Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Coronaviridae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coronaviridae is a family of enveloped, positive-sense, single-stranded RNA viruses. The viral genome is 26–32 kb in length. The particles are typically decorated with large (~20 nm), club- or petal-shaped surface projections (the “peplomers” or “spikes”), which in electron micrographs of spherical particles create an image reminiscent of the solar corona. Members of this family are thus referred to as coronaviruses.
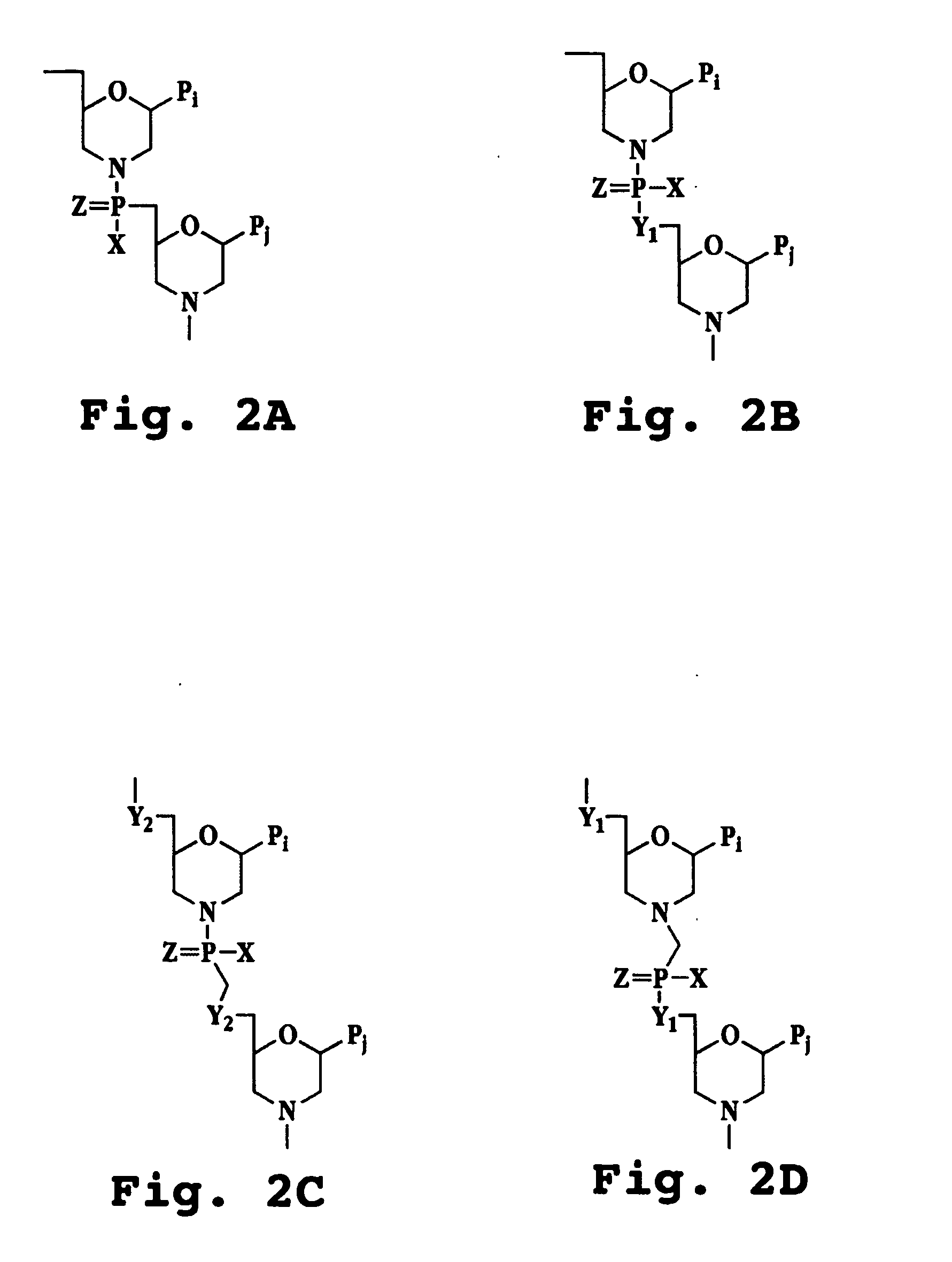
Antisense antiviral compound and method for treating ssRNA viral infection
ActiveUS20060269911A1Promote absorptionSsRNA viruses positive-senseMicrobiological testing/measurementOligonucleotideAstroviridae
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Flaviviridae, Picomoviridae, Caliciviridae, Togaviridae, Arteriviridae, Coronaviridae, Astroviridae and Hepeviridae families in the treatment of a viral infection. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of 1240 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 5′-terminal end 40 bases of the positive-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
Methods for treating arenaviridae and coronaviridae virus infections
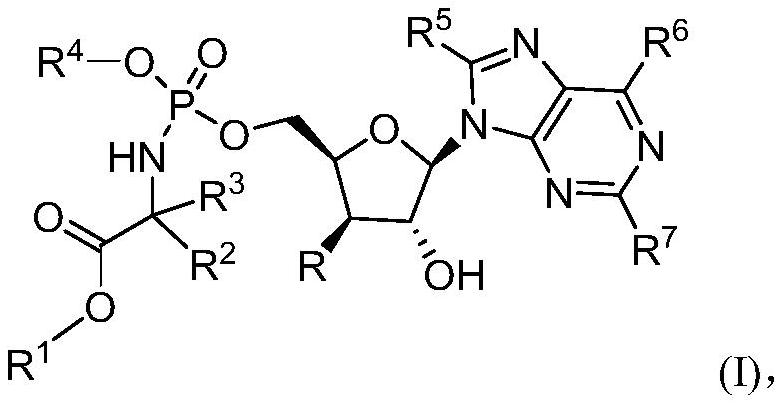
Provided are methods for treating Arenaviridae and Coronaviridae virus infections by administering nucleosides and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Lassa virus and Junin virus infections.
Owner:GILEAD SCI INC
Porcine epidemic diarrhea virus, and culture method and application thereof
ActiveCN103756974APerfect infection monitoring systemMicroorganism based processesAntiviralsSerum freeTGE VACCINE
The invention discloses a porcine epidemic diarrhea virus, and a culture method and an application thereof. The porcine epidemic diarrhea virus is called as coronaviridae coronavirus porcine epidemic diarrhea virus GDSJ / 2012, is preserved in China center for type culture collection on May 15th, 2013, and has the preservation number of CCTCC NO:V201309. The culture of the virus strain requires a serum-free DMEM culture solution containing trypsin and magnesium chloride. The virus strain can be used for preparation of a PEDV diagnostic kit and a PEDV vaccine, has good immunogenicity, and can make up for the deficiency of few conventional vaccine types.
Owner:WENS FOODSTUFF GRP CO LTD
Methods for treating arenaviridae and coronaviridae virus infections
Provided are methods for treating Arenaviridae and Coronaviridae virus infections by administering nucleosides and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Lassa virus and Junin virus infections.
Owner:GILEAD SCI INC
Antisense antiviral compound and method for treating ssRNA viral infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Flaviviridae, Picornoviridae, Caliciviridae, Togaviridae, Arteriviridae, Coronaviridae, Astroviridae and Hepeviridae families in the treatment of a viral infection. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 5′-terminal end 40 bases of the positive-sense RNA strand of the virus.
