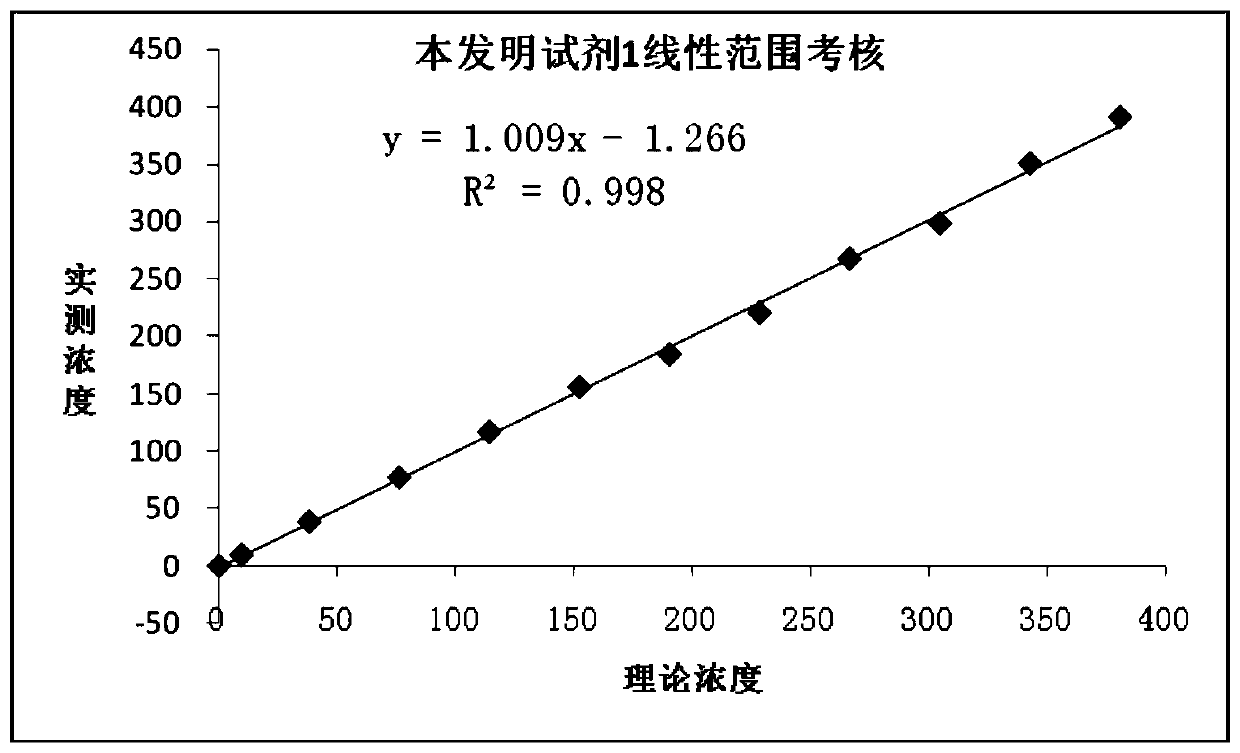
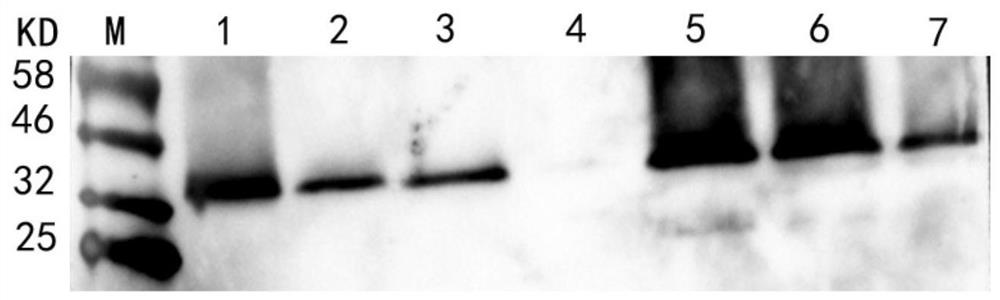
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Antigen test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In an antibody assay, the sample is taken and mixed with a material containing an antigen. Lab tests can be run on blood and urine samples to check for antibodies. Since antigens react to antibodies, specific antibodies can be used to test for the presence of infectious organisms.
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
InactiveCN101726596ASimple and fast operationHigh sensitivityBiological testingLuminescent compositionsCelluloseHepatitis B virus
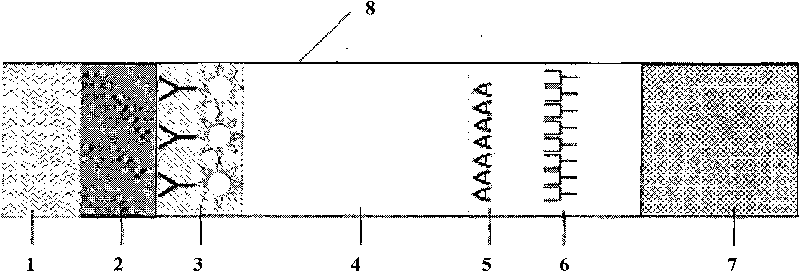
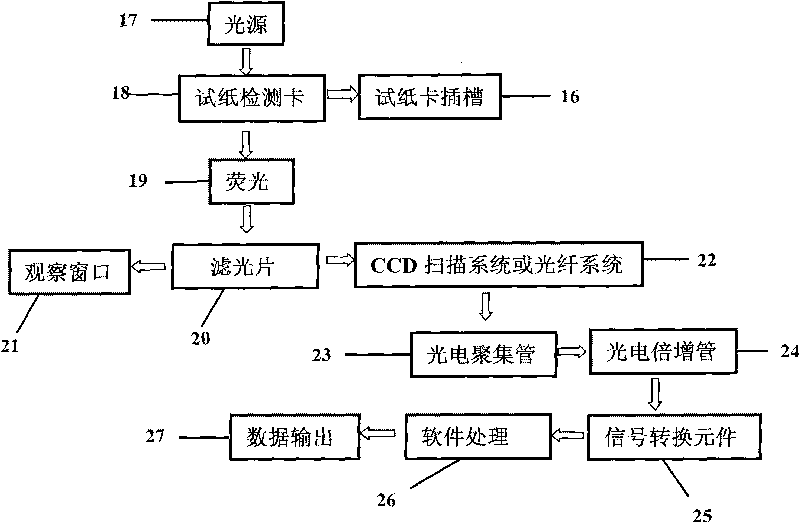
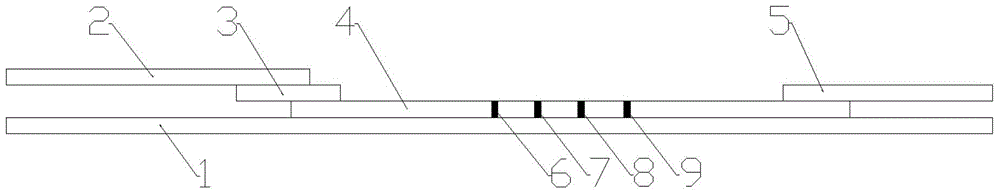
The invention discloses a fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and a method for preparing the same. The testing card comprises a hepatitis b surface antigen test paper strip, a hepatitis b e surface antigen test paper strip, a hepatitis b surface antibody test paper strip, a hepatitis b e surface antibody test paper strip, and a hepatitis b core antibody test paper strip. Each test paper strip is formed by overlapping and bonding filter paper, a sample pad, a glass fiber film spray-coated with fluorescent microspheres, a cellulose nitrate film and water absorption paper on a bottom plate by glue in sequence, wherein the cellulose nitrate film is coated with antigens serving as a testing area and anti-rabbit antibodies serving as a quality control area; and during a test, after emitted fluorescent light passes a filter, the emitted spectrum is collected, accumulated and multiplied by the CCD scanning technology and then converted into a numerical signal, the numerical signal is multiplied by a correction factor, and the strength of the corrected fluorescent light is substituted in a standard curve of a fluorescence analyzer, so that the concentrations of the five indexes of hepatitis b of the sample can be automatically worked out. The test of hepatitis b viruses by the testing card has the characteristics of specificity, sensitivity, simpleness and accuracy.
