Primers and probes, kits and methods for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-ncov
A 2019-ncov, real-time fluorescence quantitative technology, applied in biochemical equipment and methods, microbial-based methods, microbial measurement/inspection, etc., can solve the problem of no specificity, achieve short time period, strong specificity, The effect of saving diagnosis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The development of embodiment 1 real-time fluorescent quantitative PCR detection kit
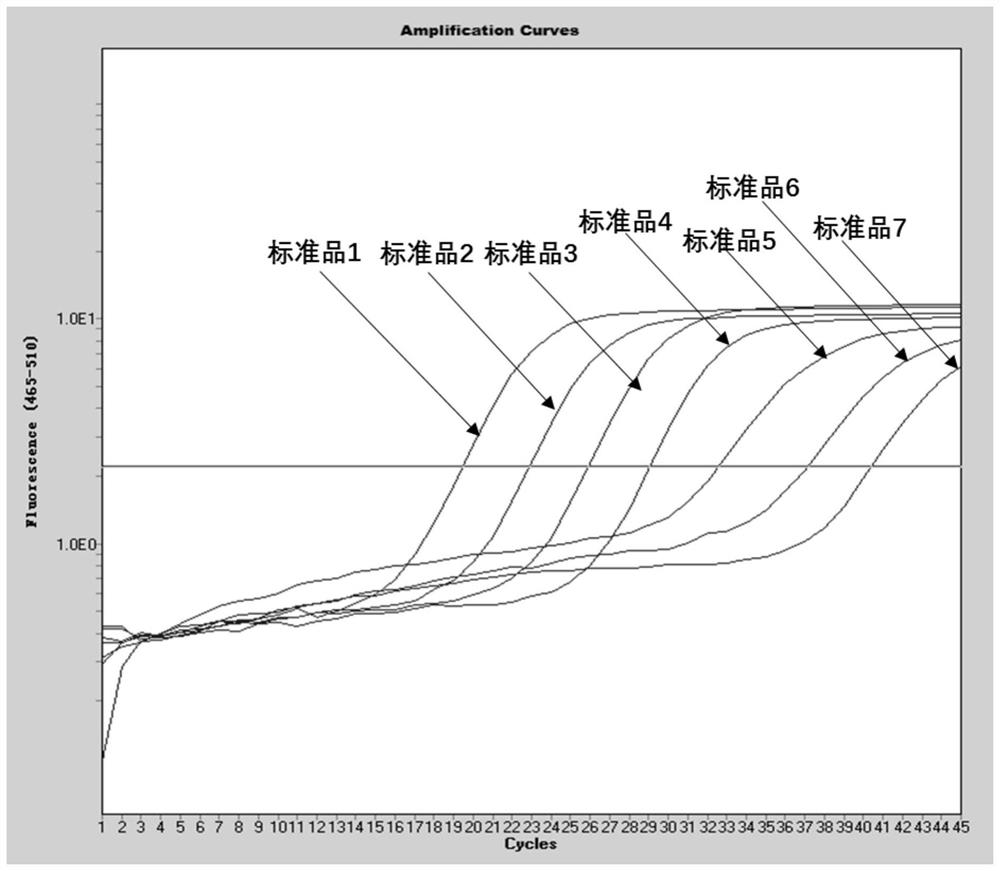
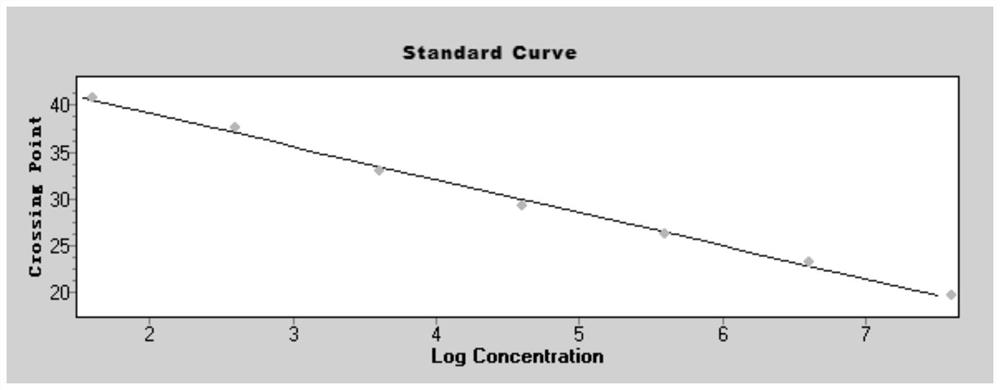
[0034] 1. In this example, a pair of specific primers and a specific fluorescent probe were designed by selecting the E gene sequence encoding the envelope protein specific to the new coronavirus 2019-nCoV, and a pair of specific fluorescent probes were designed by using the internal standard gene RnaseP. A real-time fluorescent quantitative PCR technology can be used to detect the new coronavirus 2019-nCoV. The E gene is an important structural gene of the coronavirus, and the primers and probes designed in the present invention are only 100% similar to the E gene of the new coronavirus 2019-nCoV, while other coronaviruses including SARS, MERS, 229E, OC43, NL63 and HKU1 No match, so it can specifically bind to the E gene of the new coronavirus 2019-nCoV and initiate amplification, but not amplify other types of coronavirus. The primer sequences and probe sequences designed in this e...
