Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Nasal Swab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
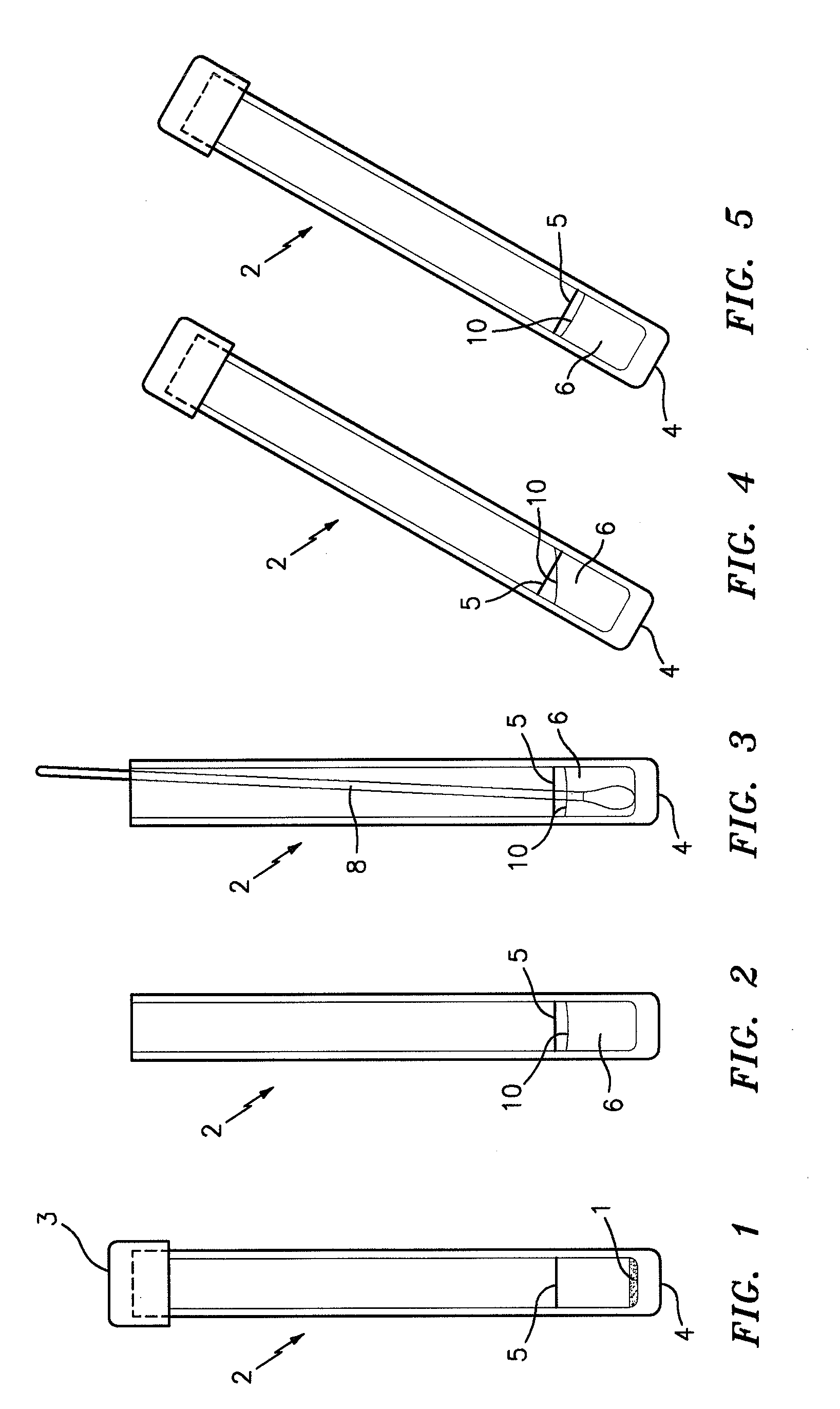
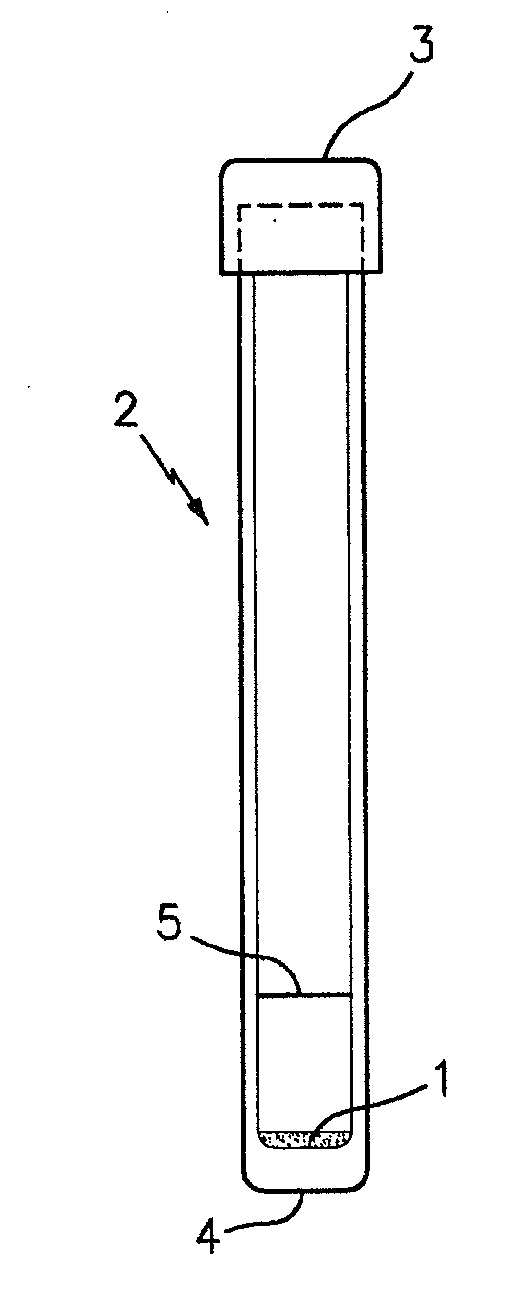
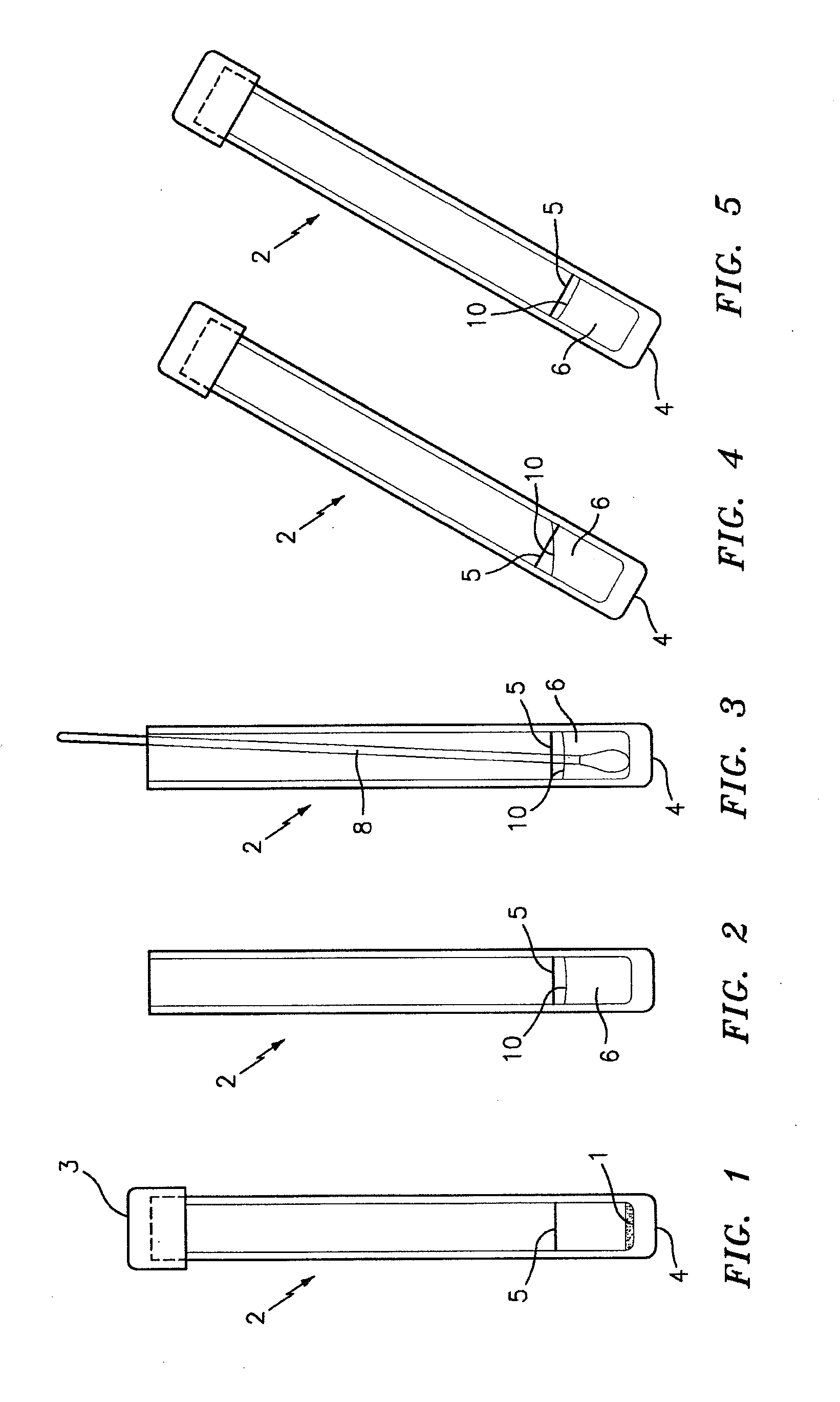
A method used to collect biological material from within the nasal passages. A cotton swab is inserted into the nasal opening and rotated against the anterior nasal mucosa and them withdrawn.
Rapid fluorescence PCR detection kit for ASFV (African swine fever virus)
PendingCN109593893ADetection fitReduce manual operationsMicrobiological testing/measurementDNA/RNA fragmentationSerum igeFluorescence
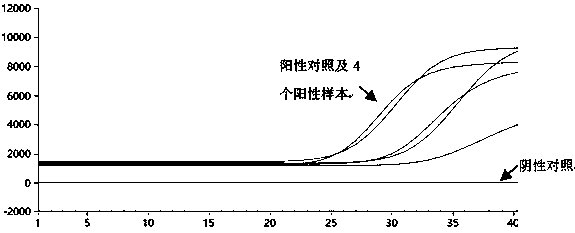
The invention discloses a rapid fluorescence PCR detection kit for ASFV (African swine fever virus). The kit comprises specific primers ASFV-F and ASFV-R as well as a TaqMan probe ASFV-P, the 5' end of the probe labels fluorescence dye as FAM and the 3' end labels a fluorescence quenching group as BHQ-1. The kit can be used for detecting the ASFV in nasal swabs, blood, serum, plasma and tissue ofswine to realize rapid detection of the ASFV. In the whole ASFV detection process, only 40 min is taken from DNA extraction to obtaining of detection results, so that the detection time is greatly shortened, and the detection efficiency is improved.