MDCK-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
A serum-free medium and suspension culture technology, which can be used in biochemical equipment and methods, medical preparations containing active ingredients, viruses, etc., and can solve the problems of tumorigenicity danger and complex culture process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare the cell line derived from MDCK by serum-free culture and suspension culture
[0034] CCL-34 in MDCK cell line provided by ATCC. In T-25 flasks, in EMEM medium supplemented with 10% serum at 37°C and 5% CO 2 CCL-34 was cultured under. After cell expansion, cells were cultured in a medium consisting of EMEM medium and serum-free medium (50%). During the culture process, confirm whether the growth of the cells is normal. When normal cell growth was confirmed, the growing cells were cultured in medium containing serum-free medium (75%). Repeat the process to obtain cell lines adapted to serum-free medium (100%). EX-CELL MDCK (Sigma), UltraMDCK (Lonza) and VP-SFM (Invitrogen) can be used as medium for serum-free culture.
[0035] Cell lines adapted to serum-free media are fully expanded in T-flasks. Then, in a spinner bottle (Corning) at 37 °C and 5% CO 2 The expanded cell line was adapted to suspension culture with agitation at a rate of 40-80 rpm. When th...
Embodiment 2
[0036] Evaluation of Cell Line Proliferation Potential
[0037] The prepared MDCK-derived cell lines cultured in a serum-free medium were cultured under the conditions shown in Table 1. The proliferative potential of MDCK-derived cell lines was assessed. MDCK cell line (ATCCCCL-34) was cultured in medium supplemented with 10% serum as a negative control.
[0038] Table 1
[0039] [Table 1]
[0040] [surface]
[0041]
[0042] At the beginning of the culture, the cell culture concentration was about 1.0×10 5 cells / ml. When the cell concentration reaches about 1×10 6 cells / ml or after 3-4 days of culture, the cells were passaged. Adjust the cell concentration at the initial stage of subculture to 1×10 5 cells / ml.
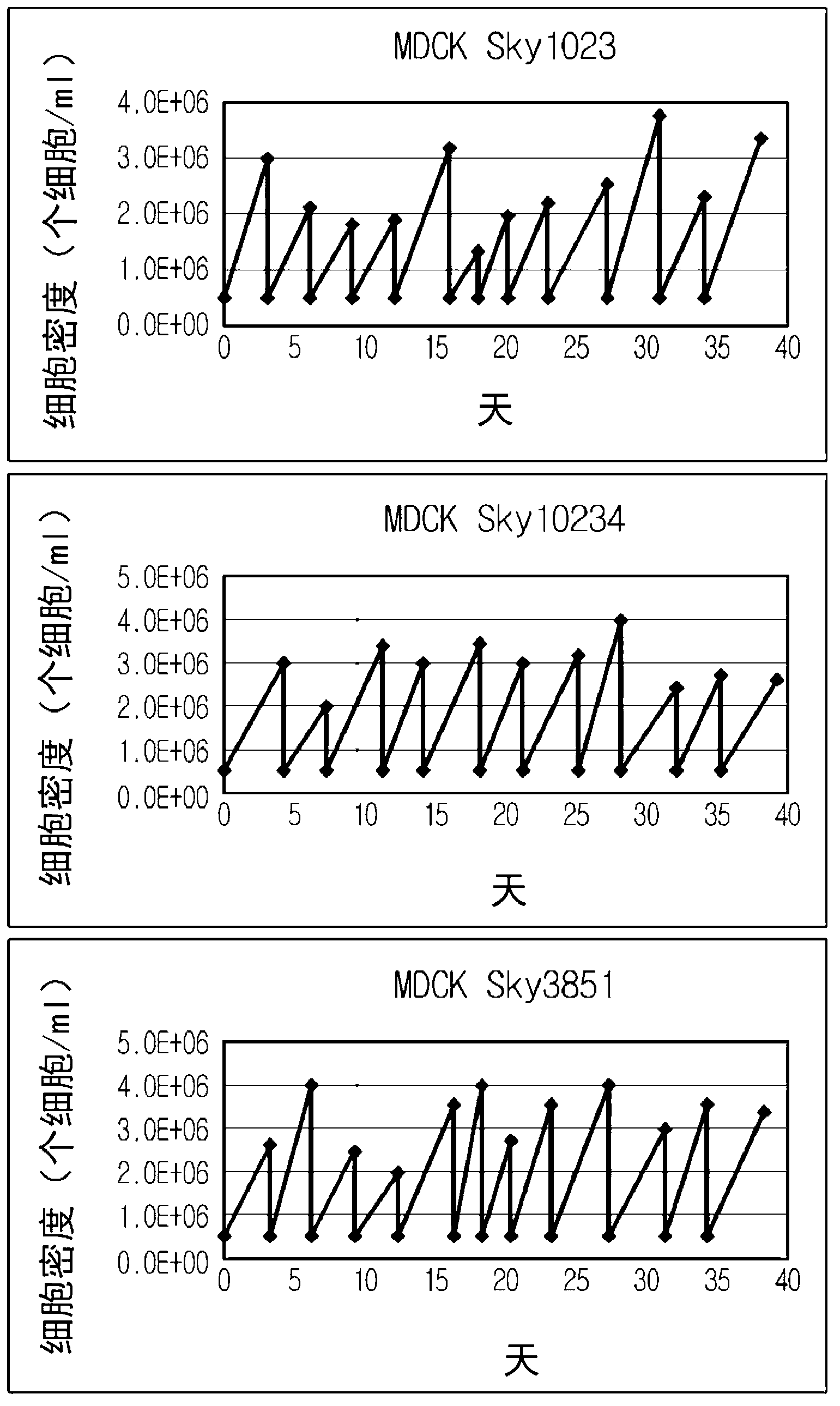
[0043] Each of the MDCK-derived cell lines grown in serum-free media exhibited comparable cell growth rates to MDCK cells grown in serum media.
Embodiment 3
[0044] Evaluation of proliferation curve and passage stability of cell lines
[0045] After adaptation to serum-free culture and suspension culture, the three cell lines were further cultured in spinner flasks, and their proliferation curves and passage stability were evaluated. Adjust the concentration of cells at the initial stage of culture to approximately 4×10 5 cells / ml. After about 3-4 days of culture, the cell concentration reached about 2×10 6 cells / ml or more. Culture was performed under the following conditions. The result is as figure 1 shown.
[0046] Starting cell concentration: 4 x 10 5 cells / ml
[0047] Culture scale: 50ml spinner bottle
[0048] Spinner rotation rate: 60rpm
[0049] Culture conditions: 37°C, 5% CO 2 , wet
[0050] Subculture conditions: 3-4 days after culture
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com