Embodiment 2
[0073] The performance measurement of the real-time fluorescent PCR kit of embodiment 2 new coronavirus 2019-nCoV
[0074] 1. Verification of accuracy
[0075] The gold standard for viral nucleic acid detection is genome sequencing. The test results of this kit are compared with viral genome sequencing to analyze its accuracy. In this example, 4 specimens determined to be novel coronavirus 2019-nCoV by genome sequencing were selected, and the results after detection with the kit provided by the present invention are shown in Table 4 below. It can be seen from the results that all 4 positive samples were detected, indicating that the accuracy of the real-time fluorescent PCR of the novel coronavirus 2019-nCoV provided by the present invention is 100%.
[0076] Accuracy analysis of the present invention of table 4
[0077]
[0078] 2. Specificity verification
[0079] The specificity of the kit is evaluated by detecting other pathogens. In this example, 32 positive samples...
Embodiment 3
[0094] Embodiment 3 clinical detection
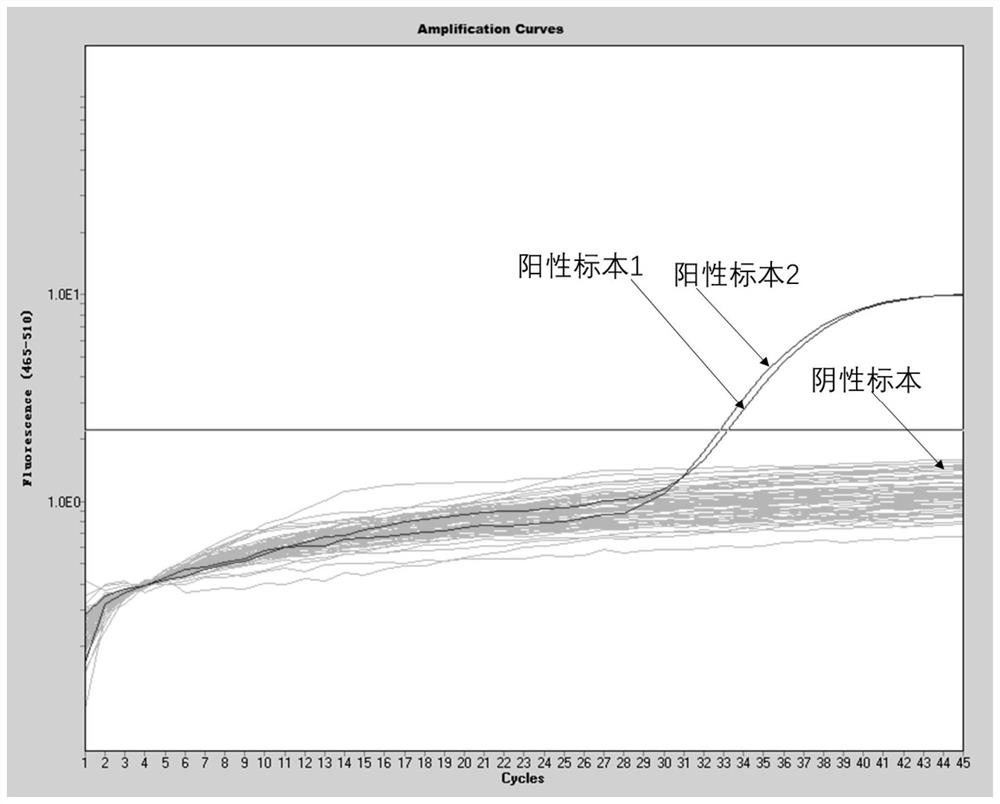
[0095] Alveolar lavage fluid, nasal swabs, throat swabs, whole blood, serum, plasma, urine and stool from patients with positive confirmed cases of novel coronavirus 2019-nCoV were tested with the above method, and the results are as follows: Image 6 As shown, the alveolar lavage fluid, nasal swabs, throat swabs, whole blood, serum, plasma, urine and stool from patients with a positive diagnosis of the new coronavirus 2019-nCoV all had amplification curves, and the alveolar lavage fluid, The virus content in nasal swabs, throat swabs and stool samples was high, and the virus positive control and virus negative control were normal. At the same time, the amplification curves of the internal reference gene Rnase P of these samples were normal, as shown in Figure 7 As shown, it shows that the extraction and amplification process of this experiment is normal, and the positive and negative results are accurate. This proves that this method ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com