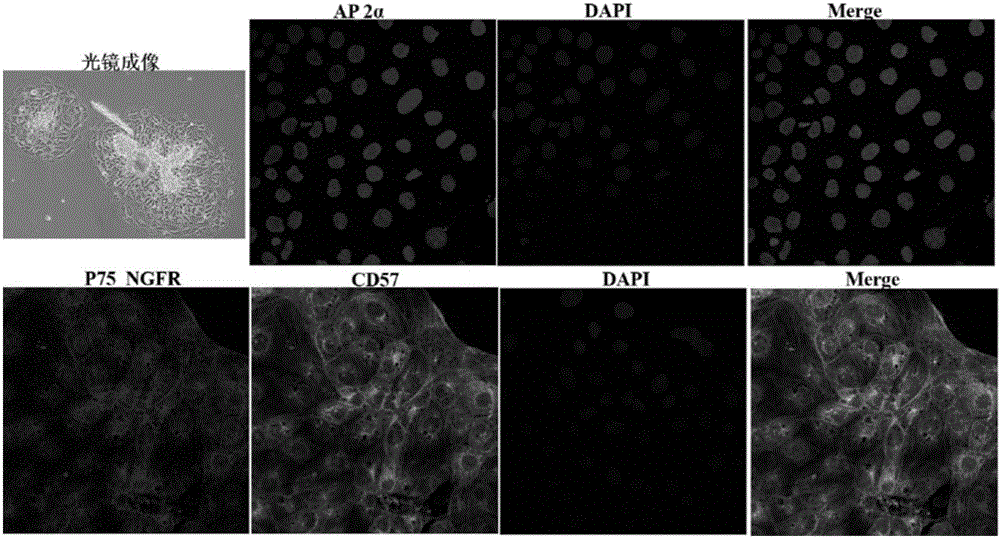
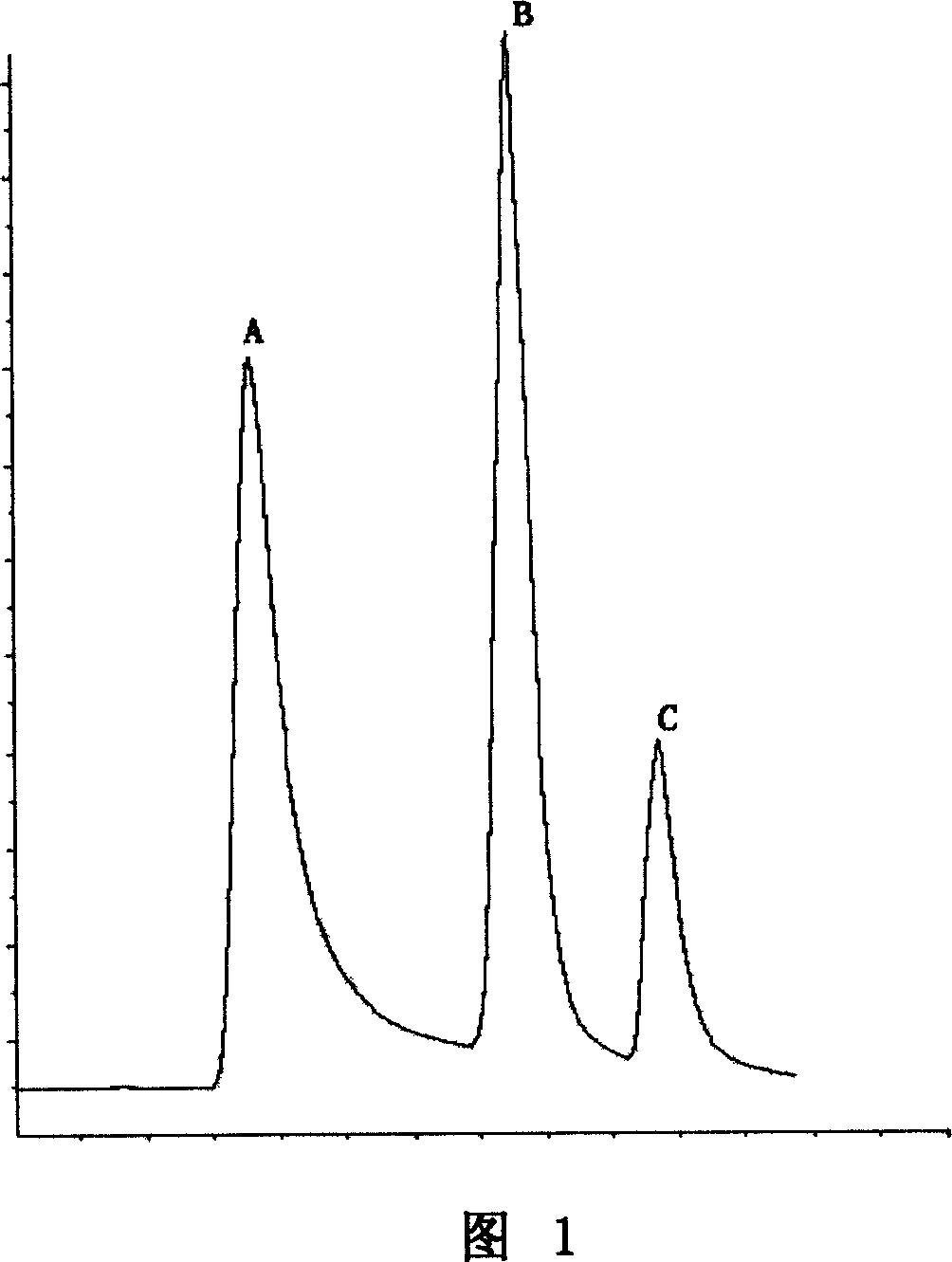
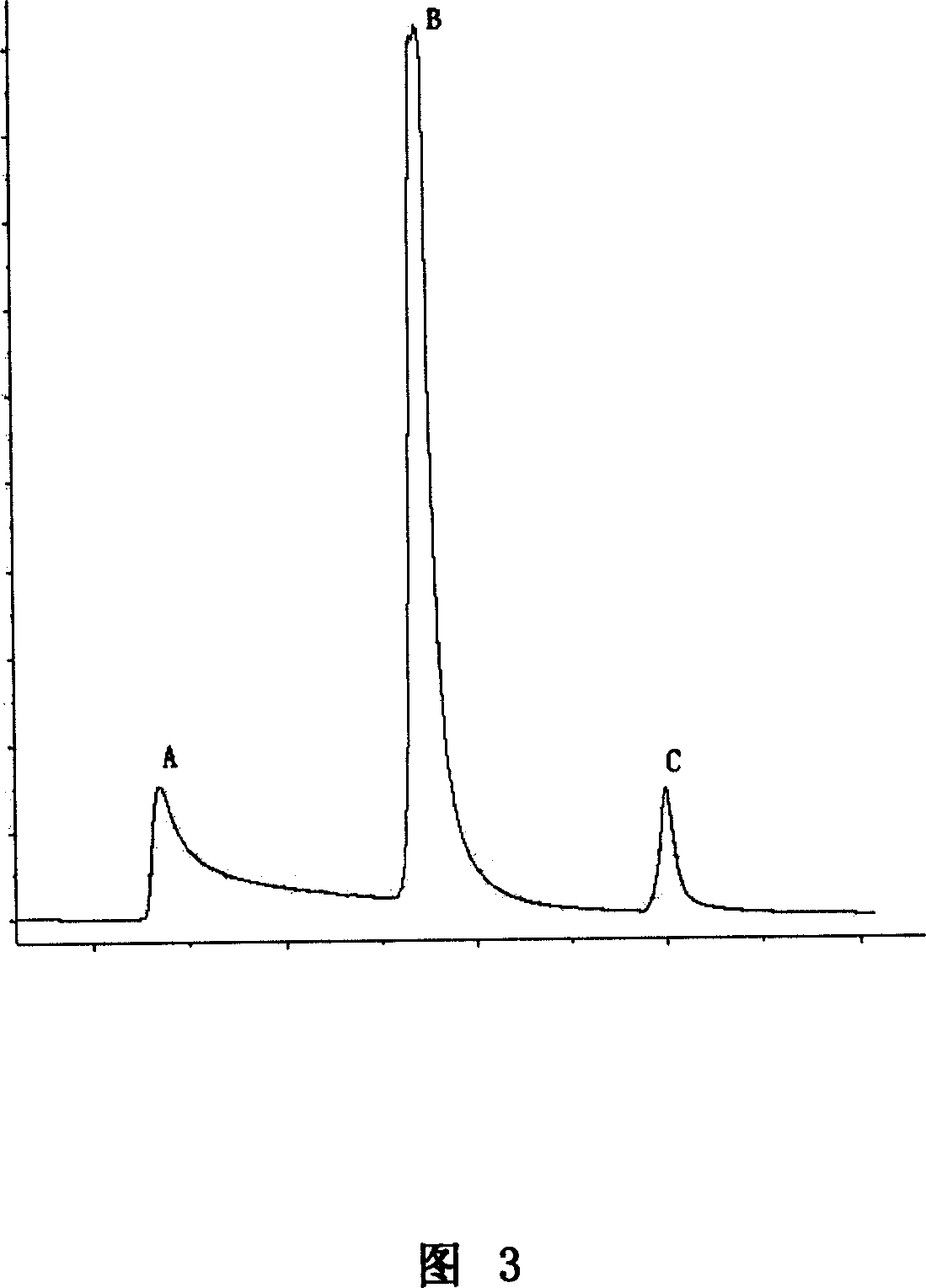
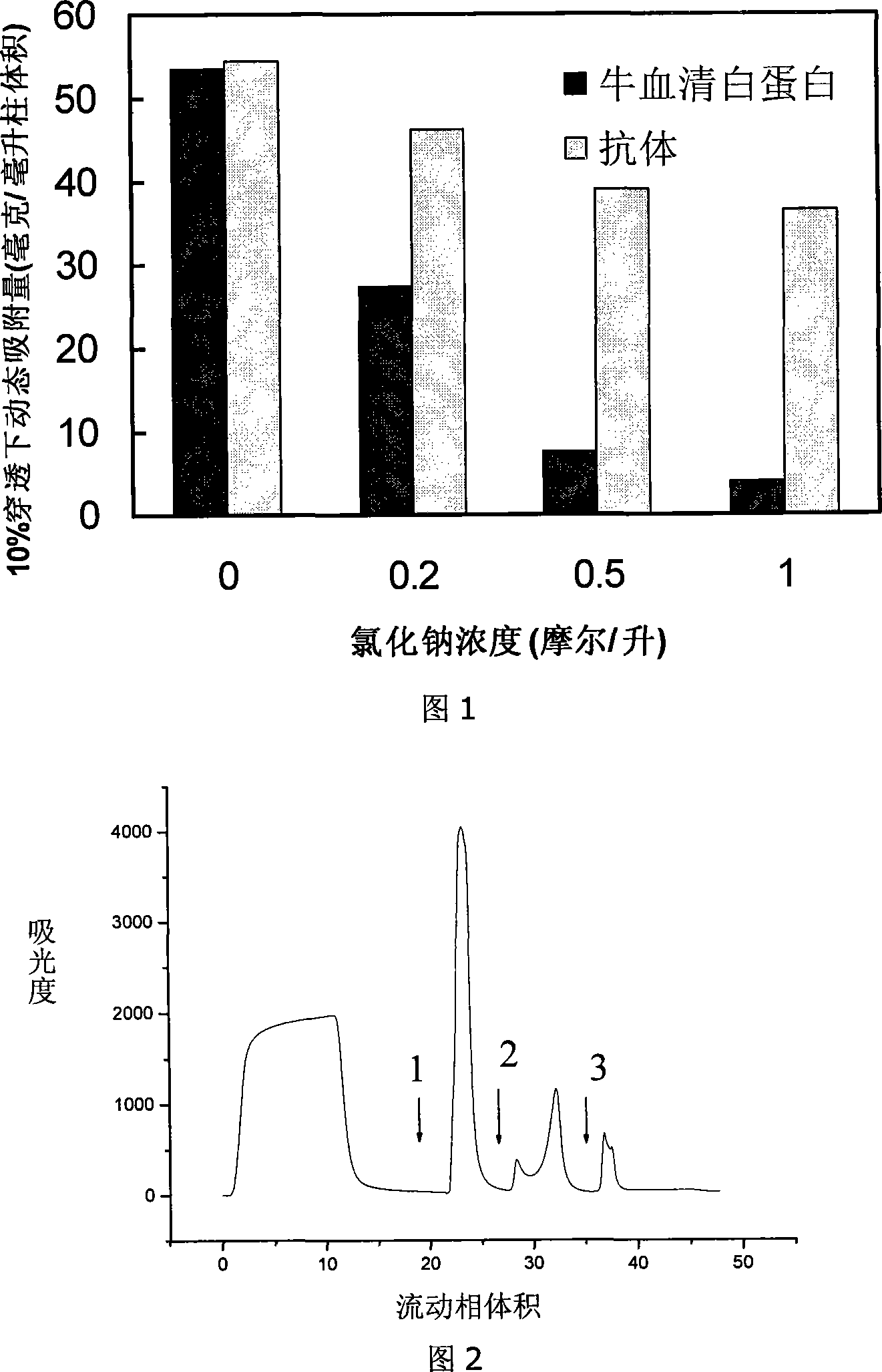
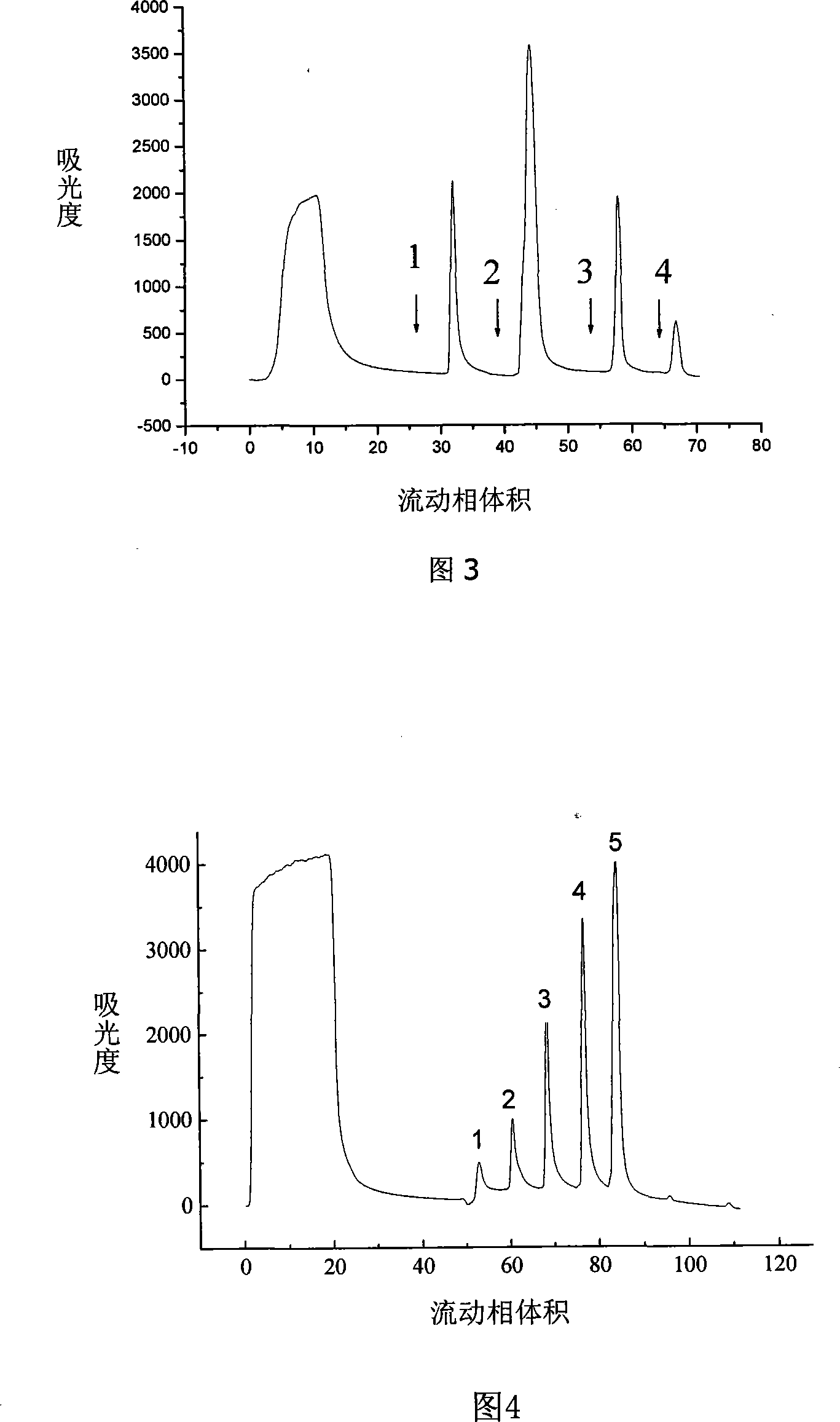
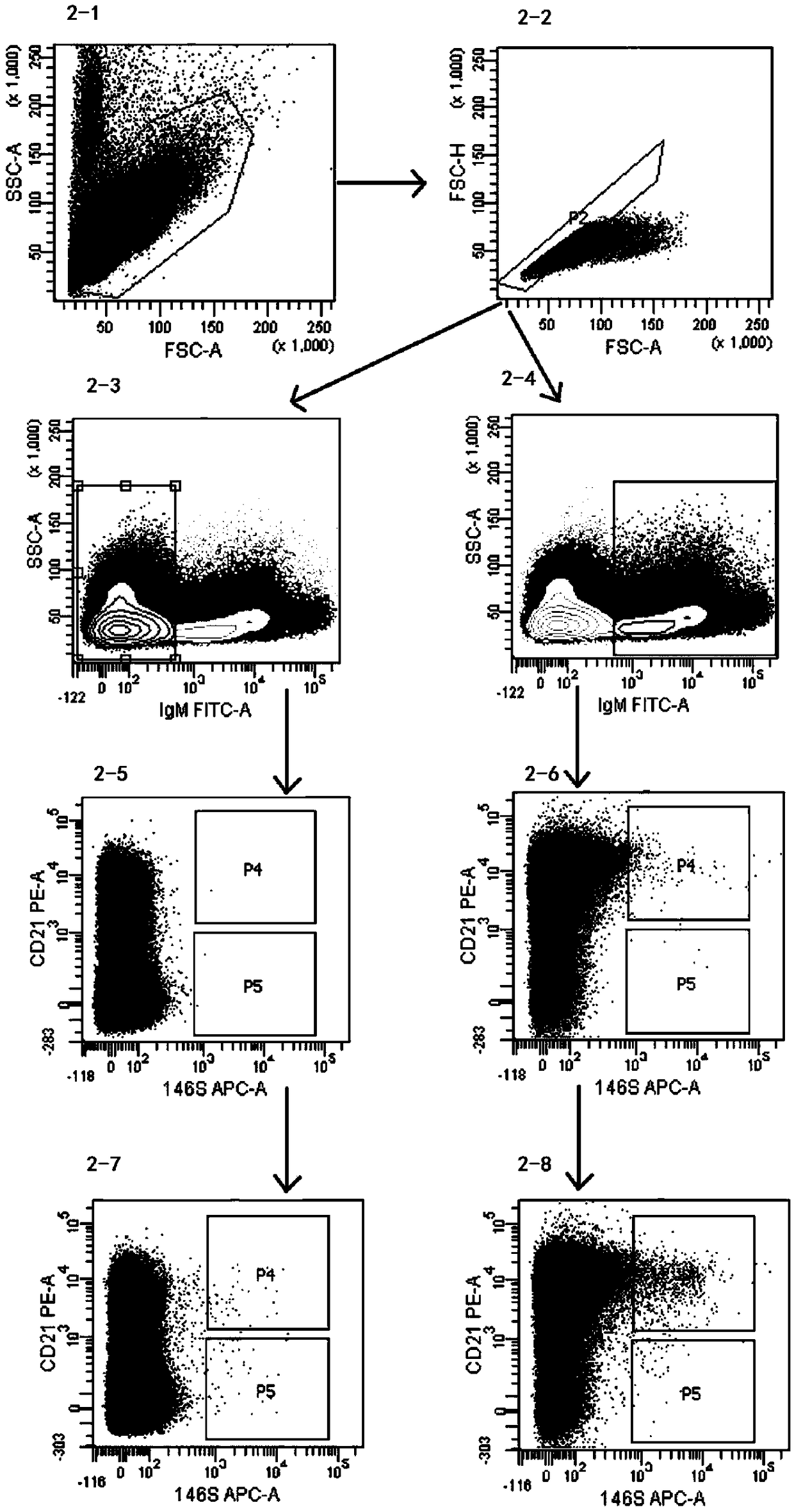
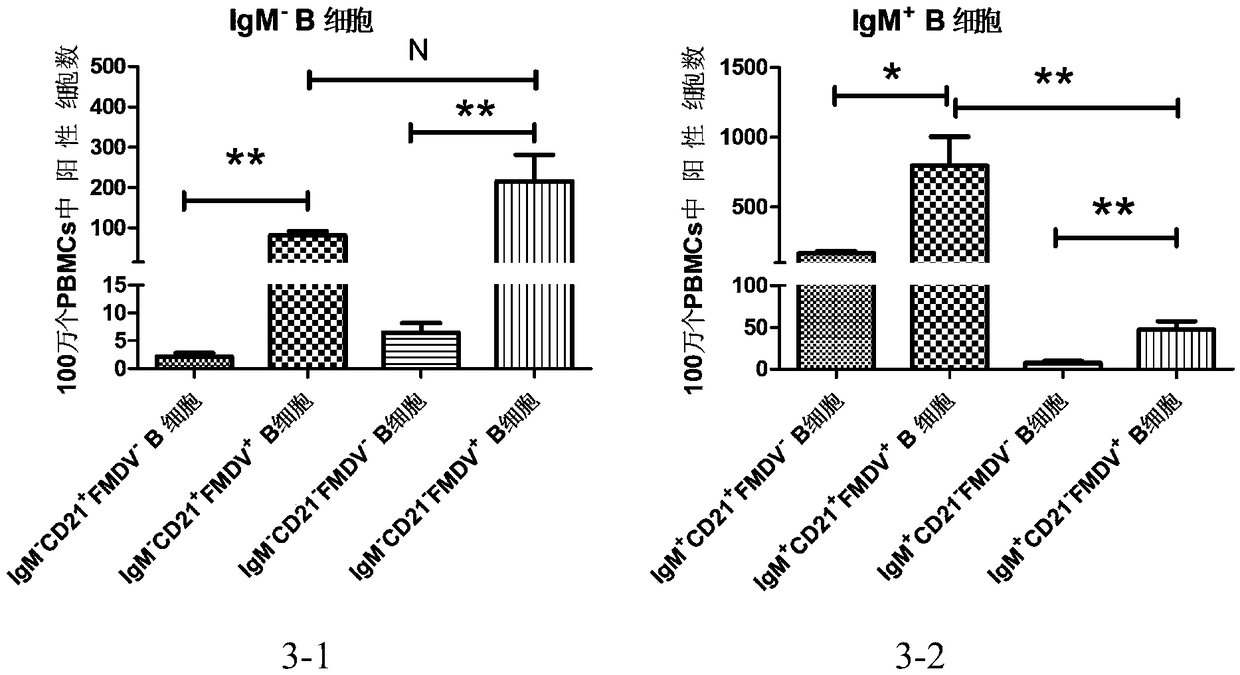
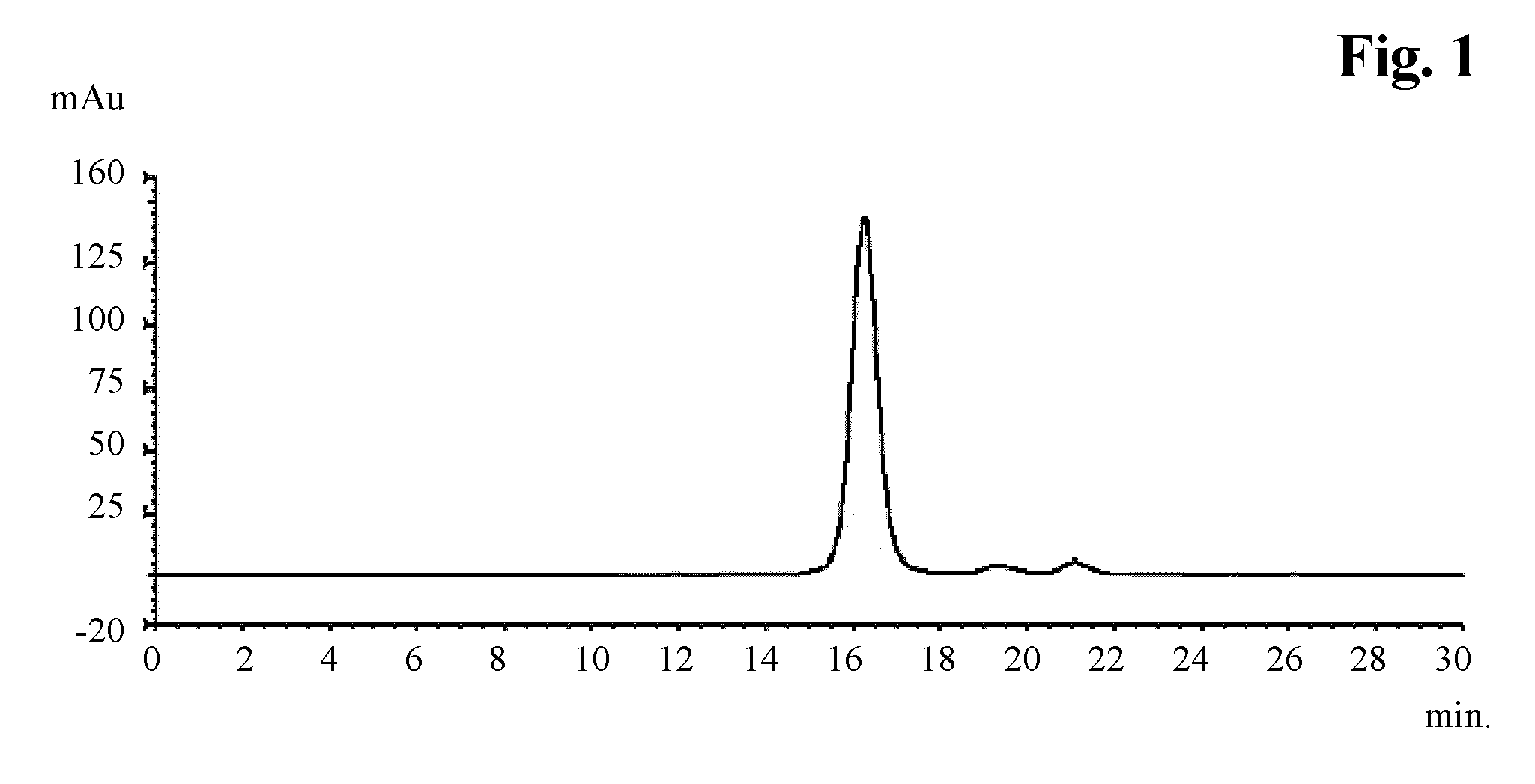
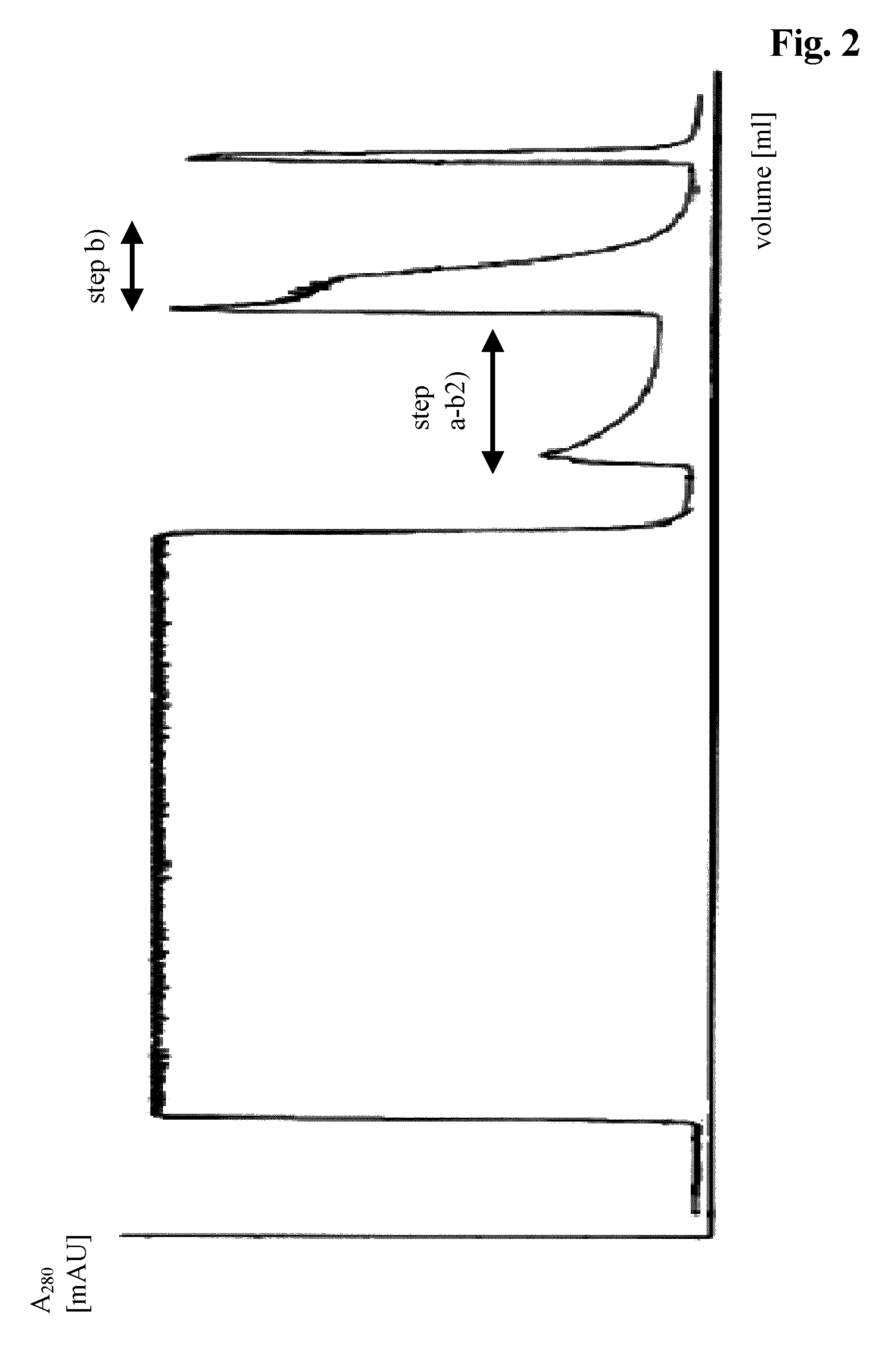