Owner:WUXI ZODOLABS BIOTECH
Mycobacterium tuberculosis antibody rapid diagnosis reagent kit and detecting method thereof
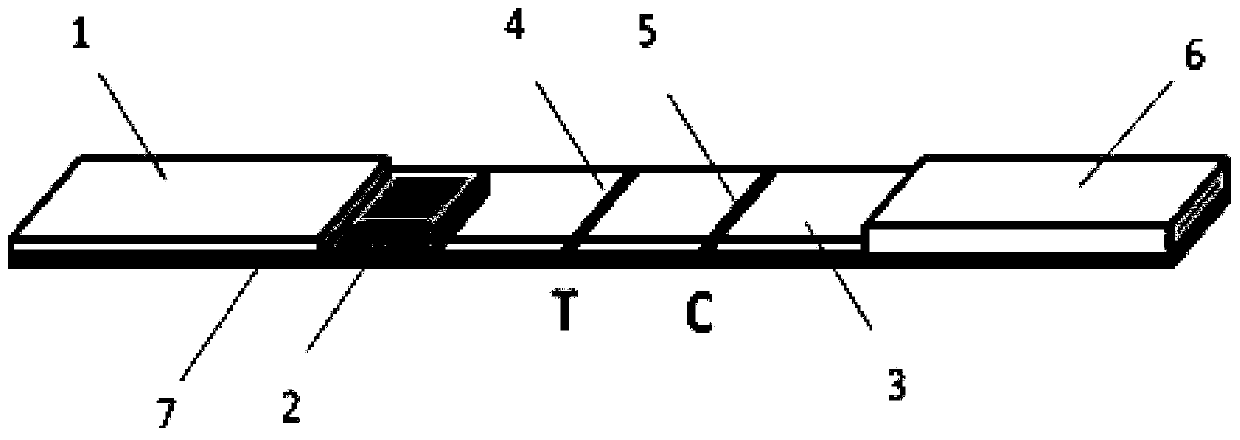
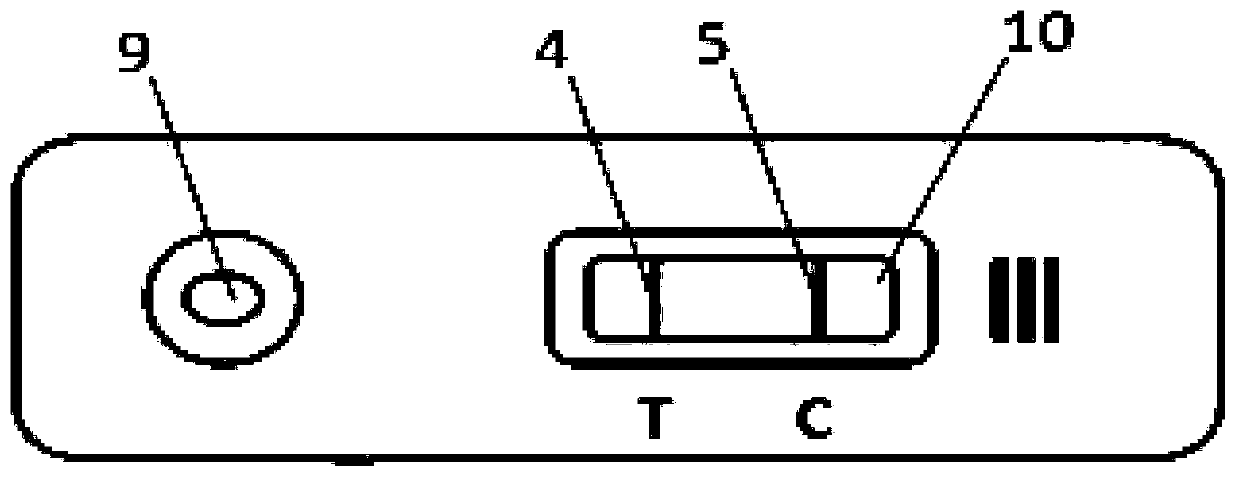
The present invention relates to a method to prepare a reagent kit to quickly diagnose mycobacterium tuberculosis antigens jointly packaged by 38KD and 16KD antigens and a test method. The blood antigen test paper of the present invention jointly packages 38 KD and 16KD of TB antigens into a lower part of a NC film to form a test line (T). Goat anti-mouse IgG is packaged into an upper part of the NC film to form a quality control line (C). In addition, anti-human IgG (mouse anti-human IgG, goat anti-human IgG or SPA) marks nanometer coloring particles (colloidal gold particles or sol particles or nanometer beads) to prepare a labeling pad. The NC film, the labeling pad, a sampling pad and a sample adsorption pad are glued and assembled onto a plastic board to form the test paper. During test, few tested sample is added onto the NC film of the test paper. And then, sample diluents are added through a sampling end. After sampling, it is necessary to observe result of immunoreaction, thus realizing quick auxiliary TB antigen diagnosis.
Owner:天津中新科炬生物制药股份有限公司
Assaying substrate with surface-enhanced raman scattering activity
InactiveUS20120115245A1Reliably carry-outGood reproducibilityRaman scatteringAnalysis by subjecting material to chemical reactionElectrode potentialAntigen
A metal substrate obtained by agglomerating 5 nm to 100 nm metal nano-particles (including clusters) having SERS activity on a metal substrate having a lower electrode potential (higher ionization tendency) than the electrode potential of the metal nano-particles, and fixing the metal nano-particles in an optimally agglomerated state that acts as hot sites, when a detection specimen is adsorbed in a non-dried state, and a predetermined laser light is irradiated, the surface enhanced Raman scattered (SERS) light of antigen detection specimen can be detected by surface Raman resonance in an optimally agglomerated state.
