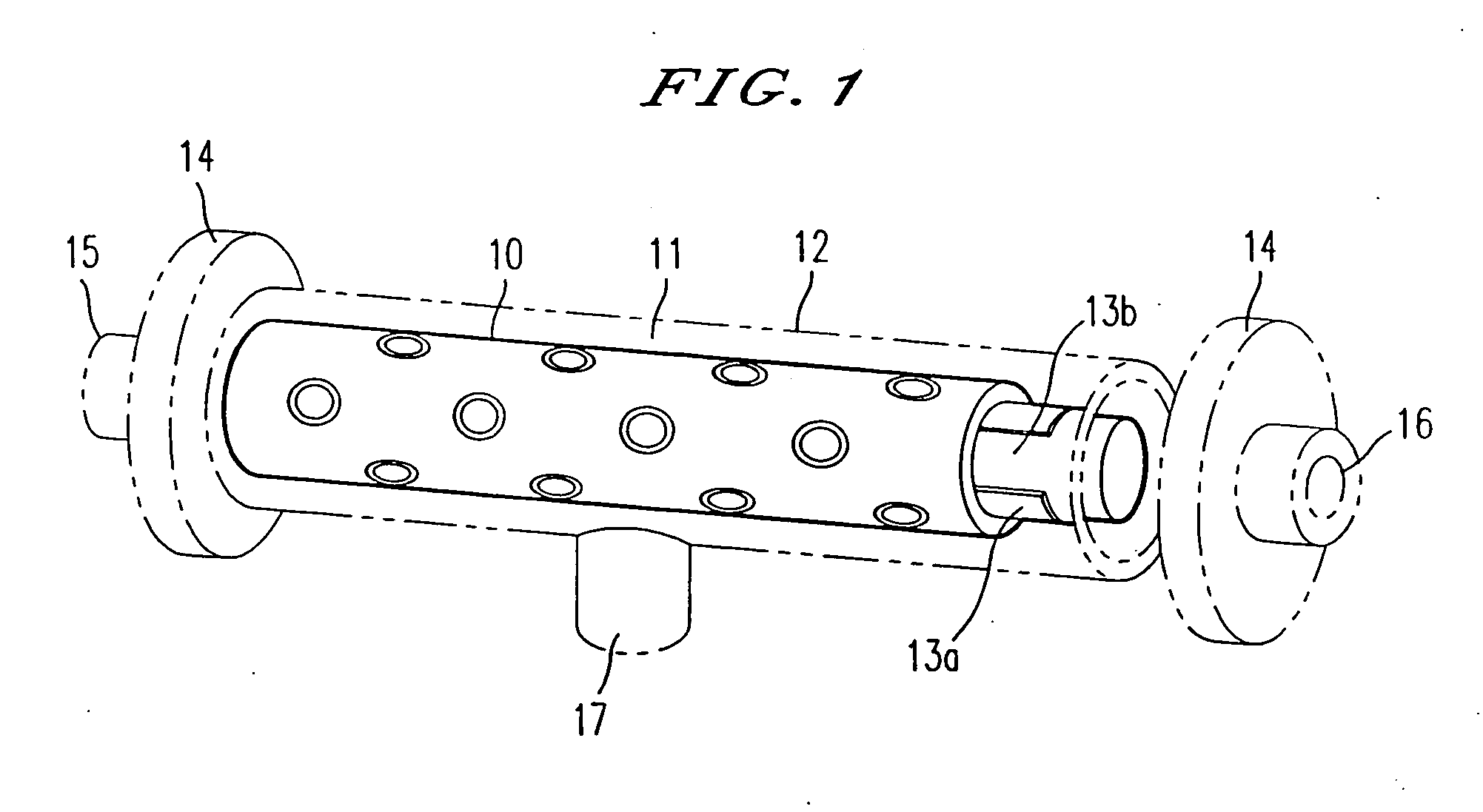
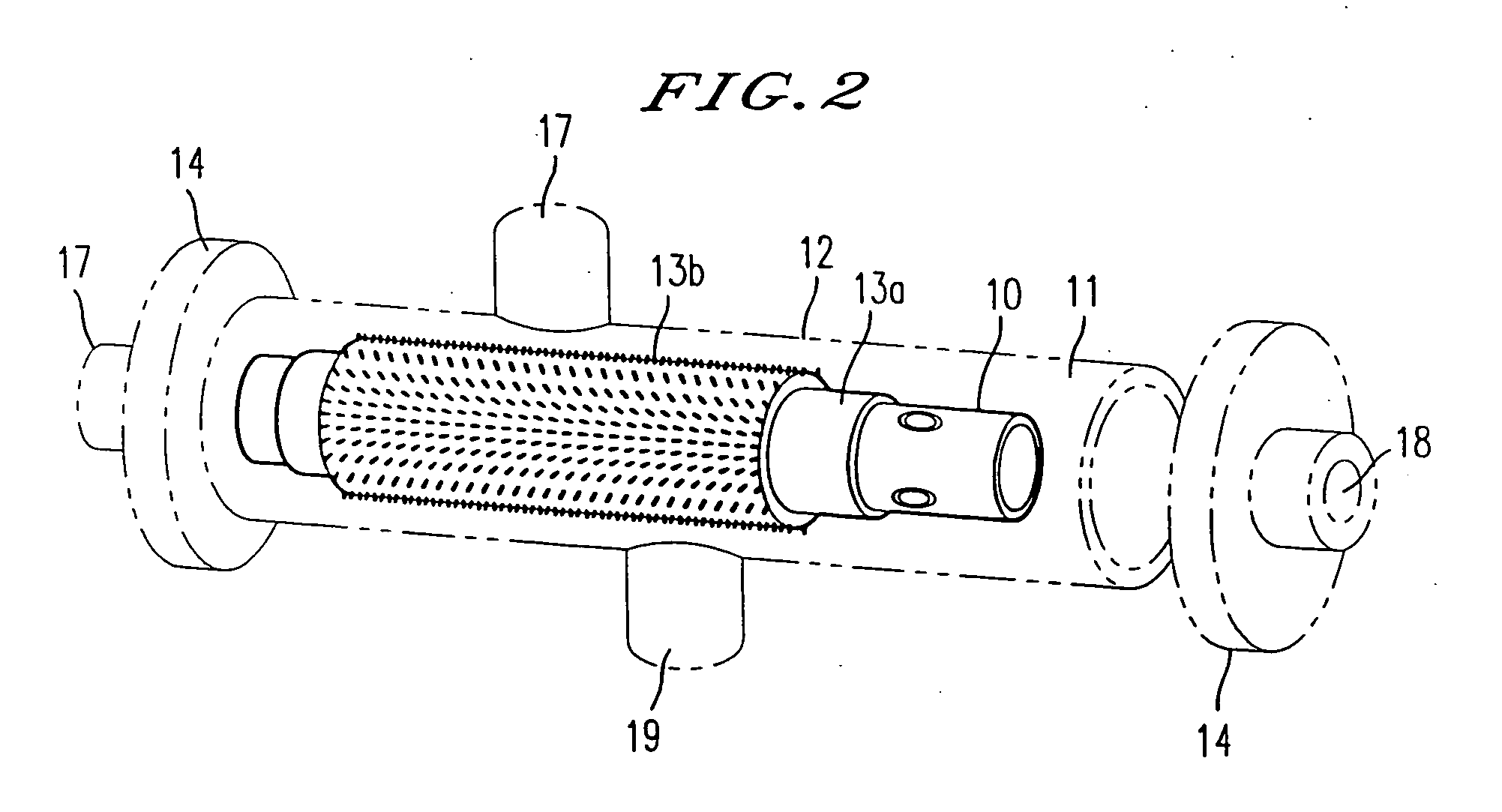
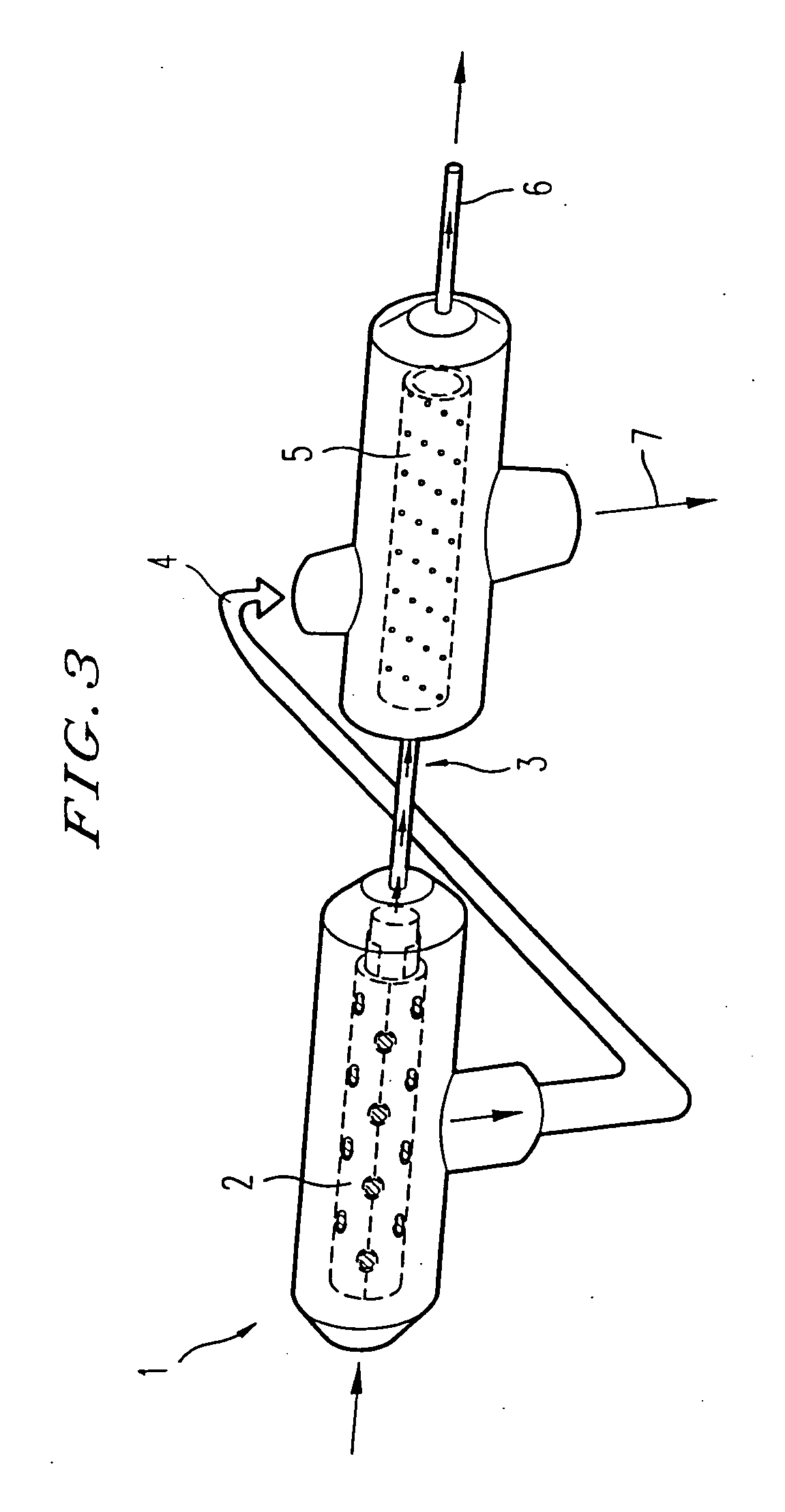
Methods and compositions of bioartificial kidney suitable for use in vivo or ex vivo
a bioartificial kidney and composition technology, applied in the field of bloartiflcial kidney, can solve the problems of insufficient time for primary cells to be used in bioengineering, immortalized cells do not possess the full range of function of primary renal cells,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0080] Endothelial Cell Culture. A permanent human endothelial cell line, designated ECV 304 (Takahashi et al, In vitro Cell Dev. Biol. 25:265-274, 1990), was used for the initial studies for ease of use. To extend the gene transfer and tissue engineering constructs, endothelial cells in primary culture from rabbit vasculature, was also developed.
[0081] ECV-304 Cell Isolation and Culture
[0082] Isolation: ECV-304 (Human Endothelial, transformed) permanent cell line was obtained from American Type Culture Collection (Rockville, Md.). Culture Conditions: ECV-3.04 cultures were maintained on tissue culture plates in a humidified, 37°, 5% CO2 incubator, and grown