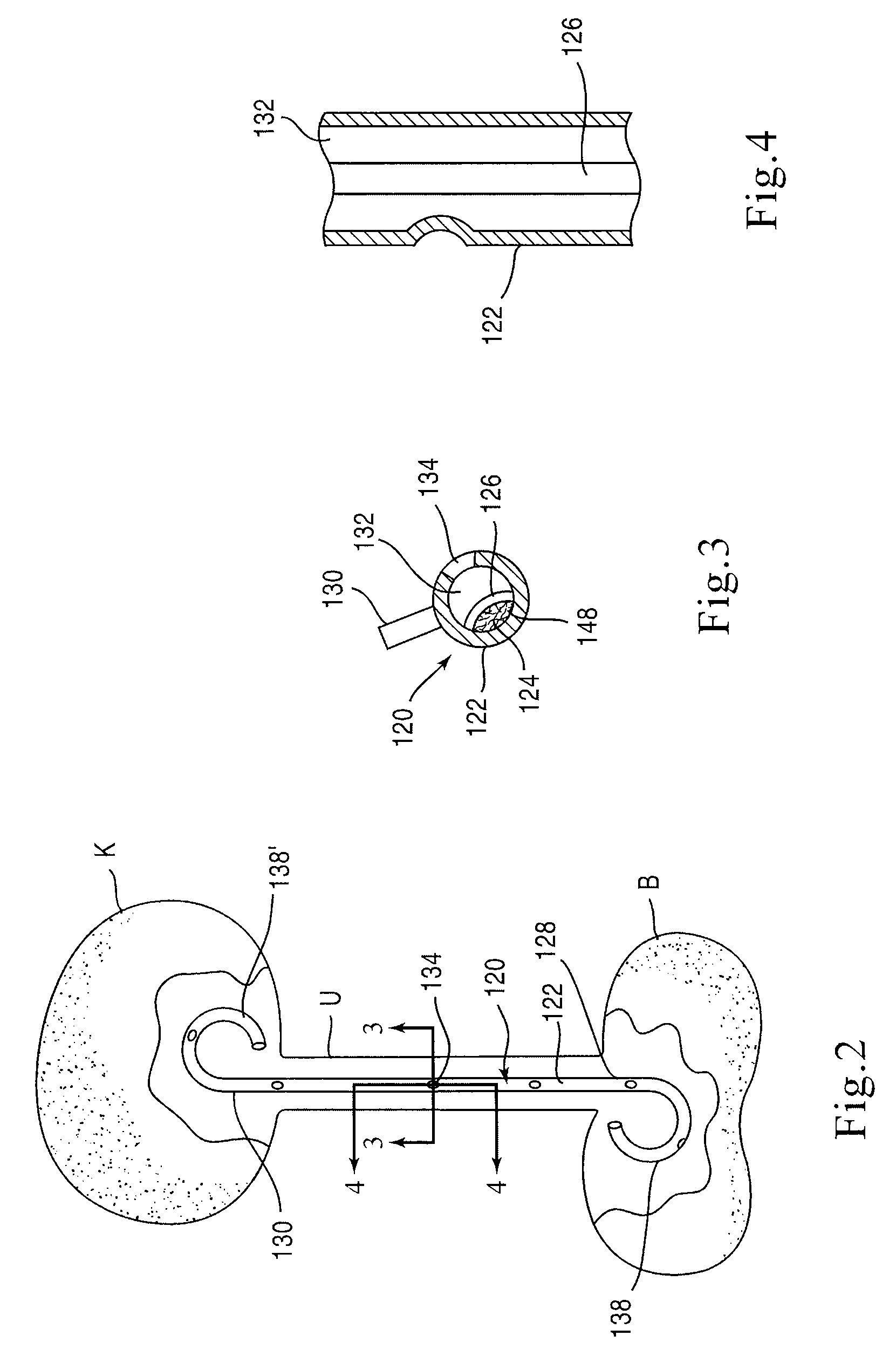
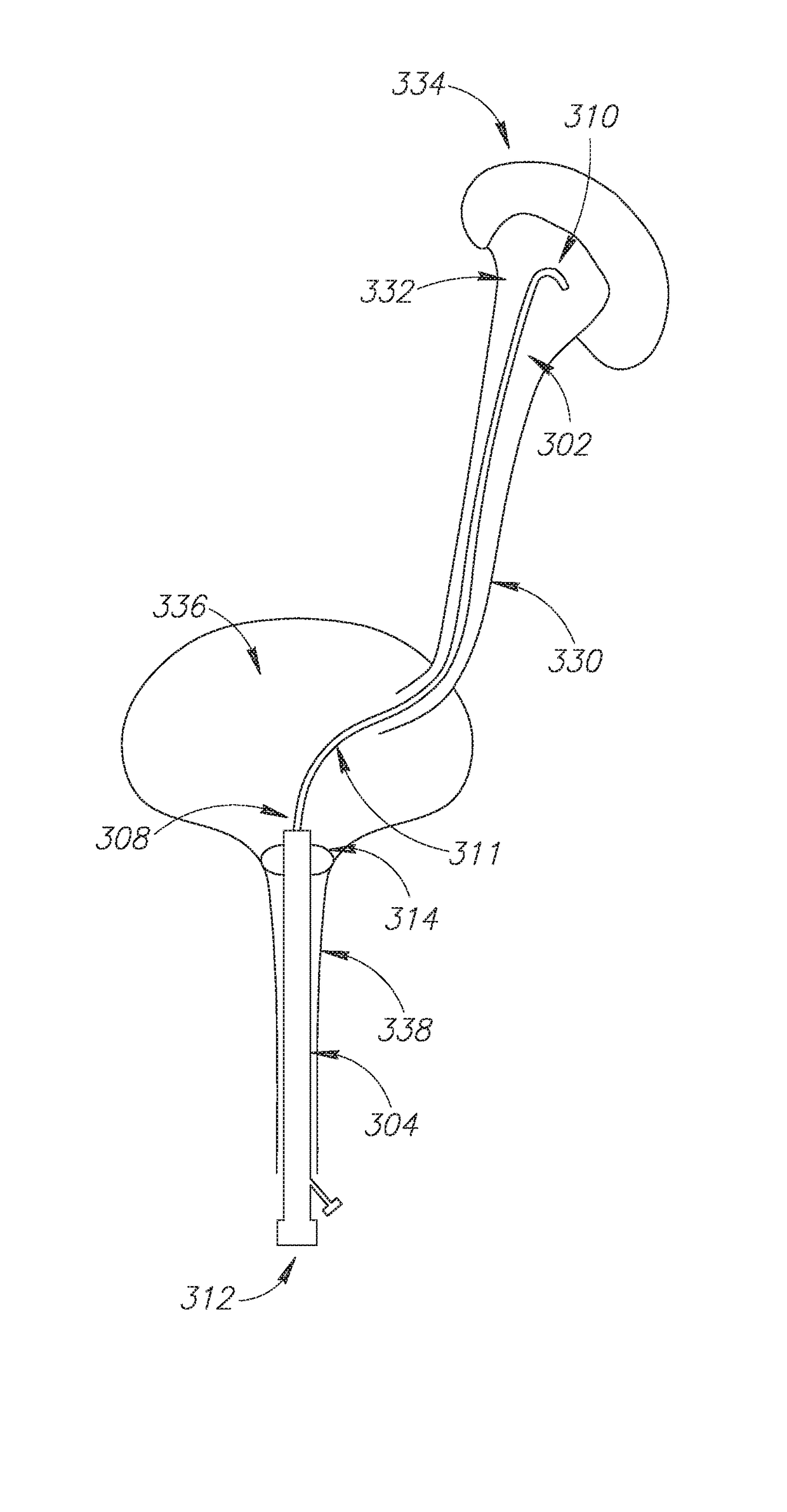
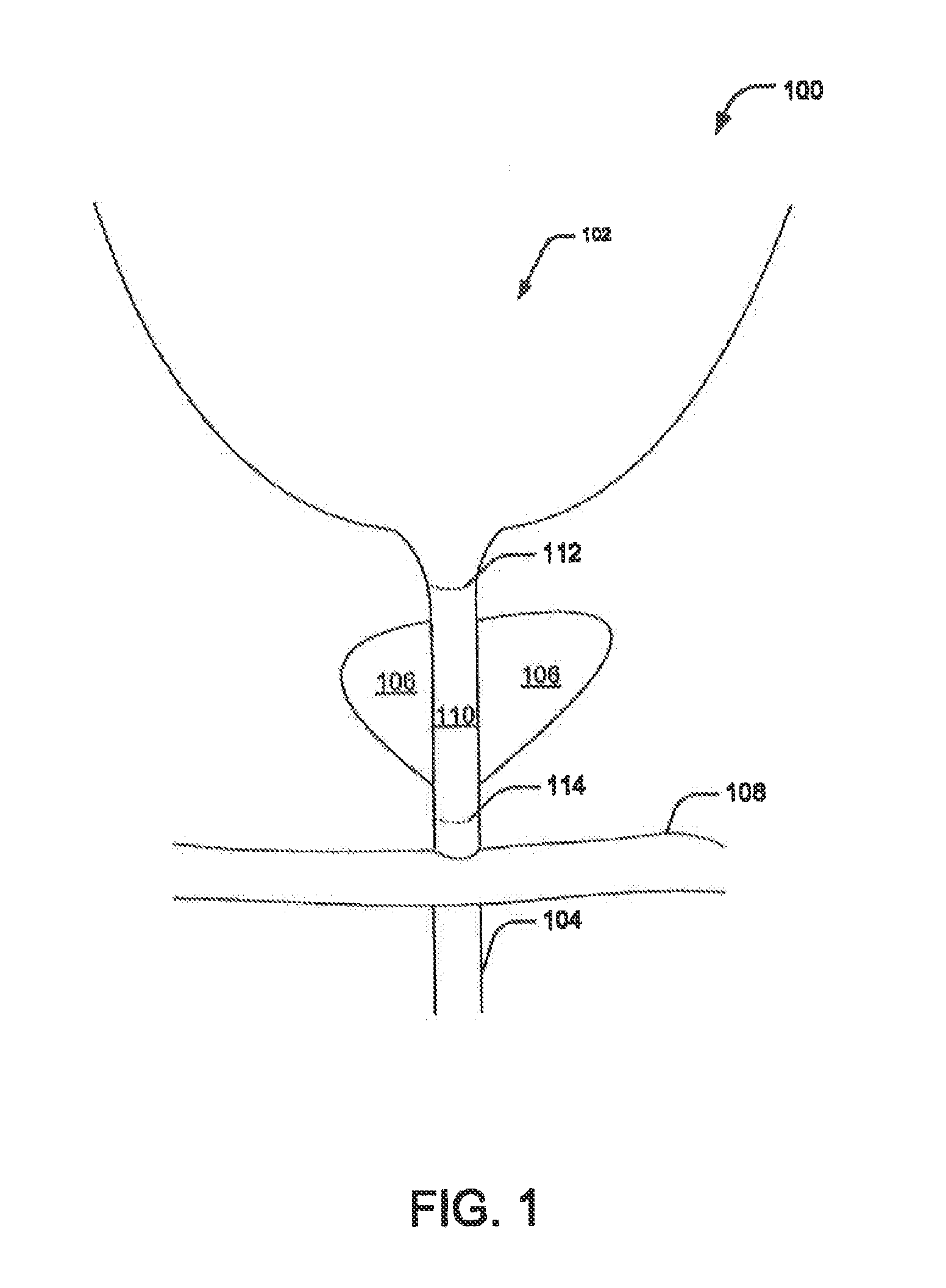
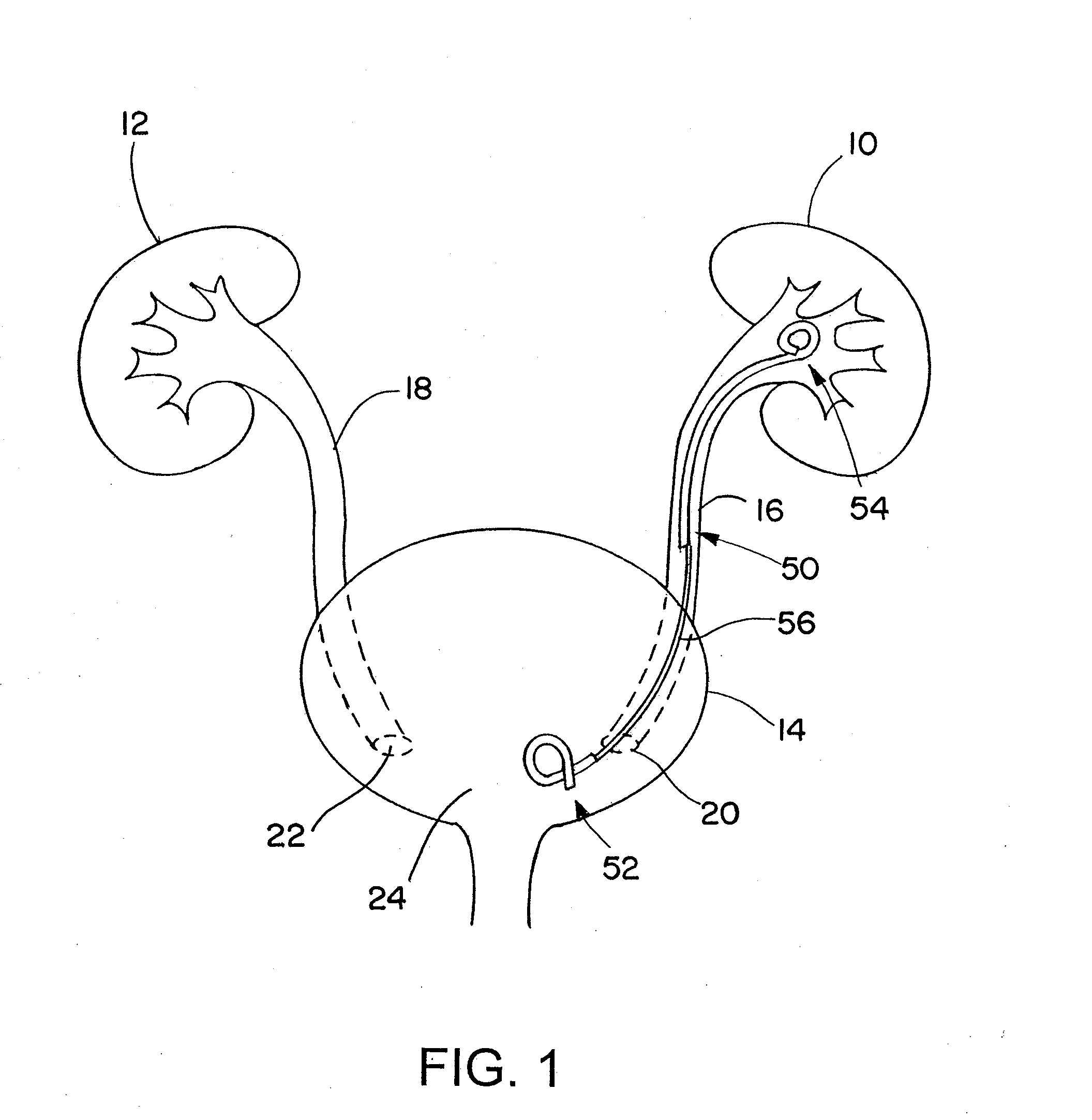
Provided is a ureteral stent (=50) including a bladder portion (=52) positioned in a bladder of a patient, a kidney portion (=54) positioned in a kidney and ureteral passageway of the patient, and one or more tethers (=56) coupling the bladder portion to the kidney portion. The ureteral stent allows urine to pass around a blockage, and allows a ureter orifice connecting the ureteral passageway to the bladder to move between a compressed state and an uncompressed state to prevent or minimize urinary reflux, flank pain, blood in the urine, etc., while allowing the bladder portion to move freely in the bladder to prevent the bladder portion from irritating the trigone muscle.
