Vaccine composition for porcine circovirus and swine influenza and its preparation method and use
A technology of porcine circovirus and vaccine composition, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation, identification and purification of porcine circovirus and swine influenza virus H1N1 fusion antigen
[0070] 1.1 PCR amplification of PCV2Cap gene
[0071]Using the gene sequence of the PCV2-SH strain (accession number: HM038027) in GeneBank (see the sequence table SEQIDNo.1 for details) as a template, a pair of primers (702bp, 233AA) were designed with the software PrimerPremier5.0 for amplifying PCV2- Cap gene, its two ends add BamHI and PstI restriction site respectively, under (PH) promoter, primer sequence is as follows:
[0072] PCV2-Cap-BamHI: CGGGATCCATGACGTATCCAAGGAGGC
[0073] PCV2-Cap-PstI: AACTGCAGTTAAGGGTTAAGTGTGGGGGC
[0074] The parameters of the PCR reaction are: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 50 s, annealing at 54.3°C for 30 s, extension at 72°C for 45 s, and after 35 cycles, extension at 72°C for 5 min; The product was identified, and PCV2-Cap gene DNA was recovered and purified with DNA Fragment ...
Embodiment 2
[0094] Example 2 Preparation, identification and purification of porcine circovirus and swine influenza virus H3N2 fusion antigen
[0095] 2.1 Sequence selection and primer setting
[0096] Using the gene sequence in GeneBank (accession number: CY107035.1) (see the sequence table SEQIDNo.5 for details) as a template, use the software PrimerPremier5.0 to design a pair of primers (663bp, 221AA) for amplifying the H3N2-HA2 gene. SmaI and KpnI restriction sites were added to both ends, located under the P10 promoter, and the primer sequences are as follows:
[0097] H3N2-HA2-SmaI upstream:
[0098] CCCCCGGGGGCATATTCGGCGCAATCGCAGGT
[0099] Downstream of H3N2-HA2-KpnI:
[0100] GGGGTACCAATGCAAATGTTGCATCTAATGTTG
[0101] The parameters of the PCR reaction are: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 50 s, annealing at 53.8°C for 45 s, extension at 72°C for 40 s, and after 35 cycles, extension at 72°C for 5 min; The product was identified, DNA was recovered ...
Embodiment 3
[0105] The preparation of embodiment 3 porcine circovirus, swine influenza virus vaccine composition
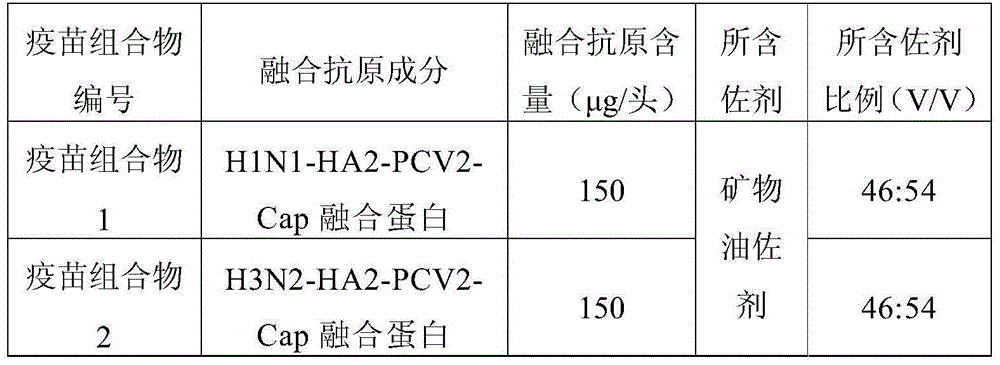
[0106] The porcine circovirus and porcine influenza virus fusion antigens prepared in Example 1-2 were diluted with PBS solution of pH 7.4 respectively, and the fusion antigens and mineral oil adjuvant diluted respectively were prepared as shown in Table 1, with 500 Stir at a speed of -800r / min for 10-15min, add thimerosal solution with a volume ratio of 1% before stopping the stirring, so that the final concentration does not exceed 1 / 10,000, fully oscillate and mix well, which is the vaccine composition 1, the vaccine composition Object 2. Store it at 2-8°C after aliquoting.
[0107] Table 1 swine influenza virus, porcine circovirus vaccine composition ratio
[0108]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com