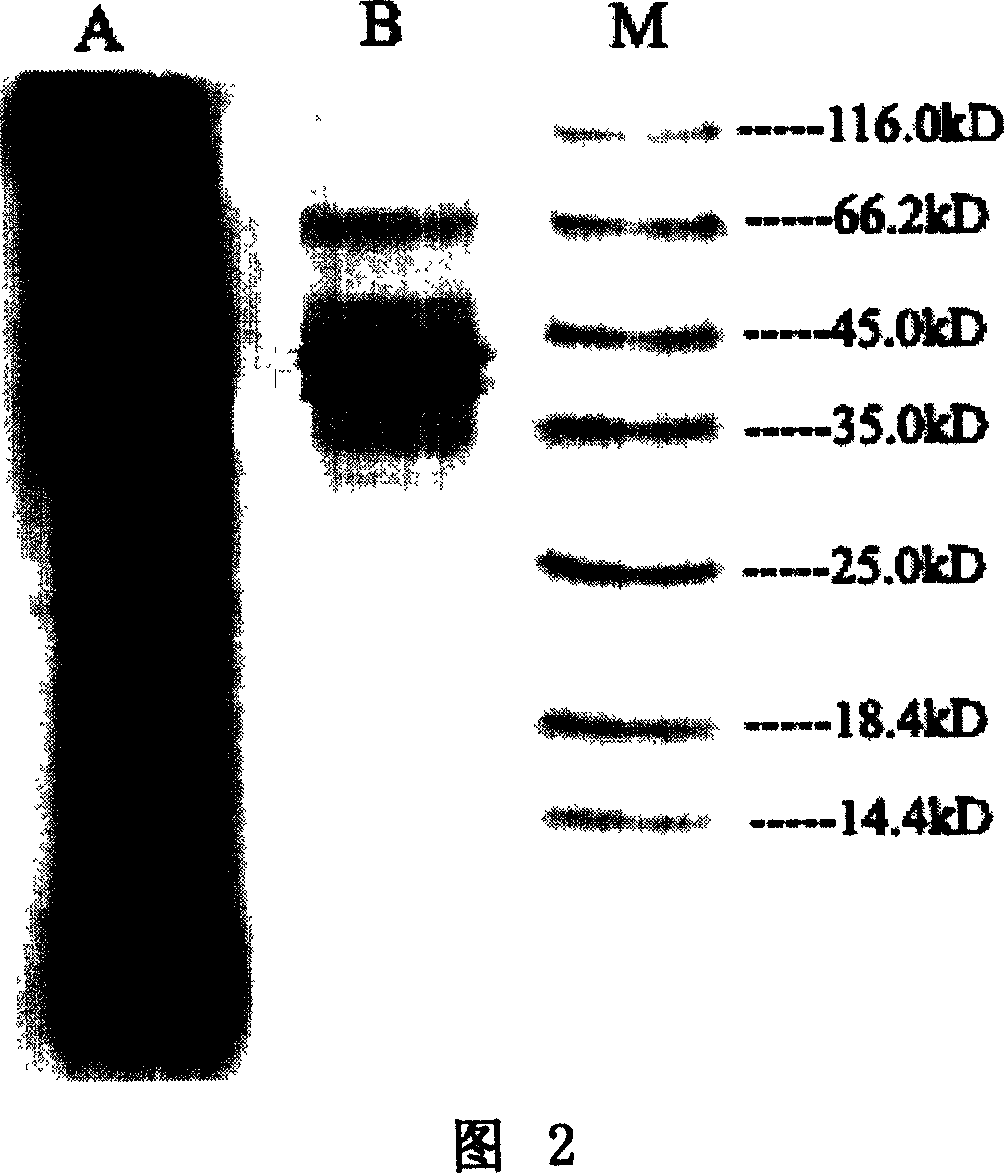Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
238 results about "Cell culture supernatant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The cell culture supernatant is the media in which the cells were growing. You may want to centrifugate it just to eliminate any debris or floating cells and take just the supernatant without any cell.