Owner:SAREPTA THERAPEUTICS INC
Sense Antiviral Compound and Method for Treating Ssrna Viral Infection
InactiveUS20080311556A1Disruption of secondary structureInhibition of replicationOrganic active ingredientsBiocideSsRNA virusesViral infection
The invention provides sense antiviral compounds and methods of their use in inhibition of growth of viruses of the Flaviviridae, Picornoviridae, Caliciviridae, Togaviridae, Coronaviridae families and hepatitis E virus in the treatment of a viral infection. The sense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of (12-40) subunits, including at least (12) subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 3′-terminal end (40) bases of the negative-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
Permissive cells and uses thereof
ActiveUS20100158947A1Compound screeningSsRNA viruses positive-senseVaccine ProductionFamily Arteriviridae
Owner:UNIV GENT ENGLISH TRANSLATION BEING GHENT UNIV
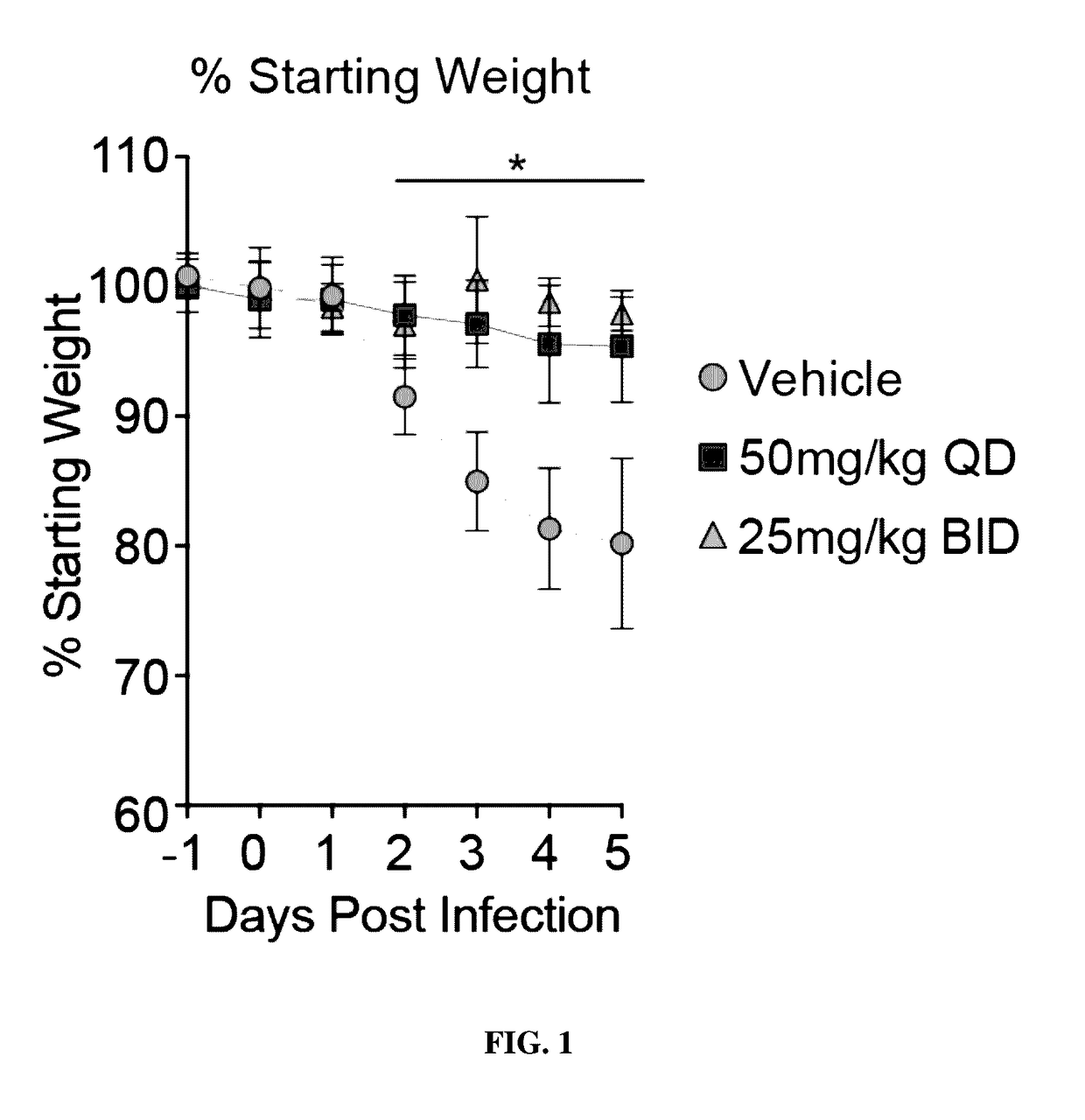
Method and compostitions for treating coronavirus infection
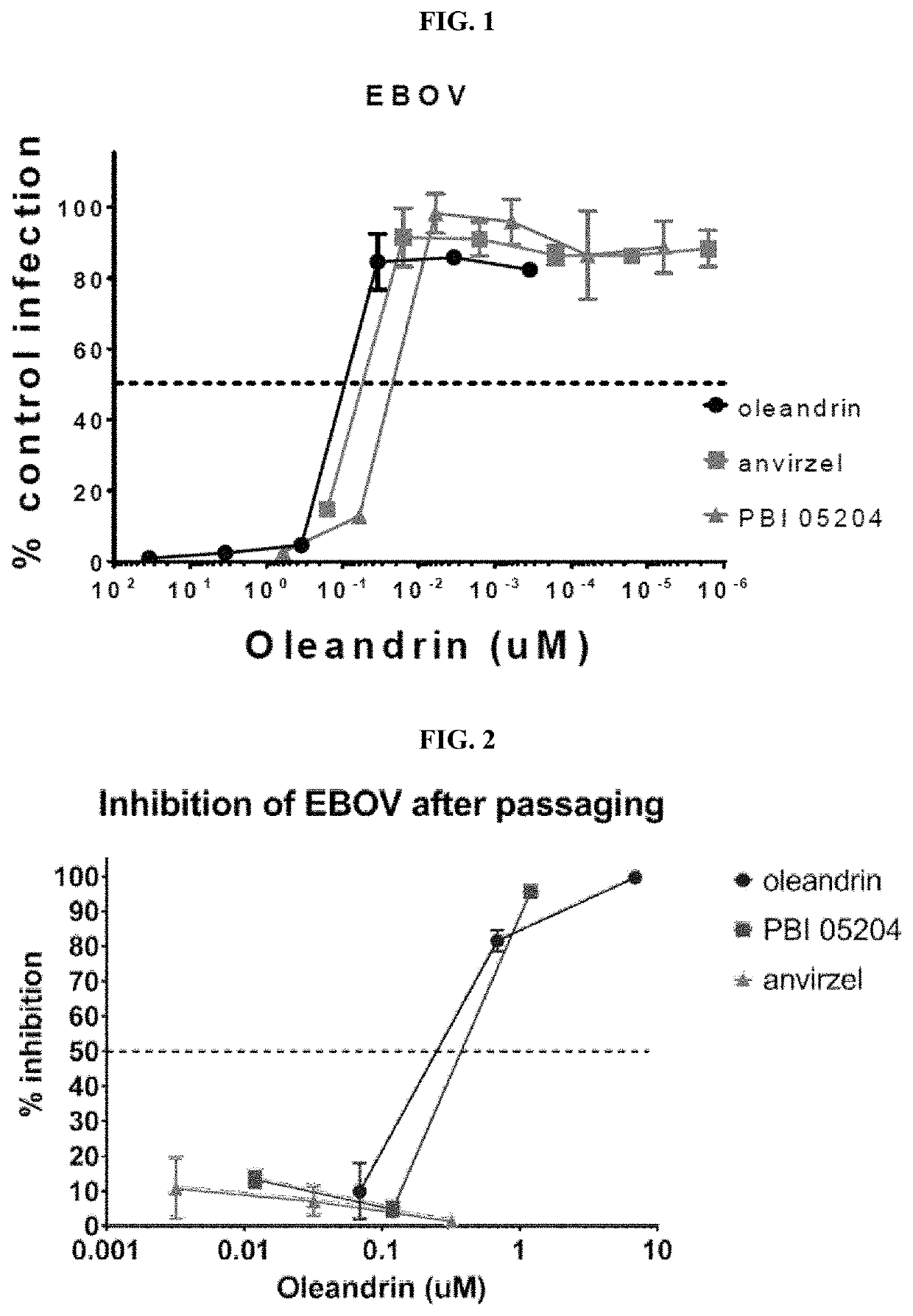
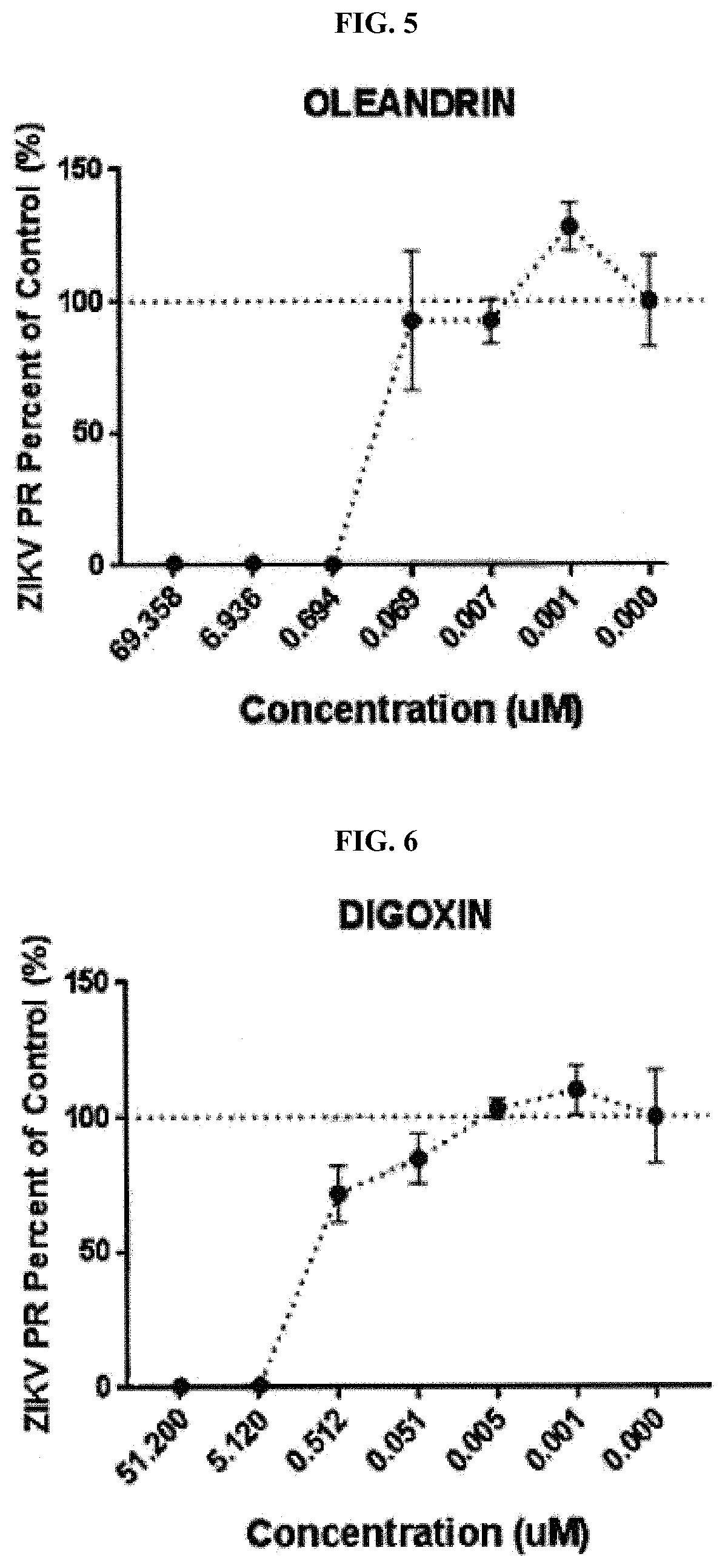
ActiveUS10729735B1Inhibiting infectivityReducing intercellular transmissionOrganic active ingredientsAntiviralsOleandrinViral infection
A method of treating viral infection, such as viral infection caused by a virus of the Coronaviridae family, is provided. A composition having at least oleandrin is used to treat viral infection.