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD +2
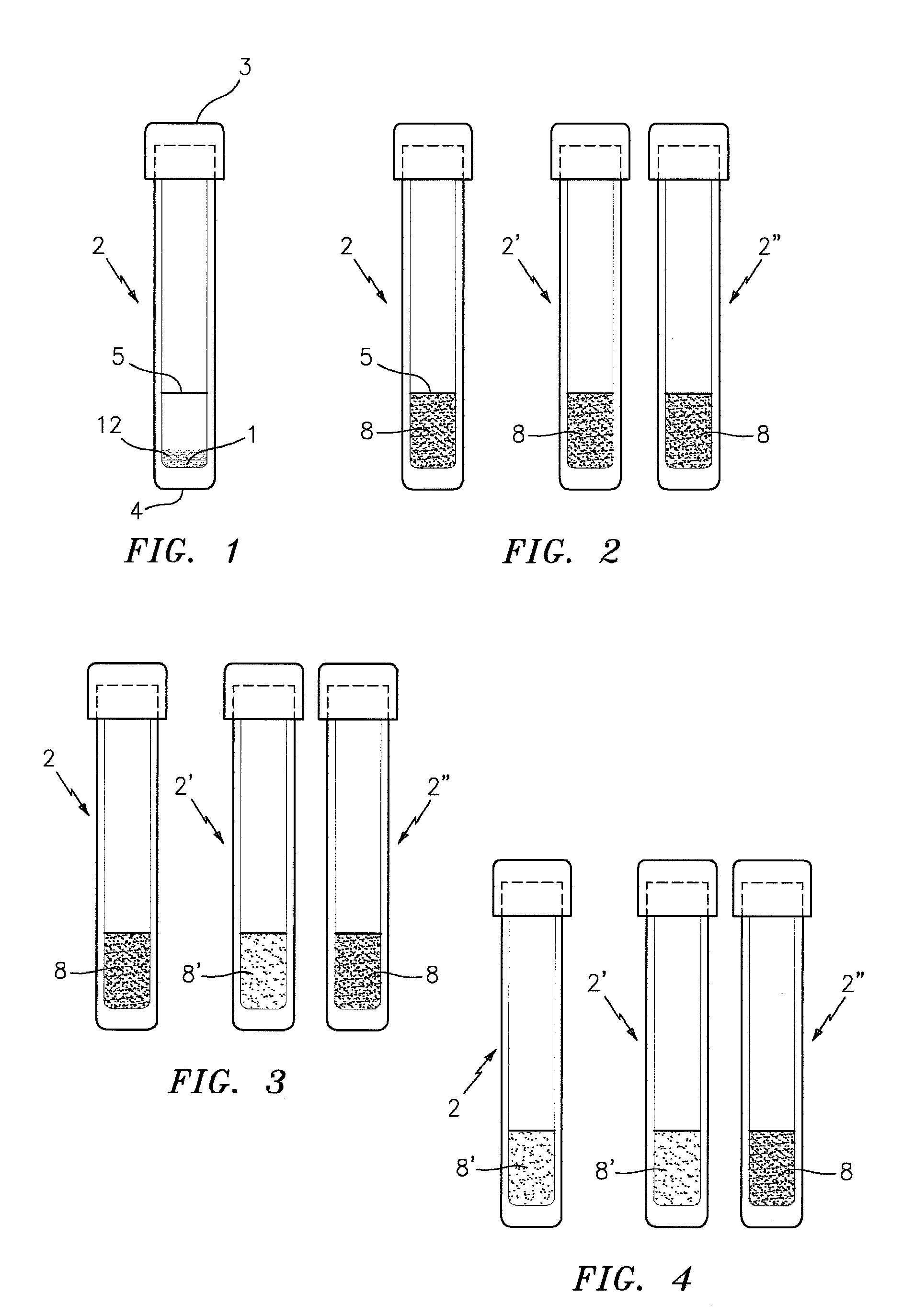
Disposable Sample Processing Unit
InactiveUS20090036665A1Analysis using chemical indicatorsHeating or cooling apparatusPoint of careRNA extraction
A low-cost, non-instrumented, easy-to-use disposable platform for extraction, stabilization, and preservation of viral RNA in specimens at the point of collection is described. The system may use chemical heating. The platform performs the following steps: specimen lysis, RNA extraction, and RNA stabilization in a modular approach. This modular approach confers versatility to the product for application to multiple targets such as avian flu, and HIV, specimens such as blood, nasal swabs, and downstream applications such as PCR or transcription-mediated amplification. The technology described is a point-of-care specimen-processing platform generically applicable to both emerging point-of-care and central-facility molecular diagnostic tests, as well as to surveillance applications.