Owner:MYTECH CO LTD
Visible protein chip for detecting poultry disease serum antibody, its preparation method and application
The invention discloses a visual protein chip for detecting serum antibody of new-castle disease virus of chickens, infectious bronchitis virus of chickens, avian influenza virus and infectious bursal disease virus of chickens , which is prepared by the following steps: purifying and diluting whole proteins of the four virus respectively; pointing samples of the positive control serum, the negative control serum and the four virus proteins onto a chip carrier respectively; drying, fixing, sealing and washing the samples to obtain the visual protein chip. The visual protein chip uses the purified whole proteins as capturing antigens to detect the virus-specific antibodies in chicken serum so as to simplify the preparation technology and reduce the production cost, and the visual protein chip has better specificity but no cross, has high reliability of results and has the advantages of quickness, simplicity and convenience, high sensitivity, good specificity and the like. When the serum is diluted by 6,400 times, the visual protein chip still can detect the antibodies, the sensitivity is 400 times of that of the prior AGP detection method. According to the detection to serum samples in-place, the detection rate of the visual protein chip is higher than the proir AGP method remarkably.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol and preparation method thereof
ActiveCN104237521AAchieving Simultaneous DetectionRapid Field DetectionMaterial analysisCellulosePolyvinyl chloride
The invention provides a three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol and a preparation method thereof, and belongs to the technical field of immunological detection. The preparation method comprises two parts, namely, the preparation of a monoclonal antibody and the preparation of a colloidal gold chromatographic test strip, wherein the monoclonal antibody can be used for recognizing thiamphenicol, chloramphenicol and florfenicol at the same time and is high in sensitivity; the colloidal gold chromatographic test strip comprises a polyvinyl chloride backing; a sample pad is arranged at the front end of the polyvinyl chloride backing and is connected with the front end of a nitrocellulose membrane; the rear end of the nitrocellulose membrane is connected with a water absorbing pad; the monoclonal antibody marked by colloidal gold is used as a combining pad; the nitrocellulose membrane is sequentially wrapped with a chloramphenicol sodium succinate-BSA (Bovine Serum Albumin) antigen test ray T and a goat-anti-mouse IgG control ray C. The three-in-one colloidal gold chromatographic test strip for detecting thiamphenicol, chloramphenicol and florfenicol is fast to detect, and the detection needs only 3 to 5 minutes; the test strip is convenient to carry, and suitable for detection on site; the operation is simple and convenient and does not need a professional technical person.