Isolation of proteins
InactiveUS20050176122A1Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
Owner:UPFRONT CHROMATOGRAPHY
Process for concentration of macromolecules
ActiveUS20060149042A1Reduce conductivityHigh final concentrationDepsipeptidesPeptide preparation methodsCell culture supernatantOragene
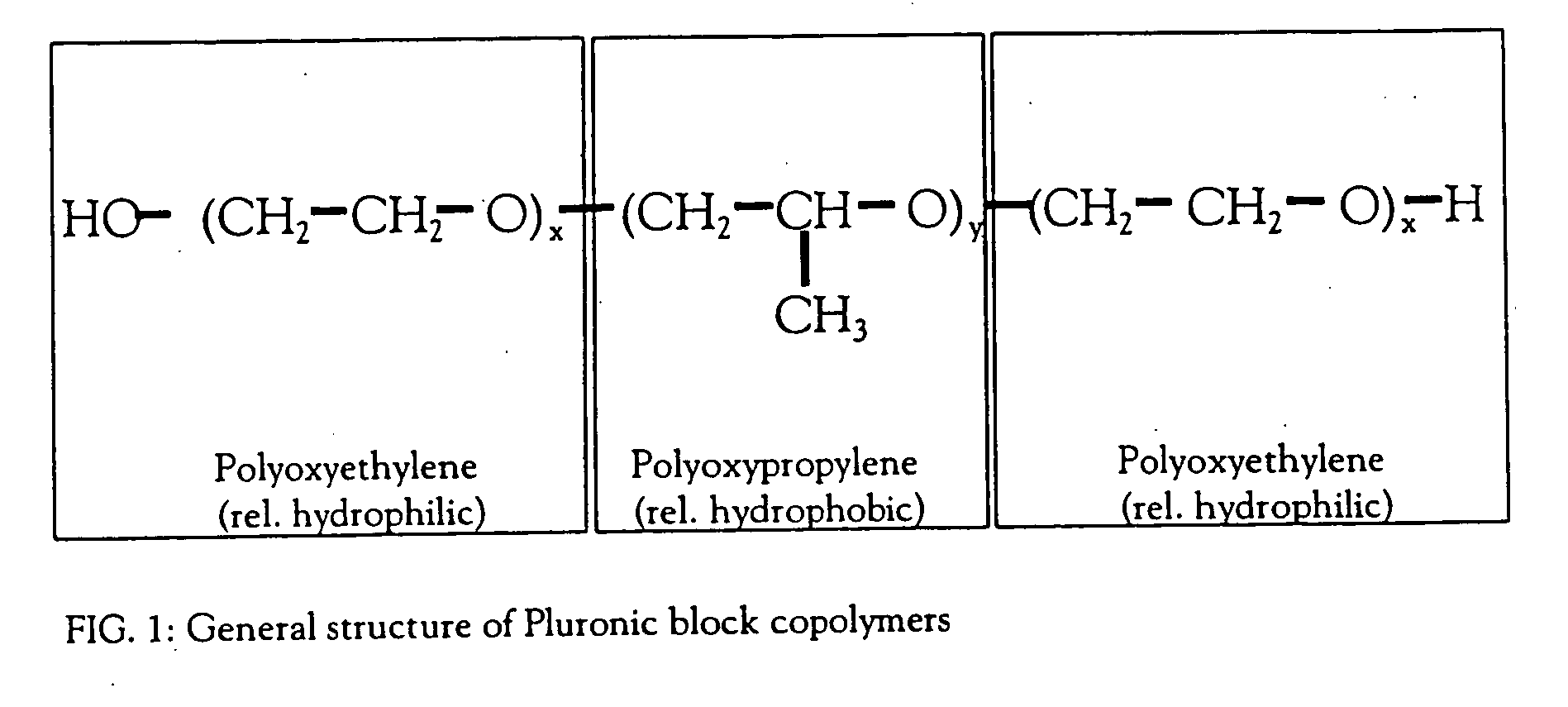
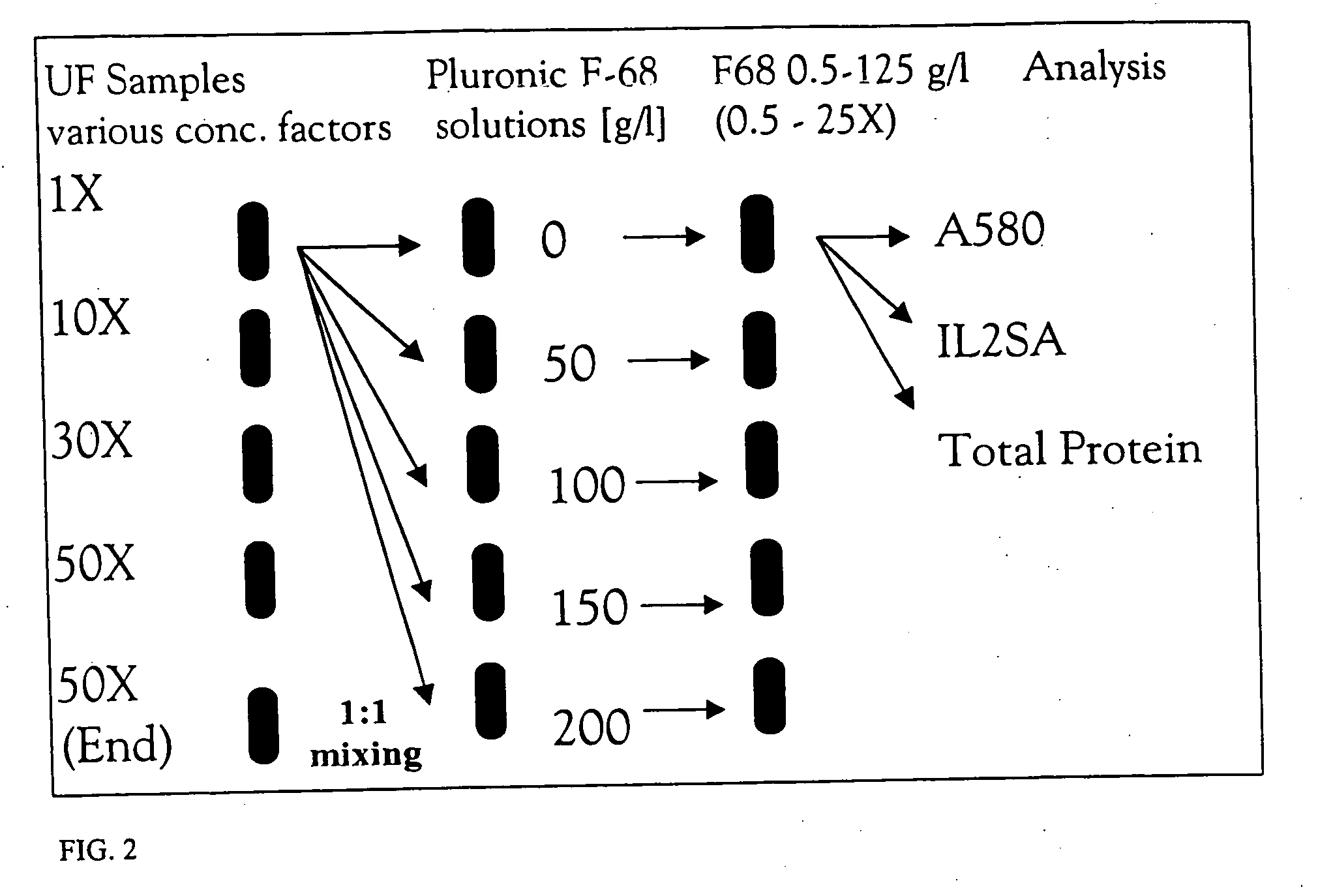
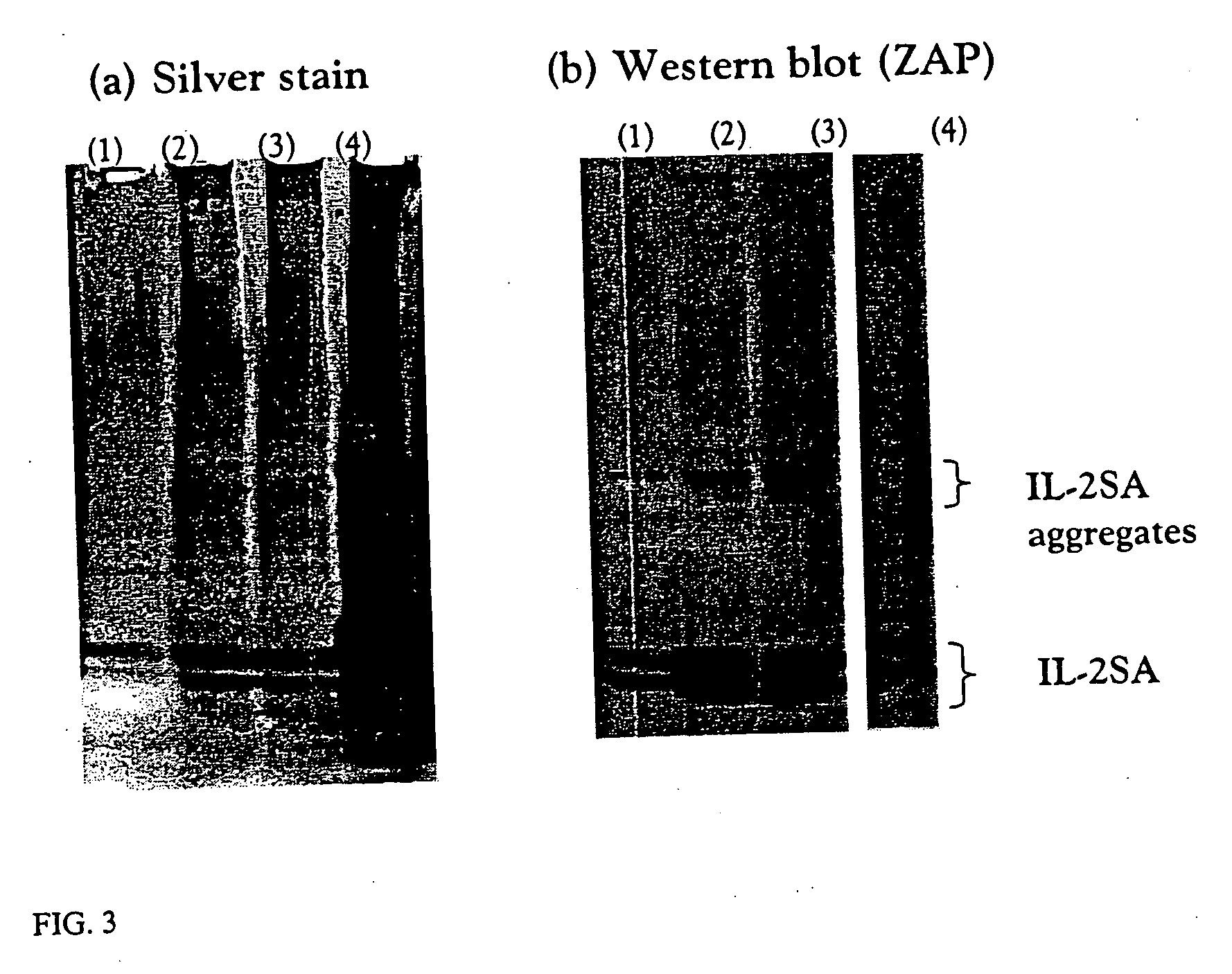
The invention provides methods for concentrating a macromolecule from a solution comprising the macromolecule and an organic polymer by first subjecting the solution to ultrafiltration to produce a first retentate solution, then adjusting the conductivity of the first retentate solution such that any protein precipitation induced by the organic polymer is essentially prevented to produce a second retentate solution, and then subjecting the second retentate solution to ultrafiltration. In a preferred embodiment, the conductivity is adjusted by diafiltration against water, suitable diluent or buffer. Preferably, the invention pertains to the concentration of solutions of native or recombinant proteins. The invention further pertains preferably to methods for the concentration of cell culture supernatant comprising a product protein and organic polymers of the Pluronic family of block co-polymers, and more preferably comprising Pluronic F-68 block co-polymer.
Owner:BAYER HEALTHCARE LLC
Method for separating exosome from human placenta-derived mesenchymal stem cell source and application thereof
PendingCN106282107AImprove acquisitionEasy to operateCell dissociation methodsMammal material medical ingredientsFiltrationCentrifugation
The invention relates to a method for separating exosome from a human placenta-derived mesenchymal stem cell source. The method comprises the following steps: when the P2-P3 placenta-derived mesenchymal stem cell convergence rate reaches 85-90%, obtaining a cell culture supernate, carrying out membrane filtration, and collecting the filtrate; centrifugating the filtrate, collecting the centrifugal supernate, adding a 10w / v%-12w / v% polyethyleneglycol culture medium supernate solution into the centrifugal supernate according to the volume ratio of 1:1, sufficiently and uniformly mixing, and carrying out secondary centrifugation, wherein the finally obtained precipitate is the exosome. The method is convenient for taking materials, is easy for enrichment amplification culture, reutilizes the P2-P3 placenta-derived mesenchymal stem cell culture medium supernatant to obtain the exosome, and does not refer to the problem of medical ethics. In the exosome separation process, membrane filtration, centrifugation, polyethyleneglycol solution addition and other means are adopted to effectively increase the exosome enrichment, so the method has the advantages of short time consumption and low cost.
Owner:章毅 +7
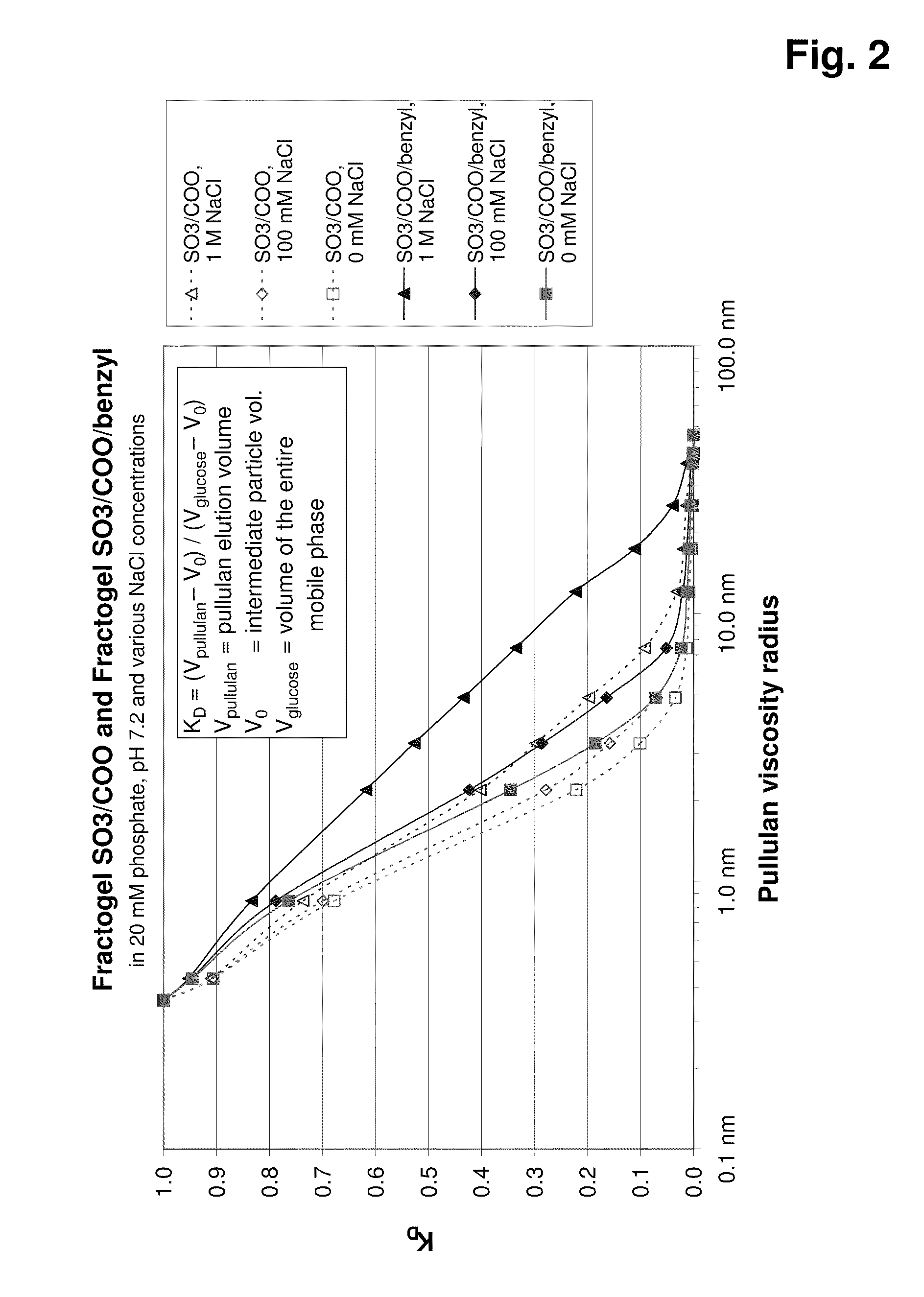
Graft copolymer for cation- exchange chromatography
InactiveUS20100181254A1High strengthChromatographic cation exchangersCation exchanger materialsHuman bodyChromatographic separation
The invention relates to chromatographic separating materials having improved binding capacity for biological constituents in cell culture supernatants, or animal or human body fluids, in particular for monoclonal antibodies. The present invention likewise relates to the preparation of separating materials of this type, and to the use thereof, in particular for the removal of charged biopolymers from corresponding liquids.
