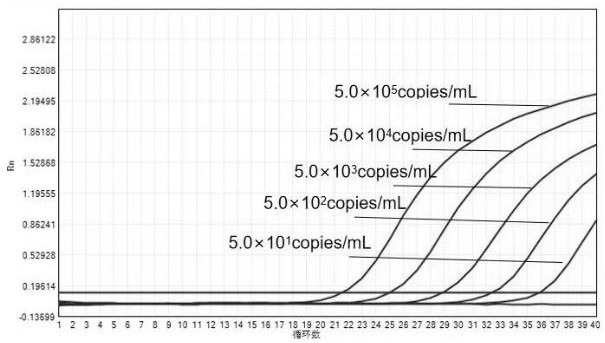
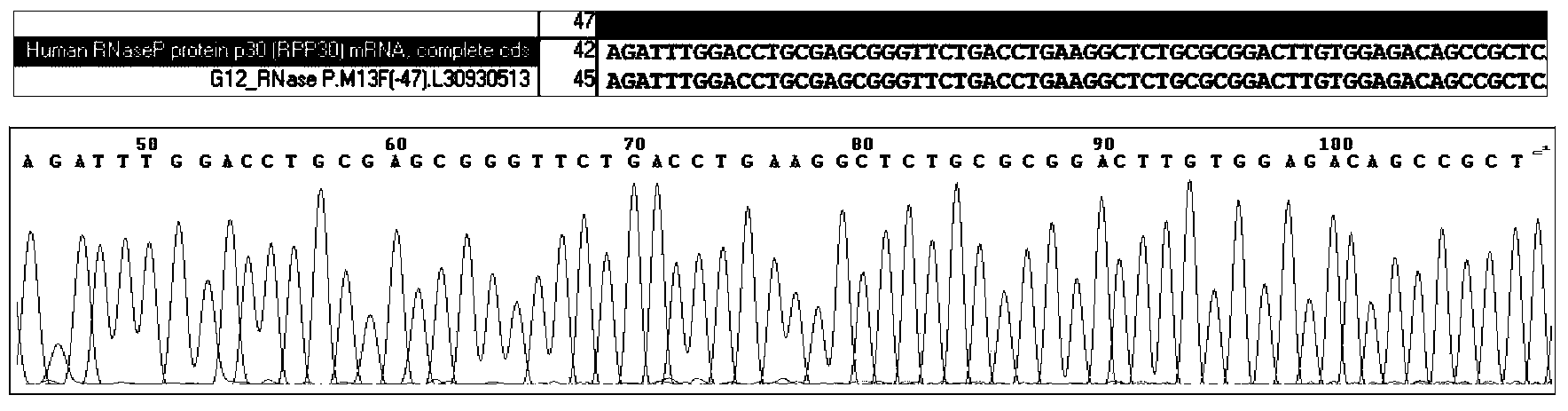
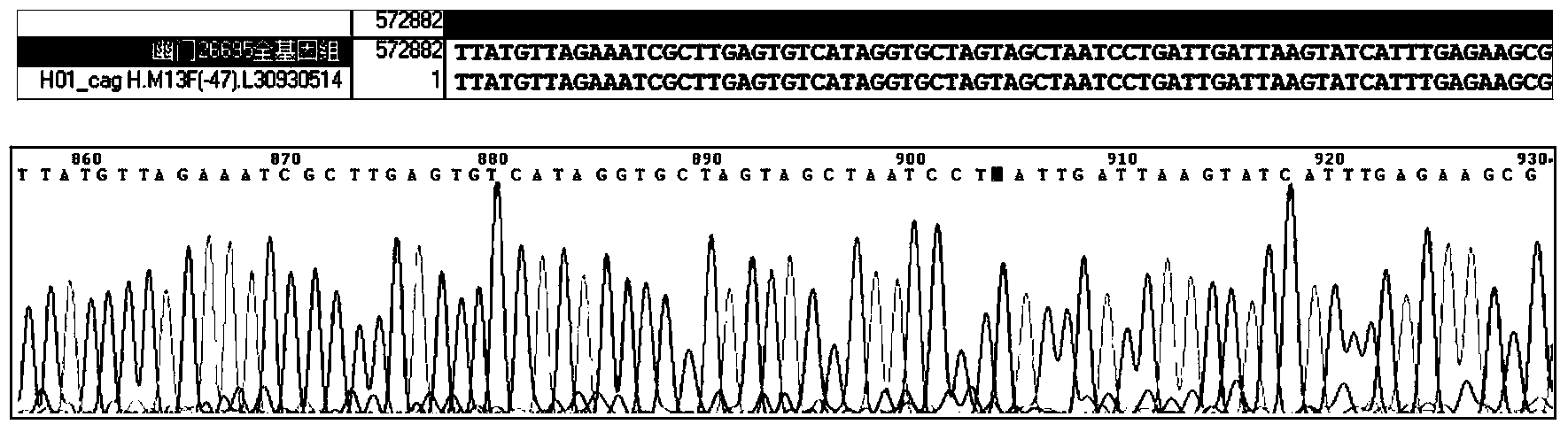
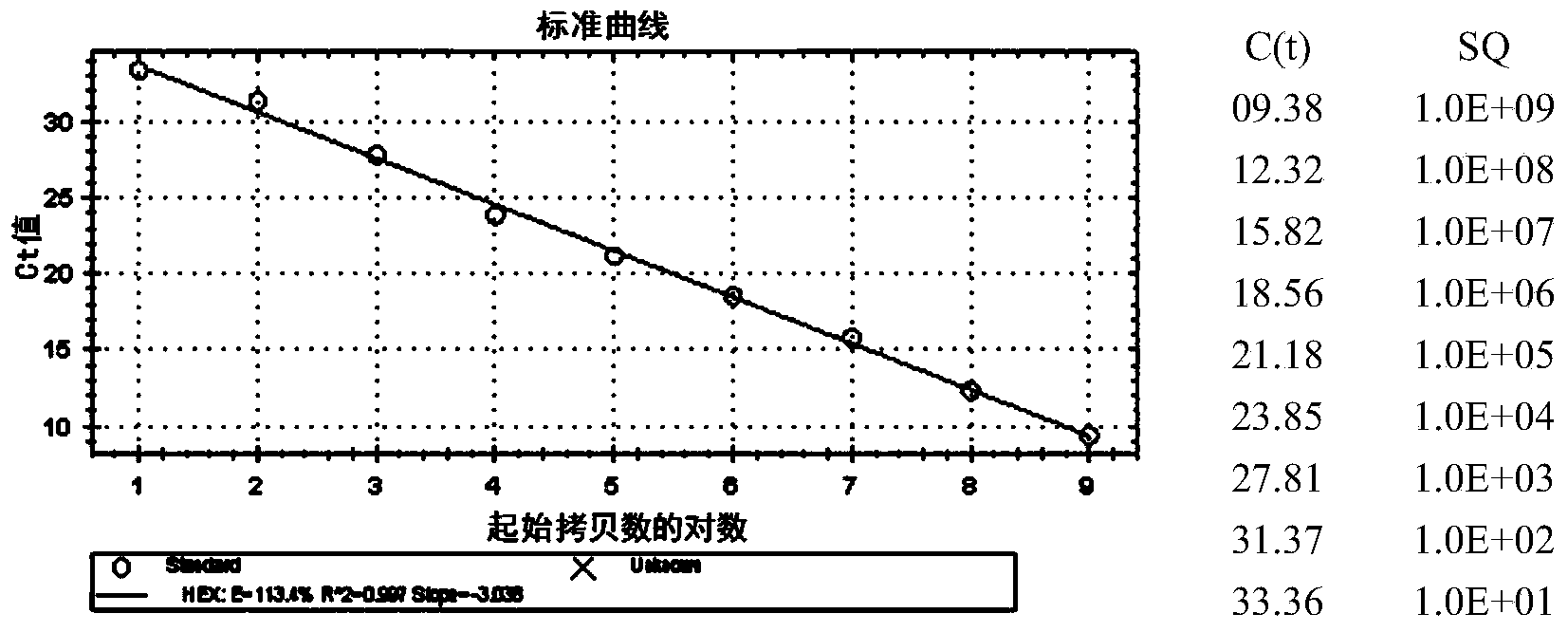
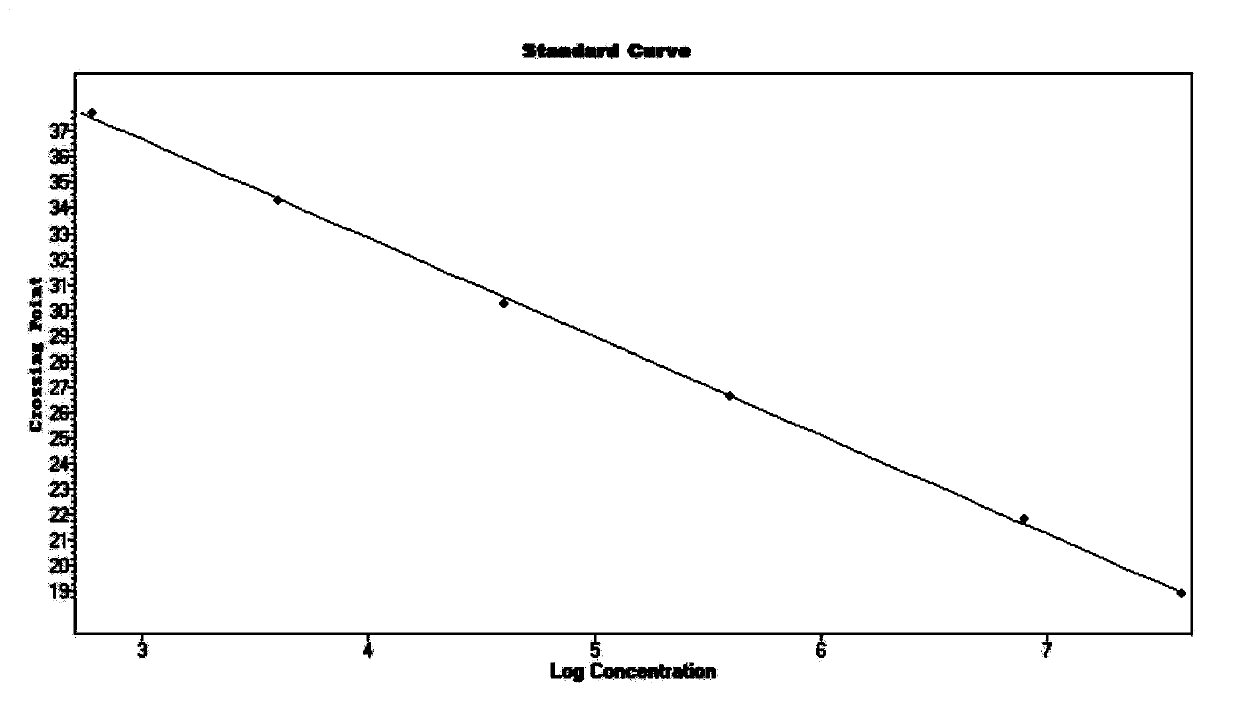
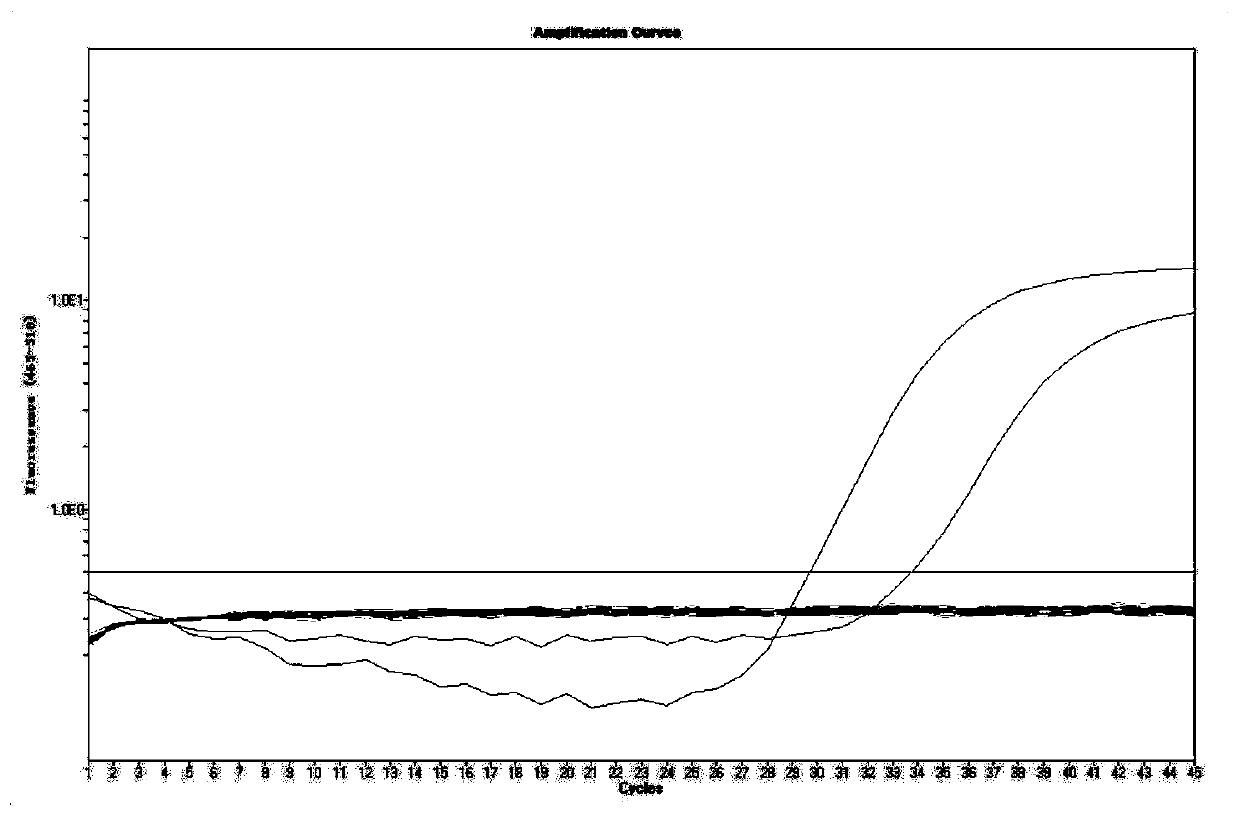
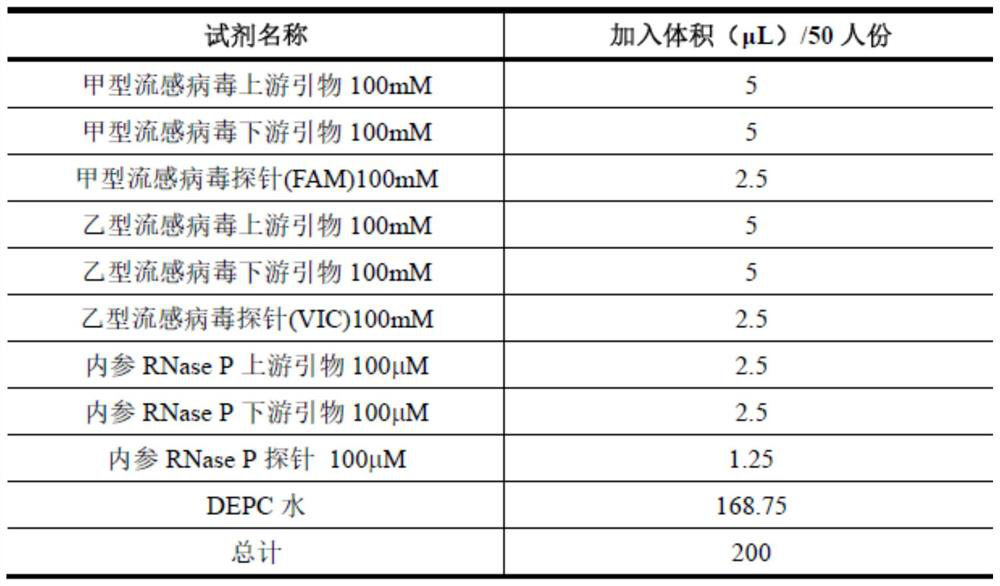
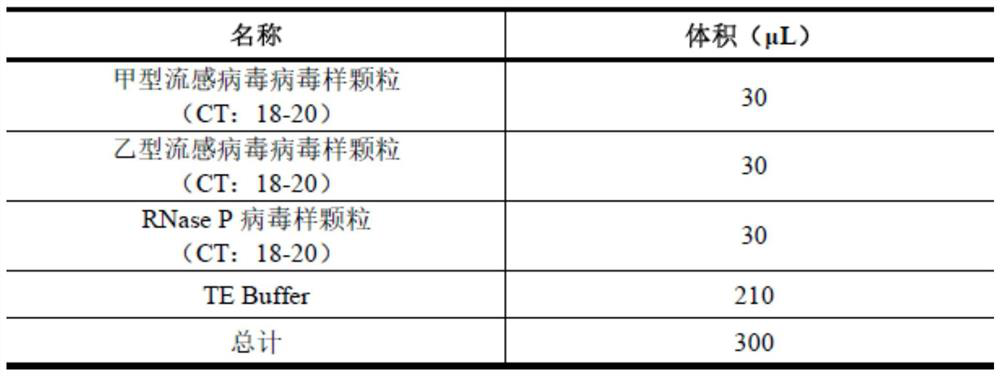
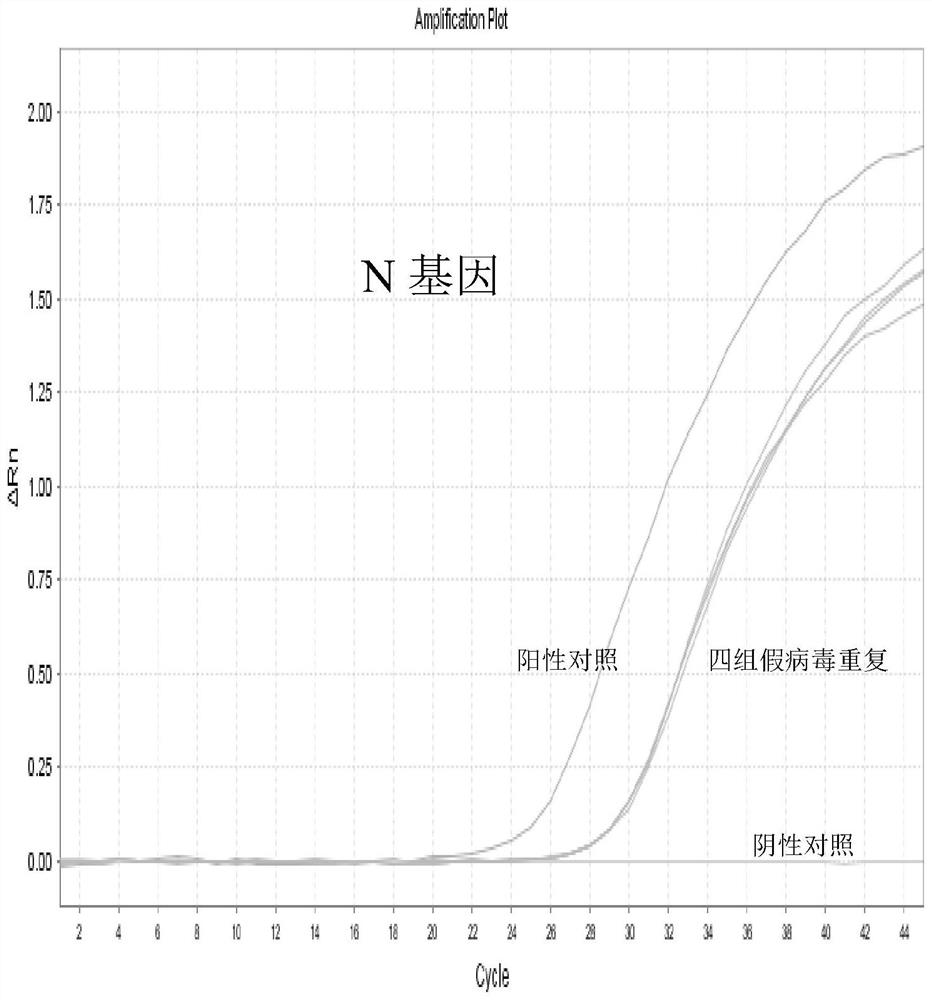
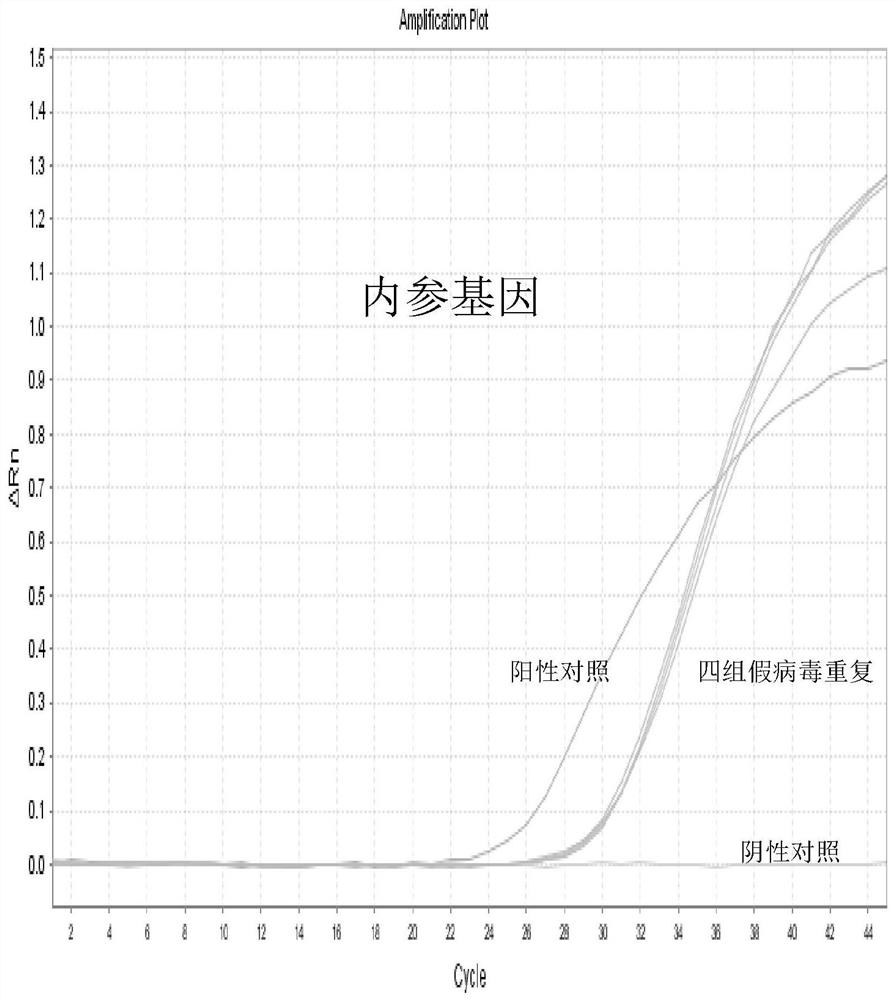
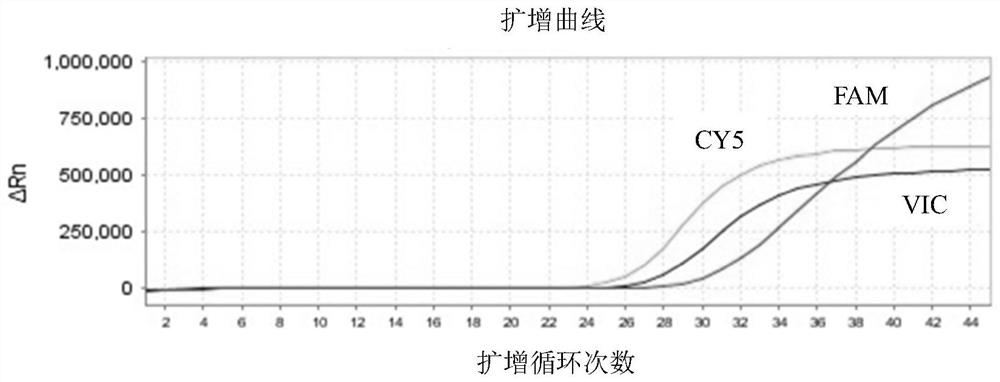
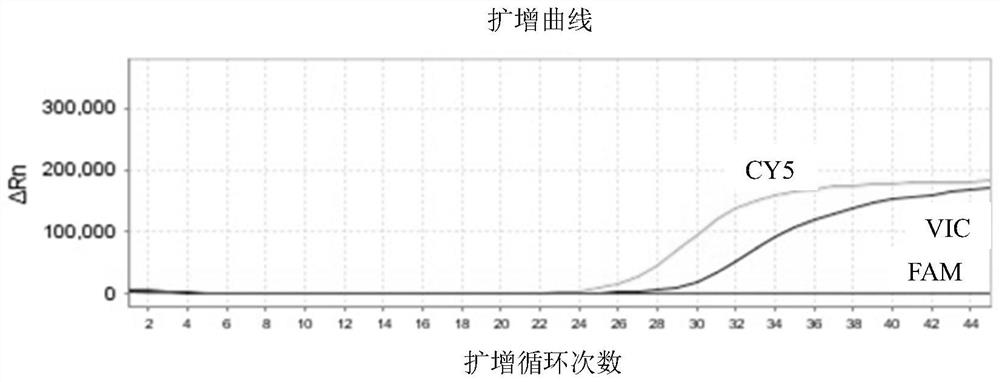
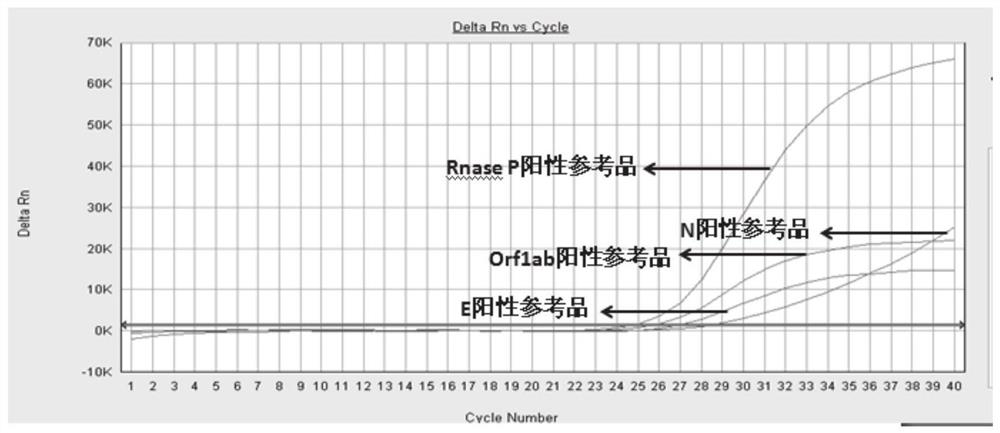
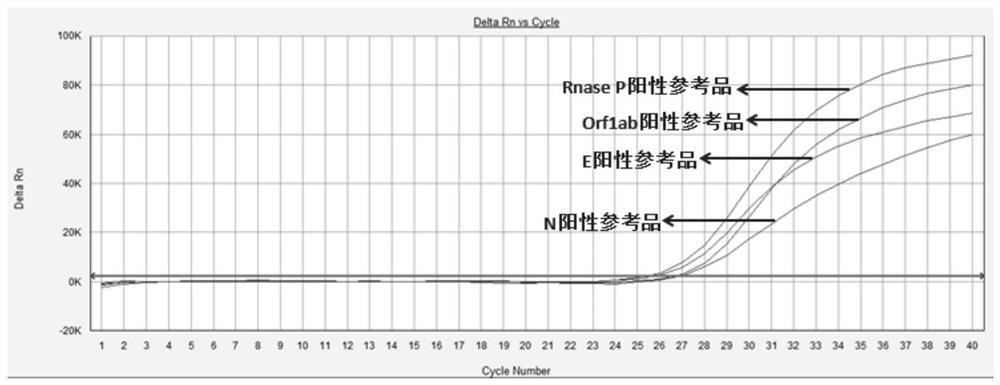
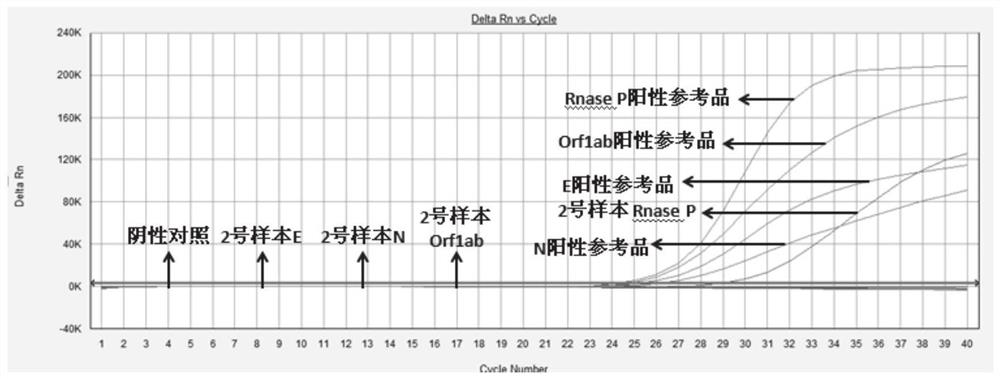
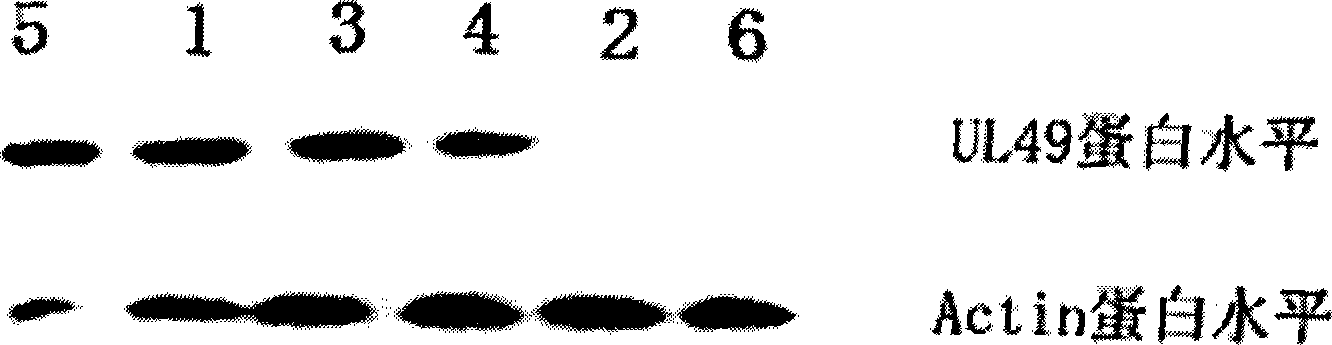Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "RNase P" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
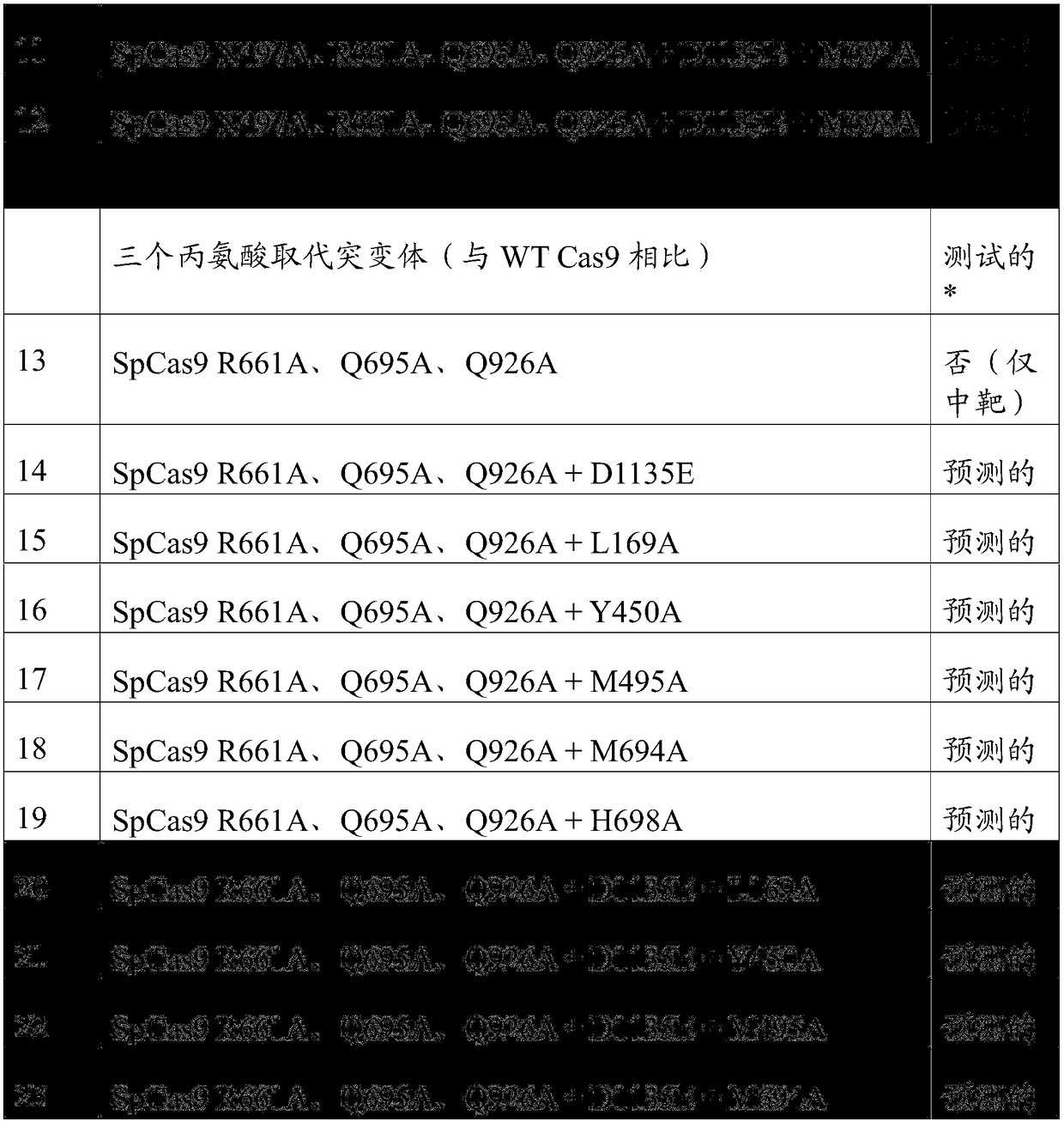
Patent Status
Application Year
Inventor
Ribonuclease P (EC 3.1.26.5, RNase P) is a type of ribonuclease which cleaves RNA. RNase P is unique from other RNases in that it is a ribozyme – a ribonucleic acid that acts as a catalyst in the same way that a protein-based enzyme would. Its function is to cleave off an extra, or precursor, sequence of RNA on tRNA molecules. Further, RNase P is one of two known multiple turnover ribozymes in nature (the other being the ribosome), the discovery of which earned Sidney Altman and Thomas Cech the Nobel Prize in Chemistry in 1989: in the 1970s, Altman discovered the existence of precursor tRNA with flanking sequences and was the first to characterize RNase P and its activity in processing of the 5' leader sequence of precursor tRNA. Recent findings also reveal that RNase P has a new function. It has been shown that human nuclear RNase P is required for the normal and efficient transcription of various small noncoding RNAs, such as tRNA, 5S rRNA, SRP RNA and U6 snRNA genes, which are transcribed by RNA polymerase III, one of three major nuclear RNA polymerases in human cells.