Owner:PROGRAM FOR APPROPRIATE TECHNOLOGY IN HEALTH
Novel coronavirus 2019-nCoV fluorescent RPA detection primers, probe, kit and method
PendingCN111500776AHigh precisionGood repeatabilityMicrobiological testing/measurementMicroorganism based processesReference genesAlveolar lavage fluid
The invention discloses novel coronavirus 2019-nCoV fluorescent RPA detection primers, a probe, a kit and a method. The kit comprises novel coronavirus 2019-nCoV specific primers shown in SEQ ID NO. 1-2 and a probe shown in SEQ ID NO. 3. According to the invention, the existence of the novel coronavirus 2019-nCoV ORF1ab gene and the internal reference gene RNase P is simultaneously detected by adopting a single-tube double-fluorescence channel, and the existence of the novel coronavirus 2019-nCoV RNA in pulmonary alveolar lavage fluid, nasal swab, pharyngeal swab, sputum and other samples canbe detected. The method is short in detection time and suitable for clinical and bedside virus nucleic acid rapid screening, the kit has extremely high sensitivity and specificity, an internal reference gene is added into the detection system, and quality monitoring is performed on the extraction and amplification process of the sample according to the detection result of the internal reference gene.
Owner:湖南润美基因科技有限公司
Method and medium for detecting the presence or absence of staphylococcus aureus in a test sample
InactiveUS20110269160A1Facilitates handling and packaging and storingFavorable source of coagulase substrateBacteriaMicrobiological testing/measurementLesion swabTest sample
Owner:PILOTS POINT LLC
Method and medium for detecting the presence or absence of staphylococcus aureus in a test sample
InactiveUS20090191577A1Small amountFacilitates handling and packaging and storingBacteriaMicrobiological testing/measurementLesion swabTest sample
A presence / absence test for Staphylococcus aureus (S. aureus) involves placing a first generation test sample in a solution that will clot in the presence of S. aureus. The solution contains components that will selectively grow S. aureus and also contains clotting factors that will react with S. aureus, if S. aureus is present in the sample, to clot the solution. Examples of specimen samples that can be tested include nasal swabs and lesion swabs, among others. The test can also be modified to detect the presence or absence of methicillin resistant S. Aureus (MRSA).
Owner:PILOTS POINT LLC
Method and medium for detecting the presence or absence of staphylococcus aureus in a test sample
InactiveUS20110269163A1Facilitates handling and packaging and storingFavorable source of coagulase substrateBacteriaMicrobiological testing/measurementLesion swabStaphylococcus cohnii
A presence / absence test for Staphylococcus aureus (S. aureus) involves placing a first generation test sample in a solution that will clot in the presence of S. aureus. The solution contains components that will selectively grow S. aureus and also contains clotting factors that will react with S. aureus, if S. aureus is present in the sample, to clot the solution. Examples of specimen samples that can be tested include nasal swabs and lesion swabs, among others. The test can also be modified to detect the presence or absence of methicillin resistant S. Aureus (MRSA).
Owner:PILOTS POINT LLC
Detection method and detection kit for porcine type A foot-and-mouth disease virus specific IgA antibody
The invention discloses a detection method for a porcine type A foot-and-mouth disease virus specific IgA antibody; a prokaryotic expression system-expressed foot-and-mouth disease virus VP1 protein is used as a coating antigen, a mouse anti porcine IgA monoclonal antibody is used as a secondary antibody, an enzyme-labeled goat anti mouse IgG antibody is used as an indicator, a to-be-tested sample is subjected to reactions with the coating antigen, the monoclonal antibody and the enzyme-labeled antibody successively, and then is compared with a reference positive sample and a negative sample, and the result can be used for evaluating the level of the porcine foot-and-mouth disease virus specific IgA antibody; and a detection kit is provided. The detection method has the beneficial effects that the invention provides an effective method for evaluating the immune effect of foot-and-mouth disease mucosa and provides a new method for early diagnosis of infection of foot-and-mouth disease, and the foot-and-mouth disease virus specific IgA antibody in porcine nasal swabs can be quickly and accurately detected, sample collection operation is simple, manpower consumption is low, and the stress on animals is small.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for detecting the presence or absence of pathogenic Staphylococci in a test sample, including test mixture with micro particles
InactiveUS8546103B2Favorable source of coagulase substrateImprove performance consistencyMicrobiological testing/measurementStaphylococcus cohniiFirst generation
Owner:PILOTS POINT LLC
Real-time fluorescence RT-PCR detection kit for H1N1 type A swine influenza virus and application of detection kit
InactiveCN104450956AStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceTissue sample
The invention discloses a fluorescent quantitative RT-PCR detection kit for an H1N1 type A swine influenza virus, and an application of the detection kit. Through multiple sequence alignment, a primer and a probe with high specificity for detecting the H1N1 type A swine influenza virus is designed aiming at conservative gene segments of the H1N1 (2009) type A swine influenza virus, a Eurasian avian-like H1N1 swine influenza virus, a classical type H1N1 swine influenza virus and a human-derived H1N1 swine influenza virus, and is applied to real-time fluorescence RT-PCR detection. An experiment result proves that the specific PCR primer and TaqMan fluorescence probe disclosed by the invention are high in specificity when being used for detecting the H1N1 type A swine influenza virus; the sensitivity can reach 2.6*10<-5>ng; tissue samples such as nasal swabs, lungs and tracheas of to-be-detected swinery can be detected; the chick embryo allantoic fluid can also be detected; the detection kit is simple to operate and easy to popularize; basic operation and application are facilitated; and the detection kit can become a useful detection tool for diagnosis of H1N1 type A swine influenza virus diseases and epidemiological survey.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for detecting the presence or absence of methicillin resistant staphylococcus aureus (MRSA) in a test sample
InactiveUS8524468B2Facilitates handling and packaging and storingFavorable source of coagulase substrateMicrobiological testing/measurementBiological material analysisThroatLesion swab
A dry mixture, a liquid menstrum, and a method, are described for use in detecting the presence or absence of Methicillin Resistant Staphylococcus aureus (“MRSA”) in a first generation biological or environmental specimen sample. First generation specimen samples that can be analyzed include nasal swabs, lesion swabs, skin swabs, throat swabs, food swabs, tanning salon swabs, gym swabs, restaurant swabs, hotel swabs, and the like. The menstrum and method include an anti-ribosomal antibiotic component that will selectively prevent Methicillin Susceptible Staphylococcus aureus (“MSSA”) from growing in the menstrum, while allowing MRSA to grow in the menstrum. The menstrum also includes components which will stimulate growth of MRSA, plus coagulase reacting factors which will cause the menstrum to clot in the event that MRSA is present in the sample. The menstrum also includes components which will produce a detectable signal in the clot, which signal indicates the presence of MRSA in the sample.