Owner:MERCK PATENT GMBH
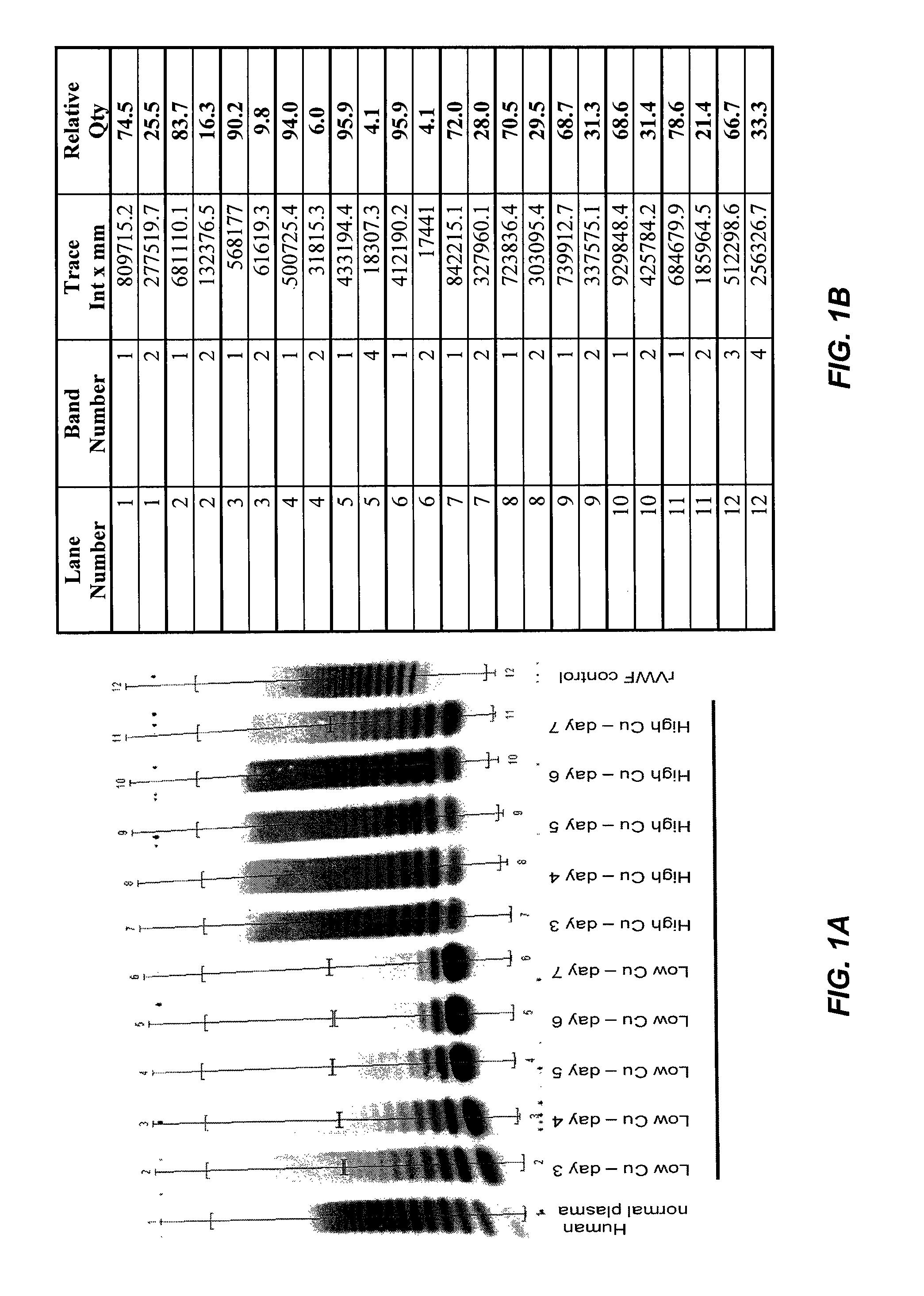
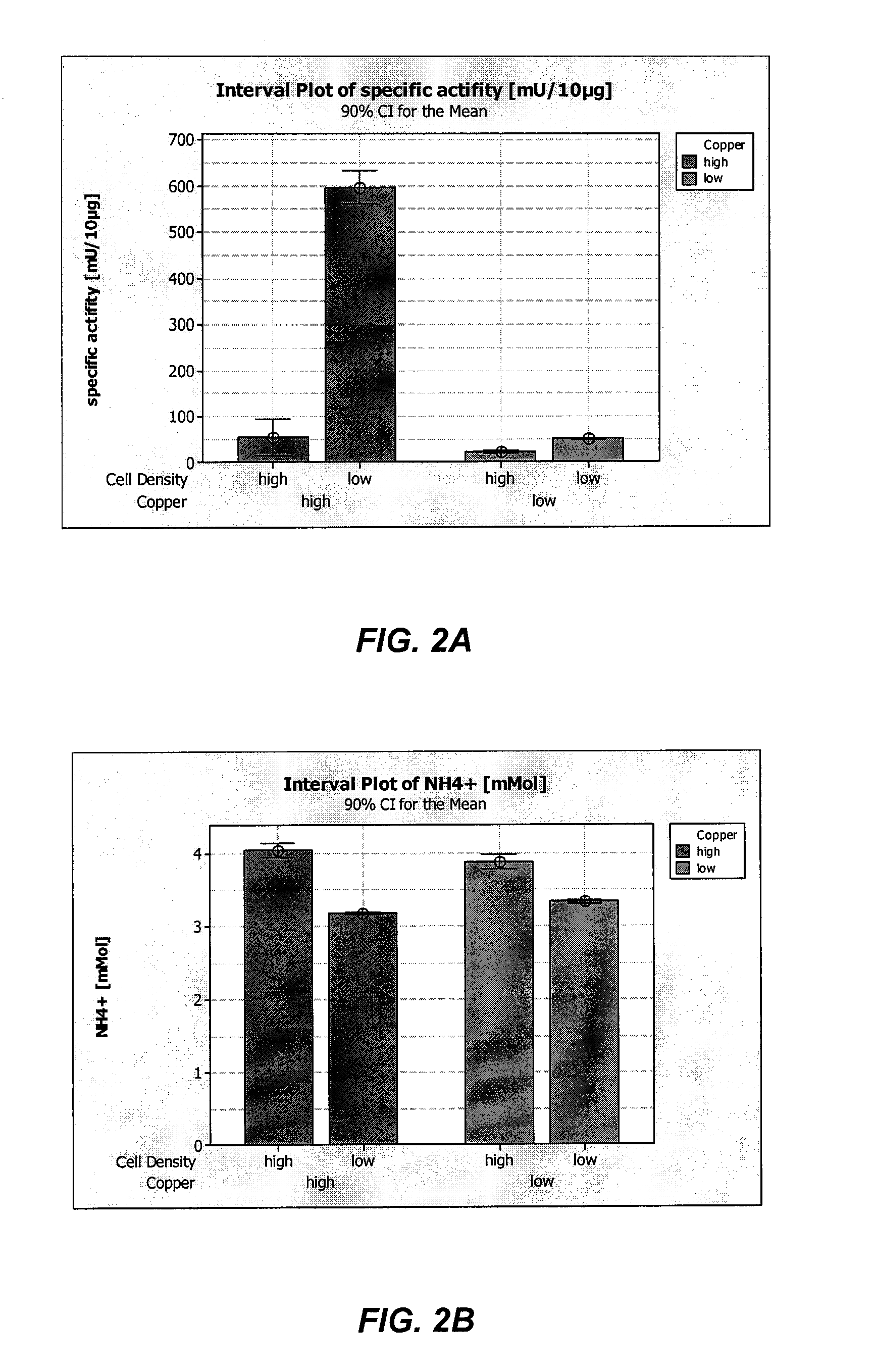
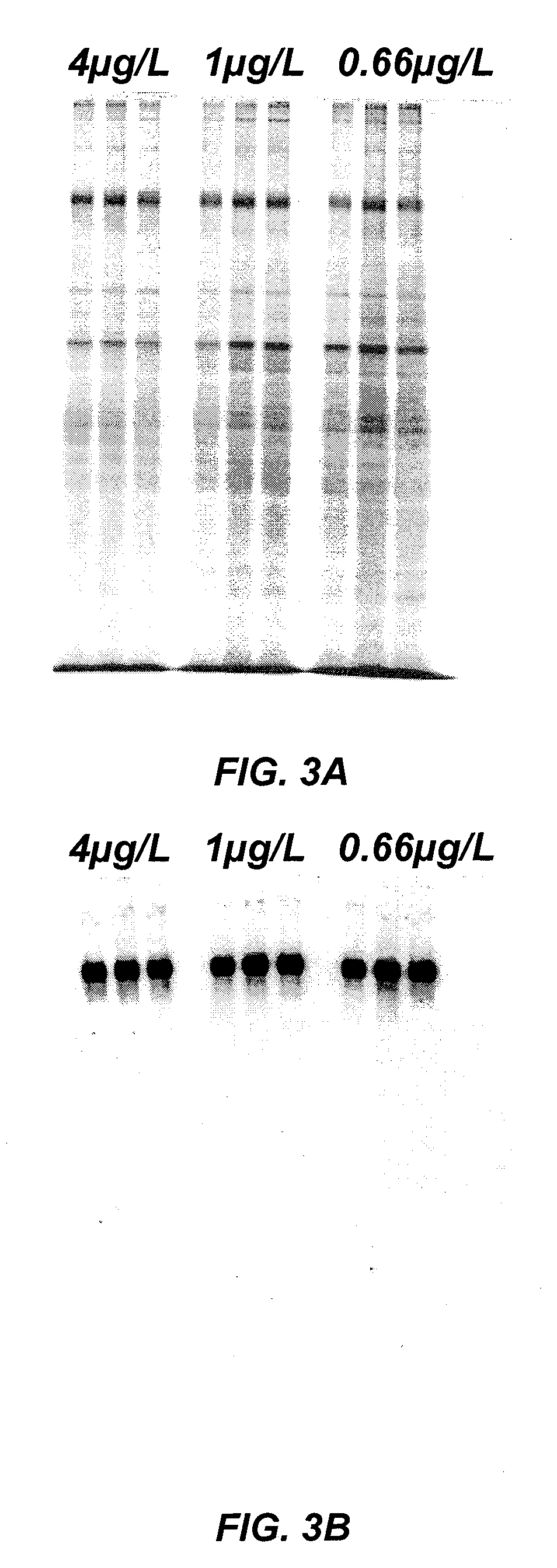
Method of producing recombinant high molecular weight vWF in cell culture
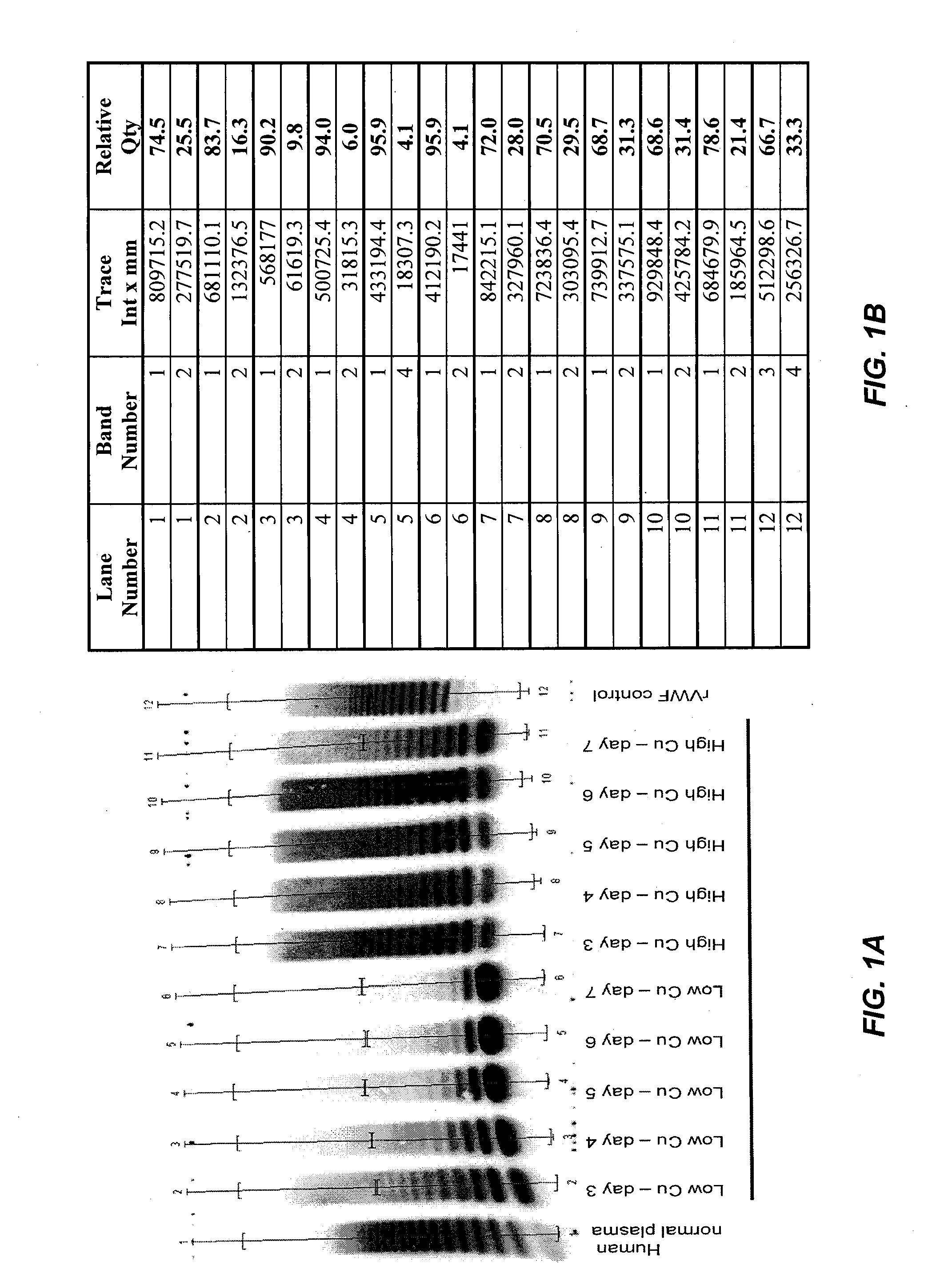
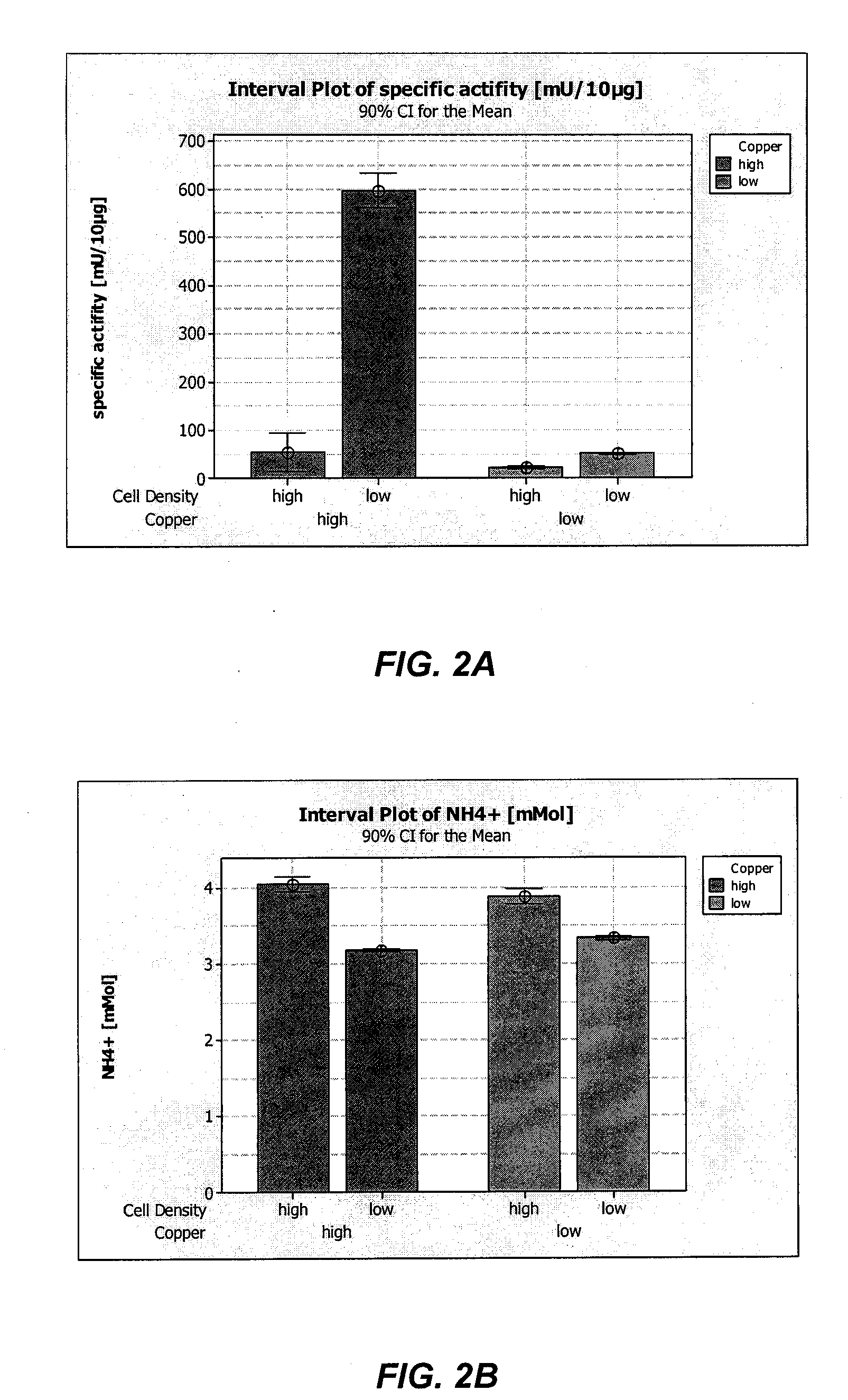
ActiveUS8852888B2High activityHigh expressionFactor VIIPeptide/protein ingredientsCell culture mediaCell culture supernatant
Among other aspects, the present invention relates to cell culture conditions for producing high molecular weight vWF, in particular, highly multimericWF with a high specific activity and ADAMTS13 with a high specific activity. The cell culture conditions of the present invention can include, for example, a cell culture medium with an increased copper concentration and / or cell culture supernatant with a low ammonium (NH4+) concentration. The present invention also provides methods for cultivating cells in the cell culture conditions to express high molecular weight vWF and rA13 having high specific activities.