Owner:PHOENIX BIOTECH INC
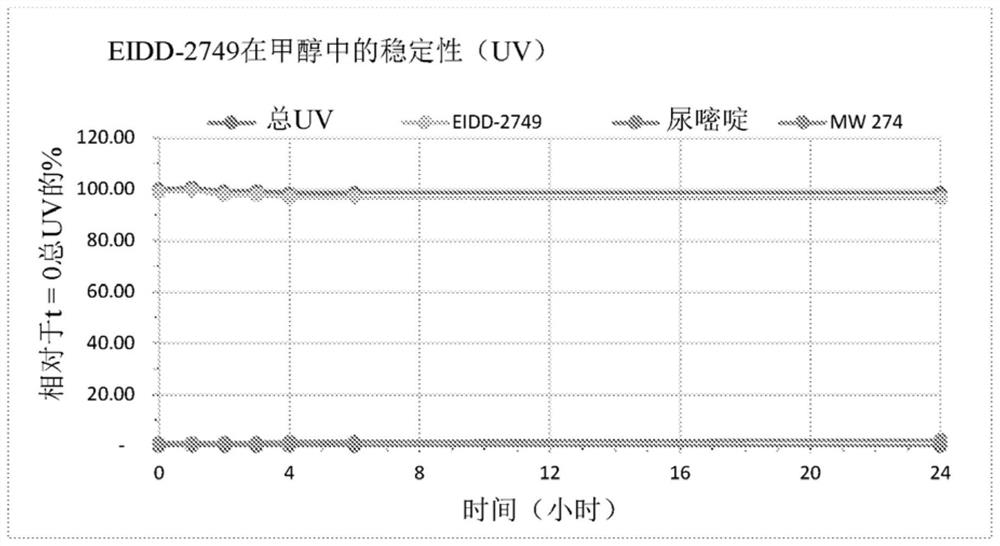
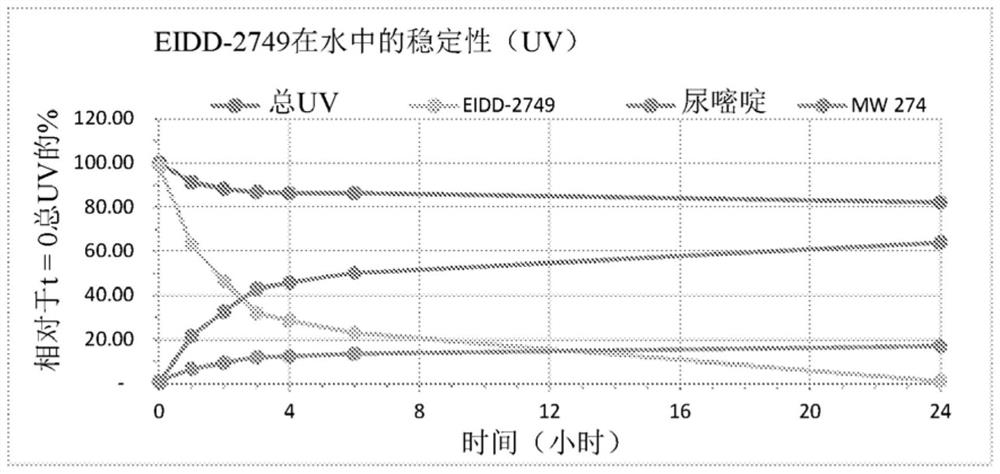
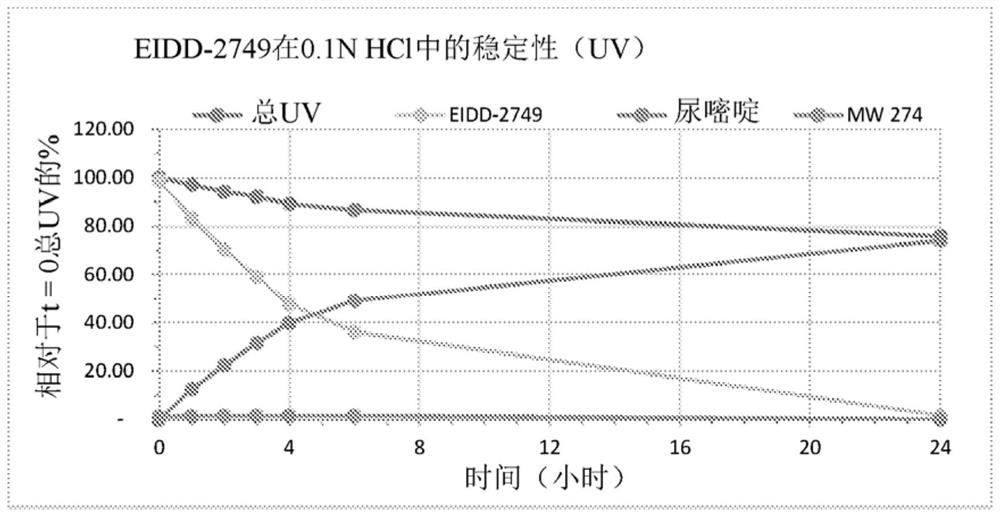
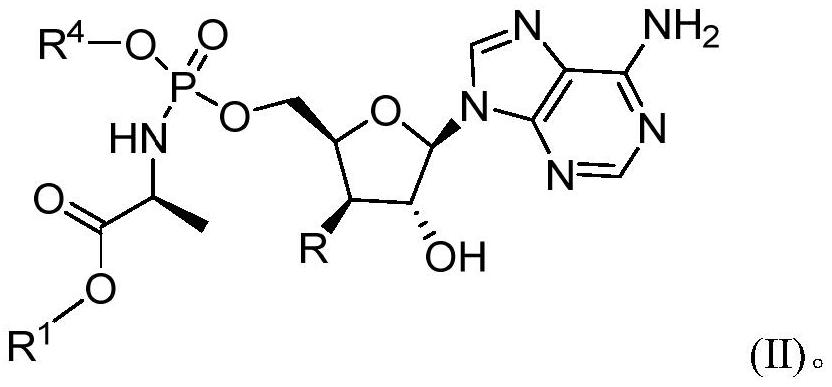
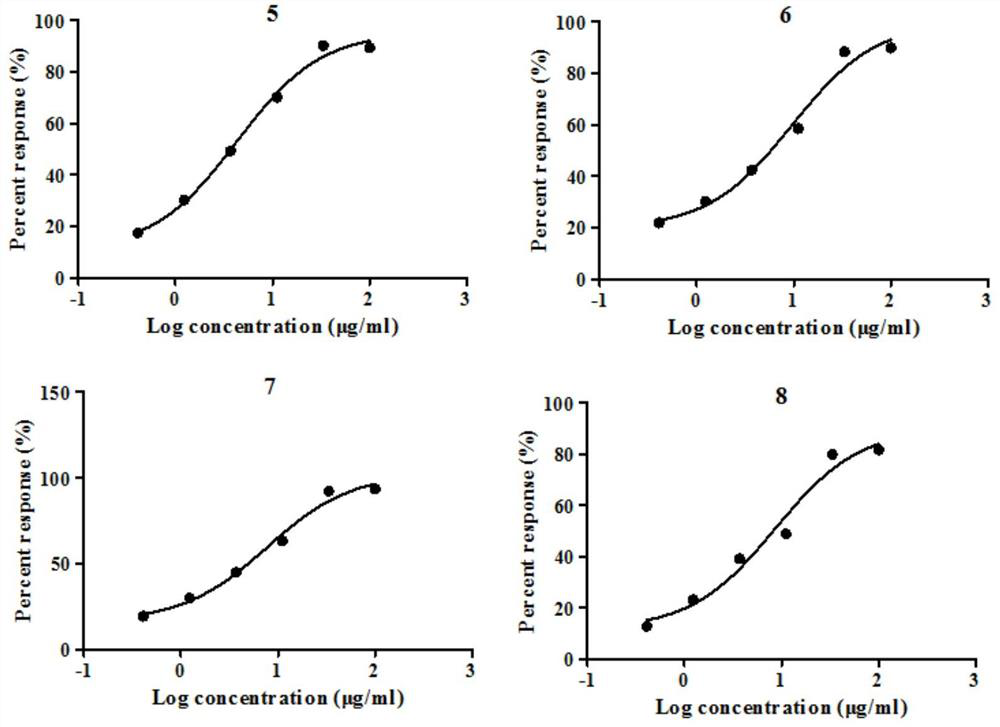
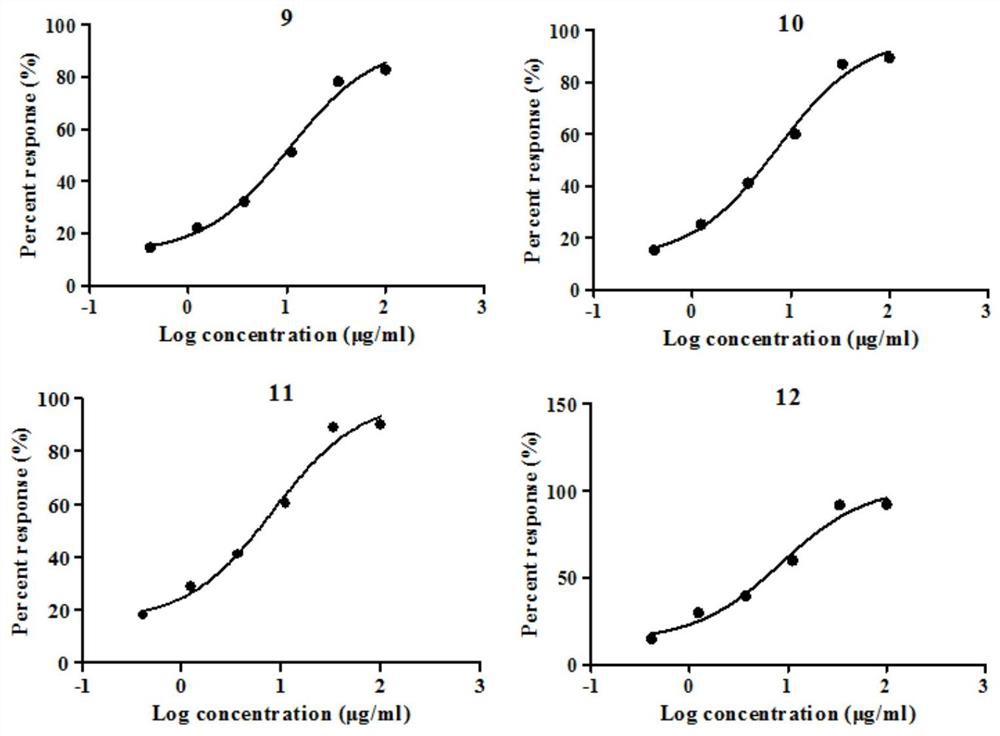
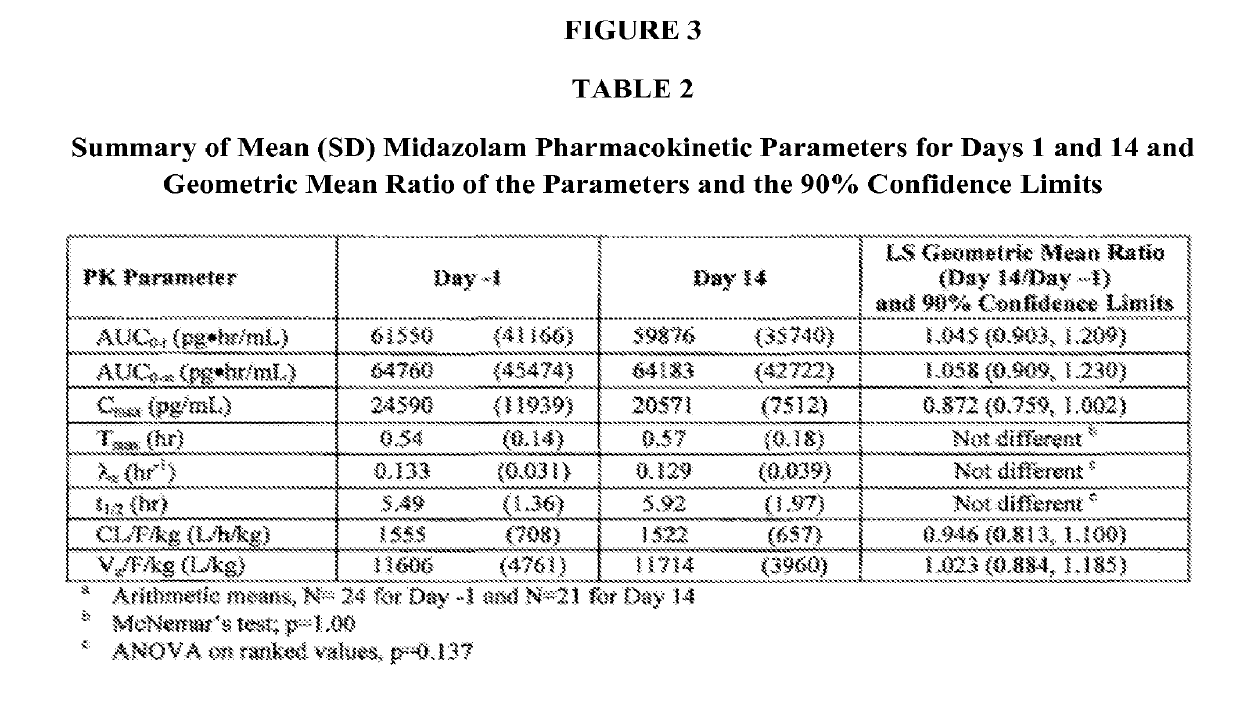
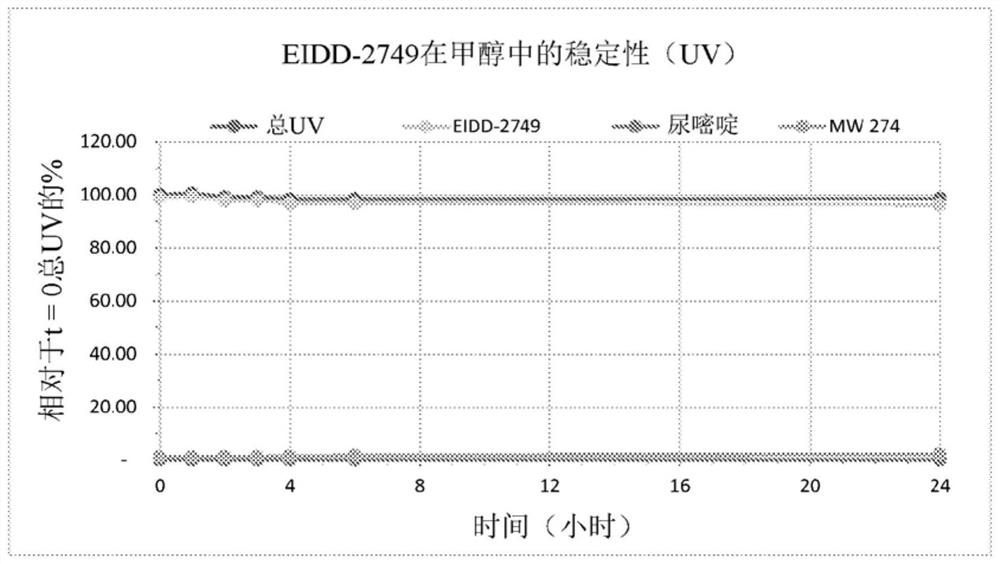
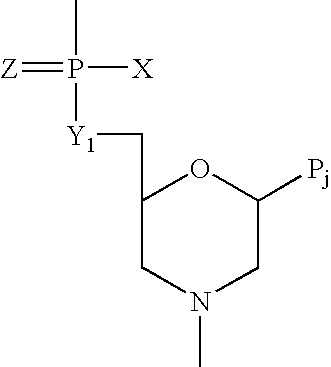
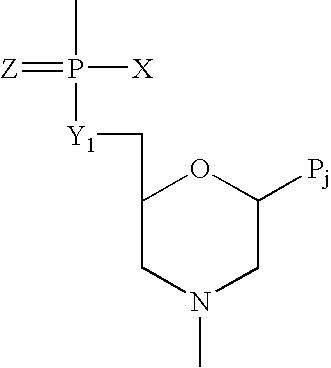
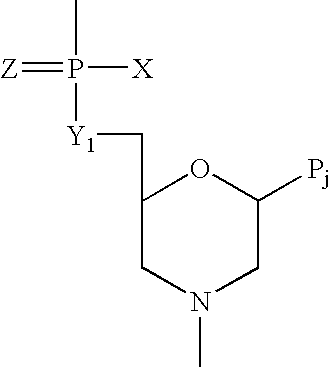
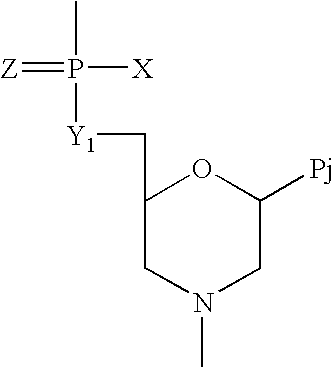
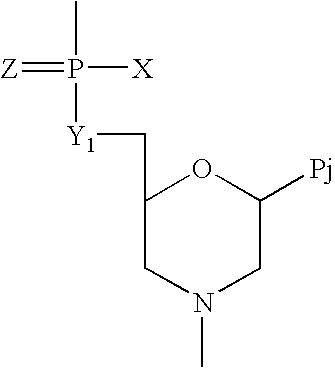
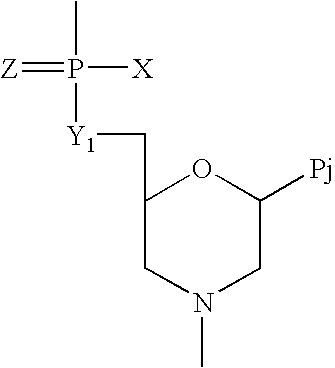
Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate
The present invention relates to novel salts and crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate having the structure:for use in treating viral infections. One crystalline form of the compound can be characterized by an X-ray powder diffraction (XRPD) pattern having peaks at 22.3°, 16.9°, and 16.2° 2-θ±0.2° 2-θ. In some embodiments, the viral infection is caused by a virus selected from the group consisting of Arenaviridae, Coronaviridae, Filoviridae, Flaviviridae, and Paramyxoviridae.