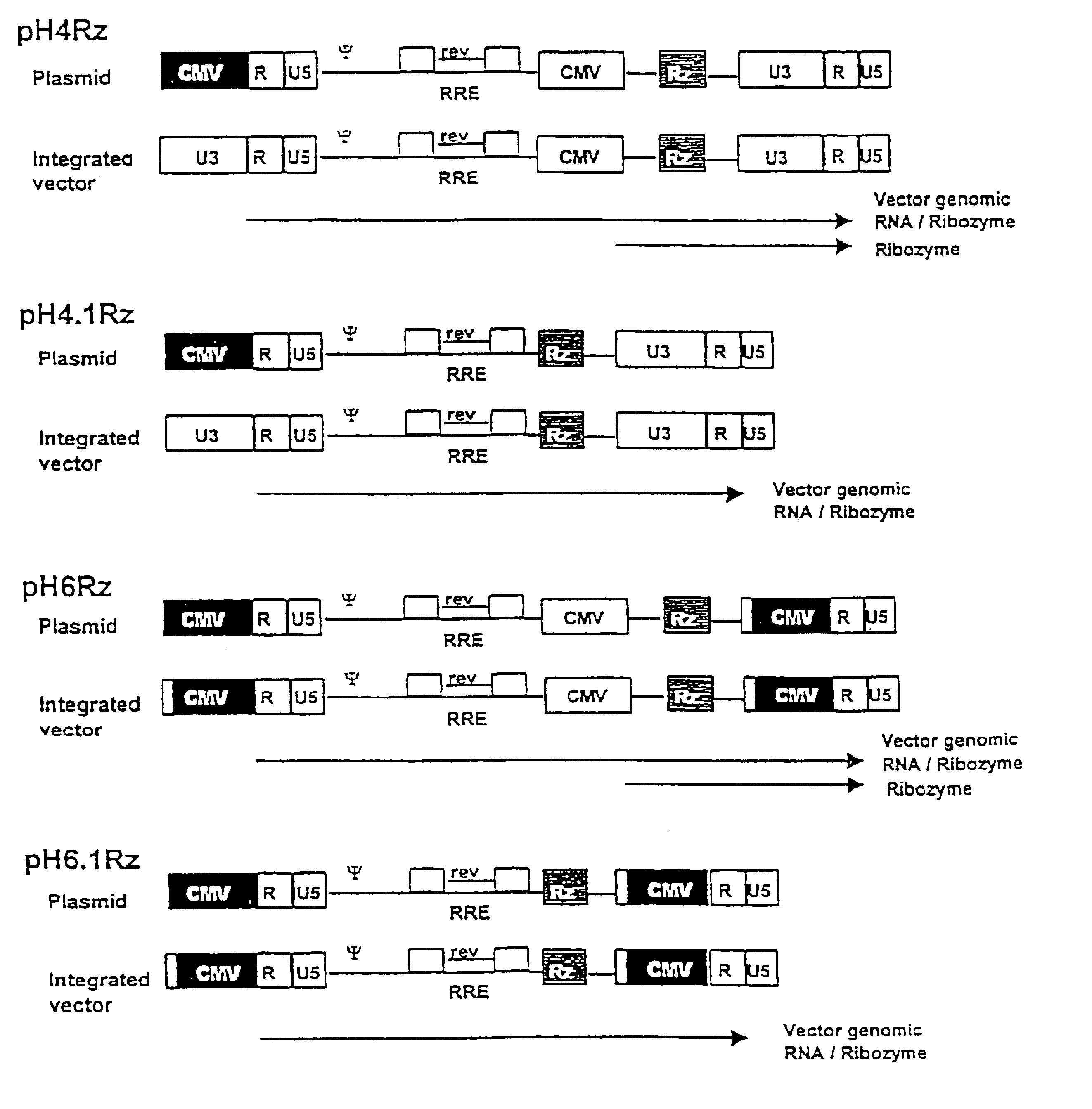
Anti-viral vectors
InactiveUS6783981B1Efficient exportPeptide/protein ingredientsGenetic material ingredientsNucleotideRNase P
A viral vector production system is provided which system comprises: (i) a viral genome comprising at least one first nucleotide sequence encoding a gene product capable of binding to and effecting the cleavage, directly or indirectly, of a second nucleotide sequence, or transcription product thereof, encoding a viral polypeptide required for the assembly of viral particles, (ii) a third nucleotide sequence encoding said viral polypeptide required for the assembly of the viral genome into viral particles, which third nucleotide sequence has a different nucleotide sequence to the second nucleotide sequence such that said third nucleotide sequence, or transcription product thereof, is resistant to cleavage directed by said gene product; wherein at least one of the gene products is an external guide sequence capable of binding to and effecting the cleavage by RNase P of the second nucleotide sequence. The viral vector production system may be used to produce viral particles for use in treating or preventing viral infection.
Owner:OXFORD BIOMEDICA (UK) LTD
Primer, probe, test kit and method for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-nCoV
ActiveCN111057797AGood repeatabilityQuick checkMicrobiological testing/measurementMicroorganism based processesNasopharyngeal aspirateVirus detection
The invention belongs to the technical field of virus detection, and particularly relates to a primer, probe, test kit and method for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-nCoV. A single-tube double-fluorescence channel is adopted to simultaneously detect the existence of the novel coronavirus 2019-nCoV and reference gene Rnase P, and the existence of the novelcoronavirus 2019-nCoV RNA in specimens such as alveolar lavage fluid, nasopharyngeal swab, whole blood, serum, feces and tissues can be detected. The method is short in detection time period, and issuitable for clinical and bedside rapid detection of diagnosis; the virus detection specificity is high, and the accuracy is high; virus qualitative analysis and quantitative analysis are carried out,and the quantitative linear range is good; the detection sensitivity is high; the experimental result is good in repeatability and high in precision; and the reference gene is added into a detectionsystem, so that the quality of the whole process of extracting and amplifying the sample can be monitored through the detection result of the reference gene.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Novel coronavirus 2019-nCoV fluorescent RPA detection primers, probe, kit and method
PendingCN111500776AHigh precisionGood repeatabilityMicrobiological testing/measurementMicroorganism based processesReference genesAlveolar lavage fluid
The invention discloses novel coronavirus 2019-nCoV fluorescent RPA detection primers, a probe, a kit and a method. The kit comprises novel coronavirus 2019-nCoV specific primers shown in SEQ ID NO. 1-2 and a probe shown in SEQ ID NO. 3. According to the invention, the existence of the novel coronavirus 2019-nCoV ORF1ab gene and the internal reference gene RNase P is simultaneously detected by adopting a single-tube double-fluorescence channel, and the existence of the novel coronavirus 2019-nCoV RNA in pulmonary alveolar lavage fluid, nasal swab, pharyngeal swab, sputum and other samples canbe detected. The method is short in detection time and suitable for clinical and bedside virus nucleic acid rapid screening, the kit has extremely high sensitivity and specificity, an internal reference gene is added into the detection system, and quality monitoring is performed on the extraction and amplification process of the sample according to the detection result of the internal reference gene.
Owner:湖南润美基因科技有限公司
Method for detection of pathogenic organisms
A method for detection of pathogenic organisms wherein the method includes differentiation between species. The method is especially suitable to detect and to diagnose infection by pathogenic organisms which are hard and / or laborious to detect with conventional methods. The method relies upon analysis of specific variable regions of the RNase P RNA gene, namely the P3 and / or P19 region(s).
Owner:HERRMANN BJORN +2
High-sensitivity novel coronavirus 2019-nCoV nucleic acid detection kit and use method
PendingCN113322348AHigh detection sensitivityImprove the detection rateMicrobiological testing/measurementAgainst vector-borne diseasesNucleic acid detectionRNase P
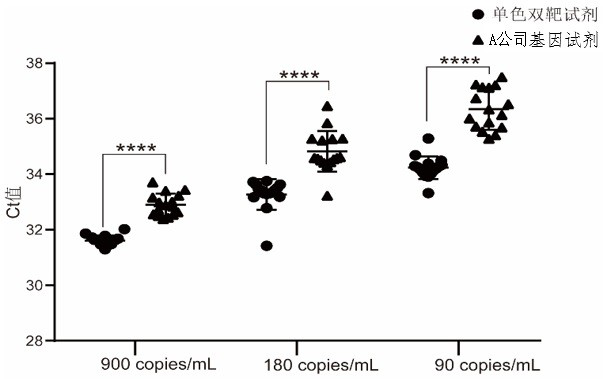
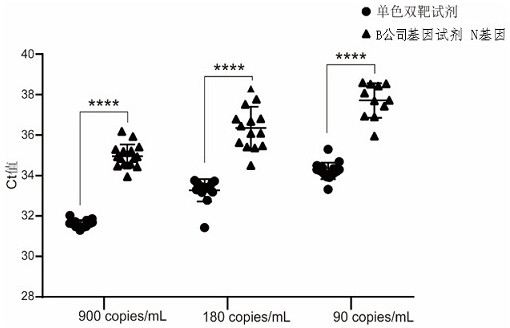
The invention discloses a high-sensitivity novel coronavirus 2019-nCoV nucleic acid detection kit and a use method. The high-sensitivity novel coronavirus 2019-nCoV nucleic acid detection kit comprises an ORF1ab gene primer pair, an N gene primer pair, an endogenous reference gene primer pair and a probe set. Novel coronavirus (2019-nCoV) ORF1ab and N genes are used as target areas, specific primers and probes are designed; by detecting the change of fluorescence signals, the novel coronavirus 2019-nCoV nucleic acid in a sample is subjected to qualitative detection; the 5' ends of the ORF1ab gene probe and the N gene probe are modified by a fluorophore FAM to form a monochromatic double-target detection system, and a large-volume system higher than the conventional system is used for amplification, so that when nucleic acid detection is carried out on the novel coronavirus 2019-nCoV, the detection sensitivity is high and can reach 50 copies / mL; and moreover, the nucleic acid extraction and detection process is monitored by taking a ribonuclease P (RNase P, RNP) gene widely existing in normal human epithelial cells as an internal target gene, and false negative and false positive are avoided, so that the nucleic acid detection kit is wide in market popularization prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Gastric juice multiple real-time polymerase chain reaction (PCR) detection-based helicobacter pylori (HP) individualized treatment auxiliary diagnosis method
InactiveCN103911446AMicrobiological testing/measurementMicroorganism based processesReference genesIndividualized treatment
The invention discloses a gastric juice multiple real-time polymerase chain reaction (PCR) detection-based helicobacter pylori (HP) individualized treatment auxiliary diagnosis method. According to the method, specific genes of HP, HP clarithromycin drug-resistant mutation sites A2143G and A2142G, and polymorphism of CYP2C19 gene sites such as CYP2C19*2(G681A) and CYP2C19*3(G636A) of corresponding patients can be simultaneously detected from 10-150mu l of gastric juice samples, and human epithelial cell ribonuclease P(Rnase P) is taken as a reference gene. The detection method is rapid, accurate and small in sample demand amount, whether HP infection exists can be detected from the 10-150mu l of gastric juice samples in a single test, the HP clarithromycin drug-resistant conditions and genotypes of CYP2C19 of the patients can be reported, and medication guide is provided for individualized eradication therapy of HP infection.
Owner:ICDC CHINA CDC
Nucleic acid composition, kit and method for simultaneously detecting multiple mutant strains of SARS-CoV-2
ActiveCN113897460AReduce detection timesReduce testing costsMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionWild type
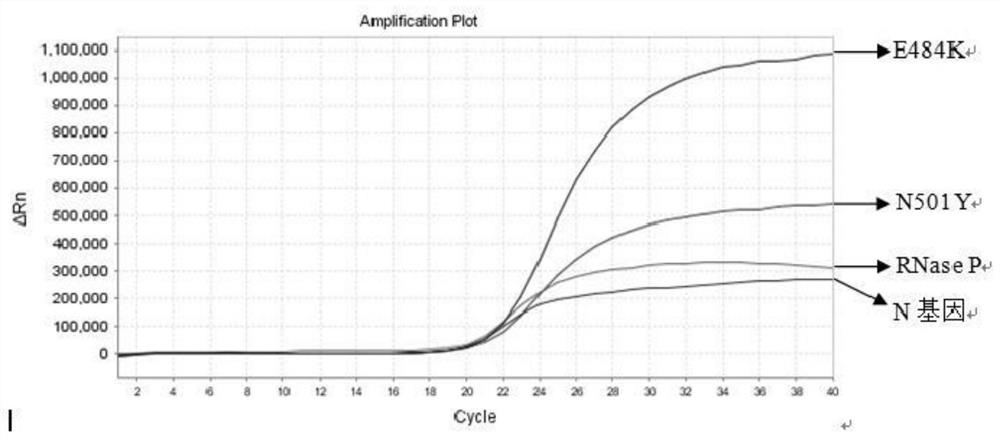
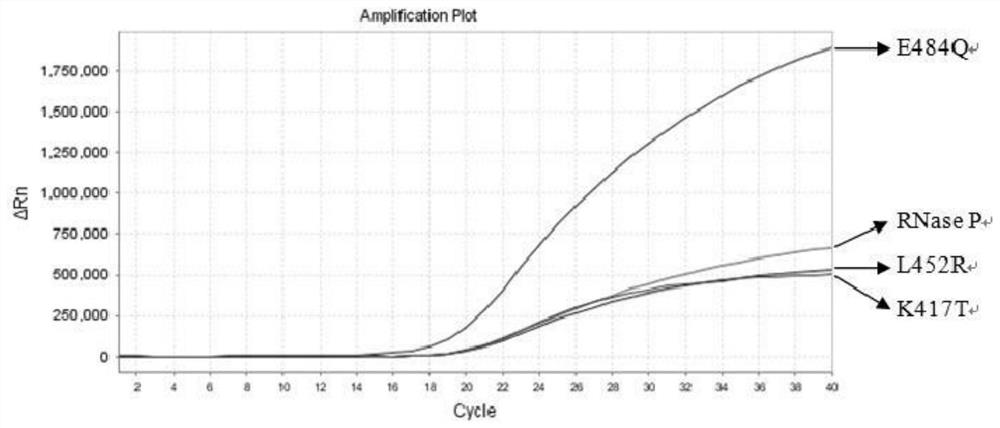
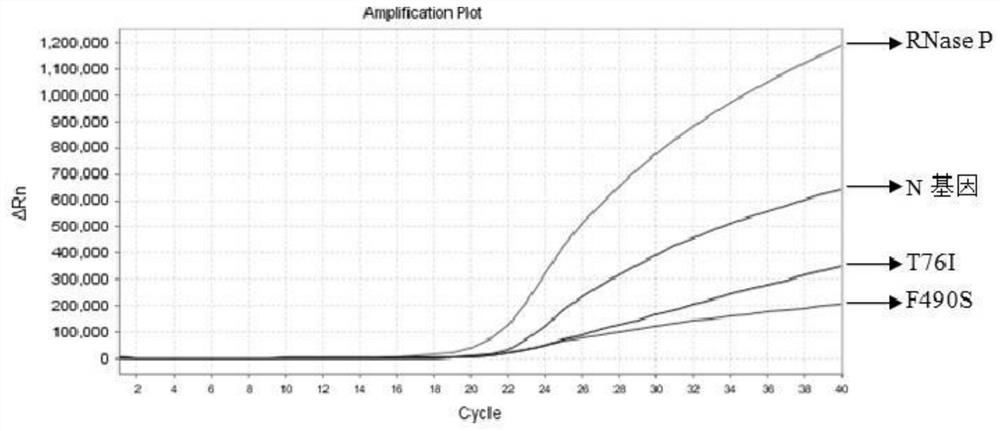
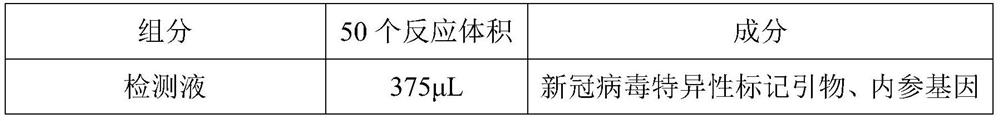
The invention relates to the field of nucleic acid detection, and particularly discloses a nucleic acid composition, a kit and a method for simultaneously detecting multiple mutant strains of SARS-CoV-2. The SARS-CoV-2 comprises a wild type and Alpha mutant strain, a Beta mutant strain, a Gamma mutant strain, an Eta mutant strain, a Delta mutant strain, a lota mutant strain, a Kappa mutant strain and a Lambda mutant strain, and the design sites of a primer probe composition comprise N gene, RNase P gene and S gene N501Y, E484K, K417T, L452R, E484Q, F490S, T76I, L452Q and 246-253del mutant types. The nucleic acid composition, the kit and the method disclosed by the invention can be used for simultaneously and rapidly detecting the SARS-CoV-2 and the main epidemic mutant strain thereof, and have the advantages of high sensitivity and good specificity.
Owner:SHENZHEN ZIJIAN BIOTECH
Novel coronavirus nucleic acid PCR-colloidal gold immunochromatography detection kit
InactiveCN113186346AQuick checkSensitive and accurate detectionMicrobiological testing/measurementMicroorganism based processesOpen reading frameReverse transcriptase
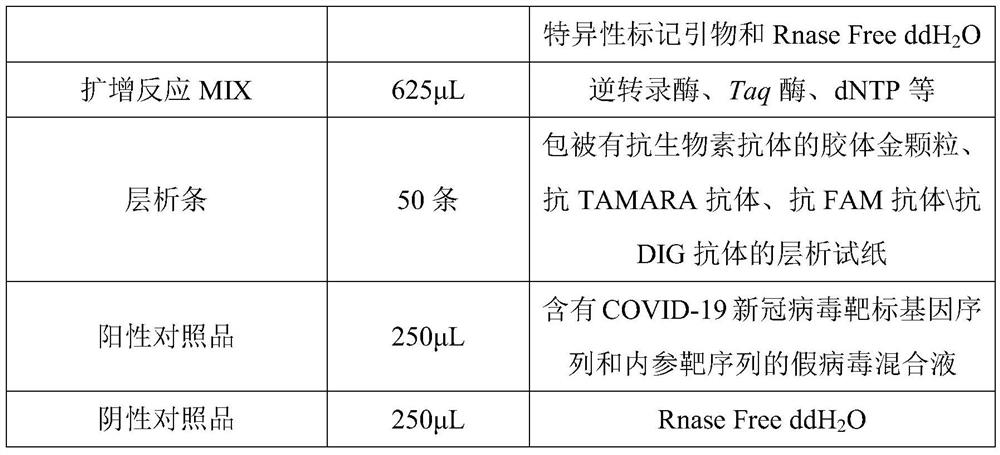
The invention discloses a novel coronavirus nucleic acid PCR-colloidal gold immunochromatography detection kit. The kit comprises an amplification reaction solution, a CoV detection solution, a positive reference substance and a negative reference substance, and the CoV detection liquid comprises a specific marker primer aiming at a new coronavirus open reading frame 1ab and a coding nucleocapsid protein N gene, a specific marker primer of a reference gene RNase P and RNase Free ddH2O. According to the kit, after nucleic acid is extracted from a collected cell sample, a specific fragment is amplified under the action of reverse transcriptase and Taq DNA polymerase, then the nucleic acid detection of the new coronavirus is realized by utilizing colloidal gold immunochromatography, in addition, the kit adopts a reference gene RNase P to carry out tracking quality control on sample collection, nucleic acid extraction and PCR amplification processes, and the whole experiment process is ensured to be within a controllable range.