Owner:PILOTS POINT LLC
Human nasal swab alpha ray dose fast detector with automatic sample change function
InactiveCN104808235ARealize automatic continuous sample changeShorten the timeX-ray spectral distribution measurementDosimetersVacuum pumpingAutomatic control
The invention relates to a human nasal swab alpha ray dose fast detector with an automatic sample change function. The fast detector is characterized by automatic sample change and continuous rapid detection, and belongs to the technical field of radiation dose protection. The detector is mainly composed of an alpha ray detection system, a sample frame, a shell, a vacuum system, a servo motor, and a computer. The sample frame disposed in the shell is equally divided into five chambers, and the sample frame can be rotated to make all the chambers reach five positions one after another, namely, a sample introduction position, a vacuum pumping position, a detection position, a vacuum degassing position and a sample discharge position. In the sample introduction and discharge positions, a nasal swab equipped with a bracket is put into an I-shaped slot of the sample or leave the slot and enter a nasal swab collecting box by gravity. The chamber in the vacuum pumping position is connected with a mechanical pump through a three-way pipe, and the other end of the three-way pipe is connected with the chamber in the detection position through a rapid vacuum protection valve. Vacuum sealing is realized through sealing contact between the sample frame and the shell. Alpha ray detection is automatically controlled by the computer. After detection of a sample is completed, the sampling frame is rotated by the servo motor to implement automatic sample change.
Owner:BEIJING NORMAL UNIVERSITY
Assembled and visualized nasal swab detection system
InactiveCN111839608AVisually observe the environmentVisually observe the sampling effectSurgical needlesVaccination/ovulation diagnosticsNasal Cavity EpitheliumSurgery
Owner:蔡贞渝
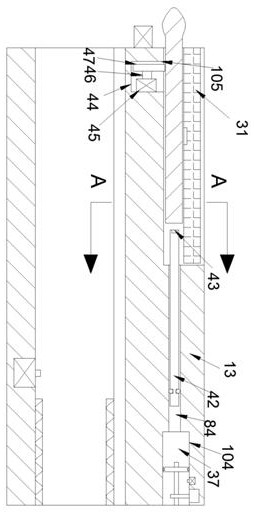
Full-self-service nasal swab sampler and use method thereof
PendingCN111317513AEasy accessEasy to markSurgeryVaccination/ovulation diagnosticsElectric machineryEngineering
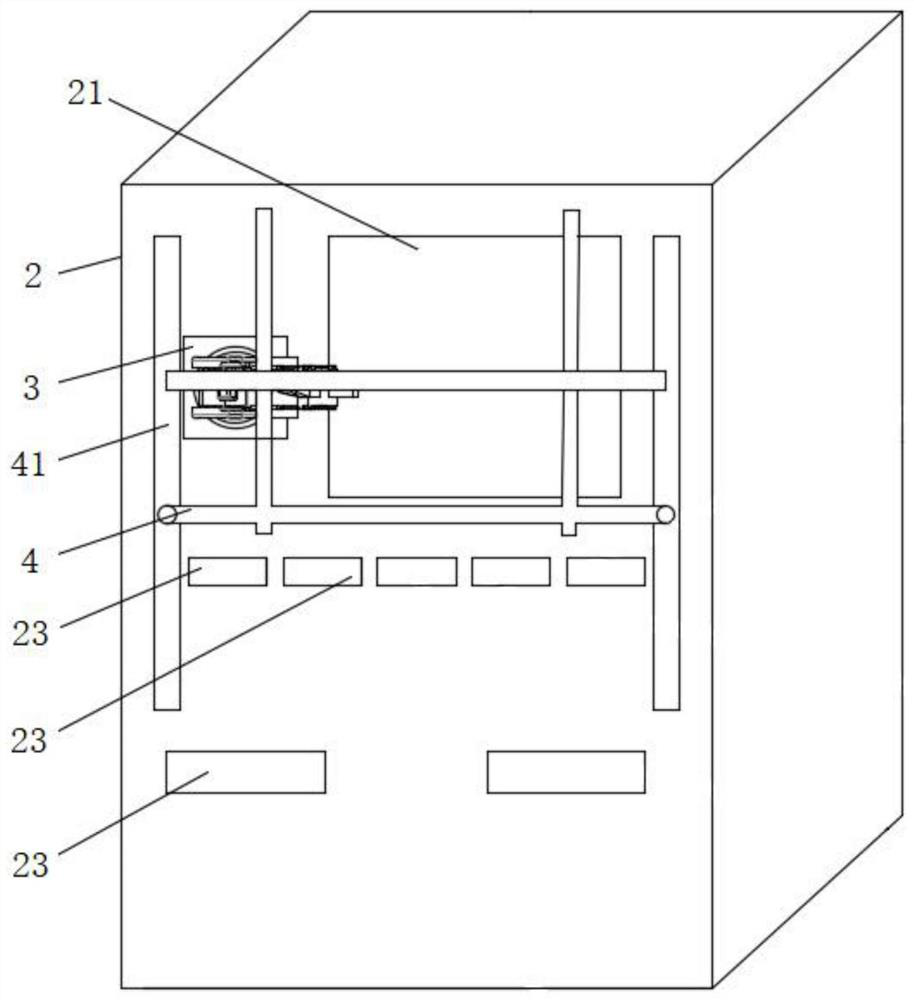
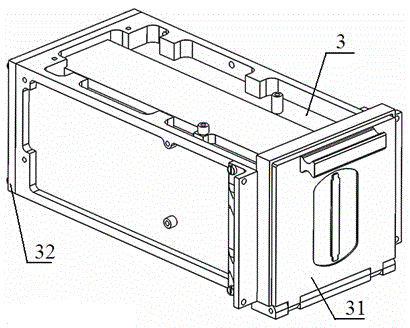
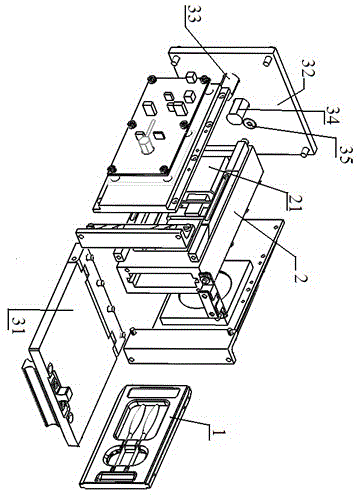
The invention discloses a full-self-service nasal swab sampler and a use method thereof. The full-self-service nasal swab sampler comprises a cabinet, wherein a first electric telescopic rod is fixedly connected to the left side of the bottom of an inner cavity of the cabinet; a placement plate is fixedly connected to the top of the first electric telescopic rod; a nasal swab storage mechanism isarranged at the top of the placement plate; a feeding mechanism is fixedly connected to the left side of the inner cavity of the cabinet; and a driving motor is fixedly connected to the back of the inner cavity of the cabinet. By designing a touch display screen, the use method of the nasal swab sampler can be displayed on the touch display screen, and the working efficiency is improved; by meansof cooperation of the first electric telescopic rod, the placement plate, the feeding mechanism and a sampling door, a tester can conveniently take the nasal swab sampler, and the operation is convenient; and by means of cooperation of the driving motor, a rotating wheel and code paper, each nasal swab sampler can be conveniently marked, later searching is facilitated, and therefore the effect ofhigh working efficiency is achieved, the problem that an existing device is low in working efficiency is solved, and medical workers can be prevented from being infected.