Owner:GILEAD SCI INC
Glycopeptide antibiotic derivatives
InactiveUS20050250677A1Decreasing and removing antibacterial activityMaintain antiviral activityBiocideDigestive systemHerpes zoster virusGlycopeptide
Novel glycopeptide antibiotic derivatives, processes for their preparation, their use as a medicine, their use to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections are provided. The present invention relates to the use of glycopeptide antibiotics and their semisynthetic derivatives to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections of subjects, more in particular infections with viruses belonging to Retroviridae, Herpes viridae, Flaviviridae and the Coronaviridae, like HIV (human immunodeficiency virus), HCV (hepatitis C virus), BVDV (bovine viral diarrhoea virus), SARS (severe acute respiratory syndrome) causing virus, FCV (feline coronavirus), HSV (herpes simplex virus), VZV (varicella zoster virus) and CMV (cytomegalovirus).
Owner:BALZARINI JAN +2
Preventive or therapeutic composition for viral infectious disease
InactiveUS20060189542A1Preventing flavivirus infectious diseasesOrganic active ingredientsBiocideOxidized GlutathioneFlaviviridae
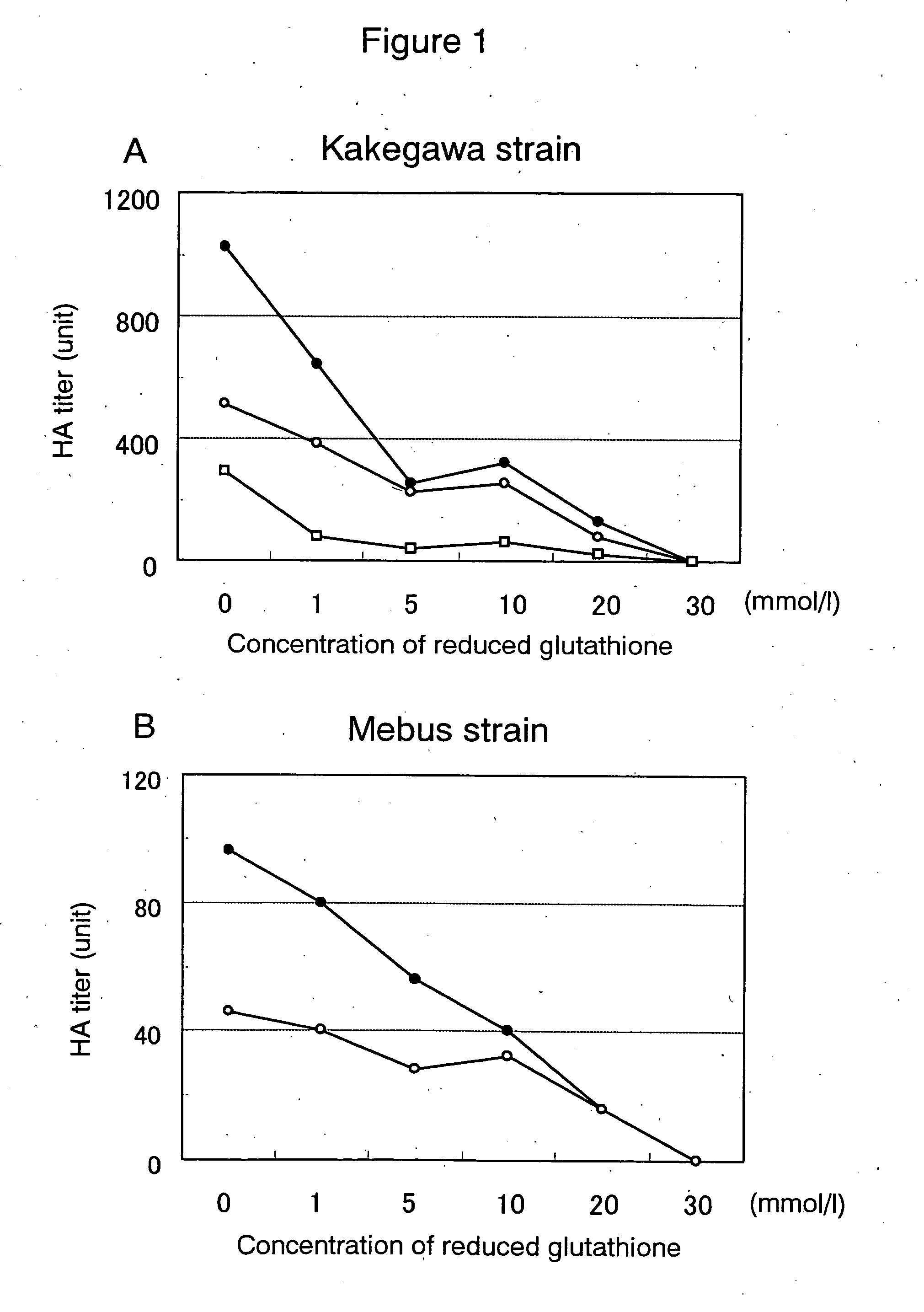
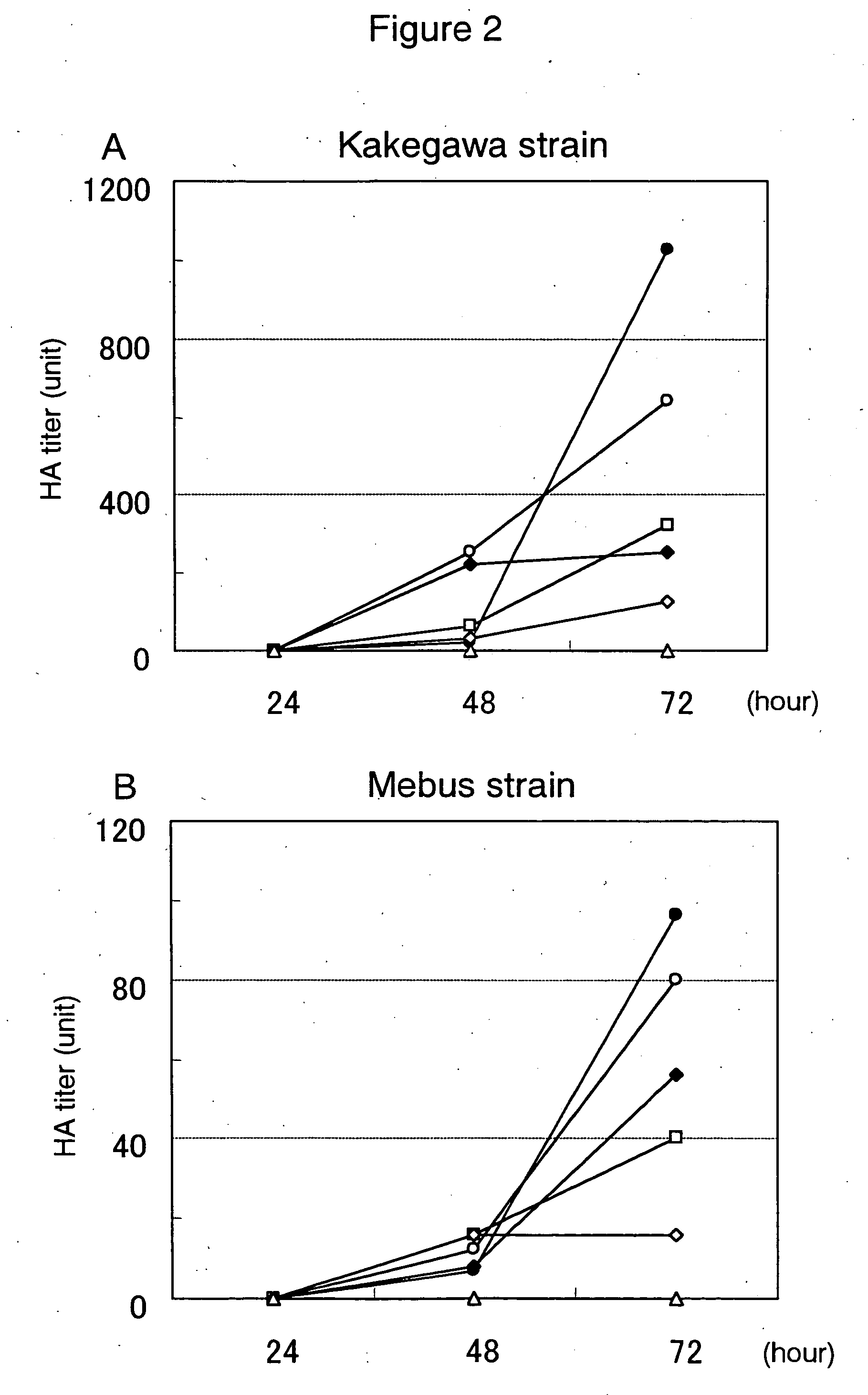
The invention provides a composition for preventing or treating infectious diseases of a virus belonging to the Coronavirus family or Flavivirus family, the composition containing one or more substances selected from reduced glutathione or oxidized glutathione or pharmaceutically acceptable salts thereof and catechin, and a composition for preventing or treating infectious diseases of a virus belonging to the Coronavirus family or Flavivirus family, the composition containing reduced glutathione or oxidized glutathione or pharmaceutically acceptable salts thereof and catechin.