Owner:JIANGNAN UNIV
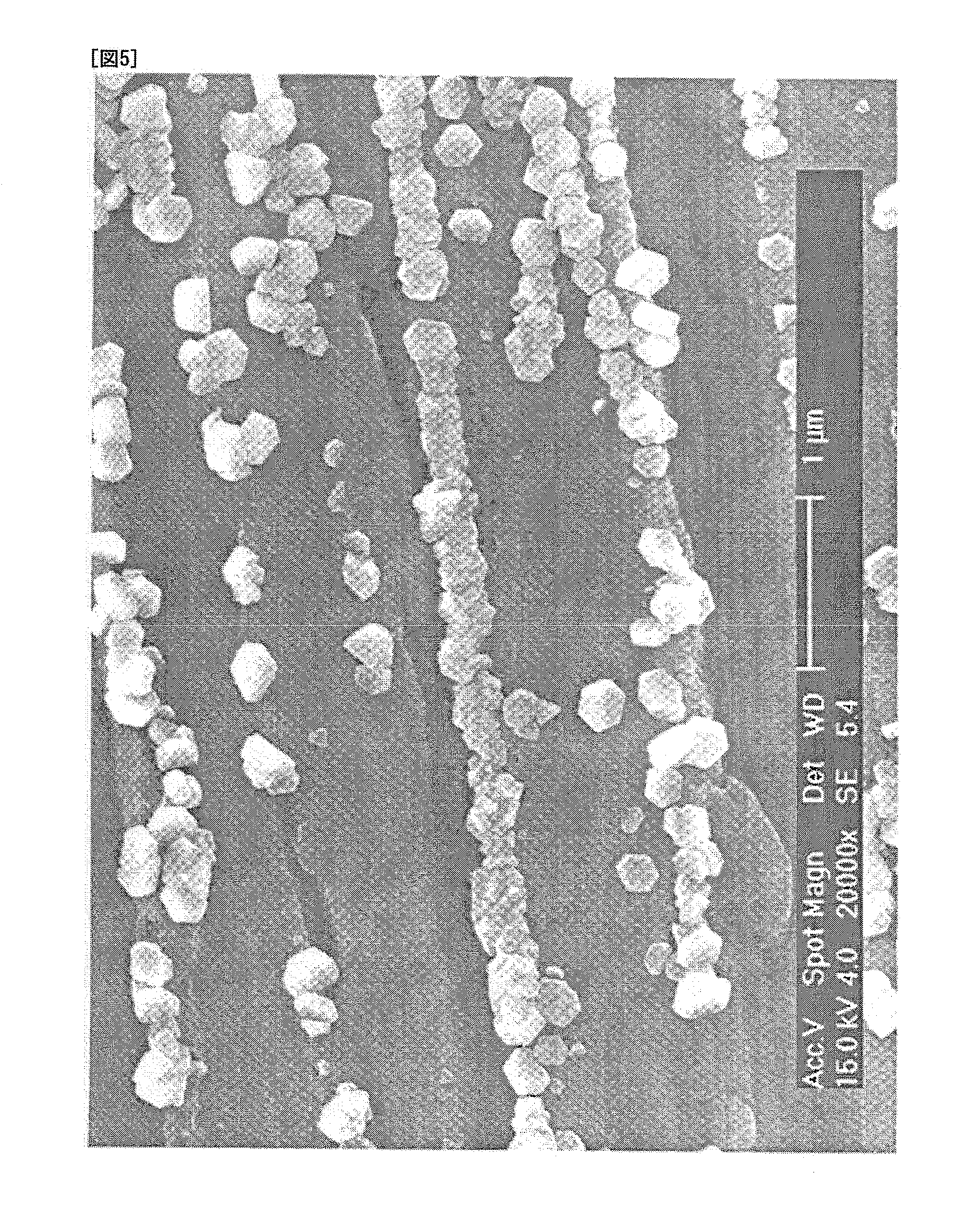
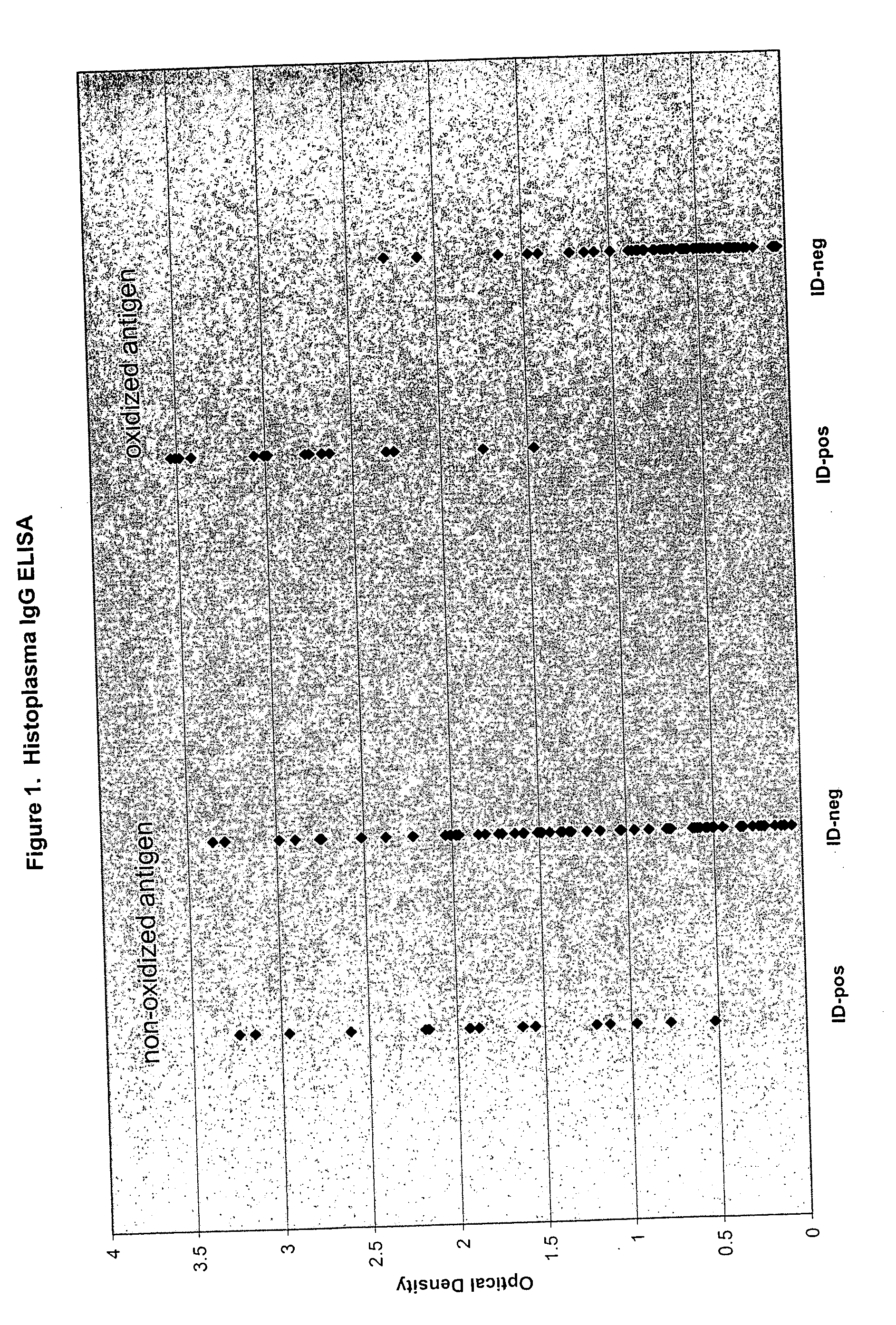
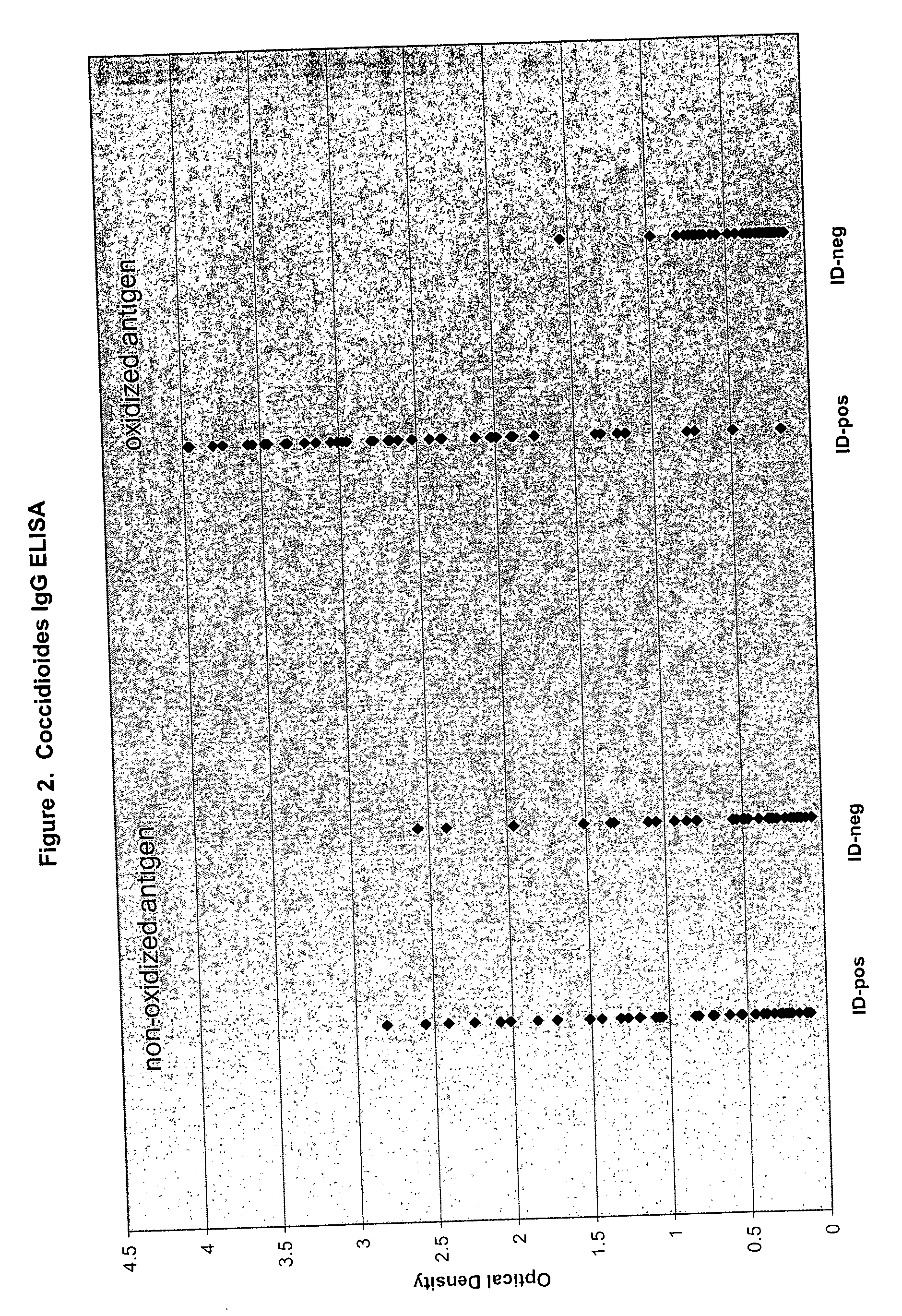
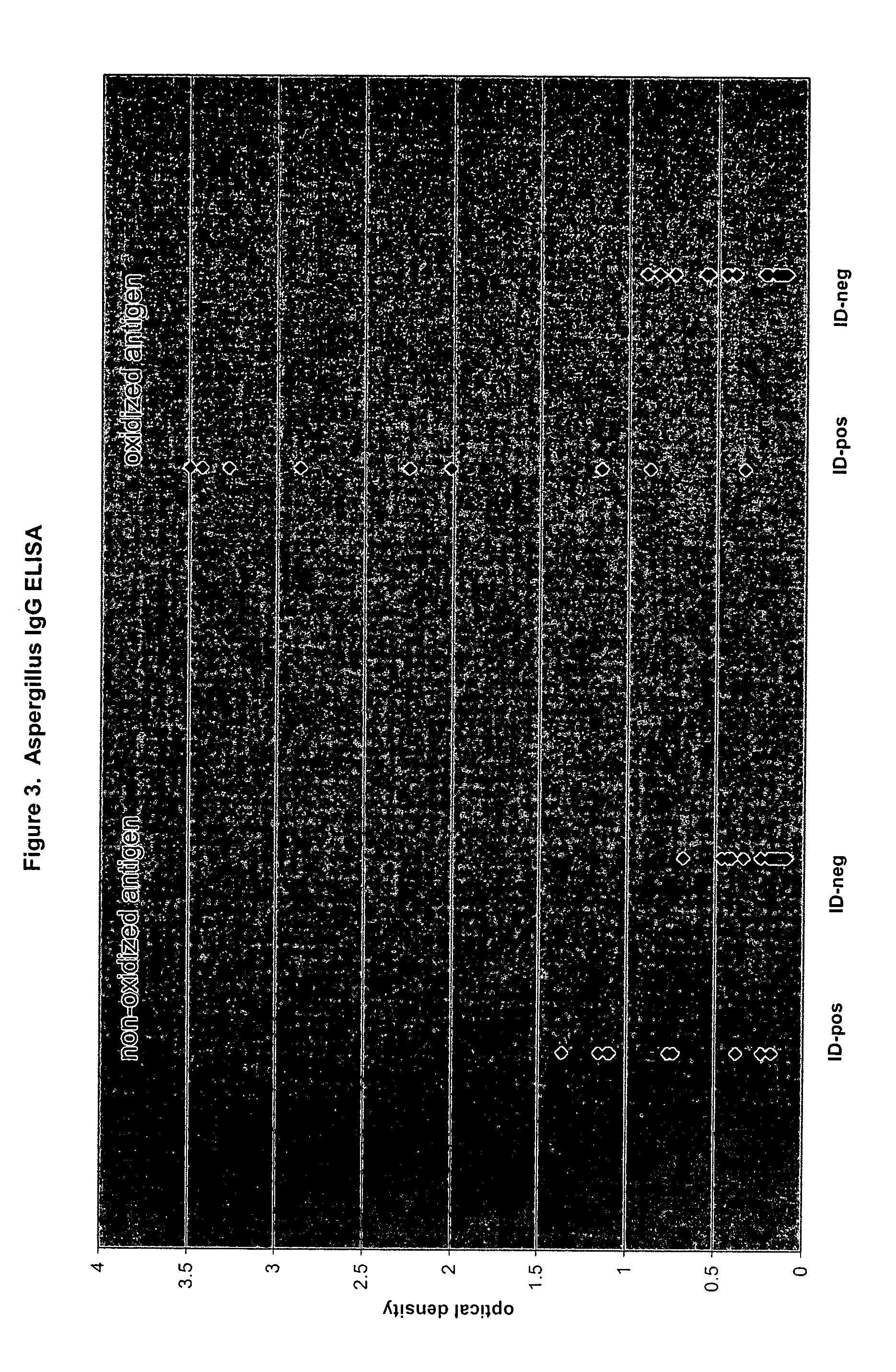
Oxidized fungal antigens and methods of making and using thereof
Owner:FOCUS TECH
Reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies
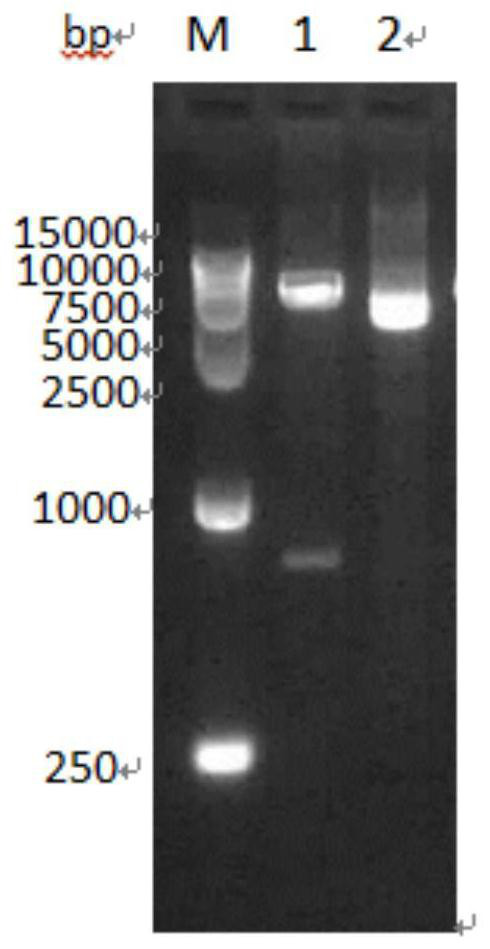
The invention relates to a reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies and belongs to the technical field of hygiene inspection and medical inspection. The reagent kit is characterized by comprising the steps of: 1, primer design; 2, polymerase chain reaction (PCR) amplification; 3, agarose gel electrophoresis reaction conditions; 4, target deoxyribonucleic acid (DNA) segment cutting; 5, heat shock conversion method application; 6, reagent kit purification and plasmid preparation by a sodium dodecyl sulfate (SDS) cracking method; 7, protein induction expression; 8, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE); 9, required strip cutting from the SDS-PAGE; and 10, colloidal gold preparation. The regent kit has the beneficial effect that the limitation aiming at the single-clostridium and single-antigen test in the prior art is avoided. The antibody is pure, the valence is high, and the sensitivity and the accurate degree are improved. The single added Listeria bacterium is detected. Compared with an enzyme-linked immuno sorbent assay (ELISA) method and a PCR method, the reagent kit has the advantage that the Listeria bacterium detection is faster and more convenient by the immune colloidal gold chromatography.
Owner:何鑫 +4
Cryptosporidium parvum recombinant antigen for diagnosis
InactiveCN103740733AImprove featuresGenetic engineeringFermentationProtein detectionCryptosporidium parvum
The invention discloses a cryptosporidium parvum recombinant antigen gene (i) MUCIN ( / i) and an expressed fusion protein recombinant antigen rMUCIN. 520 parts of healthy human serum are randomly taken, the recombinant antigens rMUCIN and rCp23 are used as detection antigens, a human serum IgG antibody is detected by ELISA (enzyme-linked immunosorbent assay), spss software is used for analysis of data, and the results show that the rMUCIN protein and the rCp23 protein have the same positive rate in detection of unknown serum (XC2=0.370, and P=0.543>0.05), while the positive rate of the rMUCIN protein in detection of the unknown serum is higher than that of a cryptosporidium parvum crude antigen (XC2=5.222, and P=0.02<0.05). The invention further provides a kit for ELISA detection of the anti-cryptosporidium parvum IgG antibody, which takes the recombinant antigen rMUCIN as the detection antigen, takes the rCp23 as a positive control and has stronger specificity, sensitivity and reliability.