Owner:TAKEDA PHARMA CO LTD
Chromatographic Method For Purifying FC-Containing Proteins
ActiveUS20130197197A1High purityProcess development can be shortenedPeptide preparation methodsImmunoglobulinsCell culture supernatantBiology
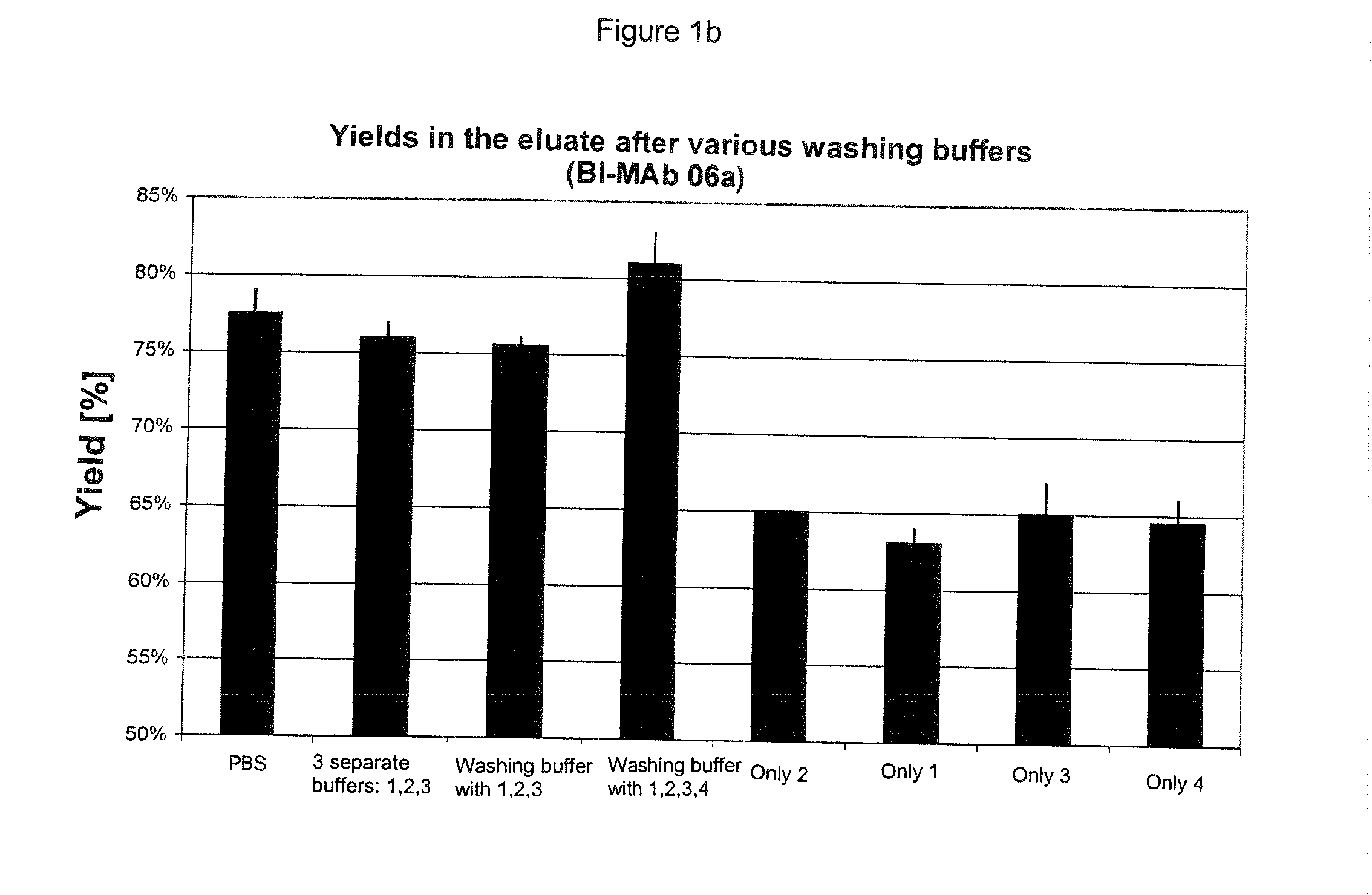
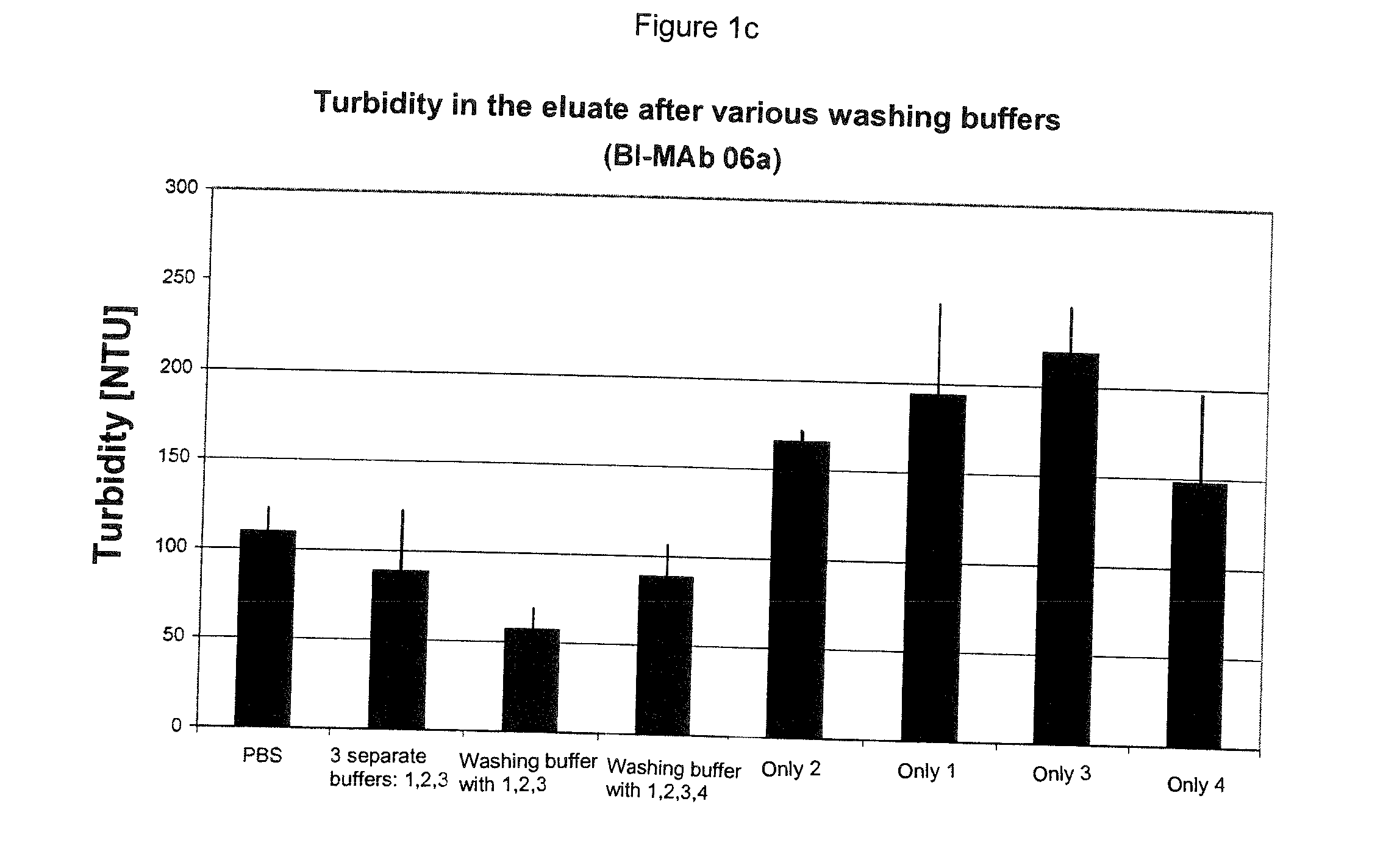
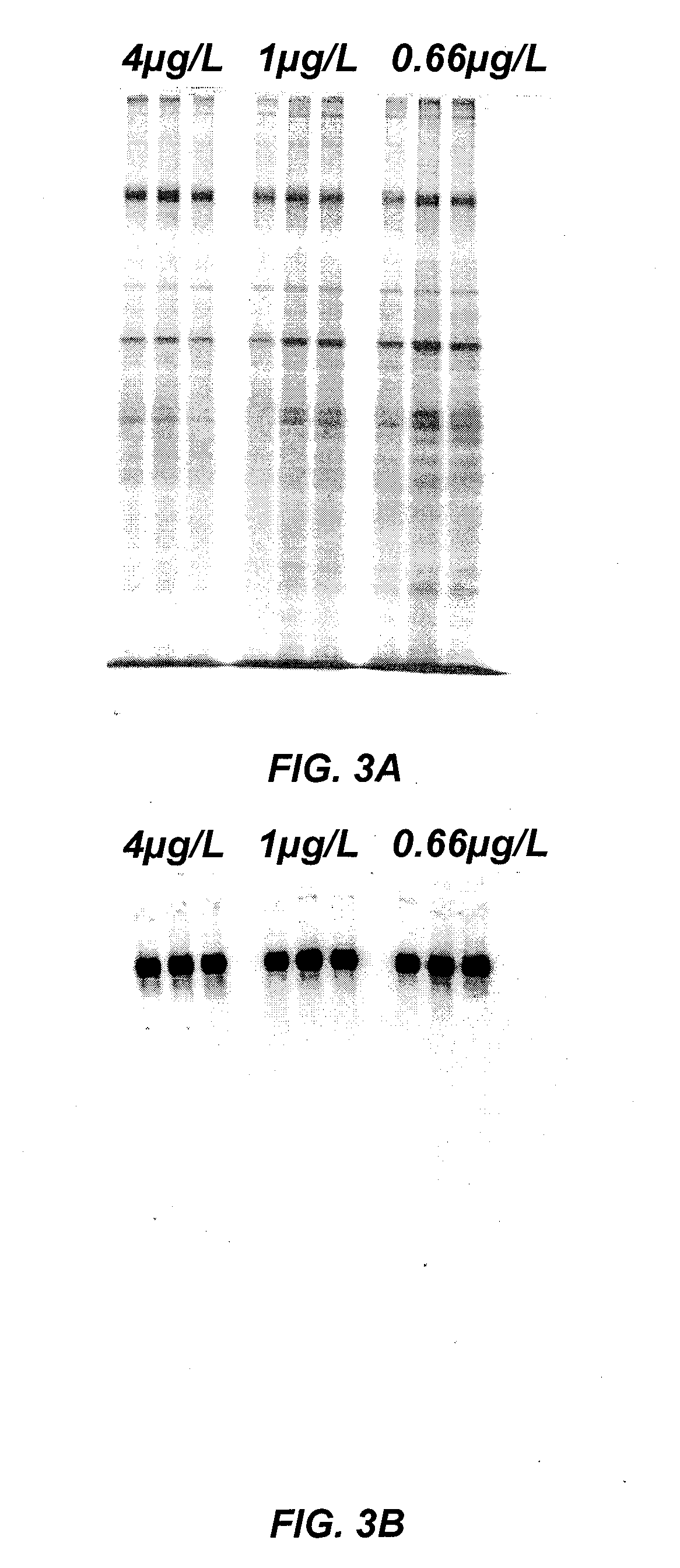
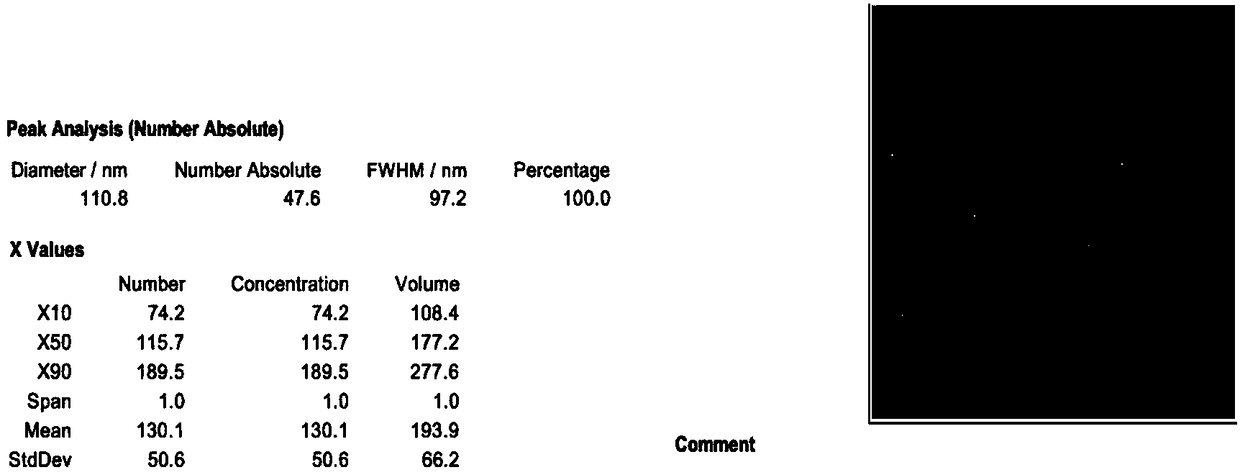
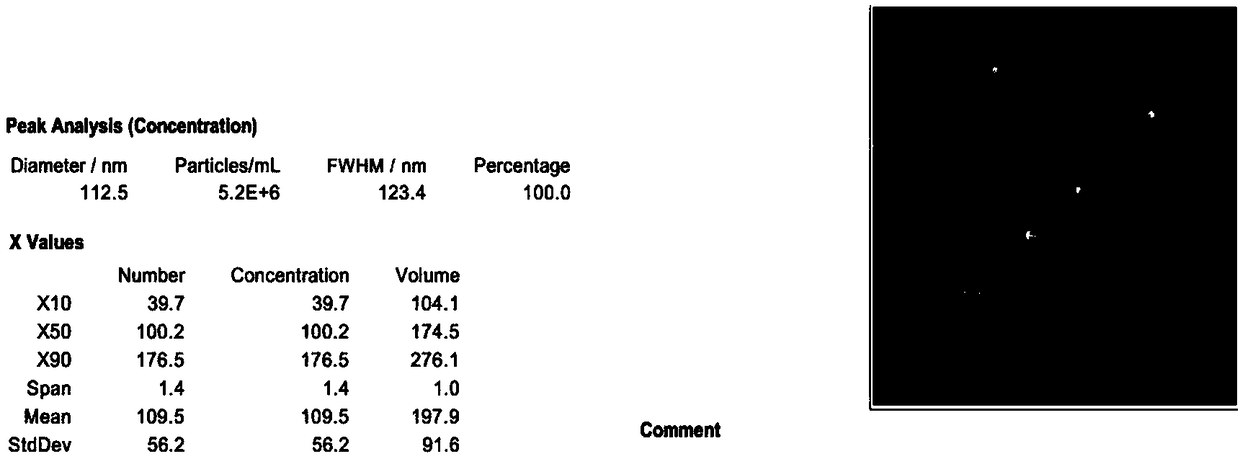
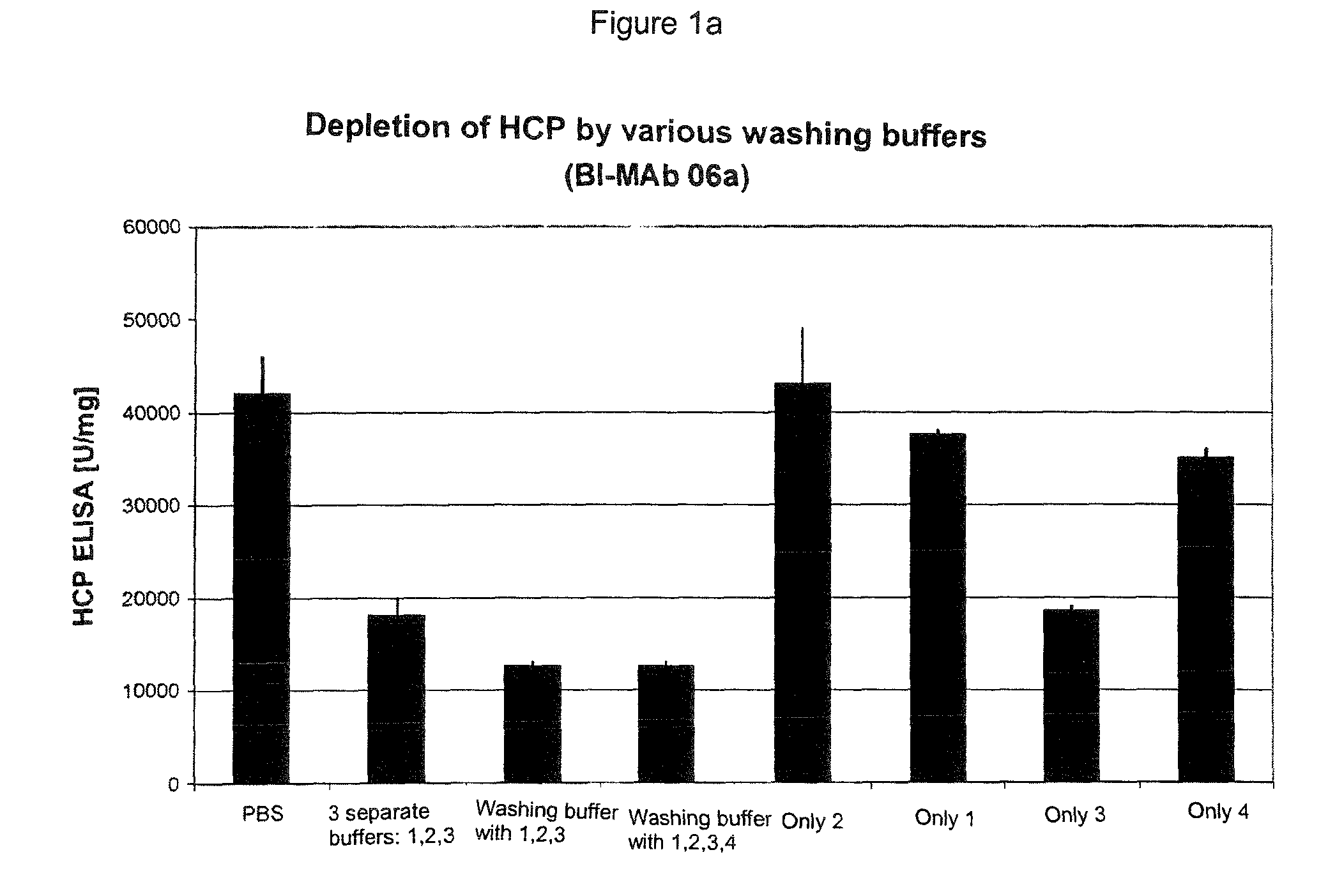
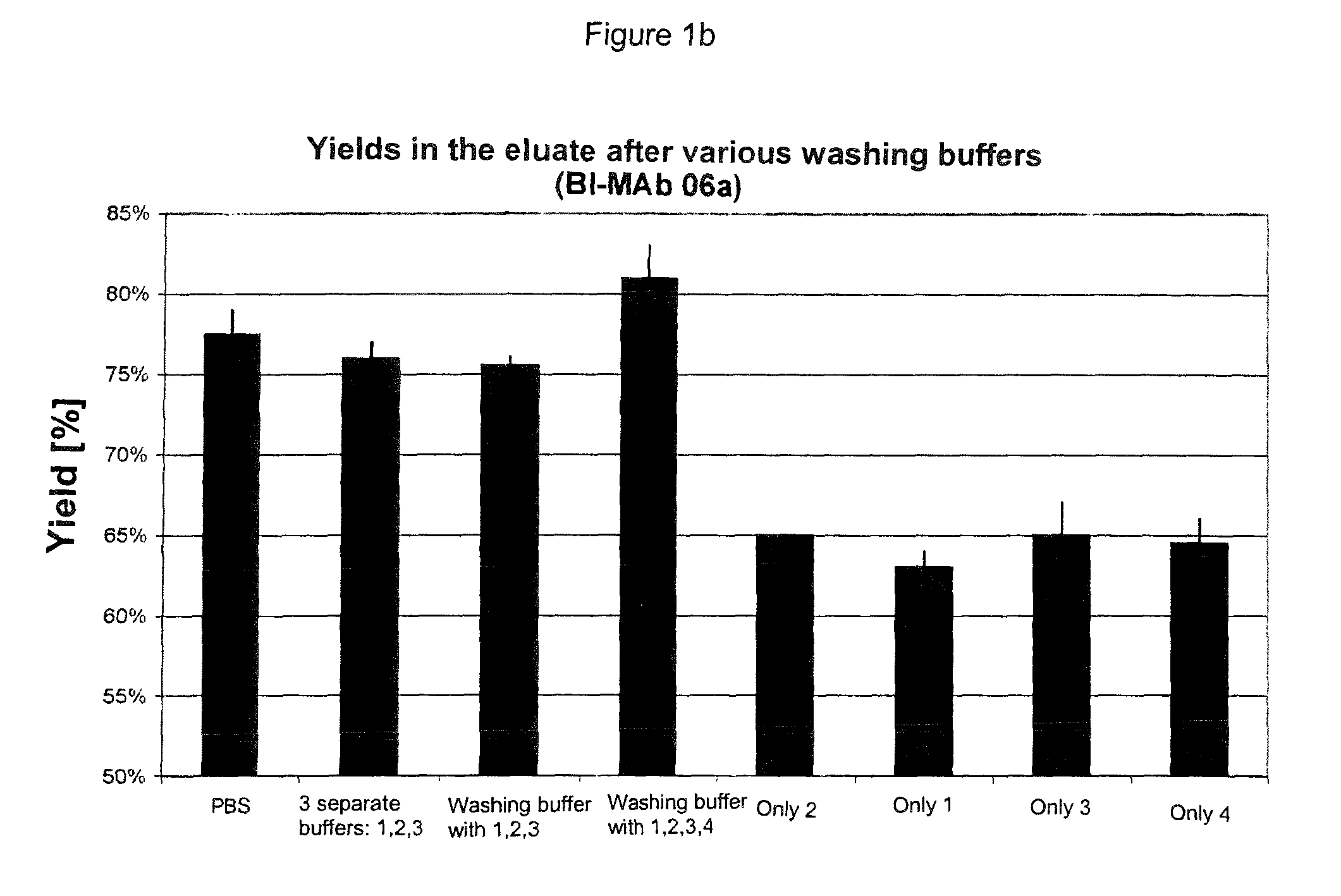
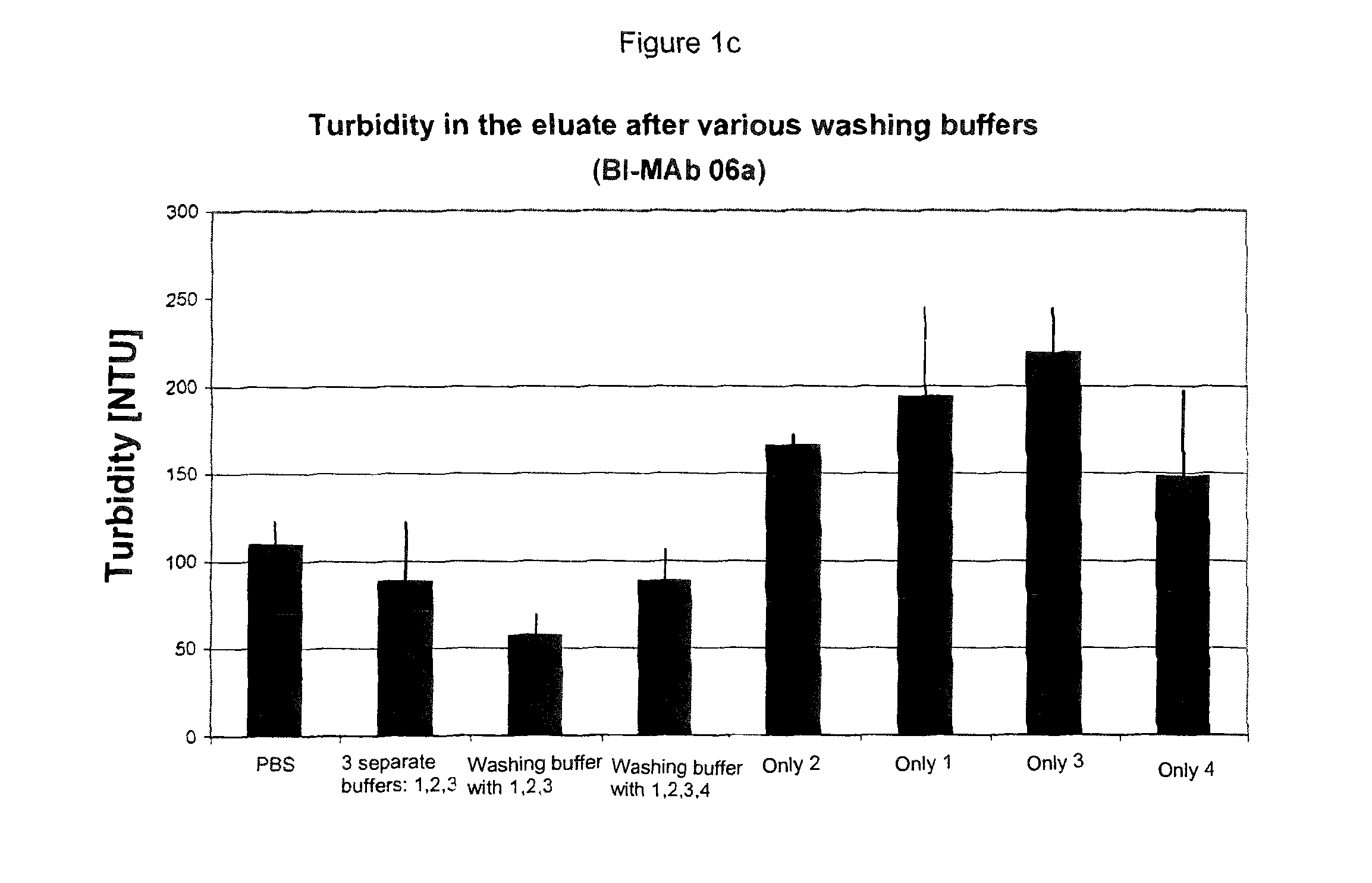
The present invention relates to methods of depleting impurities, in particular host cell proteins (HCP) and DNA from cell culture supernatants by means of protein A chromatography using a novel washing buffer.
