Colloidal gold chromatography reagent strip, preparation method thereof and novel crown antigen detection kit
A technology of colloidal gold and reagent strips, which is applied in the field of new crown antigen detection kits, can solve the problems of missed detection of virus antigens such as the antibody binding ability is not outstanding, and achieve the effect of enhancing the visual effect of the naked eye, enhancing the effect of color development, and reducing the phenomenon of missed detection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] A method for preparing a colloidal gold chromatography reagent strip 10 for detecting a new crown antigen, comprising the steps of:
[0053] a) Preparation of colloidal gold particle solution: HAuCl 4 Heat to 120°C while stirring in an intelligent magnetic stirrer, add 1 / 20 to 1 / 4 volume of sodium citrate (so that the amount of sodium citrate added is 1wt%), and continue heating for 10 to 30 minutes until the color of the solution is stable It is ruby red, and it can be considered that stable colloidal gold particles have been produced. After cooling to room temperature, store at 2°C to 8°C.
[0054] b) Preparation of colloidal gold nanoflower solution: add the colloidal gold particle solution prepared in step a) to 60 to 100 times the volume of purified water, heat to 60°C to 80°C, and then add HAuCl 4 (making HAuCl 4 The amount added is 1 wt%), sodium citrate (so that the added amount of sodium citrate is 1 wt%) and hydroquinone solution, stirred continuously at r...
Embodiment 1
[0065] This embodiment provides a colloidal gold chromatography reagent strip 10 for detecting the new crown antigen, and its preparation method comprises the following steps:
[0066] a) Preparation of colloidal gold particle solution: HAuCl 4 Heat to 120°C while stirring in an intelligent magnetic stirrer, add 1 / 10 volume of sodium citrate (1wt%), and continue heating for 20 minutes until the color of the solution is stable and appears as ruby, it can be considered that stable colloidal gold particles have been produced , cooled to room temperature and stored at 4°C.
[0067] b) Preparation of colloidal gold nanoflower solution: add the colloidal gold particle solution prepared in step a) to 80 times the volume of purified water, add HAuCl after heating to 60°C 4 (1wt%), sodium citrate (1wt%) and hydroquinone solution, stirred continuously at room temperature, centrifuged at a speed of 10000rpm for 20min, then removed the supernatant, collected the synthesized colloidal gol...
Embodiment 2
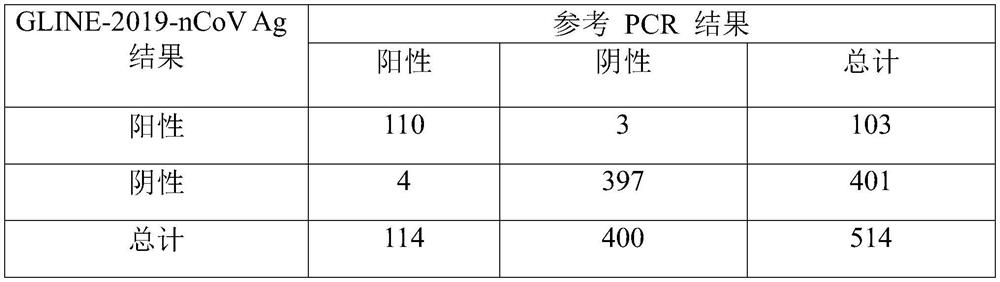
[0076] In this embodiment, the colloidal gold chromatography reagent strip 10 for detecting the new crown antigen prepared in Example 1 is used to quickly detect the new coronavirus antigen in the fresh sample and match the PCR result to evaluate the colloidal gold chromatography reagent strip 10 for detecting the new crown antigen sensitivity and specificity.
[0077] By testing 514 fresh nasopharyngeal swabs in 3 different locations, the results were compared with the PCR results. Add the nasopharyngeal swab to 450 μL of virus lysate, rotate and squeeze it up and down 3-10 times, take 80 μL after fully lysed and add it to the sample hole 1062 of the colloidal gold chromatography reagent strip 10 for detecting the new crown antigen, about 3 drops, after waiting for 15 minutes, observe the test results, the test results are shown in Table 1, as can be seen from Table 1, the matching results are, the sensitivity is 96.49%; the specificity is 99.25%, and the accuracy is 98.6%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com