Owner:北京华诺奥美医学检验实验室有限公司
Six respiratory virus nucleic acid detection kit and use method
InactiveCN112195278AImprove accuracyAvoid False Negative Interpretation ResultsMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionRNase P
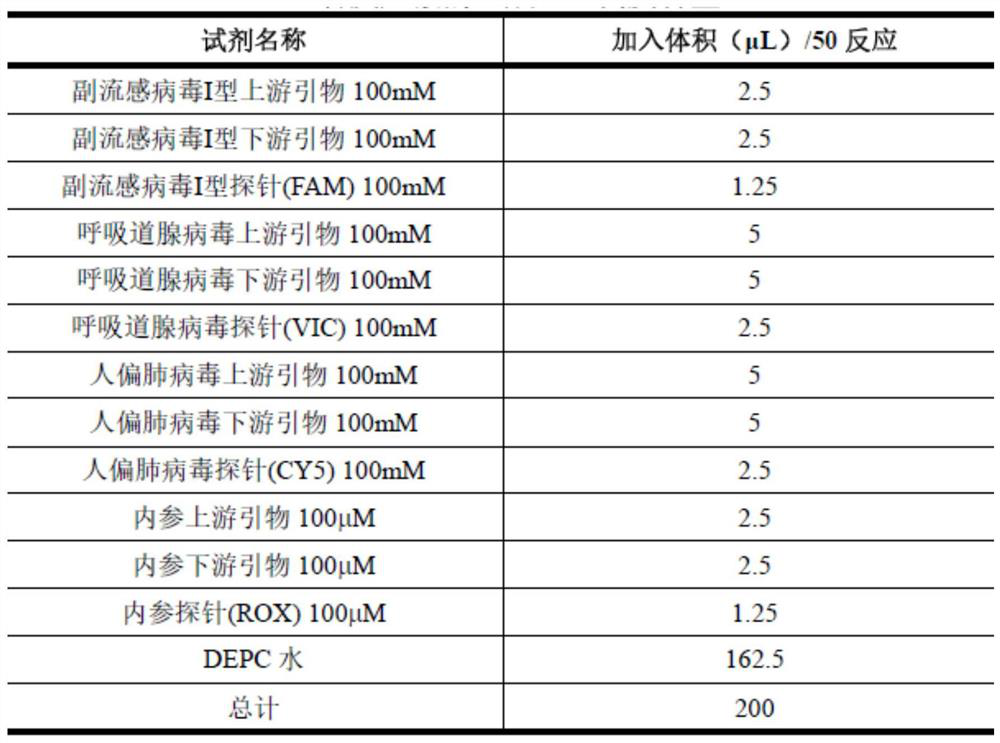
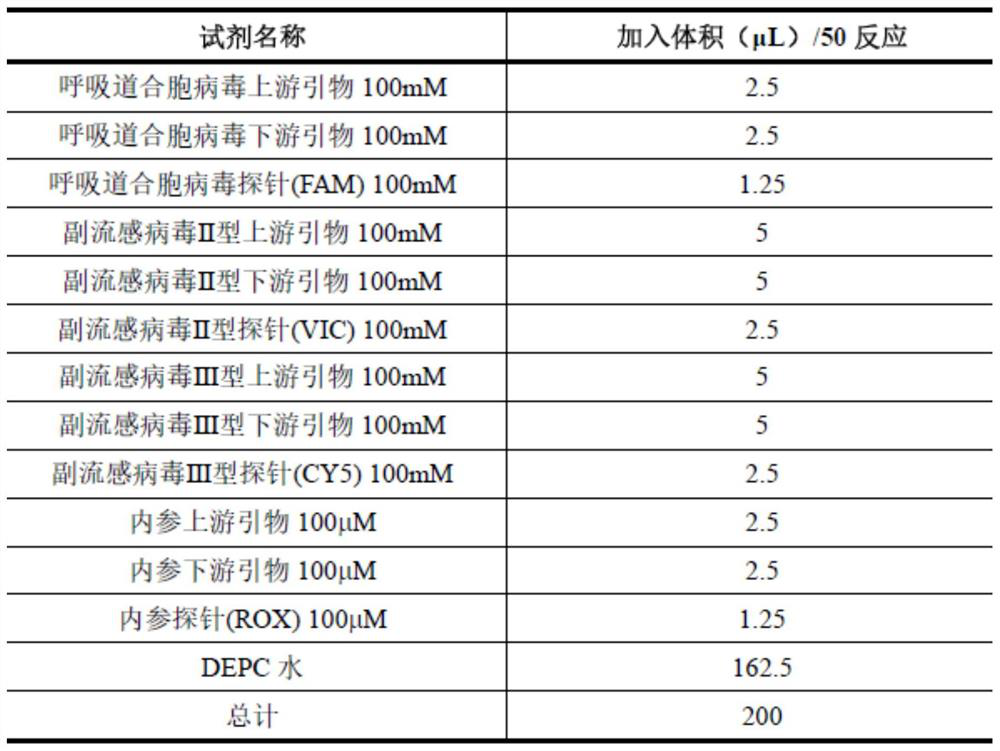
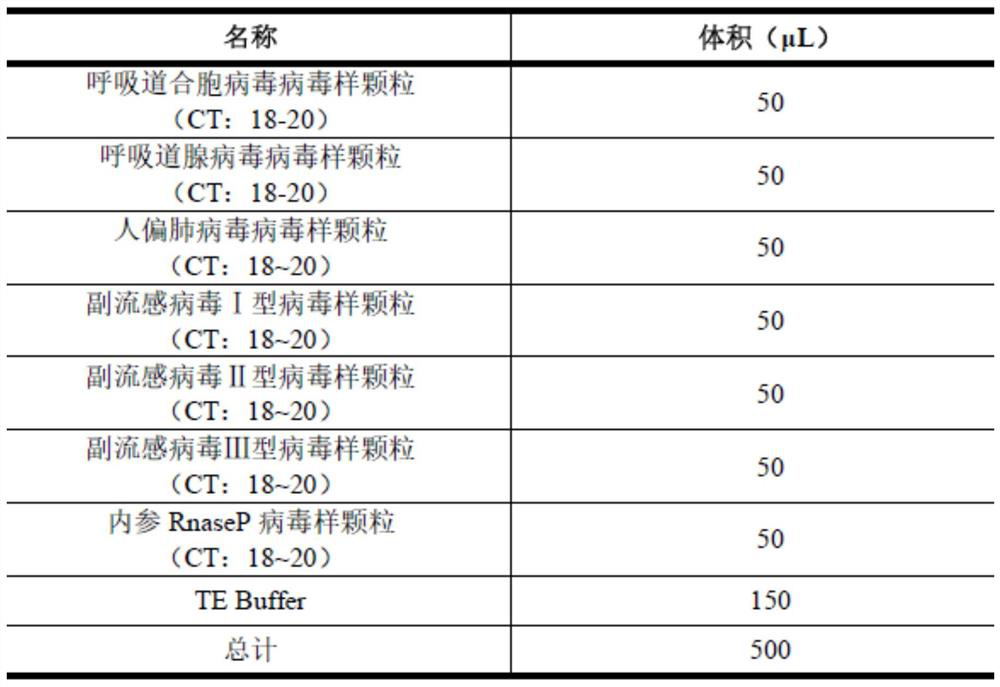
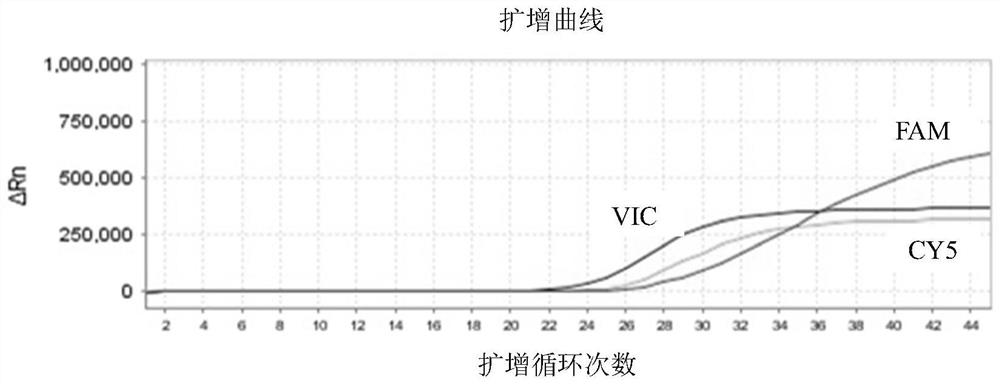
According to a six respiratory virus nucleic acid detection kit and a use method, whether respiratory syncytial virus, respiratory adenovirus, human metapneumovirus, parainfluenza virus type I, parainfluenza virus type II and parainfluenza virus type III exist or not is detected in an A tube and a B tube of the same reaction system, the A tube is used for detecting parainfluenza virus type I, respiratory adenovirus and human metapneumovirus, and comprises probes which are respectively marked with FAM, VIC and CY5, the B tube is used for detecting respiratory syncytial virus, parainfluenza virus type II and parainfluenza virus type III, and comprises probes which are respectively marked with FAM, VIC and CY5, the A / B tubes are provided with an internal reference control, the internal reference control is a human conservative gene RNase P fragment, an internal reference probe is marked with ROX, and the internal reference control can display the false negative, so that the occurrence offalse negative interpretation results caused by inhibitors existing in a sample or operation errors is avoided, and thereby the accuracy of PCR detection is improved.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD
Reference gene for respiratory tract RNA virus PCR detection and detection product thereof
PendingCN111286560AImprove diagnostic accuracyTruly Monitor IntegrityMicrobiological testing/measurementMicroorganism based processesReference genesRNase P
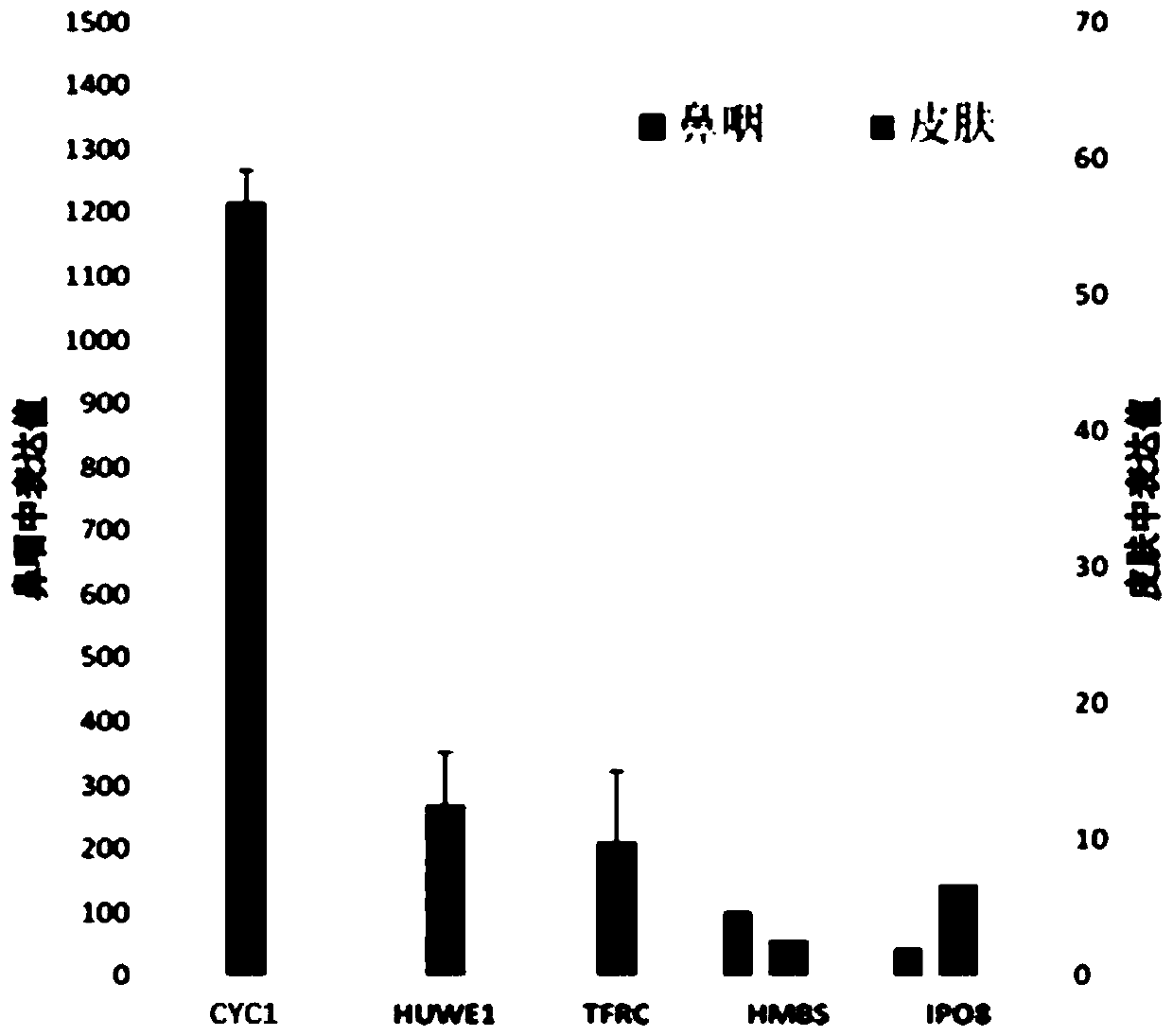
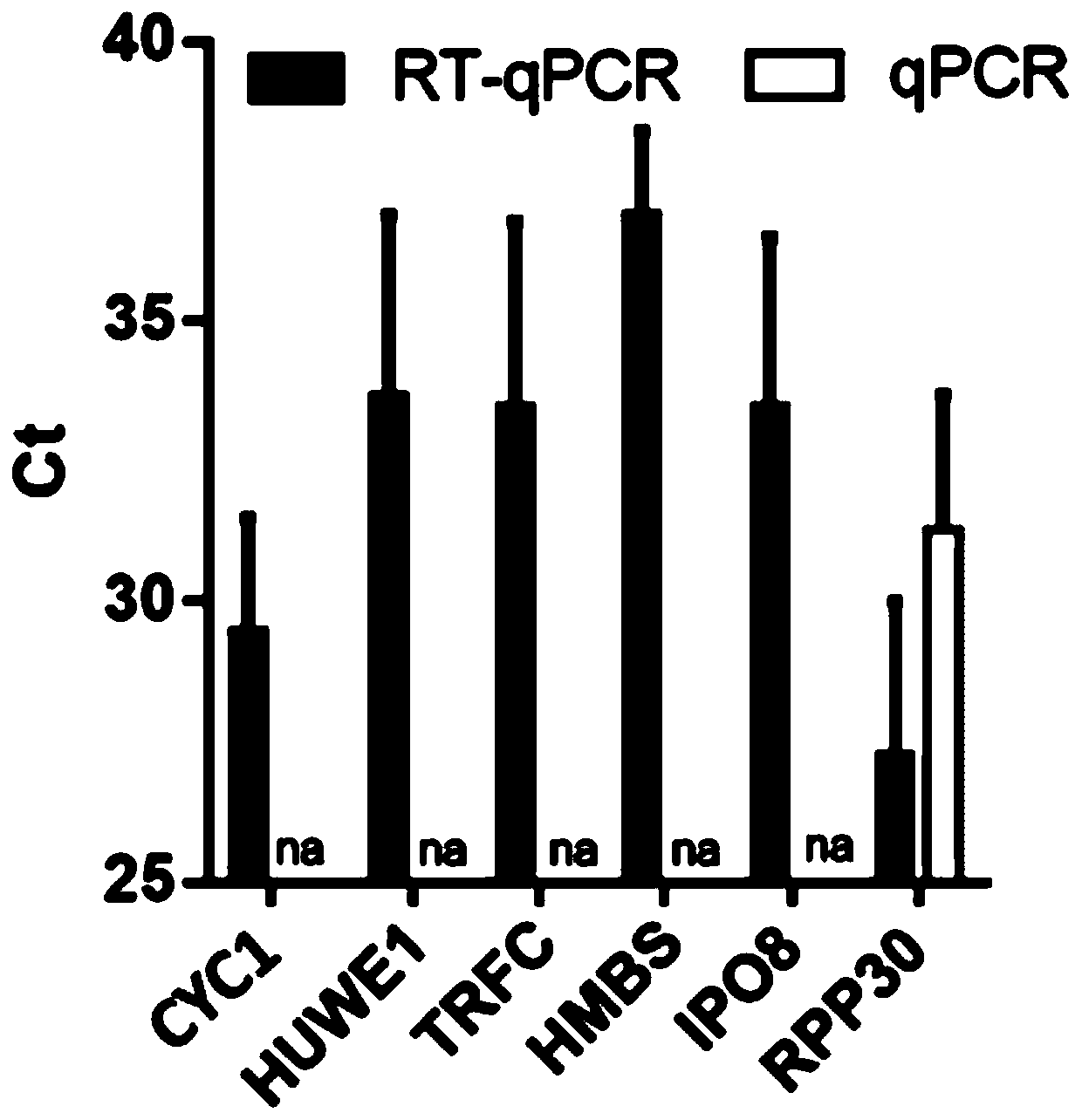
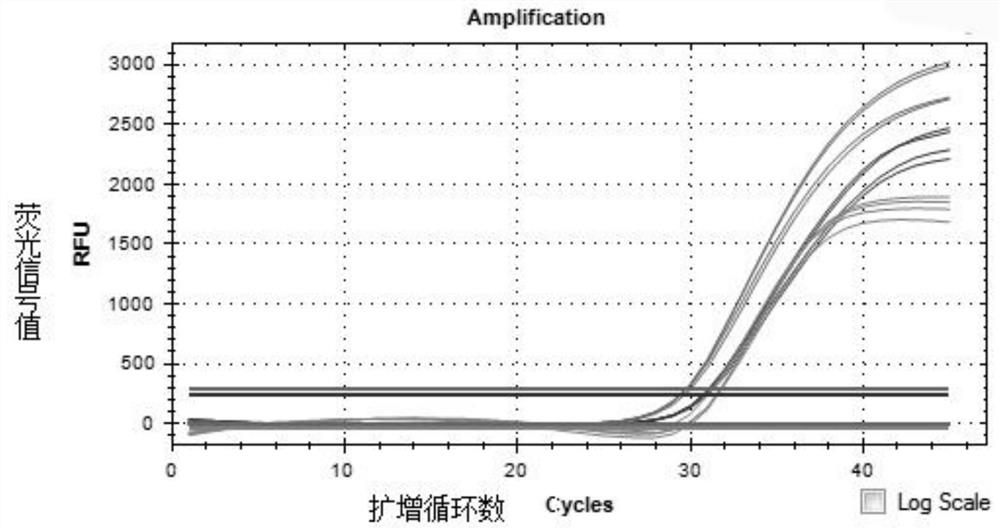
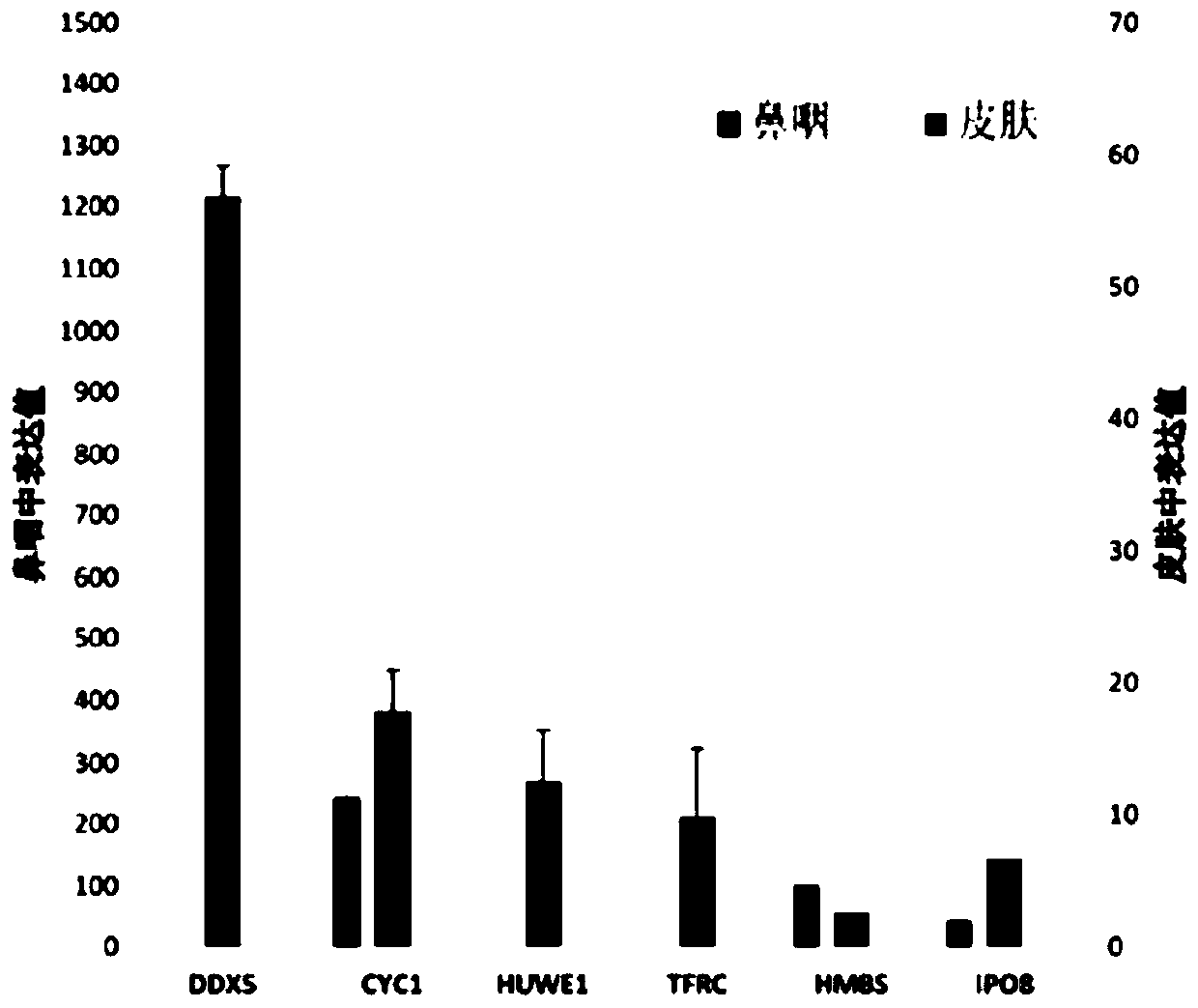
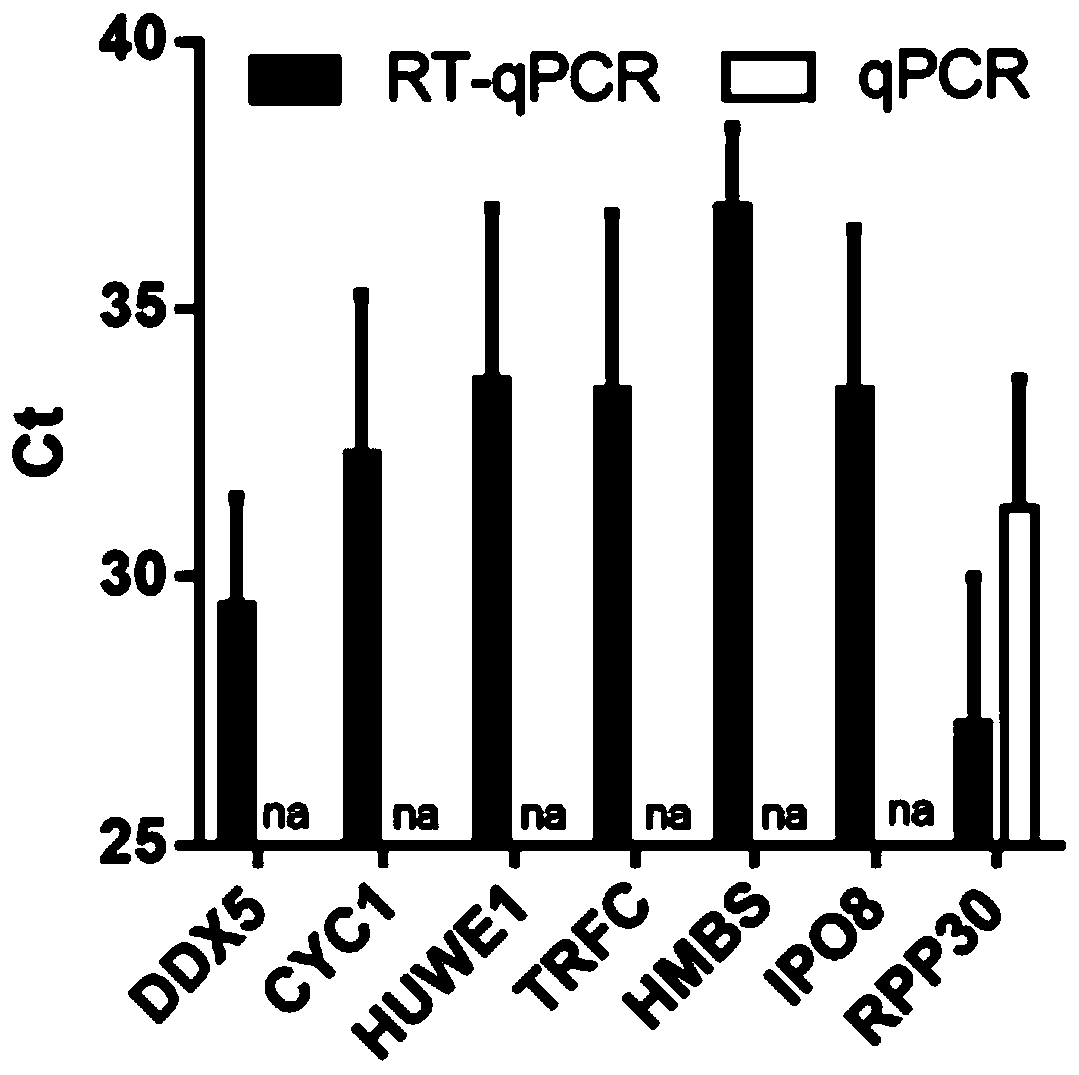
The invention provides a reference gene for respiratory tract RNA virus PCR detection and a detection product thereof. The reference gene is at least one of CYC1, HUWE1, TFRC and IPO8 genes. The invention also designs a primer and a probe for the reference gene. Compared with an internal reference gene RNase P (RPP30) and a corresponding primer and probe used by the American CDC, the primer and probe for the internal reference gene designed and screened by the invention are highly specific, only mRNA amplification is carried out, and genome DNA amplification is not carried out; and the reference gene can be stably detected in people.