Owner:BOEHRINGER INGELHEIM INT GMBH
Humanized cell factor hair agent and preparing method thereof
InactiveCN101199837APromote growthSpeed up entryPowder deliveryPeptide/protein ingredientsLipid formationCuticle
The invention discloses a human-derived cell-factor germinal preparation and the preparation method, relating to the technical filed of the healthcare product. The invention contains lecithin which is the lipid membrane-forming material, cholesterin and active component germinal cell factor, with the proportion by weight between them being 2-6:1-4:1-5; the lecithin is selected from egg yolk lecithin or soybean lecithin; the germinal cell factor is selected from the albumen freeze-dry powder of the culture supernatant of human fibroblasts, placental cells, cuticle cells or dermal papilla cells, with abundant cell factors for the hair growth in the culture supernatant; the germinal cell factors are enveloped in the lipid membrane. The rotary evaporating method is adopted in the process to make the product the lipidosome medicament form which can easily permeate the skin barrier. In addition, the product is frozen and dried into the more stable powder, which needs only to be dissolved by the distilled water containing 2% penetrating enhancer azone while in use. The invention is of easy penetration, high skin retention and good curative effect and free of side effect.
Owner:何荫良
Human umbilical cord mesenchymal stem cell extract freeze-dried powder and preparation method
ActiveCN106109496AIncrease SOD activityAgainst oxidative damagePowder deliveryAntinoxious agentsIrritationFreeze-drying
The invention relates to human umbilical cord mesenchymal stem cell extract freeze-dried powder and a preparation method. The preparation method of the human umbilical cord mesenchymal stem cell extract freeze-dried powder comprises the following steps: obtaining human umbilical cord mesenchymal primary stem cells, which are relatively high in purity, by virtue of density gradient centrifugation, conducting subculture, collecting a culture supernatant of the 3rd-18th generations of cells, and mixing the supernatant with trehalose, so that the freeze-dried powder is prepared. The human umbilical cord mesenchymal stem cell extract freeze-dried powder prepared by the invention has good appearance characteristics; the freeze-dried powder, when redissolved, is relatively rapid to dissolve and is completely dissolved; and animal experiments prove that the freeze-dried powder is low in irritation, high in safety and reliable to use. In addition, the freeze-dried powder disclosed by the invention can effectively preserve various bioactive cell factors in the human umbilical cord mesenchymal stem cell culture supernatant, protect skin from ultraviolet damage and promote the synthesis of skin collagen, and the freeze-dried powder has functions of whitening the skin and delaying aging.
Owner:广东省华桑丽皙生物技术有限公司
Method of producing recombinant adamts13 in cell culture
ActiveUS20120034674A1High activityHigh expressionFactor VIIPeptide/protein ingredientsCell culture mediaADAMTS13
Among other aspects, the present invention relates to cell culture conditions for producing high molecular weight vWF, in particular, highly multimericWF with a high specific activity and ADAMTS13 with a high specific activity. The cell culture conditions of the present invention can include, for example, a cell culture medium with an increased copper concentration and / or cell culture supernatant with a low ammonium (NH4+) concentration. The present invention also provides methods for cultivating cells in the cell culture conditions to express high molecular weight vWF and rA13 having high specific activities.
Owner:TAKEDA PHARMA CO LTD
Preparation method of exosome capable of simultaneously loading chemical drug and nano-material
InactiveCN108114290AImprove solubilityImprove stabilityEnergy modified materialsKetone active ingredientsDiseaseIn vivo
The invention discloses a preparation method of exosome capable of simultaneously loading a chemical drug and a nano-material. The preparation method comprises the following steps: 1) extracting cellsecreted exosome from cell culture supernatant by utilizing a classic differential ultra-speed centrifuging method; 2) simultaneously loading curcumin and nano ferroferric oxide in a water solution into the exosome by utilizing an electroporation method; 3) measuring OD260nm (an RNA (Ribonucleic Acid) characteristic absorption peak) to identify whether perforation is successful or not; 4) after finishing the perforation, immediately putting a mixed solution into a cell culture box and hatching for 1h, so as to facilitate exosome membrane repairing; 5) carrying out 100000g ultra-speed centrifuging to remove free curcumin and nano ferroferric oxide, so as to obtain the compound exosome capable of simultaneously loading the curcumin and the nano ferroferric oxide. The method disclosed by themethod is simple and feasible and high in success rate; in the prepared compound exosome, the loading amounts of the curcumin and the nano ferroferric oxide can meet in vivo and in vitro treatment andimaging requirements and a novel tool is provided for targeting diagnosis and treatment of various chronic diseases including tumors.
Owner:SOUTHEAST UNIV
Chromatographic method for purifying FC-containing proteins
ActiveUS9284347B2Efficient separationHigh purityPeptide preparation methodsAntibody ingredientsCell culture supernatantBiology
The present invention relates to methods of depleting impurities, in particular host cell proteins (HCP) and DNA from cell culture supernatants by means of protein A chromatography using a novel washing buffer.
Owner:BOEHRINGER INGELHEIM INT GMBH
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
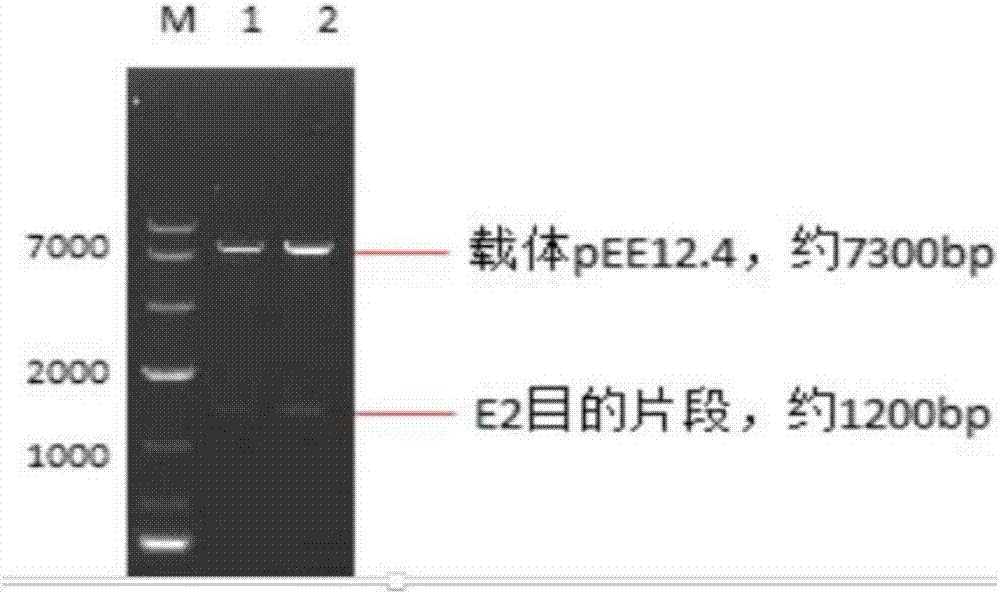
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Lyophilized powder of human mesenchymal stem cell culture supernatant and preparation method thereof
ActiveCN103251649AEffective preservationAddress the bottleneck of limited shelf lifePowder deliveryMammal material medical ingredientsMesenchymal stem cellStem cell culture
The invention relates to lyophilized powder of human mesenchymal stem cell culture supernatant and a preparation method of the lyophilized powder. The preparation method comprises the following steps of: culturing a sub-culturing human mesenchymal stem cell by using a mesenchymal stem cell culture medium containing autologous serums; collecting a cell culture supernatant with low sub-culturing times; and preparing the collected supernatant into the lyophilized powder. The lyophilized powder prepared by the preparation method of the lyophilized powder of the human mesenchymal stem cell culture supernatant effectively preserves various cell factor mixtures with biological activities in the human mesenchymal stem cell culture supernatant, is capable of effectively solving the problem of short storage life of the supernatant and lays a foundation for an industrial application of the human mesenchymal stem cell culture supernatant.
Owner:冯文峰
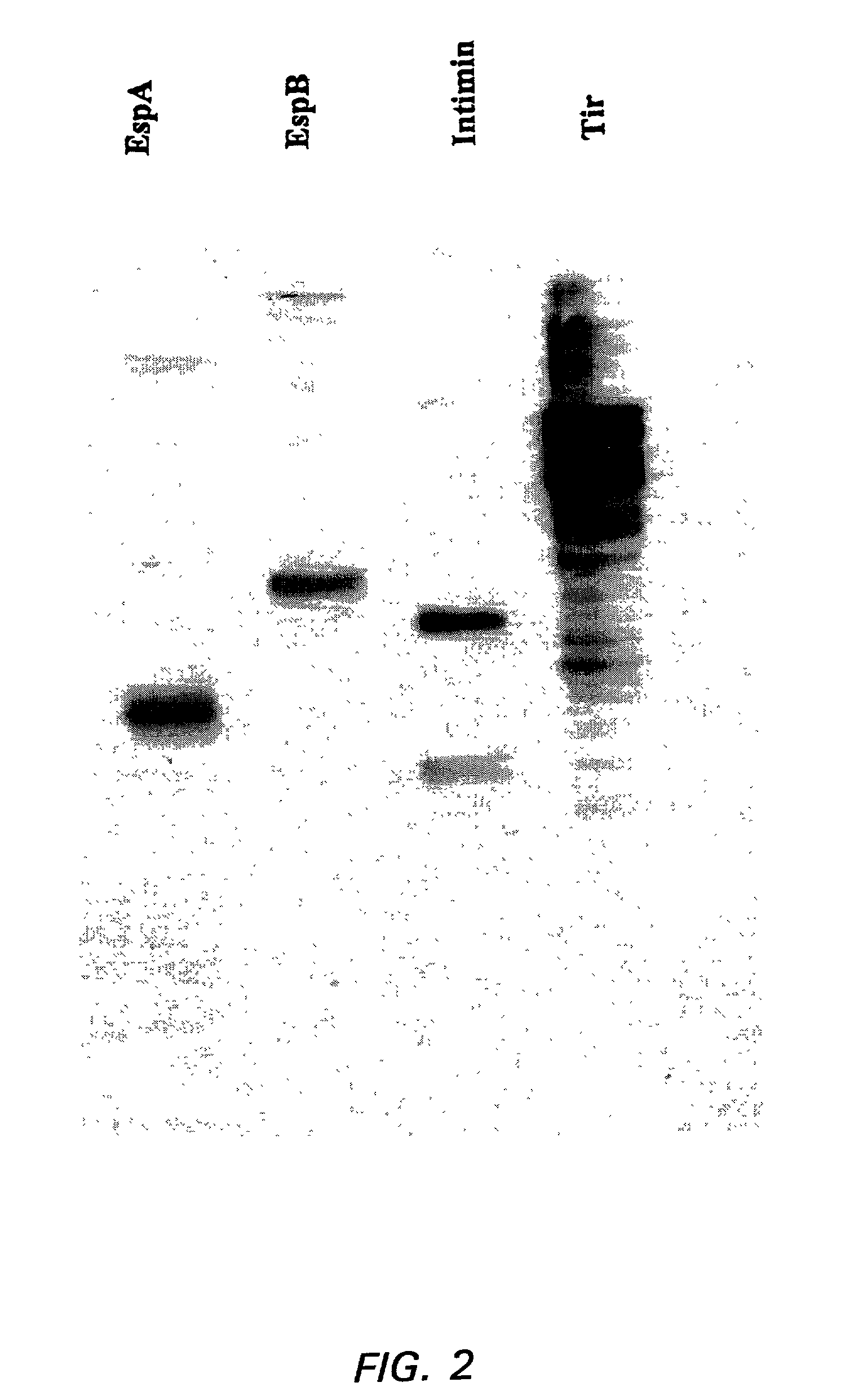
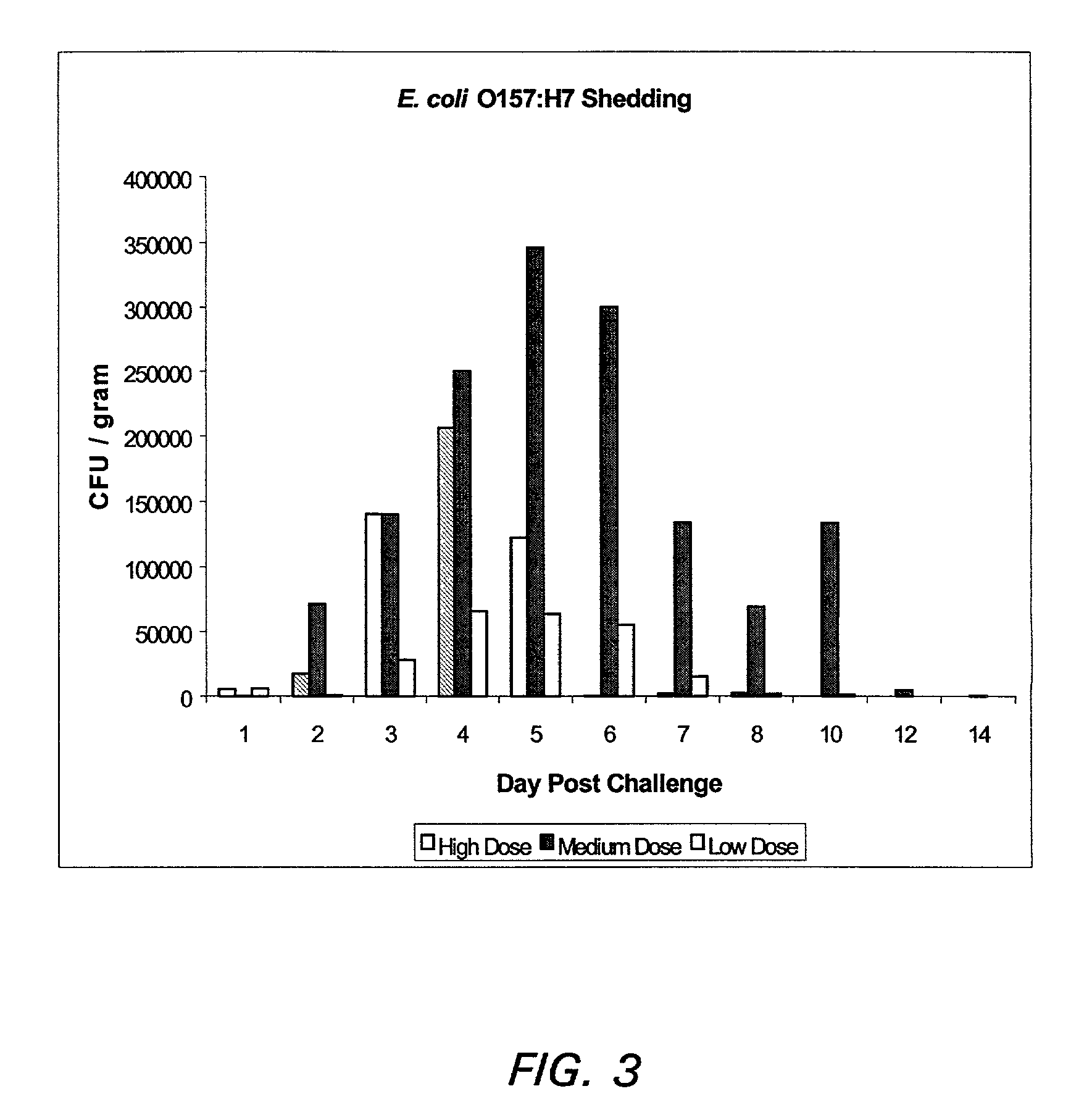
Enterohemorrhagic Escherichia coli vaccine
InactiveUS7300659B2Reducing EHEC colonizationEasy and relatively inexpensive to prepareAntibacterial agentsBacterial antigen ingredientsAntigenEnterobacteriales
Compositions and methods for stimulating an immune response against a secreted enterohemorragic Escherichia coli (EHEC) antigen are disclosed. The compositions comprise EHEC cell culture supernatants.
Owner:THE UNIV OF BRITISH COLUMBIA +1
Preparation methods and application of recombinant swine fever E2 protein and subunit vaccine of recombinant swine fever E2 protein
PendingCN107674883AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetVaccine Production
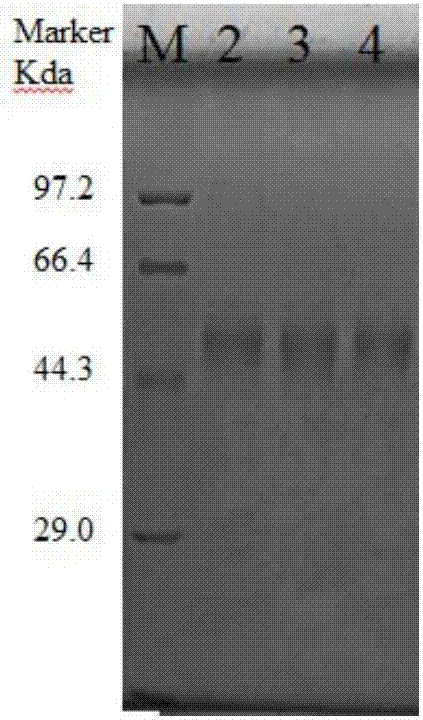
The invention discloses preparation methods and application of recombinant swine fever E2 protein and a subunit vaccine of the recombinant swine fever E2 protein. The preparation method of the recombinant swine fever E2 protein comprises the following steps that (1) a swine fever E2 protein coding gene is cloned into an eukaryotic expression vector to obtain recombinant plasmid containing the swine fever E2 protein coding gene; (2) then, the recombinant plasmid containing the swine fever E2 protein coding gene is transfected into a CHO cell strain; (3) the CHO cell strain obtained in the step(2) is cultured, screened and domesticated; and (4) the cell strain in the step (3) is fermented and cultured; and the recombinant swine fever E2 protein is obtained after purification. The methods provided by the invention have the advantages that the target protein can be obtained from cell culture supernatant; the yield reaches up to 1g / L; the protein purification time is shortened; the vaccineproduction steps are simplified; and the vaccine production cost is also greatly reduced.