Owner:JILIN UNIV
Sample dilution composition for detecting sugar antigen 72-4, detection reagent and kit containing detection reagent
InactiveCN110824175AShorten the timeEliminate distractionsBiological material analysisBiological testingDiseaseEnzyme binding
The invention relates to the field of medical examination, and particularly relates to a sample dilution composition for detecting a sugar antigen 72-4, a detection reagent and a kit containing the detection reagent. The kit mainly comprises a magnetic particle suspension coated with a CA72-4 antibody, an enzyme conjugate, a sample diluent and a calibrator. The sample dilution composition can be used for auxiliary diagnosis and postoperative monitoring of tumor diseases such as gastric cancer, ovarian cancer, colorectal cancer, pancreatic cancer and cervical cancer. The kit is short in detection time, and the time of an inspector is greatly saved; the kit adopts a double-antibody sandwich method, is wide in linear range and high in clinical practicability; the kit adopts the specific sample diluent, so that the difference between new and old samples can be eliminated, false positive interference caused by HAMA is effectively reduced, and the detection accuracy of CA72-4 is greatly improved; and the kit adopts a two-step method, so that the risk of HOOK in clinical practice is reduced.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Calprotectin combined with lactoferrin antigen test strip and preparation method thereof
PendingCN109682979AHigh sensitivityHigh speedDisease diagnosisBiological testingCalprotectinProtein detection
The invention discloses a calprotectin combined with lactoferrin antigen test strip and a preparation method thereof, and aims to provide a calprotectin combined with lactoferrin test strip with highdetection speed, simple operation and high sensitivity and also provides a preparation method of the calprotectin combined with lactoferrin test strip with simple process, good repeatability, stable finished structure and good performance. The calprotectin combined with lactoferrin antigen test strip comprises a bottom plate, a sample pad, a marker pad, a test pad and an absorbent pad. The preparation method comprises the steps that firstly the sample pad, the marker pad and the test pad are prepared, and then the sample pad, the marker pad, the test pad and the absorbent pad are in lap jointon the bottom plate in sequence. The calprotectin combined with lactoferrin antigen test strip is applied to the technical field of intestinal inflammatory disease marker protein detection test strips.