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Fully-premixed freeze-drying multi-fluorescent PCR detection kit for novel coronavirus, influenza A virus and influenza B virus and detection method thereof
PendingCN112760415ALow level of operation requiredQuick filterMicrobiological testing/measurementAgainst vector-borne diseasesFreeze-dryingRespiratory pathogen
The invention discloses a multi-fluorescent PCR rapid detection kit for novel coronavirus, influenza A virus and influenza B virus. The kit comprises freeze-dried solid RT-PCR Mix, liquid redissolution Buffer, freeze-dried solid positive control and freeze-dried solid negative control, wherein the freeze-dried solid RT-PCR Mix contains a primer group corresponding to primer sequences of a SARS-CoV-2 specific gene ORF1ab, an influenza A M gene, an influenza B M gene and a human reference gene RNAse P. The kit combines a multi-fluorescent quantitative PCR technology and a freeze-drying process, utilizes three pairs of special primers and human reference genes to amplify specific sequences of three pathogens in vitro, and performs real-time detection in combination with a fluorescent probe. The detection method is simple and convenient to operate, has low requirements for the operation level of detection personnel, and can detect three common respiratory pathogens at a time, the detection time and the detection cost are greatly saved, rapid screening of large-batch samples is realized, the whole detection process only takes 40 minutes to 1 hours, and results are accurate and reliable.
Owner:青岛巴特菲科技发展有限公司
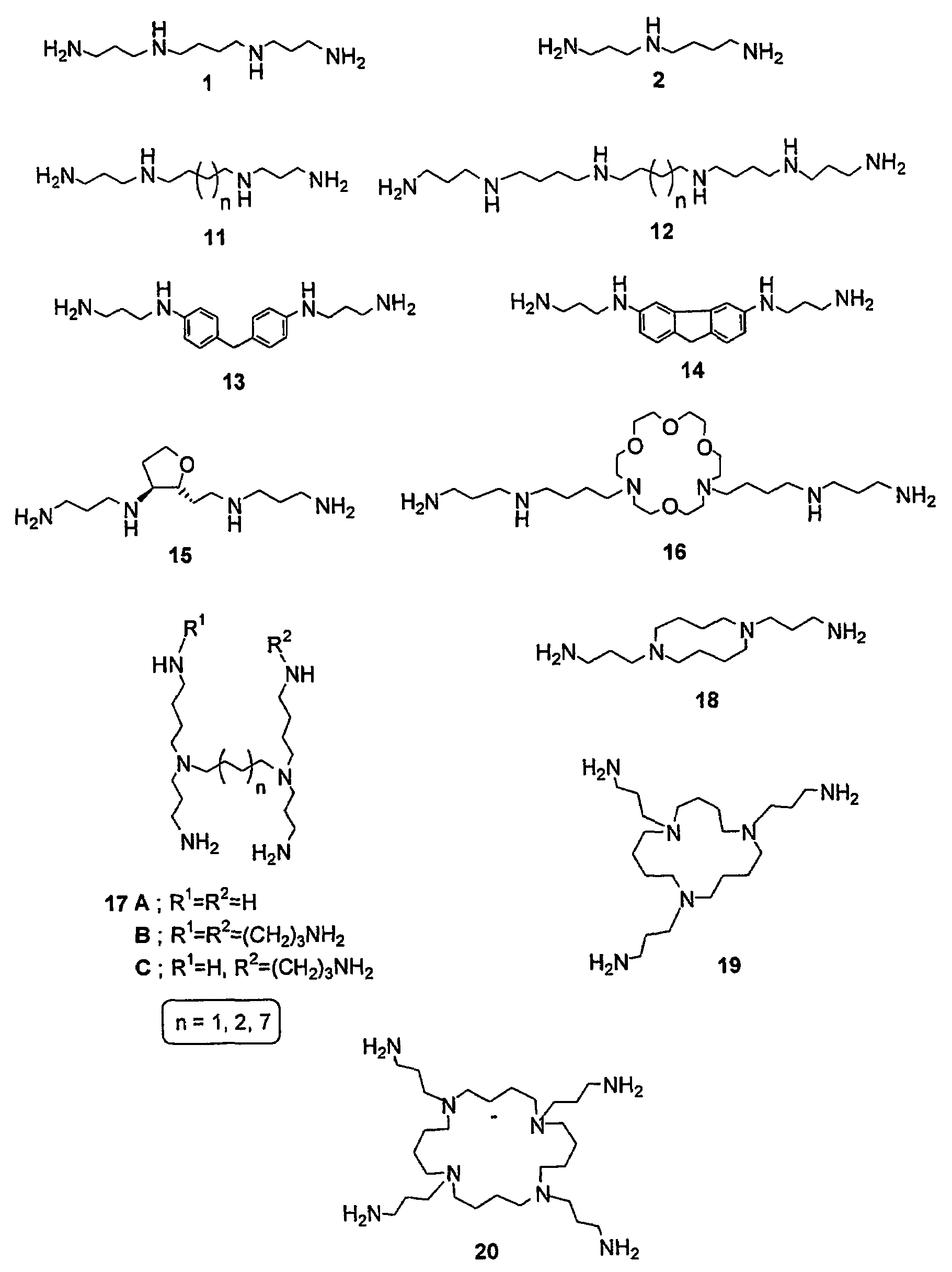
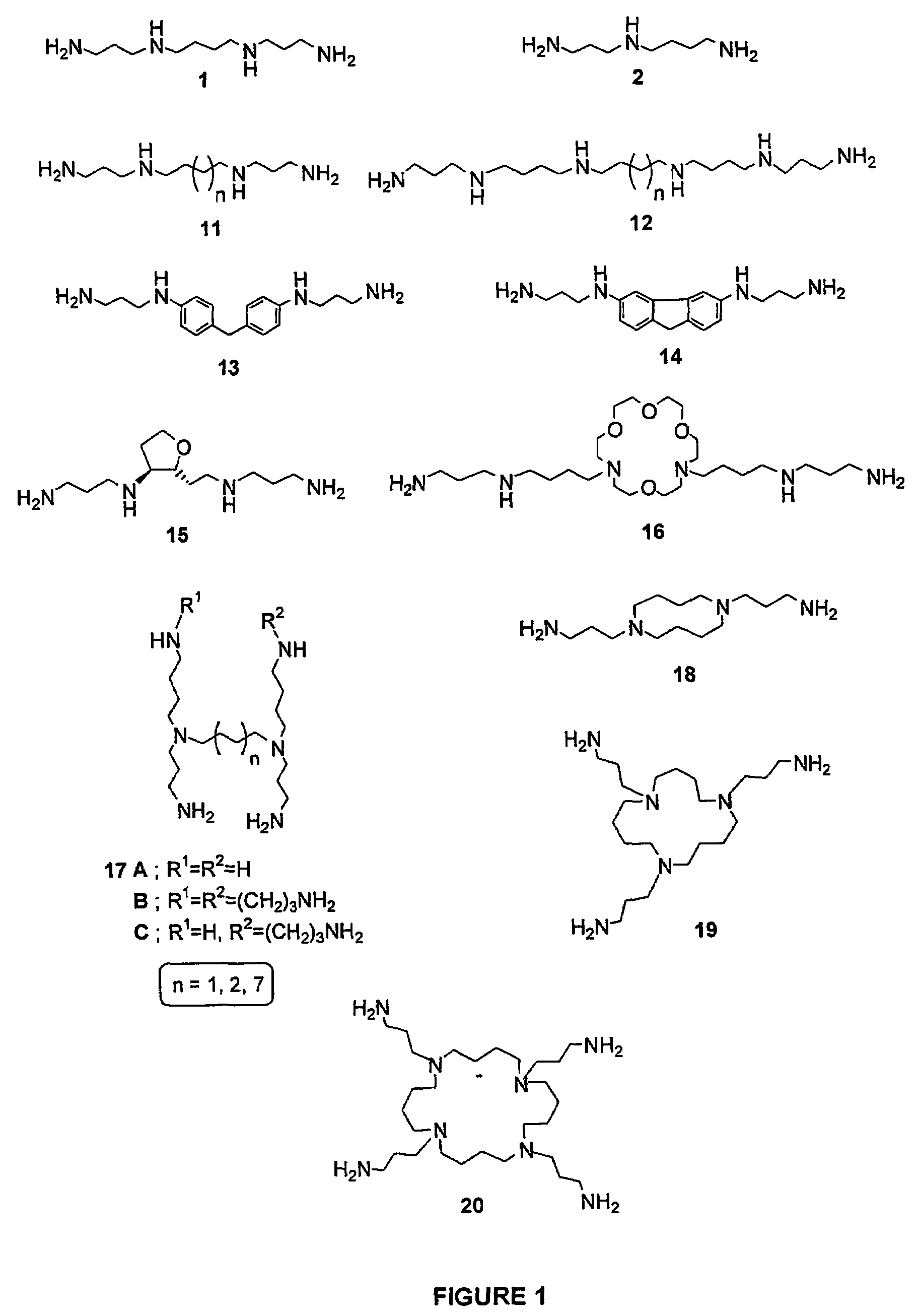
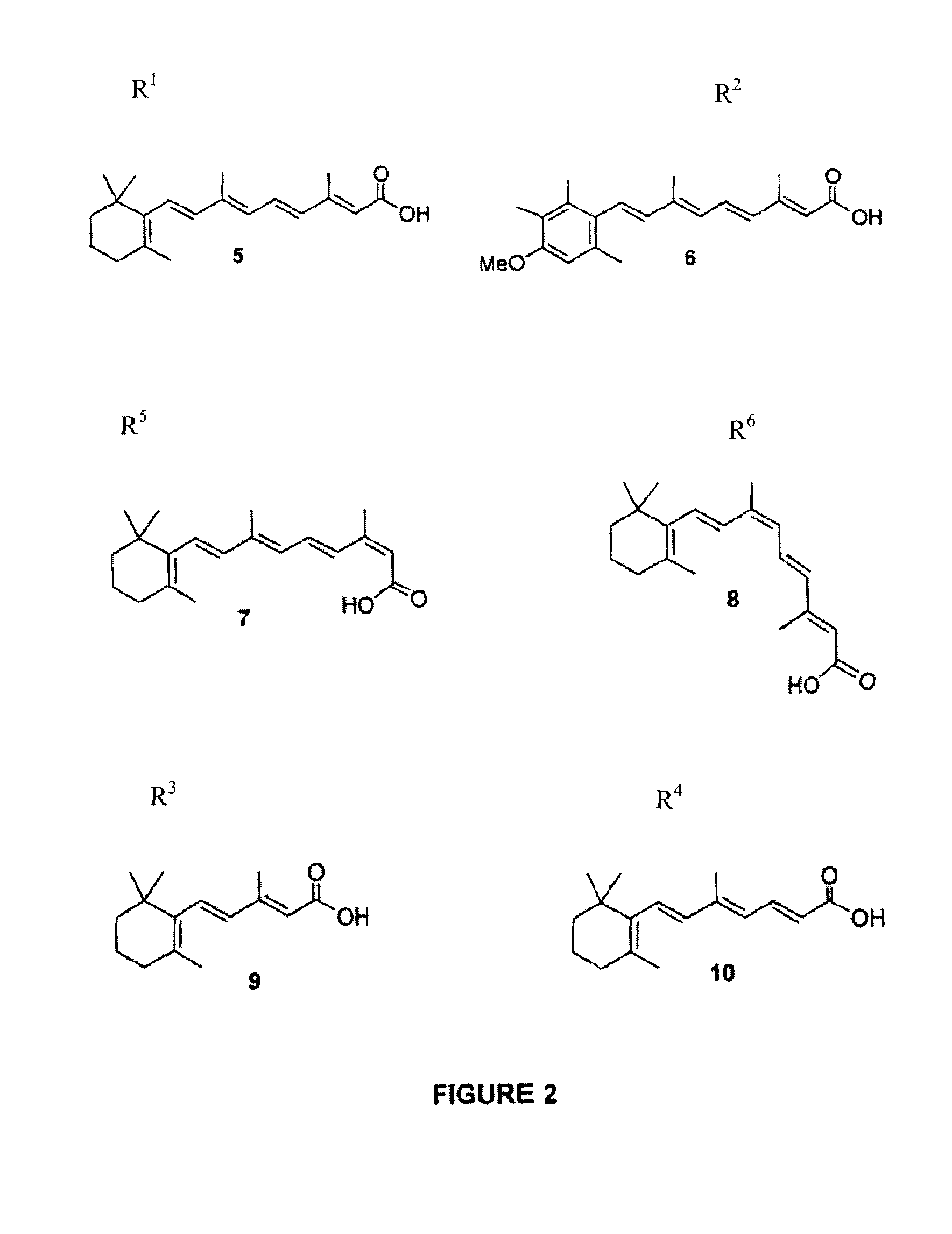
Polyamine conjugates with acidic retinoids and preparation thereof
Invented are novel polyamine conjugates which have been readily obtained using as key-step the condensation of linear, conformationally restricted, cyclic and branched polyamines or suitably protected derivatives with vitamin A derivatives. These compounds inhibit the ribozyme ribonuclease P (RNase P) and the production of interleukin-2 (IL-2) and interferon-γ (INF-γ) by peripheral blood mononuclear cells in vitro.
