Permissive cells and uses thereof
a technology of permissive cells and cells, applied in the field ofvirology, can solve the problems of loss of induction of important neutralizing antibodies, inability to produce and release infectious progeny viruses, and even inability to carry out some cell types, so as to increase the permissivity of partially susceptible cells and enhance the production of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Methods
Cell Culture and Transfection
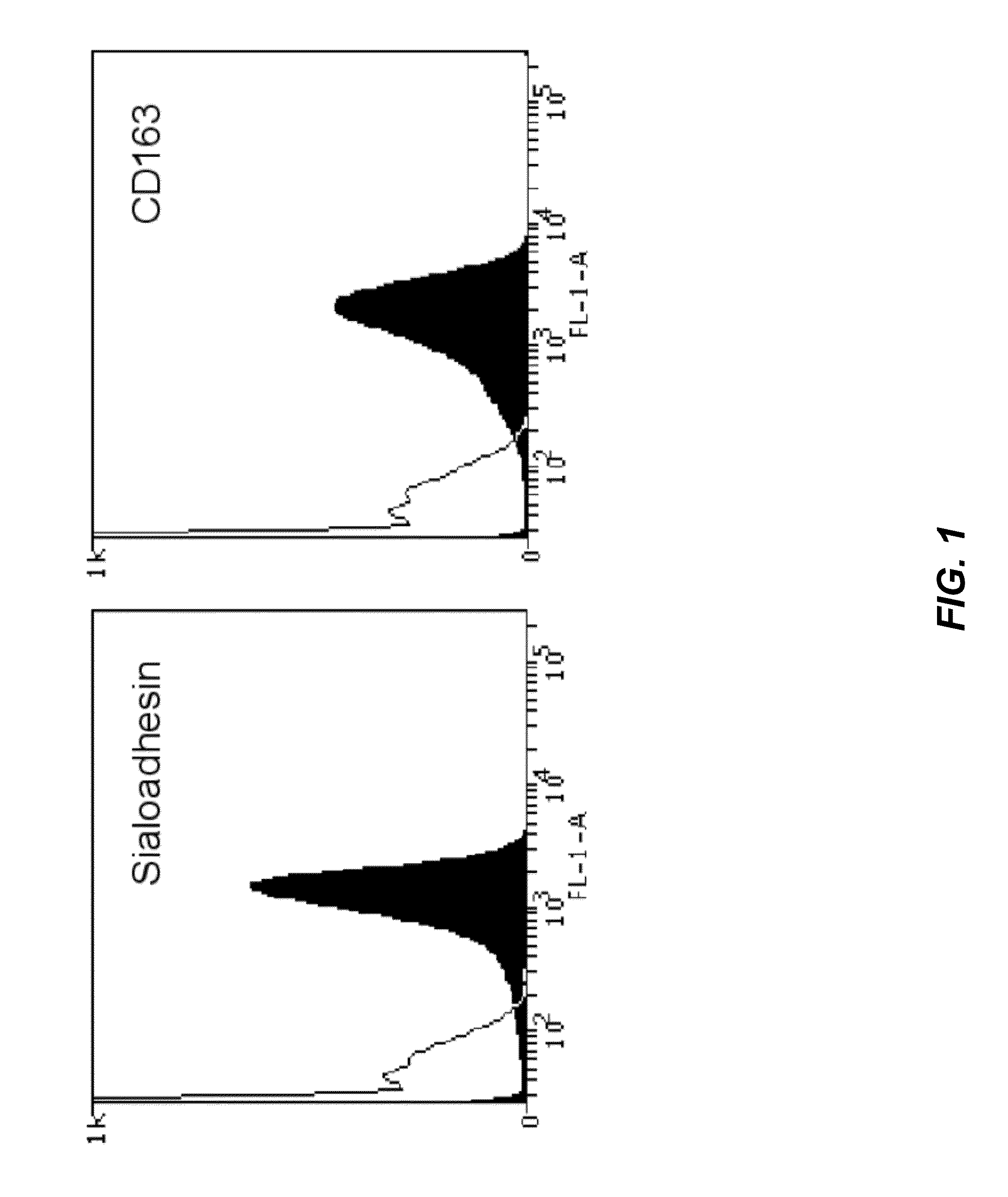
[0102]Primary alveolar macrophages were obtained from 4- to 6-week old conventional Belgian Landrace pigs from a PRRSV-negative herd as described by Wensvoort et al. (Wensvoort et al., 1991), and cultivated in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids and 1 mM sodium pyruvate. Marc-145 cells were cultivated in Minimum Essential Medium with Earle's salts (MEM) supplemented with 5% FBS. PK-15 cells were grown in MEM supplemented with 10% FBS. BHK-21 cells were cultivated in MEM supplemented with 10% FBS, 1% nonessential amino acids and 1 mM sodium pyruvate. CHO-K1 cells were cultivated in F-12 medium supplemented with 10% FBS and 1 mM sodium pyruvate. All cells were grown in their specific medium supplemented with 2 mM L-glutamine and a mixture of antibiotics in a humified 5% CO2 atmosphere at 37° C. PK-15, BHK-21 and CHO-K1 cells were transfected respectively with lipofectamine (Invitrogen), lipofectamine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com