Owner:THE SEVENTH AFFILIATED HOSPITAL SUN YAT SEN UNIV SHENZHEN
African swine fever virus P72 gene fluorescent probe PCR detection primer probe set, kit and method thereof
PendingCN112877476AHigh detection sensitivityShort detection timeMicrobiological testing/measurementMicroorganism based processesForward primerClassical swine fever virus CSFV
The invention provides an African swine fever virus P72 gene fluorescent probe PCR detection primer probe set, the nucleotide sequence of a forward primer of the primer probe group is shown as SEQ ID NO.1, the nucleotide sequence of a reverse primer of the primer probe group is shown as SEQ ID NO.2, and the nucleotide sequence of a specific fluorescent probe is shown as SEQ ID NO.3, the 5'end of the specific fluorescent probe is marked with a fluorescent reporter group, and the 3 'end of the specific fluorescent probe is marked with a fluorescent quenching group. The invention further provides the African swine fever virus P72 gene fluorescent probe PCR detection kit and the method thereof. The kit and the detection method thereof disclosed by the invention have the advantages of wide coverage range, short detection time and high detection efficiency, and can be used for detecting the African swine fever virus in samples such as saliva, nasal swabs, blood and tissues of pigs.
Owner:LONGYAN UNIV
Method and reagents for detecting the presence or absence of staphylococcus aureus in a test sample
InactiveUS20110027823A1Small amountFavorable source of coagulase substrateMicrobiological testing/measurementStaphylococcus cohniiStaphylococcus pseudintermedius
A presence / absence test for Staphylococcus aureus (S. aureus) involves placing a first generation test sample in a solution that will clot in the presence of S. aureus. The solution contains components that will selectively grow S. aureus and also contains clotting factors that will react with S. aureus, if S. aureus is present in the sample, to clot the solution. Examples of specimen samples that can be tested include nasal swabs and lesion swabs, among others. The test can also be modified to detect the presence or absence of methicillin resistant S. Aureus (MRSA). The addition of micro particles having a size in the range of about 0.1 micron to about 1.0 mm provides localities where the bacteria agglomerate, thereby significantly decreasing the clotting time, and providing a significantly stronger clot. The micro particles can be used in other bacteria tests to accelerate the production of an end result. Such other tests can include a vancomycin-resistant enterococcus test; a Group B Streptococcus test; a test for hemolytic E. coli; and a test for Listeria monocytogenes, to name a few. These tests are all performed in a liquid broth-type reagent mixture and do not necessarily involve clotting of the broth.
Owner:PILOTS POINT LLC
PCR primer, kit and method for detecting African swine fever virus MGF-505-1R gene
PendingCN112831609AStrong specificityHigh amplification efficiencyMicrobiological testing/measurementMicroorganism based processesNucleotideClassical swine fever
The invention provides a PCR (Polymerase Chain Reaction) primer for detecting an African swine fever virus MGF-505-1R gene. The PCR primer is selected from any one of the following two pairs of primers: a primer 1 consisting of nucleotide sequences as shown in SEQ ID No.1 and SEQ ID No.2; a primer 2 is composed of nucleotide sequences as shown in SEQ ID No. 3 and SEQ ID No. 4. The invention also provides a kit for detecting the African swine fever virus MGF-505-1R gene, the kit comprises a PCR mixed solution, an enzyme mixed solution, a negative control and a positive control, the PCR mixed solution comprises the primer, and the primer is the primer 1 or the primer 2. The kit provided by the invention has the advantages of high amplification efficiency, high detection sensitivity and high specificity, and can be used for detecting the African swine fever virus MGF-505-1R gene in samples such as saliva, nasal swab, blood, tissue and the like of pigs.
Owner:LONGYAN UNIV
Fluorescence quantification RT-PCR (reverse transcription-polymerase chain reaction) detection kit of Eurasian avian-like type H1N1 swine influenza virus and application thereof
ActiveCN104450955AStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceReverse transcriptase
The invention discloses a fluorescence quantification RT-PCR (reverse transcription-polymerase chain reaction) detection kit of a Eurasian avian-like type H1N1 swine influenza virus and an application thereof. By virtue of multiple alignments of sequences, and aiming at conserved gene segments of the Eurasian avian-like type H1N1 swine influenza virus, an A(H1N1) (2009) swine influenza virus, a classical type H1N1 swine influenza virus and a human-derived H1N1 swine influenza virus, a primer and a probe with strong specificity for detecting the Eurasian avian-like type H1N1 swine influenza virus are designed and are used for performing real-time fluorescence RT-PCR detection. Experiment results show that by using the kit provided by the invention to detect the Eurasian avian-like type H1N1 swine influenza virus, the specificity is high, and the detection sensitivity to virus RNA (ribonucleic acid) can reach 4.6*10<-7>ng / reaction, so that tissue samples of nasal swabs, lungs, tracheas and the like of a to-be-detected swine herd can be detected, and chick embryo allantoic fluid can also be detected. The kit is simple to operate, easy to popularize and convenient in basic-level operation and application, and can become a useful detection tool for epidemic disease diagnosis of the Eurasian avian-like type H1N1 swine influenza virus and epidemiologic investigations.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Rapid and highly fieldable viral diagnostic
ActiveUS20140272943A1Lower requirementMicrobiological testing/measurementInfected cellWhite blood cell
The present invention relates to a rapid, highly fieldable, nearly reagentless diagnostic to identify active RNA viral replication in a live, infected cells, and more particularly in leukocytes and tissue samples (including biopsies and nasal swabs) using an array of a plurality of vertically-aligned nanostructures that impale the cells and introduce a DNA reporter construct that is expressed and amplified in the presence of active viral replication.
Owner:UT BATTELLE LLC
COVID-19 antigen detection card as well as preparation method and application thereof
ActiveCN112730832AHigh fluorescence intensityEliminate distractionsBiological testingLuminescent compositionsSaliva sampleAntigen testing
The invention discloses a COVID19 antigen detection card as well as a preparation method and application thereof, and belongs to the field of nano materials and nano medicine, an adopted rare earth nano probe is erbium fluoride sodium coated sodium yttrium fluoride with a core-shell structure, the particle size is 20nm-30nm, the composition of the rare earth nano probe is NaErF4@NaYF4, and NaErF4 is a full erbium doped core structure, NaYF4 is a shell layer, and @ represents that the NaYF4 is coated on the surface of the NaErF4. The nano-probe is a rare earth fluoride nano-material and has the advantages of low background, long luminescence life, strong fluorescence signal, high signal-to-noise ratio and the like, the probe is connected with a new crown monoclonal antibody through a covalent bond for marking, a marking product is stable, and the COVID19 antigen detection card has the characteristics of high sensitivity, high accuracy, rapid and simple detection and the like, and can be used for screening and detection of early-stage COVID19 suspected patient throat swabs, nasal swabs, nasopharyngeal swabs and saliva samples, so that clinical definite diagnosis can be quickly and specifically assisted.