Owner:PAPAIOANNOU DIONYSIOS +2
Real-time fluorescent quantitative PCR detection primer, probe, kit and method for Ljungan virus and application thereof
PendingCN110699492AQuick checkSensitive and accurate detectionMicrobiological testing/measurementDNA/RNA fragmentationReference genesRNase P
The invention belongs to the technical field of virus detection, and particularly relates to a real-time fluorescent quantitative PCR detection primer, probe, kit and method for Ljungan virus and application thereof. Two single-tube fluorescence channels are adopted to simultaneously detect the existence of the Ljungan virus and reference gene Rnase P, and can detect the existence of the RNA of the Ljungan virus in specimens such as whole blood, serum, cerebrospinal fluid, excrement and tissues. According to the invention, the detection time period is short and the detection efficiency is high; the virus detection specificity is high, and the accuracy is high; virus quantitative analysis can be carried out while virus qualitative analysis is carried out, and the quantitative linear range is good; the detection sensitivity is high; operation is simple, and popularization is easy; experimental results are good in repeatability and high in precision; and the quality of the whole process of extracting and amplifying the specimens can be monitored through the detection result of the reference gene.
Owner:广东龙帆生物科技有限公司
LTL (leukocyte telomere length) detecting method based on qPCR (quantitative polymerase chain reaction) method
InactiveCN106480218ALow concentration requirementThe results are highly reliableMicrobiological testing/measurementRepetitive SequencesDisease
The invention discloses an LTL (leukocyte telomere length) detecting method based on a qPCR (quantitative polymerase chain reaction) method. The method comprises steps as follows: 1) extracting genomic DNA of different tissue, diluting DNA samples to 5 ng / ul, taking 5 ul of each tissue sample, and mixing the tissue samples to prepare a whole genomic DNA template; 2) performing primary real-time qPCR detection and detecting a telomere primer of a telomere repetitive sequence; 3) performing secondary real-time qPCR detection and transcribing a single-copy reference RNase P gene of RNase P enzyme; 4) calculating the relative telomere length T / S. The LTL detecting method based on the qPCR method has the benefits as follows: the requirement for the samples and genomic DNA concentration is not high, the experiment operation process is simple and feasible, the result credibility is high, and the method is adaptable to scientific research and clinical popularization and application and plays an important role in preventing aging, cancer and diseases.
Owner:浙江指针基因科技有限公司
Influenza A and B virus nucleic acid detection kit and use method
InactiveCN112176109AAvoid False Negative Interpretation ResultsMicrobiological testing/measurementNucleic acid detectionRNase P
The invention discloses an influenza A and B virus nucleic acid detection kit. The influenza A and B virus nucleic acid detection kit comprises a nucleic acid amplification reaction solution, an enzyme mixed solution, an influenza A / B virus mixed reaction solution, a positive control solution, a weakly positive control solution and a negative control solution, wherein the influenza A / B virus mixedreaction solution comprises an influenza A virus upstream primer, an influenza A virus downstream primer, an influenza A virus probe, an influenza B virus upstream primer, an influenza B virus downstream primer, an influenza B virus probe, an internal reference RNase P upstream primer, an internal reference RNase P downstream primer, an internal reference RNase P probe and DEPC water, and the internal reference means the detection of the internal reference of the product. The internal reference of the embodiment participates in the process from nucleic acid extraction to RT-PCR, and can be used for monitoring the collection, storage and transportation of samples and the nucleic acid extraction process and amplification process, so that false negative interpretation results are avoided.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD
External leader sequence for guiding RNase P ribozyme and use thereof in anti-HCMV medicament preparation
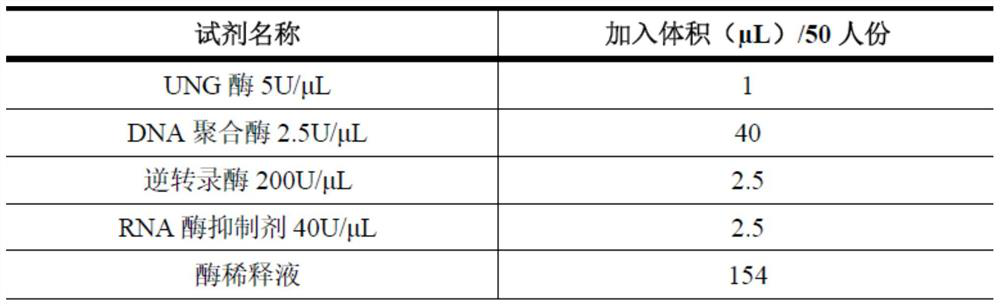
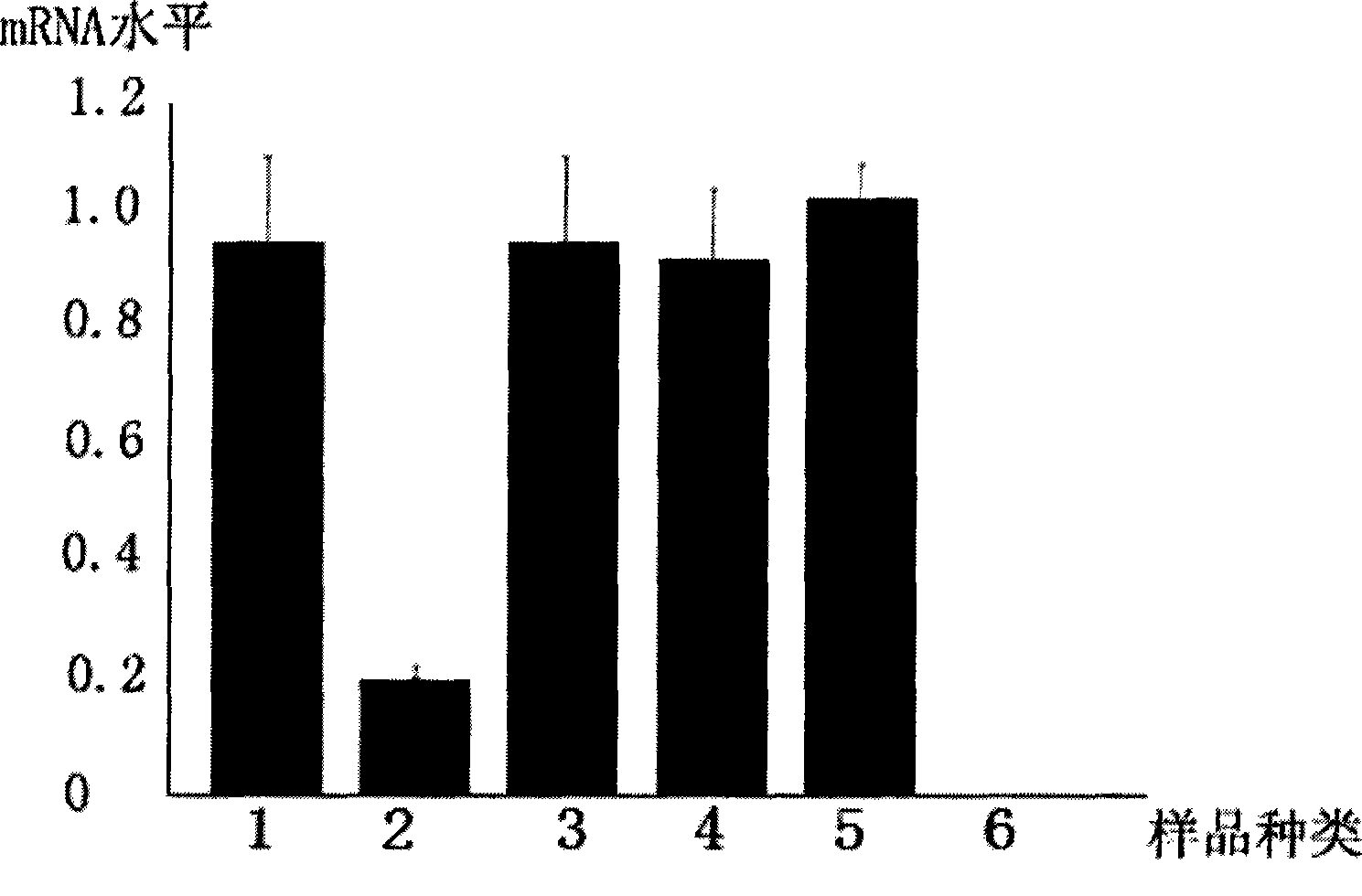
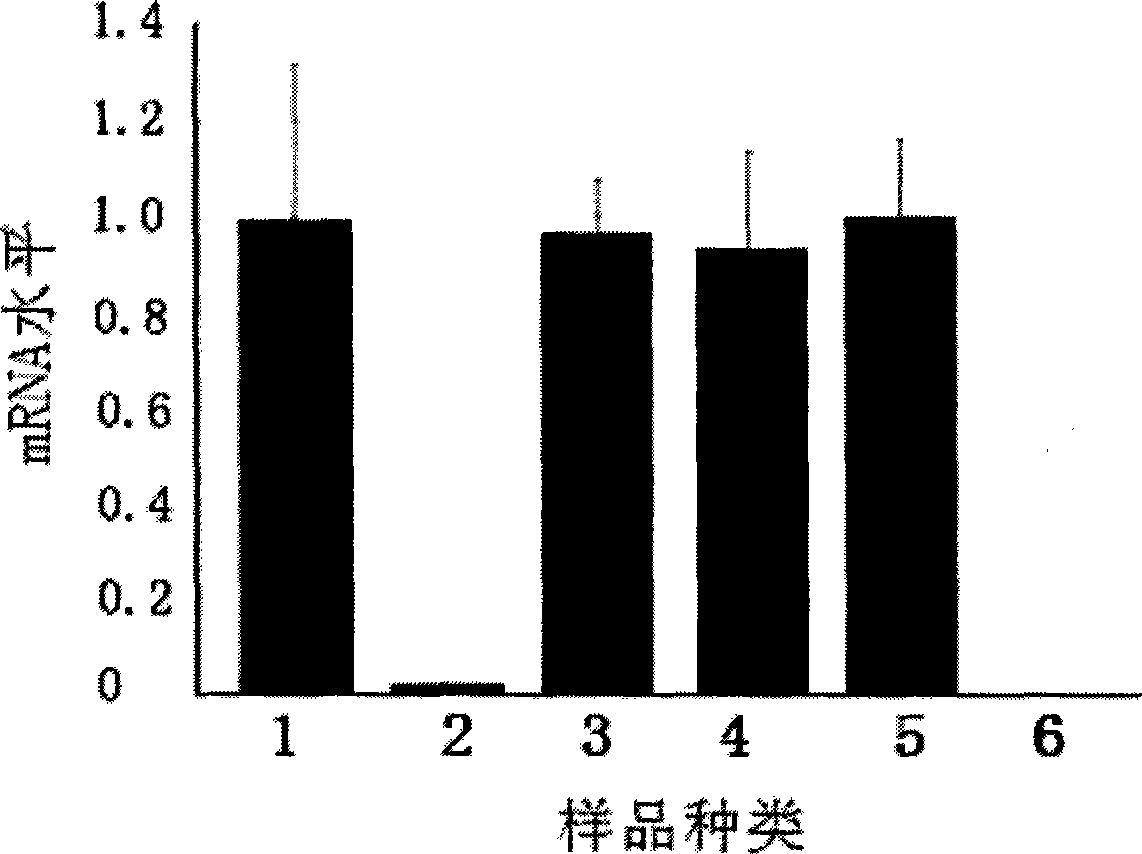
InactiveCN101463351AEfficient expressionDelay drug resistanceOrganic active ingredientsAntiviralsNucleotideRNase P
The invention discloses an EGS of 12nt, which can conduct RNase P ribozyme and comprises EGS which is based on RNA and has the nucleotide sequence to be shown in SEQ ID NO: 1, and EGS that is based on DNA and has the nucleotide sequence to be shown in SEQ ID NO: 2. The EGS can effectively inhibit the expression of HCMV UL49 gene. The EGS with the characteristic of DNA has the silent gene efficiency of 99%, and the EGS with the characteristic of RNA has the silent gene efficiency of 98%. Compared with the existing EGS which can cause the UL49 gene to be silent, the EGS of the invention has the sequence which only contains 12 basic groups, thus not only having the shortest nucleic acid molecules, but also effectively and specially leading the expression of the UL49 gene to be silent. The EGS of the invention is used for researching and developing the anti-HCMV drug, and can greatly reduce the cost when the drug is researched and applied.
Owner:JINAN UNIVERSITY
Fluorescent RT-PCR reagent and method for detecting influenza A virus, influenza B virus and coronavirus SARS-CoV-2
InactiveCN112410469AAvoid risk of failureAvoid temperatureMicrobiological testing/measurementMicroorganism based processesFreeze-dryingLate gene
The invention provides a freeze-dried type multiple fluorescent RT-PCR reagent and a method for detecting and distinguishing influenza A virus, influenza B virus and coronavirus SARS-CoV-2. Primers and probes of the fluorescent RT-PCR reagent for detecting the influenza A virus, the influenza B virus and the coronavirus SARS-CoV-2 provided by the invention are obtained by designing and screening based on the latest gene sequence information of the influenza A virus, the influenza B virus and the coronavirus SARS-CoV-2 in a public gene sequence database, and the reagent has good inclusivity, specificity and reaction performance. According to the reagent, a human RNase P nucleic acid fragment is used as a detection internal reference. In addition, the fluorescent RT-PCR detection reagent forthe influenza A virus, the influenza B virus and the coronavirus SARS-CoV-2 is prepared into a freeze-dried form capable of being stored at normal temperature through freeze-drying technology, thus avoiding the risk that the conventional liquid fluorescent PCR detection reagent is invalid due to the change of storage temperature or repeated freezing and thawing, and the transportation and management cost is reduced.