in media M 199 supplemented with 10% heat inactivated fetal calf serum plus 0.1 % fungi-bact, with media changed twice a week. Cells were subcultured at a ratio of 1:6 by using a solution of 1° endothelial cells 0.25% trypsin, 1 mM EDTA.
[0083] Mesangial Cell Isolation and Culture
[0084] Isolation: Mesangial cells were isolate...
example 2
[0087] Gene transfer of hirudin into endothelial cells. A full length cDNA encoding for the hirudin variant HV-2 (Johnson et al, Seminars in Thrombosis 15:302-315, 1989) has been constructed utilizing dual asymmetric polymerase chain reaction (PCR) in which four adjacent oligonucleotides of 76 to 89 bases in length having short overlaps of 14 bases were used as primers in a PCR mixture (Sander et al, Biotechniques 12:14-16, 1992). In constructing this cDNA, a signal sequence for von Willebrand factor (vWF) was—incorporated in frame 5′ to the hirudin gene to ensure secretion of hirudin from transduced cells; protein coding sequences were selected based upon optimal codon usage in rabbit and human genetic sequence data (Wada et al, Nucl. Acids Res. 19:1981-1986, 1991), and appropriate restriction enzyme cut sites 5′ and 3′ to the cDNA encoding the vWF signal peptide and hirudin HV-2 for ease of transfer into the retroviral vector. The sequence of the constructed cDNA was confirmed to ...
example 3
[0090] Improved Retroviral Gene Transfer for Hirudin Production. To improve hirudin protein production and secretion by endothelial cells, a different retroviral construct was developed, as detailed in FIG. 4. In this regard, introduction of a drug-selectable marker that is coexpressed with the hirudin gene was planned. Previous strategies have been employed for the coexpression of drug-selectable genes with a second nonselectable gene with the utilization of independent promoters, such as a retroviral LTR and another promoter (Emerman et al, Cell 39:459467, 1984). With this approach, a replication-defective retroviral vector was constructed capable of efficient constitutive expression of the hirudin gene in mammalian cells by using the LTR promoter. A selectable neomycin (neo) gene, conferring G418 resistance, is under the control of the SV40 promoter. The optimal codon usage in human genetic sequence data was selected to design the hirudin cDNA sequence. A signal sequence for plas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com