Owner:ZHUHAI ENCODE MEDICAL ENG
Human blood type test kit and preparation method thereof
The invention discloses a human blood type test kit which comprises a test strip. The test strip comprises a gold conjugate pad and a reaction membrane, and the gold conjugate pad is coated with a colloidal gold-labelled mouse anti-human A antigen monoclonal antibody, a colloidal gold-labelled mouse anti-human B antigen monoclonal antibody and a colloidal gold-labelled mouse anti-human D antigen monoclonal antibody; the reaction membrane comprises a B antigen test line coated with the mouse anti-human B antigen monoclonal antibody, an A antigen test line coated with the mouse anti-human A antigen monoclonal antibody, an Rh test line coated with the mouse anti-human D antigen monoclonal antibody and a quality control line coated with a goat anti-mouse IgG polyclonal antibody. The invention further provides a preparation method of the kit. The preparation method comprises the steps of reaction membrane preparation, gold conjugate pad preparation, cutting assembly and the like. The human blood type test kit can simultaneously test A, B, O and Rh blood types, is intuitive in test result, can be used for testing hemolysis samples and has the high sensitivity, specificity and stability.
Owner:北京中检安泰诊断科技有限公司
Group A streptococcus antigen detection test strip and kit and preparation methods thereof
InactiveCN110568187AReduce distractionsRule out false positivesMaterial analysisAntigen testImmunolabeling
The invention discloses a group A streptococcus antigen detection test strip and kit and preparation methods thereof, and relates to the technical field of group A streptococcus detection. The group Astreptococcus antigen test strip is prepared by sequentially attaching a nitrocellulose membrane coated with an anti-group A streptococcus detection antibody, an immune binding pad coated with an anti-group A streptococcus immunolabeled antibody, absorbent paper and a sample pad to a polyvinyl chloride bottom plate; a detection card can detect whether a group A streptococcus antigen is present ina sample to be detected or not by detecting a marker; and the test strip and the detection card comprising the test strip can specifically and quickly detect and diagnose early infection of group A streptococcus, thereby reducing the cost, realizing rapid detection and meeting the requirements of clinical use.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Porcine circovirus type 4 ELISA antibody detection kit, application and method for detecting porcine circovirus type 4 antibody
PendingCN112345767AStrong characteristicHigh sensitivityBiological testingImmunoassaysCircovirusSerum samples
The invention provides a porcine circovirus type 4 ELISA antibody detection kit, application and a method for detecting a porcine circovirus type 4 antibody, and relates to the technical field of biology. The porcine circovirus type 4 ELISA antigen detection kit provided by the invention comprises an elisa plate coated with porcine circovirus type 4 Cap protein. The ELISA antibody detection kit isestablished by using the circovirus type 4 Cap protein as an antigen, and the Cap protein as a circovirus conservative protein has good antigenicity and can be specifically combined with a circovirustype 4 antibody in a serum sample, so that the kit provided by the invention has very strong specificity and sensitivity to the circovirus type 4.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Colloidal gold immunochromatography test paper for rapidly diagnosing brucellosis
The invention relates to the technical field of immunodiagnosis, and discloses colloidal gold immunochromatography detection test paper for rapidly diagnosing brucellosis and a preparation method of the colloidal gold immunochromatography detection test paper for rapidly diagnosing brucellosis in order to solve the problem of inaccurate detection results caused by antigen detection of brucellosisantibodies in the prior art. The method specifically comprises the following steps: 1) preparing a detection line T and a quality control line C; 2) preparing a gold-labeled pad; 3) preparing the testpaper: sequentially connecting and assembling the water absorption pad, the sample pad, the microsphere probe treatment pad, the antibody-coated modified nitrocellulose membrane and the PVC bottom plate to obtain a finished product. Whether brucellosis exists or not can be accurately detected, the product adopts an antibody antigen detection mode, and the antigen has no incubation period; compared with a product for detecting an antigen, the test paper is more accurate, the antigen detection accuracy is higher than that of a product for detecting the antibody, the detection efficiency is high, and the preparation process is simple.
Owner:HANGZHOU ALLTEST BIOTECH
Preparation and application of renewable terahertz biological sample detection cell
ActiveCN110658154ALimit sensitivityEasy to popularize and applySamplingMaterial analysis by optical meansOxidized low density lipoproteinBiological macromolecule
The invention belongs to the technical field of biological sample detection, and particularly discloses an ultra-sensitive, unlabeled and renewable specific biological macromolecule detection method.The method comprises the following steps of (1) preparing the renewable terahertz biological sample detection cell, namely cleaning a substrate, carrying out deep silicon etching, coating, gumming, exposing, developing, carrying out post-baking, carrying out wet etching and cutting; (2) modifying a high polymer membrane strip through a polyethyleneimine-glutaraldehyde crosslinking method to prepare an antibody cross-linked detection membrane strip; (3) carrying out THz-TDs detection; (4) adding an antigen for incubation; (5) carrying out THz-TDs detection; and computing a frequency shift variation deltaf; and (6) repeating the steps (3) to (5) to detect biomolecular solutions of different concentrations. The perpetration and the application are mainly used for detecting oxidized low-density lipoprotein solutions of different concentrations, the problems of insufficient terahertz detection sensitivity and difficulty in metamaterial cleaning are solved, and the specific and unlabeled antigen detection is realized.
Owner:张阳
Helicobacter pylori vaccination
InactiveUS20050175629A1Longer timescale of immunotherapyAntibacterial agentsAmpoule syringesAdjuvantVaccination
A sterile immunogenic preparation of three purified H. pylori antigens (CagA, VacA and NAP) adjuvanted with alum in an isotonic buffer solution for intramuscular injection. The antigens may be administered in conjunction with antibiotics and / or antisecretories. Urease breath testing, stool antigen testing, and / or immunological analysis may be used as correlate(s) of protection against H. pylori infection. Urea may be used to improve VacA solubility.
Owner:CHIRON CORP
ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for goose astrovirus Capsid protein antigen, detection method and application
PendingCN111289751AHigh detection sensitivityThe detection process is fastImmunoassaysBiotechnologyAvastrovirus
The invention discloses an ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for a goose astrovirus Capsid protein antigen, a detection method and application. The kit comprises: an ELISA platecoated with a goose astrovirus Capsid protein polyclonal antibody, a confining liquid, a sample diluent, a purified goose astrovirus Capsid recombinant protein antigen standard substance, an HRP labeled anti-goose astrovirus Capsid monoclonal antibody, a washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution; meanwhile, the invention further discloses amethod for accurately and quantitatively detecting the goose astrovirus Capsid protein antigen in a sample by adopting the kit. The kit contains an antigen standard substance, can detect the accuratecontent of the goose astrovirus Capsid protein in a sample, and is suitable for quantitative detection of the goose astrovirus Capsid gene subunit vaccine.