Owner:NOVO BIOTECH CORP
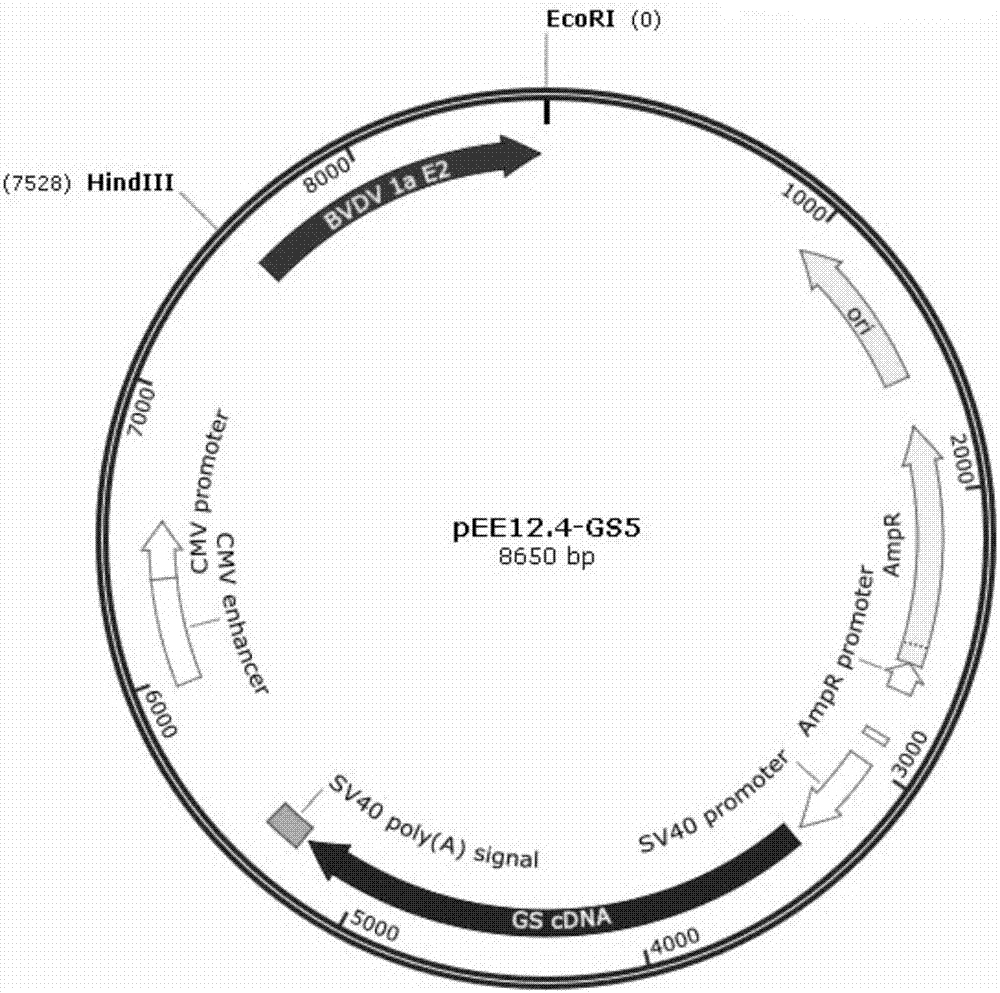
Preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and subunit vaccine and application
ActiveCN107973841AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetBovine Viral Diarrhea Viruses
The invention discloses preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and a subunit vaccine and applications and belongs to the technical fields of animal vaccines and veterinary biologicals. The object of the invention is to provide a preparation method capable of industrially producing the bovine viral diarrhea virus recombinant subunit vaccine ona large scale. The preparation method for the recombinant subunit vaccine, provided by the invention, comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein E2coding gene; 2) transfecting CHO cells, and performing selection, screening and acclimatizing to obtain suspending CHO cell strains, which stably and efficiently express the protein E2; 3) subjectingthe cell strains obtained in the step 2) to fermentation culture, and carrying out purification, so as to obtain recombinant protein E2; and 4) uniformly mixing the recombinant protein E2 and ISA 201VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches upto 500mg / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
A directional differentiation induction method of human embryonic stem cells to obtain corneal endothelial cells
ActiveCN106167790AConsistent proliferative abilityCulture processNervous system cellsCorneal endothelial cellNeural crest
A directional differentiation induction method of human embryonic stem cells to obtain corneal endothelial cells is provided. The method includes inducing the human embryonic stem cells to differentiate into human neural crest stem cells, inducing the human neural crest stem cells to differentiate into the human corneal endothelial cells, and other steps. The method adopts a primary human corneal stroma cell culture supernatant fluid, a human corneal endothelial cell culture supernatant fluid and a human lens cell culture supernatant fluid as a conditioned medium. Through adding retinoic acid and a plurality of recombinant proteins into the medium, and combining three-dimensional and two-dimensional culture methods, a corneal endothelial cell development process is simulated to induce the human embryonic stem cells to directionally differentiate into the human corneal endothelial cells, the corneal endothelial cells morphological structures of which are similar to those of normal human corneal endothelial cells can be obtained, and in-vitro subculture results show that proliferation of the obtained human corneal endothelial cells is consistent with proliferation of the normal human corneal endothelial cells.
Owner:GENERAL HOSPITAL OF PLA
Method of purifying and combining human interleukins 12
ActiveCN101033254AReduce the cost of separation and purificationSimple and fast operationInterleukinsMolecular sieveAnion-exchange chromatography
This invention discloses a method to purify rhIL-12, belonging to the protein purification technology, which technical points are that the cell culture supernatant of rhIL-12 is conducted filtering, cation or anion exchange chromatography, precipitation of ammonium sulfate, anion or cation exchange chromatography and molecular sieve chromatography, and during the precipitation of ammonium sulfate, pH is far away from the isoelectric point of rhIL-12. This method uses ammonium sulfate precipitation to remove most of hybridproteins, making the future separation simple and effective.
Owner:广州市茵良强生物科技有限公司
Preparation method and application of NK cytokine mixture
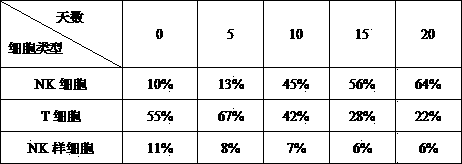
InactiveCN103923879AEfficient and safe cultivation methodMammal material medical ingredientsBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsFreeze-drying
The invention belongs to the field of tumor biotherapy, and relates to a preparation method and an application of a NK cells-cytokines mixture prepared by using an activation method for NK cells. NK cells obtained according to the invention are tumor-killing cells which are cultured in vitro, activated and amplified and has a cytotoxic effect, and can directly kill tumor cells and enhance immunity after being fed back to the bodies of patients; NK cell culture supernatant prepared according to the invention and freeze-dried powder preparations prepared by freeze-drying and concentrating the NK cell culture supernatant contain a series of natural cytokine mixtures with bioactivity, and the mixture of natural cytokines has good antitumor activity in anti-tumor treatment.
Owner:湖北华赛生物医药技术有限公司
Anti-rabbit hemorrhagic disease virus VP60 albumen monoclonal antibody
InactiveCN101519447AThe preparation method is simple and feasibleStrong specificityImmunoglobulins against virusesTissue cultureBALB/cCell culture supernatant
The invention relates to an anti-rabbit hemorrhagic disease virus (RHDV)VP60 albumen monoclonal antibody, and belongs to the technical field of biology. An SP2 / 0 myeloma cell and a BALB / c mouse splenic cell immunized by utilizing RHDV to recombine VP60 albumen undergo cell fusion, are selectively cultured by an HAT culture medium and undergo double ELISA screening by utilizing the recombined VP60 albumen and RHDV; the obtained cell culture supernatant is checked up and screened respectively to obtain a hybrid tumor cell strain A3C which can stably excrete the anti-RHDV VP60 albumen monoclonal antibody; the ascitic fluid ELISA titer of the A3C is detected to be 1:327,600; and according to identification, the monoclonal antibody can specifically combine the expressed recombined VP60 albumen as well as the RHDV, and one single reaction strip appears in both specific combinations, thereby proving that the anti-RHDV VP60 albumen monoclonal antibody is a VP60 specific antibody of the RHDV capsid albumen.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Tumor-targeted delivery carrier based on cell-derived micro-vacuoles, preparation method and application
ActiveCN106692984AGood biological stabilityHigh biosecurityEnergy modified materialsGenetic material ingredientsDspe pegCell culture supernatant
The invention discloses a tumor-targeted delivery carrier based on cell-derived micro-vacuoles, a preparation method and an application. The preparation method comprises the following steps of: (A) preparing a conditioned medium: supplementing fetal bovine serum, antibiotics, DSPE-PEG-Biotin and DSPE-PEG-Folate into a basal medium; (B) using the obtained conditioned medium in cell culture, and collecting cell culture supernatant for subsequent separation; (C) carrying out low-speed centrifugation on the obtained culture supernatant to remove cell debris and apoptotic bodies, then adding SA-IONPs, mixing uniformly, incubating, then separating by a magnet, using PBS for re-suspension, eluting for multiple times to obtain the cell-derived micro-vacuoles with membrane surfaces modified by folic acid and iron oxide nano-particles, and freezing for storage; and (D) loading chemotherapeutic drugs or therapeutic genes into the functionalized micro-vacuoles doubly-modified by an electroporation mode, and carrying out re-suspension after separation with the magnet. The tumor-targeted delivery carrier based on cell-derived micro-vacuoles, the preparation method and the application disclosed by the invention are applicable to specific targeting delivery of multiple chemotherapeutic drugs and therapeutic genes, and have the advantages of enhancing the anti-tumor effect, reducing the systemic toxicity and improving the clinical effect of the current therapeutic selection, so that a new hope is brought for clinical therapy of tumors.
Owner:珈泌生物科技(武汉)有限责任公司
Chromatogram medium for immunoglobulin class protein separation purification and preparation method thereof
InactiveCN101185881AImprove adsorption capacityHigh purityOther chemical processesPeptide preparation methodsCell culture supernatantChromatography column
The invention relates to a chromatography which is used for isolating and purifying immunoglobulin protein and a preparation method and belongs to the preparation technology of isolating and purifying a chromatography medium of the immunoglobulin. The chromatography medium refers to the end of a space arm that is coupled with a double-loop compound molecular which has an imidazol group and a benzene ring as a chromatography medium matrix. The preparation method uses an allylic chromatography medium and a bromide alchoholization reaction of N-bromosuccinimide to get an activated chromatography medium which reacts with the amino of the double-loop compound to complete the coupling of the matrix in dimethyl sulfoxide solution. The chromatography medium has higher dynamic adsorption capacity to the antibody within the ionic strength scope from 0.02 mol / L to 2.0 mol / L and is directly applied to the recycling of the antibody in the solid to liquid; at he same time, the medium has more moderate elution condition and can purify the antibody from serum, ascites, cell culture supernatant and other solid to liquid having the antibody. The chromatography medium has the wide application prospect in the process of antibody preparation.
Owner:TIANJIN UNIV
Method for preparing adipose derived stem cell bioactive peptide freeze-dried powder
InactiveCN107034183AFor long-term storageEasy to transportPeptide preparation methodsSkeletal/connective tissue cellsFreeze-dryingUltrafiltration
The invention provides a method for preparing adipose derived stem cell bioactive peptide freeze-dried powder. The preparation method includes the following steps that S1, autologous fat tissue is extracted; S2, primary-generation fat stem cells are prepared from the autologous fat tissue; S3, fifth-generation fat stem cells are prepared from the primary-generation fat stem cells, and cell culture supernatant fluid is obtained; S4, the cell culture supernatant fluid is centrifuged, a supernatant solution is collected, and the supernatant solution is filtered; S5, after ultrafiltration and concentration are carried out, a freeze-drying protective additive is added for freeze drying, and the adipose derived stem cell bioactive peptide freeze-dried powder is obtained. Through a series of operating steps, an active factor mixture in adipose derived stem cell culture supernatant is preserved in the form of freeze-dried powder, obtained factors are completely derived from autografts, use is safe and reliable, and the possibility of allergy and infection of exogenous pathogens in the using process is eliminated.
Owner:安徽瑞杰赛尔生物科技有限公司
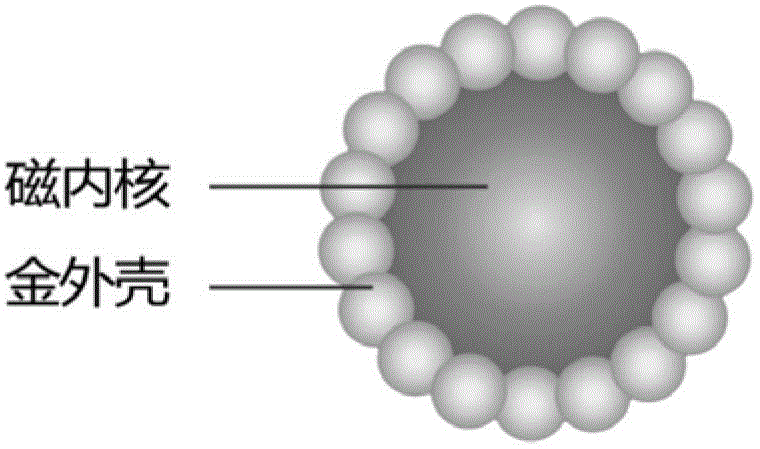
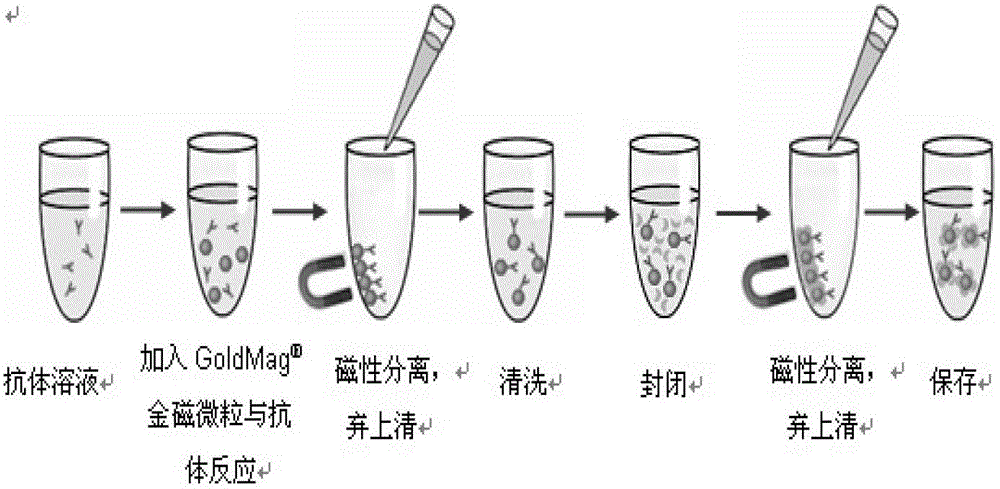
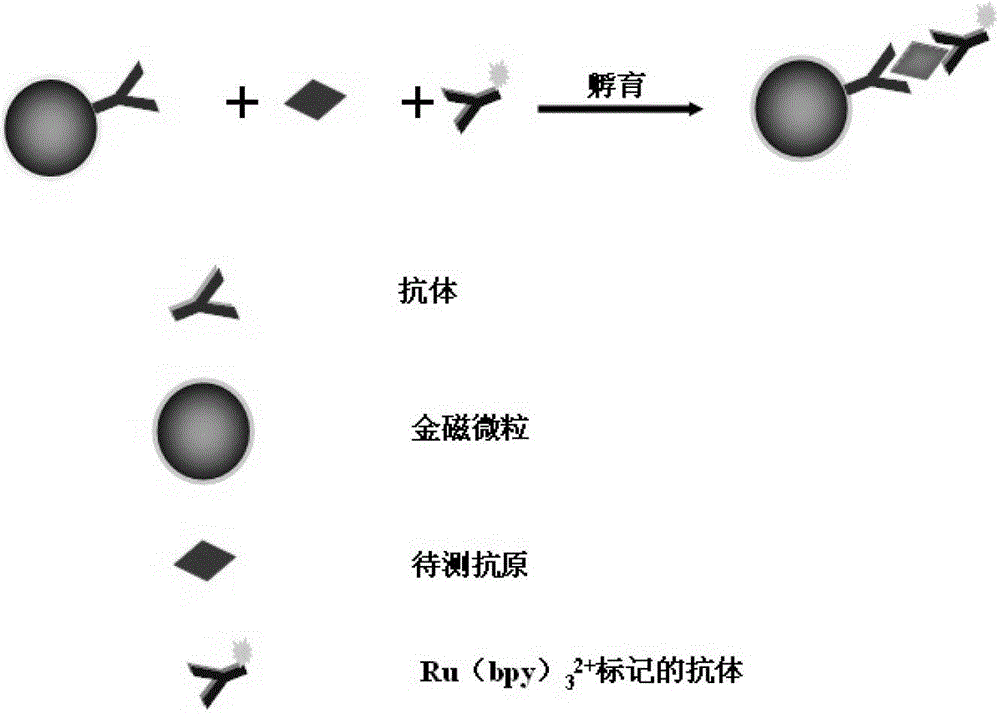
Electrical chemiluminescence immunoassay based on gold magnetic particles
The invention provides an electrical chemiluminescence immunoassay method based on gold magnetic particles, which realizes the quantitative detection on the content of antigen / antibody in human serum, blood plasma, cell culture supernatant or other related biological liquid so as to solve the problems of high cost, complicated steps, poor reaction controllability, no generality and the like in the prior art. The method mainly comprises the steps of 1) preparing immunomagnetic beads by gold magnetic particles; 2) labeling the antigen / antibody by Ru(bpy) <2+>3; 3) establishing a standard curve for quantitatively detecting the antigen / antibody; and 4) detecting a sample to be detected. A novel electrical chemiluminescence immunoassay technology is adopted, and the electrical chemiluminescence immunoassay method based on gold magnetic particles has the advantages that the operation is simple and convenient, the valence of antibody can not be affected in the process that the antibody is labeled by Ru(bpy) <2+>3, the labeling cost is low, and the universality is realized.
Owner:XIAN GOLDMAG NANOBIOTECH
Umbilical cord mesenchymal stem cell preparation and preparation method and application thereof
InactiveCN110269833ARich in effective factorsImprove product qualityCell dissociation methodsCosmetic preparationsActive proteinEngineering
The invention discloses an umbilical cord mesenchymal stem cell preparation and a preparation method and application thereof. Through exploration and verification by a large quantity of experiments, the 3rd-10th-generation umbilical cord mesenchymal stem cells are selected as raw materials, and by a method of dispersing tissue by a mechanical method and then performing treatment with mixed enzymes containing collagenase I, collagenase II and trypsin for a short time for many times, the vitality of the separated umbilical cord mesenchymal stem cells is guaranteed. A culture medium special for the umbilical cord mesenchymal stem cell preparation, containing DMEM / F12 (without phenol red), 10% FBS, 20 kinds of amino acids and 8 kinds of vitamins, is adopted, mixed liquor of repeated cell culture supernatant and cell lysate supernatant is collected, more umbilical cord mesenchymal stem cell active protein and active factors are obtained, the preparation cost is reduced, and industrial requirements for a stem cell product preparation are met. The prepared umbilical cord mesenchymal stem cell preparation can promote proliferation of epidermic cells and skin fibroblast, is safe and is free from toxic and side effects, an allergy does not exist, and when the umbilical cord mesenchymal stem cell preparation is applied to feature-beautifying skin-care products, the effects are notable.