Owner:KYOWA HAKKO BIO CO LTD
Application of gallic acid as well as derivatives and structural analogues thereof in preparation of anti-coronavirus drugs
InactiveCN111658631ASignificant anti-coronavirus effectDefinite curative effectOrganic active ingredientsAntiviralsBiotechnologyMiddle East respiratory syndrome
The invention belongs to the technical field of biological medicines, and particularly relates to application of gallic acid as well as derivatives and structural analogues thereof in preparation of anti-coronavirus drugs. Researches show that gallic acid as well as derivatives and structural analogues thereof have an obvious antivirus effect on coronaviridae viruses including 2019 novel coronaviruses (2019-nCoV or SARS-CoV-2), Middle East respiratory syndrome coronaviruses (MERS-CoV) and the like. Gallic acid substances are natural, widely exist in various plants, are polyphenol compounds existing in the nature, are widely applied to the fields of food, biology and medicine, and are very safe, so that the substances have very good application value in the aspect of preparation and development of antiviral drugs for coronaviridae.
Owner:GUANGZHOU SHENGPU INVESTMENT MANAGEMENT
Novel Antiviral Therapies
The field of the invention relates to the use of carbohydrate binding compounds as a medicine, their use to treat or prevent viral infections, their use to manufacture a medicine to treat or prevent viral infections and their use in a vaccination strategy. The present invention relates to the use of said compounds to manufacture a medicine to treat or prevent viral infections of subjects, more in particular infections with viruses having glycosilated envelop proteins such as Retroviridae (i.e. Lentivirinae), like HIV (human immunodeficiency virus), Flaviviridae, like HCV (hepatitis C virus), Hepadnaviridae, like HBV (hepatitis B virus), Coronaviridae, like SARS corona virus, and Orthomyxoviridae, like influenza A, B or C.
Owner:K U LEUVEN RES & DEV +1
Viral inactivation process
ActiveUS20110020406A1Reduce viremiaSsRNA viruses positive-senseViral antigen ingredientsFamily ArteriviridaePorcine reproductive and respiratory syndrome virus
The invention relates generally to the field of virology. More particularly, the present invention relates to methods for determining the effect of a viral inactivation procedure on the antigenicity of the inactivated virus. In particular for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). The invention further provides methods to determine the antigenicity of an inactivated virus as well as methods to screen for anti-viral compounds using any one of the aforementioned methods Methods of using the inactivated and immunogenic virus thus obtained, in particular in the manufacture of a vaccine are also provided by the present invention.
Owner:UNIV GENT
4'-halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto
Disclosed are halogen containing nucleotide and nucleoside therapeutic compositions and uses related thereto. In certain embodiments, the disclosure relates to the treatment or prophylaxis of viral infections. Such viral infections can include tongaviridae, bunyaviridae, arenaviridae, coronaviridae, flaviviridae, picornaviridae, Eastern, Western, and Venezuelan Equine Encephalitis (EEE, WEE and VEE, respectively), Chikungunya fever (CHIK), Ebola, Influenza, RSV, and Zika virus infections.
Owner:EMORY UNIVERSITY
Phosphoramidate derivatives of nucleoside compounds and uses of derivatives
ActiveCN112010916AInhibition of DiffusionExcellent anti-2019-nCoV activityOrganic active ingredientsSugar derivativesPhosphoramidateCoronavirus
The invention belongs to the technical field of medicines, and relates to phosphoramidate derivatives of nucleoside compounds, uses of the phosphoramidate derivatives, and a pharmaceutical compositioncontaining the compounds. The derivatives and pharmaceutical composition can be used as an antiviral reagent, especially an anti-novel coronavirus (SARS-CoV-2) reagent. The invention also relates toa method for preparing the compounds and the pharmaceutical composition, and the use of the derivatives and pharmaceutical composition to prevention or treatment of viral infections, including but notlimited to, Flaviviridae viral infections, Filoviridae viral infections, Enteroviridae viral infections, Orthomyxoviridae viral infections, Paramyxoviridae viral infections, coronaviridae viral infections, particularly novel coronaviridae (SARS-CoV-2) infections.
Owner:SUNSHINE LAKE PHARM CO LTD
Antiviral therapies
The field of the invention relates to the use of carbohydrate binding compounds as a medicine, their use to treat or prevent viral infections, their use to manufacture a medicine to treat or prevent viral infections and their use in a vaccination strategy. The present invention relates to the use of said compounds to manufacture a medicine to treat or prevent viral infections of subjects, more in particular infections with viruses having glycosilated envelop proteins such as Retroviridae (i.e. Lentivirinae), like HIV (human immunodeficiency virus), Flaviviridae, like HCV (hepatitis C virus), Hepadnaviridae, like HBV (hepatitis B virus), Coronaviridae, like SARS corona virus, and Orthomyxoviridae, like influenza A, B or C.
Owner:K U LEUVEN RES & DEV +1
Porcine epidemic diarrhea virus and separation culture method
InactiveCN106399259AMicroorganism based processesViruses/bacteriophagesPorcine epidemic diarrhoea virusImmunogenicity
The present invention discloses a strain of porcine epidemic diarrhea virus and a separation culture method. According to the present invention, the virus is Coronaviridae Coronavirus porcine epidemic diarrhea virus, is named PEDV / CH / BJ / 2014, and has the preservation number of CGMCC No.10111; the culture of the virus requires a blank DMEM culture liquid containing trypsin; and the porcine epidemic diarrhea virus has good immunogenicity, is the current domestic epidemic variant, can be used for preparing PEDV diagnosis kit, PEDV attenuated vaccines and inactivated vaccines, and enriches the existing PEDV vaccine resources.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Permissive cells and uses thereof
InactiveUS20140186395A1Highly efficient to sustain viral replicationReduce and avoid virus adaptationCompound screeningSsRNA viruses positive-senseVaccine ProductionFamily Arteriviridae
Described are methods for determining the permissiveness of a cell for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular, for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Further described are methods and compositions related to the generation of host cells permissive for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular, for PRRSV. Methods of utilzing the cells thus identified or thus generated, in preparing a culture of a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, as well as the use of the virus for the purpose of vaccine production or diagnosis, are also described.
Owner:UNIV GENT ENGLISH TRANSLATION BEING GHENT UNIV
COVID-19 data acquisition and analysis system
PendingCN111681727AGuaranteed data transferRealize data fusionMedical data miningDatabase management systemsCollection analysisScientific study
The invention discloses a COVID-19 data acquisition and analysis system, and belongs to the field of data acquisition and analysis. The system provides service and assistance for medical staff and government decision makers and provides important support for scientific research of novel coronavirus by fully utilizing various information including treatment schemes, medication data, inspection data, test data, image data, epidemiological data and the like. The system is constructed according to the characteristics of the COVID-19 novel coronavirus pneumonia, has strong specialty, connects epidemiological data with treatment schemes, inspection data, test data, medication data and image data of patients according to the identification numbers or identification numbers of the patients in thedata acquisition and analysis process, realizes the data fusion of public health data and internal information systems of hospitals, and is favorable for comprehensively knowing the COVID-19 novel coronavirus pneumonia.
Owner:CENT SOUTH UNIV
Application of gallic acid and derivatives and structural analogs thereof in preparation of anti-coronavirus medicines
InactiveCN112336709ASignificant anti-coronavirus effectDefinite curative effectOrganic active ingredientsAntiviralsMiddle East respiratory syndromeGallic acid ester
The invention belongs to the technical field of biological medicines, and particularly relates to application of gallic acid and derivatives and structural analogs thereof in preparation of anti-coronavirus medicines. Researches of the invention show that gallic acid and derivatives and structural analogs thereof have obvious antiviral effects on various coronaviridae viruses, including 2019-novelcoronaviruses (2019-nCoV or SARS-CoV-2), Middle East Respiratory Syndrome-Coronavirus (MERS-CoV), etc. Moreover, gallic acid and derivatives and structural analogs thereof are natural in source, widely exist in various plants, can be widely applied to the fields of food, biology and medicines, and are very safe, so that gallic acid and derivatives and structural analogs thereof have very good application value in the aspect of preparing and developing antiviral drugs against coronaviridae.