Owner:厦门奥德生物科技有限公司
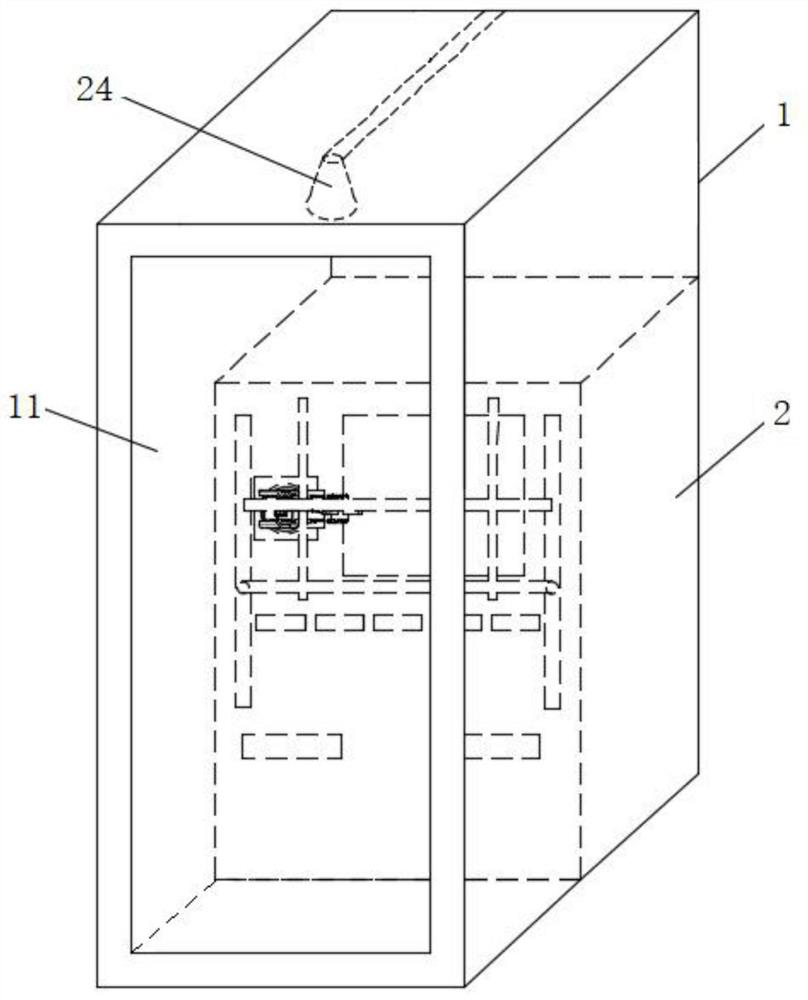
Nasopharyngeal oropharyngeal swab collection robot
PendingCN112641472AEase of recovery testingAvoid infectionVaccination/ovulation diagnosticsInstruments for stereotaxic surgeryNoseIdentity recognition
The invention provides a nasopharyngeal oropharyngeal swab collection robot. The robot comprises a box body; a cavity is formed in the box body; the cavity is divided into a control cavity, a storage cavity and a recovery cavity; a processor is arranged in the control cavity; the processor is connected with an identity recognition device; a swab, a bottle cap and a bottle body are sequentially stored in the storage cavity; the recovery cavity comprises a nose swab recovery cavity and a throat swab recovery cavity; and manipulators are arranged in front of the box body, provided with recognition devices and connected with the processor. According to the invention, nylon flocking swab is grabbed through the first manipulator and enters the nasal cavity of a patient, the recognition device recognizes the bottom of the nasopharynx, the nylon flocking swab is placed at the position, the first manipulator rotates by a certain angle and then is taken out, the second manipulator obtains the bottle body, the first manipulator places the nylon flocking swab into the bottle body to obtain the bottle cap, the bottle cap and the bottle body are screwed, the second manipulator obtains a patient identity label and adheres the patient identity label to the bottle body in the first manipulator, and the first manipulator places a stored nose swab into the nose swab recovery cavity, so that the infection of a medical staff is avoided.
Owner:ZHEJIANG MEDICAL COLLEGE
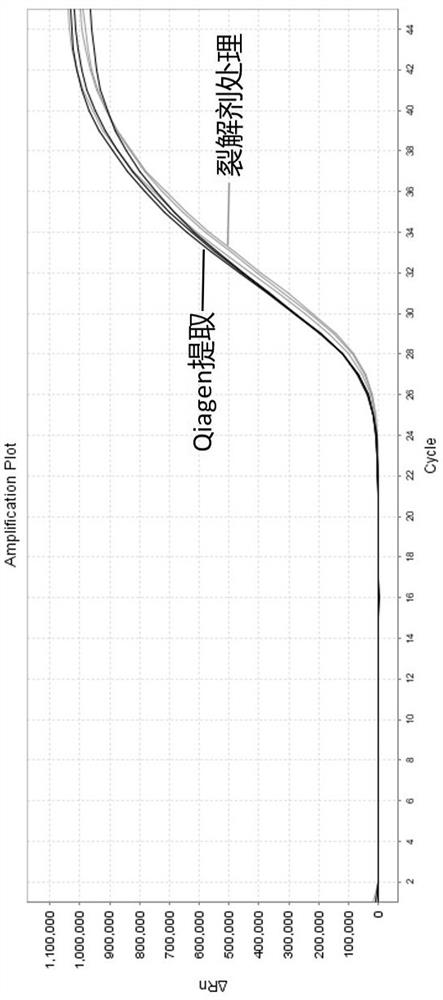
Cracking agent suitable for direct amplification of DNA or RNA virus, kit and application of cracking agent and kit in PCR detection of virus
PendingCN113444771AQuick releaseEfficient releaseMicrobiological testing/measurementMicroorganism based processesPharyngeal swabActive agent
The invention discloses a cracking agent suitable for direct amplification of DNA or RNA viruses. The cracking agent comprises Tris-HCl, chaotropic salt, a surfactant, a reducing agent, EDTA and an RNA enzyme inhibitor Rnasin. When the lysing agent is used for treating samples such as serum, plasma, nasal swabs, pharyngeal swabs, sputum and alveolar lavage fluid, virus nucleic acid can be rapidly and effectively released under the room temperature condition, lysate can be directly used for qPCR amplification, a nucleic acid extraction step is not needed, the detection efficiency is greatly improved, and the whole time is shortened from 3 hours to 1.5 hours.
Owner:YEASEN BIOTECHNOLOGY (SHANGHAI) CO LTD
Nasal swab sampling robot and use method thereof
InactiveCN113057682AEasy to transportEasy to detectSurgeryVaccination/ovulation diagnosticsPhysical medicine and rehabilitationElectric machinery
The invention discloses a nose swab sampling robot and a using method thereof. The nose swab sampling robot comprises a shell, wherein a first cavity is formed in the shell, a first through hole penetrating leftwards is formed in the left wall of the first cavity, a first motor is fixedly arranged on the right wall, on the right side of the first through hole, of the first cavity, a first threaded shaft extending leftwards into the first through hole is installed on the left side face of the first motor, an integrated block is connected to the rear wall of the first cavity in a left-right sliding mode, a second through hole is formed in the integrated block in a left-right penetrating mode, a gear ring is arranged in the annular wall of the second through hole in the right side, the gear ring is in threaded connection with the first threaded shaft, and a first sensor is arranged in the position, on the left side of the gear ring, of the bottom wall of the second through hole. According to the invention, nasal swab sampling can be automatically conducted on a patient, and the labor intensity of medical workers is relieved; and sampled cotton swabs can be automatically packaged, so that the cotton swabs are convenient to transport and intensively detect.