Owner:扬州优邦生物药品有限公司
Colloidal gold chromatography reagent strip, preparation method thereof and novel crown antigen detection kit
ActiveCN113607944AIncrease surface areaReduce missed detectionBiological material analysisAgainst vector-borne diseasesReagent stripThroat swab
The invention discloses a colloidal gold chromatography reagent strip for detecting a new crown antigen, a preparation method thereof and a kit. The colloidal gold chromatography reagent strip comprises a bottom plate, a nitrocellulose membrane, a coupling pad, a sample pad and absorbent paper, the sample pad, the coupling pad, the nitrocellulose membrane and the absorbent paper are sequentially connected to the bottom plate in the horizontal direction, and avidin biotin amplified colloidal gold nanoflowers are coupled to the coupling pad. The nitrocellulose membrane is modified by nanocellulose, a detection line coated with a new crown antigen capture antibody and a quality control line coated with a quality control molecule capture antibody are arranged on the nitrocellulose membrane, and the detection line and the quality control line are sequentially distributed in the chromatography direction. The colloidal gold chromatography reagent strip can be used for conveniently and quickly detecting new crown antigens of nasopharynx swabs and throat swabs with high sensitivity.
Owner:SHENZHEN YHLO BIOTECH +1
Device for collecting supernate in dog manure antigen ELISA (Enzyme Linked Immunosorbent Assay) detection
PendingCN106353143AOvercoming impedimentReduce exposure timeWithdrawing sample devicesAntigen testBiology
The invention discloses a device for collecting supernate in dog manure antigen ELISA (Enzyme Linked Immunosorbent Assay) detection. The device comprises a filtering tube and an outer-layer casing, wherein holes are formed in the filtering tube; the filtering tube is arranged inside the outer-layer casing. When being used, the outer-layer casing is filled with a dog manure sample and a diluent; after being sufficiently and uniformly mixed, the dog manure sample and the diluent are not centrifuged or centrifuged for 10 minutes at a speed of 3000rpm / min, subsequently, the filtering tube is added into the outer-layer casing, and then the supernate can be directly detected or is sucked out for detection. The device can be adopted to collect the supernate after centrifugation in the pretreatment process of the dog manure sample, the supernate is not affected by floating impurities in the sample, the supernate can be collected without obstacle, the testing process can be accelerated, and the labor and the time can be saved.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
A novel coronavirus antigen detection test strip
ActiveCN112557654BAvoid stickinessHigh detection specificityBiological material analysisBiological testingBlood filmFiltration membrane
A novel coronavirus antigen detection test strip provided by the present invention comprises: a substrate, and a sample pad, a colloidal gold adsorption pad, a blood filter membrane, an antibody-carrying membrane and a water-absorbent pad are sequentially overlapped and overlapped on the substrate; The antibody carrying membrane is provided with a detection line and a quality control line at intervals, the detection line is close to the colloidal gold adsorption pad, and the quality control line is close to the water absorption pad; the coating of the colloidal gold adsorption pad Colloidal gold-labeled novel coronavirus monoclonal antibody B and colloidal gold-labeled mouse IgG antibody; the detection line is coated with novel coronavirus monoclonal antibody A; the quality control line is coated with anti-mouse IgG polyclonal antibody; novel coronavirus The virus monoclonal antibody B is different from the new coronavirus monoclonal antibody A; by setting a blood filter membrane, it can prevent the sample to be tested from being viscous and not running, and can also filter out other macromolecular interfering substances to improve specificity.
Owner:NANTONG EGENS BIOTECH CO LTD
Integrated antigen detection device and antigen detection process quality control method thereof
PendingCN114878806AMake sure to modifyAccurate identificationSurgeryVaccination/ovulation diagnosticsNasal passagesNasal Cavity Epithelium
The invention belongs to the technical field of antigen detection, and particularly relates to an integrated antigen detection device and an antigen detection process quality control method thereof, and the method comprises the following steps: obtaining a first image of a nasal swab part inserted into a nasal cavity; first judgment is carried out based on the first image, and whether sample sampling of the detected person is qualified or not is judged. The problem that an infected person cannot be found in time due to the fact that the self-testing process of the detected person is not standard, misoperation or intentional cheating exists in the prior art is solved; the method has the advantages that the self-test process of the subject is standardized, misoperation or intentional cheating behaviors of the subject in use are effectively prevented, and therefore the infected person is effectively found in time.
Owner:孙非 +1
Rapid Lyme antigen test for detection of Lyme disease
ActiveUS9500648B1Detect presenceEarly diagnosisBiological material analysisAntigen assaysCapture antibody
A method for detecting B. burgdorferi antigens in body fluid samples, such as urine. Polyclonal antibodies are used that bind to 31, 34, and 39 kDa B. burgdorferi antigens, wherein the polyclonal antibodies function as immobilized capture antibodies. Detection antibodies are used, having an enzyme linked thereto, which also bind to the B. burgdorferi antigens. A body fluid sample is reacted with the detection antibodies to form complexes between the detection antibodies and the B. burgdorferi antigens in the body fluid sample. The complexes are reacted with the immobilized capture antibodies, wherein the complexes become linked to the capture antibodies. A substrate is added to the complexes linked to the capture antibodies, wherein the substrate is converted by the enzyme to a visual and / or detectable product if B. burgdorferi antigens are present in the body fluid sample.
Owner:TATE JR ROBERT M
Method and device for realizing COVID-19Ag antigen detection result identification based on vision, processor and storage medium thereof
PendingCN114742993AReduce the possibility of inaccuracyReduce manual review costsCharacter and pattern recognitionMedical imagesComputer hardwareMachine vision
The invention relates to a method for identifying and processing a COVID-19Ag antigen detection result based on machine vision. The method comprises the following steps: (1) identifying and acquiring an antigen detection kit body as a target detection object by mobile terminal equipment; (2) the mobile terminal device creates a target connection entity and carries out cloud uploading; (3) receiving a to-be-detected object by the cloud service, and performing target and result identification on the antigen detection kit through an AI target detection algorithm; (4) returning an identification detection result to the mobile terminal equipment in an array form; and (5) the mobile terminal device determines a final antigen detection result according to the returned array result. The invention further relates to a corresponding device, a processor and a computer readable storage medium thereof. By adopting the method and the device for realizing COVID-19Ag antigen detection result identification processing based on machine vision, the processor and the computer readable storage medium thereof, a large amount of manual checking cost can be efficiently and conveniently saved through batch automatic processing.
Owner:上海瞳熵科技有限公司
Antigen detection kit with good stability
ActiveCN110404606BImprove stabilityPrevent movementBiological testingEnclosures/chambersBiomedical engineeringTest tube
Owner:南京帝基生物科技有限公司
Fluorescence detection card for aflatoxin B1, preparation method of fluorescence detection card and method for detecting aflatoxin B1 in grain and oil
PendingCN114525200AHigh sensitivityEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsMurine antibodyBiochemistry
The invention discloses a fluorescence detection card for aflatoxin B1, a preparation method of the fluorescence detection card and a method for detecting the aflatoxin B1 in grain and oil. A fluorescent microsphere labeled aflatoxin B1 monoclonal antibody conjugate is embedded in a sample combination pad of the detection card, an aflatoxin B1 antigen is coated on a quality control line of a chromatography membrane, and a goat anti-mouse antibody is coated on a detection line. The preparation method of the detection card comprises the following steps: adding a fluorescent microsphere labeled aflatoxin B1 monoclonal antibody conjugate into the sample combination pad for embedding treatment to obtain the fluorescent detection card with high sensitivity. According to the method for detecting the aflatoxin B1 in the grain and oil, a sample pretreatment method is limited, a specific extracting solution is selected, the extraction rate of the aflatoxin B1 in the grain and oil can be increased, aflatoxin B1 residues in a sample can be effectively extracted, and therefore measured data are more accurate.