Owner:深圳市赛格诺生物科技有限公司
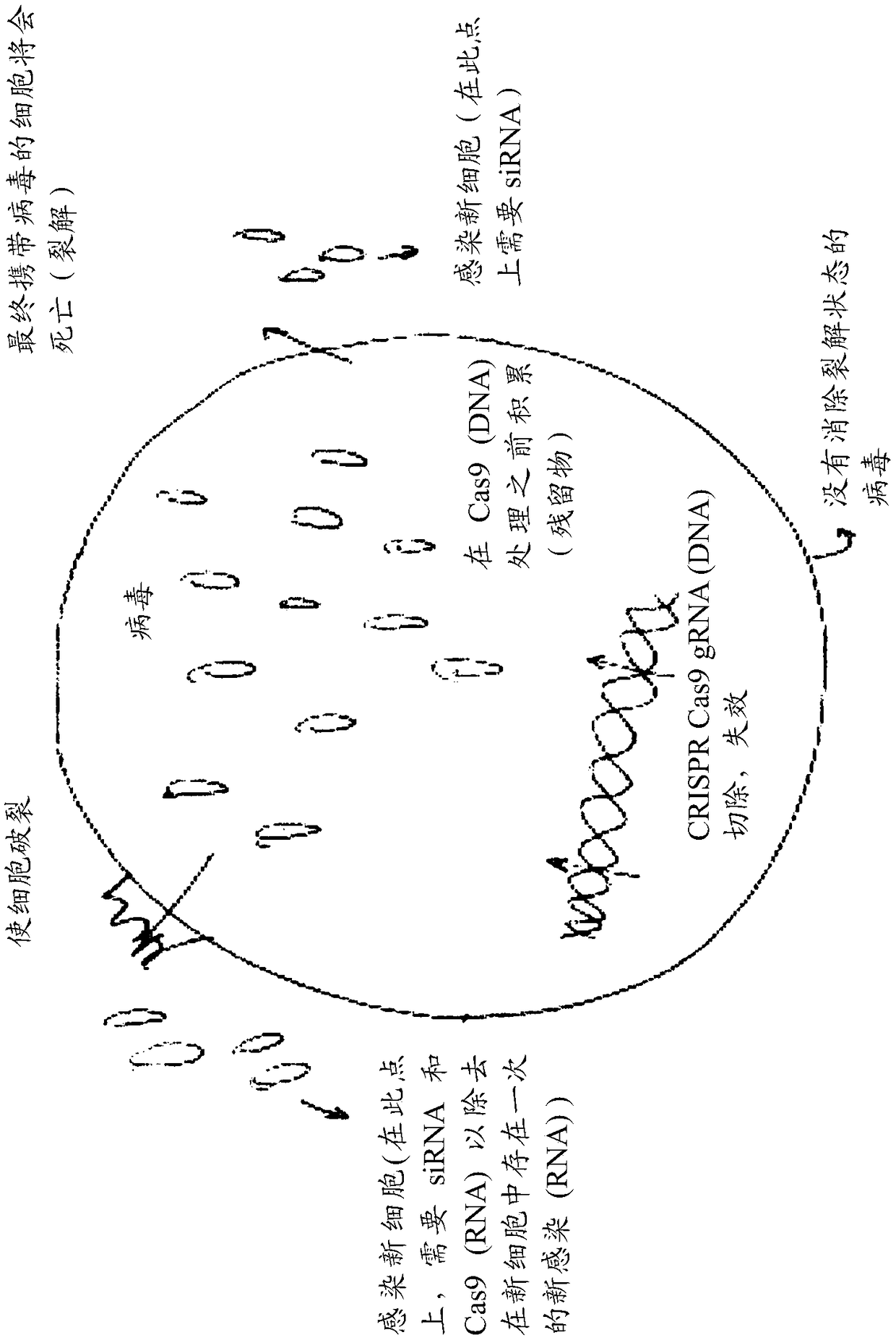
Compositions and methods of treatment for lytic and lysogenic viruses
Owner:EXCISION BIOTHERAPEUTICS INC
Reference gene for respiratory tract RNA virus PCR detection and detection product thereof
PendingCN111363848AImprove diagnostic accuracyTruly Monitor IntegrityMicrobiological testing/measurementMicroorganism based processesReference genesGenomic DNA
The invention provides a reference gene for respiratory tract RNA virus PCR detection and a detection product thereof. The reference gene is a DDX5 gene. The invention also designs a primer and a probe aiming at the reference gene. Compared with a reference gene RNase P (RPP30) for American CDC and corresponding primers and probes, the primers and the probes which are designed and screened according to the invention and aim at the reference gene are highly specific, only mRNA is amplified, and genomic DNA is not amplified; and the reference gene can be stably detected in people.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Synergistic compositions for treating microbial infections
Synergistic pharmaceutical compositions including an RNase P inhibitor and a tRNA synthetase inhibitor are provided, as well as methods for their use in treating infections. Also provided herein are methods of using the compositions to inhibit a bacterial tRNA synthetase in a cell and to decolonize bacteria on a surface.
Owner:伊沃克纳米公司
Mycoplasma genitalium nucleic acid real-time fluorescence PCR detection primer, probe and kit
InactiveCN113186316ASensitive and accurate detectionGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionRNase P
The invention discloses a mycoplasma genitalium nucleic acid real-time fluorescence PCR detection primer, a probe and a kit. The kit comprises an amplification reaction solution, a mycoplasma genitalium nucleic acid detection solution, a positive reference substance and a negative reference substance, wherein the mycoplasma genitalium nucleic acid detection liquid comprises specific primers and probes aiming at mycoplasma genitalium and a human RNase P gene, the sequences of the primers aiming at the mycoplasma genitalium are as shown in SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe aiming at the mycoplasma genitalium is as shown in SEQ ID NO.3. According to the primer, the probe and the kit for real-time fluorescence PCR detection of the mycoplasma genitalium, provided by the invention, the mycoplasma genitalium can be rapidly, accurately and sensitively detected, the repeatability of an experimental result is good, the precision is high, the detection time period is short, and the detection can be completed within 70 minutes as short as possible. Therefore, the detection time is greatly saved, and the clinical diagnosis efficiency can be accelerated.
Owner:北京华诺奥美基因生物科技有限公司
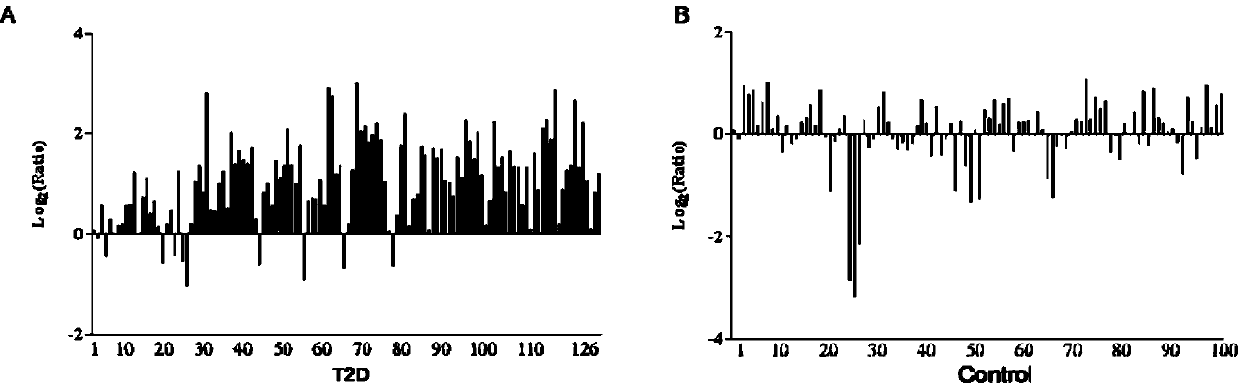
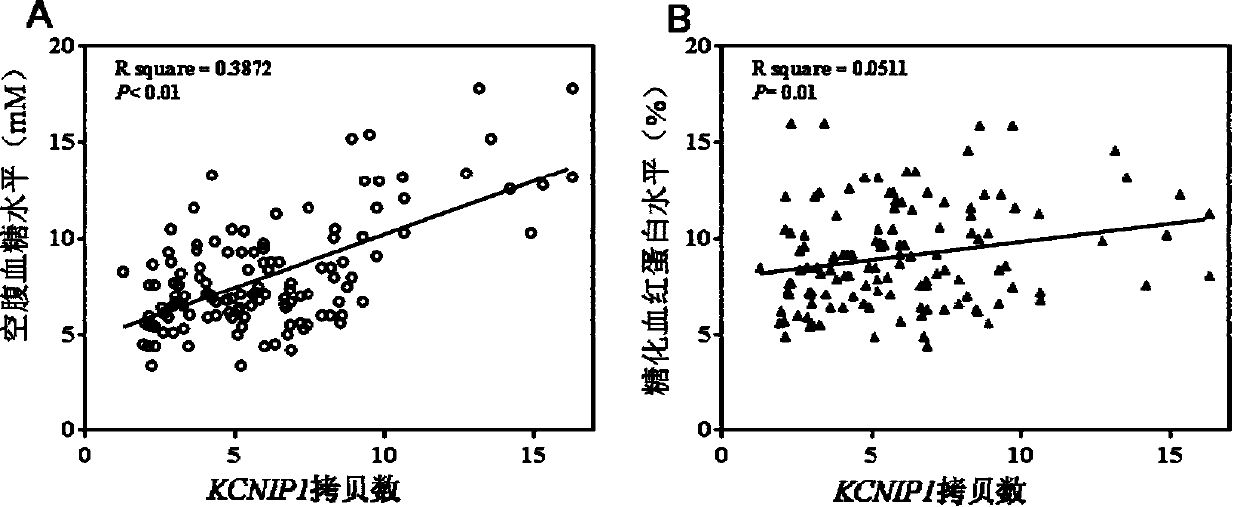
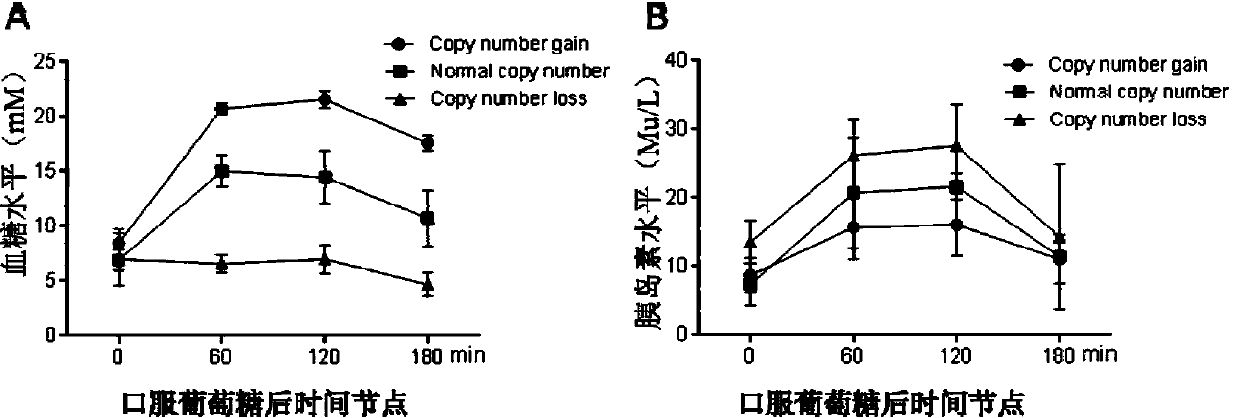
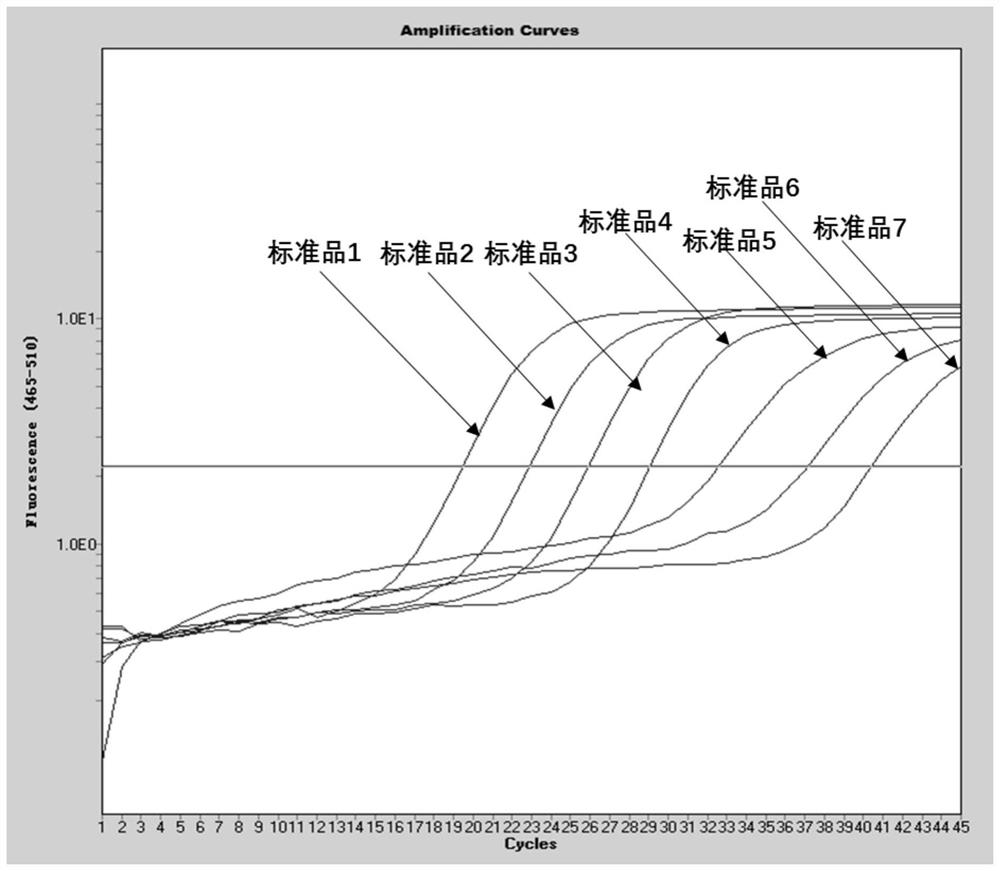
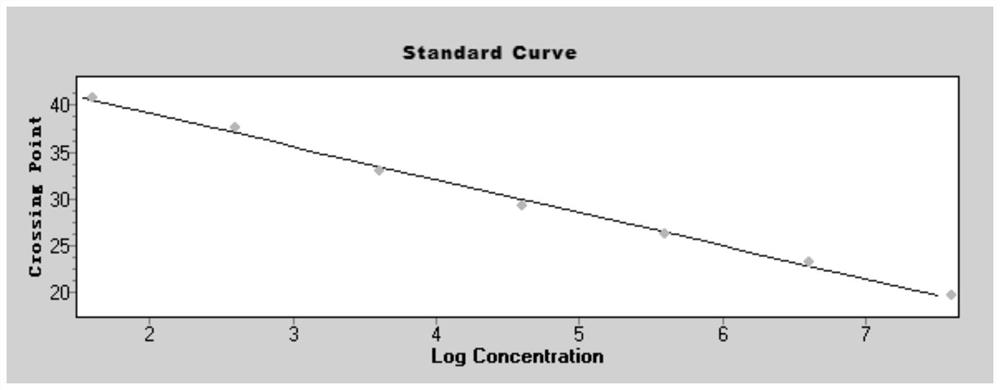
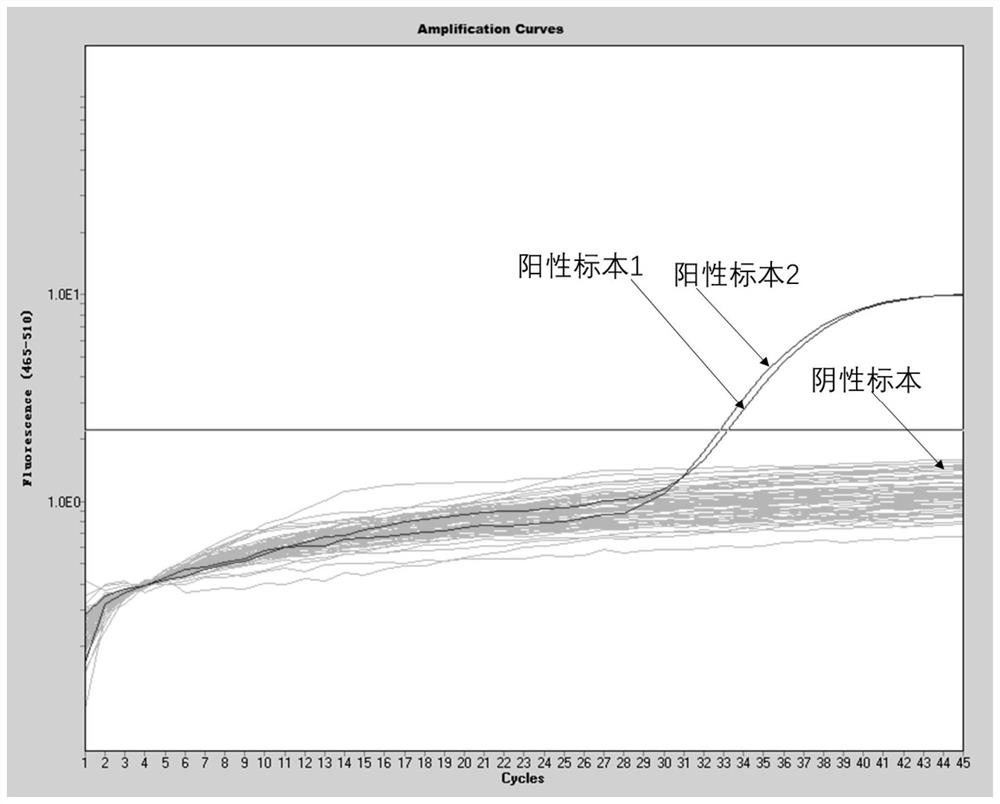
Method for detecting type II diabetes related gene KCNIP1 copy number variation, and application thereof
InactiveCN107119114ASimple and fast operationLow costMicrobiological testing/measurementReference genesMolecular diagnostics
The invention discloses a method for detecting type II diabetes related gene KCNIP1 copy number variation (CNV), and application thereof. The position of CNV on a chromosome is Chr5q35.1:170062275-170064128. According to the detection method, blood genome DNA of patients suffering from type II diabetes and normal individuals serve as templates, primer pairs P1(F1,R1) and P2(F2,R2) serve as primers, a KCNIP1 gene CNV area and a reference gene RNase P are amplified separately through real-time fluorescent quantitative PCR, the CNV is divided into an inserting type, a deletion type and a normal type according to Log2<2-delta delta Ct>, and the distribution difference of the KCNIP1 gene CNV in the patients suffering from type II diabetes and the normal individuals can be identified. The method can serve as a molecular diagnostic mark of the type II diabetes and provides important scientific basis for research and development of molecular targeted medicines.