Owner:苏州晓岩居贸易有限公司
Method and medium for detecting the presence or absence of methicillin resistant staphylococcus aureus (MRSA) in a test sample
InactiveUS20110275114A1Facilitates handling and packaging and storingEasy to testMicrobiological testing/measurementThroatLesion swab
A dry sample analyzing mixture, a liquid sample analyzing medium, and a sample analyzing method, are described for use in detecting the presence or absence of Methicillin Resistant Staphylococcus Aureus (“MRSA”) in an incubated first generation biological or environmental specimen sample. First generation specimen samples that can be analyzed include nasal swabs, lesion swabs, skin swabs, throat swabs, food swabs, tanning salon swabs, gym swabs, restaurant swabs, and the like. The medium and method include an anti-ribosomal antibiotic component that will selectively prevent Methicillin Susceptible Staphylococcus Aureus (“MSSA”) from growing in the medium, while allowing MRSA to grow in the medium. The medium also includes components which will stimulate growth of MRSA. The medium also includes components which will produce a detectable signal, which signal indicates the presence of MRSA in the incubated sample.
Owner:PILOTS POINT LLC
Novel nucleic acid extraction-free preservation solution
PendingCN112608978ACause harmMicrobiological testing/measurementAgainst vector-borne diseasesAntifungalActive agent
The invention relates to a novel nucleic acid extraction-free preservation solution, and discloses the preservation solution capable of being directly used for detection without subsequent nucleic acid extraction and a preparation method of the preservation solution. The preservation solution comprises the following components: 0.1-2% of a surfactant, 0.1-2% of an acid-base buffer agent, 1-5% of citrate, 0.1-5% of weak acid, 0-10% of guanidine salt, 0-0.1% of protease K, 0.1-1% of a chelating agent. 0.1-2% of an antiseptic agent, with the balance being water. The preservation solution is used for preserving tissue and cell analysis samples, and comprises coronaviruses, influenza viruses, hand-foot-and-mouth viruses, measles rubella viruses and other types of viruses, mycoplasma and chlamydia. After a nasal swab and a pharynx swab are placed in the preservation solution, the unique components of the preservation solution can protect nucleic acid RNA from being degraded by RNA enzyme and protect deoxyribonucleic acid DNA from being degraded by DNA enzyme; and the preservation solution has strong antibacterial and antifungal effects through combined use of multiple preservatives.
Owner:安徽雷根生物技术有限公司
Nasopharynx swab collector
PendingCN113633492AGuaranteed accuracyAvoid direct contactBreathing protectionTreatment roomsThroat swabEngineering
A nasopharynx swab collector comprises an isolation cabin, a collection box body is arranged in the isolation cabin, a display screen with a built-in microprocessor and a manipulator with a recognizer are arranged on the collection box body, and a height-adjustable head fixing frame is obliquely arranged in front of the display screen; a plurality of storage cavities are arranged in the isolation cabin, electric control doors communicated with the storage cavities are respectively arranged on the front surface of the collection box body, a spraying device is arranged in the isolation cabin, and the microprocessor controls the manipulator to complete collection, packaging and recovery of throat swabs or nose swabs and control starting of the spraying device. According to the invention, intelligent machinery is adopted to replace manpower to collect nasopharyngeal swabs of a patient, so that the accuracy of sample collection is ensured, and the collection efficiency is improved; meanwhile, the isolation cabin adopts single-person isolation sampling and is disinfected in time, so that direct contact between medical personnel and patients is avoided, cross infection is prevented, and the infection probability is reduced.
Owner:WUHAN UNIV
Integrated antigen detection device and antigen detection process quality control method thereof
PendingCN114878806AMake sure to modifyAccurate identificationSurgeryVaccination/ovulation diagnosticsNasal passagesNasal Cavity Epithelium
The invention belongs to the technical field of antigen detection, and particularly relates to an integrated antigen detection device and an antigen detection process quality control method thereof, and the method comprises the following steps: obtaining a first image of a nasal swab part inserted into a nasal cavity; first judgment is carried out based on the first image, and whether sample sampling of the detected person is qualified or not is judged. The problem that an infected person cannot be found in time due to the fact that the self-testing process of the detected person is not standard, misoperation or intentional cheating exists in the prior art is solved; the method has the advantages that the self-test process of the subject is standardized, misoperation or intentional cheating behaviors of the subject in use are effectively prevented, and therefore the infected person is effectively found in time.
Owner:孙非 +1
Nasal swab detection device for alpha radioactive internal contamination
ActiveCN105549061AImprove detection efficiencyLow detection limitX/gamma/cosmic radiation measurmentNasal cavitySignal processing circuits