Owner:GUANGZHOU ANNUO FOOD SCI & TECH CO LTD
Colloidal gold test paper for detecting A-beta antigen in senile dementia serum and use method of colloidal gold test paper
The invention discloses colloidal gold test paper for detecting an A-beta antigen in senile dementia serum and a use method of the colloidal gold test paper, and relates to the technical field of whole blood sample pretreatment, the colloidal gold test paper comprises a labeling pad, and is characterized in that two detection lines are arranged on the labeling pad respectively and used for detecting the A-beta antigen, one detection line is coated with an antibody capable of recognizing A-beta40, and the other detection line is coated with an antibody capable of recognizing A-beta42. According to the colloidal gold test paper, a whole blood sample is pretreated by using a brand new designed Tris buffer solution, so that A-beta40 and 42 of the blood sample to be detected can be detected on the colloidal gold test paper, and the colloidal gold test paper is suitable for senile dementia patients who need to monitor the course of Alzheimer's disease at any time or are in the period of medicine replacement.
Owner:瑞吉瑞得医药生物科技(无锡)有限公司
Chlamydia pneumoniae antigen detection plate and detection box
PendingCN114397451ADetection lateHigh sensitivityFluorescence/phosphorescenceImmunoassaysCelluloseNitrocellulose
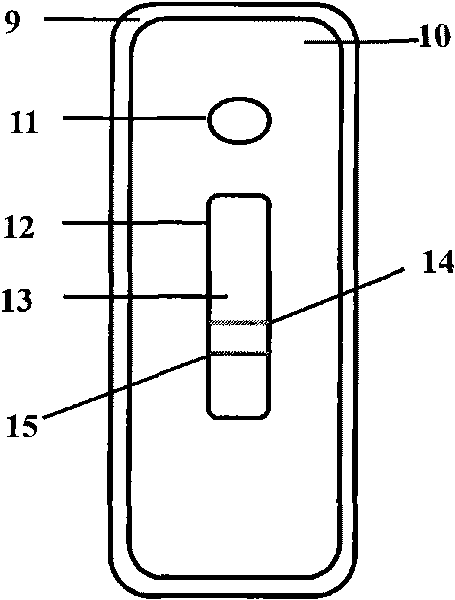
The invention provides a chlamydia pneumoniae antigen detection plate and a detection kit, the chlamydia pneumoniae antigen detection plate comprises an upper cover and a lower cover which are sealed and buckled, the upper cover is provided with a sample adding hole and an observation window, the lower cover is provided with a clamping groove, and a test strip is placed in the clamping groove, the chlamydia pneumoniae antigen detection plate is characterized in that the test strip comprises a PVC (polyvinyl chloride) batten, the PVC batten is provided with a nitrocellulose membrane, and the nitrocellulose membrane is connected with the upper cover. The position of the nitrocellulose membrane corresponds to the position of the observation window, absorbent paper is arranged at one end, far away from the sample adding hole, of the nitrocellulose membrane, a combination pad is arranged at the other end of the nitrocellulose membrane, the combination pad is completely covered by the upper cover, a sample adding pad is arranged at the free end of the combination pad, and the sample adding pad is arranged at the free end of the upper cover. The position of the sample adding hole corresponds to that of the sample adding pad; according to the invention, antigen detection is adopted, the antigen detection can be detected in an incubation period, an acute period or an initial disease course, and the antibody detection is later.
Owner:山东康华生物医疗科技股份有限公司
Antigen test kit suitable for self-service detection and data acquisition system
PendingCN114778837AGuaranteed correctnessRealize centralized managementCo-operative working arrangementsBiological testingPsa testAntigen assays
The invention discloses an antigen test kit suitable for self-service detection and a data acquisition system. A chromatography detection card of the antigen test kit is arranged in a test kit card shell; a serial number window, a result observation window and a sample adding opening are sequentially formed in the top surface of the test kit clamping shell upper cover; the sample pad and the water absorption pad are arranged at the two ends of the reaction pad, and the sample pad, the water absorption pad and the reaction pad are fixedly arranged between the test kit clamping shell upper cover and the test kit clamping shell bottom cover. And a background database of the antigen test data acquisition system stores a serial number of an antigen test kit, text information of a detection bar code and a corresponding detection result. The detection result is displayed in an encrypted pattern mode, the pattern or the decrypted text cannot be used for judging the detection result, the detection result can be obtained only by matching the pattern or the decrypted text with the serial number in a data acquisition system, detection is accurate and rapid, the device is simple and easy to popularize, and accurate and rapid centralized acquisition of the detection result can be achieved.
Owner:倪晟
Neutralizing epitope peptide in RBD region of SARS-CoV-2 S protein and application of neutralizing epitope peptide
PendingCN114163504ABinding can be blockedMultivalent bindingSsRNA viruses positive-senseAntibody mimetics/scaffoldsNeutralising antibodySite-directed mutagenesis
On the basis of the neutralizing antibody in the RBD region of the SARS-CoV-2S protein, the Fab segment of the neutralizing antibody is prepared through recombinant expression, the Fab segment reacts with the RBD region of the SARS-CoV-2S protein to form an antigen-antibody compound, and purification, crystallization and X-crystal diffraction analysis are performed. The method comprises the following steps: analyzing an antigen-antibody compound structure by using a homologous compound three-dimensional structure in a PDB database as a model to obtain amino acid residues interacting with a neutralizing antibody on SARS-CoV-2S protein RBD, carrying out site-specific mutagenesis on key amino acid residues, and detecting the affinity of an SARS-CoV-2S protein RBD mutant and the neutralizing antibody Fab. And determining the epitope peptide fragment according to the influence degree of the RBD point mutation on the affinity. The invention also provides application of the epitope peptide fragment in preparation of a fusion antigen for detecting SARS-CoV-2, and application of the epitope peptide fragment in preparation of an immunogen for preparing a vaccine or an antibody.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD
FRET-based homogeneous immunodetection method and detection composition
PendingCN114137217ALow background fluorescence interferenceHigh detection sensitivityBiological testingChemical physicsImmuno detection
The invention relates to an FRET (Fluorescence Resonance Energy Transfer)-based homogeneous immunodetection method and a detection composition, and belongs to the technical field of analytical chemistry. The technical problems that an existing FRET-based homogeneous immunodetection method is low in sensitivity, high in background fluorescence and the like are solved. The detection composition comprises a bismuth-based material marked with an antigen and a rare earth doped luminescent nanocrystal marked with an antibody, or the bismuth-based material marked with the antibody and the rare earth doped luminous nanocrystal marked with the antigen are included; wherein the antigen and the antibody are antigen-antibody pairs capable of carrying out specific binding reaction; the bismuth-based material is a bismuth-based two-dimensional nanosheet material or a metal ion doped bismuth-based two-dimensional nanosheet material. The invention also provides a method for antibody or antigen detection by using the detection composition. The FRET-based homogeneous immunodetection method has the advantages of small background fluorescence interference, high detection sensitivity, low cost, simplicity, convenience in operation and the like.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com