Preparation methods and application of recombinant swine fever E2 protein and subunit vaccine of recombinant swine fever E2 protein
A subunit vaccine, swine fever technology, applied in biochemical equipment and methods, vaccines, veterinary vaccines, etc., can solve the problem that protein expression, folding and modification are not as good as mammalian cell expression systems, and it is difficult to achieve 500mg/L output, The differences in the structure of the natural antigen protein of the virus, etc., achieve the effect of no pollution risk, easy mass production, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
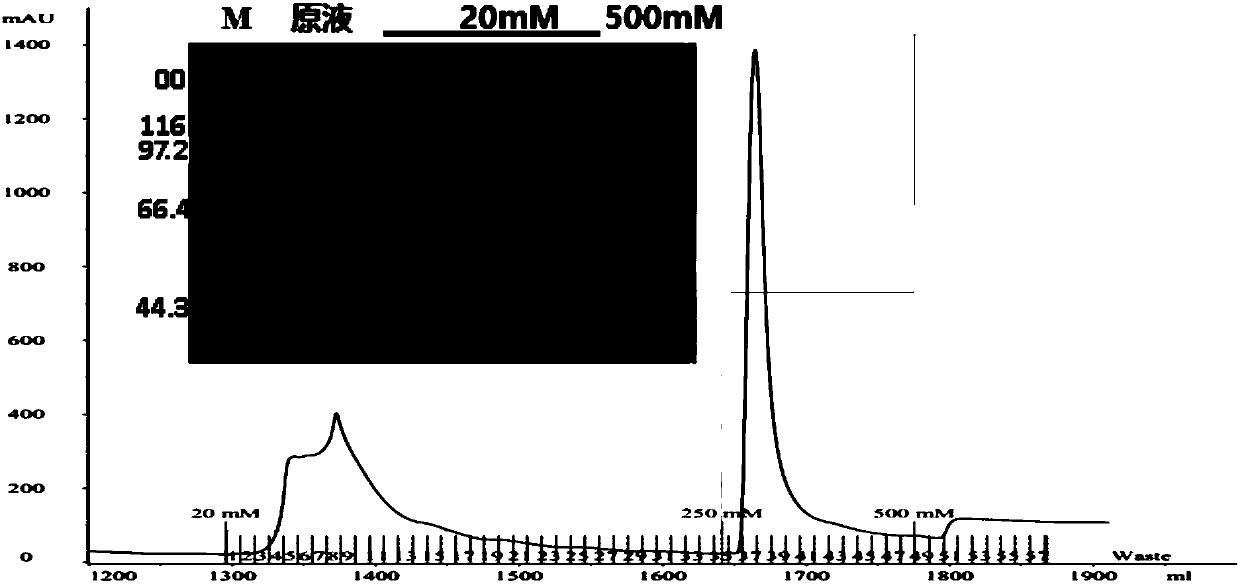
Image
Examples
Embodiment 1
[0041] Embodiment 1: Codon optimization of classical swine fever E2 protein
[0042] Through codon optimization on the nucleotide sequence of the classical swine fever E2 protein, the sequence of OPTI-E2 was obtained, as shown in SEQ ID NO.2. This work was entrusted to Suzhou Jinweizhi Biotechnology Co., Ltd. to complete.
[0043] Comparing the sequences of SEQ ID NO.2 and SEQ ID NO.3, it is found that the nucleotide sequence of the classical swine fever E2 protein after codon optimization is 21.3% higher than the nucleotide sequence of the non-codon-optimized classical swine fever E2 protein , that is, 222 nucleotides out of 1044 nucleotides are different. see details Figure 8 shown.
Embodiment 2
[0044] Example 2: Construction of pEE12.4-OPTI-E2 recombinant plasmid
[0045] 2.1 PCR amplification of the target fragment OPTI-E2
[0046] 2.1.1 PCR reaction
[0047] (1) Primer design and synthesis
[0048] Upstream primer: 5'-CCAAGCTTGCCGCCACCATGAAAGTGCTGAGGGGCCAG-3'
[0049] Downstream primer: 5'-CCGGAATTCTTAGTGATGGTGATGGTGATGAGC-3'
[0050] (2) Add 50 μL of the sample system, as shown in the table below:
[0051]
[0052] PCR amplification program:
[0053]
[0054] 2.1.2 Gel recovery of PCR products
[0055] (1) Mark the sample collection EP tube, adsorption column and collection tube;
[0056] (2) Take the weight of the marked empty EP tube, and record the value;
[0057] (3) Carefully cut out a single target DNA band from the agarose gel with a scalpel on a gel cutter and put it into a clean 1.5mL centrifuge tube;
[0058] (4) Add 600 μL PC buffer to the 1.5mL centrifuge tube in step (3), place in a 50°C water bath for about 5 minutes, and gently turn th...
Embodiment 3
[0121] Embodiment 3: pCDNA3.1-OPTI-E2 recombinant plasmid construction
[0122] According to the steps in Example 2, the results of the double enzyme digestion identification experiment are shown in Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com