Owner:UNIV HOSPITALS HEALTH SYST
Essentially tubular body passage lengthening device and method
InactiveUS20050010191A1Easily adjustable externallyExtension of timeBiocideDiagnosticsStomaIntestinal structure
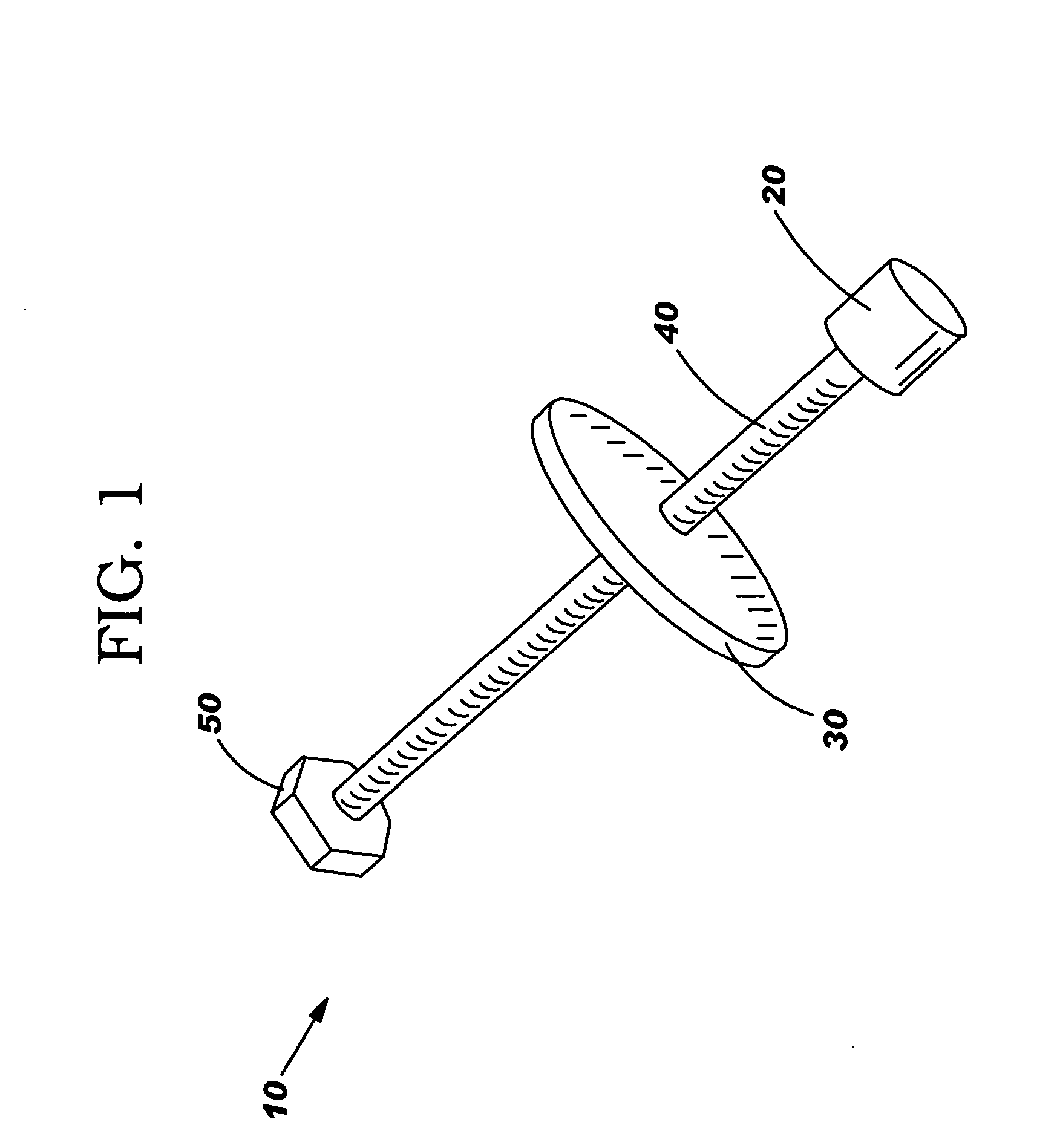
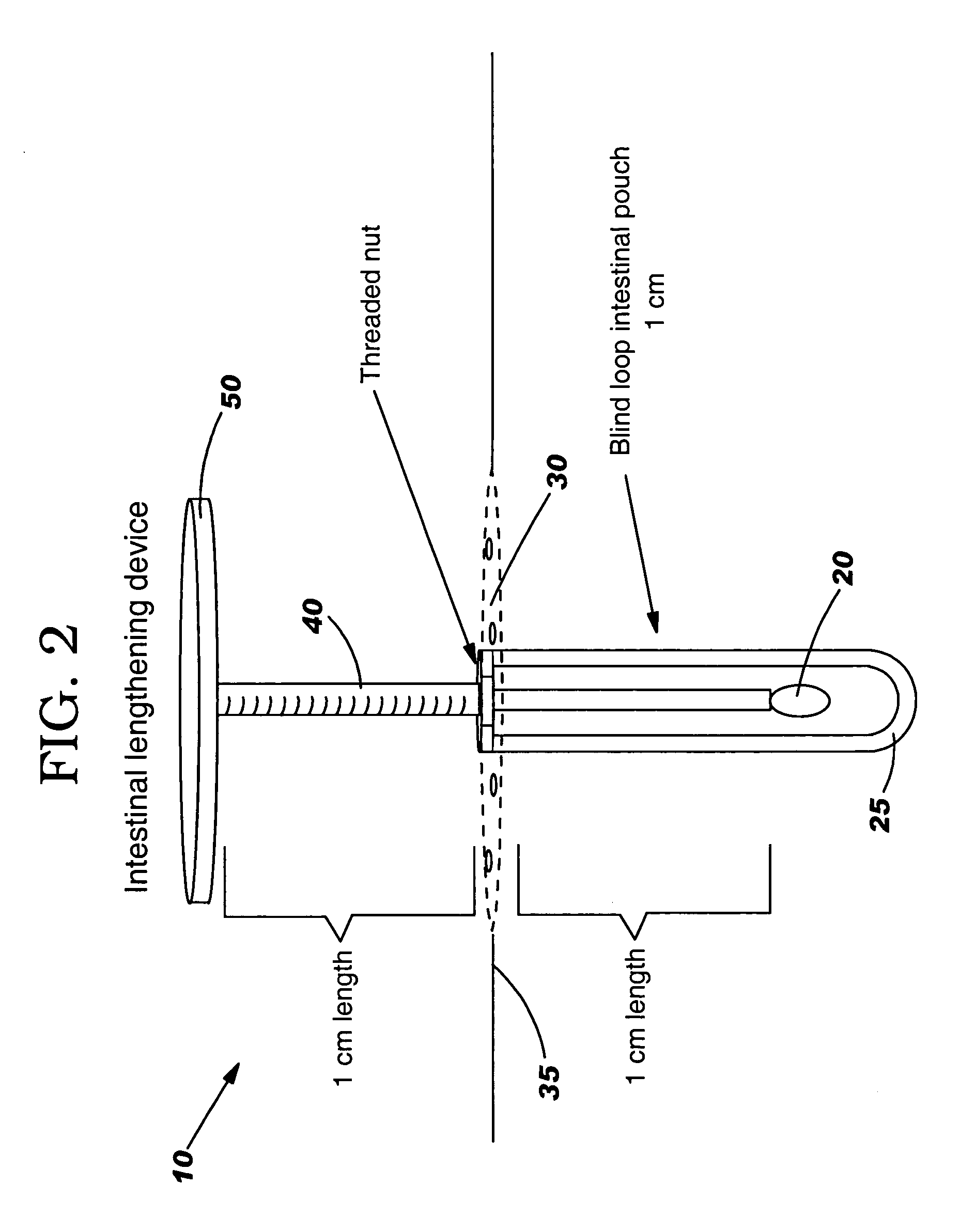
An intestinal lengthening device and method for lengthening the intestine of infants and neonates. The method steps include: resecting a section of intestine to be lengthened; closing the one end of the intestine section, preferably the proximal end, and attaching the other end to an opening in the abdominal wall to form a fistula with stoma; allowing the attachments to heal; attaching the intestinal lengthening device; applying and maintaining 5-20 kPa of tension to the device; allowing the gut to rest for 7 days when the desired length is reached; removing the intestinal lengthening device; reattaching the intestinal section to the intestine. The device includes an inserted end that attaches to the mucosal surface of an intestinal section, an abdominal end that attaches to the abdomen of the patient, and a tension rod to incrementally increase the distance between the two ends. An in situ device includes a sealed tension rod between 2 expansion tips.
Owner:SKINNER MICHAEL A +1
Ureteral stent with end-effector and related methods
InactiveUS20060015190A1Less irritatingReduce retentionStentsBalloon catheterControl releaseInsertion stent
A system and related methods for maintaining the patentcy of the ureter comprising a pusher tube having a pusher tube lumen and an inflate lumen disposed within a wall of the pusher tube and a urinary stent having a proximal and distal portions with an elongated body portion therebetween configured to fit the ureter of the patient and defining a lumen. The system further includes an end-effector that may comprise an inflatable balloon positioned at the proximal portion of the urinary stent for retaining the proximal portion in the urinary bladder. At the distal portion, a retention end-piece is positioned for retaining the distal portion of the stent in the renal pelvis. The end-effector and the retention end-piece of the stent maintain the elongated body portion in situ. The end-effector may also include an inflatable balloon and may contain pharmaceutical or biologic agents for controlled release into the bladder.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com