Owner:湖南丰晖生物科技有限公司
Preparation method for fully-bovine-derived broad-spectrum neutralizing antibody against O-type foot-and-mouth disease viruses
ActiveCN108997493AImprove efficiencyIncrease genetic diversityImmunoglobulins against virusesVector-based foreign material introductionCell culture supernatantNeutralizing antibody
The invention relates to a preparation method for a fully-bovine-derived broad-spectrum neutralizing antibody against O-type foot-and-mouth disease viruses, belonging to the technical field of preparation of antibodies. The preparation method comprises the following steps: 1) performing primary, secondary and tertiary immunization on cattle by using O-type foot-and-mouth disease viruses; 2) screening antigen-specific individual B cells of the O-type foot-and-mouth disease viruses; 3) amplifying heavy-chain and light-chain variable genes of a bovine antibody; 4) acquiring the heavy-chain and light-chain constant region sequences of the bovine antibody; 5) preparing a full-length heavy-chain vector of a fully-bovine-derived monoclonal antibody and a full-length light-chain vector of the fully-bovine-derived monoclonal antibody; and 6) applying the full-length heavy-chain vector and the full-length light-chain vector of the fully-bovine-derived monoclonal antibody to cotransfection of cells, taking a cell culture supernatant, and purifying the supernatant so as to obtain the fully-bovine-derived broad-spectrum neutralizing antibody against O-type foot-and-mouth disease viruses. The preparation method of the invention utilizes different foot-and-mouth disease virus strains for infecting cattle and carries out screening to obtain the neutralizing antibody capable of neutralizing O-type foot-and-mouth disease viruses of three pedigrees; and the preparation method can prepare the fully-bovine-derived broad-spectrum neutralizing antibody.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
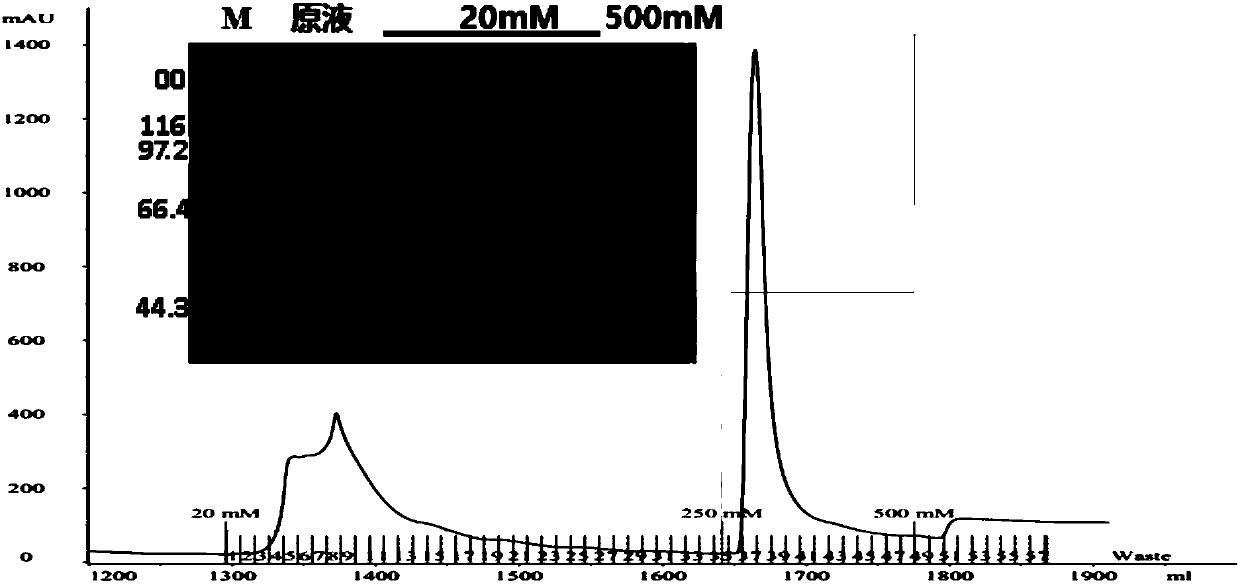
Optimized method for antibody capturing by mixed mode chromatography
ActiveUS9422329B2Serum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCapture antibodyCell culture supernatant
Herein is reported a method for the purification of an antibody directly captured from clarified cell culture supernatants using Streamline CST and / or Capto MMC, wherein especially product related (aggregates and fragments) and process related impurities (host cell protein, media components) could efficiently be removed, resulting in a preparation with a purity comparable to classical protein A affinity chromatography.
Owner:F HOFFMANN LA ROCHE INC
Method for albumin purification
ActiveUS20050214902A1Conveniently performedEasy to disassembleSerum albuminPeptide preparation methodsSensuIon exchange
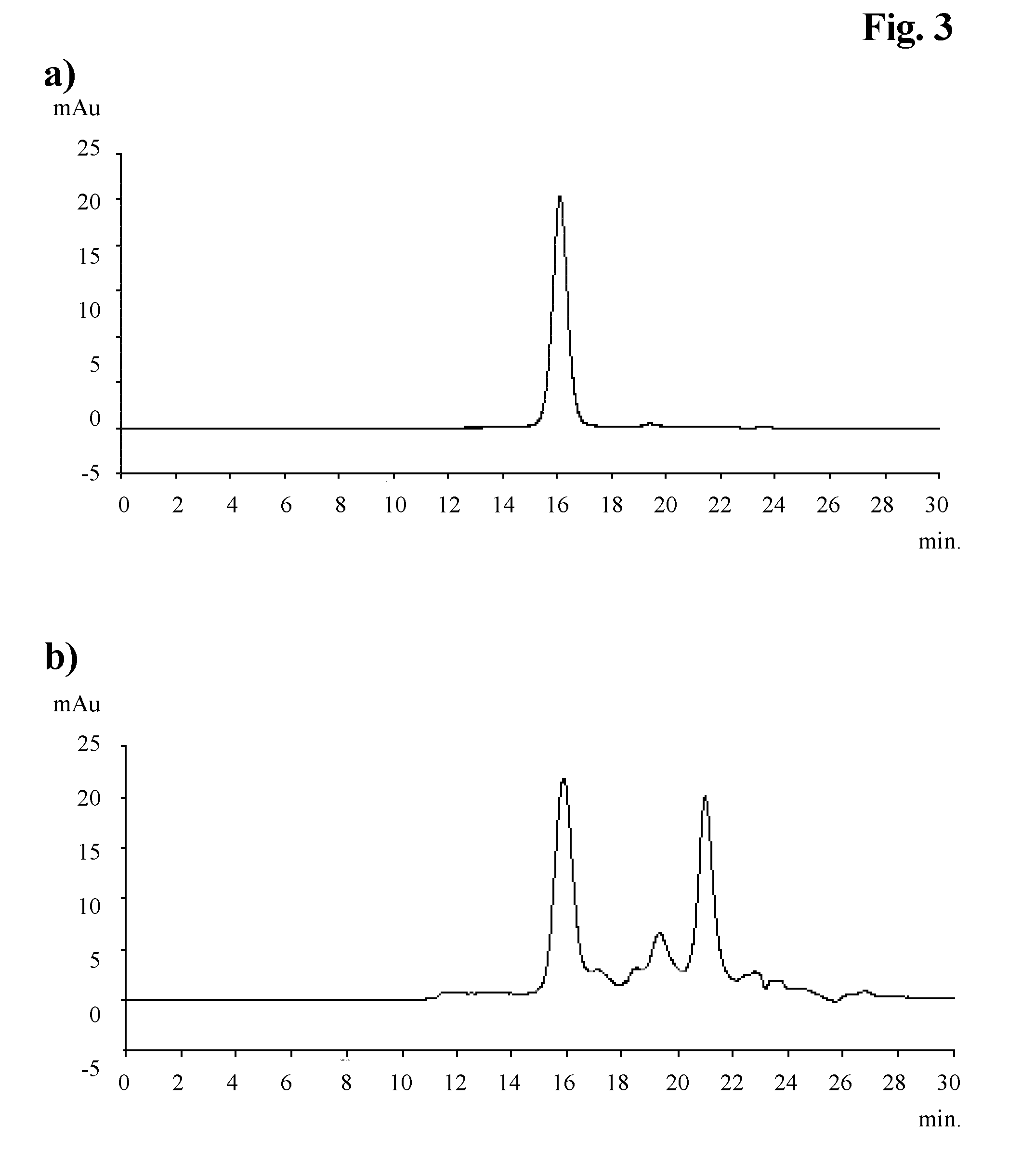
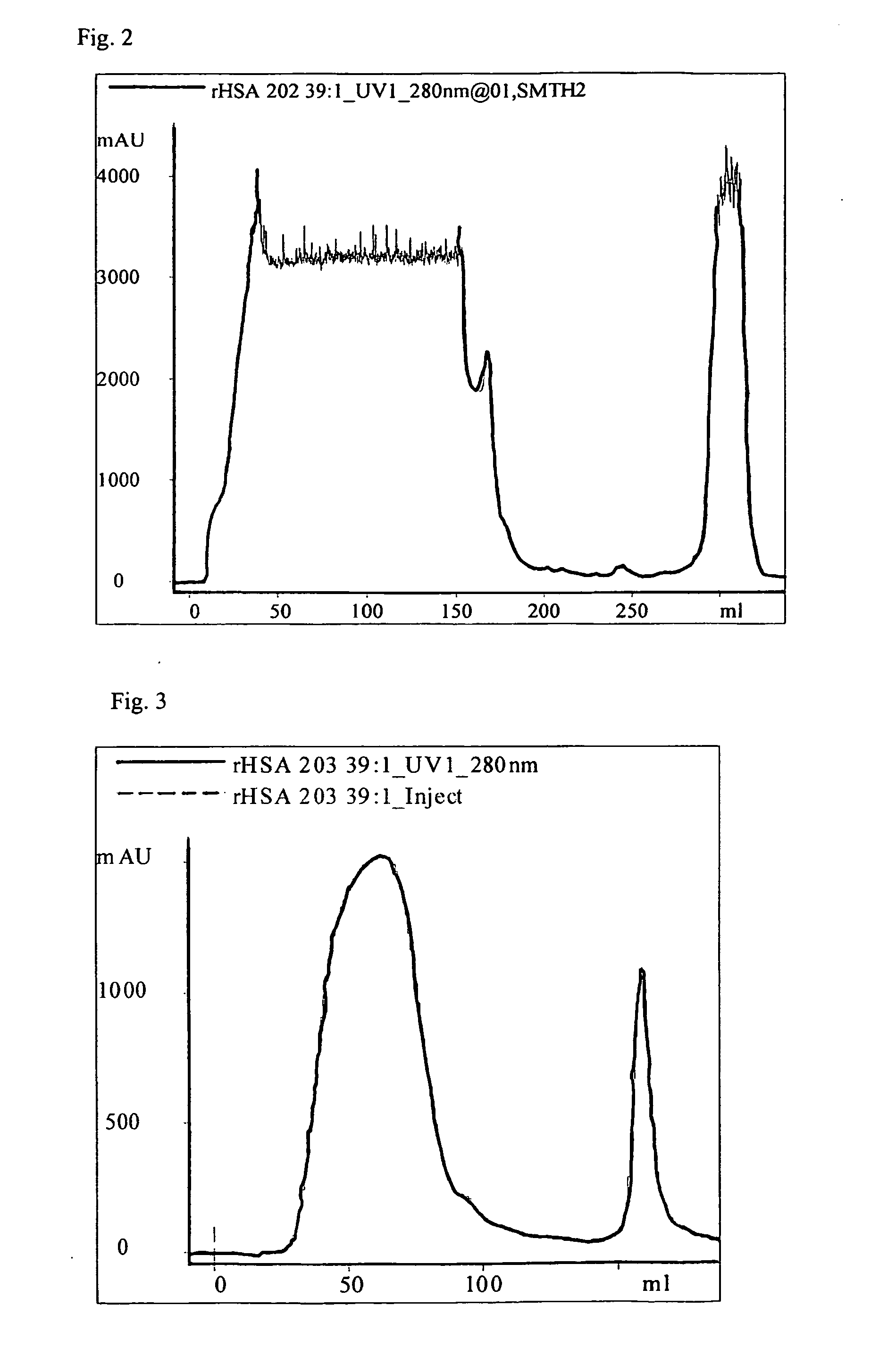
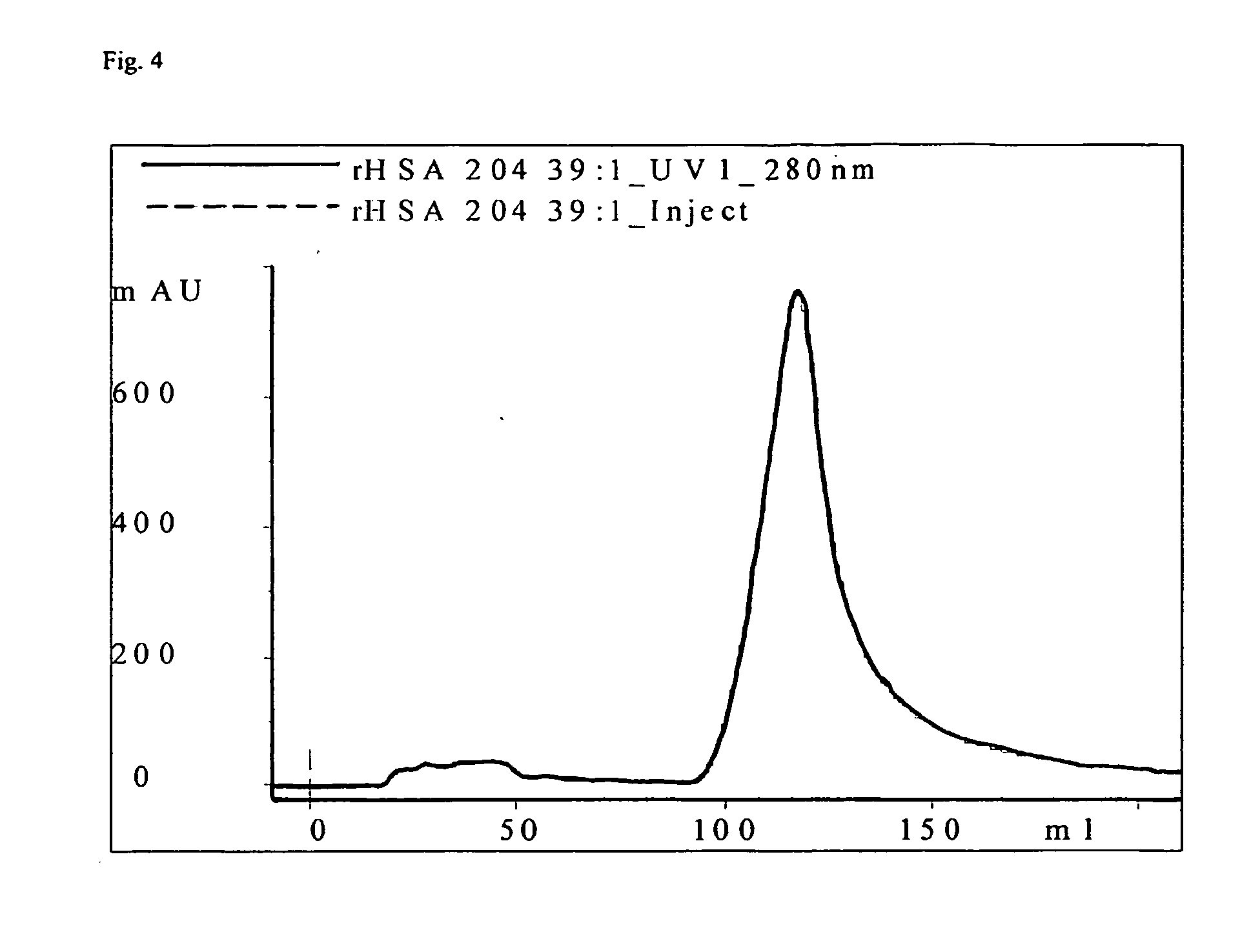
The present invention is a method of purifying recombinant human serum albumin (rHSA) from a solution, which method comprises to subject a cell culture supernatant (CCS) comprising rHSA cation exchange on a bimodal high salt tolerant matrix; hydrophobic interaction chromatography (HIC); anion exchange; and recovering the purified rHSA. The bimodal high salt tolerant cation exchange matrix used enables performing a purification of a cell culture supernatant directly, in the sense that no further dilution thereof is necessary. Due to its high binding capacity, said bimodal cation exchange matrix also allows use of a smaller amount of matrix as compared to a corresponding conventional cation exchanger matrix. Accordingly, the present invention allows substantial savings as regards volumes and consequently operation costs.
Owner:NORTH CHINA PHARMA GROUP CORP +1
Annexin V-FITC exosome capture affinity magnetic beads, preparation method thereof and method for extracting exosome by using affinity magnetic beads
ActiveCN110540961AComplete formHigh purityCell dissociation methodsTumor/cancer cellsExosomeCell culture supernatant
The invention provides Annexin V-FITC exosome capture affinity magnetic beads, a preparation method thereof and an extraction method. The extraction method mainly comprises the following steps: (1) expression and purification of Annexin V, preparation of Annexin V-FITC, and construction of Annexin V-FITC exosome capture affinity magnetic beads; (2) cell culture and supernatant separation and concentration: separating out cell culture supernatant after the cell culture, and carrying out concentration treatment; (3) exosome extraction: co-incubating the cell culture supernatant obtained in the step (2) with the exosome capture affinity magnetic beads to obtain an affinity magnetic bead-exosome compound; and (4) exosome eluting: co-incubating the obtained affinity magnetic bead-exosome compound with an eluent, and collecting the eluent so as to obtain an extracted and separated exosome product. The exosome capture affinity magnetic beads disclosed by the invention can be repeatedly used and are convenient to extract, and the purity of the extracted exosome is high.
Owner:ZHENGZHOU UNIV
Method for screening monoclonal antibodies on different binding sites of antigen
ActiveCN104020289ARapid determinationSimplify the screening stepsBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsBinding siteAntigen binding
The invention discloses a method for screening monoclonal antibodies on different antigen binding sites. The method comprises the following steps: preparing an antigen specific monoclonal antibody by an antigen immune animal with two or more antigenic determinants through a hybridoma monoclonal technology; carrying out in-vitro cell culture on positive clone cell strains to obtain cell culture liquid supernatant containing the antigen specific monoclonal antibody; adding the hybridoma cell culture liquid supernatant into a solution of rubber latex grains which are covalently cross-linked with protein A or G in a two-and-two combined manner; and incubating and adding antigens to obtain a monoclonal antibody set with the increased light absorbance, namely obtain the monoclonal antibody with the different antigen binding sites.
Owner:NINGBO ACCUTECH BIOSCI LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com