Owner:GUANGZHOU SHENGPU INVESTMENT MANAGEMENT
Methods and compositions for increasing the effectiveness of antiviral agents
InactiveUS20190321340A1High treatment rateGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsCommon coldDisease
Compositions and method of the present invention comprise novel formulations for increasing the effectiveness of antiviral agents and for preventing and treating symptoms associated with the common cold and viral infections. The present invention is directed to treating symptoms resulting from viral infections and diseases associated with Picomaviridae, Coronaviridae, Orthomyxoviridae, Paramyxovirinae, Reoviridae, and Adenoviridae. The novel formulations provided herein improve the therapeutic ratio of antiviral agents such as pleconaril.
Owner:ANTIVIRUS THERAPEUTICS
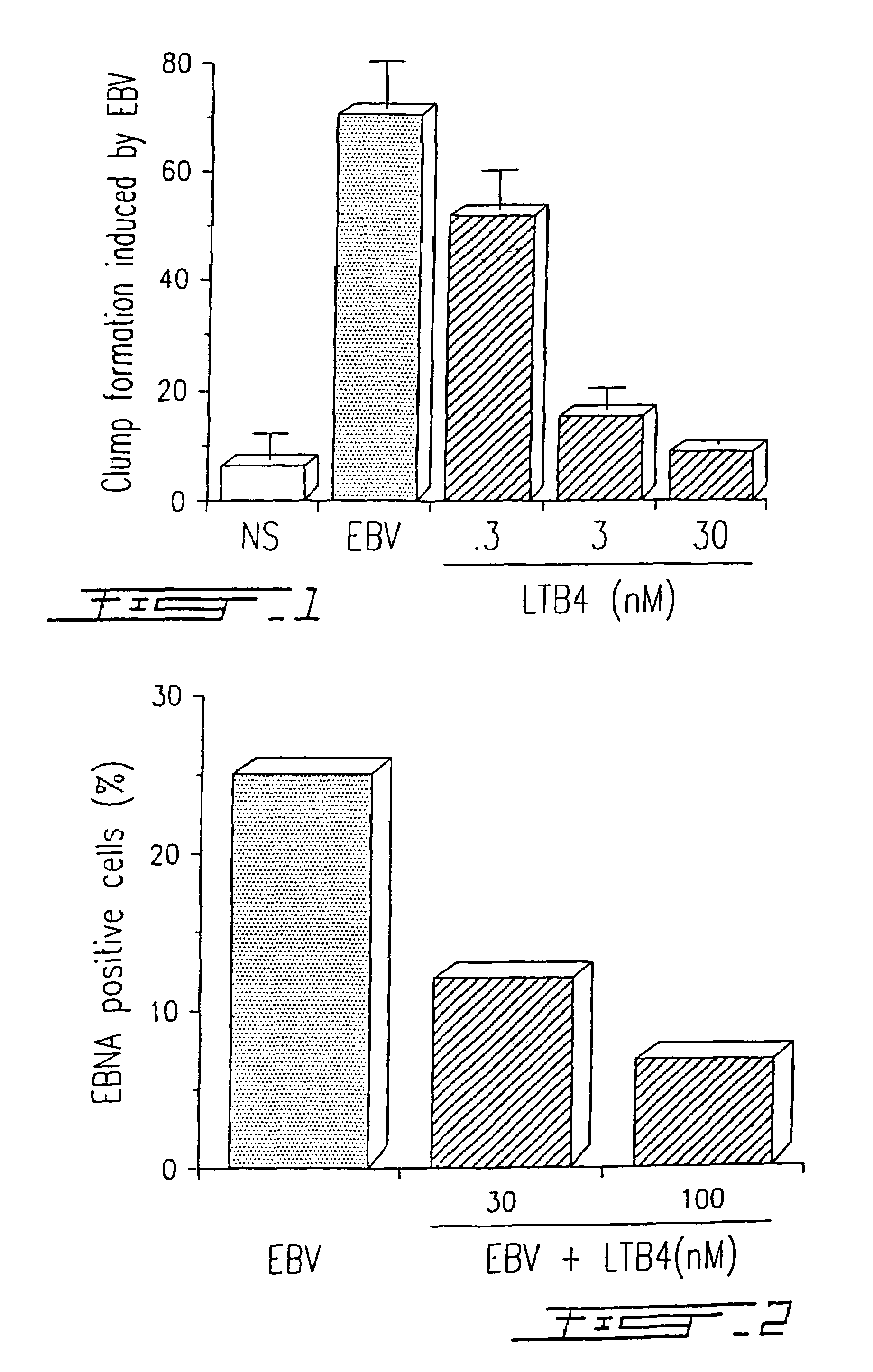
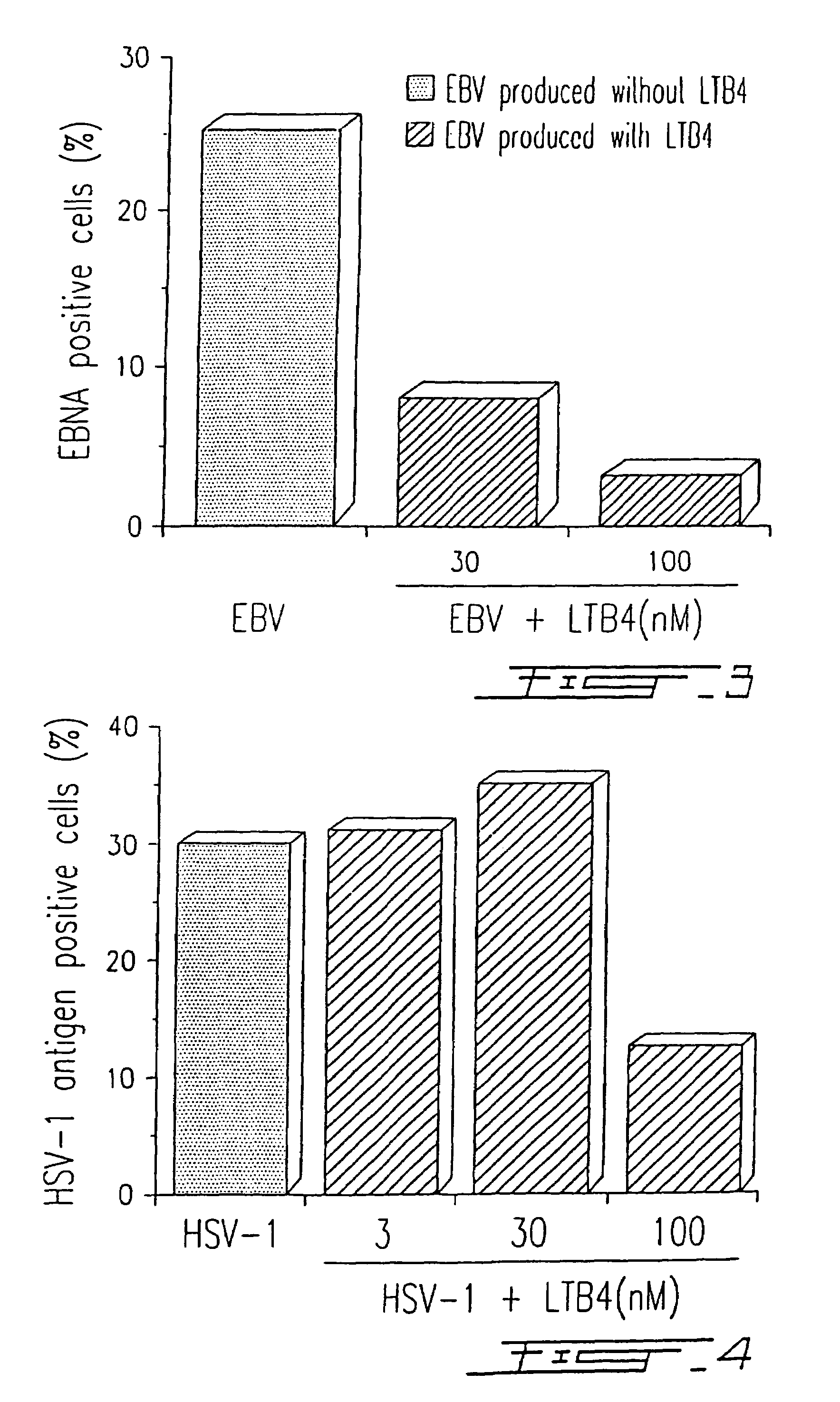
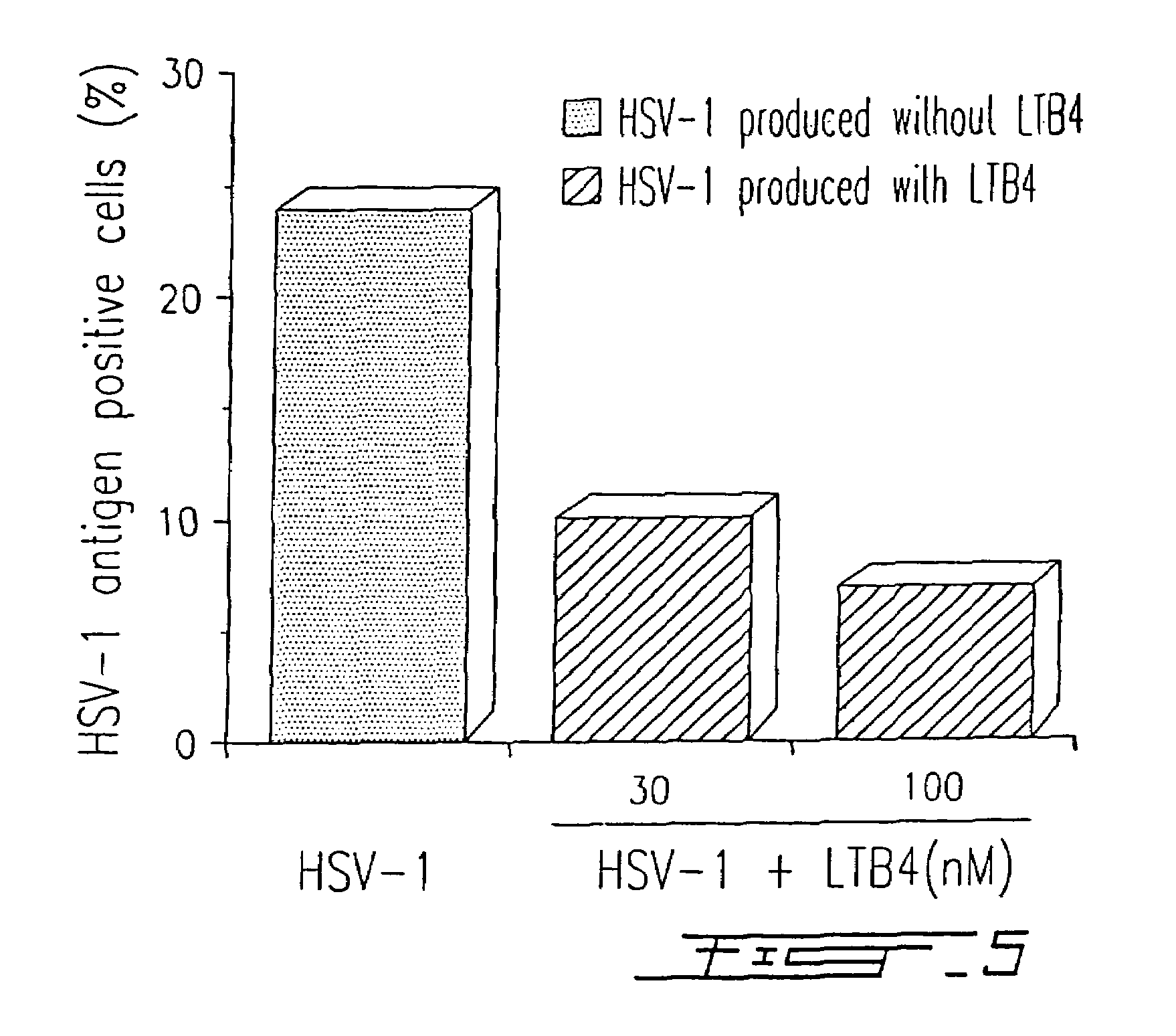
Agents with leukotriene B4-like antiviral (enveloped RNA) activities
The present invention relates to the use of the antiviral activity of exogenous leukotriene B4 (LTB4), variants and derivatives thereof as a therapeutic agent in viral infections caused by human and animal enveloped RNA viruses. The human and animal enveloped RNA viruses are RNA viruses, such as togaviridae, orthomyxoviridae, paramyxoviridae, coronaviridae, filoviridae, arenaviridae, bunyaviridae, rhabdoviridae and flaviviridae in general, and Retroviridae such as HIV-1 and HIV-2.
Owner:LTBE4 SWEDEN
Nanoparticle based coronavirus vaccine
ActiveCN113121704AAvoid infectionGood broad-spectrum immunitySsRNA viruses positive-senseBacteriaCoronavirus vaccinationNanoparticle
The invention provides a nanoparticle based coronavirus vaccine. The vaccine includes nanoparticles, the nanoparticles include fusion protein, wherein the fusion protein includes at least one immunogenic part of S protein of viruses in coronaviridae and at least one part of a self-assembled monomer subunit connected to the at least one immunogenic part of the S protein; and the at least one immunogenic part of the S protein is shown on the surfaces of the nanoparticles. The coronavirus vaccine can be used for preventing and / or treating coronavirus infections.
Owner:CHINA SCI XINYUN BIOTECH BEIJING CO LTD
Use of vMIP and congeneric E protein ligand in pharmacy
InactiveCN1470284AImprove medicinal effectPeptide/protein ingredientsAntiviralsDiseaseInfection rate
The invention refers to the new use of HHV8 chemotactic factor vMIP and the ramification and other albumen factor in the pharmacy field, especially refers to the use in preventing and curing the SARScorresponding Coronaviridae infection. The invention uses vMIP-I, vMIP-II, vMIP-III of recombined HHV8, combines the process that the E albumen of SARS coronaviridae can resist the virus infecting the cell, the invention has prominent effect on decreasing the infection rate of SARS coronaviridae to kidney cell Vero E6, the mechanism is that the combination with homologous E albumen receiver prevents the infection, so it is possible that human control SARS disease effectively.
Owner:JINAN UNIVERSITY
A strain of porcine epidemic diarrhea virus and its culture method and application
ActiveCN103756974BPerfect infection monitoring systemMicroorganism based processesAntiviralsSerum freeTGE VACCINE