Owner:WUHAN UNIV OF SCI & TECH
Primers and probes, kits and methods for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-ncov
ActiveCN111057797BGood repeatabilityQuick checkMicrobiological testing/measurementMicroorganism based processesNasopharyngeal aspirateVirus detection
The invention belongs to the technical field of virus detection, and particularly relates to a primer, probe, test kit and method for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-nCoV. A single-tube double-fluorescence channel is adopted to simultaneously detect the existence of the novel coronavirus 2019-nCoV and reference gene Rnase P, and the existence of the novelcoronavirus 2019-nCoV RNA in specimens such as alveolar lavage fluid, nasopharyngeal swab, whole blood, serum, feces and tissues can be detected. The method is short in detection time period, and issuitable for clinical and bedside rapid detection of diagnosis; the virus detection specificity is high, and the accuracy is high; virus qualitative analysis and quantitative analysis are carried out,and the quantitative linear range is good; the detection sensitivity is high; the experimental result is good in repeatability and high in precision; and the reference gene is added into a detectionsystem, so that the quality of the whole process of extracting and amplifying the sample can be monitored through the detection result of the reference gene.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Multiplex fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting hematogenous infectious viruses
ActiveCN113373267AQuick screeningLow costMicrobiological testing/measurementDNA/RNA fragmentationMultiplexSerum samples
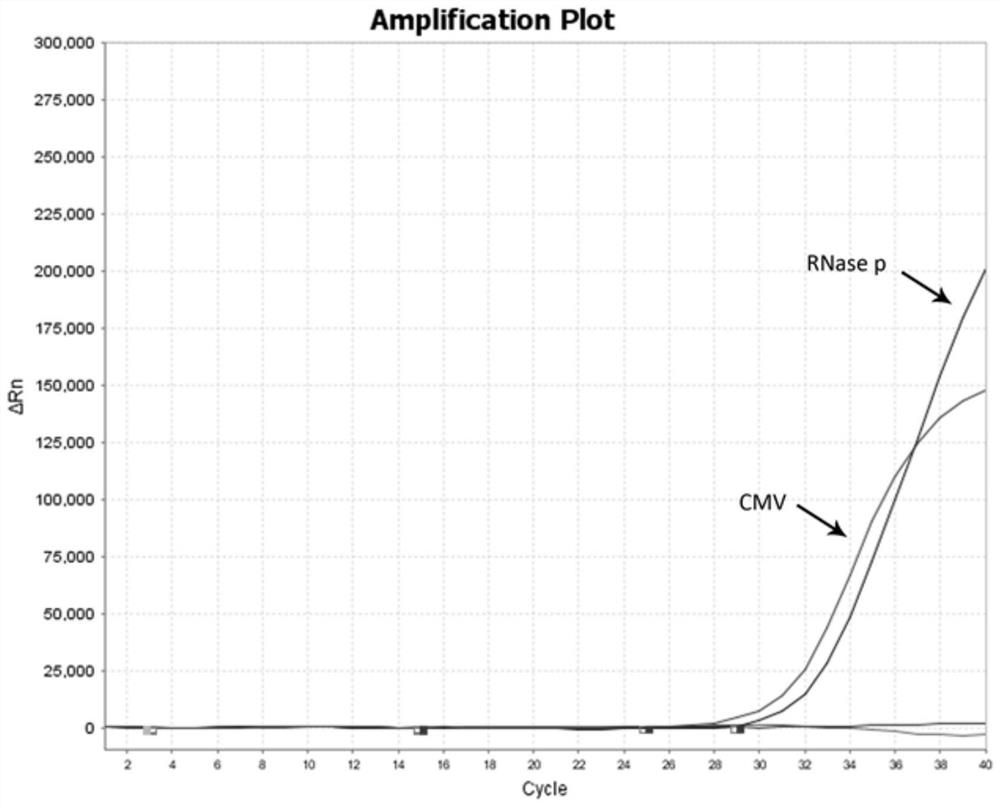
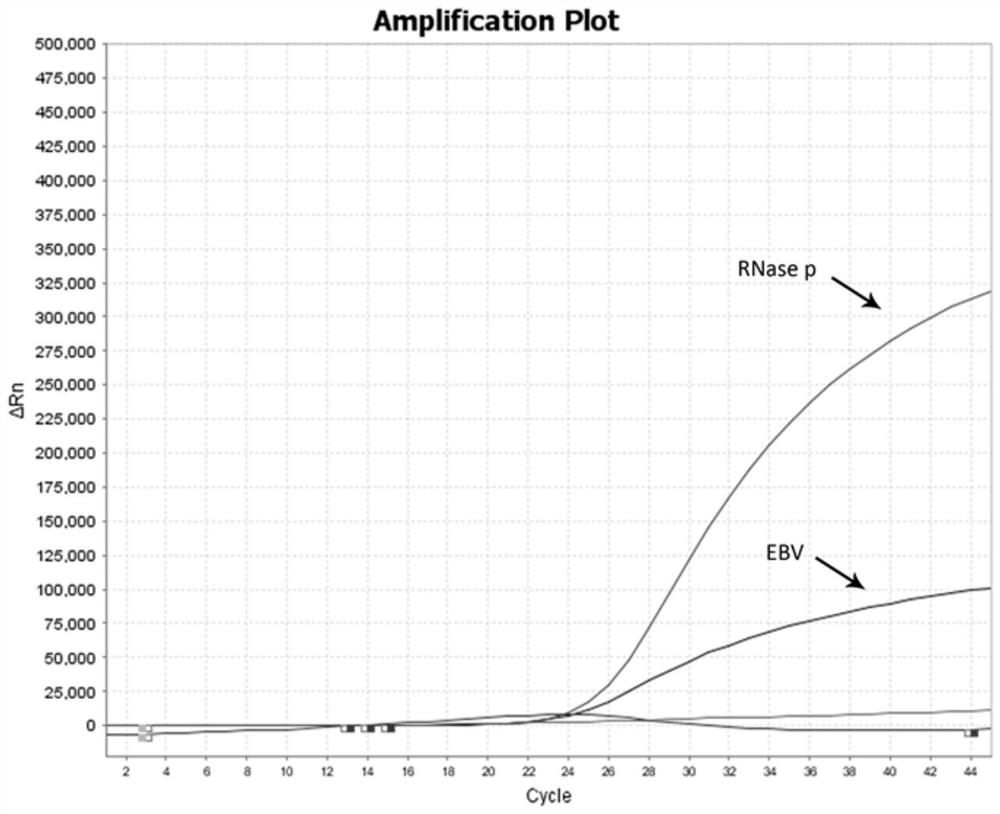
The invention provides a multiplex fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting hematogenous infectious viruses. The multiplex fluorescent quantitative RT-PCR kit comprises quantitative RT-PCR reaction liquid, enzyme Mix liquid, primer probe Mix liquid, standard substances (cytomegalovirus, Epstein-Barr virus, herpes simplex virus and RNase P standard substances), positive reference substances and negative reference substances. The primer probes comprise four groups of primers and corresponding probes with different fluorescence labels. According to the kit, high-specificity primer probes are designed according to high-conservative proteins of cytomegalovirus, Epstein-Barr virus, herpes simplex virus and RNase P correspondingly, and whether the viruses exist in a serum sample or not is simultaneously detected through a PCR reaction. The kit disclosed by the invention is simple, convenient and rapid, cost-saving, good in repeatability and capable of effectively and rapidly screening the hematogenous infectious viruses. The kit can be applied to rapid screening of the hematogenous infectious viruses and research of hematogenous infectious virus epidemiology.
Owner:ZHEJIANG UNIV
Novel coronavirus 2019-nCoV real-time fluorescent PCR detection primer and probe, kit and method
PendingCN112266980AQuick checkSensitive and accurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesWhite blood cellNucleic acid
The invention discloses a novel coronavirus 2019-nCoV real-time fluorescent PCR detection primer and probe, kit and method. Two loci of nucleic acid after 2019-nCoV replication are selected for detection; the two loci include a 2019-nCoV specific locus with high specificity, and the locus has no cross reaction with other common pathogens of which infection site are the same as the locus or have similar infection symptoms and the total nucleic acid of human leukocytes. A single tube with three fluorescence channels are adopted to simultaneously detect the presence of novel coronavirus 2019-nCoVand an internal reference gene Rnase P, and can also detect the presence of the RNA of the novel coronavirus 2019-nCoV in specimens such as bronchoalveolar lavage fluid, nose swabs and throat swabs.Through the primer, probe, kit and method, detection time is short and can be shortened to 1.5 h, so that the primer, probe, kit and method are suitable for rapid clinical and bedside detection and diagnosis; and the primer, probe, kit and method are high in detection of virus specificity, and high in repeatability, sensibility and accuracy rate.
Owner:ZHENGZHOU UNIV +1
Primer and probe composition for detecting helicobacter pylori 23SrRNA gene mutation as well as application and kit of primer and probe composition
PendingCN114574601AEfficient identificationImprove the detection rateMicrobiological testing/measurementMicroorganism based processesGenes mutationHelicobacter
The invention relates to the technical field of biological detection, and particularly discloses a primer and probe composition for detecting helicobacter pylori 23S rRNA gene mutation, application of the primer and probe composition and a kit. The primer and probe composition comprises a mutant primer and probe composition for detecting an A2142C site, an A2142G site and an A2143G site of a helicobacter pylori 23S rRNA gene, a primer and probe composition for detecting a helicobacter pylori 16S rRNA gene, a primer and probe composition for detecting a human RNAse P gene, and a blocking primer. The primer and probe composition is applied to preparation of a reagent or a kit for detecting helicobacter pylori 23S rRNA gene mutation. The kit comprises the primer and the probe composition. According to the application, the detection efficiency of helicobacter pylori 23S rRNA gene mutation is improved.
Owner:BEIJING XINJI YONGKANG BIOTECH
Circular RNA knockdown method and application thereof
ActiveCN112126645AHigh knockdown efficiencyLess off-targetHydrolasesAntibody mimetics/scaffoldsRNase PBiochemistry
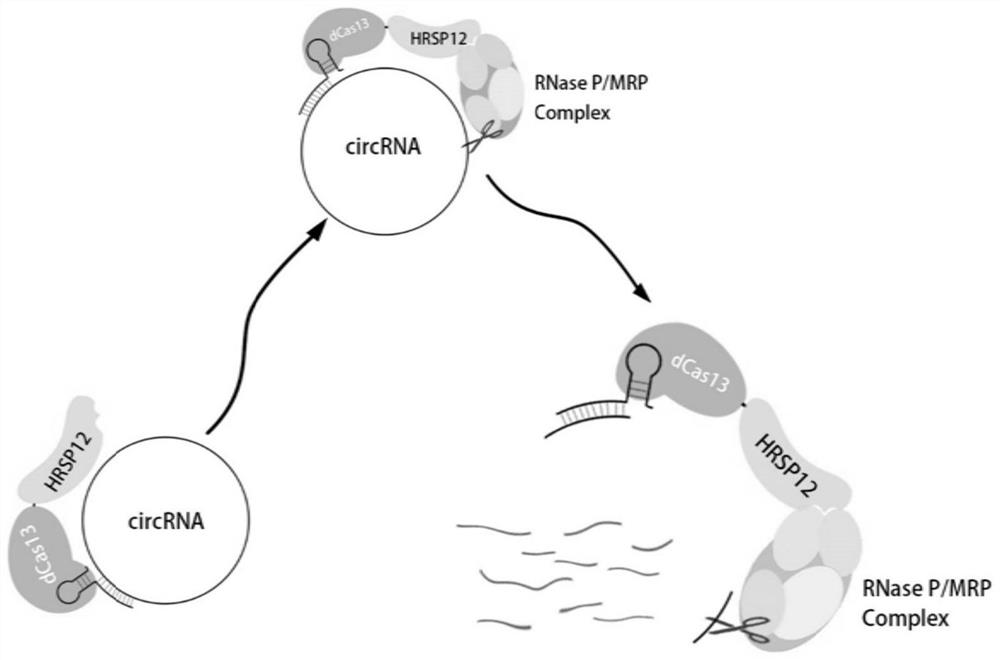
The invention relates to the field of molecular biology, in particular to a circular RNA targeted knockdown method of and application thereof. According to the circular RNA knockdown method, dCas13 protein is used for fusion expression of HRSP12, dCas13 and matched gRNA are used for specific targeting of circular RNA, RNase P / MRP compounds are recruited through dCas13-HRSP12 fusion protein, specific targeting degradation of circular RNA is achieved, and the method has the beneficial effects that interference to in-vivo steady-state environment is little, experiment results are more real and reliable, and specific targeting knock-down efficiency is high.
Owner:GUANGZHOU GENESEED BIOTECH +1
Novel coronavirus fluorescent PCR (Polymerase Chain Reaction) detection kit and use method thereof
PendingCN114250324ARapid test diagnosisSensitive and accurate detectionMicrobiological testing/measurementMicroorganism based processesGlycerolRNase P
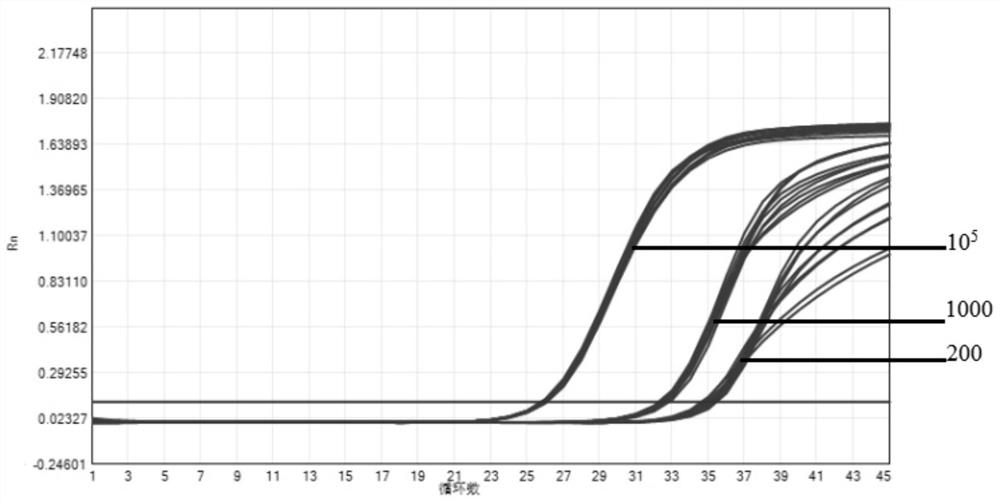
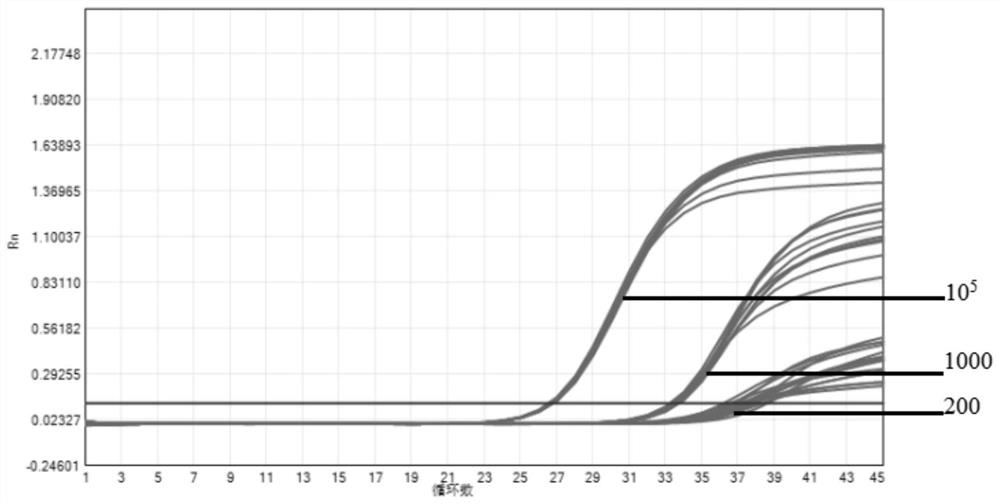
The invention provides a novel coronavirus fluorescent PCR detection kit and a use method thereof. The novel coronavirus fluorescent PCR detection kit comprises primers, probes, Taq DNA polymerase, UNG enzyme, dNTPs, Tris-HCl, MgSO4, glycerol and gelatin, wherein the primers and the probes are used for detecting a novel coronavirus ORFlab gene and an N gene and an internal standard virus RNase P gene; the probes of the ORFlab gene, the N gene and the RNase P gene are all probes modified by locked nucleic acid. The detection limit of the kit is 200 copies / mL, the locked nucleic acid probe, UNG enzyme, glycerol and gelatin are adopted, false negative results can be effectively avoided, and the kit has the advantages of being good in specificity, high in sensitivity and good in reproducibility.
Owner:WUHAN BIOTECH GENE ENG +1
Exterior guiding gene C6 concurrently used with RnaseP ribozyme and its uses
InactiveCN1807619AImprove efficiencySmall chemical doseGenetic material ingredientsAntiviralsDiseaseRNase P
This invention discloses an external guiding gene and its application, wherein the gene is used combining with the RNase P ribozyme. The related external guiding sequence is based on the RNA, which has the sequence of the SEQ ID NO: 1, and it also includes the external guiding sequence based on the DNA, which has the sequence of the SEQ ID NO: 2, all of the sequences has the special guiding cutting activity that guides RNase P to cut HCMV polyenzyme mRNA flake, and the target point is at 1760-1776 of the UL54 gene. The EGS in this invention is to research and exploit the HCMV infection disease treatment and the gene drugs of related disease.
Owner:JINAN UNIVERSITY
Primers and method for simultaneously detecting Orf1ab, E and N regions of 2019-nCov virus in one tube
PendingCN112501352AImprove precision and specificityMonitor AccuracyMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionRNase P
The invention provides primers, probes and a detection method for nucleic acid detection of 2019-nCov or SARS-Cov-2 virus, including upstream and downstream primers and probes used for amplifying Orf1ab, E and N regions covering 2019-nCov virus nucleic acid, and upstream and downstream primers and probes used for detecting internal standard gene RNase P. The four probes are labeled by different fluorophores, and all detection can be completed in one tube through condition optimization, so that the detection cost and the detection time are greatly saved. The detection reagent and the detectionmethod have the advantages of good detection specificity, high sensitivity and simple operation, and can realize rapid and accurate detection of 2019-nCov or SARS-Cov-2 viruses.
Owner:杭州艾迪康医学检验中心有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com