The invention discloses a nasal swab detection device for alpha radioactive internal contamination. The device comprises a nasal swab frame, a sample frame and a vacuum cavity, wherein wherein the nasal swab frame is used for mounting nasal swabs, the sample frame is used for mounting the nasal swab frame, at least one nasal swab can be mounted on the nasal swab frame, and the heads of the nasal swabs cannot be shielded; an ejection mechanism is mounted on the rear end surface in the sample frame, and the nasal swab frame can be inserted from the front end surface of the sample frame and is tightly matched with the ejection mechanism; detectors are symmetrically arranged on the left side and the right side of the sample frame and connected with one preset signal processing circuit respectively, and the sample frame is located in the vacuum cavity. According to the nasal swab detection device for alpha radioactive internal contamination, a nasal cavity wiping object of a to-be-tested person is taken as a target detection object, the nasal swabs are inserted into the sample frame, detection circuits are arranged on two sides of the sample frame, the whole is placed into the vacuum cavity to be vacuumized for measuring, the distances of a to-be-tested sample and the detectors are fixed, the double detectors form double surface stereo-measurement, and vacuumizing is combined, so that the detection efficiency is improved greatly, and the minimum detectable limit is reduced.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY +1
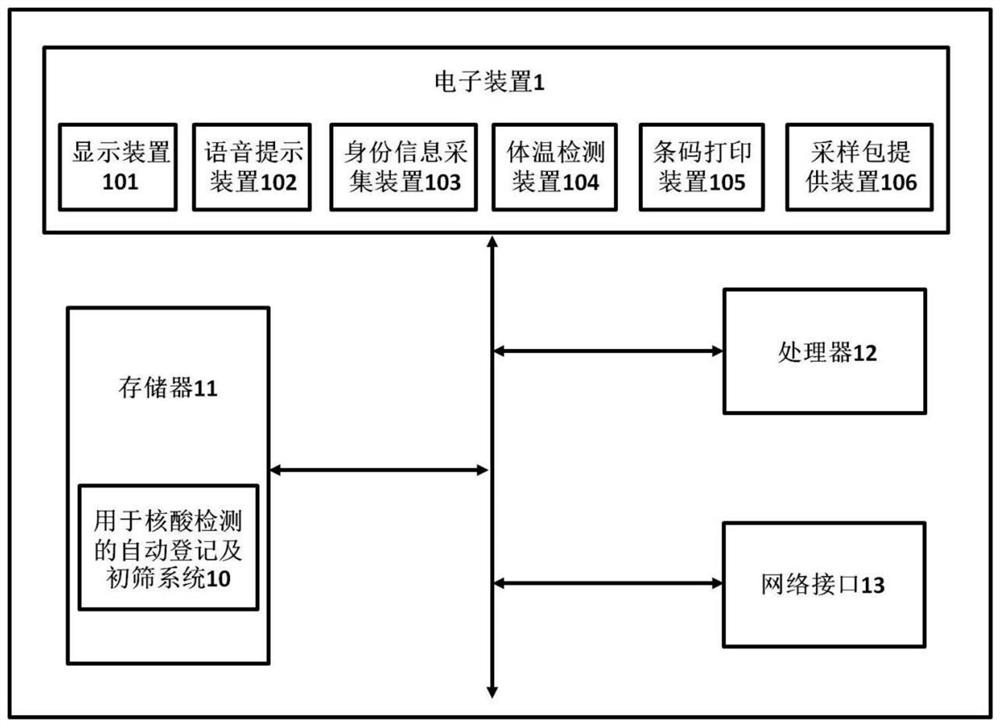
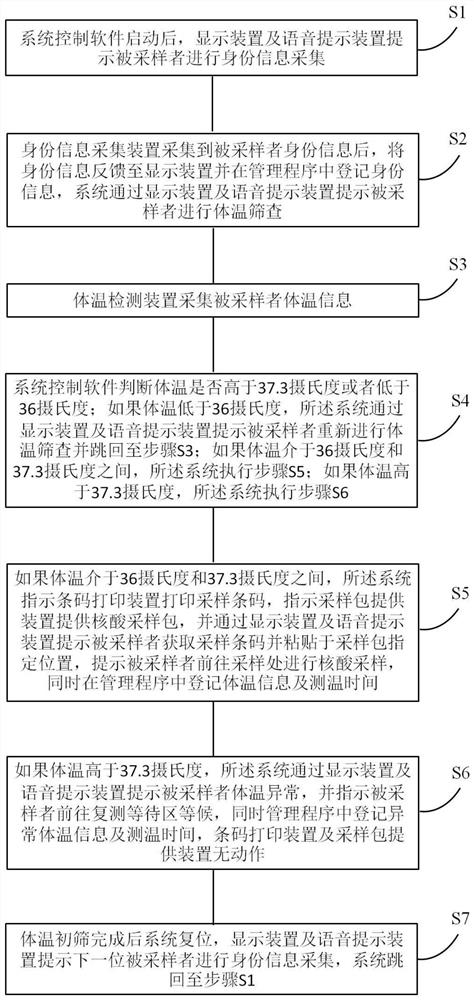
Nucleic acid self-registration and preliminary screening system operation method, electronic device and storage medium
PendingCN112890876AImprove work efficiencyAvoid mutual contactEpidemiological alert systemsSurgeryComputer hardwareThroat swab
The invention discloses a nucleic acid self-registration and preliminary screening system operation method, an electronic device and a storage medium. The electronic device comprises a memory, a processor, a display device, a voice prompt device, an identity information acquisition device, a body temperature detection device, a bar code printing device and a sampling package providing device, and a self-service registration and preliminary screening system which can run on the processor and is used for nucleic acid sampling is stored on the memory. According to the nucleic acid self-registration and preliminary screening system operation method, the electronic device and the storage medium, identity information registration of a sampled person and issuing of a nucleic acid sampling package are completed in a self-service manner, so that the consumption of human resources for sampling is effectively reduced, and the working efficiency of nucleic acid sampling is improved. Meanwhile, in the whole self-service registration process, mutual contact between sampled personnel and between the sampled personnel and sampling personnel can be avoided, the fever personnel are primarily screened and excluded in a body temperature detection mode, and cross infection possibly caused by the fact that the fever personnel and other healthy personnel conduct nucleic acid sampling of nasal swabs, throat swabs and the like at the same time is effectively avoided.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
A kind of method and detection kit for nasal flora detection
ActiveCN108866220BReduce distractionsDesigned for high amplification efficiencyMicrobiological testing/measurementLibrary creationHigh homologyFlora
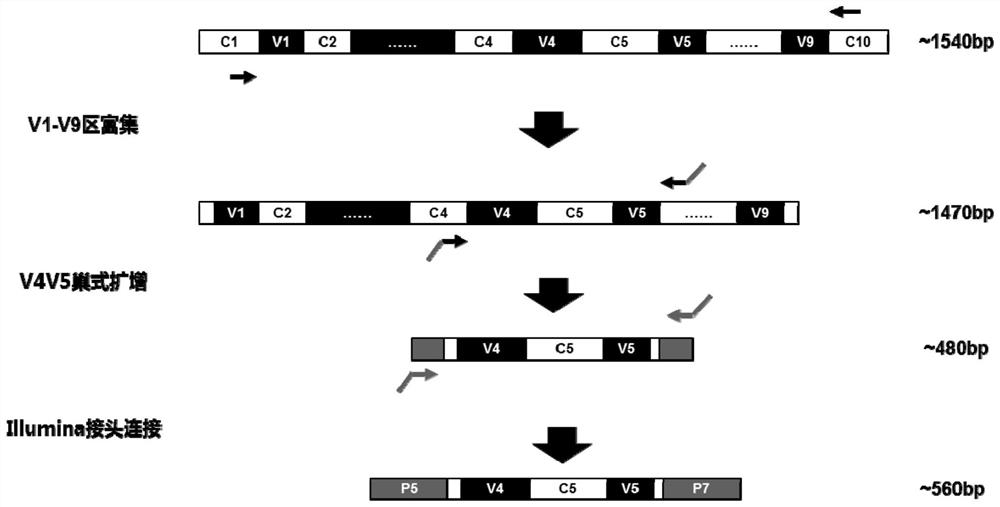
The invention discloses a method for detecting nasal flora and a detection kit. The present invention provides a set of primers for the detection of sample flora, including primers for amplifying the V4-V5 region of bacterial 16S rDNA and primers for amplifying the V1-V9 region of bacterial 16S rDNA; the present invention first uses the amplification efficiency The V1‑V9 primers with high specificity and low human homology first enrich the 16S rDNA fragment, and then use nested PCR to amplify the target segment V4V5 double fragment, which overcomes the need to directly amplify the V4V5 segment. The amplification primers have high homology with the human genome sequence. When amplifying nasal swab samples with high human gDNA content, there will be serious non-specific amplification problems.
Owner:CAPITALBIO CORP
Fluorescent quantitative RT-PCR detection kit for Eurasian avian h1n1 swine influenza virus and its application
ActiveCN104450955BStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceVirus influenza
The invention discloses a fluorescence quantification RT-PCR (reverse transcription-polymerase chain reaction) detection kit of a Eurasian avian-like type H1N1 swine influenza virus and an application thereof. By virtue of multiple alignments of sequences, and aiming at conserved gene segments of the Eurasian avian-like type H1N1 swine influenza virus, an A(H1N1) (2009) swine influenza virus, a classical type H1N1 swine influenza virus and a human-derived H1N1 swine influenza virus, a primer and a probe with strong specificity for detecting the Eurasian avian-like type H1N1 swine influenza virus are designed and are used for performing real-time fluorescence RT-PCR detection. Experiment results show that by using the kit provided by the invention to detect the Eurasian avian-like type H1N1 swine influenza virus, the specificity is high, and the detection sensitivity to virus RNA (ribonucleic acid) can reach 4.6*10<-7>ng / reaction, so that tissue samples of nasal swabs, lungs, tracheas and the like of a to-be-detected swine herd can be detected, and chick embryo allantoic fluid can also be detected. The kit is simple to operate, easy to popularize and convenient in basic-level operation and application, and can become a useful detection tool for epidemic disease diagnosis of the Eurasian avian-like type H1N1 swine influenza virus and epidemiologic investigations.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com