The invention discloses a strain of porcine epidemic diarrhea virus and its cultivation method and application. The strain of porcine epidemic diarrhea virus is named Coronaviridae Coronaviridae Porcine Epidemic Diarrhea Virus GDSJ / 2012 (Porcine epidemic diarrhea virus). It was deposited in the China Center for Type Culture Collection on May 15, 2013, with the preservation number CCTCC NO:V201309. The culture of this strain requires the use of serum-free DMEM medium containing trypsin and magnesium chloride. It can be used to prepare PEDV diagnostic reagents and PEDV vaccines, has good immunogenicity, and can make up for the shortage of existing vaccines.
Owner:WENS FOODSTUFF GRP CO LTD
Methods and compositions for treating coronavirus infections
ActiveCN112689510AHydroxy compound active ingredientsPharmaceutical delivery mechanismOleandrinEngineering
Provided is a method for treating a viral infection such as a viral infection caused by a coronaviridae virus, for example. Compositions having at least oleandrin are used in the treatment of viral infections.
Owner:PHOENIX BIOTECH INC
Human medical prophylaxis of coronaviridae pathogenic infection by topical application of immune coronaviridae immunoglobulin a
A method of inhibiting or treating infection by Coronaviridae virus of a susceptible host is provided that includes the administration of immune anti-Coronaviridae secretory IgA having a recombinant secretory component to at least one of tissue of the susceptible host. The administration being prior to, or after the susceptible host is exposed to the Coronaviridae virus. A composition for such administration is also provided.
Owner:OLSON ROBERT SCOTT +1
Application of recombinant cytokine gene derived protein or fragment thereof
PendingCN113289006AInhibitory activityOrganic active ingredientsPeptide/protein ingredientsDiseasePharmaceutical drug
The invention provides an application of a recombinant cytokine gene-derived protein or a fragment thereof in inhibiting the activity of a coronaviridae virus. The recombinant cytokine gene-derived protein comprises an amino acid sequence SEQ ID NO: 1, or an amino acid sequence having at least 90% identity with the amino acid sequence SEQ ID NO: 1. In addition, the invention also provides application of the recombinant cytokine gene derived protein or the fragment thereof in preparation of drugs for preventing or treating diseases or symptoms related to coronaviridae viruses in subjects. The recombinant cytokine gene derived protein or fragment thereof according to the present invention has enhanced anti-coronaviridae virus activity compared to human interferon [alpha]2b (HuIFN-[alpha]2b).
Owner:GENOVA BIOTECH QINGDAO CO LTD +1
4 '-halogen-containing nucleotide and nucleoside therapeutic compositions and uses related thereto
Halogen-containing nucleotide and nucleoside therapeutic compositions and related uses thereof are disclosed. In certain embodiments, the disclosure relates to the treatment or prevention of viral infections. Such viral infections may comprise a tapiviridae, a bunyaviridae, an arenaviridae, a coronaviridae, a flaviviridae, a picornaviridae, eastern equine encephalitis, western equine encephalitis, and Venezuela equine encephalitis (EEE, WEE, and VEE, respectively), Chikungunya fever (CHIK), Ebola, influenza, RSV, and Zika viral infections.
Owner:EMORY UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff2b4087-9dcd-48db-bfe0-8cbe99326db5/US10836787-D00001.png)
![Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff2b4087-9dcd-48db-bfe0-8cbe99326db5/US10836787-D00002.png)
![Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate Crystalline forms of (S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5- (4-aminopyrrolo[2,1-f] [1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy) phosphoryl)amino)propanoate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff2b4087-9dcd-48db-bfe0-8cbe99326db5/US10836787-D00003.png)